Abstract
Aim
We designed a study to evaluate the cardioprotective effect of two soluble epoxide hydrolase (sEH) inhibitors, 1-(1-propanoylpiperidin-4-yl)-3-(4-trifluoromethoxy)phenyl)urea (TPPU) and trans-4-{4-[3-(4-trifluoromethoxyphenyl)-ureido]cyclohexyloxy}benzoic acid (t-TUCB), in ischemia-reperfusion (IR) model.
Methods
Cardioprotective effects of the sEH inhibitors were evaluated against IR-induced myocardial damage in hearts from normal, hypertensive and diabetic rats using Langendorff’s apparatus. In addition, the effect of sEH inhibitors on endothelial function was evaluated in vitro and ex vivo using isolated rat thoracic aorta.
Results
IR increased the myocardial damage in hearts from normal rats. IR-induced myocardial damage was augmented in hearts isolated from hypertensive and diabetic rats. Myocardial damage as evident from increase in the activities of lactate dehydrogenase (LDH) and creatine kinase-MB (CK-MB) in heart perfusate was associated with significant decrease in the heart rate and developed tension, and increase in the resting tension in isolated heart. Both sEH inhibitors protected the heart in normal, hypertensive and diabetic rats subjected to IR injury. The sEH inhibitor t-TUCB relaxed phenylephrine pre-contracted aorta from normal rats. Relaxant effect of acetylcholine (ACh) was reduced in aortas from diabetic and hypertensive rats compared to normal rats. Pre-treatment of sEH inhibitors to diabetic and hypertensive rats increased relaxant effect of ACh on aortas isolated from these rats.
Conclusions
Prophylactic treatment with sEH inhibitors decreased myocardial damage due to IR, hypertension and diabetes, and decreased endothelial dysfunction created by diabetes and hypertension. Therefore, inhibitors of sEH are useful probes to study cardiovascular pathology and inhibition of the sEH is a potential approach in the management of IR-induced cardiac damage and endothelial dysfunction related cardiovascular disorders.
Keywords: Ischemia/reperfusion-induced myocardial damage, sEH inhibitor TPPU and t-TUCB, endothelial dysfunction, Langendorff’s apparatus, lisinopril, metformin
Introduction
Cardiovascular disease is one of the most serious diseases throughout the world in terms of reported deaths and cost of hospitalization [1, 2]. Ischemic heart disease / coronary artery disease (IHD/CAD) is one of the major cardiovascular diseases and results from imbalance between the myocardial supply and demand for oxygenated blood. Myocardial damage such as myocardial infarction is not fully reversible and increases the pressure overload of the heart. The heart can manage limited pressure overload by contracting faster but will fail if pressure over load is not decreased by minimizing myocardial damage and augmenting supply of oxygenated blood [3, 4]. One of the causative factors for deleterious contraction of blood vessels is endothelial dysfunction which is commonly observed in patients with diabetes and hypertension [5–8]. Endothelial dysfunction is associated with the degree of oxidative stress [10, 11].
Endothelial dysfunction also plays a role in hypertension [12]. Despite numerous therapeutic approaches for cardiovascular diseases, endothelial dysfunction remains to be a problem. Recent reports indicate the potential utility of sEH inhibitors for cardiovascular diseases. These molecules relax blood vessels under some conditions [13] and decrease high blood pressure [14]. The sEH metabolizes the arachidonic acid derived epoxyeicosatrienoic acids (EETs) to less active dihydroxy metabolites. EETs seem to have homeostatic roles in cardiac and renal physiology in addition to being potently anti-inflammatory molecules. Therefore, inhibition of sEH exerts vasodilatory effect by minimizing the degradation of and stabilizing the levels of EETs.
In this study, we evaluated the cardioprotective activity of two structurally different sEH inhibitors, TPPU and t-TUCB ex vivo, using Langendorff’s apparatus where cardiac damage was induced by ischemia and reperfusion. In addition, we expanded to diabetic and hypertensive models to test the hypothesis that inhibition of sEH would be beneficial for cardiac damage induced by IR. These experiments were supported by in vitro assays on isolated blood vessels. Endothelial damage was elicited in thoracic aorta by inducing diabetes and hypertension in Wistar rats. The effect of sEH inhibition was evaluated on endothelial function ex vivo using acetylcholine (ACh) as a marker of endothelial function.
Material and methods
Chemicals and materials
Streptozotocin (Sigma-Aldrich Co., USA), CK-MB Kit (Span diagnostic, India), LDH kit (Span diagnostic, India) ketamine hydrochloride (Neon Labs, India), xylazine hydrochloride (Indian Immunologicals Ltd, India), heparin (Gland Pharma, India) were purchased. The sEH inhibitors were synthesized as previously reported [15, 16]. Other reagents were of analytical grade.
Animals
The animal experiment was conducted after obtaining the permission of Institutional Animal Ethics Committee and complied with the guideline set by Committee for the Purpose of Care and Supervision on Experimental Animals, India. Male Wistar rats weighing 200–250 g were used for this experiment. The animals were bred at the animal house of Al-Ameen College of Pharmacy and reared as per the standard laboratory conditions.
Induction of hypertension
Hypertension was induced by providing 10 % of fructose in drinking water orally for 2 months. The development of hypertension in rats was confirmed by measuring blood pressure using tail cuff apparatus. Data were recorded and saved in a data acquisition system (AD Instrument, Australia) [17]. TPPU and t-TUCB (0.1 and 0.3 mg/kg, p.o.) were used as experimental compounds, whereas lisinopril (15 mg/kg, p.o.) was used as a standard of care. The administration of compounds was initiated on the same day following confirmation of hypertension (day 1) and continued for 4 weeks. Administration of fructose solution was also continued to maintain hypertensive state of the animals.
Induction of diabetes
Type 1 diabetes was induced by injecting overnight fasted rats with a single dose of streptozotocin (dissolved in cold citrate buffer, pH 4.5, 52 mg/k.g., i.p.) [18, 19]. The development of hyperglycemia was monitored by quantification of fasting serum glucose after 96 h post STZ injection using glucose assay kit (Span Diagnostics Ltd., India) [20]. The animals were fasted again for 14 h before blood withdrawal from the retro orbital plexus. The rats with glucose levels of 250–350 mg/dl at 96 h after STZ injection were considered diabetic. Inhibitors of sEH were used as test drugs (0.1 and 0.3 mg/kg, p.o.), whereas metformin (500 mg/kg, p.o.) was used as a standard of care. The administration of compounds was initiated on the same day following confirmation of diabetes (day 1) and continued for 4 weeks.
Isolated heart in vitro and ex vivo study
For the in vitro study, rat was given heparin (100 units/rat, i.p.) prior to anesthesia. After 15 minutes, rat was anesthetized with ketamine hydrochloride (65 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.). Heart was isolated and washed in Krebs-Henseleit (K-H) solution saturated with carbogen gas (95% O2 and 5% CO2) to remove blood. The heart was then mounted on a cannula and perfused with K-H solution gassed with carbogen gas at 37 °C at a constant flow rate of 5 ml/min by means of peristaltic pump (Master Flex, USA) using a modified Langendorff’s setup. A fine thread was tied to the apex of the heart and passed through a pulley to the force transducer (MLT500, AD Instruments, Australia) connected to ADInstrument data acquisition system. Two-gram tension was applied to the heart. In the thermostatic chamber, the heart was allowed to equilibrate for 10 minutes. TPPU and t-TUCB were dissolved in PEG400 (30 mg/mL) and then diluted in distilled water to give a true solution. The effect of TPPU and t-TUCB (1 ng – 10 μg/100 μL) were observed on heart rate and developed tension after injecting the inhibitor solutions into the latex injection port of modified Langendorff’s apparatus under normal conditions of perfusion [21]. This latex injection port was present outside the thermostatic chamber, and just before cannula. Two different concentrations of the sEH inhibitors were injected with the intervals of 5 minutes.
For ex vivo studies, the heart was isolated from rat treated with vehicle, sEH inhibitors, metformin or lisinopril and normal function was recorded as detailed above. Global ischemia was induced by stopping the flow of K-H solution for 15 min followed by 30 minutes of reperfusion. Changes in heart beat/min, resting tension (gram) and developed tension (gram) were recorded and compared among the groups.
Estimation of cardiac damage
Activities of LDH and CK-MB in heart perfusate samples were determined for assessing cardiac damage or injury, upon reperfusion. Samples of the perfusate were collected from the isolated perfused heart at 0–5 minutes and 10–15 minutes of reperfusion in control as well as treatment groups and used for estimation of the enzyme activity. Mean LDH and CK-MB activities were reported.
The LDH activity was determined by monitoring the rate of reduced nicotinamide adenine dinucleotide (NADH) oxidation in presence of pyruvate [22, 23]. To 2.5 ml of phosphate buffer (100 mM), 1 ml of perfusate and 100 μL of NADH (2.5 mg/ mL of phosphate buffer) were added. The mixture was allowed to stand for 20 min. After transferring it into a cuvette, the absorbance was measured using a UV-Visible spectrophotometer (SHIMADZU, UV-16100, Japan) at 340 nm for 30 sec after the addition of 100 μL of sodium pyruvate (22.7 mM) solution, (the initial reading at zero time) and the rate of change in extinction was recorded every minute for 3 minutes.
Where ΔE 340/min = Average change in absorbance/min; 3.7 = Total reaction volume expressed in ml; 1000 = Conversion of units/ml to units/l; 1 = sample volume in ml; 6.3 = mM absorptivity of NADH.
CK-MB activity in perfusate was estimated by using CK-MB lab kit (Span Diagnostics, India) according to manufacturer instruction. Briefly, to 500 μL of working reagent solution, 25 μL of anti CK-MM reagent was added and the solution was mixed thoroughly. Fifty microliter of perfusate containing CK-MB was added and vortexed. The mixture was aspirated immediately into Semi-Auto Analyzer (ARTOS, India) at 37 °C and with 600 seconds delay time absorbance was measured at 340 nM every minute starting from 0 to 3 minute and change in absorbance was calculated [24].
Where, ΔE/340 nm = average change in absorbance at 340 nm.
In vitro and ex vivo blood vessel study
The thoracic aorta of anesthetized rat was isolated, washed in K-H solution maintained at 37 °C and then mounted between two steel hooks in a 4-channel organ bath containing 20 mL K-H solution. This chamber was continuously aerated with carbogen gas. The aorta was stretched to 2 g tension and washed with K-H solution 4 times within 1 hour. After stabilization of the aorta, different concentrations (1 ng/ml- 100 μg/mL, final concentration) of sEH inhibitors were added to organ bath and possible contractile effect of sEH inhibitors were evaluated.
For evaluating possible relaxant effect, first the aorta was contracted with sub-maximal dose of adrenergic α1 receptor agonist, phenylephrine, and then lisinopril, metformin, TPPU or t-TUCB were added separately at increasing concentrations and relaxant effects were monitored.
To evaluate the effect of sEH inhibitors in diabetes-induced endothelial dysfunction, the relaxant effect of ACh on the aorta from diabetic rat treated with vehicle, metformin or sEH inhibitors was recorded and compared with that from normal rat. Similarly, to evaluate the effect of sEH inhibitors in hypertension-induced endothelial dysfunction, the relaxant effect of ACh on aorta of hypertensive rat treated with vehicle, lisinopril or sEH inhibitors was recorded and compared with that of aorta from normal rat [20].
For the in vitro and ex vivo aorta studies, a series of concentrations of the sEH inhibitors and ACh were prepared and 1μL-100 μL volumes were added before each measurement with a time interval of 5 minutes. The total volume of added solution never exceeded 1% volume of K-H solution in organ bath.
Statistical analysis
Data are expressed as mean ± SEM. The results related to the heart study were subjected to a one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison post hoc test. The results of the aorta study were subjected to two-way ANOVA followed by Bonferroni’s post-hoc test for statistical evaluation. GraphPad Prism (version: 5.03) statistical program was used for statistical calculation and generation of the graphs.
Results
Rats which received STZ displayed expected increase in the level of glucose in blood. Normal rats had 110 ± 10 mg/dL blood glucose, whereas diabetic rats had 280 ± 30 mg/dL. Systolic blood pressure of rats treated with fructose solution (140 ± 5 mmHg) were higher (p < 0.05) compared to that of rats treated with water and vehicle (118 ± 2 mmHg). The inhibitors of sEH did not affect cardiac function of normal rat in vitro but restored altered cardiac function in IR model. Significant endothelial dysfunction was observed (p < 0.05) in the aortas obtained from these hypertensive and diabetic rats in comparison to untreated disease control rats. Treatment with sEH inhibitors significantly (p < 0.05) reduced endothelial dysfunction.
Treatment with sEH inhibitors minimize decrease in heart rate
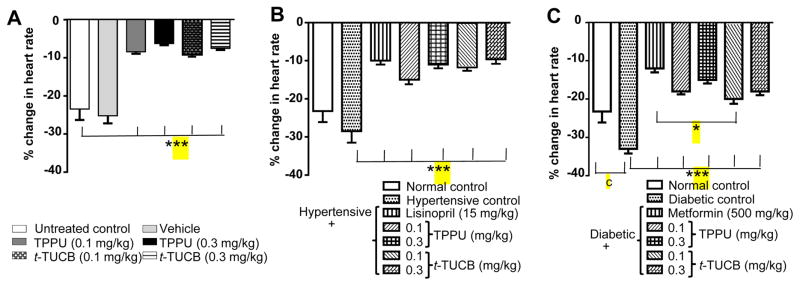
A hallmark of IR injury in both rodents and men is a decrease in heart rate due to reduced contractility. Ischemia-reperfusion injury expectedly led to a significant decrease in mean heart rate (244/min, without IR vs 198/min, after IR; p < 0.05) in normal rats. Vehicle (PEG400 in water) did not have any effect on heart rate. In parallel, hypertensive rats displayed significantly (p < 0.05) lower mean heart rate both pre- (244/min vs 216/min, p< 0.05) and post-IR (198/min vs 168/min). Similarly, diabetic rats displayed significantly decreased (p < 0.05) pre-IR (244/min vs 192/min, p< 0.05) and post-IR (198/min vs 144/min, p< 0.05) mean heart rate. Overall, both TPPU and t-TUCB significantly (p < 0.001) reduced the IR driven decrease in heart rate in the isolated hearts from normal, hypertensive and diabetic rats (Figure 1). These data suggest inhibition of sEH promotes the maintenance of normal heart rate.
Figure 1.
Treatment with sEH inhibitors reduces IR-induced decrease in the heart rate. (A) Treatment with vehicle did not change heart rate after IR injury, whereas treatment with sEH inhibitors caused a significant decrease (*** p < 0.001) in heart rate in comparison to normal control rats treated with water. (B) Hypertension did not decrease heart rate significantly after IR. Treatment with lisinopril and both doses of sEH inhibitors significantly reduced (*** p < 0.001) the hypertension driven decrease in heart rate in comparison to untreated hypertensive rats. (C) Diabetes significantly decreased heart rate (c p < 0.001) after IR in comparison to normal rats treated with water. Treatment with metformin and both doses of sEH inhibitors significantly reduced (*** p < 0.001) the diabetes driven decrease in heart rate in comparison to untreated diabetic rats. Metformin was more effective (* p < 0.05) than t-TUCB (0.1 mg/kg). Data are presented as mean ± SEM of 6 observations. One-way ANOVA followed by Tukey’s multiple comparison test was used for statistical calculation.
Treatment with sEH inhibitors minimize increase in resting tension
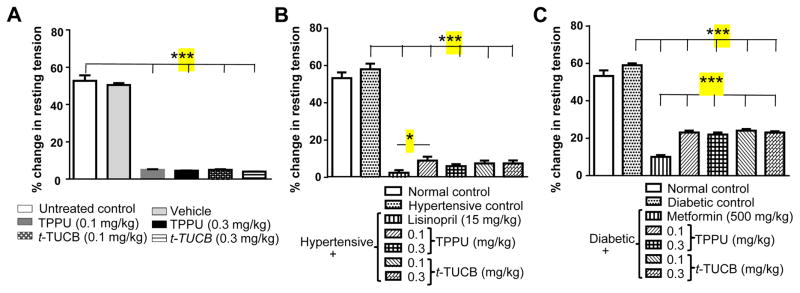
Mean resting tension, a parameter of muscle contractility increased (2 g, pre-IR vs 3 g, post-IR; p < 0.05) significantly during reperfusion. Vehicle did not have significant effect on resting tension. Hypertension and diabetes did not affect post-IR resting tension significantly in this study. Treatment with both sEH inhibitors significantly minimized (p < 0.001) the IR driven increase in the resting tension (Figure 2).
Figure 2.
Treatment with sEH inhibitors decreases IR-induced increase in resting tension. (A) Treatment with vehicle did not change resting tension after IR injury, whereas treatment of sEH inhibitors reduced significant (*** p < 0.001) increase in resting tension in comparison to normal control rats treated with water. (B) Hypertension did not further increase resting tension significantly after IR, but treatment with lisinopril and sEH inhibitors could significantly reduce (*** p < 0.001) hypertension driven increase in resting tension in comparison to untreated hypertensive rats. Lisinopril (15 mg/kg) was more effective (p < 0.05) than TPPU (0.1 mg/kg). (C) Diabetes did not further affect resting tension after IR in comparison to normal rats treated with water, but treatment with metformin and sEH inhibitors significantly reduced (*** p < 0.001) increase in resting tension in comparison to untreated diabetic rats. Metformin (500 mg/kg) was more effective (*** p < 0.001) than sEH inhibitors. Data are presented as mean ± SEM of 6 observations. One-way ANOVA followed by Tukey’s multiple comparison test was used for statistical calculation.
Treatment with sEH inhibitors minimize decrease in developed tension
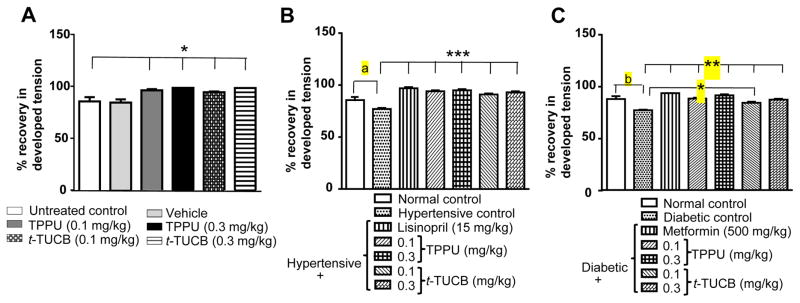
As would be expected from IR injury, mean developed tension was significantly (6 g, pre-IR vs 5 g, post-IR; p < 0.05) lower after IR. Hypertensive animals had significantly lower (5 g vs 4 g, p <0.05) developed tension. Similarly, a significant decrease (5 g vs 3 g, p < 0.05) in the developed tension was observed in the hearts from diabetic rats post-IR. Treatment with sEH inhibitors significantly (p < 0.05) minimized this IR driven decrease in developed tension (Figure 3).
Figure 3.
Treatment with sEH inhibitors reduces IR-induced decrease in the developed tension. (A) Treatment with vehicle did not change the tension developed after IR injury, whereas treatment with sEH inhibitors reduced the significant decrease (* p < 0.05) in developed tension in comparison to normal control rats treated with water. (B) Hypertension decreased developed tension significantly (a p < 0.05) after IR in comparison to normotensive, and treatment with lisinopril significantly reduced (*** p < 0.001) hypertension driven decrease in the developed tension in comparison to untreated hypertensive rats. Both doses of sEH inhibitors significantly reduced (*** p < 0.001) high blood pressure-induced decrease in developed tension in comparison to untreated hypertensive rats. (C) Diabetes significantly decreased (b p < 0.01) developed tension after IR in comparison to normal rats treated with water, and treatment with metformin and both sEH inhibitors significantly reduced (** p < 0.01) diabetes driven decrease in developed tension in comparison to untreated diabetic rats. Metformin (500 mg/kg) was more effective (* p < 0.05) than t-TUCB (0.1 mg/kg). Data are presented as mean ± SEM of 5–6 observations. One-way ANOVA followed by Tukey’s multiple comparison test was used for statistical calculation.
Treatment with sEH inhibitors decrease cardiac damage
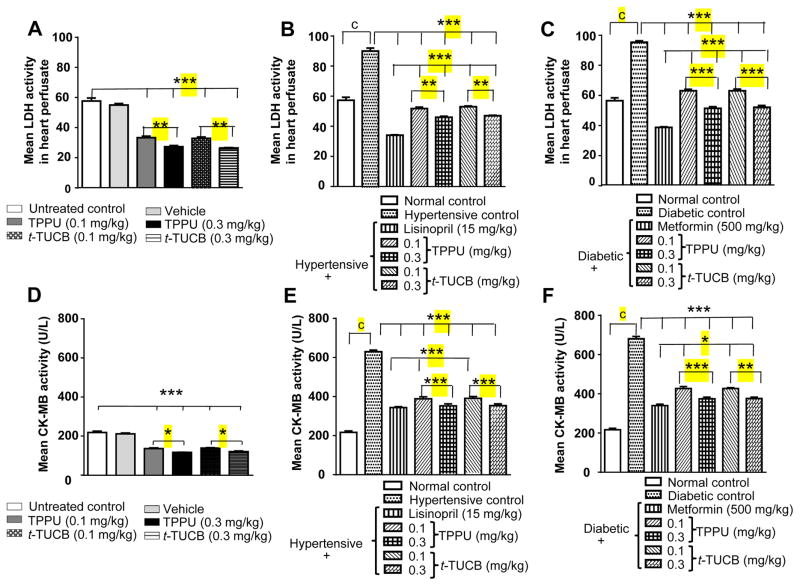
A major consequence of IR injury is cardiac damage and this was evident in hearts subjected to IR throughout this study. Activities of LDH and CK-MB were higher in perfusate samples of hearts from hypertensive and diabetic rats in comparison to normal rats treated with vehicle (p < 0.05). This implies that hypertension and diabetes increase susceptibility to myocardial infarction, a well-established outcome. Treatment with sEH inhibitors significantly decreased (p < 0.01) activities of LDH and CK-MB in perfusate of hearts (Figure 4) demonstrating a cardioprotective effect.
Figure 4.
Treatment with sEH inhibitors reduces the IR-induced increase in the activity of LDH and CK-MB in heart perfusate. An increase in the level of LDH and CK-MB suggests cardiac damage (A) treatment with vehicle did not change activity of LDH after IR injury, whereas treatment with sEH inhibitors reduced the significant (*** p < 0.001) increase in activity of LDH in comparison to normal control rats treated with water. TPPU and t-TUCB dose dependently (** p < 0.01) protected the heart. (B) Hypertension significantly increased activity (c p < 0.01) of LDH after IR. Treatment with Lisinopril and both doses of sEH inhibitors significantly reduced (*** p < 0.001) hypertension driven increase in activity of LDH in comparison to untreated hypertensive rats. The effect of lisinopril (15 mg/kg) was better (*** p < 0.001) than sEH inhibitors. TPPU and t-TUCB dose dependently (** p < 0.01) protected heart. (C) Diabetes significantly increased (c p < 0.001) the activity of LDH after IR in comparison to normal rats treated with water. Treatment with metformin and both doses of sEH inhibitors significantly reduced (*** p < 0.001) the diabetes driven increase in activity of LDH in comparison to untreated diabetic rats. The effect of metformin (500 mg/kg) was better (*** p < 0.001) than sEH inhibitors. TPPU and t-TUCB dose dependently (*** p < 0.01) protected heart (D) Treatment with vehicle did not change activity of CK-MB after IR injury, whereas treatment with sEH inhibitors reduced the significant increase (*** p < 0.001) in activity of CK-MB in comparison to normal control rats treated with water. TPPU and t-TUCB dose dependently (* p < 0.05) protected the heart. (E) Hypertension significantly increased (c p < 0.001) activity of CK-MB after IR. Treatment with lisinopril and both doses of sEH inhibitors significantly reduced (*** p < 0.001) hypertension driven increase in the activity of CK-MB in comparison to untreated hypertensive rats. Lisinopril (15 mg/kg) was more effective (***p < 0.001) than the 0.1 mg/kg dose of both sEH inhibitors (F) Similarly, diabetes significantly increased (c p < 0.001) activity of CK-MB after IR in comparison to normal rats treated with water. Treatment with metformin (500 mg/kg) (*** p < 0.001) and both doses of sEH inhibitors significantly reduced (** p < 0.01) diabetes induced increase in the activity of CK-MB in comparison to untreated diabetic rats. TPPU (*** p < 0.01) and t-TUCB (** p < 0.01) dose dependently protected heart. Metformin at 500 mg/kg was better effective (*p < 0.05) than TPPU (0.1 mg/kg) and t-TUCB (both doses). Data are presented as mean ± SEM of 6 observations. One-way ANOVA followed by Tukey’s multiple comparison test was used for statistical calculation.
Treatment with sEH inhibitors decrease endothelial dysfunction
In addition to the observed cardioprotective effects on isolated hearts we tested if the inhibitors of sEH modulate the contractility of the aorta preparations. Although the inhibitors did not have any effects on baseline contractility, t-TUCB at 10 μM significantly relaxed phenylephrine (3 μM) pre-contracted aorta. Endothelial dysfunction was apparent in the aortas from hypertensive and diabetic rats because the relaxant effect of ACh was significantly diminished (p < 0.05) in comparison to aorta from normal rats treated with vehicle. Treatment with TPPU and t-TUCB at both doses (0.1 and 3 mg/kg) reduced hypertension-induced endothelial dysfunction, whereas treatment with TPPU (0.3 mg/kg) and t-TUCB (0.1 mg/kg) significantly reduced diabetes induced endothelial dysfunction (Figure 5).
Figure 5.
Effect of TPPU and t-TUCB on endothelial function. (A) The t-TUCB at 10 and 100 μM significantly relaxed phenylephrine (3 μM) pre-contracted aorta. (B) Aortas from hypertensive rats exhibited significant endothelial dysfunction in comparison to aorta from normal rats. Endothelial function was measured in terms of relaxant effect of ACh on aorta. A significant decrease (# p < 0.01, hypertensive rat vs normal rat) in the relaxant effect of ACh was considered as endothelial dysfunction. Treatment (0.1 and 0.3 mg/kg/day for 28 days) with TPPU and t-TUCB to hypertensive rat significantly increased relaxant effect of ACh on aortas from hypertensive rats when compared with that of untreated hypertensive rats. Lisinopril also decreased (*p < 0.01) endothelial dysfunction. (C) Aortas from diabetic rats exhibited significant (# p < 0.01) endothelial dysfunction in comparison to aortas from normal rats. Treatment with TPPU (0.3 mg/kg/day) and t TUCB (0.1 mg/kg/day) for 28 days to diabetic rats significantly increased relaxant effect of ACh on aortas from diabetic rats when compared with that of untreated diabetic rats. Metformin did not decrease endothelial dysfunction in this model. * p < 0.05, **p <0.01, *** p < 0.001.
Discussion
In this study, we evaluated the possible cardiovascular effects of inhibiting sEH. Two structurally different and potent inhibitors were employed. These inhibitors prevent the degradation of bioactive lipid mediators, the EETs and other epoxy fatty acids. Earlier work demonstrated the doses used are sufficient to stabilize the EETs. After administration of 0.1 and 0.3 mg/kg doses, blood levels of TPPU reach much higher than its IC90 value on recombinant rat sEH [25, 26]. In addition, here we maintained the animals on daily doses of sEH inhibitors which should sustain a high degree of inhibition. In ex vivo experiments, we measured changes in multiple important parameters of cardiovascular function. The sEH inhibitors did not alter baseline heart rate and developed tension. This is consistent with previous publications.
Effect of IR on cardiac function was evaluated by quantifying heart rate, resting tension and developed tension. A significant decrease in heart rate and developed tension and an increase in resting tension after IR injury precede pathological changes in a failing heart. Resting tension represents the force experienced by heart whereas developed tension represents work performed by heart. Here consistent with earlier work we observed that hypertension and diabetes increase workload of heart and decrease performance of the heart. Though hypertension and diabetes did not increase resting tension significantly in this model, the anti-hypertensive (lisinopril) and anti-diabetic (metformin) medicines significantly minimized hypertension and diabetes driven increase in resting tension respectively. This signifies the role of hypertension and diabetes on increasing resting tension in IR model. Lisinopril and metformin reduced hypertension and diabetes-induced decrease in developed tension. These findings are in line with the impact of hypertension and diabetes on cardiac function. However, treatment with the sEH inhibitors reversed these pathological changes in cardiac function. The increase in the activity of LDH and CK-MB in the organ perfusate after ischemia is direct evidence of cardiac damage. Higher levels of cardiac damage were observed in the hearts of rat with diabetes and high blood pressure in comparison to normal, and healthy rats. Similarly, endothelial dysfunction was also observed in the aorta of rat with diabetes or hypertension in comparison to the normal rat. Prophylactic treatment with sEH inhibitors at 100 and 300 μg/kg daily dose for 4 weeks significantly protected the heart from cardiac injury and endothelial dysfunction.
The inhibitors of sEH have been reported to decrease hypertension in numerous animal models including angiotensin-induced, salt-sensitive and maternally fructose-fed hypertension in addition to genetically hypertensive Goto-Kakizaki rats [27–31]. The anti-hypertensive effect of sEH inhibitors are thought to be mediated through increasing the levels of epoxy fatty acids, more specifically the EETs and epoxydocosapentaenoic acids derived from DHA [25, 32]. [31]. A stable synthetic analog of EET also decreased angiotensin-induced hypertension [33].
Typically, cardiac damage is more evident in hearts of hypertensive rats than healthy rats and lisinopril, an inhibitor of angiotensin-converting enzyme, reduces cardiac damage in hypertensive animals. We observed the same phenomena here and inhibition of sEH was highly effective in reversing this damage. These observations suggest a fundamental role for the EETs and other epoxy fatty acids in maintaining homeostasis and reducing cardiac damage.
Cardiovascular abnormalities are reported in diabetic patients. Heart rates of diabetics are reported to be decreased in comparison to non-diabetics [34]. Data from a Finnish population-based study suggests that diabetic patients are at a significant risk of developing myocardial infarction in comparison to non-diabetic patients [35]. Myocardial infarction leads to congestive heart failure. The risk of congestive heart failure and coronary artery disease is highest in diabetics as suggested by the Framingham study [36]. The American Heart Association recognizes a significant risk of cardiovascular disease in diabetes and considers diabetes as a cardiovascular disease [37]. Here, cardiac damage was expectedly more significant in hearts of diabetic rats compared to healthy rats. As would be expected from earlier body of work, metformin reduced cardiac damage. Furthermore, inhibition of sEH largely reduced cardiac damage in hearts of diabetic rats suggesting that sEH inhibitors are cardioprotective compounds. Inhibition of sEH and genetic deletion are reported to decrease hyperglycemia, promote insulin secretion, and reduce islet apoptosis [38]. The cardioprotective effect of sEH inhibitors in diabetic rats could be due to decrease in hyperglycemia and apoptosis of islets, and increase in the secretion of insulin [38–40]. However, given that similar effects are observed in hypertensive animals it is likely that the sEH inhibitors have a direct protective effect.
Endothelial dysfunction is an important hallmark of both hypertension and diabetes. In this study, we evaluated the endothelial dysfunction using responses of the aorta to relaxation by ACh as a marker of endothelial function. We observed an increase in endothelial dysfunction with diabetes and hypertension and treatment with sEH inhibitors ameliorated the endothelial dysfunction. Endothelial dysfunction increases the propensity of eventual myocardial damage and improvement of endothelial function in diabetes [41, 42] and hypertension [43] minimizes the possibility of myocardial damage. Thus, improvement of endothelial function could be the basis of the cardioprotective effects of sEH inhibitors in ischemia-reperfusion injury both in diabetic and hypertensive rats.
Earlier, we reported the cardioprotective effect of t-TUCB at 3–30 mg/kg against isoproterenol induced myocardial infarction in normal rats [44]. In this study, we demonstrate that both t-TUCB and TPPU protect hearts at very low doses (i.e., 0.1–0.3 mg/kg). More importantly, the sEH inhibitors ameliorated cardiac damage in both hypertension and diabetes models. One mechanism related to IR-induced cardiac damage seems to be endoplasmic reticulum (ER) stress [45–48]. The ER is a complex organelle on which most protein synthesis occurs. Stressors of ER function such as misfolded proteins or calcium imbalance are detected by membrane bound sensors PERK, IRE1 and ATF6. These sensors signal and initiate transcriptional and translational programs related to amelioration of the stress, including silencing of most protein synthesis and augmenting the synthesis of chaperone molecules, promoting proteasome mediated degradation. In cases where these unfolding protein response elements (UPREs: PERK, IRE1 and ATF6) are unable to overcome the stress, apoptotic pathways are activated [49–54]. Chemical inhibition of sEH and genetic deletion has been demonstrated to decrease ER stress [54–56]. The cardioprotective effect of sEH inhibitors in this model could therefore be due to mitigation of ER stress. Another possible mechanism for these effects could be the known decrease in inflammation by the inhibitors of sEH. Inflammatory responses are reported in IR with increase in the levels of TNFα and IL-6 [57, 58]. The minimization of cardiac damage also could be due to elevation of anti-inflammatory epoxy fatty acids and decrease in inflammatory mediators by sEH inhibitors [26, 59, 60].
A limitation of this research is that we did not quantify the effect of treatment on hypertension and blood sugar level at the end of treatment. Effect of lisinopril and metformin were not evaluated on isolated heart in vitro. Moreover, we have quantified only surrogate markers (LDH and CK-MB) of cardiac damage. Myocardial staining with 2,3,5-triphenyltetrazolium chloride (TTC) could have provided conclusive evidence about effect of treatment on cardiac damage. Echocardiography and in vivo ischemia reperfusion injury also would have provided stronger data.
Conclusions
The sEH inhibitors minimize cardiovascular damage in heart from normal, diabetic and hypertensive rats due to ischemia-reperfusion injury suggesting the importance of epoxy fatty acids as chemical mediators in cardiac health. Therefore, sEH inhibitors such as TPPU and t-TUCB can be useful in the ischemia-reperfusion related cardiac damage. These findings could be applicable to both human and companion animals suffering from cardiac pathology. Treatment with both the inhibitors ameliorated endothelial dysfunction. Further studies are warranted for elucidating mechanism of cardiac protection.
References
- 1.Tarride JE, Lim M, DesMeules M, et al. A review of the cost of cardiovascular disease. Can J Cardiol. 2009;25:e195–e202. doi: 10.1016/s0828-282x(09)70098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaziano TA. Cardiovascular disease in the developing world and its cost-effective management. Circulation. 2005;112:3547–3553. doi: 10.1161/CIRCULATIONAHA.105.591792. [DOI] [PubMed] [Google Scholar]
- 3.Weber KT, Janicki JS. The metabolic demand and oxygen supply of the heart: physiologic and clinical implications. Am J Cardiol. 1979;44:722–729. doi: 10.1016/0002-9149(79)90294-7. [DOI] [PubMed] [Google Scholar]
- 4.De Boer RA, Pinto YM, Van Veldhuisen DJ. The imbalance between oxygen demand and supply as a potential mechanism in the pathophysiology of heart failure: the role of microvascular microvascular growth and abnormalities. Microcirculation. 2003;10:113–126. doi: 10.1038/sj.mn.7800188. [DOI] [PubMed] [Google Scholar]
- 5.Hadi ARH, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. 2005;1:183–198. [PMC free article] [PubMed] [Google Scholar]
- 6.Hadi ARH, Al Suwaidi J. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag. 2007;3:853–876. [PMC free article] [PubMed] [Google Scholar]
- 7.Mudau M, Genis A, Lochner A, Strjjdom H. Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovasc J Afr. 2012;23:222–231. doi: 10.5830/CVJA-2011-068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dharmashankar K, Widlansky ME. Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep. 2010;12:448–455. doi: 10.1007/s11906-010-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alberti KG, Zimmet PZ. Definition, diagnosis, and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1988;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 10.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 11.Su Y, Liu XM, Sun YM, et al. The relationship between endothelial dysfunction and oxidative stress in diabetes and prediabetes. Int J Clin Pract. 2008;62:877–882. doi: 10.1111/j.1742-1241.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- 12.Silva BR, Pernomian L, Bendhack LM. Contribution of oxidative stress to endothelial dysfunction in hypertension. Front Physiol. 2012;3:441. doi: 10.3389/fphys.2012.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olearczyk JJ, Field MB, Kim IH, Morisseau C, Hammock BD, Imig JD. Substituted adamantyl-urea inhibitors of the soluble epoxide hydrolase dilate mesenteric resistance vessels. J Pharmacol Exp Ther. 2006;318:1307–1314. doi: 10.1124/jpet.106.103556. [DOI] [PubMed] [Google Scholar]
- 14.Koeners MP, Wesseling S, Ulu A, et al. Soluble epoxide hydrolase in the generation and maintenance of high blood pressure in spontaneously hypertensive rats. Am J Physiol Endocrinol Metab. 2011;300:E691–E698. doi: 10.1152/ajpendo.00710.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rose TE, Morisseau C, Liu JY, et al. 1-Aryl-3-(1-acylpiperidin-4-yl)urea inhibitors of human and murine soluble epoxide hydrolase: structure-activity relationships, pharmacokinetics, and reduction of inflammatory pain. J Med Chem. 2010;53:7067–7075. doi: 10.1021/jm100691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang SH, Tsai HJ, Liu JY, et al. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem. 2007;50:3825–3840. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zemančíková A, Török J. Cardiovascular effects of high-fructose intake in rats with nitric oxide deficiency. Interdiscip Toxicol. 2014;7:159–164. doi: 10.2478/intox-2014-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Yan L-J. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab Syndr Obes. 2015;8:181–188. doi: 10.2147/DMSO.S82272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Junod A, Lambert AE, Stauffacher W, Renold AE. Diabetogenic action of streptozotocin: relationship of dose to metabolic response. J Clin Invest. 1969;48:2129–2139. doi: 10.1172/JCI106180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanakpura VK, Goswami SK, Dethe S, Inamdar MN. Efficacy of methanolic extract of Piper longum on endothelial function in vitro and ex vivo. Manipal J Pharm Sci. 2016;2:26–32. [Google Scholar]
- 21.Inamdar MN, Venkataraman BV, Aleem Md A. A simple and improved perfusion apparatus for isolated hearts. Ind J Pharmacol. 1994;26:262–265. [Google Scholar]
- 22.Wroblewski F, LaDue JS. Lactate dehydrogenase activity in blood. Proc Soc Exp Med. 1978;90:210. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]
- 23.Varley H, Growenlock AH, Bell M. Practical Clinical Biochemistry. 5. Vol. 1. London: William Heinman Medical Books Ltd; 1980. Determination of serum lactate dehydrogenase activity; pp. 715–723. [Google Scholar]
- 24.Momin FN, Kalai BR, Shikalgar TS, Naikwade NS. Cardioprotective effect of methanolic extract of Ixora coccinea Linn. leaves on doxorubicin-induced cardiac toxicity in rats. Indian J Pharmacol. 2012;44:178–183. doi: 10.4103/0253-7613.93844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulu A, Stephen Lee KS, Miyabe C, et al. An omega-3 epoxide of docosahexaenoic acid lowers blood pressure in angiotensin-II-dependent hypertension. J Cardiovasc Pharmacol. 2014;64:87–99. doi: 10.1097/FJC.0000000000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goswami SK, Wan D, Yang J, et al. Anti-ulcer efficacy of soluble epoxide hydrolase inhibitor TPPU on diclofenac-induced intestinal ulcers. J Pharmacol Exp Ther. 2016;357:529–536. doi: 10.1124/jpet.116.232108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–694. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- 28.Imig JD, Carpenter Margaret A, Shaw Sean. The soluble epoxide hydrolase inhibitor AR9281 decreases blood pressure, ameliorates renal injury and improves vascular function in hypertension. Pharmaceuticals. 2009;2:217–227. doi: 10.3390/ph2030217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imig JD, Zhao X, Zaharis CZ, et al. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension. 2005;46:975–981. doi: 10.1161/01.HYP.0000176237.74820.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olearczyk JJ, Quigley JE, Mitchell BC, et al. Administration of a substituted adamantyl urea inhibitor of soluble epoxide hydrolase protects the kidney from damage in hypertensive Goto-Kakizaki rats. Clin Sci (Lond) 2009;116:61–70. doi: 10.1042/CS20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tain YL, Lee WC, Wu KL, Leu S, Chan JY. Targeting arachidonic acid pathway to prevent programmed hypertension in maternal fructose-fed male adult rat offspring. J Nutr Biochem. 2016;38:86–92. doi: 10.1016/j.jnutbio.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Imig JD. Cardiovascular therapeutic aspects of soluble epoxide hydrolase inhibitors. Cardiovasc Drug Rev. 2006;24:169–188. doi: 10.1111/j.1527-3466.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 33.Khan AH, Falck JR, Manthati VL, Campbell WB, Imig JD. Epoxyeicosatrienoic acid analog attenuates angiotensin II hypertension and kidney injury. Front Pharmacol. 2014;5:216. doi: 10.3389/fphar.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ewing DJ, Campbell IW, Clarke BF, et al. Heart rate changes in diabetes mellitus. Lancet. 1981;317:183–186. doi: 10.1016/s0140-6736(81)90061-1. [DOI] [PubMed] [Google Scholar]
- 35.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 36.Kannel WB, McGee DL. Diabetes and Cardiovascular Disease: The Framingham Study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 37.Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 38.Luo P, Chang HH, Zhou Y, et al. Inhibition or deletion of soluble epoxide hydrolase prevents hyperglycemia, promotes insulin secretion, and reduces islet apoptosis. J Pharmacol Exp Ther. 2010;334:430–8. doi: 10.1124/jpet.110.167544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luria A, Bettaieb A, Xi Y, et al. Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance. Proc Natl Acad Sci USA. 2011;108:9038–9043. doi: 10.1073/pnas.1103482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L, Fan C, Zhang Y, et al. Beneficial effects of inhibition of soluble epoxide hydrolase on glucose homeostasis and islet damage in a streptozotocin-induced diabetic mouse model. Prostaglandins Other Lipid Mediat. 2013;104–5:42–48. doi: 10.1016/j.prostaglandins.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ciccone MM, Scicchitano P, Cameli M, et al. Endothelial Function in Pre-diabetes, Diabetes and Diabetic Cardiomyopathy: A Review. J Diabetes Metab. 2014;5:4. [Google Scholar]
- 42.Farhangkhoee H, Khan ZA, Kaur H, Xin X, Chen S, Chakrabarti S. Vascular endothelial dysfunction in diabetic cardiomyopathy: pathogenesis and potential treatment targets. Pharmacol Ther. 2006;111:384–99. doi: 10.1016/j.pharmthera.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial Dysfunction, arterial Stiffness, and heart Failure. J Am Coll Cardiol. 2012;60:1455–69. doi: 10.1016/j.jacc.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 44.Shrestha A, Krishnamurthy PT, Thomas P, Hammock BD, Hwang SH. Soluble epoxide hydrolase inhibitor, t-TUCB, protects against myocardial ischaemic injury in rats. J Pharm Pharmacol. 2014;66:1251–1258. doi: 10.1111/jphp.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu XH, Zhang ZY, Sun S, Wu XD. Ischemic postconditioning protects myocardium from ischemia/reperfusion injury through attenuating endoplasmic reticulum stress. Shock. 2008;30:422–427. doi: 10.1097/SHK.0b013e318164ca29. [DOI] [PubMed] [Google Scholar]
- 46.Miki T, Miura T, Hotta H, et al. Endoplasmic reticulum stress in diabetic hearts abolishes erythropoietin-induced myocardial protection by impairment of phospho–glycogen synthase kinase-3β–mediated suppression of mitochondrial permeability transition. Diabetes. 2009;58:2863–2872. doi: 10.2337/db09-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bai S, Cheng L, Yang Y, et al. C1q/TNF-related protein 9 protects diabetic rat heart against ischemia reperfusion injury: role of endoplasmic reticulum stress. Oxid Med Cell Longev. 2016;2016:1902025. doi: 10.1155/2016/1902025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin JK, Blackwood EA, Azizi KM, et al. ATF6 decreases myocardial ischemia/reperfusion damage and links ER stress and oxidative stress signaling pathways in the heart. Circ Res. 2016 doi: 10.1161/CIRCRESAHA.116.310266. pii: CIRCRESAHA.116.310266. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao, Yu LM, Gao WL, et al. Berberine protects rat heart from ischemia/reperfusion injury via activating JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress. Acta Pharmacol Sin. 2016;37:354–367. doi: 10.1038/aps.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Plaisance V, Brajkovic S, Tenenbaum M, et al. Endoplasmic reticulum stress links oxidative stress to impaired pancreatic beta-cell function caused by human oxidized LDL. PLoS ONE. 2016;11:e0163046. doi: 10.1371/journal.pone.0163046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Engin F. ER stress and development of type 1 diabetes. J Investig Med. 2016;64:2–6. doi: 10.1097/JIM.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun Y, Zhang T, Li L, Wang J. Induction of apoptosis by hypertension via endoplasmic reticulum stress. Kidney Blood Press Res. 2015;40:41–51. doi: 10.1159/000368481. [DOI] [PubMed] [Google Scholar]
- 53.Guo XF, Yang XJ. Endoplasmic reticulum stress response in spontaneously hypertensive rats is affected by myocardial ischemia reperfusion injury. Exp Ther Med. 2015;9:319–326. doi: 10.3892/etm.2014.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bettaieb A, Nagata N, AbouBechara D, et al. Soluble epoxide hydrolase deficiency or inhibition attenuates diet-induced endoplasmic reticulum stress in liver and adipose tissue. J Biol Chem. 2013;288:14189–14199. doi: 10.1074/jbc.M113.458414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harris TR, Bettaieb A, Kodani S, et al. Inhibition of soluble epoxide hydrolase attenuates hepatic fibrosis and endoplasmic reticulum stress induced by carbon tetrachloride in mice. Toxicol Appl Pharmacol. 2015;286:102–11. doi: 10.1016/j.taap.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inceoglu B, Bettaieb A, Trindade da Silva CA, Lee KS, Haj FG, Hammock BD. Endoplasmic reticulum stress in the peripheral nervous system is a significant driver of neuropathic pain. Proc Natl Acad Sci USA. 2015;112:9082–9087. doi: 10.1073/pnas.1510137112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duran WN. The double-edge sword of TNF-α in ischemia-reperfusion injury. Am J Physiol Heart Physiol. 2008;295:H2221–H2222. doi: 10.1152/ajpheart.01050.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kukielka GL, Smith CW, Manning AM, Youker KA, Michael LH, Entman ML. Induction of iterleukin-6 synthesis in the myocardium: potential role in post-reperfusion inflammation injury. Circulation. 1995;92:1866–1875. doi: 10.1161/01.cir.92.7.1866. [DOI] [PubMed] [Google Scholar]
- 59.Goswami SK, Inceoglu B, Yang J, et al. Omeprazole increases the efficacy of a soluble epoxide hydrolase inhibitor in a PGEα induced pain model. Toxicol Appl Pharmacol. 2015;289:419–27. doi: 10.1016/j.taap.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang W, Yang AL, Liao J, et al. Soluble epoxide hydrolase gene deficiency or inhibition attenuates chronic active inflammatory bowel disease in IL-10(-/-) mice. Dig Dis Sci. 2012;57:2580–91. doi: 10.1007/s10620-012-2217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]