Abstract
Background
Hepatitis E virus (HEV) infection causes significant morbidity and mortality worldwide, particularly among pregnant women. In clinical settings blood-based testing protocols are commonly used to diagnose HEV infection, but in community settings such invasive sampling can hinder study participation and limit discovery of the ecology and natural history of HEV infection. Oral fluid is a non-invasive biospecimen that can harbor pathogen-specific antibodies and has the potential to replace blood-based testing protocols.
Objectives
To develop an immunoassay to assess past and recent HEV infection that uses oral fluid instead of serum or plasma.
Methods
The assay was validated using paired oral fluid and serum samples collected from 141 patients who presented either with (n=76) or without (n=65) symptoms of acute viral hepatitis at a clinical diagnostics center in Dhaka, Bangladesh. The sensitivity and specificity of the oral fluid-based immunoassay for HEV IgG (past HEV infection) and HEV IgA (recent HEV infection) antibodies was calculated in reference to Wantai’s (Beijing Wantai) serum-based HEV enzyme-linked immunosorbent assay (ELISA) kits for IgG and IgM antibodies, respectively.
Results
The sensitivity and specificity of the oral fluid-based immunoassay for HEV-IgG antibodies were 98.7% and 98.4%, respectively. The sensitivity and specificity of the oral fluid-based immunoassay for HEV IgA were 89.5% and 98.3%, respectively.
Conclusions
The high concordance of our non-invasive oral fluid-based immunoassays (HEV IgG and HEV IgA) with commercial high-performance serum HEV ELISA kits (IgG and IgM) means that population-based surveillance of past and recent HEV infection could be expanded to improve our understanding of its ecology and natural history.
Introduction
Hepatitis E virus (HEV) is one of the leading global causes of acute viral hepatitis [1, 2]. HEV infections result in serious morbidity and mortality, particularly among pregnant women [3, 4], and have significant economic costs. Epidemics of hepatitis E are particularly problematic in areas of South Asia where seasonal floods lead to frequent contamination of drinking water supplies with HEV [5, 6]. Whereas case-fatality rates in the general population can vary from 0.1%–3.0% in South Asia, elevated mortality (10%–40%) in pregnant women infected with HEV genotype 1 has been demonstrated consistently. HEV infection during pregnancy frequently leads to miscarriage, preterm delivery and poor neonatal survival, stillbirth and neonatal death. Given its well-documented epidemic potential, with tens of thousands of hepatitis E cases reported annually, rapid, reliable diagnostic testing for hepatitis E is important. Rapid and reliable hepatitis E testing during outbreaks and epidemics could trigger preventive interventions (e.g., provision of safe drinking water, vaccination) to reduce the duration and severity of disease [7, 8].
Current methods to diagnose HEV infection rely on enzyme-linked immunosorbent assays (ELISAs) that test for antibodies to HEV (anti-HEV) in serum or on RT-PCR of HEV RNA in serum or stool. The collection of blood or stool samples is routine in the clinical setting where individuals are generally sick and seeking care. Such invasive sampling methods tend to be acceptable in clinical settings because they inform diagnoses and decisions that affect patients’ treatment. Filling knowledge gaps in the complex epidemiology and natural history of hepatitis E, including past exposure and asymptomatic infection, will remain challenging if screening methods rely on invasive sampling and testing among populations seeking care in clinical settings. Population-based surveillance of high-risk populations (pregnant women) and regions (South Asia; southern France) are needed. Participation rates in population-based studies can suffer depending on the invasiveness and discomfort caused by some biospecimen collections (blood, stool), particularly for disease inferences that require longitudinal repeated-measurements. Oral fluid collection requires fewer resources than blood collection since no clinically trained personnel are needed. Moreover, oral fluid can be self-collected and returned by mail/courier [9]. These attributes could help fill gaps in infectious disease surveillance in populations typically underrepresented in health research including those in remote and resource-limited settings and children and pregnant women. Tests using oral fluid have been developed for human immunodeficiency virus (HIV) [10, 11], hepatitis A virus [12], hepatitis C virus [12–14], norovirus [15, 16], Cryptosporidium parvum [15–17], cytomegalovirus [18], Helicobacter pylori [15], and Toxoplasma gondii [19, 20] and been shown to yield similar sensitivities and specificities as serum-based ELISAs to assess past and recent infection.
Oral fluid is composed of secretions from salivary glands, transudate from the capillary bed and crevicular fluid (flows from between the gums and the teeth) [21]. Crevicular fluid is the component of oral fluid that is particularly rich in IgA and IgG and IgM [21]. Most IgG in oral fluid is derived from serum and enters the oral cavity via crevicular fluid whereas most IgA in oral fluid is produced in the salivary glands and thus reflects mucosal as well as systemic immunity [22]. IgM is present at lower concentrations in oral fluid than IgG and IgA [23]. Collection devices, such as the Oracol saliva collection sponge, have been specifically designed to collect oral fluid that is enriched with crevicular fluid containing antibodies.
Little is known about the temporal dynamics of the humoral immune response to HEV in oral fluid. However, studies in rhesus monkeys infected with HEV and clinical studies among humans showed that virus shedding in stool starts about 2 weeks post infection [24]. HEV RNA in stool remains detectable up to about 8 weeks post infection. HEV RNA in blood is detectable at 2–3 weeks and remains detectable up to about 6 weeks post infection [24]. HEV-IgM antibody is detected at about 4 weeks and peaks at about 6 weeks post infection. HEV-IgM antibody is closely followed by rising HEV-IgG antibodies. Serum HEV-IgM antibodies remain detectable for at least 2–3 months, whereas serum HEV-IgG antibodies are detectable for years [25, 26]. Few studies have investigated the temporal dynamics of HEV-IgA antibody, but HEV-IgA antibody levels are believed to rise at the same time as HEV-IgM and remain detectable for about the same period of time [27].
Optimization and integration of non-invasive biomarkers of HEV infection into population-based epidemiologic studies could improve participation and retention and enable more frequent follow-ups to elucidate time-windows, routes of exposure, and the frequency and distribution of symptomatic vs. asymptomatic HEV infections [28]. The goal of this study was to develop oral fluid-based immunoassays to assess past and recent HEV infection among patients visiting a clinical diagnostics center in Dhaka, Bangladesh, to validate the oral fluid immunoassays’ performance compared to commercially available serum ELISAs [29], and to assess the acceptability and reliability of participants’ self-collection of oral fluid.
Materials and methods
Study population
Study enrollment took place at a clinical diagnostics center in Dhaka, Bangladesh. Two groups were eligible for enrollment into this study: 1) Participants who were referred to the diagnostics center by their physician because of symptoms of acute viral hepatitis (AVH group; N=76); and 2) Participants who were referred to the diagnostics laboratory because of reasons unrelated to acute viral hepatitis (hereafter “referent group”; N=65). First, potential participants were invited to participate in the study and asked to provide informed oral consent. If they agreed to participate, they were asked to provide a blood sample and two oral fluid samples (hereafter “saliva”). Participants were asked to self-collect the first saliva sample using an Oracol saliva collection device (Malvern Medical Developments, Worcester, UK) and to have a second saliva sample collected by trained clinical staff using the same saliva collection device and procedure. To collect a saliva sample, first, participants were instructed to rinse their mouth with cold water and then to rub the Oracol sponge along their gum line for one minute, in a motion similar to brushing their teeth (except rubbing the gums and cheek rather than the teeth). Clinical staff followed the same sample collection instructions to collect a second saliva sample from each participant. Upon completion of the sample collection, participants were asked to fill out a short questionnaire assessing (1) preference of providing a blood vs. a saliva sample and preference of self-collecting a saliva sample vs. having a saliva sample collected by trained staff; (2) drinking water source, sanitation and other household characteristics at the participant’s home; (3) whether they had symptoms of acute viral hepatitis and the time since onset of these symptoms; (4) smoking and betel nut chewing habits; (5) and demographics. The Johns Hopkins Bloomberg School of Public Health (JHSPH) Institutional Review Board approved this study (IRB00004424). The Bangladesh Institute for Child Health served as the local IRB for this study.
Sample collection and processing
Blood samples were allowed to clot at room temperature for 20 min before centrifugation for 15 min at 1,500 g. Then serum was transferred into cryovials and stored at −80°C. The Oracol sponge of each saliva collection device was turned upside down (sponge up) using sterile forceps, and was then centrifuged for 10 min at 1,500 g to spin the saliva out of the sponge. After centrifugation the sponge was removed and the saliva transferred into cryovials that were also stored at −80°C. Saliva and serum samples were shipped to JHSPH in a vapor phase liquid nitrogen cryomover.
Immunoassays to assess HEV infection status
Beijing Wantai HEV ELISA kits were chosen as the reference kits to validate the serum- and saliva-based HEV assays developed in this study (referred to as EHMIL [Environmental Health, Microbiology and Immunology Lab] assays). Serum samples were tested for IgG antibodies against HEV to evaluate past infection and IgM antibodies against HEV to evaluate recent infection using anti-HEV IgG and anti-HEV IgM ELISA kits (Beijing Wantai Biological Pharmacy, Beijing, China). The assays were performed according to the manufacturer’s instructions. For assay validation we used the Wantai HEV IgG and HEV IgM results as reference regardless of the participant group (AVH vs. referent group). To date there are no FDA-approved HEV IgG and HEV IgM assays, but previous research has shown that Wantai’s ELISAs are more [30] or equally sensitive and specific than other commercially available HEV ELISAs [31, 32].
EHMIL serum and saliva immunoassays to assess HEV infection status
The EHMIL immunoassays developed in this study are based on Luminex technology. For both, the serum-based and the saliva-based assay, HEV antigen was coupled covalently to magnetic beads. The magnetic beads serve as solid phase and are allowed to react with the serum or saliva sample. HEV-specific antibodies in the sample will bind to the immobilized HEV antigen and can be detected using fluorophore-labeled Ig class-specific antibodies (e.g. fluorophore-labeled anti-human IgG, anti-human IgM or anti-human IgA antibody).
Coupling of antigens to magnetic beads
The HEV antigen used in this study (HEV ORF2 protein, DevaTal Inc, Hamilton, NJ) is a glutathione S-transferase (GST) tagged fusion protein. Prior to coupling, the HEV antigen was purified by size exclusion chromatography (Bio-Spin 6 columns, buffer exchange protocol, Bio-Rad, Hercules, CA) to remove any amine-containing substances that may interfere with the carbodiimide coupling reaction (e.g. Tris, urea) and eluted in phosphate buffer saline (PBS, pH 7.4). Next, HEV antigen was coupled covalently to carboxylated magnetic MagPlex Microspheres (Luminex Corp., Austin, TX), hereafter “beads”, according to the Luminex xMAP Cookbook 2nd edition with the following modifications: 1) After activation with N-hydroxysulfosuccinimide sodium salt (Sulfo-NHS) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) beads were washed once (instead of twice) with 250 μL 50 mM 2-morpholinoethanesulfonic acid (MES) buffer, pH 5.0, 2) without sonication (instead of with) and 3) HEV antigen was diluted in 400 μL PBS (instead of MES) prior to addition to the activated beads.
Coupling of HEV antigen to the magnetic beads was confirmed using a serum sample containing anti-HEV IgG antibodies (confirmed by Wantai HEV IgG ELISA) and R-phycoerythrin (PE) labeled anti-human IgG antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA).
Human antibodies may bind to GST tags and adjusting the HEV antigen specific signal for the GST specific signal may improve assay performance [33]. Therefore, GST antigen (Pierce, ThermoFisher Scientific) was coupled to a different bead set using the coupling procedure described above. Coupling of GST to the magnetic beads was confirmed using mouse anti-GST antibody (Pierce, ThermoFisher Scientific) and PE-labeled anti-mouse IgG antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA).
EHMIL duplex serum HEV assay
Serum samples were tested for HEV IgG, HEV IgM and HEV IgA antibodies by a duplex assay that measures the HEV antigen and GST response in the same sample. Two sets of beads (one coupled with GST-tagged HEV antigen, the other one coupled with GST antigen) were vortexed, sonicated and diluted in assay buffer (PBS with 0.05% Tween20 and 1% bovine serum albumin, hereafter “buffer”). Then 25 μL bead mix (1,500 beads of each set) and 25 μL serum sample diluted 1:500 in buffer were added to each well of a microtiter plate. The plate was covered and allowed to incubate for 1 h on a plate shaker at 200 rpm. Then the beads were washed three times using an automated magnetic bead wash station (Bio-Plex Pro Wash Station, Bio-Rad, Hercules, CA). Next, 50 μL of PE-labeled anti-human IgG or anti-human IgA antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) diluted 1:100 in buffer were added to each well. The plate was allowed to incubate for 1 h as described above. Then the magnetic beads were washed once more, suspended in 100 μL sheath fluid (Luminex, Austin, TX) and the fluorescence signal was measured on a Bio-Plex 200 instrument (Bio-Rad, Hercules, CA). To test serum samples for anti-HEV IgM antibodies, 50 μL biotinylated anti-human IgM antibody diluted 1:3,000 in buffer were added to each well, the plate was allowed to incubate as described above and the beads were washed three times. Next, 50 μL of PE-labeled streptavidin (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) diluted 1:100 in buffer were added to each well. After another incubation and wash step the beads were suspended in 100 μL sheath fluid and the fluorescence signal was measured as described above.
EHMIL duplex saliva HEV assay
Saliva samples were centrifuged for 5 min at 10,000 g to remove any debris and precipitated mucins. Undiluted supernatants were then tested for anti-HEV IgG antibodies using the procedure described above. Each well contained 25 μL bead mix and 25 uL saliva; the final saliva dilution was 1:2 in buffer. To test saliva samples for anti-HEV IgA antibodies, saliva samples were assayed at a final dilution of 1:8 in buffer.
EHMIL HEV assay performance
Assay performance was determined using the Wantai HEV IgG and HEV IgM ELISA results as the reference method. To calculate EHMIL serum- and saliva-based HEV assay sensitivity and specificity we considered the Wantai HEV IgG and HEV IgM ELISA results to be correct (100% sensitive and 100% specific). Five serum samples were classified as “borderline” (signal to cut-off ratio was between 0.9 and 1.1) using the Wantai HEV IgM ELISA and were therefore excluded from the analysis. We defined cut-off values by calculating the mean signals for each EHMIL HEV assay using the corresponding Wantai HEV ELISA negative samples and adding three standard deviations (SDs). More specifically, we used three methods to calculate the mean signal. For example, to determine the cut-offs for the EHMIL serum HEV IgG assay the mean signal in method (1) was defined as the mean EHMIL serum HEV IgG median fluorescence intensity (MFI) of the Wantai HEV IgG negative samples plus three SDs. The mean signal in method (2) was defined as the mean of the ratios of serum HEV IgG MFI divided by serum GST IgG MFI of Wantai HEV IgG negative samples plus three SDs. The mean signal in method (3) was defined as the mean of the difference between the serum HEV IgG MFI and the serum GST IgG MFI (HEV MFI minus GST MFI) of Wantai HEV IgG negative samples plus three SDs. We used the same three methods to calculate cut-off values for the EHMIL saliva HEV IgG, serum HEV IgM and HEV IgA and saliva HEV IgA assays. To define the cut-off values for the saliva HEV-IgG assay we used the saliva sample MFIs that corresponded to the Wantai HEV IgG negative samples. To define the cut-off values for the EHMIL serum HEV IgM and IgA assays, we used the serum sample IgM and IgA MFIs, respectively, that corresponded to the Wantai HEV IgM negative samples. For the EHMIL saliva HEV-IgA assay we used the saliva sample IgA MFIs that corresponded to the Wantai HEV IgM negative samples.
The sensitivity of the EHMIL serum and saliva HEV assays was calculated by dividing the number of correctly classified positive samples by EHMIL assay by the number of Wantai anti-HEV IgG or IgM ELISA positive samples. The specificity of the EHMIL serum and saliva assays was calculated by dividing the number of correctly classified negative samples by EHMIL assay by the number of Wantai HEV IgG or IgM ELISA negative samples.
EHMIL duplex saliva HEV assay validation
We validated the duplex saliva HEV assay by measuring intra- and inter-assay reproducibility, recovery of spiked saliva samples, linearity of dilution and functional sensitivity. Acceptable criteria for intra- and inter-assay variability were defined as coefficient of variation (CV) <10% and <15%, respectively. Acceptable criteria for functional sensitivity were CV <20% and for dilution recovery and linearity we defined a recovery between 80% and 120% of each dilution as acceptable.
Statistical analyses
We used chi-squared tests to estimate associations between the AVH and referent group by anti-HEV IgG positive status for the following demographic and household characteristics: age (in quartiles), household drinking water source, household sanitation, other household characteristics, level of education, and habit of chewing betel nut or tobacco use. Additionally, we used two-sample t-tests to examine the variability of the continuous age distributions by anti-HEV IgG positive vs. negative status.
Results
Study population
One-hundred and forty-one individuals participated in this study, with 76 participants enrolled in the AVH group and 65 enrolled in the referent group (Table 1). Approximately half were female (49%); the median age was 35 years (range: 18–66 years). The majority of participants (96.5%) preferred to self-collect the saliva sample over having the sample collected by clinical staff; 90.8% preferred to provide a saliva sample over a blood sample and 92.2% would have preferred to self-collect the saliva sample at home and mail or drop it off at a hospital rather than having it collected by clinical staff at the hospital. Self-collected saliva specimens yielded a slightly higher mean saliva volume than staff-collected samples (392 μL vs. 374 μL), although this difference was not statistically significant (p<0.15). Table 1 provides additional information about the study participants’ demographics, household characteristics and habits of betel nut or tobacco use stratified by participant group.
Table 1.
Study participant demographics, serologic HEV infection status results, and water, sanitation and household characteristics stratified by enrollment group.
| AVHa group | Referent groupb | χ2 | |
|---|---|---|---|
| N (%) | 76 (53.9%) | 65 (46.1%) | |
| Age, median (SD) | 33.5 (12.2) | 35.0 (11.1) | 0.62 |
| Female, N (%) | 25 (32.9%) | 44 (67.7%) | <0.01 |
|
| |||
| Past (IgG) and recent (IgM) HEV infection status | |||
| Wantai HEV-IgG ELISA (+), N (%) | 50 (65.8%) | 28 (43.1%) | <0.01 |
| Wantai HEV-IgM ELISA (+), N (%) | 17 (22.4%) | 2 (3.1%) | <0.01 |
|
| |||
| Water, sanitation & household (HH) characteristics | |||
| N (%) | N (%) | ||
| HH Sanitation | 0.65 | ||
| Septic tank, modern toilet | 74 (97.4%) | 64 (98.7%) | |
| Pit latrine (water sealed) | 1 (1.3%) | 0 (0%) | |
| No facility, bush, field | 1 (1.3%) | 1 (1.6%) | |
| HH drinking water source | 0.34 | ||
| Piped water boiled | 34 (44.7%) | 29 (44.6%) | |
| Piped water not boiled | 3 (4.0%) | 0 (0%) | |
| Pump Water | 0 (0%) | 1 (1.5%) | |
| Tube well | 37 (48.7%) | 35 (53.9%) | |
| Other | 2 (2.6%) | 0 (0%) | |
| HH roof | 0.34 | ||
| Cement, concrete | 47 (61.8%) | 35 (53.9%) | |
| Tin, iron sheeting, metal | 29 (38.2%) | 30 (46.2%) | |
| HH wall | 0.46 | ||
| Plaster | 57 (75.0%) | 44 (67.7%) | |
| Tiles | 0 (0%) | 1 (1.5%) | |
| Tin, iron sheeting, metal | 17 (22.37%) | 17 (26.2%) | |
| Mud, bamboo | 2 (2.6%) | 3 (4.6%) | |
| Floor type | 0.25 | ||
| Mosaic | 8 (10.5%) | 2 (3.1%) | |
| Tiles | 13 (17.1%) | 16 (24.6%) | |
| Cement | 39 (51.3%) | 30 (46.2%) | |
| Wood | 1 (1.3%) | 0 (0%) | |
| Mud | 15 (19.7%) | 17 (26.2%) | |
|
| |||
| Education | 0.28 | ||
| Higher education | 23 (30.3%) | 18 (27.7%) | |
| Higher secondary | 18 (23.7%) | 9 (13.9%) | |
| Secondary | 22 (29.0%) | 18 (27.7%) | |
| Primary | 7 (9.2%) | 13 (20.0%) | |
| No formal education | 6 (7.9%) | 7 (10.8%) | |
|
| |||
| Betel nut | 18 (23.7%) | 11 (16.9%) | 0.32 |
| Chew tobacco | 11 (14.5%) | 5 (7.7%) | 0.21 |
| Smoke tobacco | 25 (32.9%) | 6 (9.2%) | <0.01 |
Patients visiting the clinical diagnostics laboratory for symptoms of acute viral hepatitis (AVH) or
for reasons unrelated to acute viral hepatitis (referent group).
Prevalence of past and recent HEV infection among study participants
Paired serum and saliva samples from 141 study participants were collected; two saliva samples were damaged during transportation. Serum samples from participants presenting with (AVH group) and without symptoms of acute viral hepatitis (referent group) were tested for past (Wantai HEV IgG ELISA) and recent (Wantai HEV IgM ELISA) HEV infection (see Table 1). Fifty-five percent of participant samples tested positive for anti-HEV IgG antibodies while 19 samples (13%) tested positive for HEV-IgM antibodies. The presence of HEV-IgM antibodies is consistent with acute or recent infection. All HEV IgM positive serum samples tested also positive for HEV IgG.
Factors associated with AVH vs. referent group and with anti-HEV IgG status
We found no statistically significant association between age, household characteristics or betel nut use and AVH vs. referent group. However, more males and tobacco smokers were in the AVH compared to the referent group (χ2 p<0.01). Only male participants reported smoking tobacco, i.e. the association between smoking and AVH group overlaps that with gender. As expected, there were more HEV IgG and HEV IgM positive individuals in the AVH group (Table 1; χ2 p<0.01); however, two individuals in the referent group tested positive for HEV IgM (a marker of recent infection). HEV-IgG positive status was associated with the household drinking water source (χ2 p<0.01; data not shown). Significantly more participants who reported piped water as their drinking water source tested HEV-IgG positive (45/66; 68%) than participants who reported tubewell water or “pump water” (31/73; 42%) as their drinking water source. Anti-HEV IgG positive status was also positively associated with increasing age, although not statistically significantly, likely due to the small sample size (p<0.06; data not shown).
Performance of EHMIL serum and saliva HEV IgG assays
Serum and saliva samples were tested using the EHMIL duplex HEV and GST immunoassay for HEV IgG antibodies. Sensitivity and specificity of the EHMIL serum HEV IgG assay was calculated using Wantai HEV IgG ELISA positive (n=78) and negative (n=63) results and ranged from 98.7% to 100% and 96.8% to 100%, respectively, depending on the method of signal adjustment used (Table 2). Method 1 yielded the highest sensitivity and specificity (Table 2). Sensitivity and specificity of the EHMIL saliva HEV IgG assay ranged between 93.5% and 98.7% and 93.5% and 98.4%, respectively, depending on the signal adjustment method used (Table 2). Method 2 yielded the highest sensitivity and specificity for the EHMIL saliva HEV IgG assay.
Table 2.
Performance of EHMIL serum and saliva HEV IgG assays compared to Wantai HEV IgG ELISA.
| HEV immunoassay | Samples | Cut-off | Positive | Negative | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Wantai HEV IgG | 141 | 0.19 OD | 78/78 | 63/63 | Reference | Reference |
|
| ||||||
| EHMIL seruma HEV IgG | ||||||
| (1) No adjustment | 141 | 182 MFI | 78/78 | 63/63 | 100.0% | 100.0% |
| (2) Ratio | 141 | 2.3 | 77/78 | 62/63 | 98.7% | 98.4% |
| (3) Difference | 141 | 70 MFI | 78/78 | 61/63 | 100.0% | 96.8% |
|
| ||||||
| EHMIL salivab HEV IgG | ||||||
|
| ||||||
| (1) No adjustment | 139c | 570 MFI | 73/77 | 58/62 | 94.8% | 93.5% |
| (2) Ratio | 139c | 1.7 | 76/77 | 61/62 | 98.7% | 98.4% |
| (3) Difference | 139c | 80 MFI | 72/77 | 60/62 | 93.5% | 96.8% |
Serum samples were diluted 1:1000 for EHMIL assay;
saliva samples were diluted 1:2 for EHMIL assay;
two saliva samples were missing.
OD=optical density; MFI=Median fluorescence intensity.
Note: HEV antigen used in EHMIL assays is fused with GST tag. Cut-off value to calculate sensitivity and specificity of EHMIL HEV assays determined using (1) only HEV specific signal, (2) ratio of HEV to GST specific signal, (3) difference between HEV and GST specific signal.
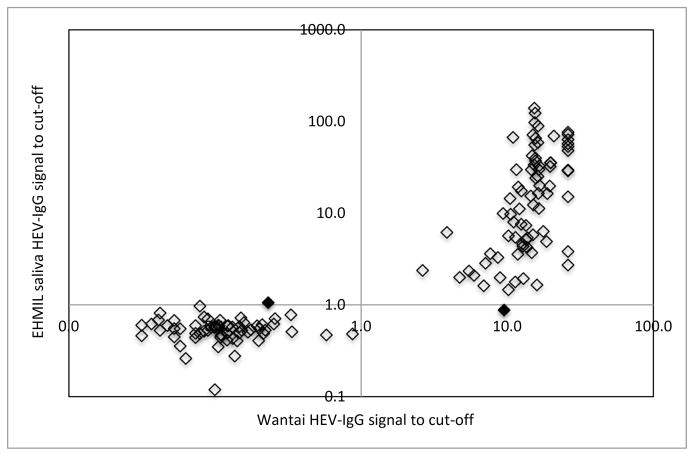
To better visualize the concordance between the EHMIL saliva HEV IgG assay and the Wantai HEV IgG assay we plotted the EHMIL HEV IgG signal to cut-off ratios of all saliva samples (method 2) against the Wantai HEV IgG signal to cut-off ratios of each corresponding serum sample (Figure 1). Samples in the upper right quadrant are classified as positive by both assays; samples in the lower left quadrant are classified as negative by both assays. The two samples that were discrepant by the EHMIL saliva HEV IgG assay are denoted by black filled in diamonds in the upper left and lower right quadrants (see Figure 1).
Figure 1.
Performance of EHMIL saliva HEV IgG immunoassay compared to Wantai serum HEV-IgG ELISA.
Note. Open diamonds indicate concordant classification between the EHMIL saliva HEV IgG assay and the Wantai HEV IgG assay. Black filled in diamonds (one in the upper left and one in lower right quadrant) denote the two samples that were discrepant by the EHMIL saliva HEV IgG assay and the Wantai HEV IgG assay.
Performance of EHMIL serum HEV IgM and HEV IgA and saliva HEV IgA assays
Serum and saliva samples were tested using the EHMIL duplex HEV and GST assay for HEV IgM (serum only) and IgA antibodies (serum and saliva). Both the EHMIL HEV IgM and IgA assays were compared to Wantai HEV IgM ELISA to determine sensitivity and specificity. The sensitivity of the EHMIL serum HEV IgM assay was 94.7% (one discrepant sample), and the specificity was 98.3% (2 discrepant samples), regardless of the signal adjustment method (Table 3). The sensitivity of the EHMIL serum HEV IgA assay ranged between 94.7% and 100%, and the specificity ranged between 98.3% and 99.1%, depending on the signal adjustment method (Table 3). Method 3 yielded the highest sensitivity and specificity for detection of HEV IgA (Table 3). The sensitivity of the EHMIL saliva HEV IgA assay ranged between 36.8% and 89.5% and the specificity ranged between 98.3% and 99.1%, depending on signal adjustment method, compared to Wantai HEV IgM ELISA (Table 3). Method 2 yielded the highest overall sensitivity and specificity for detection of salivary HEV IgA (Table 3).
Table 3.
Performance of EHMIL serum HEV IgM and HEV IgA and saliva HEV IgA assays.
| HEV immunoassay | Samples | Cut-off | Positive | Negative | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Wantai HEV IgM | 136a | 0.26 OD | 19/19 | 117/117 | Reference | Reference |
|
| ||||||
| EHMIL serumb HEV IgM | ||||||
| (1) No adjustment | 136 | 2857 MFI | 18/19 | 115/117 | 94.7% | 98.3% |
| (2) Ratio | 136 | 4.3 | 18/19 | 115/117 | 94.7% | 98.3% |
| (3) Difference | 136 | 1437 MFI | 18/19 | 115/117 | 94.7% | 98.3% |
|
| ||||||
| EHMIL serumb HEV IgA | ||||||
| (1) No adjustment | 136 | 641 MFI | 18/19 | 116/117 | 94.7% | 99.1% |
| (2) Ratio | 136 | 11.4 | 19/19 | 115/117 | 100.0% | 98.3% |
| (3) Difference | 136 | 543 MFI | 19/19 | 116/117 | 100.0% | 99.1% |
|
| ||||||
| EHMIL salivac HEV IgA | ||||||
| (1) No adjustment | 134d | 571 MFI | 7/19 | 113/115 | 36.8% | 98.3% |
| (2) Ratio | 134d | 2.3 | 17/19 | 113/115 | 89.5% | 98.3% |
| (3) Difference | 134d | 238 MFI | 13/19 | 114/115 | 68.4% | 99.1% |
Five serum samples were classified as “borderline” (neither positive nor negative) by Wantai HEV IgM assay and were therefore excluded from the sensitivity and specificity analysis.
Serum samples were diluted 1:1000 for EHMIL assay;
saliva samples were diluted 1:8 for EHMIL assay;
two saliva samples were missing.
OD=optical density; MFI=Median fluorescence intensity.
Note: HEV antigen used in EHMIL assays is fused with GST tag. Cut-off value to calculate sensitivity and specificity of EHMIL HEV assays determined using (1) only HEV specific signal, (2) ratio of HEV to GST specific signal, (3) difference between HEV and GST specific signal.
Comparison of saliva HEV-IgG assay results between participant-collected and clinical staff-collected saliva samples
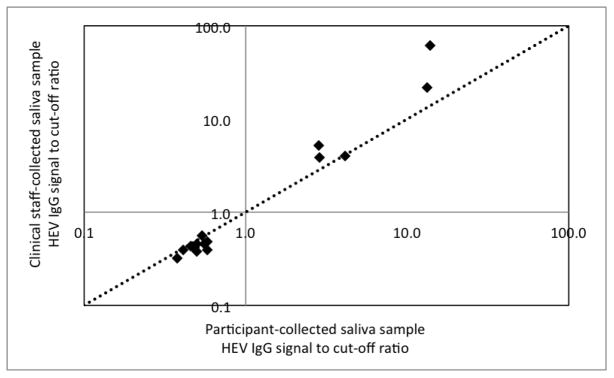
According to study procedures the participant self-collected the first saliva sample and clinical staff collected the second saliva from the participant. A subset (approximately 10%) of participant- and staff-collected saliva samples was tested for HEV-IgG to assess potential differences in measurement outcomes. The subset included 10 HEV-IgG negative and 5 HEV-IgG positive saliva samples. The HEV-IgG signal to cut-off ratios (signal adjustment method 2) of participant vs. clinical staff-collected samples is shown in Figure 2. All self-collected and staff-collected saliva samples were correctly classified (HEV-IgG positive or negative compared to Wantai serum HEV IgG ELISA). We observed only minimal differences between self- and staff-collected HEV-IgG negative samples. However, four out of five staff-collected HEV-IgG positive samples yielded higher signal to cut-off ratios than the corresponding self-collected saliva samples. A diagonal dotted line representing equal signal to cut-off ratios on both axes is shown in Figure 2 for visualization.
Figure 2.
Comparison between participant- and clinical staff-collected saliva samples measured by EHMIL saliva HEV IgG immunoassay
EHMIL saliva HEV assay validation
The intra-assay variability of a negative, low and high positive HEV-IgG saliva sample (8 replicates each) was 5%, 4% and 4%, respectively. Inter-assay variability of a negative, low and high positive saliva sample (2 replicates each tested in 3 independent assays) was 20%, 11% and 4%, respectively. The functional sensitivity of the assay (18 replicates of a low positive sample) was 6%. Linearity of dilution and recovery of a spiked sample ranged between 96–104% and 82–99%, respectively.
Discussion
We observed good concordance between our EHMIL serum and saliva HEV immunoassays and commercial serum HEV ELISAs. To calculate the sensitivities and specificities of the EHMIL assays we used the emerging global research standard HEV ELISA [34], which is produced by Wantai Biological. The reported sensitivity of the Wantai HEV IgG ELISA is 98% to 100% and the specificity is 99.99%. To calculate the assay sensitivity, Wantai tested samples from patients clinically diagnosed with hepatitis E and assumed that a true positive sample is any sample that yielded a positive result either using the Wantai HEV IgG ELISA or another manufacturer’s HEV IgG ELISA [35]. The specificity calculation of the Wantai HEV IgG assay is based on the assumption that samples of the normal population (blood donors) will form a frequency distribution with two peaks. The first peak they observed is a log-normal distribution (representing the HEV IgG negative population); the second peak is a negative skewed distribution (representing the HEV IgG positive population). Wantai chose a cut-off point between those two peaks and calculated that any sample that yields a signal lower than the cut-off point will have a false-positive rate of 0.01% [35].
The reported sensitivity and specificity of the Wantai HEV IgM assay is between 96.4% and 97.9% and between 95.3% and 100%, respectively. The sensitivity of the Wantai HEV IgM ELISA was determined in a similar manner to the Wantai HEV IgG ELISA sensitivity, i.e., using samples from patients clinically diagnosed with hepatitis E and assuming that any sample that yielded a positive result either using the Wantai HEV IgM assay or another manufacturer’s HEV IgM assay is a true positive. To determine the Wantai HEV IgM ELISA specificity, samples obtained from patients infected with hepatitis A, B and C virus, from hepatitis B virus vaccine recipients and from individuals representing the normal population were tested.
In our saliva assays, signal adjustment method 2 (ratio of the HEV specific MFI to the GST specific MFI) yielded the best performance because saliva samples exhibited a much stronger GST specific signal than serum samples. Some saliva samples yielded higher GST specific MFI than HEV antigen specific MFI, therefore not adjusting the HEV signal for GST (method 1) would be inappropriate, whereas method (3) yielded negative signals for some samples and was therefore sub-optimal. The cut-off values used in this study are based on 63 Wantai HEV IgG ELISA confirmed negative samples and on 117 Wantai HEV IgM ELISA negative samples. We believe that the same cut-off values could be applied in future HEV screening studies in other study populations if the same assay conditions (sample dilution, incubation times, antibody source and concentration, etc.) were used. Because immunoassay conditions influence the cut-off values it would be advisable to use a panel of known negative samples to confirm existing and/or re-establish cut-off values if assay conditions are modified.
One limitation of this study is the small number of confirmed HEV-IgM positive samples, which likely had a negative bearing on the performance characterization of the EHMIL saliva HEV IgA assay. To determine the sensitivity of the EHMIL serum HEV IgA and IgM and the saliva HEV IgA assays more accurately we will need to test a larger number of HEV IgM positive samples. Another limitation is the use of a GST-tagged antigen. The use of a GST-tagged antigen in salivary assays requires signal adjustment, which introduces variability into the assay performance. Ideally, we should have used an HEV antigen without tag or with a different tag that exhibits minimal cross-reactivity with antibodies in saliva. We attempted first to develop a salivary HEV ELISA, but had difficulties separating the HEV-specific vs. GST-derived assay signal. Additionally, more sample volume is required in the ELISA format compared to the duplex bead-based assays since each sample must be measured in at least two separate wells to obtain the signal attributable to the (GST-tagged) HEV antigen and the signal attributable to GST alone. Furthermore, the ELISA plate has to be coated with equimolar amounts of GST and GST-tagged HEV for reliable signal adjustment. The binding efficiency of antigens to microtiter plates is affected by multiple parameters, and, although theoretically feasible, we found it challenging to accurately separate the HEV antigen signal from signal that is derived from the GST tag and from signal that may be due to other non-specific binding. We observed less non-specific binding using the bead-based assay format and, as long as both bead sets are coupled with an excess of antigen (GST-tagged HEV and GST, respectively), it is relatively straightforward to discern the HEV-specific signal from the GST tag signal using the ratio of the signals of the two bead sets for saliva assays.
To measure past and recent immune responses to pathogens in saliva it is important to use a saliva collection device that is specifically designed to collect crevicular fluid rather than whole saliva, since this component of oral fluid has the highest concentration of antibodies derived from serum. However, in order to implement the here developed oral fluid HEV assay in population-based studies or in disease surveillance programs a simpler assay format is needed. Most labs are equipped with ELISA readers, but not necessarily with Luminex technology based platforms. In order to transition the test into an ELISA format, HEV antigens with minimal non-specific binding to human antibodies in oral fluid are needed. This will eliminate the need for a duplex assay and will also enable the transition of the test into a field device. A lab-based ELISA would enable researchers and clinicians to screen large populations non-invasively and repeatedly for evidence of past and recent HEV infection. This may elucidate time windows of exposure and deepen our understanding of infection and transmission patterns and of the burden of HEV in general. A field device to test for HEV would be of particular significance in outbreak situations where the etiologic agent needs to be identified quickly and reliably to decide about best approaches to mitigate and limit transmission of the disease.
Our study demonstrated participants’ preference to self-collect saliva rather than having clinical staff collect their sample. Participants also indicated a preference for saliva over blood collection and that they would prefer to collect saliva at home and submit their sample for testing via mail. Because all self-collected and staff-collected saliva samples were correctly classified (HEV-IgG positive or negative compared to Wantai serum HEV IgG ELISA), self-collection of saliva could have utility in population-based HEV surveillance. Interestingly, among HEV-IgG positive saliva samples collected by clinical staff we observed slightly higher signal-to-cutoff values. It is not clear whether this could be related to the order of sample collection, which was determined by the study procedure, or to potential differences in the collection technique between staff- and participant self-collected swabs. It is possible that stimulation of the oral mucosa by the participant’s self-collected sample resulted in slightly higher signal-to-cutoff values in the clinical staff-collected saliva, which followed the first swab.
Despite the significant global burden of disease and mortality now attributed to HEV, this emerging pathogen remains neglected by clinicians and public health professionals alike. This is partly due to unreliable and highly variable serologic hepatitis E assay performance [34, 36, 37] and understanding of hepatitis E etiology. Our oral fluid hepatitis E assays fulfilled all validation criteria with the exception of the inter-assay variability of the negative saliva sample. The fluorescence signal of the negative saliva sample chosen for the validation assay was near the lower limit of the measurable signal. A slightly higher variability in the very low fluorescence signal range is not uncommon and does not affect the sensitivity or specificity of the overall assay.
Even if saliva-based hepatitis E testing is not more accurate than existing serologic hepatitis E tests, it opens the door for large-scale population-based cross-sectional and longitudinal studies of hepatitis E incidence and prevalence. Saliva-based hepatitis E incidence and prevalence estimation could reveal the underlying immunological landscape of HEV infection and inform novel prevention strategies in endemic and resource limited settings that experience large-scale epidemics.
In summary, the EHMIL serum and saliva HEV antibody assays showed good concordance with the Wantai ELISAs. Future epidemiologic studies should include saliva collection to further improve saliva-based assay performance characteristics and to promote the application of saliva-based diagnostics in population-based and clinical research settings.
Highlights.
Non-invasive sampling could improve community-based research participation
We developed saliva-based assays to assess past and recent hepatitis E virus (HEV) infection
The sensitivity and specificity of the salivary assays are comparable to serum based ELISAs
Salivary assays could improve our knowledge of the ecology and natural history of HEV
Acknowledgments
Funding Sources and IRB approval
Funding for this study was provided by the National Institute for Occupational Health and Safety (NIOSH) grant 1K01OH010193-01A1, the Johns Hopkins Center for Global Health and the Johns Hopkins Water Institute. NP was supported by the National Institute of Environmental Health Sciences (NIEHS) award no. 5T32ES007141-30. CDH was supported by NIOSH grant 1K01OH010193-01A1. This study was approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board (IRB00004424). The Bangladesh Institute for Child Health served as the local IRB for this study.
Footnotes
Disclosure Statement: In the interest of full disclosure, DAG is Founder and Chief Scientific and Strategy Advisor at Salimetrics and SalivaBio and these relationships are managed by the policies of the committee’s on conflict of interest at Johns Hopkins University School of Medicine and the University of California, Irvine. SAG is Chief Scientific Officer at Salimetrics. No other author has conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Labrique AB, et al. Hepatitis E: an emerging infectious disease. Epidemiol Rev. 1999;21(2):162–79. doi: 10.1093/oxfordjournals.epirev.a017994. [DOI] [PubMed] [Google Scholar]
- 2.Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13(3):145–54. doi: 10.1002/rmv.384. [DOI] [PubMed] [Google Scholar]
- 3.Tsega E, et al. Acute sporadic viral hepatitis in Ethiopia: causes, risk factors, and effects on pregnancy. Clin Infect Dis. 1992;14(4):961–5. doi: 10.1093/clinids/14.4.961. [DOI] [PubMed] [Google Scholar]
- 4.Hamid SS, et al. Fulminant hepatic failure in pregnant women: acute fatty liver or acute viral hepatitis? J Hepatol. 1996;25(1):20–7. doi: 10.1016/s0168-8278(96)80323-0. [DOI] [PubMed] [Google Scholar]
- 5.Labrique AB, et al. Epidemiology and risk factors of incident hepatitis E virus infections in rural Bangladesh. Am J Epidemiol. 2010;172(8):952–61. doi: 10.1093/aje/kwq225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ippagunta SK, et al. Presence of hepatitis E virus in sewage in Northern India: frequency and seasonal pattern. J Med Virol. 2007;79(12):1827–31. doi: 10.1002/jmv.21017. [DOI] [PubMed] [Google Scholar]
- 7.Labrique AB, et al. Hepatitis E, a Vaccine-Preventable Cause of Maternal Deaths. Emerging Infectious Disease journal. 2012;18(9):1401. doi: 10.3201/eid1809.120241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krain LJ, Nelson KE, Labrique AB. Host Immune Status and Response to Hepatitis E Virus Infection. Clin Microbiol Rev. 2014;27(1):139–65. doi: 10.1128/CMR.00062-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris-Cunnington MC, et al. A population-based seroprevalence study of hepatitis A virus using oral fluid in England and Wales. Am J Epidemiol. 2004;159(8):786–94. doi: 10.1093/aje/kwh107. [DOI] [PubMed] [Google Scholar]
- 10.Delaney KP, et al. Performance of an oral fluid rapid HIV-1/2 test: experience from four CDC studies. Aids. 2006;20(12):1655–60. doi: 10.1097/01.aids.0000238412.75324.82. [DOI] [PubMed] [Google Scholar]
- 11.Morris M, et al. Prevalence of HIV infection among young adults in the United States: results from the Add Health study. Am J Public Health. 2006;96(6):1091–7. doi: 10.2105/AJPH.2004.054759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amado LA, et al. Detection of hepatitis A, B, and C virus-specific antibodies using oral fluid for epidemiological studies. Mem Inst Oswaldo Cruz. 2006;101(2):149–55. doi: 10.1590/s0074-02762006000200006. [DOI] [PubMed] [Google Scholar]
- 13.Cha YJ, et al. Performance Evaluation of the OraQuick Hepatitis C Virus Rapid Antibody Test. Annals of Laboratory Medicine. 2013;33(3):184–189. doi: 10.3343/alm.2013.33.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drobnik A, et al. Public health implications of rapid hepatitis C screening with an oral swab for community-based organizations serving high-risk populations. Am J Public Health. 2011;101(11):2151–5. doi: 10.2105/AJPH.2011.300251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin SM, et al. Development of a multiplex microsphere immunoassay for the quantitation of salivary antibody responses to selected waterborne pathogens. J Immunol Methods. 2011;364(1–2):83–93. doi: 10.1016/j.jim.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Moe CL, et al. Diagnosis of norwalk virus infection by indirect enzyme immunoassay detection of salivary antibodies to recombinant norwalk virus antigen. Clin Diagn Lab Immunol. 2004;11(6):1028–34. doi: 10.1128/CDLI.11.6.1028-1034.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egorov AI, et al. Recent diarrhea is associated with elevated salivary IgG responses to Cryptosporidium in residents of an eastern Massachusetts community. Infection. 2010;38(2):117–23. doi: 10.1007/s15010-009-9323-4. [DOI] [PubMed] [Google Scholar]
- 18.Chaushu G, et al. Salivary immunoglobulins in recipients of bone marrow grafts. III. A longitudinal follow-up of CMV specific antibodies. Bone Marrow Transplant. 1996;17(2):237–41. [PubMed] [Google Scholar]
- 19.Sampaio BF, et al. Saliva as a source of anti-Toxoplasma gondii IgG for enzyme immunoassay in human samples. Clin Microbiol Infect. 2014;20(1):O72–4. doi: 10.1111/1469-0691.12295. [DOI] [PubMed] [Google Scholar]
- 20.Hajeer AH, et al. Toxoplasma gondii: detection of antibodies in human saliva and serum. Parasite Immunol. 1994;16(1):43–50. doi: 10.1111/j.1365-3024.1994.tb00303.x. [DOI] [PubMed] [Google Scholar]
- 21.McKie A, Vyse A, Maple C. Novel methods for the detection of microbial antibodies in oral fluid. The Lancet Infectious Diseases. 2002;2(1):18–24. doi: 10.1016/s1473-3099(01)00169-4. [DOI] [PubMed] [Google Scholar]
- 22.Brandtzaeg PER. Do Salivary Antibodies Reliably Reflect Both Mucosal and Systemic Immunity? Annals of the New York Academy of Sciences. 2007;1098(1):288–311. doi: 10.1196/annals.1384.012. [DOI] [PubMed] [Google Scholar]
- 23.Brandtzaeg P. Secretory immunity with special reference to the oral cavity. Journal of Oral Microbiology. 2013;5 doi: 10.3402/jom.v5i0.20401.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalton HR, et al. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis. 2008;8(11):698–709. doi: 10.1016/S1473-3099(08)70255-X. [DOI] [PubMed] [Google Scholar]
- 25.Kamar N, et al. Hepatitis E. The Lancet. 379(9835):2477–2488. doi: 10.1016/S0140-6736(11)61849-7. [DOI] [PubMed] [Google Scholar]
- 26.Hoofnagle JH, Nelson KE, Purcell RH. Hepatitis E. New England Journal of Medicine. 2012;367(13):1237–1244. doi: 10.1056/NEJMra1204512. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi M, et al. Simultaneous Detection of Immunoglobulin A (IgA) and IgM Antibodies against Hepatitis E Virus (HEV) Is Highly Specific for Diagnosis of Acute HEV Infection. Journal of Clinical Microbiology. 2005;43(1):49–56. doi: 10.1128/JCM.43.1.49-56.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Exum NG, et al. Use of Pathogen-Specific Antibody Biomarkers to Estimate Waterborne Infections in Population-Based Settings. Current Environmental Health Reports. 2016:1–13. doi: 10.1007/s40572-016-0096-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kmush BL, et al. Two Generations of “Gold Standards”: The Impact of a Decade in Hepatitis E Virus Testing Innovation on Population Seroprevalence. Am J Trop Med Hyg. 2015;93(4):714–7. doi: 10.4269/ajtmh.15-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou H, et al. Comparison of the reliability of two ELISA kits for detecting IgM antibody against hepatitis E virus. Zhonghua Yu Fang Yi Xue Za Zhi. 2008;42(9):667–71. [PubMed] [Google Scholar]
- 31.Pas SD, et al. Diagnostic performance of selected commercial HEV IgM and IgG ELISAs for immunocompromised and immunocompetent patients. J Clin Virol. 2013;58(4):629–34. doi: 10.1016/j.jcv.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Avellon A, et al. Comparative sensitivity of commercial tests for hepatitis E genotype 3 virus antibody detection. J Med Virol. 2015;87(11):1934–9. doi: 10.1002/jmv.24251. [DOI] [PubMed] [Google Scholar]
- 33.Griffin SM, et al. Application of salivary antibody immunoassays for the detection of incident infections with Norwalk virus in a group of volunteers. J Immunol Methods. 2015;424:53–63. doi: 10.1016/j.jim.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bendall R, et al. A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. Journal of Medical Virology. 2010;82(5):799–805. doi: 10.1002/jmv.21656. [DOI] [PubMed] [Google Scholar]
- 35.Beijing Wantai Biological Pharmacy Enterprise Co., L. WANTAI HEV-IgG ELISA. 2014 [cited 2016; V. 2014-01:[DIAGNOSTIC KIT FOR IgG ANTIBODY TO HEPATITIS E VIRUS (ELISA)]. Available from: http://www.ystwt.cn/IFU/HEV/HEV-IgG_CE.pdf.
- 36.Wenzel JJ, et al. Test Performance Characteristics of Anti-HEV IgG Assays Strongly Influence Hepatitis E Seroprevalence Estimates. Journal of Infectious Diseases. 2012 doi: 10.1093/infdis/jis688. [DOI] [PubMed] [Google Scholar]
- 37.Drobeniuc J, et al. Serologic Assays Specific to Immunoglobulin M Antibodies against Hepatitis E Virus: Pangenotypic Evaluation of Performances. Clinical Infectious Diseases. 2010;51(3):e24–e27. doi: 10.1086/654801. [DOI] [PubMed] [Google Scholar]