Abstract
Children with sickle cell disease (SCD) are at risk for working memory deficits due to multiple disease processes. We assessed working memory abilities and related functions in 32 school-age children with SCD and 85 matched comparison children using Baddeley’s working memory model as a framework. Children with SCD performed worse than controls for working memory, central executive function, and processing/rehearsal speed. Central executive function was found to mediate the relationship between SCD status and working memory, but processing speed did not. Cognitive remediation strategies that focus on central executive processes may be important for remediating working memory deficits in SCD.
Children with sickle cell disease (SCD) are at high risk for deficits in neurocognitive functioning (Berkelhammer et al., 2007; Schatz & McClellan, 2006). Over the past two decades there has been increased attention paid to more subtle forms of neurocognitive deficits that occur in this population, even in the absence of overt stroke. Among these deficits, working memory is a prominent area of impairment that has important implications for competency in a range of other cognitive and academic skills. The specific components of working memory in children with SCD that lead to problems in overall working memory functioning are not well understood. In order to effectively remediate problems in neurocognitive functioning in this population, neuropsychological research needs to better identify these specific factors that can be targeted though techniques such as cognitive training (Klingberg et al., 2005).
SCD is a chronic, genetic health condition, which affects the blood by producing S-type hemoglobin instead of the typical A-type hemoglobin (Rees, Williams, & Gladwin, 2010). Due to the rigidity and stickiness of the blood cells, blockage within the blood vessels can occur, causing a vaso-occlusive pain episode, the hallmark symptom of SCD. SCD affects 1 in 400 African American births and 1 in 1200 Hispanic-American births, but is also found in individuals from South or Central America, the Caribbean islands, Mediterranean countries, India and Saudi Arabia (Rees et al., 2010). In addition to pain, children with SCD are also at risk for other complications including priapism, hip necrosis, anemia, jaundice, spleen damage, and eye problems (Rees et al., 2010). Neurologic morbidity also occurs in approximately 25–35% of children by the age of 21 years, with only about 5% of these cases due to overt stroke (Adams, Frempong, and Wang, 2001; Schatz & McClellan, 2006). In most cases involving neurologic complications, silent cerebral infarction, sleep-disordered breathing, and other sources of altered cerebral blood flow create mild neurocognitive deficits that are often missed in routine clinical care and educational settings (Adams et al., 2001).
Despite the range of specific medical morbidities and associated neurocognitive deficits, there are some common neurocognitive patterns related to working memory that occur. Cortical and subcortical structures at the distal portions of the anterior and middle cerebral artery distribution are often affected, resulting in tissue injury and/or disrupted oxygen delivery in deep white matter, basal ganglia, middle and superior frontal gyrus, and dorsal parietal regions (Pavlakis et al., 1988; Powars et al., 1999). Among the associated cognitive deficits, the impact of these mechanisms of injury on working memory and executive function has been of interest due to the prevalence of these deficits in SCD, their association with the vulnerable brain regions described above, and the importance of working memory for quality of life outcomes such as school attainment and academic skills (DeBaun et al., 1998; Gathercole & Pickering, 2000).
For the present study we used Baddeley’s model of working memory as a framework for understanding contributing factors to working memory deficits in children with SCD (Baddeley 1992; Baddeley, 2003). According to the multiple component model put forth by Baddeley (1992, 2003), working memory can be divided into modality specific rehearsal buffers (the phonological loop supporting verbally-mediated working memory and the visuospatial sketchpad supporting visual-spatial working memory) and a modality-independent central executive. The phonological loop is important for comprehension of speech under taxing situations, the visuospatial sketchpad measures the ability to hold and manipulate visuospatial representations, and the central executive coordinates information from both of these systems (Baddeley, 1992; Baddeley, 2003).
Prior studies of working memory in SCD have provided some insights into specific component cognitive processes that may be responsible for working memory deficits. Most prior studies showing working memory deficits in SCD have relied exclusively on verbally-mediated working memory tasks, thus, there is strong evidence of verbal working memory deficits, but little data to demonstrate a deficit in visual-spatial working memory. In fact, a prior study involving visual-spatial (Corsi block-tapping) and verbal (digit) span tasks indicated a selective deficit in backward span for verbal material that was correlated with phonological processing ability, but no observed deficits in forward or backward visual-spatial span or a visual-spatial self-ordered pointing task (Schatz & Roberts, 2005). This observed pattern of co-occurring auditory-verbal deficits and auditory-verbal working memory deficits with intact visual-spatial working memory is contrary to other data from children with SCD, which showed greater disruption of visual-spatial skills than for auditory-verbal skills for children with cerebral vascular complications (Schatz, Craft, Koby, & DeBaun, 2004). Only one research study has shown evidence of deficits specifically for visual-spatial working memory. Hijmans and colleagues (2011) used a computerized visual-spatial N-back paradigm that showed the group with SCD (selected for high risk genotypes) showed poorer visual-spatial working memory than demographically-matched comparison children; in addition, deficits were found in visual-spatial construction and planning tasks. However, comparisons with verbal working memory were limited by the sample, which was composed of children who had immigrated from a range of different countries with different first languages (Hijmans et al., 2011).
Several studies have implicated prefrontally-mediated attention and rehearsal processes that may be important for working memory deficits in SCD (White, Salorio, Schatz, & DeBaun, 2000, Brandling-Bennett, White, Armstrong, & Christ, 2003). A limitation of these studies is that they have not directly assessed the component processes that were inferred to be responsible for lower working memory performance in children with SCD (i.e. dysfunction in the phonological loop, poorer central executive control over the manipulation of information). Thus, deficits in specific processes were inferred from comparisons across working memory task conditions, but convergent validity for the deficit was not collected from additional tasks to establish the reliability and generalizability of the inferred deficit. For example, it is unclear if reduced word length effects from verbal span tasks found by White et al. (2000) represents issues with a slowed rehearsal rate of information or increased susceptibility to interference (Cowan, Wood, Nugent, & Treisman, 1997). Assessing cognitive abilities that support working memory, such as processing speed, would allow for a clearer distinction between executive and non-executive functions involved in working memory deficits. Processing speed is frequently affected in SCD and could lead to slowed rehearsal speed and poorer working memory independent of other executive function skills (Armstrong et al., 1996; Wang et al., 2001).
In the present study we evaluated working memory in children with SCD using validated tasks of verbal and spatial working memory from the Executive Abilities: Methods and Instruments for Neurobehavioral Evaluation and Research (EXAMINER) Battery. The EXAMINER Battery was developed to create a flexible set of methods that can be used to define and measure executive functioning in clinical investigations and clinical trials (Kramer et al., 2014). We used tasks from this battery to assess working memory in two modalities (verbal, visual-spatial) and measures of central executive processes and processing speed. Processing speed measures, as used in this study, have previously been shown to be a major determinant of speed of rehearsal for short-term memory and were used as proxy measures for this construct (Kail, 1991). Central executive processes were assessed using the Cognitive Control factor from the EXAMINER battery, which assesses response inhibition and set shifting constructs similar to those described by Miyake et al. (2000). In addition to these cognitive measures, structural magnetic resonance imaging (MRI) exams were used to assess midsagittal corpus callosum area (as an index of healthy-appearing white matter) as well as lesion volume and location for children with visible cerebral infarction.
Four hypotheses were tested. First, we hypothesized that children with SCD would show deficits relative to a demographically-matched comparison group on measures of working memory, measures of processing speed, and measures of central executive processes. Second, we predicted the magnitude of the deficit in verbal and spatial working memory in SCD would be similar, which we defined as an effect-size difference for the two tasks of less than 5% explained variance. The lack of modality-specific deficit was predicted due to the better task characteristics of the visual-spatial working memory task used by Hijmans et al. (2011) as compared with Schatz & Roberts (2005). Precision control over stimuli appears to be important for visual-spatial working memory tasks (Berch, Krikorian, & Huha,1998; Fischer, 2001); also, the reverse condition of the Corsi Block Tapping task may not show increased attentional load in children as expected to tax working memory (Isaacs & Vargha-Khadem, 1989). Third, we also expected both processing speed and central executive deficits would be mediators of working memory ability in SCD, consistent with previous data indicating these component processes are important for working memory and often affected by SCD (Baddeley, 2003; Berkelhammer et al., 2007). Fourth, we expected there to be different structural brain findings associated with processing speed and central executive deficits. Processing speed was expected to be most strongly related to midsagittal corpus callosum area due to the importance of white matter integrity and efficient interregional communication between task relevant brain regions for this cognitive function (Rypma et al., 2006; Penke et al., 2012). Central executive processes were expected to be most strongly related to the total volume of lesions in frontal and parietal cortical regions (D’Esposito et al., 1995; Collette & Van der Linden, 2002).
Methods
Participants and Recruitment
Participants were recruited as part of a multi-site study to develop a new battery of executive function measures for use with children and adults experiencing neurologic disease (Kramer, 2014). Children diagnosed with SCD genotypes at high risk for neurologic complications included HbSS and HbS-beta-thal0 (n =32), and demographically-matched controls without neurologic disease (n = 85) were recruited for this study and used for these analyses. Children with SCD at higher risk for neurocognitive deficits were emphasized in the recruitment process to provide criterion validity for the battery of measures. Exclusion criteria consisted of diagnosis of a co-morbid major medical, psychiatric, or developmental condition (e.g., autism, bipolar mood disorder, cancer, intellectual disability) or cognitive/motor limitations that would prohibit the child from participating in the cognitive testing. A detailed description of the neurologic history of the SCD group was reported previously (Schatz et al., 2014). Among the 32 children with SCD, 20 children had a history of sleep apnea, 12 children had a history of abnormal cerebral blood flow on transcranial Doppler ultrasound, 16 children had cerebral vessel narrowing on magnetic resonance angiography, 15 children had cerebral vascular lesions evident on structural MRI, and 7 children had a history of overt stroke. Seventeen children were currently receiving chronic blood transfusion treatment and six children were receiving oral medication treatment (hydroxyurea) to reduce SCD complications. Routine blood labs on the day of testing showed a mean hemoglobin level of 7.1 (SD = 0.9), white blood cell count of 11.2 (SD = 3.0), and platelet count of 449.7 (SD = 112.7).
Healthy controls were recruited from local after school care and summer care programs. These children were selected based on similar demographics as our SCD clinic population, who are primarily African-American and about half of whom are of lower socioeconomic status. Healthy controls were demographically similar to the SCD group with the exception of family income, which had a significantly higher representation of families earning more than $40,000 per year. Lower rates of employment, despite the similar education levels among the two groups, is an expected finding since parents of children with serious chronic health conditions are less likely to be employed outside the home (Kuhlthau & Perrin, 2001). We statistically controlled for family income in analyses to mitigate concerns about this factor as an unexplained variable for findings.
Parents at the summer and after-school programs were sent a flyer with an accompanying letter, consent form, and demographic questionnaire for the study to return, if interested. Children diagnosed with SCD were recruited at their clinic appointment at a southeastern Children’s Hospital. All children reported English as their primary language. Other descriptive statistics are presented in Table 1.
Table 1.
Descriptive Information for Children with SCD and the Comparison Group (Non-SCD)
| Variable | SCD (n = 32) | Non-SCD (n = 85) | Statistic |
|---|---|---|---|
| Age (M ± SD; range) | 12.7± 3.1 (8.0 – 17.7) | 12.9 ± 2.5 (8.0 – 18.0) | t(1,115) = 0.41 |
| Gender (n) | |||
| Males | 18 (56%) | 38 (45%) | X2(1)= 1.24 |
| Females | 14 (44%) | 47 (55%) | |
| Race/Ethicity (n) | |||
| African-American/Black | 32 | 80 | X2(1) = 1.97 |
| Hispanic and African-American/Black | 0 | 5 | |
| WJ-III Scaled Scores (M ± SD) | |||
| Verbal Comprehension | 85.9 ± 13.7 | 95.4 ± 10.8 | t(1,115) = 3.92** |
| Visual Matching | 77.3 ± 21.6 | 95.4 ± 13.1 | t(1,115) = 5.52** |
| Numbers Reversed | 82.8 ± 18.6 | 95.2 ± 16.3 | t(1,115) = 3.53** |
| Parent education (n) | |||
| 9–11 years | 3 (9%) | 4 (5%) | X2(3) = 1.93 |
| High school | 8 (25%) | 17 (20%) | |
| Some college | 17 (53%) | 45 (53%) | |
| College degree | 4 (12%) | 18 (21%) | |
| Family income (n) | |||
| <$10,000/year | 8 (25%) | 7 (8%) | X2(4) = 12.83* |
| $10–20,000/year | 6 (19%) | 9 (11%) | |
| $20–30,000/year | 9 (28%) | 16 (19%) | |
| $30–40,000/year | 4 (12%) | 16 (19%) | |
| >$40,000/year | 5 (16%) | 37 (44%) | |
| Adults in the home (M ± SD) | 1.8 ± 0.5 | 1.9 ± 0.6 | t(1,115) = 0.72 |
| Age of primary caregiver (M ± SD) | 38.9 ± 9.5 | 37.4 ± 9.2 | t(1,115) = −0.76 |
Note.
p < .05;
p < .01. If there are multiple parents in the home, parent education is based on the highest education of any one parent. Adults in the home includes anyone 18 years or older (other than child participant) who resides in the same home as the participant.
Procedures
Parents completed informed consent and children assented to participating in the study. Children participated in a single session of cognitive testing with a trained examiner. Two children with SCD and four healthy control children completed the testing in two sessions due to time constraints. Parents completed a demographic questionnaire and a behavioral report measure. If a family with a child with SCD wished to participate in the study, but the child was experiencing pain or other illness related complications, an assessment was scheduled for a later date. All children with SCD were offered the opportunity to complete an optional magnetic resonance (MR) imaging study. Eight participants completed the research MR exam. Those participants that did not wish to complete the research MR exam had a clinical MR/MRA exam within eighteen months of cognitive testing, however the majority of participants had MR exams within 3 months of testing (n = 27) and 3 participants had exams within 12 months of cognitive testing. Medical record reviews were conducted for the children with SCD to determine clinical history after cognitive testing and research MR exams had been completed. Reports from clinical exams were coded for study variables; a neuroradiologist reviewed the eight research scans to code the same variables.
Measures
EXAMINER Battery
The EXAMINER Battery provides raw factor scores that are created from 11 core variables used to compute a three-factor model (Fluency, Cognitive Control, Working Memory) and a Composite score based on a one-factor model. The factor scores, which are not corrected for age, are generated from eight tasks including phonemic and category fluency tasks, a computerized flanker task, a computerized continuous performance test (CPT), a computerized dot counting task, a computerized spatial n-back task, a computerized anti-saccade task, a computerized set-shifting task; in addition, there is a behavioral rating form completed by the test administrator. Test–retest reliability for the battery was evaluated in 122 normal adult controls at an average interval of 25 days, which is the source of reliability data presented below (Kramer et al., 2014). Validity data for the association of factor scores with degree of neurocognitive risk in SCD was published previously with the present sample (Schatz Stancil, Katz, & Sanchez, 2014). We used the Working Memory factor and the component tests to evaluate working memory and the Cognitive Control factor to represent central executive functions. In order to compute a processing speed variable, we computed the mean reaction time across baseline conditions from the flanker task and the set-shifting task.
Working Memory Factor
The Working Memory factor is based on three variables derived from two tasks: The dot counting task and the n-back test. Test–retest reliability for the Working Memory factor was r = .78. The dot counting task measures verbal working memory. The participant counts the number of colored dots on the screen among distractors and then states the number of dots in each display. The total score is calculated by summing the total number of correct responses across the trials. Higher scores indicate better performance on the task. This is considered a reliable measure of verbal working memory, r =.81. The n-back test measures spatial working memory. In this task children press a button whenever a square is presented in the same spatial location as the previous trial (“yes trials”) and a different button if the square is presented in a different location as the previous trial (“no trials). Both total correct and d-prime are computed. Higher scores indicate better performance on the task. This is considered a reliable measure of visual-spatial working memory, r =.64.
Processing Speed measure
The Processing Speed measure was computed as a mean of the mean response time scores from the Flanker task congruent trials and the Set Shifting non-shift trials. Within the non-SCD comparison group, the response time measure derived from these tasks showed a strong fit (R2 = .97) to the exponential function for processing speed based on chronological age as described by Kail (1991).
Cognitive Control Factor
The Cognitive Control factor is based on measures of inhibitory control, set shifting, and behaviors associated with the dysregulation of executive function. Within this factor are four variables including the total Flanker score, total Set Shifting score, anti-saccade total, and total dysexecutive errors. For the Flanker test, Set Shifting test, and Anti-saccade test scores are based on performance on conditions taxing executive function relative to baseline tasks and described further below (also see Kramer et al., 2014). Higher scores on the cognitive control factor score indicate poorer performance. The test–retest reliability for the Cognitive Control factor was r =.88.
In the Flanker Task, the participant focuses on a small cross in the center of the screen. After a short variable duration, a row of five arrows is presented in the center of the screen either above or below the fixation point. On half the trials, the flanking arrows are congruent with the direction of the center arrow and on half of the trials the flanking arrows are incongruent with the direction of the center arrow. The total Flanker score is derived from both error and response time data that is used to contrast performance on incongruent versus congruent trials.
The set shifting task measures accuracy and reaction time. In this task, participants match a stimulus on the top of the screen to one of two stimuli in the bottom corners of the screen. There are task-consistent blocks in which participants perform the task with only one element (either classifying shapes or classifying colors). In variant blocks participants switch back and forth between the two tasks pseudo-randomly with the target dimension expressed via the word “shape” or “color” presented at the bottom of the screen. Performance on the task-consistent and task-variant blocks are compared to measure the performance differences between consistent and variant blocks (expressed in latency and accuracy) and the differences between switch and non-switch trials within the variant block (also expressed in latency and accuracy).
In the anti-saccade task there are three blocks of trials in which subjects look at a fixation point in the center of a computer screen and move their eyes in response to a peripherally-presented stimulus. The first block is pro-saccade trials and the second and third blocks are anti-saccade trials. The primary outcome measure is the total number of correct responses on the two anti-saccade trials as observed by the examiner.
Dysexecutive errors is a composite variable derived from: false alarm responses on a CPT, rule violations on the verbal fluency tasks, the tendency to make errors on Flanker incongruent trials relative to congruent trials, the tendency to make errors on the Set Shifting shift trials relative to the non-shift trials, and the total score on the Behavior Rating Scale completed the test administrator. For the CPT, participants complete 100 experimental trials on a computer in which they push a button only for a target shape. The CPT is designed to elicit false alarm errors with 80% of trials show the target. The primary dependent measure is the total number of false alarm errors. The Behavioral Rating Scale contains Likert-type ratings of the following behaviors: agitated, stimulus-bound, perseverative, decreased initiation, motor stereotypes, lack of social/emotional engagement, impulsiveness, and socially inappropriate behavior with these behaviors defined in the EXAMINER manual.
Norm-referenced Measures of Cognitive Functions
Measures of verbal-comprehension, processing speed, and short-term/working memory domains from the Woodcock-Johnson Tests of Cognitive Ability, 3rd edition (WJ-III) were administered. Verbal Comprehension is a measure of crystallized knowledge and verbal reasoning involving four subtests: picture naming, generating synonyms, generating antonyms, and verbal analogies. Visual Matching is a measure of processing speed in which the child identifies matching numbers among rows of numbers over a three-minute period. Numbers Reversed is a measure of short-term/working memory in which the child hears a series of digits and repeats back the sequence in reverse order. Each test was administered and scored according to the test manual and converted to age-adjusted standard scores.
Magnetic Resonance (MR) Exams and CC Measurement
All scans were collected without sedation. A total of twenty-eight children completed clinical MR, eight children completed research MR exams and four completed both scans. T1-weighted scans in the sagittal plane were used for CC measures. All of the clinical scans were collected on 1.5T scanners using a 2D T1-weighted spin echo sequence with a matrix size of 256×192. A three-dimensional (3D) MP-RAGE sequence was used with a matrix of 256×256 for research scans. All participants also had axial T2-weighted and T2-weighted FLAIR sequences that were used to identify regions with apparent cerebral infarction and a 3D time of flight MRA sequence to visualize the major cerebral arteries. Midsagittal CC measurements were completed as described previously (Schatz & Buzan, 2006). CC measurements were completed by two raters with high reliability (r = .98). Clinical and research scans showed comparable rank order and absolute size of the measurements for the participants completing both types of scans (e.g., total CC area was M = 471.5 mm2 for the clinical scans and M = 467.3 mm2 for the research scans).
Lesion quantification was conducted as described previously (Schatz et al., 2002; 2006). T2-weighted images were viewed as 16-bit images and areas of hyperintense tissue were outlined and measured using NIH v.1.63 software (Rasband, 2002). Two raters independently measured lesion area on each MRI image twice in separate sessions and assigned lesion location using standard anatomical maps (Damasio & Damasio, 1989). Average area measurements across all ratings were summed for each participant and multiplied by slice thickness plus the gap to create a volume measurement. Lesion volume was computed for total volume, volume in frontal and parietal cortex, and volume in subcortical regions (e.g., deep white matter, basal ganglia structures). Participants with SCD who had no visible lesions were assigned lesion volume scores of zero for the purpose of the correlation/regression analyses described later. Inter-rater reliability for the lesion volume measurements was r = .87 for frontal-parietal lesion volume and r = .82 for subcortical lesion volume. Mean CC area for the group was 564.3 mm2 (SD = 85.6; range 415.5 – 714.3), mean lesion volume in frontal-parietal cortical areas was 13.4 mm3 (SD = 28.7; range 0.0 – 104.0), and mean lesion volume in subcortical regions was 1.4 mm3 (SD = 1.9; range 0 – 5.4).
Statistical Methods
For hypothesis one, we used univariate ANOVA to determine whether normal controls performed better on the EXAMINER Working Memory, Processing Speed, and Cognitive Control factors than children with SCD. Age was included as a covariate to reduce unexplained variance because EXAMINER variables were not age-corrected. In addition, family income level was included as a covariate to eliminate variance that could be due to this potential confound. For hypothesis two, the similarity of the magnitude of the deficit in verbal and spatial working memory in SCD was examined using a mixed factor repeated measures ANOVA with the task by group interaction used to assess the hypothesis. The within subjects factor was performance on each of the two working memory tasks (total score for Dot Counting task and d-prime from the N-back task). The between subjects factor was group (SCD, control). Covariates were the same as for hypothesis one. For hypothesis three, we used a regression approach to examine mediation effects to determine if including either processing speed or central executive (cognitive control) measures into statistical models will significantly reduce the difference in working memory scores between children with SCD and controls. The Sobel test was used to assess the extent of mediation. Finally, for hypothesis four we examined Pearson correlations to determine if, within the SCD group, EXAMINER Processing Speed and Cognitive Control variables showed the expected pattern of correlations with MR variables. For descriptive and comparative purposes we also examined the correlation of WJ-III norm-referenced measures and the EXAMINER Working Memory variables with MR data. We used partial correlations controlling for age to reduce unexplained variance for the EXAMINER variables, which are not age-corrected. We used a standard alpha level of .05 for statistical significance in each analysis.
Results
Results of key study measures by group are shown in Table 2. To test our first hypothesis, we assessed for group differences on the key study measures of working memory, central executive processes, and processing speed while statistically controlling for age and family income levels. The ANCOVA indicated the two groups showed statistically significant differences for the EXAMINER Working Memory Composite (F(1, 108) = 16.24, p = .000; partial η2 = .131), EXAMINER Processing Speed (F(1, 108) = 17.61, p = .000; partial η2 = .135), and EXAMINER Cognitive Control Composite (F(1, 108) = 25.87, p = .000; partial η2 = .193). The age covariate accounted for 15–40% of variance in the statistical models whereas family income accounted for 2–5% of the variance in the statistical models. Estimated marginal means indicated poorer performance for the SCD group across all three measures.
Table 2.
Mean Scores (± SD) for Key Study Measures.
| Variable | SCD (n = 32) |
Non-SCD (n = 85) |
Statistic t(115) |
|---|---|---|---|
| EXAMINER Working Memory Composite (raw score) | −.68 ± .55 | −.14 ± .66 | 4.86** |
| Dot Counting (# correct) | 10.91 ± 3.59 | 13.36 ± 4.70 | 2.68** |
| N-back (d-prime) | 1.08 ± 1.79 | 1.79 ± 0.75 | 4.70** |
|
| |||
| Examiner Cognitive Control Composite (raw score) | .73 ± .70 | −.09 ± .85 | −4.79** |
| Flanker Test (total score) | 6.99 ± 1.17 | 8.24 ± 1.28 | 4.82** |
| Dysexecutive Errors (total) | 12.50 ± 6.76 | 9.80 ± 6.53 | −1.97 |
| Antisaccade Test (total score) | 24.78 ± 8.95 | 27.69 ± 4.52 | 2.32* |
| Set Shifting Task (total score) | 5.64 ± 0.95 | 6.76 ± 1.22 | 4.73** |
|
| |||
| EXAMINER Processing Speed | 1.07 ± 0.24 | 0.83 ± 0.25 | −4.73** |
Note.
p < .05;
p < .01. For the Cognitive Control Composite, Dysexecutive Errors, and Processing Speed scores higher scores represent poorer performance. Mean response time for EXAMINER processing speed is calculated in seconds.
To test our second hypothesis, relative differences in performance for the verbal (Dot Counting) and visual-spatial (N-back) working memory tasks was tested with a mixed factor repeated measures ANCOVA controlling for age and family income to examine the magnitude of the task by group interaction effect. The task by group interaction term was not statistically significant and accounted for a small percentage of variance, F(1, 113) = 3.21, p = .08, partial η2 = .028. An examination of the estimated marginal means indicated the SCD group performed more poorly on both tasks (N-back, M = 1.08 vs. 1.79; Dot Counting, M = 10.91 vs. 13.36), with a trend toward a larger difference for the N-back task as compared with the Dot Counting task. Thus, the effect size (partial η2 = .028) was smaller than hypothesized (5% explained variance) to support a meaningful difference between verbal and spatial working memory deficits.
To test our third hypothesis, hierarchical linear regression models were used to test for the extent to which the EXAMINER Processing Speed score or the EXAMINER Cognitive Control Composite score functioned as mediators of the deficit in working memory found in the SCD group. In order to test for mediation, we followed Baron and Kenny’s statistical guidelines (1986). For the pattern of associations to be consistent with a mediation model, the independent variable (presence or absence of SCD) must be associated with both the mediator (central executive, processing speed) and the dependent variable (i.e., working memory). These associations were established in testing Hypothesis 1 (see above). In addition, there should be a significant decrease in explained variance between the independent variable and dependent variable when adding the potential mediation.
First, we conducted two separate hierarchical linear regression analyses to determine the strength of the relationship between the independent variable (SCD vs. non-SCD group membership) and each mediator (EXAMINER Processing Speed and Cognitive Control). In the first step age and family income variables were entered as predictors of the mediator variable. In the second step, group (SCD, non-SCD) was entered as the independent variable. Second, we conducted two separate hierarchical linear regression analyses to determine the extent to which group differences in the mediator accounted for variance in working memory. In the first step age and family income variables were entered as predictors of the EXAMINER Working Memory Composite. In the second step, both group and the mediator variable were entered as independent variables predicting the working memory score. The raw regression coefficients and their standard error terms are used to compute the Sobel statistic, which tests whether the indirect effect of the independent variable (group) on the dependent variable (working memory) via the mediator (cognitive control, processing speed) is significantly different from zero. All regression analyses had overall statistically significant models with all F’s > 13 and all p’s < .001.
For the mediation models to test our third hypothesis, mediation of the Working Memory Composite via Processing Speed did not show evidence of mediation, Sobel = 0.58, p = .56, but the Cognitive Control Composite was consistent with mediation of Working Memory, Sobel = 2.70, p = .007. As shown in Table 2, three of the component variables making up the Cognitive Control Composite score showed statistical differences between the study groups. We conducted exploratory analyses for these scores (the Flanker Task, Anti-saccade Task, and Set-Shifting Task variables used for the Cognitive Control factor score) using parallel regression analyses as described above and computed the Sobel statistic to examine if this overall mediation effect for the Cognitive Control factor score was driven by specific tasks making up the composite score. The Set-Shifting Task score showed the most robust effect, Sobel = 2.03, p = .042. The Flanker Task score was just above the study alpha, Sobel = 1.92, p = .055. Finally, the Anti-saccade task showed the least evidence for a mediation effect, Sobel = 1.13, p = .260. Standardized regression coefficients from these regression models were used to assess the strength of individual pathways, as shown in Figure 1.
Figure 1.
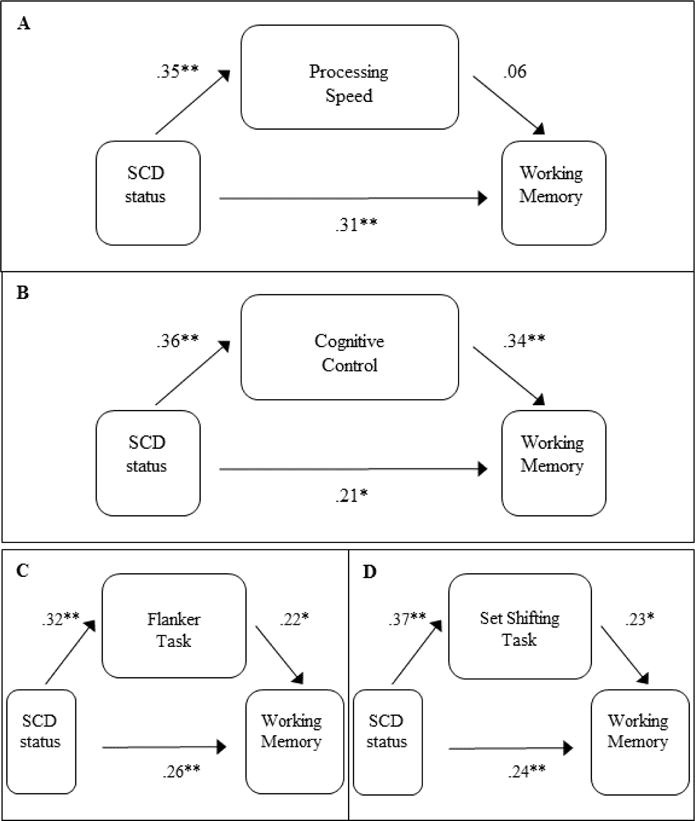
Standardized regression coefficients for the potential mediating role of processing speed (panel A) and cognitive control (panel B) deficits in SCD influencing working memory performance. Component variables for the cognitive control factor score with the largest contribution to the mediation effect are shown in panels C and D. Note that although the component paths in panel C were statistically significant, the overall Sobel test was not. *p < .05; **p<.01.
To examine our fourth hypothesis, the correlation values are shown in Table 3. There were moderate-sized and statistically significant associations between Processing Speed and midsagittal CC area and also between Cognitive Control and the volume of frontal-parietal cortical lesions; both associations were in the expected direction from the a priori predictions. Overall, there appeared to be the strongest association between increased volume of visible tissue injury in subcortical regions and decreased processing speed (for both the EXAMINER and WJ-III measures). The next strongest associations appeared to be between greater frontal-parietal cortical lesion volume and decreased performance on working memory (for both the EXAMINER and WJ-III measure) and WJ-III Verbal Comprehension. Finally, midsagittal CC area showed the strongest association with the EXAMINER Processing Speed measure, but a much smaller and statistically non-significant association with the WJ-III measure of processing speed.
Table 3.
Age-Adjusted Partial Correlations among MRI Measurements and Cognitive Variables
| Cognitive variable | CC area - total | Frontal-parietal cortical lesion volume | Subcortical lesion volume |
|---|---|---|---|
| EXAMINER Working Memory | .34 (p = .067) |
−.56 (p = .002) |
−.38 (p = .043) |
| EXAMINER Processing Speed | −.37 (p = .046) |
.15 (p = .445) |
.54 (p = .003) |
| EXAMINER Cognitive Control | −.26 (p = .170) |
.37 (p = .049) |
−.29 (p = .110) |
| WJ-III Verbal Comprehension | .22 (p = .248) |
−.58 (p = .001) |
−.38 (p = .043) |
| WJ-III Visual Matching | .16 (p = .400) |
−.33 (p = .078) |
−.50 (p = .006) |
| WJ-III Numbers Reversed | .28 (p = .147) |
−.52 (p = .004) |
−.24 (p = .214) |
Note. Correlations shown in bold type were a priori expected associations. Scores are for SCD participants (n=32).
Discussion
The present study examined working memory in children with SCD as well as the contribution of processing speed and central executive processes to working memory deficits in this population. Results of the study hypotheses were mixed, but generally supported. As expected, children with SCD showed deficits relative to the demographically-matched, non-SCD comparison group on measures of working memory (in both the verbal, and visual-spatial modalities), processing speed, and central executive functioning. This is not surprising considering evidence from previous studies that demonstrate that individuals with SCD show deficits in these cognitive functions compared with peers without SCD (DeBaun et al. 1998; White, et al., 2000). Central executive processes appeared to be an important contributing factor to the working memory deficits as shown by the mediation models; however, contrary to our predictions, processing speed deficits did not appear to be a significant contributing factor to the SCD-related working memory deficits. Finally, although the predicted associations between central executive processes and processing speed with measures of brain structure were found, processing speed showed the strongest association with visible tissue injury in subcortical regions, and midsagittal corpus callosum area was not consistently correlated with processing speed across measures of this cognitive construct.
Central executive processes, but not processing speed, emerged as a mediator to explain working memory deficits associated with SCD. It is unclear from our data if this mediation was due to a global deficit in the cognitive control tasks or to deficits in more specific executive skills. Individual tasks showed different degrees of association with the mediation effect such that there could be specific central executive components that have a greater impact on working memory for this group. Specifically, the Set-Shifting task showed the most robust effect, whereas the Flanker Task and Anti-saccade task showed more modest contribution to the mediation effect. Consistent with the Unity and Diversity model of executive function, mental set shifting taps into a distinct neurocognitive circuit from the other tasks that were more dependent on inhibitory control mechanisms (Miyake et al., 2000). Alternately, the different outcomes for individual tasks may have to do with task-specific psychometric issues. Different tasks believed to tap into a specific executive function skill often show surprisingly low inter-correlation, suggesting that some classic neuropsychological tasks may be less robust measures of the presumed construct (Testa, Bennett, & Ponsford, 2012). The tasks in the present study were selected to be good measures of an overall cognitive control construct, not more specific constructs.
The absence of a mediation effect for processing speed stands in contrast to other pediatric conditions that have shown a more prominent role of processing speed and related rehearsal mechanisms in working memory deficits (Schatz, Kramer, Ablin, & Matthay, 2000; Swanson, 1999). These different patterns of contributing factors to working memory deficits across studies of pediatric disorders highlights the individual and group-level heterogeneity that can lead to working memory deficits. Geary and colleagues (2012) found that first grade children with higher central executive capacity used more strategies to correctly solve addition problems that taxed working memory in comparison to children with lower central executive capacity (Geary, Hoard, & Nugent, 2012). Thus, strategy training, which has previously shown promise for cognitive remediation in SCD, may be an important area of focus for future clinical investigation of working memory in SCD (Yerys et al., 2003).
Collectively, looking at data in this and previous studies of working memory in SCD, there is little evidence of a modality-specific deficit in working memory in SCD as suggested by Schatz & Roberts (2005). This may mean that, similar to work with adolescents with ADHD, training in either verbal or spatial working memory may be equally effective in improving working memory functioning (Gibson et al., 2011). Alternately, some researchers have suggested modality-specific central executive processes may be present, contrary to Baddeley’s model (Myerson, Hale, Rhee, & Jenkins, 1999). Analyses of the component tasks making up the EXAMINER Cognitive Control factor in the present data suggested measures of mental set shifting and response inhibition may be important to consider for future study of potential modality-specific central executive processes.
The lack of mediation effects for processing speed explaining working memory deficits was unexpected. There are several methodological factors that should be considered for the null finding. The present study used a heterogeneous sample of children with SCD-related neurocognitive deficits. It is possible there are subgroups of children for whom processing speed is a more important factor for working memory. For example, subcortical lesions, as often found with silent cerebral infarcts, were related to processing speed deficits and this type of morbidity is also associated with working memory deficits (Debaun et al., 1998). It is possible that a study focusing on this specific morbidity might find different results. In addition, we found different patterns of relationships with lesion effects for the EXAMINER Processing Speed measure based on computerized response time tasks and the norm-referenced paper-and-pencil measure. Though correlated, there are a range of methods used for assessing processing speed and these differences may have influenced our study outcome (Roberts & Stankov, 1999). However, we were able to find another non-peer-reviewed study that showed a similar pattern of results suggesting that processing speed may not be as critical of a factor as central executive processes in determining working memory deficits in SCD (Salorio, 2000). Finally, the present study did not include domain-specific measures of rehearsal speed. Though processing speed has been shown to be a major determinant of rehearsal speed, future studies should consider more refined measures of these processes.
There are other limitations of our study that are important for considering the results. The present study over-recruited children with SCD at high risk for neurocognitive deficits and the results should not be viewed as representative of typical cognitive or working memory performance in all children with SCD. Rather, the data on a high-risk sample were used to assess which processes are most responsible for working memory deficits when they occur in this population. In addition, the mediation effects for processing speed and central executive function were examined using cross-sectional data with regression models, which is one of the weakest methods for assessing mediation. Studies involving manipulation of these abilities (e.g., with cognitive training) would provide a stronger methodological bases for making inferences about the importance of these specific skills for working memory deficits in SCD. The present data, however, provide preliminary data to help guide future studies.
This study further highlights the importance of working memory in children with SCD. Routine screening of children with SCD who are more likely to exhibit problems in working memory (i.e. more severe genotypes) will allow neuropsychologists to efficiently identify problems earlier in development and establish a remediation plan. In addition to working memory measures, this study demonstrates the need to include measures of central executive processes to understand these children’s deficits. Children low in this domain are likely to be more susceptible to problems in working memory and therefore associated problems in fluid reasoning and academic functioning. Future research should explore whether adding additional methods of assessment (central executive measures) is more accurate than traditional cognitive screening methods to detect children with neurocognitive issues. For example, there are a lack of well-established, norm-referenced measures of executive function to use with preschool age children, which may limit early detection of neurocognitive concerns. In addition, future research studies using working memory training in children with SCD may consider adding strategies that address issues with specific central executive processes.
Acknowledgments
The authors thank the families who participated in this research. This work was completed in partial fulfillment of the first author’s Master’s degree requirements. This work was supported in part by a grant from the National Institutes of Health (NIH-NINDS-05-02; Kramer, P.I., Schatz, site P.I.). There are no known financial or other relationships that could be interpreted as a conflict of interest for this manuscript.
References
- Adams RJ, Ohene-Frempong K, Wang W. Sickle cell and the brain. Hematology. 2001;2001(1):31–46. doi: 10.1182/asheducation-2001.1.31. [DOI] [PubMed] [Google Scholar]
- Armstrong FD, Thompson RJ, Wang W, Zimmerman R, Pegelow CH, Miller S, Hurtig A. Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease. Pediatrics. 1996;97(6):864–870. [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255(5044):556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: Looking back and looking forward. Nature Reviews: Neuroscience. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Berch DB, Krikorian R, Huha EM. The Corsi block-tapping task: Methodological and theoretical considerations. Brain and Cognition. 1998;38(3):317–338. doi: 10.1006/brcg.1998.1039. [DOI] [PubMed] [Google Scholar]
- Berkelhammer LD, Williamson AL, Sanford SD, Dirksen CL, Sharp WG, Margulies AS, Prengler RA. Neurocognitive sequelae of pediatric sickle cell disease: a review of the literature. Child Neuropsychology. 2007;13(2):120–131. doi: 10.1080/09297040600800956. [DOI] [PubMed] [Google Scholar]
- Brandling-Bennett EM, White DA, Armstrong MM, Christ SE. Patterns of verbal long-term and working memory performance reveal deficits in strategic processing in children with frontal infarcts related to sickle cell disease. Developmental Neuropsychology. 2003;24(1):423–434. doi: 10.1207/S15326942DN2401_01. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der Linden M. Brain imaging of the central executive component of working memory. Neuroscience and Behavioral Review. 2002;26:105–125. doi: 10.1016/s0149-7634(01)00063-x. [DOI] [PubMed] [Google Scholar]
- Cowan N, Wood NL, Nugent LD, Treisman M. There are two word-length effects in verbal short-term memory: Opposed effects of duration and complexity. Psychological Science. 1997;8(4):290–295. [Google Scholar]
- Damasio H, Damasio AR. Lesion analysis in neuropsychology. Oxford University Press; USA: 1989. [Google Scholar]
- DeBaun MR, Schatz J, Siegel MJ, Koby M, Craft S, Resar L, Noetzel M. Cognitive screening examinations for silent cerebral infarcts in sickle cell disease. Neurology. 1998;50(6):1678–1682. doi: 10.1212/wnl.50.6.1678. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- Fischer MH. Probing spatial working memory with the Corsi Blocks task. Brain and Cognition. 2001;45(2):143–154. doi: 10.1006/brcg.2000.1221. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Pickering SJ. Working memory deficits in children with low achievements in the national curriculum at 7 years of age. British Journal of Educational Psychology. 2000;70:177–194. doi: 10.1348/000709900158047. [DOI] [PubMed] [Google Scholar]
- Geary DC, Hoard MK, Nugent L. Independent contributions of the central executive, intelligence, and in-class attentive behavior to developmental change in the strategies used to solve addition problems. Journal of Experimental Child Psychology. 2012;113:49–65. doi: 10.1016/j.jecp.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson BS, Gondoli DM, Johnson AC, Steeger CM, Dobrzenski BA, Morrissey RA. Component analysis of verbal versus spatial working memory in adolescents with ADHD: A randomized, controlled trial. Child Neuropsychology. 2011;17(6):546–563. doi: 10.1080/09297049.2010.551186. [DOI] [PubMed] [Google Scholar]
- Hijmans CT, Fijnvandraat K, Grootenhuis MA, van Geloven N, Heijboer H, Peters M, Oosterlaan J. Neurocognitive deficits in children with sickle cell disease: a comprehensive profile. Pediatric blood & cancer. 2011;56(5):783–788. doi: 10.1002/pbc.22879. [DOI] [PubMed] [Google Scholar]
- Isaacs EB, Vargha-Khadem F. Differential course of development of spatial and verbal memory span: A normative study. British Journal of Developmental Psychology. 1989;7:377–380. [Google Scholar]
- Kail R. Developmental change in speed of processing during childhood and adolescence. Psychological bulletin. 1991;109(3):490. doi: 10.1037/0033-2909.109.3.490. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Mungas D, Possin KL, Rankin KP, Boxer AL, Rosen HJ, Widmeyer M. NIH EXAMINER: conceptualization and development of an executive function battery. Journal of the International Neuropsychological Society: JINS. 2014;20(1):11. doi: 10.1017/S1355617713001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Westerberg H. Computerized training of working memory in children with ADHD: A randomized, controlled trial. Journal of the American Academy of Child & Adolescent Pyschiatry. 2005;44(2):177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Kuhlthau KA, Perrin JM. Child health status and parental employment. Archives of pediatrics & adolescent medicine. 2001;155(12):1346–1350. doi: 10.1001/archpedi.155.12.1346. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Myerson J, Hale S, Rhee SH, Jenkins L. Selective interference with verbal and spatial working memory in young and older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 1999;54(3):P161–P164. doi: 10.1093/geronb/54b.3.p161. [DOI] [PubMed] [Google Scholar]
- Pavlakis SG, Bello J, Prohovnik I, Sutton M, Ince C, Mohr JP, De Vivo DC. Brain infarction in sickle cell anemia: magnetic resonance imaging correlates. Annals of neurology. 1988;23(2):125–130. doi: 10.1002/ana.410230204. [DOI] [PubMed] [Google Scholar]
- Penke L, Munoz Maniega S, Bastin ME, Valez Hernandez MC, Murray C, Deary IJ. Brain white matter tract integrity as a neural foundation for general intelligence. Molecular Psychiatry. 2012;17:1026–1030. doi: 10.1038/mp.2012.66. [DOI] [PubMed] [Google Scholar]
- Powars DR, Conti PS, Wong WY, Groncy P, Hyman C, Smith E, Hiti AL. Cerebral vasculopathy in sickle cell anemia: diagnostic contribution of positron emission tomography. Blood. 1999;93(1):71–79. [PubMed] [Google Scholar]
- Rasband W. NIH Image v.1.63 (software program) Research Services Branch, National Institutes of Health; 2002. [Google Scholar]
- Rees DC, Williams TN, Gladwin MT. Sickle cell disease. The Lancet. 2010;376:2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- Roberts RD, Stankov L. Individual differences in speed of mental processing and human cognitive abilities: Toward a taxonomic model. Learning and Individual Differences. 1999;11(1):1–120. [Google Scholar]
- Rypma B, Berger JS, Prabhakaran V, Bly BM, Kimberg DY, D’Esposito M. Neural correlates of cognitive efficiency. Neuroimage. 2006;33:969–979. doi: 10.1016/j.neuroimage.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Salorio CF. Doctoral dissertation. 2000. The role of frontal lobe function in working memory, inhibitory control, and processing speed in children with sickle cell disease. Retrieved from ProQuest Dissertations and Theses. [Google Scholar]
- Schatz J, Buzan R. Decreased corpus callosum size in sickle cell disease: Relationship with cerebral infarcts and cognitive functioning. Journal of the International Neuropsychological Society. 2006;12:24–33. doi: 10.1017/S1355617706060085. [DOI] [PubMed] [Google Scholar]
- Schatz J, Craft S, Koby M, DeBaun MR. Asymmetries in visual-spatial processing following childhood stroke. Neuropsychology. 2004;18(2):340. doi: 10.1037/0894-4105.18.2.340. [DOI] [PubMed] [Google Scholar]
- Schatz J, Kramer JH, Ablin A, Matthay KK. Processing speed, working memory, and IQ: A developmental model of cognitive deficits following cranial radiation therapy. Neuropsychology. 2000;14(2):189–200. doi: 10.1037//0894-4105.14.2.189. [DOI] [PubMed] [Google Scholar]
- Schatz J, McClellan CB. Sickle cell disease as a neurodevelopmental disorder. Mental retardation and developmental disabilities research reviews. 2006;12(3):200–207. doi: 10.1002/mrdd.20115. [DOI] [PubMed] [Google Scholar]
- Schatz J, Roberts C. Short-term memory in children with sickle cell disease: Executive versus modality-specific processing deficits. Archives of Clinical Neuropsychology. 2005;20:1073–1085. doi: 10.1016/j.acn.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Schatz J, Stancil M, Katz T, Sanchez CE. EXAMINER Executive Function Battery and Neurologic Morbidity in Pediatric Sickle Cell Disease. Journal of the International Neuropsychological Society: JINS. 2014;20(1):29. doi: 10.1017/S1355617713001239. [DOI] [PubMed] [Google Scholar]
- Swanson HL. Reading comprehension and working memory in learning-disabled readers: Is the phonological loop more important than the executive system? Journal of Experimental Child Psychology. 1999;72(1):1–31. doi: 10.1006/jecp.1998.2477. [DOI] [PubMed] [Google Scholar]
- Testa R, Bennett P, Ponsford J. Factor analysis of nineteen executive function tests in a healthy adult population. Archives of Clinical Neuropsychology. 2012;27:213–224. doi: 10.1093/arclin/acr112. [DOI] [PubMed] [Google Scholar]
- Wang W, Enos L, Gallagher D, Thompson R, Guarini L, Vichinsky E, Cooperative Study of Sickle Cell Disease Neuropsychologic performance in school-aged children with sickle cell disease: a report from the Cooperative Study of Sickle Cell Disease. The Journal of Pediatrics. 2001;139(3):391–397. doi: 10.1067/mpd.2001.116935. [DOI] [PubMed] [Google Scholar]
- White DA, Salorio CF, Schatz J, DeBaun M. Preliminary study of working memory in children with stroke related to sickle cell disease. Journal of Clinical and Experimental Neuropsychology. 2000;22(2):257–264. doi: 10.1076/1380-3395(200004)22:2;1-1;FT257. [DOI] [PubMed] [Google Scholar]
- Yerys BE, White DA, Salorio CF, McKinstry R, Moinuddin A, DeBaun M. Memory strategy training in children with cerebral infarcts related to sickle cell disease. Journal of Pediatric Hematology/Oncology. 2003;25(6):495–498. doi: 10.1097/00043426-200306000-00014. [DOI] [PubMed] [Google Scholar]


