Abstract
The essential oils of Citrus sinensis and Citrus latifolia showed antimycotic activity against Candida spp. isolated from the oral cavity; they are neither mutagenic on the Ames test nor cytotoxic. Their main components are R-(+)-limonene, β-thujene, α-myrcene and γ-terpinene. The aim of this work was to evaluate their antimutagenic and antioxidant capacities. Antimutagenic properties were evaluated against MNNG and ENNG on S. typhimurium TA100; against 2AA on strain TA98 and in front of 4NQO and NOR on strain TA102. Both were antimutagenic against MNNG (p < 0.001) but only C. latifolia was antimutagenic against ENNG (p < 0.001). Both presented antimutagenic activity against 2AA (p < 0.001). They were antioxidant against the ROS-generating compound 4NQO (p < 0.001) and the antibiotic NOR (p < 0.001). In the antioxidant evaluation, the activity in DPPH assay was in a range of 6–23% for C. sinensis and of 22–71% for C. latifolia. Both were antioxidant compared with BHT in β-carotene bleaching assay and were able to decreased apoptosis in HaCat cells stimulated with H2O2. The levels of intracellular superoxide ion were lower in the presence of both oils. In conclusion, the essential oils of C. sinensis and C. latifolia are antimutagenic against at least three types of mutagens and have antioxidants properties.
Introduction
Essential oils (EOs) of natural origin are volatile liquid fractions, generally, steam-distillable containing substances responsible for the aroma of plants. EOs are important ingredients in the cosmetic industry, in food as flavorings or condiments and in the pharmaceutical production1. In particular, the Citrus genus is a source of vitamin C, flavonoids and terpenoids that have been the subject of study due to their demonstrated beneficial properties. The whole oils and its different components have been recognized as generally safe (GRAS) by the Food and Drug Administration (FDA)2.
We previously demonstrated that the EOs of Citrus sinensis and Citrus latifolia are antimycotic against Candida albicans, Candida tropicalis, Candida guilliermondii, Candida glabrata and Candida lusitaniae strains isolated from the oral cavity of elder patients. Both EOs were not mutagenic in the Ames test and not cytotoxic to the human oral epithelium. Based on these results, we are extending the analysis on their properties by testing its antimutagenic and antioxidant activities3.
The main components of these EOs were R-(+)-limonene and α-myrcene for C. sinensis, and R-(+)-limonene, β-thujene and γ-terpinene for C. latifolia, these molecules may have further biological activities. Antonella et al. 2013 showed that the R-(+)-limonene, α-terpineol and its chemical derivative 1,8-cineol4, were able to inhibit mutagenesis induced by 2-amino-anthracene, 2-amino-fluorene and alkylating agent Methyl-metano-sulfonate on S. typhimurium TA98, TA100 and E. coli uvrA strains5. R-(+)-Limonene and β-pinene, have been described as the bioactive components of several herbs and spices used in food preparation6. The concentration and proportion of these components are variable, but several epidemiological reports indicate that their consumption may reduce gastric cancer risk6.
Thujene, contained in these EOs has shown to be a good antioxidant, able to efficiently quenching singlet oxygen7. The monoterpenes such as R-(+)-limonene, γ-terpinene and β-pinene were identified as good antioxidants by the DPPH method8.
R-(+)-Limonene and perillyl alcohol have been described as dietary monoterpenes that may inhibit post-transcriptional isoprenylation of cell growth regulatory proteins such as ras9. The use of these antioxidants and antimutagens in cancer prevention has been widely evaluated, and it has been reported that these dietary monoterpenes have chemotherapeutic activity in pancreatic, mammary and prostatic tumours10. Another example of chemotherapeutic compounds is the juice of Citrus medica, which supplementation was antimutagenic and anticarcinogenic in human astrocytoma cell lines11.
Several studies have been developed with whole essential oils of several plants. Bhalla et al. (2013) reported that antimutagenesis of EOs might be due to antioxidant quenching reaction, induction of detoxification enzymes and inhibition of CYP450 involved in activation of pre-carcinogens. The antimutagenesis of EOs involving DNA repairing mechanisms is perhaps the less studied process12. Other study had shown that some EOs might be implicated with DNA repairing systems. The EO of basil (Ocimun basilicum L.) protects Escherichia coli from ultraviolet light-induced mutagenesis, only on a DNA excision repairing proficient strain13. In fact, there are other works that had stated that DNA repairing improvement mechanisms by essential oils and extracts would have to do with the antimutagenic mechanisms14. Nonetheless, the antioxidant capability of natural compounds is perhaps the action mechanism most studied and documented in the literature due to the importance of oxidative reactions on cell damage15.
One method that is a standard to evaluate genotoxic risk is the Ames test, which is accepted by multiple organizations (IARC, EPA, FDA, IVGT, REAC, MHW-Japan) as a screening test of compounds for human consumption and use. It is based on strains harboring a series of mutations that are reverted in the presence of a mutagen; each strain used in this methodology allows the identification of mutations by frameshifting (TA98), base substitution (TA100) or damage by free radicals (TA102). Additionally, it has been used to prove the antimutagenic activity of compounds from diverse origins in front of a known mutagen, by measuring the decrease in the number of revertants (His+). It is an approach to elucidate the mechanism of action of both mutagenic and antimutagenic molecules16.
The aim of this work was to further evaluate the properties of essential oils from Citrus sinensis and Citrus latifolia such as antimutagenic and antioxidant activities.
Results
Antimutagenesis
Antimutagenic effects frequently are dependent on the mutagen and antimutagen doses used. The doses of EOs used in this work were not toxic for the strains, when evaluated alone.
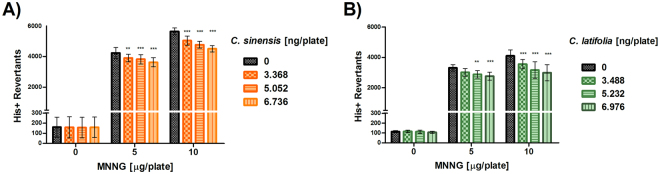
Both essential oils were able to inhibit alkylating mutations induced by MNNG. C. sinensis (Fig. 1A) and C. latifolia (Fig. 1B) were antimutagenic at all its doses, even when the strain was exposed to 10 μg of MNNG (p < 0.001). Nonetheless, the EO of C. latifolia had not activity in front of 5 µg/pl of MNNG when applied at its lowest concentration.
Figure 1.
Antimutagenesis in S. typhimurium TA100. (A) Antimutagenesis of Citrus sinensis against mutations induced by MNNG. Spontaneous reversion 161 ± 80 **p < 0.01, ***p < 0.001. (B) Antimutagenesis of Citrus latifolia against mutations induced by MNNG. Spontaneous reversion: 114 ± 10, **p < 0.01, ***p < 0.001. Each point represents the mean of 15 replicas on five independent studies.
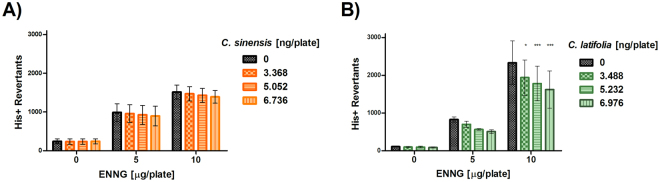
For the ethylating agent ENNG, there was not a significant reduction in the number of His+ revertants at neither C. sinensis doses used in front of 5 and 10 µg/plate of this alkylating agent (Fig. 2A). A gradual reduction in the number of His+ revertants was observed with increasing amounts of C. latifolia against 10 µg/plate of ENNG (p < 0.05 for 3.488 ng/pl; p < 0.001 for 5.232 and 6.976 ng/pl). However, when the TA100 strain was exposed to 5 µg/pl of ENNG there was not a difference with and without the EO of C. latifolia (Fig. 2B).
Figure 2.
Antimutagenesis in S. typhimurium TA100. (A) Antimutagenesis of Citrus sinensis against mutation induced by ENNG. Spontaneous reversion 239 ± 10. (B) Antimutagenesis of Citrus latifolia, against mutation induced by ENNG. Spontaneous reversion: 239 ± 10. Each point represents the mean of 15 replica on five independent studies, *p < 0.05, ***p < 0.001.
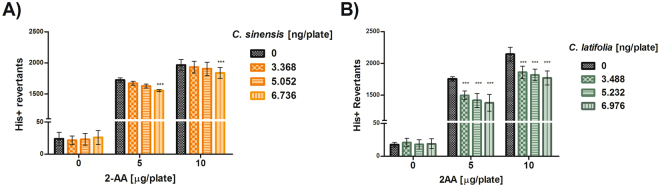
Antimutagenesis against 2AA was significant only at the highest concentration of C. sinensis against both doses of this pre-mutagen (p < 0.001) (Fig. 3A). In contrast, C. latifolia presented an evident antimutagenic activity at all its concentration (p < 0.001) and with both concentrations of 2AA (Fig. 3B).
Figure 3.
Antimutagenesis in S. typhimurium TA98. (A) Antimutagenesis of Citrus sinensis against mutation induced by 2AA. Spontaneous reversion 16 ± 3. ***p < 0.001. (B) Antimutagenesis of Citrus latifolia. Spontaneous reversion 17 ± 5, ***p < 0.001. Each point represents the mean of 15 replicas on five independent studies.
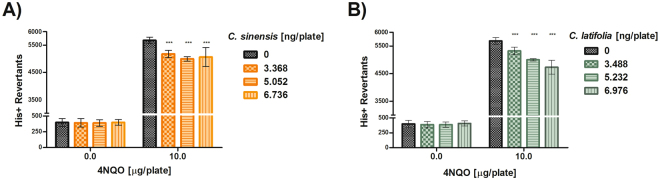
Mutations induced by 4NQO were reduced with both EOs (Fig. 4). C. sinensis (Fig. 4A) or C. latifolia (Fig. 4B) had a statistically significant antimutagenic effect at every proved concentration (p < 0.001).
Figure 4.
Antimutagenesis in S. typhimurium TA102. (A) Antimutagenesis of Citrus sinensis, against 4-NQO. Spontaneous reversion 266 ± 30 ***p < 0.001. (B) Antimutagenesis of Citrus latifolia, against 4NQO. Spontaneous reversion 266 ± 30 ***p < 0.001. Each point represents the mean of 9 replicas on three independent studies.
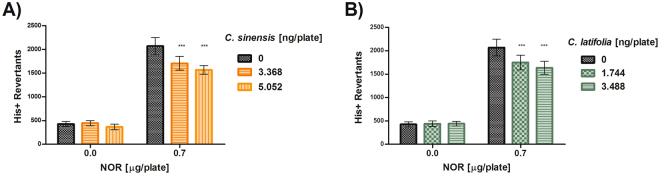
The antioxidant effect of these essential oils was also evaluated against NOR (Fig. 5A and B). Both EOs shown a dose-dependent and statistically significant reduction of mutations induced by this ROS-generating antibiotic.
Figure 5.
Antimutagenesis in S. typhimurium TA102. (A) Antimutagenesis of Citrus sinensis, against NOR. Spontaneous reversion 412 ± 23 ***p < 0.001. (B) Antimutagenesis of Citrus latifolia, with NOR. Spontaneous reversion 412 ± 23 ***p < 0.001. Each point represents the mean of 9 replicas on three independent studies.
Antioxidation
DPPH free radical scavenging
To ratify whether the results on the TA102 strain were related to the antioxidant activity of these EOs, we performed a DPPH assay compared with EGCG, a flavonoid with reported antioxidant activity from green tea. The Figure 6 shows the percentage of the antioxidation of the EGCG (38–88%), C. latifolia (22–71%) and C. sinensis (6–23%). It could be observed that the EO of C. latifolia had a better activity than the EO of C. sinensis, although both were lower than EGCG.
Figure 6.
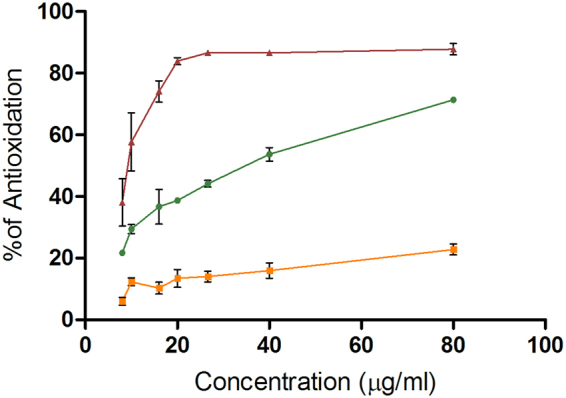
Graphic of the % of antioxidation versus concentration of  EGCG,
EGCG,  C. sinensis and
C. sinensis and  C. latifolia.
C. latifolia.
Bleaching of β-carotene in linoleic acid system
The antioxidant activity of the essential oils of Citrus sinesis and Citrus latifolia by bleaching of β-carotene with the linoleic acid is shown in Fig. 7. The more effective an antioxidant the slower the depletion of color. The increase in the absorbance of β-carotene indicates less oxidation by linoleic acid. The control BHT showed a lower exhaustion of color than the essential oils. Based on this, the results were BHT > C. latifolia > C. sinensis.
Figure 7.
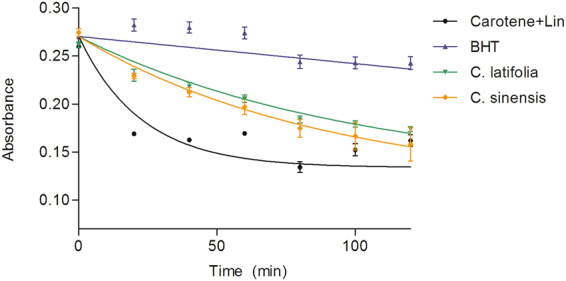
Effect of the EOs in the Inhibition of β-carotene bleaching.  Control (25 µL Linoleic acid + 0.8 mg β-carotene),
Control (25 µL Linoleic acid + 0.8 mg β-carotene),  BHT (400 mg),
BHT (400 mg),  C. sinensis (400 mg) and
C. sinensis (400 mg) and C. latifolia (400 mg). Each point is the mean ± S.D. of three biological experiments performed by triplicate.
C. latifolia (400 mg). Each point is the mean ± S.D. of three biological experiments performed by triplicate.
Detection of apoptosis using flow cytometry
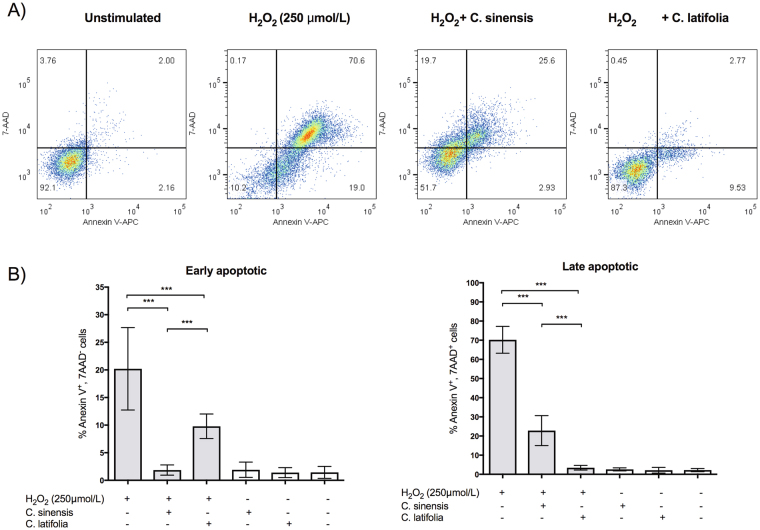
To demonstrate the antioxidant activity of the EOs in vivo, the number of cells in early and late apoptosis was measured by flow cytometry using Annexin V staining and 7-ADD as cell membrane integrity exclusion dye. Figure 8A shows representative density plots where C. latifolia maintains the cell integrity in presence of H2O2 in a higher proportion (87.3% of survival) than C. sinensis (51.7% of survival), compared with damage cells (10.2% of survival). Figure 8B illustrate the quantitation of early and late apoptosis with and without the EOs. C. sinensis reduces the late apoptosis from 70.2% to 22.8% and C. latifolia from 70.2% to 3.4%. Meanwhile the early apoptosis was reduced from 20.2% to 1.84% and 9.79%, respectively.
Figure 8.
Cell apoptosis measured by flow cytometry. (A) Representative density plots in the presence and absence of the EOs. (B) Quantization of early and late apoptosis as % Annexin V-7AAD positive cells. Results are presented as mean ± S.D. ***p < 0.0001.
Measurement of intracellular reactive oxygen species
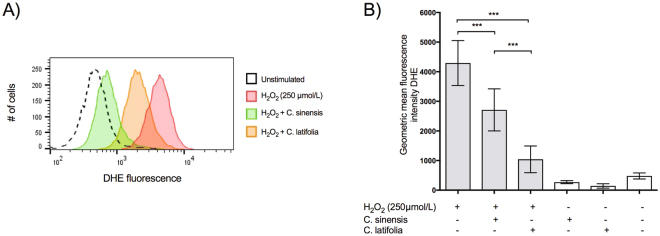
To test the formation of ROS inside the cells, an analysis was performed by flow cytometry. Dihydroethidium (DHE) was used to detect intracellular •O2−. Given its ability to freely permeate cell membranes, DHE has extensively been used to monitor •O2− production. Upon reaction with •O2−, DHE is rapidly oxidized to form ethidium, a red fluorescent product that intercalates DNA and amplifies the red fluorescence signal. Figure 9A shows the decrease in the signal of DHE within HaCaT cells in the presence of the EOs of C. sinensis (orange curve) and C. latifolia (green curve), compared with cultures stimulated only with H2O2 (red curve). Cells treated with the EOs were significant different from untreated cells (9B, p < 0.0001).
Figure 9.
Inhibition of intracellular superoxide formation. (A) Histogram of the ethidium fluorescence with and without EOs. (B) Quantization of the mean intensity in treated and untreated cells. ***p < 0.0001.
Discussion
Essential oils are a mixture of volatile compounds, generally classified in two major biosynthetic groups: terpenes/terpenoids and aromatic/aliphatic molecules17. In a precedent work, we found that EOs from C. sinensis and C. latifolia are antimycotic and besides are neither mutagenic nor cytotoxic to human oral epithelium3. Added up to these qualities, in here we demonstrated that these EOs also have antimutagenic properties. The use of antimutagens is a good alternative to reduce genotoxic risk to the exposure to xenobiotic compounds, and to therapeutic drugs18. These studies are worthwhile as they may offer an alternative for the prevention of genetic damage and even more to reduce carcinogenic risks19.
The reduction of mutagenesis induced by methyl or ethyl N-nitroso guanidines using the essential salmonella battery of the Ames test, suggests that these essential oils might have an antimutagenic effect against alkylating agents. The Citrus genus has previously been studied that reduce DNA damage by alkylating mutagens like methyl-methane-sulfonate (MMS), cyclophosphamide (CP) and ifosfamide. Orange juice prevented and repaired the damage induced by MMS and CP in comet assay and the grapefruit juice reduced the micronucleus formation and sister chromatid exchanges in vivo 20–22. The antimutagenic properties of the EOs of C. sinensis and C. latifolia observed in the present work are in accordance with the aforementioned studies on the antimutagenic effect against alkylating agents.
Our results demonstrated a slight reduction of the mutagenic effect of the pre-mutagen 2AA, which requires to be activated mainly by CYP1A1 (present on the S9 mix used in here) to be metabolized into a mutagenic product23. C. sinensis was only anti-mutagenic at a dose of 6.736 ng/plate with exposures of 5 and 10 μg/plate of 2AA. The EO of C. latifolia prevented the mutagenic transformation of 2AA at all concentrations. This result is in accordance with previous reports founding that grapefruit juice, which contains organic components like bergamottin, is able to reduce mutagenesis of pre-mutagen genotoxic compounds such as benzo(a)pyrene in the Ames test24. The consumption of C. sinensis and C. latifolia may be very useful as protection against the mutagenic effect of pre-mutagens polycyclic aromatic compounds. Nevertheless, antimutagens that are able to inhibit the cytochrome activity should be taken carefully. It has been discussed that they may also inhibit other members necessary to metabolize clinical drugs, and interfere with its therapeutic metabolism25, 26. Further studies in the application of these antimutagens should be advisable.
The main components of the tested citrus EOs are terpenes/terpenoids, as we previously analyzed by GC-MS3, which have been attributed with antioxidant properties. In fact, there are several references reporting antioxidant properties of medical plants. For instance, Bouchekrit et al. (2016) found that the EO of Elaeoselinum asclepium has a 43.9% of α-pinene and has not only antibacterial but antioxidant properties27. Castella texana, an antiamoebic plant, also presented antioxidant properties through a reduction in the genotoxic DNA damage induced by ROS, generated by fluoroquinolone NOR28. In regard to the citrus genus, Loizzo et al.8, reported antioxidant effects of the essential oil of Citrus x limon cv Femminello comune, which contains limonene, γ-terpinene and β-pinene as its major components.
In this work we demonstrated the antioxidant properties of our essential oils, although the plotted curves where lower than the reference compound (EGCG, 38–87%). It is worth mentioning that EGCG is a flavonoid from green tea considered as a potent antioxidant29 hence the effect of C. sinensis and C. latifolia (6–23% and 22–71%, respectively) is not despicable, especially for the latter. This effect has been studied more frequently in C. sinensis than C. latifolia although some were performed using oils or extracts obtained in different conditions30–32.
These results were corroborated using a lipophilic method (β-carotene bleaching) which proved that the essential oil of C. latifolia is more potent antioxidant than C. sinensis suggesting that the effect depends on the composition of each oil. C. sinensis is essentially R-(+)-limonene (96%), although the EO of C. latifolia has a lower concentration of this terpene, its second most abundant component, β-thujene (14.85%), absent in C. sinensis, might be contributing to the observed antioxidant activity. On the other hand, the annexin V/7AAD assays showed that the EO of C. sinensis decreased the early apoptosis while C. latifolia reduced the late apoptosis. In fact the essential oil of C. latifolia is able to prevent the total apoptosis in a higher percentage (85% of viable cells) than C. sinensis, which has not been previously described.
Other works had measured the antioxidant activity of some components of the Citrus genus (limonene and others) and had demonstrated that are able to protect epithelial cells from oxidative stress, which partially explains the effect of our EOs.
The measurement of the intracellular superoxide ion corroborated that the EOs act like free radical scavengers since the fluorescence intensity is lower when added to the cells, which could explains its effect in apoptosis.
All together, these results suggest that there is a possible contribution (synergic or additive) of the components present in C. latifolia with the R-(+)-limonene in the antioxidant activity of this essential oil.
Antioxidation is perhaps the most frequently evaluated mechanism of action for natural occurring molecules, and it might be part of the antimutagenic capacity of our EOs, based on the observations made on TA102 strain, HaCaT cells and for the formation of intracellular superoxide.
In comparison with other species, previous studies have established an antimutagenic activity for the essential oil of Myrtus communis, a small evergreen shrub from northwestern Europe belonging to the same taxonomic division, class and subclass than Citrus genus. M. communis contains a terpenes/terpenoids composition3, 33 similar to C. sinensis and C. latifolia, suggesting that some of the shared molecules could be attributed with this observed quality. We hypothesized that the β-pinene could be also an antimutagenic component since its abundance in each oil seems to concur with their antimutagenic potency (0.358% in C. sinensis y 12.79% in C. latifolia 3). The γ-terpinene is another homologous component in M. communis that is relatively abundant (12.8%), although is present only in the essential oil of C. latifolia. Finally, α-pinene, myrcene, and α-thujene are also compounds in common, nonetheless they were found in minimal abundance in our EOs. In contrast, the abundance of R-(+)-limonene, the most concentrated element in our citrus oils, is inversely proportional to their activity, suggesting that there is a limited participation of R-(+)-limonene in the antimutagenic, antioxidant and antifungal activities3 of C. sinensis and C. latifolia. Although, more studies on the capacities of each component of these essential oils are necessary to reach a more accurate conclusion.
Conclusions
The evaluated EOs from C. sinensis and C. latifolia, presented in this work can act by several antimutagenic mechanisms; they are able to reduce alkylated DNA damages through a reduction in the expression of base-substitution mutations; also, both are antimutagenic by reducing the activation of pre-mutagens like 2AA, and, finally, against quinolones (NOR and 4NQO) as possible ROS-scavenging mixtures as proven by DPPH, β-carotene bleaching and oxidative stress assays.
Methods
Reagents
Essential Oils from C. sinensis and C. latifolia were obtained by hydro-distillation of the peel, and were kindly donated by Frutech International Corporation Cargee Additives, Montemorelos Nuevo León, México. Methyl-N ´nitro-N-nitrosoguanidine (MNNG), Ethyl-N-nitro-N-nitrosoguanidine (ENNG), 2-amino-anthracene (2AA), 4-nitro-quinone-oxide (4NQO), and norfloxacin (NOR) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Dimethyl sulfoxide (DMSO) was obtained from J.T. Baker Xalostoc, México. Aroclor-1254 was obtained from Supelco Bellefonte, PA; S9 arochlor-1254 induced rat liver homogenate (S9 mix), was prepared as described by Maron and Ames29. Diphenyl-2-picrylhydrazyl (DPPH) and the reference standards Epigallocatchin-3-gallate (EGCG) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). β-carotene was kindly donated by Dra. Rosa Martha Pérez-Gutiérrez. Chloroform and Linoleic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). RPMI-1640 was purchased from Life Technologies (TermoFisher, Waltham, MA, USA). Annexin V-Allophycocyanin (APC) and 7-amino-actinomycin D (7-AAD) Apoptosis Detection kit (Biolegend, USA) was provided by Dr. Mario Adán Moreno-Eutimio. DHE was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
HaCaT normal human epidermal keratinocyte cell line were cultured in RPMI-1640 supplemented with 10% Fetal Bovine Serum (FBS) and 1% penicillin-streptomycin (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) at 37 °C in an atmosphere containing 5% CO2.
Antimutagenesis Assays
Antimutagenesis assays were performed with the Ames test on Salmonella typhimurium 29. Briefly, overnight cultures of S. typhimurium TA98, TA100 or TA102 strains were prepared on nutrient broth Oxoid No. 2 for 16 hrs at 37°C onto a water bath (American Scientific Products BT-23). Antimutagenic activity of both EOs was evaluated against 2AA (5 or 10 μg/Petri dish) on S. typhimurium TA98; MNNG and ENNG (5 or 10 μg/Petri dish) on TA100; against 4NQO (5 or 10 μg/Petri dish) on TA102. 100 μL of the exposed tester strain, both mutagens and essential oils were poured on sterile tubes containing 2.0 mL of soft-agar at 45 °C; 1.68 to 8.2 ng /Petri dish of C. sinensis and 1.74 to 6.97 ng/Petri dish of C. latifolia were used, these concentrations were previously described as non-toxic for the Ames test1. 2AA was activated with 500 μL of S9 mix, the mixture was then poured onto Vogel-Bonner plates and incubated for 48 hrs. Antimutagenesis of essential oils against NOR was evaluated with the pre-incubation method, previously described16, 29. EOs, NOR (0.07 to 7.0 ng) and 500 μL (S9 mix) were poured onto an sterile tube along with 100 μL of the tester strain and then incubated at 37 °C for 60 min, afterwards 2.0 mL of soft agar were added into the mixture and finally poured onto Vogel-Bonner Petri dishes for a 48 hrs incubation. Blanks were the strains with 10 μL of DMSO, and negative controls were all EOs doses without mutagen. Positive controls were the appropriate mutagen without the EOs12.
Antioxidant activity
DPPH free radical-scavenging assay
Antioxidant activity of the EOs was determined through the production of DPPH radicals. Briefly, reaction mixtures of each oil were prepared with 200 µM DPPH in ethanol in a final volume of 180 µL. Serial dilutions of the EOs of C. sinensis or C.latifolia (80, 40, 26.66, 20, 16, 10 and 8 µg/mL) were measured. Epigallocatchin-3-gallate (EGCG) was used as a reference antioxidant at the same concentration that the EOs. After incubation at room temperature for 30 min, the absorbance was acquired at 517 nm using a microplate reader (Eon™Microplate Spectrophotometer, bioTek, VT, USA). The results were calculated as inhibition percentage of DPPH radical formation (% of antioxidation).
Bleaching of β-carotene in linoleic acid system
The antioxidant activity of the essential oils of C. latifolia and C. sinensis was also evaluated by β-carotene/linoleic acid bleaching following the recommendations of Prieto et al.34 and as previously reported in Koleva et al.35. Briefly, a stock was prepared of an emulsion of β-carotene-linoleic acid by dissolving β-carotene at 0.8 mg/ml in chloroform, 25 μl of linoleic acid and 200 mg Tween 40. The chloroform was removed under vacuum on a centrifuge (V-AL; vacofuge plus; eppendorf) at 200 x g. The content of each tube was emulsified in 50 ml of mili-Q water saturated with oxygen (30 min, 18.2 mΩ) with vigorous stirring. 1.0 ml of this reaction was mixed with 200 μl of the essential oil (400 mg) or standard (BHT, 400 mg) dilutions and 300 μl were dispensed by triplicate in a microplate for analysis. The absorbance was immediately (t = 0) measured at 470 nm in a Eon™Microplate Spectrophotometer (bioTek, VT, USA) against a blank, consisting of an emulsion only with β-carotene. Afterwards, the emulsion was incubated for 120 min at 45 °C, and the absorbance was recorded every twenty minutes.
Detection of apoptosis using flow cytometry
HaCaT cells (2.5 × 105) were seeded in 24-well plates and treated with 1 µL of the EO of C. sinensis (0.842 µg) or C. latifolia (0.872 µg) with 250 µM of H2O2 for 24 h; afterwards, cultures were dissociated with 0.1% trypsin/EDTA in PBS 1X. The cells were then washed in PBS 1X and resuspended in binding buffer, and the apoptotic cell death rate was examined using Annexin V-APC and 7AAD double staining (incubation with 5 µl Annexin V-APC and 10 µl 7AAD for 15 min in the dark), according to the manufacturer’s protocol. Subsequently, the cells stained with Annexin V-APC/7-AAD were detected using a BD Accury C6 flow cytometer (BD Biosciences, CA, USA). Data acquisition and analyses were performed using Flowjo v10.3 software. Three independent biological replicates were performed by triplicate.
Measurement of intracellular reactive oxygen species
DHE (150 mM) and 1 µL of the EOs of C. sinensis (0.842 µg) or C. latifolia (0.872 µg) was added to HaCaT cell (2.5 × 105 in 24-well plates), which were then incubated at 37 °C for 30 min in the dark, to determine the intracellular •O2− concentrations. Samples were treated with 250 µM H2O2 to create oxidative stress. Cells were then washed, resuspended in PBS 1X, and maintained on ice for immediate detection by flow cytometry (BD Accury C6, CA, USA). Data were analyzed using the Flowjo v10.3 software. For fluorescence quantification samples were acquired in triplicate, and 10,000 events were used for each measurement. Cells were excited at 488 nm, and DHE fluorescence was detected using 585/42 (DHE) bandpass filters. Data were expressed as the geometric mean fluorescence intensity (MFI).
Statistical analysis
Statistical analysis was performed using Bonferroni correction of a two-way ANOVA test using Graph-Pad Prism 5.0 software.
Acknowledgements
We thank TLC María Gabriela Aguilera Hernández for her help in preparing culture medium and reagents required for this work. Authors recognize Sandra Luz Hernández -Ojeda for her contribution to this work. We thank Dra. Rosa Martha Pérez-Gutiérrez for the donated reagents and her valuable contribution.
Author Contributions
M.A.A. and N.J.R.P. made substantial contributions to conception and design, M.M.G. performed acquisition of data, N.J.R.P. and J.S.N. contributed to the analysis and interpretation of data; J.F.E. and M.A.M.E. contributed to the experimental design for the antioxiodiant activity determination and performance of figures 6–8. J.E.A. and J.D.T.G. participated in drafting the article and revising it critically for important intellectual content and technical writing. M.G.A. participated in the obtaining of the essential oils. All authors gave final approval of the version to be submitted and any revised version.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Proteggente AR, Saija A, De Pasquale A, Rice-Evans CA. The compositional characterisation and antioxidant activity of fresh juices from sicilian sweet orange (Citrus sinensis L. Osbeck) varieties. Free Radical Res. 2003;37:681–687. doi: 10.1080/1071576031000083198. [DOI] [PubMed] [Google Scholar]
- 2.Espina L, et al. Chemical composition of commercial citrus fruit essential oils and evaluation of their antimicrobial activity acting alone or in combined processes. Food control. 2011;22:896–902. doi: 10.1016/j.foodcont.2010.11.021. [DOI] [Google Scholar]
- 3.Ruiz-Perez NJ, et al. Antimycotic Activity and Genotoxic Evaluation of Citrus sinensis and Citrus latifolia Essential Oils. Sci Rep. 2016;6:25371. doi: 10.1038/srep25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farina L, Boido E, Carrau F, Versini G, Dellacassa E. Terpene compounds as possible precursors of 1,8-cineole in red grapes and wines. J Agric Food Chem. 2005;53:1633–1636. doi: 10.1021/jf040332d. [DOI] [PubMed] [Google Scholar]
- 5.Antonella DS, Federico D, Grazia SM, Gabriela M. Antimutagenic and antioxidant activities of some bioflavours from wine. Food Chem Toxicol. 2013;60:141–146. doi: 10.1016/j.fct.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 6.Kaefer CM, Milner JA. The role of herbs and spices in cancer prevention. J Nutr Biochem. 2008;19:347–361. doi: 10.1016/j.jnutbio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouamri ME, Gorrichon J, Braun A, Oliveros E. The reactivity of citronellol and α-thujene with singlet oxygen. Rate constants of chemical reaction and physical quenching. Photochem Photobiol. 1991;54:619–623. [Google Scholar]
- 8.Loizzo MR, et al. Chemical profile and antioxidant properties of extracts and essential oils from Citrus × limon (L.) burm. Cv. Femminello comune. Chemistry & biodiversity. 2016;13:571–581. doi: 10.1002/cbdv.201500186. [DOI] [PubMed] [Google Scholar]
- 9.Crowell PL, Siar Ayoubi A, Burke YD. Antitumorigenic effects of limonene and perillyl alcohol against pancreatic and breast cancer. Adv Exp Med Biol. 1996;401:131–136. doi: 10.1007/978-1-4613-0399-2_10. [DOI] [PubMed] [Google Scholar]
- 10.Crowell PL. Prevention and therapy of cancer by dietary monoterpenes. The J Nutr. 1999;129:775S–778S. doi: 10.1093/jn/129.3.775S. [DOI] [PubMed] [Google Scholar]
- 11.Entezari M, et al. Antimutagenicity and anticancer effects of Citrus medica fruit juice. Acta Med Iran. 2009;47:373–377. [Google Scholar]
- 12.Bhalla Y, Gupta VK, Jaitak V. Anticancer activity of essential oils: a review. J Sci Food Agric. 2013;93:3643–3653. doi: 10.1002/jsfa.6267. [DOI] [PubMed] [Google Scholar]
- 13.Stanojević J, et al. The effect of essential oil of basil (Ocimum basilicum L.) on UV-induced mutagenesis in Escherichia coli and Saccharomyces cerevisiae. Arch Biol Sci. 2008;60:93–102. doi: 10.2298/ABS0801093S. [DOI] [Google Scholar]
- 14.Llana-Ruiz-Cabello M, et al. In vitro toxicological evaluation of essential oils and their main compounds used in active food packaging: a review. Food Chem Toxicol. 2015;81:9–27. doi: 10.1016/j.fct.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Teixeira B, et al. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind Crop Prod. 2013;43:587–595. doi: 10.1016/j.indcrop.2012.07.069. [DOI] [Google Scholar]
- 16.Arriaga-Alba M, Montero-Montoya R, Aguirre-Espinoza JJ. The Ames test in twenty-first century. Res Rev. 2012;2:23–37. [Google Scholar]
- 17.Bassole IH, Juliani HR. Essential oils in combination and their antimicrobial properties. Molecules. 2012;17:3989–4006. doi: 10.3390/molecules17043989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arriaga-Alba M, et al. Antimutagenesis of beta-carotene to mutations induced by quinolone on Salmonella typhimurium. Arch Med Res. 2000;31:156–161. doi: 10.1016/S0188-4409(00)00046-1. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad MS, Ahmad S, Ali A, Afzal M. Anticarcinogenic and antimutagenic activity of Alstonia scholaris on the albino mice bone marrow cells and peripheral human lymphocyte culture against methyl methane sulfonate induced genotoxicity. Adv Biomed Res. 2016;5:92. doi: 10.4103/2277-9175.183140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarez-Gonzalez I, Madrigal-Bujaidar E, Sanchez-Garcia VY. Inhibitory effect of grapefruit juice on the genotoxic damage induced by ifosfamide in mouse. Plant Foods Hum Nutr. 2010;65:369–373. doi: 10.1007/s11130-010-0193-1. [DOI] [PubMed] [Google Scholar]
- 21.Franke SI, Pra D, Erdtmann B, Henriques JA, da Silva J. Influence of orange juice over the genotoxicity induced by alkylating agents: an in vivo analysis. Mutagenesis. 2005;20:279–283. doi: 10.1093/mutage/gei034. [DOI] [PubMed] [Google Scholar]
- 22.Al–Taai EMF. Protective Effects of Sweet Orange Peel (Citrus Sinensis L.) The Induction of Micronuclei Induced by Cyclophosphamide in Human Peripheral Lymphocytes. J Food Technol Res. 2016;3:28–35. doi: 10.18488/journal.58/2016.3.1/58.1.28.35. [DOI] [Google Scholar]
- 23.Pan, S. T. et al. Computational Identification of the Paralogs and Orthologs of Human Cytochrome P450 Superfamily and the Implication in Drug Discovery. Int J Mol Sci17 (2016). [DOI] [PMC free article] [PubMed]
- 24.Olguín-Reyes S, Camacho-Carranza R, Hernández-Ojeda S, Elinos-Baez M, Espinosa-Aguirre J. Bergamottin is a competitive inhibitor of CYP1A1 and is antimutagenic in the Ames test. Food Chem Toxicol. 2012;50:3094–3099. doi: 10.1016/j.fct.2012.05.058. [DOI] [PubMed] [Google Scholar]
- 25.Owira PM, Ojewole JA. The grapefruit: an old wine in a new glass? Metabolic and cardiovascular perspectives. Cardiovasc J Afr. 2010;21:280–285. doi: 10.5830/CVJA-2010-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White, C. Inhibitory effect of selected herbal supplements on CYP450-mediated metabolism: an in vitro approach, Stellenbosch: Stellenbosch University, (2016).
- 27.Bouchekrit M, et al. Essential oils from Elaeoselinum asclepium: Chemical composition, antimicrobial and antioxidant properties. Asian Pac J Trop Biomed. 2016;6:851–857. doi: 10.1016/j.apjtb.2016.07.014. [DOI] [Google Scholar]
- 28.Reyes-Lopez M, et al. The amoebicidal aqueous extract from Castela texana possesses antigenotoxic and antimutagenic properties. Toxicol In Vitro. 2005;19:91–97. doi: 10.1016/j.tiv.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82:1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ubando-Rivera J, Navarro-Ocaña A, Valdivia-López M. Mexican lime peel: Comparative study on contents of dietary fibre and associated antioxidant activity. Food Chem. 2005;119:851–858. [Google Scholar]
- 31.Singh P, et al. Chemical profile, antifungal, antiaflatoxigenic and antioxidant activity of Citrus maxima Burm. and Citrus sinensis (L.) Osbeck essential oils and their cyclic monoterpene, dl-limonene. Food Chem Toxicol. 2010;48:1734–1740. doi: 10.1016/j.fct.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Casquete R, et al. Evaluation of the effect of high pressure on total phenolic content, antioxidant and antimicrobial activity of citrus peels. Innovative Food Science & Emerging Technologies. 2015;31:37–44. doi: 10.1016/j.ifset.2015.07.005. [DOI] [Google Scholar]
- 33.Mimica-Dukic N, et al. Essential oil of Myrtus communis L. as a potential antioxidant and antimutagenic agents. Molecules. 2010;15:2759–2770. doi: 10.3390/molecules15042759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prieto MA, Rodriguez-Amado I, Vazquez JA, Murado M. A. beta-Carotene assay revisited. application to characterize and quantify antioxidant and prooxidant activities in a microplate. J Agric Food Chem. 2012;60:8983–8993. doi: 10.1021/jf302218g. [DOI] [PubMed] [Google Scholar]
- 35.Koleva I, van Beek TA, Linssen JP, de Groot A, Evstatieva LN. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal. 2002;13:8–17. doi: 10.1002/pca.611. [DOI] [PubMed] [Google Scholar]