Abstract
Background
Reasons for trends in venous thromboembolism (VTE) incidence are uncertain.
Objectives
To determine VTE incidence trends and risk factor prevalence, and estimate population-attributable risk (PAR) trends for each risk factor.
Patients/Methods
In a population-based cohort study of all residents of Olmsted County, MN, 1981–2010, annual incidence rates were calculated using incident VTE cases as the numerator and age- and sex-specific Olmsted County population estimates as the denominator. Poisson regression models were used to assess the relationship of crude incidence rates to year of diagnosis, age at diagnosis, and sex. Trends in annual prevalence of major VTE risk factors prevalence were estimated using linear regression. Poisson regression with time-dependent risk factors (person-years approach) was used to model the entire population of Olmsted County and derive the PAR.
Results
The age- and sex-adjusted annual VTE incidence, 1981–2010, did not change significantly. Over the time period, 1988–2010, the prevalence of obesity, surgery, active cancer and leg paresis increased. Patient age, hospitalization, surgery, cancer, trauma, leg paresis and nursing home confinement jointly accounted for 79% of incident VTE; obesity accounted for 33% of incident idiopathic VTE. The increasing prevalence of obesity, cancer and surgery accounted in part for the persistent VTE incidence. The PAR of active cancer and surgery, 1981–2010, signficiantly increased.
Conclusions
Almost 80% of incident VTE events are attributable to known major VTE risk factors and one-third of incident idiopathic VTE events are attributable to obesity. Increasing surgery PAR suggests that concurrent efforts to prevent VTE may have been insufficient.
Keywords: venous thrombosis, pulmonary embolism, thrombophlebitis, phlebitis, epidemiology
Introduction
In 2008 the U.S. Surgeon General categorized VTE as a major public health problem, issued a “call to action” to reduce VTE occurrence, and identified “reasons for the persistent occurrence of VTE over time” as key gaps in VTE knowledge [1]. From a list of over 80 evidence-based practices the U.S. Agency for Healthcare Research and Quality identified DVT prevention as the number one priority with the greatest potential to improve patient safety in hospitals [2]. Consequently, authoritative bodies recommend universal anticoagulant-based DVT prophylaxis for major surgery and hospitalization for acute medical illness [3–5]. However there are few contemporary data on trends in DVT and PE incidence, and the few available studies report conflicting results [6–10]. Moreover, there are essentially no data on trends in the prevalence of major VTE risk factors or the burden of VTE disease in the community attributable to each risk factor. To address these important gaps in knowledge, we performed a population-based cohort study to estimate trends in the incidence of symptomatic VTE and the prevalence of VTE risk factors, and the population-attributable risk for each risk factor.
Materials and Methods
Study Design and Setting
Using the longitudinal and population-based resources of the Rochester Epidemiology Project (REP) [11–13], we identified the inception cohort of all Olmsted County, MN residents with incident symptomatic DVT and/or PE over the 45-year period, 1966–2010, as previously described [14–16]. For this study, we restricted our cohort study analyses to residents with incident VTE over the 30-year period, 1981–2010, because the autopsy rate (~30%) was relatively constant over this timeframe and over 90% of VTE cases were objectively-diagnosed. The study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
Cohort Identification and Incidence Criteria
A master list of potential Olmsted County residents with DVT, PE, pulmonary infarction or similar diagnoses, or who had any diagnostic test or procedure used in the diagnosis of DVT or PE, was constructed as previously described [14]. Residency in Olmsted County at the time of first diagnosis of VTE was confirmed by the REP. We had access to a mean of 36.0 ± 20.7 years (median, 36 years) of documented medical history prior to the first diagnosis of VTE in these patients. Incident events were distinguished from recurrent events.
Measurements
Using explicit criteria and a standardized electronic data collection instrument with appropriate data checks, trained and experienced nurse abstractors reviewed all medical records in the community [17] and recorded the date and type (arm or leg DVT, PE) of incident VTE, baseline characteristics, and vital status at last clinical contact, as previously described [14].
To estimate trends in the prevalence of major VTE risk factors among Olmsted County residents, we used the REP Data Warehouse to retrieve ICD-9 codes for trauma/fracture, leg paresis, and surgery, and to retrieve nursing home visits and hospitalizations. We derived and validated ICD-9-CM code algorithms for trauma/facture and leg paresis (see Supplementary Material, Table 1). We used data from the Mayo Clinic Cancer Registry to estimate the prevalence of active cancer as previously defined [18]. To estimate the prevalence of body mass index (BMI) categories, we used a predicted probability of each BMI category based on Mayo electronic medical record data as well as population survey data from five previously published REP studies (see Supplementary Material, Table 2).
Analyses
Overall VTE Incidence Analyses: Denominator, Method 1. Recent upgrades in the methodology for the REP census allow the REP to track residency status of anyone seen at any medical facility in Olmsted County who has received a medical diagnosis. Each individual ever resident in Olmsted County 1966 to present who had a medical diagnosis has a timeline [12]. Each change in residency (Olmsted, non-Olmsted) is tracked. These timelines incorporate data on more than 1.16 million medical records from over 493,000 unique individuals and allows individual movement to be tracked into and out of the county, providing reliable Olmsted County population counts on any date from January 1, 1966 through present. The population at risk was determined using two methods. In method 1, for models including the risk factors age, sex and calendar year with or without BMI, the population living in Olmsted County on July 1st of each year as determined by the REP census was used as the denominator in the incidence calculation overall and as the denominator in the incidence calculation adjusted by BMI prevalence (see Supplementary Material, Table 3). Denominator, Method 2. Because the REP is a compilation of data from many different medical providers, a patient may have diagnoses recorded as both a resident and a non-resident of Olmsted County. To include persons whose Olmsted County residency appears to change multiple times in a short period, we used a moving window smoothing algorithm which incremented daily and changed Olmsted County residency status when over 50% of the time in the window was resident or non-resident; the most stable window was 364 days. Using this smoothed residency enabled us to follow a patient’s entire residency in Olmsted County; this was used as the denominator (method 2) in the person-years analysis of VTE, analysis of attributable risk, and in analysis of time to VTE (see Supplementary Material, Table 3). The method 2 denominator allowed us to evaluate the relationship of risk factors available through Olmsted County medical providers or the Mayo Clinic Cancer Registry (such as hospital stay or the occurrence of cancer) and VTE, for the entire population as well as to determine the true prevalence of any of these risk factors, and thus the true attributable risk (see Supplementary Material, Tables 4 and 5). Annual incidence rates (per 100,000 person-years) were calculated using incident cases of DVT or PE as the numerator and age-sex-specific and age-sex-BMI category specific estimates of the population (method 1) as the denominator. These rates were calculated overall and for each calendar year from 1981–2010, with confidence intervals based on a Poisson error assumption. General linearized regression with Poisson error, and a log link, with log person-years as an offset, was used to model the relationship of crude VTE incidence (count) to age, sex and calendar year. Modeling including BMI category was done similarly (see Supplementary Material, Table 3).
Attributable Risk (AR) due to BMI
To estimate AR due to BMI we created a second dataset with VTE count set to missing and BMI set to ≤ 24.9 kg/m2. We set the original and second datasets together and fit the Poisson Model to get an expected predicted value. We then expresed this as the proportion of risk (see Supplementary Material, Table 3).
Prevalence Estimates
Estimates of the prevalence of major VTE risk factors [19] were determined for each calendar year/age/sex category using method 2 for the denominator and the person-years at risk for the numerator (see Supplementary Material, Table 4). Trends in the prevalence of each risk factor were estimated using linear regression with age, sex, and calendar year as independent variables.
Poisson Regression (Person-Years) Analysis
Using the REP census, we analyzed the occurrence of incident VTE, 1995–2008, using a person-years approach (denominator from method 2). An individual’s timeline while an Olmsted County resident was subdivided into periods of risk with start and stop dates for each combination of risk factors. In addition, calendar year and age (in 5 year groups) were updated at each change in risk interval, and the occurrence/non-occurrence of a VTE event was attributed to each interval. We fit a full generalized linear model, Poisson error, log link, log-person-years offset, on incident VTE, 1995–2008, with all risk variables characterized as to when they occurred (see Supplementary Material, Table 3).
Attributable Risk (AR) Analysis
To determine the AR, we used he estimated model from the person-years analysis, but created auxiliary data sets in which we set each risk factor in turn, or the combination of all risk factors, to zero for the purpose of calculating model-predicted counts. For assessment of age-attributable risk, age was set to 20 years. Thus we were able to re-estimate the predicted risk of VTE in the absence of each risk factor, and to express this change as a proportion of the total risk. However, individual PARs are not additive. The delta method was used to determine the standard errors of these various AR estimates [20] (see Supplementary Material, Table 3).
Cox Proportional Hazards Analysis
As a validation of the person-years approach and also using the REP census, each individual was followed forward in time from their first medical encounter within the time frame of the study. An individual’s timeline while an Olmsted County resident was subdivided into periods of risk with start dates and stop dates for each risk factor as defined above. This technique, done on all Olmsted County residents (method 2), 1/1/1988 to 12/31/ 2008, allowed us to analyze time to incident VTE using Cox time-dependent hazard modeling of the entire population of Olmsted County, using a counting process approach (see Supplementary Material, Table 3).
Results
Between January 1, 1981 and December 31, 2010, 3293 residents developed a first lifetime VTE, with a mean (±SD) patient age at VTE onset of 64.3 ± 18.8 years (median 66.7 years; range 0–103 years); 1,785 (54%) were female. The mean (±SD) patient age at VTE onset for females and males was 65.9 ± 20.0 (median 70.5 years; range 0–103 years) and 62.4 ± 17.1 (median 64.1 years; range 0–98 years), respectively. The distribution by VTE event type was 1,655 (50.3%) leg DVT only, 1638 (49.7%) PE with or without (±) DVT (excluding isolated subsegmental PE). Of those with incident DVT, the distribution by DVT location was 1277 (77%) proximal ± distal leg and 378 (23%) distal leg DVT only (excluding isolated soleal and/or gastrocnemius DVT); 279 (16.9%) PE ± DVT were discovered solely at autopsy.
Incidence of VTE, 1981–2010
The average annual age- and sex-adjusted incidence of VTE over the 30-year period, adjusted to U.S. White 2010 census data, was 123 (95% CI: 118, 127) per 100,000 person-years (Table [see Supplementary Material, Table 7, for incidence by 5-year age and sex group]). The age- and sex-adjusted incidence rates for proximal ± distal leg DVT and PE ± DVT were 47 (95% CI: 45, 50) and 62 (95% CI: 59, 65) per 100,000, respectively; distal leg DVT alone was14 (95%CI: 12, 15). The age-adjusted VTE incidence rates for females and males were 113 (95% CI: 108, 119) and 134 (95% CI: 127, 141) per 100,000 person-years, respectively (male-female ratio, 1.19:1). The average annual incidence rates for secondary VTE: overall, leg DVT and PE ± DVT were 87 (95% CI, 84, 91), 42 (95% CI, 39, 44) and 45 (95% CI, 43, 48), respectively. Similarly, the average annual incidence rates for idiopathic VTE as previously defined [21]: overall, leg DVT and PE ± DVT were 35 (95% CI, 33, 38), 18 (95% CI, 17, 20) and 17 (95% CI, 15, 19), respectively.
Table.
Annual Incidence per 100,000 of All Venous Thromboembolism (VTE), Leg Deep Vein Thrombosis (DVT) Only and Pulmonary Embolism with or without Arm or Leg DVT (PE ± DVT) Among Olmsted County, Minnesota, Residents for the Study Period, 1981–2010, and for the Most Recent 5-Year Period, by Age and Sex
| Age & Sex | 1981–2010 | 2006–2010 | ||||||
|---|---|---|---|---|---|---|---|---|
| Leg DVT Only | PE ± Arm
or Leg DVT |
All VTE | Leg DVT Only | PE ± Arm
or Leg DVT |
All VTE | |||
| Proximal ± Distal |
Distal Only | Proximal ± Distal |
Distal Only | |||||
| Females | ||||||||
| 0–9 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 2 |
| 10–19 | 3 | 1 | 2 | 5 | 2 | 0 | 4 | 6 |
| 20–29 | 12 | 4 | 12 | 27 | 16 | 5 | 11 | 31 |
| 30–39 | 18 | 8 | 17 | 43 | 29 | 12 | 25 | 67 |
| 40–49 | 28 | 12 | 36 | 75 | 26 | 26 | 46 | 98 |
| 50–59 | 38 | 14 | 51 | 102 | 26 | 12 | 45 | 84 |
| 60–69 | 80 | 29 | 101 | 210 | 66 | 30 | 99 | 194 |
| 70–79 | 140 | 38 | 212 | 390 | 118 | 36 | 191 | 345 |
| 80–89 | 260 | 59 | 347 | 666 | 230 | 127 | 262 | 619 |
| ≥90 | 323 | 63 | 513 | 900 | 228 | 28 | 228 | 484 |
| Subtotal | 43 | 13 | 57 | 113 | 38 | 18 | 52 | 108 |
| 95% CI | 40, 46 | 11, 15 | 53, 61 | 108, 119 | 32, 45 | 13, 22 | 44, 60 | 97, 119 |
| Males | ||||||||
| 0–9 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 2 |
| 10–19 | 2 | 1 | 1 | 3 | 0 | 2 | 0 | 2 |
| 20–29 | 9 | 2 | 7 | 19 | 8 | 2 | 11 | 21 |
| 30–39 | 11 | 7 | 13 | 30 | 10 | 4 | 16 | 31 |
| 40–49 | 37 | 13 | 43 | 93 | 51 | 12 | 59 | 122 |
| 50–59 | 56 | 18 | 71 | 145 | 67 | 11 | 85 | 163 |
| 60–69 | 104 | 26 | 144 | 273 | 126 | 30 | 137 | 292 |
| 70–79 | 198 | 41 | 243 | 481 | 153 | 43 | 146 | 342 |
| 80–89 | 257 | 65 | 366 | 688 | 256 | 38 | 320 | 615 |
| ≥90 | 423 | 47 | 587 | 1056 | 84 | 84 | 168 | 336 |
| Subtotal | 52 | 14 | 68 | 134 | 53 | 12 | 61 | 126 |
| 95% CI | 48, 57 | 12, 16 | 63, 73 | 127, 141 | 44, 61 | 8, 16 | 52, 71 | 113, 140 |
| Total | 47 | 14 | 62 | 123 | 45 | 15 | 56 | 117 |
| 95% CI | 45, 50 | 12, 15 | 59, 65 | 118, 127 | 40, 51 | 12, 18 | 50, 62 | 108, 126 |
Directly age-adjusted to the 2010 United States white population.
Directly age- and sex-adjusted to the 2010 United States white population.
Trends in VTE Incidence, 1981–2010 (Poisson modeling)
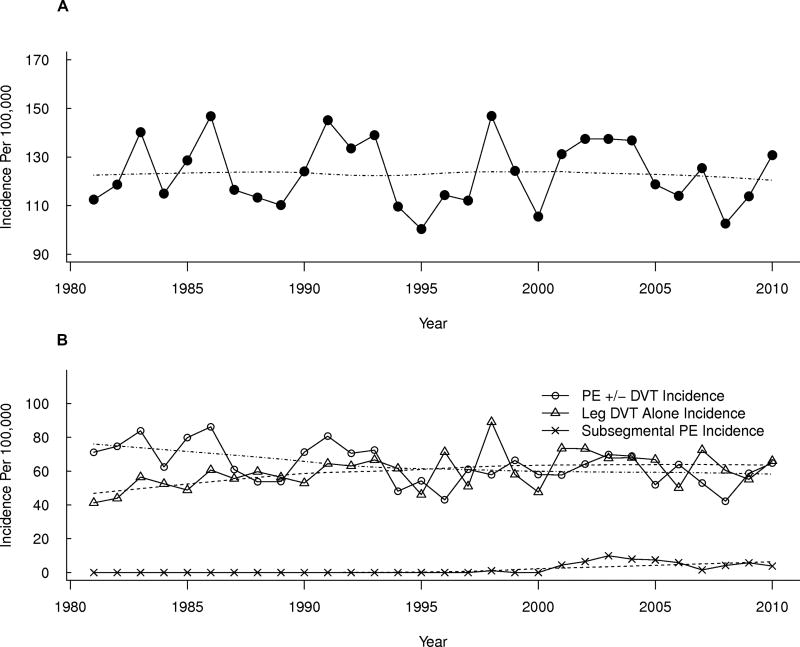
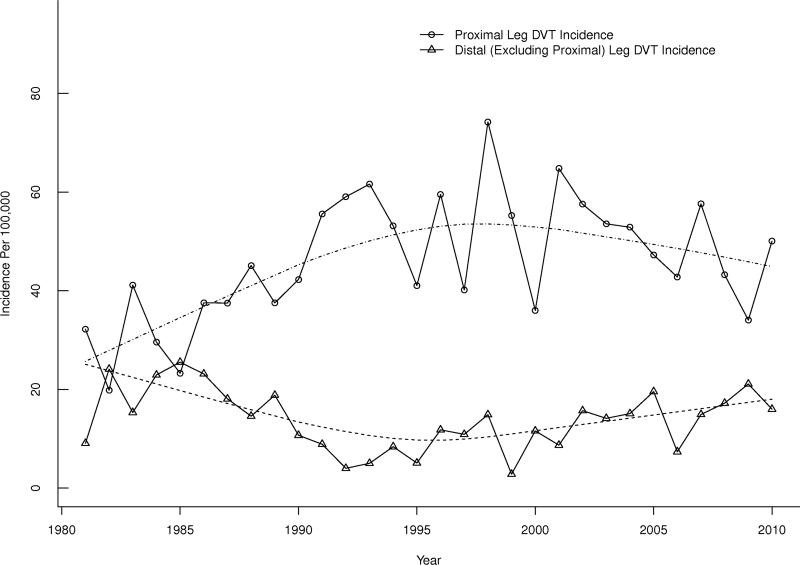
The age- and sex-adjusted annual VTE incidence did not change significantly over the 30-year period, 1981–2010 (p=0.70; Figure 1A). The annual leg DVT alone incidence increased modestly by about 7% per decade (p=0.01), and was offset by a 6% per decade decrease in annual PE ± DVT incidence(p=0.05; Figure 1B). Of the increase in “leg DVT alone” incidence, most was due to an increase in isolated distal leg DVT. The incidence rates for proximal (± distal) leg DVT decreased significantly by 16% per decade (p=0.04) in contrast to a 45% increase per decade for distal leg DVT alone (p=0.005; Figure 2).
Figure 1.
Age- and sex-adjusted annual Incidence of (A) Venous Thromboembolism and (B) Pulmonary Embolism (PE) with or without (±) Arm or Leg Deep Vein Thrombosis (DVT), Leg DVT Alone, and Subsegmental PE Alone, Among Olmsted County, Minnesota Residents, 1981–2010, by Calendar Year.
Figure 2.
Age- and sex-adjusted annual Incidence of Proximal with or without (±) Distal Leg Deep Vein Thrombosis (DVT) and Distal Leg DVT Alone, Among Olmsted County, Minnesota Residents, 1981–2010, by Calendar Year.
Trends in the Prevalence of Major VTE Risk Factors, 1988–2010
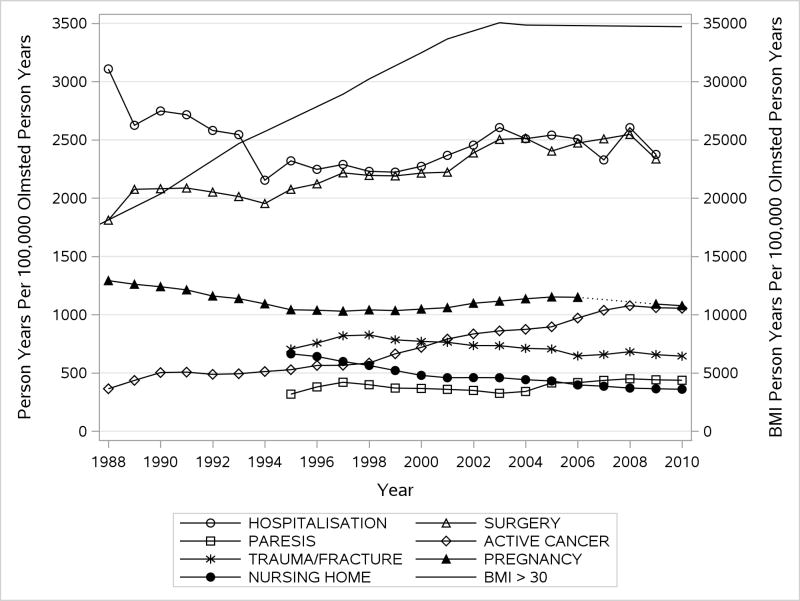
In order to use available study and electronic data on major VTE risk factors, we subset our cohort to the 23-year time period, 1988–2010 (2727 total VTE [1409 DVT, 1319 PE ± DVT]; 790 idiopathic). Over this time period, the prevalence of obesity and severe obesity increased dramatically for both sexes among the entire population of Olmsted County residents (Figure 3). The prevalence of surgery, active cancer and leg paresis per 100,000 person-years of the Olmsted County population also increased, while the prevalence of hospitalization, trauma/fracture, nursing home placement, and, among women, number of pregnancies, per 100,000 person-years of the Olmsted County population decreased. The number of hospitalizations per Olmsted County population was relatively constant but the duration of hospitalization decreased.
Figure 3.
Trends in the Prevalence of Major Venous Thromboembolism Risk Factors, 1988–2010, by Calendar Year; Person-years of Indicated Risk Factor per 100,000 Person-years of Olmsted County Residency.
Reasons for trends in VTE incidence, 1981–2010
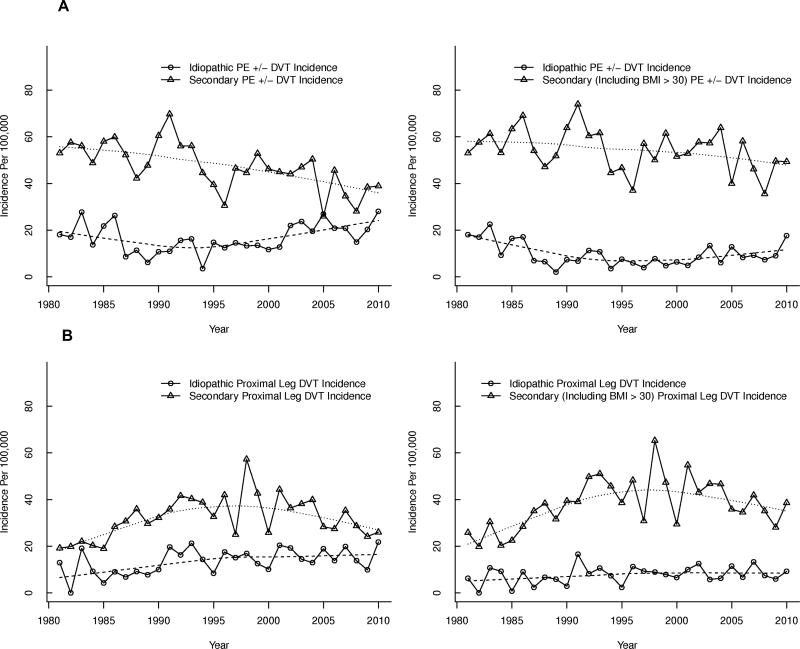
We identified several reasons for the observed secular trends in VTE incidence over this 30-year time period. Figure 4 presents trends in the annual incidence of secondary and idiopathic PE ± DVT (Figure 4A) and secondary and idiopathic proximal ± distal leg DVT (Figure 4B) where secondary events either exclude (first panel) or include (second panel) obesity (BMI>30 kg/m2). When obesity was excluded from secondary VTE events, the incidence rates of idiopathic PE ± DVT increased significantly by about 20% per decade (p=0.0007), while the rates for secondary PE ± DVT decreased significantly by about 13% per decade(first panel of Figure 4A). Similarly, the incidence rate for idiopathic proximal ± distal leg DVT increased significantly by about 16% per decade (p=0.01), while the incidence of secondary proximal ± distal leg DVT did not change significantly (first panel of Figure 4B), respectively. However, when secondary events included obesity, the incidence rates of idiopathic and secondary PE ± DVT did not change significantly over time (second panel of Figure 4A); the incidence of idiopathic proximal ± distal leg DVT also did not change significantly, while the incidence of secondary proximal ± distal leg DVT increased significantly by about 8% per decade (p=0.03; second panel of Figure 4B). These analyses suggest that the increasing prevalence of obesity partly accounted for the observed secular trends in VTE incidence. To further test this hypothesis, we used the cohort subset, 1988–2010 (method 1), to construct a Poisson regression model of VTE incidence, including age, male sex, calendar year and idiopathic versus secondary VTE where the latter is the usual definition of secondary VTE (excluding BMI). In this model, BMI was not associated with incident VTE (p=0.21). However, in a model examining the interaction of type of VTE (secondary/idiopatic) with all other variables, idiopathic VTE strongly interacted with all the variables in the model except patient age (interaction p-value<0.0001 for male sex, calendar year and BMI category; interaction p=0.30 for age). In a separate model of idiopathic VTE alone, male sex, increasing calendar year and increasing age were all strongly associated with an increased risk of incident idiopathic VTE (p<0.0001 for all). In this model, compared to normal BMI (<25 kg/m2)), all non-ideal BMI categories (25–29 kg/m2, 30–34 kg/m2, 35+ kg/m2) had an increased risk for incident idiopathic VTE, with the highest risk in the top two BMI categories (p<0.0001 for all). In a model of secondary VTE alone, female sex, increasing calendar year and age were associated with an increased risk of incident secondary VTE (p=0.004, p=0.06, p<0.001, respectively). BMI was modestly associated with an increased risk of incident secondary VTE (p=0.04) but with the lowest BMI category (<25 kg/m2) having the highest risk, likely reflecting the inclusion of active cancer in secondary VTE.
Figure 4.
Age- and sex-adjusted annual Incidence of Secondary vs. Idiopathic (A) Pulmonary Embolism (PE) with or without (±) Arm or Leg Deep Vein Thrombosis (DVT) and (B) Secondary vs. Idiopathic Leg DVT Alone, Among Olmsted County, Minnesota Residents, 1981–2010, by Calendar Year.
Population attributable risk due to BMI (1988–2010)
The population attributable risk due to above-normal BMI (relative to entire population at normal BMI), based on the Poisson model of incident VTE with BMI as an independent variable, (and including an auxiliary dataset with only normal BMI and unknown counts to be predicted) was 33% among the idiopathic subgroup, adjusting for age, sex, and calendar year. The population attributable risk due to above-normal BMI for secondary VTE was −8%. Paralleling the differences seen in the overall model and as seen when modifying the definition of secondary VTE as above, this analysis indicates a large contrast in attributable risk due to BMI for incident idiopathic VTE as compared to incident secondary VTE.
Modeling Relationship of Risk Factors and VTE Incidence in the entire Olmsted County Population, 1995–2008
The 1995–2008 time frame (1765 incident VTE [928 DVT, 837 PE]), where we had complete electronic prevalence data for the entire county, was used to estimate attributable risk for all major VTE risk factors except pregnancy/post-partum. The person-years analysis on the complete population data and risk factor prevalence showed a 1.2-fold increased risk of VTE per increasing 5-year patient age group (see Supplementary Material, Table 6), 37-, 5.9- and 1.2-fold increases in the risk of incident VTE for “in–hospital”, “within 92 days post hospital discharge” and nursing home residency, respectively, relative to residing in the community. Active cancer, surgery, trauma/fracture and leg paresis showed 3.3-, 3.2-, 2.1- and 2.2-fold increases in risk of incident VTE, respectively, compared to the absence of each condition. Cox proportional hazards versions of this same data based on an age-scale and a calendar-year scale, showed similar results except for slightly higher risk estimates for in-hospital and 92 days post hospital discharge, active cancer risk and nursing-home risk and slightly lower risk estimate for surgery (see Supplementary Material, Table 6).
Estimation and Trends of Population-Attributable Risk (PAR) of VTE Factors, 1995–2008
Joint Attributable Risk. Using the entire population of Olmsted County, 1995–2008, and the Poisson person-years approach, the joint attributable risk of patient age (using the 20–24 year old age-group as the referent), hospitalization (jointly in-hospital and 92 days post hospital discharge), surgery, active cancer, trauma/fracture, leg paresis, and nursing home stay was 79% (95% CI: 77, 82). Individual Attributable Risk. Using the 20–24 year old age-group population as the referent and adjusting for other risk factors, the VTE risk attributable to increasing patient age was 70% (95% CI: 68%, 73%). The individual attributable risks over this time frame (adjusted for the other risk factors) for in-hospital, <92 days post hospital discharge, surgery, active cancer, trauma/fracture, leg paresis, and nursing home confinement were: 20.0% (95% CI: 18.1, 22.0); 20.5% (95% CI: 18.2, 22.7); 21.5% (95% CI: 18.8, 24.1); 11.3% (95% CI: 9.4, 13.2); 5.7% (95% CI: 4.0, 7.3); 4.0% (95% CI: 2.7, 5.3); and 2.1% (95% CI: 0.3, 4.0), respectively. Individual Attributable Risk over Time. When the attributable risks were estimated separately for each year (adjusting for age) and time trends were assessed, only active cancer and surgery showed a significant increase (P<0.0001 and p=0.0002, respectively) in attributable risk over time; nursing home residency showed a significant decrease (p=0.005; Figure 5).
Figure 5.
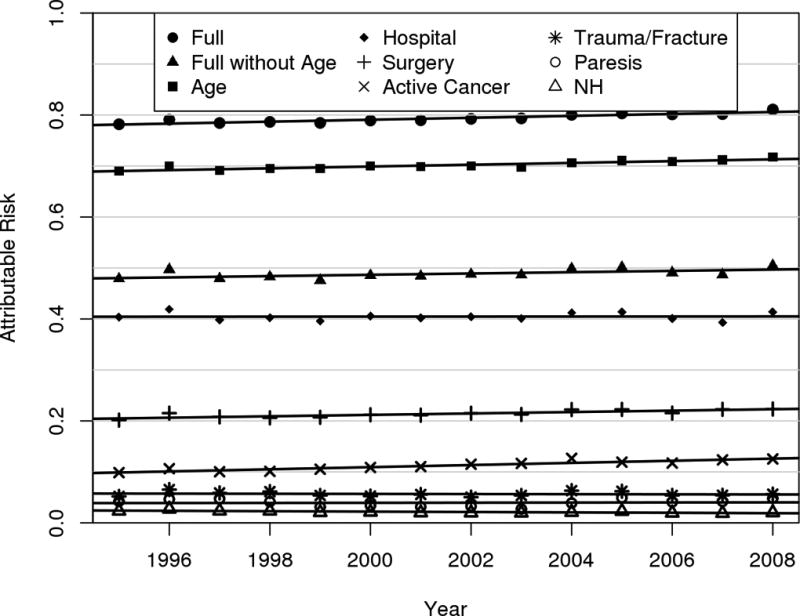
Trends in the Independent Population-Attributable Risk of Hospitalization, Surgery, Active Cancer, Trauma/Fracture, Leg Paresis and Nursing Home Confinement for Venous Thromboembolism
Discussion
Over the 30-year period, 1981–2010, we found average annual age- and sex-adjusted overall VTE, proximal ± distal leg DVT and PE ± DVT incidence rates of 123, 47 and 62 per 100,000 person-years, respectively; isolated distal leg DVT incidence was 14 per 100,000. Our observed rates were similar to those of other contemporary population-based studies where cases were confirmed by direct medical record review [6, 22, 23]. Overall VTE incidence was higher in males (134) than females (113; male:female ratio 1.19:1), and secondary VTE incidence (87) was over two-fold higher than idiopathic VTE incidence (35). Overall VTE incidence increased with increasing patient age for both sexes.
Despite identification of independent VTE risk factors, improved primary methods of prevention and widespread dissemination of evidence-based prophylaxis guidelines, the incidence of VTE did not change significantly over the study period. However, trends in incidence differed by VTE event type; annual leg DVT incidence increased by about 7% per decade while PE incidence decreased by 6% per decade. Of the increase in leg DVT incidence, most was due to isolated distal leg DVT; proximal leg DVT incidence decreased by 16% per decade while isolated distal leg DVT incidence increased by 45% per decade; the latter observation may reflect improved imaging sensitivity for distal leg DVT over time (e.g., whole leg venous duplex ultrasonography). Moreover, the clinical significance of isolated distal leg DVT is uncertain [24]. Given that PE is an independent predictor of reduced survival compared to DVT alone [25], one might predict that the number of deaths solely due to PE also decreased over this timeframe. However, the apparent decrease in PE incidence over time should be interpreted with caution as this observation could reflect a reduction in autopsy-discovered PE. While Olmsted County autopsy rates were much higher than the U.S. national average (ranging from ~35–40 and 15–30 per 100 deaths for men and women, respectively), older men and women were infrequently autopsied and the incidence of PE increases with increasing patient age for both sexes relative to DVT alone [13, 14, 26, 27].
Using hospital discharge diagnosis (ICD9-CM) codes from U.S. state [7] or national [28] administrative databases, previous studies reported a significant increase in contemporary PE incidence and suggested that PE was over-diagnosied and -treated [8] due to increased utlization of more sensitive imaging (i.e., identification of isolated subsegmental PE by CT pulmonary angiography) [7, 8, 29]. However, we found the incidence of isolated subsegmental PE, 2001–2010, to be only 5.6 (95% CI: 4.3, 7.0) per 100,000. Our and other’s [22] finding of a decrease and/or no change in contemporary PE incidence rates based on direct medical record review further illustrates the hazard of inferences based on indirect data such as administrative (claims) data [30–33].
There are several potential reasons for the persistent incidence of VTE over time, including: (a) exposure to new and as yet, unrecognized risk factors, (b) an increasing prevalence of known VTE risk factors, (c) incorrect or underutilization of effective prophylaxis, and (d) prophylaxis failure. Exposure to new, unrecognized risk factors would appear as an increase in the incidence of idiopathic VTE, and we did observe significant increases in idiopathic PE ± DVT and idiopathic proximal ± distal leg DVT incidence rates over time when obesity was not included in the definition of secondary VTE. We found a dramatic increase in the prevalence of obesity and severe obesity among both sexes of the Olmsted County population, 1988–2010, and obesity was significantly associated with the incidence of idiopathic VTE, accounting for about one-third of the increase in idiopathic VTE incidence (obesity PAR=0.33 for idiopathic VTE). These findings confirm and extend previous observations of a significant association of overweight and obese BMI with idiopathic VTE [34]. In contrast, the PAR due to above-normal BMI for secondary VTE was “−8%”, suggesting that forcing the population to return to normal BMI confers no benefit with respect to incident secondary VTE. This analysis indicates a large contrast in attributable risk due to BMI for idiopathic vs. secondary VTE.
Among other major VTE risk factors, the Olmsted County prevalence of active cancer increased, 1988–2010; active cancer was associated with a 3.3-fold increased odds of VTE and accounted for 11.3% of the VTE burden (PAR=0.11). The prevalence of surgery and leg paresis also increased, 1988–2010; surgery and leg paresis were associated with 3.2- and 2.2-fold increased odds of VTE and PARs of 21.5% and 4.0%, respectively. While recognizing that population attributable risk must be interpreted cautiously when all the risk factors are highly interrelated, as these are here, we noted that the PARs of active cancer and surgery increased, 1988–2010, indicating that active cancer and surgery accounted for part of the persistent VTE incidence. The Olmsted County prevalence of hospitalization, trauma/fracture, nursing home confinement, and, among women, number of pregnancies, decreased over time; the number of hospitalizations, 1988–2010, was relatively constant but the duration of hospitalization decreased. Nevertheless, being “in-hospital” and being within “92 days (i.e., 365 days ÷ 4, or about three months) after hospital discharge” were associated with 37- and 5.9-fold increased odds of VTE and PARs of 20.0% and 20.5%, respectively, indicating that hospitalization along with surgery also account for part of the persistent VTE incidence and suggesting that efforts to prevent VTE related to hospitalization and/or surgery may have been insufficient. The joint VTE PAR, including patient age, “in-hospital”, “92 days post hospital discharge”, surgery, active cancer, trauma/fracture, leg paresis and nursing home confinement (1995–2008) was 79%, indicating that the vast majority of VTE events are secondary and thus, potentially preventable (except for patient age).
In conclusion, the overall incidence of VTE over the 30-year period, 1981–2010, was relatively constant. VTE incidence continues to increase with age for both men and women, with a higher incidence among women during childbearing years. Most incident VTE events are secondary to known VTE risk factors and potentially preventable. Past and current guidelines recommend VTE prophylaxis solely for patients hospitalized for acute medical illness, acute trauma or surgery [35–37]. We found the PAR of surgery over this timeframe significantly increased; increasing prevalence of surgery may explain some of this, but it is possible that concurrent efforts to prevent VTE may have been insufficient. The persistent incidence of VTE also appears to be due, in part, to the increasing prevalence of obesity and active cancer. Better VTE risk assessment tools are needed that identify the individual at risk, especially the individual with active cancer and undergoing surgery.
Supplementary Material
What is known on this topic
Venous thromboembolism (VTE) is a common and potentially fatal disease that recurs frequently
Whether prophylaxis measures have reduced the occurrence of VTE is unknown
Reasons for any persistent occurrence of VTE also are unknown
What this paper adds
VTE incidence, 1981–2010, did not change significantly
Almost 80% of incident VTE events were attributable to known major VTE risk factors
One-third of incident idiopathic VTE events were attributable to obesity
The increasing obesity, cancer and surgery prevalence accounted, in part, for the persistent VTE incidence
Increasing surgery population-attributable risk suggests that concurrent efforts to prevent VTE may have been insufficient.
Acknowledgments
J.A. Heit conceived and designed the study, collected, analyzed and interpreted the data, and wrote the manuscript.
A.A. Ashrani participated in the study design, data collection, analyses and interpretation, and manuscript preparation.
D.J. Crusan performed the statistical analyses and participated in manuscript preparation.
R. D. McBane participated in the study design, data collection, analyses and interpretation, and manuscript preparation.
T.M. Petterson participated in the study design, data collection, analyses and interpretation, and manuscript preparation.
K.R. Bailey participated in the study design, data collection, analyses and interpretation, and manuscript preparation.
Research reported in this publication was supported by grants from the National Heart, Lung and Blood Institute under Award Number R01HL066216 to JAH, and the National Institute on Aging under Award Number R01AG034676, of the National Institutes of Health, and by Mayo Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures
Dr. Heit declares no conflict of interest.
Dr. Ashrani declares no conflict of interest.
Mr. Crusan declares no conflict of interest.
Ms. Petterson declares no conflict of interest.
Dr. Bailey declares no conflict of interest.
References
- 1.The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. U.S. Public Health Service 2008. U.S. Department of Health and Human Services. 2008 [Google Scholar]
- 2.Shojania K, Duncan B, McDonald K-SU, Wachter R. Making Health Care Safer: A Critical Analysis of Patient Safety Practices: UCSF-Stanford Evidence-Based Practice Center. Agency for Healthcare Research and Quality. 2001 Jul 20; Publication no. 01-E058, Evidence report no. 43. www.ahrq.gov/clinic/ptsafety/summary/htm; 2001. [PMC free article] [PubMed]
- 3.Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, Cook DJ, Balekian AA, Klein RC, Le H, Schulman S, Murad MH. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e195S–226S. doi: 10.1378/chest.11-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, Samama CM. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e227S–277S. doi: 10.1378/chest.11-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, Ortel TL, Pauker SG, Colwell CW., Jr Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e278S–325S. doi: 10.1378/chest.11-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrom J. Incidence and mortality of venous thrombosis: A population-based study. J Thromb Haemost. 2007;5:692–699. doi: 10.1111/j.1538-7836.2007.02450.x. [DOI] [PubMed] [Google Scholar]
- 7.DeMonaco NA, Dang Q, Kapoor WN, Ragni MV. Pulmonary embolism incidence is increasing with use of spiral computed tomography. Am J Med. 2008;121:611–617. doi: 10.1016/j.amjmed.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: Evidence of overdiagnosis. Archives of Internal Medicine. 2011;171:831–837. doi: 10.1001/archinternmed.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tagalakis V, Wharin C, Kahn SR. Comprehensive update on the prevention and treatment of venous thromboembolism in cancer patients. Seminars in Thrombosis and Hemostasis. 2013;39:127–140. doi: 10.1055/s-0032-1333537. [DOI] [PubMed] [Google Scholar]
- 10.Huang W, Goldberg RJ, Anderson FA, Kiefe CI, Spencer FA. Secular trends in occurrence of acute venous thromboembolism: The Worcester VTE Study (1985–2009) Am J Med. 2014;127:829–839. e825. doi: 10.1016/j.amjmed.2014.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melton L. History of the Rochester Epidemiology Project. Mayo Clinic Proceedings. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 12.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: The Rochester Epidemiology Project. American Journal of Epidemiology. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: An illustration from the Rochester Epidemiology Project. Mayo Clinic Proceedings. 2012;87:151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: A 25-year population-based study. Archives of Internal Medicine. 1998;158:585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 15.Heit JA, Melton LJ, 3rd, Lohse CM, Petterson TM, Silverstein MD, Mohr DN, O'Fallon WM. Incidence of venous thromboembolism in hospitalized patients vs community residents. Mayo Clinic Proceedings. 2001;76:1102–1110. doi: 10.4065/76.11.1102. [DOI] [PubMed] [Google Scholar]
- 16.Heit JA. Venous thromboembolism: Disease burden, outcomes and risk factors. J Thromb Haemost. 2005;3:1611–1617. doi: 10.1111/j.1538-7836.2005.01415.x. [DOI] [PubMed] [Google Scholar]
- 17.Kurland LT, Molgaard CA. The patient record in epidemiology. Scientific American. 1981;245:54–63. doi: 10.1038/scientificamerican1081-54. [DOI] [PubMed] [Google Scholar]
- 18.Ashrani AA, Gullerud RE, Petterson TM, Marks RS, Bailey KR, Heit JA. Risk factors for venous thromboembolism among active cancer patients: a population-based case-control study. Thromb Res. 2016;139:29–37. doi: 10.1016/j.thromres.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Archives of Internal Medicine. 2000;160:809–815. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 20.Cramér H. Mathematical methods of statistics. Princeton, NJ: Princeton University Press; 1946. p. 353. [Google Scholar]
- 21.Heit JA, Leibson CL, Ashrani AA, Petterson TM, Bailey KR, Melton LJ., 3rd Is diabetes mellitus an independent risk factor for venous thromboembolism?: A population-based case-control study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:1399–1405. doi: 10.1161/ATVBAHA.109.189290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spencer FA, Gore JM, Reed G, Lessard D, Pacifico L, Emery C, Crowther MA, Goldberg RJ. Venous thromboembolism and bleeding in a community setting. The Worcester Venous Thromboembolism Study. Thrombosis and Haemostasis. 2009;101:878–885. [PMC free article] [PubMed] [Google Scholar]
- 23.Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, Folsom AR. Deep vein thrombosis and pulmonary embolism in two cohorts: The Longitudinal Investigation of Thromboembolism Etiology. Am J Med. 2004;117:19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Palareti G. How I treat isolated distal deep vein thrombosis (IDDVT) Blood. 2014;123(12):1802–9. doi: 10.1182/blood-2013-10-512616. [DOI] [PubMed] [Google Scholar]
- 25.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd Predictors of survival after deep vein thrombosis and pulmonary embolism: A population-based, cohort study. Archives of Internal Medicine. 1999;159:445–453. doi: 10.1001/archinte.159.5.445. [DOI] [PubMed] [Google Scholar]
- 26.Ahronheim JC, Bernholc AS, Clark WD. Age trends in autopsy rates. Striking decline in late life. JAMA. 1983;250:1182–1186. [PubMed] [Google Scholar]
- 27.Hoyert DL. The changing profile of autopsied deaths in the United States, 1972–2007. NCHS Data Brief. 2011:1–8. [PubMed] [Google Scholar]
- 28.Stein PD, Matta F, Dalen JE. Is the campaign to prevent VTE in hospitalized patients working? Chest. 2011;139:1317–1321. doi: 10.1378/chest.10-1622. [DOI] [PubMed] [Google Scholar]
- 29.Burge AJ, Freeman KD, Klapper PJ, Haramati LB. Increased diagnosis of pulmonary embolism without a corresponding decline in mortality during the CT era. Clin Radiol. 2008;63:381–386. doi: 10.1016/j.crad.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Leibson CL, Needleman J, Buerhaus P, Heit JA, Melton LJ, 3rd, Naessens JM, Bailey KR, Petterson TM, Ransom JE, Harris MR. Identifying in-hospital venous thromboembolism (VTE): A comparison of claims-based approaches with the Rochester Epidemiology Project VTE cohort. Medical Care. 2008;46:127–132. doi: 10.1097/MLR.0b013e3181589b92. [DOI] [PubMed] [Google Scholar]
- 31.Sarrazin MS, Rosenthal GE. Finding pure and simple truths with administrative data. JAMA. 2012;307:1433–1435. doi: 10.1001/jama.2012.404. [DOI] [PubMed] [Google Scholar]
- 32.Haut ER, Pronovost PJ, Schneider EB. Limitations of administrative databases. JAMA. 2012;307:2589. doi: 10.1001/jama.2012.6626. author reply 2589–2590. [DOI] [PubMed] [Google Scholar]
- 33.Ashrani AA, Heit JA. Caution on interpreting the time trends in pulmonary embolism as "overdiagnosis". Archives of Internal Medicine. 2011;171:1962. doi: 10.1001/archinternmed.2011.549. author reply 1962–1963. [DOI] [PubMed] [Google Scholar]
- 34.Delluc A, Mottier D, Le Gal G, Oger E, Lacut K. Underweight is associated with a reduced risk of venous thromboembolism. Results from the EDITH case-control study. J Thromb Haemost. 2009;7:728–729. doi: 10.1111/j.1538-7836.2009.03280.x. [DOI] [PubMed] [Google Scholar]
- 35.Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, Cook DJ, Balekian AA, Klein RC, Le H, Schulman S, Hassan Murad M. Prevention of VTE in nonsurgical patients. Chest. 2012;141(2 suppl):e195S–e226S. doi: 10.1378/chest.11-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, Samama CM. Prevention of VTE in nonorthopedic surgical patients. Chest. 2012;141(2 suppl):e227S–e277S. doi: 10.1378/chest.11-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, Ortel TL, Pauker SG, Colwell CW., Jr Prevention of VTE in orthopedic surgery patients. Chest. 2012;141(2 suppl):e278S–e325S. doi: 10.1378/chest.11-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.