Abstract
AIM
To investigate markers for high-grade dysplasia for the optimal timing of liver transplantation in patients with primary sclerosing cholangitis (PSC).
METHODS
Earlier data support a dysplasia-carcinoma sequence, even low- to high-grade dysplasia, in PSC-associated cholangiocarcinoma (CCA). Surveillance using endoscopic retrograde cholangiography (ERC) and brush cytology aims to detect cases of biliary dysplasia, and liver transplantation is an option in cases with suspicion of malignancy in brushing. This study investigated markers to identify patients with high-grade biliary dysplasia for optimal timing in early liver transplantation. Patients undergoing surveillance using ERC and brush cytology during 2008-2014 and who were diagnosed with biliary dysplasia in explanted liver or CCA until February 2016 were included in the study. Demographic data, cholangiography findings, laboratory values, cytological morphology and DNA ploidy were analysed.
RESULTS
Thirty PSC patients had biliary neoplasia in the explanted liver during the study period. Sixteen of these patients had low-grade dysplasia, 10 patients had high-grade dysplasia, and 4 patients had CCA. Fifteen PSC patients diagnosed with CCA were not transplanted. Patients with low-grade dysplasia were younger. Alkaline phosphatase or carcinoembryonic antigen values did not differ between groups during surveillance, but carbohydrate antigen 19-9 was higher in CCA patients. No difference in PSC duration, ERC scores, suspicious cytology, or ploidy analysis was found between groups. No difference was observed between fibrosis stage in explanted livers. Low- and high-grade dysplasia could not be differentiated before liver transplantation based on liver enzymes, tumour markers, ERC scores, brush cytology or DNA ploidy.
CONCLUSION
Repeated suspicion of neoplasia in brush cytology should be an indication for evaluations of liver transplantation prior to the development of CCA.
Keywords: Endoscopic retrograde cholangiography, Brush cytology, Cholangiocarcinoma, Biliary dysplasia
Core tip: We investigated markers of high-grade dysplasia for the optimal timing of early liver transplantation (LT) in patients with primary sclerosing cholangitis (PSC). PSC patients in our unit undergo surveillance with endoscopic retrograde cholangiography (ERC) and brush cytology (BC) to identify evidence of dysplasia before progression to cholangiocarcinoma. Carbohydrate antigen 19-9 was higher in patients with cholangiocarcinoma, but no other differences between laboratory values, ERC scores, BC or ploidy analysis between the low-grade, high-grade or CCA groups were observed. Repeated suspicion of neoplasia in BC should be an indication for the evaluation for LT prior to the development of cholangiocarcinoma.
INTRODUCTION
Primary sclerosing cholangitis (PSC) is a chronic cholestatic disease that often presents in conjunction with inflammatory bowel disease (IBD). PSC leads to bile duct strictures and liver fibrosis. PSC also markedly increases the risk for cholangiocarcinoma (CCA), with a lifetime risk of 5%-10%[1,2]. Bile duct dysplasia is a precursor for CCA[3] in PSC. Brush cytology (BC) and imaging are used for the detection of malignancy in PSC.
The incidence of PSC is high in Nordic countries. The primary diagnosis of PSC in our hospital uses endoscopic retrograde cholangiography (ERC) with BC in all patients for individual risk stratification to estimate disease progression and risk for dysplasia[4]. PSC patients also undergo a regular surveillance programme using ERC and BC according to a previously described protocol[5], which demonstrated that the frequency of ERCs depends on disease severity in ERC and earlier results in BC and ploidy analysis. The indications for liver transplantation (LT) in PSC patients include end-stage liver disease or symptoms of the disease (e.g., recurrent cholangitis). CCA is also an indication for LT in combination with chemoradiation in select centres[6]. CCA is generally a contraindication for LT in our centre. However, LT may be considered to prevent progression to CCA[4,7] in cases with biliary dysplasia.
This study evaluated risk factors in PSC patients with histologically confirmed biliary dysplasia or CCA.
MATERIALS AND METHODS
Patients
This study included PSC patients with verified biliary dysplasia or CCA. There were 588 PSC patients under surveillance at the Helsinki University Hospital during 2008-2014. PSC patients in the study population who were diagnosed with CCA or biliary dysplasia until February 2016 were included in this study. All explanted PSC livers were re-evaluated, and cases with biliary dysplasia or CCA (n = 30) were included. All PSC patients who were diagnosed with CCA during the aforementioned period who did not undergo liver transplantation were included (n = 15). At least one ERC was performed for each patient during the study period. Low-grade dysplasia (LGD) of the bile ducts was observed in 16 transplanted patients, and high-grade dysplasia (HGD), including carcinoma in situ, was observed in 10 patients with LT. CCA was observed in four patients with LT and 15 patients without LT. Suspicion of biliary dysplasia was considered an important decision criterion for evaluation for liver transplantation in 7/10 patients with HGD, 12/16 patients with LGD, and 4/4 patients with CCA. Thirty-two patients (71%) were male, and 13 patients were female.
Laboratory results
Laboratory parameters [carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA), alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, bilirubin, and international normalised ratio (INR)] were collected one day before the latest ERC.
Imaging studies
Images from the latest ERC were scored according to a modified Amsterdam score[4]. An MRI and/or CT were performed prior to LT.
Brush cytology and ploidy analysis
All brush samples and DNA flow cytometry specimens until LT or detection of CCA were included. Brush samples were re-evaluated and graded as benign (including atypical due to inflammation or regeneration), suspicious for neoplasia, or malignant (Figure 1). Cytocentrifuge slides and slides from cellblocks were evaluated when cellblocks were available. Ploidy analyses were performed as previously described[7]. Briefly, a separate sample for DNA flow cytometry was obtained before the BC sample and added to RPMI 1640 medium (Gibco/Thermo Scientific, MA, United States) supplemented with 1% L-glutamine, 1% penicillin-streptomycin, 0.5% heparin and 0.5% human serum albumin. Cells were centrifuged, washed, treated with RNAse (Sigma Chemical Company, MO, USA) and stained with ethidium bromide (Sigma Chemical Company, MO, United States) in Tris-EDTA buffer. HL-60 cells were used as a normal control, and goose and salmon trout red blood cells were used as internal controls. DNA flow cytometry was performed using a FACSCalibur flow cytometer (BD Biosciences, Oxford, United Kingdom). The results were analysed using ModFit LT software (Becton Dickinson Immunocytometry Systems, Becton, Dickinson and Company, Franklin Lakes, NJ, United States), and a DNA index over 1.1 was considered aneuploidy.
Figure 1.
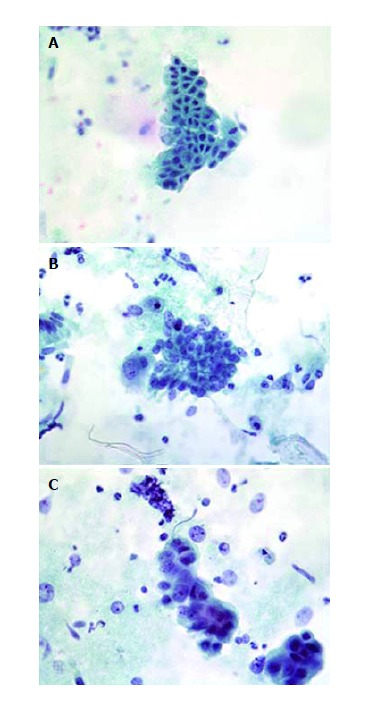
Representative examples of bile duct brushings. A: Benign brush cytology with inflammatory atypia; B: Brush cytology with suspicion of malignancy; C: Malignant brush cytology. Patient was diagnosed with cholangiocarcinoma.
Histology
Two pathologists, who were blinded to the BC or original interpretation, re-evaluated explanted livers and bile ducts or other histological samples (Table 1, Figure 2). Biliary dysplasia was graded as high-grade or low-grade. Patients were divided into groups (LGD, HGD, CCA) using the worst histological finding.
Table 1.
Histological findings in 30 explanted livers of primary sclerosing cholangitis patients with biliary low-grade dysplasia or high-grade dysplasia/cholangiocarcinoma
| LGD | HGD + CCA2 | P value | |
| n | 16 | 14 | |
| Early LT1, n | 12 | 11 | 1.000 |
| Histology intrahepatic | |||
| Liver weight, g (IQR) | 1380 (1190-1717) | 1408 (1262-1487) | 0.910 |
| Fibrosis stage 1-4 (IQR) | 3.5 (3.0-4.0) | 3.0 (2.0-4.0) | 0.473 |
| Stage 1-2, n | 3 | 4 | 0.675 |
| Stage 3-4, n | 13 | 10 | 0.675 |
| Histology extrahepatic, n | |||
| Purulent cholangitis | 12 | 6 | 0.142 |
| Ulceration | 11 | 6 | 0.224 |
| Intestinal metaplasia | 2 | 1 | 0.822 |
| Squamous metaplasia | 1 | 0 | 0.790 |
In early LTs, suspicion of biliary neoplasia was an important decision criterion for LT;
CCA four patients; HGD ten patients. Values are median values unless otherwise indicated (IQR). P values: Mann-Whitney U-test for continuous variables, Fisher's exact test for dichotomous variables. CCA: Cholangiocarcinoma; HGD: High-grade dysplasia; IQR: Interquartile range; LGD: Low-grade dysplasia; LT: Liver transplantation.
Figure 2.
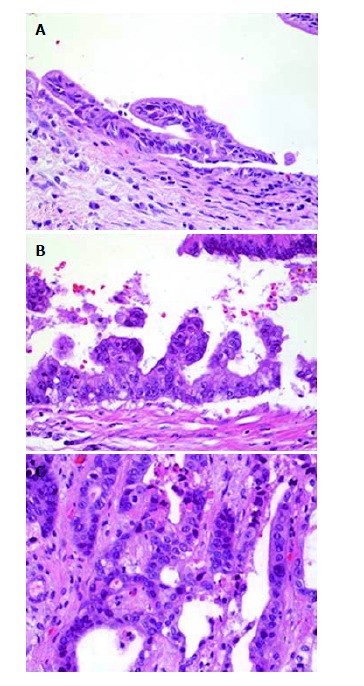
Histology from explanted livers diagnosed with biliary neoplasia. A: Bile duct from explanted liver with low-grade dysplasia; B: Bile duct from explanted liver with high-grade dysplasia; C: Cholangiocarcinoma from explanted liver.
Statistical analysis
Statistical analyses were performed using the IBM SPSS statistical software package version 24 (IBM, New York, NY, United States). The Jonckheere-Terpstra and Mann-Whitney U-tests were used to evaluate differences in continuous variables between groups. Fisher’s exact test was used for dichotomous variables. Bonferroni correction was used in pairwise tests, and adjusted values are presented.
Ethics
The local ethics committee approved the study protocol.
RESULTS
Demographics
There was no statistically significant gender difference between groups (Table 2), but all groups included a majority of male patients (32 males and 13 females in total). Patients with LGD were significantly younger than other patients (P = 0.003). There was no difference in PSC duration before dysplasia or CCA diagnosis. Thirty (67%) patients had IBD. Twenty-two of these 30 patients had ulcerative colitis, seven patients had Crohn’s disease, and one patient had indeterminate colitis. The presence of IBD was not significantly different between groups.
Table 2.
Demographics, laboratory results and endoscopic retrograde cholangiography findings in patients with primary sclerosing cholangitis and low-grade dysplasia, high-grade dysplasia and cholangiocarcinoma
| LGD | HGD | CCA | P value | P value (LGD vs HGD) | P value (HGD vs CCA) | P value (LGD vs CCA) | ||
| n | 16 | 10 | 19 | . | . | . | . | |
| Male/female | 10/6 | 8/2 | 14/5 | 0.701 | . | . | . | |
| Age, yr | 32 (26-48) | 48 (35-59) | 55 (37-60) | 0.003 | 0.056 | 0.896 | 0.004 | |
| PSC duration, yr | 4.1 (2.2-8.3) | 4.4 (2.3-7.9) | 4.0 (0.3-17.1) | 0.948 | . | . | . | |
| Symptoms, n | 12 | 8 | 16 | 0.890 | . | . | . | |
| Icterus, n | 2 | 4 | 12 | 0.014 | 0.483 | 1.000 | 0.024 | |
| Pruritus, n | 9 | 3 | 8 | 1.000 | . | . | . | |
| Cholangitis/fever, n | 4 | 2 | 8 | 0.415 | . | . | . | |
| Fatigue, n | 7 | 3 | 4 | 1.000 | . | . | . | |
| Pain, n | 5 | 3 | 4 | 0.863 | . | . | . | |
| MELD score | 7.6 (7.4-11.0) | 7.6 (6.4-8.7) | 7.7 (6.4-10.4) | 0.883 | . | . | . | |
| IBD, n | 10 | 5 | 15 | 0.307 | . | . | . | |
| ALP, U/L | 175 (90-324) | 89 (69-176) | 237 (158-396) | 0.214 | . | . | . | |
| AST, U/L | 45 (32-118) | 51 (34-64) | 59 (38-94) | 0.618 | . | . | . | |
| ALT, U/L | 61 (31-113) | 56 (30-102) | 55 (46-112) | 0.717 | . | . | . | |
| Bilirubin, μmol/L | 23 (13-32) | 11 (8-22) | 25 (15-47) | 0.354 | . | . | . | |
| CA19-9, KU/L | 10 (3-22) | 10 (5-16) | 120 (14-415) | 0.002 | 1.000 | 0.006 | 0.006 | |
| CEA, μg/L | 1.3 (1.0-2.7) | 2.4 (1.4-2.9) | 2.1 (1.4-5.8) | 0.081 | . | . | . | |
| mERC score | ||||||||
| Intrahepatic (IQR) | 6 (4-6) | 6 (5-7) | 6 (6-6) | 0.440 | . | . | . | |
| Extrahepatic (IQR) | 3 (2-6) | 4 (2-6) | 4 (2-5) | 0.613 | . | . | . | |
| Total score (IQR) | 9 (7-11) | 11 (8-12) | 9 (8-11) | 0.523 | . | . | . | |
Values are presented as median (IQR) unless otherwise indicated. Jonckeere-Terpstra test was used to analyse differences of continuous variables, and Fisher’s exact test was used for dichotomous variables. Significance values were adjusted using the Bonferroni correction for multiple tests. CCA: Cholangiocarcinoma; HGD: High-grade dysplasia; IQR: Interquartile rate; LGD: Low-grade dysplasia; mERC score: Modified Amsterdam ERC score; MELD score: Model for end-stage liver disease score; IBD: Inflammatory bowel disease; ALP: Alkaline phosphatase; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; PSC: Primary sclerosing cholangitis; CA19-9: Carbohydrate antigen 19-9; CEA: Carcinoembryonic antigen.
Laboratory values
ALP, bilirubin, AST and ALT values were not significantly different between the three groups at the latest ERC before LT or detection of CCA. CA19-9 was higher in CCA patients (P = 0.002). CEA values did not significantly differ between groups (P = 0.081).
ERC findings
There were no significant differences in ERC scores between patient groups (Table 2). Intrahepatic disease without extrahepatic changes was observed in ERC in only two patients, and both of these patients exhibited LGD. Only mild extrahepatic ERC changes (modified Amsterdam ERC score 1-2) were observed in 11 patients, and five of these patients exhibited CCA. Abundant extrahepatic ERC changes (score ≥ 3, corresponding to dominant stricture) were observed in 32 patients. Fourteen (14/32) of these patients were diagnosed with CCA.
Brush cytology
All patients underwent ERC with BC (1-9 BC/patient, median 3 BCs). The number of available BCs did not significantly differ between groups (Table 3). Suspicious BC was observed in 11/16 patients with LGD, 8/10 patients with HGD and 4/4 patients with CCA in liver transplanted patients. Eleven of 15 patients without LT had suspicious or malignant BC (malignant 2 patients, suspicious 9 patients). Suspicious or malignant BC was observed in 34/45 patients (76%), with no difference between the neoplastic groups.
Table 3.
Brush cytology and DNA flow cytometry in patients with low-grade dysplasia, high-grade dysplasia or cholangiocarcinoma
| LGD | HGD | CCA | P value | |
| Patients, n | 16 | 10 | 19 | |
| Liver transplanted, n | 16 | 10 | 4 | |
| BCs, n (IQR) | 4.0 (2.3-5.0) | 4.5 (1.8-5.3) | 2.5 (1.8-5.3) | 0.219 |
| BC suspicious/malignant | 11 | 8 | 15 | 0.746 |
| Flow cytometry, n (IQR) | 3.0 (2.3-4.0) | 3.0 (1.8-5.0) | 2.0 (1.0-4.3) | 0.195 |
| DNA-index (highest, IQR) | 1.0 (1.0-1.2) | 1.2 (1.0-1.8) | 1.0 (1.0-1.2) | 0.745 |
| Aneuploid | 6 | 5 | 7 | 0.795 |
Numbers are median (IQR) or n. P values: Jonckeere-Terpstra test for continuous variables, and Fisher's exact test for dichotomous variables. BC: Brush cytology; CCA: Cholangiocarcinoma; HGD: High-grade dysplasia; IQR: Interquartile rate; LGD: Low-grade dysplasia.
Ploidy analyses
Ploidy analysis of a brush sample was available for all patients (1-7/patient, median 3 DNA flow cytometry analyses). Aneuploidy was observed in 18/45 patients (40%), and there were no significant differences between groups. DNA indices were between 1 and 2.41 and did not significantly vary between the three groups. The median DNA index in HGD was 1.2.
Symptoms
Eighty percent of patients were symptomatic, with no difference between groups (Table 2). However, CCA patients were more often icteric than patients with LGD (P = 0.014).
Explanted livers
The median weight of explanted livers was 1399 g (range, 768-2195 g, IQR 1244-1526 g). The median fibrosis stage was 3 (range 1-4[8]), with no difference between the groups. Only mild fibrosis (stage 1-2) was observed in 3/16 of the patients with LGD, and 4/14 patients with HGD or CCA. The remaining patients exhibited a fibrosis stage of 3-4. Histologically typical PSC morphology was observed in all liver samples, including onion-skin fibrosis around the bile ducts and cholangitis. No dysplasia in peripheric bile ducts was observed. All patients with HGD also exhibited LGD of the bile ducts. All CCA patients also exhibited HGD of the bile ducts. Hepatocellular carcinoma was observed in two patients, who also exhibited LGD.
Cholangiocarcinoma
Nineteen patients exhibited CCA. Four CCAs were diagnosed in explanted livers after LT. Two CCAs were diagnosed at laparotomy during a planned LT. Two CCAs were detected at autopsy. A histological sample from the main tumour or metastasis was obtained during surgery or core-needle biopsy in eight patients. Two CCAs were verified with imaging and (malignant) BC, and one CCA was verified using imaging only. CCAs were diagnosed a median of 4.0 years after PSC diagnosis (range, 0-19 years). Six CCAs were detected less than one year after PSC diagnosis, two of these CCAs were detected at a diagnostic ERC. The localisation of CCA was perihilar or extrahepatic in 14 cases and intrahepatic in four cases. The origin of the tumour could not be determined in one patient with peritoneal carcinosis. The median time from the latest BC until CCA diagnosis was 53 d. BC was malignant in two CCA patients, and it remained suspicious in 13 patients. BCs were benign in four CCA patients. BC was suspicious in two of the patients with intrahepatic CCA and benign in two of these patients.
DISCUSSION
Forty-two patients with histologically confirmed biliary neoplasia were found during ERC surveillance: 16 patients with LGD, 10 patients with HGD and 16 patients with CCA. An additional 3 CCAs were detected without histological confirmation. Patients with LGD were younger. AST, ALT or CEA values did not differ between the groups. CA19-9 was significantly elevated only in patients with CCA. No difference in PSC duration, ERC changes, suspicious BC, ploidy analysis, presence of IBD or histological fibrosis was found between the three groups. It was impossible to differentiate LGD from HGD based on liver enzymes, tumour markers, or BC. Evaluations for LT should already be performed when BC is repeatedly suspicious.
The number of cytological samples suspicious for neoplasia was equal in all three neoplastic groups. The sensitivity of BC to detect biliary neoplasia was quite low (43%) in earlier studies[9]. The sensitivity to detect CCA with BC (neoplasia suspicion or malignant BC) in our previous study was up to 71% in a large, unselected patient population[5]. This high accuracy was thought to be a result of the sampling method[4] and the protocol used in our hospital. Two experienced gastroenterologists perform the ERCs, and the cellular yield is generally very high. A dedicated team of cytopathologists also perform the cytological interpretation. However, biliary neoplasia is not always found in the explanted liver, even with suspicious BC. Twenty-one patients exhibited benign biliary histology in the explanted liver in our earlier study, and four of these patients had suspicious BC[5]. It would be clinically relevant to differentiate the HGD group from the other two neoplastic groups before LT because the timing of an early LT would likely exhibit the most benefit. However, we report that cytology alone did not separate three groups with neoplastic biliary changes. Neoplastic biliary changes were also not detected in liver biopsy because biliary dysplasia was not observed in the peripheral bile ducts. However, HGD may be diagnosed with BC in select cases[10].
We also used DNA flow cytometry in addition to BC in selected cases (e.g., advanced ERC changes or previously suspicious BC). Based on earlier studies[7,11], aneuploidy was not more commonly observed in CCAs. This result may reflect the fact that cancer cells in CCA may not be reachable for brushing because of their growth pattern or desmoplasia. The median DNA index was higher in patients with HGD, but this difference was likely the result of the low number of cases, and statistical significance was not observed. The demand for more cases to reach significance is also based on the fact that only a portion of the dysplasias, or even CCAs, presented with aneuploid DNA. Better markers for HGD than aneuploidy are needed. The combination of genetic abnormalities with BC may help distinguish benign and malignant bile duct strictures[12,13]. However, there are no specific markers to detect HGD without histology.
CA19-9 levels often rise with CCA, but it is not specific for CCA[14,15]. CA19-9 may be markedly elevated in up to 32% of PSC patients in the absence of CCA[15]. CA19-9 was elevated mostly in CCA patients in the present study, which supports the use of CA19-9 as a tumour marker for CCA. However, it is not suitable for biliary dysplasia screening. Several surveillance algorithms of PSC recommend the use of CA19-9[2,16,17]. Elevated CEA values were also commonly observed in CCA patients but only immediately prior to the CCA diagnosis (data not shown). These findings suggest that CA19-9 and CEA are late markers, and they cannot be used for the detection of premalignant lesions. The elevation of CA19-9 generally indicates that the dysplasia surveillance programme has actually failed to find cases with dysplasia. CA19-9 is not expressed in 5%-10% of the population who have the Lewis a- b-genotype[18], and the fucosyltransferase genotype influences CEA levels[19].
ALP is generally elevated at PSC diagnosis, and normalisation of ALP is associated with a better prognosis[20-24]. ALP levels are not helpful in differentiating HGD from LGD, which was observed in this study. CCA patients were not younger than other patients, and there was up to 19 years of time between PSC and CCA diagnosis. One-third (6/19) of CCAs were detected within one year of PSC diagnosis in the present study. Ten of the 19 CCA patients in this study were diagnosed with PSC prior to the beginning of this study period (2008). Therefore, the effect of this surveillance programme could not be evaluated for the entire PSC population. Patients with LGD were significantly younger than patients with CCA or HGD. Retrospectively, some of these patients could have waited for the LT if there were no symptoms, but this study demonstrated that it is generally impossible to diagnose HGD prior to LT.
ERC findings were similar in all patient groups, which is likely due to several end-stage PSCs with cirrhosis in the LGD group. ERC with BC is routinely performed for the screening and detecting of biliary dysplasia before LT in our hospital, even LT due to end-stage cirrhosis or symptoms. Our results demonstrate that the ERC score is not helpful for the differentiation of these three groups. Our strategy significantly differs from the practice in many US transplantation centres, where pre-liver transplant screening for CCA in patients with PSC is routinely performed in only 30% of centres[25].
LGD was observed in conjunction with HGD in all cases, and HGD of the bile ducts was observed in all four CCA patients who received LT. These CCAs were discovered after LT in the explanted liver. These findings support the dysplasia-CCA sequence as described by Lewis et al[3]. This theory of a dysplasia-cancer sequence, which was indirectly demonstrated in this study, justifies the dysplasia surveillance programme of PSC. However, further tools are needed to differentiate premalignant cases.
All HGDs and 14/19 CCAs were located in perihilar or extrahepatic bile ducts, which supports earlier studies that demonstrated that most CCAs were located in perihilar or distal bile ducts[26]. CCA or dysplasia in these locations is generally reachable using BC, and surveillance with BC may be justified. However, we observed 19 CCAs in these patients compared to 26 with premalignant lesions. One CCA was diagnosed in a patient who was dropped from the surveillance due to advanced age. Two CCAs were detected during the planned LT, and the LT was cancelled. Four CCAs were detected after LT, CCA was diagnosed in the explanted liver, and two of these patients had clean perioperative pathology samples. Two CCA patients were not under surveillance for years, but they returned with symptoms. The surveillance protocol was not optimal for all patients, and they entered the surveillance programme gradually. If HGD is considered the highest risk for CCA and patients diagnosed with CCA during the first year after PSC diagnosis are excluded, then at least 43% of CCAs were possibly prevented using early LT.
Early CCA is an indication for LT combined with chemo- and radiotherapy in select centres[6]. Surveillance of biliary dysplasia using BC provides another option, with LT being performed at an earlier phase to avoid oncological treatment. The indications of LT in PSC patients in most centres include end-stage liver disease or symptomatic disease (e.g., recurrent cholangitis). CCA is generally a contraindication for LT in our centre. LTs due to PSC have been performed since 1984 in our unit, and dysplasia in ERC has been considered a partial indication for LT in PSC patients since 2001[27].
The limitation of this study is the small number of patients, which restrains the findings of this study, and some significant differences may be missed. Some patients who subsequently developed CCA did not follow the surveillance protocol until they became symptomatic. Our hospital is the only hospital in our country that performs organ transplantations. Therefore, no liver transplantations were missed during surveillance.
In conclusion, there is currently no reliable method to differentiate LGD from HGD prior to LT. CA19-9 rises with CCA but not in biliary dysplasia. Repeated suspicious BC, which suggests dysplasia and a high risk for future CCA, may be an indication for LT.
COMMENTS
Background
Primary sclerosing cholangitis (PSC) patients exhibit an elevated risk for cholangiocarcinoma (CCA), with a lifetime risk of 5%-10%. Biliary dysplasia is a precursor of CCA, and liver transplantation (LT) may be considered to prevent progression to CCA in cases with biliary dysplasia. A more common indication for LT is end-stage liver disease or disease symptoms. CCA develops via a low-grade dysplasia (LGD) to high-grade dysplasia (HGD) to CCA sequence. PSC patients in our centre undergo regular surveillance with endoscopic retrograde cholangiography (ERC) and brush cytology (BC) to detect biliary dysplasia, and patients with repeated suspicious findings are referred for evaluation of LT. The diagnosis of HGD prior to consideration for LT would be beneficial because of the high risk of progression to CCA. This study compared ERC, BC, ploidy analysis, and laboratory values between patients diagnosed with CCA, HGD or LGD.
Research frontiers
Biliary dysplasia is an established indication for LT in few transplantation centres. BC is used to detect biliary neoplasia, but HGD may be verified from brushing only in selected cases.
Innovations and breakthroughs
This study found that differentiating HGD and LGD prior to histological verification in the explanted liver is not practically possible. BC, laboratory values and ERC scores did not differ between patients with LGD, HGD and CCA, with the exception of CA19-9, which was elevated in CCA patients.
Applications
This study suggests that the patient should be referred for evaluation of LT when biliary neoplasia is repeatedly suspected in BC of a PSC patient to prevent progression to CCA.
Terminology
ERC - an endoscopic procedure that enables an evaluation of bile ducts by injecting contrast medium to the bile ducts for radiographic visualisation.
Peer-review
The paper is novel, and an interesting study evaluating clinical and laboratory characteristics of PSC patients in order to identify subjects with biliary dysplasia that could benefit from early liver transplantation. The figures are good and eye-catching.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Finland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): b
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The Ethical committee of Helsinki University Hospital approved this study (Dnro 278/13/03/01/09).
Conflict-of-interest statement: We have no financial relationships to disclose.
Data sharing statement: No additional data are available.
Peer-review started: February 22, 2017
First decision: March 16, 2017
Article in press: June 19, 2017
P- Reviewer: Geramizadeh B, Maroni L S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
Contributor Information
Sonja Boyd, Department of Pathology, University of Helsinki and Helsinki University Hospital, HUSLAB, 00029 Helsinki, Finland. sonja.boyd@hus.fi.
Marko Vannas, Transplantation and Liver Surgery Clinic, University of Helsinki and Helsinki University Hospital, 00029 Helsinki, Finland.
Kalle Jokelainen, Clinic of Gastroenterology, University of Helsinki and Helsinki University Hospital, 00029 Helsinki, Finland.
Helena Isoniemi, Transplantation and Liver Surgery Clinic, University of Helsinki and Helsinki University Hospital, 00029 Helsinki, Finland.
Heikki Mäkisalo, Transplantation and Liver Surgery Clinic, University of Helsinki and Helsinki University Hospital, 00029 Helsinki, Finland.
Martti A Färkkilä, Clinic of Gastroenterology, University of Helsinki and Helsinki University Hospital, 00029 Helsinki, Finland.
Johanna Arola, Department of Pathology, University of Helsinki and Helsinki University Hospital, HUSLAB, 00029 Helsinki, Finland.
References
- 1.Claessen MM, Vleggaar FP, Tytgat KM, Siersema PD, van Buuren HR. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol. 2009;50:158–164. doi: 10.1016/j.jhep.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Razumilava N, Gores GJ, Lindor KD. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology. 2011;54:1842–1852. doi: 10.1002/hep.24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis JT, Talwalkar JA, Rosen CB, Smyrk TC, Abraham SC. Precancerous bile duct pathology in end-stage primary sclerosing cholangitis, with and without cholangiocarcinoma. Am J Surg Pathol. 2010;34:27–34. doi: 10.1097/PAS.0b013e3181bc96f9. [DOI] [PubMed] [Google Scholar]
- 4.Boyd S, Tenca A, Jokelainen K, Mustonen H, Krogerus L, Arola J, Färkkilä MA. Screening primary sclerosing cholangitis and biliary dysplasia with endoscopic retrograde cholangiography and brush cytology: risk factors for biliary neoplasia. Endoscopy. 2016;48:432–439. doi: 10.1055/s-0041-110792. [DOI] [PubMed] [Google Scholar]
- 5.Boyd S, Mustonen H, Tenca A, Jokelainen K, Arola J, Färkkilä MA. Surveillance of primary sclerosing cholangitis with ERC and brush cytology: risk factors for cholangiocarcinoma. Scand J Gastroenterol. 2017;52:242–249. doi: 10.1080/00365521.2016.1250281. [DOI] [PubMed] [Google Scholar]
- 6.Rosen CB, Darwish Murad S, Heimbach JK, Nyberg SL, Nagorney DM, Gores GJ. Neoadjuvant therapy and liver transplantation for hilar cholangiocarcinoma: is pretreatment pathological confirmation of diagnosis necessary? J Am Coll Surg. 2012;215:31–38; discussion 38-40. doi: 10.1016/j.jamcollsurg.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Halme L, Arola J, Numminen K, Krogerus L, Mäkisalo H, Färkkilä M. Biliary dysplasia in patients with primary sclerosing cholangitis: additional value of DNA ploidity. Liver Int. 2012;32:783–789. doi: 10.1111/j.1478-3231.2011.02672.x. [DOI] [PubMed] [Google Scholar]
- 8.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 9.Trikudanathan G, Navaneethan U, Njei B, Vargo JJ, Parsi MA. Diagnostic yield of bile duct brushings for cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc. 2014;79:783–789. doi: 10.1016/j.gie.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Boberg KM, Jebsen P, Clausen OP, Foss A, Aabakken L, Schrumpf E. Diagnostic benefit of biliary brush cytology in cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol. 2006;45:568–574. doi: 10.1016/j.jhep.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Lindberg B, Arnelo U, Bergquist A, Thörne A, Hjerpe A, Granqvist S, Hansson LO, Tribukait B, Persson B, Broomé U. Diagnosis of biliary strictures in conjunction with endoscopic retrograde cholangiopancreaticography, with special reference to patients with primary sclerosing cholangitis. Endoscopy. 2002;34:909–916. doi: 10.1055/s-2002-35298. [DOI] [PubMed] [Google Scholar]
- 12.Timmer MR, Lau CT, Meijer SL, Fockens P, Rauws EA, Ponsioen CY, Calpe S, Krishnadath KK. Genetic Abnormalities in Biliary Brush Samples for Distinguishing Cholangiocarcinoma from Benign Strictures in Primary Sclerosing Cholangitis. Gastroenterol Res Pract. 2016;2016:4381513. doi: 10.1155/2016/4381513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerr SE, Barr Fritcher EG, Campion MB, Voss JS, Kipp BR, Halling KC, Lewis JT. Biliary dysplasia in primary sclerosing cholangitis harbors cytogenetic abnormalities similar to cholangiocarcinoma. Hum Pathol. 2014;45:1797–1804. doi: 10.1016/j.humpath.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Sinakos E, Saenger AK, Keach J, Kim WR, Lindor KD. Many patients with primary sclerosing cholangitis and increased serum levels of carbohydrate antigen 19-9 do not have cholangiocarcinoma. Clin Gastroenterol Hepatol. 2011;9:434–439.e1. doi: 10.1016/j.cgh.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Venkatesh PG, Navaneethan U, Shen B, McCullough AJ. Increased serum levels of carbohydrate antigen 19-9 and outcomes in primary sclerosing cholangitis patients without cholangiocarcinoma. Dig Dis Sci. 2013;58:850–857. doi: 10.1007/s10620-012-2401-3. [DOI] [PubMed] [Google Scholar]
- 16.Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–678. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 17.Lindor KD, Kowdley KV, Harrison ME; American College of Gastroenterology. ACG Clinical Guideline: Primary Sclerosing Cholangitis. Am J Gastroenterol. 2015;110:646–659; quiz 660. doi: 10.1038/ajg.2015.112. [DOI] [PubMed] [Google Scholar]
- 18.Duffy MJ, Sturgeon C, Lamerz R, Haglund C, Holubec VL, Klapdor R, Nicolini A, Topolcan O, Heinemann V. Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann Oncol. 2010;21:441–447. doi: 10.1093/annonc/mdp332. [DOI] [PubMed] [Google Scholar]
- 19.Wannhoff A, Folseraas T, Brune M, Rupp C, Friedrich K, Knierim J, Weiss KH, Sauer P, Flechtenmacher C, Schirmacher P, et al. A common genetic variant of fucosyltransferase 2 correlates with serum carcinoembryonic antigen levels and affects cancer screening in patients with primary sclerosing cholangitis. United European Gastroenterol J. 2016;4:84–91. doi: 10.1177/2050640615581577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanich PP, Björnsson E, Gossard AA, Enders F, Jorgensen R, Lindor KD. Alkaline phosphatase normalization is associated with better prognosis in primary sclerosing cholangitis. Dig Liver Dis. 2011;43:309–313. doi: 10.1016/j.dld.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al Mamari S, Djordjevic J, Halliday JS, Chapman RW. Improvement of serum alkaline phosphatase to <1.5 upper limit of normal predicts better outcome and reduced risk of cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol. 2013;58:329–334. doi: 10.1016/j.jhep.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Lindström L, Hultcrantz R, Boberg KM, Friis-Liby I, Bergquist A. Association between reduced levels of alkaline phosphatase and survival times of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2013;11:841–846. doi: 10.1016/j.cgh.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 23.Rupp C, Rössler A, Halibasic E, Sauer P, Weiss KH, Friedrich K, Wannhoff A, Stiehl A, Stremmel W, Trauner M, et al. Reduction in alkaline phosphatase is associated with longer survival in primary sclerosing cholangitis, independent of dominant stenosis. Aliment Pharmacol Ther. 2014;40:1292–1301. doi: 10.1111/apt.12979. [DOI] [PubMed] [Google Scholar]
- 24.de Vries EM, Wang J, Leeflang MM, Boonstra K, Weersma RK, Beuers UH, Geskus RB, Ponsioen CY. Alkaline phosphatase at diagnosis of primary sclerosing cholangitis and 1 year later: evaluation of prognostic value. Liver Int. 2016;36:1867–1875. doi: 10.1111/liv.13110. [DOI] [PubMed] [Google Scholar]
- 25.Trilianos P, Selaru F, Li Z, Gurakar A. Trends in pre-liver transplant screening for cholangiocarcinoma among patients with primary sclerosing cholangitis. Digestion. 2014;89:165–173. doi: 10.1159/000357445. [DOI] [PubMed] [Google Scholar]
- 26.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215–1229. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vannas MJ, Boyd S, Färkkilä MA, Arola J, Isoniemi H. Value of brush cytology for optimal timing of liver transplantation in primary sclerosing cholangitis. Liver Int. 2017;37:735–742. doi: 10.1111/liv.13276. [DOI] [PubMed] [Google Scholar]


