Abstract
Background
Rhesus and cynomologus macaques are valuable animal models for the study of Human Immunodeficiency Virus (HIV) prevention strategies. However, for such studies focused on the vaginal route of infection, differences in vaginal environment may have deterministic impact on the outcome of such prevention, providing the rationale for this study.
Methods
We tested the vaginal environment of rhesus and cynomolgus macaques longitudinally to characterize the normal microflora based on Nugent scores and pH. This evaluation was extended after colonization of the vaginal space with Lactobacilli in an effort to recreate NHP models representing the healthy human vaginal environment.
Results and Conclusion
Nugent scores and pH differed significantly between species, though data from both species were suggestive of stable bacterial vaginosis. Colonization with Lactobacilli was successful in both species leading to lower Nugent score and pH, though rhesus macaques appeared better able to sustain Lactobacillus spp over time.
Keywords: vagina, microbiota, lactobacillus, macaque
Introduction
Worldwide, an estimated 38.8 million people currently live with HIV [1]. In the United States alone, approximately 1 million people are infected with HIV, and an estimated 14% of these individuals are undiagnosed [1, 2]. Vaginal acquisition represents the major portal of entry for HIV infection, and as such, is the focus of many studies seeking to prevent the sexual transmission of HIV [3–9]. Rhesus (Macaca mulatta) and Cynomolgus (Macaca fascicularis) macaques are valuable models for evaluating the safety and efficacy of HIV prevention strategies because of similarities to humans in their susceptibility to infection with simian immunodeficiency virus (SIV) or chimeric simian/human immunodeficiency virus (SHIV),, and the gradual development of disease similar to that seen in human HIV infection[10]. However, susceptibility to vaginal HIV acquisition is heavily influenced by multiple factors, including mucosal host defenses, the presence of local infections, pH, inflammation, hormonal changes, and the resident microbiota [11–15].
In this study we conducted a comparative evaluation of the vaginal environment of rhesus and cynomolgus macaques via the evaluation of Nugent Scores and vaginal pH to determine if there is a significant species difference in these parameters. Nugent Scores represent a rapid test of the overall microbiota and are calculated on a scale of 0–10 with lower scores (0–3) indicating a healthy vagina, and higher scores (7–10) indicative of bacterial vaginosis, characterized by a reduced population of acid-producing Lactobacillus spp, and greater proportions of Gram negative and Gram variable bacteria such as Mobiluncus, Bacteroides, Gardnerella and Mycoplasma spp [16]. This condition has consistently been accompanied by an increased vaginal pH, and has been shown to increase susceptibility to vaginal acquisition of HIV [14, 17, 18]. We compared these known human risk factors in both species to better define the respective non-human primate models for prevention of vaginal acquisition of HIV in women. In addition, we sought to colonize rhesus and cynomolgus macaque vaginas with Lactobacillus rhamnosus, a facultative anaerobe commonly found in the healthy female genital tract, and used as a probiotic for the treatment of BV [19]. We monitored the longevity of L. rhamnosis and its effect on vaginal pH in each model. We submit that these data will allow for a better definition of vaginal challenge studies in the context of vaccine and microbicide evaluations using these nonhuman primate models.
Methods and Materials
Humane Care Guidelines
All animals used in this study were housed at the Yerkes National Primate Research Center (YNPRC) in Atlanta, GA. All protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Emory University (YER-2001013). All animal care procedures were compliant with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals.
Lactobacillus growth conditions and inoculations
Lactobacillus casei var rhamnosus GR-1 (L. Rhamn. ATCC 55826) was used in this study. The nucleotide sequence of HIV gp41-64 and anti-erythromycin were cloned into the L. rhamn genome. L. rhamn was cultured using Man Rigosa Sharpe broth (Sigma Aldrich, Cat# 69966) containing 32μg/ml erythromycin. After seeding, the culture tubes were sealed and cultured without shaking at 30°C for 48 hrs. At the end of culture, the L. rhamn were washed three times with DPBS before being resuspended in 20mg/ml glycogen (Sigma Aldrich G0885) solution, and stored at 4°C overnight prior to inoculation. The colony forming units were determined on Bromo Cresol Purple agar (Himedia labs M1351) plated containing 30μg/ml erythromycin, and titrated generally around 1–2×1010 per ml.
Study animals and experimental design
Fifteen normal cycling female adult specific-pathogen-free (SPF) cynomolgus macaques (CMs, age: 5–6.6 years old; weight: 3.9–10 kg) and twenty SPF Rhesus macaques (RMs, age: 3.25–6.58 years old; weight: 4.35–9.40 kg) were used in this study. Baseline blood, BactiSwab (Remel Cat# R12100; sent to Yerkes clinical pathology lab for Nugent Score), and cotton-tip swab (Fisher brand Cat# 23-400-110, for vaginal pH) samples were collected weekly from each monkey for a minimum of 3 baseline recordings. All collection procedures were performed under anesthesia.
L. Rhamn inoculation
For L. rhamn inoculation, five cynomolgus macaques and twenty rhesus macaques were used. The CMs were tested first with 3 consecutive strategies:
The monkey’s perianal area was cleaned with gauze saturated with sterile saline solution and the vaginal mucus was removed with 1ml syringe. The monkey’s vagina was then inoculated with 1ml DPBS containing 1~2×1010 CFU/ml L. rhamn in 20mg/ml glycogen with a 1 ml needleless syringe. Animals were maintained in a supine position for 5 minutes before returning to their cage. This procedure was repeated once a week for 4 weeks, but the week of menses was skipped. Swabs were collected before inoculations for vaginal pH and Nugent Scores.
Ten weeks after the last inoculation, after perianal cleaning, the CM’s vaginas were flushed with 10ml 1% lactic acid, pH=3.8 (prepared from Sigma Aldrich, Cat# 252476, ACS reagent, ≥85%) right before inoculation of 1ml of freshly prepared 1–2×1010CFU L. rhamn weekly for 5 weeks. Samples were collected as described above.
Ten weeks later, the five CM’s vaginas were pretreated with 2ml of a suspension of 40mg/ml erythromycin one day before L. rhamn inoculation. The next day, the vaginal vaults were flushed with 10ml 1% lactic acid (pH3.8) right before inoculation of 1.5~ 2ml 1~2×1010CFU/ml L. rhamn. Swab samples were collected until 16 weeks after this inoculation.
Twenty SPF rhesus macaques were inoculated with L. rhamn according to the third strategy, preceded by vaginal erythromycin treatment followed one day later by a 10 ml 1% lactic acid flush as described above and inoculation of 1.5–2ml 1–2×1010CFU/ml L. rhamn. Swab samples were collected weekly for 7 weeks.
Determination of vaginal pH
The macaques’ vaginal pH was determined by EMD Millipore MColor pHast™ pH Test Strips and Indicator Papers (pH 0–6 and 5–10, Cat# 1095310007 and1095330001 respectively). In brief, a sterile cotton-tip applicator was inserted into the vagina, turned over 2 times, and then rolled onto pH test strips. The pH values were determined by two observers.
Nugent score determinations and calculations
Nugent scores have been used in evaluating the vaginal environment of women since 1991; scores of 7–10 indicate bacterial vaginosis whereas scores of 1–3 indicate lactobaccillus-dominated microflora [20]. In order to evaluated bacterial vaginosis in the macaques, the Gram-stained vaginal BactiSwab smear slides were evaluated by a microbiologist in the clinical pathology lab at the Yerkes National Primate Research Center, blinded to the animal’s experimental status. The evaluation scale included an Amsil test (fishy smell from the vaginal swabs after adding 1–2 drops of 10% fresh KOH), clue cells (the vaginal epithelia cells’ border obscured by adherent small coccobacilli), and scores of three bacteria: Gardnerella, Curved Gram negative (G-) bacilli, and lactobacillus spp. See Supplemental Table 1.
Statistics
Differences between CM and RM vaginal pH and Nugent scores before and after L. rhamn inoculation were analyzed by unpaired two-tail t-test in GraphPad Prism.
Results
The average baseline Nugent score of the RMs was 8.36 ± 0.26, while the average Nugent score of the CMs was 9.11 ± 0.12 (p=0.0072). These Nugent scores are indicative of bacterial vaginosis [21]. Figure 1A displays the tighter dispersion of Nugent scores amongst CMs as compared to RMs, some of which had lower Nugent scores comparable to those found in healthy humans. On the 0–10 scale, no individual CMs had a baseline Nugent score of less than 5. Baseline Nugent scores of RMs showed a higher level of variation, although vaginal cultures revealed that only 3 of 20 RMs had measurable levels of Lactobacillus spp. at baseline (data not shown). While the average Nugent scores of both species correlated with human bacterial vaginosis, the difference between the average Nugent scores of CM vs RMs was highly significant (p=0.0072) using an unpaired two-tailed t-test. The vaginal pH of both species was also evaluated and compared. The average vaginal pH of CMs was 7.14 ± 0.67, while the average vaginal pH of RMs was 6.09 ± 0.07 (p<0.0001). As seen in figure 1B, vaginal pH correlated with the differences in Nugent scores and vaginal pH values in RM were stable over 2 reproductive seasons.
Figure 1.
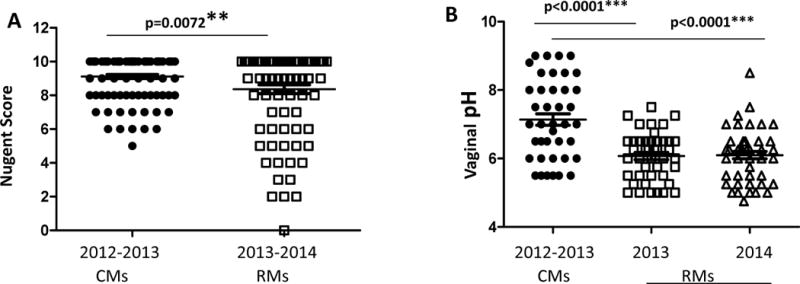
(A) Comparison of Nugent scores between cynomolgus and rhesus macaques. Rhesus macaque Nugent Scores were collected during the reproductive period (Oct.–Dec.). (B) Comparison of vaginal pH between cynomolgus and rhesus macaques. Statistical values are presented as **p=0.0072; ***p<0.0001
The initial inoculation protocol of CMs with L. rhamn consisted of a series of one inoculation per week without pre-conditioning and each preceded by the collection of samples to assess both the Nugent score and pH, to evaluate the colonization from the previous administration. As seen in Fig 2A five consecutive inoculations of 1–2×1010 CFUs of L rhamn, failed to alter the vaginal Nugent scores of CM, demonstrating failure of the L rhamn to durably colonize the vaginal vault. The repeated inoculations did however induce a lower vaginal pH (from 7.45±0.32 to 6.5±0.43) at week 2 (Fig 2B, p=0.018) with a similar but non-significant trend for the subsequent time points.
Figure 2.
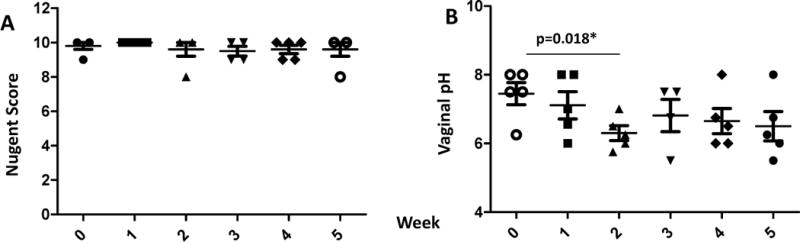
Nugent Scores (A) and vaginal pH (B) of cynomolgus macaques before and after weekly inoculation of 1~2×1010 CFU of L. rhamn without pre-conditioning. Statistical values are presented as *p=0.018.
In our second attempt to inoculate CMs, the monkey vaginal vault was flushed with 10 mL of a 1% lactic acid solution (pH3.8) in an effort to reduce and inhibit the pre-existing microflora prior to inoculation of L. rhamn, at weekly intervals (Figure 3). This approach induced a slight but non-significant decrease in Nugent scores monitored weekly, but no evidence of colonization by L rhamn. Somewhat surprisingly, the vaginal pH showed no appreciable change or perhaps even a slight increase (Fig 3B).
Figure 3.
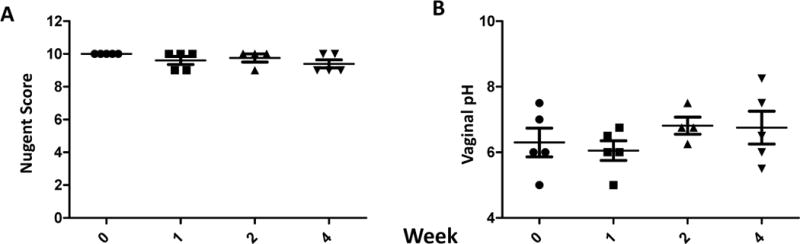
Nugent Scores (A) and vaginal pH (B) of 5 cynomolgus macaques before and after weekly inoculation of 1~2×1010 CFU of L. rhamn preceded by a 10ml 1% lactic acid flush immediately before L. rhamn. inoculation once a week. No statistically significant differences were observed.
In a third attempt to colonize CMs, the animals were pre-conditioned by the vaginal administration of 1–2 ml of Erythromycin 24 hours prior to inoculation. The next day, each animal underwent vaginal flushing with 10mL 1% lactic acid followed by inoculation of 1–2×1010 CFUs of L rhamn. Three animals received a single inoculation, while two animals received two inoculations 1 week apart. Nugent scores and pH were then monitored weekly for 12 weeks (Figure 4). Measurements starting at 1 week post inoculation revealed steep decreases in average Nugent score from 9.4±0.24 to 3.33±0.33 and pH from 6.65±0.47 to 5.1±0.08. As reflected in figure 4, differences in Nugent scores reached statistical significance in weeks 1, 3, 9 (p<0.0001), 4 (p<0.004), and 5 (p<0.01) post inoculation. The trends seen for vaginal pH mirrored those noted in Nugent score, though the pH differences were not statistically significant. Overall, the L. rhamn colonization, while fluctuating over time, was durable in CMs.
Figure 4.
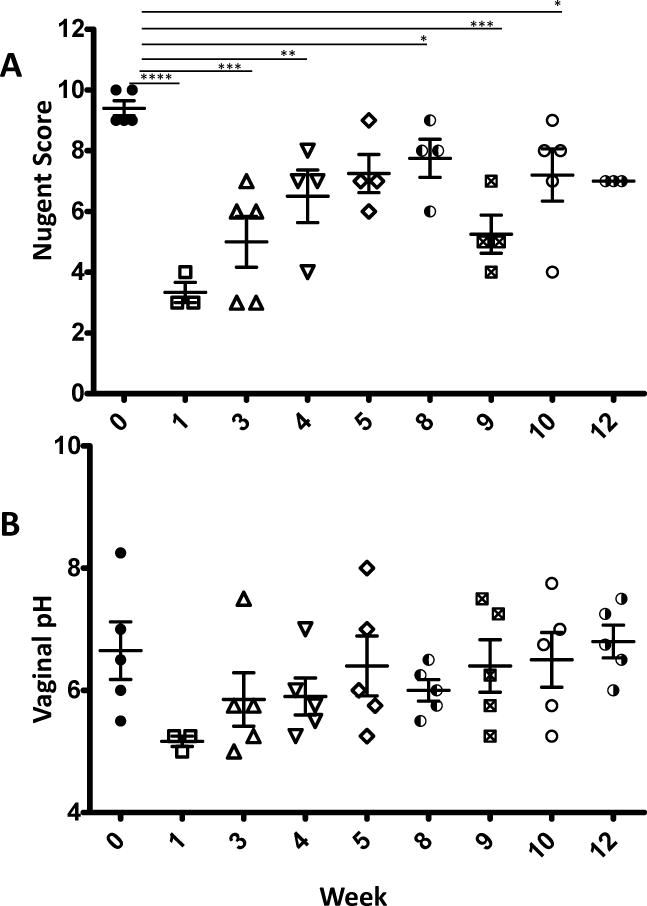
Nugent Scores (A) and vaginal pH (B) of 5 cynomolgus macaques before and after inoculation of 1~2×1010 CFU of L. rhamn preceded by the vaginal administration of 2 mls of erythromycin solution (40mg/ml) 24 hours before a 10ml 1% lactic acid flush followed by inoculation of L. rhamn. Each animal received 2 such administrations (on week 0 and 1) and were monitored weekly for up for 12 weeks. Statistical values are presented as * p<0.05; ** p<0.01; ***p<0.001; ****p<0.0001.
For the vaginal colonization of RMs, L. rhamn was administered according to the same protocol as the final inoculation of CMs. RMs also received intravaginal erythromycin 24 hours prior to inoculation, and lactic acid flushing immediately prior to inoculation. All RMs received a single administration of L. rhamn (1–2×1010 CFUs). Following inoculation, a sharp decrease in average Nugent scores was seen (6.2 to 5.6) that remained statistically significant despite small fluctuations in the average at each weekly time point for seven weeks as shown in in Figure 5A. Nugent scores were measured again at 42 weeks post inoculation, and were found to have remained significantly decreased compared to baseline (not shown) even at this late time point. Average pH was also followed weekly after inoculation, and showed statistically significant decreases in weeks 1, 2, 4, and 6 as seen in figure 5B.
Figure 5.
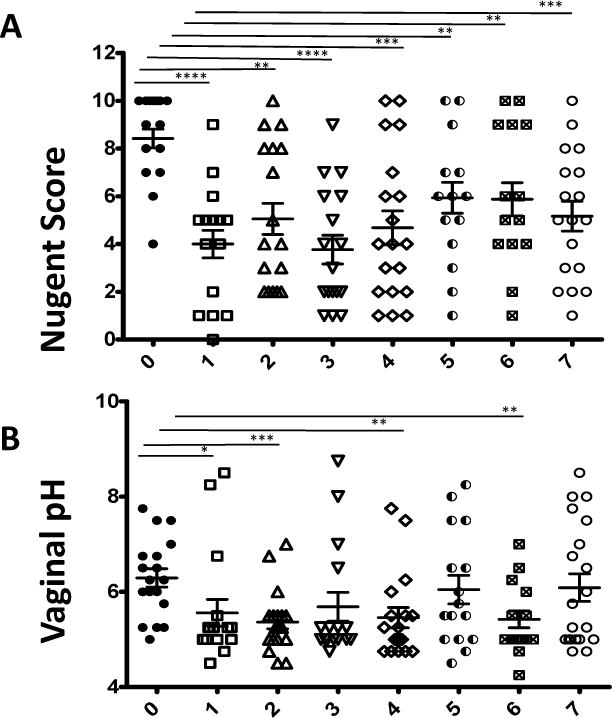
Nugent score and vaginal pH of rhesus macaques before and after L rhamn. inoculation following erythromycin administration and 1% lactic acid flushing. (A) Nugent scores; after a single L rhamn inoculation, Nugent scores sharply decreased and remained low for 7 weeks and beyond (up to week 42, data not shown).(B) Vaginal pH after a single L. Rhamn. inoculation. Statistical values are presented as * p<0.05; ** p<0.01; ***p<0.001; ****p<0.0001
To gain a better understanding of the kinetics of the microflora measured by Nugent score in the 2 species, relative values for Gardnerella, curved Gram− bacilli and Lactobacilli were plotted over time and compared between the two macaque species (Figure 6). There was a marked difference between RMs and CMs in their ability to maintain lactobacilli over time and the inhibition of bacterial strains associated with bacterial vaginosis, despite relatively comparable initial representation of Gardnerella, curved Gram− bacilli and Lactobacilli. While both species demonstrated successful colonization by L Rham, the relative representation of this mucosal protective species was markedly higher in RMs than in CMS. Conversely, Gardnerella, after an initial decrease in CMs, regained prominence by week 5 post administration of lactobacilli, whereas RMs limited their rebound over time.
Figure 6.
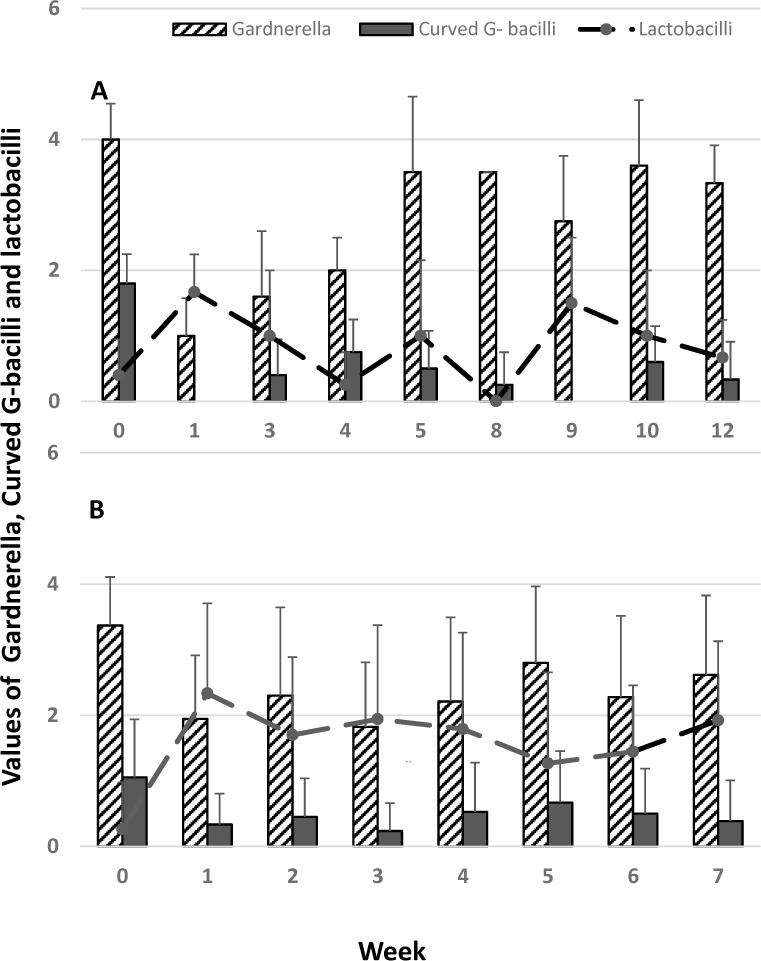
Relative abundance of Gardnerella, curved Gram− bacilli and Lactobacilli in the vaginal environment of CMs (A) and RMs (B) before and after colonization with L Rhamn. The data are representative of 5 CMs and 20 RMs. The values shown represent Nugent scoring values for Gardnerella, curved Gram− bacilli and inverse scoring for Lactobacilli (1–4).
Discussion
A correlation between bacterial vaginosis and increased HIV acquisition in women has been well established [22, 23]. Since macaques are used as a major model of vaginal HIV infection for pathogenesis, vaccine and microbicide efficacy testing, we submit that the definition of the vaginal normal environment in macaque species is a critical parameter, and the ability to recreate various versions of the human microbial environment may represent further refinement of the model. Detailed analysis of the pigtail macaque vaginal microbiome has been reported resulting in preferred use of this species in microbicide studies [24]. RM and particularly CM vaginal environments have received comparatively less attention, even though both species are used extensively for the testing of vaccine efficacy, including protection from repeated vaginal challenges [25–28]. Moreover, the ability to colonize macaques with Lactobacilli to mimic a healthy human vaginal environment has not systematically been attempted. Additionally, there are differences in the reproductive cycle between tropical macaque species (CM and pigtailed macaques) which are cycling year round, and subtropical RMs which exhibit a mating and a reproductive season. Thus, we attempted to define differences in vaginal flora between RM and CM. Of note the data presented herein represents mating season recordings in attempts to match the CMs regular cycles. Indeed, differences were noted between the two species compared. While the average Nugent scores in both species were equivalent to bacterial vaginosis in humans, vaginal Nugent scores were significantly lower in RMs than CMs. Nugent scores are strongly correlated to the prevalence of various bacteria within the vagina, particularly gram negative and gram variable species that produce bacterial vaginosis in humans, relative to Lactobacillus spp [29]. While bacterial vaginosis is associated with increased acquisition of HIV in women, it remains difficult to assert whether a normal RM or CM vaginal flora renders them more susceptible to vaginal infection with SIV, although anecdotal observations suggest that this normal flora does not amount to the level of pro-inflammatory environment that accompanies human bacterial vaginosis [22, 23, 26]. However, it suggests that the benefits of a lactobacillus dominated environment (low pH, production of lactate, inhibition of gram negative bacteria and fungi) are largely absent and as such, the macaque model is more representative of women with bacterial vaginosis as opposed to those with healthy vaginal flora [30].
A low vaginal pH, primarily determined by Lactobacillus production of lactic acid, inactivates HIV [31]. In this study we confirmed that the average vaginal pH of both macaque species studied was higher than the average vaginal pH in women, likely due to the low number of acid producing Lactobacillus spp. However, there was an additional species specific difference with RMs able to maintain a lower vaginal pH than CMs, suggesting greater resistance to vaginal SIV infection in RMs as compared to CMs with a total absence of Lactobacilli spp. The low levels of Lactobacilli spp in pigtail and RMs has been correlated with low availability of glycogen in vaginal epithelium, suggesting that the vaginal environment may not be able to sustain Lactobacilli spp dominance over time[27]. However, in our attempts to recreate a model of healthy human vaginal environment, when the macaque vagina was pretreated with a short course of antibiotic treatment, inoculation of both RMs and CMs with L. rhamn induced a lasting dominant albeit fluctuating presence of this beneficial flora more representative of a healthy human vagina.
Colonizing the RM vagina with Lactobacillus spp has been previously accomplished with strains that were either present in Chinese RMs or L. jensenii expressing cyanovirin-A, a lectin aimed at preventing HIV/SIV acquisition, though Lactobacillus spp are occasionally detected in RMs, and apparently more prevalent in Chinese RMs [32–34]. The initial decrease in pH while attempting to colonize the CM vagina by inoculation only, suggested limited and certainly subdominant colonization though there was no appreciable decrease in Nugent score, even after weekly inoculations of L. rhamn. Even flushing the vagina with 10mL 1% lactic acid immediately prior to inoculation did not appear to create an environment suitable for L. rhamn to prosper. As outlined above, upon successful colonization with L. rhamn, Nugent scores and pH dropped dramatically in CMs, but gradually climbed back up. Additionally, for reasons unknown, L. rhamn dominance transiently resumed at week 9 without external manipulation, indicating the Lactobacillus colonization was durable, but the extent of Lactobacillus dominance varied over time. In RMs, the Lactobacillus colonization and dominance appeared more durable, as it was still detectable several months after inoculation (not shown).
The results of our study outline differences between RM and CM vaginal environment, which may have to be taken into consideration when choosing a model for vaginal challenge with SIV or SHIV. While the reproductive season in RMs may be accompanied with differences in hormonal levels over the course of the year, the ability to colonize them with Lactobacillii to establish a vaginal microbiota resembling a healthy human vaginal microbiota seems readily achievable. CMs on the other hand provide a model of year round cycling similar to humans, but altering the microbiota may be more difficult than in RMs.
Acknowledgments
These studies were funded by a Public Health NIH grant 1U19AI096398 as well as support from the Yerkes Base grant OD P51OD11132. We wish to thank the Veterinary and Research support team of the Yerkes National Primate Research Center for their help in these studies.
References
- 1.Wang H, et al. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the Global Burden of Disease Study 2015. The lancet HIV. 2016;3(8) doi: 10.1016/S2352-3018(16)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall HI, et al. Prevalence of diagnosed and undiagnosed HIV infection—United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2015;64(24):657–662. [PMC free article] [PubMed] [Google Scholar]
- 3.Karim QA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Damme L, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. New England Journal of Medicine. 2008;359(5):463–472. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 5.Baeten JM, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. New England Journal of Medicine. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohan LC, Sassi AB. Vaginal drug delivery systems for HIV prevention. The AAPS journal. 2009;11(1):78–87. doi: 10.1208/s12248-009-9082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baeten JM, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. New England Journal of Medicine. 2016 doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spence P, et al. Recent work on vaginal rings containing antiviral agents for HIV prevention. Current Opinion in HIV and AIDS. 2015;10(4):264–270. doi: 10.1097/COH.0000000000000157. [DOI] [PubMed] [Google Scholar]
- 9.Woodsong C, Holt JD. Acceptability and preferences for vaginal dosage forms intended for prevention of HIV or HIV and pregnancy. Advanced drug delivery reviews. 2015;92:146–154. doi: 10.1016/j.addr.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Hatziioannou T, Evans DT. Animal models for HIV/AIDS research. Nature Reviews Microbiology. 2012;10(12):852–867. doi: 10.1038/nrmicro2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laga M, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. Aids. 1993;7(1):95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Taha TE, et al. HIV infection and disturbances of vaginal flora during pregnancy. JAIDS Journal of Acquired Immune Deficiency Syndromes. 1999;20(1):52–59. doi: 10.1097/00042560-199901010-00008. [DOI] [PubMed] [Google Scholar]
- 13.Ravel J, et al. Twice-daily application of HIV microbicides alters the vaginal microbiota. MBio. 2012;3(6):e00370–12. doi: 10.1128/mBio.00370-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sewankambo N, et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. The Lancet. 1997;350(9077):546–550. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 15.Bouvet JP, Grésenguet G, Bélec L. Vaginal pH neutralization by semen as a cofactor of HIV transmission. Clinical Microbiology and Infection. 1997;3(1):19–23. doi: 10.1111/j.1469-0691.1997.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 16.Money D. The laboratory diagnosis of bacterial vaginosis. Canadian Journal of Infectious Diseases and Medical Microbiology. 2005;16(2):77–79. doi: 10.1155/2005/230319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atashili J, et al. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS (London, England) 2008;22(12):1493. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taha TE, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. Aids. 1998;12(13):1699–1706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Recine N, et al. Restoring vaginal microbiota: biological control of bacterial vaginosis. A prospective case–control study using Lactobacillus rhamnosus BMX 54 as adjuvant treatment against bacterial vaginosis. Archives of gynecology and obstetrics. 2016;293(1):101–107. doi: 10.1007/s00404-015-3810-2. [DOI] [PubMed] [Google Scholar]
- 20.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beverly ES, et al. Utility of Amsel criteria, Nugent score, and quantitative PCR for Gardnerella vaginalis, Mycoplasma hominis, and Lactobacillus spp. for diagnosis of bacterial vaginosis in human immunodeficiency virus-infected women. Journal of clinical microbiology. 2005;43(9):4607–4612. doi: 10.1128/JCM.43.9.4607-4612.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atashili J, et al. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008;22(12):1493–501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taha TE, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS. 1998;12(13):1699–706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Spear GT, et al. Longitudinal assessment of pigtailed macaque lower genital tract microbiota by pyrosequencing reveals dissimilarity to the genital microbiota of healthy humans. AIDS Res Hum Retroviruses. 2012;28(10):1244–9. doi: 10.1089/aid.2011.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu KT, et al. Directed shift of vaginal microbiota induced by vaginal application of sucrose gel in rhesus macaques. Int J Infect Dis. 2015;33:32–6. doi: 10.1016/j.ijid.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 26.Spear G, et al. In captive rhesus macaques, cervicovaginal inflammation is common but not associated with the stable polymicrobial microbiome. PLoS ONE. 2012;7(12):e52992. doi: 10.1371/journal.pone.0052992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirmonsef P, et al. A comparison of lower genital tract glycogen and lactic acid levels in women and macaques: implications for HIV and SIV susceptibility. AIDS Res Hum Retroviruses. 2012;28(1):76–81. doi: 10.1089/aid.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spear GT, et al. Identification of rhesus macaque genital microbiota by 16S pyrosequencing shows similarities to human bacterial vaginosis: implications for use as an animal model for HIV vaginal infection. AIDS Res Hum Retroviruses. 2010;26(2):193–200. doi: 10.1089/aid.2009.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delaney ML, Onderdonk AB. Nugent score related to vaginal culture in pregnant women. Obstet Gynecol. 2001;98(1):79–84. doi: 10.1016/s0029-7844(01)01402-8. [DOI] [PubMed] [Google Scholar]
- 30.Boskey ER, et al. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum Reprod. 2001;16(9):1809–13. doi: 10.1093/humrep/16.9.1809. [DOI] [PubMed] [Google Scholar]
- 31.Aldunate M, et al. Vaginal concentrations of lactic acid potently inactivate HIV. J Antimicrob Chemother. 2013;68(9):2015–25. doi: 10.1093/jac/dkt156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lagenaur LA, et al. Prevention of vaginal SHIV transmission in macaques by a live recombinant Lactobacillus. Mucosal Immunol. 2011;4(6):648–57. doi: 10.1038/mi.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu RR, et al. A Chinese rhesus macaque (Macaca mulatta) model for vaginal Lactobacillus colonization and live microbicide development. J Med Primatol. 2009;38(2):125–36. doi: 10.1111/j.1600-0684.2008.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lagenaur LA, et al. Robust vaginal colonization of macaques with a novel vaginally disintegrating tablet containing a live biotherapeutic product to prevent HIV infection in women. PLoS ONE. 2015;10(4):e0122730. doi: 10.1371/journal.pone.0122730. [DOI] [PMC free article] [PubMed] [Google Scholar]


