Abstract
Background & aims
Hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC) is prevalent worldwide. Despite its limitations, serum alpha-fetoprotein (AFP) remains the most widely-used biomarker for the diagnosis of HCC. This study aimed to assess whether measurement of peripheral plasma Dickkopf-1 (DKK1) and Tie2-expressing monocytes (TEMs) could overcome the limitations of AFP and improve the diagnostic accuracy of HCC.
Methods
Plasma DKK1 level and the percentage of TEMs in peripheral CD14+CD16+ monocytes from HCC patients (n = 82), HBV-related liver cirrhosis (LC) patients (n = 29), chronic hepatitis B (CHB) infected patients (n = 28) and healthy volunteers (n = 31) were analyzed by ELISA and flow cytometry. Receiver operating characteristic (ROC) curves were used to analyze a single biomarker, or a combination of two or three biomarkers. Univariate and multivariate analyses were performed to assess the significance of each marker in prediction of HCC and AFP-negative HCC from LC patients.
Results
The percentage of TEMs in peripheral CD14+CD16+ monocytes and plasma level of DKK1 in HCC group were significantly higher than those in LC, CHB and healthy control groups (all P-values <0.05). The percentage of TEMs alone was also significantly higher in AFP-negative HCC group than that in LC, CHB and healthy control groups (all P-values <0.05). Plasma DKK1 level alone could not distinguish between AFP-negative HCC and LC patients. ROC curves showed that the optimal diagnostic cutoff value was 550.93 ng/L for DKK1 and 4.95% for TEMs. There was no significant difference in AUC of DKK1, TEMs and AFP in HCC diagnosis between the four groups (all P>0.05). A combination of DKK1, TEMs and AFP measurements increased the AUC for HCC diagnosis as compared with either marker alone (0.833; 95%CI 0.768–0.886). The AUC for TEMs was 0.692 (95% CI 0.564–0.819) in differentiating AFP-negative HCC from LC, with a sensitivity of 80.0% and a specificity of 65.52%. Only TEMs prevailed as a significant predictor for AFP-negative HCC differentiating from LC patients in univariate and multivariate analyses (P = 0.016, P = 0.023).
Conclusions
TEMs and DKK1 may prove to be potential complementary biomarkers for AFP in the diagnosis of HCC. TEMs rather than DKK1 could serve as a complementary biomarker for AFP in the differential diagnosis of AFP-negative HCC versus LC patients.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors and the third leading cause of cancer-related deaths worldwide[1]. Although numerous efforts have been made to discover more reliable biomarkers for the diagnosis of HCC such asα-fetoprotein (AFP)AFP-L3, DCP and GP73, serum AFP remains the most commonly used biomarker [2–3]. However, the sensitivity and specificity of serum AFP for the diagnosis of HCC were only 39–65% and 76–94% respectively [4]. To overcome the limitations of AFP, it is necessary and urgent to find novel and more reliable serum biomarkers for early detection of HCC.
In 2005, Palmaet al [5]discovered a novel subpopulation of monocytes expressing the tyrosine kinase receptor Tie2 (tyrosine kinase with immunoglobulin and epidermal growth factor homology domains 2) as a representative surface marker. Tie2-expressing monocytes (TEMs) have been found in various human tumors to form tumor blood vessels and promote tumor angiogenesis and growth by paracrine secretion of angiogenic factors such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (b-FGF), and matrix metalloproteinase-9 (MMP-9) [6–8]. In 2013, Matsubaraet al [9] found that the frequency of TEMs, as defined as CD14+CD16+TIE2+ cells in peripheral blood, was significantly higher in HCC patients than that in non-HCC patients, and that the frequency changed with the therapeutic response or recurrence.
Dickkopf-1 (DKK-1) is a secretory antagonist of the Wnt signaling pathway. Recently, Shen et al [10] reported that serum DKK1 had better sensitivity, specificity and area under the receiver operating characteristic curve (AUC) than AFP for early diagnosis of HCC, especially in HCC patients with negative AFP results and/or those in the early stage of the disease.
In this study, we analyzed the levels of peripheral plasma DKK1 and AFP and the percentage of TEMs with respect to the sensitivity, specificity and AUC of each biomarker alone and a combination of two or three biomarkers in HCC patients with hepatitis B virus (HBV) infection and patients with HBV-related liver cirrhosis (LC), patients with chronic hepatitis B infection (CHB), and healthy controls(NC). In addition, we analyzed the diagnostic performance of either DKK1 or TEMs alone, or their combination for detection of AFP-negative HCC.
Materials and methods
Ethics statement
The analyses of blood samples were approved by the Research Ethics Committee of Affiliated Nantong No. 3 Hospital of Nantong University (Nantong, China). All patients and healthy controls provided their written informed consent to participate in this study.
Sample collection and storage
HCC patients with HBV infection who were admitted to Affiliated Nantong No. 3 Hospital of Nantong University between October 2014 and June 2016 were included in this study. HCC was diagnosed based on histological findings or typical imaging characteristics as defined by the Diagnosis, Management and Treatment of Hepatocellular Carcinoma (V2011) issued by the Ministry of Health of the Chinese People’s Republic of China [11]. Tumor stages were decided according to the Barcelona Clinic Liver Cancer (BCLC) staging classification[12]. 3 mL heparinized blood was collected from each participant of the four groups, including 31 healthy volunteers (NC group), 82 HCC patients with HBV infection (HCC group), 29 patients with HBV-related liver cirrhosis (LC group), and 28 patients with chronic hepatitis B infection (CHB group). Firstly, whole blood was used for analysis of TEMs. Then, the residual whole blood was centrifugated immediately at 3000 rpm for 10 min to separate plasma, and the plasma aliquots were stored at -70°C until analysis of AFP and DKK1. Patient characteristics and disease classification are shown in Table 1. Blood samples were usually collected one day before surgery or radiofrequency ablation in the patients. Blood samples from healthy volunteers were obtained from the Health Check-up Unit, Affiliated Nantong No. 3 Hospital of Nantong University. These normal controls had normal complete blood counts, liver and kidney function tests, and had no apparent chronic inflammatory diseases.
Table 1. Clinical characteristics of the study participants.
| Clinicopathologic characteristics | HCC | LC | CHB | NC |
|---|---|---|---|---|
| Number of patients | 82 | 29 | 28 | 31 |
| Gender (male/female) | 64/18 | 25/4 | 18/10 | 20/11 |
| Mean age (SD) | 56.78(10.58) | 52.17(13.34) | 38.14(12.10) | 46.30(10.63) |
| Child-Pugh grade (A/B/C) | 71/9/2 | 17/10/2 | 25/2/1 | - |
| Tumor size, cm (≤3/>3) | 26/56 | - | - | - |
| BCLC stage (0/A/B/C/D) | 9/17/34/20/2 | - | - | - |
| AFP, ug/L (≤20/>20) | 45/37 | 19/10 | 25/3 | 31/0 |
| DKK1, ng/L (≤550.93/>550.93) | 9/73 | 11/18 | 14/14 | 21/10 |
| TEMs, % (≤4.95/>4.95) | 23/59 | 19/10 | 20/8 | 23/8 |
HCC, hepatocellular carcinoma; LC, liver cirrhosis; CHB, chronic hepatitis B virus infection; NC, normal controls
Frequency analysis of peripheral blood TEMs
Heparinized whole blood was processed at room temperature immediately after collection. TEMs were defined by their simultaneous expression of CD14, CD16 and TIE2[9]. Surface expression of CD14,CD16 and TIE2 was analyzed by direct immune-fluorescence staining followed by a lyse-wash procedure[6,13]. In brief, 100μL whole blood was blocked with 20μL human FcR blocking reagent (MACS, Miltenyi Biotec Inc. USA) for 20 min, and then incubated with fluorescence-labeled mouse anti-human Abs against CD14, CD16 and TIE2 at saturating concentrations for 20 min using CD14-FITC, CD16-PE-Cy5 (Becton-Dickinson, San Jose, CA, USA), and TIE2-PE (EMD Millipore Corporation, USA). To eliminate erythrocytes, the lyse solution was added and then wash with PBS twice. Flow cytometry was immediately performed with a Coulter Epics XL Flow Cytometer (Beckman Coulter) and Kaluza v1.2 software. IgG1-k-PE isotypic antibody (EMD Millipore Corporation, USA) was applied with all the samples as controls (Fig 1).
Fig 1. Identification of TEMs as CD14+CD16+TIE2+ cells in peripheral blood.
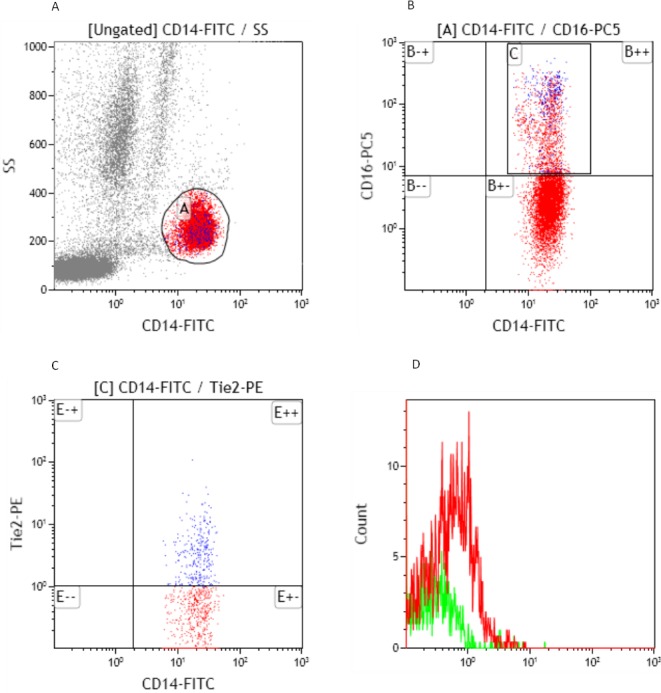
A. CD14+ PBMC obtained from HCC patients were stained and analyzed by flow cytometry. B. CD14+ monocytes were divided into two distinct subsets, CD14+CD16+ and CD14+CD16- cells. C. TEMs were examined by using TIE2-PE orIgG1-k-PEisotypic antibody. D.The percentage of TEMs in peripheral blood CD14+CD16+ monocytes for this sample was 16.14% (the percentage of TEMs in CD14+CD16+ monocytes measured by TIE2-PE staining minus that measured by IgG1-k-PE staining, 24.2% - 8.06% = 16.14%).
Analysis of plasma DKK1 and AFP
Plasma AFP was measured using I2000 automatic chemiluminescence immunoassay analyzer (Abbott Architect i2000SR, USA). DKK-1 was detected using commercially available enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions (R&D Systems, Inc. Minneapolis, MN, USA). Standard curves were generated for each ELISA plate used. The samples were analyzed in duplicate with a KHB ST-360 micro-plate Reader (Shanghai Kehua Bio-Engineering Co.,Ltd.)
Statistical analysis
Statistical analyses were done with SPSS software version 17 and MedCalc version 12.7.0.Differences between the four study groups were assessed by the Kruskal-Wallis nonparametric Test, and differences between two groups by the Mann-Whitney nonparametric U Test. ROC curves were plotted for each biomarker to investigate their capability to distinguish between HCC and non-HCC, and moreover define the cut-off value of each biomarker for HCC diagnosis by maximum sensitivity and specificity [14]. Prediction of HCC and AFP-negative HCC from LC patients by independent variables was assessed in univariate and multivariate analyses with binary logistic regression. Correlations between the investigated parameters were investigated using Pearson’s correlation coefficient. The difference in rates was evaluated by chi-square test.
To assess whether the combined use of DKK1, TEMs and AFP measurements was better than either of these three biomarkers alone, binary logistic regression analysis was further performed to create a new variable predicted probability (P1) for HCC on the basis of the following equation (HCC group versus LC, CHB and NC groups): Logit P1 = -2.635+0.002×DKK1+0.001×AFP+0.147×TEMs.
The reported P-values are results of two-sided tests. P-values <0.05 are considered statistically significant.
Results
TEMs and DKK1 are significantly increased in HCC patients
To investigate the diagnostic performance of DKK1 and TEMs for the diagnosis of HCC, plasma DKK1 level and the percentage of TEMs in peripheral CD14+CD16+ monocytes were analyzed by ELISA and flow cytometry, respectively. As shown in Table 2, the median percentage of TEMs in peripheral CD14+CD16+ monocytes and the median plasma DKK1 level in HCC group were significantly higher than those in LC, CHB and NC groups (all p <0.05). Comparisons of DKK1 and TEMs between the45 HCC patients with AFP-negative and 37 HCC patients with AFP-positive were analyzed. We did not detect any significant difference. Similar results were also found between the HCC patients with DKK1 and TEMs low and high groups as defined by the cut-off (as shown in S1 Table).
Table 2. Plasma DKK1 level and the percentage of TEMs in different groups.
| Groups | Patients(n) | DKK1 median(IQR), ng/L | P value | TEMs median(IQR),% | P value |
|---|---|---|---|---|---|
| Total HCC | 82 | 780.70 (619.68–1171.96) | 0.022* | 8.25 (3.77–13.45) | 0.012* |
| AFP negative HCC | 45 | 771.44 (642.45–933.07) | 0.083# | 8.11 (5.59–12.40) | 0.006* |
| LC | 29 | 686.91 (467.80–863.58) | 0.188## | 4.77 (2.36–9.43) | 0.350## |
| CHB | 28 | 570.88 (248.83–904.45) | 0.123### | 3.48 (2.21–5.91) | 0.564### |
| NC | 31 | 432.83 (218.84–658.49) | 0.003* | 3.54 (1.85–5.73) | 0.126# |
*P<0.05 (vs LC)
#P≥0.05 (vs LC)
## P≥0.05 (vs CHB)
### P≥0.05 (vs NC).
HCC, hepatocellular carcinoma; LC, liver cirrhosis; CHB, chronic hepatitis B virus infection; NC, normal controls; IQR, Inter-Quartile Range. Kruskal-Wallis H test was used to analyzed the statistical significance of DKK1 and TEMs between total patients, all P<0.0001. All P values were analyzed by Mann-Whitney nonparametric U test.
In addition, the median percentage of TEMs in peripheral CD14+CD16+ monocytes and the median plasma DKK1 level in the 45 AFP-negative HCC patients were analyzed. It was found that the median percentage of TEMs in peripheral CD14+CD16+ monocytes in these patients were significantly higher than that in LC, CHB and NC groups (all P<0.05). Althoughplasma DKK1 level could be used to distinguish AFP-negative HCC patients from chronic HBV infected patients and healthy controls (all P<0.05), we did not detect any significant difference between AFP-negative HCC and LC patients (P = 0.083).
Comparison of AUC, sensitivity and specificity of TEMs, DKK1 and AFP in distinguishing HCC patients from the other control groups
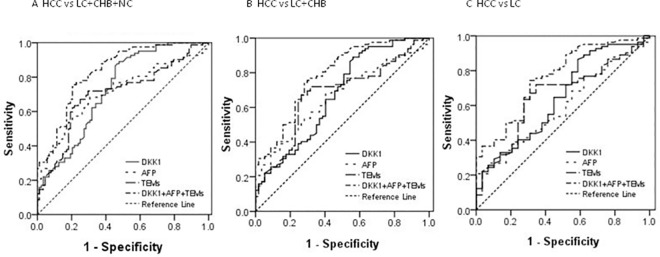
We next analyzed the ROC curves to evaluate the sensitivity and specificity of DKK1, TEMs, AFP and Logit P1 in the diagnosis of HCC patients versus the other three cohorts (Fig 2). The ROC curves showed that the optimal diagnostic cutoff value was 550.93 ng/L for DKK1 and 4.95% for TEMs. We chose 20 μg/L as the cutoff value for AFP in this study.
Fig 2. ROC curves for DKK1, TEMs, AFP and Logit P1 for the diagnosis of HCC versus different cohorts.
(A) ROC curves for DKK1, TEMs, AFP and Logit P1 for the diagnosis of HCC versus all controls, with an AUC value of 0.728, 0.701, 0.712 and 0.833 respectively. (B) ROC curves for DKK1, TEMs, AFP and LogitP1 for the diagnosis of HCC versus LC and CHB controls, with an AUC value of 0.673, 0.673, 0.664 and 0.789 respectively. (C) ROC curves for DKK1, TEMs, AFP and LogitP1 for the diagnosis of HCC versus LC controls, with an AUC value of 0.644, 0.657, 0.592 and 0.762 respectively. ROC = receiver operating characteristic. AUC = area under receiver operating characteristic. HCC = hepatocellular carcinoma. CHB = chronic hepatitis B virus infection. LC = liver cirrhosis. Logit P1 = -2.635+0.002×DKK1+0.001×AFP+0.147×TEMs.
As shown in Fig 2A, Tables 3 and 4, the AUC showed no significant difference between DKK1, TEMs and AFP. In combination, the AUC for the new variable Logit P1 of binary logistic regression analysis of triple markers (DKK1, TEMs and AFP) showed the biggest AUC (all P-values<0.05). As a single marker, DKK1>550.93 ng/L showed the highest sensitivity (89.02%) and the lowest specificity (52.27%), whereas AFP>20 μg/L showed the lowest sensitivity (45.12%) and the highest specificity (85.23%).
Table 3. Measurement results of DKK1, TEMs or AFP alone and their combinations for HCC diagnosis in different cohorts.
| Markers | AUC(95% CI) | Sn(%) | Sp(%) | Sn+Sp | PPV(%) | NPV(%) |
|---|---|---|---|---|---|---|
| HCC vs LC,CHB and NC | ||||||
| DKK1 | 0.728(0.654–0.793) | 89.02 | 52.27 | 1.41 | 63.48 | 83.64 |
| TEMs | 0.701(0.626–0.768) | 71.95 | 70.45 | 1.42 | 69.41 | 72.94 |
| AFP | 0.712(0.638–0.779) | 45.12 | 85.23 | 1.30 | 74.00 | 62.50 |
| DKK1+TEMs+AFP | 0.833(0.768–0.886) | 76.83 | 77.27 | 1.54 | 75.90 | 78.16 |
| HCC vs LC and CHB | ||||||
| DKK1 | 0.673(0.588–0.752) | 89.02 | 43.86 | 1.33 | 69.52 | 73.53 |
| TEMs | 0.673(0.589–0.750) | 71.95 | 68.40 | 1.40 | 76.62 | 62.90 |
| AFP | 0.664(0.579–0.742) | 45.12 | 77.19 | 1.22 | 74.00 | 49.44 |
| DKK1+TEMs+AFP | 0.789(0.711–0.853) | 76.83 | 68.42 | 1.45 | 77.78 | 67.24 |
| HCC vs LC | ||||||
| DKK1 | 0.664(0.548–0.733) | 89.02 | 37.93 | 1.27 | 80.22 | 55.00 |
| TEMs | 0.657(0.561–0.745) | 71.95 | 65.52 | 1.37 | 85.51 | 45.24 |
| AFP | 0.592(0.495–0.685) | 45.12 | 65.52 | 1.11 | 78.72 | 29.69 |
| DKK1+TEMs+AFP | 0.762(0.672–0.838) | 76.83 | 62.07 | 1.39 | 85.14 | 48.65 |
| AFP-negative HCC vs LC,CHB and NC | ||||||
| DKK1 | 0.709(0.624–0.795) | 91.11 | 52.27 | 1.43 | 49.40 | 92.00 |
| TEMs | 0.739(0.648–0.829) | 80.00 | 70.45 | 1.50 | 58.06 | 87.32 |
| DKK1+TEMs | 0.785(0.709–0.861) | 40.00 | 82.95 | 1.23 | 54.55 | 73.00 |
| AFP-negative HCC vs LC and CHB | ||||||
| DKK1 | 0.649(0.542–0.755) | 91.11 | 43.86 | 1.35 | 56.16 | 86.21 |
| TEMs | 0.708(0.605–0.812) | 80.00 | 68.42 | 1.48 | 66.67 | 81.25 |
| DKK1+TEMs | 0.729(0.632–0.827) | 40.00 | 77.19 | 1.57 | 58.06 | 61.97 |
| AFP-negative HCC vs LC | ||||||
| DKK1 | 0.620(0.483–0.757) | 91.11 | 37.93 | 1.29 | 69.49 | 73.33 |
| TEMs | 0.692(0.564–0.819) | 80.00 | 65.52 | 1.46 | 78.26 | 67.86 |
| DKK1+TEMs | 0.707(0.577–0.837) | 40.00 | 72.41 | 1.12 | 69.23 | 43.75 |
Table 4. Assessment of AUC values of DKK1, TEMs or AFP alone and their combinations in distinguishing HCC or AFP-negative HCC and other cohorts.
| P | P | P | |
|---|---|---|---|
| HCC vs all controls | HCC vs LC+CHB | HCC vs LC | |
| AFP vs DKK1 | 0.772 | 0.896 | 0.575 |
| AFP vs TEMs | 0.839 | 0.895 | 0.462 |
| DKK1 vs TEMs | 0.620 | 0.993 | 0.876 |
| Logit P1 vs AFP | 0.006 | 0.017 | 0.029 |
| Logit P1 vs DKK1 | 0.000 | 0.001 | 0.018 |
| Logit P1 vs TEMs | 0.000 | 0.004 | 0.021 |
| AFP-negative HCC vs all controls | AFP-negative HCC vs LC+CHB | AFP-negative HCC vs LC | |
| DKK1 vs TEMs | 0.621 | 0.392 | 0.398 |
| Logit P2 vs DKK1 | 0.012 | 0.032 | 0.101 |
| Logit P2 vs TEMs | 0.204 | 0.596 | 0.737 |
Logit P1 = -2.635+0.002×DKK1+0.001×AFP+0.147×TEMs, Logit P2 = -2.565+0.002×DKK1+0.137×TEMs.
TEMs rather than DKK1 could serve as a complementary biomarker for AFP in the differential diagnosis of AFP-negative HCC versus LC patients
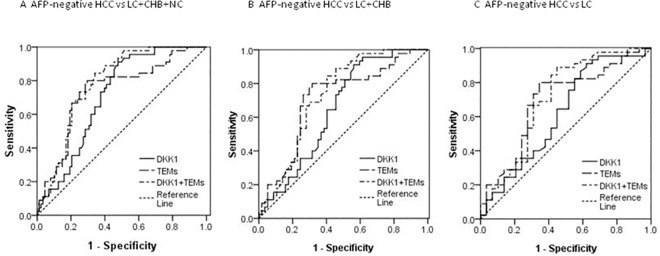
In addition, in the 45 AFP-negative HCC patients, their AUC for DKK1 and TEMs for HCC diagnosis from the other three control groups was 0.709 (95% CI 0.624–0.795, Fig 3A, Table 3, P<0.01) and 0.739 (95% CI 0.648–0.829, Fig 3A, Table 3, P<0.01) respectively. However, the AUC for DKK1 was only 0.620 (95% CI 0.483–0.757, Fig 3C, Table 3, P = 0.083) in differentiating AFP-negative HCC from LC. When the cutoff value was selected at 550.93 ng/L, its sensitivity was 89.11%, but the specificity was only 37.93%. The AUC for TEMs was 0.692 (95% CI 0.564–0.819, Fig 3C, Table 3, P<0.01) in differentiating AFP-negative HCC from LC. Its sensitivity and specificity were 80.0% and 65.52% respectively.
Fig 3. ROC curves for DKK1, TEMs and Logit P2 for the diagnosis of AFP-negative HCC versus different cohorts.
(A) ROC curves for DKK1, TEMs and LogitP2 for the diagnosis of HCC versus all controls, with an AUC value of 0.709, 0.739 and 0.785 respectively. (B) ROC curves for DKK1, TEMs and LogitP2 for the diagnosis of HCC versus LC and CHB controls, with an AUC value of 0.649, 0.708 and 0.729 respectively. (C) ROC curves for DKK1, TEMs and LogitP2 for the diagnosis of HCC versus LC controls, with an AUC value of 0.620, 0.692 and 0.707 respectively. ROC = receiver operating characteristic. AUC = area under receiver operating characteristic. HCC = hepatocellular carcinoma. CHB = chronic hepatitis B virus infection. LC = liver cirrhosis. Logit P2 = -2.565+0.002×DKK1+0.137×TEMs.
To assess whether the combined use of DKK1 and TEMs measurements was better than either of these two biomarkers alone, binary logistic regression analysis was further performed to create a new variable predicted probability (P2) for AFP-negative HCC on the basis of an equation (AFP-negative HCC versus all LC, CHB and NC groups): Logit P2 = -2.565+0.002×DKK1+0.137×TEMs. Although the combined use of DKK1 and TEMs increased the AUC (0.785; 95% CI 0.709–0.861, Fig 3A) in differentiating AFP-negative HCC from LC, chronic HBV infected patients and normal controls, the combination use of DKK1 and TEMs was no better than TEMs alone in differentiating AFP-negative HCC from the other three control cohorts, and the combined use of DKK1 and TEMs did not statistically increased the AUC (0.707; 95% CI 0.577–0.837, Fig 3C) as compared with DKK1 or TEMs alone in differentiating AFP-negative HCC from LC (Fig 3C, Table 4).
Univariate and multivariate analyses by binary logistic regression were further performed to assess the significance of each marker in prediction of HCC and AFP-negative HCC from LC patients. Univariate analysis revealed DKK1 (P = 0.043, odds ratio = 0.999) and TEMs (P = 0.018, odds ratio = 0.901) as predictors for HCC differentiating from LC patients. In multivariate analysis, Only TEMs (P = 0.011, odds ratio = 0.892) prevailed as a valuable predictor for HCC differentiating from LC patients. Only TEMs prevailed as a significant predictor for AFP-negative HCC differentiating from LC patients in univariate and multivariate analyses (P = 0.016, P = 0.023). (as shown in S2 Table).
There were no or weak correlation between plasma DKK1, AFP and the percentage of TEMs in peripheral CD14+CD16+ monocytes
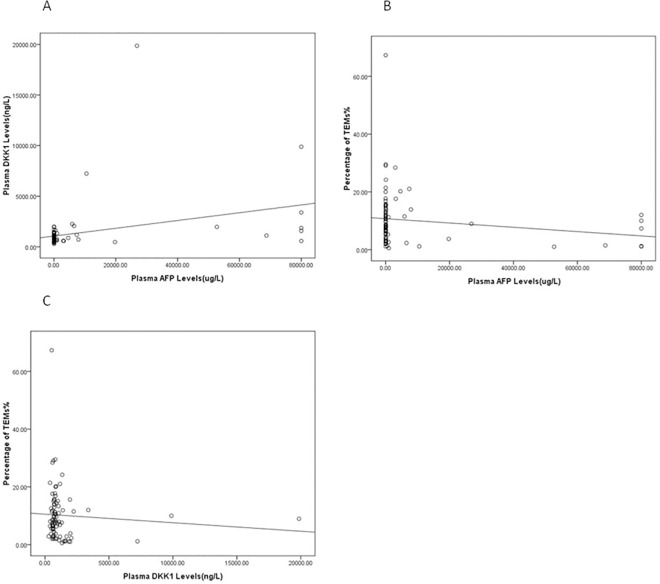
The correlations between plasma DKK1, AFP and the percentage of TEMs in peripheral CD14+CD16+ monocytes were analyzed in 82 HCC patients. It was found that there was a weak correlation between plasma DKK1 and AFP level (P = 0.002, r = 0.332; Fig 4A), Neither the plasma AFP level (P = 0.129, r = -0.169; Fig 4B) nor DKK1 (P = 0.489, r = -0.078; Fig 4C) was correlated with the percentage of TEMs in peripheral CD14+CD16+monocytes. These results were consistent with the results of S1 Table.
Fig 4. There were no or weak correlations between plasma DKK1, AFP and the percentage of TEMs.
Correlations between the percentage of TEMs, DKK1 and AFP were analyzed in 82 HCC patients. (A) Pearson’s analysis shows a low positive correlation between plasma DKK1 and AFP(n = 82, P = 0.002, r = 0.332). (B) Pearson’s analysis shows no correlation between the percentage of TEMs in peripheral CD14+CD16+ monocytes and AFP (n = 82, P = 0.129, r = -0.169). (C) Pearson’s analysis shows no correlation between the percentage of TEMs in peripheral CD14+CD16+ monocytes and DKK1 (n = 82, P = 0.489, r = -0.078).
Discussion
TEMs are a subgroup of circulating and tumor-infiltrating myeloid cells with potency to promote angiogenesis in xenotransplanted human tumors [6]. Previous studies [9, 15] defined TEMs as CD14+CD16+Tie2+ cells and reported that they were increased in HCC patients.TEMs are generally considered as a novel biomarker and potential therapeutic target for the diagnosis of HCC. However, reports about TEMs in healthy individuals have been controversial [6, 15]. In addition, the procedure of TEM analysis is complex, usually requiring as much as 10ml peripheral whole blood to isolate individual mononuclear cells by using Ficoll-Hypaque density gradient centrifugation[15]. In this study, we evaluated TEMs by using direct immunfluorescence staining as described in previous studies [6,13]. As expected, the percentage of TEMs in peripheral blood CD14+CD16+ monocytes of HCC patients was significantly increased as compared with that in healthy controls and patients with HBV-related CHB and LC. A previous study[15] reported that the median percentage of TEMs in peripheral blood CD14+CD16+ monocytes of NC group was 23.4%, which was significantly higher than that obtained in the present study (4.33%, IQR 1.85–5.73%). In addition, He et al showed that the AUC (0.800, 95% CI 0.704–0.896) for TEMs for HCC diagnosis from HBV-related LC was superior to our result (0.657, 95% CI 0.561–0.745). We hypothesize that the possible reason may be due to the different manufacturers of the Tie2+ antibody used and the different operating methods. In addition, different statistic populations and potential statistic biases may also lead to such differences.
As shown in Table 3, DKK1 was the most sensitive marker for HCC in different cohorts.Previous studies demonstrated that DKK1 was a biomarker for early diagnosis of HCC. Shen et al [10] reported that the median serum DKK1 concentration was 3.08 (IQR 1.75–4.57) ng/mL in HCC patients, with an optimal diagnostic cutoff of DKK1 of 2.153 ng/mL. This median serum DKK1 value was higher than that in the present study. The reason may be that we used plasma rather than serum for the measurement due to sample availability. According to the operating protocol of the kit used in this study, the serum level of DKK1 was higher than the plasma level of DKK1 due to platelet releasing[16,17]. Janget al [18] reported that the cutoff and median plasma DKK1 were 500 and 1497.1 (IQR 279.0–782.1) pg/mL in HCC patients, respectively, which are consistent with 550.93 and 1351.89 (IQR 619.68–1171.96) ng/L in our study. Shen et al [10] reported that the AUC, sensitivity and specificity of DKK1 in differentiating between LC, chronic HBV infected patients and healthy controls were 0.848, 69.1% and 90.6% respectively versus 0.830, 57.8% and 88.0% for AFP. However, their results were not reproducible in this study. In this study although DKK1 had the highest sensitivity (89.02%), the specificity (52.27%) is lower than AFP (85.23%). Combination of DKK1, AFP and TEMs could significantly increase the AUC (0.833; 95% CI 0.768–0.886) for HCC diagnosis, which is higher than that when any of the biomarkers was used alone. However, the sensitivity was lower than that of DKK1(χ2 = 4.307,P = 0.038), and the specificity was lower than that of AFP(χ2 = 12.05,P = 0.001).
Even with the help of advanced imaging technology, detection of early-stage and AFP-negative HCC remains difficult. A previous study [10] reported that the AUC, sensitivity and specificity of DKK1 were 0.830 (0.785–0.875), 70.4% and 84.7% in differentiating AFP-negative HCC from LC, and chronic HBV infected patients, which are superior to the results reported in the present study. As shown in Table 2 of the present study, although plasma DKK1 level could distinguish AFP-negative HCC from chronic HBV infected patients and healthy controls, it was unable to distinguish AFP-negative HCC from LC patients. Similarly, Jang et al [18] showed that the AUC of DKK1 was 0.617 in differentiating AFP-negative HCC from LC controls, which is consistent with our result (0.620, 95% CI 0.483–0.757).
We also firstly evaluated whether TEMs could be used as a marker for the diagnosis of AFP-negative HCC. The AUC for TEMs was 0.739 in differentiating AFP-negative HCC from LC, chronic HBV infected patients and healthy controls (95% CI 0.648–0.829). The AUC for TEMs was 0.692 in differentiating AFP-negative HCC from LC patients (95% CI 0.564–0.819), with a sensitivity of 80.0% and a specificity of 65.52%. Only TEMs prevailed as a significant predictor for AFP-negative HCC differentiating from LC patients in univariate and multivariate analyses (P = 0.016, P = 0.023).
We also found that there were no or weak correlations between plasma DKK1, AFP and the percentage of TEMs in peripheral CD14+CD16+ monocytes, suggesting that DKK1, TEMs and AFP could complement each other for screening and diagnosing HCC.
In conclusion, the combination of TEMs, DKK1 and AFP showed a larger AUC than each biomarker alone. TEMs and DKK1 could be complementary to AFP in the diagnosis of HCC. Only TEMs but not DKK1 could be a complementary marker to AFP in diagnosing AFP-negative HCC versus LC patients.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
The authors would like to thank Xue Zhong for his technical support, and Wei Ding and Min Jiang for their help in sample collection. The authors also thank Shengyong Huang and Yan Wu for supporting AFP measurement of the present study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by the Project of “226 Project” of Nantong City in 2014 (CR201401).
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. [DOI] [PubMed] [Google Scholar]
- 2.Chaiteerakij R,Addissie BD, Roberts LR.Update on biomarkers of hepatocellular carcinoma.Clin Gastroenterol Hepatol. 2015;13:237–45. doi: 10.1016/j.cgh.2013.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu WW, Guo JJ, Guo L, Jia HL, Zhu M, Zhang JB, et al. Evaluation of midkine as a diagnostic serum biomarker in hepatocellular carcinoma. Clin Cancer Res. 2013;19:3944–54. doi: 10.1158/1078-0432.CCR-12-3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniele B, Bencivenga A, Megna AS, Tinessa V. Alpha-fetoprotein and ultrasonography screening for hepatocellular carcinoma.Gastroenterology. 2004;127:Suppl 1:S108–12. [DOI] [PubMed] [Google Scholar]
- 5.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–26. doi: 10.1016/j.ccr.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 6.Venneri MA, De Palma M, Ponzoni M, Pucci F, Scielzo C, Zonari E, et al. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007;109:5276–85. doi: 10.1182/blood-2006-10-053504 [DOI] [PubMed] [Google Scholar]
- 7.Tanaka F, Ishikawa S, Yanagihara K, Miyahara R, Kawano Y, Li M, et al. Expression of angiopoietins and its clinical significance in non-small cell lung cancer. Cancer Res. 2002;62:7124–9. [PubMed] [Google Scholar]
- 8.Coffelt SB, Tal AO, Scholz A, De Palma M, Patel S, Urbich C, et al. Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res. 2010;70:5270–80. doi: 10.1158/0008-5472.CAN-10-0012 [DOI] [PubMed] [Google Scholar]
- 9.Matsubara T, Kanto T, Kuroda S, Yoshio S, Higashitani K, Kakita N, et al. TIE2-expressing monocytes as a diagnostic marker for hepatocellular carcinoma correlates with angiogenesis.Hepatology. 2013;57:1416–25. doi: 10.1002/hep.25965 [DOI] [PubMed] [Google Scholar]
- 10.Shen Q, Fan J, Yang XR, Tan Y, Zhao W, Xu Y, et al. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol. 2012;13:817–26. doi: 10.1016/S1470-2045(12)70233-4 [DOI] [PubMed] [Google Scholar]
- 11.Ministry of Health of the People's Republic of China. Diagnosis management, and treatment of hepatocellular carcinoam (v2011). Journal of Clinical Hepatology. 2011;27:1141–59. [Google Scholar]
- 12.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134 [DOI] [PubMed] [Google Scholar]
- 13.Schauer D, Starlinger P, Reiter C, Jahn N, Zajc P, Buchberger E, et al. Intermediate monocytes but not TIE2-expressing monocytes are a sensitive diagnostic indicator for colorectal cancer. PLoS One. 2012;7:e44450 doi: 10.1371/journal.pone.0044450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian EN, Han SY, Ding SZ, Lv X. Expression and diagnostic value of CCT3 and IQGAP3 in hepatocellular carcinoma. Cancer Cell Int. 2016;16:55 doi: 10.1186/s12935-016-0332-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He YF, Wang CQ, Yu Y, Qian J, Song K, Sun QM, et al. Tie2-Expressing Monocytes Are Associated with Identification and Prognoses of Hepatitis B Virus Related Hepatocellular Carcinoma after Resection.PLoS One. 2015;10:e0143657 doi: 10.1371/journal.pone.0143657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueland T, Otterdal K, Lekva T, Halvorsen B, Gabrielsen A, Sandberg WJ, et al. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1228–34. doi: 10.1161/ATVBAHA.109.189761 [DOI] [PubMed] [Google Scholar]
- 17.Voorzanger-Rousselot N, Goehrig D, Facon T, Clézardin P, Garnero P. Platelet is a major contributor to circulating levels of Dickkopf-1: clinical implications in patients with multiple myeloma. Br J Haematol. 2009;145:264–6. doi: 10.1111/j.1365-2141.2009.07587.x [DOI] [PubMed] [Google Scholar]
- 18.Jang ES, Jeong SH, Kim JW, Choi YS, Leissner P, Brechot C. Diagnostic Performance of Alpha-Fetoprotein, Protein Induced by Vitamin K Absence, Osteopontin, Dickkopf-1 and Its Combinations for Hepatocellular Carcinoma. PLoS One. 2016;11:e0151069 doi: 10.1371/journal.pone.0151069 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.