Abstract
Purpose
This study investigates clinical practice patterns and parent perception of intervention for children with mild hearing loss (HL).
Method
Ages at and delays between service delivery steps (first diagnostic evaluation, confirmation of HL, hearing aid [HA] fitting, entry into early intervention) were investigated for 113 children with mild HL. Comparisons were made to children with moderate-to-severe HL. Parents of children with mild HL reported reasons for delays and their perceptions of intervention and amplification for their children.
Results
Seventy-four percent of children with mild HL were identified through the newborn hearing screen; 26% were identified later due to passing or not receiving a newborn hearing screen. Ninety-four percent of children with mild HL were fit with HAs, albeit at significantly later ages than children with moderate-to-severe HL. Most parents indicated that their children benefited from HA use, but some parents expressed ambivalence toward the amount of benefit.
Conclusions
Audiologists appear to be moving toward regularly providing amplification for children with mild HL. However, delays in HA fittings indicate that further educating professionals and parents about the benefits of early amplification and intervention is warranted to encourage timely fitting and consistent use of HAs.
A prominent tenet of early intervention is that it takes advantage of early plasticity of learning systems. If children with hearing loss receive intervention during this period of early plasticity, they may experience fewer language and academic delays. This concept motivates the rationale for Early Hearing Detection and Intervention (EHDI) programs: Targeting early diagnosis through newborn hearing screening (NHS), provision of amplification, and early intervention should lead to optimal outcomes for children with hearing loss (HL). For the EHDI process to have its intended effect, it is critical that follow-up services be provided in a timely manner (Vohr, Carty, Moore, Letourneau et al., 1998; Yoshinaga-Itano, Sedey, Coulter, & Mehl, 1998). The Joint Committee on Infant Hearing (JCIH) recommends the “1-3-6” benchmarks: hearing screening by 1 month of age, confirmation of HL by 3 months of age, hearing aid (HA) fitting within 1 month of confirmation of HL, and entry into early intervention by 6 months of age (JCIH, 2007). The JCIH recommendations for early identification and intervention apply to children with any degree of HL, including children with mild HL; however, previous research has indicated that there is ambiguity among clinicians and parents regarding the appropriate recommendations for intervention for children with mild HL (Fitzpatrick et al., 2016; Walker, Holte, et al., 2015). The primary purpose of the current study is to examine identification of HL and timing of follow-up services for children with mild HL, which serves to expand the evidence base on practice patterns and management options for this group of children.
For the purposes of the present study, we are adopting a broad definition of mild HL: 20 to 45 dB HL four-frequency pure-tone average (PTA; 500, 1000, 2000, and 4000 Hz) in the better ear or a bilateral high-frequency HL (three-frequency PTA better than 25 dB HL in the better ear but thresholds greater than 25 dB HL at 3, 4, or 6 kHz). This is in slight contrast to the working definition of permanent mild bilateral HL set forth by the 2005 National Workshop on Mild and Unilateral Hearing Loss: a three-frequency PTA at 500, 1000, and 2000 Hz between 20 and 40 dB or pure-tone thresholds greater than 25 dB HL at two or more frequencies above 2000 Hz in both ears (Yoshinaga-Itano, Johnson, Carpenter, & Brown, 2008). In the current work, the four-frequency PTA inclusion criterion incorporates characteristics of the children's high-frequency hearing, which is likely to result in a slightly higher PTA value than would be found using a three-frequency PTA due to sloping configurations of HL (Pittman & Stelmachowicz, 2003). This broader definition is also consistent with previous research including children with mild HL (Blair, Peterson, & Viehwig, 1985; Davis, Elfenbein, Schum, & Bentler, 1986; Fitzpatrick, Durieux-Smith, & Whittingham, 2010; Fitzpatrick et al., 2016; Fitzpatrick, Whittingham, & Durieux-Smith, 2014; Sininger, Grimes, & Christensen, 2010).
Developmental Outcomes and Service Provision in Children With Mild HL
We have limited knowledge about the impact of appropriate intervention programs on the developmental outcomes of children with mild HL for two reasons. First, there is a paucity of research on outcomes for this group, perhaps due to the belief that mild HL does not place children at risk for delays or difficulties (Wake et al., 2006). The common use of the terms mild, minimal, and slight HL to describe this population contributes to this minimization of the need for intervention (Haggard & Primus, 1999). Second, the limited research that does exist provides mixed evidence regarding the negative impact of mild HL on outcomes. Although some authors have concluded that children with mild HL do not experience adverse effects as the result of HL in this range (e.g., Kiese-Himmel & Ohlwein, 2003; Wake et al., 2006; Wolgemuth, Kamhi, & Lee, 1998), other studies have indicated that these children face challenges at home and school (e.g., Blair et al., 1985; Đoković et al., 2014; Porter, Sladen, Ampah, Rothpletz, & Bess, 2013). For example, Wake et al. (2006) did not find significant differences between children with mild HL and children with normal hearing on measures of reading, language, and behavior. In contrast, Blair et al. (1985) found that school-age children with mild HL performed significantly below their classmates on standardized tests, and the gap increased compared with grademates at higher grade levels. It should be noted, however, that there are limitations in interpreting these prior studies with regard to the importance of early identification and intervention for children with mild HL. Much of the available research was conducted with populations of children who did not have access to NHS, and thus the children were identified later, on average, than might be expected for children with mild HL born in the post-NHS era (Blair et al., 1985; Davis et al., 1986; Đoković et al., 2014; Wake et al., 2006; Wolgemuth et al., 1998).
We currently know little about the timing of service provision (i.e., age at first diagnostic evaluation, confirmation of HL, HA fitting, and entry into early intervention) for children with mild HL in the post-NHS era. Recent evidence from the Outcomes of Children with Hearing Loss (OCHL) study suggest that consistent HA use may reduce the risk of negative outcomes in children with mild HL (Walker, Holte, et al., 2015). Walker, Holte, et al. found a positive association between amount of daily HA use and language outcomes. These results indicate that children with mild HL benefit from amplification. Walker, Holte, et al. examined only a subset of children with mild HL who participated in OCHL and did not investigate when children with mild HL receive audiological services or how many were fit with amplification. Developing a better understanding of service provision for children with mild HL in the post-NHS era is the next step toward ensuring this group is receiving optimal services.
Although little is known about timing of service delivery specifically for contemporary children with mild HL, the OCHL team recently published two studies examining timing of service delivery for children with mild-to-severe HL who referred on the NHS (NHS group; Holte et al., 2012) and children with mild-to-severe HL who either passed the screen or were not screened (later-identified group; Walker et al., 2014). The purpose of these studies was to determine when EHDI follow-up steps were occurring for the respective groups and identify factors that predicted delays in these follow-up steps. Predictors that were examined in both studies included maternal education level (as a proxy measure of socioeconomic status), degree of HL, site of testing, and gender. For the NHS group, the only factor associated with timing of confirmation of HL and HA fitting was maternal education (mothers with higher educational levels had children who completed these two follow-up steps at younger ages), and no factors were associated with age at early intervention. For the later-identified group, the only factor associated with timing of confirmation of HL and HA fitting was degree of HL (children with poorer hearing completed these two follow-up steps at younger ages); again, no factors were associated with age at early intervention. An additional finding was that children in the NHS group generally followed the expected pattern of service delivery benchmarks, with average age at confirmation of HL occurring before HA fitting, followed by entry into early intervention. It is interesting to note that the later-identified group did not follow the same pattern of service delivery as the NHS group. The children who were later identified showed an earlier average age at entry into early intervention compared with confirmation of HL and HA fitting. In other words, some of the children in the later-identified group were receiving early intervention services prior to being tested for HL. For some of the participants, HL diagnosis and HA fittings were significantly delayed even though the children were already participating in early intervention. Although these studies shed some light on service delivery practices for the current generation of children who are hard of hearing, children with mild HL were grouped with children with moderate-to-severe HL. Thus, there is a gap in the literature with reference to clinical practice patterns for children with mild HL even though they make up approximately 30% of children with HL (Fitzpatrick et al., 2010, 2014). The current article addresses this gap by looking at the factors that predict timeliness and delays in follow-up services in a large group of children with mild HL who referred on the NHS or were later identified (either due to passing the NHS or not being screened at birth).
Parental Attitudes Toward Mild HL
Another critical gap in our knowledge base involves parents' attitudes toward amplification and intervention for children with mild HL. Parent perception of the impact of mild HL on their child's outcomes may influence whether they enroll their child in early intervention and support their child's consistent use of amplification. If parents feel ambivalent regarding whether their child with mild HL benefits from HAs or intervention, they may be less inclined to advocate for consistent HA use or services. Indeed, Walker et al. (2013) found that parents of children with milder HL reported less consistent daily HA use and lower consistency of use across varying situations than parents of children with moderate-to-severe HL. It is possible that differences in use time were, in part, attributable to differences in parents' attitudes toward the perceived risk presented by their child's HL or the benefits of HAs for their child. However, Walker et al. did not address parents' attitudes toward HAs or why these parents reported less consistent HA use for their children. One recent study by Fitzpatrick et al. (2016) examined parental attitudes about the impact of HL. The study included 11 children with unilateral HL and nine children with mild HL in the better ear. The authors reported several themes that emerged from open-ended interviews with parents. Despite generally positive attitudes toward the hearing screening process, parents noted uncertainty about whether their child received benefit from HAs. This may indicate an uncertainty about the need for amplification, but given the small number of participants with mild HL, we are limited in the conclusions we can draw for this population. Increased knowledge about parental attitudes will help professionals understand the factors that relate to adoption of amplification and birth-to-3 services (i.e., Part C of the Individuals with Disabilities Education Act). This understanding should allow professionals to provide appropriate informational counseling to parents, thus facilitating parents' understanding of the impact of mild HL and consistent use of amplification.
The current study focuses on children with mild HL in the better ear, thus allowing us to address the gap in knowledge about service delivery for this group of children. We also sought to identify variables that predicted access to timely follow-up and barriers to accessing services. Last, we examined parent perception of benefit from amplification and early intervention for parents of children who received these services. This study addressed five research questions:
At what ages do children with mild HL access follow-up services (HL confirmation, HA fitting, and entry in early intervention)?
How do ages at access to follow-up services and timeliness of service delivery (delays between first evaluation and confirmation of HL, confirmation of HL and HA fitting, and confirmation of HL and entry into early intervention) differ for children with mild HL compared with children with moderate-to-severe HL?
What factors affect timing of HL confirmation, HA fitting, entry into early intervention, and delays in these benchmarks for children with mild HL?
What are the parent-reported reasons for delays in diagnostic evaluations or HA fitting for children with mild HL?
Do parents of children with mild HL report benefits from early intervention and amplification?
Method
Participants
All participants were part of the longitudinal, multisite OCHL study. To qualify for participation in the OCHL study, children were required to be between 6 months and 7 years of age at the time of enrollment in the study and present with a permanent bilateral HL (sensorineural, mixed, or permanent conductive) with a better-ear three- or four-frequency PTA no better than 25 dB HL and no poorer than 75 dB HL. Exceptions were made to include children with mild high-frequency HL (three-frequency PTA < 25 dB HL in the better ear but thresholds > 25 dB HL at 3, 4, or 6 kHz). Children with significant cognitive, visual, or motor impairments were excluded from participation. For all participants, at least one primary caregiver spoke English in the home. Children who used manually coded English or American Sign Language as their primary mode of communication were excluded.
A total of 113 children with mild HL in the better ear (PTA ≤ 45 dB HL for at least two research visits in the longitudinal OCHL study) are the focus of the current article. The participants with mild HL were separated into two groups for analyses: (a) children who were identified with HL at the NHS (NHS group) and (b) children who were later identified (later-identified group). The later-identified group consisted of children who had passed the NHS (n = 24) or did not receive an NHS (n = 5). The range of better-ear PTA (BEPTA) values for the 113 children was 6.25 to 45.00 dB HL (M = 34.21, SD = 8.23). Poorer-ear PTA (PEPTA) values were collected for 111 children and ranged from 7.5 to 120.0 dB HL (M = 41.68, SD = 12.95). Seventy-five of these children (68%) demonstrated a mild HL in both ears (i.e., mild bilateral HL). The remaining 32% of the children demonstrated thresholds greater than 45 dB HL in the poorer ear (i.e., mild asymmetric HL). Thirty-three of the participants (29%) had BEPTA between 41 and 45 dB HL. Eleven children (10%) had a high-frequency HL in the better ear.
An additional 137 children with moderate-to-severe HL in the better ear (PTA > 45 dB HL at two or more visits) are included as a comparison group in the analysis for the second research question. The range of BEPTA for the 137 children was 46.0 to 82.5 dB HL (M = 58.7, SD = 1.6). These children were also separated into two groups on the basis of identification status, with 110 children in the NHS group and 27 children in the later-identified group.
Data Collection
As part of the OCHL study, children and their families participated in an initial baseline visit. The initial visit was followed by visits twice a year for children up to 2 years of age and once a year for children older than 2 years of age. OCHL testing visits consisted of an audiologic assessment; including evaluation of HA function; and administration of age-appropriate speech, language, and cognitive measures (for a full list of specific test measures used in the OCHL study, see Tomblin et al., 2015).
Intake Interview
At the initial test visit, parents completed an intake interview with a research assistant. Intake questions documented the ages at which the child met audiologic and intervention benchmarks, including whether or not the child referred on the NHS as well as ages at (a) suspicion of HL, (b) first diagnostic audiologic evaluation, (c) confirmation of HL, (d) HA fitting, and (e) entry into early intervention. Eighty of the 113 children with mild HL were reported to receive birth-to-3 early intervention and had a documented age at entry into early intervention; thus, only those 80 children were included in statistical analyses related to early intervention. Parents provided explanations for delays between (a) NHS referral and first diagnostic evaluation or, in the case of children who passed or did not receive an NHS, suspicion of HL and first diagnostic evaluation and (b) HL confirmation and HA fitting. Parents also reported whether children were eligible for early intervention services primarily due to HL and, if not, the primary reason for eligibility.
Family Interview
Approximately 6 months after each in-person visit, parents completed a telephone interview with a research assistant concerning demographic information, service delivery, and satisfaction with services. Three versions of the interview were given across the age range of participants: birth to 3 years, preschool, and school age. During each interview, parents were asked whether they felt HAs helped their child hear better. The interview provided closed-set response options of yes, no, don't know, and doesn't wear HAs. Parents were then prompted to give an explanation for their answer. Results were taken from the earliest completed family interview, including 35 responses from the birth-to-3 version, 42 responses from the preschool version, and 36 responses from the school-age version. On the birth-to-3 version of the family interview, the interviewer also asked parents about the effect of early intervention services on their child's development (response options: very negative, some negative, no effect, some positive, very positive, too soon to tell, or don't know). See http://www.ochlstudy.org for full versions of each interview and Harrison et al. (2016) for more details regarding administration of the interview.
Audiologic Assessment
A pediatric audiologist completed all hearing assessments. A test assistant participated in assessments as needed. The audiologist attempted to obtain air-conduction and bone-conduction thresholds at 500, 1000, 2000, and 4000 Hz at a minimum using visual reinforcement audiometry, conditioned play audiometry, or conventional audiometry, depending on the age of the child. All attempts were made to obtain ear-specific thresholds utilizing insert earphones, supra-aural headphones, or the child's own earmolds paired with insert earphones. If a full audiogram could not be completed, the audiologist obtained a copy of the child's most recent unaided audiogram from the child's clinical audiologist. The BEPTA and PEPTA were calculated for subsequent analyses using four-frequency PTA when it was available and three-frequency PTA (500, 1000, and 2000 Hz) when we could not obtain four frequencies.
Data Analyses
Analyses were run separately for the NHS and the later-identified groups. For the first research question, which examined timing of service delivery for children with mild HL, results are reported descriptively. For the second research question, which compared timing of service delivery for children with mild HL and children with moderate-to-severe HL, we conducted t tests at four time points: (a) age at first evaluation, (b) age at confirmation of HL, (c) age at HA fitting, and (d) age at entry into early intervention. For the third research question, which examined predictors of timing of service delivery, the dependent variables of interest were (a) age at HL confirmation, (b) age at HA fitting, (c) age at entry into early intervention, (d) months between age at first evaluation and HL confirmation, (e) months between age at HL confirmation and HA fitting, and (f) months between age at HL confirmation and age at entry into early intervention. The independent variables were BEPTA, PEPTA, site of testing (University of Iowa, Boys Town National Research Hospital, University of North Carolina–Chapel Hill), gender, immediate family history of HL (parents, siblings), and maternal education level. As a result of the large number of maternal education categories in the data set, four arbitrary ordinal levels were introduced in the analysis: high school or less, some college, bachelor's degree, and postgraduate. The data for the third research question were heavily skewed with severe outliers. To keep the outliers from having undue influence on the statistical analysis, we performed nonparametric analyses. For continuous variables BEPTA and PEPTA, we used Spearman's rank correlation, and for categorical variables of mother's education, gender, and family history, we used the Kruskal–Wallis test. Data for the fourth and fifth questions were analyzed descriptively.
Results
At What Ages Do Children With Mild HL Access Follow-Up Services?
The first research question examined the ages at which children with mild HL received EHDI benchmark services as identified by the JCIH (2007). Median and average ages at which children received intervention services and delays between service delivery benchmarks are displayed in Table 1.
Table 1.
Pure-tone averages, ages at service delivery, and delays between service benchmarks for children with mild hearing loss (HL) identified via newborn hearing screening (NHS) or later.
| Variable | NHS group (n = 84) |
Later-identified group (n = 29) |
||||||
|---|---|---|---|---|---|---|---|---|
| M | Median | SD | Range | M | Median | SD | Range | |
| Pure-tone average (dB HL) | ||||||||
| Better ear | 36.2 | 36.9 | 7.4 | 16.3–45.0 | 28.4 | 27.5 | 8.0 | 6.3–43.0 |
| Poorer ear | 43.5 | 43.8 | 12.0 | 22.5–120.0 | 36.4 | 32.5 | 14.4 | 7.5–82.5 |
| Age at service delivery (months) | ||||||||
| Suspected HL | 28.5 | 29.0 | 16.9 | 1.0–60.0 | ||||
| First evaluation | 6.1 | 2.0 | 11.2 | 0.3–60.0 | 33.0 | 35.0 | 16.5 | 1.0–60.0 |
| Confirmation of HL | 10.8 | 3.0 | 16.5 | 0.5–70.0 | 36.8 | 37.0 | 16.3 | 1.0–62.0 |
| HA fitting | 15.3 | 7.0 | 18.2 | 1.5–72.0 | 38.5 | 40.5 | 17.8 | 3.0–68.0 |
| Entry into EI | 7.4 | 6.0 | 6.2 | 1.0–34.0 | 17.5 | 20.0 | 9.7 | 2.0–33.0 |
| Delays between benchmarks (months) | ||||||||
| Suspicion to first evaluation | 4.7 | 1.0 | 6.9 | 0.0–26.0 | ||||
| First evaluation to confirmation | 4.9 | 0.0 | 13.2 | 0.0–70.0 | 3.8 | 0.0 | 10.1 | 0.0–44.0 |
| Confirmation to HA fitting | 5.1 | 2.0 | 10.8 | 0.0–68.0 | 4.0 | 2.0 | 6.7 | 0.0–34.0 |
| EI to confirmation a , b | 6.8 | 2.0 | 10.6 | 1.0–36.0 | 11.9 | 7.0 | 9.6 | 2.0–31.0 |
| Confirmation to EI c | 2.8 | 2.0 | 3.1 | 0.0–17.0 | 3.5 | 1.0 | 5.7 | 0.0–12.0 |
Note. HA = hearing aid; EI = early intervention.
NHS group, n = 10; later-identified group, n = 9.
EI to confirmation reflects the children who were receiving EI services prior to receiving confirmation of HL.
NHS group, n = 57; later-identified group, n = 4.
Of the 113 children with mild HL, 74% (n = 84) were identified through NHS, and 26% (n = 29) were later identified. Among the children in the NHS group, 63% had a mild bilateral HL (mild HL in both ears), and 37% had a mild asymmetric HL (mild HL in the better ear). In the later-identified group, 82% had a mild bilateral HL, and 18% had a mild asymmetric HL.
Twenty-four children in the later-identified group passed the NHS, and the remaining five children were not screened or NHS status could not be verified. On the basis of parent report, five children in the later-identified group referred on the initial NHS but passed a subsequent screen. It is difficult to determine how many children in the later-identified group had a congenital HL as opposed to delayed-onset HL. Only two children in the later-identified group had a confirmed delayed-onset HL, so it is possible that a number of the children passed the NHS but had HL from birth.
HL Confirmation
As shown in Table 1, it is worth noting the skewed distribution of the data for children in the NHS group. The median age at confirmation is much earlier than the average age, suggesting that many of the children had their HL confirmed in a timely fashion. Twenty-seven families of later-identified children reported reasons for suspicion of HL. The most common reasons for suspecting HL were speech delay (n = 12) or a concern that their child was not hearing well (n = 7). Four parents had a concern for HL due to other medical conditions. Two families suspected HL due to a positive family history. Two families did not suspect HL until it was identified through school hearing screenings.
HA Fitting
The vast majority of children with mild HL in both the NHS and later-identified groups were fit with amplification (n = 82/84 [98%] and 24/29 [83%], respectively). Again, it is important to note the skewed distribution of age at HA fitting for the NHS group. Forty-three percent (n = 35/81) of children in the NHS group were fit with HAs within 1 month of confirmation of HL compared with 29% (n = 7/24) of the later-identified children.
Early Intervention
Seventy-two of the 84 children (86%) in the NHS group were eligible for early intervention. Of those 72 children, 69 received early intervention services. Three children were eligible but did not receive early intervention. One parent reported that services were not recommended because the child was “higher functioning.” A second child did not have an Individualized Family Service Plan because the HL was initially unilateral. This child was monitored until hearing progressed to mild HL in the better ear (after 36 months of age). A third child did not receive early intervention but attended Head Start. The remaining 12 children in the NHS group were reportedly ineligible for early intervention. Ten of those children were ineligible because they were older than 36 months of age by the time of diagnosis, one child's HL was “too mild,” and one parent did not indicate why the child was ineligible.
Thirteen of the 29 children in the later-identified group were eligible for early intervention. Fifteen of the 29 children were ineligible because they were older than 36 months when their HL was confirmed. The parent of the remaining child was unsure of eligibility.
Eighty-seven percent (n = 60/69) of children in the NHS group who received early intervention services were eligible for early intervention due to the presence of HL. Parents of eight children reported that HL was the secondary reason for early intervention (primary reasons included prematurity, motor delays, and/or speech delays), and one parent was unsure of the primary reason for eligibility. In contrast, only 38% (n = 5/13) of children who were later identified were enrolled due to the presence of HL. Primary causes for enrollment into early intervention before confirmation of HL for children in the later-identified group were also reported to be speech delays, motor delays, or prematurity. Table 1 displays the average and median lengths of delays between enrollment in early intervention and confirmation of HL.
How Do Ages at Access to Follow-Up Services and Timeliness of Service Deliver Differ for Children With Mild HL Compared With Children With Moderate-to-Severe HL?
Ages at Access to Services
We conducted t tests that explored between-groups differences in ages at four time points in service provision (age at first evaluation, confirmation of HL, HA fitting, and entry into early intervention) as a function of severity of HL and whether children were identified through NHS (see Table 2 and Figure 1). Four of the t tests compared children identified at NHS with mild HL to children with moderate-to-severe HL (NHS-mild and NHS > 45 dB, respectively). The other four t tests compared children identified later with mild HL to children identified later with moderate-to-severe HL (later-mild and later > 45 dB, respectively). For t tests that were significant for Levene's test for homogeneity, we used Welch's adjustment for unequal variances.
Table 2.
Results of t tests comparing ages at service delivery between severity of hearing loss groups (mild and > 45 dB pure-tone average) matched for identification type (newborn hearing screening [NHS] or later).
| Service delivery | df | t | p | Mean difference (in months) |
|---|---|---|---|---|
| First evaluation | ||||
| NHS-mild vs. NHS > 45 | 125.4 | 1.7 | .090 | 2.4 |
| Later-mild vs. later > 45 | 54.0 | 2.2 | .030 | 9.7 |
| Confirmation | ||||
| NHS-mild vs. NHS > 45 | 119.4 | 2.7 | .009 | 5.4 |
| Later-mild vs. later > 45 | 54.0 | 2.8 | .007 | 12.3 |
| HA fitting | ||||
| NHS-mild vs. NHS > 45 | 126.4 | 3.0 | .003 | 6.9 |
| Later-mild vs. later > 45 | 49.0 | 2.5 | .020 | 11.8 |
| Entry into early intervention | ||||
| NHS-mild vs. NHS > 45 | 165.0 | 0.1 | .930 | 0.1 |
| Later-mild vs. later > 45 | 33.0 | 0.2 | .880 | 0.5 |
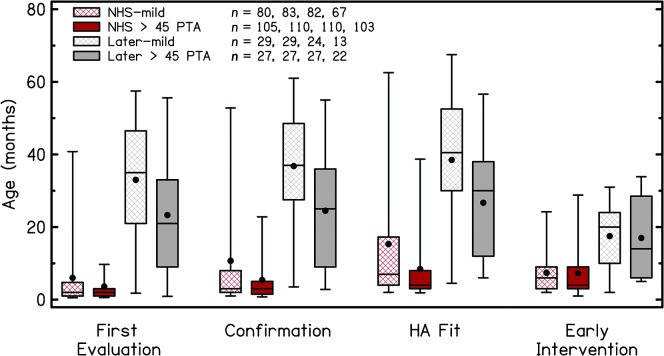
Figure 1.
Comparison of ages at receipt of services for children who referred on the newborn hearing screening (NHS) and those who were later identified with mild hearing loss or better-ear pure-tone averages (PTA) greater than 45 dB. The upper limit of the box represents the 75th percentile, and the lower limit of the box represents the 25th percentile. Lower and upper bars represent the fifth and 95th percentiles, respectively. The upper bar for early intervention for the “later > 45 PTA” group represents the 90th percentile due to low participant numbers in that subgroup. Within the boxes, filled circles represent the means and solid lines represent the median values. HA = hearing aid.
For the NHS groups, confirmation of HL and HA fitting occurred at significantly later ages for children with mild HL compared with children with moderate-to-severe HL. There were no significant differences in age at first evaluation or entry into early intervention. For the later-identified groups, first evaluation, confirmation, and HA fitting occurred at significantly later ages for children with mild HL compared with children with moderate-to-severe HL. There were no significant differences in age at entry into early intervention.
Timeliness of Service Delivery
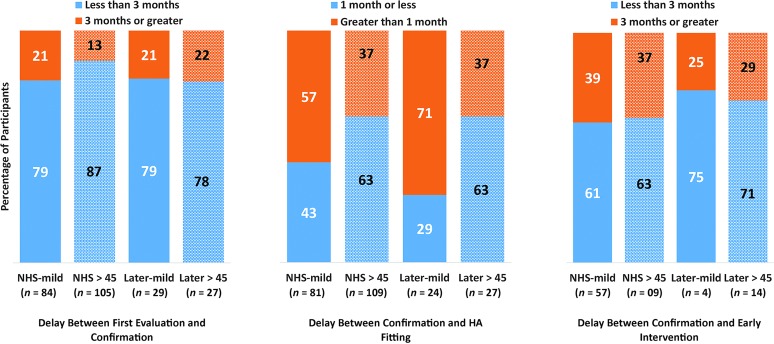
We examined timeliness of service delivery by calculating the delay between follow-up steps: (a) first evaluation and confirmation of HL, (b) confirmation of HL and HA fitting, and (c) confirmation of HL and entry into early intervention (including only children who received intervention after confirmation of HL). These calculations were made for the same four groups as in the previous analysis: NHS-mild, later-mild, NHS > 45 dB, and later > 45 dB. In examining access to service delivery, we compared the proportion of children who had acceptable periods of time between service provision milestones to children who had lengthier delays on the basis of JCIH goals (JCIH, 2007). Children who had delays of 3 months or greater between the first evaluation and confirmation, greater than 1 month between confirmation and HA fitting, and 3 months or greater between confirmation and entry into early intervention were considered to have longer-than-acceptable delays between service provision benchmarks. Figure 2 shows these proportions as a function of severity of HL and identification group. The majority of children, regardless of degree of HL or identification group, experienced timely access to confirmation of HL after their first diagnostic evaluation. However, a slightly larger percentage of the NHS group with moderate-to-severe HL met this service delivery benchmark compared with children with moderate-to-severe HL in the later-identified group or children with mild HL. The highest percentage of delays occurred between confirmation of HL and HA fitting. This was especially evident in the two groups of children with mild HL. All four groups were fairly equivalent in timeliness of early intervention services. The later-identified groups had the largest proportion of children receiving early intervention within the JCIH goals; however, these results should be interpreted with caution because there were few children in those subgroups (as a result of low numbers of children in the later-identified groups receiving early intervention services after confirmation of HL).
Figure 2.
Percentage of children having acceptable delays between service delivery milestones (blue bars) or longer delays (orange bars) on the basis of Joint Committee on Infant Hearing recommendations. Children with mild hearing loss are indicated with solid bars, and children with better-ear pure-tone averages greater than 45 dB HL are indicated with patterned bars. In the second set of bars, “greater than 1 month” equals 6 weeks or greater. NHS = newborn hearing screening.
What Factors Affect Timing of HL Confirmation, HA Fitting, Entry Into Early Intervention, and Delays Between These Benchmarks for Children With Mild HL?
We used nonparametric statistical methods (Spearman's rank correlation and Kruskal–Wallis test) to evaluate which independent variables were related to the dependent variables of interest: (a) age at HL confirmation, (b) age at HA fitting, (c) age at entry into early intervention, (d) months between age at first evaluation and HL confirmation, and (e) months between age at HL confirmation and HA fitting in the NHS- and later-identified groups with mild HL. Although we also initially intended to include months between age at HL confirmation and age at entry into early intervention as a dependent variable, there were too few participants enrolled in intervention after HL confirmation in the later-identified group to complete the analysis for delay between HL confirmation and age at entry into early intervention. The independent variables were BEPTA, PEPTA, maternal education level, site of testing, gender, and immediate family history of HL. For children in the NHS group, there were no significant predictors for any of the five dependent variables. For the later-identified group, only one dependent variable had any significant predictors: PEPTA was a significant predictor for the delay between age at confirmation of HL and HA fitting (r = −.45, p = .03). Children with higher (worse) PEPTA values were fit with HAs at younger ages.
What are Parent-Reported Reasons for Delays in Diagnostic Evaluations or HA Fitting for Children With Mild HL?
The following section describes the parent-reported reasons for delays in follow-up services. Follow-up services were considered delayed on the basis of the JCIH 1-3-6 goals (JCIH, 2007). Parents identified one primary reason for delays from a closed set of responses, including the option for an “other” open-ended response (for response options, see Holte et al., 2012).
Forty-four percent (37/84) of the children in the NHS group experienced delays of 3 months or greater from NHS referral to the first diagnostic test. Of those families experiencing delays, 24 parents reported reasons for delays. The most reported reason that the first diagnostic evaluation was delayed was due to multiple rescreenings (n = 12). Parents of 11 children reported the number of follow-up screenings after NHS. The reports ranged from one to seven, with an average of 2.6 rescreens. Less common causes included difficulty getting an appointment quickly (n = 2), delay due to treatment of middle ear problems (n = 2), delay because family was assured that the failed screening was likely caused by something other than HL (n = 1), family choosing to wait before scheduling a diagnostic test (n = 1), and family not being told to get a diagnostic auditory brainstem response (ABR) test (n = 1). In addition, five parents responded in the “other” category and reported reasons including medical delays (prematurity, child in neonatal intensive care unit, or other medical issues that prevented follow-up) and being too young for a sedated ABR as causes for delay.
Another goal of JCIH (2007) is that HA fitting should take place within 1 month of confirmation of HL. Of the 106 children (NHS and later-identified groups combined) who were fit with HAs, 59% (63/106) were reported by parents to experience a delay greater than 1 month from HL confirmation to HA fitting. Forty-six parents reported reasons for delay. The most prevalent responses for delays to HA fitting were difficulty obtaining clinic appointment for HA fitting (n = 8), HAs were not initially recommended (n = 8), and family decided not to proceed with HA fitting right away (n = 7). Fewer parents indicated delay in obtaining approval for insurance or other third-party funding for HAs (n = 4), other medical conditions that prevented follow-up for HA fitting (n = 2), delay in obtaining an appointment for medical clearance for HAs (n = 2), or recurrent ear infections or other middle ear problems (n = 1). Fourteen parents responded in the “other” category, including waiting until behavioral thresholds were obtained, coordinating appointments, setting an appointment for the HA fitting more than 1 month later, and medical intervention (e.g., pressure-equalization tubes).
In cases where HAs were not recommended, one child had a fluctuating loss due to enlarged vestibular aqueduct syndrome that resulted in multiple follow-up diagnostic tests before fitting amplification. In other cases where HAs were not recommended, several parents reported that professionals recommended waiting until more confirmatory tests had been performed (n = 2) or until the children were at least 6 months of age before fitting (n = 2). Other parents reported encountering professionals who gave conflicting recommendations on the necessity of amplification or who would not fit HAs (n = 3).
Do Parents of Children With Mild HL Perceive a Benefit From Early Intervention and Amplification?
Thirty-two of the 35 parents who were administered the birth-to-3 family interview provided responses regarding the effect of early intervention on their child. Twenty-nine responses related to home-based intervention and three related to locations other than home. Most parents (88%, n = 28/32) felt that early intervention had a very positive (n = 17) or some positive (n = 11) effect on their child's development. The remaining parents (13%, n = 4/32) reported that services had no effect (n = 3) or that it was too soon to tell (n = 1).
A total of 101 parents responded to the question “Do you think hearing aids help your child hear better?” The majority of parents indicated that they felt that HAs helped their child hear better (93%, n = 94/101). The remaining seven parents reported do not know. Of the 94 families that reported yes, 92 provided an explanation. Of those families, 86% (n = 79/82) provided explanations that were consistent with HAs benefiting their child (e.g., “Oh definitely! We can't stand it if he doesn't have them in. Conversations are confusing without them.”). Thirteen explanations (14%) indicated that the parent was unsure of the amount of benefit or listed a positive comment with a caveat that the child can hear without the HAs or that the HAs are not worn consistently (“Yes [the HAs provide benefit], but not a significant amount. She doesn't want to wear them at school and she doesn't wear them at home much.”). The following explanations of the seven families reporting do not know elicited similar information regarding parents' doubts about the benefit of HAs due to the child's young age or the child's alertness or responsiveness without the HAs on:
I don't know, we still have a hard time noticing she has a hearing loss.
He does respond to quieter sounds with the aids on but he is alert with them off too. We need to get better at putting them on.
I know it is necessary, but he is so young and he takes them off a lot. I think it helps and see the value in it.
I don't know. I can't tell a big difference.
I cannot say yes or no. It is hard to tell. She responds well without her aids.
He does not always wear his aids. I doubt sometimes that he hears better with them on. He doesn't like his aids but I make him wear them.
I can't imagine that they do. He is so young now to know.
Discussion
Evidence-based protocols and guidelines recommend the provision of intervention and amplification for children with mild HL (American Academy of Audiology [AAA], 2013; JCIH, 2007); however, audiologists have demonstrated uncertainty about appropriate clinical recommendations for this population (Fitzpatrick et al., 2010, 2014). The purpose of the present study was to address these issues of clinical equipoise by documenting current practice patterns for children with mild HL. We also explored factors that may influence timing of service delivery and examined parents' opinions about intervention and amplification for these children.
NHS and Mild HL
Prior to the advent of NHS, children with mild HL demonstrated an average age at identification of 4 to 5 years (Coplan, 1987; Fitzpatrick et al., 2010). Approximately 98% of eligible infants born in the United States are now being screened for HL at birth (U.S. Centers for Disease Control, 2011), which has led to dramatic reductions over the past two decades in the age at identification of HL for children who are hard of hearing (Harrison & Roush, 1996; Holte et al., 2012). Although the vast majority of infants are being screened for HL, an often stated concern is that children with mild HL are more likely to be underidentified or later identified compared with children with moderate-to-profound HL (Johnson et al., 2005; Ross et al., 2008). Late identification leads to later intervention (or no intervention at all), which puts children with mild HL at risk for academic, language, and social delays (Bess, Dodd-Murphy, & Parker, 1998; Blair et al., 1985; Most, 2004; Walker, Holte, et al., 2015; Yoshinaga-Itano et al., 2008).
Of the 113 children in the current study, nearly three quarters (74%) were identified through NHS. On the surface, these data may assuage concerns that a large proportion of children with mild HL are being missed on the NHS because a considerably larger percentage of children were identified through NHS rather than not. These results should be interpreted with some caution, however. Children in the OCHL study had a confirmed HL as the primary criteria for participation, and most of the participants were recruited through clinical settings (for a description of recruitment methods, see Tomblin, Walker et al., 2015). Thus, the participants represent a clinically based sample rather than a population-based sample and are biased toward inclusion of children who were identified by the NHS. The current study also used a broad definition of mild HL, including children with PTAs less than or equal to 45 dB HL (as opposed to 40 dB HL). Children with asymmetric HL (i.e., mild HL in the better ear and moderate HL or worse in the poorer ear) were also included in this investigation. As a result of the higher thresholds in the poorer ear, it would be unlikely that they would pass the NHS if they had HL at birth. The current results would appear to support this hypothesis in that a higher percentage of the NHS group had an asymmetric mild HL compared with the later-identified group (37% and 18%, respectively). Children with symmetric mild bilateral HL may pass the NHS despite having a congenital HL because the screening pass/fail criteria are often designed to identify moderate HL or worse (Dedhia, Kitsko, Sabo, & Chi, 2013). Although all of the children in the later-identified group with verified NHS results passed the screening, it is difficult to determine how many of these children had a congenital HL as opposed to delayed-onset HL. We could confirm only that two children in the later-identified group had a delayed onset through retrospective data, so it is possible that a number of the children had congenital HL that went undetected.
Results from the current study and others (Dedhia et al., 2013; Johnson et al., 2005; Lü et al., 2011; Weichbold, Nekahm-Heis, & Welzl-Mueller, 2006) promote the need for continued surveillance of hearing status during childhood. Johnson et al. (2005) recommended that children who fail the initial otoacoustic emission screen but pass the subsequent automated ABR should continue to be monitored as part of EHDI high-risk registries if the goal of the NHS program is to identify children with mild HL or worse. Another recommendation is extending screening for permanent HL into early childhood by conducting screenings during well child checks or early childhood programs. Early childhood programs such as Head Start have implemented screening protocols to identify possible HL. These programs have successfully demonstrated that the screening protocols are feasible (Eiserman et al., 2008). A counterargument to continued monitoring in early childhood is the cost of such programs and the risk of referring children with normal hearing for additional testing. Best practices for conducting expanded surveillance programs effectively are still undetermined, and further research is needed regarding the value of implementing steps even after NHS to ensure the detection of mild HL as part of a comprehensive EHDI program.
Timing of Follow-Up Services
Confirmation of HL
In addition to examining how many children with mild HL are identified via NHS, another goal of this article was to examine the timing of follow-up steps (confirmation, HA fitting, early intervention) for children with mild HL. Follow-up diagnostic tests and confirmation of HL guide management decisions by providing knowledge about the degree, configuration, and type of HL and whether it is permanent or transient. McKay, Gravel, and Tharpe (2008) pointed out the difficulty in confirming HL in the mild range due to a lack of sensitivity in current electrophysiologic and behavioral tests. Thus, we may predict that confirmation of HL would be delayed in children with mild HL. Because NHS programs have been initiated, studies have indicated that the median age of confirmation for children with mild HL was 9.6 months (Fitzpatrick et al., 2014). In the current study, the median age for children identified through NHS was 3 months, which matches the JCIH recommendation of confirmation of HL by 3 months of age. For children who were later identified, the median age at confirmation was 37 months. Once HL was suspected by the parents, however, there was an average delay between suspicion and confirmation of HL of 4.7 months (median = 1). Thus, when mild HL is detected by NHS or suspected by caregivers, confirmation appears typically to be occurring in a timely fashion for many children. These results may alleviate concerns about the difficulty in confirming mild HL using current objective and behavioral measures (McKay et al., 2008; White & Muñoz, 2008).
Fitting of HAs
Although these results would suggest that many children with mild HL are meeting JCIH benchmarks for confirmation of HL, perhaps the larger concern is the appropriate path to take regarding amplification. In a report on the implementation of universal NHS in New York state, Dalzell et al. (2000) indicated that children with mild HL were delayed in HA receipt compared with children with more severe HL and were less likely to receive HAs. Fitzpatrick et al. (2010) reported that 60% of children with mild HL in the study received an initial recommendation for HAs. A follow-up study by Fitzpatrick et al. (2014) found that 87% of children with mild HL eventually received a recommendation for amplification. The results from the current study suggest that there may have been less ambiguity for the current group of children with regards to clinical recommendations for HAs compared with the earlier studies mentioned previously. The majority of children were fit with HAs (98% in the NHS group and 83% in the later-identified group). Thus, these findings may be an indication of greater recognition of the difficulties children with mild HL face and increased acceptance of clinical intervention via amplification for this population. It should also be noted, however, that we did not address configuration of HL (specifically flat, mild HL compared with high-frequency HL) in the current article as a result of a limited number of participants with high-frequency HL. Further research on evidence-based guidelines for different configurations, particularly high-frequency bilateral HL, is warranted.
Another way to examine uncertainty regarding clinical recommendations is to examine the length of delay between age at confirmation of HL and HA fitting. JCIH recommends that HA fittings occur within 1 month of confirmation of HL. Durieux-Smith, Fitzpatrick, and Whittingham (2008) found that children with mild HL who were identified early (diagnosis by 6 months of age) were substantially delayed in HA receipt compared with children with moderate HL or worse, with an average delay between confirmation of HL and HA fitting of 22.8 months for children with mild HL (compared with 3.4 months for children with moderate-to-profound HL). Although the differences were not as large in the current study, our results mirror the finding that delays between these two benchmarks are longer for children with mild HL than for children with moderate-to-severe HL, with delays of 5.1 and 2.8 months, respectively, for children identified via NHS and 4.0 and 2.5 months, respectively, for children identified later. Furthermore, the current data indicate that there was a lower percentage of children with mild HL who were fit with HAs within the JCIH guidelines compared with children with moderate-to-severe HL. Sixty-four percent of the mild HL cohort (NHS and later-identified groups combined) experienced a delay of greater than 1 month in the HA fitting process compared with 29% of the children with moderate-to-severe HL. Thus, our evidence suggests that although HAs are being recommended more often than in the past, the HA fitting process continues to be more prolonged in the mild HL group. These results differ from results for the full cohort of children in the OCHL study identified via NHS, in which Holte et al. (2012) did not find that degree of HL influenced timing of confirmation of HL or HA fitting. The discrepancy may be a result of Holte et al. treating degree of HL as a continuous variable, whereas we examined the impact of severity of HL by examining group differences between children with mild losses and children with moderate-to-severe losses.
Audiologists and families may be uncertain whether to proceed with HAs immediately after confirmation of mild HL. Early in development, it is difficult to determine which children will follow a typical course of development without intervention and which will require hearing technology to mitigate the negative impact of reduced auditory access. The AAA pediatric amplification guidelines recommend HA provision to children with mild HL (AAA, 2013), but there is little else in the way of evidence-based guidelines for when to fit HAs. Because of the lack of guidance, Bagatto and Tharpe (2014) described a case-by-case method for considering amplification for children with mild HL. Their clinical decision guide includes five factors for audiologists to consider: (a) configuration and degree of HL, (b) ear canal and earmold acoustics, (c) HA gain and noise floor, (d) child factors (e.g., additional disabilities, ambulatory status, and listening environment), and (e) family factors (e.g., readiness and motivation to pursue HAs). Bagatto and Tharpe also recommend that the decision to pursue amplification should not be made in isolation. Rather, family-centered counseling should also take place in conjunction with comprehensive early intervention, as needed.
Early Intervention
Early intervention services support later academic achievement and prevent speech and language delays regardless of degree of HL (Moeller, 2000; Yoshinaga-Itano et al., 2008). Early intervention has not been fully explored in prior research on children with mild HL. One strength of the current project was that we were able to examine enrollment in early intervention. The majority of children in the NHS group received early intervention services (82%), with 64% starting early intervention by 6 months of age. The delays between confirmation and entry into early intervention were similar for the children with mild HL and the children with more severe HL. Thus, for the OCHL cohort, early intervention for children with mild HL appears to be meeting the JCIH recommendations in the majority of cases in which HL is identified at birth. As a caveat, however, the OCHL study did not recruit children from across the United States (recruitment was focused on the Midwest and Mid-Atlantic regions), and it is important to note that this picture may differ depending on individual states. States vary in eligibility criteria for early intervention: Some states require only that a child have a diagnosed condition that places them at risk for delays, whereas other states require that the child demonstrate a documented delay in speech, language, or cognition. In states where a documented delay is required to receive services, some children with mild HL may not qualify if they do not show delays relative to normative samples on standardized tests. Previous results indicate that comparisons to normative data for standardized tests may underestimate how many children with mild HL are failing to reach their full potential, as developmental delays are more readily apparent when children are compared with peers matched for socioeconomic background (Blair et al., 1985; Walker, Holte, et al., 2015). Early intervention programs that target children who are at risk for delays due to HL offer the best opportunities for allowing children to reach their full potential and reducing the likelihood of delays becoming apparent at later ages.
Although the majority of children identified through NHS received early intervention services, this was not true for children in the later-identified group, of whom only 45% received early intervention services. The primary reason children in this group did not receive services is that their HL was not confirmed until after 36 months, making them ineligible for birth-to-3 services that include valuable opportunities for family involvement. One critical observation for the later-identified group is that nine out of the 13 children in early intervention started those services before their first hearing evaluation (as shown in Table 1). In some cases, the delay between enrollment in early intervention and the first hearing evaluation was quite long, with 29 months being the longest delay. Although we cannot confirm that these children had HL when they started early intervention, the delays between entry into early intervention and first diagnostic evaluation and confirmation of HL are quite disconcerting. For those children who had permanent HL when they started early intervention, it is likely that the intervention would have been more effective if the HL had been addressed. These results parallel the findings in Walker et al. (2014) and again highlight the fact that hearing screenings are not a required component of Part C/birth-to-3 services in the federal Individuals with Disabilities Education Act. JCIH (2007) recommends that medical care providers refer patients for audiological testing in any cases of children who display speech and language delays regardless of whether they passed the NHS. This recommendation may be costly and difficult to implement given the shortage of pediatric audiologists in the United States and other countries (White, Forsman, Eichwald, & Munoz, 2010). At the same, there are also consequences, both financial and otherwise, to providing intervention services to children with HL that are limited in their effectiveness as a result of the services not addressing the unique developmental risks faced by children with HL. At a minimum, hearing screenings should automatically be included during the evaluation process for an Individualized Family Service Plan to rule out HL as a contributing factor to developmental delay. This step will facilitate appropriate delivery of intervention to children receiving birth-to-3 services.
Factors That Influence Timeliness of Service Delivery
Previous studies have found that both family- and child-specific factors influence the timeliness of service delivery for children with HL. When considering maternal education, degree of HL, gender, and test site for children in the OCHL study identified at NHS, maternal education level was the only variable that predicted unique variance in age at first diagnostic evaluation, confirmation of HL, and HA fitting in children with congenital mild-to-severe HL (Holte et al., 2012). For later-identified children with mild-to-severe HL in the OCHL study , Walker et al. (2014) found that degree of HL in the better ear predicted age at service delivery (first evaluation, confirmation, HA fitting). In another study, Fitzpatrick et al. (2014) also found that BEPTA influenced HA receipt for children with mild HL who were earlier and later identified. Taken together, these previous studies suggest that when children who are hard of hearing (mild-to-severe HL) refer on the NHS, socioeconomic status drives timing of follow-up services, whereas degree of HL is important for children who are hard of hearing who are identified later (potentially due to late-onset or progressive HL or passing the NHS due to insensitive tests). It is surprising that none of the factors that have been shown to influence service delivery for children who are hard of hearing were significant in the current data set (i.e., BEPTA or maternal education). Examining only children with mild HL removed variability in PTA that we might expect otherwise in a larger cohort of children who are hard of hearing. The sole exception was that hearing in the poorer ear predicted age at HA fitting for later-identified children. The current study did not find an association between timing of services and maternal education, as found in previous research. This result leads us to believe that all families of children with mild HL—regardless of socioeconomic status—may be at risk for delays in service delivery. It is unclear what other factors are contributing to timing of service delivery for children with mild HL, although the parent-reported reasons for delays between service delivery milestones for NHS- and later-identified groups of children with mild HL were similar to those reported from the larger OCHL cohorts in Holte et al. (2012) and Walker et al. (2014).
Of particular concern, and specific to the population of children with mild HL, is the report of conflicting professional recommendations on the necessity of amplification and encountering professionals who would not fit HAs on a child with mild HL. These reports emphasize the need for consistent guidelines regarding fitting amplification and increased awareness regarding the positive outcomes associated with early and consistent use of amplification. An additional concern is the parental report of multiple rescreenings as a cause of delay between the first diagnostic evaluation and confirmation of HL. These data must be interpreted with some caution because we had no means of verifying parents' report of the number of times rescreens occurred. Nevertheless, multiple rescreenings can have particular clinical significance for children with mild HL. Besides postponing a timely diagnosis and confirmation of HL, if children are repeatedly receiving otoacoustic emission or automated ABR screens before referral for a diagnostic ABR, it is likely that there could be a sporadic “pass” for a child with mild HL, which is then interpreted as the child having hearing within normal limits. Coordinators of state EHDI programs are strongly advised to follow the JCIH (2007) recommendations for minimizing the number of rescreens due to the statistical probability of obtaining a pass through chance alone.
In addition to delay to confirmation of HL caused by rescreens, parents anecdotally reported delays due to difficulty obtaining an appointment with the professionals or due to the professionals postponing a diagnostic test until a child was older. Professionals have a primary role to provide appropriate, timely services to families, and it is worth examining why priority appointments were not allotted to children immediately following NHS referral or confirmation of HL for HA fitting. Further examination of professional and parental attitudes surrounding intervention may be important in further explaining differences in timing of service delivery.
Parental Attitudes Toward Intervention and Amplification
It is important to examine parental attitudes toward intervention and amplification for children with mild HL because this information can inform our approach to counseling families. Appropriate counseling is key to understanding the implications of mild HL, particularly in the case of young children who are in the process of acquiring speech and language skills. Parents must make decisions about participating in intervention and pursuing amplification, usually without experiencing the challenges of mild HL themselves. On the basis of prior research (Haggard & Primus, 1999), we may predict that parents and caregivers will underestimate the effects of mild HL simply because the term mild denotes reduced magnitude of disability and therefore less need for intervention. In the present study, however, 96% of children with mild HL participated in early intervention when they were eligible for services due to HL. In addition, the majority of parents expressed satisfaction with early intervention services for their children with mild HL. Only 13% of parents indicated that they saw no effect of early intervention services or that it was too early to tell whether there was an impact. Fitzpatrick et al. (2016) did not directly query parents of children with mild HL about their attitudes toward early intervention, but they found that parents did express a need for continual support and ongoing monitoring of speech and language development. Thus, these results appear to support the JCIH (2007) recommendations that all children with confirmed permanent HL should receive early intervention services by 6 months of age, even in the case of mild HL. Indeed, there seems to be little argument for delaying early intervention because these family-centered services should provide the best opportunity for educating families on the risk mild HL poses as well as speech and language development.
Another reason to consider parent attitudes toward amplification is that they may influence how much children wear HAs. Recent studies, including several from the OCHL study, have shown that children with mild HL tend to wear HAs less often than children with more severe HL (Gustafson, Davis, Hornsby, & Bess, 2015; Walker, McCreery, et al., 2015; Walker et al., 2013). Few studies have investigated why there is less consistent HA use in this population. One plausible explanation for reduced HA use is that parents may feel unsure about the benefit that HAs provide. The current data reflect some of the ambiguity parents may feel about amplification. When asked a yes/no question regarding whether HAs helped their child hear better, the majority of parents (93%) replied in the affirmative. Parental explanations about benefit demonstrate that there is a gray area, however, in that some of the open-ended explanations came with a caveat. Some parents commented that they had difficulty noticing the HL or seeing a difference in behavior with or without HAs. Some parents also acknowledged that they needed to be better about having their child wear HAs or remarked that it was difficult to note any benefit from HAs due to the young age of their child. Therefore, although parents may report that HAs help their child hear better, they may also deemphasize consistent HA use because they are not seeing an immediate impact on behavior or language development. As a result, children may wear their HAs only in specific situations, such as at preschool, but not at home or in public, thus reducing opportunities for language learning. Less consistent HA use may not appear to be a cause for alarm because children with mild HL have significant residual hearing and some access to the speech spectrum even when unaided. On the other hand, evidence suggests that children with mild HL show as much benefit from HAs as children with moderate-to-severe HL on measures of articulation and language (Tomblin, Oleson, Ambrose, Walker, & Moeller, 2014). Further research has shown that daily HA use influences language outcomes in early school-age children with mild HL, particularly for morphosyntactic acquisition (Walker, Holte, et al., 2015). Access to high-frequency information is especially important because many English morphological markers consist of high-frequency, low-intensity phonemes (Koehlinger, Van Horne, & Moeller, 2013; Koehlinger, Van Horne, Oleson, McCreery, & Moeller, 2015; Stelmachowicz, Pittman, Hoover, & Lewis, 2002; Stelmachowicz, Pittman, Hoover, Lewis, & Moeller, 2004). Furthermore, children with mild HL will frequently encounter situations in which background noise and distance could have negative effects on the ability to learn language. Compared with unaided hearing, amplification can provide better access to the speech signal in degraded listening situations. On the basis of these findings, additional counseling about the long-term benefits of early, consistent access to sound appears to be warranted. Such counseling is especially applicable to children with mild HL, who may appear to be able to fully perceive and understand speech without amplification. Parents may not recognize how difficult auditory perception and comprehension is in challenging listening situations or how much information their child is missing in daily conversational interactions.
Counseling on HA use should be situated within parental education on the importance of early, consistent access to high rates of quality linguistic input. Research has clearly demonstrated that cumulative auditory–linguistic experience has a positive impact on later language and academic outcomes (Moeller & Tomblin, 2015; Nittrouer & Burton, 2001, 2005). This experience is shaped by a variety of factors, including amount of HA use, the quality of the HA fit, and the quality and quantity of linguistic input in the child's environment (Ambrose, Walker, Unflat-Berry, Olsen, & Moeller, 2015; Moeller & Tomblin, 2015). Although early intervention services typically focus on supporting families in providing their children with high rates of linguistic input (Decker & Vallotton, 2016), children's cumulative auditory–linguistic experiences cannot be fully maximized if children do not have optimal auditory access to that input through well-fit and consistently worn amplification.
Conclusions
Mild HL (defined in the current study as BEPTA ≤ 45 dB HL) is highly prevalent in children with bilateral HL (Fitzpatrick et al., 2014), and children with mild HL are now often being identified through NHS (Holte et al., 2012). Early identification creates an opportunity to develop evidence-based clinical interventions for this population. Thus, it is important to understand service delivery practices in children with mild HL. The clinical implications from the current study are that (a) audiologists are moving toward greater confidence in providing amplification, as the majority of children with mild HL received amplification, and (b) children with mild HL are at risk for delays in HL-related intervention, especially when later identified. Further efforts toward educating professionals and parents about the benefits of amplification are warranted to reduce delays in services and encourage consistent fitting and use of HAs.
Acknowledgments
This work was supported by National Institutes of Health Grants 5R01DC009560 (coprincipal investigators J. Bruce Tomblin, University of Iowa, and Mary Pat Moeller, Boys Town National Research Hospital) and 5R01DC013591 (principal investigator Ryan W. McCreery, Boys Town National Research Hospital). The content of this project is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Deafness and Other Communication Disorders or the National Institutes of Health. The following people provided support, assistance, and feedback at various points in the project: Mary Pat Moeller, Wendy Fick, and Marlea O'Brien. Special thanks go to the families and children who participated in the research and to the examiners at the University of Iowa, Boys Town National Research Hospital, and the University of North Carolina–Chapel Hill.
Funding Statement
This work was supported by National Institutes of Health Grants 5R01DC009560 (coprincipal investigators J. Bruce Tomblin, University of Iowa, and Mary Pat Moeller, Boys Town National Research Hospital) and 5R01DC013591 (principal investigator Ryan W. McCreery, Boys Town National Research Hospital).
References
- Ambrose S. E., Walker B., Unflat-Berry L. M., Olsen J., & Moeller M. P. (2015). Quantity and quality of caregivers' linguistic input to 18-month and 3-year-old children who are hard of hearing. Ear and Hearing, 36(Suppl. 1), 48–59. doi:10.1097/AUD.0000000000000209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Audiology. (2013). Clinical practice guidelines: Pediatric amplification. Retrieved from http://audiology-web.s3.amazonaws.com/migrated/PediatricAmplificationGuidelines.pdf_539975b3e7e9f1.74471798.pdf
- Bagatto M. P., & Tharpe A. M. (2014). Decision support guide for hearing aid use in infants and children with minimal/mild bilateral hearing loss. In A Sound Foundation through Early Amplification: Sixth International Conference Proceedings (pp. 145–151). Stäfa, Switzerland: Phonak AG. [Google Scholar]
- Bess F. H., Dodd-Murphy J., & Parker R. A. (1998). Children with minimal sensorineural hearing loss: Prevalence, educational performance, and functional status. Ear and Hearing, 19, 339–354. [DOI] [PubMed] [Google Scholar]
- Blair J. C., Peterson M. E., & Viehwig S. H. (1985). The effects of mild sensorineural hearing loss on academic performance of young school-age children. The Volta Review, 87, 87–93. [Google Scholar]
- Coplan J. (1987). Deafness: Ever heard of it? Delayed recognition of permanent hearing loss. Pediatrics, 79, 206–213. [PubMed] [Google Scholar]
- Dalzell L., Orlando M., MacDonald M., Berg A., Bradley M., Cacace A., … Gross S. (2000). The New York State universal newborn hearing screening demonstration project: Ages of hearing loss identification, hearing aid fitting, and enrollment in early intervention. Ear and Hearing, 21, 118–130. [DOI] [PubMed] [Google Scholar]
- Davis J. M., Elfenbein J., Schum R., & Bentler R. A. (1986). Effects of mild and moderate hearing impairments on language, educational, and psychosocial behavior of children. Journal of Speech and Hearing Disorders, 51, 53–62. [DOI] [PubMed] [Google Scholar]
- Decker K. B., & Vallotton C. D. (2016). Early intervention for children with hearing loss: Information parents receive about supporting children's language. Journal of Early Intervention, 38, 151–169. doi:10.1177/1053815116653448 [Google Scholar]
- Dedhia K., Kitsko D., Sabo D., & Chi D. H. (2013). Children with sensorineural hearing loss after passing the newborn hearing screen. JAMA Otolaryngology—Head & Neck Surgery, 139, 119–123. [DOI] [PubMed] [Google Scholar]
- Đoković S., Gligorović M., Ostojić S., Dimić N., Radić-Šestić M., & Slavnić S. (2014). Can mild bilateral sensorineural hearing loss affect developmental abilities in younger school-age children? Journal of Deaf Studies and Deaf Education, 19, 484–495. [DOI] [PubMed] [Google Scholar]
- Durieux-Smith A., Fitzpatrick E., & Whittingham J. (2008). Universal newborn hearing screening: A question of evidence. International Journal of Audiology, 47(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Eiserman W. D., Hartel D. M., Shisler L., Buhrmann J., White K. R., & Foust T. (2008). Using otoacoustic emissions to screen for hearing loss in early childhood care settings. International Journal of Pediatric Otorhinolaryngology, 72, 475–482. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick E. M., Durieux-Smith A., & Whittingham J. (2010). Clinical practice for children with mild bilateral and unilateral hearing loss. Ear and Hearing, 31, 392–400. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick E. M., Grandpierre V., Durieux-Smith A., Gaboury I., Coyle D., Na E., & Sallam N. (2016). Children with mild bilateral and unilateral hearing loss: Parents' reflections on experiences and outcomes. Journal of Deaf Studies and Deaf Education, 21, 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick E. M., Whittingham J., & Durieux-Smith A. (2014). Mild bilateral and unilateral hearing loss in childhood: A 20-year view of hearing characteristics, and audiologic practices before and after newborn hearing screening. Ear and Hearing, 35, 10–18. [DOI] [PubMed] [Google Scholar]
- Gustafson S. J., Davis H., Hornsby B. W., & Bess F. H. (2015). Factors influencing hearing aid use in the classroom: A pilot study. American Journal of Audiology, 24, 563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggard R. S., & Primus M. A. (1999). Parental perceptions of hearing loss classification in children. American Journal of Audiology, 8, 83–92. [DOI] [PubMed] [Google Scholar]
- Harrison M., Page T. A., Oleson J., Spratford M., Berry L. U., Peterson B., … Moeller M. P. (2016). Factors affecting early services for children who are hard of hearing. Language, Speech, and Hearing Services in Schools, 47, 16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M., & Roush J. (1996). Age of suspicion, identification, and intervention for infants and young children with hearing loss: A national study. Ear and Hearing, 17, 55–62. [DOI] [PubMed] [Google Scholar]
- Holte L., Walker E., Oleson J., Spratford M., Moeller M. P., Roush P., … Tomblin J. B. (2012). Factors influencing follow-up to newborn hearing screening for infants who are hard of hearing. American Journal of Audiology, 21, 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., White K. R., Widen J. E., Gravel J. S., James M., Kennalley T., … Vohr B. R. (2005). A multicenter evaluation of how many infants with permanent hearing loss pass a two-stage otoacoustic emissions/automated auditory brainstem response newborn hearing screening protocol. Pediatrics, 116, 663–672. [DOI] [PubMed] [Google Scholar]
- Joint Committee on Infant Hearing. (2007). Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics, 120, 898–921. [DOI] [PubMed] [Google Scholar]
- Kiese-Himmel C., & Ohlwein S. (2003). Characteristics of children with permanent mild hearing impairment. Folia Phoniatrica et Logopaedica, 55, 70–79. [DOI] [PubMed] [Google Scholar]
- Koehlinger K. M., Van Horne A. J. O., & Moeller M. P. (2013). Grammatical outcomes of 3-and 6-year-old children who are hard of hearing. Journal of Speech, Language, and Hearing Research, 56, 1701–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehlinger K. M., Van Horne A. O., Oleson J., McCreery R., & Moeller M. P. (2015). The role of sentence position, allomorph, and morpheme type on accurate use of s-related morphemes by children who are hard of hearing. Journal of Speech, Language, and Hearing Research, 58, 396–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü J., Huang Z., Yang T., Li Y., Mei L., Xiang M., … Yao G. (2011). Screening for delayed-onset hearing loss in preschool children who previously passed the newborn hearing screening. International Journal of Pediatric Otorhinolaryngology, 75, 1045–1049. [DOI] [PubMed] [Google Scholar]
- McKay S., Gravel J. S., & Tharpe A. M. (2008). Amplification considerations for children with minimal or mild bilateral hearing loss and unilateral hearing loss. Trends in Amplification, 12, 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller M. P. (2000). Early intervention and language development in children who are deaf and hard of hearing. Pediatrics, 106(3), e43. [DOI] [PubMed] [Google Scholar]
- Moeller M. P., & Tomblin J. B. (2015). An introduction to the Outcomes of Children with Hearing Loss study. Ear and Hearing, 36, 4S–13S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most T. (2004). The effects of degree and type of hearing loss on children's performance in class. Deafness and Education International, 6, 154–166. [Google Scholar]
- Nittrouer S., & Burton L. T. (2001). The role of early language experience in the development of speech perception and language processing abilities in children with hearing loss. The Volta Review, 103, 5–37. [Google Scholar]
- Nittrouer S., & Burton L. T. (2005). The role of early language experience in the development of speech perception and phonological processing abilities: Evidence from 5-year-olds with histories of otitis media with effusion and low socioeconomic status. Journal of Communication Disorders, 38, 29–63. [DOI] [PubMed] [Google Scholar]
- Pittman A. L., & Stelmachowicz P. (2003). Hearing loss in children and adults: Audiometric configuration, asymmetry, and progression. Ear and Hearing, 24, 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter H., Sladen D. P., Ampah S. B., Rothpletz A., & Bess F. H. (2013). Developmental outcomes in early school-age children with minimal hearing loss. American Journal of Audiology, 22, 263–270. [DOI] [PubMed] [Google Scholar]
- Ross D. S., Holstrum W. J., Gaffney M., Green D., Oyler R. F., & Gravel J. S. (2008). Hearing screening and diagnostic evaluation of children with unilateral and mild bilateral hearing loss. Trends in Amplification, 12, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sininger Y. S., Grimes A., & Christensen E. (2010). Auditory development in early amplified children: Factors influencing auditory-based communication outcomes in children with hearing loss. Ear and Hearing, 31, 166–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelmachowicz P. G., Pittman A. L., Hoover B. M., & Lewis D. E. (2002). Aided perception of /s/ and /z/ by hearing-impaired children. Ear and Hearing, 23, 316–324. [DOI] [PubMed] [Google Scholar]
- Stelmachowicz P. G., Pittman A. L., Hoover B. M., Lewis D. E., & Moeller M. P. (2004). The importance of high-frequency audibility in the speech and language development of children with hearing loss. Archives of Otolaryngology—Head & Neck Surgery, 130, 556–562. [DOI] [PubMed] [Google Scholar]
- Tomblin J. B., Oleson J. J., Ambrose S. E., Walker E., & Moeller M. P. (2014). The influence of hearing aids on the speech and language development of children with hearing loss. JAMA Otolaryngology—Head & Neck Surgery, 140, 403–409. doi:10.1001/jamaoto.2014.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin J. B., Walker E. A., McCreery R. W., Arenas R. M., Harrison M., & Moeller M. P. (2015). Outcomes of children with hearing loss: Data collection and methods. Ear and Hearing, 36, 14S–23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Centers for Disease Control. (2011). Summary of 2009 National CDC EHDI data. Retrieved from http://www.cdc.gov/ncbddd/hearingloss/2009-Data/2009_EHDI_HSFS_Summary_508_OK.pdf
- Vohr B. R., Carty L. M., Moore P. E., & Letourneau K. (1998). The Rhode Island hearing assessment program: Experience with statewide hearing screening (1993–1996). The Journal of Pediatrics, 133, 353–357. [DOI] [PubMed] [Google Scholar]
- Wake M., Tobin S., Cone-Wesson B., Dahl H.-H., Gillam L., McCormick L., … Ukoumunne O. C. (2006). Slight/mild sensorineural hearing loss in children. Pediatrics, 118, 1842–1851. [DOI] [PubMed] [Google Scholar]
- Walker E. A., Holte L., McCreery R. W., Spratford M., Page T., & Moeller M. P. (2015). The influence of hearing aid use on outcomes of children with mild hearing loss. Journal of Speech, Language, and Hearing Research, 58, 1611–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E. A., Holte L., Spratford M., Oleson J., Welhaven A., & Harrison M. (2014). Timeliness of service delivery for children with later-identified mild-to-severe hearing loss. American Journal of Audiology, 23, 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E. A., McCreery R. W., Spratford M., Oleson J. J., Van Buren J., Bentler R., … Moeller M. P. (2015). Trends and predictors of longitudinal hearing aid use for children who are hard of hearing. Ear and Hearing, 36, 38S–47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E. A., Spratford M., Moeller M. P., Oleson J., Ou H., Roush P., & Jacobs S. (2013). Predictors of hearing aid use time in children with mild-to-severe hearing loss. Language, Speech, and Hearing Services in Schools, 44, 73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichbold V., Nekahm-Heis D., & Welzl-Mueller K. (2006). Universal newborn hearing screening and postnatal hearing loss. Pediatrics, 117, e631–e636. [DOI] [PubMed] [Google Scholar]
- White K. R., Forsman I., Eichwald J., & Munoz K. (2010). The evolution of early hearing detection and intervention programs in the United States. Seminars in Perinatology, 34, 170–179. [DOI] [PubMed] [Google Scholar]
- White K. R., & Muñoz K. (2008). Screening for mild and unilateral hearing loss. Seminars in Hearing, 29, 149–158. [Google Scholar]
- Wolgemuth K. S., Kamhi A. G., & Lee R. F. (1998). Metaphor performance in children with hearing impairment. Language, Speech, and Hearing Services in Schools, 29, 216–231. [DOI] [PubMed] [Google Scholar]
- Yoshinaga-Itano C., Johnson C. D., Carpenter K., & Brown A. S. (2008). Outcomes of children with mild bilateral hearing loss and unilateral hearing loss. Seminars in Hearing, 29, 196–211. [Google Scholar]
- Yoshinaga-Itano C., Sedey A. L., Coulter D. K., & Mehl A. L. (1998). Language of early- and later-identified children with hearing loss. Pediatrics, 102, 1161–1171. [DOI] [PubMed] [Google Scholar]