Abstract
A series of studies investigated the effects of hedonic content, brightness, and contrast on pupil diameter during free viewing of natural scenes, assessing the amplitude of the initial light reflex and subsequent, sustained pupil diameter change. Hedonic picture content varied from highly arousing scenes of erotica and violence, to scenes depicting nature, babies, loss, contamination, food, and more. Despite equivalent overall picture brightness and contrast, pupil diameter still varied as a function of the local brightness of central vision at fixation. Statistical (Experiment 1) and methodological (Experiments 2 & 3) solutions produced complimentary data indicating that scenes of erotica and violence reliably attenuate the amplitude of the initial light reflex and prompt enhanced late diameter pupil changes, compared to other scene contents. A principal components analysis supported the hypothesis that a single sympathetically-mediated process enhances pupil dilation during picture viewing, modulating both initial constriction and late diameter changes. Rather than being a subtle index of “liking”, pupil diameter is primarily sensitive to events that reliably elicit measurable sympathetic nervous system activity.
Keywords: emotion, pupil, pictures, brightness, contrast, sympathetic nervous system
Pupil diameter is reliably modulated by perceptual, cognitive and affective factors, including brightness, contrast, cognitive task, memory, and emotionality (e.g., Granholm & Steinhauer, 2004). During free viewing of natural scenes, the onset of a picture prompts a rapid constriction that covaries highly with brightness; in contrast, pupil diameter during subsequent viewing is modulated by emotionality, with increased dilation for pleasant or unpleasant, compared to neutral, scenes (Bradley, Miccoli, Escrig, & Lang, 2008; Snowden et al., 2016; Ferrari et al., 2016). Emotional intensity can also modulate the amplitude of the initial light reflex, however. For instance, when a flash of light is presented in the context of threat of shock, the initial reflexive constriction is attenuated, compared to safe periods, eliciting a “fear-inhibited light reflex” (Bitsios, Szabadi, & Bradshaw, 1996). More recent data indicate that viewing highly arousing scenes of erotica and violence also attenuates the initial light reflex, compared to scenes of neutral people (Henderson, Bradley & Lang, 2014). The primary goal of the current series of studies is to determine how early and late modulation of pupil diameter varies with specific picture content to further elucidate the mechanisms underlying changes in pupil diameter during scene perception.
Because brightness is a major factor controlling the amplitude of the initial light reflex (e.g., Lowenfeld, 1999; Barbur, 2004), psychological studies employing visual stimulation must contend with a number of critical issues concerning its control. In a recent study (Henderson et al., 2014), emotionally engaging scenes prompted significant attenuation of the initial light reflex, whereas scrambled versions (identical in overall brightness to the intact scenes) did not, suggesting that differential brightness was not mediating attenuation of the light reflex. Nonetheless, intact scenes varied in brightness (albeit matched across hedonic valence), which might still contribute to differences in early constriction. In the current studies, pictures were altered (using computer software) to be identical in overall brightness. Even when brightness is equated, however, contrast can vary, with scenes composed of brighter whites and darker darks equivalent in mean brightness to scenes composed of more muted grays, potentially affecting pupil diameter. To explicitly investigate effects of scene contrast on pupil diameter during scene viewing, scenes presented in Experiment 1 were identical in brightness, but were presented in either a high or low contrast version to different participants. To the extent that brighter whites affect pupil diameter during scene viewing, we expected smaller pupil diameter changes (e.g., more constriction) when viewing high, compared to low, contrast scenes.
Whereas the modulatory effects of hedonic valence on the amplitude of the initial light reflex are somewhat modest (Henderson et al., 2014), changes in pupil diameter elicited later in the picture viewing interval can be quite dramatic, and covary significantly with skin conductance activity (Bradley et al., 2008). Whereas skin conductance is primarily controlled by sympathetic nervous system activity, pupil diameter is dually controlled by the action of the dilator and sphincter muscles, controlled by sympathetic and parasympathetic nervous system activity, respectively. Thus, it is possible that pupil diameter will be sensitive to scenes that are rated as emotionally engaging (i.e. pleasant or unpleasant), but that do not strongly engage sympathetic activity. Across a series of studies, pictures depicting a variety of semantic and emotional content were presented to investigate modulation of the pupil during scene viewing.
The experimental series
In Experiment 1, twelve different contents were presented that included highly arousing scenes of erotica and violence (threat, mutilation) as well as less arousing unpleasant (i.e., loss and contamination) and pleasant content (i.e., nature, food, and babies), together with neutral objects, scenes, or people. Based on previous studies (e.g., Bradley et al., 2008; Henderson et al., 2014), we expected highly arousing scenes of erotica and violence to significantly modulate late pupil diameter. Of particular interest was whether modulatory effects of viewing sex and violence on initial constriction remain when brightness and contrast are equated across exemplars, and whether other hedonic contents also show early or late pupil modulation. In addition, to determine the extent to which the pupil changes when viewing erotica may be due to the presence of unclothed bodies, scenes of babies either with or without clothes were included in the stimulus set. Although each exemplar in Experiment 1 was identical in brightness, one group of participants viewed low contrast versions of each scene, whereas another group viewed high contrast scenes. Scene contrast was varied between subjects to avoid large changes in contrast within the experimental session.
The data from Experiment 1 suggested that differences in local brightness - the brightness of the portion of the image in central vision for each fixation -- affects pupil diameter during scene viewing. To control for differences in local brightness at fixation, in Experiments 2 & 3, scenes were reduced in size and presented in a perceptual array such that local brightness did not vary with fixation. In Experiment 2, early and late changes in pupil diameter were re-assessed using the same set of 12 hedonic contents presented in Experiment 1. In Experiment 3, four new scene contents were added to assess alternative hypotheses for large modulatory differences found for scenes of erotica in Experiments 1 and 2. A final set of analyses combined data from Experiments 2 and 3 to investigate issues regarding gender, spatial frequency, autonomic reactivity and a single process interpretation of pupil modulation during affective scene viewing.
Experiment 1
Participants
Fifty-one students (25 women) from General Psychology courses at the University of Florida students participated for course credit.
Materials and Design
Stimuli were 60 pictures selected from the International Affective Picture System1 (IAPS: Lang, Bradley, & Cuthbert, 2008) and other sources that were 1024x768 and landscape in orientation. Pictures were converted to 8-bit grayscale using GraphicConverter (version 5.9.5) and modified using GIMP software (version 2.8.10) so that brightness was identical for each picture. Brightness was set to 140 on the 0–255 8-bit (white to black) grayscale range, and contrast, defined as the root mean square (RMS) of the image pixels, was set to 25 (max11 lux on screen) for the low contrast versions. A high contrast version of 50 RMS (max 15 lux on screen) was then created for each scene in which the mean brightness was identical to the low contrast version of each image. Approximately half of the participants (n=26) viewed low contrast versions of each picture and the remaining participants (n=25) viewed high contrast versions.
Of the 60 pictures, there were 20 unpleasant scenes that included contents depicting mutilation (5), contamination (5), attack (5), and loss (5), 15 neutral scenes depicting people (5), objects (5), and scenes (5), and 25 pleasant scenes depicting erotic heterosexual couples (5), food (5), nature (5), and babies (10); pictures of babies included those with (5) and without (5) clothes.
Each picture was displayed for 6 s and preceded by a 7–9 s intertrial interval, during which a small black fixation cross (37 x 39 mm) was displayed in the center of the screen on an 8-bit grayscale patterned image with the same brightness and contrast as the content pictures. Pictures were arranged in blocks of 12, with 5 pleasant, 4 unpleasant, and 3 neutral pictures in each block and no more than two pictures of the same valence presented consecutively. Pictures were presented to participants in one of two orders; across orders, specific pictures were presented in either the first half or second half of the study.
Apparatus
Each 1024 x 768 picture was displayed on a 380 x 300 mm screen (19 in monitor; Samsung SyncMaster 191T) placed at a distance of approximately 76 cm from the participant’s eye, subtending 28 x 22 degrees of visual angle. Pupil diameter was recorded using an ASL 6000 series EyeTrac6 control unit and user interface software (Applied Science Laboratories, Bedford, MA) which allows free movement of the head, and consists of a video camera and an infrared light source pointed at the participant’s right eye. Head movement is accounted for using face tracking via a camera mounted in the recording apparatus. Pupil diameter was recorded for 60 Hz for 2s prior to picture onset and for the duration of picture presentation with a resolution of 0.1 mm.
Procedure
Upon arrival at the laboratory, each participant signed a consent form and was seated in a small, sound-attenuated, dimly lit (approximately 60 lux) room. Each participant was instructed that a series of pictures would be displayed and that each picture should be viewed the entire time it was on the screen.
Data Reduction and Analysis
Samples in which the pupil was obscured due to blinking were identified using ASL Results software and linear interpolation was used to estimate pupil size. Each sample was subsequently deviated from a 1 s baseline preceding picture onset for each participant and trial. Data from 6 participants (3 in each group) were not used due to unsuccessful pupil discrimination on more than one-third of the trials. For the remaining 46 participants, average trial loss was .05.
ANOVAs conducted on each timepoint with content as a factor resulted in significant effects from 750 ms after picture onset until picture offset. The window in which the amplitude2 of initial constriction was estimated therefore began at 750 ms and continued until 1000 ms, and a window from 2 to 3 s following picture onset was used to estimate late pupil diameter changes3.
Mixed ANOVAs included contrast (high, low) as a between subject variable and interval (early, late) and either hedonic valence (3) or specific content (12) as repeated measures. Multivariate (Wilk’s lambda) statistics are reported when relevant.
Results
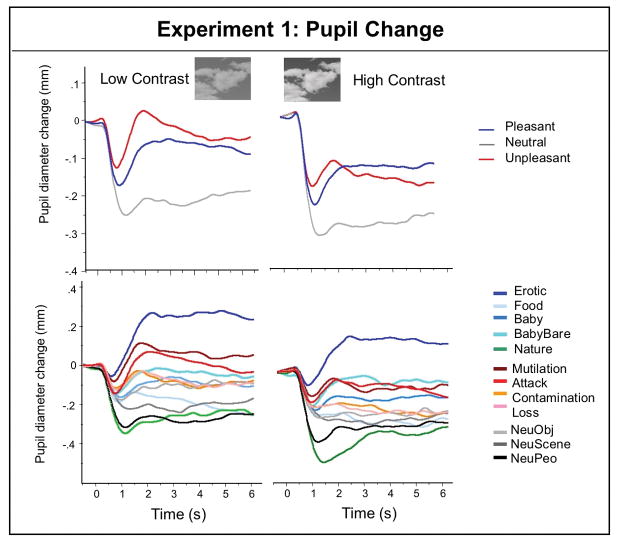
Figure 1 illustrates pupil waveforms measured in Experiment 1 when participants were viewing low (top left) or high (top right) contrast pictures depicting pleasant, neutral or unpleasant scenes that were identical in brightness. A main effect of contrast, F(1,43) = 5.3, p < .03, ηρ2 = .11, indicated that high contrast scenes, which included brighter whites, prompted generally smaller pupil diameter 4 than low contrast scenes throughout picture viewing, which, however, did not differ as a function of hedonic valence.
Figure 1.
Experiment 1. Top Pupil waveforms for low contrast (left) and high contrast (right) scenes of equivalent brightness, averaged over pleasant, neutral, and unpleasant content. Insets (top) illustrate examples of the low and high contrast version of a scene. Bottom: Pupil waveforms for low contrast (left) and high contrast (right) images for each of 12 scene contents.
Significant main effects of hedonic valence were found for both early F(2,42) = 27, p < .0001, ηρ2 = .56 and late diameter change, F(2,42) = 35, p < .0001, ηρ2 = .62 that replicated the pattern found in previous studies. Thus, compared to neutral scenes, both pleasant and unpleasant scenes elicited less initial constriction (F’s(1,43) = 36 and 54, respectively, p’s < .0001) and larger late diameter (F’s(1,43) = 71 & 48, respectively, p’s < .0001). An interaction of interval and hedonic content, F(2,42) = 17.8, p < .0001, ηρ2 = .46, indicated that, whereas pupil diameter when viewing neutral pictures did not change from early to late in the viewing interval, pupil diameter was larger later, compared to earlier, in the viewing interval, for both pleasant F(1,43) = 33, p < .0001, ηρ2 = .44, and unpleasant scenes, F(1,43) = 17.3, p < .0001, ηρ2 = .29.
Figure 1 illustrates pupil waveforms when viewing scenes of different hedonic content in the low (bottom left) and high (bottom right) contrast versions. A significant main effect of contrast, F(1,43) = 6.14, p = .02, ηρ2 = .13, again did not interact with specific hedonic content. Thus, Figure 2 (left panel) plots the mean change during initial constriction and later in the viewing interval as a function of hedonic content, averaged over participants viewing low and high contrast images. Main effects of hedonic content F(11, 33) = 16.4, p < .0001, ηρ2 = .85, and interval, F(1, 43) = 15, p < .0001, ηρ2 = .26, were accompanied by a significant interaction of content and interval, F(11, 33) = 7.8, p < .0001, ηρ2 = .72.
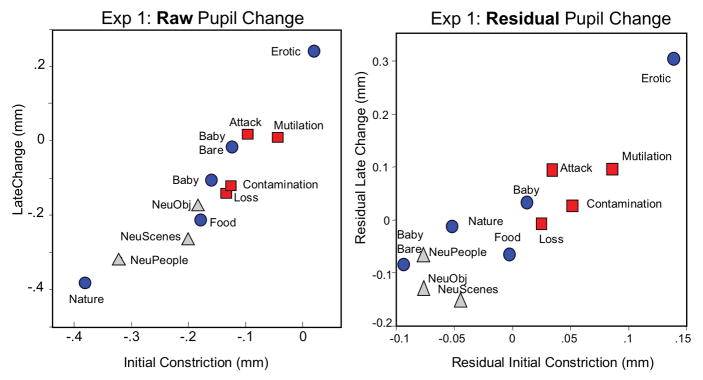
Figure 2.
Experiment 1. LeftMean changes (mm) in pupil diameter during initial constriction (.75 – 1 s) and later in the viewing interval (2–3 s) for each hedonic content; Experiment 1. Right: Residual changes in pupil diameter during initial constriction (.75–1 s) and later in the viewing interval (2–3 s) following an analysis in which local brightness at fixation served as a covariate. Blue=Pleasant scenes; Gray=Neutral scenes; Red=Unpleasant scenes.
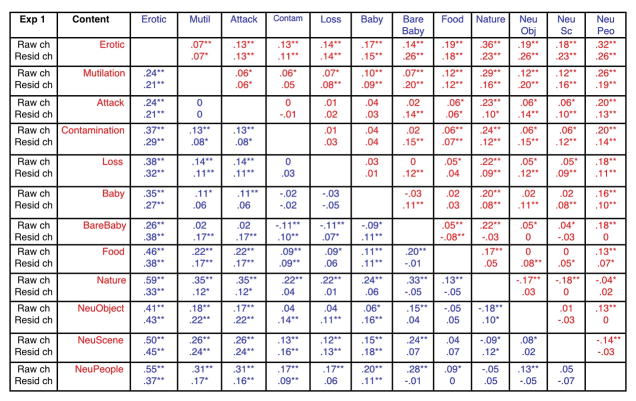
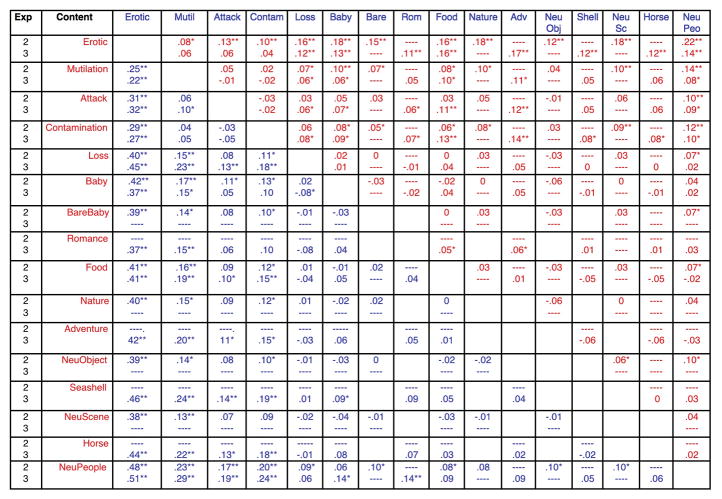
Simple main effects tests indicated that hedonic content affected pupil diameter for both initial constriction, F(11, 33) = 16, p < .0001, ηρ2 = .84 and late diameter changes, F(11,33) = 15.4, p < .0001, ηρ2 = .84. Followup protected t-tests were conducted to assess the reliability of differences between specific contents, and Figure 3 (top row in each cell) lists the difference in pupil diameter between each pair of contents for initial constriction (upper red diagonal) and later diameter changes (lower blue diagonal). Viewing scenes of erotica prompted significantly less initial constriction and greater late diameter changes, compared to all of the other picture contents. On the other hand, nature scenes (which are rated highly pleasant) unexpectedly prompted the greatest initial constriction and least late diameter change, compared to all of the other scenes. When considering the exemplars among nature pictures, a number of exemplars included relatively bright objects, such as white clouds (see example in Figure 3) or snow-covered mountains, raising a question of whether, despite the fact that overall brightness and contrast were identical for each picture, the local brightness at fixation could be contributing to differences in the amplitude of pupil changes, particularly in the region of the light reflex.
Figure 3.
Experiment 1Mean difference in pupil change (mm) between each pair of scene contents and statistical significance for initial constriction (red; top diagonal) and late diameter changes (blue; bottom diagonal) using either raw pupil change (top entry in each cell) or residual change (bottom entry in each cell) following a regression analysis using local brightness at fixation as a covariate. The direction of the subtraction for the difference score is Row Content (red) minus Column Content (blue) for the red (upper) diagonal and Column Content (blue) minus Row Content (red) for the blue (lower) diagonal. ** p < .005, * p < .05, --- no comparison.
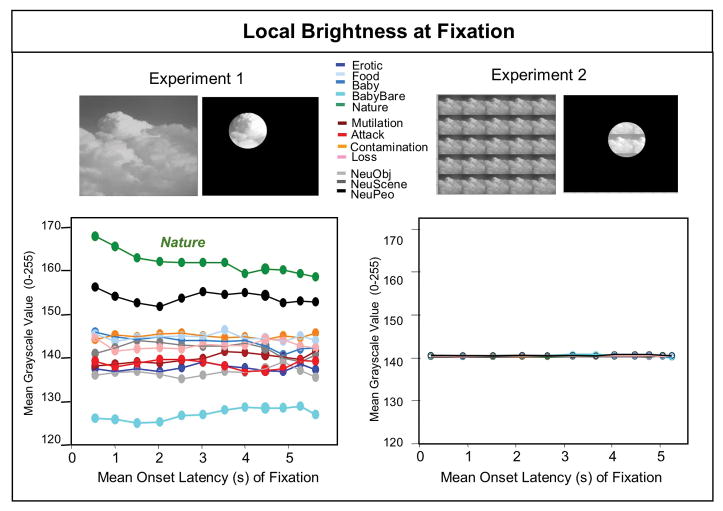
To assess this possibility, the local brightness at each fixation was determined by using a 10-degree circular mask to represent the portion of the scene available to central (macular5) vision on each fixation, as illustrated in the top of Figure 4. The mean local brightness in this region was computed for each participant, trial, and fixation. Figure 4 (bottom left) illustrates the mean local brightness for each of the first 12 fixations, averaged over participant and content. A significant main effect of hedonic content, F(11,34) = 34, p < .0001, ηρ2 = .92 supports the hypothesis that the local brightness at fixation varied with hedonic content, despite the fact that each scene was identical in overall brightness and contrast.
Figure 4.
Experiment 2. Top left Local brightness at central fixation was determined by overlaying a 10 degree circular mask on each scene for each fixation to estimate brightness of central vision. Bottom left: Data for the first 12 fixations are plotted as a function of the mean onset latency of each fixation averaged across participants for each content. The local brightness at fixation was significantly higher for nature scenes, compared to other content, and significantly less for scenes of babies (without clothes). Experiment 3. Top right:Local brightness at fixation was again determined by overlaying a 10 degree circular mask on the 5x5 matrices to estimate the brightness of central vison for each fixation. Bottom right: Data for the first 12 fixations are again plotted as a function of the mean onset latency of each fixation averaged across participants for each content. Unlike Experiment 2, there are no differences in local brightness at fixation as a function of content, confirming that the 5 x 5 display reduces or eliminates differences in local brightness.
Consistent with the data illustrated in Figure 4 (bottom left), followup protected t-tests indicated that significantly brighter regions were fixated when viewing nature scenes, compared to all other content, and significantly darker regions were fixated when viewing pictures of babies without clothes, compared to all other contents. In fact, even small differences in local brightness among the scenes tended to be significantly different, with only pictures of clohted babies, contamination and food not different from one another.
To statistically remove effects of local brightness on pupil change, a repeated measures analysis of covariance was conducted in which the average local brightness for fixations made in the first 3 s of the viewing interval6 for each content served as the covariate in separate analyses of early and late pupil changes. Figure 2 (right panel) plots the mean residuals resulting from this analysis. Residual pupil changes were most different from raw changes for scenes of nature and babies without clothes, which had garnered the brightest and darkest local brightness at fixation, respectively. Despite these differences, the overall pattern of modulation across content is strikingly similar to the pattern found with the raw changes, with a significant main effect of hedonic content F(11,34) = 22.7, p < .0001, ηρ2 = .88 and an interaction of content and interval, F(11,34) = 7.7, p < .0001, ηρ2 = .71.
Again, significant simple main effects of content were found both during initial constriction, F(11,34) = 12.1, p < .0001, ηρ2 = .80 and later in the viewing interval, F(11,34) = 22, p < .0001, ηρ2 = .88. Figure 3 (bottom row in each cell) lists the mean differences between each hedonic content for residual pupil changes and the results of t-tests for initial constriction (red; top diagonal) and late diameter change (blue; bottom diagonal). Similar to the effects found for raw pupil changes, erotica again prompted significantly less initial constriction than all other picture contents. Scenes of mutilation and contamination did not significantly differ in the amplitude of initial constriction, and were similar in size to that found for scenes of attack, with each prompting significantly less constriction than scenes of babies (without clothes), food, nature, or neutral images. Although the differences were much smaller in magnitude, pictures of loss, babies (with clothes), and food prompted slightly less constriction than neutral objects, scenes or people. On the other hand, initial constriction for nature scenes and babies without clothes did not differ in size from that measured when viewing neutral scenes.
Late diameter residual changes, like late raw changes, indicated that scenes of erotica prompted significantly larger late diameter changes, compared to each of the other picture contents. Scenes of mutilation and attack prompted the next largest late changes, which did not differ from one another and were both slightly larger then found for scenes of contamination. Scenes of babies (with clothes) prompted greater late diameter changes than all of the neutral contents, whereas scenes of nature and loss were somewhat more variable, showing significantly larger late residual changes compared to neutral objects and scenes. Late pupil diameter when looking at pictures of food did not differ from that measured when looking at neutral pictures. Unlike the raw changes, when differences in brightness at local fixation were removed, scenes of babies (without clothes) did not prompt late changes that differed from any neutral scene content.
Discussion
High contrast scenes prompted greater initial constriction and smaller late diameter changes than low contrast scenes of identical brightness, consistent with a hypothesis that brighter whites in the visual display affect pupil diameter, regardless of equivalent overall brightness. More importantly, even when scenes were identical in brightness and contrast, the local brightness at fixation varied as a function of specific content, with brighter regions fixated for nature exemplars (i.e., clouds, snow-covered mountains) and darker regions for scenes depicting babies without clothes. These differences were simply due to variations in the brightness of perceptual features in individual scenes.
A statistical solution controlled differences in local brightness at fixation by assessing residual pupil changes following a regression analysis in which the brightness at central vision was regressed on raw pupil change, separately for initial constriction and late diameter changes. Not surprisingly, differences between residual and raw pupil change were greatest for specific contents that had varied greatly from the others in local brightness at fixation (e.g., nature, babies). Nonetheless, effects of specific content on initial constriction and late diameter changes remained fairly similar to those found for the unadjusted raw changes, in which scenes of erotica, mutilation, attack, and, to some extent, contamination, most reliably prompted less initial constriction and larger late pupil diameter changes, compared to other scenes.
Experiment 2
In Experiment 2, we re-assessed effects of hedonic scene content using a methodological, rather than statistical, solution to control differences in local brightness at fixation. One obvious method is to reduce the size of the image so that pixels differing in local brightness tend to be represented in every fixation. A very small image at a distance from the viewer would satisfy this requirement. On the other hand, small scenes are more difficult to perceptually parse, requiring finer oculomotor adjustments during viewing, and could potentially alter semantic or affective processing.
Thus, as a compromise, each picture was reduced in size and then repeated 25 times in a 5x5 perceptual array, as illustrated in Figure 3 (top right). In these displays, affective and semantic information is still relatively easy to apprehend, due to the repetition of scenes, but the decrease in picture size should reduce differences in local brightness at each fixation. If these perceptual arrays are successful in reducing the effects of local brightness on pupil diameter, we expected that, unlike in Experiment 1, local brightness at fixation would no longer differ as a function of specific content. To further reduce effects of local brightness, the low contrast images from Experiment 1 were used.
The same 12 picture contents were presented as in Experiment 1. Heart rate and skin conductance were measured as additional indices of emotional reactivity and these data are reported in the combined analysis in Experiment 3.
Method
Participants
Participants were 35 students (19 female) from General Psychology courses at the University of Florida who participated for course credit.
Materials and Design
Scenes were identical to those used in Experiment 1 except that each scene was reduced in size to 205x154 pixels and presented 25 times in a 5x5 matrix (see Figure 3, top right).
Autonomic measurement
Heart rate was measured using two 22 mm Ag/Cl sensors placed on the inside surface of each forearm. The cardiac signal was input to a Coulbourn S75–01 bioamplifer with a bandpass filter of 8–40 Hz and then to a Coulbourn bipolar comparator module that sent a digital trigger each time an R-wave was detected. Interbeat intervals were recorded in ms and then edited offline and converted to beats-per-minute (BPM) using VPM software (Cook, 2001).
Skin conductance was measured using two 22 mm Ag/Ag-Cl electrodes filled with appropriate paste and placed adjacently over the hypothenar eminence of the nondominant palm. A constant current (.5 V) was generated between the electrodes using a Coulbourn S71–22 coupler, and the resulting activity was sampled and digitized at 10 Hz. Both skin conductance and heart rate change were quantified by subtracting the average activity in the 1 s preceding picture onset from each data point. Based on the resulting waveforms, cardiac orienting was defined as the average heart rate change in the first 3 s of picture viewing; skin conductance reactivity was defined as the average skin conductance change from 3–6 s post picture onset. A log transform was conducted on skin conductance data prior to analysis.
Procedure and Data Analysis
The procedure was identical to that described in Experiment 1, as were data acquisition and reduction. Data from four participants were not used due to unsuccessful pupil discrimination on more than one-third of the trials. For the remaining 31 participants, average trial loss was .03.
ANOVAs conducted on pupil change for each timepoint using content as a factor again resulted in significant effects from 750 ms after picture onset until picture offset, and early constriction was again estimated in a window from 750–1000 ms after picture onset and late diameter changes in a window from 2–3 s after picture onset.
Results
Figure 5 (top) illustrates pupil waveforms when viewing the scenes presented in the 5x5 matrices. As a method check, the local brightness of central vision for each fixation was again computed and is illustrated in Figure 4 (right panel). Unlike Experiment 1, analysis of the local brightness at fixation did not produce any significant differences as a function of hedonic content, with the mean identical to the manipulated brightness of the overall scene (i.e. 140 on 0–256 grayscale). These data confirm that the selected perceptual matrix at the utilized distance eliminated differences in local brightness at fixation.
Figure 5.
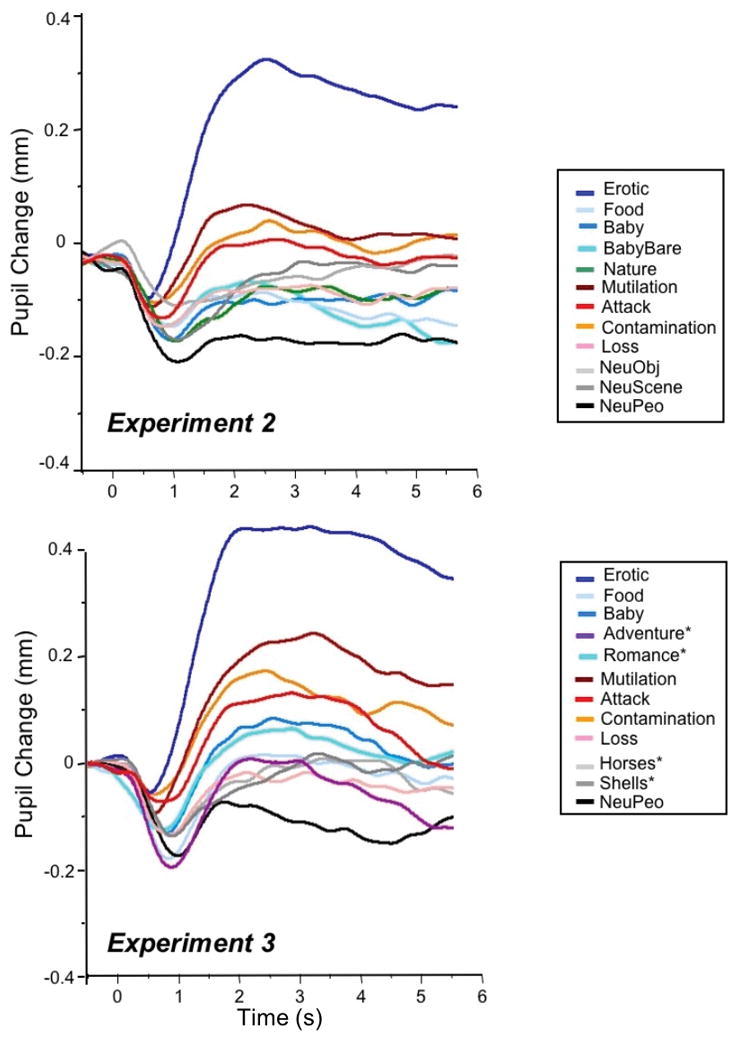
Top: Pupil waveforms for each of the 12 picture contents presented in Experiment 2. Bottom: Pupil waveforms for each of the 12 picture contents presented in Experiment 3.
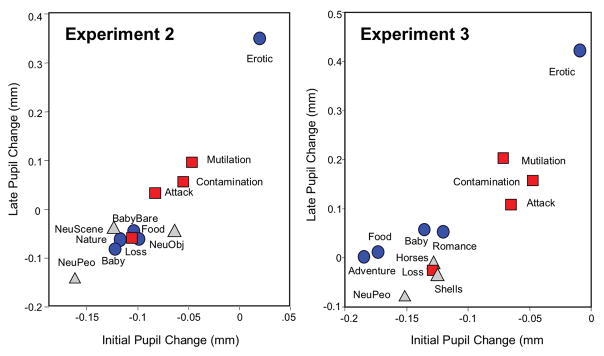
Figure 6 (left panel) illustrates early and late pupil changes as a function of specific content in Experiment 2. Similar to Experiment 1, main effects of hedonic content, F(11, 20) = 6.9, p < .0001, ηρ2 = .79, and interval, F(1, 30) = 41, p < .0001, ηρ2 = .58, were accompanied by a significant interaction of content and interval, F(11,20) = 9.4, p < .0001, ηρ2 = .84. Again, significant simple main effects of content were found for both for initial pupil constriction, F(11,20) = 2.84, p = .02, ηρ2 = .61, and for later pupil diameter changes, F(11,20) = 8.2, p < .0001, ηρ2 = .82. Figure 7 (top row in each cell) lists the difference in pupil diameter between each pair of contents for initial constriction (upper, red diagonal) and later changes (lower, blue diagonal), together with the results of protected t-tests assessing the size of the differences for initial constriction (red diagonal) and late diameter change (blue diagonal).
Figure 6.
Left: Mean change (mm) in pupil diameter during initial constriction (.75 - 1 s) and later in the viewing interval (2–3 s) for each hedonic content; Experiment 2. Right: Mean change (mm) in pupil diameter during initial constriction (.75 - 1 s) and later in the viewing interval (2–3 s) for each hedonic content; Experiment 3. Blue=Pleasant scenes; Gray=Neutral scenes; Red=Unpleasant scenes.
Figure 7.
Mean difference in pupil change (mm) between each pair of scene contents and statistical significance for initial constriction (red; top diagonal) and late diameter changes (blue; bottom diagonal) for scenes presented in Experiment 2 (top entry in each cell) and Experiment 3 (bottom entry in each cell). The direction of the subtraction for the difference score is Row Content (red) minus Column Content (blue) for the red (upper) diagonal and Column Content (blue) minus Row Content (red) for the blue (lower) diagonal. ** p < .005, * p < .05, --- no comparison.
The main differences in pupil diameter between specific contents were similar to those found in analyses of both the raw and residual data in Experiment 1. Thus, for initial constriction, scenes of erotica again resulted in significantly less initial constriction than all other contents. Scenes of mutilation did not differ from scenes of attack or contamination in initial constriction and all of these contents showed significantly less constriction than most of the other contents. Aside from these effects, however, differences in initial constriction were small and somewhat variable among the remaining contents, with scenes of loss, food and babies (without clothes) showing slightly, but significantly, less initial constriction than scenes of neutral people.
For late pupil diameter changes, scenes of erotica again prompted large differences that were significantly from each of the other contents, whereas scenes of mutilation, attack and contamination did not differ from one another and prompted the second largest increases in pupil diameter, after erotica. Other contents were similar in the amplitude of late diameter changes with only scenes of loss, food, and babies (without clothes) prompting slightly larger changes than when neutral people.
Experiment 3
In Experiment 3, we explored a number of different possible explanations for the heightened modulation found for scenes of erotica. First, data from both Experiments 1 & 2 suggest that the large modulatory changes for erotica are probably not due to the presence of nude bodies, as, once local brightness was controlled, scenes of babies without clothes did not prompt reactions similar to those found for erotic scenes. To determine whether the effects are specific to erotica or are a more general feature of arousing pleasant scenes, two additional categories of pleasant scenes that garner relatively high ratings of arousal were presented, including scenes of romance (which replaced bare babies) and adventure (which replaced nature). Scenes of romance depict clothed couples in loving contexts (e.g. hugging, kissing) that are not sexually explicit, and adventure scenes include exciting physical activities such as ski jumping, surfing, etc.
Second, in addition to ratings of high arousal, erotic scenes are perceptually more similar to one another, and, because of this, are also functionally repeated across the experiment. Previous data indicate that late pupil diameter in particular can sometimes increase for repeated items (Bradley & Lang, 2015; Goldinger & Papesh, 2012). To determine the extent to which feature similarity or recognition, rather than emotional arousal, could be contributing to these large changes in pupil diameter, low arousal scenes in which specific features were very similar across exemplars were presented, including pictures of seashells (which replaced neutral objects) and pictures of horses (which replaced neutral scenes).
Scenes were identical in brightness and contrast and presented in the 5x5 perceptual arrays used in Experiment 2.
Method
Participants
Thirty-eight students (23 female) from General Psychology courses at the University of Florida who participated for course credit.
Method, Procedure, and Data Analysis
The design was identical to Experiment 2 except that 4 new categories of 5 pictures each were presented, including adventure, romance, seashells, & horses, together with the 5 pictures in 8 of the categories presented in Experiment 2, including erotica, mutilations, attack, contamination, loss, babies, food, and everyday people. Pictures were again presented in 5x5 matrices that were identical in brightness (mean grayscale = 140) and contrast (RMS = 25).
The procedure was identical to that described in Experiments 1 & 2, as were data acquisition and reduction. Data from three participants were not used due to unsuccessful pupil discrimination on more than one-third of the trials. For the remaining 35 participants, average trial loss was .04.
ANOVAs conducted on pupil change for each timepoint using content as a factor again resulted in significant effects from 750 ms after picture onset until picture offset, and early constriction was therefore again estimated in a window from 750–1000 ms after picture onset and late diameter changes in a window from 2–3 s after picture onset.
Results
Figure 5 (bottom) illustrates pupil waveforms as a function of specific content in Experiment 3, and Figure 6 (right) plots early and late pupil changes. The pattern of modulation is again similar to that found in Experiments 1 & 2, with significant effects for hedonic content, F(11,24) = 10.7, p < .0001, ηρ2 = .83, interval, F(1,34) = 61, p < .0001, ηρ2 = .64 and their interaction, F(11,24) = 15.3, p < .0001, ηρ2 = .88. Effects of hedonic content were significant both for initial constriction, F(11,24) = 3.6, p = .004, ηρ2 = .62, and for late diameter changes, F (11,24) = 19.4, p < .0001, ηρ2 = .90.
Figure 7 (bottom row in each cell) lists differences in pupil change between each content and the results of significance tests for initial constriction (red; upper diagonal) and late diameter changes (blue; lower diagonal). For initial constriction, scenes of erotica, mutilation, attack, and contamination did not differ from one another and all prompted less initial constriction than scenes of neutral people. For late diameter changes, erotica continued to prompt significantly larger changes than all other contents, and scenes of mutilation, attack, and contamination showed larger late diameter than scenes of loss, food, adventure, shells, horses, and neutral people.
Neither adventure scenes or scenes of romance prompted attenuated initial constriction compared to neutral scenes, and late diameter changes when looking at romantic pictures did not differ from that elicited when viewing adventure, babies, or food. On the other hand, pictures of romance and babies showed some evidence of larger late changes, compared to when viewing neutral people.
Scenes with similar features -- seashells and horses- did not prompt a pattern similar to that found for erotic scenes, but instead prompted changes in initial constriction and late diameter changes that were not different from when looking at neutral scenes of people.
Combined analysis: Experiments 2 & 3
Data from the 66 participants7 in experiments 2 & 3 (which utilized the same type of perceptual array) were aggregated using the common hedonic contents that were presented (i.e., erotic, mutilation, attack, contamination, loss, food, baby, and neutral people) to investigate a number of additional issues concerning pupil modulation during picture viewing.
Spatial frequency
Although brightness, contrast, and local brightness at fixation were controlled for the 5x5 matrices presented in Experiments 2 and 3, spatial frequency could still vary between visual displays. Using picture, rather than participant, as the unit in this analysis, a multiple regression analysis including various measures of spatial frequency (e.g., JPEG size, the percentage of the image explained by first singular value [first eigenimage] following a singular value decomposition) found no significant effects of spatial frequency on changes in pupil diameter.
Gender
Previous studies have found that men often show heightened reactions to erotica, compared to women (e.g., Bradley, Codispoti, Sabatinelli, & Lang, 2001) and previous data (Hess & Polt, 1960) have suggested that women are more reactive to scenes depicting babies. Table 1 lists the mean pupil response for late pupil diameter changes and initial constriction separately for men and women for the common contents presented in Experiments 2 & 3. Changes in pupil diameter, both early and late, were strikingly similar for men and women, with no significant differences, and no interactions involving gender.
Table 1.
Mean (SE) of pupil diameter changes (late and early), skin conductance change, and heart rate change as a function of specific content for all participants, and separately for men and women. Combined Analysis of Experiment 2 & 3.
| Hedonic Content | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | Erotic | Mutil | Attack | Contam | Food | Loss | Baby | NeuPeo |
| Late Pupil Diameter Change (2–3 s; mm) | ||||||||
|
Women n=42 |
.39 (.03) | .15 (.04) | .07 (.08) | .15 (.06) | −.03 (.05) | −.04 (.02) | −.02 (.04) | −.10 (.04) |
|
Men n=24 |
.37 (.05) | .16 (.05) | .07 (.07) | .03 (.06) | −.01 (.04) | −.05 (.04) | .02 (.02) | −.16 (.05) |
|
All n=66 |
.38 (.03) | .15 (.04) | .07 (.08) | .10 (.06) | −.03 (.04) | −.04 (.03) | −.01 (.03) | −.12 (.04) |
| Initial Pupil Constriction (.75-1 s; mm) | ||||||||
|
Women n=42 |
−.03 (.03) | −.08 (.01) | −.10 (.02) | −.07 (.02) | −.17 (.04) | −.12 (.02) | −.17 (.03) | −.16 (.02) |
|
Men n=24 |
−.03 (.04) | −.07 (.02) | −.06 (.03) | −.08 (.03) | −.10 (.03) | −.13 (.02) | −.10 (.02) | −.17 (.02) |
|
All n=66 |
−.03 (.03) | −.07 (.01) | −.08 (.02) | −.06 (.02) | −.14 (.03) | −.13 (.02) | −.14 (.02) | −.16 (.01) |
| Heart Rate Change (bpm) | ||||||||
|
Women n=41 |
−2.42 (.45) | −2.47 (.15) | −1.41 (.54) | −1.84 (.06) | −.83 (.41) | −.60 (.27) | .31 (.41) | .31 (.41) |
|
Men n=23 |
−2.08 (.26) | −2.36 (.56) | −1.44 (.75) | −1.75 (.91) | −1.08 (.51) | −.75 (.67) | .11 (.62) | −.25 (.37) |
|
All n=64 |
−2.22 (.25) | −2.30 (.24) | −1.46 (.55) | −1.68 (.30) | −.93 (.24) | −.70 (.41) | .26 (.35) | −.06 (.36) |
| Skin Conductance Change (Log μS) | ||||||||
|
Women n=41 |
.043 (.007) | .048 (.007) | .043 (.009) | .018 (.005) | .016 (.002) | .023 (.006) | .022 (.005) | .021 (.004) |
|
Men n=24 |
.064 (.005) | .032 (.013) | .026 (.007) | .015 (.006) | .014 (.006) | .016 (.002) | .015 (.006) | .011 (.003) |
|
All n=65 |
.051 (.006) | .042 (.008) | .036 (.007) | .017 (.005) | .015 (.003) | .021 (.004) | .020 (.004) | .018 (.002) |
Autonomic response
The pupil is dually controlled by parasympathetically mediated constriction via the pupillary sphincter muscle and sympathetically mediated dilation via the dilator muscle. Because the slowing of heart rate following stimulus onset in animals is often attributed to parasympathetic activation (e.g., Campbell, Wood, & McBride, 1997) whereas skin conductance is attributed to a sympathetically-mediated reaction (e.g., Martini & Bartholomew, 2001), we measured heart rate and skin conductance during picture viewing in Experiments 2 & 3 as indices of parasympathetic and sympathetic activation, respectively.
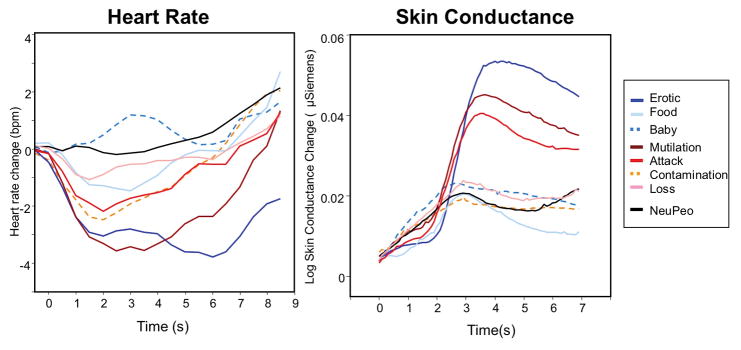
Figure 8 illustrates the heart rate and skin conductance change waveforms during picture viewing. Skin conductance reactivity was defined as the mean change 3–6s following picture onset, and cardiac orienting as the mean change over the first 3 s of picture viewing (see Table 1). Significant differences as a function of specific content were found for each measure (skin conductance (F[7,57] = 2.8, p = .014, ηρ2 = .26; cardiac orienting, F[7, 55] = 6.8, p < .0001, ηρ2 = .46). Pairwise comparisons showed that skin conductance changes were enhanced when viewing scenes of erotica and mutilations, compared to scenes of neutral people (p’s < .005). A similar pattern was found for cardiac orienting, with greater heart rate deceleration when viewing scenes of erotica and mutilation, compared to scenes of neutral people (p’s < .005). There were no main effects or interactions involving gender in analyses of either heart rate or skin conductance change (see Table 1).
Figure 8.
Heart rate (left) and skin conductance (right) change as a function of hedonic content for participants in Experiments 2 and 3.
Principal components analysis
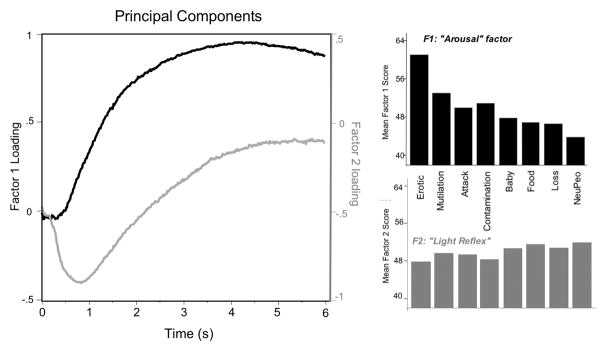
Effects of hedonic content were similar for both early and late pupil diameter changes, but larger later in the viewing interval, suggesting a single modulatory process whose impact increases over time. To explore this hypothesis, a principal components analysis was calculated in which the timecourse pupil changes from 0–6 s for each content (8) and participant (66) served as the input matrix. Two components (following varimax rotation) accounted for 86% of the variance. As illustrated in Figure 9 (left panel), the first factor (accounting for 74% of the variance) shows no change in pupil diameter for the 500 ms following picture onset and then increases in amplitude across the viewing interval, consistent with sympathetically-mediated action on the iris dilator muscle. The second component (12% of the variance) identifies a rapid constriction in the initial second of picture viewing (peaking around 800 ms after picture onset), consistent with the action of the sphincter muscle on pupil diameter in the light reflex window.
Figure 9.
Left: A principal component analysis on pupil waveforms resulted in two components in which the primary component (black) is a pupil dilation that begins around 500 ms after picture onset and continues throughout the viewing interval, consistent with an effect of emotional arousal on the dilator muscle, and a second component (gray) that is an initial rapid constriction, consistent with the light reflex. Right: Hedonic content had a significant effect on the factor scores for the first, arousal, component (top), with significantly higher scores for emotionally arousing, compared to neutral, content, whereas hedonic content did not significantly affect factor scores for the second, light reflex, component (bottom). The mean factor scores for each component were algebraically transformed ((score x10) + 50) to be above zero.
Separate repeated measures analysis of variance on the factor scores indicated a significant effect of hedonic content only for the primary dilation component, F(7,59) = 34.8, p < .0001, ηρ2 = .81, with erotica, mutilation and threat showing higher factor scores than the other contents (see Figure 9, top right panel). For the second, light reflex, component however, there were no differences in factor scores as a function of hedonic content8 (Figure 9, bottom left panel; F(7,59) = 1.2, p = .22).
General Discussion
Large, reliable changes in pupil diameter were found when participants viewed natural scenes of erotica and violence, including significant attenuation of initial pupil constriction (in the region of the light reflex), as well as larger diameter changes later in the viewing interval. Despite equivalence in scene brightness and contrast, pupil diameter was somewhat influenced by the local brightness of central fixation which was successfully dealt with statistically (Experiment 1) and methodologically (Experiments 2 & 3). A principal component analysis on the combined data from Experiments 2 & 3 supported the hypothesis that pupillary changes during affective picture perception reflect modulation by a single process in which pupil dilation begins around 500 ms after picture onset and continues until offset, consistent with sympathetically-driven action of the iris dilator muscle. Supporting this, factor scores on this primary component were significantly elevated for emotionally arousing, compared to neutral, scenes.
Brightness and contrast
High, compared to low, contrast versions of scenes that were identical in brightness prompted more initial constriction and less late diameter change, consistent with the expected impact on pupil diameter of the brighter whites in high contrast images. On the other hand, equating scenes for overall brightness and contrast did not unequivocably prompt an identical sensory experience, particularly in terms of the initial light reflex. Rather, depending upon the semantic and affective content of brighter and darker regions in specific pictures, the local brightness at fixation varied with specific content. Thus, in Experiment 1, the local brightness at fixation was elevated for nature scenes depicting white clouds or snow-covered mountains, which was due solely to the brightness of the interesting and/or informative information in the visual array.
In Experiment 1, a statistical analysis assessed residual pupil change after covarying the local brightness at fixation and demonstrated that, notwithstanding effects of local brightness, the pattern of pupil modulation for raw and residual changes was very similar, discrepant only for the specific contents that showed the largest divergence in local brightness from the intended grayscale mean (e.g., nature). In Experiment 2, differences in local brightness at fixation were methodologically controlled by reducing picture size and presenting these images in perceptual arrays in which each fixation included all brightness levels. A manipulation check confirmed that, for fixations on these scenes, mean local brightness did not differ as a function of specific content and was indeed identical to the computed (and intended) mean brightness of each scene. Pupil modulation as a function of content when viewing these local brightness-controlled perceptual arrays was similar in both initial constriction and late pupil diameter - greatest for scenes of sex and violence - as previously found in analysis of residual pupil change in Experiment 1.
Considering that differences in local brightness at fixation can affect pupil diameter, it will be important to control this factor in studies assessing pupil changes using visual displays. One solution is to reduce the size of the image so that, at the established viewing distance, each fixation tends to include all variations in local brightness. A second solution is to simultaneously measure eye fixations and to subsequently remove differences in the brightness at central vision from measured pupil changes. In either case, differences in pupil diameter due to brightness are reduced or eliminated. In an early study that considered the potential effects of local brightness on pupil diameter, Hess, Beaver & Shrout (1975) presented a single slide containing squares of different brightness, instructing participants to view different portions for 8s, with results suggesting that the relationship between brightness at fixation and pupil diameter was small. Similarly, in the studies reported here, changes in pupil diameter due to local brightness had a minimal effect on the pattern of pupil modulation as a function of hedonic content. Nonetheless, to the extent that interesting or informative areas systematically vary in brightness, the critical signal will include more noise, if left uncontrolled in studies using visual stimuli.
Hedonic content
Enhanced pupil diameter found when viewing scenes of erotica - either early or late - was not due to the presence of naked bodies, since, when brightness was controlled, pictures of babies did not prompt pupil diamter changes similar to that found for erotic couples. In addition, presenting specific contents that shared features across exemplars (i.e., seashells, horses) did not result in late dilation changes that were similar to those found for erotica, reducing the possibility that this effect reflects perceptual similarity or recognition. And, neither arousing scenes of adventure and romance or pleasant pictures of food, babies, and nature scenes strongly modulated pupil diameter; effects for each of these contents were smaller, less reliable, and more variable in pattern across the three experiments. Scenes of babies, for example, sometimes prompted late changes that were slightly larger than when viewing neutral people (Experiment 3) and sometimes did not (Experiment 2). In an early study involving a small sample of 3 men and 3 women, Hess & Polt (1960) reported that women, compared to men, showed greater pupil dilation when viewing babies, but, in the larger sample investigated here, gender differences were negligible for all contents.
Although earlier studies suggested that the pupil constricted when viewing unpleasant scenes (Hess & Polt; 1960; Nunnally, Knott, Duchnowski, and Parker, 1967), there was no evidence of pupil constriction when viewing aversive content in the current studies. Rather, for arousing scenes of mutilation and attack, pupil diameter increased substantially, and modulation was apparent both in attenuated initial constriction and larger late diameter changes. Scenes of contamination, on the other hand, often, but not always, prompted modulatory effects similar to those found with mutilation and attack. Followup analysis of individual exemplars suggested that images that depict people actively vomiting was a key element in determining the specific scenes scenes that elicited enhanced pupil diameter and skin conductance changes. For less arousing unpleasant scenes of loss (sadness), on the other hand, neither pupil diameter nor autonomic reactions were strongly modulated.
Finding that emotionally arousing contents of sex and violence and, to some extent, highly disgusting images are those that most reliably induce changes in pupil diameter is consistent with previous studies finding that only some picture contents that are rated emotionally arousing reliably prompt psychophysiological reactivity. Thus, in studies that have explicitly compared specific hedonic content, it is scenes depicting sex and violence that elicit the most startle modulation (Bradley, Codispoti, Cuthbert, & Lang, 2001), the largest late positive brain potentials (Schupp et al., 2004, Weinberg & Hajcak, 2010), the largest attenuation of startle probe P3 amplitude (Schupp et al., 2004), enhanced scanning (Bradley, Costa, & Lang, 2015), and heightened BOLD activity (Bradley et al., 2003). That pupil diameter is also similarly selectively sensitive to arousing scenes of sex and violence is consistent with these findings. After more than 25 years of research using emotional pictures, it is now clear that evaluative (arousal) ratings of natural scenes are not synonymous with the degree of engagement of defensive or appetitive activation measured in the brain or body.
From a motivational perspective, we have suggested that it is cues that strongly engage fundamental systems of appetite and defense -- those that would demand action if encountered in the natural environment -- that prompt measurable reactions in the laboratory psychophysiological context (e.g., Lang, Bradley & Cuthbert, 1997; Bradley & Lang, 2007; Bradley, Codispoti, Cuthbert, & Lang, 2001). At least for the healthy, relatively young participants in the current studies, it is scenes of sex and violence and, to some extent disgusting scenes, that reliably engage sympathetic arousal, prompting modulation of pupil diameter and other measures of sympathetic nervous system action. Evaluative reports, on the other hand, are more likely to also be influenced by cultural or societal norms regarding appropriate emotional arousal. Specific contents of sex and violence, or, more prosaically, those cuing love or death, are, however, the most reliable inducers of emotion in the laboratory environment, and studies comparing responses to pleasant and unpleasant stimuli will vary to the extent that either or both of these contents are not included in the experimental array.
Autonomic response
The dilator and sphincter muscles that control pupil diameter are innervated by sympathetic and parasympathetic action, which, respectively, increase or decrease pupil diameter (Beatty & Lucero-Wagner, 2000; Loewenfeld, 1958; Yoshitomi, Ito, & Inomata, 1985). Previously, a strong covariation between skin conductance change and late changes in pupil diameter suggested that both were mediated by sympathetic nervous system activity (Bradley et al., 2008). On the other hand, the initial light constriction is often found to be parasympathetically mediated (Campbell, Wood, & McBride, 1997; Steinhauer, Siegle, Condray, & Pless, 2004), suggesting that the attenuated light reflex for emotionally arousing scenes could index decreased parasympathetic activity. Skin conductance and heart rate were measured as indices of autonomic nervous system activity in Experiments 2 & 3. Replicating previous studies (e.g., Bradley et al., 2001; Bradley et al., 2008), scenes of erotica and violence prompted the greatest skin conductance activity, simultaneous with a parasympathetically mediated increase in cardiac orienting (e.g., heart rate deceleration).
Finding both parasympathetically-mediated cardiac deceleration and sympathetically-mediated pupillary dilation during emotional picture viewing was initially described as an instance of “directional fractionation” by Libby, Lacey, & Lacey (1973) who interpreted it as indexing a mode of autonomic co-activation. More recently, we have suggested that different components of the orienting response accompany separate processes, with cardiac deceleration indexing initial information intake, and skin conductance activity indexing co-occurring preparation for action, which are accompanied by different temporal profiles of habituation (e.g., Bradley, 2009). Because both of these processes are functional in the context of motivationally relevant cues, the directional fractionation of orienting results. Finding enhanced cardiac deceleration for emotionally arousing scenes, however, is not consistent with an interpretation of parasympathetic modulation of the differences in pupil diameter found during initial constriction.
Single process sympathetic modulation
To more clearly elucidate the mechanics of pupil modulation during picture viewing, a principal components analysis using the pupillary timecourse data as the input matrix was conducted. The results support the view that a single process modulates pupil diameter during affective picture perception. The primary component is a pupillary dilation that begins around 500 ms after picture onset and continues until picture offset, consistent with the sympathetically mediated action of the dilator muscle. Significantly larger factor scores were found for this dilation component when viewing emotionally arousing scenes of erotica, mutilation, and attack than for scenes of neutral people. On the other hand, analysis of factor scores on a second, light reflex, factor, which involved a rapid pupil constriction in the initial second of picture viewing, did not show a significant difference as a function of hedonic content.
Importantly, these data suggest that the initial light reflex elicited by the low-light scenes in the current studies-- which were matched for brightness, contrast, and local brightness - did not differ substantively. Rather, the amplitude difference in the window of the initial light reflex instead reflects modulation by a dilation process that begins around 500 ms after picture onset, effectively attenuating initial constriction in the window of the light reflex. Taken together, a parsimonious account is that modulation of the pupil during arousing picture viewing reflects early co-active sympathetic nervous activity on the dilator muscle that reduces initial constriction and increases in amplitude over time. This hypothesis could be directly tested by applying a topical agent that blocks the sympathetic activity of the dilator muscle (e.g., Giakoumaki, Hourdaki, Grinakis, Theou,& Bitsios, 2005; Steinhauer, Siegle, Condray, & Pless, 2004), which should reduce or eliminate differences in pupil dilation during emotional picture viewing.
Whereas the data suggest that both pupil diameter and skin conductance index sympathetic arousal, pupil change likely represents a more reliable measure, as over 92% of the participants in the studies reported here evidenced a pattern of numerically larger late diameter changes when viewing emotionally arousing, compared to neutral, scenes, whereas this pattern was found for only 67% of the participants in skin conductance. Because skin conductance measurement relies on creating an electrical circuit on the surface on the skin, through which a weak current is conducted, numerous individual difference factors (e.g., skin type, dampness, lotions, callouses, etc.) loom large, affecting the probability of obtaining a measurable response (Isen, Iacono, & Malone, 2013). Moreover, the experimental sample will typically include a number of participants with no measurable activity at all (i.e. “nonresponders”; Venables & Mitchell, 1996) which is not an issue when measuring pupil diameter, further encouraging the use of pupil measurement to index sympathetically elicited emotional arousal.
Conclusion
Like many measures of emotion in the brain and body, pupil diameter is not a sensitive index of subtle differences in “liking”, but instead is primarily engaged in appetitive and defensive contexts that are capable of eliciting measurable sympathetic arousal which, we have suggested, are those that demand action in the natural environment (Bradley, 2009; Lang & Bradley, 2010). Although controlling the brightness of the visual field at central fixation is important for reducing noise in picture viewing studies, pupil modulation was, nonetheless, strikingly similar across studies, with scenes of erotica and violence reliably eliciting attenuated initial constriction and enhanced late pupil diameter changes, as well as larger skin conductance changes and greater cardiac orienting. A principal component analysis provided strong support for a single sympathetically-mediated process that modulates pupil diameter via enhanced dilation that begins shortly after picture onset and continues throughout affective picture viewing. As a relatively easy-to-measure, reliable, and noninvasive biological assay of sympathetic arousal during picture viewing, measurement of pupil diameter holds promise for use in clinical, developmental, neurological, and other investigations of emotional reactivity.
Acknowledgments
This work was supported in part by NIMH grants MH098078.
Thanks to Vincent Costa for developing the Matlab routine that computed local brightness for each fixation, and to Evelyn Besier for assisting in data acquisition in Exp 3.
Footnotes
IAPS catalog numbers: Adventure: 8001, 8041, 8193, 8208; Attack: 1302, 1931, 6231, 6550; Baby: 2045, 2070, 2071; Contamination: 9302, 9320, 9322, 9325; Erotc: 4668, 4680, 4690, 4695; Food:7250, 7451, 7470, 7480; Mutilation: 3030, 3063, 3069, 9405; Nature: 5040, 5660, 5870, 5991; Neutral Objects: 7009, 7034, 7050, 7705; Neutral People: 2019, 2104, 2107, 2512; Neutral Scenes: 7019, 7034, 7050, 7705; Romance: 4597, 4610, 4641, 4660, Loss: 2301, 2455, 2700, 9332.
The pattern of findings does not rely on the specific time windows chosen here. Using a larger window to estimate either initial constriction (eg., .5 – 1.5 s) or late diameter changes (e.g., 2–6 s) does not change the results in any substantive manner.
We also analyzed the latency of initial peak constriction in each experiment, which produced statistically similar findings to the amplitude measures, both in terms of inferential and descriptive measures. Because latency relies on averaging waveforms across content, we opted to report the averages based on the trial-by-trial amplitude data. Nonetheless, as is clear in the waveforms, the peak constriction latency was longer for larger amplitude light reflexes, and contents significantly differed from one another in ways similar to those found for the amplitude measure.
Pupil size can be affected by gaze angle, although only extreme angles will have palpable effects on measured diameter. When we corrected for horizontal and vertical gaze angle on pupil diameter changes, results were identical to those described without correction. In addition, the correlation between gaze angle and pupil diameter was small and not significant when assessed for each participant and content, or fixation by fixation, indicating negligible effects of gaze angle on pupil diameter in these studies.
The macula is the light sensitive layer of tissue in the retina that receives light information in central vision, a field typically consisting of the central 13–18 degrees of the total visual field. Results were essentially the same if larger (e.g., 18) or smaller (e.g., 3,6,10) masks were used to estimate the brightness of fixations.
Results were essentially the same when separate estimates of local brightness were computed for fixations occurring during initial constriction (.75– 1 s) and later viewing (2–3 s), prompting the average measure reported here.
For heart rate, data from 2 participants was lost due to equipment error, resulting in 64 participants with 31 (19 female) from Experiment 2 and 33 (22 female) from Experiment 3. For skin conductance, data was lost for 1 participant due to equipment error resulting in 65 participants with 31 (19 female) from Experiment 2 and 34 (22 female) from Experiment 3.
When pupil diameter data from Experiments 2 and 3 were analyzed in a 0–500 ms for initial constriction(i.e. before the dilation process begins), the effect of hedonic content was no longer significant, consistent with the hypothesis that differences in initial constriction reflect a dilation process. Moreover, when a principal component analysis was conducted using data from Bradley et al. (2009) in which 27 participants viewed pleasant, neutral or unpleasant scenes that varied in brightness, the same two primary components were found. Repeated measures analysis on the factor scores for the first, arousal, component replicated the effect of hedonic content, F(2,25) = 5.6, p < .0001, ηρ2 = .31. More importantly, consistent with the interpretation of the second primary component as an index of the light reflex, only scene brightness (low, moderate, high) was significant in analysis of factor scores of this initial constriction component, F(2,25) = 84, p < .0001, ηρ2 = 87.
References
- Barbur JL. Learning from the pupil: Studies of basic mechanisms and clinical applications. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. Cambridge, MA: MIT Press; 2004. pp. 641–656. [Google Scholar]
- Beatty J, Lucero-Wagner B. The pupillary system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 2. Cambridge: University Press; 2000. pp. 142–162. [Google Scholar]
- Bitsios P, Szabadi E, Bradshaw CM. The inhibition of the light reflex by the threat of an electric shock: A potential laboratory model of human anxiety. Journal of Psychopharmacology. 1996;10(4):279–287. doi: 10.1177/026988119601000404. [DOI] [PubMed] [Google Scholar]
- Bradley MM. Natural selective attention: Orienting and emotion. Psychophysiology. 2009;46:1–11. doi: 10.1111/j.1469-8986.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Emotion and motivation. In: Cacioppo JT, Tassinary LG, Berntson G, editors. Handbook of Psychophysiology. 2. New York: Cambridge University Press; 2007. pp. 581–607. [Google Scholar]
- Bradley MM, Lang PJ. Memory, emotion, and pupil diameter: Repetition of natural scenes. Psychophysiology. 2015;52(9):1186–93. doi: 10.1111/psyp.12442. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Costa VD, Lang PJ. Selective looking at natural scenes: Hedonic content and gender. International Journal of Psychophysiology. 2015;98:54–8. doi: 10.1016/j.ijpsycho.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1(3):276–98. doi: 10.1037/1528-3542.1.3.276. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: Sex differences in picture processing. Emotion. 2001;1(3):300–19. doi: 10.1037/1528-3542.1.3.300. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45:602–7. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P. Activation of the visual cortex in motivated attention. Behavioral Neuroscience. 2003;117(2):369–80. doi: 10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P. Activation of the visual cortex in motivated attention. Behavioral Neuroscience. 2003;117(2):369–80. doi: 10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Wood G, McBride T. Origins of orienting and defensive responses: An evolutionary perspective. In: Lang PJ, Simons RF, Balaban M, editors. Attention and orienting: Sensory and motivational processes. Erlbaum; Mahwah, NJ: 1997. pp. 41–67. [Google Scholar]
- Cook EW., III . VPM Reference Manual. Birmingham, AL: Author; 2001. [Google Scholar]
- Ferrari V, De Cesarei A, Mastria S, Lugli L, Baroni G, Nicoletti R, Codispoti M. Novelty and emotion: Pupillary and cortical responses during viewing of natural scenes. Biological Psychology. 2016;113:75–82. doi: 10.1016/j.biopsycho.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Giakoumaki SG, Hourdaki E, Grinakis V, Theou K, Bitsios P. Effects of peripheral sympathetic blockade with dapiprazole on the fear-inhibited light reflex. Journal of Psychopharmacology. 2005;19:139–148. doi: 10.1177/0269881105048994. [DOI] [PubMed] [Google Scholar]
- Goldinger SD, Papesh MH. Pupil dilation reflects the creation and retrieval of memories. Current Directions in Psychological Science. 2012;21(2):90–95. doi: 10.1177/0963721412436811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, Steinhauer SR. Pupillometric measures of cognitive and emotional processes. International Journal of Psychophysiology. 2004;52(1):1–6. doi: 10.1016/j.ijpsycho.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Henderson R, Bradley MM, Lang PJ. Modulation of the initial light reflex during affective picture viewing. Psychophysiology. 2014;51:815–818. doi: 10.1111/psyp.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess EH, Beaver PW, Shrout PE. Brightness contrast effects in a pupillometric experiment. Perception & Psychophysics. 1975;18:125–127. doi: 10.3758/BF03204099. [DOI] [Google Scholar]
- Hess EH, Polt JM. Pupil size as related to interest value of visual stimuli. Science. 1960;132:349–350. doi: 10.1126/science.132.3423.349. [DOI] [PubMed] [Google Scholar]
- Ison JD, Iaconi WG, Malone SM. Characterizing electrodermal response habituation: a latent class approach with application to psychopathology. Psychophysiology. 2013;50:954–962. doi: 10.1111/psyp.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM. Emotion and the motivational brain. Biological Psychology. 2010;84(3):437–50. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: Affect, activation, and action. In: Lang PJ, Simons RF, Balaban M, editors. Attention and orienting. Mahwah, NJ: Erlbaum; 1997. pp. 97–135. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. University of Florida; Gainesville, FL: 2008. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Libby WL, Lacy BC, Lacey JI. Pupillary and cardiac activity during visual attention. Psychophysiology. 1973;10:270–291. doi: 10.1111/j.1469-8986.1973.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Loewenfeld IE. Mechanisms of reflex dilation of the pupil: Historical review and experimental analysis. Documenta Opthalmol. 1958;12:185–448. doi: 10.1007/BF00913471. [DOI] [PubMed] [Google Scholar]
- Loewenfeld IE. The pupil: Anatomy, physiology, and clinical applications. Boston: Butterworth and Heinemann; 1999. [Google Scholar]
- Martini FH, Bartholomew EF. Essentials of anatomy & physiology. San Francisco: Benjamin Cummings; 2001. [Google Scholar]
- Metalis SA, Hess EH. Psychophysiological index attenuation to pictures. Psychobiology. 1981;9:235–243. doi: 10.3758/BF03332930. [DOI] [Google Scholar]
- Nunnally JC, Knott PD, Duchnowski A, Parker R. Pupillary response as a general measure of activation. Perception & Psychophysics. 1967;2:149–155. doi: 10.3758/BF03210310. [DOI] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Hillman CH, Hamm AO, Lang PJ. Brain processes in emotional perception: Motivated attention. Cognition and Emotion. 2004;18:593– 611. doi: 10.1080/02699930341000239. [DOI] [Google Scholar]
- Snowdon RJ, O’Farrell K, Burely D, Erichsen JT, Newton NV, Gray NS. The pupil’s response to affective pictures: role of image duration, habituation, and viewing mode. Psychophysiology. 2016;53:1217–1223. doi: 10.1111/psyp.12668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer S, Siegle G, Condray R, Pless M. Sympathetic and parasympathetic innervation of pupillary dilation during sustained processing. International Journal of Psychophysiology. 2004;52:77–86. doi: 10.1016/j.ijpsycho.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Venables PH, Mitchell DA. The effects of age, sex and time of testing on skin conductance activity. Biological Psychology. 1996;43:87–101. doi: 10.1016/0301-0511(96)05183-6. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Beyond good and evil: the time-course of neural activity elicited by specific picture content. Emotion. 2010;10(6):767–82. doi: 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]
- Yoshitomi T, Ito Y, Inomata H. Adrenergic excitatory and cholinergic inhibitory innervations in the human iris dilator. Experimental Eye Research. 1985;40:453–459. doi: 10.1016/0014-4835(85)90158-7. [DOI] [PubMed] [Google Scholar]