Abstract
Background and objective
Systemic sclerosis (SSc) is a complex autoimmune disease commonly associated with pulmonary hypertension (PH). When associated with elevated pulmonary artery wedge pressure (PAWP), pulmonary artery pressure (PAP) is either in-proportion (post-capillary PH) or higher than expected (combined PH) relative to the increased PAWP.
Methods
Patients from the PHAROS registry (a prospective observational cohort of SSc-PH patients) who had mean PAP≥25 and PAWP>15 on right heart catheterization were stratified based on diastolic pressure gradient (DPG). Kaplan-Meier analysis was performed to compare survival and PH-related hospitalization. Baseline factors were compared between patients dying and those who survived using Cox regression analysis.
Results
Fifty-nine patients were included; twenty-one (36%) patients were classified as combined PH and 38 (64%) had post-capillary PH. No baseline characteristics were significantly different between the 2 groups. There were no differences in survival or PH-related hospitalization between the groups. The only baseline factor independently associated with death was lower six-minute walk distance (6MWD) (HR 1.33 per 25 meter decrease, 95% CI 1.11–1.59, p=0.002). PH-specific medications were started during follow-up in significantly more patients in the combined PH group compared to the post-capillary group (86% vs 50%, p=0.01).
Conclusion
Outcomes were similar between SSc patients with post-capillary PH and combined pre- and post-capillary PH. 6MWD at baseline can predict risk for death in SSc patients with PH and an elevated PAWP. More patients with combined PH were started on PH-specific medications, and the clinical benefit of treating this subgroup specifically in SSc patients needs further exploration.
Keywords: systemic sclerosis, scleroderma, pulmonary hypertension, treatment, diastolic dysfunction
INTRODUCTION
Systemic sclerosis (SSc, scleroderma) is a heterogeneous multi-organ disease that is commonly associated with pulmonary hypertension (PH). SSc-PH may be pulmonary arterial hypertension (PAH, WHO Group 1) or PH caused by left-heart disease (LHD) (WHO Group 2) or interstitial lung disease (ILD)/hypoxia (WHO Group 3). SSc patients with Group 2 PH can have either elevated pulmonary arterial pressures (PAP) due to systolic left ventricular (LV) dysfunction or from diastolic LV dysfunction (also known as heart failure with preserved ejection fraction, HFpEF).1 Diastolic dysfunction is a common complication of SSc, with an estimated prevalence of 55%, which is higher than in persons without SSc.2 Left heart disease is the most common cause of PH in the general population3 and at least 10% of SSc-PH patients have pulmonary hypertension from LHD4, but this may be underestimated.5 Patients with Group 2 SSc-PH are defined by a mean pulmonary artery pressure (mPAP) of ≥25mmHg with an elevated pulmonary artery wedge pressure (PAWP, also known as pulmonary capillary wedge pressure [PCWP]) of >15mmHg, and can be further characterized as having post-capillary PH or combined post-capillary and pre-capillary PH (formerly known as “out-of-proportion PH,” heretofore referred to as “combined PH”).3 Characteristics and survival of SSc patients with an elevated PAWP has not been well described, and reasons why some patients have post-capillary PH and others have “combined PH” are undefined. We undertook this analysis to characterize SSc-PH patients with an elevated PAWP and to explore differences between post-capillary PH and combined PH, with a specific hypothesis that those with combined PH have a poorer survival.6
METHODS
Patient selection
This study utilized the PHAROS registry, an ongoing prospective, longitudinal multi-center observational study of SSc patients at risk for or with incident PH.4 Twenty-nine participating SSc centers in the United States obtained institutional review board approval and all patients provided written informed consent prior to enrollment in the registry. Inclusion criteria for analysis were mPAP≥25mmHg and PAWP>15mmHg on their diagnostic right heart catheterization (RHC) performed within 6 months of enrollment.
The baseline time point was defined as the date of the patient’s inclusion RHC. This analysis contained subjects enrolled in the registry by May 14, 2014. A hemodynamic definition was selected for this analysis rather than WHO classification; therefore, patients with ILD were included if their PAWP was >15mmHg.
Right heart catheterization measurements and definitions
Data collected by RHC included measurement of systolic, diastolic, and mean pulmonary artery pressures, PAWP and cardiac output (CO). Pulmonary vascular resistance (PVR) was calculated as:
Transpulmonary gradient (TPG) was calculated as:
Diastolic pressure gradient (DPG) was calculated as:
Using a classification scheme suggested for left heart disease-associated PH in the general population, the group as a whole was divided into subgroups based on DPG, with an elevated gradient suggesting that the pulmonary artery pressure was increased “out-of-proportion” to the PAWP, whereas a normal DPG implies that the PAP elevation was accounted for by the high PAWP.7 Patients with post-capillary PH were defined as having a DPG of <7mmHg, whereas those with combined PH were defined as having a DPG of ≥7mmHg.3 A sensitivity analysis was conducted using TPG to define groups (TPG≤12mmHg=post-capillary PH, TPG>12mmHg=combined PH).
Statistical analysis
Baseline characteristics (demographics, medication use, pulmonary function tests, hemodynamics, and SSc disease characteristics) were compared between the post-capillary PH and the combined PH groups using unpaired t-tests or Chi squared. In a post hoc analysis, baseline characteristics of those started on PH-specific medications (endothelin receptor antagonist, prostacyclin analog, PDE-5 inhibitor) during follow-up were compared to those not receiving these medications.
In patients with ≥6 months of follow-up, we investigated the elevated PAWP group as a whole, comparing baseline factors between patients dying and those who survived during follow-up using Cox regression analysis. Examining the proportionality assumptions of the Cox proportional hazards model using both log-log curves and Schoenfeld residuals demonstrated that all assumptions were met. Multivariate analysis was performed with all univariate factors with a p<0.05 included; stepwise regression with backward elimination was conducted, with a p threshold for elimination=0.20. Collinearity between hemodynamic variables was assessed using variance inflation factors (VIF), with a VIF of >10 indicating that variables were collinear.8 There was no significant collinearity between mean PAP, CO, and PVR. Survival and time to first PH-related hospitalization between the post-capillary and the combined PH groups were compared with Kaplan-Meier analysis. A hospitalization was considered PH-related if it was for heart failure, volume overload, initiation of PH-specific medication, or a complication of PH-specific medication.
All analyses were performed using Graph Pad Prism (version 5, La Jolla, CA) and Stata (version 13, College Station, TX), and a p value <0.05 was considered to be statistically significant. Institutional review board approval was obtained for this analysis (Tulane IRB #685867). Some of these results have been previously published as an abstract.9
RESULTS
Baseline characteristics
Of 298 patients in PHAROS with PH on RHC, 59 met inclusion criteria, with 21 (36%) being classified as combined PH based on their DPG, and 38 (64%) classified as post-capillary PH. Only 2 patients (3%) had an LVEF<45%. Patients with a DPG≥7mmHg had higher mean PAP, TPG, and PVR (Table 1) compared to those with a DPG<7mmHg. There were no significant differences in any of the measured baseline characteristics between the 2 groups (all p>0.05). Although not significantly different, those with combined PH had a higher forced vital capacity (p=0.09) and were more likely to have a limited disease subtype (p=0.17). During follow-up, 50% of patients in the post-capillary PH group were started on PH-specific medications, compared to 86% of patients in the combined PH group (n=0.01). The median time on medication during the observation period was 1.8 years (IQR 1.1, 3.8). Patients were treated with endothelin-receptors antagonists (n=20), phosphodiesterase-5 inhibitors (n=28), inhaled prostanoids (n=5), and parenteral prostanoids (n=7). Eleven were treated with two medications, and 5 were on triple therapy. When TPG was used to define combined PH (TPG>12mmHg) vs. post-capillary PH (TPG≤12mmHg), the results were similar to those obtained using DPG to dichotomize groups (Supplementary Table S1).
Table 1.
Baseline characteristics for the group as a whole and when dichotomized by DPG
| Parameter | Whole group (n=59) |
Combined PH (n=21) |
Post-capillary PH (n=38) |
p value |
|---|---|---|---|---|
| Age (years) | 55.1 ± 11.7 | 57.3 ± 11.2 | 53.9 ± 12.0 | 0.31 |
| Disease duration (years) | 8.5 ± 9.1 | 9.0 ± 12.9 | 8.2 ± 6.9 | 0.76 |
| Body mass index (kg/m2) | 29 ± 7.8 | 30.3 ± 7.7 | 28.5 ± 7.9 | 0.42 |
| Sex (% female) | 70% | 70% | 69% | 1 |
| SSc subtype (%) | ||||
| Limited | 55% | 68% | 49% | 0.17 |
| Diffuse | 45% | 32% | 51% | |
| NYHA functional class (%) | ||||
| I/II | 54% | 58% | 51% | 0.79 |
| III/IV | 46% | 42% | 49% | |
| MMF use (%)* | 14% | 5% | 21% | 0.22 |
| PH-specific med use (%) + | 63% | 86% | 50% | 0.01 |
| Long term oxygen use (%) | 29% | 14% | 37% | 0.08 |
| FVC (% predicted) | 67.1 ± 19.3 | 73.4 ± 20.6 | 63.7 ± 17.9 | 0.09 |
| DLCO (% predicted) | 41.4 ± 17.2 | 44.6 ± 15.3 | 39.7 ± 18.2 | 0.33 |
| FVC/DLCO ratio | 1.79 ± 0.60 | 1.76 ± 0.51 | 1.80 ± 0.65 | 0.79 |
| 6MWD (meters) | 320 ± 122 | 314 ± 121 | 323 ± 124 | 0.82 |
| Left ventricular EF (%) | 59 ± 9 | 61 ± 8 | 59 ± 10 | 0.48 |
| Left atrial diameter (cm) | 4.0 ± 1.0 | 3.9 ± 0.8 | 4.0 ± 1.1 | 0.94 |
| Mean PAP (mmHg) | 36.8 ± 12.1 | 48.5 ± 13.0 | 30.3 ± 4.0 | <0.0001 |
| PAWP (mmHg) | 19.8 ± 3.7 | 19.7 ± 3.8 | 19.9 ± 3.7 | 0.82 |
| DPG (mmHg) | 7.4 ± 8.6 | 16.0 ± 8.8 | 2.7 ± 2.8 | <0.0001 |
| TPG (mmHg) | 17.0 ± 11.8 | 28.8 ± 12.2 | 10.4 ± 3.5 | <0.0001 |
| Cardiac output (L/min) | 5.5 ± 1.7 | 5.2 ± 2.0 | 5.7 ± 1.4 | 0.27 |
| PVR (Wood units) | 4.0 ± 3.4 | 6.6 ± 4.3 | 2.6 ± 1.5 | <0.0001 |
PH=pulmonary hypertension; SSc=systemic sclerosis; NYHA=New York Heart Association; MMF=mycophenolate; FVC=forced vital capacity; DLCO=diffuse capacity for carbon monoxide; EF=ejection fraction; 6MWD=six minute walk distance; PAP=pulmonary artery pressure; PAWP=pulmonary artery wedge pressure; DPG=diastolic pressure gradient; TPG=transpulmonary gradient; PVR=pulmonary vascular resistance
: MMF use must have been for ≥6 months prior to baseline RHC;
: PH-specific medication use during follow-up
Patients started on PH-specific medications (n=37) had a significantly higher mPAP (40.0 ± 13.8 vs. 31.3 ± 5.2mmHg, p=0.006, Table 2), DPG (9.5 ± 9.9 vs. 3.8 ± 3.5, p=0.01), TPG (20.1 ± 13.5 vs. 11.6 ± 4.6, p=0.006), and PVR (5.0 ± 3.9 vs. 2.3 ± 1.4 Wood units, p=0.003) compared to those not started on these medications (n=22). There was no difference in terms of cardiac output or PAWP between these groups. No measured baseline characteristics (demographics, SSc disease characteristics, or pulmonary function test variables) were associated with likelihood to start PH-specific medications. Interestingly, NYHA functional class at baseline did not correlate with whether a PH-specific medication was started or not (p=0.82).
Table 2.
Baseline characteristics dichotomized by PH medication use during follow up
| Parameter | PH meds (n=37) | No PH meds (n=22) | p value |
|---|---|---|---|
| Age (years) | 53.9 ± 12.7 | 57.2 ± 9.8 | 0.31 |
| Disease duration (years) | 8.5 ± 9.7 | 8.3 ± 8.2 | 0.93 |
| Sex (% female) | 69% | 71% | 1.00 |
| SSc subtype (%) | |||
| Limited | 56% | 55% | 0.61 |
| Diffuse | 44% | 45% | |
| NYHA functional class (%) | |||
| I/II | 45% | 45% | 0.82 |
| III/IV | 55% | 55% | |
| DLCO (% predicted) | 40.2 ± 18.0 | 43.7 ± 16.0 | 0.49 |
| 6MWD (meters) | 322 ± 119 | 312 ± 135 | 0.82 |
| Left atrial diameter (cm) | 3.7 ± 1.2 | 4.2 ± 0.8 | 0.16 |
| Mean PAP (mmHg) | 40.0 ± 13.8 | 31.3 ± 5.2 | 0.006 |
| PAWP (mmHg) | 19.9 ± 4.0 | 19.7 ±3.2 | 0.84 |
| DPG (mmHg) | 9.5 ± 9.9 | 3.8 ± 3.5 | 0.01 |
| TPG (mmHg) | 20.1 ± 13.5 | 11.6 ± 4.6 | 0.006 |
| Cardiac output (L/min) | 5.3 ± 1.7 | 5.8 ± 1.5 | 0.30 |
| PVR (Wood units) | 5.0 ± 3.9 | 2.3 ± 1.4 | 0.003 |
See Table 1 for abbreviations
Survival Analysis
For the survival analysis, there were a total of 163.3 patient-years at risk; the median time at risk was 2.3 years. Over a 5-year observation period, 18 patients (31%) died, with survival rates of 84.7% at 1 year, 64.7% at 3 years, and 61.2% at 5 years (Figure 1). For the PAWP>15 group as a whole, univariate factors associated with death included male sex (HR 2.94, 95% CI 1.11–7.69, p=0.03, Table 3), NYHA functional class (HR 2.50, 95% CI 1.30–4.79, p=0.006), lower six-minute walk distance [6MWD] (HR 1.33 per 25 meter decrease, 95% CI 1.15–1.54, p<0.0001), and higher PAWP (HR 1.16 per 1mmHg change, 95% CI 1.06–1.28, p=0.002). In a multivariate analysis, the only baseline parameter independently associated with death was 6MWD (HR 1.33 per 25 meter decrease, 95% CI 1.11–1.59, p=0.002. Table 3 and Figure 2).
Figure 1.
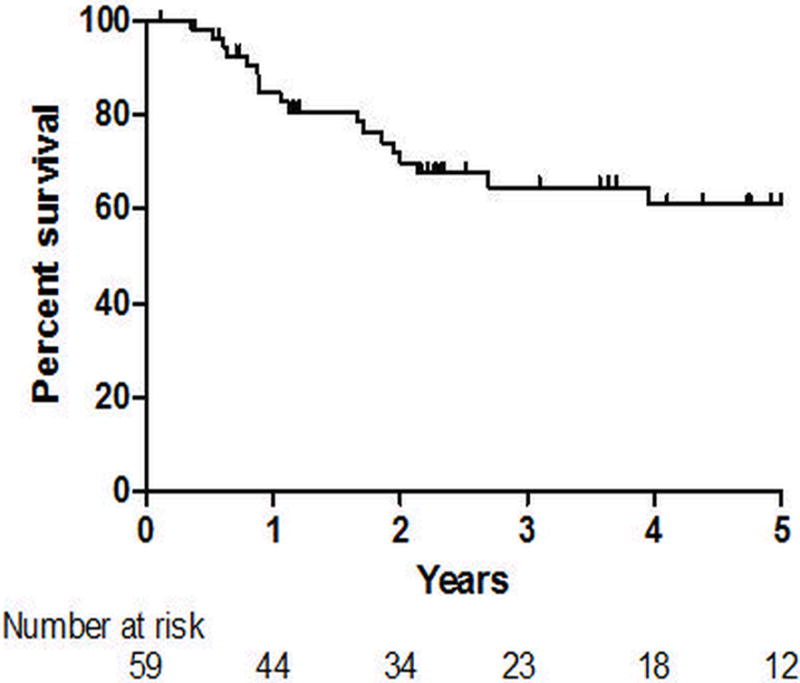
Kaplan-Meier survival curve for the elevated pulmonary artery wedge pressure group as a whole.
Table 3.
Baseline factors associated with survival
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age, per 10 year change | 1.30 | 0.86–1.96 | 0.22 | – | – | – |
| Disease duration, per year change | 0.99 | 0.94–1.05 | 0.83 | – | – | – |
| Sex, male vs. female | 2.94 | 1.11–7.69 | 0.03 | – | – | – |
| SSc subtype, limited vs. diffuse | 1.05 | 0.47–2.37 | 0.90 | – | – | – |
| NYHA functional class | 2.50 | 1.30–4.79 | 0.006 | 2.14 | 0.86–5.37 | 0.10 |
| PH medication use | 0.95 | 0.56–1.64 | 0.89 | – | – | – |
| Long term oxygen use | 1.84 | 0.71–4.76 | 0.21 | |||
| Creatinine, per 1 mg/dL change | 2.05 | 0.79–5.33 | 0.14 | – | – | – |
| Pericardial effusion | 2.16 | 0.80–5.88 | 0.13 | – | – | – |
| FVC, per 10% predicted change | 0.83 | 0.64–1.08 | 0.16 | – | – | – |
| DLCO, per 10% predicted change | 0.66 | 0.42–1.01 | 0.06 | – | – | – |
| FVC/DLCO ratio | 1.58 | 0.72–3.5 | 0.25 | – | – | – |
| 6MWD, per 25 meter decrease | 1.33 | 1.15–1.54 | <0.0001 | 1.33 | 1.11–1.59 | 0.002 |
| Mean PAP, per 10 mmHg change | 1.30 | 0.95–1.77 | 0.10 | – | – | – |
| PAWP, per 1 mmHg change | 1.16 | 1.06–1.28 | 0.002 | 1.10 | 0.96–1.26 | 0.17 |
| DPG, per 1 mmHg | 1.01 | 0.96–1.06 | 0.71 | – | – | – |
| Cardiac output, per 1 L/min change | 0.92 | 0.67–1.25 | 0.57 | – | – | – |
| PVR, per 1 Wood unit change | 1.12 | 0.95–1.32 | 0.19 | – | – | – |
See Table 1 for abbreviations. Bolded variables were included in the multivariate model. Sex was removed from the stepwise backward regression since p<0.20
Figure 2.
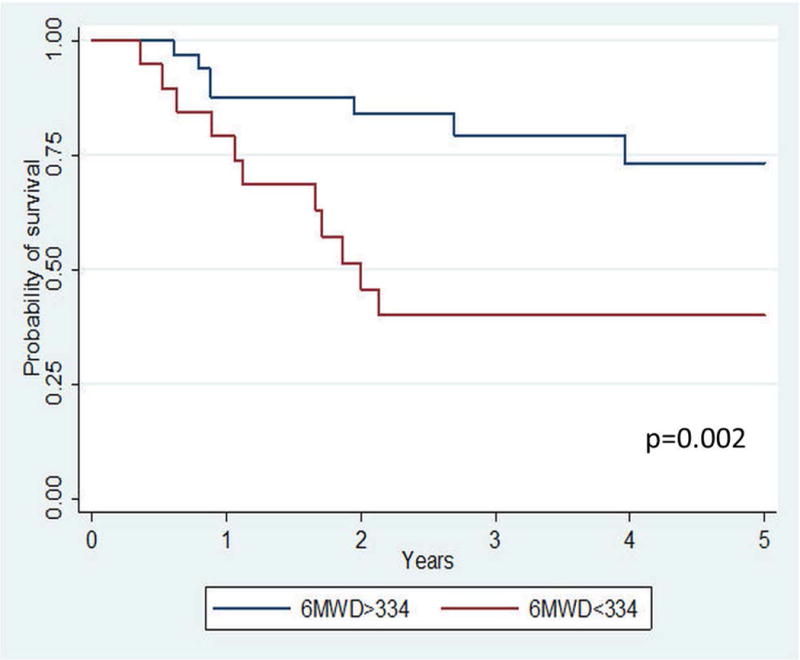
Kaplan-Meier survival curves for the group stratified by median six-minute walk distance (6MWD). Those with a lower 6MWD at baseline had significantly worse survival.
Twelve (32%) patients in the post-capillary PH group and 6 (29%) of those in the combined PH group died over a 5-year observation period. There was no significant difference in survival between the two groups (p=0.66, Figure 3A). There was also no difference in death or PH-related hospitalization between the post-capillary PH and the combined PH groups (p=0.27, Figure 3B).
Figure 3.
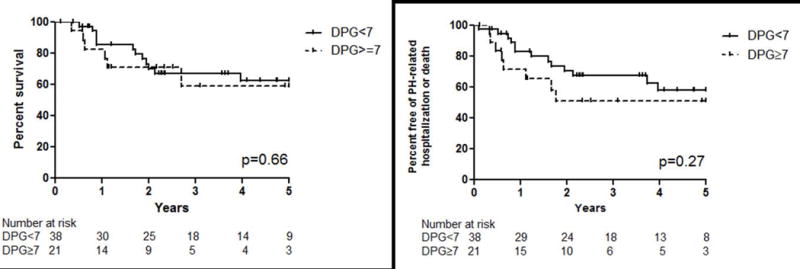
Kaplan-Meier curves for survival (A) and PH-related hospitalization or death (B) for the group stratified by diastolic pressure gradient (DPG).
DISCUSSION
In this analysis, which is the first study investigating post-capillary PH and combined PH in SSc patients, we found that baseline characteristics were similar between these two groups, although the combined PH group was more likely to be started on a PH-specific medication. We also found that there was no difference in outcomes between those with post-capillary PH and combined PH. Lastly, this study demonstrates that lower baseline 6MWD is an independent predictor of death in SSc-PH patients with an elevated PAWP.
As only two patients had an LV ejection fraction<45%, the majority of patients in this study can be considered to have diastolic dysfunction, which is consistent with prior literature10–12. In echocardiographic studies of unselected patients, diastolic dysfunction is present in 23–62% of SSc subjects, with most estimates around 50%, which is a higher prevalence than healthy controls2, 10, 13–17. In SSc patients, diastolic dysfunction tends to worsen over time13, 15, parameters of diastolic function correlate with exercise performance14, 18, and LV diastolic dysfunction is independently associated with risk for death.15, 17 Most of these studies, however, did not have a large number of PH patients, so the contribution of left heart disease to pulmonary hypertension in SSc patients is less well-defined. Prior interim analysis of the PHAROS cohort demonstrated that 10% of the PH patients were classified as having WHO Group 2 disease4, but with a larger dataset we have shown that 20% of SSc-PH patients in this cohort have an elevated PAWP.
It is not clear why some patients with an elevated PAWP have post-capillary PH and others develop combined pre- and post-capillary PH. It is assumed that in susceptible patients, long-standing left atrial hypertension leads to remodeling in the pulmonary arteries, resulting in a pulmonary artery pressure that is “out-of-proportion” to the PAWP. In SSc, the case may be more complex since patients with LHD may also have a component of PAH (WHO group 1) or ILD (WHO group 3). On the other hand, SSc patients with PAH might have mild diastolic dysfunction with an elevated PAWP. In comparison to other patients with HFpEF-PH, patients with SSc-PH and a high PAWP appear to be younger19, 20 and are less likely to be in functional class III/IV20. Hemodynamically, mPAP and CO seem to be similar, but our cohort appears to have lower PAWP and higher PVR compared to a large cohort of HFpEF-PH patients without SSc.20
Interestingly, there were no differences in any of the measured baseline characteristics between the post-capillary PH and the combined PH groups. We expected outcomes to be worse in those with combined PH6, but found that survival and time to PH-related hospitalization were similar in both groups. We recently found that survival was similar between SSc patients with group 1 PH (“pre-capillary”) and group 2 PH21.
In the high PAWP group as a whole, the only baseline parameter that was independently associated with risk for death was a low 6MWD. For each 25 meter decrease in baseline 6MWD, there was a 33% increase in risk for death over the 5 year observation period. Although well-accepted as an important measure of functional status and outcome in other forms of PAH22, the use of the 6-minute walk test (6MWT) is controversial in SSc. This is because there are many SSc manifestations that can lead to an impairment in 6MWD, including heart, lung, joint, and muscle disease.23 6MWD has been shown to correlate with disease activity and quality of life scores, but poorly with lung function parameters.24 Additionally, the 6MWD appears insensitive to change in hemodynamics when SSc patients are treated with PH-specific medications.25 However, there is excellent reproducibility of the 6MWT in SSc patients26 and as a global marker of functional status it may be helpful in determining prognosis. We demonstrate for the first time that in SSc-PH patients with an elevated PAWP, 6MWD at the time of PH diagnosis may be useful to identify patients at higher risk for death.
Treatment with PH-specific medications (endothelin receptor antagonists, prostacyclin analogs, PDE-5 inhibitors, soluble guanylate cyclase stimulators) is generally advised against in patients with left heart disease and an elevated PAWP.3 This recommendation is based on the fact that most clinical studies of PH-specific medications in left heart disease have failed to show benefit.27–29 In our observational study, the majority of SSc patients with PH and an elevated PAWP were treated with PH-specific medications, with more being treated in the combined PH group. As pulmonary hypertension pathogenesis in SSc may be a combination of diastolic dysfunction, pulmonary arterial vasculopathy, and PH associated with parenchymal lung disease, treatment paradigms for patients with standard HFpEF-PH may not apply to patients with systemic sclerosis. However, randomized trials are needed in SSc patients with an elevated PAWP to determine the clinical utility of treatment with PH-specific medications.
Our study has limitations that must be acknowledged. Although 59 patients is a reasonable number of patients for a rare condition (i.e. SSc with PH and an elevated PAWP), this is a small sample size and the risk for Type 2 error exists. With a larger sample size, a baseline factor such as FVC may have been significantly different between the groups. While the patients included here were cared for in specialized SSc centers, we do not feel that this is a limitation since SSc patients with PH are commonly seen in referral centers. The PHAROS registry database did not collect some variables that may be important when comparing patients with an elevated PAWP, such as comorbidities (e.g. sleep apnea, coronary artery disease), nor did it collect in-depth echocardiographic diastolic dysfunction parameters. Right atrial pressure, a hemodynamic parameter known to predict outcomes30, was also not collected in this study. RHC tracings were not centrally reviewed and there may have been mismeasurements of PAWP, particularly in patients with ILD and/or obesity. However, RHCs were done in referral centers and any potential mismeasurements are likely reflective of “real world” practice. Additionally, although PH-related hospitalizations were defined a priori, cause of hospitalization was not centrally adjudicated. Following current recommendations3, we used a DPG of 7 as a cutoff between post-capillary PH and combined PH, rather than TPG. However, as mirrored in this analysis, neither has been consistently shown to predict mortality.31
Conclusions
Survival and pulmonary hypertension-related hospitalizations were similar between SSc patients with post-capillary PH and combined pre- and post-capillary PH. Therefore, using the diastolic pressure gradient in SSc-PH patients with an elevated PAWP was not able to accurately phenotype or prognosticate, likely due to the mixed etiologies of elevated PAP in SSc. Since many patients with SSc-PH have concomitant ILD, proper PAWP measurement is critical when interpreting their hemodynamics. Six-minute walk distance at the time of diagnosis can be used to predict risk for death in SSc patients with PH and an elevated PAWP. Patients with combined PH were more likely to be started on PH-specific medications, although 50% of patients with post-capillary PH were also treated at some point during follow-up. The clinical benefit of treating this subgroup specifically in SSc patients needs further exploration, as the multifactorial etiology of their PH may be different than those with HFpEF-PH.
Supplementary Material
Summary at a glance.
We analyzed data on 59 patients with systemic sclerosis-pulmonary hypertension (PH) and an elevated wedge pressure and found that there was no difference in clinical outcomes between those with post-capillary PH and combined pre- and post-capillary PH. 6-minute walk distance was a predictor of death in this group.
Acknowledgments
This study was supported in part by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health (NIH), which funds the Louisiana Clinical and Translational Science Center (MRL). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Support was also in part provided by COBRE Pilot Program grant number 1P306M106392-01A1 (MRL).
References
- 1.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Ciurzynski MBP, Lichodziejewska B, Szewczyk A, Glinska-Wielochowska M, Jankowski K, Kurnicka K, Kurzyna M, Glinski W, Pruszcyk P. Assessment of left and right ventricular diastolic function in patients with systemic sclerosis. Kardiol Pol. 2008;66:269–76. [PubMed] [Google Scholar]
- 3.Vachiery JL, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, De Marco T, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62:D100–8. doi: 10.1016/j.jacc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 4.Hinchcliff M, Fischer A, Schiopu E, Steen VD. Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma (PHAROS): baseline characteristics and description of study population. J Rheumatol. 2011;38:2172–9. doi: 10.3899/jrheum.101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox BD, Shimony A, Langleben D, Hirsch A, Rudski L, Schlesinger R, Eisenberg MJ, et al. High prevalence of occult left heart disease in scleroderma-pulmonary hypertension. Eur Respir J. 2013;42:1083–91. doi: 10.1183/09031936.00091212. [DOI] [PubMed] [Google Scholar]
- 6.Gerges C, Gerges M, Lang MB, Zhang Y, Jakowitsch J, Probst P, Maurer G, Lang IM. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest. 2013;143:758–66. doi: 10.1378/chest.12-1653. [DOI] [PubMed] [Google Scholar]
- 7.Naeije R. Physiology of the pulmonary circulation and the right heart. Curr Hypertens Res. 2013;15:623–31. doi: 10.1007/s11906-013-0396-6. [DOI] [PubMed] [Google Scholar]
- 8.Hair JFAR, Jr, Tatham RL, Black WC. Multivariate Data Analysis. New York: Macmillan Publishing Company; 1995. [Google Scholar]
- 9.Lammi MRSL, Gordon JK, Lauto P, Steen VD. Scleroderma Patients With Pulmonary Hypertension and Increased Pulmonary Capillary Wedge Pressure In The Pulmonary Hypertension Assessment and Recognition Of Outcomes In Scleroderma (PHAROS) Cohort. Am J Resp Crit Care Med. 2014;189:A4771. [Google Scholar]
- 10.Hachulla AL, Launay D, Gaxotte V, de Groote P, Lamblin N, Devos P, Hatron PY, Beregi JP, Hachulla E. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis. 2009;68:1878–84. doi: 10.1136/ard.2008.095836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poanta L, Dadu R, Tiboc C, Rednic S, Dumitrascu D. Systolic and diastolic function in patients with systemic sclerosis. Eur J Intern Med. 2009;20:378–82. doi: 10.1016/j.ejim.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Allanore Y, Meune C, Vonk MC, Airo P, Hachulla E, Caramaschi P, Riemekasten G, et al. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis. 2010;69:218–21. doi: 10.1136/ard.2008.103382. [DOI] [PubMed] [Google Scholar]
- 13.Ciurzynski M, Bienias P, Irzyk K, Kostrubiec M, Szewczyk A, Demkow U, Siwicka M, Kurnicka K, Lichodziejewska B, Pruszczyk P. Heart diastolic dysfunction in patients with systemic sclerosis. Arch Med Sci. 2014;10:445–54. doi: 10.5114/aoms.2014.43739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akdogan A, Kaya EB, Sahin A, Okutucu S, Yakut E, Kalyoncu U, Aksoy H, et al. Relationship between left ventricular diastolic dysfunction and six minute walk test in patients with systemic sclerosis. Int J Rheum Dis. 2011;14:379–83. doi: 10.1111/j.1756-185X.2011.01672.x. [DOI] [PubMed] [Google Scholar]
- 15.Faludi R, Kolto G, Bartos B, Csima G, Czirjak L, Komocsi A. Five-year follow-up of left ventricular diastolic function in systemic sclerosis patients: determinants of mortality and disease progression. Semin Arthritis Rheum. 2014;44:220–7. doi: 10.1016/j.semarthrit.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Meune C, Avouac J, Wahbi K, Cabanes L, Wipff J, Mouthon L, Guillevin L, Kahan A, Allanore Y. Cardiac involvement in systemic sclerosis assessed by tissue-doppler echocardiography during routine care: A controlled study of 100 consecutive patients. Arthritis Rheum. 2008;58:1803–9. doi: 10.1002/art.23463. [DOI] [PubMed] [Google Scholar]
- 17.Hinchcliff M, Desai CS, Varga J, Shah SJ. Prevalence, prognosis, and factors associated with left ventricular diastolic dysfunction in systemic sclerosis. Clin Exp Rheumatol. 2012;30:S30–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Plazak W, Gryga K, Sznajd J, Tomkiewicz-Pajak L, Suchon E, Wilisowska J, Musial J, Podolec P. Diastolic heart dysfunction, increased pulmonary capillary wedge pressure and impaired exercise tolerance in patients with systemic sclerosis. Kardiol Pol. 2011;69:243–9. [PubMed] [Google Scholar]
- 19.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–26. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thenappan T, Shah SJ, Gomberg-Maitland M, Collander B, Vallakati A, Shroff P, Rich S. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2011;4:257–65. doi: 10.1161/CIRCHEARTFAILURE.110.958801. [DOI] [PubMed] [Google Scholar]
- 21.Gordon JK, Szymonifka J, Lammi MR, Steen VD. Clinical Characterization of Patients with World Health Organization Group 2 Pulmonary Hypertension in the Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma Cohort [abstract] Arthritis Rheumatol. 2016;68(suppl 10) [Google Scholar]
- 22.Miyamoto S, Nagaya N, Satoh T, Kyotani S, Sakamaki F, Fujita M, Nakanishi N, Miyatake K. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2000;161:487–92. doi: 10.1164/ajrccm.161.2.9906015. [DOI] [PubMed] [Google Scholar]
- 23.Schoindre Y, Meune C, Dinh-Xuan AT, Avouac J, Kahan A, Allanore Y. Lack of specificity of the 6-minute walk test as an outcome measure for patients with systemic sclerosis. J Rheumatol. 2009;36:1481–5. doi: 10.3899/jrheum.081221. [DOI] [PubMed] [Google Scholar]
- 24.Deuschle K, Weinert K, Becker MO, Backhaus M, Huscher D, Riemekasten G. Six-minute walk distance as a marker for disability and complaints in patients with systemic sclerosis. Clin Exp Rheumatol. 2011;29:S53–9. [PubMed] [Google Scholar]
- 25.Sanges S, Launay D, Rhee RL, Sitbon O, Hachulla E, Mouthon L, Guillevin L, et al. A prospective study of the 6 min walk test as a surrogate marker for haemodynamics in two independent cohorts of treatment-naive systemic sclerosis-associated pulmonary arterial hypertension. Ann Rheum Dis. 2016;75:1457–65. doi: 10.1136/annrheumdis-2015-207336. [DOI] [PubMed] [Google Scholar]
- 26.Wilsher M, Good N, Hopkins R, Young P, Milne D, Gibson A, Suppiah R, Ly J, Doughty R, Dalbeth N. The six-minute walk test using forehead oximetry is reliable in the assessment of scleroderma lung disease. Respirology. 2012;17:647–52. doi: 10.1111/j.1440-1843.2012.02133.x. [DOI] [PubMed] [Google Scholar]
- 27.Anand I, McMurray J, Cohn JN, Konstam MA, Notter T, Quitzau K, Ruschitzka F, Luscher TF. Long-term effects of darusentan on left-ventricular remodelling and clinical outcomes in the EndothelinA Receptor Antagonist Trial in Heart Failure (EARTH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:347–54. doi: 10.1016/S0140-6736(04)16723-8. [DOI] [PubMed] [Google Scholar]
- 28.Packer M, McMurray J, Massie BM, Caspi A, Charlon V, Cohen-Solal A, Kiowski W, et al. Clinical effects of endothelin receptor antagonism with bosentan in patients with severe chronic heart failure: results of a pilot study. J Card Fail. 2005;11:12–20. doi: 10.1016/j.cardfail.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Califf RM, Adams KF, McKenna WJ, Gheorghiade M, Uretsky BF, McNulty SE, Darius H, et al. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: The Flolan International Randomized Survival Trial (FIRST) Am Heart J. 1997;134:44–54. doi: 10.1016/s0002-8703(97)70105-4. [DOI] [PubMed] [Google Scholar]
- 30.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–9. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 31.Tampakakis E, Tedford RJ. Reply: characterization of pulmonary hypertension in heart failure using the diastolic pressure gradient: the conundrum of high and low diastolic pulmonary gradient. JACC Heart Fail. 2015;3:426–7. doi: 10.1016/j.jchf.2015.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


