Abstract
Protein phosphatase 2A (PP2A), a tumor suppressor protein, has been implicated in cell cycle and apoptosis. Additionally, studies have illustrated its crucial roles in transformation of normal human cells to tumorigenic status. PPP2CA, which encodes the alpha isoform of the catalytic subunit of PP2A, has been recently reported to be associated with several types of cancers. Therefore, we hypothesized that genetic variants in PPP2CA might influence susceptibility of gastric cancer. To test this hypothesis, three tagging single nucleotide polymorphisms (SNPs) in PPP2CA were genotyped in a case-control study including 1,113 cases and 1,848 controls in a Chinese population. Three tagging SNPs in PPP2CA were genotyped using Illumina Human Exome BeadChip. We observed that the A allele of rs13187105 was associated with an increased risk of gastric cancer (adjusted odds ratio (OR) = 1.14, 95% confidence interval (CI): 1.02–1.28, P = 0.017). Further analyses showed that rs13187105 [A] was associated with decreased expression of PPP2CA mRNA (P = 5.1 × 10−6), and PPP2CA mRNA was significantly lower in gastric tumor tissues when comparing that in their adjacent normal tissues (P = 0.037). These findings support our hypothesis that genetic variants in PPP2CA may be implicated in gastric cancer susceptibility in Chinese population.
Introduction
Gastric cancer is a major public health problem around the world, which led to 951,000 incident cases and 721,000 deaths worldwide in 20121. In China, the problem is more serious, with estimates of 679,100 new cases and 498,000 deaths in 20152. Various risk factors are involved in gastric carcinogenesis, for instance, Helicobacter pylori infection, nitrites consumption and processed meat intake have been associated with risk of gastric cancer3–8. Besides these environmental and life style factors, host genetic factors may also play vital roles in the process of gastric cancer development, so that some individuals are prone to develop gastric cancer than the others9. Recently, genome-wide association studies (GWASs) have shown huge advantages in uncovering germline common variants associated with malignant tumors. Multiple susceptibility loci of gastric cancer, such as 1q22, 5p13.1, 3q13.31 and 10q23, have been identified in previous GWASs10–12. However, the findings of GWASs could only account for a fringe of genetic predisposition of gastric cancer because of the strict selection criteria and the limited number of single nucleotide polymorphisms (SNPs) in the arrays. Therefore, candidate gene strategy is still an important method to identify susceptibility loci in vital genes involved in carcinogenesis13.
Protein phosphatase 2A (PP2A), a heterotrimeric holoenzyme complex, is one of the major serine/threonine phosphatases and participates in a large proportion of phosphatase activity in eukaryotic cells14–16. It has been demonstrated in previous studies that the activation of telomerase and Ras, along with the inactivation of tumor suppressor proteins p53 and retinoblastoma protein are sufficient to immortalize the majority of human cells. However, these immortalized cells can hardly transform to tumorigenic status without inhibiting PP2A activity17–20. The PP2A core enzyme, which consists of a 36 kDa C catalytic subunit and a 65 kDa A scaffold subunit, can combine with a regulatory B subunit. There are various kinds of B subunits and different B subunits play different cellular function21. The C catalytic subunit of PP2A has two subtypes, α and β, and the α isoform of the catalytic subunit (PPP2Cα) is encoded by the gene PPP2CA. Previous studies have demonstrated that PPP2Cα plays important roles in regulating PP2A activity through mediating the selection of PP2A regulatory subunits21–23. PPP2CA has been reported to be associated with several types of cancer, including prostate cancer, non-small cell lung cancer and acute myeloid leukemia24–26. Bhardwaj A et al. observed the attenuate of migration and invasion potential of prostate cancer cells after they overexpressed PPP2CA 27. Moreover, they found that overexpression of PPP2CA could inhibit prostate tumor growth and metastasis in an orthotopic mouse model27.
However, studies of the association between genetic variants in PPP2CA and risk of gastric cancer are lacking. In the present study, we conducted a case-control study of 1,113 patients with gastric cancer and 1,848 cancer-free controls in a Chinese population to evaluate the associations of three tagging SNPs in PPP2CA with risk of gastric cancer. Furthermore, we used public databases to compare mRNA level of PPP2CA in gastric tumor tissues with that in adjacent normal tissues, and to explore potential function of the SNPs on gastric cancer development.
Results
The characteristics of patients with gastric cancer and cancer-free controls included in the present study were summarized in Supplementary Table S1. Age, sex and drinking status were comparable between the cases and the controls. However, there were more smokers in the control group than in cases (52.07% vs 48.71%). In addition, the results of multivariate logistic regression analysis for age, sex, smoking and drinking status were also shown in Supplementary Table S1. The detailed information of the 3 tag SNPs genotyped was listed in Supplementary Table S2. The call rates for all three variants were 100%. Besides, they were all in Hardy-Weinberg equilibrium (HWE) among controls (P = 0.637 for rs13187105, P = 0.769 for rs2292283 and P = 0.819 for rs254057).
The genotype distributions of the 3 tag SNPs and their associations with risk of gastric cancer were summarized in Table 1. Logistic regression analyses showed that the A allele of rs13187105 was significantly associated with an increased risk of gastric cancer under the additive model (adjusted odds ratio (OR) = 1.14, 95% confidence interval (CI): 1.02–1.28, P = 0.017). However, no statistically significant association was observed for rs2292283 and rs254057 under the additive models. To further explore the association between rs13187105 and risk of gastric cancer, we performed stratified analyses by age, sex, smoking status, drinking status and tumor site. As shown in Table 2, the association between rs13187105 and gastric cancer remained significant in the subgroups of participants ≥60 years old, men and smokers. Significant heterogeneity was observed between smokers and non-smokers (P for heterogeneity = 0.040). The minor allele [A] of rs13187105 was associated with an increased risk of gastric cancer among smokers (adjusted per-allele OR = 1.29, 95% CI: 1.09–1.51, P = 0.002), but no significant association was observed in non-smokers (adjusted per-allele OR = 1.01, 95% CI: 0.86–1.19, P = 0.873).
Table 1.
Genotype frequencies of the 3 tag SNPs in PPP2CA and their associations with gastric cancer risk.
| genotypes | Cases(n = 1,113) | Controls(n = 1,848) | OR(95% CI)a | P valuea | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| rs13187105 | ||||||
| CC | 324 | 29.11 | 572 | 30.95 | 1.00 | |
| CA | 515 | 46.27 | 922 | 49.89 | 0.97(0.81–1.16) | 0.708 |
| AA | 274 | 24.62 | 354 | 19.16 | 1.34(1.08–1.67) | 0.008 |
| Additive model | 1.14(1.02–1.28) | 0.017 | ||||
| rs2292283 | ||||||
| GG | 399 | 35.85 | 692 | 37.45 | 1.00 | |
| GA | 501 | 45.01 | 883 | 47.78 | 0.95(0.80–1.13) | 0.599 |
| AA | 213 | 19.14 | 273 | 14.77 | 1.30(1.04–1.64) | 0.024 |
| Additive model | 1.10(0.99–1.23) | 0.080 | ||||
| rs254057 | ||||||
| GG | 981 | 88.14 | 1653 | 89.45 | 1.00 | |
| GA | 127 | 11.41 | 189 | 10.23 | 1.20(0.93–1.54) | 0.158 |
| AA | 5 | 0.45 | 6 | 0.32 | 1.38(0.40–4.77) | 0.607 |
| Additive model | 1.20(0.95–1.51) | 0.136 | ||||
aAdjusted for age, sex, smoking status, drinking status and the top ten principal components.
Table 2.
Stratified analyses of associations between rs13187105 and gastric cancer risk.
| Variables | Case | Control | OR(95% CI)a | P a | P b |
|---|---|---|---|---|---|
| CC/CA/AA | CC/CA/AA | ||||
| Age (years) | |||||
| <60 | 139/226/101 | 250/416/161 | 1.07(0.90–1.27) | 0.441 | 0.354 |
| ≥60 | 185/289/173 | 322/506/193 | 1.19(1.03–1.38) | 0.021 | |
| Sex | |||||
| Male | 246/387/206 | 424/673/246 | 1.17(1.03–1.34) | 0.016 | 0.378 |
| Female | 78/128/68 | 148/249/108 | 1.04(0.83–1.31) | 0.717 | |
| Smoking status | |||||
| Never | 176/267/135 | 255/439/180 | 1.01(0.86–1.19) | 0.873 | 0.040 |
| Ever | 147/248/139 | 317/483/174 | 1.29(1.09–1.51) | 0.002 | |
| Drinking status | |||||
| Never | 191/314/158 | 344/551/220 | 1.12(0.97–1.29) | 0.125 | 0.752 |
| Ever | 133/201/116 | 228/371/134 | 1.16(0.97–1.39) | 0.100 | |
| Tumor site | |||||
| Cardia | 163/243/140 | 572/922/354 | 1.15(1.00–1.32) | 0.056 | 0.911 |
| Non-cardia | 161/272/134 | 572/922/354 | 1.14(0.99–1.30) | 0.074 | |
aDerived from additive models using logistic regression analyses with adjustments for age, sex, smoking status, drinking status and the top ten principal components. b P for heterogeneity test based on χ2-based Q test.
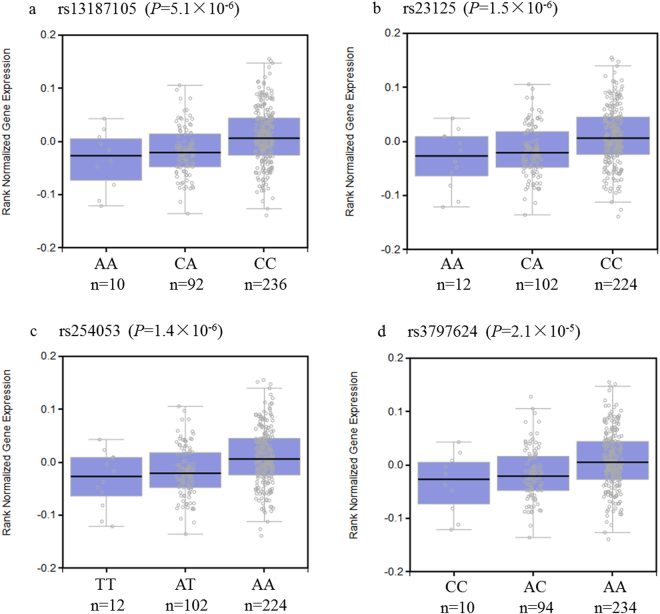
Based on Genotype-Tissue Expression project (GTEx) portal, we compared the expression of PPP2CA among individuals with different genotypes and found that the risk allele of rs13187105 was associated with reduced expression of PPP2CA (P = 5.1 × 10−6) in peripheral blood samples (Fig. 1a)28. Moreover, using The Cancer Genome Atlas (TCGA) database, we found that the mRNA level of PPP2CA was decreased in gastric cancer tissues compared to the adjacent normal tissues (P = 0.037) (Fig. 2)29. These findings suggested that PPP2CA is a potential suppressor gene of gastric cancer, and its expression might be regulated by rs13187105 or some other SNPs in strong linkage disequilibrium (LD) with rs13187105. Therefore, using HaploReg v4.1 database, we subsequently analyzed 81 SNPs in strong LD (r2 > 0.8) with rs13187105 to explore the potential causal SNPs with real biological function. We found that 12 SNPs are located in the promoter region, and 5 of them (rs254051, rs2284317, rs23125, rs254053, rs3797624) are predicted to be bound with proteins (Supplementary Table S3). We also annotated these SNPs based on UCSC genome browser, and the findings were shown in Supplementary Fig. S1. Briefly, according to the Encyclopedia of DNA Elements (ENCODE) data, 3 SNPs (rs23125, rs254053 and rs3797624) are located in the peak of H3K4me3 chromatin modification (7 cell lines) and the region is also enriched with DNase Hypersensitivity clusters and transcription factor ChIP-seq signals. Data from the Roadmap Epigenomics also reveals that these 3 SNPs are located in the peak of H3K4me3 chromatin modification and candidate active transcription start site in stomach mucosa. In addition, results from GTEx portal showed that the minor alleles of all these 3 SNPs were associated with reduced PPP2CA expression (P = 1.5 × 10−6 for rs23125, P = 1.4 × 10−6 for rs254053 and P = 2.1 × 10−5 for rs3797624) (Fig. 1b–d). These results provided evidence that the 3 potential causal SNPs (rs23125, rs254053 and rs3797624), in complete LD with rs13187105 (Supplementary Table S3), are located in the functional promoter region of PPP2CA gene and may regulate the expression of PPP2CA.
Figure 1.
Expression quantitative trait loci (eQTL) analyses of rs13187105 (a), rs23125 (b), rs254053 (c), rs3797624 (d) with PPP2CA mRNA expression levels in the whole blood samples. The results indicated that minor alleles of rs13187105, rs23125, rs254053 and rs3797624 were significantly correlated with decreased expression levels of PPP2CA. The p values were derived from linear regression model. The data was obtained from Genotype-Tissue Expression project (GTEx V6p) Portal.
Figure 2.
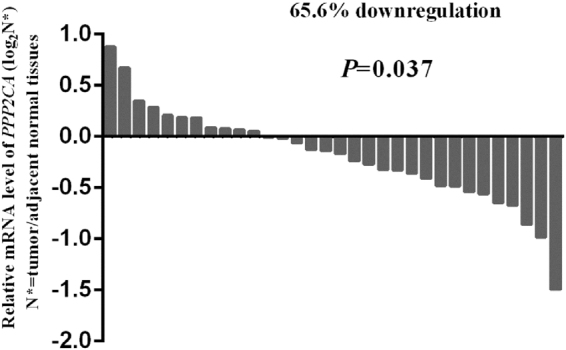
PPP2CA mRNA expression levels in 32 paired gastric tumor and adjacent normal tissues. Compared with adjacent normal controls, the expression level of PPP2CA was decreased in tumor tissues. The p value was derived from paired Student’s t-test. The data was obtained from The Cancer Genome Atlas (TCGA) database.
Discussion
In this study, we genotyped 3 tag SNPs in PPP2CA and evaluated their associations with risk of gastric cancer in a case-control study including 1,113 cases and 1,848 controls in a Chinese population. We found that rs13187105 in PPP2CA was significantly associated with an altered risk of gastric cancer.
In addition, in silico analyses showed that the minor allele [A] of rs13187105 was significantly associated with reduced PPP2CA mRNA expression in peripheral blood samples. Moreover, PPP2CA expression was lower in gastric tumor tissues compared to that in adjacent normal tissues. Further analyses indicated that 3 potential causal SNPs (rs23125, rs254053and rs3797624) in complete LD with rs13187105 are located in the functional promoter region of PPP2CA gene and may regulate the expression of PPP2CA. We also evaluated the associations between the 4 SNPs (rs13187105, rs23125, rs254053 and rs3797624) and the expression of PPP2CA in gastric mucosa samples, and the data was shown in Supplementary Fig. S2 28. Though the p values did not reach statistical significance (P < 0.05), the directions of the associations were consistent when comparing with that in whole blood samples. The possible reason is that the sample size of gastric mucosa (n = 170) is much smaller than that of whole blood (n = 338).
In Table 1, We also found that rs2292283 AA genotype was associated with the risk of gastric cancer compared with GG genotype (adjusted OR = 1.14, 95% CI: 1.04–1.64, P = 0.024). The association between rs2292283 AA genotype and risk of gastric cancer might due to the strong LD between rs2292283 and rs13187105 (r2 = 0.71).
In our study, after Bonferroni correction, the p value of rs13187105 in additive model was 0.051, which was marginal associated with risk of gastric cancer. We also used recessive model to access the association between rs13187105 and the risk of gastric cancer, and we found that the AA genotype of rs13187105 was significantly associated with an increased risk of gastric cancer compared with CC+CA genotypes (adjusted odds ratio (OR) = 1.37, 95% confidence interval (CI): 1.14–1.66, P = 0.001), and after Bonferroni correction, the p value of rs13187105 under recessive model was 0.003.
PP2A is a bona fide tumor suppressor protein which plays essential roles in the regulation of cell cycle, apoptosis, transcription and DNA repair. PP2A holoenzymes are estimated to be responsible for 30%–50% of total cellular serine/threonine dephosphorylating activity30–32. In addition, PP2A is an important protein in malignant transformation. PPP2CA encodes the alpha isoform of the catalytic subunit of PP2A, and its methylation and phosphorylation play essential roles in the selection of PP2A regulatory B subunits, which are important for PP2A activity. Previous study indicated that the activity of PP2A was downregulated after decreasing the expression level of PPP2CA 33. Therefore, it is biological plausible that decreased expression level of PPP2CA might affect PP2A activity in stomach tissues and then further influence the process of carcinogenesis.
According to our findings, the risk allele of rs13187105 was significantly associated with the reduced expression of PPP2CA, and the 3 candidate casual SNPs (rs23125, rs254053 and rs3797624) in complete LD with rs13187105 are located within the putative promoter regulatory regions. Therefore, the decreased expression of PPP2CA might be partially responsible for the observed association between rs13187105 and the risk of gastric cancer.
Our study was the first to explore the associations between genetic variants in PPP2CA and risk of gastric cancer. Some potential limitations of our study merit discussion. First, there were more smokers in the control group than in cases (52.07% vs 48.71%). The smoking status of gastric patients was collected in hospitals and some patients had changed their lifestyle since they were diagnosed with gastric cancer or they were likely to falsify their smoking history when information collection, as a result, bias might exit in our case-control study. To control the influence of bias, smoking status was adjusted when calculated the associations between SNPs and gastric cancer risk. Therefore, the bias might exert less impact to the results of the associations. Second, we did not have access to other independent study populations to confirm our findings. However, the sample size of the current study was relatively large, which at some level ensure the study quality. What’s more, the annotations of the SNPs were all based on public databases. Further functional studies are warranted to clarify the underlying biological mechanisms.
Taken together, our study found that the minor allele [A] of rs13187105 was associated with an increased risk of gastric cancer and meanwhile associated with decreased PPP2CA expression. These findings might stimulate further research investigating the roles of PPP2CA in the development of gastric cancer.
Materials and Methods
Study subjects
The details of the study participants including 1,113 gastric cancer cases and 1,848 cancer-free controls had been described previously34. Briefly, the cases were histopathologically or cytologically confirmed primary gastric carcinoma, and were recruited from the hospitals in Jiangsu province, China between January 2004 and December 2011. Those with previous history of malignancies or had undergone radiotherapy or chemotherapy were excluded. The control subjects were randomly selected from healthy individuals in a community-based screening program for chronic non-communicable diseases conducted in Jiangsu province and were frequency matched to cases by age and sex. All subjects were unrelated Chinese Han populations. After written informed consent was obtained, each participant was interviewed to collect demographic information and related risk factors of gastric cancer. After the interview, peripheral blood sample was collected from each subject. Individuals who smoked at least once per day for more than one year were classified as smokes, and those who drank more than twice a week for no less than a year were considered as drinkers. The study was approved by the institutional review board of Nanjing Medical University and all procedures were performed in accordance with relevant guidelines and regulations. Written informed consent was obtained from every participant.
Selection of tag SNPs and genotyping
The HapMap database (HapMap Data Rel 27 phase II+III, Feb09) was used to retrieve SNP information in PPP2CA (including 10kb upstream region) in the Chinese Han population (CHB). Haploview 4.2 software was used to select tag SNPs with r2 threshold of 0.80 and minor allele frequency (MAF) ≥ 0.05. Finally, 3 SNPs including rs13187105 (C > A), rs2292283 (G > A) and rs254057 (G > A) were selected for further analyses.
Genomic DNA was extracted from peripheral leukocytes using phenol-chloroform method. The tag SNPs were genotyped in all participants using Illumina Human Exome BeadChip, in which these three loci were customer-designed. Genotype calling was done using the Illumina GenomeStudio software.
Public database searching
The HapMap database (http://hapmap.ncbi.nlm.nih.gov/cgi-perl/gbrowse/hapmap27_B36/) was used to download the genotype information of PPP2CA in the CHB population. The Expectation-Maximization (RSEM) normalized read counts of PPP2CA in 32 paired gastric tumors and adjacent normal tissues were downloaded from the TCGA database (https://cancergenome.nih.gov/, accessed on April 8, 2015). The GTEx V6p Portal (http://www.gtexportal.org/home/) was used to perform the expression quantitative trait loci (eQTL) analyses in the whole blood samples (n = 338) and in the stomach mucosa samples (n = 170). The LD information of rs13187105 was calculated based on the data from 1000 Genomes project (phase3) (https://www.ncbi.nlm.nih.gov/variation/tools/1000genomes/) in CHB population. Functional annotations were performed using the HaploReg v4.1 database (http://archive.broadinstitute.org/mammals/haploreg/haploreg.php) and UCSC genome browser (https://genome.ucsc.edu/cgi-bin/hgGateway).
Statistical analysis
Departures from HWE were tested by the goodness-of-fit χ2 test to compare the observed genotype frequencies to the expected ones among the control subjects. Differences in the distribution of categorical variables including age, sex, smoking and drinking status were evaluated by χ2 test and age was compared by Welch’s t-test when regarded as a continuous variable. Multivariate logistic regression analysis for age, sex, smoking and drinking status was also done to assess their effects on gastric cancer susceptibility. To evaluate the associations between the tag SNPs and gastric cancer risk, unconditional logistic regression models were used to calculate ORs and their 95% CIs adjusted for age, sex, smoking status, drinking status and the top ten principal components. The detailed information about the top ten principal components could be obtained from our previous study34. In brief, the population structure was evaluated using principal component analysis using EIGENSOFT4.2 based on 4,861 autosomal scaffold markers included in the exome array. No population outliers were detected, suggesting that the study subjects were genetically matched. Therefore, to avoid the effect of the population structure, the top ten principal components were included in the logistic regression model as covariates. The p values of eQTL were obtained from linear regression model. Paired Student’s t-test was used to compare the PPP2CA expression levels of 32 gastric tumor and adjacent normal tissue pairs. All analyses were performed using R3.2.3 and plink1.07. Two-sided P-values less than 0.05 were considered as statistically significant.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
This work was supported by grants from the Key Grant of Natural Science Foundation of Jiangsu Higher Education Institutions(15KJA330002); the National Natural Science Foundation of China (81602917, 81402489, 81602927); Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (PPZY2015A067); Priority Academic Program for the Development of Jiangsu Higher Education Institutions (Public Health and Preventive Medicine); Natural Science Foundation of Jiangsu Province (BK20161031) and Key Program of Science and Technology Development Foundation of Nanjing Medical University (2015NJMUZD019).
Author Contributions
D.J. and L.L. designed research and amended the manuscript; H.T. and H.K. performed statistical analyses and wrote the manuscript; M.Y. contributed to the discussion and review of the manuscript; Z.M. helped conduct quality control; Y.C., Y.F., Q.Q., W.T. and W.Y. participated in the sample collection. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Tongtong Huang and Kexin He contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-12040-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jiangbo Du, Email: dujiangbo@njmu.edu.cn.
Li Liu, Email: kit9178@sina.com.
References
- 1.Ferlay J, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, et al. Cancer statistics in China, 2015. CA: A Cancer Journal for Clinicians. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Lochhead P, El-Omar EM. Helicobacter pylori infection and gastric cancer. Best practice & research. Clinical gastroenterology. 2007;21:281–297. doi: 10.1016/j.bpg.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Wroblewski LE, Peek RM, Jr., Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clinical microbiology reviews. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song P, Wu L, Guan W. Dietary Nitrates, Nitrites, and Nitrosamines Intake and the Risk of Gastric Cancer: A Meta-Analysis. Nutrients. 2015;7:9872–9895. doi: 10.3390/nu7125505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lochhead P, El-Omar EM. Gastric cancer. British Medical Bulletin. 2008;85:87–100. doi: 10.1093/bmb/ldn007. [DOI] [PubMed] [Google Scholar]
- 7.Correa P. Gastric Cancer. Gastroenterology Clinics of North America. 2013;42:211–217. doi: 10.1016/j.gtc.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez CA, et al. Meat Intake and Risk of Stomach and Esophageal Adenocarcinoma Within the European Prospective Investigation Into Cancer and Nutrition (EPIC) JNCI Journal of the National Cancer Institute. 2006;98:345–354. doi: 10.1093/jnci/djj071. [DOI] [PubMed] [Google Scholar]
- 9.El-Omar EM. Role of host genes in sporadic gastric cancer. Best practice & research. Clinical gastroenterology. 2006;20:675–686. doi: 10.1016/j.bpg.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Saeki N, et al. A functional single nucleotide polymorphism in mucin 1, at chromosome 1q22, determines susceptibility to diffuse-type gastric cancer. Gastroenterology. 2011;140:892–902. doi: 10.1053/j.gastro.2010.10.058. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y, et al. A genome-wide association study identifies new susceptibility loci for non-cardia gastric cancer at 3q13.31 and 5p13.1. Nature genetics. 2011;43:1215–1218. doi: 10.1038/ng.978. [DOI] [PubMed] [Google Scholar]
- 12.Abnet CC, et al. A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nature genetics. 2010;42:764–767. doi: 10.1038/ng.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen H, Jin G. Human genome epidemiology, progress and future. Journal of biomedical research. 2013;27:167–169. doi: 10.7555/JBR.27.20130040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. The Biochemical journal. 2001;353:417–439. doi: 10.1042/bj3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wera S, Hemmings BA. Serine/threonine protein phosphatases. The Biochemical journal. 1995;311(Pt 1):17–29. doi: 10.1042/bj3110017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu WJ, Shen Y, Ding J. [Protein phosphatase 2A: its structure, function and activity regulation] Sheng wu hua xue yu sheng wu wu li xue bao Acta biochimica et biophysica Sinica. 2003;35:105–112. [PubMed] [Google Scholar]
- 17.Junttila MR, et al. CIP2A inhibits PP2A in human malignancies. Cell. 2007;130:51–62. doi: 10.1016/j.cell.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 18.Janssens V, Goris J, Van Hoof C. PP2A: the expected tumor suppressor. Current opinion in genetics & development. 2005;15:34–41. doi: 10.1016/j.gde.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Hahn WC, et al. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 20.Rangarajan A, Hong SJ, Gifford A, Weinberg RA. Species- and cell type-specific requirements for cellular transformation. Cancer cell. 2004;6:171–183. doi: 10.1016/j.ccr.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Longin S, et al. Selection of protein phosphatase 2A regulatory subunits is mediated by the C terminus of the catalytic Subunit. J Biol Chem. 2007;282:26971–26980. doi: 10.1074/jbc.M704059200. [DOI] [PubMed] [Google Scholar]
- 22.Bryant JC, Westphal RS, Wadzinski BE. Methylated C-terminal leucine residue of PP2A catalytic subunit is important for binding of regulatory Balpha subunit. The Biochemical journal. 1999;339(Pt 2):241–246. doi: 10.1042/bj3390241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tolstykh T, Lee J, Vafai S, Stock JB. Carboxyl methylation regulates phosphoprotein phosphatase 2A by controlling the association of regulatory B subunits. The EMBO journal. 2000;19:5682–5691. doi: 10.1093/emboj/19.21.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan X, Chen M. MYLK and MYL9 expression in non-small cell lung cancer identified by bioinformatics analysis of public expression data. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:12189–12200. doi: 10.1007/s13277-014-2527-3. [DOI] [PubMed] [Google Scholar]
- 25.Park MH, et al. Gene expression profile related to prognosis of acute myeloid leukemia. Oncology reports. 2007;18:1395–1402. [PubMed] [Google Scholar]
- 26.Singh AP, et al. Genome-wide expression profiling reveals transcriptomic variation and perturbed gene networks in androgen-dependent and androgen-independent prostate cancer cells. Cancer letters. 2008;259:28–38. doi: 10.1016/j.canlet.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhardwaj A, et al. Restoration of PPP2CA expression reverses epithelial-to-mesenchymal transition and suppresses prostate tumour growth and metastasis in an orthotopic mouse model. British journal of cancer. 2014;110:2000–2010. doi: 10.1038/bjc.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Consortium GT. The Genotype-Tissue Expression (GTEx) project. Nature genetics. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cancer Genome Atlas Research, N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature513, 202-209, doi:10.1038/nature13480 (2014). [DOI] [PMC free article] [PubMed]
- 30.Mumby M. PP2A: unveiling a reluctant tumor suppressor. Cell. 2007;130:21–24. doi: 10.1016/j.cell.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 31.Neviani P, et al. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer cell. 2005;8:355–368. doi: 10.1016/j.ccr.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Chowdhury D, et al. gamma-H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair. Molecular cell. 2005;20:801–809. doi: 10.1016/j.molcel.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Bhardwaj A, et al. Modulation of protein phosphatase 2A activity alters androgen-independent growth of prostate cancer cells: therapeutic implications. Molecular cancer therapeutics. 2011;10:720–731. doi: 10.1158/1535-7163.MCT-10-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu, M. et al. Exome Array Analysis Identifies Variants in SPOCD1 and BTN3A2 That Affect Risk for Gastric Cancer. Gastroenterology, https://doi.org/10.1053/j.gastro.2017.02.017 (2017). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.