Abstract
Purpose
The benefits of annual surveillance mammography in older breast cancer survivors with limited life expectancy are not known, and there are important risks; however, little is known about mammography use among these women.
Materials and Methods
We used National Health Interview Study data from 2000, 2005, 2008, 2010, 2013, and 2015 to examine surveillance mammography use among women age ≥ 65 years who reported a history of breast cancer. Using multivariable logistic regression, we assessed the probability of mammography within the last 12 months by 5- and 10-year life expectancy (using the validated Schonberg index), adjusting for survey year, region, age, marital status, insurance, educational attainment, and indicators of access to care.
Results
Of 1,040 respondents, 33.7% were age ≥ 80 years and 88.6% were white. Approximately 8.6% and 35.1% had an estimated life expectancy of ≤ 5 and ≤ 10 years, respectively. Overall, 78.9% reported having routine surveillance mammography in the last 12 months. Receipt of mammography decreased with decreasing life expectancy (P < .001), although 56.7% and 65.9% of those with estimated ≤ 5-year and ≤ 10-year life expectancy, respectively, reported mammography in the last year. Conversely, 14.1% of those with life expectancy > 10 years did not report mammography. In adjusted analyses, lower (v higher) life expectancy was significantly associated with lower odds of mammography (odds ratio, 0.4; 95% CI, 0.3 to 0.8 for ≤ 5-year life expectancy and OR, 0.4; 95% CI, 0.3 to 0.6 for ≤ 10-year life expectancy).
Conclusion
Many (57%) older breast cancer survivors with an estimated short life expectancy (< 5 years) receive annual surveillance mammography despite unknown benefits, whereas 14% with estimated life expectancy > 10 years did not report mammography. Practice guidelines are needed to optimize and tailor follow-up care for older patients.
INTRODUCTION
The US population is aging, and the number of older women who will develop breast cancer is expected to increase by 50% between 2010 and 2030.1 Currently, approximately 70,000 US women age ≥ 70 years are diagnosed with breast cancer annually,2 many living years beyond their cancer diagnoses and most dying from non–breast cancer causes.3,4
Recommendations for screening mammography in the United States have evolved in recent years, with increasing acknowledgment of the limitations in applying uniform screening guidelines to all women.5,6 For average-risk women without a history of breast cancer, the American Cancer Society currently recommends cessation of screening mammography for women with < 10-year life expectancy.6,7 Despite these guidelines, discussions surrounding cessation of screening mammography are challenging and happen infrequently.8-11 Likely as a result, screening mammography rates for women have remained stable over time, regardless of age,12,13 even among those with limited life expectancy. In 2010, 36% of women with < 5-year life expectancy reported having had screening mammography within the last 2 years.14,15
With regard to surveillance mammography, recently updated guidelines from the Older Adult Oncology National Comprehensive Cancer Network state that mammography decisions in older breast cancer survivors should be primarily based on patient preference and life expectancy, with likely no benefit to screening women with ≤ 5-year life expectancy.16 In contrast, and as a result of a lack of prospective data to guide strategies, Breast Cancer Survivorship guidelines from the American Cancer Society and ASCO currently recommend annual mammography for all breast cancer survivors who have residual breast tissue, regardless of age or life expectancy.17,18 There are no studies specifically examining the benefits of surveillance mammography in older breast cancer survivors, let alone those with limited life expectancy, and the use of mammography in this growing population of patients has been questioned.14,19-21
Among women without a history of breast cancer, it is estimated to take > 10 years before one breast cancer death is prevented among 1,000 women age 50 to 74 years mammographically screened.22 Although the lag time to benefiting from mammography screening among breast cancer survivors is not known, it is likely similar. However, there are risks of screening that occur immediately, including anxiety and complications related to the evaluation of false-positive tests and overdiagnosis (detection of tumors that are of no threat).6,14,23-27 Therefore, older women with limited life expectancies who undergo surveillance mammography are unlikely to experience benefit and may instead place themselves at risk for harm.
Given the lack of data to clearly guide surveillance mammography in older breast cancer survivors, it is not surprising that mammography use in older breast cancer survivors is highly variable.28,29 In a study of older women with stage I or II breast cancer during 1992 to 1999 who were insured by Medicare, 77.6% underwent mammography during months 7 to 18 after diagnosis, and 56.7% had mammography annually over the 3 years after diagnosis. Lower use of mammography was observed with increasing age, black race, more comorbidity, unmarried status, and certain geographic regions, whereas those with more frequent oncology provider visits had more imaging.28 High rates of surveillance mammography in the first year after treatment of in situ breast cancers (91.3%) with lower rates over time were also observed in a Medicare population (age ≥ 65 years) during 1992 to 2005, with highest rates among those with more provider visits.29 However, the use of surveillance mammography by life expectancy has not been previously described.
To better understand use of surveillance mammography among older women with varying life expectancy, we used National Health Interview Survey (NHIS) data from 2000 to 2015 to examine the proportion of older breast cancer survivors having routine surveillance mammography in the past year by 5- and 10-year life expectancy.15,30-32
MATERIALS AND METHODS
Data Source
The NHIS is a large, nationally representative, in-person household interview survey of noninstitutionalized US civilians conducted annually by the Census Bureau for the National Center for Health Statistics.33 NHIS collects information on participants’ demographics, health history, and medical services used. The NHIS sampling design uses stratification, clustering, and oversampling of specific subgroups.34 Within households, one adult per family is randomly selected to complete questionnaires. We used NHIS data on sampled adults from 2000, 2005, 2008, 2010, 2013, and 2015 because of the availability of the Person, Sample Adult, and/or Sample Adult Cancer files during these years, which contain information on cancer history, mammography use, and other health information relevant to this analysis. The final sample adult response rates (interviewed sample adults/eligible sample adults from interviewed families × final family response rate) by year were 72.1% (2000), 69.0% (2005), 62.6% (2008), 60.8% (2010), 61.2% (2013), and 55.2% (2015).33 Proxy respondents are not allowed in the adult sample except in extreme cases (< 1% annually) wherein the sampled adults are mentally or physically unable to respond for themselves. We excluded these cases and included only self-responders. The study was deemed exempt from review by the Office for Human Research Studies at Dana-Farber Cancer Institute (Boston, MA).
Participants
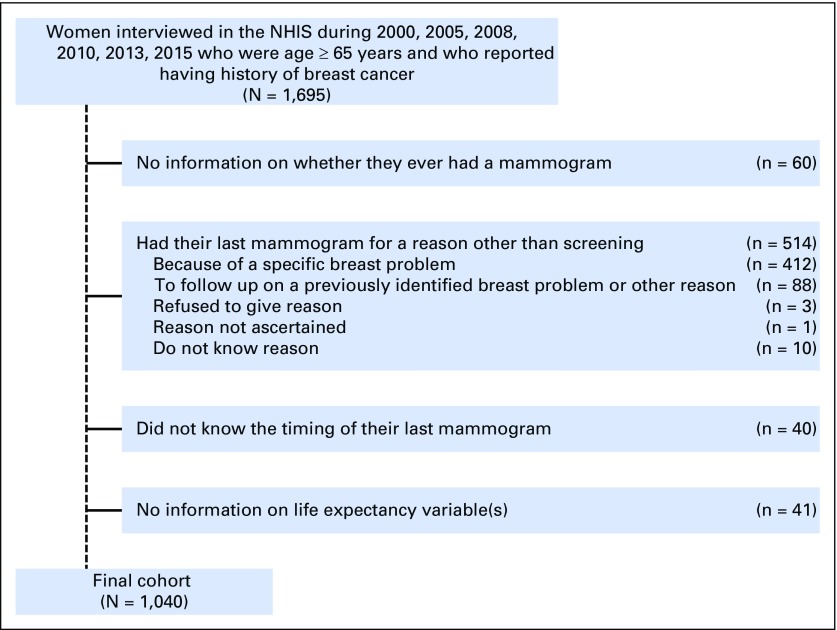
Among 180,969 respondents to the sample adult questionnaire during 2000, 2005, 2008, 2010, 2013, and 2015, we identified 1,695 women who were age ≥ 65 years and who reported having a history of breast cancer (defined reporting a history of breast cancer, having a cancerous lump removed from the breast, or by answering yes to the question: “as a result of these additional tests after your mammogram[s], were you diagnosed with cancer?”). We excluded women who refused to answer, had missing information, or said “I don’t know” to whether they had a mammogram (n = 60) and 514 women who stated they had a mammogram in the last year for reasons other than for screening (“because of a problem” [n = 412], “other reason” or “to follow up on a previously identified breast problem” [n = 88], “refused” [n = 3], “not ascertained” [n = 1], or “don’t know” [n = 10]). Last, we excluded 40 women who did not report a clearly defined time interval since their last mammogram and 41 women who had missing/unknown information for any variable required to calculate life expectancy. The final analytic cohort included 1,040 women (Fig 1).
Fig 1.
Cohort inclusions/exclusions. NHIS, National Health Interview Survey.
Outcome of Interest
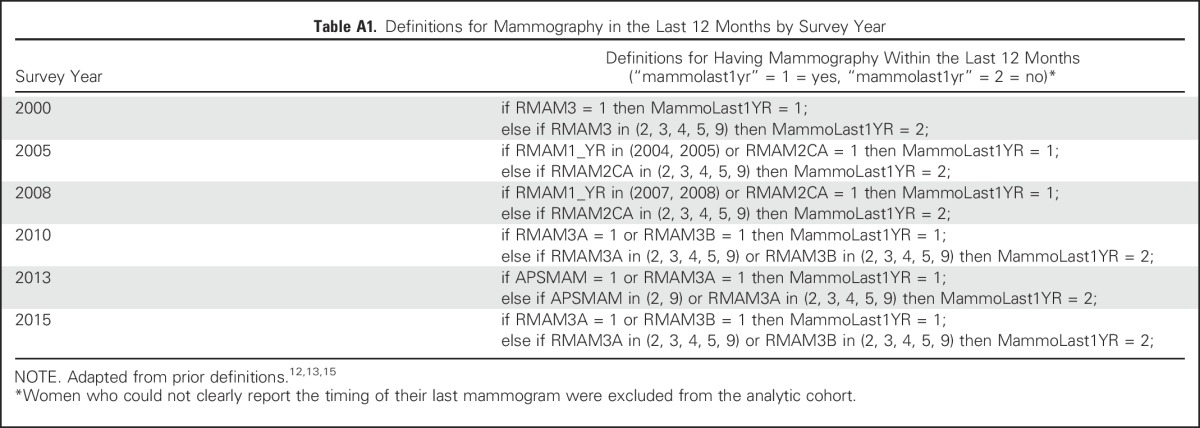
Our primary outcome of interest was self-reported receipt of mammography within the last 12 months. The variables used to construct this outcome by year are shown in Appendix Table A1 (online only). We adapted prior definitions of mammography use within the NHIS.12,13,15
Independent Variable of Interest
For each woman, we estimated 5- and 10-year life expectancy using the validated Schonberg index.15,30-32 This index was developed and validated using NHIS data and includes 11 risk factors independently and significantly associated with mortality, including age, sex, cigarette use, body mass index, functional limitations, difficulty with mobility, the number of hospitalizations in the past year, perceived health, and history of emphysema, diabetes, and cancer (excluding nonmelanoma skin cancers). In addition, because our study focused exclusively on women with breast cancer, we were interested in a woman’s non–breast cancer–related mortality risk, and thus in our primary analyses we did not include history of breast cancer in our measure for history of cancer. However, in sensitivity analyses, we repeated analyses including history of breast cancer in our measure for history of cancer. On the basis of the presence or absence of these 11 risk factors, we calculated a life expectancy score for each respondent. Because life expectancy is the average survival of a population, we considered women with > 50% 5-year mortality risk (defined as ≥ 15 points) to have a life expectancy ≤ 5 years, and we considered women with > 50% 10-year mortality risk (≥ 10 points) to have a life expectancy ≤ 10 years.15,30-32
Covariates
We considered the following factors that have previously been shown to be associated with mammography use among older women, including age, race/ethnicity, educational attainment, geographical region, marital status, insurance, number of provider visits in the last year, and the usual source of care in the last 12 months (ie, specialists or primary care clinicians).14,15 We also included survey year in analyses, categorizing this into three intervals because of the relatively smaller sample sizes in some years (2000 and 2005, 2008 and 2010, 2013 and 2015). All variables were categorized as presented in Table 1.
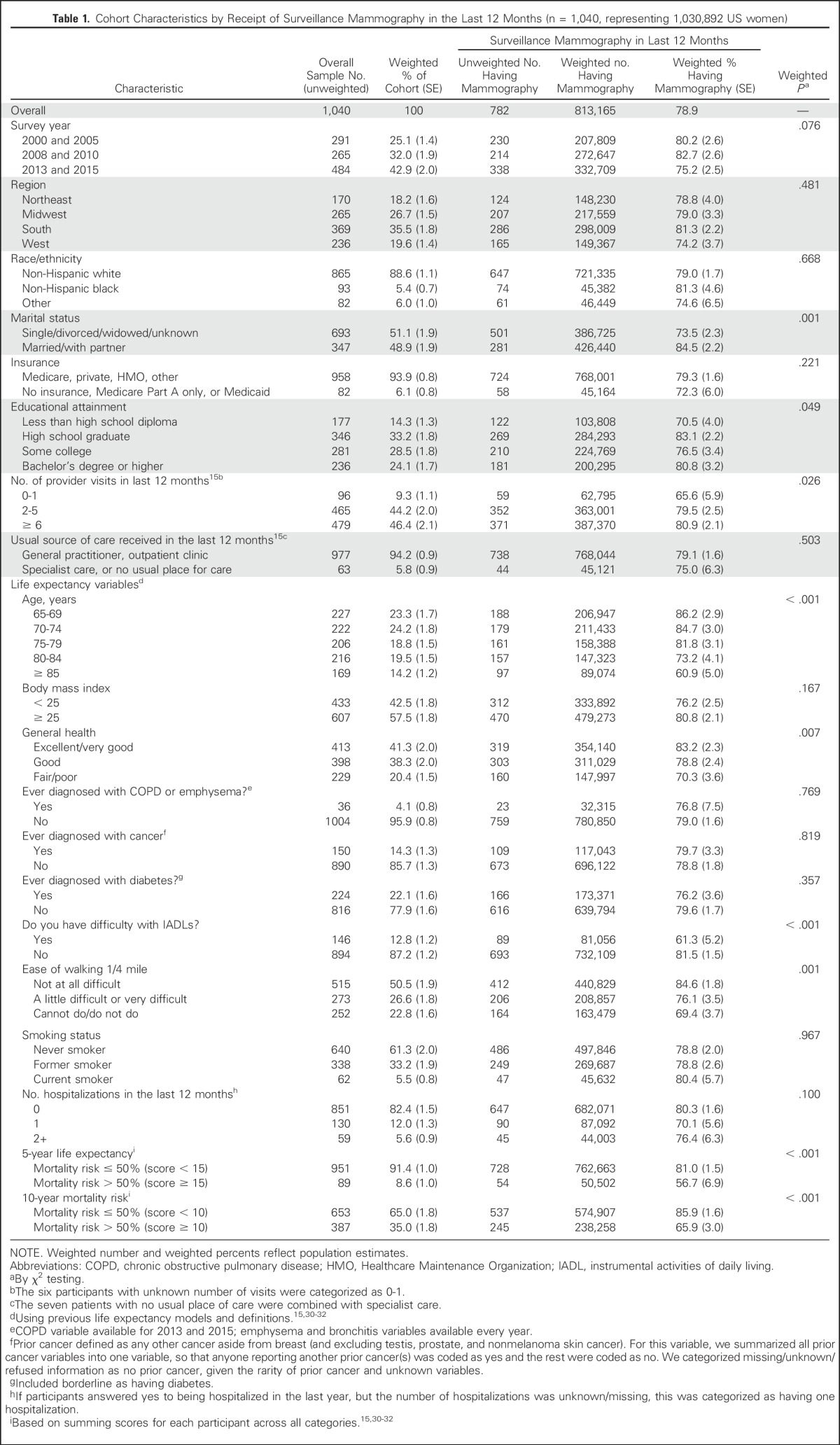
Table 1.
Cohort Characteristics by Receipt of Surveillance Mammography in the Last 12 Months (n = 1,040, representing 1,030,892 US women)
Statistical Analysis
We compared mammography receipt within the last 12 months for all women by each patient characteristic, including estimated life expectancy and each of its contributing factors, using χ2 tests. We then performed multivariable logistic regression to estimate the probability of having mammography in the last 12 months by 5- and 10-year life expectancy, adjusting for the covariates listed above. In sensitivity analyses, we first repeated models after categorizing all women in the cohort as having a prior cancer. To account for any issues in ascertainment of reasons for mammography (eg, if a woman interpreted having a routine mammogram after breast cancer as having testing to address a breast problem), we also repeated the models after including the 490 women excluded because they provided reasons other than screening for their mammography and who met other eligibility criteria (ie, 24 of 513 women were still excluded in sensitivity analyses for having incomplete mortality index variables). Analyses were performed using SAS survey procedures version 9.4 (SAS Institute, Cary, NC) to account for the complex sampling design, and data were weighted to reflect national estimates.34
RESULTS
The cohort characteristics for the 1,040 women with a history of breast cancer are shown in Table 1. Most women were white (88.6%) and at least high school graduates (85.8%), and many were actively engaged in health care, with 46.4% reporting having seen a health care provider six or more times over the last year. With regard to the variables contributing to life expectancy calculations, 33.7% were age ≥ 80 years, 41.3% reported excellent/very good health, and 82.4% had not been hospitalized in the last year. Few reported having chronic lung disease, diabetes, or prior non-breast cancer. About half (50.5%) of women reported no difficulty walking one fourth of a mile. After summing life expectancy scores, 8.6% and 35.0% had life expectancies of ≤ 5 years and ≤ 10 years, respectively.
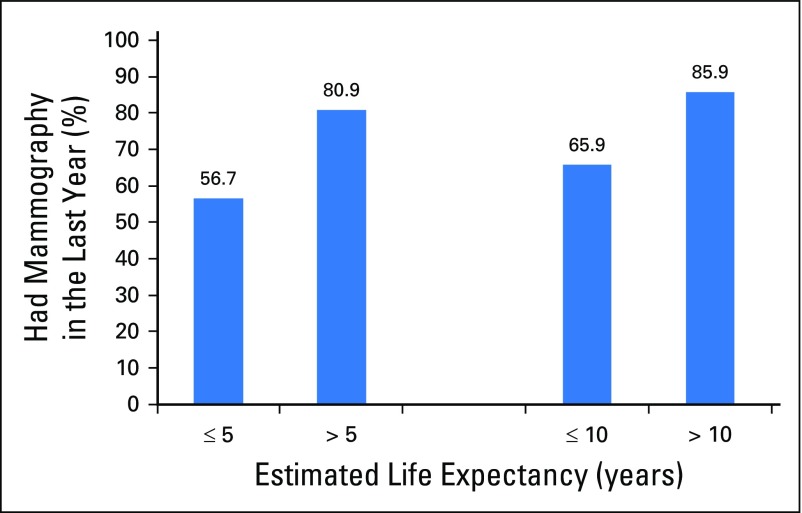
Overall, 78.9% of women reported having surveillance mammography in the last 12 months (Table 1). Married status, having more provider visits, increasing age, reporting excellent/very good health, and reporting no difficulty with instrumental activities of daily living and no difficulty with walking one fourth of a mile were all significantly associated with higher mammography use (all P < .05). More than half (56.7%) of women with ≤ 5-year life expectancy and 65.9% of women with ≤ 10-year life expectancy had surveillance mammography in the past year. Conversely, 14.1% of women with > 10-year life expectancy did not report recent surveillance mammography (Fig 2).
Fig 2.
Proportion of women who received surveillance mammogram in the last year by life expectancy (n = 1,040, representing 1,030,892 US women). P < .001 for both comparisons of surveillance mammography by life expectancy using the χ2 test.
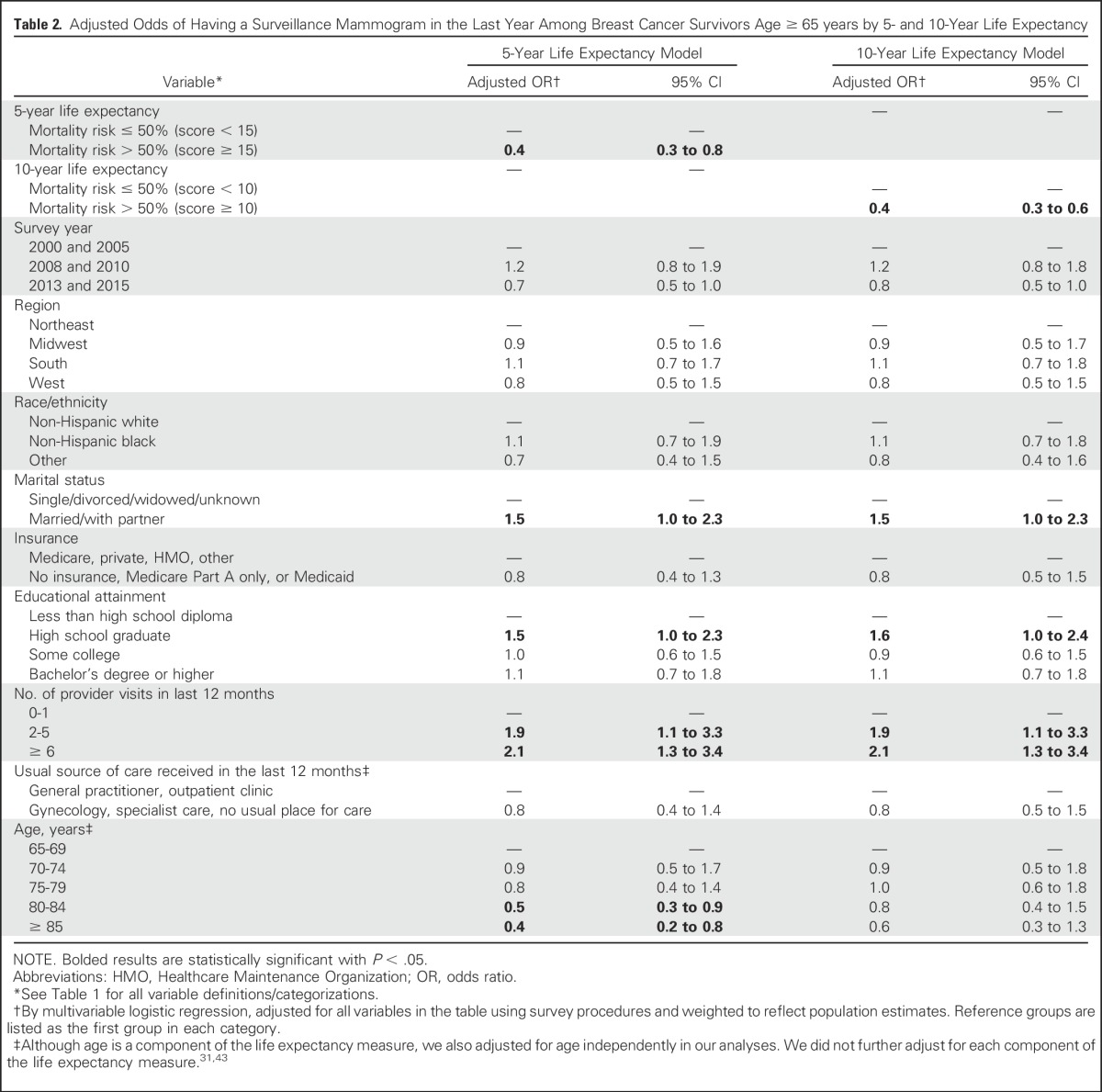
In adjusted analyses (Table 2), having a lower estimated life expectancy remained significantly associated with lower odds of surveillance mammography in the last 12 months (adjusted odds ratio [OR], 0.4; 95% CI, 0.3 to 0.8 for those with ≤ 5-year life expectancy and OR, 0.4; 95% CI, 0.3 to 0.6 for those with ≤ 10-year life expectancy [both v higher estimated life expectancy]). Other factors associated with higher odds of surveillance mammography included being married/partnered (v single), having a high school diploma (v not), and having more provider visits. Increasing age was associated with lower odds of having mammography but was significant in models examining 5-year life expectancy only. In sensitivity analyses done after recalculation of mortality scores, even more women with limited life expectancy (60.8% and 70.0% of women with ≤ 5-year and ≤ 10-year life expectancy) had mammography. After including women having mammography regardless of reasoning, 56.1% and 66.3% with ≤ 5-year and ≤ 10-year life expectancy had mammography. Results from adjusted models were similar to primary analyses (data not shown).
Table 2.
Adjusted Odds of Having a Surveillance Mammogram in the Last Year Among Breast Cancer Survivors Age ≥ 65 years by 5- and 10-Year Life Expectancy
DISCUSSION
In this population-based analysis of older breast cancer survivors, we found that surveillance mammography use decreased with advancing age and declining life expectancy, with nearly 80% of women having surveillance mammography in the last year. However, 56.7% of women with ≤ 5-year life expectancy (who are unlikely to benefit from mammography) and 65.9% of those with ≤ 10-year life expectancy (who likely have little chance of benefit) reported having a mammogram in the past year. Meanwhile, 14.1% of older breast cancer survivors with > 10-year life expectancy did not have surveillance mammography, even though they are likely to live long enough to benefit from testing. Our findings suggest the need to improve the tailoring of recommendations for surveillance mammography among older women with a history of breast cancer, especially for those with limited life expectancy. Moreover, strategies are needed to inform breast cancer survivors with a short life expectancy when they may stop being screened without a detrimental effect on breast cancer–related mortality.
Previous studies have also reported high use of mammography in older breast cancer survivors.28 Use of surveillance mammography may be high among older women with limited life expectancy because guidelines have not provided consistent strategies for cessation of surveillance mammography16-18 (and in particular the National Comprehensive Cancer Network Older Adult Guidelines16 did not provide input on surveillance until 2016). Also, women with a history of breast cancer (and their providers) often find annual mammograms reassuring because of concerns for an increased risk for in-breast events or increased anxiety related to past diagnoses. Furthermore, providers may not extrapolate the uncertainties of benefit for mammographic screening in older women to older breast cancer survivors, leading to indefinite, annual mammography without dialogue in many cases. However, many clinically important breast cancers will present by physical examination alone,19 and it remains unclear whether the addition of mammography over physical examination alone among the oldest and frailest women meaningfully improves outcomes.
Although we acknowledge the lack of prospective data to guide these discussions, the benefits of routine surveillance breast imaging have recently been called into question for those with limited life expectancy as well as those with a history of lower-risk, hormone receptor–positive breast cancers who are taking hormonal therapy (whose long-term risk for bilateral in-breast events is likely ≤ 10%).20 In addition, some data suggest that the risk for in-breast and contralateral breast events decreases with increasing age,35-38 likely further lowering the utility of mammography in many women. Moreover, there are potential and immediate harms of mammography in an aging population that must be considered, including false positives, unnecessary biopsies, and, perhaps most importantly, overdiagnosis, all of which have been well documented in screening populations and may be even more likely in breast cancer survivors because of the lower threshold to evaluate indeterminate/new findings on mammogram for these patients.5,23-27,39-41
To truly individualize follow-up care appropriately for breast cancer survivors, better evidence is needed about the benefits and risks of mammography in older survivors with varying mortality risk to develop consensus and uniform practice with close collaboration between specialists and primary care providers. This will require larger, prospective evaluations of how having or not having mammography impacts breast cancer outcomes and quality of life in this setting. In the meantime, clinicians should make a concerted effort to discuss and personalize the pros and cons of surveillance mammography, focusing on the importance of continued ongoing follow-up, breast awareness and physical examinations, a patient’s individualized health priorities, and a promotion of a healthy lifestyle, even if mammography is stopped (or continued at a reduced frequency). We recognize that conversations on cessation of mammography with breast cancer survivors may be particularly challenging because of their personal experiences with cancer. However, if women understand their individualized risks and benefits of testing in this setting, they will at least have the opportunity to make informed decisions rather than have the false security that routine mammograms may indefinitely improve their longevity.
To our knowledge, this large, population-based analysis is the first to examine the use of surveillance mammography by life expectancy and provides important information on current patterns of care and opportunities for improvement. However, we acknowledge several limitations. First, because the timing and use of mammography for routine purposes was ascertained by self-report, it is possible that women did not accurately recall this information, although we found similar results when examining mammography use regardless of reason. Second, we lacked information on patient preferences or conversations with providers about mammography. Third, some patient subgroups were small, limiting generalizability to all survivors, such as nonwhite women and those who were underinsured. Fourth, the measures for life expectancy were validated in the general community-dwelling population and have not been specifically validated in women with a history of breast cancer, where the accuracy of general mortality risk measures have been questioned,42 although we examined life expectancy with and without inclusion of breast cancer in calculations with similar findings. Fifth, we did not have information on mammography use before the most recent mammogram. Sixth, we lacked information on the timing of breast cancer diagnosis in relation to when mammography occurred, tumor characteristics (including stage), the risk for in-breast recurrences, and use of mammography in the setting of metastatic breast cancer. However, the time since diagnosis should not influence the duration of mammography use, as steady rates of in-breast events occur over time after a diagnosis, without a clear plateau.35 Finally, the NHIS interviews community-dwelling adults who agree to a lengthy interview, perhaps skewing toward a more engaged and healthier population of cancer survivors. However, our sample included adequate numbers of women with short life expectancy to examine mammography in these women. Furthermore, our results are consistent with others28,29 that have shown a higher likelihood of mammography with more frequent provider visits, regardless of health status.
In conclusion, we observed high use of surveillance mammography in women with limited life expectancy among a national sample of > 1,000 older breast cancer survivors. Our findings highlight the urgent need for more data on the risks and benefits of mammography surveillance among older women with limited life expectancy so we can better inform patients. Future studies should focus on developing strategies on how best to engage older women with a history of breast cancer in shared decision making and how to best tailor surveillance mammography. This will allow for evidence-based guidelines on use of surveillance mammography in older breast cancer survivors that emphasize who is unlikely to derive benefit from mammography and who is more likely to experience potential harm. Such guidelines can help oncologists and primary care providers engage patients in decision making and help focus our interventions on those that may better promote longevity and well-being.
ACKNOWLEDGMENT
We thank the Centers for Disease Control and Prevention for conducting these surveys and for maintaining data for the National Health Interview Survey. We also thank all survey participants for offering their valuable time and personal/health-related information.
Appendix
Table A1.
Definitions for Mammography in the Last 12 Months by Survey Year
Footnotes
Supported by American Cancer Society Grants No. 125912-MRSG-14-240-01-CPPB (R.A.F.) and RSGT 10-080-01-CPHPS (E.P.M.); Susan G. Komen Grant No. CCR14298143 (R.A.F.); National Cancer Institute Grants No. K24CA181510 (N.L.K.) and R01 CA181357 (M.A.S.); and National Institute on Aging Grant No. R01 AG041869 (M.A.S.).
AUTHOR CONTRIBUTIONS
Conception and design: Rachel A. Freedman, Nancy L. Keating, Lydia E. Pace, Mara A. Schonberg
Financial support: Rachel A. Freedman
Administrative support: Rachel A. Freedman, Mara A. Schonberg
Collection and assembly of data: Rachel A. Freedman, Joyce Lii, Ellen P. McCarthy
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Use of Surveillance Mammography Among Older Breast Cancer Survivors by Life Expectancy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Rachel A. Freedman
Research Funding: Puma Biotechnology (Inst), Genentech (Inst), Eisai (Inst)
Nancy L. Keating
No relationship to disclose
Lydia E. Pace
No relationship to disclose
Joyce Lii
No relationship to disclose
Ellen P. McCarthy
No relationship to disclose
Mara A. Schonberg
No relationship to disclose
REFERENCES
- 1.Smith BD, Smith GL, Hurria A, et al. : Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol 27:2758-2765, 2009 [DOI] [PubMed] [Google Scholar]
- 2. American Cancer Society: Breast Cancer Facts and Figures 2015-2016. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-046381.pdf.
- 3.Schonberg MA, Marcantonio ER, Ngo L, et al. : Causes of death and relative survival of older women after a breast cancer diagnosis. J Clin Oncol 29:1570-1577, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yancik R, Wesley MN, Ries LA, et al. : Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA 285:885-892, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Walter LC, Schonberg MA: Screening mammography in older women: A review. JAMA 311:1336-1347, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schonberg MA: Decision-making regarding mammography screening for older women. J Am Geriatr Soc 64:2413-2418, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oeffinger KC, Fontham ET, Etzioni R, et al. : Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 314:1599-1614, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schonberg MA, Ramanan RA, McCarthy EP, et al. : Decision making and counseling around mammography screening for women aged 80 or older. J Gen Intern Med 21:979-985, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torke AM, Schwartz PH, Holtz LR, et al. : Older adults and forgoing cancer screening: “I think it would be strange”. JAMA Intern Med 173:526-531, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman RM, Elmore JG, Pignone MP, et al. : Knowledge and values for cancer screening decisions: Results from a national survey. Patient Educ Couns 99:624-630, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Fairfield KM, Gerstein BS, Levin CA, et al. : Decisions about medication use and cancer screening across age groups in the United States. Patient Educ Couns 98:338-343, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Pace LE, He Y, Keating NL: Trends in mammography screening rates after publication of the 2009 US Preventive Services Task Force recommendations. Cancer 119:2518-2523, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Breen N, Gentleman JF, Schiller JS: Update on mammography trends: Comparisons of rates in 2000, 2005, and 2008. Cancer 117:2209-2218, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schonberg MA, Silliman RA, Marcantonio ER: Weighing the benefits and burdens of mammography screening among women age 80 years or older. J Clin Oncol 27:1774-1780, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schonberg MA, Breslau ES, McCarthy EP: Targeting of mammography screening according to life expectancy in women aged 75 and older. J Am Geriatr Soc 61:388-395, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Comprehensive Care Network (NCCN) Guidelines : Older Adult Oncology http://www.nccn.org/professionals/physician_gls/pdf/senior.pdf
- 17.Runowicz CD, Leach CR, Henry NL, et al. : American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. J Clin Oncol 34:611-635, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Runowicz CD, Leach CR, Henry NL, et al. : American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. CA Cancer J Clin 66:43-73, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Massimino KP, Jochelson MS, Burgan IE, et al. : How beneficial is follow-up mammography in elderly breast cancer survivors? Ann Surg Oncol 23:3518-3523, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freedman RA, Keating NL, Partridge AH, et al. : Surveillance mammography in older patients with breast cancer-can we ever stop?: A review. JAMA Oncol 3:402-409, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobsen PB, Rowland JH, Paskett ED, et al. : Identification of key gaps in cancer survivorship research: Findings from the American Society of Clinical Oncology survey. J Oncol Pract 12:190-193, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Lee SJ, Boscardin WJ, Stijacic-Cenzer I, et al. : Time lag to benefit after screening for breast and colorectal cancer: Meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ 346:e8441, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schonberg MA, Silliman RA, Ngo LH, et al. : Older women’s experience with a benign breast biopsy—A mixed methods study. J Gen Intern Med 29:1631-1640, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welch HG, Black WC: Overdiagnosis in cancer. J Natl Cancer Inst 102:605-613, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Welch HG, Prorok PC, O’Malley AJ, et al. : Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med 375:1438-1447, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Hubbard RA, Kerlikowske K, Flowers CI, et al. : Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: A cohort study. Ann Intern Med 155:481-492, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marmot MG, Altman DG, Cameron DA, et al. : The benefits and harms of breast cancer screening: An independent review. Br J Cancer 108:2205-2240, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keating NL, Landrum MB, Guadagnoli E, et al. : Factors related to underuse of surveillance mammography among breast cancer survivors. J Clin Oncol 24:85-94, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Brawarsky P, Neville BA, Fitzmaurice GM, et al. : Use of annual mammography among older women with ductal carcinoma in situ. J Gen Intern Med 27:500-505, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. doi: 10.1111/jgs.14805. Schonberg MA, Li V, Marcantonio ER, et al: Predicting mortality up to 14 years among community-dwelling adults aged 65 and older. J Am Geriatr Soc 65:1310-1315, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schonberg MA, Davis RB, McCarthy EP, et al. : Index to predict 5-year mortality of community-dwelling adults aged 65 and older using data from the National Health Interview Survey. J Gen Intern Med 24:1115-1122, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schonberg MA, Breslau ES, Hamel MB, et al. : Colon cancer screening in U.S. adults aged 65 and older according to life expectancy and age. J Am Geriatr Soc 63:750-756, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control : National Health Interview Survey. http://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
- 34.National Health Interview Survey: Variation Estimation Guidance, NHIS 2006-2015 (Adapted from the 2006-2015 NHIS Survey Description Documents). http://www.cdc.gov/nchs/data/nhis/2006var.pdf
- 35.Rasmussen CB, Kjær SK, Ejlertsen B, et al. : Incidence of metachronous contralateral breast cancer in Denmark 1978-2009. Int J Epidemiol 43:1855-1864, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Darby S, McGale P, et al. : Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378:1707-1716, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Thompson W, Semenciw R, et al. : Epidemiology of contralateral breast cancer. Cancer Epidemiol Biomarkers Prev 8:855-861, 1999 [PubMed] [Google Scholar]
- 38.Nichols HB, Berrington de González A, Lacey JV, Jr, et al. : Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol 29:1564-1569, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braithwaite D, Zhu W, Hubbard RA, et al. : Screening outcomes in older US women undergoing multiple mammograms in community practice: Does interval, age, or comorbidity score affect tumor characteristics or false positive rates? J Natl Cancer Inst 105:334-341, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harding C, Pompei F, Burmistrov D, et al. : Breast cancer screening, incidence, and mortality across US counties. JAMA Intern Med 175:1483-1489, 2015 [DOI] [PubMed] [Google Scholar]
- 41. Welch HG, Schwartz L, Woloshin S: Over-diagnosed: Making People Sick in the Pursuit of Health. Boston, MA, Beacon Press, 2012. [Google Scholar]
- 42.Kimmick GG, Major B, Clapp J, et al. : Using ePrognosis to estimate 2-year all-cause mortality in older women with breast cancer: Cancer and Leukemia Group B (CALGB) 49907 and 369901 (Alliance A151503). Breast Cancer Res Treat 163:391-398, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schonberg MA, Davis RB, McCarthy EP, et al. : External validation of an index to predict up to 9-year mortality of community-dwelling adults aged 65 and older. J Am Geriatr Soc 59:1444-1451, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]