Abstract
Aims
Paired-like homeobox 2b (PHOX2B) is a transcription factor with expression outside of the central nervous system restricted to neurons and chromaffin cells of the autonomic nervous system. Germline mutations cause congenital central hypoventilation syndrome and predispose to neuroblastoma and Hirschsprung disease. Among pediatric small round cell tumors, PHOX2B is neuroblastoma-specific. Two studies of adult autonomic nervous system tumors (n=62) produced conflicting results (all tumors stained in one; expression was restricted to 40% of paragangliomas in the other). We examined PHOX2B expression in a large cohort of pheochromocytomas and paragangliomas, as well as well-differentiated neuroendocrine tumors (WDNET) and poorly differentiated neuroendocrine carcinomas (PDNEC).
Methods and results
Tissue microarrays were constructed from 609 tumors: 111 pheochromocytomas, 146 paragangliomas, 250 WDNETs, and 102 PDNECs. PHOX2B immunohistochemistry was scored for extent (%) and intensity (0–3+), with an H-score (extent*intensity) calculated. PHOX2B expression was seen in 32% of pheochromocytomas and 47% of paragangliomas. Mean/median H-scores for these tumors were in the 30–55 range (i.e., weak to moderate staining). No WDNETs and only 7% of PDNECs stained, the latter often strongly so. In a representative cohort of corresponding whole sections (n=55), results in WDNETs and PDNECs were unchanged, while half of pheochromocytomas/paragangliomas negative on TMA became focally, weakly positive.
Conclusions
We found frequent, weak to moderate PHOX2B expression in pheochromocytomas/paragangliomas and no expression in WDNETs, which could be diagnostically useful in the distinction of these tumors. Expression in a minority of PDNECs likely reflects the transcription factor lineage infidelity characteristic of this tumor class.
Keywords: PHOX2B, immunohistochemistry, transcription factor, neuroendocrine, pheochromocytoma, paraganglioma, differential diagnosis
Introduction
The transcription factor paired-like homeobox 2b (PHOX2B) has been referred to as the master regulator of the autonomic nervous system and of the noradrenergic phenotype. Among neural crest derivatives, PHOX2B is expressed by sympathetic, parasympathetic, and enteric ganglia; adrenal and extra-adrenal chromaffin cells; and by glomus cells.(1–5) In the Phox2b homozygous knockout mouse, sympathetic and parasympathetic ganglia degenerate; enteric ganglia undergo migration arrest in the foregut and subsequently degenerate; and although the adrenal medulla forms, tyrosine hydroxylase and dopamine-β-hydroxylase (key enzymes in the synthesis of norepinephrine) are not expressed.(2) Germline PHOX2B mutations cause congenital central hypoventilation syndrome(3, 6, 7) and predispose to neuroblastoma(3, 7–11) and Hirschsprung disease.(3, 7, 8, 10)
Neuroblastoma is a primitive tumor of sympathoadrenal lineage. Given PHOX2B’s central role in determining this lineage, it is not surprising that it has been investigated as a NB diagnostic, in which it has been shown to be highly sensitive and specific as an immunohistochemical marker, including in decalcified bone marrow specimens(12–14), and in quantitative real-time PCR panels as a marker of minimal residual disease.(15–17)
PHOX2B has been similarly investigated as a marker of the neuroendocrine autonomic nervous system tumors, namely pheochromocytoma and paraganglioma, with conflicting results in two studies. While one group found PHOX2B expression in 6 of 6 such tumors(12), another found expression in 40% of 35 paragangliomas and none of 21 pheochromocytomas.(13) We sought to examine PHOX2B expression in a large cohort of pheochromocytomas and paragangliomas, as well as in well-differentiated neuroendocrine tumors (WDNETs) and poorly differentiated neuroendocrine carcinomas (PDNECs), which represent key differential diagnostic considerations due to substantial morphologic and immunophenotypic overlap.
Materials and methods
Pheochromocytomas, paragangliomas, WDNETs, and PDNECs were identified from the surgical pathology archives of the University of Iowa Hospitals and Clinics. WDNETs included gastroenteropancreatic (i.e., NET G1 and G2), bronchopulmonary (i.e., typical and atypical carcinoid tumor), and thyroid (i.e., medullary thyroid carcinoma) tumors meeting standard WHO 2010 gastrointestinal, 2015 lung, and 2004 endocrine tumor “Blue Book” criteria, respectively, while PDNECs (including visceral small and large cell neuroendocrine carcinoma and Merkel cell carcinoma) met criteria set forth in the 2010 gastrointestinal, 2015 lung, and 2005 skin tumor “Blue Books.”(18–21) Original glass slides were reviewed, the diagnosis was confirmed, and a “best tumor block” was identified. Tissue microarrays (TMAs) were constructed using the Manual Tissue Arrayer MTA-1 (Beecher Instruments; Sun Prairie, WI), with the following 609 tumors arrayed as triplicate 1 mm cores: 111 pheochromocytomas, 146 paragangliomas (112 head and neck, 34 thoracoabdominal), 250 WDNETs (32 thyroid, 46 lung, 8 duodenum, 67 pancreas, 93 jejunoileum, 4 appendix), and 102 PDNECs (36 lung, 21 extrapulmonary visceral, 45 Merkel cell).
PHOX2B immunohistochemistry was performed manually on 4-μm-thick tissue sections after deparaffinization, rehydration, and pressure cooker heat-induced epitope retrieval in Target Retrieval Solution (pH 6.1; Dako; Carpinteria, CA) using a rabbit monoclonal antibody (clone EPR14423; 1:500 dilution; Abcam; Cambridge, MA) and the polymer-based Dako EnVision+ detection system. A neuroblastoma served as the positive control, while a multi-tissue block including normal skin, skeletal muscle, and colon served as the negative control. PHOX2B expression was evaluated for extent (0–100%) and intensity (0–3+) in each TMA core and an overall H-score (mean extent*intensity) was calculated for each tumor, with any non-zero H-score considered positive.
The results of PHOX2B staining in pheochromocytomas and paragangliomas in TMAs were correlated with the results of previously performed SDHB immunohistochemistry.(22) SDHB immunohistochemistry was performed similar to above, utilizing a mouse monoclonal antibody (clone 21A11AE7; 1:100; Abcam). Staining was assessed as intact (any cytoplasmic staining) or lost (entirely absent in tumor with intact internal control).
The results of PHOX2B staining in the TMAs were validated in a representative cohort of corresponding whole sections, including cases with null (H-score=0), low (H-score<100), and high (H-score≥100) expression on TMA. This cohort included 10 WDNETs (all null), 15 PDNECs (8 null, 3 low, 2 high), 15 pheochromocytomas (5 null, 5 low, 5 high), and 15 paragangliomas (5 null, 5 low, 5 high). Whole section PHOX2B immunohistochemistry was again scored for extent (0–100%) and intensity (0–3+) of staining, with an H-score calculated.
Two-sided Fisher’s exact and Kruskal-Wallis tests were used to analyze categorical and continuous data, respectively. Linear regression was used to describe the relationship between PHOX2B results in TMA and whole sections. p<0.05 was considered significant. This research was conducted with University of Iowa Institutional Review Board approval.
Results
PHOX2B Expression in TMA
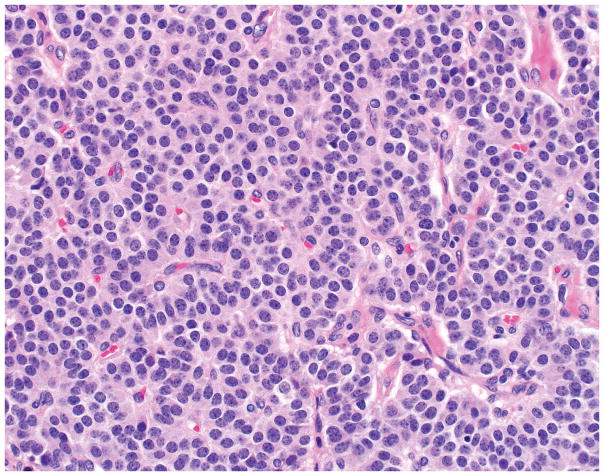
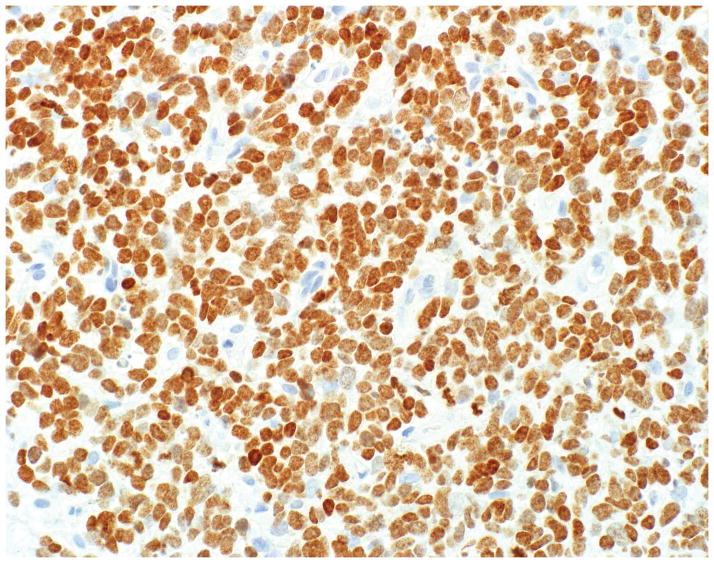
PHOX2B expression was seen in 32% (35/111) of pheochromocytomas and 47% (69/146) of paragangliomas (p=0.015), including 52% of head and neck and 32% of thoracoabdominal tumors (p=0.052). Mean and median H-scores for these tumor types were in the 30–55 range (p=0.798), corresponding to weak to moderate staining (Figure 1A–B). No WDNETs (0/250) and only 7% (7/102; 1 lung, 3 extrapulmonary visceral, 3 Merkel cell) of PDNECs expressed PHOX2B, in the latter tumor type often strongly so (mean and median H-scores in positives of 140 and 210) (Figure 1C–F). Moderate to strong PHOX2B expression was also noted in enteric ganglion cells and was not seen in enteroendocrine cells of the ileum or in islets of Langerhans (Figure 2A–B). Expression data are summarized in Table 1.
Figure 1.
A–F, PHOX2B Staining in Neuroendocrine Neoplasms. A significant minority of pheochromocytomas (depicted in A) and paragangliomas demonstrate weak to moderate PHOX2B expression (B); neuroendocrine tumors (C) are uniformly PHOX2B-negative (D), while rare neuroendocrine carcinomas (F) are PHOX2B-positive, typically strongly so (F) (original magnification of each image 400x).
Figure 2.
A–C, PHOX2B Staining in Cognate Normal Cells. Enteric ganglia are moderately to strongly PHOX2B-positive, while enteroendocrine cells residing in the bases of overlying crypts are uniformly negative (A). Islets of Langerhans are also uniformly negative (B). Adrenal medulla demonstrates weak to moderate PHOX2B staining (C) (original magnification of each image 400x).
Table 1.
PHOX2B Expression in Adult Neuroendocrine Neoplasms
| Tumor Type | n | % positive | Mean (median) H-score, if positive | Range of H-scores, among positives |
|---|---|---|---|---|
| Pheochromocytoma | 111 | 32 | 48 (33) | 1.7–270 |
| Paraganglioma (all) | 146 | 47 | 52 (30) | 2.5–250 |
| Paraganglioma (head and neck) | 112 | 52 | 55 (30) | 2.5–250 |
| Paraganglioma (thoracoabdominal) | 34 | 32 | 34 (27) | 3.3–75 |
| Well-differentiated neuroendocrine tumor | 250 | 0 | NA | NA |
| Poorly differentiated neuroendocrine carcinoma | 102 | 7 | 140 (210) | 1.3–263 |
Correlation of PHOX2B TMA Results with SDHB
There was no relationship between PHOX2B-positivity and succinate dehydrogenase status (p=0.68 for pheochromocytomas, p=0.64 for paragangliomas, p=0.31 for all pheochromocytomas and paragangliomas). Succinate dehydrogenase deficiency, defined by loss of SDHB expression, was seen in 6% of pheochromocytomas and 14% of paragangliomas.
Relationship Between PHOX2B in TMA and Corresponding Whole Sections
PHOX2B expression in TMAs and corresponding whole sections was significantly related (r2=0.81; p<0.0001; Figure 3). Considering the categories of null, low, and high PHOX2B expression, 8/55 (15%) tumors’ PHOX2B status was reclassified in corresponding whole sections; 7 were upgraded and 1 was downgraded, all only 1 class (i.e., no tumor went from null to high and vice versa). All 10 WDNETs remained negative on whole sections, and only 1/15 (7%) of PDNECs were reclassified (H-score=33 on TMA→H-score=180 on whole section). Seven of thirty (23%) pheochromocytomas/paragangliomas were reclassified, with 5/10 going from PHOX2B-null to low (with whole section H-scores of 2, 2, 5, 5, and 20; corresponding to “rare cells, weak” staining); in addition, 1 tumor each went from low to high (H-score=30→H-score=160) and high to low (H-score=140→H-score=80). On whole sections of pheochromocytoma, weak to moderate staining of non-neoplastic adrenal medulla was observed (Figure 2C).
Figure 3.
Relationship of PHOX2B Staining in TMAs and Corresponding Whole Sections. Scatter plot of H-score data from 55 cases with regression line (r2=0.81; p<0.0001).
Discussion
We detected PHOX2B expression in a significant fraction of pheochromocytomas and paragangliomas, no expression in WDNETs, and infrequent expression in PDNECs using a rabbit monoclonal antibody. In TMA, the overall rate of positivity in pheochromocytomas and paragangliomas was around 40%; this rate likely would reflect the frequency of positivity in small biopsies of these tumors. In a representative set of corresponding whole sections, PHOX2B results for WDNETs and PDNECs were essentially unchanged. Among a subset of 10 pheochromocytomas/paragangliomas that were PHOX2B-null on TMA, 5 were upgraded to PHOX2B-low (i.e., rare cells, weak) on corresponding whole sections. Assuming this finding to be representative (i.e., that half of TMA-null cases are in fact low-positive), the estimated rate of PHOX2B-positivity in whole sections of resection specimens is 70%. This discrepancy in TMA and whole section-positivity is not entirely surprising given the generally weak to moderate staining seen in these two tumor types (and in non-neoplastic adrenal medulla).
Bielle and colleagues initially reported PHOX2B expression in 100% of a limited number of pheochromocytomas (n=4), a paraganglioma (n=1), sympathetic ganglia neurons (n=2), enteric nervous system neurons (n=1), and adrenal medulla chromaffin cells (n=6).(12) Staining in these tumors was noted to be weaker than in neuroblastomas and ganglioneuroblastomas, and staining in the normal anatomic tissues/structures was noted to be weak. The authors utilized a goat polyclonal antibody (H-20). Nonaka and colleagues, utilizing the same goat polyclonal, subsequently reported expression in 40% of 35 paragangliomas, noting expression to be variable and generally weak, and in no pheochromocytomas (n=21); ganglion cells and chromaffin cells were also negative.(13) Regarding neuroendocrine autonomic nervous system tumors, we attribute the discrepancy in these studies to a “small numbers bias” in the Bielle and Nonaka studies and a relatively insensitive assay in the Nonaka study, given lack of staining in the normal tissues/structures expected to express PHOX2B. Regarding the discrepancy in the frequency of positivity in paragangliomas (40%) and pheochromocytomas (0%) in the Nonaka study, it is of note that while 10/35 (29%) paragangliomas were examined in whole sections (with the remaining in TMAs), only 3/21 (14%) pheochromocytomas were.
A genome-wide association study identified PHOX2B as a Crohn’s disease susceptibility locus.(23) In this same study, normal mouse ileum and human colon samples were immunostained for PHOX2B with the authors reporting staining in a “subset of epithelial cells, possibly neuroendocrine cells;” the antibody source was not specified. Of note, multiple subsequent genome-wide association studies failed to replicate the significance of PHOX2B as a Crohn’s susceptibility locus.(24–27) Moran and colleagues, examining the significance of enteroendocrine cells in ileal Crohn’s disease, reported PHOX2B co-localization with glucagon-like peptide-1 (GLP-1) and chromogranin A in a subset (~15%) of ileal enteroendocrine cells.(28) These findings, in addition to differential diagnostic considerations, led us to include WDNETs in our study cohort. We found no PHOX2B expression in 250 WDNETs or in any ileal enteroendocrine cells or islets of Langerhans. Similarly, Nonaka and colleagues found no PHOX2B expression in 89 WDNETs.(13) Of note, the Moran study utilized a rabbit polyclonal PHOX2B antibody and avidin-biotin-based detection chemistry, both of which could have contributed to a non-specific result.
We detected PHOX2B expression in a fraction (7%) of PDNECs, including 3% of lung, 14% of extrapulmonary visceral, and 7% of cutaneous neoplasms. We attribute this expression to the “transcription factor lineage infidelity” characteristic of this class of tumors (i.e., PDNECs tend to express multiple transcription factors, irrespective of their site of origin).(29) Nonaka and colleagues had previously failed to demonstrate PHOX2B expression in 50 PDNECs.(13) Diffuse, strong PHOX2B expression would favor a diagnosis of PDNEC over pheochromocytoma/paraganglioma. Four of seven (57%) PHOX2B-positive PDNECs were high expressors, all with H-scores>200, while only 13% and 4% of PHOX2B-positive pheochromocytomas/paragangliomas had H-scores ≥100 and ≥200, respectively.
Pheochromocytomas, paragangliomas, WDNETs, and PDNECs are all neuroendocrine neoplasms, characterized by the expression of general neuroendocrine markers (i.e., chromogranin A and/or synaptophysin) and the production of peptide hormones and/or biogenic amines. WDNETs and PDNECs are generally distinguished from the former based on their epithelial nature (i.e., they express keratins), though keratin expression may be patchy(30, 31); although pheochromocytomas and paragangliomas classically do not express keratins(32, 33), there is a rare report to the contrary.(34) Pheochromocytomas and paragangliomas classically contain sustentacular cells(35, 36), though WDNETs often do, too(31, 37, 38), an underrecognized fact that we have seen lead to diagnostic errors. In the setting of a neuroendocrine neoplasm, PHOX2B expression, though relatively insensitive, would support a diagnosis of pheochromocytoma/paraganglioma over a diagnosis of WDNET or PDNEC.
Other markers we have previously employed in this differential include SDHB(39, 40) and GATA-3.(13, 41, 42) Succinate dehydrogenase (SDH) is inactivated in 5–10% of pheochromocytomas and 15% and 30% of paragangliomas of head and neck and thoracoabdominal origin, respectively, which leads to loss of expression of the SDHB subunit. The multispecific transcription factor GATA-3 has been found to be expressed by the vast majority of pheochromocytomas (93%; 42/45) and paragangliomas (83%; 86/104) in 3 studies(13, 41, 42), and not by WDNETs (0/133) or PDNECs (0/84).(13, 42) Of note, expression of the pancreatic WDNET marker Islet 1 was also noted in 6 of 6 paragangliomas/pheochromocytomas in one study.(43) Given these latter findings, it should not be surprising to the reader that, like PHOX2B, both GATA-3(44–47) and Islet 1(48) are critical to sympathoadrenal development. Immunohistochemical markers potentially useful in the differential diagnosis of pheochromocytoma/paraganglioma, WDNET, and PDNEC are summarized in Table 2.
Table 2.
Markers in the Differential Diagnosis of Adult Neuroendocrine Neoplasms
| Marker | Pheochromocytoma/ Paraganglioma | WDNET | PDNEC |
|---|---|---|---|
| Broad-spectrum keratin (e.g., CAM5.2, AE1/AE3) | - | + (CK7 usually −) | + (CK7 usually −) |
| S-100/SOX10 (sustentacular cells) | Often + | Sometimes + | - |
| PHOX2B | Often + | - | Rarely +* |
| GATA-3 | Usually + (limited data) | − (limited data) | Sometimes +* |
| Islet 1 | + (limited data) | + (pancreas, rectum, thyroid) | Often +* |
| SDHB | Occasionally lost | Intact | Intact |
Note:
likely as a manifestation of transcription factor lineage infidelity
Conclusion
PHOX2B, the master regulator of the autonomic nervous system and of the noradrenergic phenotype, is expressed by a significant fraction of pheochromocytomas and paragangliomas and is not expressed by WDNETs. In laboratories employing PHOX2B as a neuroblastoma marker, expression may also be used to support a diagnosis of pheochromocytoma/paraganglioma over that of WDNET/PDNEC. Compared to staining in neuroblastomas, which is typically diffuse, strong, staining in pheochromocytomas and paragangliomas is more likely to be of weak to moderate intensity and of more limited extent, such that the estimated rate of positivity in small biopsy material (40%) is less than that in whole sections of resected tumors (70%). Otherwise, among low to intermediate grade neuroendocrine neoplasms, the single best marker to support a diagnosis of pheochromocytoma/paraganglioma would appear to be GATA-3, while broad spectrum keratin expression would support a diagnosis of WDNET. Rare PHOX2B expression, often strong, in PDNECs may be a manifestation of the “transcription factor lineage infidelity” typical of this class of tumors.
Acknowledgments
This work was supported by NIH grant P50 CA174521-01A1 (TMO, JRH, AMB).
Footnotes
Author Contributions:
AMB and JLH designed the study; AMB, JPL, and YPH performed the research; AMB and JPL analyzed the data; AMB wrote the paper; TMO and JRH contributed patients; all authors edited the paper.
Presented in part at the 106th Annual Meeting of the United States and Canadian Academy of Pathology (USCAP), San Antonio, TX; March 2017.
Nothing To Disclose – The authors have indicated that they have no conflicts of interest that relate to the content of this manuscript.
References
- 1.Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development. 1997;124(20):4065–75. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- 2.Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature. 1999;399(6734):366–70. doi: 10.1038/20700. [DOI] [PubMed] [Google Scholar]
- 3.Amiel J, Laudier B, Attie-Bitach T, Trang H, de Pontual L, Gener B, et al. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nature genetics. 2003;33(4):459–61. doi: 10.1038/ng1130. [DOI] [PubMed] [Google Scholar]
- 4.Dauger S, Pattyn A, Lofaso F, Gaultier C, Goridis C, Gallego J, et al. Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development. 2003;130(26):6635–42. doi: 10.1242/dev.00866. [DOI] [PubMed] [Google Scholar]
- 5.Huber K, Karch N, Ernsberger U, Goridis C, Unsicker K. The role of Phox2B in chromaffin cell development. Developmental biology. 2005;279(2):501–8. doi: 10.1016/j.ydbio.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Weese-Mayer DE, Berry-Kravis EM, Zhou L, Maher BS, Silvestri JM, Curran ME, et al. Idiopathic congenital central hypoventilation syndrome: analysis of genes pertinent to early autonomic nervous system embryologic development and identification of mutations in PHOX2b. American journal of medical genetics Part A. 2003;123A(3):267–78. doi: 10.1002/ajmg.a.20527. [DOI] [PubMed] [Google Scholar]
- 7.Trochet D, O’Brien LM, Gozal D, Trang H, Nordenskjold A, Laudier B, et al. PHOX2B genotype allows for prediction of tumor risk in congenital central hypoventilation syndrome. American journal of human genetics. 2005;76(3):421–6. doi: 10.1086/428366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trochet D, Bourdeaut F, Janoueix-Lerosey I, Deville A, de Pontual L, Schleiermacher G, et al. Germline mutations of the paired-like homeobox 2B (PHOX2B) gene in neuroblastoma. American journal of human genetics. 2004;74(4):761–4. doi: 10.1086/383253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Limpt V, Schramm A, van Lakeman A, Sluis P, Chan A, van Noesel M, et al. The Phox2B homeobox gene is mutated in sporadic neuroblastomas. Oncogene. 2004;23(57):9280–8. doi: 10.1038/sj.onc.1208157. [DOI] [PubMed] [Google Scholar]
- 10.McConville C, Reid S, Baskcomb L, Douglas J, Rahman N. PHOX2B analysis in non-syndromic neuroblastoma cases shows novel mutations and genotype-phenotype associations. American journal of medical genetics Part A. 2006;140(12):1297–301. doi: 10.1002/ajmg.a.31278. [DOI] [PubMed] [Google Scholar]
- 11.Raabe EH, Laudenslager M, Winter C, Wasserman N, Cole K, LaQuaglia M, et al. Prevalence and functional consequence of PHOX2B mutations in neuroblastoma. Oncogene. 2008;27(4):469–76. doi: 10.1038/sj.onc.1210659. [DOI] [PubMed] [Google Scholar]
- 12.Bielle F, Freneaux P, Jeanne-Pasquier C, Maran-Gonzalez A, Rousseau A, Lamant L, et al. PHOX2B immunolabeling: a novel tool for the diagnosis of undifferentiated neuroblastomas among childhood small round blue-cell tumors. The American journal of surgical pathology. 2012;36(8):1141–9. doi: 10.1097/PAS.0b013e31825a6895. [DOI] [PubMed] [Google Scholar]
- 13.Nonaka D, Wang BY, Edmondson D, Beckett E, Sun CC. A study of gata3 and phox2b expression in tumors of the autonomic nervous system. The American journal of surgical pathology. 2013;37(8):1236–41. doi: 10.1097/PAS.0b013e318289c765. [DOI] [PubMed] [Google Scholar]
- 14.Hata JL, Correa H, Krishnan C, Esbenshade AJ, Black JO, Chung DH, et al. Diagnostic utility of PHOX2B in primary and treated neuroblastoma and in neuroblastoma metastatic to the bone marrow. Archives of pathology & laboratory medicine. 2015;139(4):543–6. doi: 10.5858/arpa.2014-0255-OA. [DOI] [PubMed] [Google Scholar]
- 15.Cheung IY, Feng Y, Gerald W, Cheung NK. Exploiting gene expression profiling to identify novel minimal residual disease markers of neuroblastoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(21):7020–7. doi: 10.1158/1078-0432.CCR-08-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stutterheim J, Gerritsen A, Zappeij-Kannegieter L, Kleijn I, Dee R, Hooft L, et al. PHOX2B is a novel and specific marker for minimal residual disease testing in neuroblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(33):5443–9. doi: 10.1200/JCO.2007.13.6531. [DOI] [PubMed] [Google Scholar]
- 17.Stutterheim J, Gerritsen A, Zappeij-Kannegieter L, Yalcin B, Dee R, van Noesel MM, et al. Detecting minimal residual disease in neuroblastoma: the superiority of a panel of real-time quantitative PCR markers. Clinical chemistry. 2009;55(7):1316–26. doi: 10.1373/clinchem.2008.117945. [DOI] [PubMed] [Google Scholar]
- 18.Rindi G, Arnold R, Bosman FT, Capella C, Klimstra DS, Kloppel G, et al. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. 4. Lyon: IARC; 2010. pp. 13–4. [Google Scholar]
- 19.Brambilla E, Beasley MB, Austin JHM, Capelozzi VL, Chirieac LR, Devesa SS, et al. Neuroendocrine tumours. In: Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG, editors. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4. Lyon: IARC; 2015. pp. 63–8. [Google Scholar]
- 20.Kohler S, Kerl H. Merkel cell carcinoma. In: LeBoit PE, Burg G, Weedon D, Sarasin A, editors. WHO Classification of Tumours of the Skin. 3. Lyon: IARC; 2005. pp. 272–3. [Google Scholar]
- 21.Matias-Guiu X, DeLellis R, Moley JF, Gagel RF, Albores-Saavedra J, Bussolati G, et al. Medullary thyroid carcinoma. In: DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. WHO Classification of Tumours of Endocrine Organs. 3. Lyon: IARC; 2004. pp. 86–91. [Google Scholar]
- 22.Holleran E, Boldt B, McKissic D, Hornick JL, Bellizzi AM. Succinate dehydrogenase deficiency in pheochromocytoma and paraganglioma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2016;29(Supplement 2):149A. [Google Scholar]
- 23.Rioux JD, Xavier RJ, Taylor KD, Silverberg MS, Goyette P, Huett A, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nature genetics. 2007;39(5):596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wellcome Trust Case Control C. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–78. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franke A, Balschun T, Karlsen TH, Hedderich J, May S, Lu T, et al. Replication of signals from recent studies of Crohn’s disease identifies previously unknown disease loci for ulcerative colitis. Nature genetics. 2008;40(6):713–5. doi: 10.1038/ng.148. [DOI] [PubMed] [Google Scholar]
- 26.Roberts RL, Hollis-Moffatt JE, Gearry RB, Kennedy MA, Barclay ML, Merriman TR. Confirmation of association of IRGM and NCF4 with ileal Crohn’s disease in a population-based cohort. Genes and immunity. 2008;9(6):561–5. doi: 10.1038/gene.2008.49. [DOI] [PubMed] [Google Scholar]
- 27.Glas J, Seiderer J, Pasciuto G, Tillack C, Diegelmann J, Pfennig S, et al. rs224136 on chromosome 10q21.1 and variants in PHOX2B, NCF4, and FAM92B are not major genetic risk factors for susceptibility to Crohn’s disease in the German population. The American journal of gastroenterology. 2009;104(3):665–72. doi: 10.1038/ajg.2008.65. [DOI] [PubMed] [Google Scholar]
- 28.Moran GW, Pennock J, McLaughlin JT. Enteroendocrine cells in terminal ileal Crohn’s disease. Journal of Crohn’s & colitis. 2012;6(9):871–80. doi: 10.1016/j.crohns.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Czeczok TW, Gailey MP, Hornick JL, Bellizzi AM. High-grade neuroendocrine carcinomas are characterized by marked transcription factor lineage infidelity: an evaluation of 36 diagnostic markers in 83 tumors. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2014;27(Supplement 2):152A. [Google Scholar]
- 30.Chu PG, Lau SK, Weiss LM. Keratin expression in endocrine organs and their neoplasms. Endocrine pathology. 2009;20(1):1–10. doi: 10.1007/s12022-009-9061-7. [DOI] [PubMed] [Google Scholar]
- 31.Bellizzi AM. Assigning site of origin in metastatic neuroendocrine neoplasms: a clinically significant application of diagnostic immunohistochemistry. Advances in anatomic pathology. 2013;20(5):285–314. doi: 10.1097/PAP.0b013e3182a2dc67. [DOI] [PubMed] [Google Scholar]
- 32.Miettinen M, Lehto VP, Virtanen I. Immunofluorescence microscopic evaluation of the intermediate filament expression of the adrenal cortex and medulla and their tumors. The American journal of pathology. 1985;118(3):360–6. [PMC free article] [PubMed] [Google Scholar]
- 33.Hoefler H, Denk H, Lackinger E, Helleis G, Polak JM, Heitz PU. Immunocytochemical demonstration of intermediate filament cytoskeleton proteins in human endocrine tissues and (neuro-) endocrine tumours. Virchows Archiv A, Pathological anatomy and histopathology. 1986;409(5):609–26. doi: 10.1007/BF00713428. [DOI] [PubMed] [Google Scholar]
- 34.Chetty R, Pillay P, Jaichand V. Cytokeratin expression in adrenal phaeochromocytomas and extra-adrenal paragangliomas. Journal of clinical pathology. 1998;51(6):477–8. doi: 10.1136/jcp.51.6.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lloyd RV, Blaivas M, Wilson BS. Distribution of chromogranin and S100 protein in normal and abnormal adrenal medullary tissues. Archives of pathology & laboratory medicine. 1985;109(7):633–5. [PubMed] [Google Scholar]
- 36.Schroder HD, Johannsen L. Demonstration of S-100 protein in sustentacular cells of phaeochromocytomas and paragangliomas. Histopathology. 1986;10(10):1023–33. doi: 10.1111/j.1365-2559.1986.tb02539.x. [DOI] [PubMed] [Google Scholar]
- 37.Barbareschi M, Frigo B, Mosca L, Carboni N, Arrigoni GP, Leonardi E, et al. Bronchial carcinoids with S-100 positive sustentacular cells. A comparative study with gastrointestinal carcinoids, pheochromocytomas and paragangliomas. Pathology, research and practice. 1990;186(2):212–22. doi: 10.1016/S0344-0338(11)80538-8. [DOI] [PubMed] [Google Scholar]
- 38.Tsuta K, Raso MG, Kalhor N, Liu DC, Wistuba II, Moran CA. Sox10-positive sustentacular cells in neuroendocrine carcinoma of the lung. Histopathology. 2011;58(2):276–85. doi: 10.1111/j.1365-2559.2011.03747.x. [DOI] [PubMed] [Google Scholar]
- 39.van Nederveen FH, Gaal J, Favier J, Korpershoek E, Oldenburg RA, de Bruyn EM, et al. An immunohistochemical procedure to detect patients with paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: a retrospective and prospective analysis. The Lancet Oncology. 2009;10(8):764–71. doi: 10.1016/S1470-2045(09)70164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gill AJ, Benn DE, Chou A, Clarkson A, Muljono A, Meyer-Rochow GY, et al. Immunohistochemistry for SDHB triages genetic testing of SDHB, SDHC, and SDHD in paraganglioma-pheochromocytoma syndromes. Human pathology. 2010;41(6):805–14. doi: 10.1016/j.humpath.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 41.So JS, Epstein JI. GATA3 expression in paragangliomas: a pitfall potentially leading to misdiagnosis of urothelial carcinoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2013;26(10):1365–70. doi: 10.1038/modpathol.2013.76. [DOI] [PubMed] [Google Scholar]
- 42.Miettinen M, McCue PA, Sarlomo-Rikala M, Rys J, Czapiewski P, Wazny K, et al. GATA3: a multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelial tumors. The American journal of surgical pathology. 2014;38(1):13–22. doi: 10.1097/PAS.0b013e3182a0218f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agaimy A, Erlenbach-Wunsch K, Konukiewitz B, Schmitt AM, Rieker RJ, Vieth M, et al. ISL1 expression is not restricted to pancreatic well-differentiated neuroendocrine neoplasms, but is also commonly found in well and poorly differentiated neuroendocrine neoplasms of extrapancreatic origin. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2013;26(7):995–1003. doi: 10.1038/modpathol.2013.40. [DOI] [PubMed] [Google Scholar]
- 44.Lim KC, Lakshmanan G, Crawford SE, Gu Y, Grosveld F, Engel JD. Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nature genetics. 2000;25(2):209–12. doi: 10.1038/76080. [DOI] [PubMed] [Google Scholar]
- 45.Tsarovina K, Pattyn A, Stubbusch J, Muller F, van der Wees J, Schneider C, et al. Essential role of Gata transcription factors in sympathetic neuron development. Development. 2004;131(19):4775–86. doi: 10.1242/dev.01370. [DOI] [PubMed] [Google Scholar]
- 46.Moriguchi T, Takako N, Hamada M, Maeda A, Fujioka Y, Kuroha T, et al. Gata3 participates in a complex transcriptional feedback network to regulate sympathoadrenal differentiation. Development. 2006;133(19):3871–81. doi: 10.1242/dev.02553. [DOI] [PubMed] [Google Scholar]
- 47.Tsarovina K, Reiff T, Stubbusch J, Kurek D, Grosveld FG, Parlato R, et al. The Gata3 transcription factor is required for the survival of embryonic and adult sympathetic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(32):10833–43. doi: 10.1523/JNEUROSCI.0175-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huber K, Narasimhan P, Shtukmaster S, Pfeifer D, Evans SM, Sun Y. The LIM-Homeodomain transcription factor Islet-1 is required for the development of sympathetic neurons and adrenal chromaffin cells. Developmental biology. 2013;380(2):286–98. doi: 10.1016/j.ydbio.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]