Abstract
The rhizosphere, the narrow zone of soil around plant roots, is a complex network of interactions between plants, bacteria, and a variety of other organisms. The absolute dependence on host-derived signals, or xenognosins, to regulate critical developmental checkpoints for host commitment in the obligate parasitic plants provides a window into the rhizosphere’s chemical dynamics. These sessile intruders use H2O2 in a process known as semagenesis to chemically modify the mature root surfaces of proximal host plants and generate p-benzoquinones (BQs). The resulting redox-active signaling network regulates the spatial and temporal commitments necessary for host attachment. Recent evidence from non-parasites, including Arabidopsis thaliana, establishes that reactive oxygen species (ROS) production regulates similar redox circuits related to root recognition, broadening xenognosins’ role beyond the parasites. Here we compare responses to the xenognosin dimethoxybenzoquinone (DMBQ) between the parasitic plant Striga asiatica and the non-parasitic A. thaliana. Exposure to DMBQ simulates the proximity of a mature root surface, stimulating an increase in cytoplasmic Ca2+ concentration in both plants, but leads to remarkably different phenotypic responses in the parasite and non-parasite. In S. asiatica, DMBQ induces development of the host attachment organ, the haustorium, and decreases ROS production at the root tip, while in A. thaliana, ROS production increases and further growth of the root tip is arrested. Obstruction of Ca2+ channels and the addition of antioxidants both lead to a decrease in the DMBQ response in both parasitic and non-parasitic plants. These results are consistent with Ca2+ regulating the activity of NADPH oxidases, which in turn sustain the autocatalytic production of ROS via an external quinone/hydroquinone redox cycle. Mechanistically, this chemistry is similar to black and white photography with the emerging dynamic reaction-diffusion network laying the foundation for the precise temporal and spatial control underlying rhizosphere architecture.
Introduction
The plant rhizosphere is so richly populated by mutualistic associations that it has been dubbed the second plant genome [1]. With an estimated 1011 microbial cells/gm of root tissue [2] containing as many as 104 different species [3], the plant root must recruit, maintain, and defend this complex extracellular microbiome. Bacterial colonization depends on a rich and diverse chemical language where the rates of exudation, the inherent physical and biological stability of the agents, and the differential responses of members of the rhizosphere all contribute to this information rich and biologically dynamic signaling landscape [4–8]
Beyond their role in regulating plant-microbial associations, insights into the plant’s contribution to this signaling landscape has emerged from studies on: (i) host recognition by parasitic plants [9–12], (ii) kin recognition/selection [13–16], and (iii) allelopathy [17,18]. Our understanding of the redox dynamics of these chemical networks has expanded significantly with the discovery of semagenesis [19,20]. In this process, reactive oxygen species (ROS) exuded from the root tip of the parasite initiates a mild ‘wound’ response that ultimately leads to oxidation of a prospective host’s cell wall phenols into p-benzoquinones (BQs) [19,21]. Persistent exposure of the parasite root meristem to BQs is necessary and sufficient to induce organogenesis of the parasite’s attachment organ, the haustorium [9,22,23], and serves as a signature for viable host roots.
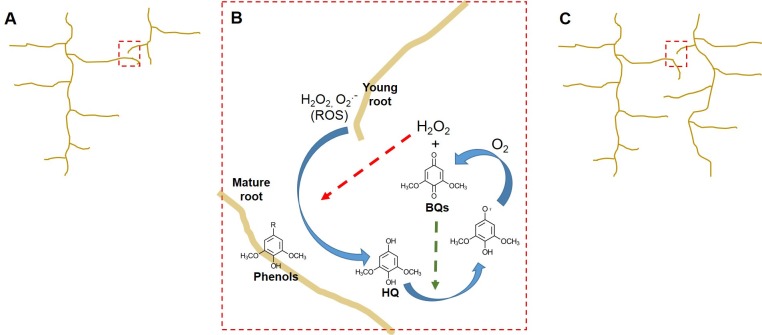
Evidence that non-parasitic members of the Eudicotidae clade, including Nicotiana tabacum and Arabidopsis thaliana [24], also produce ROS at the growing root tip suggested that semagenesis may serve a broader role in the rhizosphere. Indeed, the same chemical network which generates BQs via the oxidation of host derived phenols by parasite derived ROS, could reasonably be recreated by replacing the parasite root with any root tip. We propose that the pairing of the roots between non-parasites would provide a mechanism for younger roots to detect a more established neighbor and adjust root system architectures (RSA) accordingly (Fig 1).
Fig 1. ROS/phenol/BQ reaction network in plants.
Simple model for ‘quorum’ or collision sensing in Arabidopsis. (A) A growing lateral root from a young seedling encounters an established plant (red box). (B) Within a certain distance, ROS produced by the tip of the growing seedling contributes to oxidation of cell wall associated phenols to yield BQs (red arrow). DMBQ is shown here as an example. These BQs accumulate, contribute to the autocatalytic production of new ROS intermediates, amplify the signal (green arrow). (C) Evidence of an established root system arrests root elongation in a quorum-like process, regulating global root system architecture.
The half-life of hydrogen peroxide (H2O2) in biotic matrices is estimated to be on the order of milliseconds, with other ROS intermediates decomposing even faster [25–27], consistent with the oxidation of phenols occurring only within 50 μm of the growing root tip [9,22,23]. In the mixed-microbial biofilms where H2O2 is used as an antimicrobial agent by streptococci, its diffusion zone may be limited to 4 μm [28], providing an even more precise ruler in those matrices. In addition, the hydroquinone (H2Q) intermediates, which accumulate during the reaction between root-tip derived ROS and cell wall phenols, can further react with molecular oxygen to generate additional H2O2 (Fig 1B). Thus H2O2 concentrations may amplified chemically outside the root cells [29] as shown in Fig 1. Here, H2O2 initiates phenol decomposition yielding the hydroquinone, H2Q, which in the presence of oxygen generates BQ and more H2O2 [30]. In this scenario, the concentration and spatial distribution of H2Q, BQ, and ROS, all critical members of this autocatalytic cycle, are co-dependent and defined by the oxygen tension in the rhizosphere.
We now report a simple assay to evaluate the impact of root density on growth of A. thaliana seedlings and provide evidence that BQ exposure recapitulates the root density phenotype [15,31,32]. Further, we report a comparative study of calcium fluctuations that connect BQ exposures in Striga and Arabidopsis spp. Taken together, these results support semagenesis as an active quorum sensing-like strategy. Further, since both pathogenic and mutualistic Rhizobium spp depend on the very phenols involved in this dynamic chemical network, the redox circuit should also impact their symbiotic behaviors. We argue that these dynamic redox chemical networks provide critical environmental information to a broad array of inhabitants of the rhizosphere. Finally, we propose that semagenesis provides quorum sensing (QS) information on root density, a process that has been effectively re-deployed by parasitic members for the purposes of host detection.
Materials and methods
Materials
Agarose, sucrose, and MES were obtained from Merck (Darmstadt, Germany). H2DCF-DA was purchased from Molecular Probes (Carlsbad, CA). All other chemicals were purchased from Sigma Aldrich (St. Louis, MO) or Caisson Labs (Logan, UT).
Pre-treatment & germination of Striga
Striga asiatica seeds were obtained from Drs. R.E. Eplee and Rebecca Norris (U.S. Department of Agriculture, Witchweed Methods Development Laboratory; Oxford, NC). All S. asiatica work was done under the auspices of the USDA quarantine licenses awarded to Emory University. S. asiatica seedlings were pre-treated by washing the seedlings in the following order: 3% chromic acid for 3 minutes, a solution of 1% Tween-20 and 7% bleach for 7 minutes, and finally 70% ethanol for 1 minute. The seeds were then rinsed and placed in ddH2O for 10–14 d in capped Erlenmeyer flasks. Following incubation, the seeds were germinated by 24 h exposures to 10−9 M strigol in 0.1 mM KCl [21].
Sterilization and growth of Arabidopsis seedlings
Arabidopsis thaliana seedlings (50 μl) were sterilized by successive 3 minute treatments with: (i) 5% Bleach + 0.05% Tween-20 solution and (ii) 70% ethanol. Samples were rinsed 3 times with an equal volume of sterile distilled water after both treatments. Sterile seeds were suspended in 1 mL of dH2O and spread on MS plates (0.25X Murashige & Skoog salts, 1% sucrose, and 0.8% agar, pH: 5.6). Seedlings were plated with approximately 50 seedlings/plate, stored in the dark at 4°C for 3–4 days, and then grown for 5–7 days in a temperature controlled growth chamber (22°C) with a 16 h photoperiod.
Haustorial induction and inhibition
All Striga experiments are initiated one day post-germination. Striga seedlings were placed in 6 well plates (30 seedlings/well) with 5 mL of 0.1 mM KCl, 10 μM DMBQ, and the indicated concentration of LaCl3. Seedlings are scored as having successfully formed a haustorium if the root tip displays both swelling as well as haustorial hair formation. The entire process of haustorium development requires 24 hours, so hair formation can only be observed in two day-old seedlings. For timed exposure assays, the treatment was applied for the indicated time interval, after which the liquid was removed from the well and the seedlings were washed three times with 0.1 mM KCl. Seedlings were then placed in 5 mL of 0.1 mM KCl and scored for haustorial development at 24 hours. Reversibility of LaCl3 inhibition was accomplished by triplicate washings with 100 μM CaCl2 followed by returning seedlings to the 0.1 mM KCl buffer. Unless otherwise stated, the data are expressed as the average of three experiments ± standard deviation.
H2DCF-DA imaging for ROS
Seedlings of S. asiatica and A. thaliana were analyzed for ROS production using H2DCFDA as a fluorescent probe as previously described [19]. Bovine liver catalase was used to scavenge ROS in the media by adding 250 units/ml of culture, incubating for 1 hour at ambient temperature, and inactivation by heating the sample to 100°C on a heat block for 15 minutes. Seedling density varied from 1 seedling per well to a maximum of 15 seedlings per well (5 seedlings/1 ml).
Ca2+ imaging in Striga asiatica
One day-old seedlings of Striga asiatica were loaded with 50 μM Fluo-4 AM for 20 minutes at 4°C then returned to room temperature for 10 minutes. Seedlings were then washed in triplicate with ddH2O, transferred to a well of an 8 well microscope slide, resuspended in 250 μl of phosphate buffer (pH: 6.1) and imaged for basal Ca2+ via an epifluorescent microscope at set time points (Ex: 488/Em: 535 nm). The effect of DMBQ was evaluated by the addition of sufficient compound from stock to produce a 10 μM solution. Images were collected at the indicated time intervals to evaluate changes in fluorescence.
LaCl3 exposures in A. thaliana
Following the growth period, A. thaliana seedlings were transferred to 6 or 12-well plates and treated with 3 ml of 0.25X MS media (0.25X Murashige & Skoog salts, 1% sucrose, and 0.8% agar, pH: 5.6) with or without LaCl3 (standard concentration of LaCl3 used was 10 μM). Seedling density varied from 1 seedling per well to a maximum of 15 seedlings per well.
Imaging YC3.6 seedlings
7–10 day-old seedlings of A. thaliana expressing the fluorescence resonance energy transfer (FRET)-based Ca2+ sensor YC3.6 [33,34] were transferred to deep-well depression slides for visualization along with 1 ml of 0.25X Murashige & Skoog media. Ratiometric imaging was performed on a Nikon C1 laser scanning confocal microscope by exciting the cyan fluorescent protein (CFP) at 457 nm. Emission from CFP (λem = 473–505 nm) and from FRET-dependent Venus (λem = 526–536 nm) was collected at 480 nm and 528 nm.
Data processing
Data analysis was conducted using the statistical program R (version 2.13.1). YC 3.6, Fluo4-AM, and H2DCF-DA fluorescence intensities were analyzed using Fiji (ImageJ).
Results
Calcium mediates haustorial organogenesis and ROS production in S. asiatica
BQ-initiated reduction of ROS production in S. asiatica has been correlated with down-regulation of the NADPH oxidase 1, SaNOX1 [35], which contains two Ca2+-binding EF-hand domains. The activity of EF-hand containing NADPH oxidases has been positively correlated with Ca2+ concentrations in A. thaliana, supporting a link between BQ-mediated ROS production and calcium regulation [36–38]. Furthermore, given the morphological signatures of haustorial growth, swelling of the root tip and development of the haustorial hairs [39,40], the general role of calcium dynamics on ROS production [36,41–43], as well as polar growth of root hairs [34,41], we reasoned that Ca+2 signaling may integrate BQ exposures to internal biochemical events in plants.
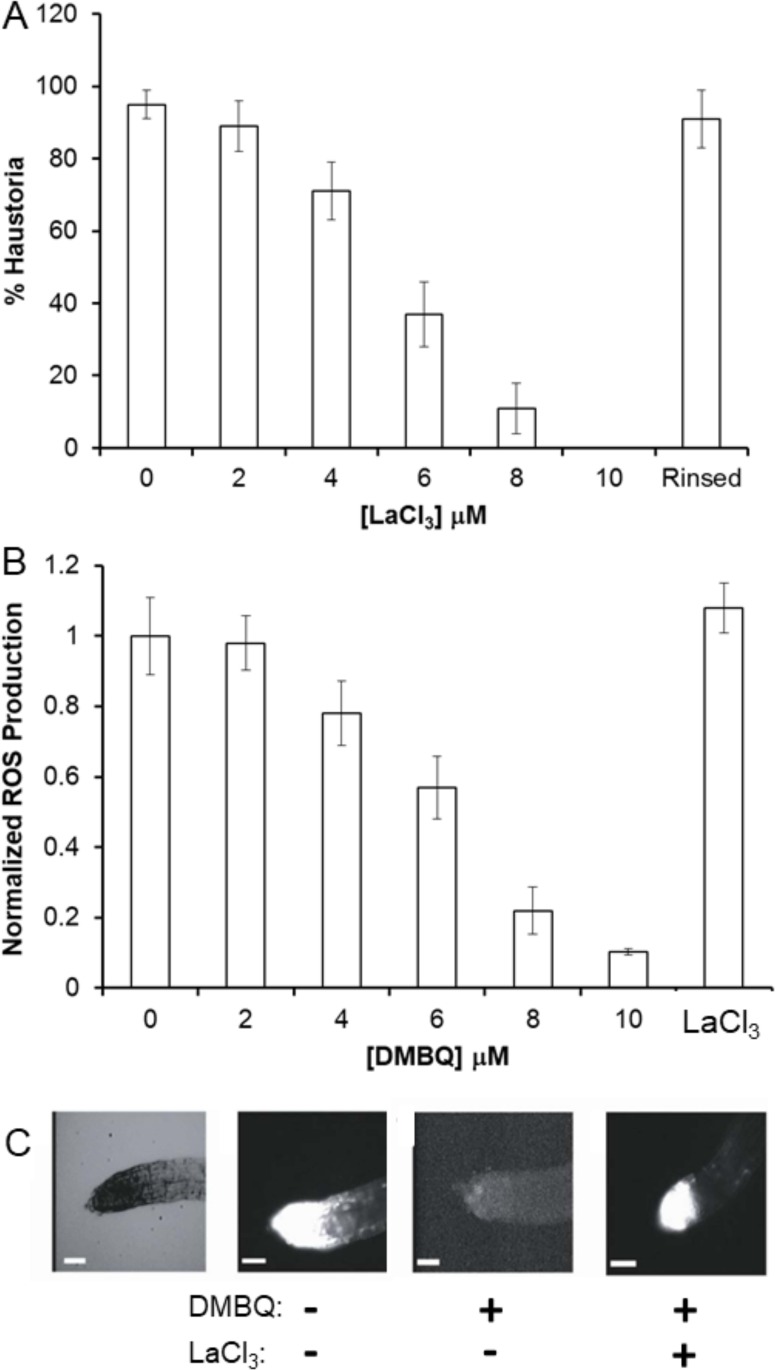
We began by evaluating whether the downstream response to BQ exposures is mediated through cellular calcium dynamics despite the differential phenotypic responses between parasites and non-parasites. Fig 2A shows the effect of the non-specific Ca2+-channel inhibitor lanthanum chloride (LaCl3) [44] on S. asiatica haustorial development when induced with 2,6-dimethoxy-p-benzoquinone (DMBQ). Under these conditions, half-maximal inhibition occurs at 5 μM LaCl3 but is reversed by rinsing the seedlings with CaCl2 (Fig 2A). The effects of DMBQ on ROS production can be observed using the cell-permeable ROS-sensitive fluorophore H2DCF-DA (2’,7’-dichlorodihydrofluorescein diacetate) [19]. DMBQ significantly reduces ROS accumulation in S. asiatica root tips (Fig 2B and 2C), and 10 μM LaCl3 effectively blocks DMBQ-induced down-regulation of ROS production. In the absence of these xenogenosins (BQs), no haustorial formation occurs.
Fig 2. LaCl3 inhibits DMBQ perception.
(A) Seedlings were treated with 10 μM DMBQ and the indicated concentration of LaCl3, then scored for haustorium formation after 24 h. ‘Rinsed’ seedlings were subsequently washed with 100 μM CaCl2, re-exposed to 10 μM DMBQ and scored for haustorium development after 24 h. (B) Seedlings treated with the indicated concentration of DMBQ for 2 hours were then incubated with H2DCF-DA and visualized for ROS production. LaCl3 seedlings were treated with 10 μM DMBQ and LaCl3 simultaneously, incubated for 2 hours then imaged for ROS production. (C) Bright field (light background) and H2DCF fluorescence images (dark background) of S. asiatica seedlings 2 hours after treatments with (+) or without (-) 10 μM DMBQ and/or LaCl3. Bar = 100 microns. All experiments conducted on day-old seedlings of Striga asiatica in triplicate with results expressed as average ± SD.
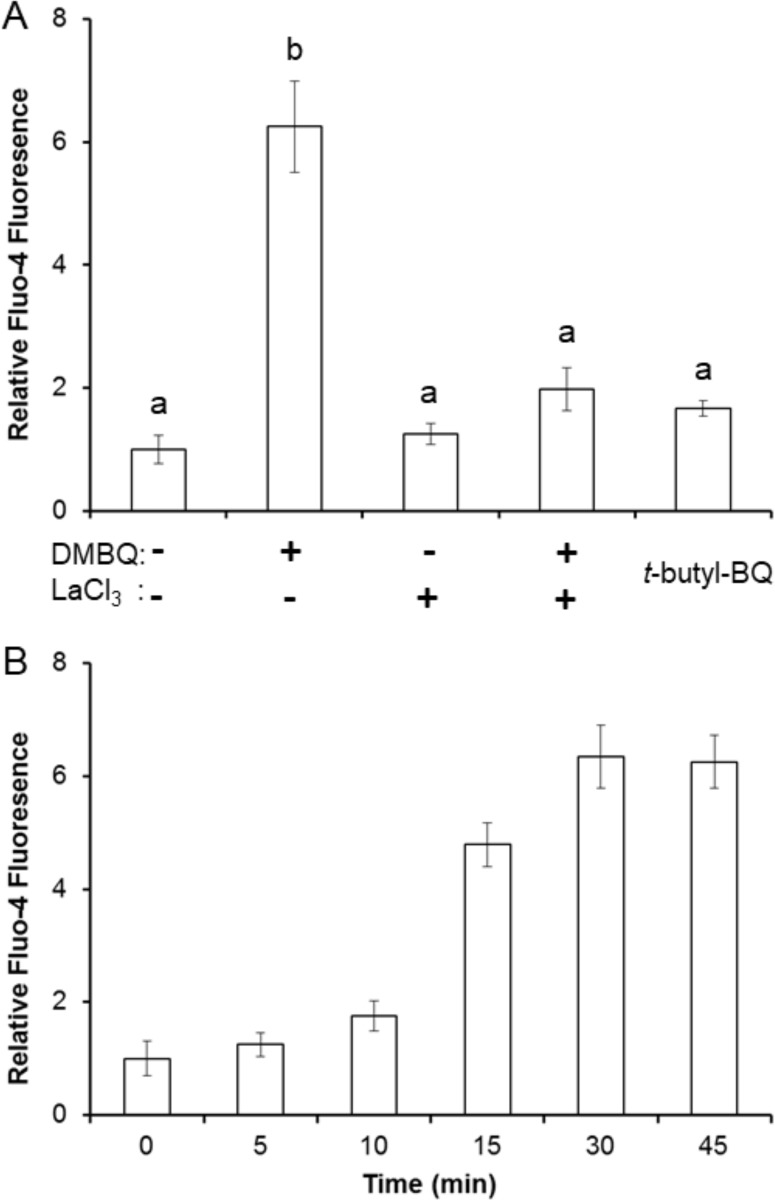
To directly visualize changes in cytoplasmic calcium concentrations ([Ca2+]cyt) in S. asiatica seedlings, we utilized the cell permeable fluorescent Ca2+-indicator Fluo4-AM [45]. Ten minute exposures to 10 μM DMBQ significantly increases Fluo4 fluorescence (Fig 3A) at the root tips of S. asiatica seedlings and this increase persists for at least 30 minutes (Fig 3B). LaCl3 inhibits terminal haustorial commitment and ROS production, consistent with a BQ-mediated influx of cytoplasmic calcium as a critical step early in the response pathways. The observation that the non-haustorial-inducing tert-butyl-p-benzoquinone does not increase [Ca2+]cyt supports the effect being specific to haustorial inducing quinones.
Fig 3. LaCl3 inhibits DMBQ perception in Striga asiatica.
(A) Seedlings were treated with 10 μM DMBQ and/or 10 μM LaCl3 or with t-butyl-BQ for 1 hour then loaded with Fluo-4 AM and imaged for calcium-dependent fluorescence. (B) Seedlings were incubated with 10 μM DMBQ for the indicated time period then loaded with Fluo-4 AM and imaged for calcium dependent fluorescence. All experiments conducted on day-old seedlings of S. asiatica in triplicate with results expressed as average ± SD. Samples with different letters are statistically distinct from one another (p<0.05) based on a Tukey’s post-hoc test.
BQs regulate ROS and calcium flux in the root tips of A. thaliana
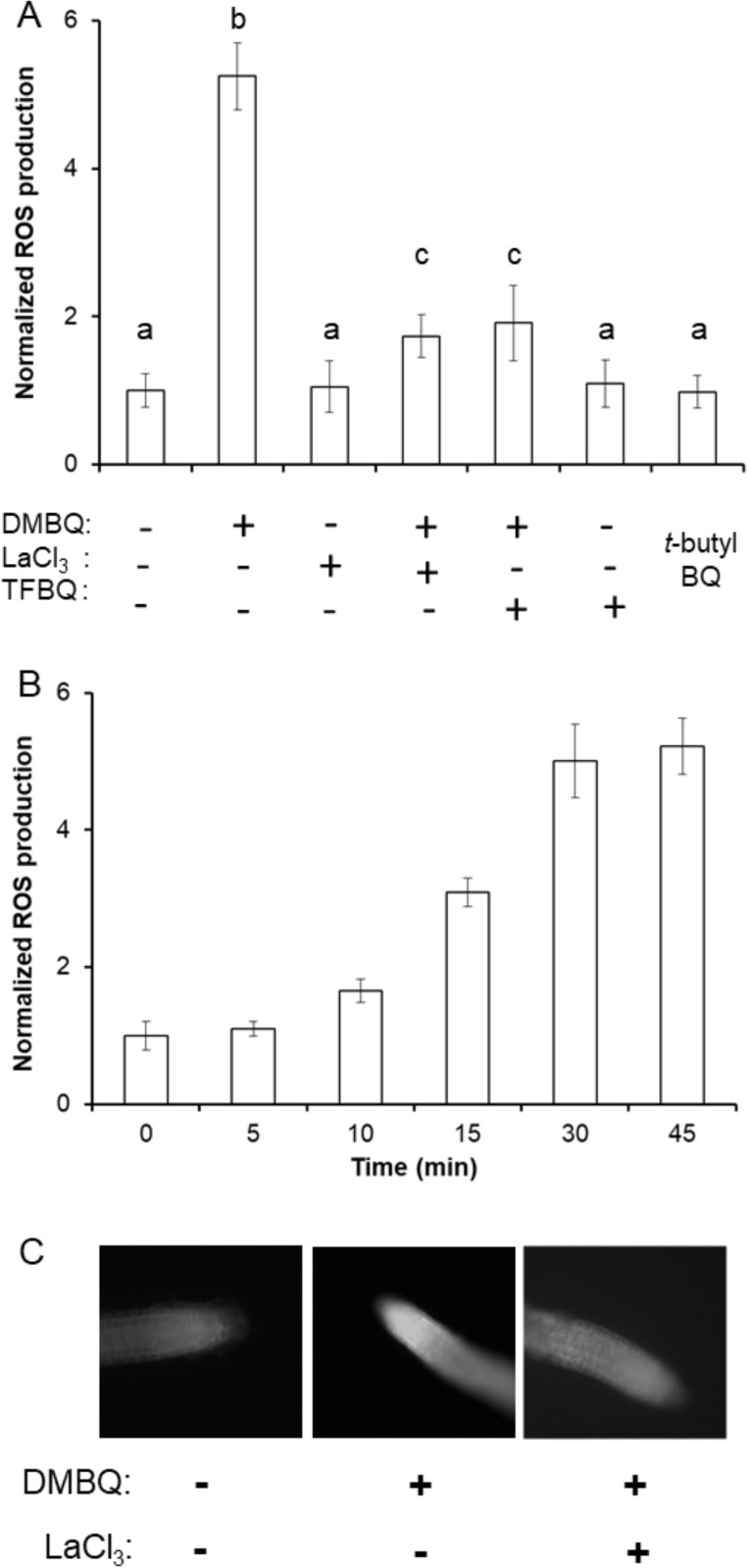
H2DCF-DA treatments confirm that DMBQ increases (Fig 4A), rather than decreases, ROS accumulation in the root tips of both N. tabaccum and A. thaliana. This is consistent with previous studies, that observed this increase in N. tabaccum seedlings with the ROS sensitive stain nitroblue tetrazolium [24]. Increased H2DCF-DA fluorescence is not seen with the non-haustorial inducing t-butyl BQ and is inhibited by LaCl3 (Fig 4A). Most importantly, ROS accumulation is inhibited by the BQ structural analog tetrafluorobenzoquinone (TFBQ), designed specifically to inhibit the redox events associated with haustorial-inducing BQs in S. asiatica (Fig 4A)[46,47]. DMBQ-induced production of ROS steadily increases over 1 hour (Fig 4B), consistent with the time previously shown to be required for haustorial induction in S. asiatica [19,47].
Fig 4. DMBQ induction of ROS depends on Ca2+ in A. thaliana seedlings.
(A) Seedlings were treated with 10 μM DMBQ, LaCl3, and TFBQ as indicated for 1 hour then visualized for ROS production with H2DCF-DA. (B) Seedlings were treated with 10 μM DMBQ for the indicated period of time then visualized for ROS production with H2DCF-DA. Relative ROS production is normalized to DMBQ-free seedlings treated with H2DCF-DA, and all experiments are conducted in triplicate and expressed as average ± SD. Samples with different letters are statistically distinct from one another (p<0.05) based on a Tukey’s post-hoc test.
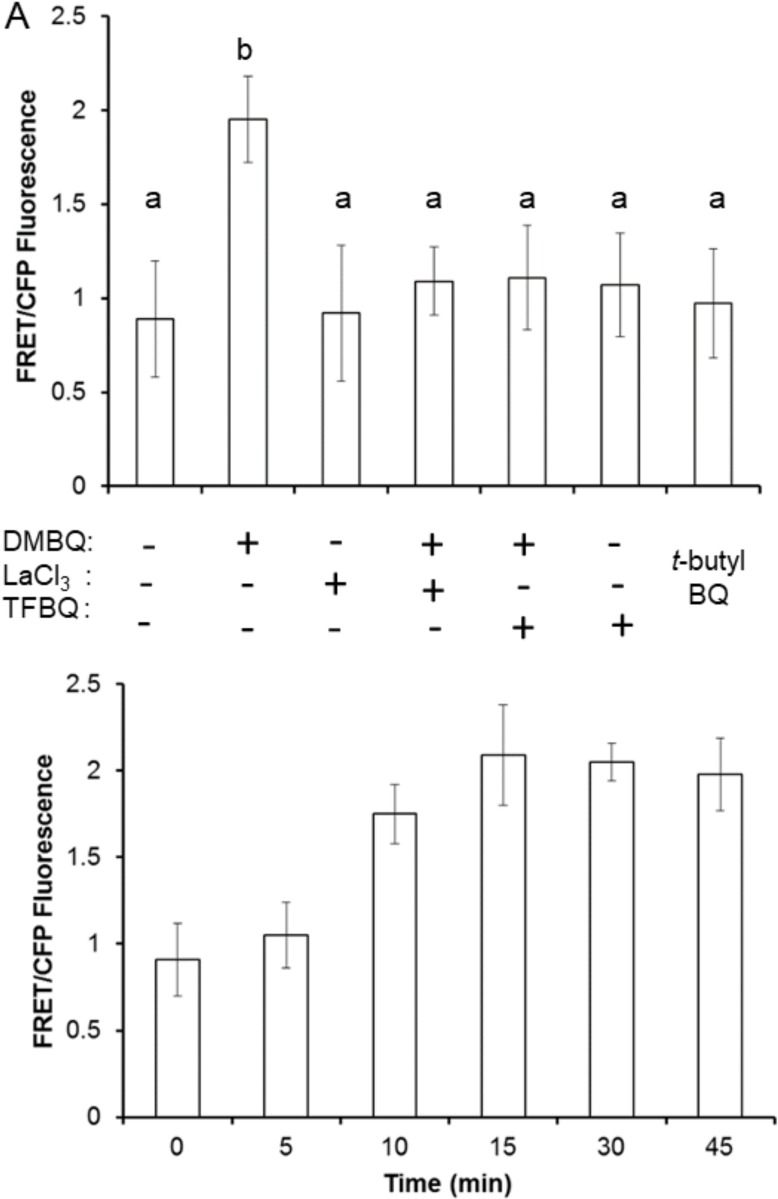
Calcium dynamics in A. thaliana was specifically evaluated in strains expressing the YC3.6 FRET-based calcium reporter. This fusion protein consists of a cyan fluorescent protein (CFP - λem = 473–505 nm) linked by a calcium-sensitive calmodulin domain to a circularly permutated Venus (cpVENUS) fluorescent protein (λem = 526–536 nm)[34]. Calcium-binding to calmodulin brings these two fluorophores into contact, increasing resonance energy transfer from CFP to cpVENUS, creating a real-time, highly sensitive probe of [Ca2+]cyt. FRET-dependent VENUS fluorescence in this strain increases nearly two-fold on exposure to 10 μM DMBQ, consistent with an increase in [Ca2+]cyt. As in S. asiatica, increased [Ca2+]cyt is inhibited by LaCl3 and TFBQ (Fig 5A). A statistically significant increase in [Ca2+]cyt is detectable within 10 min of DMBQ exposure, plateaus after 15 min, and is sustained for at least 45 min (Fig 5B). Again, the non-haustorial-inducing tert-butyl-p-benzoquinone does not increase fluorescence (see Fig 5A).
Fig 5. [Ca2+]cyt -dynamics in A. thaliana YC3.6 seedlings in response to DMBQ.
(A) Seedlings are treated with 10 μM DMBQ, LaCl3,.t-butyl BQ, and/or TFBQ as indicated for 1 hour then visualized for calcium changes. (B) Seedlings are loaded with 10 μM DMBQ and incubated for the indicated time before being scored for calcium-dependent fluorescence changes. All experiments are conducted on seedlings of A. thaliana YC3.6 in triplicate and the results are expressed as average ± SD. Samples with different letters are statistically distinct from one another (p<0.05) based on a Tukey’s post-hoc test.
BQs simulate the effects of high root density
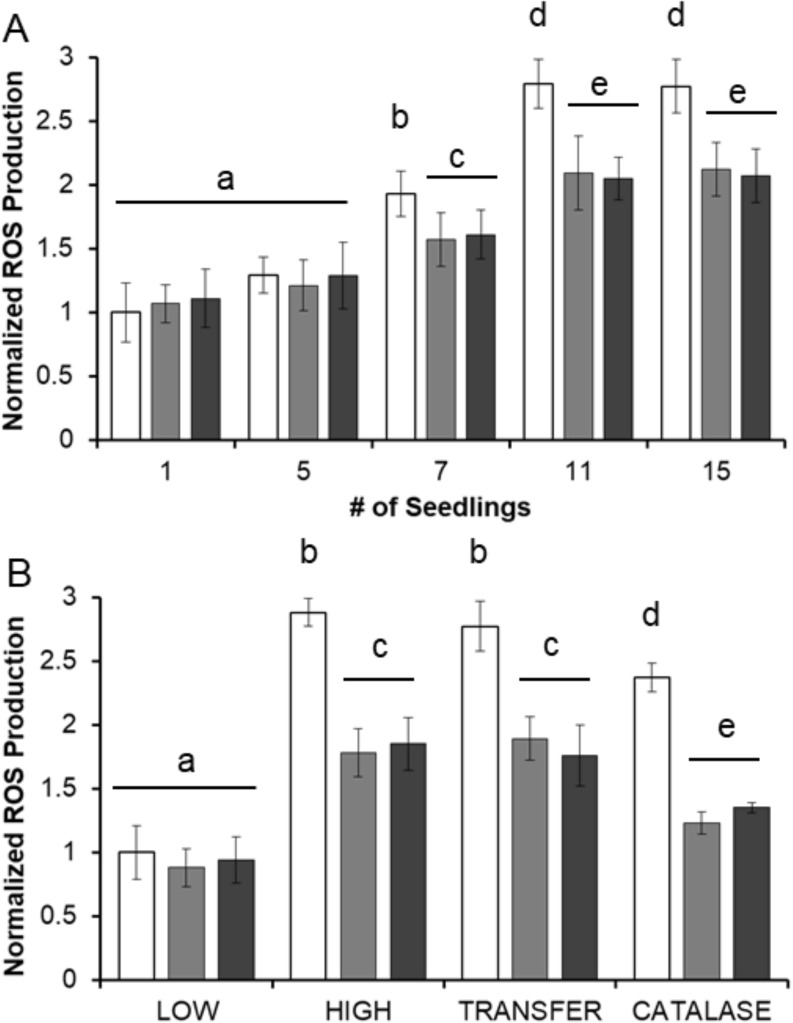
To determine if semagenesis plays a functional role in non-parasite communities, a series of plant ‘density’ assays are outlined in Fig 6. A. thaliana seedlings are incubated at increasing densities (1, 5, 7, 11, or 15 seedlings) in 3 ml of MS media, and then evaluated for ROS production by H2DCF-DA. As seen in Fig 6A, ROS production positively correlates with seedling density. Density-dependent increases in ROS production are partially but not completely inhibited by LaCl3 and TFBQ in any of these treatments. Higher concentrations (>50 μM) of the inhibitors induce leaf chlorosis and/or necrosis within 24 h, limiting their usable concentration range. Placing a single seedling in 200 μl of buffer for 2 hours, the same density as 5 seedlings/ml, gave similar fluorescence intensities as with 15 seedlings in 3 ml (Fig 6B).
Fig 6. Root density impacts ROS production in A. thaliana.
(A) The indicated number of seedlings are placed in 3 ml of MS media containing: 10 μM DMBQ (white), 10μM DMBQ and 10 μM LaCl3 (light grey), or 10 μM DMBQ and 10μM TFBQ (dark grey). After 2 hours the seedlings were visualized for ROS production with H2DCF-DA. (B) Individual seedlings were stored at ‘low density’ (1 seedling in 3 ml), ‘high density’ (1 seedling in 200 μl), or ‘transfer’ media (3 ml of media previously used to store 15 seedlings) with or without catalase treatment. Media was either supplemented with just DMSO stock (white) or with the addition of 10 μM LaCl3 (light grey) or 10μM TFBQ (dark grey). After 2 hours samples were visualized for ROS production with H2DCF-DA. All experiments conducted on 30 seedlings of A. thaliana in triplicate with results expressed as average ± SD. Samples with different letters are statistically distinct from one another (p<0.05) based on a Tukey’s post-hoc test.
Oxidation of wall phenols is expected to yield a range of BQs with DMBQ as a representative example. While ROS are not stable in a biological matrix, the BQs, notably DMBQ, might accumulate in these matrices. To test that possibility, seedlings maintained at ‘low’ density (1 seedling in 3 ml) are incubated for 2 hours in ‘high’ density media recovered from separate solutions prepared by pre-incubating 5 seedlings/ml for 2 hours. ROS production increases in the transferred media and LaCl3 and TFBQ exposures again partially inhibit this increase (Fig 6B). However, it is possible that observed increases in ROS production in transferred media are due to carry over of ROS. To control for ROS transfer, the high-density media is treated for 1 h with catalase to scavenge H2O2 then inactivated by heat. As seen in Fig 6B, catalase-treated samples from ‘high density seedling’ media increase ROS production and are attenuated by both LaCl3 and TFBQ treatments, reflecting the transfer of a stable inducing signal. The exogenous addition of 5 μl/ml H2O2 solution (3% w/v) to these samples resulted in increased fluorescence accumulation (from ≈2.37 of control to 3.25 +/- 0.2 normalized ROS production), arguing against the lower value arising from complete reaction of the available dye or reduced dye incorporation.
Discussion
Opportunistic parasitic plants provided the first clear evidence of a redox-based, dynamic chemical network functioning along the plant root surface [23,47,48]. Here we show that this process may be far more widespread across diverse rhizospheres. This redox circuit alters the overall RSA of plants in close proximity to one another, a phenomenon not unlike the density-dependent phenotypic switching observed in many bacteria and some fungi known as quorum sensing [49–51]. However, unlike traditional quorum sensing signals (autoinducers) that are synthesized by individuals, ROS/quinone networks arise from a dynamic chemical reaction that depends on two distinct partners. The growing plant root meristem, which has relatively low cell wall phenol densities [24,35], provides reactive oxygen species, while another more mature root provides the requisite phenols, allowing this process to serve as an active detection system for the presence of neighboring mature root surfaces.
In both S. asiatica and A. thaliana, BQ perception leads to an increase in cytoplasmic calcium concentrations in approximately the same time frame (≥ 15 min). Blocking the Ca2+ channels with La3+ ions attenuates the plant’s sensitivity to BQ exposure. Similarly, the antioxidant quinone TFBQ, which does not generate H2O2 via the mechanisms described in Fig 1, disrupts both Ca2+ dynamics and BQ signaling. While both plants respond to the same chemical signal with an increase of [Ca2+]cyt, the other biochemical and phenotypic responses are remarkably different between parasites and non-parasites. While in parasites the pathway regulates the vegetative/parasitic transition, in non-parasites the pathway functions in a traditional quorum sensing mode with signal (BQ) perception stimulating ROS production to further enhance signal production. Similar autocatalytic behaviors are well-documented components of most QS systems ensuring population-wide responses [50,51].
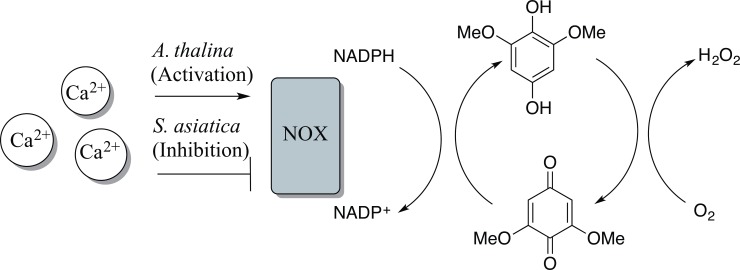
Detailed examination of the reaction mechanism of ROS generation in the presence of DMBQ can explain these differences (Fig 7). NADH readily reduces quinones to hydroquinones via hydride transfer reactions [52]. Hydroquinones are oxidized by molecular oxygen in a reaction pathway that involves semiquinone and superoxide radical intermediates and results in the production of hydrogen peroxide [53–55]. This reaction scheme is indirectly supported by the insensitivity of A. thaliana to the antioxidant t-butyl benzoquinone (TBBQ) that cannot chemically produce H2O2 via autoxidation. A BQ’s specificity as a haustorial inducer also correlates with the quinone’s redox potential, with a narrowly defined window between -280 and +20mV [47], consistent with the redox cycling model and providing indirect evidence for an operating quinone-mediated reaction diffusion network.
Fig 7. Proposed role of Ca2+ balance in BQ regulated root recognition in parasitic and non-parasitic plants.
DMBQ reduction by plant’s NADPH- oxidase is needed to maintain the production of hydrogen peroxide via redox cycling. Ca2+ assisted NADPH down-regulation of NADPH oxidase in Sriga leads to decrease the rate of hydrogen production while up-regulation of the enzyme in A. thaliana leads to rise in ROS concentration along the root surface that ultimately is perceived as a signal to shut down the root growth.
Up-regulation of NOX enzyme activity, triggered by increases in Ca2+ concentration in Arabidopsis [38], would increase the rate of hydroquinone production, and subsequently, produce higher H2O2 concentrations. In this positive feedback loop, even low initial concentrations of the benzoquinone may result in high final H2O2 concentrations. In soil, metal ions and biotic enzymes, including cell wall catalases, may rapidly decompose H2O2. However, if hydroquinone regeneration by NADH is maintained, a steady state concentration of H2O2 around the root surface could persist for extended durations. In S. asiatica, however, the initial increase in ROS concentration is followed by a slower decay. The hour-long timing under these conditions is consistent with BQ exposure necessary to induce the vegetative/parasite transition in S. asiatica [19,23,47].
Our recent investigations of the differential expression of S. asiatica genes following exposure to DMBQ have shown a dramatic decrease in expression of a specific Ca2+-regulated NADPH oxidase (SaNOX1) during haustorial development [35]. Consistent with the mechanism proposed in Fig 7, reduction of SaNOX1 activity will decrease the rate of DMBQ cycling and ROS generation. While SaNOX1 expression is down regulated after sufficient BQ exposure, ROS production declines in the parasite faster than the loss of gene expression. How the production of ROS by SaNOX1 is limited in the presence of increased calcium remains unclear but certainly represents a fundamental point of divergence between parasitic and non-parasitic plants. This simple change, and the subsequent variations to the dynamic chemical network in the rhizosphere, allows the parasites to utilize this process to gauge host proximity. Calcium sensitivity in plant-related NOX proteins may be regulated by phosphorylation, suggesting a redox-sensitive kinase could be responsible for this differential regulation [56,57].
The source of the observed calcium influx will be critical to understanding this network, but has yet to be determined as LaCl3 is a non-specific inhibitor of calcium channels. Future work will attempt to map both the origin of the observed calcium influx as well as the regulatory elements and organization of these processes in both plants. However, the ability of these channel inhibitors to prevent BQ induced regulation of ROS production, either up or down, places calcium regulation as an early determinant in this response network.
Oxygen is another critical component of the circuit shown in Fig 7. Recent visualizations of oxygen concentration with planar optodes show sharp concentration gradients along the mature plant roots [58]. Accordingly, the plant root may be able to direct the spatial and temporal distribution of both oxidant and reductant, creating an “external metabolism” of functional significance [59] for plant-plant and plant-microbe signaling. Such redox-mediated reaction-diffusion networks can be complex, generating chemical waves, steady state pattern formation, bistability, and oscillations [60,61], and the details of how such processes operate in the rhizosphere can now be further investigated.
This proposed external redox metabolism may be just one of many existing in the rhizosphere. For example, the monocot hosts of S. asiatica are known to exude specific electron-rich hydroquinones that accumulate at high concentrations along their root surface [48]. The sorghum-derived germination stimulant spatially restricts S. asiatica seed germination to the host root surface. Given that this electron rich hydroquinone is readily autoxidized, these reactions might be an even more critical part of dynamic reaction-diffusion rhizosphere networks [9].
The autoinducers associated with quorum sensing regulate a wide variety of bacterial phenotypes, including bioluminescence, virulence factor production, biofilm formation, and motility [4], providing physiological benefits to other members of the rhizosphere ranging from pathogenesis to nitrogen fixation in legume-rhizobia mutualisms [62–64]. Many of these behaviors are also regulated by plant phenols, potentially coupling the redox pathways defined here to bacterial behaviors. Further, since both pathogenic and mutualistic Rhizobium spp depend on the phenols, the redox circuit may well impact many of these symbiotic behaviors. Much as cell wall fragments [65] and membrane fatty acids [66] serve as secondary signals for coordinating a variety of eukaryotic cellular responses [67], this external redox signaling network may coordinate significant regional architectures broadly across the rhizosphere.
Finally, it should be noted that the chemistry of hydroquinone auto-oxidation has long been exploited in black and white photography to generate high resolution 2D images. These 2D images are generated when silver salt grains, partially activated by exposure to sunlight, are reduced to silver in the presence of hydroquinones. An initially weak signal is amplified several orders of magnitude by two autocatalytic processes: surface-catalyzed silver reduction and hydroquinone autoxidation. At the same time, the spatial propagation of the signal remains tightly regulated due to the high reaction order and presence of inhibitors, such as sulfite, in developing solutions (69). Building on similar chemistry, the 3D spatial resolution around the plant root could be remarkably precise, providing a dynamic chemical ‘movie’ of the complex multi-component rhizosphere dance[68]. Defining reaction networks that regulate the plant’s second genome are even more necessary and invaluable in this time of our rapidly changing physical climate.
Acknowledgments
We thank Emory University for undergraduate student research fellowships to AWF and PY, the Florida Institute of Technology for graduate fellowship support of SL, the NIH FIRST Fellowship to BDP, and Emory University’s Department of Neurology for fellowship support to OT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper. There are no supplemental figures.
Funding Statement
We thank Emory University for undergraduate student research fellowships to AWF and PY, the Florida Institute of Technology for graduate fellowship support of SL, the NIH FIRST Fellowship to BDP, and Emory University’s Department of Neurology for fellowship support to OT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Berendsen RL, Pieterse CMJCMJ, Bakker PAHM. The rhizosphere microbiome and plant health. Vol. 17, Trends in Plant Science. 2012. p. 478–86. doi: 10.1016/j.tplants.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 2.Mendes R, Kruijt M, de Bruijn I, Dekkers E, van der Voort M, Schneider JHM, et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science (80-). 2011;332:1097–100. doi: 10.1126/science.1203980 [DOI] [PubMed] [Google Scholar]
- 3.Egamberdieva D, Kamilova F, Validov S, Gafurova L, Kucharova Z, Lugtenberg B. High incidence of plant growth-stimulating bacteria associated with the rhizosphere of wheat grown on salinated soil in Uzbekistan. Environ Microbiol. 2008;10(1):1–9. doi: 10.1111/j.1462-2920.2007.01424.x [DOI] [PubMed] [Google Scholar]
- 4.Waters CM, Bassler BL. Quorum Sensing: Communication in Bacteria. Annu Rev Cell Dev Biol [Internet]. 2005. January [cited 2012 Oct 27];21(1):319–46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16212498 [DOI] [PubMed] [Google Scholar]
- 5.Palmer AG, Senechal AC, Mukherjee A, Ané J-M, Blackwell HE. Plant responses to bacterial N-acyl L-homoserine lactones are dependent on enzymatic degradation to L-homoserine. ACS Chem Biol [Internet]. 2014. August 15 [cited 2015 Apr 28];9(8):1834–45. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24918118 doi: 10.1021/cb500191a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binns AN, Joerger RD, Banta LM, Lee K. Molecular and Chemical Analysis of Signal Perception by Agrobacterium In: Advances in Molecular Genetics of Plant-Microbe Interactions Vol 2 Kluwer Academic Publishers; 1993. p. 51–61. [Google Scholar]
- 7.Sergeevich SA, Alexandrovich ZV, Yurievna SO, Yurievich BA, Anatolievich TI. Nod-Factor Signaling in Legume-Rhizobial Symbiosis In: El-Shemy H, editor. Plants for the Future. InTECH; 2015. p. 135–60. [Google Scholar]
- 8.Lin YH, Fang F, Pierce BD, L. D.G. A Rhizobium radiobacter histidine kinase can employ both Boolean AND and OR logic gates for initiating pathogenesis. ChemBioChem. 2015;16:2183–90. doi: 10.1002/cbic.201500334 [DOI] [PubMed] [Google Scholar]
- 9.Fate G, Chang M, Lynn DG. Control of Germination in Striga asiatica: Chemistry of Spatial Definition. Plant Physiol [Internet]. 1990. May;93(1):201–7. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1062489&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynn DG, Chang M. Phenolic Signals in Cohabitation: Implications for Plant Development. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:497–526. [Google Scholar]
- 11.Yoder JI. Host-plant recognition by parasitic Scrophulariaceae. Vol. 4, Current Opinion in Plant Biology. 2001. p. 359–65. [DOI] [PubMed] [Google Scholar]
- 12.Palmer AG, Gao R, Maresh J, Erbil WK, Lynn DG. Chemical biology of multi-host/pathogen interactions: chemical perception and metabolic complementation. Annu Rev Phytopathol [Internet]. 2004. January [cited 2012 Nov 5];42(70):439–64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15283673 [DOI] [PubMed] [Google Scholar]
- 13.Depuydt S. Arguments for and against self and non-self root recognition in plants. Front Plant Sci [Internet]. 2014;5(November):614 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4222137&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biedrzycki ML, V L, Bais HP. Transcriptome analysis of Arabidopsis thaliana plants in response to kin and stranger recognition. Vol. 6, Plant Signaling & Behavior. 2011. p. 1515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudley SA, File AL. Kin recognition in an annual plant. Biol Lett. 2007;3(4):435–8. doi: 10.1098/rsbl.2007.0232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer AG, Ali M, Yang S, Parchami N, Bento T, Mazzella A, et al. Kin recognition is a nutrient-dependent inducible phenomenon. Plant Signal Behav. 2016;11(9):e1224045 doi: 10.1080/15592324.2016.1224045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang D ju, Zhang J, Yang W qin, Wu F zhong. Potential allelopathic effect of Eucalyptus grandis across a range of plantation ages. Ecol Res. 2010;25(1):13–23. [Google Scholar]
- 18.Golisz A, Sugano M, Hiradate S, Fujii Y. Microarray analysis of Arabidopsis plants in response to allelochemical L-DOPA. Planta. 2011;233(2):231–40. doi: 10.1007/s00425-010-1294-7 [DOI] [PubMed] [Google Scholar]
- 19.Keyes WJ, Palmer AG, Erbil WK, Taylor J V, Apkarian RP, Weeks ER, et al. Semagenesis and the parasitic angiosperm Striga asiatica. Plant J. 2007;51(4):707–16. doi: 10.1111/j.1365-313X.2007.03171.x [DOI] [PubMed] [Google Scholar]
- 20.Palmer AG, Liu Y, Adkins SM, Zhang X, Wu I-L, Chen X, et al. The molecular language of semagenesis. Plant Signal Behav [Internet]. 2008;3(8):560–1. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2634496&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim D, Kocz R, Boone L, Keyes WJ, Lynn DG. On becoming a parasite: evaluating the role of wall oxidases in parasitic plant development. Chem Biol. 1998;5(2):103–17. [DOI] [PubMed] [Google Scholar]
- 22.Fate GD, Lynn DG. Xenognosin methylation is critical in defining the chemical potential gradient that regulates the spatial distribution in Striga pathogenesis. J Am Chem Soc. 1996;118(46):11369–76. [Google Scholar]
- 23.Smith CE, Dudley MW, Lynn DG. Vegetative/Parasitic transition: control and plasticity in striga development. Plant Physiol [Internet]. 1990;93(1):208–15. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1062490&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer AG, Chen MC, Kinger NP, Lynn DG. Parasitic angiosperms, semagenesis and general strategies for plant-plant signaling in the rhizosphere. Pest Manag Sci [Internet]. 2009. May [cited 2012 Nov 3];65(5):512–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19235134 doi: 10.1002/ps.1717 [DOI] [PubMed] [Google Scholar]
- 25.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol [Internet]. 2008;4(5):278–86. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18421291 doi: 10.1038/nchembio.85 [DOI] [PubMed] [Google Scholar]
- 26.Gechev TS, Hille J. Hydrogen peroxide as a signal controlling plant programmed cell death. Vol. 168, Journal of Cell Biology. 2005. p. 17–20. doi: 10.1083/jcb.200409170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharjee S. The Language of Reactive Oxygen Species Signaling in Plants. J Bot. 2012;2012:1–22. [Google Scholar]
- 28.Stacy A, Everett J, Jorth P, Trivedi U, Rumbaugh KP, Whiteley M. Bacterial fight-and-flight responses enhance virulence in a polymicrobial infection. Proc Natl Acad Sci. 2014. May;111(21):7819–24. doi: 10.1073/pnas.1400586111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song Y, Buettner GR. Thermodynamic and kinetic considerations of semiquinone radicals to form superoxide and hydrogen peroxide. Free Radic Biol Med. 2011;49(6):919–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sella E, Shabat D. Hydroquinone-quinone oxidation by molecular oxygen: a simple tool for signal amplification through auto-generation of hydrogen peroxide. Org Biomol Chem. 2013;11(31):5074–8. doi: 10.1039/c3ob40962g [DOI] [PubMed] [Google Scholar]
- 31.Murphy GP, Dudley SA. Kin recognition: Competition and cooperation in Impatiens (Balsaminaceae). Am J Bot. 2009;96(11):1990–6. doi: 10.3732/ajb.0900006 [DOI] [PubMed] [Google Scholar]
- 32.Casper BB, Jackson RB. PLANT COMPETITION UNDERGROUND. Vol. 28, Annual Review of Ecology and Systematics. 1997. p. 545–70. [Google Scholar]
- 33.Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A. Expanded dynamic range of fluorescent indicators for Ca(2+) by circularly permuted yellow fluorescent proteins. Proc Natl Acad Sci U S A. 2004;101(29):10554–9. doi: 10.1073/pnas.0400417101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monshausen GB, Messerli M a, Gilroy S. Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol [Internet]. 2008. August [cited 2012 Nov 5];147(4):1690–8. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2492656&tool=pmcentrez&rendertype=abstract doi: 10.1104/pp.108.123638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang L, Liu Y, Jariwala J, Lynn DG, Palmer AG. Detection and Adaptation in Parasitic Angiosperm Host Selection. Am J Plant Sci. 2016;7(8):1275–90. [Google Scholar]
- 36.Torres MA, Onouchi H, Hamada S, Machida C, Hammond-Kosack KE, Jones JDG. Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91(phox)). Plant J. 1998;14(3):365–70. [DOI] [PubMed] [Google Scholar]
- 37.Potocký M, Jones MA, Bezvoda R, Smirnoff N, Žárský V, Zárský V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol. 2007;174(4):742–51. doi: 10.1111/j.1469-8137.2007.02042.x [DOI] [PubMed] [Google Scholar]
- 38.Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422(6930):442–6. doi: 10.1038/nature01485 [DOI] [PubMed] [Google Scholar]
- 39.Dorr I. How Striga Parasitizes its Host: a TEM and SEM Study. Ann Bot [Internet]. 1997;79(5):463–72. Available from: http://aob.oxfordjournals.org/content/79/5/463 [Google Scholar]
- 40.Hood ME, Condon JM, Timko MP, Riopel JL. Primary Haustorial Development of Striga asiatica on Host and Nonhost Species. Phytopathology. 1998;88(1):70–5. doi: 10.1094/PHYTO.1998.88.1.70 [DOI] [PubMed] [Google Scholar]
- 41.Lohar DP, Haridas S, Gantt JS, VandenBosch KA. A transient decrease in reactive oxygen species in roots leads to root hair deformation in the legume-rhizobia symbiosis. New Phytol. 2007;173(1):39–49. doi: 10.1111/j.1469-8137.2006.01901.x [DOI] [PubMed] [Google Scholar]
- 42.Mazars C, Thuleau P, Lamotte O, Bourque S. Cross-talk between ROS and calcium in regulation of nuclear activities. Mol Plant. 2010;3(4):706–18. doi: 10.1093/mp/ssq024 [DOI] [PubMed] [Google Scholar]
- 43.Yan Y, Wei CL, Zhang WR, Cheng HP, Liu J. Cross-talk between calcium and reactive oxygen species signaling. Vol. 27, Acta Pharmacologica Sinica. 2006. p. 821–6. doi: 10.1111/j.1745-7254.2006.00390.x [DOI] [PubMed] [Google Scholar]
- 44.Knight H, Trewavas AJ, Knight MR. Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J [Internet]. 1997;12(5):1067–78. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9418048 [DOI] [PubMed] [Google Scholar]
- 45.Gee KR, Brown KA, Chen WN, Bishop-Stewart J, Gray D, Johnson I. Chemical and physiological characterization of fluo-4 Ca(2+)-indicator dyes. Cell Calcium [Internet]. 2000;27(2):97–106. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10756976 doi: 10.1054/ceca.1999.0095 [DOI] [PubMed] [Google Scholar]
- 46.Zeng Z, Cartwright CH, Lynn DG. Chemistry of cyclopropyl-p-benzoquinone: A specific organogenesis inhibitor in plants. J Am Chem Soc. 1996;118(5):1233–4. [Google Scholar]
- 47.Smith CE, Ruttledge T, Zeng Z, O’Malley RC, Lynn DG. Redox Control of Development: The Haustorium of Striga asiatica. Proc Natl Acad Sci United States Am. 1996;93:6986–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang M, Netzly DH, Butler LG, Lynn DG. Chemical regulation of distance. Characterization of the first natural host germination stimulant for Striga asiatica. J Am Chem Soc. 1986. November;108(24):7858–60. doi: 10.1021/ja00284a074 [DOI] [PubMed] [Google Scholar]
- 49.Hogan DA. Talking to Themselves: Autoregulation and Quorum Sensing in Fungi MINIREVIEW Talking to Themselves: Autoregulation and Quorum Sensing in Fungi. Eukaryot Cell. 2006;5(4):613–9. doi: 10.1128/EC.5.4.613-619.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Long T, Tu KC, Wang Y, Mehta P, Ong NP, Bassler BL, et al. Quantifying the integration of quorum-sensing signals with single-cell resolution. PLoS Biol. 2009;7(3):e68 doi: 10.1371/journal.pbio.1000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ng W-L, Bassler BL. Bacterial quorum-sensing network architectures. Annu Rev Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carlson BW, Miller LL. Mechanism of the oxidation of NADH by quinones. Energetics of one-electron and hydride routes. J Am Chem Soc. 1985. January;107(2):479–85. [Google Scholar]
- 53.Roginsky VA, Pisarenko LM, Michel C. The kinetics and thermodynamics of quinone–semiquinone–hydroquinone systems under physiological conditions. 1999;871–6.
- 54.Roginsky VA, Bruchelt G, Stegmann HB. Fully reversible redox cycling of 2,6-dimethoxy-1,4-benzoquinone induced by ascorbate. Biochem. 1998;63:200–6. [PubMed] [Google Scholar]
- 55.Roginsky V, Barsukova T. Kinetics of oxidation of hydroquinones by molecular oxygen. Effect of superoxide dismutase. J Chem Soc Perkin Trans 2. 2000;(7):1575–82. [Google Scholar]
- 56.Kimura S, Kaya H, Kawarazaki T, Hiraoka G, Senzaki E, Michikawa M, et al. Protein phosphorylation is a prerequisite for the Ca2+-dependent activation of Arabidopsis NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca2+ and reactive oxygen species. Biochim Biophys Acta—Mol Cell Res [Internet]. 2012. February [cited 2017 Jun 8];1823(2):398–405. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22001402 [DOI] [PubMed] [Google Scholar]
- 57.Huala E, Oeller PW, Liscum E, Han I-S, Larsen E, Briggs WR. Arabidopsis NPH1: A Protein Kinase with a Putative Redox-Sensing Domain. Science (80-) [Internet]. 1997. [cited 2017 Jun 8];278(5346). Available from: http://science.sciencemag.org/content/278/5346/2120 [DOI] [PubMed] [Google Scholar]
- 58.Tschiersch H, Liebsch G, Borisjuk L, Stangelmayer A, Rolletschek H. An imaging method for oxygen distribution, respiration and photosynthesis at a microscopic level of resolution. New Phytol. 2012;196(3):926–36. doi: 10.1111/j.1469-8137.2012.04295.x [DOI] [PubMed] [Google Scholar]
- 59.Steinbock O, Tth A, Showalter K. Navigating Complex Labyrinths: Optimal Paths from Chemical Waves. 1995;(February). [DOI] [PubMed]
- 60.Zhou L, Aon MA, Almas T, Cortassa S, Winslow RL, O’Rourke B. A Reaction-Diffusion Model of ROS-Induced ROS Release in a Mitochondrial Network. PLOS Comput Biol. 2010. January;6(1):e1000657 doi: 10.1371/journal.pcbi.1000657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Panayotaros J-GC and GC-P and P. Bistable reaction–diffusion on a network. J Phys A Math Theor. 2015;48(7):75102. [Google Scholar]
- 62.González JE, Marketon MM. Quorum Sensing in Nitrogen-Fixing Rhizobia Quorum Sensing in Nitrogen-Fixing Rhizobia. Microbilogy Mol Biol Rev. 2003;67(4):574–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhatt M V., Khandelwal A, Dudley SA. Kin recognition, not competitive interactions, predicts root allocation in young Cakile edentula seedling pairs. New Phytol. 2011;189(4):1135–42. doi: 10.1111/j.1469-8137.2010.03548.x [DOI] [PubMed] [Google Scholar]
- 64.Biedrzycki ML, Jilany TA, Dudley SA, Bais HP. Root exudates mediate kin recognition in plants. Commun Integr Biol [Internet]. 2014;3(1):28–35. Available from: http://www.tandfonline.com/doi/abs/10.4161/cib.3.1.10118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smertenko A, Bozhkov P V. Somatic embryogenesis: Life and death processes during apical-basal patterning. Vol. 65, Journal of Experimental Botany. 2014. p. 1343–60. doi: 10.1093/jxb/eru005 [DOI] [PubMed] [Google Scholar]
- 66.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–5. doi: 10.1126/science.294.5548.1871 [DOI] [PubMed] [Google Scholar]
- 67.Zipfel C. Plant pattern-recognition receptors. Vol. 35, Trends in Immunology. 2014. p. 345–51. doi: 10.1016/j.it.2014.05.004 [DOI] [PubMed] [Google Scholar]
- 68.Keyes WJ, Taylor J V., Apkarian RP, Lynn DG. Dancing Together. Social Controls in Parasitic Plant Development. Plant Physiol [Internet]. 2001. [cited 2017 Jun 29];127(4). Available from: http://www.plantphysiol.org/content/127/4/1508 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper. There are no supplemental figures.