Abstract
Glucagon like peptide-1 (GLP-1) stimulates glucose-dependent insulin secretion. Dipeptidyl peptidase-4 (DPP-4) inhibitors, which block inactivation of GLP-1, are currently in clinical use for type 2 diabetes mellitus. Recently, GLP-1 has also been reported to have neuroprotective effects in cases of cerebral ischemia. We therefore investigated the neuroprotective effects of GLP-1 receptor (GLP-1R) agonist, exendin-4 (ex-4), after cerebral ischemia-reperfusion injury. Transient middle cerebral artery occlusion (tMCAO) was induced in rats by intracerebroventricular (i.c.v.) administration of ex-4 or ex9-39. Oxygen-glucose deprivation was also induced in primary neurons, bEnd.3 cells, and BV-2. Ischemia-reperfusion injury reduced expression of GLP-1R. Additionally, higher oxidative stress in SOD2 KO mice decreased expression of GLP-1R. Downregulation of GLP-1R by ischemic injury was 70% restored by GLP-1R agonist, ex-4, which resulted in significant reduction of infarct volume. Levels of intracellular cyclic AMP, a second messenger of GLP-1R, were also increased by 2.7-fold as a result of high GLP-1R expression. Moreover, our results showed that ex-4 attenuated pro-inflammatory cyclooxygenase-2 (COX-2) and prostaglandin E2 after MCAO. C-Jun NH2 terminal kinase (JNK) signaling, which stimulates activation of COX-2, was 36% inhibited by i.c.v. injection of ex-4 at 24 h. Islet-brain 1 (IB1), a scaffold regulator of JNK, was 1.7-fold increased by ex-4. GLP-1R activation by ex-4 resulted in reduction of COX-2 through increasing IB1 expression, resulting in anti-inflammatory neuroprotection during stroke. Our study suggests that the anti-inflammatory action of GLP-1 could be used as a new strategy for the treatment of neuroinflammation after stroke accompanied by hyperglycemia.
Keywords: Neuroinflammation, Cerebral ischemia, Glucagon like peptide-1 receptor, Exendine-4, Islet-brain 1, c-Jun NH2 terminal kinase
INTRODUCTION
Stroke is the leading cause of death for the elderly, and causes permanent neurological damage, complications, and can eventually lead to death. In clinical research, approximately 30~40% of acute ischemic stroke patients with hyperglycemia, type 2 diabetes mellitus (T2DM) show increased frequency of stroke than those without [1]. This correlation suggests that a shared mechanism exists in these two disorders, stroke and T2DM. Various pathophysiological mechanisms have been proposed to explain the detrimental effects of hyperglycemia on the ischemic brain [2]. In addition, large clinical trials, including the United Kingdom Prospective Diabetes Study (UKPDS), the Action to Control Cardiovascular Risk in Diabetes trial (ACCORD), and the PROspective pioglitAzone Clinical Trial in macroVascular Events 04 (PROactive) have been conducted to reduce the risk of stroke through glycemic control [3,4,5]. However, these trials failed to show a clinical benefit, and had several limitations, such as induction of hypoglycemia and deficient research of basic mechanisms.
Glucagon like peptide-1 (GLP-1), responsible for glucose-induced insulin secretion through binding and activation of the G-protein coupled GLP-1 receptor (GLP-1R), is in clinical use for treatment of T2DM [6]. GLP-1 has been found to play a significant neuroprotective role in the brain [7]. Specifically, GLP-1R is involved in learning and memory, which is associated with prevention of hippocampal neuronal apoptosis [8]. It also has been previously reported that stimulation of GLP-1R by its agonist, exendin-4 (ex-4), reduces brain damage and improves functional outcomes in stroke [9]. With respect to the pathogenic factors involved in ischemic reperfusion injury, ex-4 suppresses oxidative stress, inducible nitric oxide synthase (iNOS) expression, and cell apoptosis after reperfusion [10]. Even though GLP-1 has therapeutic potential in neurodegenerative ischemic conditions, there has been little success in neuroprotective treatments targeting GLP-1R. During ischemia, the inflammatory response producing pro-inflammatory factors, such as cyclooxygenase-2 (COX-2), has been shown to lead to the neuronal death [11]. This response is known to be associated with increased expression of the c-Jun NH2-terminal kinase (JNK) signaling pathway, which phosphorylates the activation domain of a COX-2 transcription factor, thereby regulating COX-2 transcriptional activity [12]. It has been found that ex-4 attenuates the inflammatory response induced by lipopolysaccharides (LPS) in cardiomyoblasts [13]. However, the anti-inflammatory effects of ex-4 through modulation of COX-2/JNK signaling in cerebral ischemia have not yet been reported.
Islet brain 1 (IB1), also called JNK-interacting protein 1 (JIP1), is a scaffold protein for JNK signaling. JIP1 is selective for the MLK-MKK7-JNK MAP kinase module, which facilitates the sequential interaction of this cascade, and regulates JNK downstream activity [14]. IB1/JIP1 is expressed in neurons and β-cells. Under normal conditions, it is possible that IB1/JIP1 constitutively retains JNK in the cytoplasm, perhaps triaging it for non-stress related functions [15,16]. However, certain stresses, including cytokines, UV irradiation, and N-Methyl-D-aspartic acid exposure are associated with down-regulation of IB1/JIP1, which results in translocation of phospho-JNK into the nucleus where it activates transcriptional factors [17,18]. The effects of stimulating IB1/JIP1, and inhibiting JNK signaling, are well-characterized in β-cells, especially the effects of GLP-1R agonist, ex-4, treatment [19]. The inverse relationship of JNK and IB1/JIP1 has also been reported in neuronal stress models. Increased JNK activation and death of hippocampal neurons were observed in mice under-expressing IB1/JIP1 upon kainic acid insult [20]. Conversely, in neurons transfected with IB1/JIP1 there was inhibited nuclear translocation of activated JNK [21]. However, there have been few reports on the relationships between IB1/JIP1 and JNK signaling in stroke. Furthermore, clarification of neuroprotective mechanism of ex-4 through IB1/JIP1, particularly after transient middle cerebral artery occlusion (tMCAO), is necessary. In the present study, the mechanism by which ex-4 exerts its neuroprotective effects through IB1/JIP1 after cerebral ischemia-reperfusion injury was investigated.
MATERIAL AND METHODS
Animals
Experiments were carried out in 8-week old male Sprague-Dawley rats, weighing 280~300 g at the time of ischemia. All procedures were in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care of Sookmyung Women's University. All animals were euthanized with an overdose of the anesthetic with isoflurane after experiments were completed to minimize pain or discomfort.
Transgenic (Tg) mice of the TgHS/SF-218 strain, which carries the superoxide dismutase 1 (SOD1) gene with a CD1 background, were derived from the founder stock [22]. There were no observable phenotypic differences between the Tg mice and their wild-type (WT) littermates. Superoxide dismutase 2 knockout (SOD2 KO) mutants (heterozygous) with a CD1 background were backcrossed with CD1 mice for at least 10 generations, and genotypes were determined as previously described [23]. Littermate WT mice with an identical genetic background were used in each experiment. There were no significant differences in physiological parameters between SOD1 Tg or SOD2 KO and WT [24,25].
Ex-4 (1 nmole, Sigma-Aldrich, St. Louis, MO, USA), ex9-39 (exendin9-39, 1 nmole, Sigma-Aldrich), or vehicle (saline) was administrated into the left lateral ventricle of the brain 30 min before tMCAO. The doses of ex-4 and ex9-39 were selected based on previously published information. The coordinates for intraventricular administration were 0.8 mm posterior to bregma, 1.5 mm lateral to the midline, and 4.0 mm below the dura surface. Body temperature was maintained at 37℃ during and after injection.
Transient focal cerebral ischemia
Sprague-Dawley rats were anesthetized and maintained with 2.0% isoflurane in 30% oxygen and 70% nitrous oxide using a face mask. Body temperature was maintained at 37℃ throughout surgery using a heating blanket. The right common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were exposed through a ventral midline incision. A 2.5 cm length of 3-0 nylon suture with a round tip was introduced into the CCA. The suture was gently advanced from the ECA to the beginning of the middle cerebral artery (MCA) until slight resistance was felt, a length of 19.5 mm. 1 h after MCA occlusion, the suture was withdrawn slowly to restore blood flow.
TTC staining
After reperfusion, the brains were removed and cut into 2 mm-thick coronal sections. The sections were stained with 1.5% 2, 3, 5-triphenyltetrazolium hydrochloride (TTC; Sigma-Aldrich) in saline and then fixed in 4% paraformaldehyde in saline. The stained brains were scanned, and the infracted areas were measured, by image analysis system, Image J (NIH, Bethesda, Maryland, USA). Infarct volumes were expressed as percentages of the total brain volume.
Primary cortical neuron culture
Primary neurons were prepared from the cortex of embryonic day 16 ICR mice. Brains were harvested, and the pure layers of neurons forming the cortex were dissected from the hemisphere under a microscope. The cortical neurons were dissociated in growth medium (Gibco 11090, plus glutamax, 0.02 M glucose and 5% horse serum, Invitrogen, Carlsbad, CA, USA). The cells were seeded into 6 well plates coated with poly-D-lysine (Sigma-Aldrich), and the media was replaced with fresh neurobasal medium (Gibco 21103, plus glutamax and antibiotics) with B27 (Gibco 26050) supplement on the next day. Neurobasal medium (Gibco Invitrogen) was replaced every 2~3 days, and the cultures were maintained at 37℃ in 95% air / 5% CO2 in a humidified incubator.
Oxygen glucose deprivation
Primary neuron cultures were subjected to in vitro hypoxicischemia by exposure to oxygen-glucose deprived conditions. The chamber (Plas Labs, Lansing, MI, USA) was deoxygenated with anaerobic gas mixture (95% N2-5% CO2-5% H2) before use. Primary neurons were subjected to oxygen glucose deprivation (OGD) by replacing culture medium with Dulbecco modified Eagle medium (DMEM) without glucose (Invitrogen), and then placing them in the anaerobic chamber flushed with the anaerobic gas mixture at 37℃. After 4 h OGD, the medium was replaced again with medium with glucose and the culture was moved to a 5% CO2/95% air incubator for different reoxygenation periods. Primary neurons were treated with ex-4 (50 nM) or ex9-39 (50 nM) during reoxygenation. IB1/JIP1 siRNA were transfected to the neurons 24 h prior to OGD, when necessary.
Western blotting
After ischemia-reperfusion injury, the cultured cells, or sliced brain tissue from infracted hemisphere, were dissected and homogenized in lysis buffer. The protein extracts were separated on SDS-polyacrylamide gel and electroblotted onto polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). Membranes were incubated overnight at 4℃ in a buffer with 0.1% Tween with primary antibodies specific to GLP-1 (Abcam, Cambridge, MA, USA), GLP-1R (Abcam), IB-1 (BD Transduction Laboratories, San Diego, CA, USA), phospho-SAPK/JNK (Cell Signaling Technology, Beverly, MA, USA), SAPK/JNK (Cell Signaling Technology), phospho-c-Jun (Cell Signaling Technology), p-MKK3/MKK6 (Cell Signaling Technology), phospho-p38 MAPK (Cell Signaling Technology), p38 (Cell Signaling Technology), p44/42 MAPK (Cell Signaling Technology), MMP-9 (Millipore), COX-2 (Cayman, Ann Arbor, MI, USA), β-actin (Sigma-Aldrich), and Transcription factor IID (TFIID) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Following this, membranes were then incubated with appropriate secondary antibodies. The signal was then detected using a chemiluminescent kit (Thermo Scientific, Rockford, IL, USA). Signals were normalized with β-actin or TFIID to standardize equal protein loading.
Quantitative polymerase chain reaction
Total cellular RNA was isolated using TRIzol reagent (Life Technologies, Carlsbad, CA, USA), and the cDNA was synthesized. Real-time quantitative PCR assays were carried out using the 7500 Software version 2.0.5 (Applied Biosystems, Foster City, CA, USA) as the amplification system with 0.4 pM primers, 2 µL template (RT product), 10 µL SYBR Green Real time PCR Master Mix (Toyobo, Osaka, Japan) in 20 µL PCR volume. The sequences of primers were as follows: IB1/JIP-1 forward: 5′-ATGTCTTCATGAGTGGCCC-3′, and reverse: 5′-GATTTCAAGGACACAGCTGG-3′; GLP-1R forward: 5′-AGTAGTGTGCTCCAAGGGCAT-3′, and reverse: 5′-AAGAAAGTGCGTACCCCACCG-3′; COX-2 forward: 5′-CCATGTCAAAACCGTGGTGAATG-3′, and reverse: 5′-ATGGGAGTTGGGCAGTCATCAG-3′. The reverse transcription reaction steps for each cycle consisted of a 10 s initial denaturation step at 95℃, and were followed by 20 s at 60℃ for annealing, and then 30 s at 72℃ for extension.
Cyclic AMP concentrations
To determine adenylyl cyclase activity, rats were treated with ex-4, ex9-39, or vehicle (saline) 30 min prior to MCAO. Dissected brain tissue was lysed in 0.1 M HCl. Direct cAMP measurements were performed using a direct cAMP enzyme immunoassay kit (Enzo Life Sciences, Farmingdale, NY, USA), according to the manufacturer's protocol. The mean value was recorded in pg/ml.
Prostaglandin E2 concentrations
To assess the COX-2 activity, prostaglandin E2 (PGE2), the product of COX-2, concentrations were measured. The infarcted brain treated with ex-4 or ex9-39 was homogenized in a buffer solution (0.1 M phosphate buffer pH 7.4, 1 mM EDTA, 10 µM indomethacin). The PGE2 levels in homogenized lysates were determined by an enzyme-linked immunosorbent assay kit (Cayman, Ann Arbor, MI, USA), according to the manufacturer's instructions, which is based on the competition between endogenous PGE2 and PGE2-acetylcholinesterase conjugate (PGE2 tracer) for a limited amount of PGE2 monoclonal antibody. The mean value was recorded in pg/ml.
Analysis of COX-2 promoter activities
Primary neurons were transfected with a COX-2 luciferase reporter gene construct (500 ng/well, Oxford Biomedical Research, Oxford MI, USA) and β-galactosidase (200 ng/well) using the Lipofectamine 2000 reagent (Invitrogen), which was added to antibiotic-free medium (Gibco). After 24 h, cells were subjected to OGD and were subsequently reoxygenated. Before measuring luciferase activity, cells were washed twice with phosphate-buffered saline. Luciferase activity was measured using a Luciferase Assay System (Promega, Madison, WI, USA) according to the manufacturer's instructions. The relative COX-2 promoter activities were normalized to that of β-galactosidase.
Immunoprecipitation assay (IP)
To determine protein-protein interactions, brain tissues were homogenized in lysis buffer (20 mM Tris-Cl pH 8.0, 5 mM MgCl2, 10% glycerol, 0.1% Tween 20, 0.3 M KCl, 1/1000 2-mercaptoethanol) containing phosphatase inhibitors and protease inhibitors (Sigma-Aldrich). A total of 1000 µg of the protein sample was incubated overnight at 4℃ with anti-IB1/JIP1 antibody (Santa Cruz Biotechnology) conjugated to protein A beads (Bio-Rad Laboratories, Hercules, CA, USA). The beads were washed three times and then boiled to release the captured proteins. After centrifugation, the supernatant was immunoblotted with anti-GLP-1R (Ser10) antibody as described in the Western blot method.
Statistical analysis
Results are expressed as means±S.E. Analysis of variance was used for multiple comparisons followed by one-way ANOVA with appropriate Student Newman-Keuls test (GraphPad Prism; Oberlin, San Diego, CA, USA). p-values <0.05 were considered statistically significant.
RESULTS
Cerebral ischemia reduced the expression of GLP-1 receptor
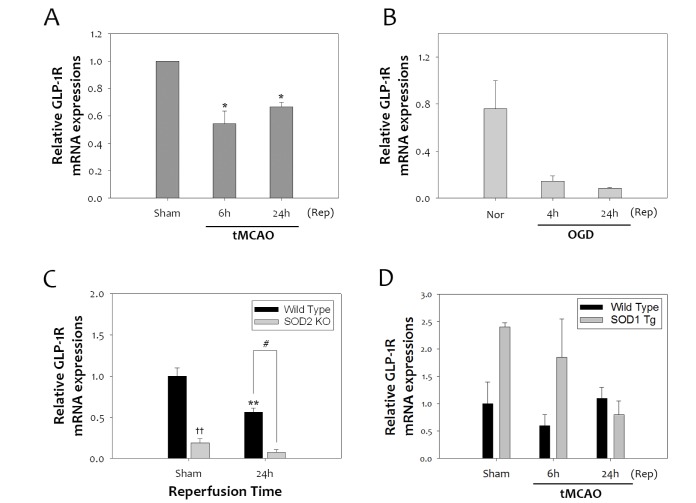
Oxidative stress has a harmful effect on stroke pathogenesis due to the high susceptibility of the brain to the reactive oxygen species (ROS)-induced damage [26]. In a previous report, activation of GLP-1R by agonist ex-4 reduces accumulation of oxidative DNA damage and lipid peroxidation caused by ischemic brain damage [10]. To determine how oxidative stress affects GLP-1R expression, GLP-1R mRNA levels were quantified in rat brains subjected to 1 h tMCAO. Cerebral ischemic reperfusion injury significantly reduced the transcript levels of GLP-1R at 6 h and 24 h after tMCAO (Fig. 1A). Primary neurons subjected to 4 h OGD also showed decreases in GLP-1R mRNA levels, compared to normoxia (Nor) at 4 h and 24 h (Fig. 1B). To clarify the effect of ROS on GLP-1R after ischemic reperfusion injury, we investigated the changes in GLP-1R mRNA levels in SOD2 KO mice that have high ROS levels, and SOD1 Tg mice that have low ROS levels. The expression of GLP-1R was significantly lower in SOD2 KO mice compared to WT mice (Fig. 1C) at 24 h. However, in SOD1 Tg mice, GLP-1R expression was significantly higher than in WT mice (Fig. 1D). Taken together, this suggests that GLP-1R expression is highly correlated with oxidative stress in cerebral ischemia.
Fig. 1. Reduction of GLP-1 receptor levels after tMCAO, and its relevance to oxidative stress in mice. (A) One-hour tMCAO reduced GLP-1R mRNA expression at 6 h and 24 h reperfusion (Rep) in mice (n =4, *p<0.05, compared to sham-operated group). (B) Four-hour OGD was performed in primary cultured neurons, and showed decreased GLP-1R mRNA levels compared to Nor. (C) SOD 2 KO mice, which have a high superoxide level, had significantly lower expression of GLP-1R than WT mice under normal or tMCAO (n =3, **p<0.01, compared to sham-operated WT group, ††p<0.01, comparison between sham-operated WT and KO group, #p<0.05, comparison between WT and KO mice at 24 h). (D) In SOD1 Tg mice, GLP-1R mRNA levels were higher than WT mice under sham or tMCAO.
Activation of GLP-1 receptor by ex-4 reduces infarct size and improves functional deficit after tMCAO
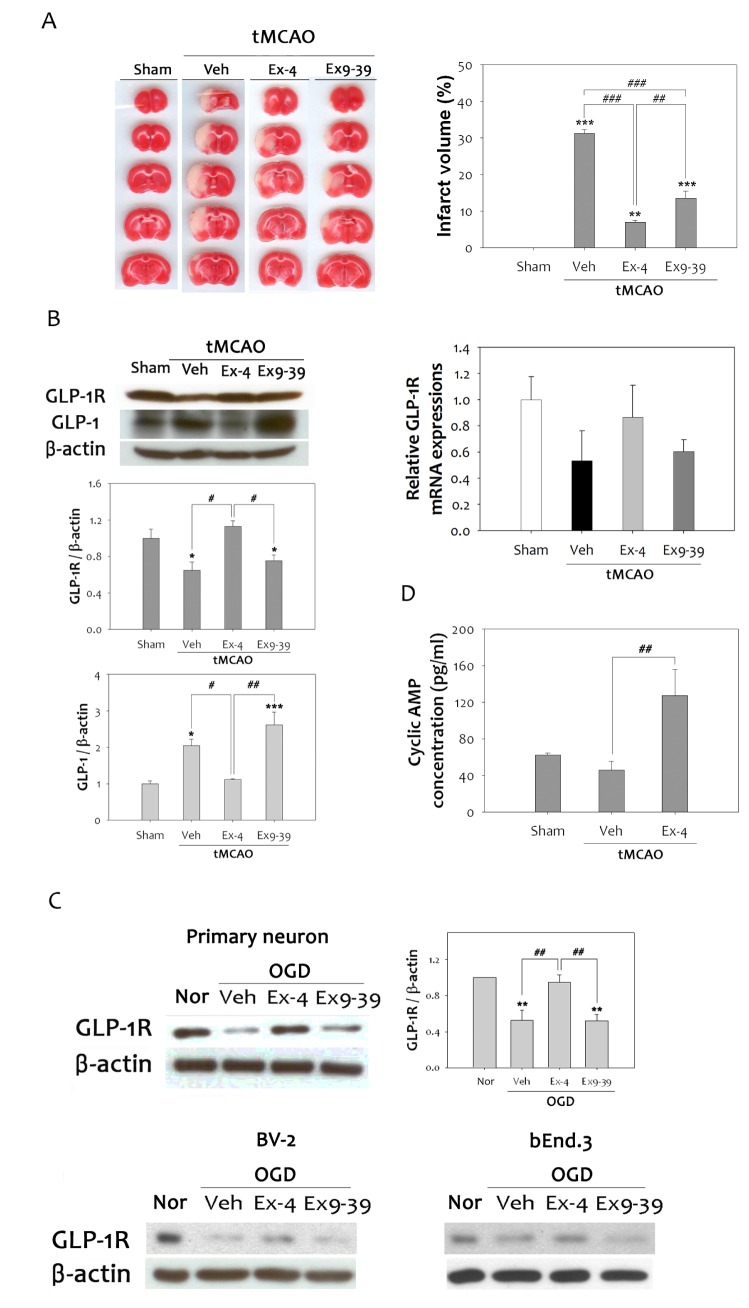
GLP-1R agonists have been reported to protect neurons from brain injury induced by oxidative damage [27]. Furthermore, repeated ex-4 administration resulted in reduction of focal cerebral ischemia-induced infarction [28]. However, the neuroprotective mechanism by which this compound operates during cerebral ischemia needs to be elucidated. To further characterize the neuroprotective effect of GLP-1R, ex-4 (GLP-1R agonist) or ex9-39 (GLP-1R antagonist) was administered to the rat brain at 1 h tMCAO. The infarct volume, as measured in 2 mm-thick brain slices, was reduced by more than 75% in ex-4 treated rats compared to vehicle group, but not to the extent that it was reduced in the ex9-39 treated group (Fig. 2A). Immunoblotting revealed that ischemic injury significantly reduced GLP-1R expressions by 36% in rat brains, but ex-4 treatment increased the expression levels of GLP-1R compared to vehicle group, while ex9-39 treatment reduced expression levels. In contrast, GLP-1 was up-regulated by ischemic insults. Ex-4 treatment was able to reduce GLP-1 expression to the basal level, but ex9-39 actually increased the expression (Fig. 2B). Relative GLP-1R mRNA expression was decreased by ischemic insult in the rat brain, but was significantly increased by ex-4 treatment compared to the vehicle group. It was confirmed in two types of primary neurons, BV-2 and bEnd.3, that hypoxic injury reduced GLP-1R expression after OGD (Fig. 2C). Likewise, ex-4 treatment up-regulated the expression of GLP-1R, but ex9-39 treatment reduced GLP-1R levels. Activation of GLP-1R elevates cAMP levels and activates downstream signaling pathways [29]. To validate activation of GLP-1R signaling by ex-4, cyclic AMP concentration was quantified in the rat brain. A cyclic AMP enzymatic immunoassay showed a 2.8-fold increase in cyclic AMP activity in the ex-4 treated group, compared to the vehicle group (Fig. 2D). These results indicate that activation of GLP-1R protects neurons against damage caused by cerebral ischemic insult.
Fig. 2. Neuroprotective effects of ex-4 through the G protein-coupled GLP-1 receptor. (A) Infarct volume was assessed by TTC staining after tMCAO in rats. Treatment with ex-4, GLP-1R agonist, resulted in a significant reduction of brain tissue damage after ischemic injury. Ex9-39, a GLP-1R antagonist, reduced brain infarction volume; however, it was less than that of ex-4 (n =3, **p<0.01, ***p<0.001, compared to sham-operated group, ##p<0.01, ###p<0.001, compared to each chemical-treated group). (B) Reduction of GLP-1R protein and mRNA expression were noted at 48 h after 1 h MCAO. Ex-4 efficiently counteracted decreases in GLP-1R in the ischemic rat brain. Treatment of ex9-39 reduced the level of GLP-1R as much as the vehicle group. Ischemic reperfusion injury significantly increased GLP-1 levels (n =5, *p<0.05, ***p<0.001, compared to sham-operated group, #p<0.05, ##p<0.01, compared to each chemical-treated group). (C) In primary neurons, mouse brain microglia cells, and endothelial cells, ex-4 increased the reduced GLP-1R protein levels after OGD compared to the vehicle group (n =5, **p<0.01, compared to Nor, ##p<0.01, compared to each chemical-treated group). (D) Cyclic AMP levels were evaluated to investigate GLP-1R signaling. Cyclic AMP levels were decreased after 1 h tMCAO, but showed a significant increase in the ex-4 treated animal group (n =4, ##p<0.01, compared to vehicle group).
Ex-4 inhibited inflammatory COX-2 expressions through suppressing phospho-JNK
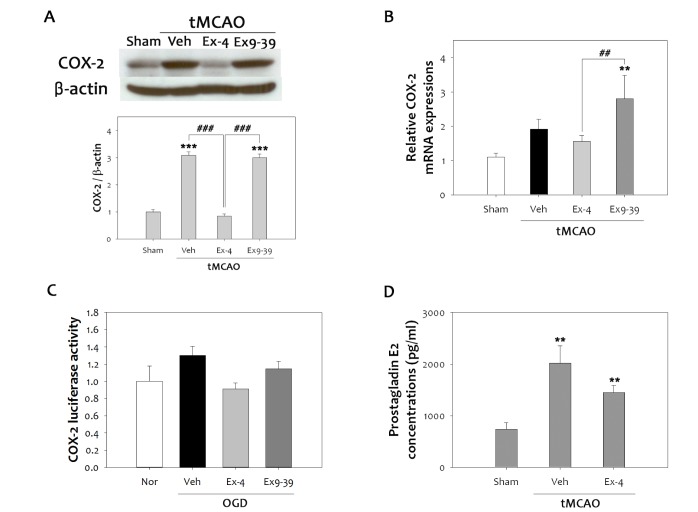
Inflammation has been known to contribute to the progression of brain damage following ischemic reperfusion injury, and COX-2 is a major factor in oxidative damage [30]. To determine whether activation of GLP-1R is related to anti-inflammatory effects in cerebral ischemia, we focused on the changes in COX-2 expression after tMCAO in rats treated with ex-4 or ex9-39. Ischemic injury significantly increased COX-2 expression after 48 h reperfusion (Fig. 3A). However, ex-4 lowered elevated COX-2 levels by as much as 70% after ischemic reperfusion injury in the rat brain. This effect was lost in the GLP-1R antagonist, ex9-39, treated group, indicating that the anti-inflammatory effect of ex-4 was mediated by GLP-1R. Quantitative PCR results also showed increased COX-2 mRNA expression elicited by ischemic reperfusion injury, as well as attenuation of this expression in ex-4 treated rat brains (Fig. 3B). To characterize the effect of ex-4 on the COX-2 promoter, COX-2/luciferase plasmid was transfected to bEnd.3 cells, and cells were treated with ex-4 or ex9-39 (Fig. 3C). Increased COX-2 promoter activity following 6 h OGD was reduced by ex-4 treatment, but not by ex-9-39 treatment. PGE2 is a major product of COX-2 at inflammatory sites, where it acts to potentiate edema formation [31]. To confirm reduction of COX-2 activity by ex-4, PGE2 levels in the brain were measured at 48 h after tMCAO (Fig. 3D). The production of PGE2 was increased 2.7 fold, indicating cerebral ischemic reperfusion injury provoked COX-2-induced inflammation and COX-2 formation, which was strongly inhibited by ex-4 treatment.
Fig. 3. Anti-inflammatory effects of ex-4 on COX-2 and PGE2. (A) The level of COX-2 was significantly increased at 48 h after tMCAO. Treatment with ex-4 restored COX-2 to the basal level after tMCAO in the rat brain. Ex9-39 treatment increased COX-2 levels as much as vehicle group. (B) Ex-4 also reduced the COX-2 mRNA levels in the rat brain after tMCAO (n =5, **p<0.01, compared to sham-operated group, ##p<0.01, compared to chemical-treated group). (C) Results were consistent with the COX-2 promoter assay. Ex-4 attenuated COX-2 luciferase activity after OGD in bEnd.3 cells. Ex9-39 increased COX-2 activity as much as vehicle group. (D) The level of PGE2, which is product of COX-2 activity, was increased by 1 h tMCAO, but this level was attenuated by ex-4 (n =5, **p<0.01, compared to the sham-operated group).
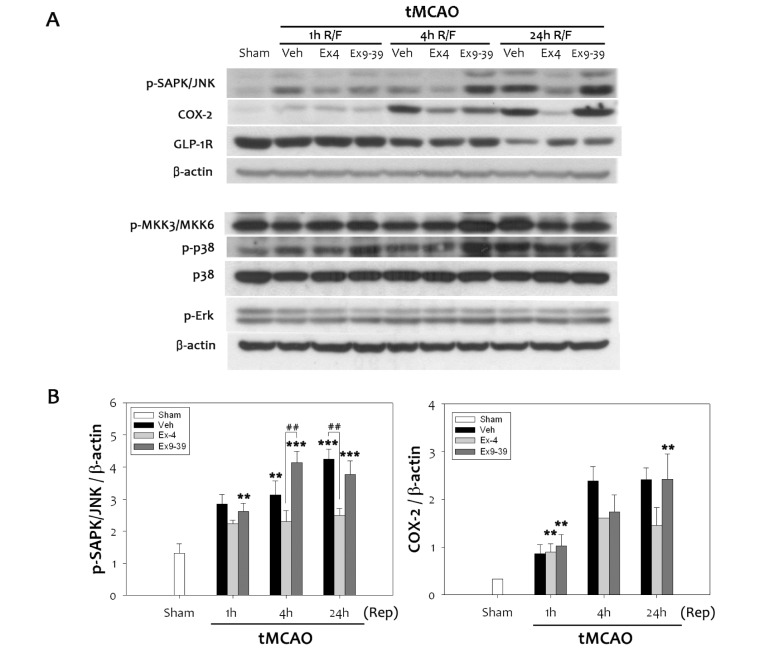
Oxidative cerebral ischemia-reperfusion injury mediates various mitogen-activated protein (MAP) kinases including MKK, p38, Erk (42/44), and JNK. To determine which kinases were involved in regulating inflammatory COX-2, phosphorylation levels of a number of MAP kinases were analyzed in the infarcted brain treated with ex-4 or ex9-39. Although there were no significant changes in p38 and Erk (42/44), cerebral ischemia-reperfusion injury increased activation of JNK. Ex-4 inhibited phosphorylation of JNK at 24 h reperfusion, which was reversed by ex9-39 (Fig. 4A). Increased COX-2 expression due to cerebral ischemic injury was attenuated by ex-4 treatment, but not by ex9-39 treatment. Changes in phospho-JNK levels were coincident with those in COX-2 levels at 24 h after tMCAO (Fig. 4B). This showed that phospho-JNK activated the COX-2 promoter, and increased expression of COX-2. Our results indicate that activation of GLP-1R inhibited COX-2 expression, which was mediated through inhibition of the JNK pathway in response to ex-4 after cerebral ischemia.
Fig. 4. Attenuation of the SAPK/JNK pathway by ex-4 through the GLP-1R. (A) Phosphorylation of SAPK/JNK was elevated following ischemic reperfusion injury in rats. Treatment with ex-4 prior to 1 h transient middle cerebral artery occlusion inhibited up-regulation of phospho-JNK and COX-2, while treatment with ex-9-39 increased phospho-JNK levels. Ex-4 did not significantly change other MAPK signaling. (B) Quantitative analyses showed significant augmentation in JNK-mediated COX-2 expression in rat (n =4, **p<0.01, ***p<0.001, compared to sham-operated group, ##p<0.01, compared to chemical-treated group at the same time point).
Enhanced IB1/JIP1 by ex-4 showed neuroprotection after cerebral ischemia
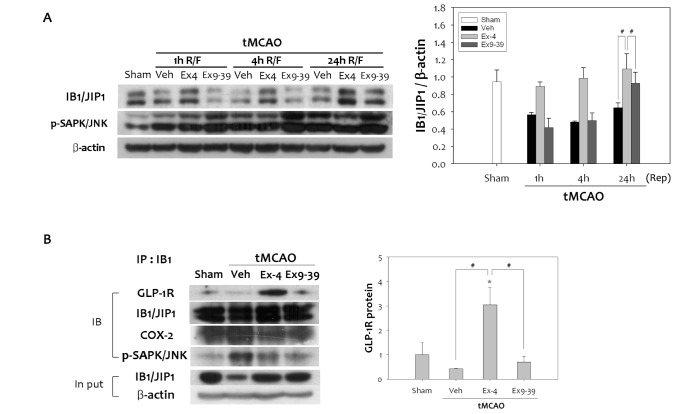
To explain the decrease in phospho-JNK caused by treatment with ex-4, the scaffold protein IB1/JIP, an upstream protein in the JNK pathway, was examined. IB1/JIP1 inhibits substrate phosphorylation and gene regulation by JNK, and suppresses the biological actions of JNK signal transduction pathway [15]. Overexpressing IB1/JIP, or its JNK-binding domain, prevents activation of c-Jun [32]. We assessed the possibility that ex-4 could modulate the levels of IB1/JIP1 to block JNK-mediated activation of COX-2. tMCAO reduced levels of IB1/JIP1 compared to the sham group (Fig. 5A). However, ex-4 treatment was able to abrogate the decrease in IB1/JIP1 elicited by tMCAO. The rise in IB1/JIP1 protein levels in the ex-4 treated group continued to increase until 24 h after tMCAO. Conversely, in the ex9-39 treated group, the levels of IB1/JIP1 were the same as the vehicle group. Increased IB1/JIP1 negatively regulated phospho-JNK, which suggests that the increase in IB1/JIP1 caused by activation of the GLP-1R is required for inhibition of JNK signaling. To further validate the effect of ex-4 on augmentation of IB1/JIP1 via GLP-1R, we investigated whether IB1/JIP1 interact with GLP-1R. We captured endogenous IB1/JIP1 from lysates of rat brains subjected to tMCAO by immunoprecipitation with the IB1/JIP1 antibody, and GLP-1R was immunoblotted. There was a significant increase in GLP-1R levels in the ex-4 treated group compared to the vehicle group (Fig. 5B). However, phospho-JNK was less recruited in the ex-4 treated group. Treatment with ex9-39 reduced the interaction between GLP-1R and IB1/JIP. Equal amounts of IgG-captured IB1/JIP1 and input proteins were confirmed. These results verify that IB1/JIP1 has a role as the negative regulator of JNK through interaction with GLP-1R in response to ex-4 treatment after cerebral ischemia in rats.
Fig. 5. Analysis of the interaction between IB1/JIP1 and GLP-1R after tMCAO in rat. (A) IB1/JIP1 protein levels were down-regulated by 1 h tMCAO, and following reperfusion. Ex-4 treatment dramatically increased IB1/JIP1 levels in the rat brain, while ex9-39 did not. Up-regulation of IB1/JIP1 by ex-4 treatment led to reduced expression of phospho-JNK (n =4, #p<0.05, compared to chemical treated group at the same time point). (B) Endogenous IB1/JIP1 was captured by immunoprecipitation with an anti-IB1/JIP1 antibody in the total fraction of infarcted brain. Results were detected by immunoblotting using antibodies to GLP-1R, COX-2, and phospho-SAPK/JNK. Decreased GLP-1R was detected in the vehicle group, while the ex-4 treated group showed significant interactions between GLP-1R and IB1/JIP1 compared to the sham group. Input lysates showed no differences (n =4, *p<0.05, compared to sham-operated group, #p<0.01, compared to chemical-treated group).
DISCUSSION
It has been proposed that shared pathophysiological mechanisms underlie hyperglycemia and stroke [1]. GLP-1 is currently in clinical use for lowering glucose levels, and has been reported to have functions as a neuropeptide, including: stimulation of neurite outgrowth, promotion of cell survival, and modulation of food and water intake [33,34,35]. Thus, we examined the effects of GLP-1 in a stroke model to investigate the neuroprotective properties.
GLP-1R is expressed in a wide range of tissues in humans and rodents, including the brain, liver, lung, kidney, spleen, thymus, adrenal glands, pancreas, and throughout the gastrointestinal tract [36,37]. Our group has detected GLP-1R in neurons, microglia, and endothelial cells of mice brain. It has been demonstrated that GLP-1R stimulation conferred neuroprotection in stroke and Parkinsonism [9,10]. The neuroprotective effect of ex-4 was absent in Glp1r-/- neurons, indicating that this effect is GLP-1R-mediated [9]. These findings are consistent with our results showing that cerebral ischemia reduced GLP-1R expression in rat brains. Furthermore, administration of GLP-1R agonist, ex-4, to in vivo and in vitro models proved to be protective after tMCAO or OGD. Treatment with ex-4 was accompanied by increased expression of GLP-1R, while treatment with the GLP-1R antagonist, ex9-39, did not show these neuroprotective effects in stroke. These findings suggest that following oxidative stress after cerebral ischemia, there is a neuroprotective effect through GLP-1R. It has been reported that ex-4 prevents the generation of ROS and suppresses oxidative stress after myocardial infarction [27,38]. Our results indicate that GLP-1R levels are highly related to the level of oxidative stress, as SOD2 KO mice had much lower expression of GLP-1R in contrast to the higher expression seen in SOD1 Tg mice.
Activation of GLP-1R elevates cAMP levels and activates the protein kinase A (PKA) signal transduction system [39]. Adding GLP-1 to primary neurons induces a time–dependent elevation in cAMP, indicative of a functional receptor [9]. Moreover, ex-4 treatment resulted in an increase of cAMP and a significant activation of CREB compared with the vehicle group in mice with transient focal cerebral ischemia [10]. This is consistent with our results that show increased cAMP concentrations in the ex-4 treatment group, as well as reduced ischemic injury. Previous work demonstrated a clear involvement of PKA, as Rp-cAMP, which blocks cAMP activation of PKA, abolished the neuroprotective effect of GLP-1 [33]. The pathways that underpin the actions of many endogenous neuroprotective agents against ischemic insults commonly converge on the transcription factor cAMP response element-binding (CREB) protein, which is triggered by increased cAMP levels [40]. In a mouse model of stroke, we confirmed that activation of GLP-1R by ex-4 was mediated by increased levels of cAMP.
As ex-4 treatment reduced infarct volume via activation of GLP-1R signaling after cerebral ischemia, we focused on the anti-inflammatory effect of ex-4. COX-2, in particular, is implicated in ischemic neuronal death [41]. Our results showed that ex-4 suppressed COX-2 expression in the ischemic brain and as well as the subsequent release of PGE2, responsible for neuroinflammation in ischemia reperfusion injury. In addition, ex-4 inhibited COX-2 promoter activity at a transcriptional level in bEnd.3 brain endothelial cells. According to previous reports, ex-4 attenuates LPS induced-inflammatory mediators TNF-α, COX-2, and MMP-9 in cardiomyoblasts [13]. In the brain, ex-4 has been shown to inhibit neuroinflammation due to microglia activation, and subsequent induction of iNOS [10]. Together with our findings, these results indicate an anti-inflammatory effect of ex-4 through modulation of COX-2 in cerebral ischemia-reperfusion injury.
The mechanism by which ex-4 causes COX-2 suppression is not fully known. Several possible regulatory kinases that are known to induce COX-2, p38 MAPK, Erk1/2, PI3K, and SAPK/JNK, were activated following ischemia reperfusion injury [42]. We showed a significant suppression of phospho-JNK specifically by ex-4 treatment. Regulation of COX-2 expression by the JNK signaling pathway was supported by the presence of putative AP-1 sites in the COX-2 gene promoter region. JNK is responsible for phosphorylating the AP-1 complex and thereby regulating its transcriptional activity. Our results demonstrated that COX-2 reduction was consistent with the significant inhibition of the phospho-JNK by ex-4 treatment after cerebral ischemia. We believe the decrease in COX-2 activation by ex-4 was induced by suppression of phospho-JNK levels, again suggesting the anti-inflammatory action of ex-4 after tMCAO in rats.
IB1, the rat homologue of JNK-interacting protein 1, has been identified as a scaffolding protein that tethers components of the JNK pathway [15,17,43]. As indicated by its name, IB1/JIP1 expression is mainly in the insulin-secreting β-cells of the pancreas, and neurons, particularly in the hippocampus, cortex, and the cerebellum [44]. The role of this protein in pancreatic islet has been studied extensively, specifically the role of IB1/JIP1 as a negative regulator of the JNK pathway [19]. In Alzheimer's model, IB1/JIP1 binds the β-amyloid precursor protein (APP), and scaffolds with JNK, providing novel insight into the role of APP as an intracellular functional molecule in the progression of Alzheimer's disease [45,46,47,48]. Dickens et al. observed that IB1/JIP1 binds more tightly to JNK than the transcriptional factors which signal for neuronal death [15]. Thus, sequestration of JNK in the cytoplasm enhances cellular resistance to stress signals and cell survival. Heterozygous mutant mice underexpressing IB1/JIP1 show greater vulnerability to epilepsy elicited by kainic acid, as well as increased activation of JNK in the mouse hippocampus [20]. In the brain, exposure to NMDA induces degradation of IB1/JIP-1 in cortical neurons [18]. Increased IB1/JIP1 delayed translocation of p-JNK to the nucleus after 8 h reperfusion in stroke, and there was perinuclear colocalization of JNK and IB1/JIP1 in neurons following stimulation, suggesting a functional interaction of these proteins [21,49]. Likewise, in our results, the inverse relationship of JNK and IB1/JIP1 expression was found in cerebral ischemia; IB1/JIP1 levels were spared after exposure to ischemia, in contrast to normal brain, with relatively more abundant IB1/JIP1 and less activated JNK. In insulin secreting cells, it has been reported that GLP-1R stimulation induced IB1/JIP1 [19]. The regulatory function of IB1/JIP1 on the JNK pathway in the brain is believed to be closely regulated by oxidative stress.
In this report, we demonstrated increased interaction between GLP-1R and IB1/JIP1 in the ex-4 treated group after tMCAO, compared to the vehicle group. These results suggest a possible contribution of ex-4 to JNK regulation, mediated by the interaction of IB1/JIP1 with GLP-1R.
Taken together, we provide evidence that oxidative cerebral ischemic insult leads to reduction of GLP-1R. Furthermore GLP-1R activation by ex-4 substantially inhibits COX-2 through modulating JNK signaling-mediated stimulation of IB1/JIP1. These findings indicate that GLP-1R stimulation is an effective neuroprotective strategy and would be valuable for the treatment of neuroinflammation as well as hyperglycemia after cerebral ischemia.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea grant (NRF- 2016K1A1A8A01939090, 2017M3C7A1031102) funded by the Korean government (MSIP).
References
- 1.Béjot Y, Giroud M. Stroke in diabetic patients. Diabetes Metab. 2010;36(Suppl 3):S84–S87. doi: 10.1016/S1262-3636(10)70472-9. [DOI] [PubMed] [Google Scholar]
- 2.Kruyt ND, Biessels GJ, Devries JH, Roos YB. Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol. 2010;6:145–155. doi: 10.1038/nrneurol.2009.231. [DOI] [PubMed] [Google Scholar]
- 3.Cull CA, Neil HA, Holman RR. Changing aspirin use in patients with Type 2 diabetes in the UKPDS. Diabet Med. 2004;21:1368–1371. doi: 10.1111/j.1464-5491.2004.01328.x. [DOI] [PubMed] [Google Scholar]
- 4.Riddle MC. Effects of intensive glucose lowering in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation. 2010;122:844–846. doi: 10.1161/CIRCULATIONAHA.110.960138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilcox R, Bousser MG, Betteridge DJ, Schernthaner G, Pirags V, Kupfer S, Dormandy J PROactive Investigators. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04) Stroke. 2007;38:865–873. doi: 10.1161/01.STR.0000257974.06317.49. [DOI] [PubMed] [Google Scholar]
- 6.Yusta B, Baggio LL, Estall JL, Koehler JA, Holland DP, Li H, Pipeleers D, Ling Z, Drucker DJ. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 2006;4:391–406. doi: 10.1016/j.cmet.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Holst JJ, Burcelin R, Nathanson E. Neuroprotective properties of GLP-1: theoretical and practical applications. Curr Med Res Opin. 2011;27:547–558. doi: 10.1185/03007995.2010.549466. [DOI] [PubMed] [Google Scholar]
- 8.During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9:1173–1179. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, Brossi A, Lahiri DK, Mattson MP, Hoffer BJ, Wang Y, Greig NH. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teramoto S, Miyamoto N, Yatomi K, Tanaka Y, Oishi H, Arai H, Hattori N, Urabe T. Exendin-4, a glucagon-like peptide-1 receptor agonist, provides neuroprotection in mice transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2011;31:1696–1705. doi: 10.1038/jcbfm.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nogawa S, Zhang F, Ross ME, Iadecola C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroer K, Zhu Y, Saunders MA, Deng WG, Xu XM, Meyer-Kirchrath J, Wu KK. Obligatory role of cyclic adenosine monophosphate response element in cyclooxygenase-2 promoter induction and feedback regulation by inflammatory mediators. Circulation. 2002;105:2760–2765. doi: 10.1161/01.cir.0000018127.10968.34. [DOI] [PubMed] [Google Scholar]
- 13.Chen TH, Wo HT, Wu CC, Wang JL, Wang CC, Hsieh IC, Kuo CY, Liu CT. Exendin-4 attenuates lipopolysaccharides induced inflammatory response but does not protects H9c2 cells from apoptosis. Immunopharmacol Immunotoxicol. 2012;34:484–490. doi: 10.3109/08923973.2011.630398. [DOI] [PubMed] [Google Scholar]
- 14.Whitmarsh AJ, Cavanagh J, Tournier C, Yasuda J, Davis RJ. A mammalian scaffold complex that selectively mediates MAP kinase activation. Science. 1998;281:1671–1674. doi: 10.1126/science.281.5383.1671. [DOI] [PubMed] [Google Scholar]
- 15.Dickens M, Rogers JS, Cavanagh J, Raitano A, Xia Z, Halpern JR, Greenberg ME, Sawyers CL, Davis RJ. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science. 1997;277:693–696. doi: 10.1126/science.277.5326.693. [DOI] [PubMed] [Google Scholar]
- 16.Nihalani D, Wong HN, Holzman LB. Recruitment of JNK to JIP1 and JNK-dependent JIP1 phosphorylation regulates JNK module dynamics and activation. J Biol Chem. 2003;278:28694–28702. doi: 10.1074/jbc.M304212200. [DOI] [PubMed] [Google Scholar]
- 17.Bonny C, Oberson A, Steinmann M, Schorderet DF, Nicod P, Waeber G. IB1 reduces cytokine-induced apoptosis of insulin-secreting cells. J Biol Chem. 2000;275:16466–16472. doi: 10.1074/jbc.M908297199. [DOI] [PubMed] [Google Scholar]
- 18.Centeno C, Repici M, Chatton JY, Riederer BM, Bonny C, Nicod P, Price M, Clarke PG, Papa S, Franzoso G, Borsello T. Role of the JNK pathway in NMDA-mediated excitotoxicity of cortical neurons. Cell Death Differ. 2007;14:240–253. doi: 10.1038/sj.cdd.4401988. [DOI] [PubMed] [Google Scholar]
- 19.Ferdaoussi M, Abdelli S, Yang JY, Cornu M, Niederhauser G, Favre D, Widmann C, Regazzi R, Thorens B, Waeber G, Abderrahmani A. Exendin-4 protects beta-cells from interleukin-1 beta-induced apoptosis by interfering with the c-Jun NH2-terminal kinase pathway. Diabetes. 2008;57:1205–1215. doi: 10.2337/db07-1214. [DOI] [PubMed] [Google Scholar]
- 20.Magara F, Haefliger JA, Thompson N, Riederer B, Welker E, Nicod P, Waeber G. Increased vulnerability to kainic acid-induced epileptic seizures in mice underexpressing the scaffold protein Islet-Brain 1/JIP-1. Eur J Neurosci. 2003;17:2602–2610. doi: 10.1046/j.1460-9568.2003.02701.x. [DOI] [PubMed] [Google Scholar]
- 21.Dong Z, Zhou L, Del Villar K, Ghanevati M, Tashjian V, Miller CA. JIP1 regulates neuronal apoptosis in response to stress. Brain Res Mol Brain Res. 2005;134:282–293. doi: 10.1016/j.molbrainres.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 22.Epstein CJ, Avraham KB, Lovett M, Smith S, Elroy-Stein O, Rotman G, Bry C, Groner Y. Transgenic mice with increased Cu/Zn-superoxide dismutase activity: animal model of dosage effects in Down syndrome. Proc Natl Acad Sci U S A. 1987;84:8044–8048. doi: 10.1073/pnas.84.22.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Copin JC, Reola LF, Calagui B, Gobbel GT, Chen SF, Sato S, Epstein CJ, Chan PH. Reduced mitochondrial manganese-superoxide dismutase activity exacerbates glutamate toxicity in cultured mouse cortical neurons. Brain Res. 1998;814:164–170. doi: 10.1016/s0006-8993(98)01082-8. [DOI] [PubMed] [Google Scholar]
- 24.Kinouchi H, Epstein CJ, Mizui T, Carlson E, Chen SF, Chan PH. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci U S A. 1991;88:11158–11162. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim GW, Kondo T, Noshita N, Chan PH. Manganese superoxide dismutase deficiency exacerbates cerebral infarction after focal cerebral ischemia/reperfusion in mice: implications for the production and role of superoxide radicals. Stroke. 2002;33:809–815. doi: 10.1161/hs0302.103745. [DOI] [PubMed] [Google Scholar]
- 26.Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4:461–470. doi: 10.1111/j.1747-4949.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- 27.Perry T, Greig NH. The glucagon-like peptides: a double-edged therapeutic sword? Trends Pharmacol Sci. 2003;24:377–383. doi: 10.1016/S0165-6147(03)00160-3. [DOI] [PubMed] [Google Scholar]
- 28.Briyal S, Gulati K, Gulati A. Repeated administration of exendin-4 reduces focal cerebral ischemia-induced infarction in rats. Brain Res. 2012;1427:23–34. doi: 10.1016/j.brainres.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 29.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Candelario-Jalil E, González-Falcón A, García-Cabrera M, Alvarez D, Al-Dalain S, Martínez G, León OS, Springer JE. Assessment of the relative contribution of COX-1 and COX-2 isoforms to ischemia-induced oxidative damage and neurodegeneration following transient global cerebral ischemia. J Neurochem. 2003;86:545–555. doi: 10.1046/j.1471-4159.2003.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams TJ, Peck MJ. Role of prostaglandin-mediated vasodilatation in inflammation. Nature. 1977;270:530–532. doi: 10.1038/270530a0. [DOI] [PubMed] [Google Scholar]
- 32.Bonny C, Oberson A, Negri S, Sauser C, Schorderet DF. Cell-permeable peptide inhibitors of JNK: novel blockers of beta-cell death. Diabetes. 2001;50:77–82. doi: 10.2337/diabetes.50.1.77. [DOI] [PubMed] [Google Scholar]
- 33.Perry T, Lahiri DK, Chen D, Zhou J, Shaw KT, Egan JM, Greig NH. A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther. 2002;300:958–966. doi: 10.1124/jpet.300.3.958. [DOI] [PubMed] [Google Scholar]
- 34.Luciani P, Deledda C, Benvenuti S, Cellai I, Squecco R, Monici M, Cialdai F, Luciani G, Danza G, Di Stefano C, Francini F, Peri A. Differentiating effects of the glucagon-like peptide-1 analogue exendin-4 in a human neuronal cell model. Cell Mol Life Sci. 2010;67:3711–3723. doi: 10.1007/s00018-010-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang-Christensen M, Larsen PJ, Göke R, Fink-Jensen A, Jessop DS, Møller M, Sheikh SP. Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. Am J Physiol. 1996;271:R848–R856. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- 36.Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995;7:2294–2300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 37.Dunphy JL, Taylor RG, Fuller PJ. Tissue distribution of rat glucagon receptor and GLP-1 receptor gene expression. Mol Cell Endocrinol. 1998;141:179–186. doi: 10.1016/s0303-7207(98)00096-3. [DOI] [PubMed] [Google Scholar]
- 38.Timmers L, Henriques JP, de Kleijn DP, Devries JH, Kemperman H, Steendijk P, Verlaan CW, Kerver M, Piek JJ, Doevendans PA, Pasterkamp G, Hoefer IE. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–510. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 39.Hui H, Nourparvar A, Zhao X, Perfetti R. Glucagon-like peptide-1 inhibits apoptosis of insulin-secreting cells via a cyclic 5’-adenosine monophosphate-dependent protein kinase A- and a phosphatidylinositol 3-kinase-dependent pathway. Endocrinology. 2003;144:1444–1455. doi: 10.1210/en.2002-220897. [DOI] [PubMed] [Google Scholar]
- 40.Walton M, Sirimanne E, Williams C, Gluckman P, Dragunow M. The role of the cyclic AMP-responsive element binding protein (CREB) in hypoxic-ischemic brain damage and repair. Brain Res Mol Brain Res. 1996;43:21–29. doi: 10.1016/s0169-328x(96)00144-1. [DOI] [PubMed] [Google Scholar]
- 41.Masferrer JL, Zweifel BS, Manning PT, Hauser SD, Leahy KM, Smith WG, Isakson PC, Seibert K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci U S A. 1994;91:3228–3232. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chun KS, Surh YJ. Signal transduction pathways regulating cyclooxygenase-2 expression: potential molecular targets for chemoprevention. Biochem Pharmacol. 2004;68:1089–1100. doi: 10.1016/j.bcp.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 43.Abderrahmani A, Niederhauser G, Favre D, Abdelli S, Ferdaoussi M, Yang JY, Regazzi R, Widmann C, Waeber G. Human high-density lipoprotein particles prevent activation of the JNK pathway induced by human oxidised low-density lipoprotein particles in pancreatic beta cells. Diabetologia. 2007;50:1304–1314. doi: 10.1007/s00125-007-0642-z. [DOI] [PubMed] [Google Scholar]
- 44.Pellet JB, Haefliger JA, Staple JK, Widmann C, Welker E, Hirling H, Bonny C, Nicod P, Catsicas S, Waeber G, Riederer BM. Spatial, temporal and subcellular localization of islet-brain 1 (IB1), a homologue of JIP-1, in mouse brain. Eur J Neurosci. 2000;12:621–632. doi: 10.1046/j.1460-9568.2000.00945.x. [DOI] [PubMed] [Google Scholar]
- 45.Helbecque N, Abderrahamani A, Meylan L, Riederer B, Mooser V, Miklossy J, Delplanque J, Boutin P, Nicod P, Haefliger JA, Cottel D, Amouyel P, Froguel P, Waeber G. Islet-brain1/C-Jun N-terminal kinase interacting protein-1 (IB1/JIP-1) promoter variant is associated with Alzheimer's disease. Mol Psychiatry. 2003;8:413–422. 363. doi: 10.1038/sj.mp.4001344. [DOI] [PubMed] [Google Scholar]
- 46.Taru H, Iijima K, Hase M, Kirino Y, Yagi Y, Suzuki T. Interaction of Alzheimer’s beta -amyloid precursor family proteins with scaffold proteins of the JNK signaling cascade. J Biol Chem. 2002;277:20070–20078. doi: 10.1074/jbc.M108372200. [DOI] [PubMed] [Google Scholar]
- 47.Matsuda S, Yasukawa T, Homma Y, Ito Y, Niikura T, Hiraki T, Hirai S, Ohno S, Kita Y, Kawasumi M, Kouyama K, Yamamoto T, Kyriakis JM, Nishimoto I. c-Jun N-terminal kinase (JNK)-interacting protein-1b/islet-brain-1 scaffolds Alzheimer’s amyloid precursor protein with JNK. J Neurosci. 2001;21:6597–6607. doi: 10.1523/JNEUROSCI.21-17-06597.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheinfeld MH, Roncarati R, Vito P, Lopez PA, Abdallah M, D'Adamio L. Jun NH2-terminal kinase (JNK) interacting protein 1 (JIP1) binds the cytoplasmic domain of the Alzheimer’s beta-amyloid precursor protein (APP) J Biol Chem. 2002;277:3767–3775. doi: 10.1074/jbc.M108357200. [DOI] [PubMed] [Google Scholar]
- 49.Hayashi T, Sakai K, Sasaki C, Zhang WR, Warita H, Abe K. c-Jun N-terminal kinase (JNK) and JNK interacting protein response in rat brain after transient middle cerebral artery occlusion. Neurosci Lett. 2000;284:195–199. doi: 10.1016/s0304-3940(00)01024-7. [DOI] [PubMed] [Google Scholar]