Abstract
Metabolic engineering of various industrial microorganisms to produce chemicals, fuels, and drugs has raised interest since it is environmentally friendly, sustainable, and independent of nonrenewable resources. However, microbial metabolism is so complex that only a few metabolic engineering efforts have been able to achieve a satisfactory yield, titer or productivity of the target chemicals for industrial commercialization. In order to overcome this challenge, 13C Metabolic Flux Analysis (13C-MFA) has been continuously developed and widely applied to rigorously investigate cell metabolism and quantify the carbon flux distribution in central metabolic pathways. In the past decade, many 13C-MFA studies have been performed in academic labs and biotechnology industries to pinpoint key issues related to microbe-based chemical production. Insightful information about the metabolic rewiring has been provided to guide the development of the appropriate metabolic engineering strategies for improving the biochemical production. In this review, we will introduce the basics of 13C-MFA and illustrate how 13C-MFA has been applied via integration with metabolic engineering to identify and tackle the rate-limiting steps in biochemical production for various host microorganisms
Keywords: Bottleneck, isotope, cofactor imbalance, cell metabolism, synthetic biology, biofuels
1. Introduction
Using microorganisms to produce various chemicals from renewable resources could be environmentally friendly and reduce strong dependence on petroleum. Recently, with the rapid development of metabolic engineering and synthetic biology, a wide range of bulk chemicals [1,2,3,4], biofuels [5,6,7,8,9], and drugs [10,11,12,13,14,15,16] from renewable feedstock have been produced by many industrial microorganisms such as Escherichia coli [17,18,19,20,21,22] and Saccharomyces cerevisiae [23,24,25,26]. However, only a few of these biosynthesized chemicals are able to be industrially commercialized due to low production levels with unsatisfactory titers, yields, and productivities [27,28]. Therefore, it is pivotal to develop novel strategies in metabolic engineering to improve microbe-based chemical production.
One of the main reasons for the low production level of engineered microorganisms is the high complexity of cell metabolism[28]. Microbial production of chemicals is more than the enzymatic conversion of the precursors to the products. Instead, to achieve the production of target chemicals at a high level, controls over microbial metabolism must coordinate the carbon flux [29,30], cofactor supply [31,32,33], cell maintenance [10,34,35], as well as other factors [36,37,38,39]. In general, many of the metabolic engineering strategies adopted to manipulate microbial metabolism for biochemical production only focus on a few known challenges (e.g., poor gene expression). However, attempting to overcome these challenges could result in new problems in host cells (e.g., metabolic burden) and hence prevent the microorganisms from achieving high-level chemical production. The lack of knowledge on such complex behavior of microbial physiology presents one of the most significant issues in improving the microbe-based chemical production.
To demystify the complex metabolic rewiring of engineered microorganisms and more importantly, derive the appropriate strategies to engineer microorganisms for better biochemical production, a technology named 13C-Metabolic Flux Analysis (13C-MFA) has been in development since the 1990s [40,41,42,43,44,45]. Basically, in 13C-MFA, carbon isotopes have been used to trace the cell metabolism. The carbon flux distributions in metabolic network of microorganisms can be determined using computational algorithms with the development of a metabolic model and the measurement of 13C-labeling patterns of the key metabolites [40,41,46,47,48,49]. By comparing variations of metabolic fluxes among different engineered microorganisms, the key issues, such as the bottleneck pathway, could often be revealed and hence guide the metabolic engineers to develop more appropriate strategies [35,50,51,52,53,54,55] for further improvement of chemical production. In the past decade, we have witnessed that numerous valuable biological insights pinpointed by 13C-MFA successfully helped to enhance the microbial production of chemicals [30,34,51,53,56,57]. Therefore, 13C-MFA has been widely considered as one of the most important tools to diagnose complex microbial metabolism and develop novel metabolic engineering strategies [29,30,32,34,35,53].
In this review, we aim to summarize the integrated tactics of 13C-MFA and metabolic engineering from cases that attempted to improve microbe-based chemical production in the past decade. We will first briefly introduce the techniques of 13C-MFA, and then categorize studies that integrated 13C-MFA with metabolic engineering in different industrial microorganisms, including (1) Saccharomyces cerevisiae; (2) Escherichia coli; (3) Bacillus subtilis; (4) Corynebacterium glutamicum; and (5) other microorganisms. Finally, we envision the emerging areas where breakthroughs of 13C-MFA could potentially promote rational metabolic engineering for improved microbe-based chemical production in future.
2. Techniques of 13C Metabolic Flux Analysis
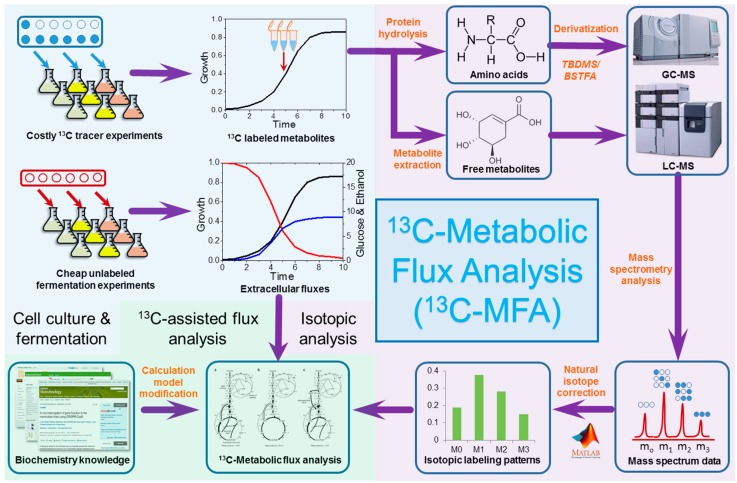
The techniques of 13C-MFA have been developed for more than two decades [40,41,42,43,44,45]. The development of advanced mathematical algorithms and high-throughput mass spectrometry technology enables accurate and quantitative analysis of metabolic fluxes for various microorganisms. Since several protocols have been published to thoroughly describe the procedures for both model and non-model organisms [46,58], in this review we will only provide a concise introduction on 13C-MFA, which includes three steps in general: cell cultivation, isotopic analysis of metabolites, and 13C-assisted pathway and flux analysis (Figure 1).
Figure 1.
The scheme of 13C-Metabolic Flux Analysis (13C-MFA).
Cell culture on 13C-labeled carbon substrate is the first step of 13C-MFA and plays a vital role for the entire flux analysis. The choice of 13C-labeled substrate is a key factor for 13C-MFA, which depends on the choice of the target microorganism and the objectives of the experiment. In general, to elucidate the flux distribution accurately, a well-studied glucose mixture, i.e., 80% [1-13C] and 20% [U-13C] glucose (w/w), has often been used to guarantee that high 13C abundance will be observed in various metabolites [46,59]. On the other hand, pure and singly labeled carbon substrate is more suitable to discover novel pathways because it is easier to trace labeled carbons in intermediates [60]. A strictly minimal medium with only the selected 13C-labeled substrate as the sole carbon source is often required for the 13C-labeling experiments. Two culture modes, i.e., batch mode and chemostat mode, are most often used for 13C-MFA to reach the required steady states for sampling, namely, metabolic and isotopic steady states, in which the concentration and isotopic labeling of intracellular metabolites are both constant. Next, cell biomass and culture medium will be used for further isotopic analysis.
The measurement of 13C-labeling in metabolites is often achieved by using mass spectrometry, such as gas chromatography–mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS). When using GC-MS for isotopic analysis, a derivatization process using TBDMS or BSTFA as the derivatization agents is always required to render molecules (e.g., over-produced chemicals [61] and proteinogenic amino acids) volatile enough to be analyzed by GC-MS. When using LC-MS for isotopic analysis, metabolites with trace amounts or high instabilities can be directly analyzed because of the high sensitivity of LC-MS. Since the effects of naturally labeled isotopes cannot be ignored when analyzing the 13C-labeling of metabolites, systematic correction of such natural isotopic effects is taken to generate the isotopic distributions e.g., mass distribution vector (MDV), for the metabolites of interest by using several well-established algorithms [62,63,64]. Such isotopic distributions, i.e., MDV, will then be used for the pathway and flux analysis [46].
Based on the MDV of various metabolites, the metabolic behaviors of microorganisms can be elucidated both qualitatively (i.e., pathway analysis) and quantitatively (i.e., flux analysis). On one hand, the 13C-assisted pathway analysis often aims to answer whether or not a metabolic pathway is active in non-model microorganisms by tracking the 13C-labeling patterns (i.e., MDVs) in key metabolites and the fate of biomolecule synthesis in these denoted biochemical pathways. On the other hand, the 13C-assisted flux analysis aims to quantify the carbon fluxes in multiple metabolic pathways by simulating the 13C-labeling patterns (i.e., MDVs) in key metabolites and searching for the “real” metabolic fluxes that could lead to the best fit of the measured MDV. In short, while 13C-assisted pathway analysis is suitable for pathway discovery in non-model microorganisms, 13C-assisted flux analysis is more useful in identifying the metabolic rewiring in industrial microorganisms.
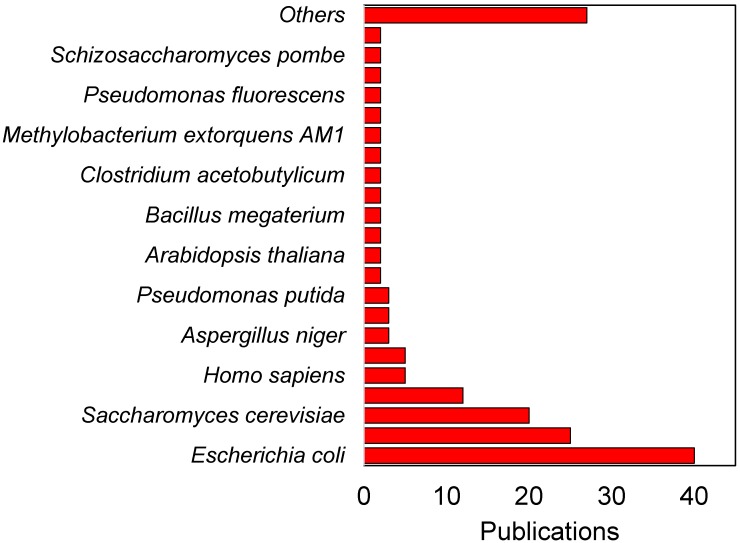
In the past decade, 13C-assisted flux analysis has been widely applied to uncover the central metabolisms of various microorganisms. According to a curated database [64] that was developed to collect the metabolic fluxes investigated by 13C-MFA, over 500 cases of metabolic flux analysis have been accomplished so far for 36 organisms (Figure 2). Most of the 13C-MFA studies focus on investigating metabolism of E.coli and S. cerevisiae. However, there is a trend that other industrial microorganisms, such as Clostridium and Cyanobacteria, will initiate more 13C-MFA studies due to their importance in biochemical production and relatively less well-known cell metabolism. Also, with the wide application of 13C-MFA, many 13C-MFA software packages, such as OpenFLUX2 [65], 13CFLUX2 [66], Metran [67], INCA [68], FiatFLUX [69], and Biomet Toolbox 2.0 [70], have been developed with highly efficient mathematical algorithms (e.g., elementary metabolite unit, EMU [67,71]) to calculate carbon fluxes in various metabolic networks (Table 1). As a result, some of the difficulties, especially the computational load, of 13C-MFA have been dramatically decreased [71]. It is reasonable to believe that the number of 13C-MFA studies could increase by orders of magnitudes in the next decade or two.
Figure 2.
Summary of current 13C-MFA studies on different organisms.
Table 1.
Summary of 13C-MFA software.
| Name | Capabilities | Labeled Pattern | Key Solver (Algorithm) | Platform | Developer |
|---|---|---|---|---|---|
| 13CFLUX2 [66] | Steady-state 13C-MFA | EMU [67] d | IPOPT | UNIX/Linux | Wiechert’s group |
| Metran [67] | Steady-state 13C-MFA | EMU | fmincon | MATLAB | Antoniewicz’s group |
| FIA [177] | Steady-state 13C-MFA | Fluxomer | SNOPT [178] | UNIX/Linux | Young’s group |
| influx_s [179] | Steady-state 13C-MFA | Cumomer | NLSIC [179] | UNIX/Linux | Portais’s group |
| C13 [180] | Steady-state 13C-MFA | SFL [181] e | fmincon | MATLAB | Nielsen’s group |
| OpenFLUX2 [65] | Steady-state 13C-MFA with PLEs a | EMU | fmincon | MATLAB | Mashko’s group |
| FiatFLUX [69] | METAFoRA b, steady-state 13C-MFA | MDV f | fmincon | MATLAB | Sauer’s group |
| INCA [68] | Steady-state 13C-MFA and INST-13C-MFA c | EMU | Customized Differential Equation Solver [71] | MATLAB | Young’s group |
| OpenMebius [182] | Steady-state 13C-MFA and INST-13C-MFA c | EMU | Levenberg-Marquardt method [183] | MATLAB | Shimizu’s group |
a PLEs: Parallel isotopic experiments; b METAFoRA: Metabolic flux ratio analysis; c INST-13C-MFA: isotopic nonstationary 13C-metabolic flux analysis; d EMU: elementary metabolite unit; e SFL: summed fractional labeling; f MDV: mass distribution vectors.
3. Integrating 13C Metabolic Flux Analysis and Metabolic Engineering for Different Industrial Microorganisms
The ultimate goal of metabolic engineering is to design and build engineered biological systems that can produce chemicals, materials, food, and drugs at high yield using the appropriate microorganisms [72]. However, the lack of fundamental understanding of cellular responses during industrial bioprocesses often prevents metabolic engineers from achieving satisfactory goals in biochemical production. In the past decade, 13C-MFA has been widely used to provide insightful information on metabolism of various microorganisms, thus helping metabolic engineers to successfully improve biochemical production. In the following sections, we have summarized recent successes on integrating 13C-MFA and metabolic engineering (Table 2) based on different host organisms: (1) Saccharomyces cerevisiae; (2) Escherichia coli; (3) Bacillus subtilis; (4) Corynebacterium glutamicum; and (5) other host microorganisms.
Table 2.
Summary of synergistic tactics of 13C-MFA and metabolic engineering a.
| Organism | Key Issues in Metabolic Engineering | Final Product (Objective) | Major Results of 13C-MFA | Strategies of Metabolic Engineering | Results of Metabolic Engineering |
|---|---|---|---|---|---|
| S. cerevisiae | Bottleneck step: Cytosolic acetyl-CoA supply | n-Butanol |
|
|
|
| Bottleneck step: Cytosolic acetyl-CoA supply | Isoprenoid-derived drugs [184] |
|
|
|
|
| Bottleneck step: Cytosolic acetyl-CoA supply | Various industrially relevant products |
|
|
|
|
| Bottleneck step: Pentose phosphate pathway |
|
|
|
|
|
| S. cerevisiae | Cofactor imbalance issue | Ethanol (Xylose utilization) [35] |
|
||
| S. cerevisiae | High maintenance energy | S-Adenosyl-L-methionine [34] |
|
|
|
| High maintenance energy | Xylose utilization [35] |
|
|||
| S. cerevisiae | Stress response: Furfural [38] | Growth (Survival) |
|
|
|
| E. coli | Bottleneck step: Cytosolic acetyl-CoA supply, reduction power supply. | Fatty acid and fatty acid derived chemicals [56,57] |
|
|
|
| E. coli [186] | Cofactor imbalance issue | NADPH-dependent compounds [52,93,117] (Lycopene, fatty acid, etc.) |
|
|
|
| E. coli | Stress response: Octanoic acid [37] | Growth (Survival) |
|
|
|
| Stress response: Super-oxidative (paraquat induced) | Growth (Survival) |
|
Suggested strategies: |
|
|
| |||||
| B. subtilis | Bottleneck step: biosynthesis pathways [126] | Riboflavin | Suggested strategies: |
|
|
| |||||
| B. subtilis | High maintenance energy [128,129] | Riboflavin |
|
|
|
| C. glutamicum | Cofactor imbalance issue | L-lysine |
|
|
|
| Cofactor imbalance issue | L-valine [53] |
|
|
|
|
| P. pastoris | High maintenance energy | R. oryzae lipase [185] |
|
|
|
| A. niger | Cofactor imbalance issues | Fructofuranosidase [151] |
|
Suggested strategies: |
|
| |||||
| P. chrysogenum | Cofactor imbalance issues[153] | Penicillin-G |
|
Suggested strategies: |
|
| |||||
| R. palustris | Cofactor imbalance issues [154] | Hydrogen |
|
Suggested strategies: |
|
| |||||
| B. succiniciproducens | Bottleneck steps in precursor supply [155] | Succinate |
|
|
|
a Abbreviations: ACC: Acetyl-CoA carboxylase, ACL: ATP citrate lyase, ACS: Acetyl-CoA synthetase, ADH: Alcohol dehydrogenase, ALD: Acetaldehyde dehydrogenase, ED: Entner-Doudoroff pathway, FAS: Fatty acid synthesis enzymes, FAD: Fatty acids degradation enzymes, G6PDH: G6P dehydrogenase, GAPDH: Glyceraldehyde 3-phosphate dehydrogenase, PDC: Pyruvate Decarboxylase, PDH: Pyruvate dehydrogenase, PP: Pentose phosphate pathway, XR: Xylose reductase, XDH: Xylitol dehydrogenase; b This value is estimated by flux values.
3.1. Saccharomyces Cerevisiae
The yeast Saccharomyces cerevisiae, an important microbial cell factory [73] and a model eukaryotic organism [24,74], has been widely used to produce various biofuels [8,75], bulk chemicals [50,76,77,78], pharmaceuticals [79,80], proteins, and other value-added products [81,82,83] by using various renewable feedstock. Although the knowledge on physiology of S. cerevisiae has been accumulated for over three decades, the complex metabolism in S. cerevisiae still impairs the improvement of chemical productivity in various bioprocesses. For example, the insufficient supply of several key precursors could be a bottleneck in biochemical production and the introduction of a heterologous sugar utilization pathway could lead to serious cofactor imbalance issues. In addition, high maintenance energy required by host cells will compete for the limited carbon source that keeps cells alive. Besides, various inhibitors in industrial fermentation process could dramatically inhibit cell growth, and even lead to cell death. To elucidate the complex yeast metabolism, 13C-MFA has been widely applied on S. cerevisiae to provide biological insights behind all above obstructions. Following these insights, metabolic engineers have developed versatile strategies to overcome rate-limiting steps for further improvement of biochemical production. In this section, as shown in Figure 3, we will summarize the findings from 13C-MFA and the corresponding metabolic engineering work for S. cerevisiae.
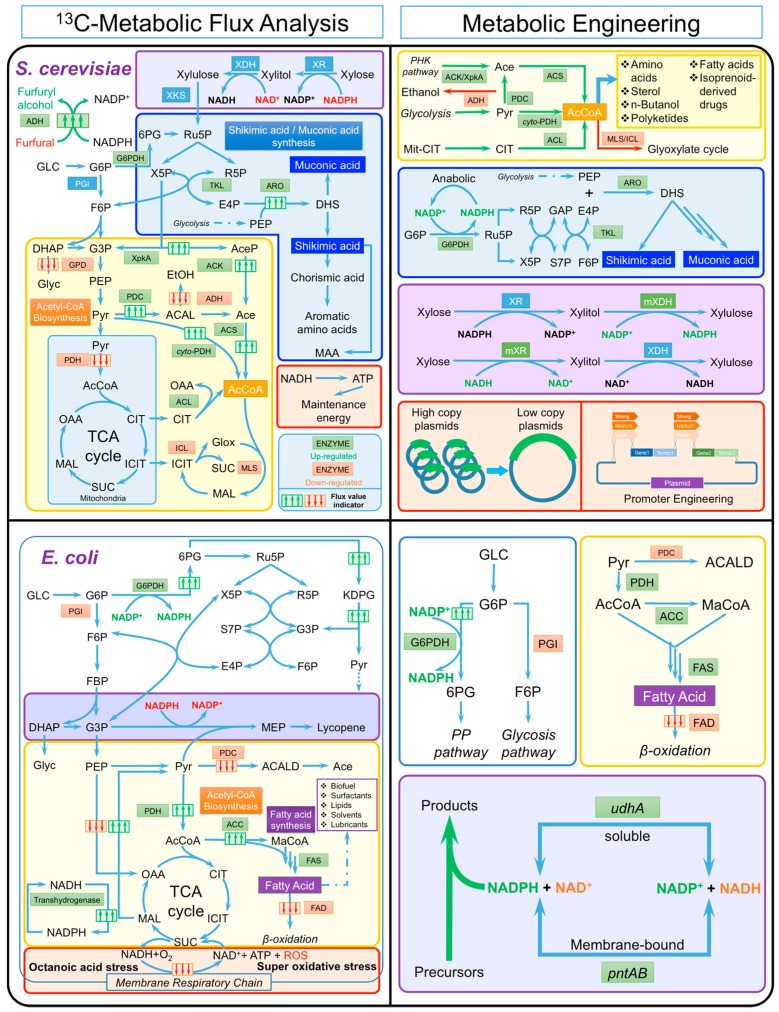
Figure 3.
Integration of the major discoveries from 13C-MFA and corresponding metabolic engineering strategies for S. cerevisiae and E. coli. Abbreviations: G6P: Glucose 6-Phosphate, F6P: Fructose 6-phosphate, 6PG: 6-Phosphogluconate, Ru5P: Ribulose 5-Phosphate, X5P: Xylulose 5-phosphate, R5P: Ribose 5-Phosphate, E4P: Erythrose 4-Phosphate, PEP: Phosphoenolpyruvate, SA: Shikimic acid, MA: Muconic acid, MAA: Mycosporine-like Amino Acids, AA: Amino Acids. PGI: Phosphoglucose isomerase, G6PDH: G6P dehydrogenase, TKL: Transketolase, ARO: Pentafunctional protein ARO1p. DHAP: Dihydroxyacetone phosphate, G3P: Glyceraldehyde 3-phosphate, Glyc: Glycerol, AceP: Acetyl-P, EtOH: Ethanol, Pyr: Pyruvate, ACAL: Acetaldehyde, Ace: Acetate, AcCoA: Acetyl-CoA, OAA: Oxaloacetate, CIT: Citrate, ICIT: Isocitrate, SUC: Succinate, MAL: Malate, Glox: Glyoxylate, GPD: Glycerol-3-phosphate dehydrogenase. XpkA: Phosphoketolase, ACK: acetate kinase, PDC: Pyruvate Decarboxylase, ADH: Alcohol dehydrogenase, PDH: Pyruvate dehydrogenase, cyto-PDH: Cytosolic pyruvate dehydrogenase, ACS: Acetyl-CoA synthetase, ACL: ATP citrate lyase, ICL: Isocitrate lyase, MLS: Malate synthetase. MaCoA: Malonyl-CoA, ACC: Acetyl-CoA carboxylase, FAS: Fatty acid synthesis enzymes, FAD: Fatty acids degradation enzymes, MEP: 2-methylerythritol 4-phosphate, mXR: Mutated xylose reductase, mXDH: Mutated xylitol dehydrogenase.
3.1.1. Bottleneck Steps
Improving the supply of key precursors is one of the most effective strategies to boost the biochemical production in metabolic engineering. 13C-MFA analyzes the metabolic flux distribution in industrial microorganisms at steady state. By using 13C-MFA to elucidate the flux distributions of genetically perturbed cells, the bottleneck step in supplying key precursors for biochemical production can be identified. These elucidated major bottlenecks would provide direct instruction to design appropriate strategies for different biochemical production. For example, as a central metabolite, acetyl-CoA is a key precursor in the biosynthesis of sterols, amino acids, fatty acid-derived chemicals, polyketides, and isoprenoid-derived drugs [50]. To accommodate the cellular requirement, microorganisms use a variety of routes for acetyl-CoA synthesis (Figure 3). 13C-MFA has been applied to investigate the acetyl-CoA biosynthesis in S. cerevisiae. By comparing S. cerevisiae strains growing under purely oxidative, respiro-fermentative and predominantly fermentative conditions [84], the main pathway to supply cytosolic acetyl-CoA in S. cerevisiae was found to be the activated pyruvate bypass pathway. In this pyruvate bypass pathway, pyruvate is converted to acetaldehyde and then to acetate for synthesis of cytosolic acetyl-CoA. Although the pyruvate bypass pathway was activated, it was not strong enough to supply sufficient cytosolic acetyl-CoA when S. cerevisiae is engineered to produce acetyl-CoA-derived chemicals, such as n-butanol.
To enhance the cytosolic acetyl-CoA availability, various metabolic engineering strategies have been adopted in S. cerevisiae (Figure 3), including the disruption of competing pathways [50], such as malate synthetase (MLS1) and all alcohol dehydrogenases (ADH), as well as the introduction of heterologous biosynthetic pathways with higher catalytic efficiency and lower energy input requirement, such as cytosolic localized PDHs (cytoPDHs) [50] and ATP-citrate lyase (ACLs) [85]. A successful example of improving the biochemical production by enhancing acetyl-CoA supply is overexpressing the cytoPDHs, which led to 3-fold increase in n-butanol production in the engineered S. cerevisiae [50].
In addition to elucidating the bottleneck of cytosolic acetyl-CoA biosynthesis for wild-type yeast, 13C-MFA was also used to uncover the effect of a heterologous acetyl-CoA overproducing pathway, i.e., phosphoketolase pathway (PHK), in a genetically engineered yeast strain with the expression of genes xpkA and ack from Aspergillus nidulans [51]. The PHK pathway was an alternative to the Embden-Meyerhof-Parnas (EMP) pathway for glucose dissimilation in several bacterial species [86] and filamentous fungi. For example, in A. nidulans, the utilization of this metabolic pathway increased carbon flow towards acetate and acetyl-CoA through the action of a phosphotransacetylase [87]. Flux distribution in the central metabolic pathways demonstrated the positive role of the PHK pathway on improving the supply of cytosolic acetyl-CoA in a S. cerevisiae strain, which was also accounted for the improved acetate production. Encouraged by this discovery, co-expression of the same PHK pathway and a wax ester synthase successfully improved the titer of fatty acid ethyl esters by 1.7 fold [88]. Such proof-of-concept study indicated that the PHK pathway could be established as a stand-alone route to divert flux from glycolysis to cytosolic acetyl-CoA supply, and holds great potential for improving the production of acetyl-CoA-derived chemicals in the future.
In addition to the central metabolite acetyl-CoA, several important precursors in the pentose phosphate pathway (PP pathway) also play a vital role in the synthesis of value-added compounds. For example, several chemicals, such as shikimic acid (a valuable drug precursor) and muconic acid (an important biopolymer precursor) could be produced from metabolites in the PP pathway [82]. To investigate the bottleneck in the PP pathway for the biosynthesis of shikimic acid, 13C-MFA was recently applied to four different engineered S. cerevisiae strains that were engineered to produce shikimic acids at different amounts (manuscript in preparation). By comparing flux distributions of the four strains, a higher flux through PP pathway was positively correlated with higher production of shikimic acid. This suggested that the low flux into the PP pathway, especially a limited supply of the precursor E4P from the PP pathway, could be the bottleneck for producing the shikimic acid. Indeed, it was found that when removing the original phenylalanine and tyrosine synthesis pathway, and overexpressing aro1, aro4 and tkl genes to improve the metabolic fluxes in PP pathway (Figure 3), shikimic acid titer was increased by nearly 2-fold in S. cerevisiae. The similar strategy was applied to an engineered S. cerevisiae strain with a heterologous pathway for muconic acid synthesis. A 24-fold increase of muconic acid production has been achieved, compared to the initial strain containing only the muconic acid synthesis pathway [82].
3.1.2. Cofactor Imbalance
Cofactors, e.g., NADH/NAD+ and NADPH/NADP+, play a key role as the redox carriers for catabolic and anabolic reactions, as well as important intermediates in cell energetics. However, the utilization of some cofactor-dependent production systems may lead to over-production or depletion of certain cofactors, and hence, break the original balances of cofactor usage in the cell metabolism. For example, one of the commonly used strategies to engineer S. cerevisiae for xylose utilization is introducing a fungal xylose pathway from native xylose-utilizing yeasts, such as Pichia stipites. Specifically, xylose could be converted to fermentable xylulose through the consecutive redox reactions catalyzed by NADPH-dependent xylose reductase (XR) and NAD+-dependent xylitol dehydrogenases (XDH), with xylitol produced as the intermediate (Figure 3). The usage of different cofactors for these two cofactor-dependent enzymes brings a notorious cofactor imbalance issue and severely restricts the xylose utilization in S. cerevisiae. More importantly, the cofactor imbalance issue is not standalone as shown by 13C-MFA, which is one of few analytical tools that can rigorously determine the cofactor usage in cell metabolism. It was found that the cofactor imbalance issue was intertwined with the central metabolism to induce network-level rewiring of carbon fluxes. Basically, 13C-MFA has been applied to systematically investigate xylose utilization of recombinant S. cerevisiae strains in an oxygen limited condition for ethanol production [35]. By implementing the 13C tracer experiments and running metabolic flux analysis for six recombinant strains with different origins of XR and XDH in the xylose pathway [35], the compensation of NADPH for the XR was supplied by the highly activated oxidative PP pathway. The strong activities in the TCA cycle were also observed and suggested that huge amounts of NADH needed to be consumed by oxidative phosphorylation. Concurrent with the global metabolic rewiring, only a small amount of the carbon fluxes was diverted to ethanol production.
Numerous efforts in metabolic engineering have been taken to solve the cofactor-imbalance issue in the xylose utilization of S. cerevisiae. One of the strategies is to engineer the cofactor dependent metabolic pathways that could decrease the xylitol production and enhance ethanol yield. For instance, lowering the flux through the NADPH-producing PP pathway could lead to increased ethanol yield and decreased xylitol production. This is attributed to an insufficient supplement of NADPH, which improves the NADH preference of XR, and thus, partially balance the cofactor usage of XR and XDH [89]. The other strategy used to tackle the cofactor imbalance issue is the partial alteration of the cofactor preference for these two enzymes, i.e., altering the preference of XR to use NADH or altering XDH to use NADP+ as the cofactors, which would generate a cofactor-balance cycle for the initial two steps of xylose utilization to balance the cofactor usage (Figure 3).
The cofactor engineering strategy has proved to be functional in several studies. For example, by replacing the native P. stipitis XR with a mutated XR that has an increased preference of NADH, the ethanol yield was improved by ~40% with decreased xylitol production [90,91,92,93,94,95,96]. Similar successes have also been achieved in several other attempts to increase the NADP+ preference for the XDH, which successfully improved the ethanol production by 28%~41% [97,98,99,100]. In addition, by replacing the NADPH-producing PP pathway (glucose-6-phosphate dehydrogenase) with a fungal NADP+-dependent D-glyceraldehyde-3-phosphate dehydrogenase (NADP-GAPDH), no carbon would be lost when producing NADPH for the XR, which would provide more carbons for the ethanol production [101]. Also, improving the NAD+ regeneration directly by introducing the heterologous genes could increase the xylitol consumption by XDH and improve the ethanol production by obtaining more carbons [102]. Another efficient approach for the xylose fermentation is to use xylose isomerase, which could convert xylose to xylulose without the requirement of cofactors; and hence, using xylose isomerase completely bypasses the cofactor imbalance issue. However, as shown by a recent 13C-MFA study, the lower activity of glycolysis that led to inefficient re-oxidation of NADH could be a new bottleneck step when using xylose isomerase in S. cerevisiae [103].
3.1.3. Metabolic Burden and Microbial Stress
Metabolic engineering is frequently equated with the heterologous production of a series of recombinant proteins. Nowadays, a large number of heterologous pathways have been introduced into a host cell, such as S. cerevisiae, in order to produce non-natural products with multiple genes inserted, deleted, replaced, or overexpressed. As a result, multiple kinds of chemicals can be produced with the implementation of complex genetic modification. However, the productivity achieved is often unsatisfactory due to the severe metabolic burden from the heterologous protein overexpression, a biological function that could be energetically expensive during transcription and translation. Such an issue, however, has not yet been well studied in the field of metabolic engineering.
The metabolic burden of industrial microorganisms is often reflected as elevated cell maintenance energy of industrial microorganisms shown as the increased TCA cycle fluxes by 13C-MFA studies (Figure 3). For engineered S. cerevisiae strains, it has been found that several genetic modifications could lead to the elevation of the cell maintenance energy. First, by using 13C-MFA for xylose-utilization S. cerevisiae strains, the elevated fluxes of TCA cycle were observed compared with wild-type strains in order to provide more ATP for cell maintenance [35]. The similar metabolic rewiring was observed for a S-Adenosyl-L-methionine (SAM) producing S. cerevisiae strain [34]. To avoid the introduction of extra metabolic burden or to compensate such a burden, metabolic engineers have explored three strategies: medium optimization, low-copy plasmids, and promoter engineering. Optimization of cultural medium and fermentation condition, such as the supplement of extra nutrients, could potentially remove stresses (e.g., nutrient limitation) and hence compensate the requirement for cell maintenance energy. In addition, utilization of a high-copy plasmid may increase risk of plasmid instability and metabolic burden [104], because the protein overexpression requires tremendous amounts of building blocks and energy, which could jeopardize the normal cell growth and increase the metabolic burden. The last method mentioned here to decrease metabolic burden is adjusting the promoter strengths of various genes, which could balance the pathways and avoid the accumulation of certain toxic intermediates.
In addition to the negative impact of the genetic interruption, environmental stresses, such as physical heat shock [105] and chemical acidity [106], could also lead to a seriously negative impact for biochemical production. For example, S. cerevisiae has been selected as a workhorse for lignocellulosic biofuel production, which holds promises for a sustainable fuel economy. However, the stress factors from the toxic compounds in the processed lignocellulosic hydrolysates, e.g., weak acids, furans, furfural, and phenolic compounds [107], have hampered the economic feasibility of biofuels by impacting the physiology and viability of microbial cells. Thus, the identification of the intracellular metabolic responses of S. cerevisiae to these stress factors is the key to rationally improve their resistance to inhibitors and productivity of biochemicals [39]. Compared to other commonly used approaches such as transcriptomics and proteomics analysis, 13C-MFA could provide more intuitive elucidation of the metabolic rewiring under stress conditions.
For example, furfural could be reduced to the less-toxic furfuryl alcohol with weak intrinsic capability of S. cerevisiae (Figure 3). However, the holistic view of metabolic responses to furfural is still missing. To further investigate the quantitative metabolic responses of S. cerevisiae under the increasing concentrations of furfural, 13C-MFA has been applied for wild-type and several evolved furfural-resistant strains in micro-aerobic and glucose-limited chemostats [38]. By comparing the different flux distributions under the increasing concentration of furfural stress, it is revealed that NADH-dependent oxireductases, which catalyzed the reduction of furfural, were the main defense mechanisms at a lower concentration of furfural (<15 mM), while NADPH-dependent oxireductases became the major resistance mechanism at the high concentration of furfural (>15 mM). Due to this shift of the major resistant mechanism, the carbon flux of pentose phosphate pathway increased as the main physiological response to high concentrations of furfural, which indicated that the increase of NADPH supply was the key to help S. cerevisiae better resist furfural stress. Inspired by this discovery, metabolic engineers overexpressed several NADPH-dependent oxireductases, particularly ADH7 and YKL071W, and successfully increased furfural resistance in the parent S. cerevisiae by 200% [38].
3.2. Escherichia coli
Due to the simplicity and fast growth, E. coli has been widely studied and applied for synthetic biology and metabolic engineering to produce various chemicals, such as advanced biofuels and value-added pharmaceuticals, with continuous accumulation of both knowledge and experiences in metabolic engineering. Nevertheless, many of the physiological responses in both wild type and engineered E. coli strains are not explicit due to its intricate regulation and metabolism. 13C-MFA, as a powerful approach to demystify the metabolic rewiring, has been applied to E. coli strains for two decades. Various valuable biological insights, such as key bottleneck steps in fatty acid-derived chemical production, cofactor imbalance issues, and metabolic responses to stress factor, have been elucidated, which helped metabolic engineers to design strategies for further improvement of the biochemical production. In this section, as shown in Figure 3, we summarized the recent outcomes of 13C-MFA studies on E. coli and corresponding metabolic engineering strategies.
3.2.1. Bottleneck Steps
Fatty acids and fatty-acid-derived chemicals are the precursors to produce transportation fuels and industrial chemicals including surfactants, solvents, and lubricants [56]. Several successful studies using microorganisms to produce fatty acids and fatty acid-derived chemicals have been achieved with the highest titer of 8 g/L fatty alcohol produced by engineered Rhodosporidium toruloides using sucrose as substrate [108]. E. coli is also considered as an excellent host for fatty acid production because of its fast growth, simple nutrient requirements, well-understood metabolism, and well-established genetic tools. In the wild type E. coli, only a small amount of free fatty acids are detectable under normal conditions, which indicates that several genetic modifications are necessary for E. coli to improve the fatty acid production. The native synthesis pathways of saturated fatty acid include the conversion of acetyl-CoA into malonyl-CoA catalyzed by ATP-dependent acetyl-CoA carboxylase, the transesterification of malonyl-CoA into an acyl carrier protein (ACP) catalyzed by malonyl-CoA ACP transacylase (fabD), and the cyclic chain elongation process [109].
Recently, various fatty acid over-producing strains have been created to use different strategies to boost the fatty acid production. However, most studies focus on engineering terminal enzymes in fatty acid biosynthesis pathways and little is known about the central metabolism responses to fatty acid production. To uncover the key bottleneck steps in fatty acid production, 13C-MFA has been performed by using an engineered fatty acid over-producing E. coli DH1 strain with the overexpression of tesA, and fadR genes as well as the knock-out of fadE gene [56]. Compared to the wild-type E. coli strain, the flux in the engineered strain was significantly diverted from acetate synthesis to fatty acid synthesis, which suggested that the increased supply of key precursors in the fatty acid synthesis played a crucial role to increase subsequent fatty acid synthesis. The flux of the pentose phosphate pathway also dramatically increased to compensate large amounts of reduction powers, mostly NADPH, for the fatty acid production in the engineered strain. Finally, since more carbon fluxes were required to supply cytosolic acetyl-CoA (the starting point of fatty acid biosynthesis), the flux of the anaplerotic pathway into the TCA cycle decreased 1.7-fold in the engineered strain. Overall, as indicated by 13C-MFA, the supply of fatty acid precursor, cytosolic acetyl-CoA, and the reduction power, NADPH, were recognized as the key bottlenecks in microbial engineering for fatty acid production.
Various engineering strategies have been suggested and explored (Figure 3) in order to improve fatty acid production. For example, to overcome the challenge of limited supply of the key fatty acid precursor, malonyl-CoA, the acetyl-CoA carboxylase was over-expressed to provide more malonyl-CoA, which successfully improved the production of fatty acids [110,111]. Besides the enhancement of upstream synthesis pathways, the downstream degradation pathway, namely, the fatty acid degradation pathway was removed by knocking out fadE in E. coli. Combined with the over-expression of tesA and fabF, the yield of fatty acids was increased by nearly 3-fold [112]. Similarly, co-expression fabZ and a thioesterase from Ricinus communis in a fadD (a key gene in fatty acid degradation) deletion mutant could enhance the fatty acid titer by nearly 3-fold [113]. In summary, the bottleneck of precursor supply identified by 13C-MFA has now been well addressed in metabolic engineering for fatty acid production. The insufficient supply of NADPH, another bottleneck uncovered by 13C-MFA, could be the next direction to further improve the fatty acid production and requires the attention of metabolic engineers.
3.2.2. Cofactor Imbalance
The imbalance issue of NADPH has been founded in several engineered E. coli strains via 13C-MFA. For example, 13C-MFA has been used to analyze the cell metabolism in wild type and fatty acid over-producing E. coli strains. As revealed by 13C-MFA, the engineered strain requires excessive NADPH compared to the wild-type strain, i.e., 255 units compared to 179 units NADPH when the flux of glucose uptake was normalized to 100 units. However, the sum of NADPH supplied from central metabolism could only reach 100 units [56], which is deficient by more than 150 units of NADPH. This clearly indicated that more NADPH was required to improve fatty acid production in E. coli. In order to compensate for the NADPH deficiency, the transhydrogenase pathway was activated in the engineered E. coli with flux increasing by 70% compared to that in wild type strains (i.e., from 90 units to 153 units) for fatty acid biosynthesis.
Realizing the significance of cofactor balance, particularly the NADPH supply, in engineered E. coli strains, metabolic engineers have adopted various strategies to overcome this challenge to enhance biochemical production. One strategy is switching the specificities of glycolytic enzymes, e.g., GAPDH, from NAD+-dependence to NADP+-dependence. Such a switch increased the availability of NADPH by building a NADPH-producing glycolysis pathway, and further improved the NADPH-dependent lycopene production by ~100% [52]. Also, several genetic modifications to redirect the metabolic flux from the glycolysis pathway into the PP pathway have been performed to enhance NADPH supply. These genetic modifications overexpressed zwf that encodes glucose-6-phosphate dehydrogenase (G6PDH) [114,115,116], deleted pfkA and pfkB that encode the phosphofructokinase (PFK) [117], or deleted pgi that encodes phosphoglucose isomerase [118,119] (Figure 3). In addition, transhydrogenase [120,121] or NAD kinase [52] was overexpressed to further boost the NADPH availability in E. coli. Overall, following the metabolic rewiring identified by 13C-MFA, the availability of NADPH has been increased for biochemical production in E. coli.
3.2.3. Metabolic Burden and Microbial Stress
Several strategies to reduce the metabolic burden in E. coli (mentioned in 3.1.3) were achieved to increase the biochemical productivity (Figure 3). For example, using low-copy plasmid in the engineered lycopene-producing E. coli strain could increase the cell density by approximately 24% compared to the engineered strain with high-copy plasmid [122], although the cell density of low-copy plasmid strain is still inevitably lower than the control culture. Similarly, the titer of lycopene in the E. coli with low-copy plasmid was 20% higher than that with high-copy plasmid. Another example of engineered E. coli strain to decrease metabolic burden is adjusting the promoter strengths of various genes in taxadiene pathways to improve the efficiency of taxadiene production in E. coli [10]. In general, promoter engineering was implemented to tune the expression level of two modules in the taxadiene pathway: a native upstream methylerythritol-phosphate (MEP) pathway forming isopentenyl pyrophosphate and a heterologous downstream pathway forming terpenoid. As a result, the minimal accumulation of an inhibitory intermediate compound for cell growth, indole, was achieved by expressing the upstream pathway in a very low level, which led to ~15-fold increase of the taxadiene production.
In addition to elucidating the metabolic responses to the genetic modification of engineered E. coli strains, several 13C-MFA studies have recently been performed on the metabolic responses to different environmental stresses on E. coli. First, 13C-MFA was applied to elucidate the metabolic responses of E.coli to octanoic acid stress [37] (Figure 3). A decreased flux in the TCA cycle and an increased flux in the pyruvate oxidative pathway for producing acetate were observed by comparing the flux distributions of stressed and unstressed E. coli strains. It was hypothesized that octanoic acid triggered the membrane disruption and led to NAD+ deficiency due to the destabilization of membrane-bound proteins, such as NADH dehydrogenase. The interrupted regeneration of NAD+ would repress several key NAD+ dependent pathways, such as the malate dehydrogenase pathways in the TCA cycle, and the pyruvate dehydrogenase pathway. In addition, the pyruvate pool shrank under octanoic acid stress condition, which could be attributed to the repression of the PdhR regulator that is highly sensitive to pyruvate in controlling the expressions of the PDH complex, NADH dehydrogenase II, and cytochrome bo-type oxidase [123]. Based on the 13C-MFA results, several possible strategies to further enhance the octanoic acid resistance were proposed, including the supplement of pyruvate in the medium and the replacement of NADH/NAD+-sensitive enzymes.
In addition to the acidity stress, superoxide stress is another commonly encountered stress factor, which promotes the excessive production of reactive oxygen species (ROS) that have detrimental effects on cell metabolism and other physiological activities[124]. By performing 13C-MFA for E. coli strain under paraquat-induced superoxide stress, the global flux redistribution of the central carbon metabolism was observed to resist the superoxide stress. First, the flux of oxidative PP pathway has been increased by ~2-fold, which indicated the increased NADPH requirement under the superoxide stress. In addition, the NADH production has been dramatically repressed by diverting the fluxes from the pyruvate dehydrogenase pathway into the pyruvate bypass pathway for acetate synthesis as well as from the TCA cycle into the glyoxylate shunt to reduce the NADH generation and accumulate α-ketoglutarate for protein synthesis. As a result, the NADPH/NADH ratio has been dramatically increased as the cellular responses under oxidative stress for other strains. As revealed by 13C-MFA, the overall mechanism to resist the oxidative stress was increasing the NADPH production as the preferred reduction power and decreasing the NADH production as the key electron transporter in cell respiration. This mechanism is due to the pivotal role of NADH in the generation of most of the endogenous cellular ROS during the cell respiration and the effectiveness of NADPH to maintain the reductive environment for cellular activities. Based on the flux analysis, several potential strategies to further improve the tolerance of oxidative stress were indicated, including overexpressing the zwf gene encoding the G6P dehydrogenases and the genes encoding the transhydrogenase to improve the NADPH supply.
3.3. Bacillus Subtilis
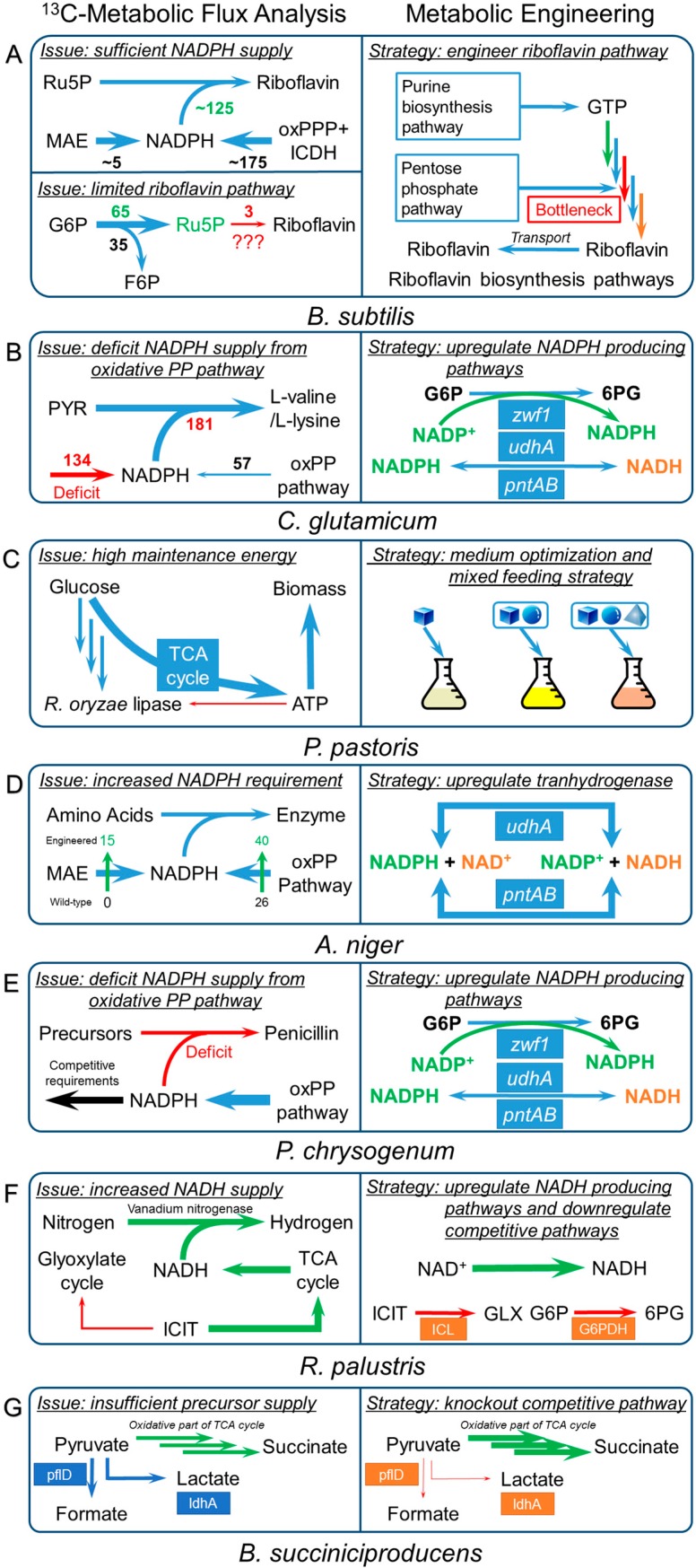
Bacillus subtilis, most well known as the microorganism engineered for commercial production of riboflavin [125], has been studied by 13C-MFA for both wild type and engineered strains in the past two decades [126,127]. Valuable insights of B. subtilis have been elucidated to better understand the cell metabolism and to further improve the biochemical production. The flux distributions of a riboflavin-producing B. subtilis strain has been rigorously investigated under three different dilution rates in chemostats [126]. As revealed by 13C-MFA, the activated PP pathway was observed, which not only supplied sufficient precursors but also produced sufficient NADPH (Figure 4A). More interestingly, the excessive NADPH production based on the flux analysis was observed under all the three dilution rates, especially in the low dilution rate without riboflavin production. In other words, the estimated amount of NADPH formation was found to be more than the NADPH requirements for both biomass and riboflavin production. The sufficient precursor supply from PP pathway could explain the high production of riboflavin and purine nucleotides. The transhydrogenase, which catalyzed the reversible conversion of NADPH to NADH, played an important role to re-oxidize the excessive NADPH that was generated due to the highly activated PP pathways.
Figure 4.
Integration of the key discoveries from 13C-MFA and the corresponding metabolic engineering strategies for (A) B. subtilis; (B) C. glutamicum; (C) P. pastoris; (D) A. niger; (E) P. chrysogenum; (F) R. palustris; and (G) B. succiniciproducens.
A complex interplay between glycolysis and the TCA cycle through the pyruvate-OAA-PEP futile cycle was also observed, which provided the sufficient replenishment of metabolites in the TCA cycle [126,127]. As a result, the energy overproduction was found under different dilution rates, i.e., the ATP formation estimated from the calculation was excessive compared to the ATP consumption. Considering that sufficient precursors, cofactors, and energy resources were supplied for the riboflavin synthesis in B. subtilis, the bottleneck for riboflavin production most likely resides in the biosynthesis pathways itself. In another large-scale 13C-MFA study on 137 null mutants of B. subtilis strains with different genetic backgrounds [128], a rigid flux distribution was observed, which is largely independent of the rate and yield of biomass. Thus, an unexpectedly stable metabolic state was maintained in B. subtilis under the given environment. This stable state was robust against the random genetic perturbations thanks to the sufficient flexibility of the acetyl-CoA branch point controlled by various transcriptional regulators. Therefore, B. subtilis could be considered as a robust host microorganism for biochemical production, with the proper extent of genetic perturbations.
Despite the advantages of using B. subtilis for biochemical production, ~50% increased requirement of maintenance energy caused by the genetic modification for riboflavin production was revealed by 13C-MFA, which could decrease the riboflavin productivity [129]. In addition, by implementing 13C-MFA for 8 strains of Bacillus species, including B. subtilis wild type, spo0A, sigE mutants and natto, B. licheniformis T218a, B. licheniformis T380B, B. amyloliquefaciens and B. pumilus, it was found that the maintenance energy required by B. subtilis was 35%~50% more than what was required by the other strains. However, consistent with the results of previous 13C-MFA studies [126,130], the excessive NADPH production was only observed in B. subtilis in contrast to other Bacillus strains with either balanced or even insufficient NADPH production (Figure 4A). This study emphasized another potential bottleneck in riboflavin production: the high maintenance energy. Since maintenance energy could be compensated by increasing ATP production from an elevated TCA cycle, the strategies mentioned in 3.1 and 3.2 to reduce or compensate the maintenance energy could also be applied to B. subtilis for improving biochemical production.
3.4. Corynebacterium Glutamicum
Corynebacterium glutamicum, the industrial workhorse for producing amino acids, such as L-lysine and L-valine, has been considered an important microorganism with extensive 13C-MFA studies [45,131,132,133,134]. Based on the insightful information provided by 13C-MFA, many metabolic engineering strategies have been developed to improve the amino acids production. For example, L-lysine is one of the major products of C. glutamicum, which is synthesized from the pyruvate and oxaloacetate requiring 4 moles NADPH to synthesize 1 mole L-lysine. By implementing 13C-MFA studies for L-lysine producing C. glutamicum strains, it was observed that the PP pathway was increased to supply NADPH, and the anaplerotic carboxylation pathway was enhanced to provide enough precursors for L-lysine synthesis [132,133,135] (Figure 4B). Following the discoveries from 13C-MFA studies, various metabolic strategies have been developed to improve the L-lysine production. First, the insufficient NADPH supply was overcome by overexpressing the enzymes in PP pathway, such as glucose-6-phosphate dehydrogenase [136], transketolase, and transaldolase [137], as well as 1, 6-bisphosphatase [138], to redirect fluxes towards PP pathway to improve lysine production. In addition to the overexpression of the native existed enzymes in PP pathway for the NADPH production, alteration of cofactor specificity of GAPDH from NAD+-dependence to NADP+-dependence successfully improved the lysine production by ~50% without decreasing cell growth rate [139]. To further investigate the responses of such alteration, 13C-MFA was performed to compare the flux distributions between wild type and mutated strains. It was found that the mutated GAPDH pathway was the major source of the NADPH in the mutated strain with the similar PP pathway flux, but with higher lysine production. Such discovery was consistent with the expectations, and demonstrated that 13C-MFA could indeed rationally guide the metabolic engineering and improve the microbial performance. In another 13C-MFA study, a strong increase in the PP pathway flux was found to be associated with L-valine production in a pyruvate decarboxylase deficient C. glutamicum strain, which again indicated that the NADPH supply was the key issue in L-valine production. By cloning a transhydrogenase from E.coli to enhance NADPH supply in C. glutamicum, L-valine yield was improved by ~200% [53].
3.5. Other Industrial Microorganisms
Although the above model microorganisms (e.g., S. cerevisiae and E. coli) have been well studied for a relatively long time with a better understanding of the cell metabolism, various non-model microorganisms have been recently highlighted for biochemical production due to their unique advantages. For example, Pichia pastoris has been used as an industrial microorganism to produce recombinant protein for more than three decades [140,141,142,143], due to the high cell density in growth, the strong and tightly regulated promoters, and partially functional post-translational modification [144,145]. Similarly, Aspergillus niger is another preferable industrial producer of various extracellular enzymes and antibiotics due to the advantages for protein secretion and post-translational modification [146]. Penicillium chrysogenum has been used to produce the penicillin G as a secondary metabolite of P. chrysogenum, which has been used as a narrow spectrum penicillin antibiotic against gram-positive organisms for more than 60 years [147,148]. In addition, Rhodopseudomonas palustris, a photosynthetic bacterium, could metabolize acetate and produce hydrogen at the non-growth stage when starved for nitrogen. Many studies also attempt to use some novel non-model microorganisms for producing versatile chemicals. For instance, Basfia succiniciproducens is found to be a novel producer with a high yield of succinate, a key platform chemical. By specifically revising the universally existing central metabolism model for various microorganisms, 13C-MFA has been performed for these non-model microorganisms. Here, as shown in Figure 4, we summarized multiple 13C-MFA studies on these non-model microorganisms that identified various issues related to biochemical production and listed the corresponding metabolic engineering strategies to solve these issues.
3.5.1. Pichia Pastoris
13C-MFA has been used for study metabolism of Pichia pastoris engineered for the production of various heterologous proteins [64,65,66,67]. Two groups of P. pastoris strains were developed and grown under the mixed methanol and glucose culture: control group that expressed a mock plasmid, and mutant group that expressed either a low-copy or a high-copy plasmid to express R. oryzae lipase. By comparing the flux redistributions between control group and mutant group, it was found that the TCA cycle fluxes of both protein-expressing P. pastoris strains were much higher than the control strain in order to produce more ATP to sustain cell growth (Figure 4C). This elevated TCA cycle confirmed that the protein folding and conformational stress indeed imposed a metabolic burden on the microbial host. It was also found that using mixed methanol and glucose culture could reduce the fluxes in TCA cycle for the reference strain with the mock plasmid, compared to using glucose as a single carbon source. Such a decreased TCA cycle indicated that the mixed feeding strategy could compensate the high cell maintenance requirements and reduce part of the metabolic burden. Indeed, in another example, by using several novel feeding strategies to cultural SAM-producing P. pastoris, the production of SAM was found to be improved by ~35% [149,150]. Thus, reduction of the metabolic burden would be an efficient way to further improve the protein productivity in P. pastoris.
3.5.2. Aspergillus Niger
Aspergillus niger is used for the recombinant production of the glycosylated enzyme fructofuranosidase, a biocatalyst of commercial interest for the synthesis of pre-biotic sugars that could induce the growth or activity of microorganisms to contribute to the well-being of humans. 13C-MFA has been applied to the recombinant strain A. niger SKAn1015, which expressed the fructofuranosidase encoding suc1 gene and secreted 45 U/mL of the target enzyme, and its parental strain, SKANip8, which did not produce the target enzyme [151]. By comparing the flux distributions in the wild-type and recombinant strain, significant redirections of metabolic fluxes were observed in the recombinant strain as below: (1) more than a 50% increase of the oxidative PP pathway (oxPP pathway); (2) activated mitochondrial malic enzyme pathway (Figure 4D); and (3) more than a 60% decrease of TCA cycle. The increased oxidative PP pathway and activated malic enzyme pathways resulted in a relative increase of 43% for the NADPH supply as compared to the wild type. The similar increase of NADPH supply in recombinant A. niger strain was also observed in other 13C-MFA studies [152]. Based on 13C-MFA studies, the high flexibility of the PP pathway and the TCA cycle was found to cope with different environmental and intracellular perturbations [151]. Thus, to further improve the heterologous enzyme production, compensation of NADPH production and cell growth could be a new focus for metabolic engineering of A. niger.
3.5.3. Penicillium Chrysogenum
Another example of 13C-MFA for non-model microorganisms is to elucidate the metabolic rewiring of P. chrysogenum in C-limited chemostats for penicillin-G production [153]. The significant differences in flux distribution through the central metabolic pathways were observed for each individual carbon source used, i.e., glucose, ethanol, and acetate, but no significant changes were found for penicillin-G production. However, when using xylose and/or nitrate as carbon and/or nitrogen source, which required additional NADPH supply, the penicillin-G production decreased (Figure 4E). This interesting metabolic rewiring showed that the potential bottleneck of penicillin production probably resided on the primary metabolism, especially the NADPH production, but not so much on precursor supply due to the similar productivity under different substrates.
3.5.4. Rhodopseudomonas Palustris
13C-MFA has also been applied to study R. palustris, a photosynthetic bacterium producing hydrogen at the non-growth condition starved for nitrogen, to elucidate the metabolic activities of the hydrogen producing and nonproducing conditions [154]. By tracking the 13C-labeled acetate through the central metabolism pathways, a shift of the acetate metabolism has been found, i.e., under the hydrogen producing condition, the cell prefers to use the TCA cycle, instead of the glyoxylate cycle, to metabolize acetate. This metabolic rewiring provides more reduction power, i.e., NADH, for hydrogen production by fully oxidizing the acetate. Therefore, improvement of NADH supply could contribute to enhancing the hydrogen production in R. palustris (Figure 4F).
3.5.5. Basfia Succiniciproducens
A 13C-MFA study was finished on Basfia succiniciproducens to improve the succinate production [155]. As elucidated by the 13C-MFA study, the metabolic flux distribution of the wild-type B. succiniciproducens strain with a high yield of succinate (0.75 mol/mol glucose) uncovered the parallel in vivo activity of the oxidative and reductive branch of the TCA cycle in B. succiniciproducens. In addition, it revealed two undesired pathways catalyzed by pyruvate-formate lyase (PflD) and lactate dehydrogenase (LdhA), which consumed the precursor pyruvate for succinate production (Figure 4G). Guided by the discovery of the 13C-MFA study, a doubly deletion ΔpflD ΔldhA B. succiniciproducens strain was developed by genetic modification and achieved 45% increase of succinate yield.
4. Perspectives of Integrating 13C Metabolic Flux Analysis with Metabolic Engineering
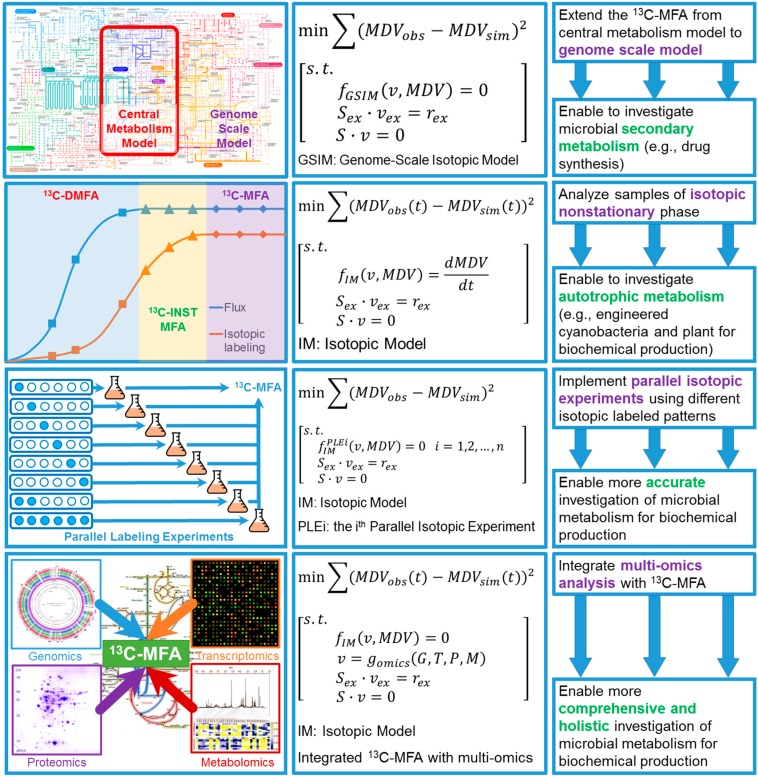
In summary, 13C-MFA, as a flexible and powerful approach to elucidate the intracellular metabolic rewiring, has been widely performed for both model and non-model microorganisms, and unraveled numerous metabolic “mysteries” inside the wild type, evolved, and engineered strains. These biological insights uncovered by 13C-MFA have profound impact on the rational design of metabolic engineering strategies to further improve the biochemical production. However, several limitations are still restricting the accuracy and flexibility of 13C-MFA. For example, the traditional 13C-MFA can only be applied at metabolic and isotopic steady states [58], which could be difficult to use when the target chemicals are produced in non-steady states (e.g., drug synthesis in the stationary growth phase). In addition, most of the conventional 13C-MFA studies can only be applied in central metabolism [156], which has very limited use when analyzing the secondary metabolism of microorganisms. To overcome these challenges, novel experimental and computational methods have recently been developed to empower 13C-MFA studies. In this section, we will summarize recent breakthroughs in 13C-MFA and provide a perspective for novel routes to achieve the integration of 13C-MFA and metabolic engineering. The details of each novel approach for 13C-MFA, including graphical flowchart, mathematical representation, and brief descriptions of recent advances of 13C-MFA were shown in Figure 5.
Figure 5.
Recent advances and perspectives of 13C-MFA, including diagrams (left column), mathematical representation (middle column), and detailed descriptions of recent advances of 13C-MFA (right column).
4.1. Expand 13C-MFA into Genome Scale
The conventional 13C-MFA was only able to be used for the determination of flux distribution in the central metabolic network, mainly because of the difficulties in (1) measuring the isotopic labeling of the numerous low-abundant metabolites to provide more information needed for expanding the model; (2) providing a high quality genome-scale model along with the detailed atom maps of every reaction in the network; and (3) the huge computational burden of simulating isotopic labeling of all metabolites in genome-scale metabolic networks. However, with the continuous efforts committed to overcome these obstacles, several successful studies have been reported to extend the 13C-MFA to genome-scale. Thanks to the rapid development of high-resolution mass spectrometry, the accurate measurement of isotopic labeling of low-abundant metabolites became possible [157,158,159]. In addition, a novel algorithm known as Canonical Labeling for Clique Approximation (CLCA) [160] has been developed to provide the atom mapping information for the reactions of genome-scale metabolic model. By using this algorithm, a new bioinformatics database, MetRxn database [161], has been built and continues to be developed, which currently contains atom mapping information for over 27,000 reactions from 112 metabolic models [162]. To use this huge metabolic network with atom mapping information for the 13C labeling patterns, an efficient network decomposing method, the elementary metabolite units (EMU) method [68], was used to decrease the computational burden by 1~2 orders of magnitude for the genome-scale mapping model.
Recently, the first 13C-MFA study based on refined genome-scale mapping model (GSMM) of E. coli strain has been reported, which showed several advantages when using the genome-scale model instead of the central metabolic model [162]. In short, using the GSMM to elucidate the flux distribution via 13C labeling patterns obtained a better prediction of 13C-labeling patterns, resolved the alternative pathways in secondary metabolism for some key metabolites, and refined the cofactor and energetic metabolism. Therefore, genome-scale 13C-MFA could provide a more thorough investigation of microbial metabolism for biochemical production. This valuable information could guide the development of rational metabolic engineering strategies in a similar way that has been used for improving chemical production.
4.2. Isotopic Nonstationary 13C-MFA (13C-INST-MFA)
13C-INST-MFA was recently developed [163,164,165,166,167] to enable the application of 13C-MFA for various autotrophic systems including cyanobacteria and plants. In brief, other than collecting 13C-labeling patterns at the isotopic steady state, 13C-INST-MFA tracks the dynamics of 13C-labeling in intracellular metabolites and applies computational algorithms to calculate the steady-state metabolic fluxes that can best fit the 13C-labeling kinetics. 13C-INST-MFA has been performed to determine the autotrophic metabolism of Synechocystis sp. PCC6803 [168] and Arabidopsis thaliana [169]. Such autotrophic metabolism is unable to be elucidated via the conventional 13C-MFA because all of the metabolites will be universally labeled at the isotopic steady state when the autotrophic organisms are fed with 13CO2 and the information about pathway usage is completely lost. The merit of 13C-INST-MFA for metabolic engineering is the capability to rigorously determine the metabolisms of numerous autotrophic systems, which used to be mysterious. Since various autotrophic systems are promising cell factories [170,171,172] that convert inorganic carbon sources, e.g., CO2, into valuable chemicals, we can envision that 13C-INST-MFA could guide metabolic engineers to rationally modify such systems for improving the production of chemical products.
4.3. 13C-Based Dynamic Metabolic Flux Analysis (13C-DMFA)
13C-DMFA has recently been developed as an approach to investigate microbial metabolism at the metabolic non-steady state [29]. One of the proof-of-concept studies for 13C-DMFA investigated the E. coli metabolism in a fed-batch fermentation process for overproduction of 1,3-propanediol. By introducing several additional parameters to describe the fed-batch fermentation process, a time-resolved flux distribution map was obtained. Based on this time-resolved flux map, it is found that the intracellular flux associated with PDO pathway increased by 10% and the split ratio between glycolysis and pentose phosphate pathway decreased from 70/30 to 50/50. 13C-DMFA has provided a way for metabolic engineers to elucidate the dynamic metabolism during industrial fermentation, especially the fed-batch fermentation. The extension of 13C-DMFA to study microbial metabolism at the stationary growth phase is also expected since numerous high-value secondary metabolites are often produced during the stationary growth phase. With the insightful information about the metabolic rewiring at non-steady states, metabolic engineers could develop more appropriate strategies to improve the biochemical production, particularly microbe-based drug production.
4.4. Improve Accuracy of 13C-MFA via Parallel Labeling Experiments (PLE)
Another recent advance in 13C-MFA is the implementation of parallel labeling experiments (PLE) by using multiple isotopic tracers to track the cell metabolism, which has been proved to improve the flux estimation and observability of 13C-MFA [173,174,175]. Parallel labeling experiments have been combined with the rapid development of the high-throughput measurement and high-performance computational algorithms [173,174,175]. The unique advantages of PLE to the conventional 13C-MFA [176] are the improved accuracy of flux estimation [173,174] and reduced time for labeling experiments. With more accurate measurement of intracellular carbon fluxes provided by PLEs, the higher resolution of microbial metabolism will be offered to metabolic engineers in the near future to develop fine-tuned engineering strategies in improving biochemical production.
5. Conclusions
Metabolic engineering has been rapidly developed for various industrial microorganisms to produce bulk chemicals, fuels, and drugs from renewable feedstock, in order to free the modern society from the depleting fossil fuel feedstock. However, the complex microbial metabolism is one of the most challenging obstacles for metabolically engineered microorganisms to reach an industrially satisfactory yield, titer or productivity. In order to overcome this challenge, 13C-MFA has been continuously developed and successfully applied to assist the rational design of metabolic strategies for both model and non-model microorganisms by rigorously quantifying the carbon flux distribution in central metabolism. As shown in this review, multiple issues in biochemical production, such as bottleneck pathways in biochemical synthesis, cofactor imbalance issue in host cells, and energetic requirements in cell maintenance, have been revealed by 13C-MFA, which guides the development of the appropriate metabolic engineering strategies for successful improvements of the target chemicals to different extents. To further advance 13C-MFA, several techniques have been recently developed to demystify the secondary metabolism, improve the accuracy and resolution of the flux distribution, and investigate the metabolism for autotrophic organisms as well as the microbial metabolism at the metabolic non-steady state. We believe that, by using 13C-MFA for various cell factories, metabolic strategies will be more rationally designed and successfully applied to improve the biochemical production.
Acknowledgments
We thank the Writing Center in Virginia Tech and Joseph Stevens for improving the language of the paper. This study was supported by start-up fund (#175323) from Virginia Tech.
Author Contributions
Xueyang Feng has the original idera for this review and with all co-authors carried out the review. Weihua Guo and Jiayuan Sheng drafted the manuscript, which was revised by Weihua Guo and Xueyang Feng. All the authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Sheng J., Feng X. Metabolic engineering of yeast to produce fatty acid-derived biofuels: Bottlenecks and solutions. Front. Microbiol. 2015;6:554. doi: 10.3389/fmicb.2015.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin J.H., Kim H.U., Kim D.I., Lee S.Y. Production of bulk chemicals via novel metabolic pathways in microorganisms. Biotechnol. Adv. 2013;31:925–935. doi: 10.1016/j.biotechadv.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Weusthuis R.A., Lamot I., van der Oost J., Sanders J.P.M. Microbial production of bulk chemicals: Development of anaerobic processes. Trends Biotechnol. 2011;29:153–158. doi: 10.1016/j.tibtech.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Hermann B.G., Blok K., Patel M.K. Producing bio-based bulk chemicals using industrial biotechnology saves energy and combats climate change. Environ. Sci. Technol. 2007;41:7915–7921. doi: 10.1021/es062559q. [DOI] [PubMed] [Google Scholar]
- 5.Stephanopoulos G. Challenges in engineering microbes for biofuels production. Science. 2007;315:801–804. doi: 10.1126/science.1139612. [DOI] [PubMed] [Google Scholar]
- 6.Peralta-Yahya P.P., Keasling J.D. Advanced biofuel production in microbes. Biotechnol. J. 2010;5:147–162. doi: 10.1002/biot.200900220. [DOI] [PubMed] [Google Scholar]
- 7.Peralta-Yahya P.P., Zhang F., del Cardayre S.B., Keasling J.D. Microbial engineering for the production of advanced biofuels. Nature. 2012;488:320–328. doi: 10.1038/nature11478. [DOI] [PubMed] [Google Scholar]
- 8.Lee S.K., Chou H., Ham T.S., Lee T.S., Keasling J.D. Metabolic engineering of microorganisms for biofuels production: From bugs to synthetic biology to fuels. Curr. Opin. Biotechnol. 2008;19:556–563. doi: 10.1016/j.copbio.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Stephanopoulos G. Metabolic engineering: Enabling technology for biofuels production. Metab. Eng. 2008;10:293–294. doi: 10.1016/j.ymben.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Ajikumar P.K., Xiao W.-H., Tyo K.E.J., Wang Y., Simeon F., Leonard E., Mucha O., Phon T.H., Pfeifer B., Stephanopoulos G. Isoprenoid pathway optimization for taxol precursor overproduction in Escherichia coli. Science. 2010;330:70–74. doi: 10.1126/science.1191652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S.Y., Kim H.U., Park J.H., Park J.M., Kim T.Y. Metabolic engineering of microorganisms: General strategies and drug production. Drug Discov. Today. 2009;14:78–88. doi: 10.1016/j.drudis.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Martin V.J.J., Pitera D.J., Withers S.T., Newman J.D., Keasling J.D. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat. Biotech. 2003;21:796–802. doi: 10.1038/nbt833. [DOI] [PubMed] [Google Scholar]
- 13.Chang M.C.Y., Keasling J.D. Production of isoprenoid pharmaceuticals by engineered microbes. Nat. Chem. Biol. 2006;2:674–681. doi: 10.1038/nchembio836. [DOI] [PubMed] [Google Scholar]
- 14.Demain A.L., Sanchez S. Microbial drug discovery: 80 Years of progress. J. Antibiot. 2009;62:5–16. doi: 10.1038/ja.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrer-Miralles N., Domingo-Espín J., Corchero J.L., Vázquez E., Villaverde A. Microbial factories for recombinant pharmaceuticals. Microb. Cell Fact. 2009;8:1–8. doi: 10.1186/1475-2859-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keasling J.D. Manufacturing molecules through metabolic engineering. Science. 2010;330:1355–1358. doi: 10.1126/science.1193990. [DOI] [PubMed] [Google Scholar]
- 17.Atsumi S., Cann A.F., Connor M.R., Shen C.R., Smith K.M., Brynildsen M.P., Chou K.J.Y., Hanai T., Liao J.C. Metabolic engineering of Escherichia coli for 1-butanol production. Metab. Eng. 2008;10:305–311. doi: 10.1016/j.ymben.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Blattner F.R., Plunkett G., Bloch C.A., Perna N.T., Burland V., Riley M., Collado-Vides J., Glasner J.D., Rode C.K., Mayhew G.F., et al. The complete genome sequence of Escherichia coli k-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 19.Huang C., Jr., Lin H., Yang X. Industrial production of recombinant therapeutics in Escherichia coli and its recent advancements. J. Ind. Microbiol. Biotechnol. 2012;39:383–399. doi: 10.1007/s10295-011-1082-9. [DOI] [PubMed] [Google Scholar]
- 20.Alper H., Miyaoku K., Stephanopoulos G. Construction of lycopene-overproducing E. Coli strains by combining systematic and combinatorial gene knockout targets. Nat. Biotech. 2005;23:612–616. doi: 10.1038/nbt1083. [DOI] [PubMed] [Google Scholar]
- 21.Farmer W.R., Liao J.C. Improving lycopene production in Escherichia coli by engineering metabolic control. Nat. Biotech. 2000;18:533–537. doi: 10.1038/75398. [DOI] [PubMed] [Google Scholar]
- 22.Clomburg J., Gonzalez R. Biofuel production in Escherichia coli: The role of metabolic engineering and synthetic biology. Appl. Microbiol. Biotechnol. 2010;86:419–434. doi: 10.1007/s00253-010-2446-1. [DOI] [PubMed] [Google Scholar]
- 23.Borneman A.R., Desany B.A., Riches D., Affourtit J.P., Forgan A.H., Pretorius I.S., Egholm M., Chambers P.J. Whole-genome comparison reveals novel genetic elements that characterize the genome of industrial strains of Saccharomyces cerevisiae. PLoS Genet. 2011;7:e1001287. doi: 10.1371/journal.pgen.1001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostergaard S., Olsson L., Nielsen J. Metabolic engineering of saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2000;64:34–50. doi: 10.1128/MMBR.64.1.34-50.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giaever G., Chu A.M., Ni L., Connelly C., Riles L., Veronneau S., Dow S., Lucau-Danila A., Anderson K., Andre B., et al. Functional profiling of the saccharomyces cerevisiae genome. Nature. 2002;418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen J., Jewett M.C. Impact of Systems Biology on Metabolic Engineering of Saccharomyces cerevisiae. FEMS Yeast Res. 2008;8:122–131. doi: 10.1111/j.1567-1364.2007.00302.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen G.-Q. New challenges and opportunities for industrial biotechnology. Microb. Cell Fact. 2012;11:111. doi: 10.1186/1475-2859-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwok R. Five hard truths for synthetic biology. Nature. 2010;463:288. doi: 10.1038/463288a. [DOI] [PubMed] [Google Scholar]
- 29.Antoniewicz M. Methods and advances in metabolic flux analysis: A mini-review. J. Ind. Microbiol. Biotechnol. 2015;42:317–325. doi: 10.1007/s10295-015-1585-x. [DOI] [PubMed] [Google Scholar]
- 30.Young J.D. 13c metabolic flux analysis of recombinant expression hosts. Curr. Opin. Biotechnol. 2014;30:238–245. doi: 10.1016/j.copbio.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y., San K.-Y., Bennett G.N. Cofactor engineering for advancing chemical biotechnology. Curr. Opin. Biotechnol. 2013;24:994–999. doi: 10.1016/j.copbio.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 32.Wasylenko T.M., Ahn W.S., Stephanopoulos G. The oxidative pentose phosphate pathway is the primary source of nadph for lipid overproduction from glucose in Yarrowia lipolytica. Metab. Eng. 2015;30:27–39. doi: 10.1016/j.ymben.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Hollinshead W.D., Henson W.R., Abernathy M., Moon T.S., Tang Y.J. Rapid metabolic analysis of rhodococcus opacus pd630 via parallel 13c-metabolite fingerprinting. Biotechnol. Bioeng. 2015;113:91–100. doi: 10.1002/bit.25702. [DOI] [PubMed] [Google Scholar]
- 34.Hayakawa K., Kajihata S., Matsuda F., Shimizu H. 13c-metabolic flux analysis in s-adenosyl-l-methionine production by saccharomyces cerevisiae. J. Biosci. Bioeng. 2015 doi: 10.1016/j.jbiosc.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Feng X., Zhao H. Investigating xylose metabolism in recombinant saccharomyces cerevisiae via 13c metabolic flux analysis. Microb. Cell Fact. 2013;12:114. doi: 10.1186/1475-2859-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lam F.H., Ghaderi A., Fink G.R., Stephanopoulos G. Engineering alcohol tolerance in yeast. Science. 2014;346:71–75. doi: 10.1126/science.1257859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu Y., Yoon J., Jarboe L., Shanks J. Metabolic flux analysis of Escherichia coli mg1655 under octanoic acid (c8) stress. Appl. Microbiol. Biotechnol. 2015;99:4397–4408. doi: 10.1007/s00253-015-6387-6. [DOI] [PubMed] [Google Scholar]
- 38.Heer D., Heine D., Sauer U. Resistance of Saccharomyces cerevisiae to high concentrations of furfural is based on nadph-dependent reduction by at least two oxireductases. Appl. Environ. Microbiol. 2009;75:7631–7638. doi: 10.1128/AEM.01649-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Çakar Z.P., Seker U.O.S., Tamerler C., Sonderegger M., Sauer U. Evolutionary engineering of multiple-stress resistant Saccharomyces cerevisiae. FEMS Yeast Res. 2005;5:569–578. doi: 10.1016/j.femsyr.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Wittmann C., Heinzle E. Mass spectrometry for metabolic flux analysis. Biotechnol. Bioeng. 1999;62:739–750. doi: 10.1002/(SICI)1097-0290(19990320)62:6<739::AID-BIT13>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 41.Wiechert W. 13c metabolic flux analysis. Metab. Eng. 2001;3:195–206. doi: 10.1006/mben.2001.0187. [DOI] [PubMed] [Google Scholar]
- 42.Dauner M., Sauer U. Gc-ms analysis of amino acids rapidly provides rich information for isotopomer balancing. Biotechnol. Prog. 2000;16:642–649. doi: 10.1021/bp000058h. [DOI] [PubMed] [Google Scholar]
- 43.de Graaf A.A. Use of 13c labelling and nmr spectroscopy in metabolic flux analysis. In: Barbotin J.N., Portais J.C., editors. Nmr in Biotechnology: Theory and Applications. Horizon Scientific Press; Norwich, UK: 2000. Chapter 4. [Google Scholar]
- 44.Christensen B., Nielsen J. Isotopomer analysis using gc-ms. Metab. Eng. 1999;1:282–290. doi: 10.1006/mben.1999.0117. [DOI] [PubMed] [Google Scholar]
- 45.SZYPERSKI T. 13c-nmr, ms and metabolic flux balancing in biotechnology research. Q. Rev. Biophys. 1998;31:41–106. doi: 10.1017/S0033583598003412. [DOI] [PubMed] [Google Scholar]
- 46.Feng X., Zhuang W.-Q., Colletti P., Tang Y. Metabolic pathway determination and flux analysis in nonmodel microorganisms through 13c-isotope labeling. In: Navid A., editor. Microbial Systems Biology. Volume 881. Humana Press; New York, NY, USA: 2012. pp. 309–330. [DOI] [PubMed] [Google Scholar]
- 47.You L., Page L., Feng X., Berla B., Pakrasi H.B., Tang Y.J. Metabolic pathway confirmation and discovery through (13)c-labeling of proteinogenic amino acids. J. Vis. Exp. 2012;59:e3583. doi: 10.3791/3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sauer U. Metabolic Networks in Motion: 13c-Based Flux Analysis. Mol. Syst. Biol. 2006;2:62–72. doi: 10.1038/msb4100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang Y.J., Martin H.G., Myers S., Rodriguez S., Baidoo E.E.K., Keasling J.D. Advances in analysis of microbial metabolic fluxes via 13c isotopic labeling. Mass Spectrom. Rev. 2009;28:362–375. doi: 10.1002/mas.20191. [DOI] [PubMed] [Google Scholar]
- 50.Lian J., Si T., Nair N.U., Zhao H. Design and construction of acetyl-coa overproducing saccharomyces cerevisiae strains. Metab. Eng. 2014;24:139–149. doi: 10.1016/j.ymben.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 51.Papini M., Nookaew I., Siewers V., Nielsen J. Physiological characterization of recombinant saccharomyces cerevisiae expressing the aspergillus nidulans phosphoketolase pathway: Validation of activity through 13c-based metabolic flux analysis. Appl. Microbiol. Biotechnol. 2012;95:1001–1010. doi: 10.1007/s00253-012-3936-0. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y., San K.-Y., Bennett G. Improvement of nadph bioavailability in Escherichia coli by replacing nad+-dependent glyceraldehyde-3-phosphate dehydrogenase gapa with nadp+-dependent gapb from bacillus subtilis and addition of nad kinase. J. Ind. Microbiol. Biotechnol. 2013;40:1449–1460. doi: 10.1007/s10295-013-1335-x. [DOI] [PubMed] [Google Scholar]
- 53.Bartek T., Blombach B., Lang S., Eikmanns B.J., Wiechert W., Oldiges M., Nöh K., Noack S. Comparative 13c metabolic flux analysis of pyruvate dehydrogenase complex-deficient, l-valine-producing corynebacterium glutamicum. Appl. Environ. Microbiol. 2011;77:6644–6652. doi: 10.1128/AEM.00575-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hou J., Vemuri G., Bao X., Olsson L. Impact of overexpressing nadh kinase on glucose and xylose metabolism in recombinant xylose-utilizing saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2009;82:909–919. doi: 10.1007/s00253-009-1900-4. [DOI] [PubMed] [Google Scholar]
- 55.Berríos-Rivera S.J., Bennett G.N., San K.-Y. Metabolic engineering of Escherichia coli: Increase of nadh availability by overexpressing an nad+-dependent formate dehydrogenase. Metab. Eng. 2002;4:217–229. doi: 10.1006/mben.2002.0227. [DOI] [PubMed] [Google Scholar]
- 56.He L., Xiao Y., Gebreselassie N., Zhang F., Antoniewicz M.R., Tang Y.J., Peng L. Central metabolic responses to the overproduction of fatty acids in Escherichia coli based on 13c-metabolic flux analysis. Biotechnol. Bioeng. 2014;111:575–585. doi: 10.1002/bit.25124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ranganathan S., Tee T.W., Chowdhury A., Zomorrodi A.R., Yoon J.M., Fu Y., Shanks J.V., Maranas C.D. An integrated computational and experimental study for overproducing fatty acids in Escherichia coli. Metab. Eng. 2012;14:687–704. doi: 10.1016/j.ymben.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Zamboni N., Fendt S.-M., Ruhl M., Sauer U. 13c-based metabolic flux analysis. Nat. Protocols. 2009;4:878–892. doi: 10.1038/nprot.2009.58. [DOI] [PubMed] [Google Scholar]
- 59.Pingitore F., Tang Y., Kruppa G.H., Keasling J.D. Analysis of amino acid isotopomers using ft-icr ms. Anal. Chem. 2007;79:2483–2490. doi: 10.1021/ac061906b. [DOI] [PubMed] [Google Scholar]
- 60.Guo W., Luo S., He Z., Feng X. 13c pathway analysis of biofilm metabolism of shewanella oneidensis mr-1. RSC Adv. 2015;5:39840–39843. doi: 10.1039/C5RA05573C. [DOI] [Google Scholar]
- 61.Iwatani S., Van Dien S., Shimbo K., Kubota K., Kageyama N., Iwahata D., Miyano H., Hirayama K., Usuda Y., Shimizu K., et al. Determination of metabolic flux changes during fed-batch cultivation from measurements of intracellular amino acids by lc-ms/ms. J. Biotechnol. 2007;128:93–111. doi: 10.1016/j.jbiotec.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Millard P., Letisse F., Sokol S., Portais J.-C. Isocor: Correcting ms data in isotope labeling experiments. Bioinformatics. 2012;28:1294–1296. doi: 10.1093/bioinformatics/bts127. [DOI] [PubMed] [Google Scholar]
- 63.Wahl S.A., Dauner M., Wiechert W. New tools for mass isotopomer data evaluation in 13c flux analysis: Mass isotope correction, data consistency checking, and precursor relationships. Biotechnol. Bioeng. 2004;85:259–268. doi: 10.1002/bit.10909. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Z., Shen T., Rui B., Zhou W., Zhou X., Shang C., Xin C., Liu X., Li G., Jiang J., et al. Cecafdb: A curated database for the documentation, visualization and comparative analysis of central carbon metabolic flux distributions explored by 13c-fluxomics. Nucleic Acids Res. 2014 doi: 10.1093/nar/gku1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shupletsov M.S., Golubeva L.I., Rubina S.S., Podvyaznikov D.A., Iwatani S., Mashko S.V. Openflux2: (13)c-mfa modeling software package adjusted for the comprehensive analysis of single and parallel labeling experiments. Microb. Cell Fact. 2014;13:152. doi: 10.1186/PREACCEPT-1256381938128538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weitzel M., Nöh K., Dalman T., Niedenführ S., Stute B., Wiechert W. 13cflux2—high-performance software suite for 13c-metabolic flux analysis. Bioinformatics. 2013;29:143–145. doi: 10.1093/bioinformatics/bts646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Antoniewicz M.R., Kelleher J.K., Stephanopoulos G. Elementary metabolite units (emu): A novel framework for modeling isotopic distributions. Metab. Eng. 2007;9:68–86. doi: 10.1016/j.ymben.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Young J.D. Inca: A computational platform for isotopically non-stationary metabolic flux analysis. Bioinformatics. 2014;30:1333–1335. doi: 10.1093/bioinformatics/btu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zamboni N., Fischer E., Sauer U. Fiatflux—A software for metabolic flux analysis from (13)c-glucose experiments. BMC Bioinf. 2005;6:209. doi: 10.1186/1471-2105-6-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garcia-Albornoz M., Thankaswamy-Kosalai S., Nilsson A., Väremo L., Nookaew I., Nielsen J. Biomet toolbox 2.0: Genome-wide analysis of metabolism and omics data. Nucleic Acids Res. 2014;42:W175–W181. doi: 10.1093/nar/gku371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Young J.D., Walther J.L., Antoniewicz M.R., Yoo H., Stephanopoulos G. An elementary metabolite unit (emu) based method of isotopically nonstationary flux analysis. Biotechnol. Bioeng. 2008;99:686–699. doi: 10.1002/bit.21632. [DOI] [PubMed] [Google Scholar]
- 72.Chopra P., Kamma A. Engineering life through synthetic biology. Silico Biol. 2006;6:401–410. [PubMed] [Google Scholar]
- 73.Hong K.-K., Nielsen J. Metabolic engineering of saccharomyces cerevisiae: A key cell factory platform for future biorefineries. Cell. Mol. Life Sci. 2012;69:2671–2690. doi: 10.1007/s00018-012-0945-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nielsen J., Jewett M.C. Impact of systems biology on metabolic engineering of saccharomyces cerevisiae. FEMS Yeast Res. 2008;8:122–131. doi: 10.1111/j.1567-1364.2007.00302.x. [DOI] [PubMed] [Google Scholar]
- 75.Bro C., Regenberg B., Förster J., Nielsen J. In silico aided metabolic engineering of saccharomyces cerevisiae for improved bioethanol production. Metab. Eng. 2006;8:102–111. doi: 10.1016/j.ymben.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 76.Overkamp K.M., Bakker B.M., Kötter P., Luttik M.A.H., van Dijken J.P., Pronk J.T. Metabolic engineering of glycerol production in saccharomyces cerevisiae. Appl. Environ. Microbiol. 2002;68:2814–2821. doi: 10.1128/AEM.68.6.2814-2821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feng X., Lian J., Zhao H. Metabolic engineering of saccharomyces cerevisiae to improve 1-hexadecanol production. Metab. Eng. 2015;27:10–19. doi: 10.1016/j.ymben.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 78.Runguphan W., Keasling J.D. Metabolic engineering of saccharomyces cerevisiae for production of fatty acid-derived biofuels and chemicals. Metab. Eng. 2014;21:103–113. doi: 10.1016/j.ymben.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 79.DeJong J.M., Liu Y., Bollon A.P., Long R.M., Jennewein S., Williams D., Croteau R.B. Genetic engineering of taxol biosynthetic genes in saccharomyces cerevisiae. Biotechnol. Bioeng. 2006;93:212–224. doi: 10.1002/bit.20694. [DOI] [PubMed] [Google Scholar]
- 80.Ro D.-K., Paradise E.M., Ouellet M., Fisher K.J., Newman K.L., Ndungu J.M., Ho K.A., Eachus R.A., Ham T.S., Kirby J., et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440:940–943. doi: 10.1038/nature04640. [DOI] [PubMed] [Google Scholar]
- 81.Yamano S., Ishii T., Nakagawa M., Ikenaga H., Misawa N. Metabolic engineering for production of β-carotene and lycopene in saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 1994;58:1112–1114. doi: 10.1271/bbb.58.1112. [DOI] [PubMed] [Google Scholar]
- 82.Curran K.A., Leavitt J.M., Karim A.S., Alper H.S. Metabolic engineering of muconic acid production in saccharomyces cerevisiae. Metab. Eng. 2013;15:55–66. doi: 10.1016/j.ymben.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 83.Jiang H., Wood K.V., Morgan J.A. Metabolic engineering of the phenylpropanoid pathway in saccharomyces cerevisiae. Appl. Environ. Microbiol. 2005;71:2962–2969. doi: 10.1128/AEM.71.6.2962-2969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Frick O., Wittmann C. Characterization of the metabolic shift between oxidative and fermentative growth in saccharomyces cerevisiae by comparative 13c flux analysis. Microb. Cell Fact. 2005;4:30. doi: 10.1186/1475-2859-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zaidi N., Swinnen J.V., Smans K. Atp-citrate lyase: A key player in cancer metabolism. Cancer Res. 2012;72:3709–3714. doi: 10.1158/0008-5472.CAN-11-4112. [DOI] [PubMed] [Google Scholar]
- 86.Meile L., Rohr L.M., Geissmann T.A., Herensperger M., Teuber M. Characterization of the d-xylulose 5-phosphate/d-fructose 6-phosphate phosphoketolase gene (xfp) from bifidobacterium lactis. J. Bacteriol. 2001;183:2929–2936. doi: 10.1128/JB.183.9.2929-2936.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Panagiotou G., Andersen M.R., Grotkjaer T., Regueira T.B., Hofmann G., Nielsen J., Olsson L. Systems analysis unfolds the relationship between the phosphoketolase pathway and growth in Aspergillus nidulans. PLoS ONE. 2008;3:e3847. doi: 10.1371/journal.pone.0003847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Jong B.W., Shi S., Siewers V., Nielsen J. Improved production of fatty acid ethyl esters in saccharomyces cerevisiae through up-regulation of the ethanol degradation pathway and expression of the heterologous phosphoketolase pathway. Microb. Cell Fact. 2014;13:39. doi: 10.1186/1475-2859-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jeppsson M., Johansson B., Hahn-Hägerdal B., Gorwa-Grauslund M.F. Reduced oxidative pentose phosphate pathway flux in recombinant xylose-utilizing saccharomyces cerevisiae strains improves the ethanol yield from xylose. Appl. Environ. Microbiol. 2002;68:1604–1609. doi: 10.1128/AEM.68.4.1604-1609.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Watanabe S., Abu Saleh A., Pack S.P., Annaluru N., Kodaki T., Makino K. Ethanol production from xylose by recombinant saccharomyces cerevisiae expressing protein-engineered nadh-preferring xylose reductase from pichia stipitis. Microbiology. 2007;153:3044–3054. doi: 10.1099/mic.0.2007/007856-0. [DOI] [PubMed] [Google Scholar]
- 91.Jeppsson M., Bengtsson O., Franke K., Lee H., Hahn-Hägerdal B., Gorwa-Grauslund M.F. The expression of a pichia stipitis xylose reductase mutant with higher km for nadph increases ethanol production from xylose in recombinant saccharomyces cerevisiae. Biotechnol. Bioeng. 2006;93:665–673. doi: 10.1002/bit.20737. [DOI] [PubMed] [Google Scholar]
- 92.Watanabe S., Pack S.P., Saleh A.A., Annaluru N., Kodaki T., Makino K. The positive effect of the decreased nadph-preferring activity of xylose reductase from pichia stipitis on ethanol production using xylose-fermenting recombinant saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 2007;71:1365–1369. doi: 10.1271/bbb.70104. [DOI] [PubMed] [Google Scholar]
- 93.Runquist D., Hahn-Hägerdal B., Bettiga M. Increased expression of the oxidative pentose phosphate pathway and gluconeogenesis in anaerobically growing xylose-utilizing saccharomyces cerevisiae. Microb. Cell Fact. 2009;8:49. doi: 10.1186/1475-2859-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petschacher B., Nidetzky B. Altering the coenzyme preference of xylose reductase to favor utilization of nadh enhances ethanol yield from xylose in a metabolically engineered strain of saccharomyces cerevisiae. Microb. Cell Fact. 2008;7:9. doi: 10.1186/1475-2859-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bengtsson O., Hahn-Hägerdal B., Gorwa-Grauslund M.F. Xylose reductase from pichia stipitis with altered coenzyme preference improves ethanolic xylose fermentation by recombinant saccharomyces cerevisiae. Biotechnol. Biofuels. 2009;2:9. doi: 10.1186/1754-6834-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Runquist D., Hahn-Hägerdal B., Bettiga M. Increased ethanol productivity in xylose-utilizing saccharomyces cerevisiae via a randomly mutagenized xylose reductase. Appl. Environ. Microbiol. 2010;76:7796–7802. doi: 10.1128/AEM.01505-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Watanabe S., Saleh A.A., Pack S.P., Annaluru N., Kodaki T., Makino K. Ethanol production from xylose by recombinant saccharomyces cerevisiae expressing protein engineered nadp+-dependent xylitol dehydrogenase. J. Biotechnol. 2007;130:316–319. doi: 10.1016/j.jbiotec.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 98.Matsushika A., Watanabe S., Kodaki T., Makino K., Inoue H., Murakami K., Takimura O., Sawayama S. Expression of protein engineered nadp+-dependent xylitol dehydrogenase increases ethanol production from xylose in recombinant saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2008;81:243–255. doi: 10.1007/s00253-008-1649-1. [DOI] [PubMed] [Google Scholar]
- 99.Krahulec S., Klimacek M., Nidetzky B. Engineering of a matched pair of xylose reductase and xylitol dehydrogenase for xylose fermentation by saccharomyces cerevisiae. Biotechnol. J. 2009;4:684–694. doi: 10.1002/biot.200800334. [DOI] [PubMed] [Google Scholar]
- 100.Matsushika A., Inoue H., Watanabe S., Kodaki T., Makino K., Sawayama S. Efficient bioethanol production by a recombinant flocculent saccharomyces cerevisiae strain with a genome-integrated nadp(+)-dependent xylitol dehydrogenase gene. Appl. Environ. Microbiol. 2009;75:3818–3822. doi: 10.1128/AEM.02636-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Verho R., Londesborough J., Penttilä M., Richard P. Engineering redox cofactor regeneration for improved pentose fermentation in saccharomyces cerevisiae. Appl. Environ. Microbiol. 2003;69:5892–5897. doi: 10.1128/AEM.69.10.5892-5897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang G.-C., Liu J.-J., Ding W.-T. Decreased xylitol formation during xylose fermentation in saccharomyces cerevisiae due to overexpression of water-forming nadh oxidase. Appl. Environ. Microbiol. 2012;78:1081–1086. doi: 10.1128/AEM.06635-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wasylenko T.M., Stephanopoulos G. Metabolomic and 13c-metabolic flux analysis of a xylose-consuming saccharomyces cerevisiae strain expressing xylose isomerase. Biotechnol. Bioeng. 2015;112:470–483. doi: 10.1002/bit.25447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Birnbaum S., Bailey J.E. Plasmid presence changes the relative levels of many host cell proteins and ribosome components in recombinant Escherichia coli. Biotechnol. Bioeng. 1991;37:736–745. doi: 10.1002/bit.260370808. [DOI] [PubMed] [Google Scholar]
- 105.Guyot S., Gervais P., Young M., Winckler P., Dumont J., Davey H.M. Surviving the heat: Heterogeneity of response in saccharomyces cerevisiae provides insight into thermal damage to the membrane. Environ. Microbiol. 2015;17:2982–2992. doi: 10.1111/1462-2920.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nugroho R.H., Yoshikawa K., Shimizu H. Metabolomic analysis of acid stress response in saccharomyces cerevisiae. J. Biosci. Bioeng. 2015;120:396–404. doi: 10.1016/j.jbiosc.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 107.Parawira W., Tekere M. Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: Review. Crit. Rev. Biotechnol. 2011;31:20–31. doi: 10.3109/07388551003757816. [DOI] [PubMed] [Google Scholar]
- 108.Fillet S., Gibert J., Suárez B., Lara A., Ronchel C., Adrio J. Fatty alcohols production by oleaginous yeast. J. Ind. Microbiol. Biotechnol. 2015;42:1463–1472. doi: 10.1007/s10295-015-1674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Feng Y., Cronan J.E. Escherichia coli unsaturated fatty acid synthesis: Complex transcription of the faba gene and in vivo identification of the essential reaction catalyzed by fabb. J. Biol. Chem. 2009;284:29526–29535. doi: 10.1074/jbc.M109.023440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Davis M.S., Solbiati J., Cronan J.E., Jr. Overproduction of acetyl-coa carboxylase activity increases the rate of fatty acid biosynthesis in Escherichia coli. J. Biol. Chem. 2000;275:28593–28598. doi: 10.1074/jbc.M004756200. [DOI] [PubMed] [Google Scholar]
- 111.Lu X., Vora H., Khosla C. Overproduction of free fatty acids in E. Coli: Implications for biodiesel production. Metab. Eng. 2008;10:333–339. doi: 10.1016/j.ymben.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 112.Zhang F., Ouellet M., Batth T.S., Adams P.D., Petzold C.J., Mukhopadhyay A., Keasling J.D. Enhancing fatty acid production by the expression of the regulatory transcription factor fadr. Metab. Eng. 2012;14:653–660. doi: 10.1016/j.ymben.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 113.Subrahmanyam S., Cronan J.E., Jr. Overproduction of a functional fatty acid biosynthetic enzyme blocks fatty acid synthesis in Escherichia coli. J. Bacteriol. 1998;180:4596–4602. doi: 10.1128/jb.180.17.4596-4602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim Y.M., Cho H.-S., Jung G.Y., Park J.M. Engineering the pentose phosphate pathway to improve hydrogen yield in recombinant Escherichia coli. Biotechnol. Bioeng. 2011;108:2941–2946. doi: 10.1002/bit.23259. [DOI] [PubMed] [Google Scholar]
- 115.Lee W.-H., Park J.-B., Park K., Kim M.-D., Seo J.-H. Enhanced production of ɛ-caprolactone by overexpression of nadph-regenerating glucose 6-phosphate dehydrogenase in recombinant Escherichia coli harboring cyclohexanone monooxygenase gene. Appl. Microbiol. Biotechnol. 2007;76:329–338. doi: 10.1007/s00253-007-1016-7. [DOI] [PubMed] [Google Scholar]
- 116.Chin J.W., Cirino P.C. Improved nadph supply for xylitol production by engineered Escherichia coli with glycolytic mutations. Biotechnol. Prog. 2011;27:333–341. doi: 10.1002/btpr.559. [DOI] [PubMed] [Google Scholar]
- 117.Wang Y., San K.-Y., Bennett G. Improvement of nadph bioavailability in Escherichia coli through the use of phosphofructokinase deficient strains. Appl. Microbiol. Biotechnol. 2013;97:6883–6893. doi: 10.1007/s00253-013-4859-0. [DOI] [PubMed] [Google Scholar]
- 118.Chemler J.A., Fowler Z.L., McHugh K.P., Koffas M.A.G. Improving nadph availability for natural product biosynthesis in Escherichia coli by metabolic engineering. Metab. Eng. 2010;12:96–104. doi: 10.1016/j.ymben.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 119.Kim S., Lee C.H., Nam S.W., Kim P. Alteration of reducing powers in an isogenic phosphoglucose isomerase (pgi)-disrupted Escherichia coli expressing nad(p)-dependent malic enzymes and nadp-dependent glyceraldehyde 3-phosphate dehydrogenase. Lett. Appl. Microbiol. 2011;52:433–440. doi: 10.1111/j.1472-765X.2011.03013.x. [DOI] [PubMed] [Google Scholar]
- 120.Sánchez A.M., Andrews J., Hussein I., Bennett G.N., San K.-Y. Effect of overexpression of a soluble pyridine nucleotide transhydrogenase (udha) on the production of poly(3-hydroxybutyrate) in Escherichia coli. Biotechnol. Prog. 2006;22:420–425. doi: 10.1021/bp050375u. [DOI] [PubMed] [Google Scholar]
- 121.Chou H.-H., Marx C.J., Sauer U. Transhydrogenase promotes the robustness and evolvability of E. Coli deficient in nadph production. PLoS Genet. 2015;11:e1005007. doi: 10.1371/journal.pgen.1005007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jones K.L., Kim S.W., Keasling J.D. Low-copy plasmids can perform as well as or better than high-copy plasmids for metabolic engineering of bacteria. Metab. Eng. 2000;2:328–338. doi: 10.1006/mben.2000.0161. [DOI] [PubMed] [Google Scholar]
- 123.King T., Lucchini S., Hinton J.C.D., Gobius K. Transcriptomic analysis of Escherichia coli o157:H7 and k-12 cultures exposed to inorganic and organic acids in stationary phase reveals acidulant- and strain-specific acid tolerance responses. Appl. Environ. Microbiol. 2010;76:6514–6528. doi: 10.1128/AEM.02392-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rui B., Shen T., Zhou H., Liu J., Chen J., Pan X., Liu H., Wu J., Zheng H., Shi Y. A systematic investigation of Escherichia coli central carbon metabolism in response to superoxide stress. BMC Syst. Biology. 2010;4:122. doi: 10.1186/1752-0509-4-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Perkins J.B., Sloma A., Hermann T., Theriault K., Zachgo E., Erdenberger T., Hannett N., Chatterjee N.P., Williams V., II, Rufo G.A., Jr., et al. Genetic engineering of bacillus subtilis for the commercial production of riboflavin. J. Ind. Microbiol. Biotech. 1999;22:8–18. doi: 10.1038/sj.jim.2900587. [DOI] [Google Scholar]
- 126.Sauer U., Hatzimanikatis V., Bailey J.E., Hochuli M., Szyperski T., Wuthrich K. Metabolic fluxes in riboflavin-producing bacillus subtilis. Nat. Biotech. 1997;15:448–452. doi: 10.1038/nbt0597-448. [DOI] [PubMed] [Google Scholar]
- 127.Dauner M., Bailey J.E., Sauer U. Metabolic flux analysis with a comprehensive isotopomer model in bacillus subtilis. Biotechnol. Bioeng. 2001;76:144–156. doi: 10.1002/bit.1154. [DOI] [PubMed] [Google Scholar]
- 128.Fischer E., Sauer U. Large-scale in vivo flux analysis shows rigidity and suboptimal performance of bacillus subtilis metabolism. Nat. Genet. 2005;37:636–640. doi: 10.1038/ng1555. [DOI] [PubMed] [Google Scholar]
- 129.Tannler S., Decasper S., Sauer U. Maintenance metabolism and carbon fluxes in bacillus species. Microb. Cell Fact. 2008;7:19. doi: 10.1186/1475-2859-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sauer U., Hatzimanikatis V., Hohmann H.P., Manneberg M., van Loon A.P., Bailey J.E. Physiology and metabolic fluxes of wild-type and riboflavin-producing bacillus subtilis. Appl. Environ. Microbiol. 1996;62:3687–3696. doi: 10.1128/aem.62.10.3687-3696.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wiechert W., de Graaf A.A. In vivo stationary flux analysis by 13c labeling experiments. In: Sahm H., Wandrey C., editors. Metabolic Engineering. Volume 54. Springer; Berlin, Germany: 1996. pp. 109–154. [DOI] [PubMed] [Google Scholar]
- 132.Wittmann C., Heinzle E. Application of maldi-tof ms to lysine-producing corynebacterium glutamicum. Eur. J. Biochem. 2001;268:2441–2455. doi: 10.1046/j.1432-1327.2001.02129.x. [DOI] [PubMed] [Google Scholar]
- 133.Klapa M.I., Aon J.-C., Stephanopoulos G. Systematic quantification of complex metabolic flux networks using stable isotopes and mass spectrometry. Eur. J. Biochem. 2003;270:3525–3542. doi: 10.1046/j.1432-1033.2003.03732.x. [DOI] [PubMed] [Google Scholar]
- 134.Quek L.-E., Wittmann C., Nielsen L., Kromer J. Openflux: Efficient modelling software for 13c-based metabolic flux analysis. Microb. Cell Fact. 2009;8:25. doi: 10.1186/1475-2859-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Krömer J.O., Sorgenfrei O., Klopprogge K., Heinzle E., Wittmann C. In-depth profiling of lysine-producing corynebacterium glutamicum by combined analysis of the transcriptome, metabolome, and fluxome. J. Bacteriol. 2004;186:1769–1784. doi: 10.1128/JB.186.6.1769-1784.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Becker J., Klopprogge C., Herold A., Zelder O., Bolten C.J., Wittmann C. Metabolic flux engineering of l-lysine production in corynebacterium glutamicum—Over expression and modification of g6p dehydrogenase. J. Biotechnol. 2007;132:99–109. doi: 10.1016/j.jbiotec.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 137.Becker J., Zelder O., Häfner S., Schröder H., Wittmann C. From zero to hero—Design-based systems metabolic engineering of corynebacterium glutamicum for l-lysine production. Metab. Eng. 2011;13:159–168. doi: 10.1016/j.ymben.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 138.Becker J., Klopprogge C., Zelder O., Heinzle E., Wittmann C. Amplified expression of fructose 1,6-bisphosphatase in corynebacterium glutamicum increases in vivo flux through the pentose phosphate pathway and lysine production on different carbon sources. Appl. Environ. Microbiol. 2005;71:8587–8596. doi: 10.1128/AEM.71.12.8587-8596.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bommareddy R.R., Chen Z., Rappert S., Zeng A.-P. A de novo nadph generation pathway for improving lysine production of corynebacterium glutamicum by rational design of the coenzyme specificity of glyceraldehyde 3-phosphate dehydrogenase. Metab. Eng. 2014;25:30–37. doi: 10.1016/j.ymben.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 140.Sreekrishna K., Brankamp R.G., Kropp K.E., Blankenship D.T., Tsay J.-T., Smith P.L., Wierschke J.D., Subramaniam A., Birkenberger L.A. Strategies for optimal synthesis and secretion of heterologous proteins in the methylotrophic yeast pichia pastoris. Gene. 1997;190:55–62. doi: 10.1016/S0378-1119(96)00672-5. [DOI] [PubMed] [Google Scholar]
- 141.Cregg J., Cereghino J., Shi J., Higgins D. Recombinant protein expression in pichia pastoris. Mol. Biotechnol. 2000;16:23–52. doi: 10.1385/MB:16:1:23. [DOI] [PubMed] [Google Scholar]
- 142.Cereghino J.L., Cregg J.M. Heterologous protein expression in the methylotrophic yeast pichia pastoris. FEMS Microbiol. Rev. 2000;24:45–66. doi: 10.1111/j.1574-6976.2000.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 143.Sreekrishna K., Potenz R.H., Cruze J.A., McCombie W.R., Parker K.A., Nelles L., Mazzaferro P.K., Holden K.A., Harrison R.G., Wood P.J. High level expression of heterologous proteins in methylotrophic yeast pichia pastoris. J. Basic Microbiol. 1988;28:265–278. doi: 10.1002/jobm.3620280410. [DOI] [PubMed] [Google Scholar]
- 144.Ahmad M., Hirz M., Pichler H., Schwab H. Protein expression in pichia pastoris: Recent achievements and perspectives for heterologous protein production. Appl. Microbiol. Biotechnol. 2014;98:5301–5317. doi: 10.1007/s00253-014-5732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Daly R., Hearn M.T.W. Expression of heterologous proteins in pichia pastoris: A useful experimental tool in protein engineering and production. J. Mol. Recognit. 2005;18:119–138. doi: 10.1002/jmr.687. [DOI] [PubMed] [Google Scholar]
- 146.Hofmann G., Diano A., Nielsen J. Recombinant bacterial hemoglobin alters metabolism of aspergillus niger. Metab. Eng. 2009;11:8–12. doi: 10.1016/j.ymben.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 147.Morikawa Y., Karube I., Suzuki S. Penicillin g production by immobilized whole cells of penicillium chrysogenum. Biotechnol. Bioeng. 1979;21:261–270. doi: 10.1002/bit.260210211. [DOI] [PubMed] [Google Scholar]
- 148.Pandey S., Ahmad T., Aryal S., Rana B., Sapkota B. Penicillin production and history: An overview. Int. J. Microbiol. Allied Sci. 2014;1:5. [Google Scholar]
- 149.Hu X.-Q., Chu J., Zhang S.-L., Zhuang Y.-P., Wang Y.-H., Zhu S., Zhu Z.-G., Yuan Z.-Y. A novel feeding strategy during the production phase for enhancing the enzymatic synthesis of s-adenosyl-l-methionine by methylotrophic pichia pastoris. Enzym. Microb. Technol. 2007;40:669–674. doi: 10.1016/j.enzmictec.2006.05.024. [DOI] [Google Scholar]
- 150.Hu X.-Q., Chu J., Zhang Z., Zhang S.-L., Zhuang Y.-P., Wang Y.-H., Guo M.-J., Chen H.-X., Yuan Z.-Y. Effects of different glycerol feeding strategies on s-adenosyl-l-methionine biosynthesis by pgap-driven pichia pastoris overexpressing methionine adenosyltransferase. J. Biotechnol. 2008;137:44–49. doi: 10.1016/j.jbiotec.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 151.Driouch H., Melzer G., Wittmann C. Integration of in vivo and in silico metabolic fluxes for improvement of recombinant protein production. Metab. Eng. 2012;14:47–58. doi: 10.1016/j.ymben.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 152.Pedersen H., Christensen B., Hjort C., Nielsen J. Construction and characterization of an oxalic acid nonproducing strain of aspergillus niger. Metab. Eng. 2000;2:34–41. doi: 10.1006/mben.1999.0136. [DOI] [PubMed] [Google Scholar]
- 153.van Gulik W.M., de Laat W.T.A.M., Vinke J.L., Heijnen J.J. Application of metabolic flux analysis for the identification of metabolic bottlenecks in the biosynthesis of penicillin-g. Biotechnol. Bioeng. 2000;68:602–618. doi: 10.1002/(SICI)1097-0290(20000620)68:6<602::AID-BIT3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 154.McKinlay J.B., Oda Y., Rühl M., Posto A.L., Sauer U., Harwood C.S. Non-growing rhodopseudomonas palustris increases the hydrogen gas yield from acetate by shifting from the glyoxylate shunt to the tricarboxylic acid cycle. J. Biol. Chem. 2014;289:1960–1970. doi: 10.1074/jbc.M113.527515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Becker J., Reinefeld J., Stellmacher R., Schäfer R., Lange A., Meyer H., Lalk M., Zelder O., von Abendroth G., Schröder H., et al. Systems-wide analysis and engineering of metabolic pathway fluxes in bio-succinate producing basfia succiniciproducens. Biotechnol. Bioeng. 2013;110:3013–3023. doi: 10.1002/bit.24963. [DOI] [PubMed] [Google Scholar]
- 156.Ravikirthi P., Suthers P.F., Maranas C.D. Construction of an E. Coli genome-scale atom mapping model for mfa calculations. Biotechnol. Bioeng. 2011;108:1372–1382. doi: 10.1002/bit.23070. [DOI] [PubMed] [Google Scholar]
- 157.Zamboni N., Sauer U. Novel biological insights through metabolomics and 13c-flux analysis. Curr. Opin. Microbiol. 2009;12:553–558. doi: 10.1016/j.mib.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 158.Büscher J.M., Czernik D., Ewald J.C., Sauer U., Zamboni N. Cross-platform comparison of methods for quantitative metabolomics of primary metabolism. Anal. Chem. 2009;81:2135–2143. doi: 10.1021/ac8022857. [DOI] [PubMed] [Google Scholar]
- 159.Christen S., Sauer U. Intracellular characterization of aerobic glucose metabolism in seven yeast species by 13c flux analysis and metabolomics. FEMS Yeast Res. 2011;11:263–272. doi: 10.1111/j.1567-1364.2010.00713.x. [DOI] [PubMed] [Google Scholar]
- 160.Kumar A., Maranas C.D. Clca: Maximum common molecular substructure queries within the metrxn database. J. Chem. Inf. Model. 2014;54:3417–3438. doi: 10.1021/ci5003922. [DOI] [PubMed] [Google Scholar]
- 161.Kumar A., Suthers P., Maranas C. Metrxn: A knowledgebase of metabolites and reactions spanning metabolic models and databases. BMC Bioinf. 2012;13:6. doi: 10.1186/1471-2105-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gopalakrishnan S., Maranas C.D. 13c metabolic flux analysis at a genome-scale. Metab. Eng. 2015;32:12–22. doi: 10.1016/j.ymben.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 163.Jazmin L., O’Grady J., Ma F., Allen D., Morgan J., Young J. Isotopically nonstationary mfa (inst-mfa) of autotrophic metabolism. In: Dieuaide-Noubhani M., Alonso A.P., editors. Plant Metabolic Flux Analysis. Volume 1090. Humana Press; New York, NY, USA: 2014. pp. 181–210. [DOI] [PubMed] [Google Scholar]
- 164.Murphy T.A., Dang C.V., Young J.D. Isotopically nonstationary 13c flux analysis of myc-induced metabolic reprogramming in b-cells. Metab. Eng. 2013;15:206–217. doi: 10.1016/j.ymben.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jazmin L., Young J. Isotopically nonstationary 13c metabolic flux analysis. In: Alper H.S., editor. Systems Metabolic Engineering. Volume 985. Humana Press; New York, NY, USA: 2013. pp. 367–390. [DOI] [PubMed] [Google Scholar]
- 166.Wiechert W., Nöh K. Isotopically non-stationary metabolic flux analysis: Complex yet highly informative. Curr. Opin. Biotechnol. 2013;24:979–986. doi: 10.1016/j.copbio.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 167.Wiechert W., Nöh K. From stationary to instationary metabolic flux analysis. In: Kragl U., editor. Technology Transfer in Biotechnology. Volume 92. Springer; Berlin, Germany: 2005. pp. 145–172. [DOI] [PubMed] [Google Scholar]
- 168.Young J.D., Shastri A.A., Stephanopoulos G., Morgan J.A. Mapping photoautotrophic metabolism with isotopically nonstationary 13c flux analysis. Metab. Eng. 2011;13:656–665. doi: 10.1016/j.ymben.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ma F., Jazmin L.J., Young J.D., Allen D.K. Isotopically nonstationary 13c flux analysis of changes in arabidopsis thaliana leaf metabolism due to high light acclimation. Proc. Natl. Acad. Sci. USA. 2014;111:16967–16972. doi: 10.1073/pnas.1319485111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Brennan L., Owende P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010;14:557–577. doi: 10.1016/j.rser.2009.10.009. [DOI] [Google Scholar]
- 171.Varman A., Yu Y., You L., Tang Y. Photoautotrophic production of d-lactic acid in an engineered cyanobacterium. Microb. Cell Fact. 2013;12:117. doi: 10.1186/1475-2859-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhou J., Li Y. Engineering cyanobacteria for fuels and chemicals production. Protein Cell. 2010;1:207–210. doi: 10.1007/s13238-010-0043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Leighty R.W., Antoniewicz M.R. Complete-mfa: Complementary parallel labeling experiments technique for metabolic flux analysis. Metab. Eng. 2013;20:49–55. doi: 10.1016/j.ymben.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 174.Crown S.B., Long C.P., Antoniewicz M.R. Integrated 13c-metabolic flux analysis of 14 parallel labeling experiments in Escherichia coli. Metab. Eng. 2015;28:151–158. doi: 10.1016/j.ymben.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Leighty R.W., Antoniewicz M.R. Parallel labeling experiments with [u-13c]glucose validate E. Coli metabolic network model for 13c metabolic flux analysis. Metab. Eng. 2012;14:533–541. doi: 10.1016/j.ymben.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 176.Crown S.B., Antoniewicz M.R. Parallel labeling experiments and metabolic flux analysis: Past, present and future methodologies. Metab. Eng. 2013;16:21–32. doi: 10.1016/j.ymben.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Srour O., Young J.D., Eldar Y.C. Fluxomers: A new approach for (13)c metabolic flux analysis. BMC Syst. Biol. 2011;5:129. doi: 10.1186/1752-0509-5-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gill P.E., Murray W., Saunders M.A. Snopt: An sqp algorithm for large-scale constrained optimization. SIAM Rev. 2005;47:99–131. doi: 10.1137/S0036144504446096. [DOI] [Google Scholar]
- 179.Sokol S., Millard P., Portais J.-C. Influx_s: Increasing numerical stability and precision for metabolic flux analysis in isotope labelling experiments. Bioinformatics. 2012;28:687–693. doi: 10.1093/bioinformatics/btr716. [DOI] [PubMed] [Google Scholar]
- 180.Cvijovic M., Olivares-Hernández R., Agren R., Dahr N., Vongsangnak W., Nookaew I., Patil K.R., Nielsen J. Biomet toolbox: Genome-wide analysis of metabolism. Nucleic Acids Res. 2010;38:W144–W149. doi: 10.1093/nar/gkq404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gombert A.K., Moreira dos Santos M., Christensen B., Nielsen J. Network identification and flux quantification in the central metabolism of saccharomyces cerevisiae under different conditions of glucose repression. J. Bacteriol. 2001;183:1441–1451. doi: 10.1128/JB.183.4.1441-1451.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kajihata S., Furusawa C., Matsuda F., Shimizu H. Openmebius: An open source software for isotopically nonstationary 13c-based metabolic flux analysis. BioMed Res. Int. 2014;2014:10. doi: 10.1155/2014/627014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Press W.H., Flannery B.P., Teukolsky S.A., Vetterling W.T. Numerical Recipes in C: The Art of Scientific Computing. Cambridge University Press; Cambridge, UK: 1988. p. 735. [Google Scholar]
- 184.Shiba Y., Paradise E.M., Kirby J., Ro D.-K., Keasling J.D. Engineering of the pyruvate dehydrogenase bypass in saccharomyces cerevisiae for high-level production of isoprenoids. Metab. Eng. 2007;9:160–168. doi: 10.1016/j.ymben.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 185.Jordà J., Jouhten P., Cámara E., Maaheimo H., Albiol J., Ferrer P. Metabolic flux profiling of recombinant protein secreting pichia pastoris growing on glucose:Methanol mixtures. Microb. Cell Fact. 2012;11:57. doi: 10.1186/1475-2859-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fuhrer T., Sauer U. Different biochemical mechanisms ensure network-wide balancing of reducing equivalents in microbial metabolism. J. Bacteriol. 2009;191:2112–2121. doi: 10.1128/JB.01523-08. [DOI] [PMC free article] [PubMed] [Google Scholar]