Abstract
Patients with cystic fibrosis, chronic obstructive pulmonary disease, severe asthma, pre-existing pulmonary lesions, and severely immunocompromised patients are susceptible to develop infections with the opportunistic pathogenic fungus Aspergillus fumigatus, called aspergillosis. Infections in these patients are associated with persistent pro-inflammatory T-helper (TH)2 and TH17 responses. Regulatory T-cells, natural suppressor cells of the immune system, control pro-inflammatory T-cell responses, but can also contribute to disease by shifting to a pro-inflammatory TH17-like phenotype. Such a shift could play an important role in the detrimental immunopathology that is seen in aspergillosis. Our study demonstrates that Aspergillus fumigatus induces regulatory T-cells with a TH17-like phenotype. We also demonstrate that these regulatory T-cells with a pro-inflammatory TH17-like phenotype can be reprogrammed to their “classical” anti-inflammatory phenotype by activating Toll-like receptor 2 (TLR2), which regulates the induction of cytotoxic T-lymphocyte-associated protein 4 (CTLA4). Similarly, soluble CTLA4 could reverse the pro-inflammatory phenotype of Aspergillus-induced regulatory T-cells. In conclusion, our results suggest a role for regulatory T-cells with a pro-inflammatory TH17-like phenotype in Aspergillus-associated immunopathology, and identifies key players, i.e. TLR2 and CTLA4, involved in this mechanism.
Introduction
Opportunistic infections caused by Aspergillus fumigatus are frequently observed in immunocompromised patients. These infections are considered to be severe complications and result from the absence of a fully functional host defence, thereby increasing susceptibility to this fungus1. Other patient groups at risk of developing disease caused by A. fumigatus are patients with cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), severe asthma, or individuals with pre-existing pulmonary lesions2–6. Clinical manifestations of such disease are called (invasive) aspergillosis, and range from hypersensitivity reactions to A. fumigatus, as is seen in allergic bronchopulmonary aspergillosis (ABPA)5, 7, to insufficient clearance of A. fumigatus with long-lasting inflammatory responses and ongoing fungal growth, as is seen in chronic pulmonary aspergillosis (CPA)2, 3.
Adequate clearance of A. fumigatus relies on T-helper cell-mediated pro-inflammatory immune responses, and particularly the T-helper (TH)1 response8–11. However, T-helper responses, in particular TH2 and TH17, are also known to play a detrimental role in the pathogenesis of ABPA and CPA12–14. These responses can cause uncontrolled inflammation, resulting in a massive influx of eosinophils and neutrophils12, 15. Although TH17-mediated recruitment of neutrophils plays an important role in the clearance of fungi, this response can also play a detrimental role in protective immunity during aspergillosis10, 11. TH17 activation by fungal growth can lead to disruption and necrosis of pulmonary tissue, thereby creating a niche for saprophytic growth of A. fumigatus 1, allowing the fungus to persist within the lungs and continue to induce persistent inflammatory responses16.
An important inhibitor of T-helper cell-driven pro-inflammatory responses are the regulatory T (Treg) cells. This endogenous immune suppressive cell controls TH2 polarization, and neutralizes TH17 responses in the lungs17. Adoptive transfer of Treg cells, or localised over-expression of Forkhead box protein P3 (FoxP3) (the classical transcription factor of Treg cells), inhibits TH2 and TH17 responses in animal models for asthma, resulting in improvement of clinical scores18, 19. Treg cells are known to modulate immune responses through both contact dependant (cytotoxic T-lymphocyte-associated protein 4; CTLA4, Indoleamine-pyrrole 2,3-dioxygenase; IDO, membrane bound Transforming growth factor β; TGFβ, and competition for major histocompatibility complex; MHC), and contact independent (Interleukin-10 and TGFβ) signalling pathways20, 21.
Recently, several studies demonstrated that Treg cells can acquire pro-inflammatory characteristics by differentiating into Interleukin (IL)-17A producing RAR-related orphan receptor γt (RORγt) expressing T-cells22, 23. This conversion was found to be involved in the pathogenesis of autoimmune arthritis and depends on pro-inflammatory cytokines such as IL-2 and IL-1523. TH17 differentiation promoting cytokines, like IL-1β, IL-6, and IL-23 further influence this conversion22. Interestingly, in CPA patients, IL-1 and IL-15 cytokine levels were found to be upregulated, suggesting a Treg – TH17 interplay in these patients14. Such a shift could play a crucial role in the IL-17 mediated immunopathology that is seen in patients with aspergillosis.
If A. fumigatus is capable of inducing Treg cells with a pro-inflammatory phenotype, this could have important implications for our understanding of the detrimental immunopathology seen in aspergillosis. In that case, reversal of pro-inflammatory Treg cells to their “classical” anti-inflammatory state could be a promising strategy for immunomodulatory therapy. This study shows that human A. fumigatus-induced Treg cells can exert this pro-inflammatory phenotype. In addition, we investigate the mechanisms involved and the potential of Treg cells to be reprogrammed to their “classical” anti-inflammatory phenotype.
Results
A. fumigatus induces regulatory T-cells with a pro-inflammatory TH17-like phenotype
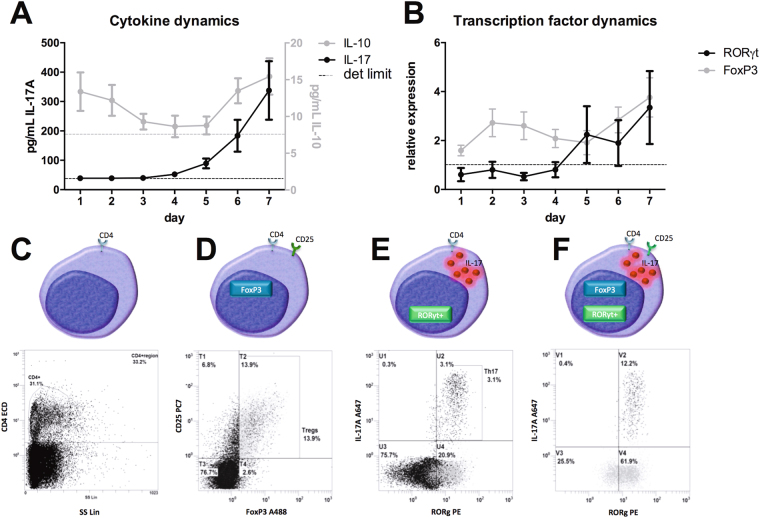
By determining the kinetics of IL-17A and IL-10 production over a course of 7 days in PBMCs simulated with A. fumigatus conidia, we determined the optimal time point to detect TH17-like pro-inflammatory Treg cells. Similar to previous studies with Candida albicans 24, A. fumigatus-induced IL-17A release was detectable at peak levels in supernatants after 7 days (Fig. 1A). The production of IL-10, an anti-inflammatory cytokine required for Treg cell differentiation25, which is also produced by Treg cells, demonstrated a biphasic response (Fig. 1A). Expression of the transcription factor of TH17 cells, RORγt, started to increase after 5 days exposure to conidia, similar to IL-17A release, whereas FoxP3 expression was already induced after 24 h and continued to increase in expression towards 7 days (Fig. 1B).
Figure 1.
Expression markers for regulatory T-cells and TH17 cells. (A) Dynamics of IL-17 and IL-10 cytokine levels in culture supernatants of PBMCs exposed to heat-inactivated A. fumigatus conidia (1 × 107/mL) for 7 days. (B) Dynamics of RORγt and FoxP3 mRNA expression in PBMCs exposed to A. fumigatus conidia for 7 days. (C–F) Gating strategy to detect regulatory T-cells with TH17 characteristics. (C) T-helper cells were selected by expression of the marker CD4 (CD4+), (D) regulatory T-cells were selected within the CD4+ population using the characteristics (CD25+ FoxP3+), (E) TH17 cell were selected within the CD4+ population using the characteristics (RORγt+ IL-17A+), and (F) TH17 cell characteristics (RORγt+ IL-17A) were selected within regulatory T-cells (CD4+ CD25+ FoxP3+) upon stimulation with heat-inactivated A. fumigatus conidia (1 × 107/mL).
To detect A. fumigatus-induced TH17-like pro-inflammatory Treg cells, PBMCs were stained and measured by flowcytometry after 7 days of stimulation with A. fumigatus conidia. T-cells were identified through CD4 (Fig. 1C). Within the CD4+ population, the number of Treg cells was quantified as the percentage of CD25+FoxP3+ cells (Fig. 1D). TH17 cells were quantified as RORγt+IL-17A+ cells within the CD4+ population (Fig. 1E). Finally, the percentage of cells with TH17 markers, i.e. RORγt / IL-17A, was determined within the Treg population, i.e. CD25+ FoxP3+ (Fig. 1F).
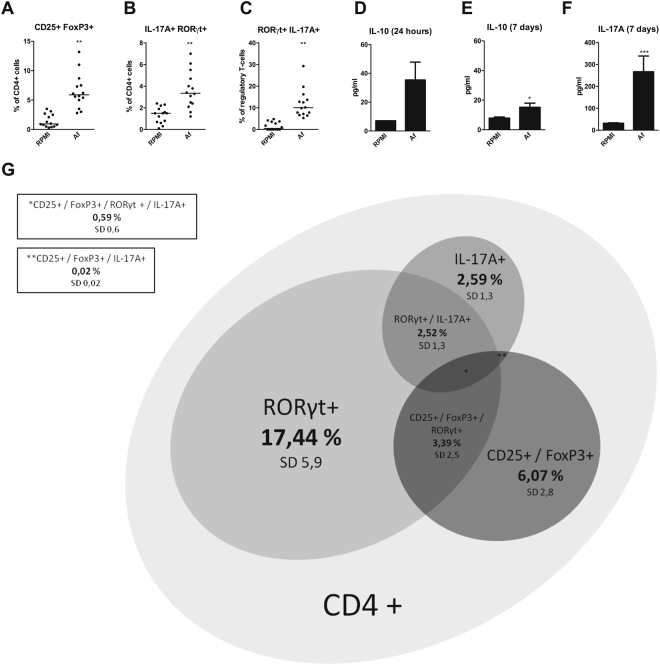
Following stimulation with A. fumigatus, a significant induction of CD25+FoxP3+ Treg cells (p = 0.0017 n = 14) (Fig. 2A), RORγt+IL-17A+ TH17 cells (p = 0.0017 n = 14) (Fig. 2B), and CD25+FoxP3+ RORγt+IL-17A+ pro-inflammatory Treg cells (p = 0.0011 n = 14) (Fig. 2C) was observed. The observed overlap between regulatory T-cell phenotype (CD25+FoxP3+), and TH17 cell-phenotype (RORγt+, IL-17A+, or RORγt+IL-17A+), within the CD4+ cell-population, following stimulation with A. fumigatus, is depicted as a Venn diagram in Fig. 2G.
Figure 2.
Aspergillus induces regulatory T-cells with a TH17-like phenotype. Scatter plots with median showing (A) Regulatory T-cell (CD25+ FoxP3+) induction after 7 days in human PBMCs stimulated with either RPMI, or heat-inactivated A. fumigatus conidia (1 × 107/mL). (B) IL-17A and RORγt expression within regulatory T-cells after 7 days in human PBMCs stimulated with either RPMI, or heat-inactivated A. fumigatus conidia (1 × 107/mL). (C) TH17 cell (IL-17A+ RORγt+) induction after 7 days in human PBMCs stimulated with either RPMI, or heat-inactivated A. fumigatus conidia (1 × 107/mL) (n = 17 donors). (D) IL-10 production after 24 hours in human PBMCs (n = 5 donors) stimulated with either RPMI, or heat-inactivated A. fumigatus conidia (1 × 107/mL). (E) IL-10 and (F) IL-17A production after 7 days in human PBMCs (n = 17 donors) stimulated with either RPMI, or heat-inactivated A. fumigatus conidia (1 × 107/mL). (G) Proportional Venn diagram showing overlap in CD4 cell-phenotype after PBMCs (n = 17 donors) were stimulated with heat-inactivated A. fumigatus conidia (1 × 107/mL) for 7 days. Results are depicted as percentage of CD4+ cell population with concomitant standard deviation (SD). Data are represented as scatter dot plots with median. Abbreviations: Af = Aspergillus fumigatus. *p-value ≤ 0.05, **p-value ≤ 0.01,***p-value ≤0.001.
In order to assess the cytokine release by these different cell populations, IL-10 production was measured in the culture supernatant after 24 hours and 7 days, and IL-17A production was measured after 7 days. After 7 days of stimulation, production of both IL-10 and IL-17A was significantly increased (p = 0.0273 n = 15 and p < 0.0001, n = 17) (Fig. 2E and F). However, this effect was not observed for IL-10 after 24 hours (Fig. 2D).
TLR2 regulates regulatory T-cells with a TH17-like phenotype
Toll-like receptor (TLR)2 is involved in the recognition of pathogen-associated molecular patterns (PAMPS) in the Aspergillus cell wall (reviewed in ref. 26), and is associated with the induction of Treg cells in response to fungi27, 28. Based on the observation that naïve splenocytes of Tlr2 −/− mice produce higher levels of IL-17A than wild type (WT) mice (Supplementary Figure 1), we hypothesized that TLR2 could have a role in shaping the population of Aspergillus-induced regulatory T-cells.
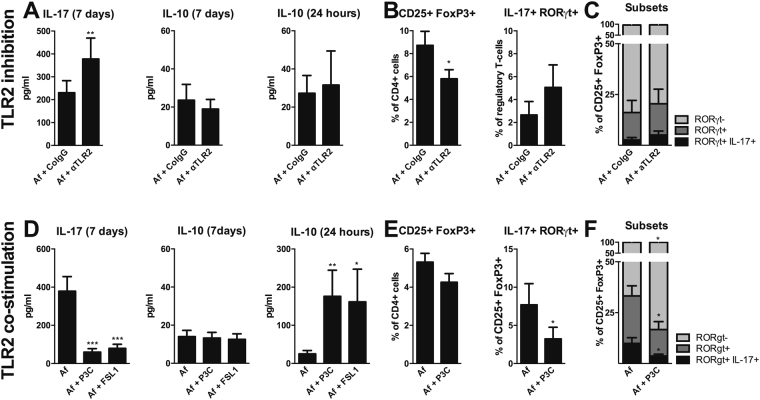
To investigate the role of TLR2 in the induction of Treg cells with a TH17-like phenotype, human PBMCs were stimulated with A. fumigatus conidia for 24 hours and 7 days while TLR2 was blocked with a neutralizing antibody.
As demonstrated previously29, blocking TLR2 before stimulating with A. fumigatus conidia resulted in a significant increase in IL-17A production (p = 0.0039 n = 9). However, no change in IL-10 production after 24 hours, and after 7 days was observed (Fig. 3A). Within the CD4+ population, the number of CD25+FoxP3+ Treg cells significantly decreased with TLR2 blockade (p = 0.0117 n = 9), while a non-significant trend towards increased expression of TH17 cell-characteristics, i.e. RORγt+IL-17A+ within these cells was observed (p = 0.1875 n = 6) (Fig. 3B). Expression of TH17 cell-characteristics, i.e. RORγt−, RORγt+, and RORγt+IL-17A+, within CD25+FoxP3+ Treg cells are depicted in Fig. 3C.
Figure 3.
TLR2 regulates Aspergillus-induced regulatory T-cells with a TH17-like phenotype. (A) IL-17A production after 7 days, IL-10 production after 24 hours and 7 days, regulatory T-cell (CD25+ FoxP3+) induction after 7 days, and IL-17A and RORγt expression within regulatory T-cells after 7 days in human PBMCs (n = 6 donors) stimulated with heat-inactivated A. fumigatus conidia (1 × 107/mL) after 1 hour pre-incubation with 10 µg/mL αTLR2, or CoIgG. (B,C) Different subsets within regulatory T-cell (CD25+ FoxP3+) induction, defined as RORγt-, RORγt+, or RORγt+ IL-17A+ regulatory T-cells (CD25+ FoxP3+), after 7 days in human PBMCs (n = 6 donors) stimulated with heat-inactivated A. fumigatus conidia (1 × 107/mL) after 1 hour pre-incubation with 10 µg/mL αTLR2, or CoIgG. Data are represented as mean ± SEM. (D) IL-17A production after 7 days, IL-10 production after 24 hours and 7 days (n = 14 donors), regulatory T-cell (CD25+ FoxP3+) induction after 7 days, and IL-17A and RORγt expression within regulatory T-cells after 7 days in human PBMCs (n = 9 donors) stimulated with heat-inactivated A. fumigatus conidia (1 × 107/mL) in the presence or absence of 10 µg/mL P3C or FSL1. (E,F) Different subsets within regulatory T-cell (CD25+ FoxP3+) induction, defined as RORγt-, RORγt+, or RORγt+ IL-17A+ regulatory T-cells (CD25+ FoxP3+), after 7 days in human PBMCs (n = 6 donors) stimulated with heat-inactivated A. fumigatus conidia (1 × 107/mL) in the presence or absence of 10 µg/ml P3C. Data are represented as mean ± SEM. Abbreviations: Af = Aspergillus fumigatus; CoIgG = Control immunoglobulin (G); αTLR2 = anti-TLR2 antibody; P3C = Pam3Cys-SKKKK; FSL-1 = Pam2Cys-SKKKK. *p-value ≤ 0.05, **p-value ≤0.01, ***p-value ≤ 0.001.
TLR2 co-stimulation reduces expansion of regulatory T-cells with a TH17-like phenotype
To further dissect the role of TLR2 in Aspergillus-induced Treg cells with pro-inflammatory TH17-like characteristics, strong TLR2 ligands, i.e. P3C (Pam3Cys-SKKKK; TLR2 and TLR1 heterodimer agonist) and FSL-1 (Pam2Cys-SKKKK; TLR2 and TLR6 heterodimer agonist), were combined with A. fumigatus stimulation assays. Co-stimulation of TLR2 with P3C and FSL-1 resulted in a significant decrease in IL-17A production after 7 days (p = 0.0002 n = 14) and a significant increase in IL-10 production after 24 hours (p = 0.0039 and p = 0.0254 n = 10), but not after 7 days (Fig. 3D). No significant change in the expansion of CD25+FoxP3+ Treg cells was observed upon co-stimulation of TLR2 with P3C, while the population of CD25+ FoxP3+ Treg cells expressing the TH17 RORγt+IL-17A+ phenotype was significantly smaller (p = 0.0313 n = 7) (Fig. 3E). Expression of TH17 cell-characteristics, i.e. RORγt−, RORγt+, and RORγt+IL-17A+, within CD25+FoxP3+ Treg cells are depicted in Fig. 3F.
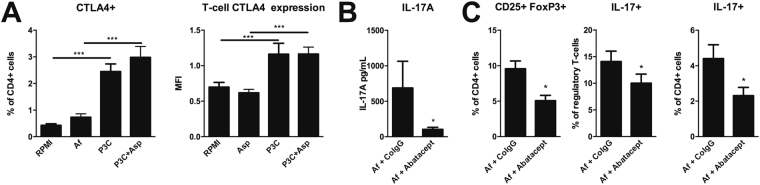
CTLA4 regulates the induction of Aspergillus-induced regulatory T-cells with a TH17-like phenotype
One of the most potent molecules that regulates the induction of pro-inflammatory T-cell subsets is CTLA4. To determine whether there is a role for CTLA4 in controlling the induction of IL-17A-expressing regulatory T-cells by Aspergillus, we investigated these cells in a patient with CTLA4 deficiency. The CTLA4 deficient patient showed slightly reduced induction of CD25+FoxP3+ regulatory T-cells and a similar induction of IL-17A+ TH17 cells, compared to the healthy control. TH17 cell-characteristics, i.e. IL-17A+, within CD25+FoxP3+ Treg cells, were substantially more induced by the patient compared to the healthy control (Supplementary Figure 2). To assess whether the capacity of TLR2 to reduce the expansion of regulatory T-cells with a TH17 phenotype was due to modulation of CTLA4, the capability to induce CTLA4 through TLR2 signalling was investigated. Activation of TLR2 by the agonist P3C resulted in a significant upregulation of CTLA4 expressing CD4+ T-cells (p = 0.0049 n = 12). Similarly, co-stimulation of TLR2 with A. fumigatus significantly upregulated the induction of CTLA4 expressing CD4+ T-cells, compared to stimulation with A. fumigatus (p = 0.0005 n = 12) (Fig. 4A). Soluble CTLA4–Ig (Abatacept) is known to inhibit the T-cell co-stimulatory molecule B7-1 (CD80), similar to endogenous CTLA430. Using soluble CTLA4, we validated whether increased CTLA4 levels could reduce Aspergillus-induced CD25+FoxP3+ regulatory T-cells with a TH17 phenotype. Addition of CTLA4-Ig to human PBMCs resulted in a decreased IL-17A production after 7 days (p = 0.0234 n = 7) (Fig. 4B). Also, induction of the number of CD25+FoxP3+ regulatory T-cells expressing a TH17 phenotype, i.e. IL-17A+, CD25+FoxP3+ regulatory T-cells and IL-17A+ TH17 cells significantly decreased upon addition of CTLA4-Ig (p = 0.0343, p = 0.0343 and p = 0.0469 n = 7) (Fig. 4D).
Figure 4.
CTLA4 regulates Aspergillus-induced regulatory T-cells with aTH17-like phenotype. (A) Percentage of CTLA4 expressing CD4+ T-cells and Mean fluorescence intensity (MFI) of CTLA4 staining following stimulation of human PBMCs (n = 13 donors) with heat-inactivated A. fumigatus conidia (1 × 107/mL), 10 µg/mL P3C or a combination of both. (B) IL-17A concentration in culture supernatants of human PBMCs (n = 6 donors) stimulated with heat-inactivated A. fumigatus conidia (1 × 107/mL) after 1 hour pre-incubation with 24 µg/mL Abatacept, or CoIgG. (C) Regulatory T-cell (CD25+FoxP3+) induction after 7 days in human PBMCs (n = 7 donors) stimulated with heat-inactivated A. fumigatus conidia (1 × 107/mL) after 1 hour pre-incubation with 24 µg/mL Abatacept, or CoIgG. TH17 cell (IL-17A + ) induction after 7 days in human PBMCs stimulated with heat- inactivated A. fumigatus conidia (1 × 107/mL) after 1 hour pre-incubation with 24 µg/mL Abatacept, or CoIgG. IL-17A+ regulatory T-cell induction after 7 days in human PBMCs stimulated with heat- inactivated A. fumigatus conidia (1 × 107/mL) after 1 hour pre-incubation with 24 µg/mL Abatacept, or CoIgG. Data are represented as mean ± SEM. Abbreviations: P3C = Pam3Cys-SKKKK; Af = Aspergillus fumigatus; CoIgG = Control immunoglobulin G. *p-value ≤ 0.05, **p-value ≤ 0.01,***p-value ≤ 0.001.
Discussion
In this study we investigated whether A. fumigatus-induced Treg cells exert a pro-inflammatory phenotype, and if this pro-inflammatory phenotype can be reprogrammed to the “classical” anti-inflammatory Treg phenotype. Human PBMCs exposed to A. fumigatus conidia showed induction of Treg cells with pro-inflammatory TH17-like characteristics. Induction of classical (CD25+FoxP3+) Treg cells and (IL-17A+RORγt+) TH17 cells was also significantly increased and related with increased production of IL-10 and IL-17A. These results indicate that A. fumigatus can indeed induce Treg cells with a pro-inflammatory TH17-like phenotype, which potentially could contribute to detrimental IL-17-mediated immunopathology.
Although the IL-17A axis plays an important role in the protective immunity against fungal pathogens such as A. fumigatus 31, it has been demonstrated that in some cases IL-17A mediated immune responses overwhelm this protective effect, promoting infection and impairing antifungal immunity13, 32. Diseases such as CPA and ABPA are characterized by a persistent hyper inflammatory state with massive influx of neutrophils and eosinophils12, 15, which may be attributed to exaggerated TH17 responses. Treg cells are potent suppressors of TH17 cells17, yet this suppressive effect is annulled when regulatory T-cells also acquire TH17 characteristics. In our study, we observed that the reduction of Treg cells with a TH17 phenotype, either through TLR2 stimulation or Abatacept, correlated with a reduction of the TH17 cytokine IL-17. Since it is technically difficult to determine the origin of IL-17 in culture supernatants we cannot ascertain that this reduction of IL-17A is strictly due to a reduction of IL-17+ Treg cells.
TLR2, together with TLR4 and TLR9, is an important Toll-like receptor in the host defence against Aspergillus (reviewed in refs 26, 33). The observation that splenocytes of Tlr2 −/− mice have an increased A. fumigatus-induced production of IL-17A, directed us towards a possible suppressive role for TLR2 in the induction of IL-17 responses. In addition, many studies have demonstrated a critical role for TLR2 in the induction of regulatory T-cells27, 28, 34. We found that blocking TLR2, while stimulating with A. fumigatus conidia, exaggerated the induction of Treg cells with pro-inflammatory TH17-like characteristics. Reversely, we observed that TLR2 co-stimulation not only increased the induction of regulatory T-cells, but also dampened the induction of regulatory T-cells with a TH17 phenotype. We suggest that altered TLR2 signalling may be involved in the aberrant induction of Treg cells with pro-inflammatory TH17-like characteristics. Whether alterations in TLR2 signalling, pathogen- or host-related, are actually involved in IL-17 mediated immunopathology needs further investigation. An attractive approach would be to investigate whether common genetic variations that slightly alter TLR2 function are associated with detrimental IL-17-mediated inflammation in aspergillosis.
TLR2 activating therapy is currently under consideration as a potential immunotherapy to increase the number of regulatory T-cells, reducing TH2 mediated hypersensitivity35, and increasing anti-tumour capacity36. However, whether such therapies are attractive to employ for treatment of aspergillosis remains to be determined. Experimental evidence highlights that TLR2 plays an important role in the antifungal host response against Aspergillus. TLR2 deficient mice for example show an impaired recruitment of neutrophils to the site of infection37, and a higher lethality and fungal burden33. Interestingly, these mice also show an increased TH2 responses compared to WT mice upon stimulation with A. fumigatus 37. This TH2 response, associated with ABPA, suppresses the protective TH1 response38. In line with our data it is tempting to speculate that the TLR2 deficient mice are more susceptible to aspergillosis due to modulation of Treg cells, as TLR2 mediated induction of Treg cells is crucial for maintaining pro-inflammatory responses and promoting fungal clearance in aspergillosis33, 37. Several studies have clearly demonstrated a protective role of Treg cells in fungal infection39, 40, and that TLR2 plays a crucial role in maintaining this population28, 34. It can however not be excluded that TLR2 has multiple lines of action in host defence against aspergillosis, such as induction of pro-inflammatory responses crucial for recruitment and activation of innate immune cells that are responsible for clearing the fungi from the lungs.
Treg cells partly exert their anti-inflammatory function through contact dependent mechanisms such as CTLA420. PBMCs of one patient who was deficient for CTLA4 were stimulated with A. fumigatus. Interestingly, a higher induction of Treg cells with a pro-inflammatory TH17-like phenotype was observed, compared to the healthy control. In the field of rheumatology, therapeutics such as Abatacept (CTLA4–Ig) are often administered to reduce pro-inflammatory responses in which the TH17 – Treg balance shifts towards the TH17 cell-response41. Therefore, such therapeutics might help reverse Treg cells with pro-inflammatory TH17-like characteristics back to their “classical” anti-inflammatory phenotype. We examined the role of Abatacept in preventing the induction of Treg cells with TH17-like characteristics, and a significantly decreased induction of Treg cells with TH17-like characteristics was seen. This indicates that Abatacept has the potential to hamper the induction of Treg cells with pro-inflammatory TH17-like characteristics. These results also indicate that CTLA4 might play a role in hampering the induction of Treg cells with pro-inflammatory TH17-like characteristics, and warrants further investigation to explore whether Abatacept could be used as a targeted therapy in patients with aspergillosis that are suffering from infection-induced immunopathology.
We observed that TLR2 stimulation increases the number of CTLA4 expressing CD4 T-cells and the level of expression on these cells. Separately we demonstrated that either TLR2 stimulation or CTLA4 could reduce the induction of Treg cells with pro-inflammatory TH17-like characteristics. The fact that TLR2 signalling boosts CTLA4 expression could suggest that TLR2 modulates the TH17 phenotype of Aspergillus-induced Treg cells through CTLA4. It should be noted that Aspergillus itself did not significantly induce CTLA4 expression. It is therefore tempting to suggest that Aspergillus induces such a pro-inflammatory Treg phenotype due to the fact that it fails to induce CTLA4 expression, but does induce the inflammatory mediators required for induction of pro-inflammatory T-cell responses.
Collectively, our study demonstrates that A. fumigatus is capable of inducing regulatory T-cells with a pro-inflammatory TH17-like phenotype. In addition, we found that TLR2 and CTLA4 play a role in regulating the induction of these cells. These findings could pave the way for novel therapeutic approaches that target Treg cells with pro-inflammatory TH17-like characteristics in order to return them to their natural immune regulatory state.
Methods
Healthy volunteers and patients
Blood was collected from healthy volunteers and patients by venous blood puncture, after informed consent was obtained. One patient that was deficient for CTLA4 was included, and for those experiment the healthy control was age (±5 years) and sex-matched. All experiments were performed and conducted in accordance to Good Clinical practice, the Declaration of Helsinki, and the approval of the Arnhem-Nijmegen Ethical Committee (nr.2010/104).
Aspergillus fumigatus
A. fumigatus V05-27, a previously characterized clinical isolate42, was used for all stimulations. Resting conidia were heat-inactivated, for 1 h at 65 °C and were checked for viability on Sabouraud agar. Conidia were stored at −80 °C until use. A concentration of 1 × 107/mL was used in the experiments, unless otherwise indicated.
Patter recognition receptor blockers and ligands
P3C (10 μg/mL) (Pam3Cys-SKKKK; TLR2/TLR1 heterodimer ligand, EMC microcollections, Tübingen, Germany), FSL-1 (1 μg/mL) (Pam2Cys-SKKKK; TLR2/TLR6 heterodimer ligand, EMC microcollections, Tübingen, Germany), and anti-TLR2 blocking antibody/control IgG (10 μg/mL) (eBioscience, Halle-Zoersel, Belgium) were used for pre-incubation and co-stimulation with heat-inactivated A. fumigatus conidia. Abatacept (CTLA4–Ig, 24 μg/mL) (inhibits the T-cell co-stimulatory molecule B7-1 (CD80), Orencia, Mulgrave, Australia) was used for pre-incubation with heat-inactivated A. fumigatus conidia.
Isolation and stimulation of peripheral blood mononuclear cells (PBMCs)
Blood was diluted in phosphate buffered saline (PBS) (1:1) and fractions were separated by Ficoll (Ficoll-Paque Plus; GE healthcare, Zeist, The Netherlands) density gradient centrifugation. Cells were washed twice with PBS and resuspended in Roswell Park Memorial Institute medium (RPMI) 1640 Dutch modification culture medium (Life Technologies/ Invitrogen, Breda, The Netherlands) supplemented with 50 μg/mL gentamicin, 2 mM Glutamax, and 1 mM pyruvate (Life Technologies). Cells were counted using a particle counter (Beckmann Coulter, Woerden, The Netherlands) after which, the concentration was adjusted to 5 × 106/mL. PBMCs were plated in 96-well round-bottom plates (Corning) at a concentration of 5 × 105/mL in a total volume of 200 µL. The samples were stimulated with A. fumigatus heat inactivated conidia (1 × 107/mL), with or without TLR2 blockers/ligands or Abatacept, or remained unstimulated for either 24 hours or 7 days at 37 °C with 5% CO2. After stimulation, supernatants were collected and stored at −20 °C until cytokine assays were performed. Cell pellets were used for flowcytometry.
qPCR
RNA was isolated using TRIzol reagent (Invitrogen) according to the protocol supplied by the manufacturer, and RNA was converted into cDNA using iScript cDNA synthesis kit (Biorad, Hercules Ca). Quantitative real-time PCR (qPCR) was performed using power SYBR Green PCR master mix (Applied Biosystems, Carlsbad, CA) and the following primers: GAPDH FWD-5′-AGGGGAGATTCAGTGTGGTG-3′ REV-5′-CGACCACTTTGTCAAGCTCA-3′, RORγt FWD-5′-TGAGAAGGACAGGGAGCCAA-3′ REV-5′-CCACAGATTTTGCAAGGGATCA-3′ and FoxP3 FWD-5′-CTGCCCCTAGTCATGGTGG-3′ REV-5′-CTGGAGGAGTGCCTGTAAGTG-3′. PCR was performed using an Applied Biosystem StepOne PCR system using PCR conditions 2 min 50 °C, 10 min 95 °C, followed by 40 cycles at 95 °C for 15 sec, and 60 °C for 1 min. RNA expression was corrected for differences in loading concentration using the signal of housekeeping protein GAPDH.
Cytokine measurements
IL-17A (R&D Systems, Mineapolis, MN) and IL-10 (Sanquin, Amsterdam, the Netherlands) were measured using commercially available ELISAs according to the protocols that were supplied by the manufacturers. Mouse IL-17A was assessed in splenocyte stimulations using the Luminex multiplex platform (Millipore, Billerica, MA).
Flowcytometry
For assessment of regulatory T-cells with TH17 characteristics, PBMCs were re-stimulated for 6 hours with phorbol 12-myristate 13-acetate (PMA) (50 ng/mL) (Sigma-Aldrich, Zwijndrecht, the Netherlands), Ionomycin (1 μg/mL) (Sigma-Aldrich), and GolgiPluginhibitor (1 µL/mL) (BD Biosciences, Breda, The Netherlands) after 7 day stimulation with A. fumigatus. Cells were stained extracellularly using Phycoerythrin(PE)–conjugated anti-CD4 (clone RPA-T4; ITK Diagnostics BV, Uithoorn, the Netherlands) or Phycoerythrin- Texas Red-X(ECD)-conjugated anti-CD4 (clone SFCI12T4D11; Beckman Coulter) and Phycoerythrin-Cyanine7–conjugated anti-CD25 (clone BC96; eBioscience) antibody. Subsequently, the cells were fixed and permeabilized with Cytofix/Cytoperm solution (eBioscience) according to the protocol that was supplied by the manufacturer. Following permeabilization, the cells were stained intracellular with Alexa Fluor 647–conjugated anti–IL-17A (clone N49-653; BD Biosciences), PE-conjugated RORγt (clone AFKJS-9; eBioscience, only used in non-patient experiments) and Alexa Fluor 488-conjugated FoxP3 (clone PCH101; eBioscience) according to the protocols supplied by the manufacturers.
For assessment of CTLA4 expression, PBMCs were stained extracellularly using Fluorescein Isothiocyanate (FITC)–conjugated anti-CD4 (clone RPA-T4; ITK Diagnostics BV) and PE- conjugated anti-CD152 (CTLA4) (clone 14D3; eBiosciences).
The cells were measured on a FC500 flow cytometer (Beckman Coulter) and the data were analysed using CXP analysis software v2.2 (Beckman Coulter).
Ex vivo Stimulation of WT and Tlr2−/− murine splenocytes
Animal studies were carried out in accordance with guidelines and regulations approved by St. Jude Children’s Research Hospital Committee on Use and Care of Animals (protocol no 482-100265-1-/13). Wild-type (WT) and Tlr2 knockout (Tlr2 −/−) C57Bl/6 mice were bred and maintained in the St. Jude Children’s Research Hospital, Memphis, TN, USA. Spleens were homogenized in 0,4 μM cell strainer (BD) and the cell number was adjusted to 1 × 107/mL. The cell suspensions (500 μL/well) were placed in a 24 well plate (Corning) and incubated with culture medium or A. fumigatus conidia for 1 or 5 days at 37 °C and 5% CO2.
Statistical analysis
Data of PBMC stimulation with either RPMI or A. fumigatus were analysed using the Wilcoxon signed rank test. P-values < 0.05 were considered statistically significant. Data are shown as scattered dot plots, columns with mean ± standard error of mean (SEM), or as a Venn diagram. All data were analysed using GraphPad Prism v5.0. The proportional Venn diagram was drawn using the eulerAPE application v2.0.343.
Electronic supplementary material
Acknowledgements
We would like to acknowledge M.S.M.A. Damen for her contribution to the revision of this paper. F.L.v.d.V. was supported by an E-Rare grant EURO-CMC.
Author Contributions
Conceived and designed the experiments: M.S.G., F.l.v.d.V. Performed the experiments: R.P.H.R., E.G.G.S., F.E.A., C.W.M.J., L.A.B.J. Analysed the data: R.P.H.R., E.G.G.S., M.S.G., C.W.M.J. Wrote the manuscript: R.P.H.R., M.S.G. Provided valuable reagents: T.D.K. Amended the manuscript: L.A.B.J., F.L.v.d.V., M.S.G., T.D.K.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-11738-4
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clinical microbiology reviews. 2009;22:447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith NL, Denning DW. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 2011;37:865–872. doi: 10.1183/09031936.00054810. [DOI] [PubMed] [Google Scholar]
- 3.Hope WW, Walsh TJ, Denning DW. The invasive and saprophytic syndromes due to Aspergillus spp. Med Mycol. 2005;43(Suppl 1):S207–238. doi: 10.1080/13693780400025179. [DOI] [PubMed] [Google Scholar]
- 4.Knutsen AP, Bellone C, Kauffman H. Immunopathogenesis of allergic bronchopulmonary aspergillosis in cystic fibrosis. Journal of cystic fibrosis: official journal of the European Cystic Fibrosis Society. 2002;1:76–89. doi: 10.1016/S1569-1993(02)00033-4. [DOI] [PubMed] [Google Scholar]
- 5.Knutsen AP, Slavin RG. Allergic bronchopulmonary aspergillosis in asthma and cystic fibrosis. Clinical & developmental immunology. 2011;2011:843763. doi: 10.1155/2011/843763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ader F, et al. Invasive pulmonary aspergillosis in chronic obstructive pulmonary disease: an emerging fungal pathogen. Clin Microbiol Infect. 2005;11:427–429. doi: 10.1111/j.1469-0691.2005.01143.x. [DOI] [PubMed] [Google Scholar]
- 7.Knutsen AP, Hutcheson PS, Slavin RG, Kurup VP. IgE antibody to Aspergillus fumigatus recombinant allergens in cystic fibrosis patients with allergic bronchopulmonary aspergillosis. Allergy. 2004;59:198–203. doi: 10.1046/j.1398-9995.2003.00310.x. [DOI] [PubMed] [Google Scholar]
- 8.Chai LY, et al. Anti-Aspergillus human host defence relies on type 1 T helper (Th1), rather than type 17 T helper (Th17), cellular immunity. Immunology. 2010;130:46–54. doi: 10.1111/j.1365-2567.2009.03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stuehler C, et al. Cross-protective TH1 immunity against Aspergillus fumigatus and Candida albicans. Blood. 2011;117:5881–5891. doi: 10.1182/blood-2010-12-325084. [DOI] [PubMed] [Google Scholar]
- 10.Cenci E, et al. Interleukin-4 causes susceptibility to invasive pulmonary aspergillosis through suppression of protective type I responses. Journal of Infectious Diseases. 1999;180:1957–1968. doi: 10.1086/315142. [DOI] [PubMed] [Google Scholar]
- 11.Cenci E, et al. Th1 and Th2 cytokines in mice with invasive aspergillosis. Infection and immunity. 1997;65:564–570. doi: 10.1128/iai.65.2.564-570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murdock BJ, et al. Interleukin-17 drives pulmonary eosinophilia following repeated exposure to Aspergillus fumigatus conidia. Infection and immunity. 2012;80:1424–1436. doi: 10.1128/IAI.05529-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zelante T, et al. Th17 cells in the setting of Aspergillus infection and pathology. Med Mycol. 2009;47(Suppl 1):S162–169. doi: 10.1080/13693780802140766. [DOI] [PubMed] [Google Scholar]
- 14.Smith NL, Hankinson J, Simpson A, Bowyer P, Denning DW. A prominent role for the IL1 pathway and IL15 in susceptibility to chronic cavitary pulmonary aspergillosis. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014;20:O480–488. doi: 10.1111/1469-0691.12473. [DOI] [PubMed] [Google Scholar]
- 15.Fei M, et al. TNF-alpha from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5360–5365. doi: 10.1073/pnas.1015476108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romani L, Puccetti P. Immune regulation and tolerance to fungi in the lungs and skin. Chem Immunol Allergy. 2008;94:124–137. doi: 10.1159/000154957. [DOI] [PubMed] [Google Scholar]
- 17.Girtsman T, Jaffar Z, Ferrini M, Shaw P, Roberts K. Natural Foxp3(+) regulatory T cells inhibit Th2 polarization but are biased toward suppression of Th17-driven lung inflammation. Journal of leukocyte biology. 2010;88:537–546. doi: 10.1189/jlb.0110044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu W, et al. Adoptive transfer of induced-Treg cells effectively attenuates murine airway allergic inflammation. PloS one. 2012;7:e40314. doi: 10.1371/journal.pone.0040314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang M, et al. Enhanced local Foxp3 expression in lung tissue attenuates airway inflammation in a mouse model of asthma. The Journal of asthma: official journal of the Association for the Care of Asthma. 2014;51:451–458. doi: 10.3109/02770903.2014.887727. [DOI] [PubMed] [Google Scholar]
- 20.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nature reviews. Immunology. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Koenen HJ, et al. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–2352. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 23.Komatsu N, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nature medicine. 2014;20:62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 24.van de Veerdonk FL, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5:329–340. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Hsu P, et al. IL-10 Potentiates Differentiation of Human Induced Regulatory T Cells via STAT3 and Foxo1. J Immunol. 2015;195:3665–3674. doi: 10.4049/jimmunol.1402898. [DOI] [PubMed] [Google Scholar]
- 26.Gresnigt MS, Netea MG, van de Veerdonk FL. Pattern recognition receptors and their role in invasive aspergillosis. Ann N Y Acad Sci. 2012;1273:60–67. doi: 10.1111/j.1749-6632.2012.06759.x. [DOI] [PubMed] [Google Scholar]
- 27.Netea MG, et al. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol. 2004;172:3712–3718. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- 28.Sutmuller RP, et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. The Journal of clinical investigation. 2006;116:485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gresnigt MS, et al. Aspergillus fumigatus-Induced IL-22 Is Not Restricted to a Specific Th Cell Subset and Is Dependent on Complement Receptor 3. J Immunol. 2013;190:5629–5639. doi: 10.4049/jimmunol.1202601. [DOI] [PubMed] [Google Scholar]
- 30.Genovese MC, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. The New England journal of medicine. 2005;353:1114–1123. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 31.van de Veerdonk FL, et al. Th17 responses and host defense against microorganisms: an overview. BMB reports. 2009;42:776–787. doi: 10.5483/BMBRep.2009.42.12.776. [DOI] [PubMed] [Google Scholar]
- 32.Zelante T, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. European journal of immunology. 2007;37:2695–2706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 33.Balloy V, et al. Involvement of toll-like receptor 2 in experimental invasive pulmonary aspergillosis. Infection and immunity. 2005;73:5420–5425. doi: 10.1128/IAI.73.9.5420-5425.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dillon S, et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. The Journal of clinical investigation. 2006;116:916–928. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai YG, Yang KD, Niu DM, Chien JW, Lin CY. TLR2 agonists enhance CD8+ Foxp3+ regulatory T cells and suppress Th2 immune responses during allergen immunotherapy. J Immunol. 2010;184:7229–7237. doi: 10.4049/jimmunol.1000083. [DOI] [PubMed] [Google Scholar]
- 36.Yamazaki S, et al. TLR2-dependent induction of IL-10 and Foxp3+ CD25+ CD4+ regulatory T cells prevents effective anti-tumor immunity induced by Pam2 lipopeptides in vivo. PloS one. 2011;6:e18833. doi: 10.1371/journal.pone.0018833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellocchio S, et al. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunol. 2004;172:3059–3069. doi: 10.4049/jimmunol.172.5.3059. [DOI] [PubMed] [Google Scholar]
- 38.Skov M, Poulsen LK, Koch C. Increased antigen-specific Th-2 response in allergic bronchopulmonary aspergillosis (ABPA) in patients with cystic fibrosis. Pediatric pulmonology. 1999;27:74–79. doi: 10.1002/(SICI)1099-0496(199902)27:2<74::AID-PPUL2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 39.Bacher P, et al. Antigen-specific expansion of human regulatory T cells as a major tolerance mechanism against mucosal fungi. Mucosal Immunol. 2014;7:916–928. doi: 10.1038/mi.2013.107. [DOI] [PubMed] [Google Scholar]
- 40.Schulze, B. et al. CD4 FoxP3 regulatory T cells suppress fatal T helper 2 cell immunity during pulmonary fungal infection. European journal of immunology, 10.1002/eji.201444963 (2014). [DOI] [PubMed]
- 41.Alunno A, et al. Altered immunoregulation in rheumatoid arthritis: the role of regulatory T cells and proinflammatory Th17 cells and therapeutic implications. Mediators of inflammation. 2015;2015:751793. doi: 10.1155/2015/751793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Netea MG, et al. Aspergillus fumigatus evades immune recognition during germination through loss of toll-like receptor-4-mediated signal transduction. The Journal of infectious diseases. 2003;188:320–326. doi: 10.1086/376456. [DOI] [PubMed] [Google Scholar]
- 43.Micallef L, Rodgers P. eulerAPE: drawing area-proportional 3-Venn diagrams using ellipses. PloS one. 2014;9:e101717. doi: 10.1371/journal.pone.0101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.