Abstract
Background
Oral tenofovir disoproxil fumarate (TDF) for HIV prevention and treatment is associated with decreases in bone mineral density (BMD). Previous reports suggest that these changes may be reversible after discontinuation of TDF.
Setting
A metabolic substudy of 498 participants in a randomized, placebo-controlled HIV prevention trial of oral co-formulated TDF with emtricitabine (TDF/FTC, Truvada®) for HIV pre-exposure prophylaxis (PrEP) enrolling a global sample of men who have sex with men and trans women.
Methods
Participants underwent dual X-ray absorptiometry (DXA) to quantify bone mineral density (BMD) in the hip and spine during PrEP and at 2 visits after stopping (median of 23 and 79 weeks post-PrEP, respectively). Results are stratified by pharmacologic measure of TDF/FTC adherence.
Results
There was no significant difference in change in hip/spine BMD at any timepoint between placebo and those with low adherence. Adherent participants had a mean (standard error) BMD change at TDF/FTC discontinuation of −1.02% (0.24) in the hip and −1.84% (0.36) in the spine. After stop, annualized BMD increases of 1.13% per year (0.27) in hip and 1.81% per year (0.36) in spine BMD were observed in adherent participants compared with 0.19% (0.16) and 0.74% (0.21) in the placebo group, respectively (p=0.003, both comparisons). On average, BMD returned to baseline levels by 1 year after PrEP stop. Recovery was consistent across age, baseline BMD z-score and treatment duration.
Conclusions
Mean BMD returns to baseline levels within 12–18 months after TDF-based PrEP discontinuation in both hip and spine with consistency across participant subgroups.
Clinical Trials Registration
clinicaltrials.gov NCT00458393.
INTRODUCTION
Randomized trials have demonstrated that pre-exposure prophylaxis (PrEP) with oral tenofovir disoproxil fumarate (TDF) or co-formulated TDF with emtricitabine (TDF/FTC, Truvada®) prevents human immunodeficiency virus (HIV) acquisition (1–6). In HIV treatment, TDF-based therapy has been associated with decreases in bone mineral density (BMD) with unclear clinical significance (7); some studies have found that TDF is associated with increased fracture risk while others have not (8–12). HIV therapy switch studies have found that substitution of TDF leads to some BMD recovery, suggesting BMD changes are reversible (13–15).
Trials of TDF-based PrEP have documented BMD decreases of 0.5 to 1.5% with no clear excess fracture signal (16–19). Bone toxicity has partially motivated the testing of alternative PrEP agents. Recently the MTN 003B PrEP trial (19), found reversible BMD changes after withdrawal of TDF-PrEP in a cohort of young African women. We examine the trajectory of BMD changes through extended follow-up of the iPrEx studies (1, 20).
METHODS
Study design
We analyzed an opt-in substudy nested in a placebo-controlled randomized clinical trial (iPrEx study) of daily oral TDF/FTC for PrEP in men who have sex with men and transgender women (MSM/TW). The bone and metabolic substudy enrolled at 7 of 11 iPrEx sites – in 5 cities (Cape Town, South Africa; Chiang Mai, Thailand; Lima, Peru; Rio de Janiero, Brazil; San Francisco, USA) with a target enrollment of 500 and achieved enrollment of 498 HIV-uninfected volunteers. The study conduct was consistent with the Declaration of Helsinki; the protocol was approved by the ethics committee at each site and all participants provided written informed consent.
Participants underwent dual X-ray absorptiometry (DXA) to quantify bone mineral density (BMD) in the hip and spine at enrollment and every 24 weeks until study drug stop (stop visit) and 24 weeks after discontinuation of study drug (post-stop visit). The scans using the same protocol across sites. The full methods and results for iPrEx have been published previously (1,20) and for the bone substudy (16). After a gap in participation, volunteers were invited to enroll in the iPrEx open label extension (OLE), which offered TDF/FTC for up to 18 months. The population and procedures for iPrEx OLE have been reported previously (20). DXA participants from iPrEx were scanned at entry into iPrEx OLE. Randomized study drug was ended for iPrEx participants in August 2010 and enrollment in iPrEx OLE occurred between June 2011 and June 2012.
Drug Levels
All DXA participants randomized to TDF/FTC underwent drug level testing for tenofovir and its metabolites in samples collected during the clinical trial (21). Of particular interest are levels of tenofovir diphosphate (TFV-DP) in viably cryopreserved peripheral blood mononuclear cells (vPBMCs) collected at the week 24 visit; these levels showed a linear association with peak loss of BMD in TDF/FTC participants (16). We dichotomized these levels as TFV-DP < 16 v. TFV-DP ≥ 16 fmol per million vPBMCs, a level which is associated with a 90% reduction in HIV incidence (21) and use of approximately 2–3 tablets per week on average (21).
Statistical Analysis
We examined BMD levels at 5 time points: iPrEx enrollment, iPrEx week 24, the iPrEx stop visit, 24 weeks post-stop, and at OLE enrollment. We compared BMD for those on placebo versus TDF/FTC randomization and then further by the week 24 TFV-DP levels in PBMCs (TFV-DP < 16 v. TFV-DP ≥ 16).
Average percentage BMD changes from baseline in hip and spine were compared across groups (TDF/FTC v. placebo and TFV-DP < 16, TFV-DP ≥ 16 v. placebo) by a linear mixed effects model with an unstructured covariance matrix (22). We examined consistency of this effect across age, baseline BMD Z-score, gender identity and duration on randomized drug.
The annual rate of BMD change (relative to baseline BMD) after the stop visit comparing TDF/FTC v. placebo and TFV-DP < 16, TFV-DP ≥ 16 v. placebo used a mixed effects model with random slopes and intercepts (22) and allowing for interactions of slope and intercept with randomization/drug exposure in the fixed model.
To address missing DXA scans (29% missed a post-stop and 42% have no OLE DXA), 200 multiple imputations (23) of the BMD values based on values at baseline, week 24, and the stop visit as well as age, race, site, height, weight, lean body mass, and date of stop scan were analyzed with the “mi” package in Stata. Demographics of those with complete and missing DXA scans were compared by the t-test for continuous variables and Fisher exact test for categorical variables. All analyses were performed in Stata 14.
RESULTS
Available Scans Baseline Characteristics
iPrEx enrolled participants from July 2007 through December 2009 with protocol-mandated study drug withdrawal in August 2010 and 24-week post stop scans completed by February 2011. Enrollment in iPrEx OLE opened in June 2011 and closed in July 2012. During that gap, oral TDF/FTC for PrEP was not approved for use in any country. The median (interquartile range, IQR) for enrollment top stop visit was 64 weeks (44–88), from stop to 24-week post-stop was 23 weeks (22–25) and from stop to OLE-enrollment was 79 weeks (60–89).
Of the 498 participants, 24-week post stop scans were available in 352 (71%), and at OLE enrollment in in 289 (58%). All participants have a stop-scan which was post-baseline in 493 (99%) at a median of 64 weeks on study (IQR: 41 to 87).
Baseline characteristics (at iPrEx enrollment) varied slightly across availability of BMD scans at the 24-week post stop visit and OLE enrollment (supplementary Table 1). Older participants were statistically significantly more likely to be scanned after drug stop (p< 0.001, 24-week post-stop; p = 0.01, OLE entry); however, median age of participants with scans at 24-week post stop (median: 25, IQR: 21 to 34) and OLE enrollment (median: 26 IQR: 21 to 34) was similar to the overall DXA population (median: 25, IQR: 21 to 33). Weeks on study medication was also higher in those with post-stop and OLE entry scan (p < 0.001 both comparisons) reflecting those who discontinued study early were less likely to return for post-drug visits or enroll in OLE. The proportion with post-stop and OLE scans varied by enrolling site ranging from 40% to 76% (p=0.05) and 26% to 67% (p < 0.001), but there was no difference by randomized arm. Among TDF/FTC participants with TFV-DP < 16 fmol, 76% had 24-week scans compared with 80% of those with TFV-DP ≥ 16 (p= 0.48). At OLE entry, it was 55% v. 69% (p=0.04) with scans, respectively. Overall, older participants, more adherent participants, and those who remained on study drug longer were more likely to be retained for scans after study drug discontinuation.
Post-Stop Changes in BMD
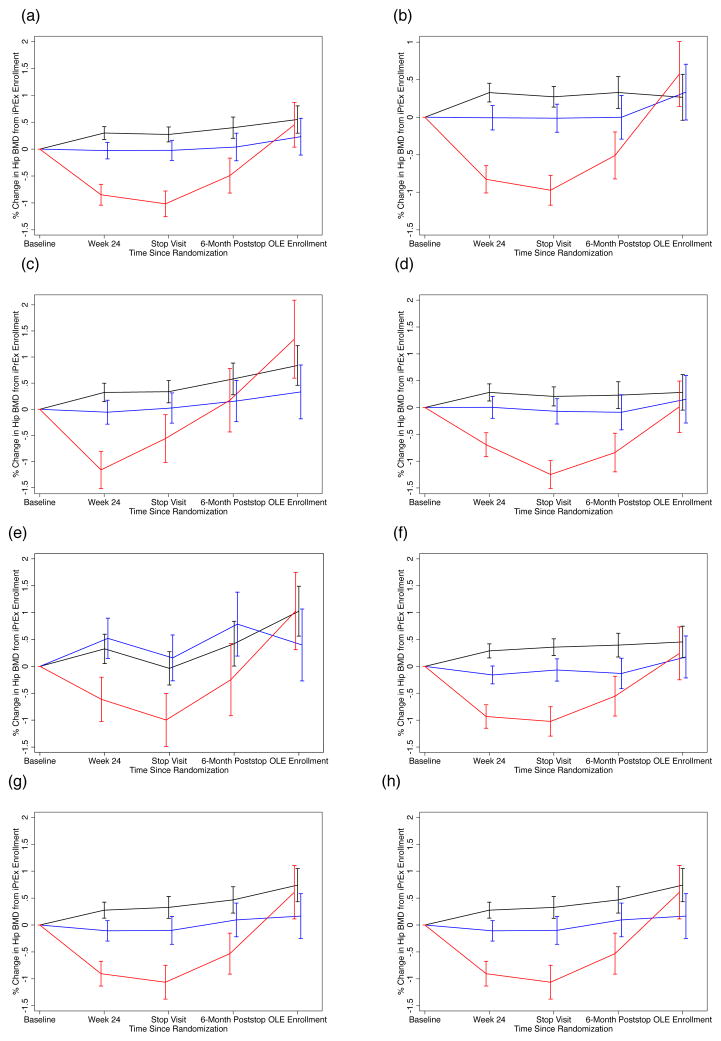
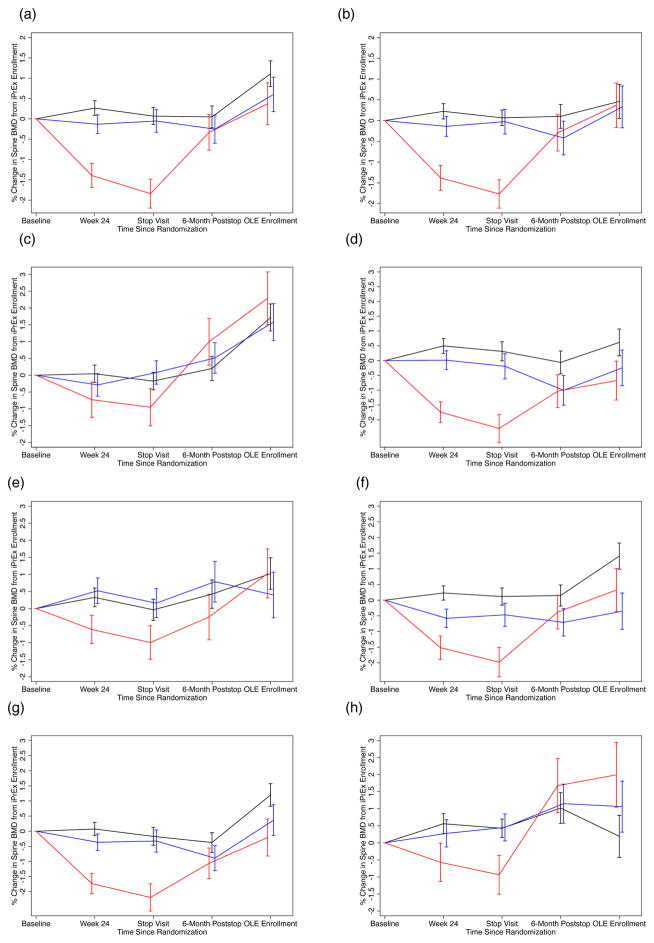
Figure 1(a) and Figure 2(a) summarize the mean (standard error) percentage change in BMD (hip and spine, respectively) from baseline to week 24, the stop visit, the 24-week post stop visit and OLE entry by placebo and TDF/FTC arms. The TDF/FTC arm is stratified by level of TFV-DP at week 24 to reflect their adherence to TDF/FTC. There is no significant difference in BMD in hip or spine at any timepoint between placebo and TDF/FTC with TFV-DP < 16 fmol/106 vPBMC. However, those with TFV-DP ≥ 16 fmol have significant decreases in both hip and spine at week 24 and the stop visit: mean (SE) of −0.85% (0.19) and −1.02%(0.24) in the hip and −1.40% (0.30) and −1.84% (0.36) in the spine. After study drug discontinuation, we observed a BMD increase of 1.13% (0.27) per year among these participants in the hip and 1.81% (0.36) in the spine compared with 0.19% (0.16) in the hip and 0.74% (0.21) in the spine in the placebo group, p=0.003 for both comparisons. At OLE entry, mean (SE) BMD in hip and spine was at or above values of the baseline scan with mean (SE) of 0.45% (0.42) and 0.37% (0.52) change (compared to baseline scan) in those with TFV-DP ≥ 16 compared to 0.55% (0.25) and 1.11% (0.32) in placebo participants, p=0.84 and p=0.22, respectively.
Figure 1.
Percentage Change in Total Hip Bone Mineral Density (BMD) from Baseline Value by Randomized Treatment and Stratified by Level of Intracellular Tenofovir Diphosphate (TFV-DP) in fmol per Million Viable Cells at Week 24 of Randomized Trial. Bars Indicate One Standard Error.
X-axis (Panels a–h): Time Since iPrEx Randomization
Y-axis (Panels a–h): Percent Change in Total Hip BMD
- All Participants – Available Scans
- All Participants – Results of Multiple Imputation
- Participants Aged < 25 years at iPrEx Entry
- Participants Aged ≥ 25 years at iPrEx Entry
- Baseline Hip Z-score < −1 at iPrEx Entry
- Baseline Hip Z-score ≥ 1 at iPrEx Entry
- Duration Randomized Treatment ≥ 1 Year
- Duration Randomized Treatment < 1 Year
Black Line: Randomized to Placebo
Red Line: Randomized to TDF/FTC with TFV-DP ≥ 16 at week 24
Blue Line: Randomized TDF/FTC with TFV-DP < 16 at week 24
Figure 2.
Percentage Change in L1–L4 Spine Bone Mineral Density (BMD) from Baseline Value by Randomized Treatment and Stratified by Level of Intracellular Tenofovir Diphosphate (TFV-DP) in fmol per Million Viable Cells at Week 24 of Randomized Trial. Bars Indicate One Standard Error.
X-axis (Panels a–h): Time Since iPrEx Randomization
Y-axis (Panels a–h): Percent Change in L1–L4 Spine BMD
- All Participants – Available Scans
- All Participants – Results of Multiple Imputation
- Participants Aged < 25 years at iPrEx Entry
- Participants Aged ≥ 25 years at iPrEx Entry
- Baseline Spine Z-score < −1 at iPrEx Entry
- Baseline Spine Z-score ≥ 1 at iPrEx Entry
- Duration Randomized Treatment ≥ 1 Year
- Duration Randomized Treatment < 1 Year
Black Line: Randomized to Placebo
Red Line: Randomized to TDF/FTC with TFV-DP ≥ 16 at week 24
Blue Line: Randomized TDF/FTC with TFV-DP < 16 at week 24
Sensitivity Analyses
To examine the effect of attrition, multiple imputation was used to infer missing scans based on baseline, week 24, and stop as well as age, race, site, height, weight, lean body mass, and date of stop scan. The results were substantially similar and appear in Figures 1(b) and 2(b) for hip and spine, respectively.
We examined consistency of BMD effects during and after PrEP by comparing subgroups by age (< 25 year at enrollment v. ≥ 25 years at enrollment, Figure 1 and 2 panels (c) and (d), respectively), baseline BMD Z-score (Z-score < −1 at enrollment v. Z-score ≥ 1, panels (e) and (f), respectively), and duration of randomized study treatment in iPrEx (< 1 year of study treatment v. ≥ 1 year, panels (g) and (h), respectively). The graphs suggest consistent evidence across subgroups of bone loss on study with clear trajectory for recovery at or above baseline levels. Of note, participants ≥ 25 years of age with TFV-DP ≥ 16 at week 24 have BMD in spine (shown in Fig. 2(d) ) 0.67% below baseline at OLE entry (95% CI: −1.94% to +0.60%); however, the change is not significantly different from placebo (p=0.10). There is no significant association between TDF/FTC duration and recovery in hip or spine BMD. Recovery did vary by site but our sites are heterogeneous in adherence, participant demographics and scan completion, complicating interpretation.
For the 7 transwoman identified participants with TFV-DP ≥ 16, changes were +0.68% (95% CI: −1.00% to +2.37%) at the stop scan and +3.33% (95% CI: +0.98% to +5.68%) at OLE entry in the hip and −1.35% (−95% CI: −3.19% to +0.49%) at the stop scan and 3.14% (95% CI: −0.64% to +6.92%) at OLE entry in the spine. We saw no evidence of an interaction between treatment and gender identity of BMD recovery (p=0.86). There was also consistent evidence of recovery stratifying the active arm using 25 fmol/106 vPBMC (consistent with at least 4 pills per week of TDF/FTC) at week 24.
DISCUSSION
We reported extended off-drug BMD follow-up in a geographically and demographically diverse sample of MSM/TW. We document increases in BMD after drug discontinuation in TDF/FTC-adherent participants of approximately 1.0% per year relative to placebo (Hip: Placebo, 0.19% per year v. TDF-DP ≥ 16, 1.13% per year; Spine: Placebo, 0.74% per year v. TDF-DP ≥ 16, 1.81 per /year). This is consistent with the observation in MTN003B that TDF-PrEP adherent young African women also experienced return to baseline BMD over the year after PrEP discontinuation (19). Both studies suggest full recovery is not evident by 24-weeks post-discontinuation but is evident after stopping oral FTC/TDF PrEP for 48 to 79 weeks.
BMD recovery appears consistent across age, duration of PrEP and baseline BMD. However, our numbers are modest overall and our power is limited to examine recovery in key PrEP populations including those who have no reached peak bone mass, those over 50, people who cycle on/off PrEP, “on demand” users or who use it for periods longer than our cohort. Our numbers are particularly small for TW participants (~7 trans women in the active arm of the substudy with evidence of high adherence); we previously reported that trans women tended to have less BMD decreases during PrEP (24) possibly due to the bone protective effects of feminizing hormones or lower adherence..
We used multiple imputation to account for missing scans. This approach is valid even with a large proportion of missing data. Its validity requires the missing at random assumption (23) that differences in missing and non-missing scan can be explained by variables used for constructing imputations. This assumption can never be fully verified.
A reviewer asked us to assess recovery of phosphorous and estimated glomerular filtration rate. The former was not reduced and hence not recovered. We previously documented the recovery of eGFR after stopping TDF/FTC (25).
This study suggests that discontinuation of TDF/FTC for PrEP will lead to BMD recovery to levels expected in the absence of PrEP in populations of MSM/TW.
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported by the National Institute of Allergy and Infectious Diseases (U01 AI106499, UM1 AI068619, U01 AI064002, R03 AI120819, R03 AI122908). Study medication was donated by Gilead Sciences.
The authors gratefully acknowledge the participants of the iPrEx Bone and Metabolic Substudy, Martin Casapia, Orlando Montoya, Esper Kallas, Valdilea Veloso, Javier Lama, Pedro Gonzales, Maria Esther Ramirez-Cardich, Sirianong Namwongprom, Piotr Chodacki and Laura Maria Carvalo de Mendonca
Footnotes
Presentations: Previously presented at the Congress of Retroviruses and Opportunistic Infections in Seattle, WA, USA on 23 February 2016.
Conflicts of interest: The iPrEx studies were sponsored by the US National Institutes of Health with co-funding from the Bill and Melinda Gates Foundation; study medication was donated by Gilead Sciences, which also supported travel expenses for non-US investigators to attend study meetings. RMG and DVG have received fees from and RMG has received a research grant from ViiV, a manufacturer of an investigational compound being investigated for use as PrEP. PLA receives study drug and contract work from Gilead Sciences. All other authors have no conflicts to declare.
Contributor Information
David V. Glidden, University of California, San Francisco, San Francisco, California, USA
Kathleen Mulligan, University of California, San Francisco, San Francisco, California, USA
Vanessa McMahan, University of Washington, Seattle Washington, USA
Peter L Anderson, University of Colorado, Denver, Aurora, Colorado, USA
Juan Guanira, Investigaciones Médicas en Salud, Lima, Peru
Suwat Chariyalertsak, Research Institute for Health Sciences, Chiang Mai University, Chiang Mai, Thailand
Susan P. Buchbinder, Bridge HIV, San Francisco Department of Public Health, San Francisco, California, USA
Linda Gail Bekker, Desmond Tutu HIV Foundation, Cape Town, South Africa
Mauro Schechter, Projeto Praça Onze, Hospital Escola São Francisco de Assis and Universidade Federal do Rio de Janeiro, Brazil
Beatriz Grinsztejn, Evandro Chagas National Institute of Infectious Diseases-Fiocruz, Rio de Janeiro, Brazil
Robert M. Grant, Gladstone Institute of Virology and San Francisco AIDS Foundation, San Francisco, California, USA
References
- 1.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 4.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 5.Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;37:2237–2246. doi: 10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]
- 6.McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;38:53–60. doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grigsby IF, Pham L, Mansky LM, et al. Tenofovir-associated bone density loss. Ther Clin Risk Manag. 2010;6:41–47. [PMC free article] [PubMed] [Google Scholar]
- 8.Bedimo R, Maalouf NM, Zhang S, et al. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS. 2012;26:825–931. doi: 10.1097/QAD.0b013e32835192ae. [DOI] [PubMed] [Google Scholar]
- 9.Horizon AA, Joseph RJ, Liao Q, et al. Characteristics of foot fractures in HIV-infected patients previously treated with tenofovir versus non-tenofovir-containing highly active antiretroviral therapy. HIV AIDS (Auckl) 2011;3:53–59. doi: 10.2147/HIV.S15588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mary-Krause M, Viard J-P, Ename-Mkoumazok B, et al. Prevalence of low bone mineral density in men and women infected with human immunodeficiency virus 1 and a proposal for screening strategy. J Clin Densitom. 2012;15:422–433. doi: 10.1016/j.jocd.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Mundy LM, Youk AO, McComsey GA, et al. Overall benefit of antiretroviral treatment on the risk of fracture in HIV: nested case-control analysis in a health-insured population. AIDS. 2012;26:1073–1082. doi: 10.1097/QAD.0b013e328351997f. [DOI] [PubMed] [Google Scholar]
- 12.Long MK, Elliott JH, Woolley IJ, et al. Low CD4 count is associated with an increased risk of fragility fracture in HIV-infected patients. J Acquir Immune Defic Syndr. 2011;57:205–210. doi: 10.1097/QAI.0b013e31821ecf4c. [DOI] [PubMed] [Google Scholar]
- 13.Negredo E, Domingo P, Pérez-Álvarez N, et al. Improvement in bone mineral density after switching from tenofovir to abacavir in HIV-1-infected patients with low bone mineral density: two-centre randomized pilot study (OsteoTDF study) J Antimicrob Chemother. 2014;69:3368–3371. doi: 10.1093/jac/dku300. [DOI] [PubMed] [Google Scholar]
- 14.Bianco C, Rossetti B, Gagliardini R, et al. Bone mineral density improvement after 48 weeks of switch to maraviroc+ darunavir/ritonavir 300/800/100 mg QD, preliminary results of GUSTA study. J Int AIDS Soc. 2014;17(4 suppl 3):19816. doi: 10.7448/IAS.17.4.19816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385:2606–2615. doi: 10.1016/S0140-6736(15)60616-X. [DOI] [PubMed] [Google Scholar]
- 16.Mulligan K, Glidden DV, Anderson PL, et al. Effects of Emtricitabine/Tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial: DXA results from iPrEx. Clin Infect Dis. 2015;61:572–580. doi: 10.1093/cid/civ324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasonde M, Niska RW, Rose C, et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One. 2014;9:e90111. doi: 10.1371/journal.pone.0090111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu AY, Vittinghoff E, Sellmeyer DE, et al. Bone mineral density in HIV-negative men participating in a tenofovir pre-exposure prophylaxis randomized clinical trial in San Francisco. PLoS One. 2011;6:e23688. doi: 10.1371/journal.pone.0023688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirembe BG, Kelly CW, Mgodi N, et al. Bone mineral density changes among young, healthy african women receiving oral tenofovir for HIV preexposure prophylaxis. J Acquir Immune Defic Syndr. 2016;71:287–294. doi: 10.1097/QAI.0000000000000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14:820–809. doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4:151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diggle PJ, Heagerty PJ, Liang KY, et al. Analysis of longitudinal data. 2. New York: Oxford University Press; 2002. pp. 81–113. [Google Scholar]
- 23.Carpenter J, Kenward M. Multiple imputation and its application. New York: John Wiley & Sons; 2012. [Google Scholar]
- 24.Deutsch MB, Glidden DV, Sevelius J, et al. HIV pre-exposure prophylaxis in transgender women: a subgroup analysis of the iPrEx trial. Lancet HIV. 2015;2:e512–519. doi: 10.1016/S2352-3018(15)00206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solomon MM, Lama JR, Glidden DV, et al. Changes in renal function associated with oral emtricitabine/tenofovir disoproxil fumarate use for HIV pre-exposure prophylaxis. AIDS. 2014;28:851–9. doi: 10.1097/QAD.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.