Pyk2 expression is upregulated upon monocyte differentiation, and promotes apoptosis in mouse monocyte populations.
Keywords: apoptosis, Ly6C, survival, mouse
Abstract
Monocytes are short-lived myeloid cells that perform functions essential for tissue homeostasis and disease resolution. However, the cellular mechanisms controlling the maintenance and turnover of monocyte populations are largely undefined. Proline-rich tyrosine kinase 2 (Pyk2) is a nonreceptor tyrosine kinase that regulates numerous immune cell functions, but its role in monocytes is currently unknown. In this study, we sought to characterize the expression and function of Pyk2 in lineage-committed monocyte populations. Here, we report that Pyk2 protein expression is increased in the Ly6C− monocyte population. Using a Pyk2 knockout mouse model (Pyk2−/−), we show that Pyk2 regulates the relative proportion of monocyte subsets normally represented in the bone marrow (BM) at steady state. In support of this conclusion, a similar phenotype was observed in the peripheral blood and spleen. Data from reciprocal BM chimera experiments indicate that the alterations in monocyte populations exhibited by Pyk2−/− mice are due to factors intrinsic to the monocytes. Lineage-tracing of monocyte populations suggests that Pyk2 promotes apoptosis in BM monocytes, thereby acting as an important homeostatic regulator of turnover in these short-lived, innate immune cells.
Introduction
Monocytes comprise a heterogeneous population of short-lived, mononuclear phagocytes that contribute to tissue homeostasis and protective immunity [1]. cMoPs continually replenish cells of this lineage through the tightly regulated process of BM monopoiesis [2–4]. Subsequent egress of BM monocytes to the vasculature results in 2 main subpopulations of PB monocytes that have been classified based on distinct phenotypic markers [5–8]. In mice, Ly6C+ “classical” monocytes circulate throughout the PB and are poised to extravasate into peripheral sites in response to inflammatory cues. Subsequent terminal differentiation of these cells into monocyte-derived Mϕs and dendritic cells is integral for the proper resolution of tissue damage and infection [9–13]. In the absence of inflammatory signals, the Ly6C+ monocytes are short-lived, precursor cells that can terminally differentiate into the second major population of blood monocytes, Ly6C− “nonclassical” monocytes [14, 15]. At steady state, it is believed that these cells function to maintain vascular integrity by “crawling” along the luminal surface of capillaries in a patrolling and scavenging capacity [16, 17]. Ly6C− monocytes also constitute a relatively small percentage of BM monocytes, although their function and cellular origin in this tissue are currently under debate [4, 14, 18]. Both subsets of monocytes also accumulate in the spleen, constituting a reservoir that can be mobilized in response to inflammatory cues [19].
Although our understanding of monocyte differentiation and function has advanced considerably, the molecular mechanisms responsible for homeostatic maintenance of these subpopulations are still unclear. Ly6C+ and Ly6C− monocytes exhibit a relatively short t1/2 of ∼18 h and 2.3 d, respectively [14]. The continuous production and rapid turnover of these cells suggest a carefully balanced orchestration of prosurvival and prodeath signaling to maintain proper subset representation. To date, multiple factors that support prolonged survival of Ly6C+ and/or Ly6C− monocytes have been identified, including M-CSF, CX3CL1, and the transcription factor NR4A1 [18, 20–23]. In the absence of these stimuli, peripheral monocytes have been shown to spontaneously undergo cell death [24–26]. Thus, it has been theorized that prosurvival signals may act to impede a continually active apoptotic program existing in monocytes at steady state. However, the specific molecular signaling pathways that drive constitutive monocyte turnover are relatively unexplored.
Pyk2 is a nonreceptor tyrosine kinase that is predominantly expressed in hematopoietic and neuronal cells [27]. Pyk2 can be activated by a variety of stimuli, including integrin engagement, growth factor signaling, intracellular calcium influx, and Ag-receptor engagement on lymphocytes [27–29]. Functionally, Pyk2 has been shown to regulate numerous cellular processes, including adhesion signaling, directional motility, immune cell activation, and proliferation (reviewed in [27, 28]). Several studies have also revealed a role for Pyk2 in controlling cell death [30–34], whereas others have linked Pyk2 up-regulation to prolonged survival of transformed cells [35–37]. Given these discrepancies, it appears that the role of Pyk2 in regulating cell survival is likely to be context specific. Notably, several of these overexpression studies were performed in vitro using immortalized cell lines or cell types that normally express low levels of endogenous Pyk2. Further clarification is, therefore, necessary to determine the role of Pyk2 in regulating the survival of cells under conditions in which it is normally expressed.
Previous studies have reported that Pyk2 is expressed at variable levels in cells of the mononuclear phagocyte system [38–41]. However, a close examination of the expression and function of this molecule across the continuum of monocyte development has not been performed. In this study, we investigated the expression of Pyk2 in monocyte lineage subsets from the BM, PB, and splenic reservoirs. We also explored the role of Pyk2 in the differentiation and accumulation of monocyte subpopulations in these tissues at steady state. We show that Pyk2 expression is elevated in the Ly6C− fraction of monocytes and that mice deficient for Pyk2 exhibit a greater representation of Ly6C− monocytes in the BM and periphery. Studies using chimeric mice indicate that this phenotype is cell autonomous. Additional evidence suggests that Ly6C− monocytes exhibit a survival benefit in the absence of Pyk2. Given those results, we propose that Pyk2 has a role during homeostasis in maintaining appropriate Ly6C− monocyte levels by promoting the turnover of these characteristically short-lived cells.
MATERIALS AND METHODS
Mice
WT C57BL/6 mice were bred and housed on site. The Pyk2−/− mouse model has been previously described [38, 42]. C57BL/6-Ly5.1 (CD45.1+) mice were purchased from Charles River Laboratories (Wilmington, MA, USA). Male and female mice (8–14 wk old) were age- and sex-matched for experiments. All studies were performed in accordance with University of Virginia Animal Care and Use Committee guidelines.
Harvest and preparation of cell suspensions from mouse tissues
Mice were euthanized, and tissues were harvested in the following order. Blood (300–700 μl/mouse) was drawn through cardiac puncture and placed in 1 ml of 5 mM EDTA/HBSS (Mg−, Ca−). Spleens were excised, pushed through a 70-μm filter, and washed in MACS buffer (0.5% BSA, 250 mM EDTA in PBS). BM was flushed from both femora and tibiae with MACS buffer, washed in MACS buffer, and filtered through a 30-μm filter. To remove erythrocytes, the tissues were incubated in ACK lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM Na2 EDTA 2 H2O in H2O) for 10 min on ice, quenched with complete medium (10% FBS/DMEM), and washed in MACS buffer.
Flow cytometry
BM, PB, and spleen cell suspensions containing ∼1 × 106 cells in 100 μl MACS buffer were incubated with the Fc-blocking Ab anti-CD16/32 (eBioscience, San Diego, CA, USA) for 10 min on ice. The cells were subsequently incubated with primary mAbs to cell-surface Ags for 25 min on ice. Abs were purchased from the following companies and used at the concentration suggested by the manufacturer: Bio-Rad Laboratories (Hercules, CA, USA): anti-F4/80 (Cl:A3-1 clone); BD Biosciences (San Jose, CA, USA): anti-F4/80 (T45-2342) and anti-CD11a (2D7); BioLegend (San Diego, CA, USA): anti-CD115 (AFS98), anti-CD117 (ACK2), anti-Ly6C (HK1.4), anti-CD11b (M1/70), anti-Ly6G (1A8), anti-CD117 (2B8), anti-CD45.1 (A20), and anti-CD192 (SA203G11); and eBioscience (San Diego, CA, USA): anti-CD115 (AFS98), anti-CD11c (N418), anti-CD45.2 (104), anti-CD3e (145-2C11), anti-CD49b (DX5), and anti-CD19 (MB19.1). Samples were stained concurrently with fluorescence minus one Ab panels. Samples were washed in MACS buffer and incubated with fixable live/dead cell stain (Thermo Fisher Scientific, Waltham, MA, USA) for 30 min on ice. Samples were then washed in MACS buffer and fixed for 20 min on ice with Cytofix (BD Biosciences), resuspended in MACS buffer, and data were acquired on the Cyan ADP LX (Beckman Coulter, Brea, CA, USA) or LSRFortessa (Becton Dickinson, Franklin Lakes, NJ, USA). FlowJo software (TreeStar, Ashland, OR, USA) was used for data analysis. Forward- and side-scatter parameters were used for exclusion of doublets from analysis. Absolute numbers were calculated using Accucount beads (Spherotech, Lake Forest, IL, USA), according to the manufacturer’s instructions. BM absolute numbers reflect counts from both femora and tibiae per mouse.
To measure cells undergoing DNA synthesis, and for BrdU lineage-tracing analyses, the BD BrdU Flow Kit (BD Biosciences) was used. Mice received a single, 2-mg BrdU pulse by i.p. injection. At the indicated time points, single-cell preparations of tissue-surface Ags were stained, followed by fixable live/dead staining, as described above. The cells were subsequently permeabilized, DNase treated, and stained with anti–BrdU-FITC, according to the manufacturer’s instructions. The percentages and absolute numbers of BrdU+ cells were determined by flow cytometry.
For ex vivo detection of Annexin V+ and dead monocytes, cell suspensions derived from BM were first stained for surface Ags to identify monocyte subpopulations. The suspensions were then incubated with Annexin V-FITC (BD Biosciences) and 0.1 μg/ml DAPI (Thermo Fisher Scientific) in Annexin V staining buffer (BD Biosciences) for 15 min at room temperature immediately before analysis by flow cytometry. Dead cells were identified as Annexin V+/DAPI+. Apoptotic cells were identified as Annexin V+ after dead-cell exclusion.
For ex vivo detection of active caspase, the Vybrant FAM Poly Caspases Assay Kit (Thermo Fisher Scientific) was used according to the manufacturer’s instructions. Briefly, cell suspensions derived from BM were first stained for surface Ags to identify monocyte subpopulations. The suspensions were then incubated with FLICA reagent (1:30 from working solution) in MACS buffer for 60 min at 37°C, followed by multiple washes in wash buffer. Immediately before analysis by flow cytometry, cells were resuspended in wash buffer/DAPI (0.1 μg/ml). Dead cells were excluded based on DAPI incorporation, and the percentage of monocyte subsets expressing active caspases was assessed by FLICA staining.
Immunomagnetic column separation of cell populations
CD115+ monocytes were isolated using the mouse MACS CD115 Microbead Kit (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer’s protocol. Briefly, single-cell suspensions were first prepared as described above. FcRs were blocked with anti-CD16/32 (eBioscience) for 10 min on ice, and the cells were subsequently incubated with anti–CD115-biotin. The CD115+ population was isolated by magnetic column after incubation with anti-biotin magnetic beads. Purity of the isolated population was assessed by flow cytometry.
FACS of monocyte subsets
Single-cell preparations of tissues were prepared and enriched for CD115+ monocytes by immunomagnetic column separation, and cells were then stained for cell surface markers, as described above. Immediately before FACS sorting, cells were resuspended in MACS buffer/DAPI (0.1 μg/ml; Thermo Fisher Scientific). FACS sorting of monocyte subpopulations was performed on the Influx Cell Sorter (Becton Dickinson).
In vitro generation of BMDMs
Whole BM was collected as described above, enumerated by hemacytometer, and added to medium preparations at 4–6 × 106 cells per 10-cm plate. Base medium (αMEM with 10% FBS and penicillin/streptomycin) was supplemented with 10% CMG14-12 conditioned medium (source of M-CSF). After 7 d in culture, the adherent cells were washed 3 times with PBS, incubated with trypsin/EDTA for 15 min, quenched with complete medium, washed with MACS buffer, and resuspended in MACS buffer.
Immunoblotting
Cell suspensions were washed in PBS, pelleted, and incubated in radioimmunoprecipitation assay lysis buffer, as described previously [39]. Immunoblotting was performed as described previously [39]. The following Abs were used: anti-Pyk2 (Thermo Fisher Scientific), anti-ERK p44/42 (Cell Signaling Technology, Danvers, MA, USA), and anti-AKT (Cell Signaling Technology).
BM chimera generation
CD45.1+ WT recipient mice were depleted of endogenous lymphoid tissue by irradiation (2 doses of 550 centigrays at 3-h intervals), and 1 d later were i.v. injected with 5 × 106 BM cells isolated from CD45.2+ WT or Pyk2−/− donor femora and tibiae. BM reconstitution was allowed to proceed in recipients for 8 wk before analysis. Recipient mice were provided oral sulfamethoxazole and trimethoprim for the duration of the experiment.
In vitro culture of BM for monocyte viability assays
Whole BM was collected as described above, enumerated by hemocytometer, and added to serum-free RPMI 1640 or RPMI 1640 supplemented with 10% FBS and penicillin/streptomycin at 5 × 106 cells per 60-mm plate. After 4 h at 37°C, nonadherent cells were collected and placed on ice. Adherent cells were washed with PBS, incubated with trypsin/EDTA for 15 min, quenched with complete medium, and collected. Nonadherent and adherent cells were combined, washed with MACS buffer and prepared for flow cytometry, as described above. Cell death was determined by DAPI incorporation.
Statistical analysis
Comparisons among groups were made using an unpaired 2-tailed Student’s t test or 1-way ANOVA. *P ≤ 0.05; **P ≤ 0.01; and ***P ≤ 0.001 were considered statistically significant.
RESULTS
Pyk2 protein expression is elevated in Ly6C− monocytes
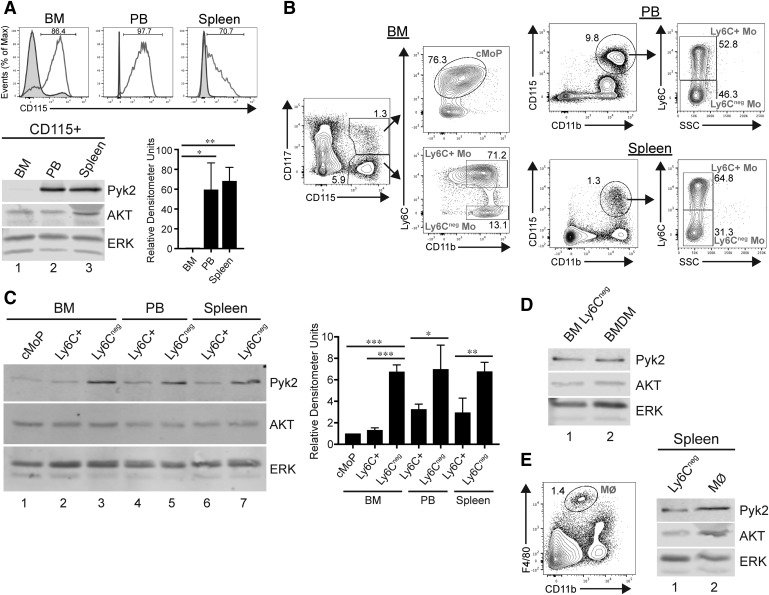
To assess protein expression of Pyk2 in monocytes during steady-state conditions, CD115+ (also known as the M-CSF receptor) cells isolated from the BM, PB, and spleen of WT mice were enriched via magnetic bead selection (Fig. 1A). Lysates were prepared, and Western blotting for Pyk2 was performed on the extracts. Pyk2 expression was markedly greater in monocytes isolated from the PB and spleen compared with BM monocytes (Fig. 1A, compare lanes 2 and 3 to lane 1). Of note, the PB and spleen have been found to contain a higher proportion of Ly6C− monocytes than the BM [7, 19].
Figure 1. Pyk2 protein expression is elevated in more-differentiated monocyte populations.
(A, top) Flow cytometry histogram plots depicting efficiency of CD115+ magnetic bead enrichment from the BM, PB, and spleen of WT mice. Plots display nonenriched cell preparations (shaded curves) and enriched fractions (unshaded curves). Numbers above the gated regions represent percentage of CD115+ in the enriched cell populations. (A, bottom left) Representative immunoblot showing Pyk2 expression in cell lysates of CD115-enriched fractions from the indicated tissues. AKT and ERK are presented as loading controls. (A, bottom right) Quantification of relative Pyk2 expression normalized to ERK is presented. Data shown are the means ± sem of 3 independent experiments. (B) Flow cytometry gating strategy to identify monocyte subpopulations in the indicated tissues. Dead cells and doublets were excluded before the displayed gating. Numbers within the outlined areas indicate percentage of the gated cell populations. (C) Representative immunoblot showing Pyk2, ERK, and AKT expression in cell lysates generated from 5 × 104 FACS-sorted monocyte subsets isolated from the indicated tissues. Quantification of relative Pyk2 expression normalized to ERK is shown in the adjacent graph. Data shown are the means ± sem of 3 independent experiments. (D) Representative immunoblot of Pyk2, AKT, and ERK expression in cell lysates from Ly6C− monocytes isolated from the BM (lane 1) or adherent BMDMs after 7 d in culture with M-CSF (lane 2). (E, left) Flow cytometry gating strategy to identify tissue-resident Mϕs in the spleen. Dead cells and doublets were excluded before the displayed gating. (E, right) Representative immunoblot comparing Pyk2, AKT, and ERK expression in cell lysates from Ly6C− monocytes (lane 1) or Mϕs (lane 2) isolated from the spleen. *P < 0.05; **P < 0.01; ***P < 0.001, compared with all other groups (1-way ANOVA).
Given those results, we hypothesized that Pyk2 expression may be developmentally regulated during monocyte differentiation. To address that question, we refined our analysis by isolating monocyte subpopulations from BM, PB, and spleen by FACS. CD115+ monocytes were magnetically enriched and FACS-sorted into cMoP, Ly6C+, and Ly6C− subsets based on established lineage markers [4, 7, 18, 19] (Fig. 1B and Supplemental Fig. 1). Back-gating analyses of these monocyte subpopulations using additional cell-surface markers (F4/80, CD11a, CD11c, and CCR2; negative for the lineage markers CD135, Ly6G, CD49b, CD3e, and CD19) confirmed expression profiles characteristically associated with the respective subsets (Supplemental Fig. 1; data not shown) [4, 18, 19]. Lysates from the sorted subsets were then immunoblotted for Pyk2. Pyk2 expression was significantly elevated in Ly6C− monocytes from the BM compared with the cMoP and Ly6C+ subsets (compare lane 3 with lanes 1 and 2 in Fig. 1C). A similar increase in Pyk2 expression was observed in Ly6C− monocytes isolated from the PB (compare lanes 4 and 5 in Fig. 1C) and spleen (compare lanes 6 and 7 in Fig. 1C) compared with Ly6C+ monocytes isolated from the same sites. Pyk2 expression has previously been characterized in mouse Mϕs but not in monocyte subsets [38]. Therefore, we directly compared Pyk2 expression between BM Ly6C− monocytes and BMDMs (Fig. 1D) and between splenic Ly6C− monocytes and resident Mϕs (defined as CD11blowF4/80hi) (Fig. 1E). In both cases, Pyk2 expression was similar between the Ly6C− monocytes and the Mϕs.
Pyk2 controls the relative proportion of monocyte subpopulations
Given the data above showing that Pyk2 expression is elevated in Ly6C− monocyte populations, we hypothesized that Pyk2 may be important for regulating the differentiation and/or maintenance of monocyte subpopulations. Although previous reports have described the overall immune cell populations in lymphoid tissues of Pyk2−/− mice as similar to those of WT mice [38], the distribution of monocyte subsets in BM and peripheral organs of these mice has not been previously determined.
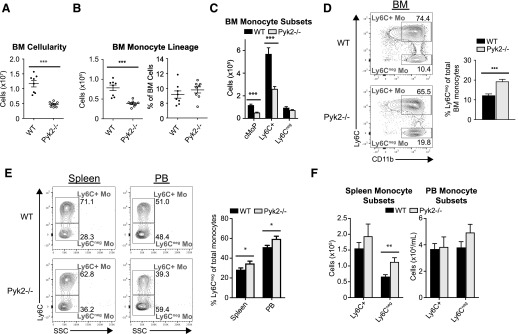
Pyk2−/− mice have been described by our group and others as being mildly osteopetrotic because of a defect in osteoclast resorption of bone and/or increased bone formation by osteoblasts [43–45]. Thus, it was not surprising that the Pyk2−/− mice exhibited significantly reduced total BM cellularity (Fig. 2A), particularly because other mouse models of osteopetrosis exhibit a similar, albeit more drastic, reduction in BM cellularity that has been attributed to a smaller BM cavity [21, 22]. However, to our knowledge, this is the first time this phenotype had been reported in Pyk2−/− mice.
Figure 2. Pyk2−/− mice exhibit altered monocyte subset representation.
Single-cell suspensions from the indicated tissues were analyzed by flow cytometry, and monocyte subsets were identified as described in Fig. 1B. (A) Comparison of the absolute number of live cells in the BM of WT and Pyk2−/− mice. Each circle represents an individual mouse. (B, left) Total numbers of BM monocytes, defined as CD115+ cells. (B, right) Percentage of monocyte lineage cells among total live BM cells. (C) Total numbers of BM monocyte subpopulations per mouse (WT, n = 8; Pyk2−/−, n = 8). (D, left) Representative FACS contour plots displaying Ly6C+ and Ly6C− monocyte subpopulations from WT and Pyk2−/− BM. (D, right) Data from (C) presented as the percentage of Ly6C− monocytes among total BM monocyte lineage cells. (E, left) Representative FACS contour plots displaying Ly6C+ and Ly6C− monocyte subpopulations from the PB and spleen of WT and Pyk2−/− mice. (E, right) Percentage of Ly6C− monocytes among total monocytes in the indicated tissues. (F) Absolute number of monocyte subpopulations per spleen (left) or per milliliters of PB (right); (spleen: WT, n = 8, Pyk2−/−, n = 8; PB: WT, n = 21, Pyk2−/−, n = 22). Data were acquired from ≥2 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001 (unpaired Student’s t test).
The decrease in total BM cellularity was accompanied by a corresponding reduction in the absolute number of monocyte-lineage cells (as defined by CD115 expression) in the BM of Pyk2−/− mice (Fig. 2B). However, despite that reduction in total monocyte numbers, Pyk2-deficient monocytes made up a similar proportion of the total BM present in these animals (Fig. 2B). As expected given the decreased number of CD115+ monocyte-lineage cells present in Pyk2−/− BM, the absolute numbers of cMoP and Ly6C+ monocyte subsets in the BM of Pyk2−/− mice were significantly reduced compared with WT controls (Fig. 2C). Surprisingly, however, the total number of Ly6C− monocytes was similar between WT and Pyk2−/− mice (Fig. 2C), leading to a disproportionate enrichment of those cells within the monocyte compartment of the BM in Pyk2−/− mice (Fig. 2D).
Unlike the BM, no reductions in total cellularity were observed in the PB and spleen of Pyk2−/− mice (data not shown). Nevertheless, Ly6C− monocytes were also proportionally overrepresented in the monocyte compartments of both the spleen and PB (Fig. 2E). This was driven by a significant increase in Ly6C− monocytes in the spleen, with no appreciable changes to Ly6C+ monocyte numbers (Fig. 2F). A similar increase in Pyk2-deficient Ly6C− monocyte numbers was not seen in the PB (Fig. 2F), possibly because of the inherent variability in absolute monocyte counts in this organ. Taken together, these data suggest that Pyk2 has a role in maintaining the proper balance of monocyte populations under homeostatic conditions.
Pyk2 regulates the accumulation of Ly6C− monocytes via a cell-autonomous mechanism
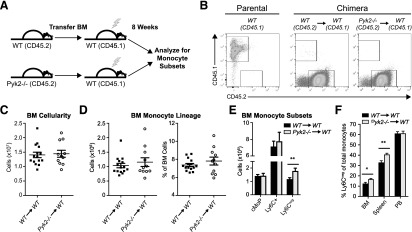
The altered representation of monocyte subsets in Pyk2−/− mice could be the result of a loss of Pyk2-dependent activities in the monocytes (cell-intrinsic) or in the stromal microenvironment that supports the development and/or survival of these cells (monocyte extrinsic). To discriminate between these possibilities, reciprocal transfers of WT or Pyk2−/− BM were performed into lethally irradiated WT hosts (Fig. 3A). Efficient reconstitution of CD45.2+ donor BM was observed 8 wk after the transfer, concomitant with complete ablation of native CD45.1+ BM in the lethally irradiated recipient mice (Fig. 3B).
Figure 3. Pyk2 regulates the accumulation of Ly6C− BM and spleen monocytes via a cell-autonomous mechanism.
(A) Schematic showing experimental design for reciprocal BM transfers. Lethally irradiated CD45.1+ WT mice received CD45.2+ WT or CD45.2+ Pyk2−/− whole BM (5 × 106 cells), followed by reconstitution for 8 wk. Single-cell preparations of BM, PB, and spleen were analyzed by flow cytometry. (B) Representative flow cytometry dot plots show CD45.1+ native BM in a nonirradiated mouse (left), compared with lethally irradiated chimeric animals 8 wk after receiving CD45.2+ WT BM (middle) or CD45.2+ Pyk2−/− BM (right). More than 99% of native BM was ablated in chimeric mice. (C) Total numbers of live cells in the BM of WT→WT and Pyk2−/−→WT mice, assessed by flow cytometry. (D, left) Total numbers of CD115-expressing BM monocytes. (D, right) Percentage of monocyte lineage cells among total live BM cells. (E) Total numbers of BM monocyte subpopulations per mouse (WT→WT, n = 15; Pyk2−/−→WT, n = 11). (F) Percentage of Ly6C− monocytes among total monocytes in the indicated tissues (BM: WT→WT, n = 15, Pyk2−/−→WT, n = 11; spleen: WT→WT, n = 18, Pyk2−/−→WT, n = 14; PB: WT→WT, n = 18, Pyk2−/−→WT n = 14). Data shown were acquired from 2 independent experiments. *P < 0.05; **P < 0.01, unpaired Student’s t test.
Within the confines of a WT host harboring a normal skeletal structure, Pyk2-deficient BM was able to reconstitute to the same level as WT BM (Fig. 3C). Thus, the deficiency in BM hematopoiesis observed in Pyk2−/− mice (Fig. 2A) likely results from the smaller BM cavity associated with osteopetrosis. In line with that possibility, the total number and percentage of monocyte lineage cells in the BM of chimeric mice was unaltered by loss of Pyk2 (Fig. 3D) and the pools of cMoP and Ly6C+ monocytes were unchanged (Fig. 3E). This indicates that the development of cMoP and Ly6C+ monocytes in the BM is not dependent on Pyk2 activity in those cells. However, a modest, but statistically significant, increase in Ly6C− monocytes was observed in the mice receiving Pyk2−/− BM (Fig. 3E), and that was accompanied by a greater representation of Ly6C− monocytes in the BM and spleen of those mice (Fig. 3F). These results, which recapitulate the disproportionate representation of Ly6C− monocytes observed in the BM and spleen of Pyk2−/− mice (Fig. 2D and E), establish that environmental (monocyte cell-extrinsic) factors are not solely responsible for those changes in monocyte subset distribution. Rather, these data indicate that loss of Pyk2 activity in Ly6C− monocytes leads to the increased accumulation of this subpopulation.
In contrast to the BM and spleen, the Ly6C− population in the PB of chimeric mice receiving Pyk2-deficient BM was identical to those receiving WT BM (Fig. 3F). This suggests that loss of Pyk2 in nonmonocytic cells may contribute to the relative representation of PB monocyte subsets observed in Pyk2−/− mice (Fig. 2E). Unfortunately, it was not possible to generate the reciprocal chimeras in Pyk2−/− hosts because of the radiosensitivity of these mice (data not shown), thus precluding our ability to directly test for environmental (nonautonomous) effects on monocyte subset accrual. Nonetheless, the data presented above indicate that the accumulation of Ly6C− monocytes in the BM and spleen is controlled, at least in part, by cell-intrinsic activities of Pyk2 in these populations. Given that Pyk2 is most highly expressed in these cells (Fig. 1C), we suggest that it functions to maintain a proper balance of Ly6C− monocytes at steady state.
Pyk2 promotes apoptosis of BM monocytes
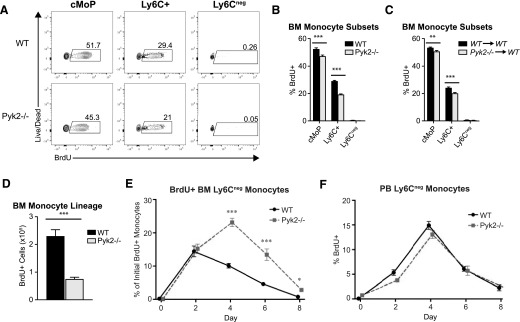
Ly6C− monocytes are derived from Ly6C+ monocytes and/or cMoPs [14, 15, 46], and are nonproliferating at steady state [4, 14, 18]. Therefore, the disproportionate accumulation of Ly6C− monocytes observed in the absence of Pyk2 could result from an increase in the proliferation rate of the precursor cells (cMoP and/or Ly6C+ cells) and/or a failure of the Ly6C− monocytes to undergo cell-cycle arrest. These possibilities were evaluated by measuring BrdU incorporation into monocyte subsets 2 h after a single i.p. injection of BrdU. Under these conditions, proliferating cells are marked with BrdU in the absence of any appreciable differentiation of the cells [14]. As expected, cMoPs contained the highest percentage of BrdU+ cells, followed by Ly6C+ monocytes (Fig. 4A and B, black bars). Like their WT counterparts, Pyk2−/− Ly6C− monocytes failed to incorporate BrdU during this 2-h pulse (Fig. 4A and B). The fraction of Pyk2-deficient cMoP and Ly6C+ monocytes incorporating BrdU was also not elevated compared with WT controls; in fact, the opposite was the case (Fig. 4B). Similar analyses performed in chimeric mice indicate that this is a monocyte-intrinsic effect (Fig. 4C). Based on these data, the greater accumulation of Ly6C− monocytes observed in Pyk2−/− mice cannot be due to increased proliferation of one or more monocyte populations.
Figure 4. Pyk2 controls the turnover of lineage-traced Ly6C− BM monocytes.
(A) The in vivo proliferative capacity of BM cMoPs, Ly6C+, and Ly6C− monocytes was assessed after a 2-h i.p. BrdU pulse. Representative contour plots display the percentage of monocyte subpopulations that incorporated BrdU, as assessed by flow cytometry. (B) Quantification showing the percentage of BrdU+ monocytes within each subpopulation of BM monocytes (WT, n = 8; Pyk2−/−, n = 8). Data shown were acquired from 2 independent experiments. (C) Percentage of BrdU+ monocytes within each subpopulation of BM monocytes from WT→WT and Pyk2−/−→WT chimeric mice after a 2-h i.p. BrdU pulse (WT→WT, n = 11; Pyk2−/−→WT, n = 11). (D) Absolute numbers of BrdU-labeled BM monocytes are presented (WT, n = 8; Pyk2−/−, n = 8). (E) Time course of BrdU+ Ly6C− monocytes that accumulate in the BM, represented as the percentage of total BrdU+ monocytes that were initially labeled on d 0 (WT, n ≥ 8; Pyk2−/−, n ≥ 8 per time point). (F) Time course of BrdU+ Ly6C− monocytes in the PB, represented as the proportion of Ly6C− cells that stained positive for BrdU by flow cytometry (WT, n ≥8; Pyk2−/−, n ≥ 8 per time point). *P < 0.05; **P < 0.01; ***P < 0.001 (unpaired Student’s t test).
We next examined the t1/2 of Ly6C− monocytes. Because these cells are nonproliferating and do not incorporate BrdU, their accumulation over time can be followed by tracing precursors (cMoP and Ly6C+ monocytes) that receive a single pulse of BrdU [14]. Coincident with the reduced numbers of cMoP and Ly6C+ monocytes present in the BM of Pyk2-deficient mice (Fig. 2C), the absolute number of BrdU-labeled cells was reduced in the Pyk2−/− BM on d 0 (Fig. 4D). The fraction of the initial pool of labeled precursors that differentiated into BrdU-labeled Ly6C− monocytes in the BM was then determined at 2, 4, 6, and 8 d (Fig. 4E). After 2 d, ∼15% of the BrdU-labeled monocytes were Ly6C− in both Pyk2−/− and WT BM, indicating that the precursor cells differentiated into Ly6C− monocytes at similar rates. In accordance with the 2–3 d t1/2 reported for these cells [14], BrdU-labeled Ly6C− monocytes in WT BM began to decrease by d 4 and were completely lost by d 8. However, BrdU+ Ly6C− monocytes continued to accumulate in Pyk2−/− BM through d 4; after which, they began to decline. As was the case for WT cells, the BrdU-labeled Ly6C− Pyk2−/− monocytes were almost completely lost by d 8.
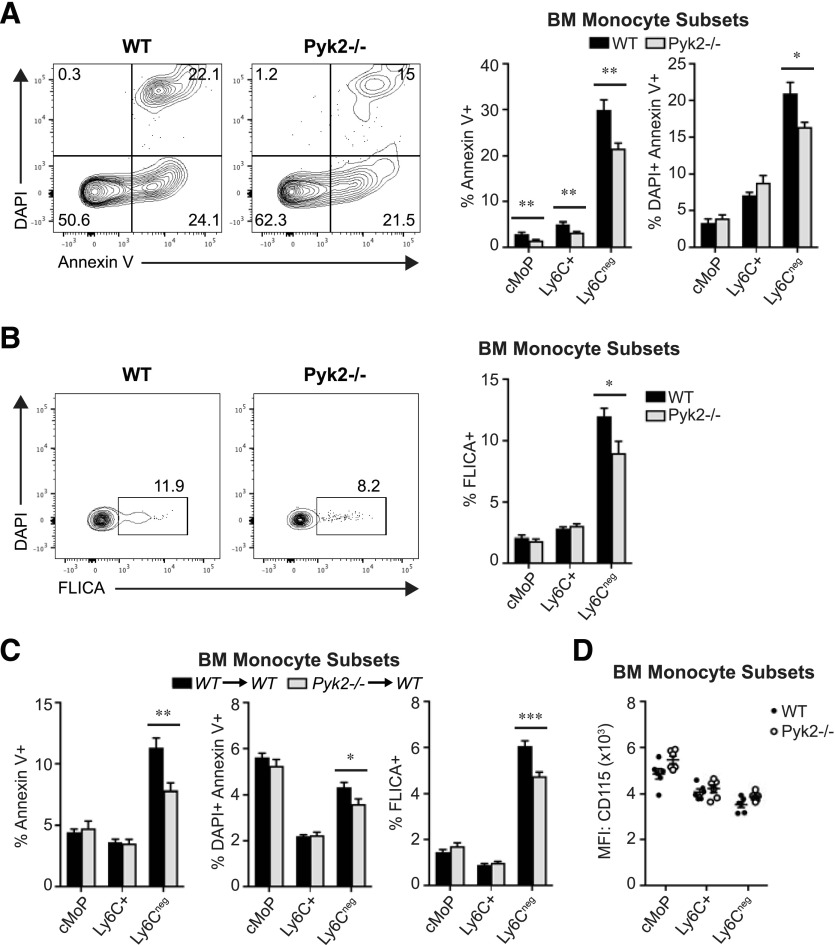
One possible explanation for the prolonged accumulation of Pyk2−/− Ly6C− monocytes in the BM is that they are less efficient at egressing to the periphery than WT cells are. However, that is unlikely because BrdU-labeled WT and Pyk2-deficient Ly6C− monocytes accumulated in the PB with similar kinetics (Fig. 4F). Alternatively, Ly6C− monocytes could have a survival benefit in the absence of Pyk2, leading to a longer t1/2 in the BM. In support of that possibility, we found that the proportion of apoptotic and dead Ly6C− monocytes was decreased in Pyk2−/− BM compared with WT BM as measured by Annexin V and DAPI incorporation, respectively (Fig. 5A). Furthermore, active caspase expression, another hallmark of apoptosis, was reduced in BM Ly6C− monocytes in the absence of Pyk2 (Fig. 5B). This reduction in apoptosis and cell death observed in Pyk2-deficient Ly6C− BM monocytes was recapitulated in chimeric WT hosts receiving Pyk2−/− BM, indicating that loss of Pyk2 confers a survival advantage for Ly6C− monocytes in a cell-autonomous manner (Fig. 5C). Notably, although M-CSF receptor (CD115) signaling is an established regulator of monocyte-lineage cell survival at homeostasis [47–49], the survival advantage for BM Ly6C− monocytes deficient in Pyk2 did not correlate with changes in surface expression of CD115 (Fig. 5D).
Figure 5. Pyk2 regulates Ly6C− BM monocyte survival in vivo.
(A) Representative contour plots display Annexin V staining and DAPI incorporation in BM Ly6C− monocytes from WT and Pyk2−/− mice, assessed by flow cytometry. The adjacent graphs show the average percentage of apoptotic (DAPI−/Annexin V+, left) or dead (DAPI+/Annexin V+, right) monocytes in BM monocyte subpopulations (WT, n = 8; Pyk2−/−, n = 8). Data were acquired from 2 independent experiments. (B) Representative contour plots display active caspase (FLICA) staining in BM Ly6C− monocytes from WT and Pyk2−/− mice, assessed by flow cytometry. The adjacent graph shows the percentage of BM monocyte subsets expressing cleaved (active) caspases, assessed by flow cytometry (WT, n = 8; Pyk2−/−, n = 8). Data were acquired from 2 independent experiments. (C) Percentage of BM monocyte subsets staining positive for Annexin V (left), DAPI (middle), or cleaved caspases (right) in WT→WT and Pyk2−/−→WT chimeric mice, assessed by flow cytometry (WT→WT, n = 12; Pyk2−/−→WT, n = 12). (D) Quantification of the mean fluorescence intensity of cell-surface CD115 (M-CSFR) for WT and Pyk2−/− BM monocyte subpopulations. *P < 0.05; **P < 0.01; ***P < 0.001 (unpaired Student’s t test).
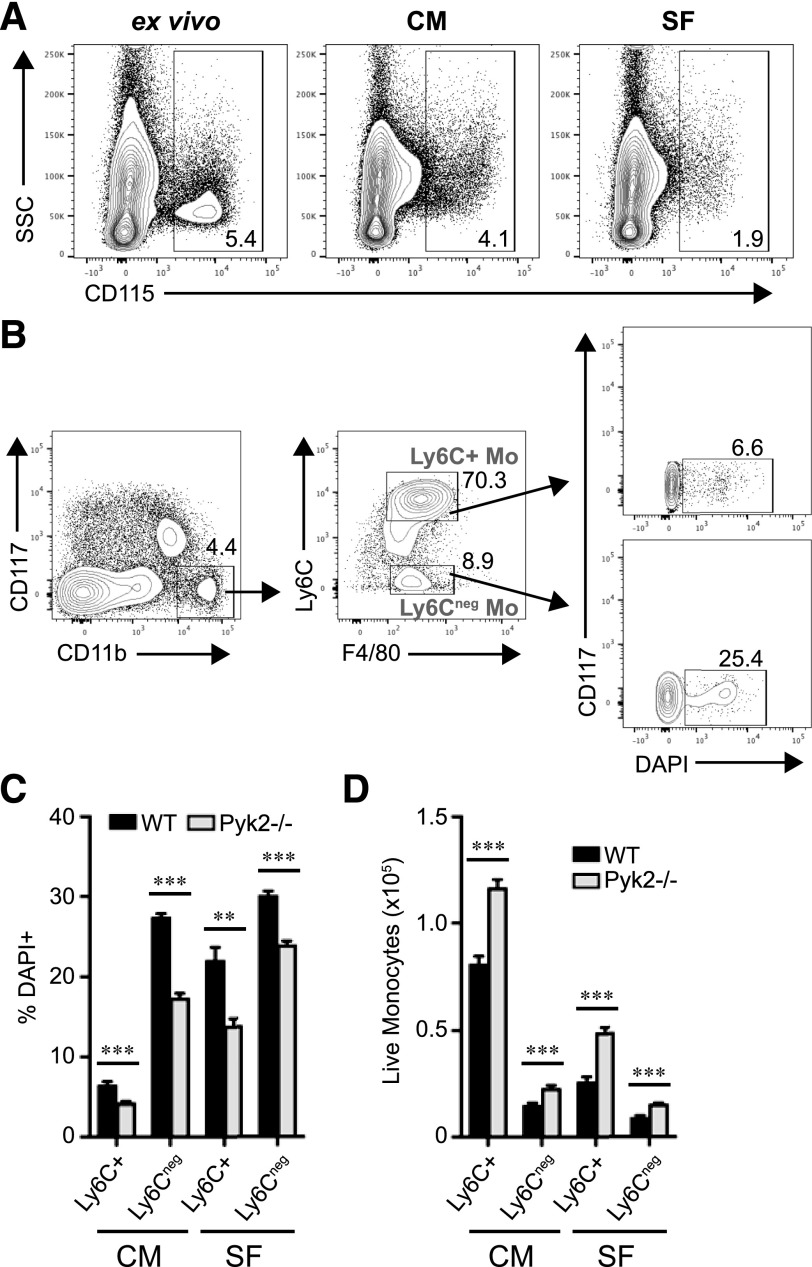
To further explore the role of Pyk2 in monocyte survival, BM cells from WT and Pyk2−/− mice were cultured in vitro for 4 h in the presence or absence of serum. Under these conditions, monocytes have been reported to undergo a rapid onset of apoptosis [23]. As was reported by Breslin et al. [50], CD115 surface staining on monocytes was diminished when the cells were maintained ex vivo at temperatures above 4°C (Fig. 6A). Consequently, monocytes cultured under these conditions were identified as CD117−CD11b+F4/80low (Fig. 6B). Under both serum-supplemented (complete media) and serum-free conditions, the percentage of dead Ly6C+ and Ly6C− monocytes was reduced in the absence of Pyk2 (Fig. 6C), whereas the absolute number of live cells was increased (Fig. 6D). Together, these data suggest that Pyk2 controls the steady-state level of monocytes by promoting the turnover of monocyte subsets under homeostatic conditions.
Figure 6. Pyk2 regulates BM monocyte survival in vitro.
WT or Pyk2−/− BM was cultured in RPMI-1640 medium supplemented with 10% FBS (complete medium; CM) or RPMI-1640 medium alone (serum-free; SF). After 4 h, nonadherent and adherent cells were collected and analyzed by flow cytometry. BM was isolated and pooled from 5 mice/genotype. Data were generated from 5 replicates/genotype. (A) Representative contour plots display CD115 expression from WT BM kept on ice (left) or cultured under CM (middle) or SF conditions (right) for 4 h. Dead cells and doublets were excluded before the displayed gating. Numbers within the outlined areas indicate the percentage of the gated cell populations. (B) Flow cytometry gating strategy to identify monocyte subpopulations in vitro. Cell death in monocyte subsets was assessed by flow cytometry using DAPI incorporation. Doublets and Ly6G+ neutrophils were excluded before the displayed gating. (C) The average percentage of dead monocytes among monocyte subpopulations. (D) Total numbers of live monocyte subsets identified as DAPI−, assessed by flow cytometry. **P < 0.01; ***P < 0.001 (unpaired Student’s t test).
DISCUSSION
The cellular factors that control the homeostatic maintenance of monocyte subsets remain poorly understood. In this study, we sought to characterize the expression of the tyrosine kinase Pyk2 and its function in the development and survival of lineage-committed monocyte subpopulations at steady state. Our data indicate that Pyk2 protein expression increases as monocytes differentiate to a Ly6C− state and that Pyk2 controls the relative proportion of monocyte populations in the BM and periphery through a monocyte-intrinsic process. Furthermore, we present both in vivo and in vitro evidence showing that Pyk2 promotes apoptosis of Ly6C− monocytes, thereby contributing to the rapid turnover of monocyte populations at steady state.
Pyk2 expression is up-regulated in Ly6C− monocytes
Although Pyk2 expression has been characterized in several myeloid lineage cell types [38, 39, 51, 52], the relative expression of this molecule across the continuum of monocyte differentiation had not been previously examined. Here, we report that, under homeostatic conditions, Pyk2 expression increases in Ly6C− monocytes. In support of our findings, Pyk2 mRNA transcript levels are reportedly elevated in Ly6C− monocytes isolated from BM and PB compared with Ly6C+ monocytes from the same tissues [53]. This suggests that the increase in Pyk2 protein expression observed in these cells may arise from transcriptional up-regulation of the gene encoding Pyk2. Although the molecular factors that govern its expression have not been established, it is tempting to speculate that the transcription factor C/EBP-β may control Pyk2 expression in monocyte populations. Although C/EBP-β protein levels have not been specifically measured in monocyte subsets, this transcription factor has long been implicated in driving gene expression programs associated with Mϕ maturation [54, 55]. Additionally, C/EBP-β association with the Pyk2 promoter was shown to induce Pyk2 expression during monocyte differentiation to Mϕs after PMA treatment of NB4 cells [56].
Pyk2-deficient mice exhibit reduced BM cellularity
Although Pyk2−/− mice were initially characterized as being mildly osteopetrotic [43–45], BM hematopoiesis had not previously been fully characterized in these mice. In this report, we show that Pyk2-deficient mice exhibit a consistent reduction in their BM cellularity compared with WT controls. This may result from a defect in BM production, considering that rapid BrdU-incorporation studies revealed a significant reduction in the percentage of proliferating monocytes and monocyte precursors present in Pyk2-deficient BM compared with WT mice (Fig. 4B). Defects in BM production have been reported in other mouse models of osteopetrosis, including mice deficient for M-CSF (op/op mice) and the M-CSF receptor [21, 22, 57]. The paucity of BM cells in both of these mice was attributed to physical limitations restricting hematopoiesis, resulting from a significantly smaller BM cavity. Our data suggest a similar phenomenon in Pyk2−/− mice because transfer of Pyk2-deficient BM into lethally irradiated WT mice resulted in reconstitution of BM cells to levels of WT control mice (Fig. 3C).
Pyk2 promotes turnover of Ly6C− monocytes through the induction of apoptotic pathways
As described above, Pyk2−/− mice manifest reduced BM cellularity compared with WT controls (Fig. 2A). This results in a reduction in the absolute numbers of cMoP and Ly6C+ monocytes, but surprisingly, this did not also result in reduced numbers of Ly6C− monocytes (Fig. 2C). In contrast to the BM, total immune cellularity is similar between WT and Pyk2−/− mice in the spleen (data not shown). In this organ, Ly6C− monocyte numbers were elevated in Pyk2−/− mice compared with WT controls (Fig. 2F). Thus, Ly6C− monocytes consistently make up a greater proportion of the total monocytes present in both the BM and periphery of Pyk2−/− animals compared with WT mice (Fig. 2D and E). We go on to demonstrate that the overrepresentation of Ly6C− monocytes observed in Pyk2−/− mice also occurs with transfer of Pyk2−/− BM into an irradiated WT host (Fig. 3F), indicating that this phenomenon is intrinsic to the monocytes. Furthermore, our data show that Ly6C− monocytes have a survival benefit in the absence of Pyk2 (Figs. 4–6), which likely accounts for the overrepresentation of these cells in the BM and periphery of Pyk2−/− animals.
Monocytes spontaneously undergo cell death in vitro when cultured in SF conditions, mediated, at least in part, by Fas death receptor signaling and caspase activation [26, 58–63]. These findings have prompted the theory that apoptosis represents the default cellular program for monocytes, although the molecular signals contributing to this remain largely undefined. Here, we demonstrate that endogenous Pyk2 functions to promote the turnover of Ly6C− monocytes during homeostatic conditions (Fig. 5) and under conditions of experimentally induced cell death (Fig. 6). This could be mediated via the default apoptosis pathway because Pyk2-deficient Ly6C− monocytes in the BM exhibited reduced caspase activation compared with WT controls (Fig. 5B). However, the Pyk2−/− Ly6C− monocytes that originated from cMoPs/Ly6C+ cells in the BM were ultimately depleted from the BM and periphery, indicating that Pyk2-independent mechanisms are also in place to control the t1/2 of these cells (Fig. 4E). Notably, ectopic overexpression of Pyk2 has been shown to induce apoptosis in multiple cell lines that do not normally express this protein [30–34]; however, before the current study, endogenous levels of Pyk2 had not been shown to trigger cell death in a steady state.
Although the paucity of cMoP and Ly6C+ monocytes observed in Pyk2−/− compared with WT mice (Fig. 2C) might, at first, suggest a role for Pyk2 in the development or survival of these populations, transfer of Pyk2−/− BM into WT hosts resulted in normal numbers of cMoP and Ly6C+ monocytes (Fig. 3E). Thus, the accumulation of these subsets was not dependent on monocyte-intrinsic activities of Pyk2. This is consistent with the low level of Pyk2 expression in these subsets (Fig. 1C). Rather, we conclude that the reduced numbers of cMoP and Ly6C+ monocytes arise because of deficiencies in overall BM production in the Pyk2−/− mice (Fig. 2A). Our data also argue against a monocyte-intrinsic role for Pyk2 in regulating cMoP and Ly6C+ monocyte survival during steady-state conditions in vivo because these cells exhibited similar levels of Annexin V and active caspase staining in the presence or absence of Pyk2 when present in a WT BM environment (Fig. 5C). This is in contrast to Pyk2−/− Ly6C− monocytes, which displayed less cell death under these conditions. It is worth noting, however, that in vitro culture of BM did reveal increased viability of Ly6C+ monocytes in the absence of Pyk2 (Fig. 6C and D). This may be due to the experimental system triggering exceptionally rapid cell death and, therefore, not reflecting a typical homeostatic environment in vivo. It would be interesting to determine whether Pyk2 controls apoptosis of Ly6C+ monocytes in response to stress or under other physiologic conditions in which the t1/2 of these cells is significantly altered.
Exogenous factors that promote the survival of monocytes have been shown to suppress the default apoptotic pathway [20–23]. During homeostasis, this is chiefly mediated through M-CSF signaling [21, 22]. Marsh and colleagues [64–66] have shown that the PI3K/AKT pathway is activated in monocytes stimulated with recombinant M-CSF, leading to repression of caspase activity and prolonged survival in culture. Moreover, inhibition of the PI3K pathway was shown to induce apoptosis in cultured human PB monocytes [58]. Together, these findings underscore the importance of M-CSF–stimulated PI3K signaling in impeding the de facto apoptotic program and prolonging monocyte lifespan. Interestingly, M-CSF administration was reported to induce tyrosine phosphorylation of Pyk2 in the THP1 human monocytic cell line cultured in vitro [67]. This study also showed that the M-CSFR, as well as PI3K, coimmunoprecipitated with Pyk2 in response to M-CSF stimulation [67]. However, the authors did not go on to specifically test the implications of those events on monocyte survival; instead, they speculated that M-CSF–mediated activation of Pyk2 was potentially linked with regulation of downstream signaling pathways that affect cell morphology. It is possible that M-CSF–dependent Pyk2 phosphorylation and interactions with M-CSFR/PI3K do not affect cell survival or that the activation of Pyk2 in response to M-CSF is unique to the THP1 cell line. Alternatively, Pyk2 may have dual roles, functioning on the one hand to promote apoptosis under conditions that favor monocyte turnover and on the other to promote survival in the presence of high levels of M-CSF. Clearly, the mechanistic underpinnings and implications regarding the interaction between Pyk2, M-CSF survival signaling and the canonical apoptotic pathway warrant further exploration.
In addition to M-CSF, prolonged survival of monocytes in culture also occurs in response to proinflammatory signals. For example, exposure of human PB monocytes to a variety of soluble inflammatory factors in vitro (including LPS, TNF-α, IL-1β, GM-CSF, and IL-18) was shown to suppress caspase activation through the PI3K/AKT pathway [61, 62, 66]. It is not clear whether inflammatory cues also extend the t1/2 of monocytes in vivo. Although our study implicates Pyk2 as a proapoptotic factor in monocytes at homeostasis, the role of Pyk2 in regulating monocyte survival during times of inflammation has not been addressed.
Given the rapid turnover of monocytes at homeostasis and the data presented above, we suggest that endogenous Pyk2 operates within the constitutive signaling program that drives apoptosis of these cells. However, in light of its increased expression and established role in adhesion signaling and migration of Mϕs and other cell types [38, 39], it is interesting to speculate that Pyk2 may have additional functions in Ly6C− monocytes. For example, Ly6C− monocytes characteristically “crawl” along the resting endothelium in a manner distinct from the typical leukocyte “rolling and adhesion” process [16, 17]. This directional motility, which is essential to Ly6C− monocyte “patrolling and scavenging” functions, is dependent on integrin signaling [16]. Because Pyk2 is highly expressed in these cells and functions in signaling pathways downstream of integrin engagement (reviewed in [27, 28]), it is uniquely positioned as a potential regulator of integrin-mediated signaling and migration of Ly6C− monocytes on the endothelium. Clearly, future studies are necessary to further address the nature and regulation of the signals that control the onset of Pyk2 expression and its subsequent functions in monocytes, both at homeostasis and in response to inflammatory insults.
AUTHORSHIP
R.A.L. performed the experiments and analyzed the data presented in all figures. He also wrote the manuscript and generated the figures. K.S.T. assisted with experiments and provided important input into the experimental design and interpretation of experiments. K.S.T. and M.F.G. reviewed and provided insightful critiques of the manuscript. A.H.B. was closely involved in experimental design, data analysis, and presentation. She also contributed to the writing and substantial revision of the manuscript.
ACKNOWLEDGMENTS
This work was supported in part by Grants R21 CA135532 (A.H.B.), P30 CA44579 [University of Virginia (UVA) Cancer Center], F31 CA180633 (R.A.L.) from the U.S. National Institutes of Health, James and Rebecca Craig Foundation, and UVA Women’s Oncology fund from the UVA Cancer Center, UVA Tobacco Research Program R&D funds, and UVA School of Medicine R&D funds. The authors would like to thank the UVA Flow Cytometry Core for their terrific expertise. We would also like to thank colleagues and laboratory members for their critical evaluation of the data.
Glossary
- ACK
ammonium/chloride/potassium
- BM
bone marrow
- BMDM
bone marrow–derived macrophage
- CD
cluster of differentiation
- cMoP
committed monocyte progenitor
- FLICA
fluorochrome-labeled inhibitors of caspases
- M-CSF
Mϕ-CSF
- M-CSFR
Mϕ-CSF receptor
- PB
peripheral blood
- Pyk2
proline-rich tyrosine kinase 2
- SF
serum-free
- WT
wild-type
Footnotes
The online version of this paper, found at www.jleukbio.org, contains supplemental information.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Gordon S., Taylor P. R. (2005) Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964. [DOI] [PubMed] [Google Scholar]
- 2.Hume D. A. (2006) The mononuclear phagocyte system. Curr. Opin. Immunol. 18, 49–53. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins S. J., Hume D. A. (2014) Homeostasis in the mononuclear phagocyte system. Trends Immunol. 35, 358–367. [DOI] [PubMed] [Google Scholar]
- 4.Hettinger J., Richards D. M., Hansson J., Barra M. M., Joschko A. C., Krijgsveld J., Feuerer M. (2013) Origin of monocytes and macrophages in a committed progenitor. Nat. Immunol. 14, 821–830. [DOI] [PubMed] [Google Scholar]
- 5.Jakubzick C., Gautier E. L., Gibbings S. L., Sojka D. K., Schlitzer A., Johnson T. E., Ivanov S., Duan Q., Bala S., Condon T., van Rooijen N., Grainger J. R., Belkaid Y., Ma’ayan A., Riches D. W., Yokoyama W. M., Ginhoux F., Henson P. M., Randolph G. J. (2013) Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity 39, 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung S., Aliberti J., Graemmel P., Sunshine M. J., Kreutzberg G. W., Sher A., Littman D. R. (2000) Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geissmann F., Jung S., Littman D. R. (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82. [DOI] [PubMed] [Google Scholar]
- 8.Palframan R. T., Jung S., Cheng G., Weninger W., Luo Y., Dorf M., Littman D. R., Rollins B. J., Zweerink H., Rot A., von Andrian U. H. (2001) Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 194, 1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller W. A., Randolph G. J. (1999) Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J. Leukoc. Biol. 66, 698–704. [DOI] [PubMed] [Google Scholar]
- 10.Nahrendorf M., Swirski F. K., Aikawa E., Stangenberg L., Wurdinger T., Figueiredo J. L., Libby P., Weissleder R., Pittet M. J. (2007) The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin S. L., Castaño A. P., Nowlin B. T., Lupher M. L. Jr., Duffield J. S. (2009) Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J. Immunol. 183, 6733–6743. [DOI] [PubMed] [Google Scholar]
- 12.Zigmond E., Varol C., Farache J., Elmaliah E., Satpathy A. T., Friedlander G., Mack M., Shpigel N., Boneca I. G., Murphy K. M., Shakhar G., Halpern Z., Jung S. (2012) Ly6Chi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 37, 1076–1090. [DOI] [PubMed] [Google Scholar]
- 13.Ziegler-Heitbrock L., Hofer T. P. (2013) Toward a refined definition of monocyte subsets. Front. Immunol. 4, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yona S., Kim K. W., Wolf Y., Mildner A., Varol D., Breker M., Strauss-Ayali D., Viukov S., Guilliams M., Misharin A., Hume D. A., Perlman H., Malissen B., Zelzer E., Jung S. (2013) Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. (published correction in Immunity 2013. 38, 1073-1079). Immunity 38, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varol C., Landsman L., Fogg D. K., Greenshtein L., Gildor B., Margalit R., Kalchenko V., Geissmann F., Jung S. (2007) Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J. Exp. Med. 204, 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auffray C., Fogg D., Garfa M., Elain G., Join-Lambert O., Kayal S., Sarnacki S., Cumano A., Lauvau G., Geissmann F. (2007) Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317, 666–670. [DOI] [PubMed] [Google Scholar]
- 17.Carlin L. M., Stamatiades E. G., Auffray C., Hanna R. N., Glover L., Vizcay-Barrena G., Hedrick C. C., Cook H. T., Diebold S., Geissmann F. (2013) Nr4a1-dependent Ly6Clow monocytes monitor endothelial cells and orchestrate their disposal. Cell 153, 362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanna R. N., Carlin L. M., Hubbeling H. G., Nackiewicz D., Green A. M., Punt J. A., Geissmann F., Hedrick C. C. (2011) The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat. Immunol. 12, 778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swirski F. K., Nahrendorf M., Etzrodt M., Wildgruber M., Cortez-Retamozo V., Panizzi P., Figueiredo J. L., Kohler R. H., Chudnovskiy A., Waterman P., Aikawa E., Mempel T. R., Libby P., Weissleder R., Pittet M. J. (2009) Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cecchini M. G., Dominguez M. G., Mocci S., Wetterwald A., Felix R., Fleisch H., Chisholm O., Hofstetter W., Pollard J. W., Stanley E. R. (1994) Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development 120, 1357–1372. [DOI] [PubMed] [Google Scholar]
- 21.Dai X. M., Ryan G. R., Hapel A. J., Dominguez M. G., Russell R. G., Kapp S., Sylvestre V., Stanley E. R. (2002) Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 99, 111–120. [DOI] [PubMed] [Google Scholar]
- 22.Wiktor-Jedrzejczak W., Bartocci A., Ferrante A. W. Jr., Ahmed-Ansari A., Sell K. W., Pollard J. W., Stanley E. R. (1990) Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc. Natl. Acad. Sci. USA 87, 4828–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landsman L., Bar-On L., Zernecke A., Kim K. W., Krauthgamer R., Shagdarsuren E., Lira S. A., Weissman I. L., Weber C., Jung S. (2009) CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood 113, 963–972. [DOI] [PubMed] [Google Scholar]
- 24.Voss O. H., Kim S., Wewers M. D., Doseff A. I. (2005) Regulation of monocyte apoptosis by the protein kinase Cδ-dependent phosphorylation of caspase-3. J. Biol. Chem. 280, 17371–17379. [DOI] [PubMed] [Google Scholar]
- 25.Parihar A., Eubank T. D., Doseff A. I. (2010) Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. J. Innate Immun. 2, 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fahy R. J., Doseff A. I., Wewers M. D. (1999) Spontaneous human monocyte apoptosis utilizes a caspase-3-dependent pathway that is blocked by endotoxin and is independent of caspase-1. J. Immunol. 163, 1755–1762. [PubMed] [Google Scholar]
- 27.Avraham H., Park S. Y., Schinkmann K., Avraham S. (2000) RAFTK/Pyk2-mediated cellular signalling. Cell. Signal. 12, 123–133. [DOI] [PubMed] [Google Scholar]
- 28.Schaller M. D. (2010) Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J. Cell Sci. 123, 1007–1013. [DOI] [PubMed] [Google Scholar]
- 29.Lipinski C. A., Tran N. L., Dooley A., Pang Y. P., Rohl C., Kloss J., Yang Z., McDonough W., Craig D., Berens M. E., Loftus J. C. (2006) Critical role of the FERM domain in Pyk2 stimulated glioma cell migration. Biochem. Biophys. Res. Commun. 349, 939–947. [DOI] [PubMed] [Google Scholar]
- 30.Xiong W., Parsons J. T. (1997) Induction of apoptosis after expression of PYK2, a tyrosine kinase structurally related to focal adhesion kinase. J. Cell Biol. 139, 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melendez J., Turner C., Avraham H., Steinberg S. F., Schaefer E., Sussman M. A. (2004) Cardiomyocyte apoptosis triggered by RAFTK/pyk2 via Src kinase is antagonized by paxillin. J. Biol. Chem. 279, 53516–53523. [DOI] [PubMed] [Google Scholar]
- 32.Chauhan D., Hideshima T., Pandey P., Treon S., Teoh G., Raje N., Rosen S., Krett N., Husson H., Avraham S., Kharbanda S., Anderson K. C. (1999) RAFTK/PYK2-dependent and -independent apoptosis in multiple myeloma cells. Oncogene 18, 6733–6740. [DOI] [PubMed] [Google Scholar]
- 33.Pandey P., Avraham S., Kumar S., Nakazawa A., Place A., Ghanem L., Rana A., Kumar V., Majumder P. K., Avraham H., Davis R. J., Kharbanda S. (1999) Activation of p38 mitogen-activated protein kinase by PYK2/related adhesion focal tyrosine kinase-dependent mechanism. J. Biol. Chem. 274, 10140–10144. [DOI] [PubMed] [Google Scholar]
- 34.Plotkin L. I., Manolagas S. C., Bellido T. (2007) Glucocorticoids induce osteocyte apoptosis by blocking focal adhesion kinase-mediated survival. Evidence for inside-out signaling leading to anoikis. J. Biol. Chem. 282, 24120–24130. [DOI] [PubMed] [Google Scholar]
- 35.Lim S. T., Miller N. L., Nam J. O., Chen X. L., Lim Y., Schlaepfer D. D. (2010) Pyk2 inhibition of p53 as an adaptive and intrinsic mechanism facilitating cell proliferation and survival. J. Biol. Chem. 285, 1743–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burdick A. D., Ivnitski-Steele I. D., Lauer F. T., Burchiel S. W. (2006) PYK2 mediates anti-apoptotic AKT signaling in response to benzo[a]pyrene diol epoxide in mammary epithelial cells. Carcinogenesis 27, 2331–2340. [DOI] [PubMed] [Google Scholar]
- 37.Meads M. B., Fang B., Mathews L., Gemmer J., Nong L., Rosado-Lopez I., Nguyen T., Ring J. E., Matsui W., MacLeod A. R., Pachter J. A., Hazlehurst L. A., Koomen J. M., Shain K. H. (2016) Targeting PYK2 mediates microenvironment-specific cell death in multiple myeloma. Oncogene 35, 2723–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okigaki M., Davis C., Falasca M., Harroch S., Felsenfeld D. P., Sheetz M. P., Schlessinger J. (2003) Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc. Natl. Acad. Sci. USA 100, 10740–10745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owen K. A., Pixley F. J., Thomas K. S., Vicente-Manzanares M., Ray B. J., Horwitz A. F., Parsons J. T., Beggs H. E., Stanley E. R., Bouton A. H. (2007) Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J. Cell Biol. 179, 1275–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glodek A. M., Le Y., Dykxhoorn D. M., Park S. Y., Mostoslavsky G., Mulligan R., Lieberman J., Beggs H. E., Honczarenko M., Silberstein L. E. (2007) Focal adhesion kinase is required for CXCL12-induced chemotactic and pro-adhesive responses in hematopoietic precursor cells. Leukemia 21, 1723–1732. [DOI] [PubMed] [Google Scholar]
- 41.Kume A., Nishiura H., Suda J., Suda T. (1997) Focal adhesion kinase upregulated by granulocyte-macrophage colony-stimulating factor but not by interleukin-3 in differentiating myeloid cells. Blood 89, 3434–3442. [PubMed] [Google Scholar]
- 42.Yu Y., Ross S. A., Halseth A. E., Hollenbach P. W., Hill R. J., Gulve E. A., Bond B. R. (2005) Role of PYK2 in the development of obesity and insulin resistance. Biochem. Biophys. Res. Commun. 334, 1085–1091. [DOI] [PubMed] [Google Scholar]
- 43.Gil-Henn H., Destaing O., Sims N. A., Aoki K., Alles N., Neff L., Sanjay A., Bruzzaniti A., De Camilli P., Baron R., Schlessinger J. (2007) Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2−/− mice. J. Cell Biol. 178, 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ray B. J., Thomas K., Huang C. S., Gutknecht M. F., Botchwey E. A., Bouton A. H. (2012) Regulation of osteoclast structure and function by FAK family kinases. J. Leukoc. Biol. 92, 1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buckbinder L., Crawford D. T., Qi H., Ke H. Z., Olson L. M., Long K. R., Bonnette P. C., Baumann A. P., Hambor J. E., Grasser W. A. III, Pan L. C., Owen T. A., Luzzio M. J., Hulford C. A., Gebhard D. F., Paralkar V. M., Simmons H. A., Kath J. C., Roberts W. G., Smock S. L., Guzman-Perez A., Brown T. A., Li M. (2007) Proline-rich tyrosine kinase 2 regulates osteoprogenitor cells and bone formation, and offers an anabolic treatment approach for osteoporosis. Proc. Natl. Acad. Sci. USA 104, 10619–10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sunderkötter C., Nikolic T., Dillon M. J., Van Rooijen N., Stehling M., Drevets D. A., Leenen P. J. (2004) Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 172, 4410–4417. [DOI] [PubMed] [Google Scholar]
- 47.Louis C., Cook A. D., Lacey D., Fleetwood A. J., Vlahos R., Anderson G. P., Hamilton J. A. (2015) Specific contributions of CSF-1 and GM-CSF to the dynamics of the mononuclear phagocyte system. J. Immunol. 195, 134–144. [DOI] [PubMed] [Google Scholar]
- 48.MacDonald K. P., Palmer J. S., Cronau S., Seppanen E., Olver S., Raffelt N. C., Kuns R., Pettit A. R., Clouston A., Wainwright B., Branstetter D., Smith J., Paxton R. J., Cerretti D. P., Bonham L., Hill G. R., Hume D. A. (2010) An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood 116, 3955–3963. [DOI] [PubMed] [Google Scholar]
- 49.Bettina A., Zhang Z., Michels K., Cagnina R. E., Vincent I. S., Burdick M. D., Kadl A., Mehrad B. (2016) M-CSF mediates host defense during bacterial pneumonia by promoting the survival of lung and liver mononuclear phagocytes. J. Immunol. 196, 5047–5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Breslin W. L., Strohacker K., Carpenter K. C., Haviland D. L., McFarlin B. K. (2013) Mouse blood monocytes: standardizing their identification and analysis using CD115. J. Immunol. Methods 390, 1–8. [DOI] [PubMed] [Google Scholar]
- 51.Rhee I., Zhong M. C., Reizis B., Cheong C., Veillette A. (2014) Control of dendritic cell migration, T cell-dependent immunity, and autoimmunity by protein tyrosine phosphatase PTPN12 expressed in dendritic cells. Mol. Cell. Biol. 34, 888–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamen L. A., Schlessinger J., Lowell C. A. (2011) Pyk2 is required for neutrophil degranulation and host defense responses to bacterial infection. J. Immunol. 186, 1656–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heng T. S., Painter M. W.; Immunological Genome Project Consortium (2008) The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 9, 1091–1094. [DOI] [PubMed] [Google Scholar]
- 54.Friedman A. D. (2007) Transcriptional control of granulocyte and monocyte development. Oncogene 26, 6816–6828. [DOI] [PubMed] [Google Scholar]
- 55.Huber R., Pietsch D., Panterodt T., Brand K. (2012) Regulation of C/EBPβ and resulting functions in cells of the monocytic lineage. Cell. Signal. 24, 1287–1296. [DOI] [PubMed] [Google Scholar]
- 56.Park M. H., Park S. Y., Kim Y. (2008) Induction of proline-rich tyrosine kinase2 (Pyk2) through C/EBPβ is involved in PMA-induced monocyte differentiation. FEBS Lett. 582, 415–422. [DOI] [PubMed] [Google Scholar]
- 57.Franzoso G., Carlson L., Xing L., Poljak L., Shores E. W., Brown K. D., Leonardi A., Tran T., Boyce B. F., Siebenlist U. (1997) Requirement for NF-κB in osteoclast and B-cell development. Genes Dev. 11, 3482–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perlman H., Pagliari L. J., Nguyen N., Bradley K., Liu H., Pope R. M. (2001) The Fas-FasL death receptor and PI3K pathways independently regulate monocyte homeostasis. Eur. J. Immunol. 31, 2421–2430. [DOI] [PubMed] [Google Scholar]
- 59.Kiener P. A., Davis P. M., Starling G. C., Mehlin C., Klebanoff S. J., Ledbetter J. A., Liles W. C. (1997) Differential induction of apoptosis by Fas-Fas ligand interactions in human monocytes and macrophages. J. Exp. Med. 185, 1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mangan D. F., Mergenhagen S. E., Wahl S. M. (1993) Apoptosis in human monocytes: possible role in chronic inflammatory diseases. J. Periodontol. 64(5 Suppl)461–466. [PubMed] [Google Scholar]
- 61.Mangan D. F., Wahl S. M. (1991) Differential regulation of human monocyte programmed cell death (apoptosis) by chemotactic factors and pro-inflammatory cytokines. J. Immunol. 147, 3408–3412. [PubMed] [Google Scholar]
- 62.Mangan D. F., Welch G. R., Wahl S. M. (1991) Lipopolysaccharide, tumor necrosis factor-α, and IL-1β prevent programmed cell death (apoptosis) in human peripheral blood monocytes. J. Immunol. 146, 1541–1546. [PubMed] [Google Scholar]
- 63.Thornberry N. A., Lazebnik Y. (1998) Caspases: enemies within. Science 281, 1312–1316. [DOI] [PubMed] [Google Scholar]
- 64.Kelley T. W., Graham M. M., Doseff A. I., Pomerantz R. W., Lau S. M., Ostrowski M. C., Franke T. F., Marsh C. B. (1999) Macrophage colony-stimulating factor promotes cell survival through Akt/protein kinase B. J. Biol. Chem. 274, 26393–26398. [DOI] [PubMed] [Google Scholar]
- 65.Bhatt N. Y., Kelley T. W., Khramtsov V. V., Wang Y., Lam G. K., Clanton T. L., Marsh C. B. (2002) Macrophage-colony-stimulating factor-induced activation of extracellular-regulated kinase involves phosphatidylinositol 3-kinase and reactive oxygen species in human monocytes. J. Immunol. 169, 6427–6434. [DOI] [PubMed] [Google Scholar]
- 66.Goyal A., Wang Y., Graham M. M., Doseff A. I., Bhatt N. Y., Marsh C. B. (2002) Monocyte survival factors induce Akt activation and suppress caspase-3. Am. J. Respir. Cell Mol. Biol. 26, 224–230. [DOI] [PubMed] [Google Scholar]
- 67.Hatch W. C., Ganju R. K., Hiregowdara D., Avraham S., Groopman J. E. (1998) The related adhesion focal tyrosine kinase (RAFTK) is tyrosine phosphorylated and participates in colony-stimulating factor-1/macrophage colony-stimulating factor signaling in monocyte-macrophages. Blood 91, 3967–3973. [PubMed] [Google Scholar]