Abstract
Binge eating disorder (BED) is defined as recurrent, distressing over-consumption of palatable food (PF) in a short time period. Clinical studies suggest that individuals with BED may have impairments in cognitive processes, executive functioning, impulse control, and decision-making, which may play a role in sustaining binge eating behavior. These clinical reports, however, are limited and often conflicting. In this study, we used a limited access rat model of binge-like behavior in order to further explore the effects of binge eating on cognition. In binge eating prone (BEP) rats, we found novel object recognition (NOR) as well as Barnes maze reversal learning (BM-RL) deficits. Aberrant gene expression of brain derived neurotrophic factor (Bdnf) and tropomyosin receptor kinase B (TrkB) in the hippocampus (HPC)-prefrontal cortex (PFC) network was observed in BEP rats. Additionally, the NOR deficits were correlated with reductions in the expression of TrkB and insulin receptor (Ir) in the CA3 region of the hippocampus. Furthermore, up-regulation of serotonin-2C (5-HT2C) receptors in the orbitoprefrontal cortex (OFC) were associated with BM-RL deficit. Finally, in the nucleus accumbens (NAc), we found decreased dopamine receptor 2 (Drd2) expression among BEP rats. Taken together, these data suggest that binge eating vegetable shortening may induce contextual and reversal learning deficits which may be mediated, at least in part, by the altered expression of genes in the CA3-OFC-NAc neural network.
Keywords: Binge eating, High Fat, Cognition, Bdnf, TrkB, Serotonin receptor
1. INTRODUCTION
Binge eating disorder (BED) involves intermittent, distressful over consumption of palatable food (PF) in brief periods of time, and this behavior, unlike bulimia or anorexia nervosa, is often not accompanied with compensatory behaviors [1]. According to DSM-V, binge episodes should be associated with at least three of the following criteria: (1) eating more rapidly than normal, (2) eating when not physically hungry, (3) eating until uncomfortably full, (4) eating alone because of shame, and (5) feeling depressed, guilty, or disgusted with oneself after overeating [1]. Factors that can influence binge eating episodes are thought to include environmental and physiological stress, dietary restraint, and intermittent exposure to energy-rich palatable food [2]. Many animal models of binge eating disorder have successfully employed these factors to mimic characteristics of human binge eating [3–7]. Regarding the food content of binges, clinical data suggests that binge eating disorder patients consume significantly more energy from fats than proteins during a binge meal [8, 9]. In a rodent model of BED, higher intermittent intake of highly PF, rich in fats and sugar, was found to predict binge eating behavior independent of body weight gain or obesity [10].
On a global scale, excessive consumption of highly palatable, and high fat (HF) foods is a major public health concern. On an individual basis, controlling ones’ consumption of these foods, given their overwhelming presence in modern western diets and innate physiological drives to consume energy-rich foods, requires intact cognitive processes including response inhibition, goal-directed learning, behavioral flexibility, attention, working memory, or decision-making [11]. Clinical data suggests that high fat intake in all age groups negatively correlates with memory, cognitive flexibility, or executive functioning [12–14]. There is abundant evidence linking high fat diet (HFD) exposure to cognitive decline in the animal models [15–18]. Interestingly, even short-term exposure to HFD (< 20days) in the rodent has been shown to significantly impair performance on spatial working memory and object recognition tasks [19, 20].
Despite clear evidence from both human and rodent studies linking high fat diet to cognitive impairments, our current understanding of cognitive impairments in BED is very limited. Behavioral disinhibition or loss of control over eating, is mainly regulated by prefrontal cortex, and post treatment relapses occur commonly in BED patients [21, 22], suggesting that some other facets of BED such as its cognitive implications need to be taken into consideration in developing effective treatment strategies. In one study, BED patients performed more poorly on a battery of neuropsychological tests for cognitive flexibility, attention, decision-making, as well as visuospatial recognition and recall memory when compared to patients with anorexia nervosa and healthy controls [23]. In another study of patients with known neurodegenerative diseases, those with co-morbid binge eating disorder had greater atrophy in right-sided orbitofrontal-insular-striatal circuit and were more likely to be diagnosed with frontotemporal dementia [24]. While BED commonly occurs in normal-weight individuals, obese individuals with BED have significantly higher rates of dietary disinhibition, psychiatric comorbidities, and cognitive dysfunction [25–27] as well as higher rates of metabolic disorder and increased inflammatory markers [28]. In one study involving body weight matched overweight women, those with BED had greater risk taking behavior, reduced utilization of feedback processing, impaired decision-making and cognitive flexibility [29]. However, another study in morbidly obese individuals with or without BED found no differences in several cognitive tests [30]. Despite these sometimes conflicting findings from human studies, there are no published reports to our knowledge that have assessed the effects of binge eating on cognitive performance and the expression of genes underlying cognition in an animal model.
Finally, although the neural mechanisms underlying cognitive impairment in BED are largely unknown, several studies have identified genes involved in the regulation of different learning and memory processes. Among the most robust findings is the observation that performance on object recognition and spatial learning and memory tasks depends on intact expression of brain derived neurotrophic factor (Bdnf) and its receptor tropomyosin receptor kinase B (TrkB) in the hippocampus (HPC) [31–34]. BDNF-TrkB binding contributes to the control of activity dependent synaptic regulation, long term potentiation, and neurogenesis, which are all critical for learning and memory formation [35]. Similar to the hippocampus, intact BDNF-TrkB expression in the prefrontal cortex (PFC) can regulate working memory, discrimination reversal learning as well as object recognition learning [36–40]. Furthermore, effects of high fat diet on disrupting both hippocampal and cortical bdnf and trkb expression has been reported in rodent models [38, 40–42]. Hippocampus and PFC interact to synchronize contextual, spatial learning and memory retrieval with working memory, decision-making, and executive functions [43, 44]. These executive functions mainly contributing to behavioral flexibility, reversal learning, and set-shifting are primarily regulated by adequate functioning of serotonergic and dopaminergic receptors signaling in the PFC and the striatum [45–51].
Though the effects of HFD and obesity on cognition have now been well studied and the underlying mechanisms are becoming more apparent, neuronal causes and consequences of BED are less understood and cannot be easily determined in human subjects. Therefore, several rodent models of BED have been developed. Some use food restriction and refeeding [3], while others employ various stressors at the end of food restriction-refeeding cycles to drive escalation in PF intake [4, 52]. In this study, we employed a previously described limited access model of BED, which involves intermittent exposure to a fat source (vegetable shortening) to induce binge eating episodes [6]. This model was adopted as it remains independent of the impact that food restriction or stress may have on the behavior or the neurochemistry of animals. Rats in our study were further divided into binge eating prone (BEP) and binge eating resistant (BER) categories based on the differences in their fat intake, as seen in some of the previous studies [7, 10], to further understand behavioral and neuronal aspects of these extreme phenotypes.
Thus, the objectives of this study were to employ the limited access rat model of BED to understand the cognitive deficits associated with binge eating shortening and to further explore the underlying neuronal mechanisms. We show differences between binge eating prone versus binge eating resistant and other control groups in fat intake, cognitive performances as well as changes in underlying patterns of neuronal gene expression. Our findings suggest that binge eating prone rats have impaired contextual and reversal learning, which may be related, at least in part, to the expression of tropomyosin receptor kinase B (TrkB) and insulin receptor (Ir) in the CA3 region of HPC and serotonin-2C (5-HT2C), dopamine receptor 1 (Drd1) or dopamine receptor 4 (Drd4) in the orbitoprefrontal cortex (OFC). We also found decreases in dopamine receptor 2 (Drd2) in the nucleus accumbens (NAc) region of BEP rats, which closely correlated with the amounts of shortening intake during binge episodes. Together, these results indicate that binge eating shortening may be associated with cognitive impairments, and to the alterations in expression of genes in the CA3-OFC-NAc neural network. Whether these cognitive deficits are a consequence of the binge eating behavior, or are preexisting and contribute to the development of binge eating will be an important focus of future studies.
2. METHODS
2.1 Animals
Forty-four male, young adult (50–55 PND at the beginning of the study) Sprague-Dawley rats (Harlan) were housed individually in tub cages in a temperature and humidity-controlled room under a 12:12 hour light-dark schedule. Throughout the experiment, all rats had ad libitum access to water and standard laboratory rodent chow (2018 Teklad, Harlan, Frederick, MD; 3.1 kcal/g: fixed formula diet of 18.6% protein, 44.2% carbohydrate, and 6.2% fat). All rats were initially habituated to the laboratory environment for one week. All procedures were approved by the Institutional Animal Care and Use Committee at Johns Hopkins University.
2.2 Limited Access Model of Binge Eating Disorder
Rats were divided into 3 experimental groups: intermittent access binge group (binge, n=28), daily access group (DAILY, n=8) and chow controls (CON, n=8). All these groups were housed in the same room for a total of eight weeks of binge paradigm, during which each group was maintained on its respective diet schedule. In our study, we employed a previously described limited access model of binge paradigm [6], using pure hydrogenated fat vegetable shortening (Crisco® brand All-Vegetable Shortening, Procter and Gamble, Cincinnati, OH; percent of calories as fat: 100%; 9.2 kcal/g) as a source of high fat. Our paradigm was slightly modified from Corwin’s limited access model such that, in our study, rats in the binge group were further divided into binge prone and resistant groups. The binge and DAILY groups were initially provided overnight shortening access to prevent neophobia during the study. Starting on PND 60, the binge group was given intermittent, restricted access to shortening on Monday, Wednesday and Friday (M/W/F) for 1 hour each day. The DAILY group was given access to shortening every day for one hour each at the same time as the binge group. Both the groups received access to shortening two hours before onset of the dark cycle. Food intake and body weight of all the rats were monitored daily. CON rats received only chow diet throughout the eight weeks of this study. After the first four weeks of exposure to the binge paradigm, the binge group rats were further classified into binge eating prone (BEP), binge eating neutral (BEN), and binge eating resistant (BER) groups based on the amounts and consistency in the kilocalories (kcals) of shortening consumed. In previous studies of BEP/BER rat models, alternative PF sources as well as different statistical methods have been employed for classifying these phenotypes (7, 10). In our study, the statistical cut-off for the classification of BEP versus BER group was defined as above or below two times the standard error of the average shortening intake of all the rats across 4 weeks. After which the rats were ranked based on the amounts of shortening intake and the consistency in the intake for all the shortening access days (M/W/F) across the 4 weeks, where in, the top 8 that consistently consumed the highest amounts of shortening over a period of 4 weeks were classified as BEP and the bottom 8 that consistently consumed the least amounts of shortening were classified as BER rats. All the rats that fell between these two groups, consumed shortening close to the group’s average amounts, were classified as BEN and were not used for further analyses. Four weeks were observed as an optimal time period to fully establish and maintain binge-like behavior in intermittent shortening access rats as compared to daily access rats in limited access BED protocol [6]. Hence, in our study, binge group was classified into BEP and BER rats and furthermore all the behavioral tests were initiated post this 4 weeks period. In this study, binge eating was operationally defined by consistently significant increased caloric intake of shortening in BEP rats as compared to both DAILY and BER groups in the 1h shortening access period over a period of 4 weeks.
2.3 Behavioral Testing
During the last four weeks of binge paradigm, cognitive tests were performed on the four experimental groups: BEP, BER, DAILY and CON. Behavioral testing was conducted according to the following schedule: first, all the rats were tested once on the novel object recognition and novel place recognition tests for 2 consecutive days. After leaving rats undisturbed for 2 days, rats were exposed twice a day for 5 days of Barnes maze testing for spatial learning acquisition and 3 days of Barnes maze reversal learning test. After which rats were left undisturbed for a week followed by a day of Barnes maze memory test. Finally, after leaving rats undisturbed for another day, they were monitored for their locomotor activity in an open field test for 3 consecutive days. Apparatuses used for cognitive testing were cleaned every time with 70% ethanol before the next rat was tested. All the rats were randomized for testing to counterbalance across all the 4 groups. To replicate the findings from the limited access protocol [6], the access to the vegetable shortening was given as close to the lights out as possible (in our study, 2 h before lights out). Therefore, all the behavioral tests were conducted in the light cycle to provide at least 4 hours between the end of the cognitive testing and the beginning of the binge episode, to avoid any effects behavioral testing may have on the shortening intake. Furthermore, performing behavioral tests before the scheduled shortening access was important to avoid any effect shortening consumption may have on the behavioral test performance, especially since the chow controls did not receive any shortening and all the other three groups consumed different amounts of it. Finally, behavioral testing was carried on both shortening access and non-access days to avoid a consistent effect of food anticipation on behavioral testing.
2.3.1 Novel Object Recognition test
A novel object recognition (NOR) test was used to evaluate hippocampal and cortical dependent contextual learning in rodents [53]. Two objects of different color, shape and size (made with Duplo-Lego blocks, Lego, USA) were placed in opposite corners of a 60cm square test-box with the center of each object 20cm from the corner of the box. The rats were placed in a corner of the box without an object, and allowed to explore for 5 minutes. This first test trial was referred to as “acquisition,” where the time spent exploring either object was recorded. On the second day of the testing, one of the objects was replaced with a distinctly different “novel object.” This trial was referred to as “recall,” where time spent exploring novel versus familiar objects for the 5 minutes was recorded. Behavior was coded by an observer blinded to the rats’ group assignment. The time spent exploring the new object was quantified as a measure of contextual learning.
2.3.2 Novel Place Recognition test
A novel place recognition (NPR) test served as the measure of hippocampal dependent short term spatial learning [54]. This is based on the rats’ inherent nature to explore displaced objects. Essentially, the ‘recall’ trial of the NOR test served as the ‘place acquisition’ phase of this test. In the second trial or ‘place recall trial’, one of the objects was placed in a new location. Rats were allowed to explore novel versus familiar locations for 5 minutes. The time spent exploring the displaced object was used to assess the short-term spatial learning.
2.3.3 Barnes Maze
2.3.3.1 Barnes Maze Acquisition
A Barnes maze (BM) acquisition test was used as a test for long term spatial learning [55]. The Barnes maze consists of a dark grey PVC circular platform (radius 61 cm), 70 cm above the floor, with 18 holes (radius 4.75 cm) around the perimeter of the platform, equally spaced out 20° from each other. A hidden escape box (38.7 x 12.1 x 14.2 cm) was placed under one of the holes. Three neutral visual cues and one aversive cue (bright light) were placed at the edge of the platform. The rats were placed in the center of the platform, and the latency to locate the escape box was measured for spatial learning. If a rat failed to find the escape box within 3 min, it was gently guided to the escape box. Two test sessions per day were conducted over a period of 5 days with an inter-trial interval of 30 min.
2.3.3.2 Barnes Maze Reversal Learning
Following BM acquisition trials, a Barnes maze reversal learning (BM-RL) test was performed. In this test, the escape box was placed at the opposite side of the maze as compared to its position during acquisition. Thus, the position of the escape box relative to the spatial cues was altered to assess behavioral flexibility. Two trails per day were conducted for 4 consecutive days. The latency to find the ‘new’ position of the escape box was scored to measure spatial reversal learning.
2.3.3.3 Barnes Maze Probe trial
One week following the reversal learning task, a probe trial was conducted. The escape box was not used in this test, rather the time spent in each quadrant of the maze was recorded. Target zone was defined as the quadrant where the escape box was previously placed in the reversal test. The time spent in the target zone was used as an index of memory consolidation.
2.3.4 Open Field Test
An open field test (OFT) was used to measure the general locomotor activity of rodents [56]. The apparatus consisted of a clear plastic box (40cm square chamber, 30cm high walls), and a computerized detection system (AccuScan Inc., Columbus, OH) that measures movement over time in the X, Y, and Z coordinates. Rats were moved to the behavioral room 1 hour prior to testing, and were allowed to acclimatize to the open field boxes for another hour. Total distance traveled, time spent immobile or moving, and time spent in the center or corners of the box were automatically recorded in 10 min intervals over a total of 30 minutes of a test session by the computerized detection system.
2.4 Tissue Collection and Sectioning
Rats were left undisturbed for two days after the behavioral testing. On PND 118, rats were killed by rapid decapitation 5 hours prior to the usual time of shortening access (that is, around the same time when behavioral tests were conducted). Brain tissue was collected and immediately frozen at −80°C. Fat pads were dissected and immediately weighed. The following brain regions were isolated from 500-μm-thick frozen coronal sections using a blunted 16-gauge stainless steel needle (inner diameter 1.25mm) based on the coordinates from Paxinos and Watson, rat brain atlas second edition [57]. All the brain regions are designated by anterior-posterior (AP), medial-lateral (ML), and dorsal-ventral (DV) stereotaxic coordinates in mm relative to bregma: orbitofrontal cortex (OFC) (3.2, ±2.4, −5.4), medial prefrontal cortex (mPFC) (3.2, ±0.4, −4.8), frontal cortex (FrC) areas (1.2, ±2.4, −1.8), dentate gyrus (DG) (−2.8, ±0.4, −4.4), region 1 of hippocampus (CA1) (−2.8, ±1.4, −3), and region 3 of hippocampus (CA3) (−2.8, 2, −3.95), nucleus accumbens (NAc) (1.2, ±1.6, −7.2), and ventral tegmental area (VTA) (−5.2, ±0.7, −8.2). It is to be noted that the primate and rodent brains, especially the prefrontal cortex, are not the same and the rodent cortical regions are not precisely characterized, however functional similarities have been drawn between the two [81].
2.5 Gene Expression
Total RNA was extracted from tissue punches using the RNeasy Lipid Tissue Mini kit (QIAGEN). cDNA was synthesized using QuantiTect Reverse Transcription kit (QIAGEN). mRNA expression of Bdnf, TrkB, leptin receptor (ObR), growth hormone secretagogue receptor or ghrelin receptor (Ghsr), insulin receptor (Ir), glucose transporter 2 (Slc2a2 or Glut2) and glucose transporter 4 (Slc2a4 or Glut4), dopamine receptor 1, 2, 4 (Drd1, Drd2, Drd4), serotonin-2A (5-HT2A), serotonin-2C (5-HT2C) and tyrosine hydroxylase (Th) were analyzed in relevant brain regions by real time qRT-PCR. Reactions were carried out in triplicate using 1× TaqMan Master Mix (Applied Biosystems) and 1× TaqMan probes for each gene (Bdnf, Ntrk2, Lepr, Ghsr, Insr, Slc2a2, Slc2a4, Drd1, Drd2, Drd4, Htr2a, Htr2c, Th) (Thermo Fisher Scientific). Real-time reactions were performed on an Applied Biosystems 7900HT Fast Real-Time PCR System with standard PCR conditions (50°C for 2 min; 95°C for 10 min; and 95°C for 15 s and 60°C for 1 min for 40 cycles). Threshold cycle (Ct) values for each set of triplicates were within 1 Ct of each other. −ΔΔCt method (Applied Biosystems) was used to determine the relative expression levels of the mRNA. Beta-actin (Actb) was used as the housekeeping gene, and its Ct values did not differ between the groups. Average Ct difference was taken for the control (CON) and test samples (BEP, BER, DAILY), and the resulting −ΔΔCt values were raised to a power of 2 to determine normalized relative expression.
2.6 Statistical analyses
Data were presented as mean plus or minus standard error of the mean. Data were analyzed by Statistica 7.0 software (StatSoft, Inc., Tulsa OK). Differences among means of the four groups in body weight, body fat, food intake, and behavior were statistically analyzed using repeated measures ANOVA. Differences in gene expression per brain region was statistically analyzed using one-way ANOVA. All the results were followed by Tukey-HSD post hoc analyses to analyze group differences. Furthermore, planned comparisons were computed to tactically compare BEP group with both BER and CON groups, to make pair-wise comparisons at specific time-points. These planned comparisons were justified based on our hypothesis that BEP group may be significantly different from the controls used in this study. These comparisons are especially useful as gene expression differences between BEP and BER groups are important from the standpoint of comparing prone versus resistant behavior to the intermittent shortening consumption. While comparisons between BEP and CON group may be important for understanding the neuronal effects of bingeing shortening as compared to consuming nutritionally balanced chow diet. Planned comparisons were performed if there was a trend indicated by the post hoc analysis. All the planned comparisons were followed by Bonferroni correction to adjust for multiple comparisons, making these analyses stringent. Pearson product-moment correlation coefficients and linear regression model were computed to assess correlations between the variables such as shortening intake, behavior, and gene expression. Food intake, body composition, and behavior analyses in this study had sample size of eight rats in each of the four groups. Gene expression analysis had at least five and at most eight rats in each of the four groups; gene expression sample points above or below two times the standard deviation of the mean were dropped. Confidence interval of 5% was used for all the statistical analyses.
3. RESULTS
3.1 Food Intake
3.1.1 Classification of binge group into BEP, BEN and BER
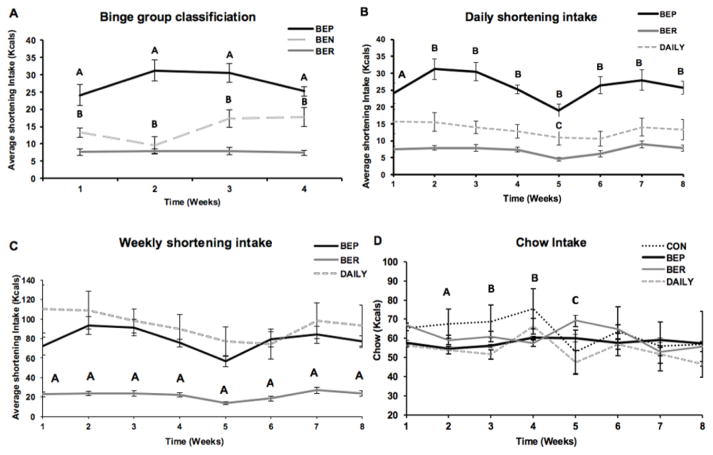
After carrying out the binge paradigm for four weeks, rats were classified as BEP (n=8), BEN (n= 12), and BER (n=8), based on kcals consumed from shortening. Repeated measures ANOVA revealed a main effect of group (F(2,25)=66.09), p<0.001), but not a significant time*group interaction effect (F(6,75)=1.8), p=0.11) on shortening intake. Post hoc analysis showed that, during the first four weeks, BEP rats consumed significantly more calories than BER and BEN rats (p<0.001); furthermore, BEN consumed more calories than BER group (p<0.05) (Fig. 1A).
Figure 1. Food Intake. A: Classification of binge rats.
The classification was based on the shortening consumption over the first 4 weeks. ‘A’- indicates a significant difference between BEP as compared to BEN (p<0.05) and BER rats (p<0.001). ‘B’- indicates a significant difference between BEN as compared to BER group (p<0.05). B: Daily shortening intake (Intake in 1h feeding period). ‘A’- indicates a significant difference between BEP and BER groups (p<0.001), ‘B’- indicates a significant difference between BEP as compared to BER and DAILY groups (p<0.001), ‘C’- indicates a significant difference between DAILY as compared to BER group (p<0.05). C: Weekly shortening intake. ‘A’- indicates a significant difference between BER as compared to BEP and DAILY groups (p<0.05). D: Chow Intake. Significant differences in the chow intake of CON rats as compared to BEP, BER and DAILY rats across 9 weeks of the study (p<0.001). ‘A’- indicates a significant difference between CON and BER groups as compared to BEP and DAILY groups (p<0.001). ‘B’- indicates a significant difference between CON as compared to BEP, BER and DAILY rats (p<0.01). ‘C’- indicates a significant difference between BER as compared to CON group (p<0.05).
3.1.2 Shortening intake over 8 weeks
Repeated measures ANOVA of average shortening intake (daily intake or intake in 1h feeding period) by BEP, BER, and DAILY groups, averaged for each week over the entire 8 weeks period showed a main group effect (F(2,21)=48.166), p<0.001), but no time*group interaction effect (F(14,147)=1.2607), p=0.2386). Post hoc analysis revealed that in most of the weeks, BEP rats consumed significantly more calories from shortening as compared to BER and DAILY groups (p<0.001), except week 1 where BEP intake was significant only compared to BER rats (p<0.001). Post-hoc analysis also showed that DAILY rats consumed more shortening as compared to BER group only in the week 5 (p<0.05) (Fig. 1B).
Repeated measures ANOVA of total weekly shortening intake showed a main group effect (F(2,21)=17.4452), p<0.001), but no time*group interaction effect (F(14,147)=1.2356), p=0.2556). Post hoc analysis revealed that the total caloric intake of BEP rats was not significantly different from that consumed by DAILY rats. However, both the BEP and DAILY groups consumed significantly more shortening than BER rats in all the weeks of binge paradigm (p<0.05) (Fig. 1C).
3.1.3 Chow Intake
Repeated measures ANOVA showed a main group effect (F(3,28)=6.507), p<0.01) and a time*group interaction (F(24,224)=3.82), p<0.001) on chow intake. Post hoc analysis showed that CON and BER rats consumed significantly more calories from chow than BEP and DAILY rats in week 2 (p<0.001), CON as compared to BEP, BER and DAILY in week 3 and 4 (p<0.01). In week 5, CON consumed more chow as compared to BER group only (p<0.05). No differences in chow intake among the four groups was found in weeks 6–8. (Fig. 1D).
3.1.4 Body Weight and Fat Composition
Repeated measures ANOVA showed no main group effect (F(3,28)= 0.63, p=0.59) or time*group interaction effects on body weight among any of the four groups (F(24,224)=1.01, p=0.454). Repeated measures ANOVA revealed no main group effect (F(3,28)=0.48), p=0.698) or time*group interaction effect (F(3,28)=0.5), p=0.686) on the body fat distribution. One-way ANOVA revealed no differences in percentage of total body fat or in percentage of body fat in the retroperitoneal, mesenteric, inguinal, epididymal, or perirenal fat pads among any of the four groups (data not shown).
3.2 Cognitive Testing
3.2.1 Novel Object Recognition Test
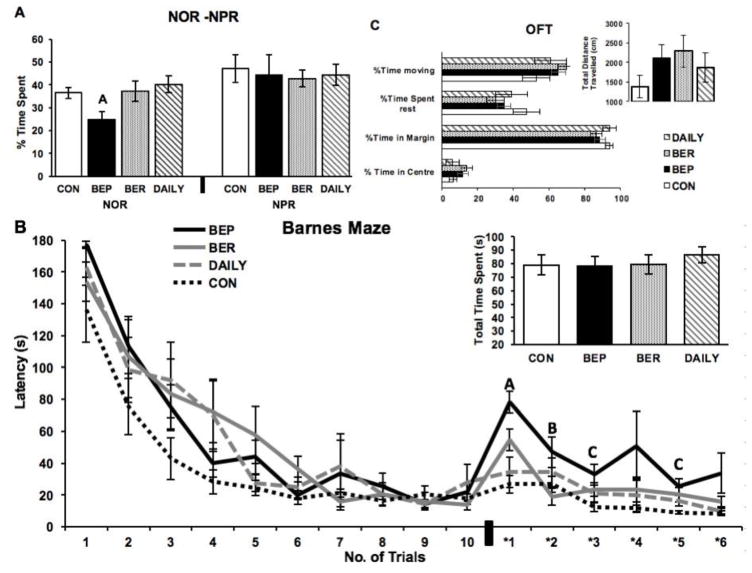
One-way ANOVA revealed that, during the recall trial, there was a group effect on the percentage time spent with the novel object (F(3,28)= 3.9, p<0.05). Post-hoc analysis showed that the BEP group spent significantly less time interacting with the novel object than the BER, DAILY and CON groups (Fig. 2A).
Figure 2. Behavior on cognitive tests. A: Novel object and novel place recognition tests.
‘A’- indicates significantly lower % of the time spent exploring the novel object in BEP rats as compared to BER, DAILY and CON groups (p<0.05). No significant difference in % of the time spent exploring the novel place in any of the four groups. B: Barnes maze test: Latency to find the escape box during the Barnes maze test. Barnes Maze acquisition (trials: 1–10): No significant differences in the latency to find the escape box between any of the four groups across any of the trials. Barnes Maze Reversal Learning (trials:*1–*6): ‘A’- indicates a significant increase in the latency to find ‘new’ position of the escape box in BEP rats as compared to CON (p<0.001), BER (p<0.05), and DAILY (p<0.001) groups in the trail *1. ‘B’- indicates a significant increase in the latency to find ‘new’ position of the escape box in BEP rats as compared to BER rats in the trial *2 (p<0.05). ‘C’- indicates a significant increase in the latency in BEP rats as compared to CON in the trials *3 (p<0.05) and *5 (p<0.01). Inset: Total time spent in the probe trial of Barnes maze test. No significant differences in the total time spent in the ‘target zone’ observed between any of the groups. C: Open field test: No differences in % time spent moving, % time spent rest, % time spent in the center of the monitor, or % time spent in the margins of the monitor was found among CON, BEP, BER and DAILY groups. No differences in the total distance travelled among any of the groups.
3.2.2 Novel Place Recognition Test
There were no differences in the percentage time spent exploring the novel place among any of the groups (F3,25)=0.1196, p=0.9477) (Fig. 2A). Three rats were dropped from this analysis as they did not spend time either at novel or familiar place.
3.2.3 Barnes Maze Test
Repeated measures ANOVA revealed a significant time effect on the latency to reach the escape-box (F(9,252)=38.6, p<0.001) such that the latency to reach the escape-box decreased over time. Also, there were no group (F(3,28)= 1.42, p= 0.257) or time*group interaction (F(27,252)= 0.88, p= 0.63) effects found on the latency to find the escape-box (Fig. 2B). However, there was an overall “high fat exposure” effect on the latency to find the escape box. This was analyzed by comparing the groups that were exposed to shortening (DAILY, BEP, and BER) to the chow controls (CON). Repeated measures ANOVA revealed an overall diet effect (F (1,30)=8.328, p<0.01), and time effect (F(9,270)=35.9, p<0.001) on the latency to reach the escape box, but no time*group interaction effect (F(9,270)=1.1293), p=0.34) was found. One-way ANOVA did not reveal significant diet effects on any of the individual trials.
In the BM reversal learning test, the latency to find the ‘new’ position of the escape box was measured. Repeated measures ANOVA revealed a significant time effect on the latency to reach the escape box (F(5,140)=11.242, p<0.001) such that the latency to reach the escape box decreased over time. There was a main group effect (F(3,28)= 7.0432), p<0.01), but no time*group interaction was found (F(15,140)=1.27), p=0.22) on the latency to find the escape box (Fig 2B). Post hoc analysis revealed significantly higher latency to find the escape box in BEP rats as compared to DAILY and CON rats in trial 1 (F(3,28)=10, p<0.001). Planned comparison revealed significantly higher latency in BEP rats as compared to BER in trials 1 and 2 (p<0.05), and as compared to CON in trial 3 (p<0.05) and trial 5 (p<0.01). Repeated measures ANOVA revealed a significant “high fat exposure” effect (F(1,30)=6.5, p<0.05) as well as time effect (F(6,180)=8.8, p<0.001) on the latency to reach the escape box in BM-RL test. Moreover, one-way ANOVA revealed a significant diet effect on individual trial 1 (p<0.05), trail 2 (p<0.01), trial 4 (p<0.05), and trial 5 (p<0.05). One-way ANOVA showed no group differences in the latency to find the escape box in the subsequent probe trial (F(3,28)=0.24528, p=0.863) (Fig. 2B inset).
3.2.4 Open Field Test
Results from the open field test did not identify any significant group effects on total distance travelled (F(3,28)=1.27, p=0.3), % time spent moving (F(3,28)=0.5, p=0.68), % time spent rest (F(3,28)=0.5, p=0.68), % time spent in center of the monitor (F(3,28)=1.05, p=0.38), or % time spent in margins of the open field test apparatus (F(3,28)=1.05, p=0.38) (Fig. 2C).
4. Gene Expression
4.1 Expression in the Hippocampus
Gene expression of Bdnf, TrkB, ObR, Ghsr, Ir, Glut2 and Glut4 in the sub-regions of the hippocampus (DG, CA1, CA3) was quantified (Fig. 3A).
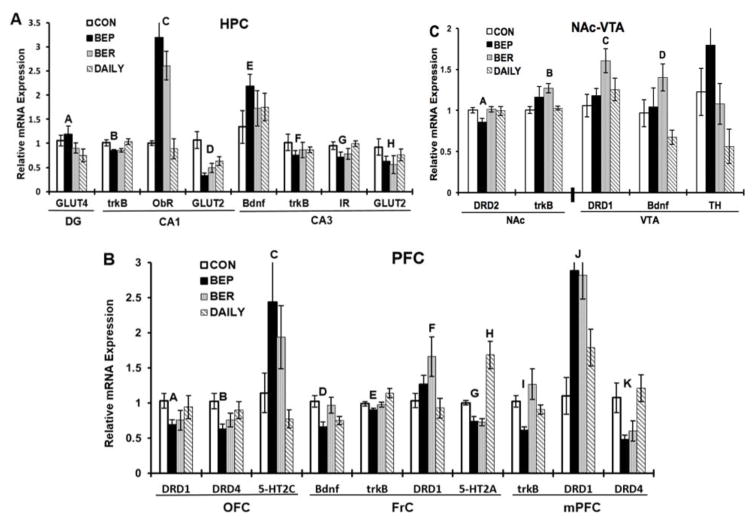
Figure 3. Gene expression.
mRNA is expressed as the normalized relative expression to the control chow fed group (CON). A: Expression in HPC: ‘A’- indicates significant increase in DG Glut4 expression in BEP group as compared to DAILY (p<0.05). ‘B’- indicates significant decrease in the CA1 TrkB expression in BEP as compared to CON group (p<0.05). ‘C’- indicates significant increase in the CA1 ObR expression in BEP and BER as compared to CON and DAILY groups (p<0.001). ‘D’- indicates significant decrease in the CA1 Glut2 expression in BEP (p<0.001), BER (p<0.01), and DAILY (p<0.05) groups as compared to CON. ‘E’- indicates significant increase in the CA3 Bdnf expression in BEP group as compared to CON (p<0.05). ‘F’- indicates significant decrease in the CA3 TrkB expression in BEP group as compared to CON (p<0.05). ‘G’- indicates significant decrease in the CA3 Ir expression in BEP group as compared to CON (p<0.05). ‘H’- indicates significant decrease in the CA3 Glut2 expression in BEP (p<0.01) and BER (p<0.05) groups as compared to CON group. B: Expression in PFC: ‘A’- indicates significant decrease in the OFC Drd1 expression in BEP group as compared to CON (p<0.05). ‘B’- indicates significant decrease in the OFC Drd4 expression in BEP group as compared to CON (p<0.05). ‘C’- indicates significant increase in OFC 5-HT2C expression in BEP group as compared to CON and DAILY groups (p<0.05). ‘D’- indicates significant decrease in the FrC Bdnf in BEP group as compared to CON (p<0.05). ‘E’- indicates significant decrease in the FrC TrkB expression in BEP as compared to DAILY group (p<0.05). ‘F’- indicates significant increase in the FrC Drd1 expression in BER group as compared to DAILY (p<0.05). ‘G’- indicates significant decrease in the FrC 5-HT2A expression in BEP as compared to CON (p<0.05) and DAILY groups (p<0.005). ‘H’- indicates significant increase in the FrC 5-HT2A expression in DAILY as compared to CON, BER, and BEP groups (p<0.005). ‘I’- indicates significant decrease in the mPFC TrkB expression in BEP group as compared to BER (p<0.005) and CON (p<0.05) groups. ‘J’- indicates significant increase in the mPFC Drd1 expression in BEP and BER as compared to CON group (p<0.05). ‘K’- indicates significant decrease in the mPFC Drd4 expression in BEP and BER as compared to DAILY groups (p<0.01) and in BEP group as compared to CON group (p<0.05). C: Expression in NAc-VTA: ‘A’- indicates significant decrease in the NAc Drd2 expression in BEP as compared to BER and CON groups (p<0.05). ‘B’- indicates significant increase in NAc TrkB expression in BER as compared to DAILY group (p<0.05). ‘C’- indicates significant increase in VTA Drd1 expression in BER group as compared to CON (p<0.05). ‘D’- indicates significant increase in VTA Bdnf in BER as compared to DAILY (p<0.005) and CON (p<0.05) groups.
4.1.1 Dentate Gyrus
One-way ANOVA showed a group effect in the relative expression levels of Glut4 in the DG region (F(3,28)=3, p<0.05). Post hoc analysis revealed a significantly greater expression of Glut4 in BEP as compared to DAILY rats (p<0.05).
4.1.2 CA1 Region
In the CA1 region, a group effect was seen in TrkB expression (F(3,27)=3.66, p<0.05). Post hoc analysis showed a trend for decreases in CA1 TrkB expression in BEP and BER rats as compared to DAILY and CON groups (p=0.07). Planned comparison revealed significantly lower expression of CA1 TrkB in BEP rats as compared to CON group (p<0.05). Likewise, a group effect was seen in the CA1 ObR expression (F(3,28)=18.1, p<0.001). Post hoc analysis revealed a significant higher expression in BEP and BER rats as compared to CON and DAILY groups (p<0.001). A group effect was also observed in the CA1 Glut2 expression (F(3,26)=8.37, p<0.001). Post hoc analyses revealed a significant lower expression in BEP (p<0.001), BER (p<0.01), and DAILY rats (p<0.05) as compared to CON group.
4.1.3 CA3 Region
One-way ANOVA showed a group effect in the expression levels of Bdnf in the CA3 region of HPC (F(3,28)=3.38, p<0.05). Post hoc analysis revealed significantly higher expression of Bdnf in BEP rats as compared to CON group. A group effect in the CA3 TrkB expression level was observed (F(3,28)=2.94, p=0.05). Post hoc analysis showed a strong trend for decrease in TrkB expression in BEP rats as compared to CON (p=0.05). Planned comparison showed significantly lower expression levels of TrkB in BEP as compared to CON rats only (p<0.05). One-way ANOVA revealed a strong trend for group effect in the CA3 Ir expression (F(3,27)=2.67, p=0.06). Post hoc analysis showed a trend for decrease in Ir expression in BEP rats as compared to CON group (p=0.07). Planned comparison revealed a significantly lower expression of Ir in BEP rats as compared to CON (p<0.05). Finally, a group effect in the Glut2 expression levels was found in the CA3 region (F(3,27)=5.41, p<0.01). Post hoc analysis revealed significantly lower Glut2 expression in BEP (p<0.01) and BER (p<0.05) groups as compared to CON group.
4.2 Expression in the Prefrontal Cortex
Gene expression of Bdnf, TrkB, Drd1, Drd2, Drd4, 5-HT2A, and 5-HT2C in sub-regions of PFC (OFC, mPFC, FrC) was quantified (Fig. 3B).
4.2.1 Orbitofrontal Cortex
One-way ANOVA (F(3,27)=2.7, p=0.06) showed a trend in the OFC Drd1 expression. Post hoc analysis showed a trend for decrease in Drd1 expression in BEP rats as compared to CON (p=0.07). Planned comparison demonstrated significantly lower expression of Drd1 in BEP as compared to CON group (p<0.05). A group effect in the OFC Drd4 expression levels (F(3,26)=2.93, p=0.05) was found. Post hoc analysis revealed a significantly lower expression of Drd4 in BEP as compared to CON group (p<0.05). One-way ANOVA showed a group effect in the OFC 5-HT2C expression (F(3,27)=4.53, p<0.05). Post hoc analysis revealed a significantly greater expression of 5-HT2C in BEP rats as compared to CON and DAILY groups (p<0.05).
4.2.2 Frontal cortex
In the FrC region, one-way ANOVA showed a trend for group effect in the relative expression levels of Bdnf (F(3,28)=2.7, p=0.06). Post hoc analysis showed a trend for decrease in Bdnf expression in BEP rats as compared to CON (p=0.06). Planned comparison revealed a significantly lower expression in BEP group as compared to CON group (p<0.05). A group effect was seen in the FrC TrkB expression (F(3,28)=3.05, p<0.05). Post hoc analysis revealed a significantly lower expression in BEP rats as compared to DAILY rats (p<0.05). A group effect was seen in Drd1 expression (F(3,26)=3.48, p<0.05). Post hoc analysis revealed a significantly greater expression in BER rats as compared DAILY (p<0.05). One-way ANOVA revealed a main group effect in the FrC 5-HT2A expression (F(3,26)=9.69, p<0.001). Post hoc analysis revealed a significantly greater expression of 5-HT2A in DAILY rats as compared to BEP, BER, and CON groups (p<0.005). Planned comparison revealed a significantly lower expression in BEP group as compared to CON group (p<0.05).
4.2.3 Medial prefrontal cortex
One-way ANOVA showed a group effect in the mPFC TrkB expression levels (F(3,28)=5.25, p<0.01). Post hoc analysis showed significantly lower expression of TrkB in BEP as compared to BER rats (p<0.005), and a strong trend as compared to CON group (p=0.05). Planned comparison resulted in significant decrease in mPFC TrkB expression in BEP as compared to CON group (p<0.05). One-way ANOVA showed a group effect in the mPFC Drd1 expression levels (F(3,25)=6.15, p<0.01). Post hoc analysis revealed significantly greater expression of Drd1 in BEP and BER rats as compared to CON group (p<0.05). Analysis of Drd4 expression levels in the mPFC region displayed a group effect (F(3,25)=5.46, p<0.01). Post hoc analysis revealed significantly lower expression of Drd4 in BEP and BER rats as compared to DAILY rats (p<0.01). Planned comparison showed a significant decrease in BEP as compared to CON group (p<0.05).
4.3 Expression in the Nucleus Accumbens and Ventral Tegmental Area
4.3.1 Nucleus accumbens
Gene expression of Drd1, Drd2, 5-HT2A, 5-HT2C, Bdnf, TrkB, and Ghsr in the NAc region was quantified (Fig. 3C). One-way ANOVA showed a group effect in the NAc Drd2 expression (F (3,27)=3.42, p<0.05). Post hoc analysis showed a lower Drd2 expression in BEP rats as compared to CON rats (p<0.05), and a strong trend as compared to BER group (p=0.06). Planned comparison revealed a significantly lower expression of NAc Drd2 in BEP rats as compared to BER group (p<0.05). One-way ANOVA revealed a group effect in TrkB expression (F(3,26)=3.63, p<0.05). Post hoc analysis revealed a significantly greater expression in BER as compared to DAILY group (p<0.05).
4.3.2 Ventral tegmental area
Gene expression of Drd1, Drd2, Th, Bdnf, and GR in the VTA region was quantified (Fig. 3C). One-way ANOVA showed a group effect (F(3,26)=3.383, p<0.05) in Drd1 expression. Post hoc analysis showed significantly greater expression levels in BER as compared to CON rats (p<0.05). A group effect (F(3,27)=5.5, p<0.005) in the VTA Bdnf expression level was observed. Post hoc analysis revealed a significantly greater expression of Bdnf in BER rats as compared to DAILY (p<0.005) and CON (p<0.05) groups. One-way ANOVA showed a trend for group effect in Th expression (F(3,28)=2.32, p=0.09). Post hoc analysis showed a strong trend for increase in Th expression in BEP rats as compared to DAILY rats (p=0.06).
5. Pearson Product-moment Correlations
Pearson product-moment correlation coefficients were computed to assess the relationships between the average shortening intake, behavior on various cognitive tasks, and gene expression data.
5.1 Correlation of average shortening intake with behavior
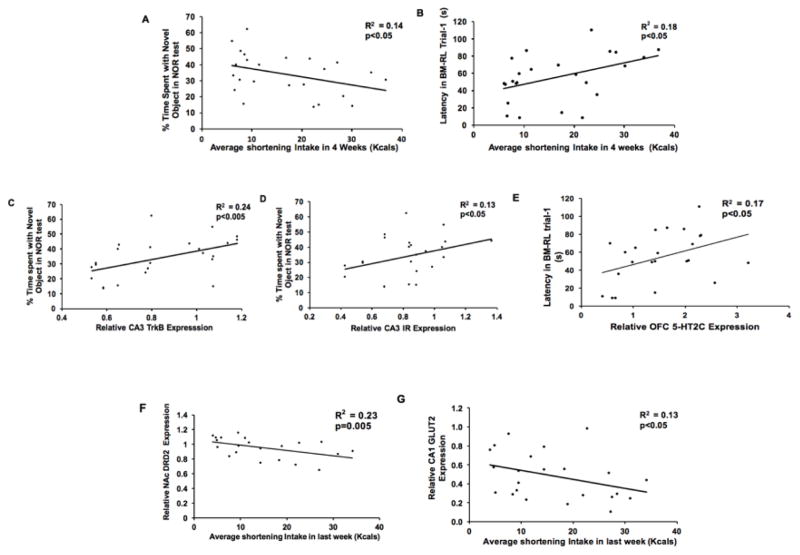
R squared values and t distribution revealed a negative correlation between the average shortening intake in first four weeks of binge paradigm and the percentage of time spent with the novel object in NOR task (n=24, r= −0.38, R2=0.14, p<0.05) (Fig. 4A). We also found a positive correlation between the average shortening intake in first 4 weeks of binge paradigm and the latency to find the new position of the escape box in trial 1 of BM-RL test (n=24, r=0.43, R2=0.18, p<0.05) (Fig. 4B). CON group was excluded from this analyses.
Figure 4. Correlations between shortening intake, behavior and gene expression.
A: Negative correlation between average shortening intake in first four weeks of binge paradigm and the % time spent with the novel object in NOR test (p<0.05). B: Positive correlation between the average shortening intake in first four weeks and the latency to find the escape box in BM-RL trial 1 (p<0.05). C: Positive correlation between the CA3 TrkB expression and the % time spent with the novel object in NOR test (p<0.005). D: Positive correlation between the CA3 Ir expression and the % time spent with the novel object in NOR test (p<0.05). E: Positive correlation between the OFC 5-HT2C expression and the latency to find the ‘new’ position of the escape box in BM-RL test trial 1 (p<0.05). F: Negative correlation between average shortening intake in last week and the NAc Drd2 expression (p=0.005). G: Negative correlation between average shortening intake in last week and the CA1 Glut2 expression (p<0.05).
5.2 Correlation of gene expression with behavior
R squared values and t distribution revealed a positive correlation between the CA3 TrkB expression % time spent with the novel object in NOR test (n=32, r= 0.49, R2=0.24, p<0.005) (Fig. 4C). Furthermore, a positive correlation between the CA3 Ir expression and % time spent with the novel object in NOR test (n=32, r=0.36, R2=0.13, p<0.05) was observed (Fig. 4D). We also found a positive correlation between the OFC 5-HT2C expression and latency to find the ‘new’ position of the escape box in BM-RL trial 1 (n=32, r=0.42, R2=0.17, p<0.05) (Fig. 4E). Similar results were observed in a linear regression model.
5.3 Correlation of average shortening intake and gene expression
We found a negative correlation between the average shortening consumed in last week of binge paradigm and the NAc Drd2 expression (n=24, r= −0.48, R2=0.23, p=0.005) (Fig. 4F). A negative correlation between the average shortening intake in last week and the CA1 Glut2 expression was also found (n=24, r= −0.36, R2=0.13, p<0.05) (Fig. 4G). CON group was excluded from this analyses.
6. DISCUSSION
In the current study, we sought to investigate the cognitive implications of bingeing shortening, employing a limited access BED model which does not incorporate food deprivation or stress. We demonstrated that BEP rats were impaired in contextual and spatial reversal learning. Disrupted expression of Bdnf-Trkb in the HPC-PFC network was observed in BEP rats. Several genes were implicated as potential contributors to this phenotype including TrkB and Ir in the CA3 region of HPC, and 5-HT2C, Drd1 and Drd4 in the OFC region of PFC. Our data also suggests that Drd2 expression in the NAc is closely correlated with binge-like behavior.
The data from behavioral tests for learning and memory presented here demonstrates that BEP rats have impaired contextual, but intact spatial learning as observed in the NOR and NPR tasks respectively. In the Barnes maze acquisition trials, which are thought to depend mostly on the hippocampus, there were no differences among BEP, BER, DAILY or CON groups. However, there was an overall effect of high fat such that rats with any shortening exposure (BEP, BER, and DAILY) were somewhat impaired relative to chow controls (CON). We found a similar shortening exposure effect in the BM-RL test, which heavily relies on cortical function in addition to intact hippocampal signaling [58, 59]. Consistent with our findings, previous studies have shown impairments in both spatial and reversal learning in rats exposed to high fat food sources [38, 60–63]. Our study demonstrated prolonged escape latency among BEP rats compared to all other groups in the BM reversal task, but not in the spatial learning task, strongly suggesting a binge intake-related deficit in reversal learning and not spatial acquisition. Importantly, no differences were found in the open field test, suggesting that the NOR and BM-RL deficits were not due to altered locomotor activity.
While the effects of chronic HFD exposure on learning and memory are well established, the implications of high fat binge eating behavior on cognition are far less well known. Here, we demonstrate impaired novel object recognition and reversal learning, specifically in BEP rats. Interestingly, these deficits appear to be independent of total caloric intake, total shortening intake, body weight and fat distribution suggesting that learning and memory may be affected by the intermittent spikes in shortening exposure and over-consumption. Consistent with other reports [64–66], food intake in BEP rats follow a caloric compensation pattern, where these rats consume significantly more shortening in the 1h feeding window as compared to DAILY and BER rats, meanwhile compensating for the extra calories obtained from the shortening by reducing their chow intake. Importantly, the total shortening intake between the BEP and DAILY rats did not differ while their behavior did. The effects of intermittent shortening overconsumption on the cognitive decline seen in BEP rats is supported by the correlations found between the average shortening intake and performances on NOR and BM-RL tests.
Among the neuronal factors that may be affecting the cognitive dysfunction of BEP rats, we found increased Bdnf and decreased TrkB expression in the CA3 region of the hippocampus in BEP rats as compared to the CON group. It is widely known that intact Bdnf and TrkB signaling in both hippocampal and cortical regions is important for object recognition memory [67–73]. A positive correlation between CA3 TrkB expression and percent time spent with the novel object suggests that CA3 TrkB may be one of the neuronal factors for BEP specific contextual learning deficit. Previous studies employing unrestricted HFD-fed rodents have found that spatial learning and memory impairments are associated with decreased hippocampal Bdnf expression [41, 42, 74, 75], which differs from the increased CA3 Bdnf expression and learning impairments found in the BEP rats in our study. The increased Bdnf expression may be a consequence of the downregulated TrkB expression in the CA3 region, or vice versa, which in part is supported by one study showing the contrasting regulation of Bdnf and its receptor, TrkB in the primary neuronal cultures [76]. Moreover, an important consideration in our model was the short-term restricted exposure and over-consumption of shortening in binge prone rats, which is a paradigm in which Bdnf pathway gene expression has not been widely studied and may explain the gene expression difference in our study. Consistent with this view, a study employing male rats fed 45% HFD for only 72 hours indicated a strong trend towards an increase in Bdnf mRNA expression in the dorsal hippocampus (p=0.059), despite no significant differences found in food intake, BW or body fat composition, as compared to the rats fed a control diet (10% fat by kcal) [77]. In another study, mice classified as diet induced obese (DIO) and diet induced resistant displayed differential expression of hippocampal Bdnf and TrkB genes when exposed to high fat, low fat or energy-restricted pair feeding diets [78]. Another study in DIO mice with 23 weeks of chronic HFD exposure found intact object recognition, but impaired object location memory with no changes in hippocampal Bdnf mRNA expression as compared to control rats [61]. We speculate that acute, intermittent, or chronic exposure to dietary fat intake may differentially affect hippocampal Bdnf and TrkB expression.
We also assessed gene expression in several sub-regions of the PFC, as high fat consumption has been previously associated with changes in cortical Bdnf expression [38]. Furthermore, the primate dorsolateral PFC and anterior cingulate cortex that regulate goal oriented planning, problem solving as well as decision-making share functional similarities with rodent mPFC and FrC [79–81]. In our study, we found significant decreases in the FrC Bdnf and TrkB expression in BEP rats as compared to CON and DAILY groups respectively, and a significant decrease in the mPFC TrkB expression in BEP as compared to BER and CON groups. Two independent studies have demonstrated that Bdnf-trkB signaling in the prefrontal cortex is involved in regulating novel object recognition memory [39, 40]. In one study, exposure to 60% HFD in juvenile mice for as short as one week was associated with impairments in the NOR task as well as Y-maze based spatial working memory task with reduced prefrontal cortex Bdnf expression, despite no differences in body weight [20]. In another, disruption of activity dependent Bdnf expression in hippocampal-PFC network was shown to impair spatial reversal learning and fear extinction without affecting spatial learning and fear acquisition [43]. Overall, hippocampal-PFC interactions are important for maintaining intact cognitive functions [44, 82]. In our study, bingeing prone rats displayed disruption of the Bdnf-Trkb expression in the hippocampal-PFC network, which may have impaired their cognitive performances.
We also examined the hippocampal expression of several genes involved in glucose homeostasis and found decreased Ir expression in the hippocampal CA3 region of BEP rats as compared to the chow control group, and a positive correlation with NOR task performance. We further found decreased Glut2 expression in all shortening exposed groups and a correlation between Glut2 expression with average shortening intake in the last week of binge paradigm. Supporting these findings, high energy diets have been previously shown to influence GLUT2 [83] as well as IR expression in the brain [84]. A positive effect of GLUT2 on hippocampal synaptic activity, neurotransmitter release, and cognition has been previously reported [85, 86], while others have suggested involvement of insulin receptors in hippocampal dependent spatial learning and memory [87–89] as well as recognition memory [90–92]. In another series of studies, intracerebroventricular injection of streptozotocin, a drug specific to GLUT2 dependent transport [93], was shown to decrease IR expression only in the CA3 region of the rat’s hippocampus [94], and was found to be associated with memory impairments [95]. Since, the CA3 region of hippocampus is critical for developing earliest acquisition of the context [96], we speculate that, in our study, decreased expression of these metabolic receptors in the CA3 region may also have played a role in affecting contextual learning of BEP rats.
We next assessed the effects of binge-like behavior on the expression of several dopamine- and serotonin-related signaling genes in the prefrontal cortical regions. In the OFC, we found increased expression of 5-HT2C in BEP rats as compared to CON and DAILY groups. Further, OFC 5-HT2C expression was negatively correlated with performance on the BM-RL task. A role for OFC serotonin signaling in reversal learning has been previously demonstrated. OFC 5HT depletion studies in marmosets and rats have indicated impairments in serial visual reversal learning [45, 97, 98], detour-reaching task [46] as well as attentional set-shifting task [99]. Likewise, 5HT markers in the rodent OFC have been shown to correlate with performances on a spatial reversal learning task while its reuptake inhibitor, citalopram was shown to improve performance [100]. In contrast, OFC specific administration of a 5-HT2C antagonist significantly enhanced performance on different reversal learning based tasks [101–103]. However, another study employing a radial maze demonstrated that administration of a 5-HT2C antagonist had no effect on spatial reversal test, where the contingency from initial spatial discrimination were simply reversed [104]; resembling more closely to the spatial reversal test used in our study. Although discrepancies in these studies can be attributed to the use of different learning paradigms, the involvement of OFC 5-HT2C receptor in the aspect of reversal learning remains consistent. Recent evidence for the involvement of 5-HT2C in the context of bingeing comes from studies employing a 5HT reuptake inhibitor, fluoxetine, as well as a 5HT agonist, lorcaserin, which suppressed high fat binge eating in wild type mice but failed to inhibit binge eating in 5-HT2C null mice indicating its potential role in bingeing [105]. Thus, OFC 5-HT2C receptors maybe an important target for high fat bingeing associated reversal learning deficits. We also found BEP specific reductions in the expression of Drd1 and Drd4 in the OFC region as compared to the CON group. Although, dopamine (DA) depletion in the OFC of marmoset monkeys did not produce any reversal learning deficits [98], and we did not find any correlations between cortical dopamine receptors and performance on cognitive tasks, there are evidences from both rodent and human studies linking D1 receptor modulation in the PFC to impairments in cognitive flexibility or spatial working memory [106–111], and to increased palatable food intake [112–114]. Likewise, several clinical studies have implicated that DRD4 gene polymorphisms are associated with executive dysfunction [115–117] as well as palatable food craving, fat intake, and binge eating behavior [118–120]. Considering that we observed changes in 5-HT2C, Drd1, and Drd4 in the cortex, it is interesting to speculate whether serotonin and dopamine-related pathways are functioning independently or in an integrated manner to affect binge eating and cognition. Some insight on this issue was provided by a clinical study suggesting that serotonin and dopamine systems are doubly dissociable in a reversal learning task [49]. Recently, it was shown that the activation of midbrain 5-HT→DA neural circuit via a 5-HT2C receptor mediated mechanism suppressed intermittent high fat binge eating in mice [105]. Several clinical studies have indicated the involvement of both serotonergic and dopaminergic genes polymorphism in BED patients [120–123]. While such data suggest a role for DA and 5-HT signaling in both binge behavior and learning and memory, we cannot attribute the gene expression pattern observed specifically to the cognitive dysfunction or binge eating behavior per se.
Finally, we measured the expression of several genes known to play important roles in the NAc-VTA “reward pathway.” The data presented here demonstrate that BEP rats had decreased NAc Drd2 expression as compared to BER and CON groups. Previously, several reviews have detailed the numerous clinical and animal studies indicating the role of the NAc-VTA pathway and DRD2 in binge eating [124–126]. In a mouse model of BED, NAc deep brain current stimulation reduced binge-like behavior, and raclopride, a selective D2 receptor antagonist, weakened this effect [127]. In our study, NAc Drd2 expression negatively correlated with shortening intake, where this correlation got stronger over the course of shortening exposure, suggesting that binge eating shortening may be affecting NAc Drd2 expression. We also found a trend for increased expression of Th in the VTA of BEP rats as compared to DAILY rats. Employing a similar limited access binge eating model for eight weeks, a recent study indicated that compared to daily shortening-fed rats, intermittent shortening rats displayed elevated Th mRNA expression levels in the VTA that restored to control levels after a bingeing episode [128]. In line with previous studies employing the animal models of BED, we show that bingeing vegetable shortening may affect dopaminergic NAc-VTA network, which may contribute further to the bingeing behavior of binge prone rats.
Behavioral differences found between BEP and BER rats in this study further validate results from previous studies that show significant fat intake differences between these two groups while maintaining similar body weights [10, 129]. Many studies have indicated underlying neuronal differences between these two groups. In one study, when subjected to foot-shock stress, BEP rats seem to increase 1h intermittent sucrose intake while maintaining a blunted corticosterone plasma levels. Furthermore, 30 min after repeated stress episodes, BEP female rats showed decreases in mRNA expression of corticotropin-releasing factor in paraventricular hypothalamic nucleus and increases in the bed nucleus of the stria terminalis, as compared to BER rats [7, 130]. In another study, intermittent access to PF pellets for 3 weeks was associated with higher Fos expression in the NAc core and shell, and in prelimbic, infralimbic and cingulate area of the mPFC in BEP rats when compared to BER rats and chow controls [131]. Likewise, in our study, BEP and BER rats differed in gene expression mainly in the reward and cortical regions; while BEP and CON differed in candidate gene expression mainly in the hippocampus. These differences in neuronal mechanisms may be underlying their different behavioral phenotypes, and may serve as molecular targets to further understand prone versus resistant behavior to bingeing as well as to explore the neuronal implications of binge eating fat versus consuming nutritionally balanced diet. Finally, other animal models of BED involving a combination of food restriction and stress, which are commonly occurring clinical features of BED, should be employed to fully understand the cognitive effects of BED.
Taken together, our data suggest that binge eating shortening for eight weeks in a rodent model of BED may be accompanied by contextual and reversal learning deficits. These cognitive deficits maybe mediated by the gene expression changes observed in the NAc, OFC, and the CA3 brain regions of bingeing prone rats, as hypothesized in Fig. 5. Overall, aberrant expression of Bdnf-TrkB in the hippocampus-prefrontal cortex network was associated with binge eating prone rats. Furthermore, genes underlying binge prone rats specific cognitive dysfunction may include, at least in part, TrkB and Ir in the CA3 region of hippocampus, 5-HT2C, Drd1 or Drd4 in the OFC region, and finally Drd2 in the NAc region, which as compared to the controls may be contributing their continued bingeing behavior. Moving forward, these results may be used to identify molecular targets for developing effective treatment strategies for BED and its associated behavioral comorbidities. It should be noted that we did not assess learning and memory in the rats prior to the exposure to the binge paradigm. Thus, while we speculate that the behavioral deficits and gene expression changes are a consequence of engaging in the binge behavior, it is possible that the rats that are binge prone have preexisting cognitive deficits and that these deficits contribute to their vulnerability when exposed to a highly palatable food source at an intermittent feeding schedule. These questions will be important areas of focus in future research.
Figure 5. Binge eating associated with cognitive deficits and gene expression changes in the NAc-OFC-CA3 neural network.
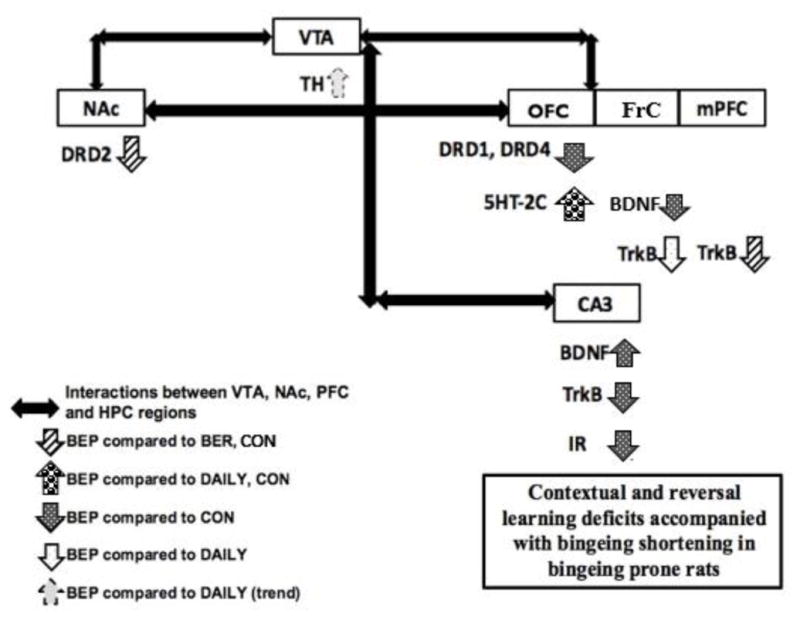
VTA-NAc mesolimbic pathway is the major “reward pathway” in the brain. Dopaminergic dysregulation in this pathway is implicated in palatable food associated reward and binge eating [124, 125]. A reduced Drd2 expression in the NAc, and a trend for increase in the VTA TH expression was associated with binge eating prone rats. Intact reversal learning is important for establishing adaptive responses or flexibility to altered contingencies [132]. Serotonin and dopamine systems in the OFC seems to be crucial in maintaining reversal learning behavior [97, 49]. We speculate that an increase in 5-HT2C expression, and decrease in Drd1 and Drd4 expression in the OFC region may have, at least in part, impaired spatial reversal learning in BEP rats. Furthermore, the interactions between the cortical-hippocampal regions are essential for intact cognitive performances [43, 44]. Bdnf-TrkB signaling disruption was observed in the HPC-PFC axis of BEP rats. Specifically, reduced TrkB and IR expression in the CA3 region of HPC were correlated with the impaired performance on novel object recognition task. Thus, alterations in expression of the genes regulating reward and cognition were accompanied with the impaired cognitive phenotype of shortening bingeing rats.
Highlights.
This study employed a rodent model to examine the effects of high-fat binge eating on cognitive performances.
Binge eating prone (BEP) rats were impaired in contextual learning and spatial reversal learning when compared to binge eating resistant and control rats.
BEP rats were found to have disrupted Bdnf-TrkB expression in the hippocampal and prefrontal cortex sub-regions as compared to control rats.
Altered expression of serotonin-2C receptor and dopamine 1 and 4 receptors in orbitofrontal cortex was associated with BEP rats.
Binge eating behavior was closely related to the decreases in the dopamine 2 receptor expression in the nucleus accumbens region.
Acknowledgments
We thank Dr. Miranda Johnson, Dr. Jennifer Albertz and Dr. Kellie Tamashiro for their technical advice and assistance with this study. We would also like to acknowledge the partial support provided from RO1DK19302 for funding this research.
Footnotes
DISCLOSURES
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Mathes WF, Brownley KA, Mo X, Bulik CM. The biology of binge eating. Appetite. 2009;52(3):545–553. doi: 10.1016/j.appet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagan MM, Moss DE. Persistence of binge-eating patterns after a history of restriction with intermittent bouts of refeeding on palatable food in rats: Implications for bulimia nervosa. Int J Eat Disord. 1997;22(4):411–420. doi: 10.1002/(sici)1098-108x(199712)22:4<411::aid-eat6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 4.Boggiano MM, Chandler PC. Binge eating in rats produced by combining dieting with stress. Curr Protoc Neurosci. 2006;Chapter 9(Unit9.23A) doi: 10.1002/0471142301.ns0923as36. [DOI] [PubMed] [Google Scholar]
- 5.Avena NM. Examining the addictive-like properties of binge eating using an animal model of sugar dependence. Exp Clin Psychopharmacol. 2007;15(5):481–491. doi: 10.1037/1064-1297.15.5.481. [DOI] [PubMed] [Google Scholar]
- 6.Corwin RL, Wojnicki FH. Binge eating in rats with limited access to vegetable shortening. Curr Protoc Neurosci. 2006;Chapter 9(Unit9.23B) doi: 10.1002/0471142301.ns0923bs36. [DOI] [PubMed] [Google Scholar]
- 7.Calvez J, Timofeeva E. Behavioral and hormonal responses to stress in binge -like eating prone female rats. Physiology & Behavior. 2016;157:28–38. doi: 10.1016/j.physbeh.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Yanovski SZ, Leet M, Yanovski JA, Flood M, Gold PW, Kissileff HR, et al. Food selection and intake of obese women with binge-eating disorder. Am J Clin Nutr. 1992;56(6):975–980. doi: 10.1093/ajcn/56.6.975. [DOI] [PubMed] [Google Scholar]
- 9.Goldfein JA, Walsh BT, Devlin MJ, Lachaussée JL, Kissileff HR. Eating behavior in binge eating disorder. Int J Eat Disord. 1993;14:427–431. doi: 10.1002/1098-108x(199312)14:4<427::aid-eat2260140405>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 10.Boggiano MM, Artiga AI, Pritchett CE, Chandler-Laney PC, Smith ML, Eldridge AJ. High intake of palatable food predicts binge-eating independent of susceptibility to obesity: An animal model of lean vs obese binge-eating and obesity with and without binge-eating. Int J Obes. 2007;31(9):1357–1367. doi: 10.1038/sj.ijo.0803614. [DOI] [PubMed] [Google Scholar]
- 11.Higgs S. Cognitive processing of food rewards. Appetite. 2016;104:10–17. doi: 10.1016/j.appet.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Eskelinen MH, Ngandu T, Helkala EL, Tuomilehto J, Nissinen A, Soininen H, et al. Fat intake at midlife and cognitive impairment later in life: A population-based CAIDE study. Int J Geriatr Psychiatry. 2008;23(7):741–747. doi: 10.1002/gps.1969. [DOI] [PubMed] [Google Scholar]
- 13.Baym CL, Khan NA, Monti JM, Raine LB, Drollette ES, Moore RD, Cohen NJ. Dietary lipids are differentially associated with hippocampal-dependent relational memory in prepubescent children. Am J Clin Nutr. 2014;99(5):1026–1032. doi: 10.3945/ajcn.113.079624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okereke OI, Rosner BA, Kim DH, Kang JH, Cook NR, Manson JE, Grodstein F. Dietary fat types and 4-year cognitive change in community-dwelling older women. Annals of Neurology. 2012;72(1):124–134. doi: 10.1002/ana.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cordner ZA, Tamashiro KLK. Effects of high-fat diet exposure on learning & memory. Physiol Behav. 2015;152(Part B):363–371. doi: 10.1016/j.physbeh.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman LR, Haley-Zitlin V, Rosenberger DS, Granholm AC. Damaging effects of a high-fat diet to the brain and cognition: A review of proposed mechanisms. Nutr Neurosci. 2014;17(6):241–251. doi: 10.1179/1476830513Y.0000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beilharz JE, Maniam J, Morris MJ. Diet-Induced Cognitive Deficits: The Role of Fat and Sugar, Potential Mechanisms and Nutritional Interventions. Nutrients. 2015;7(8):6719–6738. doi: 10.3390/nu7085307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winocur G, Greenwood CE. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol Aging. 2005;26(Suppl 1):46–49. doi: 10.1016/j.neurobiolaging.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Arnold SE, Lucki I, Brookshire BR, Carlson GC, Browne CA, Kazi H, et al. High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol Dis. 2014;67:79–87. doi: 10.1016/j.nbd.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaczmarczyk MM, Machaj AS, Chiu GS, Lawson MA, Gainey SJ, York JM, et al. Methylphenidate prevents high-fat diet (HFD)-induced learning/memory impairment in juvenile mice. Psychoneuroendocrinology. 2013;38(9):1553–1564. doi: 10.1016/j.psyneuen.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colles SL, Dixon JB, O’Brien PE. Loss of control is central to psychological disturbance associated with binge eating disorder. Obesity. 2008;16(3):608–614. doi: 10.1038/oby.2007.99. [DOI] [PubMed] [Google Scholar]
- 22.Safer DL, Lively TJ, Telch CF, Agras WS. Predictors of relapse following successful dialectical behavior therapy for binge eating disorder. The Int J Eat Disord. 2002;32(2):155–163. doi: 10.1002/eat.10080. [DOI] [PubMed] [Google Scholar]
- 23.Aloi M, Rania M, Caroleo M, Bruni A, Palmieri A, Cauteruccio MA, Segura-García C. Decision making, central coherence and set-shifting: a comparison between Binge Eating Disorder, Anorexia Nervosa and Healthy Controls. BMC Psychiatry. 2015;15:6. doi: 10.1186/s12888-015-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woolley JD, Gorno-Tempini ML, Seeley WW, Rankin K, Lee SS, Matthews BR, et al. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology. 2007;69(14):1424–1433. doi: 10.1212/01.wnl.0000277461.06713.23. [DOI] [PubMed] [Google Scholar]
- 25.Yanovski SZ. Binge eating disorder: Current knowledge and future directions. Obes Res. 1993;1(4):306–324. doi: 10.1002/j.1550-8528.1993.tb00626.x. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell JE, Mussell MP. Comorbidity and binge eating disorder. Addictive behaviors. 1995;20(6):725–732. doi: 10.1016/0306-4603(95)00095-x. [DOI] [PubMed] [Google Scholar]
- 27.Kuehnel RH, Wadden TA. Binge eating disorder, weight cycling, and psychopathology. Int J Eat Disord. 1994;15(4):321–329. doi: 10.1002/eat.2260150403. [DOI] [PubMed] [Google Scholar]
- 28.Succurro E, Segura-Garcia C, Ruffo M, Caroleo M, Rania M, Aloi M, Arturi F. Obese Patients With a Binge Eating Disorder Have an Unfavorable Metabolic and Inflammatory Profile. Medicine. 2015;94(52):e2098. doi: 10.1097/MD.0000000000002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svaldi J, Brand M, Tuschen-Caffier B. Decision-making impairments in women with binge eating disorder. Appetite. 2010;54(1):84–92. doi: 10.1016/j.appet.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Galioto R, Spitznagel MB, Strain G, Devlin M, Cohen R, Paul R, Gunstad J. Cognitive Function in Morbidly Obese Individuals With and Without Binge Eating Disorder. Compr Psychiatry. 2012;53(5):490–495. doi: 10.1016/j.comppsych.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bechara RG, Kelly AM. Exercise improves object recognition memory and induces BDNF expression and cell proliferation in cognitively enriched rats. Behav Brain Res. 2013;245:96–100. doi: 10.1016/j.bbr.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 32.Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20(18):7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizuno M, Yamada K, He J, Nakajima A, Nabeshima T. Involvement of BDNF Receptor TrkB in Spatial Memory Formation. Learn Mem. 2003;10(2):108–115. doi: 10.1101/lm.56003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minichiello L, Korte M, Wolfer D, Kühn R, Unsicker K, Cestari V, et al. Essential role for TrkB receptors in hippocampus -mediated learning. Neuron. 1999;24(2):401–414. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- 35.Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. Journal of Pharmacological Sciences. 2003;91(4):267–270. doi: 10.1254/jphs.91.267. [DOI] [PubMed] [Google Scholar]
- 36.Bimonte-Nelson HA, Hunter CL, Nelson ME, Granholm AC. Frontal cortex BDNF levels correlate with working memory in an animal model of down syndrome. Behavioural Brain Research. 2003;139(1–2):47–57. doi: 10.1016/s0166-4328(02)00082-7. [DOI] [PubMed] [Google Scholar]
- 37.Zorner B, Wolfer DP, Brandis D, Kretz O, Zacher C, Madani R, et al. Forebrain -specific trkB-receptor knockout mice: Behaviorally more hyperactive than “depressive”. Biological Psychiatry. 2003;54(10):972–982. doi: 10.1016/s0006-3223(03)00418-9. [DOI] [PubMed] [Google Scholar]
- 38.Kanoski SE, Meisel RL, Mullins AJ, Davidson TL. The effects of energy -rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behavioural Brain Research. 2007;182(1):57–66. doi: 10.1016/j.bbr.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han M, Zhang J, Yao W, Yang C, Ishima T, Ren Q, Hashimoto K. Intake of 7,8-Dihydroxyflavone During Juvenile and Adolescent Stages Prevents Onset of Psychos is in Adult Offspring After Maternal Immune Activation. Scientific Reports. 2016;6:36087. doi: 10.1038/srep36087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Camer D, Yu Y, Szabo A, Fernandez F, Dinh CH, Huang XF. Bardoxolone methyl prevents high-fat diet-induced alterations in prefrontal cortex signalling molecules involved in recognition memory. Prog Neuropsychopharmacol Biol Psychiatry. 2015;59:68–75. doi: 10.1016/j.pnpbp.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Kim TW, Choi HH, Chung YR. Treadmill exercise alleviates impairment of cognitive function by enhancing hippocampal neuroplasticity in the high-fat diet-induced obese mice. J Exerc Rehabil. 2016;12(3):156–162. doi: 10.12965/jer.1632644.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noble EE, Mavanji V, Little MR, Billington CJ, Kotz CM, Wang C. Exercise reduces diet -induced cognitive decline and increases hippocampal brain-derived neurotrophic factor in CA3 neurons. Neurobiol Learn Mem. 2014;114:40–50. doi: 10.1016/j.nlm.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakata K, Martinowich K, Woo NH, Schloesser RJ, Jimenez DV, Ji Y, Lu B. Role of activity -dependent BDNF expression in hippocampal–prefrontal cortical regulation of behavioral perseverance. Proc Natl Acad Sci USA. 2013;110(37):15103–15108. doi: 10.1073/pnas.1222872110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sigurdsson T, Duvarci S. Hippocampal-Prefrontal Interactions in Cognition, Behavior and Psychiatric Disease. Front Syst Neurosci. 2015;9:190. doi: 10.3389/fnsys.2015.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304(5672):878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- 46.Walker SC, Mikheenko YP, Argyle LD, Robbins TW, Roberts AC. Selective prefrontal serotonin depletion impairs acquisition of a detour-reaching task. Eur J Neurosci. 2006;23(11):3119–3123. doi: 10.1111/j.1460-9568.2006.04826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: Beyond working memory. Psychopharmacology. 2006;188(4):567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- 48.Winter S, Dieckmann M, Schwabe K. Dopamine in the prefrontal cortex regulates rats behavioral flexibility to changing reward value. Behav Brain Res. 2009;198(1):206–213. doi: 10.1016/j.bbr.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 49.den Ouden HE, Daw ND, Fernandez G, Elshout JA, Rijpkema M, Hoogman M, et al. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80(4):1090–1100. doi: 10.1016/j.neuron.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 50.Klanker M, Feenstra M, Denys D. Dopaminergic control of cognitive flexibility in humans and animals. Front Neurosci. 2013;7:201. doi: 10.3389/fnins.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clarke HF, Robbins TW, Roberts AC. Lesions of the Medial Striatum in Monkeys Produce Perseverative Impairments during Reversal Learning Similar to Those Produced by Lesions of the Orbitofrontal Cortex. J Neurosci. 2008;28(43):10972–10982. doi: 10.1523/JNEUROSCI.1521-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge eating: Key synergistic role of past caloric restriction and stress. Physiology & Behavior. 2002;77(1):45–54. doi: 10.1016/s0031-9384(02)00809-0. [DOI] [PubMed] [Google Scholar]
- 53.Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann -Bard P, et al. Object recognition test in mice. Nature Protoc. 2013;8(12):2531–2537. doi: 10.1038/nprot.2013.155. [DOI] [PubMed] [Google Scholar]
- 54.Jablonski SA, Schreiber WB, Westbrook SR, Brennan LE, Stanton ME. Determinants of novel object and location recognition during development. Behav Brain Res. 2013;256:140–150. doi: 10.1016/j.bbr.2013.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sunyer B, Patil S, Hoger H, Lubec G. Barnes maze, a useful task to assess spatial reference memory in mice. Nat Protoc. 2007:390. [Google Scholar]
- 56.Gould TD, Dao DT, Kovacsics CE. The open field test. In: Gould TD, editor. Neuromethods. Vol. 42. New York: Humana Press; 2009. pp. 1–20. Mood and anxiety related phenotypes in mice, Series. [Google Scholar]
- 57.Paxinos G, Watson C. The Rat Brain in Stereotoxic Coordinates. Academic Press Inc; New York: 1982. [Google Scholar]
- 58.Malá H, Andersen LG, Christensen RF, Felbinger A, Hagstrøm J, Meder D, et al. Prefrontal cortex and hippocampus in behavioural flexibility and posttraumatic functional recovery: Reversal learning and set-shifting in rats. Brain Res Bull. 2015;116:34–44. doi: 10.1016/j.brainresbull.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 59.Xue G, Xue F, Droutman V, Lu ZL, Bechara A, Read S. Common Neural Mechanisms Underlying Reversal Learning by Reward and Punishment. PLoS ONE. 2013;8(12):e82169. doi: 10.1371/journal.pone.0082169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112(4):803–814. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 61.Heyward FD, Walton RG, Carle MS, Coleman MA, Garvey WT, Sweatt JD. Adult Mice Maintained on a High-Fat Diet Exhibit Object Location Memory Deficits and Reduced Hippocampal SIRT1 Gene Expression. Neurobiol Learn Mem. 2012;98(1):25–32. doi: 10.1016/j.nlm.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kesby JP, Kim JJ, Scadeng M, Woods G, Kado DM, Olefsky JM, Semenova S. Spatial Cognition in Adult and Aged Mice Exposed to High-Fat Diet. PLoS ONE. 2015;10(10):e0140034. doi: 10.1371/journal.pone.0140034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boitard C, Cavaroc A, Sauvant J, Aubert A, Castanon N, Layé S, et al. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav Immun. 2014;40:9–17. doi: 10.1016/j.bbi.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 64.Dimitriou SG, Rice HB, Corwin RL. Effects of limited access to a fat option on food intake and body composition in female rats. Int J Eat Disord. 2000;28:436–445. doi: 10.1002/1098-108x(200012)28:4<436::aid-eat12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 65.Corwin RL, Wojnicki FH, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65(3):545–553. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- 66.Davis JF, Melhorn SJ, Heiman JU, Tschöp MH, Clegg DJ, Benoit SC. Comparison of hydrogenated vegetable shortening and nutritionally complete high fat diet on limited access -binge behavior in rats. Physiol Behav. 2007;92(5):924–930. doi: 10.1016/j.physbeh.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gareth R, Barker I, Warburton Elizabeth C. When Is the Hippocampus Involved in Recognition Memory? J Neurosci. 2011;31(29):10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Francis BM, Kim J, Barakat ME, Fraenkl S, Yücel YH, Peng S, Mount HTJ. Object recognition memory and BDNF expression are reduced in young TgCRND8 mice. Neurobiol Aging. 2012;33(3):555–563. doi: 10.1016/j.neurobiolaging.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Costa MS, Botton PH, Mioranzza S, Ardais AP, Moreira JD, Souza DO, et al. Caffeine improves adult mice performance in the object recognition task and increases BDNF and TrkB independent on phospho-CREB immunocontent in the hippocampus. Neurochem Int. 2008;53(3–4):89–94. doi: 10.1016/j.neuint.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 70.Hennigan A, Callaghan CK, Kealy J, Rouine J, Kelly ÁM. Deficits in LTP and recognition memory in the genetically hypertensive rat are associated with decreased expression of neurotrophic factors and their receptors in the dentate gyrus. Behav Brain Res. 2009;197(2):371–377. doi: 10.1016/j.bbr.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 71.Lafenêtre P, Leske O, Ma-Högemeie Z, Haghikia A, Bichler Z, Wahle P, Heumann R. Exercise Can Rescue Recognition Memory Impairment in a Model with Reduced Adult Hippocampal Neurogenesis. Front Behav Neurosci. 2009;3:34. doi: 10.3389/neuro.08.034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morici JF, Bekinschtein P, Weisstaub NV. Medial prefrontal cortex role in recognition memory in rodents. Behav Brain Res. 2015;292:241–251. doi: 10.1016/j.bbr.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 73.Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2(1):51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 74.Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112(4):803–814. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 75.Woo J, Shin KO, Park SY, Jang KS, Kang S. Effects of exercise and diet change on cognition function and synaptic plasticity in high fat diet induced obese rats. Lipids Health Dis. 2013;12:144. doi: 10.1186/1476-511X-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sommerfeld MT, Schweigreiter R, Barde YA, Hoppe E. Down-regulation of the neurotrophin receptor TrkB following ligand binding. evidence for an involvement of the proteasome and differential regulation of TrkA and TrkB. J Biol Chem. 2000;275(12):8982–8990. doi: 10.1074/jbc.275.12.8982. [DOI] [PubMed] [Google Scholar]
- 77.Gan L, England E, Yang JY, Toulme N, Ambati S, Hartzell DL, Baile CA. A 72-hour high fat diet increases transcript levels of the neuropeptide galanin in the dorsal hippocampus of the rat. BMC Neuroscience. 2015;16:51. doi: 10.1186/s12868-015-0188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu Y, Wang Q, Huang XF. Energy-restricted pair-feeding normalizes low levels of brain-derived neurotrophic factor/tyrosine kinase B mRNA expression in the hippocampus, but not ventromedial hypothalamic nucleus, in diet-induced obese mice. Neuroscience. 2009;160(2):295–306. doi: 10.1016/j.neuroscience.2009.01.078. [DOI] [PubMed] [Google Scholar]
- 79.Sul JH, Kim H, Huh N, Lee D, Jung MW. Distinct roles of rodent orbitofrontal and medial prefrontal cortex in decision making. Neuron. 2010;66(3):449–460033. doi: 10.1016/j.neuron.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: Evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27(6):555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 81.Seamans JK, Lapish CC, Durstewitz D. Comparing the prefrontal cortex of rats and primates: Insights from electrophysiology. Neurotox Res. 2008;14(2–3):249–262. doi: 10.1007/BF03033814. [DOI] [PubMed] [Google Scholar]
- 82.Jin J, Maren S. Prefrontal-Hippocampal Interactions in Memory and Emotion. Front Syst Neurosci. 2015;9:170. doi: 10.3389/fnsys.2015.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cerf ME, Williams K, van Rooyen J, Esterhuyse AJ, Muller CJ, Louw J. Gestational 30% and 40% fat diets increase brain GLUT2 and neuropeptide Y immunoreactivity in neonatal wistar rats. Int J Dev Neurosci. 2010;28(7):625–630. doi: 10.1016/j.ijdevneu.2010.07.226. [DOI] [PubMed] [Google Scholar]
- 84.Pratchayasakul W, Kerdphoo S, Petsophonsakul P, Pongchaidecha A, Chattipakorn N, Chattipakorn SC. Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sciences. 2011;88(13–14):619–627. doi: 10.1016/j.lfs.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 85.Jurcovicova J. Glucose transport in brain - effect of inflammation. Endocr Regul. 2014;48(1):35–48. doi: 10.4149/endo_2014_01_35. [DOI] [PubMed] [Google Scholar]
- 86.Salkovic-Petrisic M, Tribl F, Schmidt M, Hoyer S, Riederer P. Alzheimer-like changes in protein kinase B and glycogen synthase kinase-3 in rat frontal cortex and hippocampus after damage to the insulin signalling pathway. J Neurochem. 2006;96(4):1005–1015. doi: 10.1111/j.1471-4159.2005.03637.x. [DOI] [PubMed] [Google Scholar]
- 87.Zhao W, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol. 2001;177(1–2):125–134. doi: 10.1016/s0303-7207(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 88.Dou JT, Chen M, Dufour F, Alkon DL, Zhao WQ. Insulin receptor signaling in long-term memory consolidation following spatial learning. Learn Mem. 2005;12(6):646–655. doi: 10.1101/lm.88005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Agrawal R, Gomez-Pinilla F. “Metabolic syndrome” in the brain: deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J Physiol. 2012;590(Pt 10):2485–2499. doi: 10.1113/jphysiol.2012.230078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nistico R, Cavallucci V, Piccinin S, Macri S, Pignatelli M, Mehdawy B, et al. Insulin receptor beta -subunit haploinsufficiency impairs hippocampal late-phase LTP and recognition memory. NeuroMolecular Med. 2012;14(4):262–269. doi: 10.1007/s12017-012-8184-z. [DOI] [PubMed] [Google Scholar]
- 91.Vandal M, White PJ, Tremblay C, St-Amour I, Chevrier G, Emond V, et al. Insulin reverses the high-fat diet-induced increase in brain Aβ and improves memory in an animal model of alzheimer disease. Diabetes. 2014;63(12):4291–4301. doi: 10.2337/db14-0375. [DOI] [PubMed] [Google Scholar]
- 92.Patel SS, Gupta S, Udayabanu M. Urtica dioica modulates hippocampal insulin signaling and recognition memory deficit in streptozotocin induced diabetic mice. Metab Brain Dis. 2016;31(3):601–611. doi: 10.1007/s11011-016-9791-4. [DOI] [PubMed] [Google Scholar]
- 93.Schnedl WJ, Ferber S, Johnson JH, Newgard CB. STZ transport and cytotoxicity specific enhancement in GLUT2-expressing cells. Diabetes. 1994;43(11):1326–1333. doi: 10.2337/diab.43.11.1326. [DOI] [PubMed] [Google Scholar]
- 94.Agrawal R, Tyagi E, Shukla R, Nath C. Insulin receptor signaling in rat hippocampus: A study in STZ (ICV) induced memory deficit model. Eur Neuropsychopharmacol. 2011;21(3):261–273. doi: 10.1016/j.euroneuro.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 95.Agrawal R, Tyagi E, Shukla R, Nath C. A study of brain insulin receptors, AChE activity and oxidative stress in rat model of ICV STZ induced dementia. Neuropharmacology. 2009;56(4):779–787. doi: 10.1016/j.neuropharm.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 96.Lee I, Kesner RP. Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear-conditioning. Hippocampus. 2004;14(3):301–310. doi: 10.1002/hipo.10177. [DOI] [PubMed] [Google Scholar]
- 97.Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25(2):532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb cortex. 2007;17(1):18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- 99.Lapiz-Bluhm MDS, Soto-Pina AE, Hensler JG, Morilak DA. Chronic intermittent cold stress and serotonin depletion induce deficits of reversal learning in an attentional set -shifting test in rats. Psychopharmacology. 2009;202(1–3):329–341. doi: 10.1007/s00213-008-1224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barlow RL, Alsiö J, Jupp B, Rabinovich R, Shrestha S, Roberts AC, Dalley JW. Markers of Serotonergic Function in the Orbitofrontal Cortex and Dorsal Raphé Nucleus Predict Individual Variation in Spatial-Discrimination Serial Reversal Learning. Neuropsychopharmacology. 2015;40(7):1619–1630. doi: 10.1038/npp.2014.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boulougouris V, Robbins TW. Enhancement of spatial reversal learning by 5-HT2C receptor antagonism is neuroanatomically specific. J Neurosci. 2010;30(3):930–938. doi: 10.1523/JNEUROSCI.4312-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alsiö J, Nilsson SRO, Gastambide F, Wang RAH, Dam SA, Mar AC, Robbins TW. The role of 5-HT2C receptors in touchscreen visual reversal learning in the rat: a cross -site study. Psychopharmacology. 2015;232(21–22):4017–4031. doi: 10.1007/s00213-015-3963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nilsson SR, Ripley TL, Somerville EM, Clifton PG. Reduced activity at the 5-HT(2C) receptor enhances reversal learning by decreasing the influence of previously non -rewarded associations. Psychopharmacology. 2012;224(2):241–254. doi: 10.1007/s00213-012-2746-5. [DOI] [PubMed] [Google Scholar]
- 104.Nilsson SRO, Somerville EM, Clifton PG. Dissociable Effects of 5-HT2C Receptor Antagonism and Genetic Inactivation on Perseverance and Learned Non -Reward in an Egocentric Spatial Reversal Task. PLoS ONE. 2013;8(10):e77762. doi: 10.1371/journal.pone.0077762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu P, He Y, Cao X, Valencia-Torres L, Yan X, Saito K, et al. Activation of serotonin 2C receptors in dopamine neurons inhibits binge-like eating in mice. Biol Psychiatry. doi: 10.1016/j.biopsych.2016.06.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Calaminus C, Hauber W. Guidance of instrumental behavior under reversal conditions requires dopamine D1 and D2 receptor activation in the orbitofrontal cortex. Neuroscience. 2008;154(4):1195–1204. doi: 10.1016/j.neuroscience.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 107.Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18(4):1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Puig MV, Miller EK. The Role of Prefrontal Dopamine D1 Receptors in the Neural Mechanisms of Associative Learning. Neuron. 2012;74(5):874–86. doi: 10.1016/j.neuron.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed -response task. J Neurophysiol. 1994;71:515–28. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- 110.Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385(6617):634–636. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- 111.Takahashi H, Kato M, Takano H, Arakawa R, Okumura M, Otsuka T, et al. Differential contributions of prefrontal and hippocampal dopamine D(1) and D(2) receptors in human cognitive functions. J Neurosci. 2008;28(46):12032–12038. doi: 10.1523/JNEUROSCI.3446-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nair SG, Navarre BM, Cifani C, Pickens CL, Bossert JM, Shaham Y. Role of Dorsal Medial Prefrontal Cortex Dopamine D1-Family Receptors in Relapse to High-Fat Food Seeking Induced by the Anxiogenic Drug Yohimbine. Neuropsychopharmacology. 2011;36(2):497–510. doi: 10.1038/npp.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Land BB, Narayanan NS, Liu RJ, Gianessi CA, Brayton CE, Grimaldi D, Dileone RJ. Medial prefrontal D1 dopamine neurons control food intake. Nat Neurosci. 2014;17(2):248–253. doi: 10.1038/nn.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Touzani K, Bodnar RJ, Sclafani A. Acquisition of glucose-conditioned flavor preference requires the activation of dopamine D1-like receptors within the medial prefrontal cortex in rats. Neurobiol Learn Mem. 2010;94(2):214–219. doi: 10.1016/j.nlm.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Langley K, Marshall L, van den Bree M, Thomas H, Owen M, O’Donovan M, et al. Association of the dopamine D4 receptor gene 7-repeat allele with neuropsychological test performance of children with ADHD. Am J Psychiatry. 2004;161(1):133–138. doi: 10.1176/appi.ajp.161.1.133. [DOI] [PubMed] [Google Scholar]
- 116.Villalba K, Devieux JG, Rosenberg R, Cadet JL. DRD2 and DRD4 genes related to cognitive deficits in HIV-infected adults who abuse alcohol. Behav Brain Funct. 2015;11:25. doi: 10.1186/s12993-015-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31(2):297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- 118.Sobik L, Hutchison K, Craighead L. Cue-elicited craving for food: A fresh approach to the study of binge eating. Appetite. 2005;44(3):253–261. doi: 10.1016/j.appet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 119.Silveira PP, Portella AK, Kennedy JL, Gaudreau H, Davis C, Steiner M, Levitan RD. Association between the seven-repeat allele of the dopamine-4 receptor gene (DRD4) and spontaneous food intake in pre-school children. Appetite. 2014;73:15–22. doi: 10.1016/j.appet.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Levitan RD, Masellis M, Basile VS, Lam RW, Kaplan AS, Davis C, et al. The dopamine-4 receptor gene associated with binge eating and weight gain in women with seasonal affective disorder: An evolutionary perspective. Biol Psychiatry. 2004;56(9):665–669. doi: 10.1016/j.biopsych.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 121.Monteleone P, Tortorella A, Castaldo E, Maj M. Association of a functional serotonin transporter gene polymorphism with binge eating disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(1):7–9. doi: 10.1002/ajmg.b.30232. [DOI] [PubMed] [Google Scholar]
- 122.Davis C, Levitan RD, Kaplan AS, Carter J, Reid C, Curtis C, et al. Reward sensitivity and the D2 dopamine receptor gene: A case-control study of binge eating disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(3):620–628. doi: 10.1016/j.pnpbp.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 123.Davis C, Levitan RD, Yilmaz Z, Kaplan AS, Carter JC, Kennedy JL. Binge eating disorder and the dopamine D2 receptor: Genotypes and sub- phenotypes. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38(2):328–335. doi: 10.1016/j.pnpbp.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 124.Baik JH. Dopamine signaling in food addiction: role of dopamine D2 receptors. BMB Reports. 2013;46(11):519–526. doi: 10.5483/BMBRep.2013.46.11.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bello NT, Hajnal A. Dopamine and binge eating behaviors. Pharmacol Biochem Behav. 2010;97(1):25–33. doi: 10.1016/j.pbb.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kessler RM, Hutson PH, Herman BK, Potenza MN. The neurobiological basis of binge -eating disorder. Neurosci Biobehav Rev. 2016;63:223–238. doi: 10.1016/j.neubiorev.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 127.Halpern CH, Tekriwal A, Santollo J, Keating JG, Wolf JA, Daniels D, et al. Amelioration of binge eating by nucleus accumbens shell deep brain stimulation in mice involves D2 receptor modulation. J Neurosci. 2013;33(17):7122–7129. doi: 10.1523/JNEUROSCI.3237-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Corwin RL, Wojnicki FH, Zimmer DJ, Babbs RK, McGrath LE, Olivos DR, et al. Binge -type eating disrupts dopaminergic and GABAergic signaling in the prefrontal cortex and ventral tegmental area. Obesity (Silver Spring, Md) 2016;24(10):2118–2125. doi: 10.1002/oby.21626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Oswald KD, Murdaugh DL, King VL, Boggiano MM. Motivation for palatable food despite consequences in an animal model of binge eating. Int J Eat Disord. 2011;44(3):203–211. doi: 10.1002/eat.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Calvez J, de Avila C, Guevremont G, Timofeeva E. Stress differentially regulates brain expression of corticotropin-releasing factor in binge-like eating prone and resistant female rats. Appetite. 2016;107:585–595. doi: 10.1016/j.appet.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 131.Sinclair EB, Culbert KM, Gradl DR, Richardson KA, Klump KL, Sisk CL. Differential mesocorticolimbic responses to palatable food in binge eating prone and binge eating resistant female rats. Physiology & Behavior. 2015;152(Pt A):249–256. doi: 10.1016/j.physbeh.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ghahremani DG, Monterosso J, Jentsch JD, Bilder RM, Poldrack RA. Neural Components Underlying Behavioral Flexibility in Human Reversal Learning. Cerebral Cortex. 2010;20(8):1843–1852. doi: 10.1093/cercor/bhp247. [DOI] [PMC free article] [PubMed] [Google Scholar]