Abstract
Background
Captive rhesus macaques often exhibit hair loss.
Methods
Alopecia was quantified and behavior recorded before, during, and after fatty acid supplementation in six macaques.
Results
Fatty acid treatment was associated with a decrease in alopecia and self-grooming behavior.
Conclusions
Fatty acids may be a viable treatment for alopecia in some captive primates.
Keywords: Alopecia, hair loss, polyunsaturated fatty acids
INTRODUCTION
Alopecia, a common condition in captive non-human primates, can affect as much as 50% or more of the hair coat [1]. There are many possible causes of hair loss, including seasonality, age, hormonal imbalances, and friction [2,3]. We recently showed that significant alopecia is associated with chronic elevations of the hypothalamic-pituitary-adrenal (HPA) axis stress response system. Monkeys with greater than 30% hair loss had significantly higher hair cortisol concentrations than fully haired controls. Furthermore, cortisol concentrations were highly correlated with the total amount of hair loss [2]. In a follow up eight months later, most severely alopecic monkeys maintained their status. However, a subset of female monkeys that gained hair also showed reduced hair cortisol concentrations [4]. Severe alopecia is less common than mild alopecia. Monkeys with mild hair loss did not show HPA axis elevation and their hair loss may be related to other factors, such as low grade inflammation [5].
We examined the efficacy of polyunsaturated fatty acids (PUFAs) as a treatment for mild alopecia (<30% hair loss). Treatment with PUFAs has been shown to improve both skin and hair condition. PUFAs promoted wound healing [6], reduced skin dryness and pruritus [7], and dermatitis [8] in rodents. Dogs fed a high PUFAs diet showed improvements in hair coat quality [9] and higher hair coat condition scores [10]. In humans, PUFA supplements reduced the signs of photoaging of the skin [11]. We hypothesized that mildly alopecic monkeys receiving PUFA supplements would show regrowth of hair and a reduction in skin-related behaviors (e.g., scratching and self-grooming).
MATERIALS AND METHODS
Human Care Guidelines
Six rhesus macaques (Macaca mulatta) (4 female), ranging in age from 11 to 14 (mean = 13 yrs), participated in this experiment. The monkeys were housed indoors, in pairs (N=2) or in grooming contact (N=4), and were housed either in an Allentown cage (N=2) or a large pen (N=4) for the duration of the study. The monkeys were fed twice-daily meals of a formulated monkey chow (Lab Diet, PMI; St. Louis, MO) supplemented with vegetables, fruits, and grains. Water was available ad libitum. The monkeys were maintained under the specifications outlined by the facility’s Institutional Animal Care and Use Committee (IACUC) and the Guide to the Care and Use of Laboratory Animals. Each of the monkeys had small patchy areas of bare skin on either their legs or back. The skin appeared pale white without signs of inflammation (erythema).
Experimental Design
The experiment consisted of three phases: pre-treatment (March–August 2014), PUFA treatment (September–January or 2014), and post-treatment (March–August 2015). In the treatment phase, monkeys were given a single flax seed-derived Omega 3 chewable gummy supplement (Yummi Bears™, Hero Nutritionals; Santa Ana, CA.) each day with their morning meal of chow. During routine health exams that occurred before (January 2014), directly following (March 2015), and five months after PUFA treatment ended (January 2016), three photos were taken of each monkey’s coat (left side, right side, and back) in order to quantify the amount of alopecia.
Alopecia Scoring
Photos collected during the health exams were scored using Image J software to provide a precise assessment of amount of hair loss. A percent alopecia score was created for each monkey at each of the three time points (before, during, and after PUFA treatment) using a protocol that has been described previously [6].
Behavioral Data Collection
Five-minute behavior samples were collected M-F between 0900h and 1000h across the entire period of the study using a modified frequency scoring system. The presence of 36 behaviors were scored in 15-second intervals by trained observers with >90% inter-observer reliability. In order to assess the amelioration of skin inflammation or irritation by PUFAs, two skin related behaviors, scratching and self-grooming, were selected for analysis.
Data Analysis
The digitized alopecia scores and the averaged modified frequency scores for the two skin-related behaviors were compared across the three phases using a repeated measures Analysis of Variance (ANOVA). Post-hoc paired samples t-tests determined whether changes in alopecia score or behavior differed across phase.
RESULTS
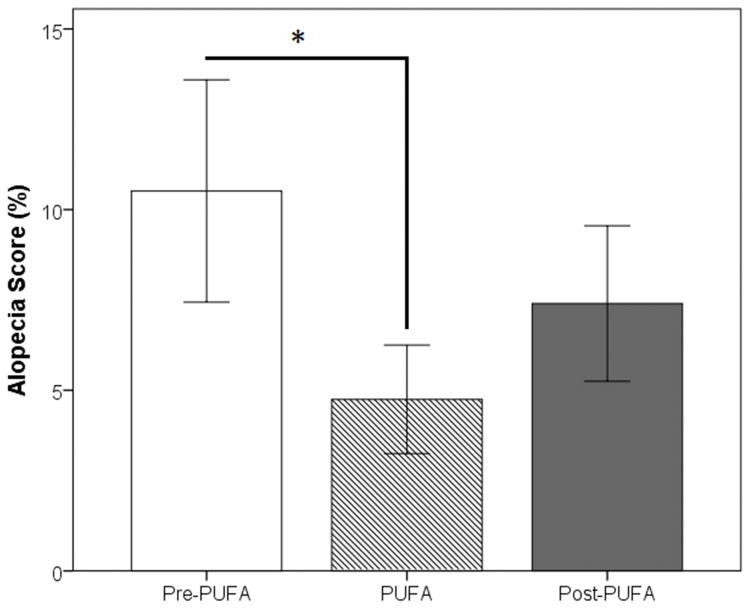
Alopecia Assessments: Overall, the subjects’ alopecia consisted of small patches along the arms, legs, and back (mean alopecia score averaged across left side, right side and prone photos: before PUFA treatment = 10.51±7.54%, during PUFA treatment = 4.75±3.68%, after PUFA treatment = 7.40±5.27%). A repeated measures ANOVA revealed a significant effect of PUFA treatment (F(2,10)=8614, p=0.007). As noted in Fig. 1, a post-hoc paired t-test demonstrated that alopecia scores decreased significantly as a function of PUFA treatment (pretreatment vs. treatment, t(5)=3.469, p=0.018) and then increased marginally during the posttreatment period (treatment vs. post-treatment, t(5)=−2.284, p=0.071).
Figure 1.
The percent alopecia score before, during, and after treatment with PUFAs (* p-value < 0.05).
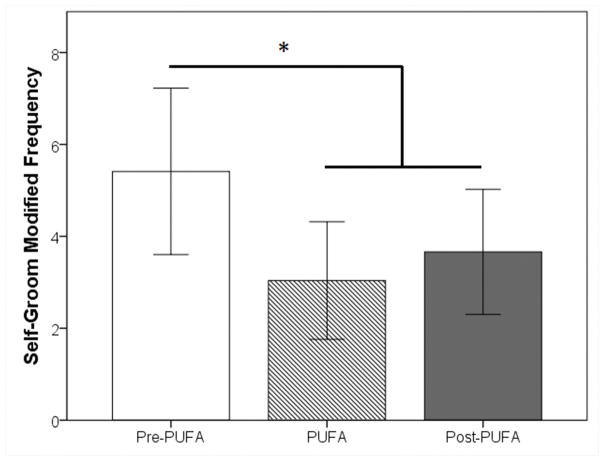
Skin Related Behaviors: Monkeys showed a significant decrease in self-grooming behavior (F(2,10)=7.368, p=0.011), but not scratching, as a function of PUFA treatment. Compared to the initial pre-treatment phase, monkeys engaged in significantly less self-grooming during the PUFA treatment (t(5)=2.951, p=0.032) and post-treatment (t(5)=3.090, p=0.027) phases (Fig. 2).
Figure 2.
The amount of self-grooming behavior observed before, during, and after treatment with PUFAs (*p-value < 0.05).
DISCUSSION
These findings indicate that PUFA administration reduced hair loss in mildly alopecic monkeys. The lack of any effect of PUFAs on scratching behavior is consistent with the absence of erythemous skin in these subjects. Nonetheless, monkeys groomed their skin and coat significantly less as a function of treatment, suggesting that some form of mild subclinical inflammation may have been present. PUFA treatment, therefore, may improve mild cases of alopecia related to minor inflammation; it is as yet unclear whether PUFAs would ameliorate more extreme forms of alopecia (>30% of the hair coat) or reduce the hair loss associated with age, seasonality, or friction.
The exact mechanism whereby PUFA influenced hair production in macaques is unknown. If the alopecia observed in these monkeys is a form of subclinical inflammation, then PUFA administration may improve skin barrier integrity. It is currently hypothesized that PUFA becomes incorporated into mast cell membranes thereby affecting membrane associated enzymes such as phospholipases D (PLD), which in turn influence mast cell exocytosis. This mechanism has been proposed to explain improvements in canine atopic dermatitis with PUFA administration (as reviewed in [12]).
However, two other possible explanations remain: stress induced hair loss or sensory-neural irritation. Chronic stress exposure is known to disrupt the hair growth cycle through the actions of cortisol on the hair follicle (see [13] for review). A link between stress and alopecia in macaques is supported by the following findings. Severe alopecia is associated with markedly elevated hair cortisol concentrations [2] and spontaneous regrowth of hair in females with this condition is associated with significantly decreased hair cortisol concentrations [14]. PUFA treatment has been shown to reduce HPA axis activity in mice [15] and hypothetically may restore hair growth cycles in alopecic animals. However, elevated cortisol concentrations are not typical of monkeys with mild alopecia and the monkeys in this study did not show increased concentrations of hair cortisol as compared to normally haired controls (data not shown).
A second alternative is sensory neural irritation, which is also not associated with erythema. In humans, the sensory manifestations are burning or tingling that appear to arise in response a wide range of stimuli that include but are not limited to ultraviolet light, heat, cold, water, chemical cleaning agents, and exogenously applied hormones [16]. It is possible that this irritation is due to increased permeability of the skin to certain agents, individual differences in epidermal neuron densities and/or greater neural activation [17]. Whether skin sensitivity is also present in macaques is largely unknown at this time.
In this study we evaluated the effects of PUFAs using a within-subject ABA experimental design in which subjects were evaluated during two control periods interspersed with a treatment period. The alternative is a between-subjects design with one group receiving treatment and the other serving as an untreated control group. Both designs have strengths and weaknesses. The within-subject design reduces individual subject variability because the same subjects are tested at all time points, but it is vulnerable to changes across time that can covary with the treatment period, and should not be used if the environment is unstable. The between-subjects design eliminates this issue with time because both experimental and control groups are studied in the same period. However, it is vulnerable to increased variability and uncontrolled differences between the experimental and control groups including, but not limited to, genetic factors, experimental history, room location, dominance status, and temperament which can only be managed with very large number of subjects that can be randomly assigned to each group. A standard within subject ABA design was used in this study to evaluate the effect of PUFA on coat condition because these animals had been housed in a very stable environment for many years (same care staff, caging environments, colony rooms, vigorous enrichment program, and no introductions of new animals, etc.). Additionally, large numbers of subjects were not available to mitigate the increased variability associated with the between-subjects design.
PUFA treatment may improve skin and hair coat condition in rhesus macaques. However, further research is needed to determine what causes mild hair loss. This information can then be used to evaluate putative mechanisms by which PUFAs reduce alopecia. Given that individual differences are present even when monkeys are housed and maintained under stable conditions for many years, it is also pertinent to identify risk factors that make some monkeys vulnerable to this condition.
Acknowledgments
Research conducted at the University of Massachusetts Amherst, Amherst, MA. 01003
Funded by NIH Grant OD011180 to MAN
References
- 1.Lutz CK, Coleman K, Worlein J, Novak MA. Hair loss and hair-pulling in rhesus macaques (Macaca mulatta) J Am Assoc Lab Anim. 2013;52:454–457. [PMC free article] [PubMed] [Google Scholar]
- 2.Novak MA, Hamel AF, Coleman K, Lutz CK, Worlein J, Menard M, Ryan A, Rosenberg K, Meyer JS. Hair loss and hypothalamic-pituitary-adrenocortical axis activity in captive rhesus macaques (Macaca mulatta) J Am Assoc Lab Anim. 2014;53:261–266. [PMC free article] [PubMed] [Google Scholar]
- 3.Novak MA, Meyer JS. Alopecia: possible causes and treatments, particularly in captive nonhuman primates. Comp Med. 2009;59:18–26. [PMC free article] [PubMed] [Google Scholar]
- 4.Novak MA, Menard MT, El-Mallah SN, Rosenberg K, Lutz CK, Worlein J, Coleman K, Meyer JS. Assessing significant (>30%) alopecia as a possible biomarker for stress in captive rhesus monkeys (Macaca mulatta) Am J Primatol. 2016 doi: 10.1002/ajp.22547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer J, Fahey M, Santos R, Carville A, Wachtman L, Mansfield K. Alopecia in rhesus macaques correlates with immunophenotypic alterations in dermal inflammatory infiltrates consistent with hypersensitivity etiology. J Med Primatol. 2010;39:112–122. doi: 10.1111/j.1600-0684.2010.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z, Wang S, Diao Y, Zhang J, Lv D. Fatty acid extracts from Lucilia sericata larvae promote murine cutaneous wound healing by angiogenic activity. Lipids Health Dis. 2010;9 doi: 10.1186/1476-511X-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barcelos RCS, de Mello-Sampayo C, Antoniazzi CTD, Segat HJ, Silva H, Veit JC, Piccolo J, Emanuelli T, Bürger ME, Silva-Lima B, Rodrigues LM. Oral supplementation with fish oil reduces dryness and pruritus in the acetone-induced dry skin rat model. J Dermatol Sci. 2015;79:298–304. doi: 10.1016/j.jdermsci.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Weise C, Ernst D, van Tol EAF, Worm M. Dietary polyunsaturated fatty acids and non-digestible oligosaccharides reduce dermatitis in mice. Pediatr Allergy Immu. 2013;24:361–367. doi: 10.1111/pai.12073. [DOI] [PubMed] [Google Scholar]
- 9.Kirby NA, Hester SL, Rees CA, Kennis RA, Zoran DL, Bauer JE. Skin surface lipids and skin and hair coat condition in dogs fed increased total fat diets containing polyunsaturated fatty acids. J Anim Physiol An A. 2009;93:501–511. doi: 10.1111/j.1439-0396.2008.00832.x. [DOI] [PubMed] [Google Scholar]
- 10.Rees CA, Bauer JE, Burkholder WJ, Kennis RA, Dunbar BL, Bigley KE. Effects of dietary flax seed and sunflower seed supplementation on normal canine serum polyunsaturated fatty acids and skin and hair coat condition scores. Vet Dermatol. 2001;12:111–117. doi: 10.1046/j.1365-3164.2001.00234.x. [DOI] [PubMed] [Google Scholar]
- 11.Latreille J, Kesse-Guyot E, Malvy D, Andreeva V, Gala P, Tschachler E, Hercberg S, Guinot C, Ezzedine K. Association between dietary intake of n-3 polyunsaturated fatty acids and severity of skin photoaging in a middle-aged Caucasian population. J Dermatol Sci. 2013;72:233–239. doi: 10.1016/j.jdermsci.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Schumann J, Basiouni S, Gück T, Fuhrmann H. Treating canine atopic dermatitis with unsaturated fatty acids: the role of mast cells and potential mechanisms of action. J Physiol Anim Nutr. 2014;98:1013–1020. doi: 10.1111/jpn.12181. [DOI] [PubMed] [Google Scholar]
- 13.Thom E. Stress and the hair growth cycle: cortisol-induced hair growth disruption. J Drugs Dermatol. 2016;15:1001–1004. [PubMed] [Google Scholar]
- 14.Novak M, Menard M, El-Mallah S, Rosenberg K, Lutz C, Worlein J, Coleman K, Meyer S. Assessing significant (>30%) alopecia as a possible biomarker for stress in captive rhesus monkeys (Macaca mulatta) Am J Primatol. 2017;79:e22547. doi: 10.1002/ajp.22547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson R, Seira Oriach C, Murphy K, Moloney G, Cryan J, Dinan T, Paul Ross R, Stanton C. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav Immun. 2017;59:21–37. doi: 10.1016/j.bbi.2016.07.145. [DOI] [PubMed] [Google Scholar]
- 16.Farage M, Maiback H. Sensitive skin: closing in on a physiological cause. Contact Dermatitis. 2010;62:137–149. doi: 10.1111/j.1600-0536.2009.01697.x. [DOI] [PubMed] [Google Scholar]
- 17.Roussaki-Schulze A, Zafiriou K, Nikoulis D, Klimi E, Rallis E, Zintzaras E. Objective biophysical findings in patients with sensitive skin. Drugs Exp Clin Res. 2005;31(Suppl):17–24. [PubMed] [Google Scholar]