A member of the θ-defensin family protects mice during infection with influenza, suggesting a new strategy for viral therapy in humans.
Keywords: innate immunity, inflammation, cytokine regulation, signal transduction
Abstract
Despite widespread use of annual influenza vaccines, seasonal influenza-associated deaths number in the thousands each year, in part because of exacerbating bacterial superinfections. Therefore, discovering additional therapeutic options would be a valuable aid to public health. Recently, TLR4 inhibition has emerged as a possible mechanism for protection against influenza-associated lethality and acute lung injury. Based on recent data showing that rhesus macaque θ-defensins could inhibit TLR4-dependent gene expression, we tested the hypothesis that a novel θ-defensin, retrocyclin (RC)-101, could disrupt TLR4-dependent signaling and protect against viral infection. In this study, RC-101, a variant of the humanized θ-defensin RC-1, blocked TLR4-mediated gene expression in mouse and human macrophages in response to LPS, targeting both MyD88- and TRIF-dependent pathways. In a cell-free assay, RC-101 neutralized the biologic activity of LPS at doses ranging from 0.5 to 50 EU/ml, consistent with data showing that RC-101 binds biotinylated LPS. The action of RC-101 was not limited to the TLR4 pathway because RC-101 treatment of macrophages also inhibited gene expression in response to a TLR2 agonist, Pam3CSK4, but failed to bind that biotinylated agonist. Mouse macrophages infected in vitro with mouse-adapted A/PR/8/34 influenza A virus (PR8) also produced lower levels of proinflammatory cytokine gene products in a TLR4-independent fashion when treated with RC-101. Finally, RC-101 decreased both the lethality and clinical severity associated with PR8 infection in mice. Cumulatively, our data demonstrate that RC-101 exhibits therapeutic potential for the mitigation of influenza-related morbidity and mortality, potentially acting through TLR-dependent and TLR-independent mechanisms.
Introduction
Influenza viruses A and B are negative-sense, single-strand RNA viruses in the Orthomyxoviridae family. To combat these pathogenic viruses, seasonal influenza vaccines are formulated, and annual vaccination is recommended by the CDC for everybody 6 mo or older for the 2016–2017 flu season [1]. During the 2014–2015 flu season, the CDC estimated that ∼150 million doses of influenza vaccine were distributed in the United States [2]. Despite the availability of these seasonal vaccines, annual estimated mortality from 1977 to 2007 from influenza ranged from 3349 to 48,614 deaths [3], peaking when influenza A (H3N2) circulated in the population [4]. These vaccines are only protective when the circulating strains of influenza closely match the vaccine strains, and the fact that many people do not get immunized can explain these statistics. Importantly, these seasonal influenza vaccines do not protect against newly emerging strains that could potentially cause large outbreaks or pandemics in the future. In addition to the mortality and morbidity associated with the viral infection, coinfections can exacerbate the outcome of influenza infection, as exemplified by the 1918 pandemic, in which most deaths were due to secondary bacterial pneumonia [5]. Secondary bacterial infection after influenza infection accounts for a significant number of deaths worldwide [6]. Therefore, it would be a valuable aid to public health to have therapeutic options for treating influenza-infected individuals. This was an unmet need in the 2014–2015 influenza season, in which the circulating influenza A (H3N2) strain was a drift variant of the seasonal vaccine strain, resulting in a vaccine with reduced efficacy [7].
In nonimmunized individuals, the first barriers to influenza infection are cells of the innate immune system. These cells are capable of recognizing the invading virus, and that innate recognition is essential for initiating an adaptive immune response. According to pattern-recognition theory [8], cells of the innate immune system identify pathogens through germline-encoded proteins called pattern-recognition receptors. These proteins identify pathogen-associated molecular patterns present in the invading microorganisms. In the case of influenza infection, the cytosolic RNA helicase RIG-I has an important role in recognition, triggering production of protective proinflammatory cytokines, such as TNF-α and antiviral type I IFNs [9]. It is theorized that the host cell can distinguish the viral RNA from cellular RNA because of a panhandle structure in its 5′-triphosphorylated terminus [10]. However, the influenza protein NS1 is capable of antagonizing RIG-I–dependent signaling [11], highlighting the need for redundancy in innate immune recognition. TLR3 can also recognize viral RNA [12] and has been shown to be activated in response to influenza infection in human lung epithelial cells to induce proinflammatory cytokines [13]. In certain cell types, such as plasmacytoid dendritic cells, TLR7 can also recognize influenza genomic RNA [14].
Innate immune recognition does not occur exclusively through recognition of pathogen substructures. Influenza infection can induce cellular stress, leading to mitochondrial dysfunction, which can culminate in NLRP3-dependent activation of caspase-1, leading to release of the proinflammatory mediators IL-1β and IL-18 [15]. Infected cells can also release DAMPs to alert neighboring cells of the danger. S100A9 is produced during in vitro infection with influenza A virus [16], whereas HMGB1 is a DAMP that is released during in vivo influenza infections in both mice and humans [17, 18]. Reactive oxygen species released by cells in the lungs of humans and animals during viral infection can cause oxidation of endogenous phospholipids to produce oxidized phosphatidylcholine [19, 20]. Importantly, oxidized phospholipids, HMGB1, and S100A9 have all been shown to signal through TLR4 [16, 21, 22]. Relevantly, we reported that TLR4−/− mice were resistant to infection with mouse-adapted influenza virus strain A/PR/8/34 (PR8) [20, 23], as well as a more potent, mouse-adapted strain ma-Ca/04 [24]. The TLR4-specific antagonist Eritoran (E5564; Eisai Co., Tokyo, Japan) blocked lethality and clinical symptoms caused by PR8 infection when given starting 2 d after infection [20]. Blocking TLR4 signaling on d 2 or on d 2 and d 4 after PR8 infection with an anti-TLR4–specific Ab also conferred protection [25]. These and other TLR4 antagonists identified by our group [26, 27] indicate that TLR4 antagonists may represent a novel class of drugs for use in treating patients with influenza.
Defensins are small, cysteine-rich, cationic peptides that primarily serve as innate immune defense mechanisms against infectious microorganisms. Unlike the α- and β-defensins, θ-defensins are circular, formed by head-to-tail ligation of 2 truncated α-defensin–like precursor peptides and possess broad antimicrobial activity, targeting bacteria, viruses, and fungi [28–32]. All 6 human θ-defensin (DEFT) pseudogenes contain a premature stop codon, preventing translation [33, 34]. However, solid-phase peptide synthesis has allowed researchers to construct artificially humanized θ-defensins, called RCs [35]. The individual RCs vary slightly in the noncysteine amino residues but bind with high affinity to N- and O-linked glycosylated proteins, such as HIV gp120 [36], and to prevent fusion mediated by influenza hemagglutinin [37]. Consequently, RCs can effectively prevent in vitro infection of human cell lines with either HIV [35, 38] or influenza A virus [39]. Similarly, RCs are protective in vitro against HSV-1/2 through binding to its surface glycoprotein [40]. In addition to their role in fighting microorganisms, defensins might also have a key regulatory role in limiting tissue-damaging inflammation. Human β-defensin 3 can inhibit TLR4-dependent signaling in vitro and in vivo [41, 42]. RTDs prevent cytokine secretion from human blood leukocytes treated with multiple TLR ligands [43], including the TLR4 agonist LPS [44], demonstrating that θ-defensins can also regulate host responses mediated by cellular glycoproteins. This also suggests a role for the structurally similar RCs in TLR antagonism. Clinically, RCs could represent a novel therapeutic against influenza infection, inhibiting the detrimental production of TLR4-dependent cytokines and blocking infection of the virus or coinfecting bacteria. The aims of our study were to determine whether RC-101 could inhibit LPS-induced TLR4 signaling and whether RC-101 could be used therapeutically against influenza-mediated disease.
MATERIALS AND METHODS
Synthesis of RC-101
RC-101, containing an arginine-to-lysine substitution from the parent RC peptide [38], was made by solid-phase synthesis. Backbone cyclization of RC101 is achieved through intramolecular native chemical ligation [45, 46] of a thioester-ending linear peptide synthesized on solid phase using Boc chemistry. Disulfide bonds were formed under oxidative conditions in the presence of DMSO. The peptide was purified by reversed-phase HPLC to homogeneity (>98% purity) and verified by electrospray ionization mass spectrometry. The final product, shown in Supplemental Fig. 1, was analyzed by reversed-phase HPLC on a Waters (Milford, MA, USA) XBridge C18 column (4.6 × 150 mm, 3.5 μm) running at 40°C with a 30-min gradient of 5–65% acetonitrile containing 0.1% trifluoroacetic acid at a flow rate of 1 ml/min. The observed molecular mass of 1889.7 Da is within the experimental error of the expected value of 1890.4 Da, which was calculated based on the average isotopic compositions of the peptide. RC-101 was solubilized in PBS before use.
Reagents
Protein-free Escherichia coli K235 LPS was prepared as described [47]. Escherichia coli 0111:B4, labeled with a biotin tag (LPS-biotin) (InvivoGen, San Diego, CA, USA), DMXAA (Sigma-Aldrich, St. Louis, MO, USA), synthetic triacylated lipoprotein, Pam3CSK4 (InvivoGen), Pam3CSK4-biotin (InvivoGen), and recombinant mouse TNF-α (eBioscience, San Diego, CA, USA) were purchased from the indicated vendors. Mouse mAb were used to visualize TLR4 proteins with the tags FLAG (M2 clone) (Sigma-Aldrich) and eCFP (clone OTI8A6) (OriGene Technologies, Rockville, MD, USA) by Western blot analysis. An anti-biotin–HRP-linked Ab (Cell Signaling Technology, Danvers, MA, USA) was used to visualize LPS-biotin. Rabbit-specific polyclonal Ab against RC-101 has been previously described [34]. All other primary and secondary Abs used in the study have been previously described [48]. The LAL Chromogenic Endotoxin Quantitation Kit (88282; Thermo Fisher Scientific, Waltham, MA, USA) was used, according to the manufacturer’s instructions to test the biologic activity of E. coli 0111:B4 LPS (included in the kit as a positive control). Chromo-LAL and control standard endotoxin (E. coli 0113:H10) were purchased from Associates of Cape Cod, Inc. (East Falmouth, MA, USA) and used according to the manufacturer’s instructions to measure the ability of RC-101 to antagonize the biologic activity of LPS at doses higher than 1 EU/ml. Recombinant HMGB1 protein was kindly provided by Dr. Kevin Tracey (Feinstein Institute for Medical Research, Manhasset, NY, USA).
Mϕ isolation, treatment, and infection
TRIF−/− mice were kindly provided by Dr. Donald Cook (U.S. National Institutes of Health, Bethesda, MD, USA), and IRAK4KDKI mice were obtained from Lilly Research Laboratories (Indianapolis, IN, USA). IRAK4 is the first enzyme recruited to MyD88, and IRAK4KDKI Mϕs behave similarly to MyD88−/− Mϕs [49]. TLR4−/− mice (N ≥10) on a C57BL/6 background were originally obtained from Dr. Shizuo Akira (Osaka University, Osaka, Japan) for breeding at University of Maryland (Baltimore, MD, USA). Thioglycollate-elicited, mouse peritoneal Mϕs were isolated from WT C57BL/6J (Jackson Laboratory, Bar Harbor, ME, USA), TLR4−/−, TRIF−/−, and IRAK4KDKI and were cultured as previously described [50]. Human monocyte-derived Mϕs were matured from elutriated PBMCs of healthy donors as previously described [51]. Mouse and human Mϕs were pretreated with either PBS control or RC-101 within 30 s of stimulation. In vitro infection with PR8 was performed as previously described [20, 23]. Mϕ lysates were harvested at the indicated times in TriPure (Roche Diagnostics, North America, Indianapolis, IN, USA) for subsequent gene expression analysis.
Gene expression analysis by qRT-PCR
cDNA was synthesized from RNA as previously described [50]. qRT-PCR reactions were monitored with the 7900HT Fast Real-Time PCR system (Thermo Fisher Scientific) using the 2× Power SYBR green mix PCR Master Mix (Thermo Fisher Scientific). Individual genes were analyzed with cytokine gene-specific human [23] and mouse [50] primers, which have previously been published. The total amount of mRNA was calculated using the comparative cycle threshold (ΔΔCt) method [52] and expressed as fold-induction compared with untreated or uninfected cells.
Transfections and pulldown assays
HEK293T cells were grown in DMEM, which was supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% FCS, as previously described [53]. One night before transfection, 2 × 105 cells were plated in a 12-well tissue-culture dish. The following day, those cells were transfected with expression plasmids using the transfection reagent SuperFect (Qiagen, Valencia, CA, USA), according to the manufacturer’s supplied instructions. Specifically, 1 μg of total plasmid (empty vector pcDNA3.1, TLR4-FLAG [53], or TLR4-CFP [54]) was mixed with SuperFect at a 1:7 ratio. For dual transfections, 0.5 μg of each plasmid was used, and the amount of SuperFect was kept constant. After transfection, the cells were allowed to incubate in growth medium for 48 h before monitoring transgene expression. After 48 h, cells were washed twice with ice-cold PBS and lysed (20 mM HEPES, 0.5% Triton X-100, 150 mM NaCl, and 1 mM PMSF). Whole-cell lysates were incubated on ice for 30 min and centrifuged at 12,000 rpm for 10 min at 4°C to pellet cellular debris. Supernatants were withdrawn and analyzed by Western blot directly (input samples) or immunoprecipitated. In the latter case, 50 μg of whole-cell lysates (in 100 μl) were incubated overnight at 4°C with gentle rotation in the presence of 1 μg of FLAG-specific Ab (Sigma-Aldrich). Protein G agarose (Thermo Fisher Scientific) bead slurries (100 μl) were added to mixtures and incubated for a further 60 min with constant rotation at room temperature. Beads were harvested by centrifugation and washed 5 times with binding buffer (25 mM Tris, 150 mM NaCl; pH 7.2). To harvest the bound proteins, beads were resuspended in 50 μl Laemmli buffer, vortexed, boiled for 10 min at 95°C, and centrifuged. Supernatants were separated by SDS-PAGE on a 10% gel and transferred to a polyvinyl difluoride membrane. For LPS pulldowns, LPS-biotin and RC-101 were incubated for 30 min at room temperature in the presence or absence of excess, unlabeled E. coli K235 LPS. Pierce Streptavidin Ultralink Resin beads (50 μl slurry; Thermo Fisher Scientific) were added to the samples and incubated for a further 60 min with constant rotation at room temperature. Beads were harvested by centrifugation and washed 5 times with binding buffer (0.1 M phosphate, 0.15 M NaCl; pH 7.2). To harvest the bound proteins, beads were resuspended in 50 μl Laemmli buffer, vortexed, boiled for 10 min at 95°C, and centrifuged. Supernatants were withdrawn and serially diluted. Samples (5 μl) were dot-blotted onto nitrocellulose membrane (0.2 μM pore size), air dried, blocked, and incubated in parallel for 1 h when probing for biotin or overnight when probing for RC-101.
Mouse infection model and clinical scoring
All mouse protocols were approved by the Institutional Animal Care and Use Committee at the University of Maryland, Baltimore. On d 0, female, 6–8-wk-old WT C57BL/6J mice were infected intranasally with ∼7500 TCID50 PR8, as we have described [20]. Mice were injected daily on d 2–6 postinfection i.v. with 100 μl of saline or RC-101 (100 μg/mouse). A similar dosing regimen with the TLR4 antagonist Eritoran was previously shown to protect mice against PR8 infection [20]. Mice were monitored daily for survival, clinical score, and weight loss for 14 d. Clinical scores ranging from 0 (no symptoms) to 5 (moribund) included piloerection, hunched posture, ruffled fur, audible lung crackling, and lethargy, as previously detailed [20]. Mice were euthanized as soon as they became moribund to prevent suffering of the animal.
Statistical analysis
Experiments were performed in duplicate for analysis of gene expression. The numerical values obtained from those assays were analyzed with GraphPad Prism 4 (GraphPad Software, La Jolla, CA, USA) with either a 1-way or 2-way ANOVA, depending on the number of experimental variables. Significance was determined to be P < 0.05. For the 1-way ANOVA, a Tukey’s post hoc test was used to look for significance among experimental groups within the data set. Survival curves of mice were analyzed for significance with a log-rank test.
Online supplemental materials
Supplemental material to this article includes 1 figure. Supplemental Fig. 1 shows the peptide sequence of RC-101 and a representative example of the purity of RC-101 after peptide synthesis.
RESULTS
RC-101 inhibits TLR4-mediated cytokine expression in human and mouse Mϕs
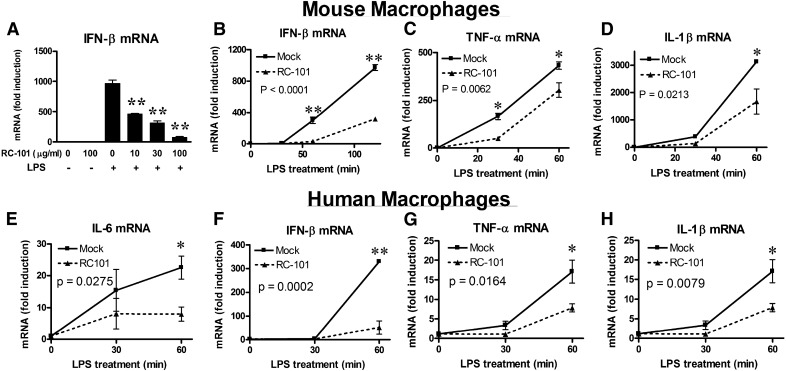
RTDs have been shown to decrease cytokine secretion in response to multiple TLR agonists [43]. Because RC-101 is structurally similar to those RTDs, we hypothesized that RC-101 would antagonize TLR4-mediated signaling, leading to cytokine induction. Consistent with that prediction, RC-101 inhibited LPS-induced IFN-β in a dose-dependent manner (Fig. 1A). Induction of IFN-β was decreased in the presence of RC-101 as early as 60 min after LPS treatment (Fig. 1B). Signal transduction in response to LPS is initiated by homodimerization of TLR4 [55]. TLR4 signal transduction contains a unique bifurcation, using both MyD88 and TRIF as adaptor proteins [56, 57]. IFN-β is considered a MyD88-independent gene induced downstream of the TLR4–TRIF pathway [56, 58]. In contrast to IFN-β, TNF-α and IL-1β represent 2 MyD88-dependent cytokines [56]. To determine whether MyD88 pathways were also affected by RC-101 treatment, expression of those MyD88-dependent genes were analyzed. Both LPS-dependent TNF-α and IL-1β were inhibited by RC-101 (Fig. 1C and D), albeit to a lesser extent than was evident for IFN-β mRNA. To determine whether RC-101–mediated TLR4 antagonism was a species-specific response, primary human monocyte-derived Mϕs were also stimulated with LPS in the presence of RC-101. Similar to that observed in mouse Mϕs, RC-101 also inhibited TRIF- and MyD88-dependent cytokine gene expression in response to LPS stimulation (Fig. 1E–H).
Figure 1. RC-101 inhibits TLR4-dependent gene expression in mouse and human Mϕs.
(A–D) Peritoneal Mϕs were treated with mock (PBS) control or RC-101 (30 μg/ml, if not otherwise indicated) immediately before treatment with LPS (100 ng/ml). Expression of IFN-β mRNA (A and B), TNF-α mRNA (C), and IL-1β mRNA (D) were determined by qRT-PCR. (E–H) Human monocyte-derived Mϕs isolated from PBMCs were treated with mock control (PBS) or the indicated concentration of RC-101 immediately before stimulation with LPS (100 ng/ml). Gene expression of the following human cytokines was determined using qRT-PCR: IL-6 (E), IFN-β (F), TNF-α (G), and IL-1β (H). The data represent the means ± sd for experimental conditions performed in duplicate. A representative of 3 independent experiments is shown. *P < 0.05; **P < 0.001. For (A), significance is compared with LPS treatment alone (0 μg/ml RC-101).
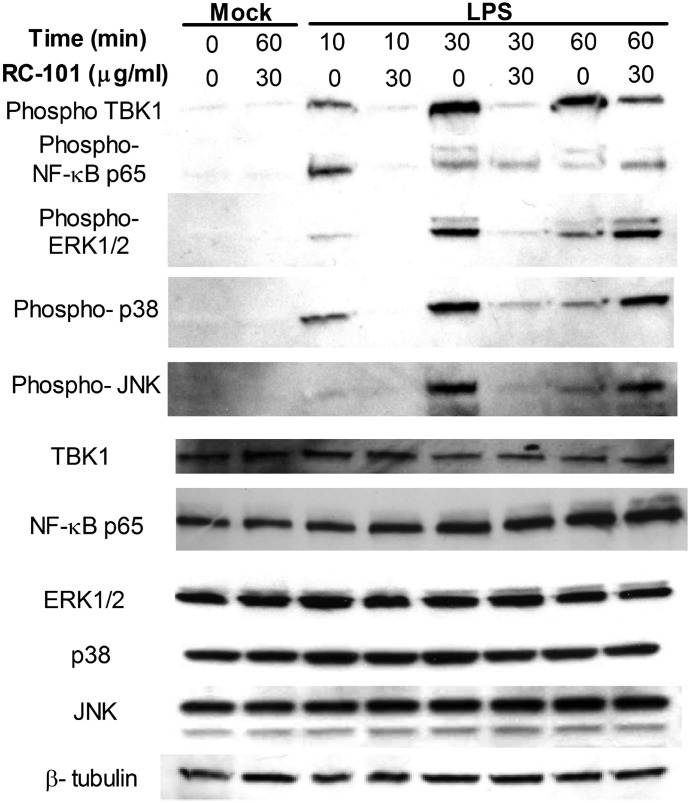
Decreased LPS-dependent cytokine gene expression from RC-101 treatment correlated with a RC-101–mediated block in activation-induced phosphorylation of the TLR4-proximal signaling elements TBK1 and NF-κB p65 at 10 and 30 min after LPS stimulation (Fig. 2). Similarly, activation of MAPK pathways, ERK, JNK, and p38, were also decreased at those time points in the presence of RC-101 (Fig. 2). TBK1 phosphorylation remained decreased at 60 min after LPS treatment in the presence of RC-101 (Fig. 2), whereas phosphorylation of MAPK and NF-κB was increased at that time point (Fig. 2), indicating that RC-101 blockade of TLR4 only delays signal transduction at that dose of LPS and does not ablate it entirely.
Figure 2. RC-101 inhibits TLR4-dependent signaling in mouse Mϕs.
Peritoneal Mϕs were treated with the indicated dose of RC-101 or mock control (PBS) immediately before treatment with LPS (100 ng/ml). Peritoneal Mϕ lysates were harvested at the indicated times, separated by denaturing SDS-PAGE, and probed with Abs directed against the following phosphorylated proteins and loading controls: TBK1, NF-κB p65, ERK1/2, JNK, p38. The housekeeping protein β-tubulin and unphosphorylated proteins were used as loading controls. A representative blot from 3 independent experiments is shown.
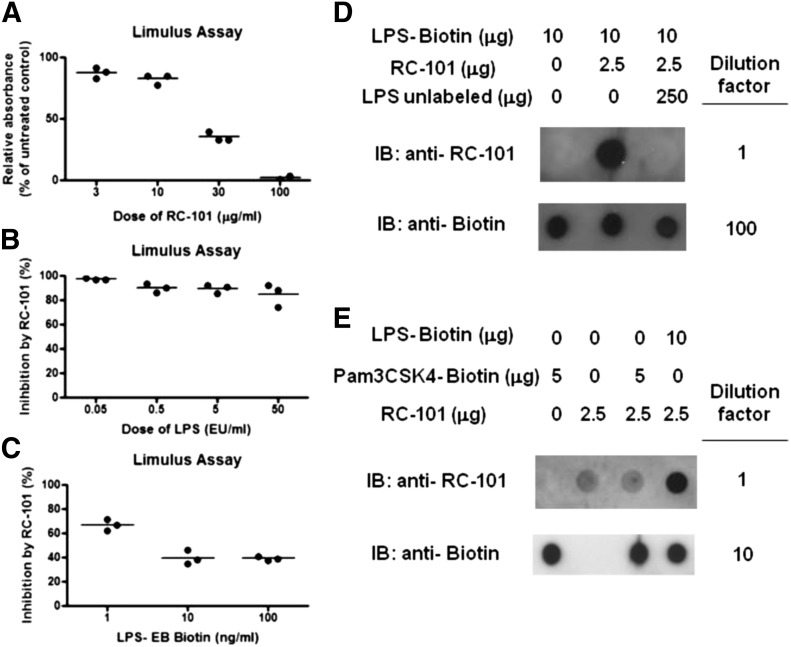
Inhibition of TLR4 signaling by RC-101 in human and mouse cells could possibly be explained by sequestration of LPS from the host TLR4 signaling complex, so we sought to test that possibility in a cell-free system. The biologic activity of LPS was measured in the LAL assay in the presence or absence of RC-101 to determine whether our cytokine expression results were likely due to a interaction between RC-101 and LPS. The biologic activity of 1 endotoxin U/ml (∼0.1 ng/ml) of LPS was partially inhibited by 30 μg/ml RC-101 and almost completely inhibited by 100 μg/ml RC-101 (Fig. 3A). The dose of LPS used in the cell-free LAL assay represents a 1000-fold lower dose of LPS than used for Mϕ TLR4 stimulation (e.g., see Fig. 1). To test more-relevant doses of LPS, we used a different LAL assay that had a linear range up to 50 EU/ml and maintained the dose of RC-101 at 30 μg/ml. Under those conditions, RC-101 (30 μg/ml) strongly inhibited the biologic activity of all LPS doses tested, including 50 EU/ml (Fig. 3B). RC-101 also inhibited the LAL activity of biotinylated LPS at doses ranging from 1 to 100 ng/ml (Fig. 3C), suggesting that it is likely that RC-101 inhibits TLR4 signaling, in part, by sequestration of LPS. To examine that possibility further, we performed an in vitro pulldown assay with biotinylated LPS in the presence or absence of RC-101. RC-101 did not impair the ability of streptavidin beads to pull down LPS-biotin (Fig. 3D). Additionally, RC-101 was recovered upon pulldown of biotinylated LPS with streptavidin beads, suggesting direct binding between RC-101 and LPS (Fig. 3D). To verify that this binding was specific, an excess of unlabeled LPS E. coli K235 was also added to the mix before pulldown. Excess unlabeled LPS prevented the pulldown of RC-101 with biotinylated LPS, suggesting that the binding of LPS and RC-101 is specific (Fig. 3D). In contrast to its interaction with LPS, RC-101 was not pulled down by the biotinylated TLR2 agonist Pam3CSK4 (Fig. 3E).
Figure 3. RC-101 is an antagonist of LPS biologic activity.
(A) Increasing doses of RC-101 were incubated with 1 endotoxin U/ml (∼0.1 ng/ml) of LPS for 10 min at 37°C before measurement of the biologic activity of LPS using the LAL assay. Each dot represents the mean from an individual experiment, and the bar represents the mean biologic activity calculated from the results of 2 or 3 independent experiments. (B and C) Increasing doses of LPS (0.05–50 EU/ml) or LPS-biotin (1–100 ng/ml) were incubated with 30 μg/ml RC-101 for 10 min at 37°C before measurement of the biologic activity of LPS using the chromo-LAL assay. For (B) and (C), each dot represents the mean from an individual experiment, and the bar represents the mean biologic activity calculated from the results of the 3 independent experiments. (D) LPS-biotin, RC-101, and unlabeled LPS were incubated in the indicated combinations for 30 min before pulldown with streptavidin-coated beads. Eluted samples were isolated, diluted, and dot-blotted onto nitrocellulose membrane and probed for biotin or RC-101. (E) LPS-biotin, RC-101, and Pam3CSK4-biotin were incubated in the indicated combinations for 30 min before pulldown with streptavidin-coated beads. Eluted samples were isolated, diluted, and dot-blotted onto nitrocellulose membrane and probed for biotin or RC-101. A representative pulldown from 3 independent experiments is shown.
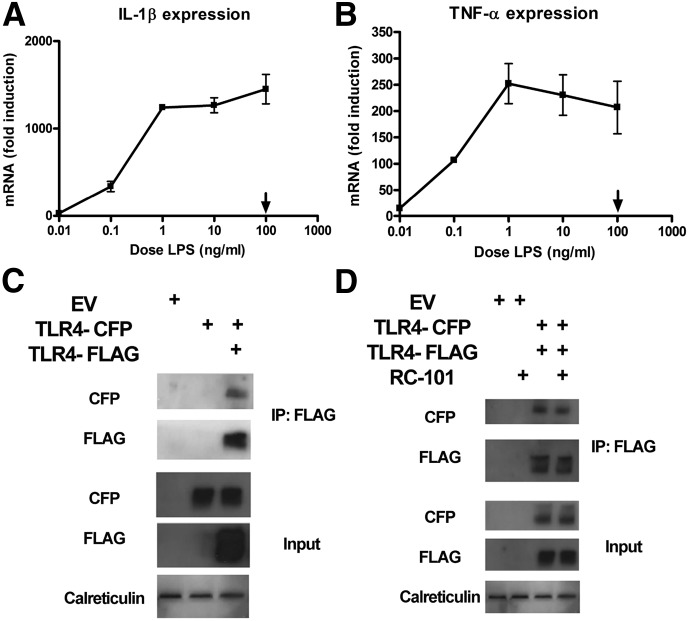
Although our data show evidence that RC-101 may mediate inhibition of TLR4-dependent signaling though sequestration of LPS, it must be noted that even 90% inhibition of LPS activity because of sequestration might not affect TLR4-mediated cytokine expression in our system because the LPS dose we used to stimulate Mϕs (100 ng/ml) was not in the linear portion of the cytokine dose–response curve (Fig. 4A and B). For that reason, we also sought to explore the capacity of RC-101 to disrupt TLR4 dimers, using a model of TLR4 oligomerization that occurs in the absence of ligands. Our laboratory has previously shown that overexpression of FLAG-tagged TLR4 in HEK293T cells can facilitate TLR4-dependent signaling in the absence of LPS [53]. Cotransfection of TLR4-FLAG and TLR4-CFP induced a physical interaction between the 2 TLR4 constructs, as evidenced by coimmunoprecipitation of the 2 epitope tags (Fig. 4C). Using that model, we examined whether RC-101 could destabilize the interaction. Addition of RC-101 failed to inhibit the coimmunoprecipitation of TLR4-CFP with TLR4-FLAG (Fig. 4D).
Figure 4. RC-101 fails to destabilize the ligand-independent TLR4 oligomerization in HEK293T cells.
(A and B) Mouse Mϕs were stimulated with increasing doses of LPS for 60 min and analyzed for expression of IL-1β (A) and TNF-α (B). The arrow represents the dose of LPS used throughout the study. The data represent the means ± sd for experimental conditions performed in duplicate. A representative of 4 independent experiments is shown. (C) HEK293T cells were transfected with the indicated expression plasmids and incubated for 48 h. Whole-cell lysates were separated by SDS-PAGE and blotted for transgene expression (input) or immunoprecipitated with anti-FLAG–specific Ab. Immunoprecipitated samples were separated by SDS-PAGE and probed with anti-FLAG or anti-CFP specific Abs. (D) Same as (C), except RC-101 (30 μg/ml) was added where noted 30 min before harvesting of the whole-cell lysates. For (C) and (D), representative blots from 1 of 3 independent immunoprecipitation reactions are shown.
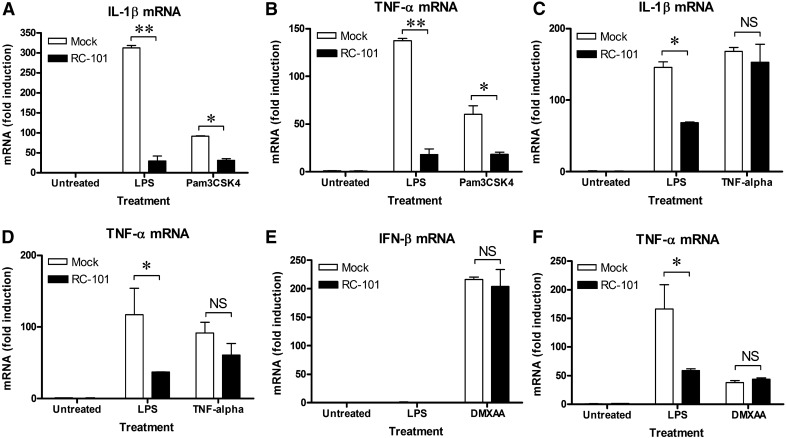
After describing the inhibitory role of RC-101 in LPS-dependent signal transduction, we sought to test whether its role was similar in other innate immune signaling pathways. TLR2-dependent cytokine secretion has been shown to be regulated by RTDs in human blood leukocytes [43], so TLR2 might be expected to be regulated by RC-101, similar to TLR4. In our laboratory, RC-101 also inhibited proinflammatory, TLR2-dependent gene induction in mouse Mϕs (Fig. 5A and B), demonstrating that RC-101–mediated inhibition is not restricted to TLR4; however, RC-101 failed to bind to biotinylated Pam3CSK4 (Fig. 3E). Therefore, inhibition of signaling is not restricted to agonists that RC-101 can bind directly. To further determine the degree of pathway specificity of RC-101–mediated inhibition, mouse Mϕs were also stimulated with non-TLR agonists in its absence or presence. RC-101 failed to inhibit cytokine gene induction in response to either recombinant TNF-α or DMXAA (Fig. 5C–F). DMXAA is a potent inducer of TBK1-dependent IFN-β in mouse Mϕs through intracellular activation of STING [50], unlike TLR4 and TLR2, which initiate signaling extracellularly. Note that the apparent lack of induction of IFN-β in response to LPS in Fig. 5E was due to a suboptimal time point for its expression (see Fig. 1). Collectively, these latter results demonstrate that RC-101 is not a universal inhibitor of innate immune signaling pathways.
Figure 5. RC-101 inhibits TLR2 signaling but is not a global inhibitor of innate signaling pathways.
Peritoneal Mϕs were treated with RC-101 (30 μg/ml) or mock control (PBS) immediately before treatment with LPS (100 ng/ml) or Pam3CSK4 (20 ng/ml) (A and B), TNF-α (100 ng/ml) (C and D), or DMXAA (100 μg/ml) (E and F). Expression of IFN-β and TNF-α at 30 min after DMXAA treatment or IL-1β, and TNF-α at 30 min after TNF-α or Pam3CSK4 treatment was determined by qRT-PCR. The data represent the means ± sd for experimental conditions performed in duplicate. A representative of 3 independent experiments is shown. *P < 0.01. NS, no significant difference.
RC-101 protects mice therapeutically from infection with PR8
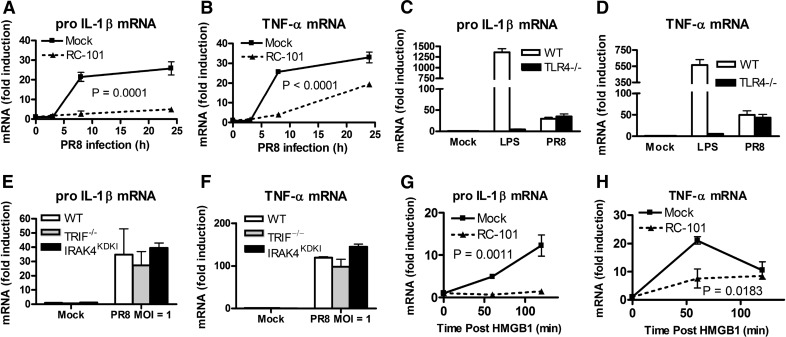
The recent discovery that Eritoran protects mice from lethal infection with PR8 [20] suggested that treatment of mice with other TLR4 antagonists, such as RC-101, may also protect against lethal influenza infection. The effect of RC-101 on PR8 infection was first examined in an in vitro infection model. In WT Mϕs, infected for 8 and 24 h in vitro, RC-101 inhibited PR8-induced expression of the proinflammatory genes encoding pro–IL-1β and TNF-α (Fig. 6A and B). Although TNF-α secretion by PR8-infected Mϕs has been shown to be partially TLR4 dependent [16], we observed that induction of pro–IL-1β and TNF-α mRNA was not significantly impaired in PR8-infected TLR4−/− Mϕs at 8 h postinfection (Fig. 6C and D). Similarly, PR8-infected TRIF−/− or IRAK4KDKI Mϕs induced similar amounts of IL-1β and TNF-α mRNA in response to PR8 as WT controls (Fig. 6E and F). IRAK4 is the first kinase recruited to MyD88 during signal transduction, and IRAK4KDKI Mϕs behave comparably to MyD88−/− Mϕs [49]. This suggests that the DAMPs that activate TLR4-dependent or other TLR-dependent signaling in vivo are likely not present in our in vitro model at the time points tested. This result also indicates that RC-101 influences cytokine induction during in vitro infection independent of TLR4, possibly by inhibiting the signaling of some other non-TLR pattern-recognition receptors, such as RIG-I, which is also capable of recognizing PR8 [9]. HMGB1 is a DAMP that has been shown to be released during influenza infection in mice and humans [17, 18]. Importantly, HMGB1 is also known to activate TLR4-dependent signaling [22]. RC-101 suppressed cytokine gene induction in response to HMGB1 (Fig. 6G and H), demonstrating that it can also block TLR4-mediated gene expression mediated by DAMPs.
Figure 6. RC-101 inhibits up-regulation of PR8-dependent and HMGB1-dependent proinflammatory cytokines in mouse Mϕs.
(A and B) Peritoneal Mϕs from WT mice were treated with RC-101 (30 μg/ml) or mock control (PBS) immediately before infection with PR8 at a multiplicity of infection of 1. At the indicated times postinfection, cells were harvested and analyzed for expression of IL-1β and TNF-α. (C and D) WT and TLR4−/− peritoneal Mϕs were infected as in (A) for 8 h or treated with LPS (100 ng/ml) for 60 min and analyzed for IL-1β and TNF-α mRNA. (E and F) WT, TRIF−/−, and IRAK4KDKI peritoneal Mϕs were infected as in (A), harvested at 8 h postinfection, and analyzed for IL-1β and TNF-α mRNA. (G and H) Peritoneal Mϕs from WT mice were stimulated with recombinant HMGB1 (2.5 μg/ml) immediately after treatment with RC-101 (30 μg/ml) or mock control and harvested at the indicated times for expression of IL-1β (G) and TNF-α (H) mRNA. Data for all panels represent the means ± sd for experimental conditions performed in duplicate. A representative of 3 independent experiments is shown.
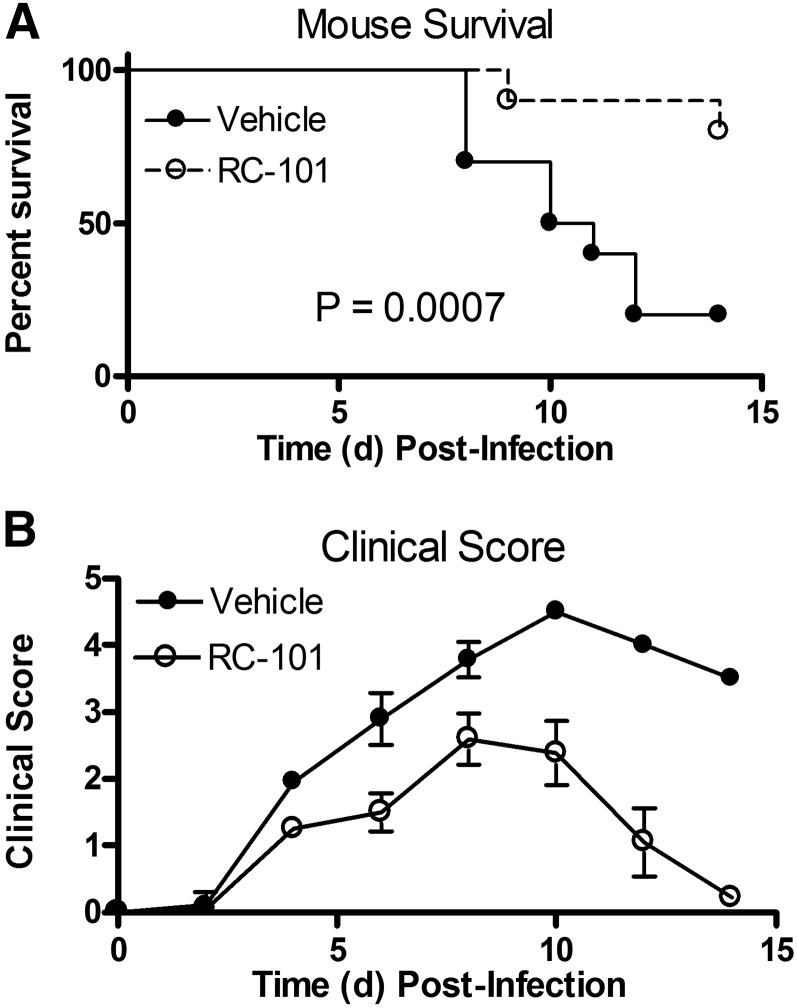
To determine whether RC-101 could be used as an antiviral and/or anti-inflammatory therapeutic in vivo, mice were infected with PR8 (∼LD90) and given 5 daily i.v. injections of RC-101 (100 μg/mouse), starting 48 h after PR8 infection and continuing until d 6 postinfection. We had previously reported that Eritoran protects 90–100% of mice using this identical dosing regimen [20]. In this study, RC-101 treatment of mice also protected from PR8-induced lethality (Fig. 7A) and significantly lessened PR8-induced clinical symptoms (Fig. 7B). This latter significant decrease in clinical symptoms suggests that RC-101 is not merely delaying the time to death because the survivors were typically healthy at the end of 2 wk. Because of the timing of the drug administration, RC-101 is not hypothesized to mediate its effect by blocking initial uptake of the virus. Together, these data suggest that RC-101 and other θ-defensins are strong candidates for future human clinical trials in the search for novel viral therapeutics.
Figure 7. RC-101 increases survival and improves clinical outcome in mice infected with PR8.
C57BL/6J mice were infected with ∼7500 TCID50 PR8 (∼LD90) and injected i.v. with saline or retrocyclin (RC-101; 100 μg/mouse) on d 2, 3, 4, 5, and 6 postinfection. Mice were monitored until d 14 postinfection. Survival (A) and mean clinical score (B) are shown. Data in (B) represent the clinical score means ± sem with a range of 0 (no symptoms) to 5 (moribund). n = 10 mice/experimental group. The data graphed represent the pooled results from 2 separate independent in vivo studies.
DISCUSSION
Although the innate immune system has a crucial role in initiating an immune response against an invading microorganism, overexuberant signaling may actually contribute to pathology during the same infection. This double-edged sword effect of inflammation in response to innate immune signaling is illustrated during infection with influenza virus. The TLR adaptor MyD88 is required for mouse survival after infection with PR8 [59]. Further illustrating the beneficial role of innate signaling pathways, pretreatment of mice with TLR4 or TLR2 agonists, before in vivo infection with influenza, has been shown to be protective [60–62]. However, the timing of those signaling pathways is crucial because oxidative stress in the lung during infection with PR8 has been linked to the development of oxidized phospholipids [20] and other DAMPs that mediate TLR4 signaling-dependent acute lung injury [19]. The detrimental role of inflammation can be further exacerbated by bacterial coinfection [63]. Because of the role of TLR4 during PR8 infection, TLR4−/− mice are refractory to lethality associated with PR8 infection [20, 23]. In further support of the role of oxidized phospholipids in mediating TLR4-dependent lethality, mortality can be prevented by inhibiting oxidized phospholipid formation by administering the antioxidant N-acetyl cysteine [64]. Additionally, we reported that mortality can be prevented by inhibiting TLR4 signaling after PR8 infection with the potent synthetic TLR4 antagonist Eritoran [20], an anti-TLR4 Ab [25], and other agents that block TLR4 signaling [26, 27]. Thus, we hypothesized that drugs that target host-cell, TLR4-dependent signaling pathways could potentially function as novel anti-influenza therapies.
In our study, RC-101 was fully capable of inhibiting LPS-dependent signaling in both mouse and human Mϕs. The impairment of both MyD88- and TRIF-signaling pathways suggests that RC-101 could function by preventing the initial interaction of LPS with TLR4, either through sequestration of LPS or blockade of the signaling event itself. Based on our study, we cannot rule out the strong possibility that a significant part of the TLR4 inhibition by RC-101 is mediated by LPS sequestration from the TLR4 signaling complex because RC-101 could both bind LPS in in vitro pulldown assays and inhibit a broad range of LPS bioactivity in cell-free LAL assays. However, the dose of LPS used to stimulate Mϕs in our study was in the saturation range, where only strong antagonists would be expected to have a significant effect on cytokine gene expression. Signal transduction in response to LPS is initiated at the plasma membrane by homodimerization of TLR4 molecules [55]. The ectodomain of human TLR4 contains 9 N-linked glycosylation sites that are required for cell-surface expression and function [65]. Removal of those sialic acid residues increases LPS-dependent signaling [66], suggesting that those sialic residues may be at the interface of TLR4 dimerization. Because RCs bind with high affinity to proteins exhibiting N- and O-linked glycosylation [36], we hypothesized that RC-101 could antagonize TLR4-mediated signaling by binding to the glycans on TLR4 to prevent ligand-induced dimerization. In line with that theory, human β-defensin 3 can also inhibit TLR4-dependent signaling through direct binding to TLR4 [67]. Using a model of ligand-independent oligomerization of TLR4 in HEK293T cells, we tested whether RC-101 could prevent or disrupt homotypic TLR4 interactions. Addition of RC-101 failed to disrupt oligomers of TLR4 in this assay, which could mean that RC-101 does not target TLR4 directly or that its binding sites were unavailable in this conformation. Unfortunately, we could not decrease the input concentrations of the TLR4 plasmids used for transfection to a level that permitted ligand-dependent signaling but that would still permit detection of coimmunoprecipitated TLR4-FLAG and TLR4-CFP molecules. The MD-2 protein is a crucial part of the LPS recognition complex [68] and is also glycosylated [65], so it is also possible that MD-2, and not TLR4, might be the primary target of RC-101 in this pathway.
In addition to inhibition of LPS-dependent signaling, we also found that Pam3CSK4-dependent activation of TLR2 signaling was inhibited by RC-101. In contrast to its direct interaction with LPS, RC-101 was unable to interact with Pam3CSK4 in vitro. This suggests that RC-101 has the capacity to act in more ways than solely by ligand sequestration. Human TLR2 also contains glycosylation sites [69], which could be a putative target of RC-101. Alternatively, TLR-dependent signal transduction is sensitive to the architecture of lipid-membrane microdomains [70]. Addition of a phosphatidylserine species can impair both TLR4 and TLR2 signaling pathways [70]. Given that the positively charged RC-101 has been found to bind to anionic membranes, such as dipalmitoyl phosphatidylglycerol [71], it is tempting to speculate that the ability of RC-101 to block both TLR4- and TLR2-dependent signaling is caused by an effect on the cell membrane itself. However, such a mechanism seems less likely because TNF-α–mediated signaling, which also requires receptor oligomerization [72], was not perturbed by RC-101.
The fusion protein for respiratory syncytial virus can be recognized in a TLR4-dependent manner [73], but no influenza protein has been identified that can function as a TLR4 ligand. In contrast, DAMPs, such as HMGB1, are released during in vivo infection, and they signal through TLR4. It is less clear whether those DAMPs are produced in vitro and how much of an effect they have on gene regulation. Secretion of proinflammatory genes at 24 h postinfection with PR8 has been shown to be MyD88-dependent in bone marrow–derived and peritoneal Mϕs [16, 26]. However, TNF-α protein secretion at 12 and 24 h was only decreased by ∼20–30% in the absence of TLR4, exhibiting only a partial TLR4 dependence [16]. Our data, using the IRAK4KDKI Mϕs, indicate that the difference in cytokine production mediated by a lack of MyD88-dependent signaling is not due to a defect in transcriptional up-regulation of MyD88-dependent genes, such as TNF-α or IL-1β, at 8 h postinfection. As part of its role as an innate immune adaptor protein, MyD88 has also been shown to be involved in stabilization of mRNA species containing adenosine- and uridine-rich elements, which are common to many cytokines [74]. So, we surmise that the TLR4–MyD88 pathway may be mediating its effect on cytokine secretion in response to PR8 through alterations in mRNA stability and not through altered transcription.
RCs offer the potential to limit inflammation mediated by DAMPs and also to target the virus itself. Although RC-101 may act by blocking initial viral entry, as previously described [37], it has been shown to be less effective against the mouse-adapted PR8 than against other influenza strains [39]. For instance, preincubating the virus with various different RCs for 30 min achieved only an ∼60% decrease in viral infectivity, as measured by a fluorescent focus formation assay [39]. Importantly, none of our experiments featured preincubation of the virus with RC-101, which would further limit the ability of RC-101 to block the virus directly. As a proof of concept that RCs can be used therapeutically, RC-101 was tested in a mouse model of influenza infection. Treatment with RC-101, starting 2 d postinfection, was shown to lessen the severity of PR8-associated disease, resulting in a highly significant increase in survival (Fig. 7). Our results on the inhibition of TLR4-mediated cytokine expression are consistent with the possibility that antagonism of TLR4 signaling accounts, at least in part, for protection in PR8-infected mice. That the protective, therapeutic effect of RC-101 against PR8 is similar to what we have previously reported for the TLR4 antagonist Eritoran [20] suggests that RC-101 may act in vivo, at least partially, through TLR4 antagonism. However, we cannot rule out the possibility of an additional direct effect on the virus itself, as has been observed by others [37, 39]. Although Eritoran does not directly kill influenza in vitro [20], Eritoran therapy was associated with decreased influenza titers in vivo, but not until d 6 after infection [20], which highlights an important connection between inflammation and viral replication in this infection model. It is quite possible that the effect of RC-101 in this system is both antiviral and anti-inflammatory.
Overall, our results in the mouse model are especially encouraging because the mouse-adapted PR8 strain used in our study lacks N-linked glycosylation on its cell-envelope hemagglutinin protein [75], making it more resistant than other influenza strains to RC-101 in vitro [39]. Therefore, RC-101 would be predicted to be more effective against human strains with increased glycosylation [76]. Similar to the efficacy demonstrated by RC-101 in our study, RTDs protected mice from a lethal lung infection with a mouse-adapted severe acute respiratory syndrome–coronavirus and limited the potentially overexuberant cytokine response occurring in the lung [77]. Together, these data suggest that θ defensins, and specifically, RC-101, are strong candidates for future human clinical trials in the search for novel viral therapeutics.
AUTHORSHIP
D.P. designed and coordinated the experiments in the study, performed and analyzed the experiments, and wrote the manuscript. K.A.S. designed and performed the experiments outlined in Fig. 7. W. Lai provided technical assistance involving PR8 infection described in Fig. 7. W. Lu provided technical assistance for all figures by synthesizing RC-101. A.M.C. provided technical assistance for detection of RC-101 by Western blot in Fig. 3. S.N.V. helped with the design and conception of the experiments, with analysis of the data, and in drafting the manuscript. A.G.D. conceived the study and helped in drafting the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the following U.S. National Institutes of Health Grants AI-18797 and AI-125215 (S.N.V.), NS-066842 (A.G.D.), and AI-072732 (W.L.) from the National Institute of Allergy and Infectious Diseases and the National Institute of Neurological Disorders and Stroke. The authors would like to thank Dr. Kevin Tracey (Feinstein Institute for Medical Research, Manhasset, NY, USA) for the kind gift of HMGB1 protein.
Glossary
- CDC
Centers for Disease Control and Prevention
- CFP
cyan fluorescent protein
- DAMP
damage-associated molecular patterns
- DMXAA
5,6-dimethylxanthenone 4-acetic acid
- HMGB1
high mobility group box-1
- IRAK
IL-1 receptor-associated kinase
- KDKI
kinase-dead knock-in
- LAL
Limulus amebocyte lysate
- Pam3CSK4
palmitoyl-3-cysteine-serine-lysine-4
- PR8
influenza virus strain A/PR/8/34
- qRT-PCR
quantitative reverse-transcription polymerase chain reaction
- RC
retrocyclin
- RIG-I
retinoic acid-inducible gene I
- RTD
rhesus macaque θ-defensin
- STING
stimulator of IFN genes
- TBK1
TANK binding kinase 1
- TCID
tissue culture–infective dose
- TRIF
TIR (Toll/IL-1R) domain-containing adapter inducing IFN-β
- WT
wild-type
Footnotes
The online version of this paper, found at www.jleukbio.org, contains supplemental information.
DISCLOSURES
The authors declare no conflicts of interest. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Grohskopf L. A., Sokolow L. Z., Broder K. R., Olsen S. J., Karron R. A., Jernigan D. B., Bresee J. S. (2016) Prevention and control of seasonal influenza with vaccines. MMWR Recomm. Rep. 65, 1–54. [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention (CDC). (2014) Seasonal influenza vaccine total doses distributed. Available at: http://www.cdc.gov/flu/professionals/vaccination/vaccinesupply.htm. Accessed November 11, 2015.
- 3.Centers for Disease Control and Prevention (CDC) (2010) Estimates of deaths associated with seasonal influenza—United States, 1976–2007. MMWR Morb. Mortal. Wkly. Rep. 59, 1057–1062. [PubMed] [Google Scholar]
- 4.Thompson W. W., Moore M. R., Weintraub E., Cheng P. Y., Jin X., Bridges C. B., Bresee J. S., Shay D. K. (2009) Estimating influenza-associated deaths in the United States. Am. J. Public Health 99(Suppl 2), S225–S230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morens D. M., Taubenberger J. K., Fauci A. S. (2008) Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J. Infect. Dis. 198, 962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chertow D. S., Memoli M. J. (2013) Bacterial coinfection in influenza: a grand rounds review. JAMA 309, 275–282. [DOI] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention (CDC). (2014) Early data suggests potentially severe flu season. Available at: http://www.cdc.gov/media/releases/2014/p1204-flu-season.html. Accessed November 11, 2015.
- 8.Janeway C. A., Jr (1989) Approaching the asymptote? evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54, 1–13. [DOI] [PubMed] [Google Scholar]
- 9.Kato H., Takeuchi O., Sato S., Yoneyama M., Yamamoto M., Matsui K., Uematsu S., Jung A., Kawai T., Ishii K. J., Yamaguchi O., Otsu K., Tsujimura T., Koh C. S., Reis e Sousa C., Matsuura Y., Fujita T., Akira S. (2006) Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105. [DOI] [PubMed] [Google Scholar]
- 10.Weber M., Gawanbacht A., Habjan M., Rang A., Borner C., Schmidt A. M., Veitinger S., Jacob R., Devignot S., Kochs G., García-Sastre A., Weber F. (2013) Incoming RNA virus nucleocapsids containing a 5′-triphosphorylated genome activate RIG-I and antiviral signaling. Cell Host Microbe 13, 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pichlmair A., Schulz O., Tan C. P., Näslund T. I., Liljeström P., Weber F., Reis e Sousa C. (2006) RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314, 997–1001. [DOI] [PubMed] [Google Scholar]
- 12.Alexopoulou L., Holt A. C., Medzhitov R., Flavell R. A. (2001) Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738. [DOI] [PubMed] [Google Scholar]
- 13.Le Goffic R., Pothlichet J., Vitour D., Fujita T., Meurs E., Chignard M., Si-Tahar M. (2007) Cutting edge: influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J. Immunol. 178, 3368–3372. [DOI] [PubMed] [Google Scholar]
- 14.Diebold S. S., Kaisho T., Hemmi H., Akira S., Reis e Sousa C. (2004) Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531. [DOI] [PubMed] [Google Scholar]
- 15.Ichinohe T., Yamazaki T., Koshiba T., Yanagi Y. (2013) Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection. Proc. Natl. Acad. Sci. USA 110, 17963–17968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai S. Y., Segovia J. A., Chang T. H., Morris I. R., Berton M. T., Tessier P. A., Tardif M. R., Cesaro A., Bose S. (2014) DAMP molecule S100A9 acts as a molecular pattern to enhance inflammation during influenza A virus infection: role of DDX21-TRIF-TLR4-MyD88 pathway. PLoS Pathog. 10, e1003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alleva L. M., Budd A. C., Clark I. A. (2008) Systemic release of high mobility group box 1 protein during severe murine influenza. J. Immunol. 181, 1454–1459. [DOI] [PubMed] [Google Scholar]
- 18.Momonaka H., Hasegawa S., Matsushige T., Inoue H., Kajimoto M., Okada S., Nakatsuka K., Morishima T., Ichiyama T. (2014) High mobility group box 1 in patients with 2009 pandemic H1N1 influenza-associated encephalopathy. Brain Dev. 36, 484–488. [DOI] [PubMed] [Google Scholar]
- 19.Imai Y., Kuba K., Neely G. G., Yaghubian-Malhami R., Perkmann T., van Loo G., Ermolaeva M., Veldhuizen R., Leung Y. H., Wang H., Liu H., Sun Y., Pasparakis M., Kopf M., Mech C., Bavari S., Peiris J. S., Slutsky A. S., Akira S., Hultqvist M., Holmdahl R., Nicholls J., Jiang C., Binder C. J., Penninger J. M. (2008) Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133, 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirey K. A., Lai W., Scott A. J., Lipsky M., Mistry P., Pletneva L. M., Karp C. L., McAlees J., Gioannini T. L., Weiss J., Chen W. H., Ernst R. K., Rossignol D. P., Gusovsky F., Blanco J. C., Vogel S. N. (2013) The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature 497, 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walton K. A., Hsieh X., Gharavi N., Wang S., Wang G., Yeh M., Cole A. L., Berliner J. A. (2003) Receptors involved in the oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine-mediated synthesis of interleukin-8: a role for Toll-like receptor 4 and a glycosylphosphatidylinositol-anchored protein. J. Biol. Chem. 278, 29661–29666. [DOI] [PubMed] [Google Scholar]
- 22.Yang H., Hreggvidsdottir H. S., Palmblad K., Wang H., Ochani M., Li J., Lu B., Chavan S., Rosas-Ballina M., Al-Abed Y., Akira S., Bierhaus A., Erlandsson-Harris H., Andersson U., Tracey K. J. (2010) A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc. Natl. Acad. Sci. USA 107, 11942–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nhu Q. M., Shirey K., Teijaro J. R., Farber D. L., Netzel-Arnett S., Antalis T. M., Fasano A., Vogel S. N. (2010) Novel signaling interactions between proteinase-activated receptor 2 and Toll-like receptors in vitro and in vivo. Mucosal Immunol. 3, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye J., Sorrell E. M., Cai Y., Shao H., Xu K., Pena L., Hickman D., Song H., Angel M., Medina R. A., Manicassamy B., Garcia-Sastre A., Perez D. R. (2010) Variations in the hemagglutinin of the 2009 H1N1 pandemic virus: potential for strains with altered virulence phenotype? PLoS Pathog. 6, e1001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirey K. A., Lai W., Patel M. C., Pletneva L. M., Pang C., Kurt-Jones E., Lipsky M., Roger T., Calandra T., Tracey K. J., Al-Abed Y., Bowie A. G., Fasano A., Dinarello C. A., Gusovsky F., Blanco J. C., Vogel S. N. (2016) Novel strategies for targeting innate immune responses to influenza. Mucosal Immunol. 9, 1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piao W., Shirey K. A., Ru L. W., Lai W., Szmacinski H., Snyder G. A., Sundberg E. J., Lakowicz J. R., Vogel S. N., Toshchakov V. Y. (2015) A decoy peptide that disrupts TIRAP recruitment to TLRs is protective in a murine model of influenza. Cell Reports 11, 1941–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perrin-Cocon L., Aublin-Gex A., Sestito S. E., Shirey K. A., Patel M. C., André P., Blanco J. C., Vogel S. N., Peri F., Lotteau V. (2017) TLR4 antagonist FP7 inhibits LPS-induced cytokine production and glycolytic reprogramming in dendritic cells, and protects mice from lethal influenza infection. Sci. Rep. 7, 40791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran D., Tran P. A., Tang Y. Q., Yuan J., Cole T., Selsted M. E. (2002) Homodimeric θ-defensins from rhesus macaque leukocytes: isolation, synthesis, antimicrobial activities, and bacterial binding properties of the cyclic peptides. J. Biol. Chem. 277, 3079–3084. [DOI] [PubMed] [Google Scholar]
- 29.Tang Y. Q., Yuan J., Osapay G., Osapay K., Tran D., Miller C. J., Ouellette A. J., Selsted M. E. (1999) A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated α-defensins. Science 286, 498–502. [DOI] [PubMed] [Google Scholar]
- 30.Leonova L., Kokryakov V. N., Aleshina G., Hong T., Nguyen T., Zhao C., Waring A. J., Lehrer R. I. (2001) Circular minidefensins and posttranslational generation of molecular diversity. J. Leukoc. Biol. 70, 461–464. [PubMed] [Google Scholar]
- 31.Arnett E., Lehrer R. I., Pratikhya P., Lu W., Seveau S. (2011) Defensins enable macrophages to inhibit the intracellular proliferation of Listeria monocytogenes. Cell. Microbiol. 13, 635–651. [DOI] [PubMed] [Google Scholar]
- 32.Rothan H. A., Han H. C., Ramasamy T. S., Othman S., Rahman N. A., Yusof R. (2012) Inhibition of dengue NS2B-NS3 protease and viral replication in Vero cells by recombinant retrocyclin-1. BMC Infect. Dis. 12, 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen T. X., Cole A. M., Lehrer R. I. (2003) Evolution of primate θ-defensins: a serpentine path to a sweet tooth. Peptides 24, 1647–1654. [DOI] [PubMed] [Google Scholar]
- 34.Venkataraman N., Cole A. L., Ruchala P., Waring A. J., Lehrer R. I., Stuchlik O., Pohl J., Cole A. M. (2009) Reawakening retrocyclins: ancestral human defensins active against HIV-1. PLoS Biol. 7, e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole A. M., Hong T., Boo L. M., Nguyen T., Zhao C., Bristol G., Zack J. A., Waring A. J., Yang O. O., Lehrer R. I. (2002) Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc. Natl. Acad. Sci. USA 99, 1813–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W., Cole A. M., Hong T., Waring A. J., Lehrer R. I. (2003) Retrocyclin, an antiretroviral θ-defensin, is a lectin. J. Immunol. 170, 4708–4716. [DOI] [PubMed] [Google Scholar]
- 37.Leikina E., Delanoe-Ayari H., Melikov K., Cho M. S., Chen A., Waring A. J., Wang W., Xie Y., Loo J. A., Lehrer R. I., Chernomordik L. V. (2005) Carbohydrate-binding molecules inhibit viral fusion and entry by crosslinking membrane glycoproteins. Nat. Immunol. 6, 995–1001. [DOI] [PubMed] [Google Scholar]
- 38.Owen S. M., Rudolph D. L., Wang W., Cole A. M., Waring A. J., Lal R. B., Lehrer R. I. (2004) RC-101, a retrocyclin-1 analogue with enhanced activity against primary HIV type 1 isolates. AIDS Res. Hum. Retroviruses 20, 1157–1165. [DOI] [PubMed] [Google Scholar]
- 39.Doss M., White M. R., Tecle T., Gantz D., Crouch E. C., Jung G., Ruchala P., Waring A. J., Lehrer R. I., Hartshorn K. L. (2009) Interactions of α-, β-, and θ-defensins with influenza A virus and surfactant protein D. J. Immunol. 182, 7878–7887. [DOI] [PubMed] [Google Scholar]
- 40.Yasin B., Wang W., Pang M., Cheshenko N., Hong T., Waring A. J., Herold B. C., Wagar E. A., Lehrer R. I. (2004) θ defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J. Virol. 78, 5147–5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semple F., MacPherson H., Webb S., Cox S. L., Mallin L. J., Tyrrell C., Grimes G. R., Semple C. A., Nix M. A., Millhauser G. L., Dorin J. R. (2011) Human β-defensin 3 affects the activity of pro-inflammatory pathways associated with MyD88 and TRIF. Eur. J. Immunol. 41, 3291–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Semple F., Webb S., Li H. N., Patel H. B., Perretti M., Jackson I. J., Gray M., Davidson D. J., Dorin J. R. (2010) Human β-defensin 3 has immunosuppressive activity in vitro and in vivo. Eur. J. Immunol. 40, 1073–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaal J. B., Tran D., Tran P., Ösapay G., Trinh K., Roberts K. D., Brasky K. M., Tongaonkar P., Ouellette A. J., Selsted M. E. (2012) Rhesus macaque θ defensins suppress inflammatory cytokines and enhance survival in mouse models of bacteremic sepsis. PLoS One 7, e51337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., Freudenberg M., Ricciardi-Castagnoli P., Layton B., Beutler B. (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088. [DOI] [PubMed] [Google Scholar]
- 45.Dawson P. E., Kent S. B. (2000) Synthesis of native proteins by chemical ligation. Annu. Rev. Biochem. 69, 923–960. [DOI] [PubMed] [Google Scholar]
- 46.Dawson P. E., Muir T. W., Clark-Lewis I., Kent S. B. (1994) Synthesis of proteins by native chemical ligation. Science 266, 776–779. [DOI] [PubMed] [Google Scholar]
- 47.McIntire F. C., Sievert H. W., Barlow G. H., Finley R. A., Lee A. Y. (1967) Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry 6, 2363–2372. [DOI] [PubMed] [Google Scholar]
- 48.Perkins D. J., Polumuri S. K., Pennini M. E., Lai W., Xie P., Vogel S. N. (2013) Reprogramming of murine macrophages through TLR2 confers viral resistance via TRAF3-mediated, enhanced interferon production. PLoS Pathog. 9, e1003479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pennini M. E., Perkins D. J., Salazar A. M., Lipsky M., Vogel S. N. (2013) Complete dependence on IRAK4 kinase activity in TLR2, but not TLR4, signaling pathways underlies decreased cytokine production and increased susceptibility to Streptococcus pneumoniae infection in IRAK4 kinase-inactive mice. J. Immunol. 190, 307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prantner D., Perkins D. J., Lai W., Williams M. S., Sharma S., Fitzgerald K. A., Vogel S. N. (2012) 5,6-Dimethylxanthenone-4-acetic acid (DMXAA) activates stimulator of interferon gene (STING)-dependent innate immune pathways and is regulated by mitochondrial membrane potential. J. Biol. Chem. 287, 39776–39788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lafferty M. K., Sun L., DeMasi L., Lu W., Garzino-Demo A. (2010) CCR6 ligands inhibit HIV by inducing APOBEC3G. Blood 115, 1564–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmittgen T. D., Livak K. J. (2008) Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108. [DOI] [PubMed] [Google Scholar]
- 53.Rallabhandi P., Nhu Q. M., Toshchakov V. Y., Piao W., Medvedev A. E., Hollenberg M. D., Fasano A., Vogel S. N. (2008) Analysis of proteinase-activated receptor 2 and TLR4 signal transduction: a novel paradigm for receptor cooperativity. J. Biol. Chem. 283, 24314–24325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toshchakov V. Y., Szmacinski H., Couture L. A., Lakowicz J. R., Vogel S. N. (2011) Targeting TLR4 signaling by TLR4 Toll/IL-1 receptor domain-derived decoy peptides: identification of the TLR4 Toll/IL-1 receptor domain dimerization interface. J. Immunol. 186, 4819–4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saitoh S., Akashi S., Yamada T., Tanimura N., Kobayashi M., Konno K., Matsumoto F., Fukase K., Kusumoto S., Nagai Y., Kusumoto Y., Kosugi A., Miyake K. (2004) Lipid A antagonist, lipid IVa, is distinct from lipid A in interaction with Toll-like receptor 4 (TLR4)-MD-2 and ligand-induced TLR4 oligomerization. Int. Immunol. 16, 961–969. [DOI] [PubMed] [Google Scholar]
- 56.Kawai T., Adachi O., Ogawa T., Takeda K., Akira S. (1999) Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11, 115–122. [DOI] [PubMed] [Google Scholar]
- 57.Hoebe K., Du X., Georgel P., Janssen E., Tabeta K., Kim S. O., Goode J., Lin P., Mann N., Mudd S., Crozat K., Sovath S., Han J., Beutler B. (2003) Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424, 743–748. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto M., Sato S., Mori K., Hoshino K., Takeuchi O., Takeda K., Akira S. (2002) Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J. Immunol. 169, 6668–6672. [DOI] [PubMed] [Google Scholar]
- 59.Seo S. U., Kwon H. J., Song J. H., Byun Y. H., Seong B. L., Kawai T., Akira S., Kweon M. N. (2010) MyD88 signaling is indispensable for primary influenza A virus infection but dispensable for secondary infection. J. Virol. 84, 12713–12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shinya K., Okamura T., Sueta S., Kasai N., Tanaka M., Ginting T. E., Makino A., Eisfeld A. J., Kawaoka Y. (2011) Toll-like receptor pre-stimulation protects mice against lethal infection with highly pathogenic influenza viruses. Virol. J. 8, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shinya K., Ito M., Makino A., Tanaka M., Miyake K., Eisfeld A. J., Kawaoka Y. (2012) The TLR4-TRIF pathway protects against H5N1 influenza virus infection. J. Virol. 86, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tan A. C., Mifsud E. J., Zeng W., Edenborough K., McVernon J., Brown L. E., Jackson D. C. (2012) Intranasal administration of the TLR2 agonist Pam2Cys provides rapid protection against influenza in mice. Mol. Pharm. 9, 2710–2718. [DOI] [PubMed] [Google Scholar]
- 63.Chen W. H., Toapanta F. R., Shirey K. A., Zhang L., Giannelou A., Page C., Frieman M. B., Vogel S. N., Cross A. S. (2012) Potential role for alternatively activated macrophages in the secondary bacterial infection during recovery from influenza. Immunol. Lett. 141, 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang R. H., Li C. H., Wang C. L., Xu M. J., Xu T., Wei D., Liu B. J., Wang G. H., Tian S. F. (2014) N-acetyl-l-cystine (NAC) protects against H9N2 swine influenza virus-induced acute lung injury. Int. Immunopharmacol. 22, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Da Silva Correia J., Ulevitch R. J. (2002) MD-2 and TLR4 N-linked glycosylations are important for a functional lipopolysaccharide receptor. J. Biol. Chem. 277, 1845–1854. [DOI] [PubMed] [Google Scholar]
- 66.Feng C., Stamatos N. M., Dragan A. I., Medvedev A., Whitford M., Zhang L., Song C., Rallabhandi P., Cole L., Nhu Q. M., Vogel S. N., Geddes C. D., Cross A. S. (2012) Sialyl residues modulate LPS-mediated signaling through the Toll-like receptor 4 complex. PLoS One 7, e32359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu C., Bao N. R., Chen S., Zhao J. N. (2016) HBD-3 regulation of the immune response and the LPS/TLR4-mediated signaling pathway. Exp. Ther. Med. 12, 2150–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nagai Y., Akashi S., Nagafuku M., Ogata M., Iwakura Y., Akira S., Kitamura T., Kosugi A., Kimoto M., Miyake K. (2002) Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat. Immunol. 3, 667–672. [DOI] [PubMed] [Google Scholar]
- 69.Weber A. N., Morse M. A., Gay N. J. (2004) Four N-linked glycosylation sites in human toll-like receptor 2 cooperate to direct efficient biosynthesis and secretion. J. Biol. Chem. 279, 34589–34594. [DOI] [PubMed] [Google Scholar]
- 70.Parker L. C., Prestwich E. C., Ward J. R., Smythe E., Berry A., Triantafilou M., Triantafilou K., Sabroe I. (2008) A phosphatidylserine species inhibits a range of TLR- but not IL-1β–induced inflammatory responses by disruption of membrane microdomains. J. Immunol. 181, 5606–5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abuja P. M., Zenz A., Trabi M., Craik D. J., Lohner K. (2004) The cyclic antimicrobial peptide RTD-1 induces stabilized lipid–peptide domains more efficiently than its open-chain analogue. FEBS Lett. 566, 301–306. [DOI] [PubMed] [Google Scholar]
- 72.Bigda J., Beletsky I., Brakebusch C., Varfolomeev Y., Engelmann H., Bigda J., Holtmann H., Wallach D. (1994) Dual role of the p75 tumor necrosis factor (TNF) receptor in TNF cytotoxicity. J. Exp. Med. 180, 445–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kurt-Jones E. A., Popova L., Kwinn L., Haynes L. M., Jones L. P., Tripp R. A., Walsh E. E., Freeman M. W., Golenbock D. T., Anderson L. J., Finberg R. W. (2000) Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1, 398–401. [DOI] [PubMed] [Google Scholar]
- 74.Datta S., Novotny M., Li X., Tebo J., Hamilton T. A. (2004) Toll IL-1 receptors differ in their ability to promote the stabilization of adenosine and uridine-rich elements containing mRNA. J. Immunol. 173, 2755–2761. [DOI] [PubMed] [Google Scholar]
- 75.Tate M. D., Brooks A. G., Reading P. C. (2011) Specific sites of N-linked glycosylation on the hemagglutinin of H1N1 subtype influenza A virus determine sensitivity to inhibitors of the innate immune system and virulence in mice. J. Immunol. 187, 1884–1894. [DOI] [PubMed] [Google Scholar]
- 76.Zhang M., Gaschen B., Blay W., Foley B., Haigwood N., Kuiken C., Korber B. (2004) Tracking global patterns of N-linked glycosylation site variation in highly variable viral glycoproteins: HIV, SIV, and HCV envelopes and influenza hemagglutinin. Glycobiology 14, 1229–1246. [DOI] [PubMed] [Google Scholar]
- 77.Wohlford-Lenane C. L., Meyerholz D. K., Perlman S., Zhou H., Tran D., Selsted M. E., McCray P. B. Jr (2009) Rhesus θ-defensin prevents death in a mouse model of severe acute respiratory syndrome coronavirus pulmonary disease. J. Virol. 83, 11385–11390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.