The identification of c-Myc as a potential PSGL-1 transcriptional regulator facilitates the determination of the physiological significance of PSGL-1 induction, and provides novel therapeutic targets.
Keywords: HIV, PSGL-1, Boolean modeling, CD40L, glutamate
Abstract
Leukocyte extravasation is a crucial feature of the normal immune response to disease and infection and is implicated in various pathologies during chronic inflammatory disease. P-Selectin glycoprotein ligand-1 (PSGL-1) is critical for leukocyte extravasation; however, despite extensive study, it remains unclear how its expression is regulated, which in turn, impedes a more precise understanding of how its expression level affects transmigration. To investigate the regulation of PSGL-1, 60 subjects, with or without HIV infection, were recruited and PSGL-1 expression in monocytes was measured. PSGL-1 was found to be up-regulated on leukocytes from HIV-infected individuals, and the physiologically relevant mediators soluble CD40 ligand (sCD40L) and glutamate were able to induce PSGL-1 transcription in human monocytes ex vivo. HIV-1 induced PSGL-1 induction, and its dependence on CD40L was validated further by use of the mouse-tropic HIV (EcoHIV) mouse model of HIV infection in C57BL/6 and CD40L knockout (KO) mice. To investigate crosstalk between the signaling cascades induced by CD40L and glutamate that lead to PSGL-1 induction, a network-based, discrete dynamic model was developed. The model reveals the MAPK pathway and oxidative stress as critical mediators of crosstalk between CD40L and glutamate-induced pathways. Importantly, the model predicted induction of the c-Myc transcription factor upon cotreatment, which was validated using transcriptomic data and pharmacologic inhibition of c-Myc. This study suggests a novel systems serology approach for translational research and reveals a mechanism for PSGL-1 transcriptional regulation, which might be leveraged to identify novel targets for therapeutic intervention.
Introduction
Excessive tissue infiltration of immune cells has been shown to be important to the pathology of various diseases, including chronic infections, such as HIV [1] and hepatitis C virus [2] infections, and CVDs, including atherosclerosis [3], among others. In many of these diseases, whereas excessive leukocyte extravasation may not be the underlying causal factor, it contributes significantly to the disease pathology and is often closely linked to disease morbidity and mortality. Thus, the targeting of leukocyte transmigration is a promising approach for the development of novel therapeutics to treat complications associated with these diseases [4].
Leukocyte transmigration is a multistep process [5]. Of note, leukocytes must first attach to the endothelium, mediated primarily by the binding of PSGL-1 (also known as CD162 or selectin P ligand), expressed on leukocytes and P-selectin, expressed on the surface of endothelial cells. In monocytes and granulocytes, the machinery required for the proper post-translation modification of PSGL-1 is constitutively expressed; however, in T cells, this machinery is induced upon exposure to activation signals [6]. Furthermore, the signaling through PSGL-1 has been reported to promote integrin expression and activation [7]. Additionally, various proinflammatory mediators have been reported to prime leukocytes for integrin activation [8, 9]. Finally, the various ligands for leukocyte-expressed transmigratory molecules are induced on endothelial cells upon endothelial cell activation [10]. Increased PSGL-1 expression has also been associated with various inflammatory conditions, including a mouse model of acute inflammation [11], individuals suffering from allergic asthma [12], and individuals with chronic obstructive pulmonary disease [13].
Whereas increased PSGL-1 expression would likely lead to increased adhesion and transmigration, this has yet to be formally proven. There are 2 potential difficulties in establishing this point adequately: 1) it is unclear what factor or factors are responsible for PSGL-1 induction in any of the contexts in which it has been reported, and 2) the mechanisms of transcriptional regulation for PSGL-1 induction are unknown. This work aims to establish what soluble factors and induced transcription factors are responsible for PSGL-1 induction during HIV infection.
HIV, the causative agent of AIDS, infects ∼36.7 million people worldwide [14]; however, with the advent of cART, the life expectancy of infected individuals is increased significantly compared with the pre-cART era [15]. Still, whereas the life expectancy of infected individuals is nearing that of uninfected individuals, it has become apparent that they are at increased risk for a variety of secondary disorders [16]. Importantly, these secondary complications are now significant contributors to morbidity and mortality in the HIV+ population, having overtaken AIDS-defining illnesses as the leading causes of death in this population [17, 18]. One complication is CVD, with HIV+ individuals at increased risk for myocardial infarction and displaying accelerated atherosclerotic plaque formation [19]. Another important complication is HAND, which is defined by psychomotor deficits, with the most severe form, dementia [20]. A related disorder, HIV encephalopathy, is defined, in part, by an accumulation of peripheral Mϕ in brain tissue (identified postmortem). Atherosclerosis is similarly understood to result from the accumulation of Mϕ in arterial walls, thought to be derived primarily from circulating monocytes upon entry into tissue [21], although there may be other sources in addition to circulating monocytes. Thus, a better understanding of leukocyte transmigration during HIV infection may facilitate efforts to target this process therapeutically for the mitigation of various HIV-associated disorders.
Here, we show that the PSGL-1 expression is increased in leukocytes following HIV infection, likely via the combined actions of glutamate and sCD40L, 2 well-characterized proinflammatory mediators that are implicated in the HIV pathogenesis. Elevated levels of PSGL-1 were also observed in monocytes from C57BL/6 mice but not CD40L-deficient mice infected with a novel EcoHIV, further confirming the role of CD40L in this process. Network-based, discrete-dynamic modeling of signaling cascades induced by sCD40L and glutamate suggests a role for c-Myc in mediating transcription of PSGL-1. The prediction was validated using transcriptomic data and pharmacologic inhibition of c-Myc. Thus, this work reveals novel, soluble factors that trigger PSGL-1 expression and sheds light on the molecular mechanisms that may influence tissue infiltration of immune cells during HIV infection.
MATERIALS AND METHODS
Ethics statement
The Research Subjects Review Board at the University of Rochester Medical Center approved studies involving human samples. All study participants were adults, and blood samples were obtained after written, informed consent, in accordance with the Declaration of Helsinki. Mouse experiments were carried out in accordance with the Animal Welfare Act and NIH guidelines, and the University Committee on Animal Resources of the University of Rochester Medical Center approved the animal protocol. The facilities and programs of the Vivarium and Division of Laboratory Animal Medicine of the School of Medicine and Dentistry are fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International.
Patient samples
Persons with (n = 32) and without (n = 28) HIV infection, without any occurrence of CVD for at least the preceding year, were recruited at the University of Rochester Medical Center. This sample size was determined based on initial power analysis in which it was found that recruiting n = 25 subjects in each group yields >90% of statistical power. The average age of these individuals was 55 ± 11 (means ± sd) yr. Subjects who reported current anti-platelet/nonsteroidal anti-inflammatory drug use were excluded from the study. All persons with HIV infection were on ART at the time of blood donation with undetectable viral loads and average T cell count in the range of 652 ± 405 per μl. The blood was drawn from these subjects into acid citrate dextrose-buffered vacutainers (BD Biosciences, San Jose, CA, USA), and various analyses were performed as outlined below.
LC-MS/MS
Whole blood from subjects was centrifuged at 250 g for 15 min, and platelet-rich plasma was collected. Following addition of PGI2 (1 μl/ml platelet-rich plasma; Cayman Chemical, Ann Arbor, MI, USA) to maintain platelet quiescence, platelet-rich plasma was centrifuged at 1000 g for 10 min to pellet the platelets. The resulting platelet-poor plasma was transferred to HPLC sample vials and analyzed with reverse-phase LC, coupled to a triple-quadrupole mass spectrometer running in negative mode (Thermo Quantum Ultra; Thermo Fisher Scientific, Waltham, MA, USA) with SRM-specific scans. The resulting data were analyzed by using the publicly available mzRock machine learning tool kit (http://code.google.com/p/mzRock), which automates SRM/HPLC feature detection, grouping, signal-to-noise classification, and comparison with known metabolite retention times. Standard solutions of glutamate were also prepared and run to allow for determination of the absolute concentration of glutamate in the plasma samples.
Monocyte isolation and culture
Human monocytes were isolated using a MACS Pan Monocyte Isolation Kit (Miltenyi Biotec, San Diego, CA, USA), per the manufacturer’s instructions. Monocytes were cultured in RPMI 1640, supplemented with 10% FBS and 2% penicillin/streptomycin/glutamine. For immunoblot assays and flow cytometric analyses, monocytes were treated for 24 h, whereas for PCR analysis, they were treated for 18 h. In both cases, cells were incubated overnight at 37°C and 5% CO2 before being treated.
Flow cytometry
Human leukocytes were analyzed using a method described previously [22] within 1 h of the blood draw. In brief, 100 μl blood was fixed, and RBCs were lysed and then stained for 30 min, with 1.25 μl CD14-APC/Cy7 (BioLegend, San Diego, CA, USA), 2.5 μl CD16-Pacific Blue (BD Biosciences), and 2.5 μl CD162-PerCP-eFluor 710 (eBioscience, San Diego, CA, USA). Following the staining, cells were acquired using a flow cytometer (BD LSRFortessa 18 color; BD Biosciences). Human monocytes, granulocytes, and lymphocytes were gated based on characteristic FSC and SSC; the monocytes were identified further via CD14 staining. The monocyte subsets were defined by CD14 and CD16 expression by using the following criteria: CD14dimCD16+ (nonclassic monocytes), CD14highCD16+ (intermediate monocytes), and CD14highCD16− (classic monocytes). Fluorescence minus one controls were used to define gates. For mouse leukocytes, 50 μl whole blood was prepared as above, but the antibodies used were as follows: 2.5 μl CD115-APC (eBioscience), 1.5 μl Ly-6C/6G-PE/Cy7, 2.5 μl CD19-AF700, 2.5 μl CD3-BV786, and 5 μl CD162-PE (BD Biosciences).
Immunoblot assay
Immunoblot assay was performed as described previously [23]. In brief, after the indicated treatments, whole-cell lysates were prepared in erythrocytes lysis buffer [50 mM HEPES, pH 7, 250 mM NaCl, 0.1% Nonidet P-40, 5 mM EDTA, 10 mM NaF, 0.1 mM Na3VO4, and 50 µM ZnCl2, supplemented with 0.1 mM PMSF, 1 mM DTT, and Phosphatase Inhibitor Cocktail 3 (P0044; Sigma-Alrich, St. Louis, MO, USA)]. After removal of cellular debris via high-speed centrifugation (13,000 rpm, 5 min), lysates were fractionalized on 7.5% SDS-PAGE gels, and protein was electrophoretically transferred to nitrocellulose membranes (GE Healthcare Bio-Sciences, Piscataway, NJ, USA). The membranes were then analyzed for immunoreactivity against the following antibodies: PSGL-1 (clone KPL1) and α-tubulin (both from Santa Cruz Biotechnology, Dallas, TX, USA). Bound antibodies were detected using species-specific infrared dye (IRDye)-conjugated secondary antibodies and subsequently developed using the Odyssey infrared imager (Li-Cor Biotechnology, Lincoln, NE, USA).
qRT-PCR
Changes in the expression of the PSGL-1 gene from treated, primary human monocytes (from healthy HIV− donors) were analyzed by qRT-PCR. Total RNA was isolated using TRIzol, according to the manufacturer’s protocol (Qiagen, Valencia, CA, USA). First-strand cDNA synthesis was then performed using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA, USA), and qRT-PCR was completed using the SYBR Green DNA polymerase (Thermo Fisher Scientific), and the following primers were used: human PSGL-1 (forward) 5′-TCCTCCTGTTGCTGATCCTACTG-3′ and (reverse) 5′-TACTCATATTCGGTGGCCTGTCT-3′ and the housekeeping gene GAPDH (forward) 5′-TGATGACATCAAGAAGGTGGTGAA-3′ and (reverse) 5′-TCCTTGGAGGCCATFTAGGCCAT-3′.
EcoHIV virus preparation
Virus stocks were prepared by transfection of plasmid DNA into 293T human embryonic kidney cells and titered for their p24 HIV core antigen by p24 ELISA (PerkinElmer, Waltham, MA, USA). In brief, 7.5 × 105 293T cells/well were cultured in a 6-well plate and transfected with 4 μg EcoHIV DNA using polyethylenimine. 293T Cell culture supernatants were harvested 48 h after transfection and concentrated by centrifugation at 22,000 g for 2 h at 4°C. The supernatant was then removed, and the pellet was resuspended in 300 μl DMEM (Thermo Fisher Scientific), aliquoted, and stored at −80°C. The EcoHIV construct was kindly provided by Dr. David Volsky (Icahn School of Medicine at Mount Sinai, New York, NY, USA).
EcoHIV infection of mice
C57BL/6 (WT) or B6.129s2-CD40lgtm1mx/J (CD40L KO) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Ten- to 17-wk-old male mice (WT, n = 6; CD40L KO, n = 6) were given i.p. injections of either saline or EcoHIV (1.0 × 105 pg p24), as described previously [24]. At 2 wk postinfection, blood was collected via cardiac exsanguinations and prepared for flow cytometric analyses, as outlined above.
Chemical and protein reagents
sCD40L, TNF-α, and IL-1β (R&D Systems, Minneapolis, MN, USA) were used at a concentration of 1 μg/μl and 10 and 1 ng/ml, respectively. L-Glutamic acid and carbamyl PAF (a nonhydrolyzable analog of PAF) were purchased from Sigma-Aldrich and used at a concentration of 5 ng/ml or 20 nM, respectively. ADP was purchased from Chrono-log (Havertown, PA, USA) and used at a concentration of 5 µM. MK801, CNQX, and 10058-F4 (Abcam, Cambridge, MA, USA) were used at concentrations of 10, 10, or 100 µM, respectively. S4CPG and actinomycin D (Tocris Bioscience, Avonmouth, Bristol, United Kingdom) were used at concentrations of 1 mM and 10 µg/ml, respectively.
Network assembly
The network was constructed by consulting a large number of primary research manuscripts and reviews, as well as signaling databases (KEGG [25]), to determine potential signaling pathways associated with CD40L. To synthesize signaling information upon induction by GSH, signaling mediators’ activity known to be modulated by ROS and GSH was used. The target nodes of the network were transcription factors that were chosen based on binding-site prediction within the 2 kb nucleotide sequence upstream of the PSGL-1 start site, as reported in the Encyclopedia of DNA Elements. However, only a subset of these was found to be connected to CD40L/glutamate-induced pathways, based on the available literature. Thus, network nodes represent proteins, and directional edges connecting nodes represent either activation or inhibition of the downstream nodes.
Discrete dynamic model implementation
The network was developed into discrete dynamical models by characterizing each node with a variable that can take the ON state when the concentration or activity is above the threshold level necessary to activate downstream immune processes or the OFF state when activity is below this threshold. The evolution of the state of each node was described by a Boolean transfer function. The operator AND was used to describe a synergistic or conditional interaction between 2 or more nodes that is necessary to activate the downstream node. When either of the nodes was sufficient for the activation of the downstream node we used the operator OR. An inhibitory effect was represented by an AND NOT operator. In cases where prior biologic information did not completely determine the transfer functions (e.g., there was no information whether 2 coincident regulatory effects are independent or synergistic), different alternative transfer functions were tested. The transfer functions that reproduced the qualitative features of the sCD40L-induced signaling were selected. For those nodes whose transfer function includes both positive and negative regulatory arguments and for which there was >1 of either, the total number of TRUE positive and negative regulatory arguments was balanced to determine the nodes’ updated state [26]. Thus, more TRUE positive regulatory arguments than negative regulatory arguments will result in the node’s update status being TRUE. The Boolean transfer functions applied in our model provide a mechanistic understanding of the crosstalk between CD40L and glutamate-induced signaling pathways.
The status of the system across time was simulated by repeatedly applying the Boolean rules for each node until a stationary state was found (e.g., no change in the activities of the following key nodes: p52, p65, STAT protein, CTCF, ERK, JNK, p38, GSH, GSSG, O2, and H2O2). As the kinetics and time scales of the individual processes represented as edges are not known, a random order asynchronous update was selected, wherein the time scales of each regulatory process were randomly chosen in such a way that the node states were updated in a randomly selected order during each time step [27–29]. The asynchronous algorithm was Xit = Fi(Xata, Xbtb, Xctc,…), where F is the Boolean transfer function for node i, and ta, tb, and tc represent the time points corresponding to the last change in the state of the input nodes a, b, c and can be in the previous or current time step. The time step (time unit) of our model corresponds approximately to 1.5 h, based on the time of PSGL-1 transcriptional induction, and predicted c-Myc activity reported here. The randomized asynchronicity of the model does not alter the steady states of the dynamic system but causes stochasticity in the trajectory between the initial conditions and the equilibria (attractors); thus, it can sample more diverse behaviors compared with traditional synchronous models. To determine the node consensus activity over time (i.e., shared trajectories with different update orders), the simulations were run 800 times. The simulated activity profiles for each node were estimated by calculating the fraction of simulations in which the nodes were in an ON state at each time step. Furthermore, network perturbations, corresponding to constitutive activation and KO simulations, were simulated by freezing the state of a node to TRUE (constitutive activation) or FALSE (KO) for the duration of the simulation. This process was conducted for each node in the network, for each treatment simulation conducted.
Our approach of using discrete dynamic modeling allowed us to sample the time scales of interactions and perform replicate simulations, as well as provide continuously varying activities of the network nodes over time, which ranged between the lower limit of 0 (below-threshold concentration in all runs) and upper limit of 1 (above-threshold concentration in all runs).
Transcriptomic data analysis
A previously published HIV infection dataset (GSE52900) was used for the transcriptomic data analysis. In brief, the study purified CD14+ monocytes from whole blood, collected from 5 HIV− donors and 4 HIV+ donors with undetectable viral loads. Total RNA was isolated and analyzed for transcript expression using HumanHT-12 v3 Expression BeadChips (Illumina, San Diego, CA, USA). Log2-transformed and robust spline-normalized data were obtained from the Gene Expression Omnibus. Lists of differentially expressed genes were obtained from a prior publication [30], which was identified by a linear model fit in conjunction with empirical Bayes statistics. These data were analyzed here for transcription factor-binding site enrichment by a hypergeometric test, and transcription factor targets identified in previous publications were used [31]. A total of 722 binding sites were tested, and the P values were corrected for multiple hypothesis testing by the Benjamini-Hochberg method. These binding sites mapped to 654 transcription factors. Additionally, Gene Set Enrichment Analysis [32] was performed using all gene sets available from the Molecular Signatures Database, and P values were corrected for multiple hypothesis testing as above. All analysis was performed using the Bioconductor software package in R [33].
Statistical analysis
Descriptive statistics are reported as means ± sd throughout the manuscript. One-way ANOVA with Bonferroni post hoc test was used in analyzing data (see Figs. 2 and 3). Two-way ANOVA F-test for main effects and interaction and Bonferroni post hoc test were applied to data (see Figs. 1, 2, and 7). Student’s t test was applied to data (see Figs. 1 and 3), and the Pearson correlation test was used to evaluate correlation between PSGL-1 and CD163 levels. These statistical analyses were conducted using the Prism statistical package (GraphPad Software, La Jolla, CA, USA).
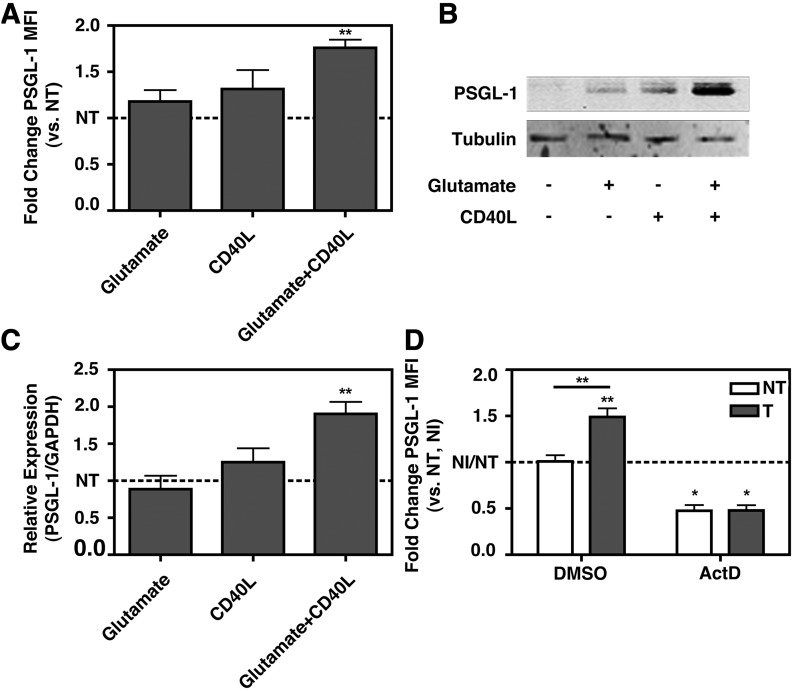
Figure 2. sCD40L and glutamate together induce monocyte PSGL-1 expression ex vivo via transcriptional up-regulation.
Monocytes were isolated from 3 HIV− donors by positive selection against CD14 after density gradient isolation of PBMCs. The monocytes were treated with glutamate, sCD40L, or both for 24 h, and PSGL-1 expression was determined by (A) flow cytometry or (B) immunoblot assay. PSGL-1 expression is increased with sCD40L + glutamate but not sCD40L or glutamate alone. (A) Data were normalized to the not-treated, without CD40L + glutamate (NT) value for each replicate and analyzed by 1-way ANOVA F-test, P = 0.0335, or 1 sample t test vs. NT (1.000), glutamate + CD40L P = 0.0135. (C) Monocytes were prepared and treated as in A and B, but total RNA was collected at 18 h post-treatment, and PSGL-1 transcript levels were determined by qRT-PCR. PSGL-1 transcription is increased only with the combination treatment. One-way ANOVA F-test, P = 0.0023, or 1 sample t test vs. NT (1.000), glutamate + CD40L P < 0.01. (D) Monocytes prepared and treated as in A and B, with or without the addition of actinomycin D, and PSGL-1 expression was analyzed by flow cytometry. Transcriptional up-regulation is required for sCD40L + glutamate-mediated PSGL-1 induction. Two-way ANOVA, treatment: P = 0.0089; inhibitor: P < 0.0001; interaction: P = 0.0098; post hoc test: NT vs. treated with CD40L + glutamate (T), **P < 0.01; 1 sample t test vs. NT (1.000), P < 0.05. *P < 0.05, **P < 0.01. NI, No inhibitor, (without actinomycin D).
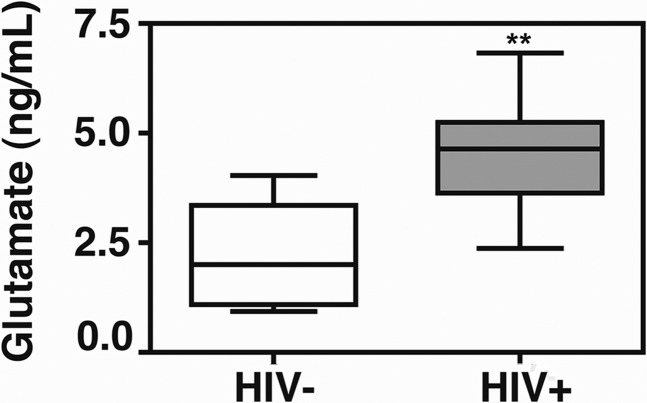
Figure 3. Plasma glutamate is increased with HIV infection.
Plasma from 8 HIV− and 8 cART-treated HIV+ donors with undetectable viral loads was collected and frozen and then analyzed by LC-MS/MS for glutamate expression. Expression was normalized to known standards. Plasma glutamate is increased significantly with HIV infection. Student’s t test, **P = 0.0036.
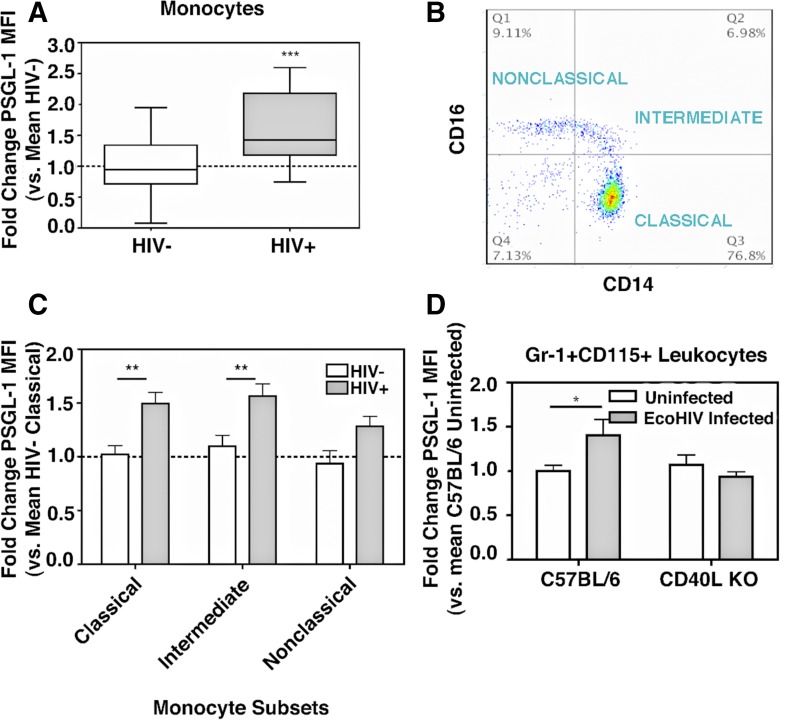
Figure 1. Monocyte PSGL-1 and plasma glutamate are induced with HIV infection and murine EcoHIV infection.
(A) Whole blood was collected from 32 HIV− donors and 28 cART-treated HIV+ donors with undetectable viral loads and analyzed by flow cytometry. Monocytes were identified by FSC/SSC and then CD14 staining and analyzed for PSGL-1 MFI. PSGL-1 is increased significantly with HIV infection. ***P = 0.0204, Student’s t-test. (B) Monocytes subsets were identified by CD14 and CD16 staining, and (C) PSGL-1 expression was determined in each subset and analyzed by 2-way ANOVA F-test. HIV+ vs. HIV−: P = 0.0011; subsets: P = 0.0068; post hoc tests: HIV+ vs. HIV−; Classic: **P < 0.01; Intermediate: **P < 0.01. (D) WT C57BL/6 mice (n = 6) and CD40 KO (n = 6) mice were infected with EcoHIV (1.0 × 105 pg p24), and blood was collected by cardiac exsanguination at 2 wk postinfection and analyzed by flow cytometry. Monocytes were identified by FSC/SSC and then by CD115 and Gr-1 staining and analyzed for PSGL-1 expression. Monocyte PSGL-1 expression is increased with EcoHIV infection in a CD40L-dependent manner. Two-way ANOVA, interaction: P = 0.0269; post hoc test: Uninfected vs. EcoHIV Infected, *P < 0.05.
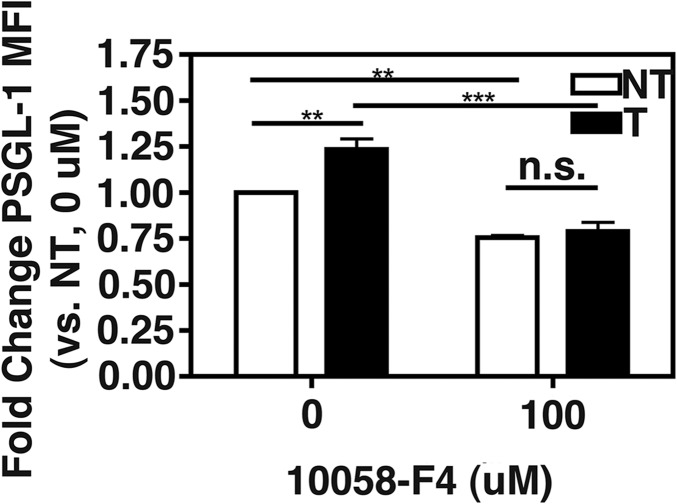
Figure 7. c-Myc-MAX dimerization inhibition by 10058-F4 prevents sCD40L + glutamate-induced PSGL-1 on monocytes ex vivo.
Monocytes were isolated from 3 HIV− donors by positive selection against CD14 after density gradient isolation of PBMCs. The monocytes were treated with glutamate and sCD40L and/or the c-Myc/MAX dimerization inhibitor, 10058-F4, for 24 h, and PSGL-1 expression was determined by flow cytometry. c-Myc/MAX is required for sCD40L + glutamate-induced PSGL-1 expression on monocytes ex vivo Two-way ANOVA, Treatment: P = 0.0078, Inhibitor: P < 0.0001, Interaction: P = 0.0303, Post-test: not treated, without CD40L + glutamate (NT) vs. treated, with CD40L + glutamate (T), P < 0.01, **P < 0.01, ***P < 0.001. NI, No inhibitor, without 10058-F4.
RESULTS
Expression of PSGL-1 is increased in monocytes of HIV-infected persons
Based on previous reports of PSGL-1 induction during other inflammatory conditions, including uveitis, diabetes, and chronic obstructive pulmonary disorder [11–13], PSGL-1 expression in monocytes from HIV− and HIV+ individuals was investigated via flow cytometry and recorded as MFI. In these experiments, monocytes were identified by FSC/SSC, followed by positive staining with CD14-specific antibodies. As shown in Fig. 1A, the monocytes derived from HIV-infected individuals exhibited greater levels of PSGL-1. Furthermore, these monocytes showed an upward trend in the expression of activation marker CD40 and a nonsignificant decrease in CD163 expression (data not shown), with no apparent correlation between PSGL-1 and CD163 levels (Supplemental Fig. 1). In addition, statistically significant increases in PSGL-1 levels were observed in lymphocytes and granulocytes of HIV+ subjects (Supplemental Fig. 2A and B, respectively). This observation contrasts with the findings reported by Liang et al. [34], who showed that the expression of PSGL-1 is down-regulated in monocyte subsets during HIV infection in a manner that is negatively correlated with the plasma levels of sCD163, although an inverse correlation between CD163 expression on monocytes and the plasma levels of sCD163 was not observed in this study, as reported by other groups [35].
As Liang et al. [34] also reported a decrease in PSGL-1 in all monocyte subsets, monocytes were gated, according to the expression levels of CD14 and CD16 (Fig. 1B), using the very same criteria defined by Liang et al. [34], and PSGL-1 levels were assessed. Consistent with the increased PSGL-1 expression observed in the bulk monocyte population (Fig. 1A), all 3 monocyte subtypes were found to contain elevated levels of PSGL-1 (Fig. 1C). One of the key differences between the cohort studied by Liang et al. [34] and the cohort studied here is the use of cART; therefore the following experiments were conducted to determine if infection alone could lead to an increase in PSGL-1 expression.
Expression of PSGL-1 in monocytes is increased following EcoHIV infection of mice in a CD40L-dependent manner
C57BL/6 mice were infected for 2 wk with the EcoHIV. This chimeric virus was developed previously by Volsky and colleagues [36] using HIV-1/NL4-3, where the segment encoding for the protein gp120, responsible for receptor binding, was replaced with the sequence for the corresponding murine leukemia virus type 1 protein, gp80. As previously reported, this virus is able to infect mice productively, including the induction of inflammatory markers and tissue infiltration of immune cells [24, 36–38]. Compared with control mice that were left uninfected, the PSGL-1 expression was found to be increased in CD115+GR1+ monocytes (WT; Fig. 1D), as well as in CD3+ T cells and CD19+ B cells (Supplemental Fig. 3A and B, respectively).
As HIV replication is restricted in monocytes, with productive infection requiring their differentiation into Mϕ [39–41], HIV may stimulate production of PSGL-1 in monocytes indirectly via the promotion of an abundance of proinflammatory mediators in the circulation. As CD40L signaling is reported to be perturbed following excess production of sCD40L in HIV-infected individuals, as well as being implicated in the pathogenesis of HAND [42–45] and atherosclerosis [46, 47], it was tested whether CD40L is required for HIV-induced PSGL-1 induction. CD40L-deficient mice (CD40L KO) were infected for 2 wk with EcoHIV, and PSGL-1 expression was determined. In the CD40L KO mice, EcoHIV infection failed to induce PSGL-1 expression in CD115+GR-1+ monocytes (CD40L KO; Fig. 1D), as well as in CD3+ T cells and CD19+ B cells (Supplemental Fig. 3A and B, respectively). Complementary to this observation, a significant interaction between CD40L and EcoHIV infection, with regard to PSGL-1 expression, was noted in CD3+ T cells of WT mice (interaction, P = 0.0269; Supplemental Fig. 3A), whereas EcoHIV-stimulated PSGL-1 expression in CD19+ B cells was found to be independent of CD40L (Supplemental Fig. 3B). Together, these data suggest that EcoHIV infection alone results in increased PSGL-1 expression in leukocytes, corroborating the observations noted in HIV-infected people (Fig. 1A–C), and that this induction is largely CD40L dependent.
Glutamate and sCD40L together are able to induce PSGL-1 expression, via transcriptional up-regulation, in primary human monocytes
To determine what factor(s) might mediate the observed increase in PSGL-1 levels in monocytes of HIV+ persons, a number of soluble factors reported to be increased with HIV infection were tested. Monocytes from healthy, HIV− donors were isolated and treated with TNF-α, IL-1β, ADP, and PAF for 24 h, after which, PSGL-1 expression was determined by flow cytometry. None of these factors alone were able to induce PSGL-1 expression in monocytes (data not shown). Additionally, both sCD40L and glutamate were tested alone or in combination. Whereas 24 h treatment with either factor individually failed to induce PSGL-1 expression, as determined by flow cytometry, the combination of sCD40L and glutamate was able to induce a significant increase in PSGL-1 expression on the surface of monocytes (Fig. 2A). This increase was confirmed by immunoblotting of whole-cell lysate (24 h post-treatment; Fig. 2B) and at mRNA level by qRT-PCR (18 h post-treatment; Fig. 2C). Finally, it was determined that this induction required transcription, as actinomycin D treatment prevents PSGL-1 induction by the combination of sCD40L and glutamate (24 h post-treatment; Fig. 2D). Thus, the combination of sCD40L and glutamate is able to induce a transcription-dependent increase of PSGL-1 in human monocytes. The data may also indicate positive feedback in PSGL-1 regulation, as basal expression was also decreased with transcriptional inhibition; alternatively, this may represent rapid turnover of PSGL-1.
To investigate how glutamate may trigger synthesis of PSGL-1, pharmacologic inhibitors that block 2 major classes of glutamate receptors (NMDA subtype and AMPA/kainate receptor) were tested. The NMDA receptor channel antagonist MK801 or the AMPA receptor antagonist CNQX failed to block PSGL-1 induction (Supplemental Fig. 4A), suggesting that glutamate is acting in a receptor-independent fashion to induce PSGL-1 expression. The levels of the glutamate receptor family member GluR1 were also assessed by immunoblot assay, and no change in the total GluR1 protein levels was detected (data not shown). As the anionic amino acid transporter xCT acts as a facilitative antiporter, the excess extracellular glutamate might antagonize the normal exchange of intracellular glutamate for extracellular cystine. To test this notion, we used the xCT inhibitor S4CPG. In conjunction with CD40L, but not alone, S4CPG is able to induce PSGL-1 expression in monocytes (Supplemental Fig. 4B). In this case, the magnitude of PSGL-1 induction was similar to what was observed in glutamate plus sCD40L-treated monocytes (see Fig. 2A). As xCT serves as a major mechanism by which cells import cystine, which in turn, is important for the synthesis of the antioxidant GSH [48], if xCT activity is impaired in HIV-infected individuals, it would be expected that there is reduced GSH levels in HIV-infected individuals. The cohort studied here does have reduced plasma GSH (Supplemental Fig. 4C). Therefore, we concluded that the glutamate is likely acting in a receptor-independent fashion to induce PSGL-1 expression in monocytes, when in combination with sCD40L. Specifically, glutamate is likely acting to impair xCT-mediate cystine import and consequently, GSH synthesis. Whereas the mechanism proposed suggests that HIV might act indirectly to impair GSH synthesis, it has been reported that in HIV-infected cells, GSH is depleted and that this is required for regular viral replication [49]. Furthermore, whereas an inhibitor of xCT was able to mimic the effects of glutamate in combination with sCD40L, there are still potential alternative routes of action. There are other glutamate receptor families besides the NMDA, AMPA, and kainate families, such as metabotropic glutamate receptors, which are expressed on a variety of cell types outside of the CNS [50]. Thus, it cannot be concluded definitively that xCT inhibition mediates glutamate and CD40L-induced PSGL-1 expression.
Plasma glutamate is increased in HIV-infected individuals
Increased sCD40L in HIV-infected individuals is well characterized [51], as is increased extracellular glutamate in the CNS of HIV-infected individuals [52]; however, it is unclear if glutamate is similarly increased in the circulation of HIV-infected subjects recruited in our studies. Thus, plasma was collected from 8 HIV+ and 8 HIV− donors, and glutamate content was determined by LC-MS/MS. Glutamate is increased significantly in HIV-infected individuals compared with healthy controls (Fig. 3). These results are consistent with previous reports in which elevated levels of glutamate were observed in the blood plasma of persons with HIV [53, 54].
Network of signaling events induced by glutamate or sCD40L reveals a highly complex signaling network
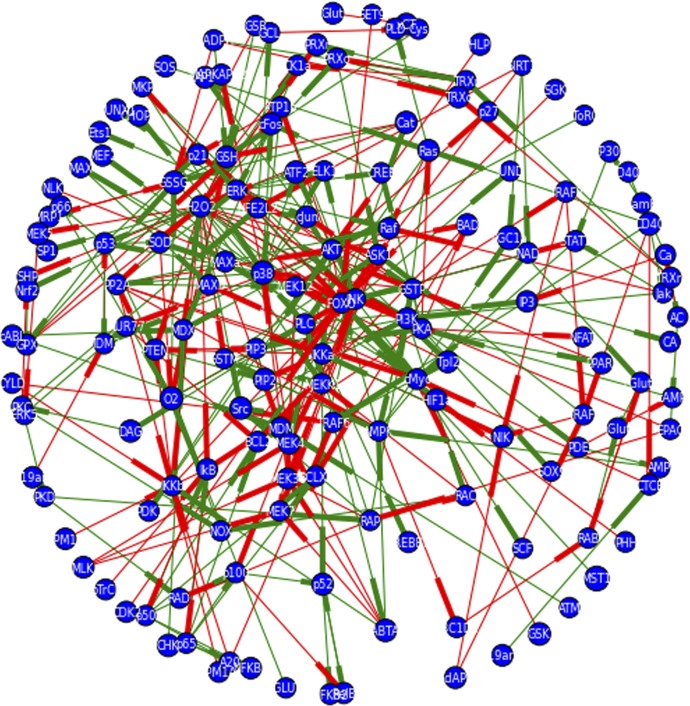
Despite the genomic organization of the PSGL-1 gene in both mice [55] and humans [56] being known for nearly 20 yr, there are no reports on the transcriptional regulation of PSGL-1. So, as to determine what factors might regulate its induction by the combination of CD40L and glutamate, a network describing signaling events downstream of CD40L and/or glutamate was constructed (see Materials and Methods). The completed network is illustrated in Fig. 4. The full network contains 155 nodes (detailed in Supplemental Table 1), or signaling mediators, and 341 edges, or regulatory interactions, and illustrates a nonassortive network (assortivity coefficient = −0.035), with an average node degree of 4.4, node clustering coefficient of 0.1982179285, and node betweeness centrality of 0.012. Thus, in this network, mediators with a large number of regulatory interactions do not preferentially interact with other mediators that also have a large number of regulatory interactions. Each mediator also has an average of 4.4 interacting partners; these partners tend not to interact with each other, and there is no 1 node that connects all other pairs of nodes. Of note, the node representing H2O2 is the node with both the highest degree (34) and betweeness centrality (0.23). Additionally, the nodes representing the forkhead box O transcription factor family and the IκB kinase α are among the top 22 nodes with highest degree (17 and 10, respectively), connectivity (0.58 and 0.83), and betweeness centrality (0.13 and 0.035). Finally, other nodes of special interest that are among the top 22 for both node degree and betweeness centrality include those representing factors related to redox stress and its regulation (H2O2, O2, GSH), factors sensitive to redox stress or GSH levels (PP2A, GSTP), MAPK family members (p38, JNK, ERK), and others (AMPK, protein kinase A, IκB).
Figure 4. Network graph of signaling pathways induced by CD40L and glutamate and transcription factors, which may be activated by these pathways that are predicted to bind near the PSGL-1 start site.
Primary and secondary sources were consulted for signaling interactions that might mediate signaling downstream of CD40L, glutamate, or GSH depletion via manual curation of search results from PubMed. Additionally, signaling databases, such as KEGG pathways, were consulted. The ENCODE database was consulted to identify transcription factors that might bind around the PSGL-1 start site, and those for which a connection was found with the signaling pathways induced by CD40L or glutamate were encoded. All signaling interactions were coded as binary interactions in Python using NetworkX and matplotlib libraries. Negative regulatory interactions are signified by a red edge, whereas positive regulatory interactions are signified by a green edge. Directionality of the interaction is indicated by the weighting of the side of the edge closest to the regulated node. The network contains 155 nodes and 341 edges.
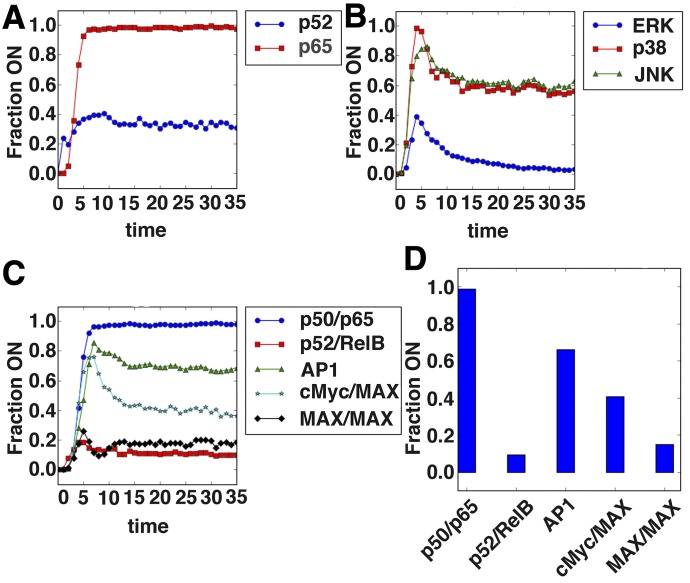
The network was simulated by developing a discrete dynamic model, described in Materials and Methods. The model was used to test whether it can recapitulate known, CD40L-induced dynamics. Simulations reveal that the canonical NFκB pathway, but not the noncanonical pathway, is strongly induced by CD40L (Fig. 5A and C), and this is indeed what has been reported as occurring in CD40L-treated cells (Pearson et al. [57]). Additionally, the model recapitulates robust activation of the MAPK ERK and weaker, transient activation of JNK, as described previously (Fig. 5B) [57, 58].
Figure 5. Boolean network model correctly predicts aspects of CD40L-induced signaling.
The network illustrated in Fig. 4 was implemented in Python using the Boolean net library, with logic rules describing the regulatory interaction between nodes, inferred based on the literature describing the regulation. A total of 800 simulations, with 80 time steps, of the CD40L-alone scenario were run using an asynchronous update schedule. The percentage of simulations in which a node was on or had a value of 1 is shown for (A) the NF-κB family members, p65 and p52; (B) the MAPK family members, ERK, JNK, and p38; and (C) the transcription factor complexes p50/p65, p52/RelB, cFos/cJun (AP-1), c-Myc/MAX, and MAX/MAX. (D) The steady-state values for these transcription factor complexes, taken as the 30th time step, are shown. The canonical NF-κB pathway is predicted to be more strongly activated than the noncanonical pathway, with moderate AP-1 and c-Myc/MAX activation.
Boolean network modeling predicts c-Myc as a likely mediator of glutamate and sCD40L-induced PSGL-1 induction
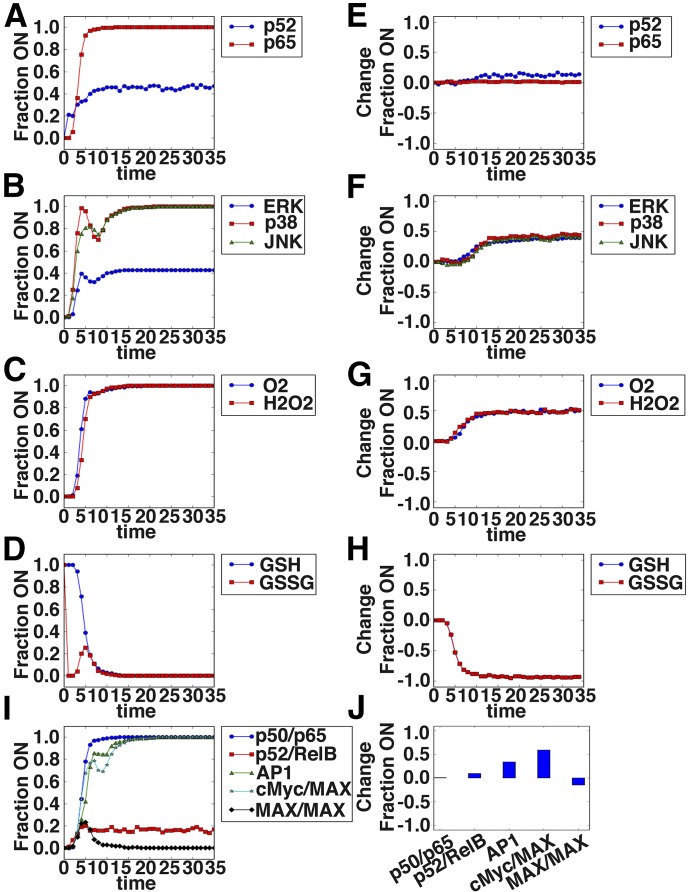
As PSGL-1 expression was induced in the joint glutamate and CD40L treatment, the model was used to predict transcription factors with differential activation in the glutamate and CD40L in silico stimulation (Fig. 2). Figure 6A–D shows the dynamics of select signaling mediators predicted in response to the combination treatment, whereas Fig. 6E–H shows the change in activation for the same set of intermediates in the combination treatment scenario compared with the CD40L alone scenario. These mediators include NF-κB members, MAPK members, ROS, GSH, and several transcription factors. One of the most notable features is a decrease in GSH and a corresponding increase in ROS. This, in turn, is predicted to result in altered transcription factor activity (Fig. 6I and J). c-Myc had the highest fold change in the joint treatment compared with the single treatment, suggesting its role in transcriptional regulation of the genes induced in the joint treatment. AP-1 also showed significant differential activity upon joint treatment.
Figure 6. Boolean network model reveals signaling dynamics downstream of sCD40L + glutamate treatment of monocytes and predicts c-Myc as a likely regulator of PSGL-1 induction.
The network illustrated in Fig. 4 was implemented in Python using the Boolean net library, with log rules describing the regulatory interaction between nodes inferred based on the literature describing the regulation. A total of 800 simulations, with 80 time steps, of the CD40L + glutamate-alone scenario were run using an asynchronous update schedule. The fraction of simulations in which a node was on or had a value of 1 is shown for selected signaling intermediates in A–D. The difference in the percentage of simulations in which a node was “on” between the combination scenario and CD40L-alone scenario is shown in E–H. A positive value indicates increased activation in the combination scenario. (A and E) The NF-κB family members p52 and p65 are shown. (B and F) The MAPK family members ERK, p38, and JNK are shown. (C and G) The reactive oxygen intermediates O2 and H2O2 are shown. (D and H) The reduced GSH and oxidized GSSG form of GSH are shown. Shown are the dynamics of the transcription factor complexes p50/p65, p52/RelB, cFos/cJun (AP-1), c-Myc/MAX, and MAX/MAX (I) and the change in the steady-state values relative to CD40L alone (J).
Glutamate and sCD40L induce c-Myc activation in primary human monocytes ex vivo, and c-Myc inhibition prevents PSGL-1 induction but not basal expression
To validate first whether c-Myc is indeed differentially activated in monocytes during HIV infection, publicly available microarray data derived from monocytes isolated from 4 HIV− donors and 5 HIV+ donors, responding well to cART [30], were analyzed. Particularly, enrichment of transcription factor-binding sites, 2 kb upstream of the start site of up-regulated genes, was estimated. Of note, c-Myc-binding sites are all enriched among up-regulated genes in monocytes from HIV+ compared with HIV− individuals (P = 0.023), as are binding sites for the c-Myc-binding partner, MAX. Thus, these observations validate the model predictions, and it is likely that c-Myc is activated in monocytes during HIV.
Next, it was determined that c-Myc does regulate glutamate and sCD40L-induced monocyte PSGL-1 induction ex vivo. c-Myc acts in complex with a number of other factors to regulate transcription. One such protein is MAX, which is well described as acting in a complex with c-Myc to promote transcription [59]. As shown in Fig. 7, the treatment with glutamate plus sCD40L in the presence of the c-Myc/MAX dimerization inhibitor 10054-F8 failed to induce PSGL-1 in monocytes. Importantly, these results suggest that c-Myc/MAX dimerization is required for PSGL-1 induction. Whereas more will need to be done to confirm the role of c-Myc in PSGL-1 regulation, these data corroborate a recent report [60] and provide evidence that c-Myc is likely activated in monocytes during HIV infection and thereby, modulates PSGL-1 levels.
Network analysis reveals potential targets for therapeutic intervention
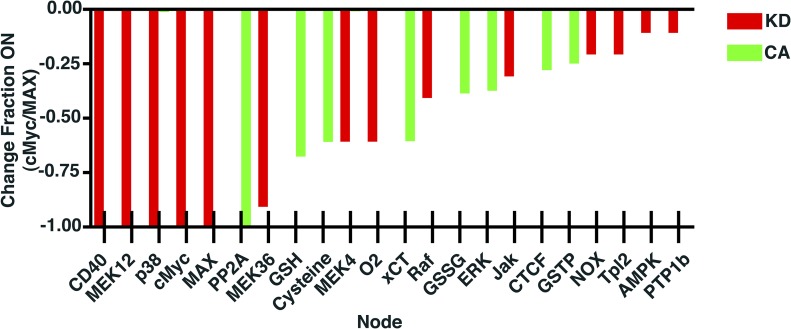
To determine which nodes or signaling intermediaries have the greatest role in c-Myc activation, simulations of systematic knockdown or constitutive activation of each node in the network was run. As seen in Fig. 8, a number of signaling intermediaries were found to have a large effect on c-Myc activation, with the top 22 being shown. Of note, the perturbation analysis suggests a crucial role for GSH, ROS, and the MAPK pathway in mediating c-Myc activation, in agreement with previous literature on oxidative stress-induced c-Myc activation [61]. Interestingly, 7 of the above nodes also have high node degree and betweeness centrality. Additionally, it would appear that c-Myc activity is primarily regulated via positive regulatory mechanisms, as only about one-third of the nodes that had a large impact on c-Myc were found to exert an effect during constitutive activation, suggesting an inhibitory role here. Interestingly, almost all of these nodes—those for which constitutive activation is predicted to lead to decreased c-Myc activity—are redox stress regulators or sensors, with the exception of the MAPK ERK and the DNA-binding protein, CTCF.
Figure 8. Knockdown and constitutive activation perturbation simulations reveal key regulatory nodes in glutamate + CD40L-induced c-Myc activation.
Each node in the network was systematically, individually forced permanently into an “off” state, knocked down (KD), or an “on” state, constitutively activated (CA), during the combination treatment scenario. Results from these simulations were then compared with the unperturbed combination treatment scenario, and changes in c-Myc/MAX activity are shown. NOX, Nitrogen oxides; Tpl2, tumor progression locus 2; PTP1b, protein tyrosine phosphatase 1b.
DISCUSSION
Here, the physiologically relevant factors CD40L and glutamate are identified as transcriptional inducers of PSGL-1 in monocytes, likely via c-Myc. That PSGL-1 is differentially regulated with HIV infection is consistent with previous reports of altered PSGL-1 expression during various inflammatory diseases [11–13]. Whereas there is another report that contradicts ours on this point [34], it is likely that this difference is associated with differences in the patient population studied.
In the work of Liang et al. [34], a cohort of men who have sex with men and acquired HIV predominantly within 12 mo of last-negative detection were shown to possess a higher proportion of nonclassic CD14dimCD16+ and intermediate CD14highCD16+ monocytes in the circulation. It was also demonstrated that these cellular subtypes, including their classic CD14highCD16− counterparts, expressed PSGL-1 at significantly lower levels [34]. The population studied here is older, has been on cART longer, and has been infected with HIV for a longer period of time than the population in the conflicting report, which likely accounts for the differences in the PSGL-1 expression levels observed. We have examined the effects of HIV infection on PSGL-1 expression in monocytes of older subjects (55 ± 11 yr) who were receiving ART, whereas Liang et al. [34] examined PSGL-1 levels in monocytes of younger patients (ranging from 27 to 33 yr of age) who were treatment naive. Thus, there may be age-specific differences in the effect of HIV infection on monocytes in the presence of ART. In support of this notion, previous work has demonstrated a positive correlation between the phenotypic alterations in monocytes and disease progression in HIV-infected persons on stable ART [62, 63].
The methods illustrated here may be generally applicable in the identification of the transcriptional regulators of genes differentially expressed on circulating cell types. The pipeline suggested here has several steps. First, a change in protein expression needs to be identified. Next, physiologically relevant mediators need to be screened to identify candidate factors. Following this, a Boolean network model will be generated to connect signaling pathways downstream of the candidate soluble mediators with transcription factors predicted to bind around the gene of interest’s start site. As these signaling interactions will be determined based on a review of the available literature, not all pathways may be amenable to such an analysis. Additionally, if relevant transcriptomic data are not available, then the data may be generated and analyzed for transcription factor binding-site enrichment so as to support the model’s predictions. Finally, the model’s predictions should be validated, even if only preliminarily, so as to determine if any further refinement of the model is necessary.
That glutamate is acting on leukocytes to modulate signaling is perhaps surprising. It seems likely that glutamate is acting by inhibiting the anionic amino acid transporter xCT, thereby impairing GSH synthesis and the ability of cells to buffer ROS, including those reported to be induced by CD40 signaling [64]. Whereas GSH antagonism has been reported to be a common feature of diverse viral infections, in the primary infected cell (reviewed in Aquilano et al. [65]), the data presented here suggest that GSH synthesis might be antagonized even in uninfected cells. This is important, as it suggests that even in cART-treated, virally suppressed, HIV-infected individuals, HIV may be able to induce PSGL-1 expression. Not only in active infection but also in suppressed infection, CD40L and glutamate are induced [42]. This reflects the continued expression of proinflammatory HIV genes, such as Tat and gp120, despite suppression of viral replication [66]. Importantly, as other proinflammatory cytokines have been reported to induce ROS generation, including TNF-α, IL-6, and IL-1β [67], glutamate may be able to act in conjunction with these as well to induce c-Myc-mediated PSGL-1 expression. It is worth noting that these cytokines have also been reported to be increased with HIV infection [68–70]. Whereas sCD40L and glutamate were able to induce PSGL-1 expression ex vivo, the role of oxidative stress in mediating this induction is likely more significant in vivo. It has been reported in HIV-infected individuals with prolonged cART use, particularly nucleotide RT inhibitor use, that mitochondrial replication is impaired, resulting in impaired respiration [71] and likely a corresponding increase in ROS production as a result of normal cellular respiration. This suggests that in the context of cART-treated HIV infection, monocytes might be even more sensitive to GSH depletion than the data here suggest.
A better understanding of PSGL-1 regulation could facilitate novel therapeutic strategies for the treatment of secondary disorders. In particular, many complications associated with HIV infection, including CVDs and neurocognitive disease, feature excessive leukocyte tissue infiltration as a significant component of their pathology. Importantly, sCD40L has been implicated in a variety of other disorders, including inflammatory bowel disease and atherosclerosis [72, 73], whereas glutamate dysregulation is common to a variety of neurocognitive disorders [74]. Thus, the mechanism outlined here may be common to many conditions.
To confirm the significance of PSGL-1 induction, it will be necessary to validate if increased PSGL-1 induction does, in fact, promote increased leukocyte transmigration. The identification here of c-Myc as being involved in PSGL-1 induction by sCD40L and glutamate, together with its predicted binding to a region around the PSGL-1 start site, calls for further investigation of the role of c-Myc in regulating PSGL-1 transcription. If c-Myc is found to bind directly to the promoter region of PSGL-1, then the role of increased PSGL-1 expression in leukocyte transmigration can be specifically interrogated via genetic manipulation.
Cancer is one such disease in which sCD40L and glutamate have been implicated, including glioblastomas, adenocarcinomas, and squamous cell carcinomas of the lung [75]. Furthermore, as sCD40L is thought to be primarily platelet derived [76], it is not surprising that as is seen with HIV infection, platelet activation is increased during cancer [77]. Increased levels of markers of oxidative stress also feature prominently in cancer pathogenesis. Thus, it seems likely that PSGL-1 may be similarly up-regulated on leukocytes during cancer. Importantly, it has been suggested that monocyte PSGL-1 expression is required for tumor metastasis, and this is thought to represent priming of the secondary site by infiltrating monocytes [78]. Additionally, c-Myc has been shown to regulate many of the genes associated with alternative activation-type Mϕ [79]. Thus, if the mechanism outlined here is also implicated in cancer pathogenesis, then it may contribute to both metastasis and immune evasion.
Whereas it appears likely that c-Myc regulates PSGL-1 expression in the system described here, c-Myc is not an ideal therapeutic target. c-Myc is well characterized as a regulator of the cell cycle and has been implicated in a variety of other cellular processes; thus, therapeutic targeting of c-Myc is likely to have variety of side effects. More amenable to targeting is either sCD40L- or glutamate-induced ROS. sCD40L is thought to be primarily platelet derived [76], and so, anti-platelet therapy may be beneficial. Not only is platelet activation important in the formation of platelet monocyte complexes, but also, the complexes have been described as promoting leukocyte transmigration. Furthermore, platelet activation has been implicated in the pathogenesis of disorders, such as atherosclerosis and HAND. The targeting of the increase in ROS may also be beneficial, particularly as the model developed here shows that c-Myc activation is especially sensitive to alterations in ROS and ROS sensors. That ROS can lead to c-Myc activation has been demonstrated previously [61], and ERK was similarly implicated in this study. NAC has been shown to be able to inhibit HIV replication in vitro [80], via inhibition of HIV-induced GSH antagonism. While the effects of NAC on HIV pathogenesis have not been well characterized in vivo, it is possible that they may inhibit the development of many HIV-associated complications, in part, as a result of the role of oxidative stress in PSGL-1 induction. Finally, it will be important to determine the primary source of the increased serum glutamate observed. Not only will this provide mechanistic insight, but it will also suggest additional targets for therapeutic intervention. Thus, there are a number of potential methods of targeting sCD40L- and glutamate-induced PSGL-1 induction in the context of HIV infection.
AUTHORSHIP
R.C. was involved in the design of all of the experiments and was primarily responsible for their execution and also prepared the manuscript. L.D.J. prepared the EcoHIV used in the mouse experiments and helped execute the mouse experiments. X.Q. conducted the power analysis and provided oversight on the use of statistical methods. J.T. contributed to the design and execution of the computational biology and bioinformatics experiments. S.B.M. contributed to the design of the human, mouse, and in vitro experiments, as well as provided guidance on their execution. He also edited the manuscript extensively.
ACKNOWLEDGMENTS
The authors acknowledge funding for this work from the U.S. National Institutes of Health (RO1 NS054578, RO1 NS066801, RO1 HL128155, RO1 HL123346, and UL1 TR002001) and also the University of Rochester Medical Center (URMC) Center for AIDS Research (P30 AI078498) for their support of core facilities. The authors thank Dr. David Volsky for his kind gift of the EcoHIV virus. The authors also thank Dr. Meera Singh for helpful discussions; the URMC Infectious Diseases Clinic, especially Elizabeth Keller and Emily Cossimano; Rochester Victory Alliance, especially Catherine Bunce; Ann Casey for help in acquiring blood samples; URMC Flow Cytometry Core for facilities; and Dr. Joshua Munger for the use of his mass spectrometer and along with Dr. Joseph Hollenbaugh, for guidance in conducting the LC-MS/MS experiments.
Glossary
- 10058-F4
(Z,E)-5-(4-ethylbenzylidine)-2-thioxothiazolidin-4-one
- AMPA
2-amino-5-hydroxy-5-methyl-4-isoxazolepropion acid
- AMPK
AMP-activated protein kinase
- APC
allophycocyanin
- ART
antiretroviral therapy
- cART
combined antiretroviral therapy
- CD40L
cluster of differentiation 40 ligand
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- CTCF
CCCTC-binding factor
- CVD
cardiovascular disease
- EcoHIV
mouse-tropic HIV
- FSC
forward-scatter
- GSH
glutathione
- GSSG
glutathione disulfide
- GSTP
glutathione S-transferase
- HAND
HIV-associated neurocognitive disorder
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- KO
knockout
- LC
liquid chromatography
- MFI
mean fluorescence intensity
- MS/MS
tandem mass spectrometry
- NAC
N-acetyl cysteine
- NMDA
N-methyl-d-aspartate
- O2
superoxide
- p38
p38 MAPK
- p52
NF-κB p52 subunit
- p65
NF-κB p65 subunit
- PAF
platelet-activating factor
- PP2A
protein phosphatase 2A
- PSGL-1
P-selectin glycoprotein ligand-1
- qRT-PCR
quantitative RT-PCR
- ROS
reactive oxygen species
- S4CPG
(S)-4-carboxyphenylglycine
- sCD40L
soluble cluster of differentiation 40 ligand
- sCD163
soluble cluster of differentiation 163
- SRM
selected reaction monitoring
- SSC
side-scatter
- WT
wild-type
- xCT
functional subunit of the cystine/glutamate transporter xc− system
Footnotes
The online version of this paper, found at www.jleukbio.org, contains supplemental information.
SEE CORRESPONDING EDITORIAL ON PAGE 949
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Anzinger J. J., Butterfield T. R., Angelovich T. A., Crowe S. M., Palmer C. S. (2014) Monocytes as regulators of inflammation and HIV-related comorbidities during cART. J. Immunol. Res. 2014, 569819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heydtmann M., Adams D. H. (2009) Chemokines in the immunopathogenesis of hepatitis C infection. Hepatology 49, 676–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osterud B., Bjorklid E. (2003) Role of monocytes in atherogenesis. Physiol. Rev. 83, 1069–1112. [DOI] [PubMed] [Google Scholar]
- 4.Kreuger J., Phillipson M. (2016) Targeting vascular and leukocyte communication in angiogenesis, inflammation and fibrosis. Nat. Rev. Drug Discov. 15, 125–142. [DOI] [PubMed] [Google Scholar]
- 5.McEver R. P., Zhu C. (2010) Rolling cell adhesion. Annu. Rev. Cell Dev. Biol. 26, 363–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlow D. A., Gossens K., Naus S., Veerman K. M., Seo W., Ziltener H. J. (2009) PSGL-1 function in immunity and steady state homeostasis. Immunol. Rev. 230, 75–96. [DOI] [PubMed] [Google Scholar]
- 7.da Costa Martins P. A., van Gils J. M., Mol A., Hordijk P. L., Zwaginga J. J. (2006) Platelet binding to monocytes increases the adhesive properties of monocytes by up-regulating the expression and functionality of beta1 and beta2 integrins. J. Leukoc. Biol. 79, 499–507. [DOI] [PubMed] [Google Scholar]
- 8.Li B., Pozzi A., Young P. P. (2011) TNFalpha accelerates monocyte to endothelial transdifferentiation in tumors by the induction of integrin alpha5 expression and adhesion to fibronectin. Mol. Cancer Res. 9, 702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Constantin G. (2008) Chemokine signaling and integrin activation in lymphocyte migration into the inflamed brain. J. Neuroimmunol. 198, 20–26. [DOI] [PubMed] [Google Scholar]
- 10.Gerdes N., Seijkens T., Lievens D., Kuijpers M. J., Winkels H., Projahn D., Hartwig H., Beckers L., Megens R. T., Boon L., Noelle R. J., Soehnlein O., Heemskerk J. W., Weber C., Lutgens E. (2016) Platelet CD40 exacerbates atherosclerosis by transcellular activation of endothelial cells and leukocytes. Arterioscler. Thromb. Vasc. Biol. 36, 482–490. [DOI] [PubMed] [Google Scholar]
- 11.Almulki L., Noda K., Amini R., Schering A., Garland R. C., Nakao S., Nakazawa T., Hisatomi T., Thomas K. L., Masli S., Hafezi-Moghadam A. (2009) Surprising up-regulation of P-selectin glycoprotein ligand-1 (PSGL-1) in endotoxin-induced uveitis. FASEB J. 23, 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dang B., Wiehler S., Patel K. D. (2002) Increased PSGL-1 expression on granulocytes from allergic-asthmatic subjects results in enhanced leukocyte recruitment under flow conditions. J. Leukoc. Biol. 72, 702–710. [PubMed] [Google Scholar]
- 13.Schumacher A., Liebers U., John M., Gerl V., Meyer M., Witt C., Wolff G. (2005) P-Selectin glycoprotein ligand-1 (PSGL-1) is up-regulated on leucocytes from patients with chronic obstructive pulmonary disease. Clin. Exp. Immunol. 142, 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.UNAIDS (2016) Global AIDS Update 2016, UNAIDS, Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland. [Google Scholar]
- 15.Nakagawa F., May M., Phillips A. (2013) Life expectancy living with HIV: recent estimates and future implications. Curr. Opin. Infect. Dis. 26, 17–25. [DOI] [PubMed] [Google Scholar]
- 16.Chu C., Selwyn P. A. (2011) Complications of HIV infection: a systems-based approach. Am. Fam. Physician 83, 395–406. [PubMed] [Google Scholar]
- 17.HIV Outpatient Study Investigators (2006) Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J. Acquir. Immune Defic. Syndr. 43, 27–34. [DOI] [PubMed] [Google Scholar]
- 18.Swiss HIV Cohort Study (2011) Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin. Infect. Dis. 53, 1130–1139. [DOI] [PubMed] [Google Scholar]
- 19.Islam F. M., Wu J., Jansson J., Wilson D. P. (2012) Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med. 13, 453–468. [DOI] [PubMed] [Google Scholar]
- 20.Watkins C. C., Treisman G. J. (2015) Cognitive impairment in patients with AIDS - prevalence and severity. HIV AIDS (Auckl.) 7, 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilgendorf I., Swirski F. K., Robbins C. S. (2015) Monocyte fate in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 35, 272–279. [DOI] [PubMed] [Google Scholar]
- 22.Singh M. V., Davidson D. C., Kiebala M., Maggirwar S. B. (2012) Detection of circulating platelet-monocyte complexes in persons infected with human immunodeficiency virus type-1. J. Virol. Methods 181, 170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davidson D. C., Schifitto G., Maggirwar S. B. (2013) Valproic acid inhibits the release of soluble CD40L induced by non-nucleoside reverse transcriptase inhibitors in human immunodeficiency virus infected individuals. PLoS One 8, e59950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones L. D., Jackson J. W., Maggirwar S. B. (2016) Modeling HIV-1 induced neuroinflammation in mice: role of platelets in mediating blood-brain barrier dysfunction. PLoS One 11, e0151702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanehisa M., Goto S. (2000) KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28, 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bornholdt S. (2008) Boolean network models of cellular regulation: prospects and limitations. J. R. Soc. Interface 5 (Suppl 1), S85–S94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson C. S., DeDiego M. L., Topham D. J., Thakar J. (2016) Boolean modeling of cellular and molecular pathways involved in influenza infection. Comput. Math. Methods Med. 2016, 7686081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albert R., Thakar J. (2014) Boolean modeling: a logic-based dynamic approach for understanding signaling and regulatory networks and for making useful predictions. Wiley Interdiscip. Rev. Syst. Biol. Med. 6, 353–369. [DOI] [PubMed] [Google Scholar]
- 29.Thakar J., Pilione M., Kirimanjeswara G., Harvill E. T., Albert R. (2007) Modeling systems-level regulation of host immune responses. PLOS Comput. Biol. 3, e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu J. Q., Sassé T. R., Saksena M. M., Saksena N. K. (2013) Transcriptome analysis of primary monocytes from HIV-positive patients with differential responses to antiretroviral therapy. Virol. J. 10, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thakar J., Hartmann B. M., Marjanovic N., Sealfon S. C., Kleinstein S. H. (2015) Comparative analysis of anti-viral transcriptomics reveals novel effects of influenza immune antagonism. BMC Immunol. 16, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subramanian A., Tamayo P., Mootha V. K., Mukherjee S., Ebert B. L., Gillette M. A., Paulovich A., Pomeroy S. L., Golub T. R., Lander E. S., Mesirov J. P. (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gentleman R. C., Carey V. J., Bates D. M., Bolstad B., Dettling M., Dudoit S., Ellis B., Gautier L., Ge Y., Gentry J., Hornik K., Hothorn T., Huber W., Iacus S., Irizarry R., Leisch F., Li C., Maechler M., Rossini A. J., Sawitzki G., Smith C., Smyth G., Tierney L., Yang J. Y., Zhang J. (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5, R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang H., Duan Z., Li D., Li D., Wang Z., Ren L., Shen T., Shao Y. (2015) Higher levels of circulating monocyte-platelet aggregates are correlated with viremia and increased sCD163 levels in HIV-1 infection. Cell. Mol. Immunol. 12, 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis B. H., Zarev P. V. (2005) Human monocyte CD163 expression inversely correlates with soluble CD163 plasma levels. Cytometry B Clin. Cytom. 63, 16–22. [DOI] [PubMed] [Google Scholar]
- 36.Potash M. J., Chao W., Bentsman G., Paris N., Saini M., Nitkiewicz J., Belem P., Sharer L., Brooks A. I., Volsky D. J. (2005) A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc. Natl. Acad. Sci. USA 102, 3760–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelschenbach J. L., Saini M., Hadas E., Gu C. J., Chao W., Bentsman G., Hong J. P., Hanke T., Sharer L. R., Potash M. J., Volsky D. J. (2012) Mice chronically infected with chimeric HIV resist peripheral and brain superinfection: a model of protective immunity to HIV. J. Neuroimmune Pharmacol. 7, 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sindberg G. M., Sharma U., Banerjee S., Anand V., Dutta R., Gu C. J., Volsky D. J., Roy S. (2015) An infectious murine model for studying the systemic effects of opioids on early HIV pathogenesis in the gut. J. Neuroimmune Pharmacol. 10, 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ancuta P., Kunstman K. J., Autissier P., Zaman T., Stone D., Wolinsky S. M., Gabuzda D. (2006) CD16+ monocytes exposed to HIV promote highly efficient viral replication upon differentiation into macrophages and interaction with T cells. Virology 344, 267–276. [DOI] [PubMed] [Google Scholar]
- 40.Crowe S., Zhu T., Muller W. A. (2003) The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J. Leukoc. Biol. 74, 635–641. [DOI] [PubMed] [Google Scholar]
- 41.Triques K., Stevenson M. (2004) Characterization of restrictions to human immunodeficiency virus type 1 infection of monocytes. J. Virol. 78, 5523–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sui Z., Sniderhan L. F., Schifitto G., Phipps R. P., Gelbard H. A., Dewhurst S., Maggirwar S. B. (2007) Functional synergy between CD40 ligand and HIV-1 Tat contributes to inflammation: implications in HIV type 1 dementia. J. Immunol. 178, 3226–3236. [DOI] [PubMed] [Google Scholar]
- 43.D’Aversa T. G., Eugenin E. A., Berman J. W. (2008) CD40-CD40 ligand interactions in human microglia induce CXCL8 (interleukin-8) secretion by a mechanism dependent on activation of ERK1/2 and nuclear translocation of nuclear factor-kappaB (NFkappaB) and activator protein-1 (AP-1). J. Neurosci. Res. 86, 630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramirez S. H., Fan S., Dykstra H., Reichenbach N., Del Valle L., Potula R., Phipps R. P., Maggirwar S. B., Persidsky Y. (2010) Dyad of CD40/CD40 ligand fosters neuroinflammation at the blood-brain barrier and is regulated via JNK signaling: implications for HIV-1 encephalitis. J. Neurosci. 30, 9454–9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davidson D. C., Hirschman M. P., Sun A., Singh M. V., Kasischke K., Maggirwar S. B. (2012) Excess soluble CD40L contributes to blood brain barrier permeability in vivo: implications for HIV-associated neurocognitive disorders. PLoS One 7, e51793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swiss HIV Cohort Study (2002) Antiretroviral therapy reduces markers of endothelial and coagulation activation in patients infected with human immunodeficiency virus type 1. J. Infect. Dis. 185, 456–462. [DOI] [PubMed] [Google Scholar]
- 47.Gremmel T., Ay C., Riedl J., Kopp C. W., Eichelberger B., Koppensteiner R., Panzer S. (2016) Platelet-specific markers are associated with monocyte-platelet aggregate formation and thrombin generation potential in advanced atherosclerosis. Thromb. Haemost. 115, 615–621. [DOI] [PubMed] [Google Scholar]
- 48.Lewerenz J., Hewett S. J., Huang Y., Lambros M., Gout P. W., Kalivas P. W., Massie A., Smolders I., Methner A., Pergande M., Smith S. B., Ganapathy V., Maher P. (2013) The cystine/glutamate antiporter system x(c)(-) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid. Redox Signal. 18, 522–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palamara A. T., Perno C. F., Aquaro S., Buè M. C., Dini L., Garaci E. (1996) Glutathione inhibits HIV replication by acting at late stages of the virus life cycle. AIDS Res. Hum. Retroviruses 12, 1537–1541. [DOI] [PubMed] [Google Scholar]
- 50.Julio-Pieper M., Flor P. J., Dinan T. G., Cryan J. F. (2011) Exciting times beyond the brain: metabotropic glutamate receptors in peripheral and non-neural tissues. Pharmacol. Rev. 63, 35–58. [DOI] [PubMed] [Google Scholar]
- 51.Sipsas N. V., Sfikakis P. P., Kontos A., Kordossis T. (2002) Levels of soluble CD40 ligand (CD154) in serum are increased in human immunodeficiency virus type 1-infected patients and correlate with CD4(+) T-cell counts. Clin. Diagn. Lab. Immunol. 9, 558–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferrarese C., Aliprandi A., Tremolizzo L., Stanzani L., De Micheli A., Dolara A., Frattola L. (2001) Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology 57, 671–675. [DOI] [PubMed] [Google Scholar]
- 53.Eck H. P., Gmünder H., Hartmann M., Petzoldt D., Daniel V., Dröge W. (1989) Low concentrations of acid-soluble thiol (cysteine) in the blood plasma of HIV-1-infected patients. Biol. Chem. Hoppe Seyler 370, 101–108. [DOI] [PubMed] [Google Scholar]
- 54.Dröge W. (1993) Cysteine and glutathione deficiency in AIDS patients: a rationale for the treatment with N-acetyl-cysteine. Pharmacology 46, 61–65. [DOI] [PubMed] [Google Scholar]
- 55.Yang J., Galipeau J., Kozak C. A., Furie B. C., Furie B. (1996) Mouse P-selectin glycoprotein ligand-1: molecular cloning, chromosomal localization, and expression of a functional P-selectin receptor. Blood 87, 4176–4186. [PubMed] [Google Scholar]
- 56.Veldman G. M., Bean K. M., Cumming D. A., Eddy R. L., Sait S. N., Shows T. B. (1995) Genomic organization and chromosomal localization of the gene encoding human P-selectin glycoprotein ligand. J. Biol. Chem. 270, 16470–16475. [DOI] [PubMed] [Google Scholar]
- 57.Pearson L. L., Castle B. E., Kehry M. R. (2001) CD40-mediated signaling in monocytic cells: up-regulation of tumor necrosis factor receptor-associated factor mRNAs and activation of mitogen-activated protein kinase signaling pathways. Int. Immunol. 13, 273–283. [DOI] [PubMed] [Google Scholar]
- 58.Ma X., Aoki T., Narumiya S. (2016) Prostaglandin E2-EP4 signaling persistently amplifies CD40-mediated induction of IL-23 p19 expression through canonical and non-canonical NF-κB pathways. Cell. Mol. Immunol. 13, 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grinberg A. V., Hu C. D., Kerppola T. K. (2004) Visualization of Myc/Max/Mad family dimers and the competition for dimerization in living cells. Mol. Cell. Biol. 24, 4294–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chao C., Silverberg M. J., Xu L., Chen L. H., Castor B., Martínez-Maza O., Abrams D. I., Zha H. D., Haque R., Said J. (2015) A comparative study of molecular characteristics of diffuse large B-cell lymphoma from patients with and without human immunodeficiency virus infection. Clin. Cancer Res. 21, 1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benassi B., Fanciulli M., Fiorentino F., Porrello A., Chiorino G., Loda M., Zupi G., Biroccio A. (2006) c-Myc phosphorylation is required for cellular response to oxidative stress. Mol. Cell 21, 509–519. [DOI] [PubMed] [Google Scholar]
- 62.Yong Y. K., Shankar E. M., Westhorpe C. L., Maisa A., Spelman T., Kamarulzaman A., Crowe S. M., Lewin S. R. (2016) Genetic polymorphisms in the CD14 gene are associated with monocyte activation and carotid intima-media thickness in HIV-infected patients on antiretroviral therapy. Medicine (Baltimore) 95, e4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chow D. C., Kagihara J. M., Zhang G., Souza S. A., Hodis H. N., Li Y., Mitchell B. I., Nakamoto B. K., Kallianpur K. J., Keating S. M., Norris P. J., Kohorn L. B., Ndhlovu L. C., Shikuma C. M. (2016) Non-classical monocytes predict progression of carotid artery bifurcation intima-media thickness in HIV-infected individuals on stable antiretroviral therapy. HIV Clin. Trials 17, 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chakrabarti S., Rizvi M., Pathak D., Kirber M. T., Freedman J. E. (2009) Hypoxia influences CD40-CD40L mediated inflammation in endothelial and monocytic cells. Immunol. Lett. 122, 170–184. [DOI] [PubMed] [Google Scholar]
- 65.Aquilano K., Baldelli S., Ciriolo M. R. (2014) Glutathione: new roles in redox signaling for an old antioxidant. Front. Pharmacol. 5, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maubert M. E., Pirrone V., Rivera N. T., Wigdahl B., Nonnemacher M. R. (2016) Interaction between Tat and drugs of abuse during HIV-1 infection and central nervous system disease. Front. Microbiol. 6, 1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sverrisson K., Axelsson J., Rippe A., Asgeirsson D., Rippe B. (2015) Acute reactive oxygen species (ROS)-dependent effects of IL-1β, TNF-α, and IL-6 on the glomerular filtration barrier (GFB) in vivo. Am. J. Physiol. Renal Physiol. 309, F800–F806. [DOI] [PubMed] [Google Scholar]
- 68.INSIGHT Strategies for Management of Antiretroviral Therapy (SMART) Study Group (2009) Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. J. Infect. Dis. 200, 973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.INSIGHT SMART Study Group (2011) Resumption of HIV replication is associated with monocyte/macrophage derived cytokine and chemokine changes: results from a large international clinical trial. AIDS 25, 1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.INSIGHT SMART Study Group (2015) Plasma levels of cytokines and chemokines and the risk of mortality in HIV-infected individuals: a case-control analysis nested in a large clinical trial. AIDS 29, 847–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Payne B. A., Wilson I. J., Hateley C. A., Horvath R., Santibanez-Koref M., Samuels D. C., Price D. A., Chinnery P. F. (2011) Mitochondrial aging is accelerated by anti-retroviral therapy through the clonal expansion of mtDNA mutations. Nat. Genet. 43, 806–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Danese S., Sans M., Fiocchi C. (2004) The CD40/CD40L costimulatory pathway in inflammatory bowel disease. Gut 53, 1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Phipps R. P. (2000) Atherosclerosis: the emerging role of inflammation and the CD40-CD40 ligand system. Proc. Natl. Acad. Sci. USA 97, 6930–6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Javitt D. C. (2004) Glutamate as a therapeutic target in psychiatric disorders. Mol. Psychiatry 9, 984–997, 979. [DOI] [PubMed] [Google Scholar]
- 75.Stepulak A., Rola R., Polberg K., Ikonomidou C. (2014) Glutamate and its receptors in cancer. J. Neural Transm. (Vienna) 121, 933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.André P., Prasad K. S., Denis C. V., He M., Papalia J. M., Hynes R. O., Phillips D. R., Wagner D. D. (2002) CD40L stabilizes arterial thrombi by a beta3 integrin--dependent mechanism. Nat. Med. 8, 247–252. [DOI] [PubMed] [Google Scholar]
- 77.Riedl J., Hell L., Kaider A., Koder S., Marosi C., Zielinski C., Panzer S., Pabinger I., Ay C. (2016) Association of platelet activation markers with cancer-associated venous thromboembolism. Platelets 27, 80–85. [DOI] [PubMed] [Google Scholar]
- 78.Hoos A., Protsyuk D., Borsig L. (2014) Metastatic growth progression caused by PSGL-1-mediated recruitment of monocytes to metastatic sites. Cancer Res. 74, 695–704. [DOI] [PubMed] [Google Scholar]
- 79.Pello O. M., De Pizzol M., Mirolo M., Soucek L., Zammataro L., Amabile A., Doni A., Nebuloni M., Swigart L. B., Evan G. I., Mantovani A., Locati M. (2012) Role of c-MYC in alternative activation of human macrophages and tumor-associated macrophage biology. Blood 119, 411–421. [DOI] [PubMed] [Google Scholar]
- 80.Roederer M., Raju P. A., Staal F. J., Herzenberg L. A., Herzenberg L. A. (1991) N-Acetylcysteine inhibits latent HIV expression in chronically infected cells. AIDS Res. Hum. Retroviruses 7, 563–567. [DOI] [PubMed] [Google Scholar]