Abstract
AIM
To investigate whether patients with refractory epilepsy and healthy infants differ in gut microbiota (GM), and how ketogenic diet (KD) alters GM.
METHODS
A total of 14 epileptic and 30 healthy infants were recruited and seizure frequencies were recorded. Stool samples were collected for 16S rDNA sequencing using the Illumina Miseq platform. The composition of GM in each sample was analyzed with MOTHUR, and inter-group comparison was conducted by R software.
RESULTS
After being on KD treatment for a week, 64% of epileptic infants showed an obvious improvement, with a 50% decrease in seizure frequency. GM structure in epileptic infants (P1 group) differed dramatically from that in healthy infants (Health group). Proteobacteria, which had accumulated significantly in the P1 group, decreased dramatically after KD treatment (P2 group). Cronobacter predominated in the P1 group and remained at a low level both in the Health and P2 groups. Bacteroides increased significantly in the P2 group, in which Prevotella and Bifidobacterium also grew in numbers and kept increasing.
CONCLUSION
GM pattern in healthy infants differed dramatically from that of the epileptic group. KD could significantly modify symptoms of epilepsy and reshape the GM of epileptic infants.
Keywords: Ketogenic diet, Cronobacter, Seizures, Gut microbiota, Epilepsy
Core tip: Many infants with epilepsy are refractory to current antiepileptic drugs, and ketogenic diet (KD) could help to moderate seizure frequency as an alternative treatment. A large number of reports have demonstrated that gut microbiota (GM) can affect children’s neurodevelopment. Concurrently, GM could be dramatically affected by diet. KD could rapidly alter GM and alleviate seizure frequency in infants with refractory epilepsy. The GM structure of epileptic infants - comprising large numbers of pathogens, such as Streptococcus - differed from that of healthy controls. After KD therapy, GM of epileptic patients changed significantly, with fewer pathogens and more beneficial bacteria.
INTRODUCTION
Pediatric epilepsy is widespread, with complications including cognitive impairment, delayed neurodevelopment and loss of bodily control[1,2]. Disequilibrium between excitation and depression of the central nervous system is acknowledged as the main factor in epilepsy incidence[3]. Prior reports have identified increased inflammatory reactions and pro-inflammatory cytokines, such as interleukin (IL)-6, IL-17 and interferon, in the cerebrospinal fluid (CSF)[4]. Anti-epileptic drugs (AEDs) and surgery are the main conventional treatments for infants with epilepsy[5]. However, there are still 30% of epileptic infants who suffered from therapeutic futility and recurrent attacks.
A growing number of reports indicated that KD is a promising therapeutic alternative for infants with refractory epilepsy, as it has been shown to ameliorate their clinical symptoms, including the frequency of seizures[6-10]. It remains unclear exactly how this occurs. Several reports implicated changed neurotransmitters after KD therapy, including γ-aminobutyric acid (GABA), monoamines and glutamate[7,11]. Dahin et al[12] and Freeman et al[13] also identified increased ketone bodies (KBs) and decreased dopamine and serotonin[12,13]. However, Sariego-Jamardo et al[14] found little change of neurotransmitters, pterins and amino acids in the CSF of KD responders as opposed to non-responders. These discrepant findings suggested a need for the further elucidation of the mechanisms of KD therapy.
Several studies showed that diet posed a significant effect on GM[8,15]. A high-fat diet induced selective enrichment of bile-metabolizing microbiota, such as Bacteroides[16], whilst high-fiber foods promoted the accumulation of plant-polysaccharide fermenting microbial organisms, including Prevotella and Clostridium[16]. A number of reports implicated involvement of GM in enteric nervous system, blood-brain barrier and glial cell development, all of which were pivotal to behavioral control and cognitive progression[17,18]. GM could produce neurotransmitters and gut hormones directly[19] or indirectly by producing signaling molecules to regulate host cells[20]. GM-derived short-chain fatty acids (SCFAs) could stimulate enterochromaffin cells to produce serotonin[21]. Wikoff et al[22] also documented decreased serotonin in peripheral serum in the absence of GM. Moreover, Clostridium sporogenes and Ruminococcus gnavus promoted decarboxylation of tryptophan to tryptamine, which modulated mood and appetite through amine-associated receptors[23]. Based on the involvement of GM in the gut-brain axis, increasing reports demonstrated imbalanced GM in neurogenic diseases (NDs), including autism-spectrum disorder, Parkinson’s disease, and depression[24]. However, GM dysbiosis in childhood epilepsy remains unexplored.
Previous studies declared that short-term dietary intake could rapidly alter human GM[8,15]. In this study, we performed a comparison between diseased infants (before and after KD treatment) and healthy controls, to explore if and how GM of infants with refractory epilepsy differed with that of age-matched healthy subjects. We also evaluated the therapeutic effect of KD on refractory epilepsy and the changes in GM after treatment. It is hoped that this research will help to bridge some gaps in the current understanding of refractory childhood epilepsy.
MATERIALS AND METHODS
Sample collection
We enrolled 14 pediatric patients with refractory epilepsy (aged 1.95 ± 3.10 years, 11 male and 3 female) in Shenzhen Children’s Hospital, according to the following inclusion criteria: Convulsion more than four times per week after treatment with ≥ 3 AEDs; no antibiotic exposure for at least 1 mo; no known genetic metabolic disorders or severe systemic illnesses; and successive KD therapy for at least 1 wk. KD was provided by Zeneca (Shenzhen, China), including Qitong ketogenic liquid milk (3.4 g protein, 8.0 g lipid and 0.6 g carbohydrate per 100 g milk), Qitong ketogenic cookies and Qitong ketogenic set-meal packages[25].
Healthy subjects (aged up to 3 years, 15 male and 15 female) were also recruited based on the following criteria: No antibiotic exposure for at least 1 mo before this study, no disease symptoms for at least 1 mo following recruitment, and no history of seizures (Supplementary Table 1). Fisher’s exact test was used to evaluate the effect of gender and age on GM composition.
DNA extraction, library construction and sequencing
The genomic DNA of microbiota was extracted from stool samples using the Power Soil DNA Isolation Kit (Mo Bio Laboratories, Carlsbad) following the manufacture’s protocol. The hypervariable V3-V4 region of the 16S rRNA gene was amplified using PCR kit (TransGenAP221-02, Peking), and DNA products were quantified by gel electrophoresis and Qubit (Thermo Fisher, Singapore). After library construction, the qualified libraries were sequenced using the Illumina MiSeq Sequencing platform (Illumina, San Diego).
Taxonomy classification and diversity detection
After filtration, overlapped paired reads were assembled as tags with FLASH (v1.2.11), and clustered to operational taxonomic units (OTUs) through USEARCH (v7.0.1090)[26]. Representative OTUs were mapped against the Greengenes database (v201305)[27] and classified with RDP classifier (v2.2)[28]. The diversity of microbiota was calculated with MOTHUR (v1.31.2)[29].
Principal component analysis and statistical analysis
PCA was performed with R software (v3.2.5). Wilcoxon rank-sum test was used to compare GM in diseased infants and healthy controls (Health group). Comparative analysis between the epileptic infants before (P1 group) and after treatment (P2 group) was conducted by Wilcoxon signed-rank test. Linear discriminant analysis Effect Size (LEfSe) analysis was used to identify microbial species which were apparently enriched in a specific group.
RESULTS
Data output and patients’ characteristics
The average number of high-quality sequencing reads produced for each sample was 117196 (range, 31900 to 305190). The number of assembled tags averaged 22800, with a range from 12655 to 27337. Both gender and age had no significant effect on GM (P = 0.069 and 0.234, respectively).
GM of healthy individuals differs dramatically with that of diseased infants
Shannon index analysis indicated higher GM diversity in healthy infants, in comparison with infants with refractory epilepsy (Figure 1, Supplementary Table 2). PCA of GM profile also identified that healthy infants could be clearly distinguished from patients (Figure 2, Supplementary Table 3). The phylum Firmicutes predominated in patients (45.82%) and was unchanged after KD therapy (47.00%) (Supplementary Table 4). Bacteroidetes accounted for 53.01% of GM in healthy infants, followed by Firmicutes (34.38%). After KD treatment, Bacteroidetes increased from 26.75% to 38.71%. Actinobacteria was enriched in healthy infants (8.49%) and occupied a lower percent in patients (2.38% before treatment and 2.92% after treatment). Proteobacteria was highly accumulated in infants with refractory epilepsy (24.34%) and decreased dramatically after KD therapy (10.77%). At the genus level, Cronobacter was dominant in the patients (23.30% vs 0.00% in the healthy group). By contrast, healthy subjects harbored more than twice Bacteroides (42.68%) than infants with refractory epilepsy (17.93%). Prevotella and Bifidobacterium also accumulated in the healthy group (7.25% and 7.84%, respectively) (Supplementary Table 5).
Figure 1.
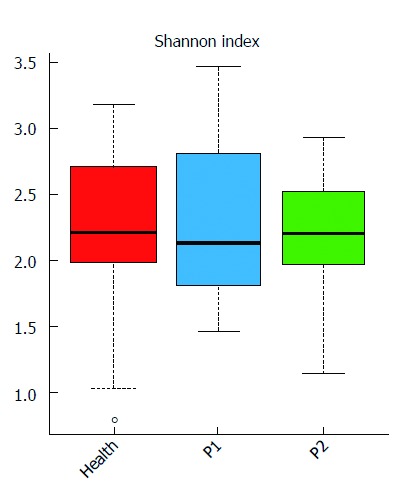
Gut microbial diversity of the three groups. Distribution of Shannon index (evenness) is shown. Red, blue, and green represent the Health, P1 and P2 groups, respectively. The gut microbiota (GM) of the healthy infants was more stable than that of the other two groups.
Figure 2.
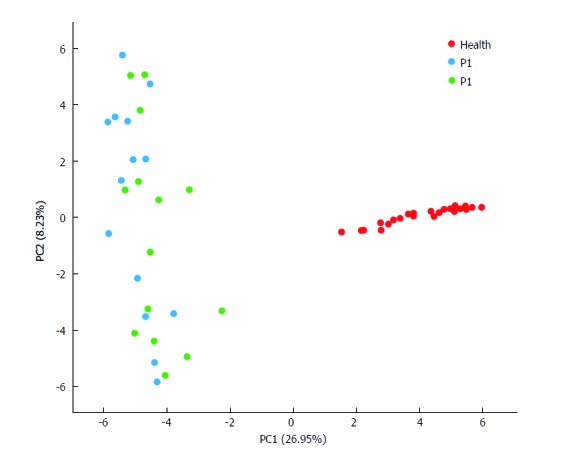
Principal component analysis. Each plot in the principal component analysis (PCA) graph stands for a sample. Red, blue and green colors represent the Health, P1 and P2 groups, respectively.
KD therapy ameliorates epilepsy and GM of patients started to improve
After a week of KD therapy, 3 (21%) patients were seizure-free and 6 (43%) had a 50% to 90% decrease of seizure frequency (Supplementary Table 1). The remaining 5 (36%) infants experienced no significant improvement in seizure control (Supplementary Table 1). GM of the P2 group was more similar to that of the Health group, by comparison with P1 group (Figures 3 and 4). After KD treatment, Bacteroides increased significantly, by 24.42%. Prevotella also increased in the P1 group from 0.37% to 1.85% after KD treatment (Figure 3 and Supplementary Table 5). Cronobacter decreased sharply in after-treatment patients, from 23.3% to 10.44 % (Figures 3 and 4 and Supplementary Table 5). KD exposure also induced a decrease in Erysipelatoclostridium (by 8.67% in the P1 group and 4.89% in the P2 group); it represented just 0.64% in healthy infants (Figures 3 and 4 and Supplementary Table 5). Streptococcus, Alistipes, Ruminiclostridium, Barnesiella and Enterococcus also decreased after KD therapy (Figures 3 and 4 and Supplementary Table 5).
Figure 3.
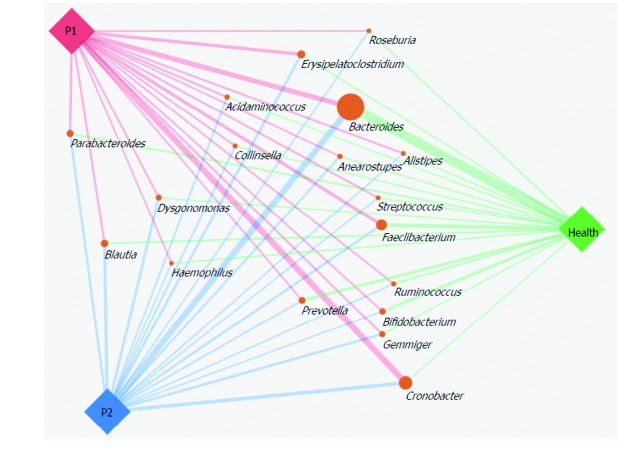
Gut microbiota structures in the Health, P1 and P2 groups at the genus level. SVG package (version 1.1) was used to produce the paragraph. The size of the circle representing each genus was determined by the relative abundance of the three groups, and the width of line linking the P1, P2 and Health groups indicates the relative abundance of each group.
Figure 4.
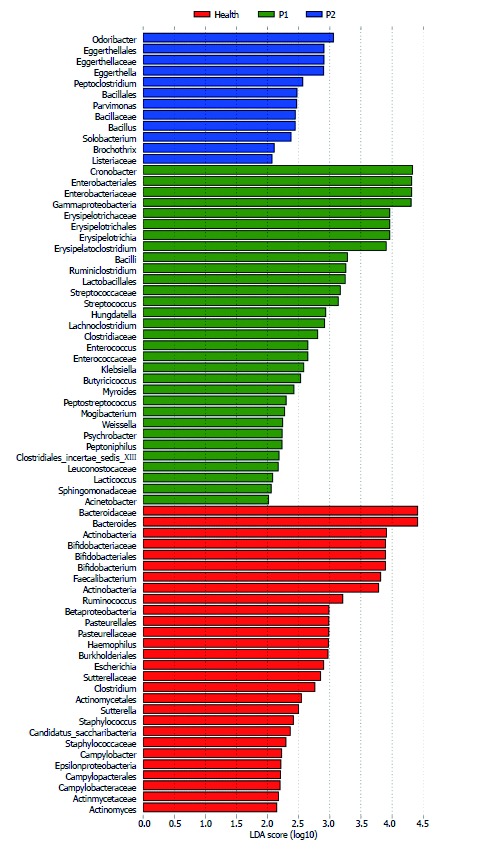
Significantly enriched gut microbiota components in the Health, P1 and P2 groups. LEfSe analysis was applied to detect the gut microbiota (GM) components in the three groups. Red, green, and blue represent the Health, P1 and P2 groups, respectively. The LDA score was set as ≤ 2. The enrichment degree is proportional to the LDA score.
DISCUSSION
KD is increasingly used for the treatment of refractory epilepsy in childhood, but the mechanism remains unclear. Previous reports indicated that GM played an important role in the gut-brain axis[24], and was affected significantly by intake of high-fat food[16]. This study focused on differed GM structures between healthy and epileptic infants, as well as altered GM in patients after one week of KD treatment. The results pointed to an imbalanced GM in patients and a significant improvement after KD therapy.
Proteobacteria comprises a variety of notorious pathogens, such as Escherichia, Salmonella and Vibrio. It accounted for 24.34% in pediatric patients and decreased dramatically after KD treatment. Bacteroidetes was dominant in healthy infants and increased largely in after-treatment patients.
We identified accumulated Bacteroides in healthy subjects as well as in patients after treatment. Bacteroides was reported to digest and metabolize high-fat food and to regulate the secretion of IL-6 and IL-17 in dendritic cells (DCs), a process strongly associated with seizure severity of epileptic patients[4,16]. However, patients-enriched Cronobacter decreased dramatically after KD therapy. Prior reports demonstrated that there were multiple virulence determinants of Cronobacter, including Cronobacter plasminogen activator and ferric ion transporter protein, which paly a detrimental role in human health[30-32]. Prevotella is a robust producer of SCFAs[33], which could protect the intestinal mucosa and function as neurotransmitters. Previous reports also indicated that SCFAs mediated nervous impulse and mitigated Parkinson’s disease[33,34]. Similarly, we identified increased Prevotella in the Health and P2 group, when compared with the P1 group. Some other genera also offer clues to epilepsy recovery, such as Erysipelatoclostridium, Blautia, Bifidobacterium and Streptococcus. Bifidobacterium was well known to be beneficial to health[35], and Streptococcus, a common pathogen, played a role especially in respiratory diseases[36]. Although GM imbalance in diseased infants was identified and GM improved after KD treatment, more exploration was needed to elucidate the contribution of a healthy GM to epilepsy onset/recovery.
This study revealed that KD can mitigate the symptoms of epilepsy and correct an imbalanced GM in epileptic infants. However, further analysis is needed to unravel how GM may be involved in epilepsy onset/recovery.
There are some limitations that need to be clarified. First, 16S rDNA analysis identified microbes at the genus level, which makes it difficult to unravel different microbes at the species or function level. Second, it would be more useful to evaluate the efficacy of KD treatment and its effect on the GM if this could be done with a longer period of follow-up. Third, an animal model might be applied to demonstrate whether GM imbalance could induce epilepsy associated symptoms. Considering these limitations, we are planning to perform metagenomic analysis on GM of healthy and epileptic infants. This will provide more insights into distinct metabolic networks in imbalanced GM.
In conclusion, we found that GM of infants with refractory epilepsy differed dramatically from that of healthy infants. Epileptic patients harbored significantly enriched pathogens and decreased beneficial bacteria. Although this study provides new insight into the involvement of GM in pediatric refractory epilepsy, the gap between KD and epilepsy recovery is still huge. To uncover the mechanism and pathogens involved in refractory infantile epilepsy, further research should underscore functional gene networks in GM.
ACKNOWLEDGMENTS
We thank the staff of WeHealthGene who contributed to the project, but whose names are not included in the author list.
COMMENTS
Background
Infants with refractory epilepsy could not be cured by several anti-epileptic drugs (AEDs) and ketogenic diet (KD) was increasingly used as an alternative therapy to refractory epilepsy. High-fat diet was reported to pose a significant impact on gut microbiota (GM), which could regulate neural systems.
Research frontiers
Previous reports demonstrated that GM could affect neural systems by secreting metabolites as neurotransmitters. In parallel, the gut-brain axis is a research hot spot in biomedicine, including the study of autism, Parkinson’s disease, and depression.
Innovations and breakthroughs
This study showed that the GM pattern of diseased infants differs significantly from that of healthy controls. The decreased number of dominant pathogens and significantly increased number of beneficial bacteria after KD treatment offer new insight into KD therapy for epilepsy.
Applications
This study found several types of bacteria altered in the GM, suggesting that these bacteria could be monitored as biomarkers to provide an important reference for epilepsy treatment.
Terminology
GM, which consists of many kinds of bacteria including pathogens, commensals, and probiotics, plays an important role in the human body.
Peer-review
The authors have performed important research in pediatric epilepsy. They discovered that the composition of the GM in healthy and diseased infants was significantly different, specifically in healthy infants as opposed to those with refractory epilepsy. Bacterial patterns were dramatically changed after KD therapy, and this was associated with a reduction in the frequency of seizures. These findings should enhance our knowledge of the relationship between epilepsy and GM and provide new insight into the clinical treatment of epilepsy.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board of Shenzhen Children’s Hospital.
Conflict-of-interest statement: The authors declare that they have no competing interests.
Data sharing statement: Sequencing data are available from the NCBI Sequence Read Archive (SRA) database (Accession number: SRP100388).
Peer-review started: May 4, 2017
First decision: June 5, 2017
Article in press: July 12, 2017
P- Reviewer: Daniel F, Prakash N S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Xu XR
Contributor Information
Gan Xie, Department of Respiratory Medicine, Shenzhen Children’s Hospital, Shenzhen 518026, Guangdong Province, China.
Qian Zhou, WeHealthGene Institute, Shenzhen 518129, Guangdong Province, China.
Chuang-Zhao Qiu, WeHealthGene Institute, Shenzhen 518129, Guangdong Province, China.
Wen-Kui Dai, WeHealthGene Institute, Shenzhen 518129, Guangdong Province, China.
He-Ping Wang, Department of Respiratory Medicine, Shenzhen Children’s Hospital, Shenzhen 518026, Guangdong Province, China.
Yin-Hu Li, WeHealthGene Institute, Shenzhen 518129, Guangdong Province, China.
Jian-Xiang Liao, Department of Pediatric Neurology, Shenzhen Children’s Hospital, Shenzhen 518026, Guangdong Province, China.
Xin-Guo Lu, Department of Pediatric Neurology, Shenzhen Children’s Hospital, Shenzhen 518026, Guangdong Province, China.
Su-Fang Lin, Department of Pediatric Neurology, Shenzhen Children’s Hospital, Shenzhen 518026, Guangdong Province, China.
Jing-Hua Ye, Department of Pediatric Neurology, Shenzhen Children’s Hospital, Shenzhen 518026, Guangdong Province, China.
Zhuo-Ya Ma, Department of Respiratory Medicine, Shenzhen Children’s Hospital, Shenzhen 518026, Guangdong Province, China.
Wen-Jian Wang, Department of Respiratory Medicine, Shenzhen Children’s Hospital, Shenzhen 518026, Guangdong Province, China. dhbk2005@163.com.
References
- 1.Nordli DR Jr. Epileptic encephalopathies in infants and children. J Clin Neurophysiol. 2012;29:420–424. doi: 10.1097/WNP.0b013e31826bd961. [DOI] [PubMed] [Google Scholar]
- 2.Nickels KC, Zaccariello MJ, Hamiwka LD, Wirrell EC. Cognitive and neurodevelopmental comorbidities in paediatric epilepsy. Nat Rev Neurol. 2016;12:465–476. doi: 10.1038/nrneurol.2016.98. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg EM, Coulter DA. Mechanisms of epileptogenesis: a convergence on neural circuit dysfunction. Nat Rev Neurosci. 2013;14:337–349. doi: 10.1038/nrn3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao LY, Ding J, Peng WF, Ma Y, Zhang YH, Fan W, Wang X. Interictal interleukin-17A levels are elevated and correlate with seizure severity of epilepsy patients. Epilepsia. 2013;54:e142–e145. doi: 10.1111/epi.12337. [DOI] [PubMed] [Google Scholar]
- 5.Vigevano F, Arzimanoglou A, Plouin P, Specchio N. Therapeutic approach to epileptic encephalopathies. Epilepsia. 2013;54 Suppl 8:45–50. doi: 10.1111/epi.12423. [DOI] [PubMed] [Google Scholar]
- 6.Kossoff EH, Zupec-Kania BA, Amark PE, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, Buchhalter JR, Caraballo RH, Helen Cross J, Dahlin MG, et al. Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia. 2009;50:304–317. doi: 10.1111/j.1528-1167.2008.01765.x. [DOI] [PubMed] [Google Scholar]
- 7.Lima PA, Sampaio LP, Damasceno NR. Neurobiochemical mechanisms of a ketogenic diet in refractory epilepsy. Clinics (Sao Paulo) 2014;69:699–705. doi: 10.6061/clinics/2014(10)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Louw E, van den Hurk D, Neal E, Leiendecker B, Fitzsimmon G, Dority L, Thompson L, Marchió M, Dudzińska M, Dressler A, et al. Ketogenic diet guidelines for infants with refractory epilepsy. Eur J Paediatr Neurol. 2016;20:798–809. doi: 10.1016/j.ejpn.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7:500–506. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- 10.Noebels JL. The Voltage-Gated Calcium Channel and Absence Epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. 4th ed. Bethesda (MD); 2012. pp. 1049–1066. [Google Scholar]
- 11.Ruskin DN, Masino SA. The nervous system and metabolic dysregulation: emerging evidence converges on ketogenic diet therapy. Front Neurosci. 2012;6:33. doi: 10.3389/fnins.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahlin M, Mansson Je Fau - Amark P, Amark P. CSF levels of dopamine and serotonin, but not norepinephrine, metabolites are influenced by the ketogenic diet in children with epilepsy. Epilepsy Res. 2012;99:132–138. doi: 10.1016/j.eplepsyres.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Freeman JM, Kossoff EH, Hartman AL. The ketogenic diet: one decade later. Pediatrics. 2007;119:535–543. doi: 10.1542/peds.2006-2447. [DOI] [PubMed] [Google Scholar]
- 14.Sariego-Jamardo A, García-Cazorla A, Artuch R, Castejón E, García-Arenas D, Molero-Luis M, Ormazábal A, Sanmartí FX. Efficacy of the Ketogenic Diet for the Treatment of Refractory Childhood Epilepsy: Cerebrospinal Fluid Neurotransmitters and Amino Acid Levels. Pediatr Neurol. 2015;53:422–426. doi: 10.1016/j.pediatrneurol.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Wu J, Zhang Y, Yang H, Rao Y, Miao J, Lu X. Intestinal Microbiota as an Alternative Therapeutic Target for Epilepsy. Can J Infect Dis Med Microbiol. 2016;2016:9032809. doi: 10.1155/2016/9032809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Ng LG, Kundu P, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins J, Borojevic R, Verdu EF, Huizinga JD, Ratcliffe EM. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol Motil. 2014;26:98–107. doi: 10.1111/nmo.12236. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto M, Kibe R, Ooga T, Aiba Y, Kurihara S, Sawaki E, Koga Y, Benno Y. Impact of intestinal microbiota on intestinal luminal metabolome. Sci Rep. 2012;2:233. doi: 10.1038/srep00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SC. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- 21.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams BB, Van Benschoten AH, Cimermancic P, Donia MS, Zimmermann M, Taketani M, Ishihara A, Kashyap PC, Fraser JS, Fischbach MA. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe. 2014;16:495–503. doi: 10.1016/j.chom.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The Central Nervous System and the Gut Microbiome. Cell. 2016;167:915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suo C, Liao J, Lu X, Fang K, Hu Y, Chen L, Cao D, Huang T, Li B, Li C. Efficacy and safety of the ketogenic diet in Chinese children. Seizure. 2013;22:174–178. doi: 10.1016/j.seizure.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 27.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson EE, Forsythe SJ. Comparative study of Cronobacter identification according to phenotyping methods. BMC Microbiol. 2016;16:146. doi: 10.1186/s12866-016-0768-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowen AB, Braden CR. Invasive Enterobacter sakazakii disease in infants. Emerg Infect Dis. 2006;12:1185–1189. doi: 10.3201/eid1208.051509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh N, Raghav M, Narula S, Tandon S, Goel G. Profiling of Virulence Determinants in Cronobacter sakazakii Isolates from Different Plant and Environmental Commodities. Curr Microbiol. 2017;74:560–565. doi: 10.1007/s00284-017-1219-9. [DOI] [PubMed] [Google Scholar]
- 33.Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30:350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 34.Miletta MC, Petkovic V, Eblé A, Ammann RA, Flück CE, Mullis PE. Butyrate increases intracellular calcium levels and enhances growth hormone release from rat anterior pituitary cells via the G-protein-coupled receptors GPR41 and 43. PLoS One. 2014;9:e107388. doi: 10.1371/journal.pone.0107388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghouri YA, Richards DM, Rahimi EF, Krill JT, Jelinek KA, DuPont AW. Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease. Clin Exp Gastroenterol. 2014;7:473–487. doi: 10.2147/CEG.S27530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krzyściak W, Pluskwa KK, Jurczak A, Kościelniak D. The pathogenicity of the Streptococcus genus. Eur J Clin Microbiol Infect Dis. 2013;32:1361–1376. doi: 10.1007/s10096-013-1914-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


