Inhibitors of NE improve survival and differentiation of iPSC and HL60 cells expressing mutant NE.
Keywords: congenital neutropenia, cyclic neutropenia, neutrophil elastase
Abstract
Mutations in ELANE, the gene for neutrophil elastase (NE), a protease expressed early in neutrophil development, are the most frequent cause of cyclic (CyN) and severe congenital neutropenia (SCN). We hypothesized that inhibitors of NE, acting either by directly inhibiting enzymatic activity or as chaperones for the mutant protein, might be effective as therapy for CyN and SCN. We investigated β-lactam–based inhibitors of human NE (Merck Research Laboratories, Kenilworth, NJ, USA), focusing on 1 inhibitor called MK0339, a potent, orally absorbed agent that had been tested in clinical trials and shown to have a favorable safety profile. Because fresh, primary bone marrow cells are rarely available in sufficient quantities for research studies, we used 3 cellular models: patient-derived, induced pluripotent stem cells (iPSCs); HL60 cells transiently expressing mutant NE; and HL60 cells with regulated expression of the mutant enzyme. In all 3 models, the cells expressing the mutant enzyme had reduced survival as measured with annexin V and FACS. Coincubation with the inhibitors, particularly MK0339, promoted cell survival and increased formation of mature neutrophils. These studies suggest that cell-permeable inhibitors of neutrophil elastase show promise as novel therapies for ELANE-associated neutropenia.
Introduction
Mutations in ELANE, the gene for NE, are the most frequent cause of CyN and SCN [1, 2]. We recently reported genotype–phenotype relationships for mutations in ELANE for a population of >300 patients with CyN and SCN followed for up to 25 y through the SCNIR [3, 4]. Most of the patients enrolled in the SCNIR are being treated long term with G-CSF. A randomized, controlled trial and longitudinal clinical observations have demonstrated the effectiveness of G-CSF for most patients [5–8]. These studies also revealed that some patients do not respond well to G-CSF and are at risk of death from infections, and some patients evolve to MDS and acute myeloid leukemia AML [9, 10]. There appeared to be “low risk” and “high risk” mutations, and there are also concerns that G-CSF may contribute to the risk of leukemia through its antiapoptotic or stimulatory properties [9]. With rare exceptions, G-CSF or hematopoietic cell transplantation are the only predictably effective treatments to correct neutropenia in these patients [11–14]. General preventive measures, care (e.g., good skin and oral hygiene) and antibiotics and supportive care when they develop infections are the other mainstays of care.
The molecular and cellular mechanisms underlying ELANE-associated neutropenia are only partially understood. Current evidence indicates that the product of mutant ELANE acts in a dominant, cell-intrinsic fashion to disrupt neutrophil production in the bone marrow and cause neutropenia [15–19]. These studies indicate that mutations in NE initiate the UPR leading to cell loss in the process of neutrophil formation in the marrow. Apoptotic death of developing myeloid cells, the cells expressing ELANE, is considered to be the critical and final cellular mechanism for neutropenia.
Neutrophil elastase is synthesized primarily at the myeloblast stage in the formation of neutrophils. Normally the enzyme is rapidly packaged in primary granules after synthesis in the endoplasmic reticulum. Several studies indicate that the mutant protein does not traffic normally [15–19]. We hypothesized that, associated with the abnormal trafficking, there is activation of the cellular mechanisms, leading to apoptosis, and that various mutant proteins are more harmful to the developing cells than others are. These differences lead to the milder disease with some mutations and more-severe disease, with a much greater risk of evolution to MDS and AML, in others [3]. We have also hypothesized that inhibitors of NE, acting either by directly inhibiting enzymatic activity or as chaperones for the mutant protein, might be effective therapeutic agents for ELANE-associated neutropenia.
To investigate that possibility, we initiated studies of inhibitors of NE, agents that have been in development for many years largely as potential anti-inflammatory drugs [20, 21]. Theoretically, the most attractive agents are cell permeable and orally absorbed. We, therefore, focused our work on a small group of cell-permeable, orally absorbed inhibitors, previously tested in clinical trials, to see whether we could advance that hypothesis from the bench to the bedside [21]. In this report, we describe development of cellular models for the study of ELANE-associated neutropenia and promising results for enhancing survival and proliferation of neutrophil progenitors expressing mutant elastase using those inhibitors.
MATERIALS AND METHODS
Patients
Patients enrolled in the SCNIR gave informed consent for results mentioned in this report under protocols approval by the Human Subjects office/Institutional Review Board of the University of Washington. For the laboratory studies, we selected a subset of mutations observed in these patients and associated with a spectrum of treatment responses and clinical outcomes, based on our previous genotype–phenotype analysis [3]. The mutations selected and a summary of associated clinical outcomes are shown in Table 1.
TABLE 1.
ELANE mutations selected for the study
| ELANE mutation | Total patients | No. of patients with AML | Percentage of patients with AML |
|---|---|---|---|
| P139L | 21 | 0 | 0 |
| C151Y | 4 | 3 | 75 |
| V174_C181del | 2 | 1 | 50 |
| G214R | 12 | 3 | 25 |
Cell lines and cell cultures
HL60 cells
HL60 cells, kindly provided by Dr. Steve Collins (Fred Hutchinson Cancer Research Center, Seattle, WA, USA). Cell lines were maintained at 37°C, 5% CO2 incubator in RPMI-1640 supplemented with 10% FBS (Gemini Bio Products, West Sacramento, CA, USA), 2 mM l-glutamine, and Pen Strep (Thermo Fisher Scientific, Waltham, MA, USA).
iPSC
We have created iPSC lines from 3 patients with neutropenia (2 brothers with P139L and another patient with G214R) and 2 healthy volunteers using dermal fibroblasts and bone marrow mesenchymal cells, created in collaboration with the University of Washington Institute for Stem Cell and Regenerative Medicine. Episomal iPSC reprogramming vectors encoding Oct3/4, Sox2, Klf4, L-Myc, Lin28, and short hairpin RNA for TP53 were used. Irradiated, growth-arrested, mouse embryonic fibroblasts were used as feeder cells. Small iPSC-like colonies started to appear after 2–3 wk and were picked, expanded, and cryopreserved. Pluripotency was examined by flow cytometry using SSEA-4 and TRA-1-60 surface markers (BD Biosciences, Franklin Lakes, NJ, USA ). The iPSC lines were evaluated for transgene integration by PCR and for the capacity to give rise to teratomas in mice. Karyotype integrity of the cell lines was analyzed at Diagnostic Cytogenetics (Seattle, WA, USA). iPSCs were cultured using feeder-free DEF-CS (Clontech Laboratories, Mountain View, CA, USA) and supplemented with mTeSR1 (StemCell Technologies, Vancouver, BC, Canada) culture systems, following manufacturer’s guidelines.
Transient expression of mutant ELANE into HL60 cells
For cloning purposes, NE cDNA was amplified by reverse transcriptase-PCR using total RNA from human U937 cells and NE-specific primers containing BamHI and EcoRI restriction sites. The cDNA was cloned into pcDNA3.1 expression vector under control of a CMV promoter (Thermo Fisher Scientific). Mutant NE cDNAs carrying WT, P139L, C151Y, V174_C181del, G214R mutations were obtained by site-directed mutagenesis using mutant NE-specific primers. To verify the preservation of an open reading frame and absence of PCR-introduced artifacts, each construct was sequenced across the entire coding region.
For transfection experiments, 5 μg of plasmid DNA containing normal or mutant NE cDNA was electroporated to 5 × 105 HL60 cells using the Neon electroporation system (Thermo Fisher Scientific). Resultant cells were cultured in RPMI-1640, supplemented with 10% FBS, for up to 3 d. For transfection efficiency, enhanced GFP expressing pcDNA3.1 plasmid was used and GFP+ cells quantified using flow cytometry. We observed 33 ± 3% transfection efficiency. (n = 7) (Supplemental Fig. 1).
Tet-regulated expression of mutant ELANE in HL60 cells
We selected a distinctive deletion mutation in ELANE for these studies. The 8–amino acid, in-frame deletion (V174_C181del) was identified in a family with 2 affected individuals, a father and a daughter. After 9 yr on G-CSF, the father developed AML and died after failing to respond to treatment. His daughter has continued G-CSF for more than 22 yr without evidence of myelodysplasia.
The plasmids used to establish the tet-regulated system were purchased from Clontech and used according to the manufacturer’s instructions. The clone positive for doxycycline-regulated enhanced GFP expression, as determined by flow cytometry or Western blot analysis using a GFP-specific mAb, was used in subsequent experiments. Tet-off cells were cotransfected with plasmids containing a hygromycin-resistance gene and human NE (normal or mutant) cDNA under control of tet-operator sequences upstream of the CMV promoter and selected by the presence of hygromycin. The resulting HL60 cells were expanded, and expression of NE was assessed by Western blot analysis at 48 or 72 h of culture in the presence or absence of doxycycline using NE–specific antibodies (anti-NE goat polyclonal IgG, Santa Cruz Biotechnology, Santa Cruz, CA, USA) (Supplemental Fig. 2).
HL60 and iPSC myeloid differentiation
Myeloid differentiation of the tet-regulated HL60 cells was stimulated by adding 2 μM ATRA to cell culture for up to 5 d. At indicated times, the resultant, conditioned cells were mounted to glass slides with Cytospin 4 (Thermo Fisher Scientific), prepared and stained with Diff-Quik (Baxter, Deerfield, IL, USA) and assessed using light microscopy. Images were acquired with a Nikon (Minato, Tokyo, Japan) digital camera.
Patient- and healthy volunteer-derived iPSCs were stimulated to differentiate with G-CSF, VEGF, BMP4, IL-3, TPO, and SCF-enriched Stemline II medium (Sigma-Aldrich, St. Louis, MO, USA) for 3 wk in 6-well plates in the presence or absence of 1 μM MK0339 inhibitor using a slightly modified and previously described methodology [22]. After 3 wk of culture, floating cells were harvested and analyzed by flow cytometry.
Flow cytometry analysis
The survival rate of tet-regulated HL60 cells cultured in the presence or absence of doxycycline was evaluated by flow cytometry. Cells were labeled with PE-conjugated Annexin V and 7‑aminoactinomycin D (BD Biosciences) and analyzed by FACS. Cell debris and necrotic cells were gated out, and the percentage of annexin V-positive cells was expressed as the number of apoptotic cells per total number of cells. Hematopoietic differentiation of iPSC was analyzed by flow cytometry using CD45 and CD11b specific antibodies (BD Biosciences).
Neutrophil elastase inhibitors
Four-cell, permeable inhibitors of NE provided by Merck & Co. (Kenilworth, NJ, USA) were tested for their effects to alter proliferation and survival of HL60 cells expressing normal and mutant NE [23]. Those orally absorbed, β-lactam–derived compounds had the following formulae: C31H40N4O6 564 688 (MK0339), C31H42N4O4 534 705 (L-910), C27H44N4O4 488 676 C4H5O5 133 081 (L-538), C27H45N4O4 489 684, and C4H5O5 133 081 (L-635). For comparison purposes, we also used sivelestat (Sigma-Aldrich), a commercially available NE inhibitor used in recent reports [17, 21, 24].
The NE proteolytic activities in cell lysates from cells exposed and not exposed to the inhibitors were determined using EnzCheck Elastase assay kit from Thermo Fisher Scientific, according to the manufacturer’s recommendations.
Confocal immunofluorescence microscopy
Sorted CD11b cells from differentiated iPSC cultures were cytospun onto Cytoslide microscope slides (Thermo Fisher Scientific), air dried, and fixed in 4% paraformaldehyde for 15 min, permeabilized with 0.1% Triton X-100 (catalog T9284; Sigma-Aldrich) for 5 min, and blocked with 1% normal goat serum for 30 min. Slides were stained with primary anti-NE (EMD Millipore, Billerica, MA, USA) and anti-MPO (Dako, Carpinteria, CA, USA) Abs at 4°C overnight, then washed and treated with AlexaFluor 647-congugated goat anti-mouse IgG and AlexaFluor 488 goat anti-rabbit IgG F(ab′) secondary Abs (Thermo Fisher Scientific) for 40 min at room temperature. The cells were washed and mounted using ProLong Gold Antifade with DAPI mounting media (Thermo Fisher Scientific). The stained cells were analyzed using a SP8X confocal microscope and ×63 NA1.4 oil immersion objective (Leica Microsystems, Mannheim, Germany).
UPR analysis
UPR was measured by using Western blot analysis. HL60 cell lines transfected to express normal and mutant ELANE were cultured for 48–72 h as described above and lysed in 1× radioimmunoprecipitation assay buffer (Thermo Fisher Scientific) containing a protease inhibitor cocktail (Thermo Fisher Scientific). The protein samples were electrophoresed through 10% SDS-PAGE gel, followed by semidry transfer to nitrocellulose membrane. The membrane was blocked and treated with primary Abs against GRP78/BiP and ATF6 (Abcam, Cambridge, MA, USA) or β-ACTIN (Santa Cruz Biotechnology) followed by washing and subsequent treatment with anti-rabbit and anti-mouse HRP tagged secondary antibodies (Abcam and Santa Cruz Biotechnology). The blots were developed using a chemiluminescence reaction.
Statistical methods
Comparisons were made using ordinary, 1-way ANOVA with Sidak’s multiple comparisons test.
RESULTS
Preliminary studies
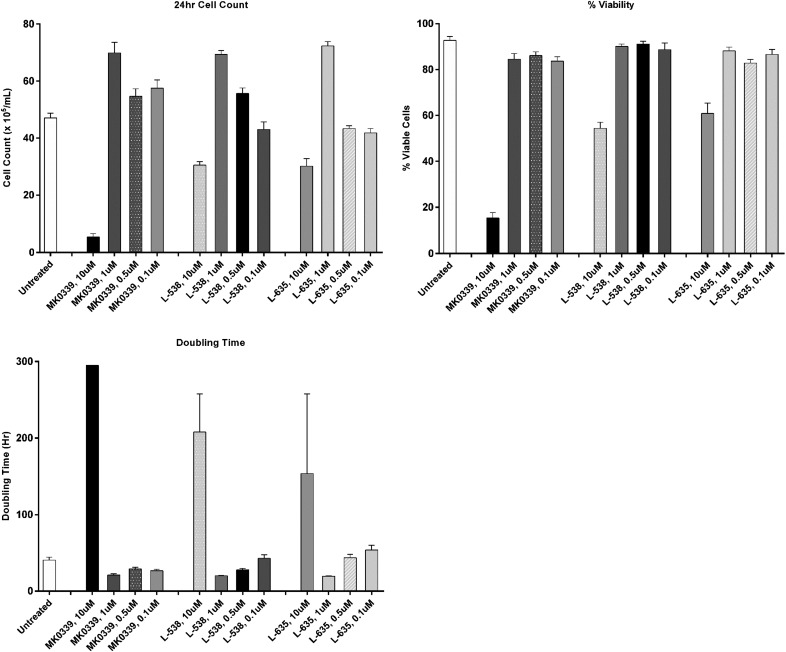
We investigated the potential toxic effects of compounds L538, L635, and MK0339 in dose–response studies. Effects on cell counts, doubling time, and viability of HL60 cells were examined in 24 h culture experiments at 37°C in a standard CO2 incubator. As shown in Fig. 1, concentrations of all these agents >1 μM inhibited both proliferation and survival; therefore, subsequent experiments used the inhibitors at a concentration of 1 μM, unless otherwise specified. In separate experiments, L910 was also found to be toxic at >1 μM.
Figure 1. The effect of Merck serine protease inhibitors (MK-0339, L-538, L-635) on proliferative capacities and viability of HL60 cells.
HL60 cells were cultured in the presence of 10, 1, 0.5, and 0.1 μM Merck inhibitors for 24 h. Cell counts and cell viability were measured with a standard light microscope and trypan-blue staining. N ≥ 3.
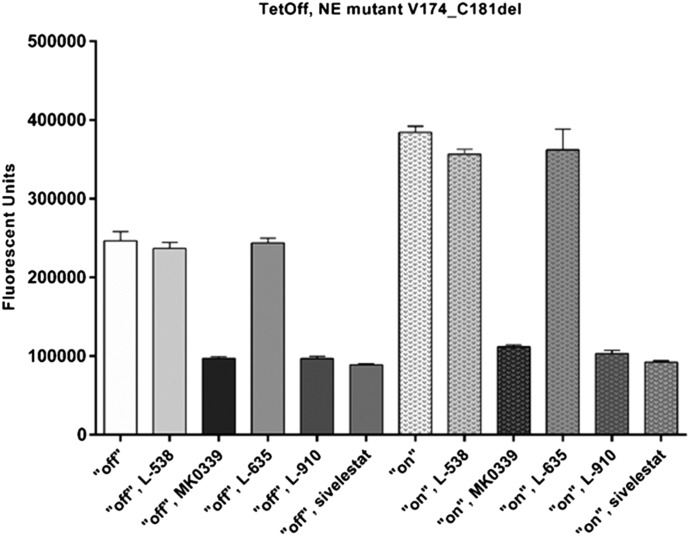
We then compared the NE inhibitor activity of L538, L635, L910, MK0339 (1 μM), and sivelestat (100 nM, the concentration used in other studies [24]) in 24-h cultures of tet-regulated HL60 cells expressing mutant NE. That study showed that MK0339, L910, and sivelestat reduced elastase activity in cell lysates by about 60% in the presence of doxycycline (mutant “off”) and by about 73% in the absence of doxycycline (mutant “on”). These differences were statistically significant (P < 0.0001 for each condition/inhibitor). L538 and L635 were inactive at these concentrations and were used throughout the rest of this investigation as inactive controls (Fig. 2).
Figure 2. The effect of Merck inhibitors and sivelestat on proteolytic activity of NE.
Cells expressing either the WT or both WT and mutant NE in the presence or absence of inhibitors were lysed and the proteolytic activity of NE was determined using Enzchek elastase assay kit and fluorescence microplate reader. N = 3.
Transient transfection experiments
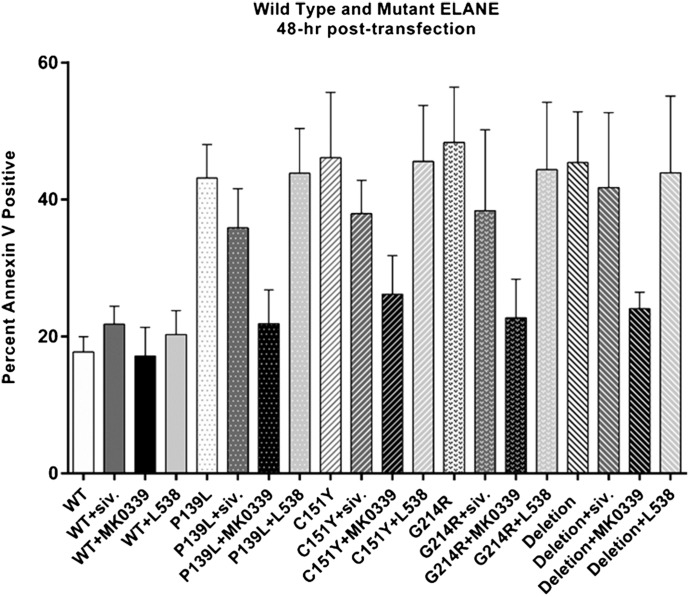
We then compared the effects of L538, MK0339, and sivelestat on survival of HL60 cells transiently expressing WT NE or 1 of 4 mutants: P139L, C151Y, V174_C181del, G214R, using annexin V as the marker for apoptosis as assessed by FACS analysis (Fig. 3).
Figure 3. The effect of MK-0339 and sivelestat inhibitors on accelerated apoptosis of human HL60 cells transiently expressing mutant NE.
HL60 cells were transfected (electroporation) with WT and mutant (P139L, G214R, C151Y, V174_C181del) NE-expressing vectors; cultured for 48 h in the presence or absence of 1 μM MK0339, 1 μM L538, and 100 nM sivelestat inhibitors; labeled with annexin V–PE; and analyzed using flow cytometry. The percentage of annexin V–positive cells is indicated. N > 3.
Expression of mutant elastase significantly (P < 0.0001) increased the proportion of annexin V–positive cells, and addition of 1 μM MK0339 at initiation of the 48 h of culture substantially blunted that effect. Comparing the cells that were or were not expressing the mutant elastases, MK0339 significantly (P < 0.0001) reduced the percentage of annexin V–positive cells for each mutant tested and as an aggregate effect, i.e., mutants with or without the inhibitor.
There was no effect of these agents on cells expressing WT NE. In addition, lower doses of MK0339 did not significantly reduce apoptosis in HL60 cells expressing the G214R mutant NE (Supplemental Fig. 3). Addition of 100 nM sivelestat was significantly effective on only 1 mutant, G214R. (P = 0.034) (Fig. 3).
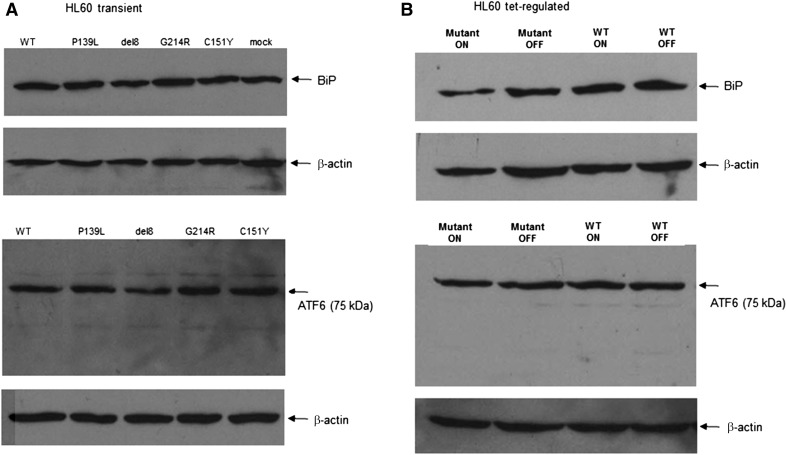
We measured UPR signature in HL60 cells transiently expressing WT or mutant NE (P139L, G214R, C151Y, and V174_C181del) by Western blot analysis. There was no significant difference in expression of UPR expression markers GRP78/BiP, ATF6 throughout the mutants, and control (Fig. 4A).
Figure 4. UPR markers in HL60 models.
(A) HL60 cells were transfected (electroporation) with WT and mutant (P139L, G214R, C151Y, V174_C181del) NE-expressing vectors, cultured for 48 h, lysed, and Western blots were performed using anti-BiP and anti-ATF6 Abs as described in Materials and methods. (B) Tet-regulated HL60 cells expressing WT or mutant NE were cultured for 72 h in the presence or absence of doxycycline, lysed, and Western blots were performed as described. Representative experiments are shown (n = 3).
Results with tet-regulated HL60 cells expressing WT and mutant NE
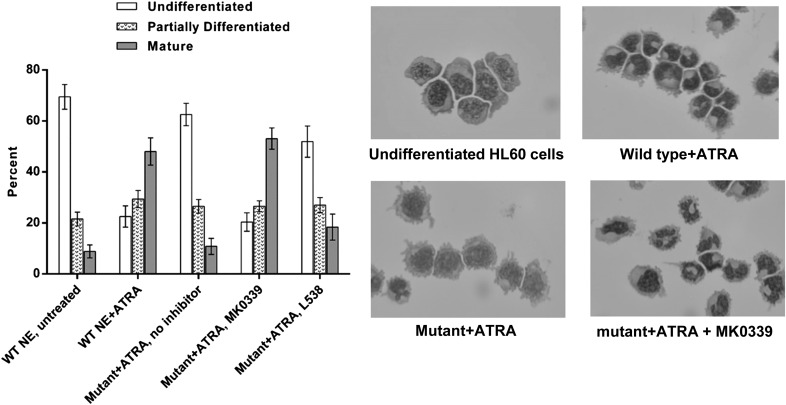
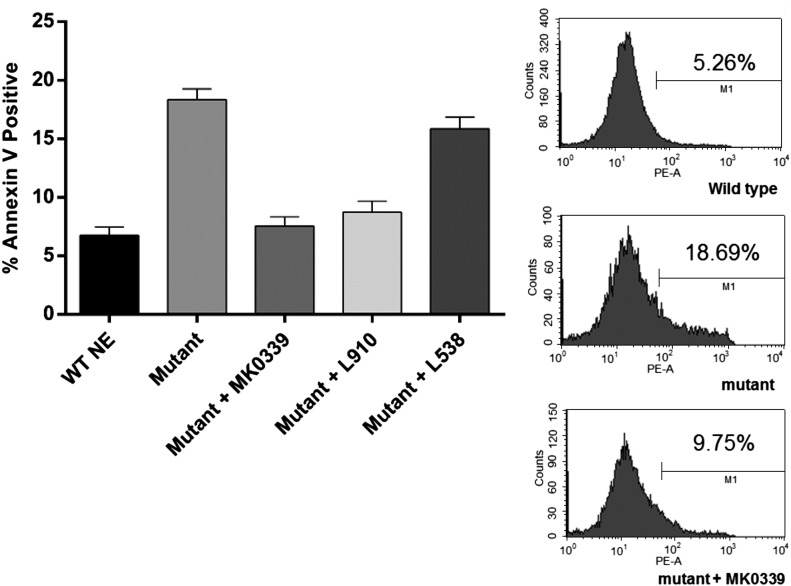
We next used HL60 cell lines expressing WT ELANE or the deletion mutant, V174_C181del, under the control of the tet-regulated promoter. Expression of the mutant elastase significantly decreased the proportion of mature myeloid cells in the cultures after 5 d of ATRA-induced differentiation (P < 0.0001). Addition of MK0339 made the cultures revert toward the control, i.e., WT with no inhibitor, pattern of differentiation (Fig. 5). In separate experiments using MK0339, L538, and L910, MK0339 had the greatest effect to reduce the proportion of annexin V–positive cells (P < 0.0001) (Fig. 6).
Figure 5. The effect of MK0339 on the impaired, ATRA-induced myeloid differentiation of tet-regulated HL60 cells expressing mutant and WT NE.
Human promyelocytic HL60 cells expressing either WT or mutant NE were induced to differentiate with 2 μM ATRA (all-trans retinoic acid) and maintained 5 d in culture, with and without 1 μM Merck inhibitors MK0339 or L538. Cell cytospins stained with Diff Quik were imaged using Nikon digital camera. Cell differentiation was evaluated by light microscope. N > 3.
Figure 6. The effect of Merck inhibitors on accelerated apoptosis of tet-regulated HL60 cells expressing mutant and wild type NE.
Cells cultured for 72 h in the presence or absence of doxycycline and MK0339, L910 and L538 were labeled with annexin V and analyzed using flow cytometry. The proportion of annexin V–positive cells is indicated. Representative experiment histograms are shown. N > 3.
UPR signature analysis (GRP78/BiP, ATF6) by Western blot revealed no significant difference between the cell lines with regulated expression of mutant or WT ELANE (Fig. 4B).
Results with iPSC derived from patients with neutropenia
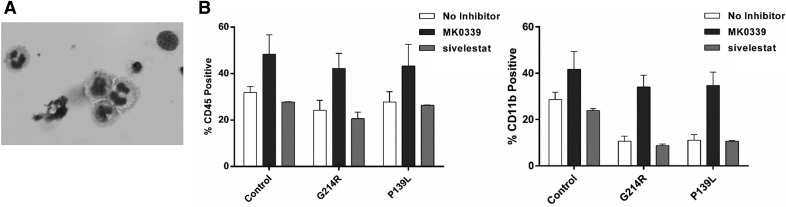
Induced pluripotent stem cells were derived from a patient with SCN and the ELANE mutation P139L and from another patient with the ELANE mutation G214R. As a control, we used similarly generated iPSC from a healthy volunteer. After 3 wk of culture in medium containing G-CSF, VEGF, BMP4, IL-3, TPO, and SCF enriched and insulin-transferrin-selenium supplemented Stemline II medium in the presence or absence of 1 μM MK0339, examination of floating cells revealed the presence of hematopoietic cells, with myeloid differentiation characteristics (Fig. 7A). There was no significant difference in the proportion of control vs. patient-derived cells expressing CD45 (P > 0.97). However, the proportion of cells differentiating to express CD11b was reduced for patient iPSCs with either the P139L or G214R mutation (P = 0.049; P = 0.043, respectively). The presence of MK0339, but not sivelestat, in the culture medium significantly increased the proportion of patient-derived iPSC differentiating to neutrophils expressing CD11b (P139L, P = 0.004; G214R, P = 0.005) (Fig. 7B).
Figure 7. iPSC myeloid differentiation.
iPSC from a healthy volunteer and 2 patients with neutropenia and 1 each with the G214R and P139L ELANE mutations were stimulated toward myeloid differentiation in 6-well plates in the presence or absence of 1 μM MK0339 and 230 μM sivelestat inhibitors, using a feeder-free culture system. After 3 wk of culture, floating cells were collected. (A) Cell cytospins stained with Diff-Quik and evaluated under a light microscope. (B) Cells were labeled with CD45 and CD11b fluorescent Abs and analyzed by flow cytometry (n = 3).
Immunostaining
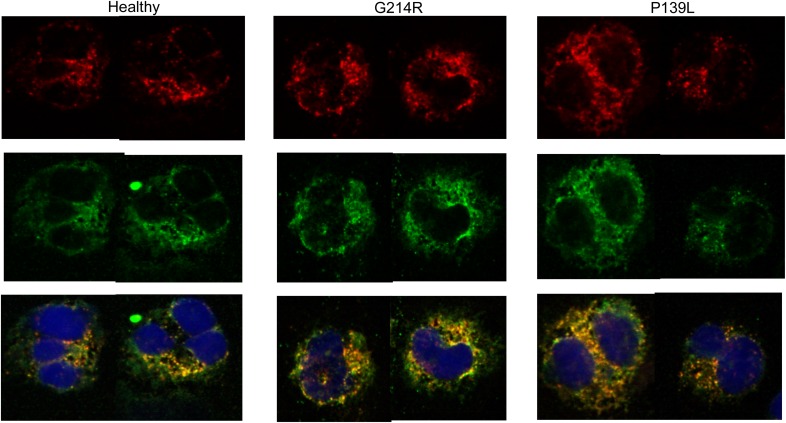
Immunostaining of CD11b sorted cells from in vitro differentiated iPSC lines showed expression of MPO and NE enzymes and normal intracellular localization in both mutant and healthy volunteer cell lines cultured without MK0339 (Fig. 8). Enzyme expression and localization were also quite similar for cells differentiating in the presence of MK0339 (images not shown).
Figure 8. iPSC myeloid differentiation.
NE/MPO intracellular localization. iPSC from a healthy volunteer and 2 patients with neutropenia and either a G214R or a P139L ELANE mutation were differentiated toward granulocytes; then, CD11b surface marker-expressing cells were sorted by flow cytometry. Cytospins of the sorted cells were stained using anti-NE and anti-MPO fluorescent Abs and evaluated using a Leica SP8X confocal microscope and ×63 NA1.4 oil immersion objective. Top row, NE. Middle row, MPO. Bottom row, NE and MPO colocalized with DAPI.
DISCUSSION
SCN and CyN are life-threatening diseases complicated by recurrent fever, painful mouth ulcers, skin and deep tissue infections, and death from infection or transformation to myeloid malignancies [25–28]. Treatment with G-CSF greatly improves the lives for most of these patients by increasing their mean blood neutrophil levels and reducing infectious complications [5–9]. The SCNIR has collected substantial data on the effectiveness and safety of longitudinal treatment of these patients with G-CSF. Those data show that, for most patients, particularly those treated with relatively low doses of daily or alternate-day G-CSF, the safety profile is very favorable [4]. Genotype–phenotype analyses based on long-term observational studies demonstrate variation in responses to G-CSF and in the risk of evolution to MDS/AML [3]. Although others have not observed all these relationships [28], our study shows that mutation G214R is consistently associated with a poor prognosis, specifically, a greater risk for failure to respond, development of serious infectious complications, and evolution to AML [3, 9]. Because most patients with SCN and CyN are now treated with G-CSF to prevent severe infections, it is not possible to determine precisely the contribution of G-CSF, if any, to the risk of AML. However, concerns persist, and experts recommend that long-term treatment with G-CSF should be administered conservatively and only to patients with recurrent fever and infections [29–31]. In addition, G-CSF must be administered s.c., usually daily or every other day, presumably for the entire life of the patient.
We began the current investigations based on the hypothesis that cell-permeable inhibitors of the mutant enzyme might alter cell survival of developing myeloid cells [32, 33]. We were aware of the long history of investigating the role of NE in the acute inflammatory response and developing drugs with that enzyme as the target of anti-inflammatory therapies [34, 35]. We decided to focus primarily on the orally absorbed β-lactam–based inhibitors of human NE developed by Merck, because we were aware that 1 synthetic inhibitor, originally called L-694,458 and now called MK0339, had been tested in early phase clinical trials, had high potency, and a favorable safety profile [36–38]. Originally, we planned to use primary bone marrow cells for our investigations, but fresh marrow samples are rarely available in sufficient quantities for research studies. Accepting that limitation, we developed 3 models as described above: patient-derived iPSC, HL60 cells transiently expressing mutant elastase, and HL60 cells with regulated expression of the mutant enzyme. In all 3 models, cells expressing the mutant enzyme have reduced survival as measured with annexin V and FACS. In the simplest of these models, transient expression of mutant elastase in HL60 cells, we did not observe substantial differences between the different mutants known to be associated with progressively more severe clinical outcomes. In this system, we found that the elastase inhibitors had similar effects, and the most potent inhibitor was MK0339. Sivelestat appeared to be less effective, but the number of comparisons was insufficient for any definitive conclusions. We were more impressed by the results of experiments with HL60 cells with regulated expression of mutant elastase. These experiments showed not only improved survival but also the potential for differentiation of the HL60 cells to form mature neutrophils. Investigations using iPSC also demonstrated that the inhibitors promoted cell survival and were permissive of complete differentiation to mature neutrophils.
We initiated these studies hypothesizing that survival of cells expressing mutant ELANE would be improved by exposure to an inhibitor of the NE protein. We did not have a panel of purified mutant NE to examine, but we did find that the inhibitors were effective at promoting cell survival in cells expressing several different mutants. Initiation of the UPR leading to cell loss by apoptosis is now widely regarded as a central mechanism for ELANE-associated neutropenia, but increases in the UPR have not been uniformly observed [16, 18]. We were a bit surprised when we did not observe effects on the UPR in our HL60-based cellular models that clearly demonstrate the effects of transfection with mutant ELANE and the effectiveness of MK0339 to promote survival and differentiation. The expression of UPR markers GRP78/BiP and ATF6 were unaltered based on Western blot analysis. As mentioned above, variability in the UPR response across the array of ELANE mutations was observed by many investigators, but we expected at least some mutations might show an effect. We were also surprised to observe that MK0339 promotes differentiation of healthy volunteer-derived iPSCs and appears to promote the survival of patients-derived iPSC harboring mutant ELANE (data not shown). These observations suggest additional effects of inhibitors of NE beyond their effects on this specific protease. We are currently investigating possible mechanisms.
Immunofluorescence microscopy reveals normal intracellular localization of primary granule proteins NE and MPO in CD11b-sorted, differentiated, mutant iPSC lines. This is an interesting observation and indicates that the defect in the mutant cell lines predominantly impairs the production and maturation of the granulocytes but does not result in morphologic, and most probably functional, defects in already mature granulocytes.
For a successful clinical trial, it is critical to have a druggable target and to be able to achieve pharmacological concentration of the drug at the target’s physiologic site. Published pharmacokinetic studies in rats indicate that MK0339 achieves a plasma concentration of <0.5 mg/ml after an oral dose of 10 mg/kg, suggesting that the drug concentrations used for these in vitro studies are sufficient to achieve at least transiently inhibitory concentrations in blood and bone marrow myeloid cells [38]. We originally thought that cells expressing only some, but not all, of the mutations of ELANE observed in SCN and CyN would be affected by the inhibitors, but the work to date suggests an effect with a broad range of ELANE mutations. We, therefore, intend to extend this work to include additional mutations with regulated expression in HL60 cells and in patient-derived iPSC. We will also examine other inhibitors of NE, particularly focusing on those with high potency and pharmacokinetic and favorable safety profiles. Recognizing the limits of our work, we are encouraged by the findings and truly hope that they will open the door to other new therapies for neutropenia.
AUTHORSHIP
V.M. and M.L.K. conducted laboratory studies and data analysis and participated in writing the report. B.F. conducted laboratory studies for this report. A.A.B. collected clinical data and participated in writing this report. A.A. participated in planning and conducting this study, and D.C.D. organized and designed this research study and participated in writing the report.
ACKNOWLEDGMENTS
We gratefully acknowledge support from Merck in providing the inhibitor drugs used for this work, the patients and physicians participating in the Severe Chronic Neutropenia International Registry, support from U.S. National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID) Grant R24AI049393, gifts from the Ella Jewell Foundation, and patients and families of the National Neutropenia Network. Nathaniel Peters of the W. M. Keck Center for Advanced Studies in Neural Signaling provided expert assistance with confocal microscopy; the Keck Center is supported by NIH/Office of Research Infrastructure Programs (ORIP) Grant S10 OD016240.
Glossary
- AML
acute myeloid leukemia
- ATRA
all-trans-retinoic acid
- CyN
cyclic neutropenia
- iPSC
induced pluripotent stem cell
- MDS
myelodysplastic syndrome
- MPO
myeloperoxidase
- NE
neutrophil elastase
- SCN
severe congenital neutropenia
- SCNIR
Severe Chronic Neutropenia International Registry
- UPR
unfolded protein response
- WT
wild-type
Footnotes
The online version of this paper, found at www.jleukbio.org, contains supplemental information.
DISCLOSURES
V.M., M.L.K., B.F., and A.A.B. declare no conflicts of interest. A.A. and D.C.D. are inventors on the joint University of Washington–Merck patent for the use of inhibitors of NE to treat inherited severe neutropenia. D.C.D. is also a consultant and receives research support from Amgen, a manufacturer of G-CSF, mentioned in the article.
REFERENCES
- 1.Xia J., Bolyard A. A., Rodger E., Stein S., Aprikyan A. A., Dale D. C., Link D. C. (2009) Prevalence of mutations in ELANE, GFI1, HAX1, SBDS, WAS and G6PC3 in patients with severe congenital neutropenia. Br. J. Haematol. 147, 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dale D. C., Link D. C. (2009) The many causes of severe congenital neutropenia. N. Engl. J. Med. 360, 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makaryan V., Zeidler C., Bolyard A. A., Skokowa J., Rodger E., Kelley M. L., Boxer L. A., Bonilla M. A., Newburger P. E., Shimamura A., Zhu B., Rosenberg P. S., Link D. C., Welte K., Dale D. C. (2015) The diversity of mutations and clinical outcomes for ELANE-associated neutropenia. Curr. Opin. Hematol. 22, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dale D. C., Cottle T. E., Fier C. J., Bolyard A. A., Bonilla M. A., Boxer L. A., Cham B., Freedman M. H., Kannourakis G., Kinsey S. E., Davis R., Scarlata D., Schwinzer B., Zeidler C., Welte K. (2003) Severe chronic neutropenia: treatment and follow-up of patients in the Severe Chronic Neutropenia International Registry. Am. J. Hematol. 72, 82–93. [DOI] [PubMed] [Google Scholar]
- 5.Dale D. C., Bonilla M. A., Davis M. W., Nakanishi A., Hammond W. P., Kurtzberg J., Wang W., Jakubowski A., Winton E., Lalezari P., Robinson W., Glaspy J. A., Emerson S., Gabrilove J., Vincent M., Boxer L. A. (1993) A randomized controlled phase III trial of recombinant human G-CSF for treatment of severe chronic neutropenia. Blood 81, 2496–2502. [PMC free article] [PubMed] [Google Scholar]
- 6.Carlsson G., Ahlin A., Dahllöf G., Elinder G., Henter J. I., Palmblad J. (2004) Efficacy and safety of two different rG-CSF preparations in the treatment of patients with severe congenital neutropenia. Br. J. Haematol. 126, 127–132. [DOI] [PubMed] [Google Scholar]
- 7.Donadieu J., Fenneteau O., Beaupain B., Mahlaoui N., Chantelot C. B. (2011) Congenital neutropenia: diagnosis, molecular bases and patient management. Orphanet J. Rare Dis. 6, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salehi T., Fazlollahi M. R., Maddah M., Nayebpour M., Tabatabaei Yazdi M., Alizadeh Z., Eshghi P., Chavoshzadeh Z., Movahedi M., Hamidieh A. A., Cheraghi T., Pourpak Z., Moin M. (2012) Prevention and control of infections in patients with severe congenital neutropenia; a follow up study. Iran. J. Allergy Asthma Immunol. 11, 51–56. [PubMed] [Google Scholar]
- 9.Donadieu J., Leblanc T., Bader Meunier B., Barkaoui M., Fenneteau O., Bertrand Y., Maier-Redelsperger M., Micheau M., Stephan J. L., Phillipe N., Bordigoni P., Babin-Boilletot A., Bensaid P., Manel A. M., Vilmer E., Thuret I., Blanche S., Gluckman E., Fischer A., Mechinaud F., Joly B., Lamy T., Hermine O., Cassinat B., Bellanné-Chantelot C., Chomienne C.; French Severe Chronic Neutropenia Study Group (2005) Analysis of risk factors for myelodysplasias, leukemias and death from infection among patients with congenital neutropenia: experience of the French Severe Chronic Neutropenia Study Group. Haematologica 90, 45–53. [PubMed] [Google Scholar]
- 10.Rosenberg P. S., Alter B. P., Bolyard A. A., Bonilla M. A., Boxer L. A., Cham B., Fier C., Freedman M., Kannourakis G., Kinsey S., Schwinzer B., Zeidler C., Welte K., Dale D. C.; Severe Chronic Neutropenia International Registry (2006) The incidence of leukemia and mortality from sepsis in patients with severe congenital neutropenia receiving long-term G-CSF therapy. Blood 107, 4628–4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferry C., Ouachée M., Leblanc T., Michel G., Notz-Carrére A., Tabrizi R., Flood T., Lutz P., Fischer A., Gluckman E., Donadieu J. (2005) Hematopoietic stem cell transplantation in severe congenital neutropenia: experience of the French SCN register. Bone Marrow Transplant. 35, 45–50. [DOI] [PubMed] [Google Scholar]
- 12.Fioredda F., Iacobelli S., van Biezen A., Gaspar B., Ancliff P., Donadieu J., Aljurf M., Peters C., Calvillo M., Matthes-Martin S., Morreale G., van 't Veer-Tazelaar N., de Wreede L., Al Seraihy A., Yesilipek A., Fischer A., Bierings M., Ozturk G., Smith O., Veys P., Ljungman P., Peffault de Latour R., Sánchez de Toledo Codina J., Or R., Ganser A., Afanasyev B., Wynn R., Kalwak K., Marsh J., Dufour C.; Severe Aplastic Anemia the Inborn Error, and the Pediatric Disease Working Parties of the European Society for Blood and Bone Marrow Transplantation (EBMT); Stem Cell Transplant for Immunodeficiencies in Europe (SCETIDE) (2015) Stem cell transplantation in severe congenital neutropenia: an analysis from the European Society for Blood and Marrow Transplantation. Blood 126, 1885–1892. [DOI] [PubMed] [Google Scholar]
- 13.Kawaguch K., Matsubara K., Uchida Y., Saito A., Miyata K., Hasegawa D., Kosaka Y., Iwata A., Nigami H., Kobayashi M. (2014) Successful treatment with allogenic hematopoietic stem cell transplantation of a severe congenital neutropenia patient harboring a novel ELANE mutation. Rinsho Ketsueki 55, 2294–2299. [PubMed] [Google Scholar]
- 14.Connelly J. A., Choi S. W., Levine J. E. (2012) Hematopoietic stem cell transplantation for severe congenital neutropenia. Curr. Opin. Hematol. 19, 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Köllner I., Sodeik B., Schreek S., Heyn H., von Neuhoff N., Germeshausen M., Zeidler C., Krüger M., Schlegelberger B., Welte K., Beger C. (2006) Mutations in neutrophil elastase causing congenital neutropenia lead to cytoplasmic protein accumulation and induction of the unfolded protein response. Blood 108, 493–500. [DOI] [PubMed] [Google Scholar]
- 16.Grenda D. S., Murakami M., Ghatak J., Xia J., Boxer L. A., Dale D., Dinauer M. C., Link D. C. (2007) Mutations of the ELA2 gene found in patients with severe congenital neutropenia induce the unfolded protein response and cellular apoptosis. Blood 110, 4179–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nayak R. C., Trump L. R., Aronow B. J., Myers K., Mehta P., Kalfa T., Wellendorf A. M., Valencia C. A., Paddison P. J., Horwitz M. S., Grimes H. L., Lutzko C., Cancelas J. A. (2015) Pathogenesis of ELANE-mutant severe neutropenia revealed by induced pluripotent stem cells. J. Clin. Invest. 125, 3103–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nustede R., Klimiankou M., Klimenkova O., Kuznetsova I., Zeidler C., Welte K., Skokowa J. (2016) ELANE mutant-specific activation of different UPR pathways in congenital neutropenia. Br. J. Haematol. 172, 219–227. [DOI] [PubMed] [Google Scholar]
- 19.Horwitz M. S., Corey S. J., Grimes H. L., Tidwell T. (2013) ELANE mutations in cyclic and severe congenital neutropenia: genetics and pathophysiology. Hematol. Oncol. Clin. North Am. 27, 19–41, vii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawabata K., Suzuki M., Sugitani M., Imaki K., Toda M., Miyamoto T. (1991) ONO-5046, a novel inhibitor of human neutrophil elastase. Biochem. Biophys. Res. Commun. 177, 814–820. [DOI] [PubMed] [Google Scholar]
- 21.Henriksen P. A. (2014) The potential of neutrophil elastase inhibitors as anti-inflammatory therapies. Curr. Opin. Hematol. 21, 23–28. [DOI] [PubMed] [Google Scholar]
- 22.Lachmann N., Ackermann M., Frenzel E., Liebhaber S., Brennig S., Happle C., Hoffmann D., Klimenkova O., Lüttge D., Buchegger T., Kühnel M. P., Schambach A., Janciauskiene S., Figueiredo C., Hansen G., Skokowa J., Moritz T. (2015) Large-scale hematopoietic differentiation of human induced pluripotent stem cells provides granulocytes or macrophages for cell replacement therapies. Stem Cell Rep. 4, 282–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies P., Ashe B. M., Bonney R. J., Dorn C., Finke P., Fletcher D., Hanlon W. A., Humes J. L., Maycock A., Mumford R. A., Navia M. A., Opas E. E., Pacholok S., Shah S., Zimmerman M., Doherty J. B. (1991) The discovery and biologic properties of cephalosporin-based inhibitors of PMN elastase. Ann. N. Y. Acad. Sci. 624, 219–229. [DOI] [PubMed] [Google Scholar]
- 24.Dokai M., Madoiwa S., Yasumoto A., Kashiwakura Y., Ishiwata A., Sakata A., Makino N., Ohmori T., Mimuro J., Sakata Y. (2011) Local regulation of neutrophil elastase activity by endogenous α1-antitrypsin in lipopolysaccharide-primed hematological cells. Thromb. Res. 128, 283–292. [DOI] [PubMed] [Google Scholar]
- 25. Dale, D. C. (2002) ELANE-related neutropenia. In GeneReviews at GeneTests: Medical Genetics Information Resource. (R. A. Pagon, T. C. Bird, C. R. Dolan, K. Stephens, eds.) University of Washington, Seattle, WA. Available at: http://www.ncbi.nlm.nih.gov/books/NBK1533/ Accessed October 10, 2016.
- 26.Boztug K., Klein C. (2013) Genetics and pathophysiology of severe congenital neutropenia syndromes unrelated to neutrophil elastase. Hematol. Oncol. Clin. North Am. 27, 43–60, vii. [DOI] [PubMed] [Google Scholar]
- 27.Bartels M., Murphy K., Rieter E., Bruin M. (2016) Understanding chronic neutropenia: life is short. Br. J. Haematol. 172, 157–169. [DOI] [PubMed] [Google Scholar]
- 28.Germeshausen M., Deerberg S., Peter Y., Reimer C., Kratz C. P., Ballmaier M. (2013) The spectrum of ELANE mutations and their implications in severe congenital and cyclic neutropenia. Hum. Mutat. 34, 905–914. [DOI] [PubMed] [Google Scholar]
- 29.Dufour C., Miano M., Fioredda F. (2016) Old and new faces of neutropenia in children. Haematologica 101, 789–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fioredda F., Calvillo M., Bonanomi S., Coliva T., Tucci F., Farruggia P., Pillon M., Martire B., Ghilardi R., Ramenghi U., Renga D., Menna G., Pusiol A., Barone A., Gambineri E., Palazzi G., Casazza G., Lanciotti M., Dufour C. (2012) Congenital and acquired neutropenias consensus guidelines on therapy and follow-up in childhood from the Neutropenia Committee of the Marrow Failure Syndrome Group of the AIEOP (Associazione Italiana Emato-Oncologia Pediatrica). Am. J. Hematol. 87, 238–243. [DOI] [PubMed] [Google Scholar]
- 31.Dale D. C., Boxer L. A. (2012) Guidelines for pediatric management of severe chronic neutropenia. Am. J. Hematol. 87, 133. [DOI] [PubMed] [Google Scholar]
- 32.Aprikyan A. A., Makaryan V., Si Q., Treonze K., Markosyn N., Finke P., Torov M., Abagyan R., Mumford R., Dale D. (2007) Small molecule inhibitor of neutrophil elastase restores impaired production of human myeloid cells observed in severe congenital neutropenia. (ASH Annual Meeting Abstracts). Blood 110, 664a [Google Scholar]
- 33.Korkmaz B., Horwitz M. S., Jenne D. E., Gauthier F. (2010) Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol. Rev. 62, 726–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janoff A. (1972) Neutrophil proteases in inflammation. Annu. Rev. Med. 23, 177–190. [DOI] [PubMed] [Google Scholar]
- 35.Ohbayashi H. (2002) Novel neutrophil elastase inhibitors as a treatment for neutrophil-predominant inflammatory lung diseases. IDrugs 5, 910–923. [PubMed] [Google Scholar]
- 36.Cvetovich R. J., Chartrain M., Hartner F. W. Jr, Roberge C., Amato J. S., Grabowski E. J. (1996) An asymmetric aynthesis of L-694,458, a human leukocyte elastase inhibitor, via novel enzyme resolution of β-lactam esters. J. Org. Chem. 61, 6575–6580. [DOI] [PubMed] [Google Scholar]
- 37.Luffer-Atlas D., Vincent S. H., Painter S. K., Arison B. H., Stearns R. A., Chiu S. H. (1997) Orally active inhibitors of human leukocyte elastase. III. Identification and characterization of metabolites of L-694,458 by liquid chromatography-tandem mass spectrometry. Drug Metab. Dispos. 25, 940–952. [PubMed] [Google Scholar]
- 38.Vincent S. H., Painter S. K., Luffer-Atlas D., Karanam B. V., McGowan E., Cioffe C., Doss G., Chiu S. H. (1997) Orally active inhibitors of human leukocyte elastase, II: disposition of L-694,458 in rats and rhesus monkeys. Drug Metab. Dispos. 25, 932–939. [PubMed] [Google Scholar]