Abstract
Background
Most prior studies assessing the prognostic value of SPECT myocardial perfusion imaging (MPI) have used semi-quantitative visual analysis. We assessed the feasibility of large-scale fully-automated quantitative analysis of SPECT MPI to predict acute myocardial infarction (AMI). Additionally, we examined the impact of attenuation correction (AC) in automated strategies.
Methods and Results
5960 patients underwent rest/stress SPECT MPI with AC. Left ventricular (LV) segmentation, contour QC check and quantitation of stress and ischemic total perfusion deficit (sTPD, iTPD) were performed. Only contours flagged for potential errors by QC were visually checked (10%). During long-term follow-up (6.1±2.7yrs), 522 patients (9%) had AMI. In Cox models, adjusted for ejection fraction (LVEF) and other relevant covariates, there was a stepwise increase in risk hazard ratios by quartile for sTPD (Q1: 1.00, Q2: 1.26, Q3: 1.66, Q4: 1.79; p<0.0001); and iTPD (Q1: 1.00, Q2: 1.26, Q3: 1.66, Q4: 1.79; p<0.0001). Area under curve for AMI prediction by automated measures was similar for AC and non-AC data (sTPD: 0.63 vs 0.64, p=0.85; iTPD: 0.61 vs 0.61, p=0.70).
Conclusions
Fully automated sTPD was an independent predictor of future AMI events even after adjusting for LVEF and other relevant covariates. AC did not significantly impact predictive accuracy.
INTRODUCTION
Myocardial perfusion imaging (MPI) with single photon emission computed tomography (SPECT) is the most widely used non-invasive technique for diagnosing coronary artery disease. Although the prognostic value of SPECT MPI has also been demonstrated, most of the prior studies have used semi-quantitative visual analysis, with relatively few studies reporting fully automated, computer-based quantitative analysis [1–4]. This is despite the fact that quantitative analysis has been shown to be more reproducible[5][6]. Furthermore, most of the prognostic studies that do utilise fully automated analysis, have not employed attenuation correction (AC), and therefore its impact on automated strategies and prognostic accuracy remains unclear [1–3]. Additionally, in recent years, there have also been software developments that allow in-built quality control (QC) checks of left ventricular (LV) segmentation during the automated process[7][8]. Therefore, in this study we evaluated fully automated analysis including QC checks, applied to SPECT MPI with AC, for the long-term prediction of future acute myocardial infarction (AMI).
METHODS
Patient population
We evaluated 8682 consecutive patients who underwent clinically indicated MPI with SPECT at St. Boniface General Hospital from February 2001 to July 2008. Examinations without both stress and rest imaging, without AC or when performed as follow up to a previous scan were excluded. The research was approved by the institutional review board.
Data sources
The Manitoba Department of Health (Manitoba Health) provides comprehensive health care coverage for residents of the province of Manitoba. Since Manitoba residents are not obliged to pay premiums for health care coverage, nonparticipation in the plan is rare. Registry data are regularly updated to capture loss of coverage due to migration or death (Vital Statistics). Manitoba Health maintains computerized databases of the physician services and hospitalizations provided for all persons registered with the system. Each physician service record includes information on the identity of the patient, the date of service, services provided, and a diagnosis, which is subsequently coded to a 3-digit International Classification of Disease-9-Clinical Modification (ICD-9-CM) code. After each hospitalization, a detailed abstract is created; prior to 2004 this included up to 16 diagnoses as 5-digit ICD-9-CM codes, and from 2004 onwards included up to 25 diagnoses as International Classification of Diseases-10-Canadian Enhancements (ICD-10-CA) codes. Pharmacy-based prescription data for the province, collected through the Drug Programs Information Network (DPIN) are now also a part of the data repository. This data repository allows for the creation of a longitudinal record of health service utilization for an individual through a unique personal health identification number, anonymized to preserve patient confidentiality. The accuracy of these administrative data has been established for a wide range of clinical disorders, including prediction of mortality after acute myocardial infarction (AMI)[9,10].
Imaging Acquisition & Reconstruction protocol
Standard 99mTc-sestamibi or tetrofosmin rest/stress protocols were employed as previously described with treadmill testing or dipyridamole infusion with low-level exercise[11]. Dual-detector scintillation cameras with low-energy high-resolution collimators (Vertex; Philips Medical Systems, Milpitas, CA) and gadolinium-153 line-source attenuation correction hardware and software (Vantage Pro; Philips Medical Systems, Milpitas, CA) were used to acquire MPI. Tomographic reconstruction was performed by AutoSPECT and Vantage Pro programs (Philips Medical Systems)[12]. Emission images were automatically corrected for non-uniformity, radioactive decay, center-of-rotation, and motion during acquisition. Filtered back-projection and Butterworth filters were applied to obtain the non-AC MPI with an order of 10 and cut-off of 0.50 for rest MPI, and an order of 5 and cut-off of 0.66 for stress MPI. The attenuation maps and the emission data were used to reconstruct the AC images with a maximum-likelihood expectation maximization algorithm incorporating scatter correction and depth-dependent resolution compensation.
Automated Image analysis
LV segmentation, LV contour quality control (QC) and quantitation of LV ejection fraction (LVEF) and global total perfusion deficit (TPD - at stress [sTPD] and rest [rTPD]) was performed with automated software without any user intervention (fully unsupervised) (QGS-QPS software, Cedars-Sinai Medical Center, Los Angeles, CA, USA). Only LV contours flagged for potential errors by QC were visually checked by an experienced technician [7][8]. LVEF was categorized as >=45% (referent), 30–44%, <30% and missing (for non-gated studies).
Automated LV segmentation and QC
Automatically derived LV contours were verified by an automated method for QC [7]. This method derives 2 parameters: the shape flag to detect the mask-failure cases, and the valve plane flag to detect the valve plane over- or under-shooting. Only LV contours with shape QC flag > 3, and valve plane flag > 0.37 or < 0.28, were visually checked by an experienced technologist (JG, > 10 yrs. experience) who then made manual adjustments based on visual judgment if deemed appropriate [7,8]. All image analyses were performed by researchers at Cedars-Sinai Medical Center using de-identified image files to ensure blinding to all clinical information and outcomes.
Automated TPD calculation
After the QC step, all studies were processed in batch mode using QPS software to calculate global sTPD and ischemic TPD (iTPD) as percentage of the myocardium. QPS software computes total perfusion deficit by extracting myocardial count density samples to polar map co-ordinates and comparing to average local values in the normal population using a pre-defined normal threshold [11]. Normal limits threshold was defined as 3.0 mean absolute deviations (approximately equivalent to 2.5 standard deviations, SD) for each polar map sample. sTPD and rTPD were measured independently for stress and rest scans respectively, and iTPD was defined as their global difference: (sTPD – rTPD). Any negative iTPD results - for example due to attenuation or differences in count statistics or filtering - were reset to zero value (i.e. no ischemia) for the analysis. Both sTPD and iTPD variables reflect a combination of extent and severity of perfusion defects[13]. For AC results, hypoperfusion severities from non-AC and AC data were derived and then averaged at each polar map location, similar to visual AC analysis where readers combine non-AC and AC data[14]. For non-AC results, TPD variables were computed only from non-AC images[13].
Study Endpoints and Covariates
Follow-up was performed through the Manitoba Population Health Research Data Repository. The primary endpoint of the study was AMI defined from hospital discharge diagnosis. Observations were censored at time of death (23.3%), migration out of province (2.5%), or end of data (March 31, 2012). The patients who moved out of the province were considered to have been lost to follow up. Early revascularization (within 90 days) was performed only in 118 patients (< 2% of the overall population) and these patients were not excluded from the overall analysis. Secondary endpoints including death and death or AMI, were also studied.
Clinical covariates were also identified through the repository. The diagnosis of diabetes mellitus was assigned if a patient had 2 physician visits or 1 hospital admission with a coded diagnosis of diabetes mellitus during the 3 y before the scan; this definition has been well validated and adopted for purposes of national diabetes surveillance.[2] Similar definitions (2 physician visits or 1 hospital admission during the 3 y before the scan) were created for congestive heart failure, cardiomyopathy, angina pectoris, dysrhythmias, chronic obstructive lung disease (COPD, a proxy for smoking), hypertension and hyperlipidemia. Hospital records were accessed to determine the number of hospitalizations during the last 3 years (a proxy for comorbidity), or any hospitalization since 1984 with a diagnosis of AMI or unstable angina, or procedure code for a surgical or percutaneous coronary revascularization. Cardiac medications used in the 12 months before scanning (at least 1 filled prescription) and during follow-up (at least 2 filled prescriptions) was obtained through review of detailed prescription records in the pharmacy database.
Statistical analysis
Categorical variables are presented as frequencies, and continuous variables as mean ± SD. Categorical variables were compared with Pearson Chi-square, and continuous variables with Student’s two sample t-test. Kaplan-Meier curves were generated in order to assess the AMI-free survival in different quartiles of sTPD and iTPD, and were compared using the log-rank test. Stratification in AMI risk was also assessed using receiver operating characteristic (ROC) analysis, and area-under-curve (AUC) comparisons were made using the DeLong method[15]. Multivariate Cox proportional hazards analysis was performed to assess MPI predictors of AMI as quartiles (sTPD without and with AC, iTPD without and with AC), initially adjusted for age and sex alone then adjusted for all clinical variables listed in Table 1. A test for linear trend across quartiles in the MPI predictors was also performed. All statistical analyses were two-tailed and a p-value of <0.05 was considered significant. Baseline comparisons and regression analyses were performed using Statistica (Version 10.0, StatSoft Inc, Tulsa, OK). ROC analyses were performed using Sigmaplot (Version 10.0, Systat Software Inc.).
Table 1.
Patient characteristics and univariate differences according to AMI outcome
| Overall (n=5960) | AMI (n=522) | No AMI (n=5438) | p | |
|---|---|---|---|---|
|
|
||||
| Demographics | ||||
| Age (years) | 64.8 ± 11.8 | 67.2 ± 11.6 | 64.6 ± 11.8 | <0.001 |
| Male | 3144 (52.8) | 334 (64) | 2810 (51.7) | <0.001 |
|
| ||||
| Clinical factors | ||||
| No. hospitalizations last 3 years | 1.5 ± 2.4 | 2.8 ± 3.6 | 1.3 ± 2.2 | <0.001 |
| Congestive heart failure | 1202 (20.2) | 199 (38.1) | 1003 (18.4) | <0.001 |
| Cardiomyopathy | 198 (3.3) | 23 (4.4) | 175 (3.2) | 0.148 |
| Angina pectoris | 1085 (18.2) | 445 (32) | 771 (16.9) | <0.001 |
| Dysrhythmias | 1109 (18.6) | 132 (25.3) | 977 (18) | <0.001 |
| Diabetes | 1889 (31.7) | 244 (46.7) | 1645 (30.3) | <0.001 |
| COPD | 769 (12.9) | 99 (19) | 670 (12.3) | <0.001 |
| Hypertension | 3510 (58.9) | 385 (73.8) | 3125 (57.5) | <0.001 |
| Hyperlipidemia | 1314 (22) | 178 (34.1) | 1136 (20.9) | <0.001 |
| Prior coronary revascularization | 1353 (22.7) | 165 (31.6) | 1188 (21.8) | <0.001 |
| Prior AMI or unstable angina | 1390 (23.3) | 198 (37.9) | 1192 (21.9) | <0.001 |
| ACE-I/ARB therapy | 3351 (56.2) | 339 (64.9) | 3012 (55.4) | <0.001 |
| Beta blocker therapy | 3150 (52.9) | 373 (71.5) | 2777 (51.1) | <0.001 |
| Nitrate therapy | 917 (15.4) | 154 (29.5) | 763 (14) | <0.001 |
| Antiarrhythmic therapy | 138 (2.3) | 17 (3.3) | 121 (2.2) | 0.134 |
| Lipid-lowering therapy | 3358 (56.3) | 359 (68.8) | 2999 (55.1) | <0.001 |
|
| ||||
| SPECT MPI | ||||
| Pharmacologic stress | 3612 (60.6) | 391 (74.9) | 3221 (59.2) | <0.001 |
| sTPD - without AC | 10.4 ± 12 | 15.2 ± 13.5 | 9.9 ± 11.7 | <0.001 |
| sTPD - with AC | 11.1 ± 11.4 | 15.7 ± 12.9 | 10.7 ± 11.2 | <0.001 |
| iTPD - without AC | 3.3 ± 5.1 | 4.9 ± 5.9 | 3.1 ± 4.9 | <0.001 |
| iTPD - with AC | 5.7 ± 5.1 | 7.4 ± 5.7 | 5.5 ± 5 | <0.001 |
| LVEF, % | 55.6 ± 14.9 | 50.6 ± 14.9 | 56.1 ± 14.8 | <0.001 |
No. = number; AMI = acute myocardial infarction; ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; MPI = myocardial perfusion imaging; sTPD = stress TPD; iTPD = ischemic TPD; TPD = total perfusion deficit; AC = attenuation correction; COPD – chronic obstructive pulmonary disease; TID = transient ischemic dilatation; LVEF = left ventricular ejection fraction (not available in 990 with ungated scans); Data are presented as mean SD or n (%) as appropriate
RESULTS
Study population
Scan data from 8682 consecutive patients undergoing clinically indicated SPECT MPI were available. Scan data were incomplete or corrupted in 1787 patients leaving 6895 with complete stress and rest image data. After exclusion of repeat scans and those without AC acquisitions, data from 5960 patients (65±12yrs, 3144 men) were available for analysis. Automation was achieved in 100% of cases. Only 10% of rest/stress studies required visual correction of contours.
During long-term follow-up (6.1±2.7yrs), 522 patients (9%) had a hospitalized AMI and 1391 patients died (23%). Patient demographics and clinical characteristics are summarized in Table 1. There were significant differences between the AMI and non-AMI groups for age, gender, pharmacological stress, congestive heart failure, diabetes, hypertension, hyperlipidemia, COPD, prior AMI or unstable angina, prior revascularization, medications, LVEF and number of hospitalizations in the last 3 years (p<0.05) (Table 1).
Multivariate Analysis for AMI Prediction
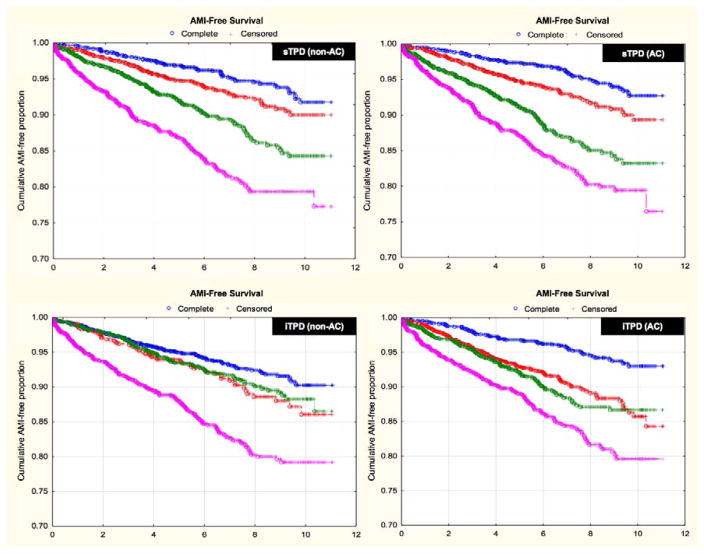
In multivariate Cox regression, adjusted for all clinical variables shown in Table 1, sTPD and iTPD were independent predictors of AMI (Table 2). Annualized AMI rate according to Kaplan-Meier curves for sTPD and iTPD are depicted in Figure 1. There was a stepwise increase in risk of AMI by sTPD quartile and by iTPD quartile - for both AC and non-AC data (p<0.001 for hazard ratio comparison across quartiles) (Figure 1, Table 2). Accordingly, crude AMI rates per 1000/year increased across successive sTPD and iTPD quartiles – for both AC and non-AC data (p<0.001) (Table 2). Similar results for secondary endpoints of death, and death or AMI are given in online supplement (Tables 3 and 4).
Table 2.
Multivariate analysis for prediction of AMI
| Crude AMI (per 1000/y) | Basic Model: Adjusted for sex and age | Fully-adjusted Model: All covariates | |||||
|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p | Hazard Ratio | 95% CI | p | ||
|
| |||||||
| sTPD | |||||||
| Q1 (ref) | 6.9 | 1.00 | - | - | 1.00 | - | - |
| Q2 | 10.2 | 1.38 | 1.01–1.89 | 0.042 | 1.09 | 0.8–1.5 | 0.575 |
| Q3 | 16.3 | 2.08 | 1.54–2.79 | <0.001 | 1.33 | 0.98–1.81 | 0.068 |
| Q4 | 26.5 | 3.21 | 2.39–4.3 | <0.001 | 1.61 | 1.17–2.23 | 0.004 |
| Linear trend | <0.001 | Linear trend | 0.001 | ||||
|
| |||||||
| sTPD AC | |||||||
| Q1 (ref) | 6.2 | 1.00 | - | - | 1.00 | - | - |
| Q2 | 10.3 | 1.54 | 1.12–2.11 | 0.008 | 1.26 | 0.91–1.73 | 0.162 |
| Q3 | 18.3 | 2.54 | 1.89–3.41 | <0.001 | 1.66 | 1.22–2.25 | 0.001 |
| Q4 | 25.7 | 3.43 | 2.57–4.58 | <0.001 | 1.79 | 1.3–2.47 | <0.001 |
| Linear trend | <0.001 | Linear trend | <0.001 | ||||
|
| |||||||
| iTPD | |||||||
| Q1 (ref) | 9.8 | 1.00 | - | - | 1.00 | - | - |
| Q2 | 13.4 | 1.32 | 0.98–1.78 | 0.065 | 1.34 | 1–1.8 | 0.054 |
| Q3 | 12.5 | 1.16 | 0.91–1.5 | 0.232 | 0.95 | 0.74–1.23 | 0.710 |
| Q4 | 24.8 | 2.09 | 1.66–2.61 | <0.001 | 1.41 | 1.12–1.78 | 0.004 |
| Linear trend | <0.001 | Linear trend | 0.018 | ||||
|
| |||||||
| iTPD AC | |||||||
| Q1 (ref) | 6.6 | 1.00 | - | - | 1.00 | - | - |
| Q2 | 13.9 | 1.91 | 1.42–2.57 | <0.001 | 1.54 | 1.14–2.08 | 0.005 |
| Q3 | 15.7 | 2.03 | 1.51–2.73 | <0.001 | 1.34 | 0.99–1.82 | 0.058 |
| Q4 | 23.1 | 2.91 | 2.19–3.85 | <0.001 | 1.71 | 1.27–2.29 | <0.001 |
| Linear trend | <0.001 | Linear trend | 0.003 | ||||
AMI = acute myocardial infarction; ref= referent; sTPD = stress TPD; iTPD = ischemic TPD; TPD = total perfusion deficit; AC = attenuation correction; Q = quartile; Lower and upper quartile thresholds were 1.9 and 14.5 (median 5.4) for non-AC sTPD; 2.8 and 15.8 (median 7.1) for AC sTPD; 0 and 4.5 (median 1.0) for non-AC iTPD; and 2.0 and 7.8 (median 4.3) for AC iTPD.
Figure 1. Cumulative survival without AMI according to automated quantitative analysis.
Cumulative survival without AMI according to automated sTPD (A, B) and automated iTPD (C, D) for both non-AC and AC data. There was a stepwise increase in risk of AMI by sTPD quartile and by iTPD quartile - for both AC and non-AC data (p<0.0001 for hazard ratio [HR] comparison across quartiles). Lower and upper quartile thresholds were 1.9 and 14.5 (median 5.4) for non-AC sTPD; 2.8 and 15.8 (median 7.1) for AC sTPD; 0 and 4.5 (median 1.0) for non-AC iTPD; and 2.0 and 7.8 (median 4.3) for AC iTPD.
Table 3.
Multivariate analysis for prediction of Death (online supplement)
| Basic Model: Adjusted for sex and age | Fully-adjusted Model: All covariates | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Hazard Ratio | 95% CI | P value | Hazard Ratio | 95% CI | P value | |
| sTPD | ||||||
| Q2 vs Q1 | 1.52 | 1.24–1.86 | <0.001 | 1.18 | 0.97–1.45 | 0.105 |
| Q3 vs Q1 | 2.30 | 1.9–2.78 | <0.001 | 1.46 | 1.2–1.78 | <0.001 |
| Q4 vs Q1 | 3.56 | 2.95–4.3 | <0.001 | 1.77 | 1.44–2.18 | <0.001 |
| Linear trend | <0.001 | Linear trend | <0.001 | |||
|
| ||||||
| sTPD AC | ||||||
| Q2 vs Q1 | 1.26 | 1.04–1.53 | 0.021 | 1.12 | 0.92–1.36 | 0.274 |
| Q3 vs Q1 | 2.00 | 1.67–2.4 | <0.001 | 1.44 | 1.2–1.74 | <0.001 |
| Q4 vs Q1 | 3.05 | 2.56–3.63 | <0.001 | 1.65 | 1.36–2.01 | <0.001 |
| Linear trend | <0.001 | Linear trend | <0.001 | |||
|
| ||||||
| iTPD | ||||||
| Q2 vs Q1 | 0.95 | 0.78–1.15 | 0.580 | 0.95 | 0.78–1.16 | 0.612 |
| Q3 vs Q1 | 1.32 | 1.15–1.53 | <0.001 | 1.07 | 0.93–1.24 | 0.355 |
| Q4 vs Q1 | 1.63 | 1.42–1.88 | <0.001 | 1.25 | 1.08–1.44 | 0.003 |
| Linear trend | <0.001 | Linear trend | 0.003 | |||
|
| ||||||
| iTPD AC | ||||||
| Q2 vs Q1 | 1.26 | 1.06–1.5 | 0.011 | 1.07 | 0.9–1.29 | 0.432 |
| Q3 vs Q1 | 1.72 | 1.45–2.03 | <0.001 | 1.21 | 1.01–1.43 | 0.034 |
| Q4 vs Q1 | 1.98 | 1.69–2.34 | <0.001 | 1.35 | 1.14–1.61 | <0.001 |
| Linear trend | <0.001 | Linear trend | <0.001 | |||
Table 4.
Multivariate analysis for prediction of Death or AMI (online supplement)
| Basic Model: Adjusted for sex and age | Fully-adjusted Model: All covariates | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Hazard Ratio | 95% CI | P value | Hazard Ratio | 95% CI | P value | |
| sTPD | ||||||
| Q2 vs Q1 | 1.40 | 1.18–1.67 | <0.001 | 1.12 | 0.93–1.33 | 0.224 |
| Q3 vs Q1 | 2.11 | 1.79–2.49 | <0.001 | 1.39 | 1.17–1.65 | <0.001 |
| Q4 vs Q1 | 3.23 | 2.74–3.8 | <0.001 | 1.68 | 1.4–2.02 | <0.001 |
| Linear trend | <0.001 | Linear trend | <0.001 | |||
|
| ||||||
| sTPD AC | ||||||
| Q2 vs Q1 | 1.30 | 1.1–1.55 | 0.002 | 1.14 | 0.96–1.36 | 0.134 |
| Q3 vs Q1 | 2.00 | 1.71–2.35 | <0.001 | 1.43 | 1.21–1.69 | <0.001 |
| Q4 vs Q1 | 2.97 | 2.55–3.47 | <0.001 | 1.64 | 1.38–1.96 | <0.001 |
| Linear trend | <0.001 | Linear trend | <0.001 | |||
|
| ||||||
| iTPD | ||||||
| Q2 vs Q1 | 1.04 | 0.88–1.24 | 0.629 | 1.06 | 0.89–1.25 | 0.537 |
| Q3 vs Q1 | 1.25 | 1.1–1.43 | <0.001 | 1.03 | 0.9–1.17 | 0.704 |
| Q4 vs Q1 | 1.65 | 1.45–1.87 | <0.001 | 1.24 | 1.09–1.42 | <0.001 |
| Linear trend | <0.001 | Linear trend | 0.003 | |||
|
| ||||||
| iTPD AC | ||||||
| Q2 vs Q1 | 1.35 | 1.15–1.58 | <0.001 | 1.14 | 0.97–1.34 | 0.100 |
| Q3 vs Q1 | 1.69 | 1.45–1.97 | <0.001 | 1.19 | 1.02–1.39 | 0.028 |
| Q4 vs Q1 | 2.05 | 1.77–2.37 | <0.001 | 1.36 | 1.17–1.59 | <0.001 |
| Linear trend | <0.001 | Linear trend | <0.001 | |||
Predictive Accuracy of sTPD and iTPD - AC vs non-AC data
For predicting the primary endpoint of AMI over the full follow-up period the AUC was similar for AC and non-AC data (sTPD: 0.63 [95% CI: 0.61–0.66] vs. 0.64 [95% CI: 061–0.66], p=0.85; iTPD: 0.61 [95% CI: 0.59–0.64] vs. 0.61 [95% CI: 0.58–0.63], p=0.70); and sTPD was superior to iTPD in all instances (all p values<0.01). Higher AUCs for both AC and non-AC data were seen for AMI occurring within the first 3 years (sTPD: 0.67 [63–70], 0.67[63–70]; iTPD: 0.64 [60–67], 0.62 [58–66]) and within the first 1 year of follow-up (sTPD: 0.71 [95% CI: 0.67–0.76], 0.72 [95% CI: 0.67–0.76]; iTPD: 0.70 [95% CI: 0.65–0.75], 0.68 [95% CI: 0.63–0.73]).
For both secondary endpoints (death; death or AMI) AC data were marginally inferior to non-AC data using sTPD (death, AUC: 0.67 [95% CI: 0.66–0.69] vs. 0.68 [95% CI: 067–0.70], respectively, p=0.017; death or AMI, AUC: 0.67 [95% CI: 0.65–0.68] vs. 0.68 [95% CI: 066–0.69], respectively p=0.04 ), but there was no significant difference when using iTPD (death, AUC: 0.61 [95% CI: 0.59–0.63] vs. 0.61 [95% CI: 0.59–0.63] respectively, p=0.88; death or AMI AUC: 0.61 [95% CI: 0.59–0.63] vs. 0.61 [95% CI: 0.59–0.62], p=0.72).
For all endpoints, primary and secondary, sTPD was superior to iTPD for both AC and non-AC data (all p values<0.01).
DISCUSSION
This is the first large-scale study to examine the prognostic value of fully-automated computer-based analysis including QC, applied to standard SPECT MPI acquisitions with AC. The main findings were that: i) large-scale fully automated analysis is highly feasible and has modest predictive accuracy for future AMI over long-term follow-up; ii) annualized AMI rates increased in proportion to the magnitude of automated sTPD or iTPD abnormality; iii) automated sTPD was a stronger predictor of AMI than automated iTPD; and iv) AC made no significant difference to the predictive accuracy of sTPD or iTPD for AMI or death; and v) both sTPD and iTPD have better immediate (1-year) than long-term (5-year) prediction of AMI.
The prognostic value of myocardial perfusion imaging is well established and provides incremental value when combined with clinical, exercise, and angiographic information[16]. A previous meta-analysis of 30,000 patients using a variety of stress protocols and radiopharmaceuticals found an annualized cardiac death rate of only 0.5% in individuals with a normal result[17]. The ability to risk stratify individuals for cardiac death and myocardial infarction can be seen in those at low, intermediate, and high likelihood for CAD [18]. MPI with SPECT can identify very low risk patients with stable chest pain syndromes and after AMI [19]. This information is incremental to what can be gained from using clinical and stress variables[19–21]. However, of note, prior studies have mostly focused in visual analysis, and there have been relatively few studies evaluating the prognostic utility of quantitative analysis[1–4].
Quantitative analysis of SPECT MPI is highly reproducible and offers the potential to eliminate observer variability and bias, providing a more standardized approach than visual analysis, as it is not dependent on the expertise of individual readers[22]. Prior diagnostic accuracy studies have demonstrated that quantitative analysis can be used to supplement visual analysis for accurate detection of CAD, but furthermore, when used independently it has been shown to have at least equivalent diagnostic accuracy as expert visual readers[8]. Previously, Leslie et al. showed that visual and quantitative categorization of scan perfusion abnormalities (using summed difference score, SDS) showed similar prognostic value for predicting acute MI or cardiac death in patients referred for SPECT MPI (n=718), but automated analysis still required a manual QC step for checking LV segmentation contours[2]. More recently, Nakazato et al. demonstrated that quantitative analysis (using sTPD and iTPD) provided similar prediction of all-cause mortality as visual analysis in patients undergoing SPECT MPI for suspected CAD (n = 1613) – but this study utilized a novel high-speed SPECT camera system with cadmium zinc-telluride detectors rather than standard widely available equipment, and again the automated analysis required a manual QC step for LV contours[4]. The only prior prognostic study evaluating fully automated quantitative analysis including contour QC, and using standard SPECT cameras, is a small case-control study (n= 81) by Xu et al. which also found visual and automated analysis to be comparable, in this case for predicting cardiac death[23]. Furthermore, none of these prior studies evaluated automated analysis using AC data which has become increasingly routine. Therefore, to our knowledge, the present study is the first large-scale study to examine the prognostic value of fully-automated computer-based analysis including QC, applied to standard SPECT MPI with AC, and to make direct comparisons with non-AC data.
Prior studies utilizing clinical reads rather than automation have shown marginal improvements in risk-stratification using AC rather than non-AC data[24–26]. Notably however, the use of fully-automated quantitative reads in the current study appears to remove this discrepancy – as the prognostic value of quantitative analysis was similar for both AC and non-AC data. Although further study and prospective validation of this finding is required, this important new observation may simplify large-scale batch processing of automated quantitative analysis and raises the possibility of providing a rapid, automated, standardized core-lab read for large trials across many centers with varying scan acquisitions.
Study Limitations
Since all imaging was performed at a single center in this study, further multi-center evaluation studies are required. However, our application of fully automated quantitative analysis increases the likelihood that our results will be widely applicable to other centers. Importantly, we did not have data regarding medical treatment that immediately followed MPI in our study. Thus, we could not evaluate whether aggressive medical therapy influenced the results of our study.
For simplicity, iTPD was calculated as the global difference in sTPD and rTPD, which can sometimes underestimate ischemia particularly in non-AC images. For example, in a patient with genuine anterior hypoperfusion but significant attenuation in the inferior wall, a simple global difference calculation may miss the ischemia. Nonetheless, as we did not find a significant difference between AC and non-AC data results, we do not believe this has a major impact on the study findings.
Finally, imaging data was collected between 2001–2008 (although clinical follow-up ran to March 2012) and ideally more contemporary data for analysis would be desirable. However, this was not possible as the line-source AC gamma camera was replaced with a SPECT/CT system after 2008. Although technological developments since then may conceivably have led to better predictive accuracy results with newer data, the fundamental principle of successfully demonstrating large-scale automated analysis for prognostic assessment is not altered.
CONCLUSION
We have demonstrated feasibility of large-scale, nearly unsupervised quantitative SPECT MPI analysis. Fully automated TPD was an independent predictor of future AMI events even after adjusting for LVEF and other relevant covariates. AC did not significantly impact predictive accuracy.
NEW KNOWLEDGE GAINED
This study shows that is possible with minimal effort to reprocess large repository of the MPI images in a near unsupervised fashion. This allows for unbiased comparison of different MPI variables with respect to prediction of myocardial infarction. Employing this strategy in a large dataset, we have learned that stress TPD variable is a better myocardial infarction predictor than the ischemic TPD and that attenuation correction does not improve prediction of myocardial infraction. As well as offering insight into automation developments that may translate into future clinical applications, the study also has significant implications for nuclear cardiology research by demonstrating the feasibility of large-scale batch processing of automated quantitative analysis. We have also learned that prediction of the myocardial infarction in the short term (1 year) is significantly more accurate than the prediction of this event in longer term (6 years).
Acknowledgments
The authors acknowledge the Manitoba Centre for Health Policy for use of data contained in the Population Health Research Data Repository under project # (HIPC# 2010/2011-55). The results and conclusions are those of the authors and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, Healthy Living, and Seniors, or other data providers is intended or should be inferred.
This research was supported in part by NIH grant R01 HL089765 (PJS); the Dowager Countess Eleanor Peel Trust, Manchester, UK (MM); and the Dickinson Trust, Manchester, UK (MM).
Daniel Berman, Guido Germano and Piotr Slomka participate in software royalties at Cedars-Sinai Medical Center for the licensing of the software for myocardial perfusion quantification.
ABBREVIATIONS
- MPI
Myocardial Perfusion Imaging
- SPECT
Single Photon Emission Computed Tomography
- AC
Attenuation Correction
- QC
Quality Control
- LVEF
Left Ventricular Ejection Fraction
- AMI
Acute Myocardial Infarction
- TPD
Total Perfusion Deficit
- sTPD
Stress TPD
- iTPD
Ischemic TPD
- CAD
Coronary Artery Disease
References
- 1.Berman D, Kang X, Van Train K, Lewin H, Cohen I, Areeda J, et al. Comparative prognostic value of automatic quantitative analysis versus semiquantitative visual analysis of exercise myocardial perfusion single-photon emission computed tomography. J Am Coll Cardiol. 1998;32:1987–95. doi: 10.1016/s0735-1097(98)00501-4. [DOI] [PubMed] [Google Scholar]
- 2.Leslie WD, Tully SA, Yogendran MS, Ward LM, Nour KA, Metge CJ. Prognostic value of automated quantification of 99mTc-sestamibi myocardial perfusion imaging. J Nucl Med. 2005;46:204–11. [PubMed] [Google Scholar]
- 3.Dakik HA, Hwang WS, Jafar A, Kimball K, Verani MS, Mahmarian JJ. Prognostic value of quantitative stress myocardial perfusion imaging in unstable angina patients with negative cardiac enzymes and no new ischemic ECG changes. J Nucl Cardiol. 2005;12:32–6. doi: 10.1016/j.nuclcard.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Nakazato R, Berman DS, Gransar H, Hyun M, Miranda-Peats R, Kite FC, et al. Prognostic value of quantitative high-speed myocardial perfusion imaging. J Nucl Cardiol. 2012;19:1113–23. doi: 10.1007/s12350-012-9619-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berman DS, Kang X, Gransar H, Gerlach J, Friedman JD, Hayes SW, et al. Quantitative assessment of myocardial perfusion abnormality on SPECT myocardial perfusion imaging is more reproducible than expert visual analysis. J Nucl Cardiol. 2009;16:45–53. doi: 10.1007/s12350-008-9018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iskandrian AE, Garcia EV, Faber T, Mahmarian JJ. Automated assessment of serial SPECT myocardial perfusion images. J Nucl Cardiol. 2009;16:6–9. doi: 10.1007/s12350-008-9020-6. [DOI] [PubMed] [Google Scholar]
- 7.Xu Y, Kavanagh P, Fish M, Gerlach J, Ramesh A, Lemley M, et al. Automated Quality Control for Segmentation of Myocardial Perfusion SPECT. J Nucl Med. 2009;50:1418–26. doi: 10.2967/jnumed.108.061333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arsanjani R, Xu Y, Hayes SW, Fish M, Lemley M, Gerlach J, et al. Comparison of Fully Automated Computer Analysis and Visual Scoring for Detection of Coronary Artery Disease from Myocardial Perfusion SPECT in a Large Population. J Nucl Med. 2013;54:221–8. doi: 10.2967/jnumed.112.108969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tu JV, Austin PC, Walld R, Roos L, Agras J, McDonald KM. Development and validation of the Ontario acute myocardial infarction mortality prediction rules. J Am Coll Cardiol. 2001;37:992–7. doi: 10.1016/s0735-1097(01)01109-3. [DOI] [PubMed] [Google Scholar]
- 10.Roos LL, Mustard CA, Nicol JP, Comm B, Mclerran DF, Malenka DJ, et al. Registries and administrative data: organization and accuracy. Med Care. 1993;31:201–12. doi: 10.1097/00005650-199303000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Slomka PJ, Fish MB, Lorenzo S, Nishina H, Gerlach J, Berman DS, et al. Simplified normal limits and automated quantitative assessment for attenuation-corrected myocardial perfusion SPECT. J Nucl Cardiol. 2006;13:642–51. doi: 10.1016/j.nuclcard.2006.06.131. [DOI] [PubMed] [Google Scholar]
- 12.Germano G, Berman DS. On the accuracy and reproducibility of quantitative gated myocardial perfusion SPECT. J Nucl Med. 1999;40:810–3. [PubMed] [Google Scholar]
- 13.Slomka PJ, Nishina H, Berman DS, Akincioglu C, Abidov A, Friedman JD, et al. Automated quantification of myocardial perfusion SPECT using simplified normal limits. J Nucl Cardiol. 2005;12:66–77. doi: 10.1016/j.nuclcard.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Fish M, Gerlach J, Lemley M, Berman DS, Germano G, et al. Combined quantitative analysis of attenuation corrected and non-corrected myocadial perfusion SPECT: Method development and clinical validation. J Nucl Cardiol. 2010;17:591–9. doi: 10.1007/s12350-010-9220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 16.Brown KA. Prognostic value of myocardial perfusion imaging: state of the art and new developments. J Nucl Cardiol. 1996;3:516–37. doi: 10.1016/s1071-3581(96)90061-6. [DOI] [PubMed] [Google Scholar]
- 17.Shaw LJ, Hendel R, Borges-Neto S, Lauer MS, Alazraki N, Burnette J, et al. Prognostic value of normal exercise and adenosine 99mTc-tetrofosmin SPECT imaging: results from the multicenter registry of 4,728 patients. J Nucl Med. 2003;44:134–9. [PubMed] [Google Scholar]
- 18.Berman DS, Hachamovitch R, Kiat H, Cohen I, Cabico JA, Wang FP, et al. Incremental value of prognostic testing in patients with known or suspected ischemic heart disease: a basis for optimal utilization of exercise technetium-99m sestamibi myocardial perfusion single-photon emission computed tomography. J Am Coll Cardiol. 1995;26:639–47. doi: 10.1016/0735-1097(95)00218-S. [DOI] [PubMed] [Google Scholar]
- 19.Hachamovitch R, Berman DS, Kiat H, Cohen I, Cabico JA, Friedman J, et al. Exercise Myocardial Perfusion SPECT in Patients Without Known Coronary Artery Disease Incremental Prognostic Value and Use in Risk Stratification. Circulation. 1996;93:905–14. doi: 10.1161/01.cir.93.5.905. [DOI] [PubMed] [Google Scholar]
- 20.Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97:535–43. doi: 10.1161/01.cir.97.6.535. [DOI] [PubMed] [Google Scholar]
- 21.Travin MI, Dessouki AMR, Cameron T, Helle GV. Use of exercise technetiumtium-99m sestamibi SPECT imagina to detect residual ischemia and for risk stratification after acute myocardial infarction. Am J Cardiol. 1995;75:665–9. doi: 10.1016/s0002-9149(99)80650-x. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y, Hayes S, Ali I, Ruddy TD, Wells RG, Berman DS, et al. Automatic and visual reproducibility of perfusion and function measures for myocardial perfusion SPECT. J Nucl Cardiol. 2010;17:1050–7. doi: 10.1007/s12350-010-9297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Y, Nakazato R, Hayes S, Hachamovitch R, Cheng VY, Gransar H, et al. Prognostic value of automated vs visual analysis for adenosine stress myocardial perfusion SPECT in patients without prior coronary artery disease: A case-control study. J Nucl Cardiol. 2011;18:1003–9. doi: 10.1007/s12350-011-9449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pazhenkottil AP, Ghadri J-R, Nkoulou RN, Wolfrum M, Buechel RR, Küest SM, et al. Improved outcome prediction by SPECT myocardial perfusion imaging after CT attenuation correction. J Nucl Med. 2011;52:196–200. doi: 10.2967/jnumed.110.080580. [DOI] [PubMed] [Google Scholar]
- 25.Baghdasarian SB, Noble GL, Ahlberg AW, Katten D, Heller GV. Risk stratification with attenuation corrected stress Tc-99m sestamibi SPECT myocardial perfusion imaging in the absence of ECG-gating due to arrhythmias. J Nucl Cardiol. 2009;16:533–9. doi: 10.1007/s12350-009-9071-3. [DOI] [PubMed] [Google Scholar]
- 26.Ardestani A, Ahlberg AW, Katten DM, Santilli K, Polk DM, Bateman TM, et al. Risk stratification using line source attenuation correction with rest/stress Tc-99m sestamibi SPECT myocardial perfusion imaging. J Nucl Cardiol. 2014;21:118–26. doi: 10.1007/s12350-013-9816-x. [DOI] [PubMed] [Google Scholar]