Abstract
Purpose
This study was designed to assess the outcome of the extracorporeal membrane oxygenation (ECMO) in liver transplantation (LT) recipients with refractory septic shock and predict the prognosis of those cases.
Methods
From February 2005 to October 2012, ECMO was used in 8 cases of refractory septic shock. Laboratory values including lactate and total bilirubin level just before starting ECMO were obtained and sepsis-related organ failure assessment (SOFA) score, acute physiology and chronic health evaluation (APACH) II score and simplified acute physiology score (SAPS) 3 were calculated. Subsequent peak serum lactate and total bilirubin level, and SOFA score after 24 hours of starting ECMO were measured.
Results
Comparisons were made between survivors and nonsurvivors. ECMO was weaned off successfully in 3 patients (37.5%) and 2 patients (25%) survived to hospital discharge. Clinical scores including SOFA, APACH II, and SAPS3 and laboratory results including lactate, total bilirubin and CRP were not significantly different between survivor and nonsurvivor groups. Lactate level and SOFA score tended to decrease after ECMO support in survivor group and total bilirubin and CRP level tended to increase in nonsurvivor group.
Conclusion
Our findings suggest that the implantation of ECMO might be considered in highly selected LT recipients with refractory septic shock.
Keywords: Liver transplantation, Septic shock, Extracorporeal membrane oxygenation
INTRODUCTION
With the recent advances in the field of the surgery and care in the intensive care unit (ICU), the outcome of liver transplantation (LT) patients has improved. All LT recipients are necessarily managed in the ICU for several days immediately after LT. During this period, patients sometimes submit to respiratory failure, cardiac failure, and sepsis, which are not only related to liver graft function but also can influence patient survival. Some of these critically ill patients prove unresponsive to all conventional treatments. Extracorporeal membrane oxygenation (ECMO) has surfaced as a salvage therapy for LT recipients [1,2,3,4,5,6,7]. Previous studies reported usefulness of veno-venous ECMO in adult LT recipients facing acute pulmonary failure by prolonging the time to recovery from the preceding critical illness [1,2]. Successful management of intraoperative severe hypoxemia and cardiac arrest with ECMO were also reported [4,6]. For septic shock unresponsive to all conventional treatment, ECMO becomes a salvage therapy to consider in neonates and children [8,9]. Furthermore, a recently published study showed good long-term clinical outcomes and suggested that ECMO might be a valuable option in adult patients with refractory cardiac dysfunction during severe bacterial septic shock by providing extra time for recovery of the failing heart and supporting the perfusion of major organs until cardiac function spontaneous recovers [7,10]. However, there is no data about the LT recipients supported by ECMO for septic shock. Therefore, we investigated the clinical outcomes of adult LT recipients supported by ECMO in response to refractory septic shock from an ECMO registry at our institution.
METHODS
Study population
From March 2005 to September 2012, 854 patients underwent LT procedures from 689 (80.7%) living and 165 (19.3%) deceased donors. Of those, 24 recipients received ECMO support due to cardiogenic shock (n = 5), septic shock (n = 8), respiratory failure (n = 10), and hypovolemic shock (n = 1). Patients were eligible for the study if they were 18 years of age or older; ECMO was implanted for sepsis, defined as clinical signs of infection and evidence of pathogenic microorganisms according to culture or serological tests. The medical records of 8 recipients who were supported by veno-arterial ECMO for septic shock were retrospectively reviewed. ECMO was indicated along with the treatment of sepsis in patients with refractory shock, defined as evidence of organ hypoperfusion (extensive skin mottling, progressive lactic acidosis, oliguria or altered mental status) despite adequate intravascular volume and the inability to maintain mean arterial pressure > 65 mmHg despite infusion of a very high-dose of catecholamines (norepinephrine > 1 µg/kg/min, dopamine > 20 µg/kg/min, or epinephrine > 1 µg/kg/min with dobutamine > 20 µg/kg/min) [7]. The institutional Review Board of Samsung Medical Center approved this study and waived the requirement for written informed consent (approval number: SMC 2016-08-038-001).
Patient management
The immunosuppression protocol and prophylaxis against viral infection after LT are described in previous reports from our center [11]. Tacrolimus, steroids, and mycophenolate mofetil (MMF) were the primary agents used for immunosuppression after LT. All transplant recipients were given 500 mg of intravenous methylprednisolone during the anhepatic phase until postoperative day 2, this was tapered to 60 mg per day for a period of 5 days, and then administered at 8 mg, twice per day, for 1 month starting on postoperative day 8. Tacrolimus treatment was started on postoperative day 3, and the optimal blood level was adjusted to maintain a trough plasma concentration of 10–15 ng/mL during the first month and was reduced to 5–10 ng/mL thereafter. MMF was used in combination with tacrolimus and steroids. Starting on postoperative day 1, 750-mg MMF was administered twice a day.
HBV prophylaxis protocol for LT recipients was combination regimen of high dose HBIG (human HBV-specific hepatitis B immunoglobulin, Greencross, Seoul, Korea) with an oral nucleotide analogue [12].
All patients enrolled in this study were managed with antibiotics, antiviral agent or antifungal agents against the identified pathogens based on in vitro susceptibility testing. Each management plan was decided after discussion with infection specialists in our institution.
ECMO implantation and management
An experienced team decided to implant ECMO for mechanical support, and cardiovascular surgeons or interventional cardiologists placed the ECMO system at bedside or in a fluoroscopy. Capiox Emergency Bypass System (Capiox EBS, Terumo Inc., Tokyo, Japan) and Permanent Life Support (PLS; MAQUET, Rastatt, Germany) were used in our hospital.
Device insertion was performed by percutaneous cannulation using the Seldinger technique. In difficult cases, the cut-down method was used for cannulation. Cannula sizes ranged from 14F to 21F for the femoral artery and from 21F to 28F for the femoral vein.
Detailed management of ECMO was previously described [13] in which continuous unfractionated heparin was infused intravenously to maintain an activated clotting time between 180 and 220 seconds. The revolutions per minute of the ECMO device were initially set to achieve an ideal cardiac index >2.2 L/min/body surface area (m2) and were adjusted to target a central mixed venous saturation >70% and a mean arterial pressure >65 mmHg. Inotropes were discontinued or reduced to minimal doses within a few hours of achieving goal-directed flows.
Echocardiography was performed daily to monitor cardiac function. If patients were hemodynamically stable and adequately oxygenated, they were considered for ECMO weaning when the flow rate was 1 l/min/m2 for 4 hours. Successful weaning was defined when the patient was disconnected from ECMO without reinsertion or death within 24 hours.
Data collection and outcome variables
Age, sex, primary liver disease, model for end-stage liver disease score, infection site, microbiology result, and laboratory data just before and during the first 24 hours of ECMO implantation were obtained through medical record review. Severity of illness at the commencement of ECMO implantation was assessed by the simplified acute physiology score (SAPS) 3, sepsis-related organ failure assessment (SOFA), and acute physiology and chronic health evaluation (APACHE) II score. Those scores were recorded as the last value before initiation of ECMO. Shock-to-ECMO duration was defined as the time from the initiation of vasoactive drugs to the start of ECMO flow. Appropriate antibiotic therapy was considered if at least one of the prescribed antibiotics on the day of the ECMO initiation had sensitivity against the identified pathogen according to in vitro susceptibility testing. The primary outcome of this study was survival to hospital discharge. Other outcome variables included successful weaning from ECMO, SOFA score on day 1, peak CRP, peak total bilirubin, and peak lactate level. Δlactate was defined using the following formula: serum lactate level just before the start of ECMO (pre-ECMO) minus the peak serum lactate level during the ECMO support period.
RESULTS
Baseline characteristics
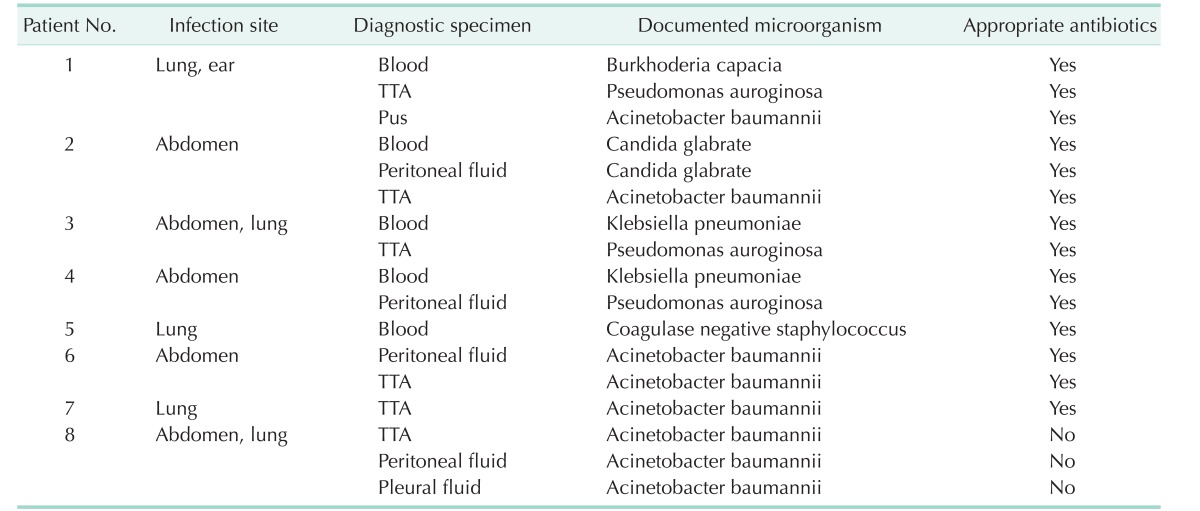
Among the 854 patients who underwent an LT procedure between March 2005 and September 2012, a total of 8 recipients aged 18 years or older with septic shock supported by venoarterial ECMO were enrolled in this study. The demographic profiles are given in Table 1. Primary liver diseases were hepatocellular carcinoma in 3 patients, HBV-related liver cirrhosis in 3 patients, alcoholic liver cirrhosis in 1 patient, and toxic hepatitis in 1 patient. These 8 patients were infected by various organisms mainly in the abdominal cavity or in the lung (Table 2) and supported by ECMO due to refractory septic shock for a median of 4 days (range, 1–13 days).
Table 1. Demographic profiles of 8 adult liver transplant recipients.
LT, liver transplantation; MELD, model for end-stage liver disease; ECMO, extracorporeal membrane oxygenation; HCC, hepatocellualr carcinoma; DDLT, deceased donor liver transplantation; LDLT, living donor liver transplantation.
a)Values are presented as median (range). b)Values are presented as number (%).
Table 2. Infection characteristics.
TTA, transtracheal aspiration.
Clinical features of the 8 adult LT recipients are summarized in Table 3. ECMO was initiated a median of 6.5 hours (range, 1–75 hours) after use of vasoactive drugs. Among the 8 patients, 6 (75%) received cardiopulmonary resuscitation and ECMO was initiated a median of 41.5 minutes (range, 20–154 minutes) later. Six patients (75%) underwent echocardiography, and 5 patients (83.3%) had left ventricular dysfunction with an ejection fraction <50%. The median SOFA score was 19 (range, 12–22), the median SAPS3 score was 86.5 (range, 68–106), and the median APACHE II score was 33.5 (range, 26–41) at just before EMCO initiation.
Table 3. Clinical features of the 8 adult liver transplant recipients at ECMO commencement.
ECMO, extracorporeal membrane oxygenation; CPR, cardiopulmonary resuscitation; LVEF, left ventricular ejection fraction; SOFA, sepsis-related organ failure assessment; SAPS, simplified acute physiology score; APACHE, acute physiology and chronic health evaluation score.
a)Values are presented as median (range). b)Values are presented as number (%).
Clinical outcomes
Of 8 patients, 7 were managed with an appropriate antibiotics regimen. Three patients (37.5%) were successfully weaned from ECMO, but only 2 (25%) survived until hospital discharge. Survivors did not experience ECMO related complications such as cannulation site bleeding, limb ischemia, or procedure-related infections. Among 5 patients who failed to attempt weaning, one suffered from graft versus host disease (GVHD), one was on a chemotherapy course against posttransplant lympho-proliferative disease (PTLD), and one had toxic cerebral dysfunction.
Comparison of survivors and nonsurvivors
Median age was 55.5 years (range, 53–58 years) in survivor group and 46.5 years (range, 19–67 years) in nonsurvivor group. Shock to ECMO duration was 25 hours (range, 7–43 hours) in survival group and 6 hours (range, 1–75 hours). At just before ECMO start, median serum lactate level, CRP, and total bilirubin were 12.8 mmol/L (range, 9.5–16 mmol/L), 10.8 mg/dL (range, 7.9–13.6 mg/dL), and 4.9 mg/dL (range, 3.1–6.7 mg/dL) in survivor group and 12.3 mmol/L (range, 6.7–20.6 mmol/L), 4.5 mg/dL (range, 0.5–14.1 mg/dL), and 2.75 mg/dL (range, 0.8–4.9 mg/dL) in nonsurvival group. Meidan SOFA score was 16 (range, 12–20) in survivor group and 19 (range, 14–22) in nonsurvivor group. In the survivor group, the lactate levels and the SOFA scores tended to decrease over the course of ECMO treatment, and in the nonsurvivor group, the total bilirubin and CRP levels tended to increase (Fig. 1).
Fig. 1. In the survivor group, the lactate level and the SOFA score tended to decrease over the course of ECMO treatment, and in the nonsurvivor group, the total bilirubin and CRP level tended to increase. SOFA, sepsis-related organ failure assessment; ECMO, extracorporeal membrane oxygenation.
DISCUSSION
The present study was dedicated to investigate how well ECMO performs as rescue therapy for adult LT recipients with refractory septic shock. The main findings of this study are as follows: (1) the survival of adult LT recipients with refractory septic shock was 25% (2 of 8) in spite of ECMO support, (2) the serum lactate level and SOFA score tended to decrease over the course of ECMO treatment in the survivor group, (3) the serum total bilirubin and CRP levels tended to increase in the non-survivor group.
Infections are the leading causes of critical illness, morbidity, and mortality after LT. It has been estimated that more than half of patients will develop an infection during the first year after LT, and many of these patients will require care in the ICU [14,15]. Bacteremia associated mortality ranges from 10%–52% in LT recipients and is higher in recipients with bacteremia due to ‘ESKAPE’ pathogens which stands for Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species [16]. These ESKAPE pathogens are emerging issues within solid organ transplantation recipients due to their wide distribution and capability of escaping the effect of antimicrobial treatment by acquisition of resistance genes, as well as formation of biofilms. Ouyang el al. [17] demonstrated that to improve the outcome of LT, more effective therapeutic treatments are of paramount importance, especially when a liver transplant recipient with an ESKAPE infection presents with septic shock. ECMO is a possible rescue therapeutic option for refractory septic shock [10,18], and it can be used in ESKAPE infection cases to improve patients outcomes. In this case, ESKAPE pathogens were isolated from the blood, peritoneal fluid, pleural fluid, and transtracheal aspiration (Table 2). The survival to hospital discharge was 25% (2 of 8) and remained low despite ECMO support. However, we believe that the 2 survivors got chance to be recovered with the ECMO support.
There are no absolute contraindications to ECMO, and each patient should be evaluated on a case-by-case basis and in the context of the individual center's experience [19]. Disseminated malignancy, advanced age, GVHD, known severe brain injury, and cardiac arrest of prolonged duration were presented as contraindications [20]. In this study, among the 6 patients who failed to survive to hospital discharge, one suffered from GVHD, one was on a chemotherapy course against PTLD, and one had toxic cerebral dysfunction. To improve the outcome of ECMO support for LT recipients with refractory septic shock, it is necessary to confirm the contraindication of our institution and implant ECMO for more selective patients.
Old age (>60 years) has been presented as risk factors of mortality in previous studies [18]. However, median ages were not significantly different between survivor and nonsurvivor groups in our study and we could not find the effect of old age (>60 years) since only 1 patient was over 60 years old. Park et al. [7] reported that none of the patients in whom ECMO was initiated more than 30.5 hours after onset of septic shock, survived. In our study, ECMO was initiated 7 and 43 hours after onset of septic shock. Even though we could not indicate absolute time of ECMO application, we recommend that ECMO should be considered when there is evidence of organ hypoperfusion despite adequate intravascular volume and the inability to maintain mean arterial pressure >65 mmHg despite infusion of a very high-dose of catecholamines as soon as possible for the better patient outcome.
During ECMO support, the trend in serum lactate level, CRP level, and SOFA score over time could be used to reflect patient response to ECMO support and offer an objective evaluation of treatment response [2,21,22]. In this study, serum lactate level, CRP level, and SOFA score showed a different trend between the survivor group and nonsurvivor group. Total bilirubin level, which has been shown to be a predictor of death after liver resection in the ICU [23], also showed a different trend between 2 groups. We believe this result reflects the importance of maintained liver function for recovery from the septic shock in LT recipients, and total bilirubin level should be monitored during ECMO support to predict the outcome. This hypothesis should be confirmed in a well-designed prospective trial or one with a large data registry.
This study had limitations that need to be addressed. This study presented a single-center experience with the application of ECMO in small number of adult LT recipients with refractory septic shock. Moreover, this was a retrospective study, using a limited number of patients. Therefore, the study setting limited the strength and generalizability of the results. Nevertheless, to the best of our knowledge, this is the first study to describe the use of ECMO for LT recipients with refractory septic shock and it will help in generating hypotheses for further research.
In conclusion, survival to hospital discharge of adult LT recipients with refractory septic shock remains low in spite of ECMO support. According to the findings of this study, we suggest that the use of veno-arterial ECMO during refractory septic shock could be considered in adult LT recipients and well-organized protocol outlining patient selection, monitoring of predictors and management of ECMO is necessary.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Choi NK, Hwang S, Kim KW, Park GC, Yu YD, Jung SH, et al. Intensive pulmonary support using extracorporeal membrane oxygenation in adult patients undergoing liver transplantation. Hepatogastroenterology. 2012;59:1189–1193. doi: 10.5754/hge10125. [DOI] [PubMed] [Google Scholar]
- 2.Park YH, Hwang S, Park HW, Park CS, Lee HJ, Namgoong JM, et al. Effect of pulmonary support using extracorporeal membrane oxygenation for adult liver transplant recipients with respiratory failure. Transplant Proc. 2012;44:757–761. doi: 10.1016/j.transproceed.2012.01.055. [DOI] [PubMed] [Google Scholar]
- 3.Stratta C, Lavezzo B, Ballaris MA, Panio A, Crucitti M, Andruetto P, et al. Extracorporeal membrane oxygenation rescue therapy in a case of portopulmonary hypertension during liver transplantation: a case report. Transplant Proc. 2013;45:2774–2775. doi: 10.1016/j.transproceed.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Yoo CS, Shin YH, Ko JS, Gwak MS, Kim GS. Anesthetic management including extracorporeal membrane oxygenation therapy of liver transplant recipient with life-threatening hypoxemia: a case report. Korean J Anesthesiol. 2013;65:151–157. doi: 10.4097/kjae.2013.65.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auzinger G, Willars C, Loveridge R, Best T, Vercueil A, Prachalias A, et al. Extracorporeal membrane oxygenation for refractory hypoxemia after liver transplantation in severe hepatopulmonary syndrome: a solution with pitfalls. Liver Transpl. 2014;20:1141–1144. doi: 10.1002/lt.23926. [DOI] [PubMed] [Google Scholar]
- 6.Tejani M, Yi SY, Eudailey KW, George I, Guarrera JV, Wagener G. Extracorporeal membrane oxygenation as a rescue device for postreperfusion cardiac arrest during liver transplantation. Liver Transpl. 2015;21:410–414. doi: 10.1002/lt.24056. [DOI] [PubMed] [Google Scholar]
- 7.Park TK, Yang JH, Jeon K, Choi SH, Choi JH, Gwon HC, et al. Extracorporeal membrane oxygenation for refractory septic shock in adults. Eur J Cardiothorac Surg. 2015;47:e68–e74. doi: 10.1093/ejcts/ezu462. [DOI] [PubMed] [Google Scholar]
- 8.Brierley J, Carcillo JA, Choong K, Cornell T, Decaen A, Deymann A, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37:666–688. doi: 10.1097/CCM.0b013e31819323c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sivarajan VB, Almodovar MC, Rodefeld MD, Laussen PC. Pediatric extracorporeal life support in specialized situations. Pediatr Crit Care Med. 2013;14(5 Suppl 1):S51–S61. doi: 10.1097/PCC.0b013e318292e16e. [DOI] [PubMed] [Google Scholar]
- 10.Brechot N, Luyt CE, Schmidt M, Leprince P, Trouillet JL, Leger P, et al. Venoarterial extracorporeal membrane oxygenation support for refractory cardiovascular dysfunction during severe bacterial septic shock. Crit Care Med. 2013;41:1616–1626. doi: 10.1097/CCM.0b013e31828a2370. [DOI] [PubMed] [Google Scholar]
- 11.Kim JM, Kwon CH, Joh JW, Ha YE, Sinn DH, Choi GS, et al. Oral valganciclovir as a preemptive treatment for cytomegalovirus (CMV) infection in CMV-seropositive liver transplant recipients. PLoS One. 2015;10:e0123554. doi: 10.1371/journal.pone.0123554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S, Kwon CH, Moon HH, Kim TS, Roh Y, Song S, et al. Antiviral treatment for hepatitis B virus recurrence following liver transplantation. Clin Transplant. 2013;27:E597–E604. doi: 10.1111/ctr.12212. [DOI] [PubMed] [Google Scholar]
- 13.Ko Y, Cho YH, Park YH, Lee H, Suh GY, Yang JH, et al. Feasibility and safety of early physical therapy and active mobilization for patients on extracorporeal membrane oxygenation. ASAIO J. 2015;61:564–568. doi: 10.1097/MAT.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 14.Blair JE, Kusne S. Bacterial, mycobacterial, and protozoal infections after liver transplantation--part I. Liver Transpl. 2005;11:1452–1459. doi: 10.1002/lt.20624. [DOI] [PubMed] [Google Scholar]
- 15.Kusne S, Blair JE. Viral and fungal infections after liver transplantation--part II. Liver Transpl. 2006;12:2–11. doi: 10.1002/lt.20667. [DOI] [PubMed] [Google Scholar]
- 16.Ye QF, Zhao J, Wan QQ, Qiao BB, Zhou JD. Frequency and clinical outcomes of ESKAPE bacteremia in solid organ transplantation and the risk factors for mortality. Transpl Infect Dis. 2014;16:767–774. doi: 10.1111/tid.12278. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang W, Li X, Wan Q, Ye Q. The risk factors for mortality and septic shock in liver transplant recipients with ESKAPE bacteremia. Hepatogastroenterology. 2015;62:346–349. [PubMed] [Google Scholar]
- 18.Huang CT, Tsai YJ, Tsai PR, Ko WJ. Extracorporeal membrane oxygenation resuscitation in adult patients with refractory septic shock. J Thorac Cardiovasc Surg. 2013;146:1041–1046. doi: 10.1016/j.jtcvs.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Ventetuolo CE, Muratore CS. Extracorporeal life support in critically ill adults. Am J Respir Crit Care Med. 2014;190:497–508. doi: 10.1164/rccm.201404-0736CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marasco SF, Lukas G, McDonald M, McMillan J, Ihle B. Review of ECMO (extra corporeal membrane oxygenation) support in critically ill adult patients. Heart Lung Circ. 2008;17(Suppl 4):S41–S47. doi: 10.1016/j.hlc.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32:1637–1642. doi: 10.1097/01.ccm.0000132904.35713.a7. [DOI] [PubMed] [Google Scholar]
- 23.Paugam-Burtz C, Janny S, Delefosse D, Dahmani S, Dondero F, Mantz J, et al. Prospective validation of the “fifty-fifty” criteria as an early and accurate predictor of death after liver resection in intensive care unit patients. Ann Surg. 2009;249:124–128. doi: 10.1097/SLA.0b013e31819279cd. [DOI] [PubMed] [Google Scholar]