Abstract
Visual perception by photoreceptors relies on the interaction of incident photons from light with a derivative of vitamin A that is covalently linked to an opsin molecule located in a special subcellular structure, the photoreceptor outer segment. The photochemical reaction produced by the photon is optimal when the opsin molecule, a seven-transmembrane protein, is embedded in a lipid bilayer of optimal fluidity. This is achieved in vertebrate photoreceptors by a high proportion of lipids made with polyunsaturated fatty acids, which have the detrimental property of being oxidized and damaged by light. Photoreceptors cannot divide, but regenerate their outer segments. This is an enormous energetic challenge that explains why photoreceptors metabolize glucose through aerobic glycolysis, as cancer cells do. Uptaken glucose produces metabolites to renew that outer segment as well as reducing power through the pentose phosphate pathway to protect photoreceptors against oxidative damage.
Keywords: Cone photoreceptor, Retinal degeneration, Aerobic glycolysis, Glucose transporter, Thioredoxin, Pentose phosphate pathway, Nucleoredoxin-like genes, Rod-derived cone viability factor
Upside down: considerations of the inverted camera type eye
The vertebrate retina, the light sensitive part of the eye, is composed of three layers of neurons and of radial Muller glial cells. The photoreceptor layer, which includes rods and cones, is located in the farthest position with respect to the incidence of light; their nuclei form what we call the outer nuclear layer as observed on retinal sections (Fig. 1). The other layers are composed of interneurons, such as bipolar cells that relay light-dependent electrochemical signals, transmitted through the photoreceptor synapses, to neurons of the ganglion cell layer. The axons of these later neurons form the optic nerve. Within the circuit, other neurons intercalated into the retina modulate the signal. The biological rational of this counterintuitive optic setting is explained by the chemical properties of the photoreceptor cellular substructure that captures the photon, the photoreceptor outer segment [1]. Most engineers would place the photoreceptors of the retina to the nearest from the incident light. The high sensitivity of the retina requires that the number of light sensing molecules, the opsins, be very high. Both for vertebrates and invertebrates, the opsin molecules are seven-transmembrane domains proteins coupled to G protein, alternatively named G protein-coupled receptors. Opsins establish a covalent link through an intra-membranal lysine residue with a chromophore derived from vitamin A. Retinal exists as two stereoisomers, 11-cis-retinal and all-trans-retinal. Upon absorption in the visible range, a photon triggers cis–trans isomerization; the chromophore is converted from a bent molecule to a straight one by the energy provided to pass the thermodynamic barrier separating the two stereoisomers. The straightening of the chromophore within the hydrophobic pocket formed by the seven-transmembrane α-helix induces a conformational change that is sensed by an intra-cellular G protein, the transducin. This molecular rearrangement is optimal within a lipid bilayer of high fluidity [2]. The fluidity of the lipid bilayer of the photoreceptor outer segment is conferred by its high proportion of lipids made of polyunsaturated fatty acids (PUFA). In mammals, docosahexaenoic acid (DHA, 22:6, n-3), an essential omega-3 fatty acid, accounts for 80 % of the PUFAs of photoreceptor outer segment. Polyunsaturation is the existence of several double bonds (C=C), which are chemically rigid. Nevertheless, C=C bonds of PUFA are flanked by two saturated bonds (C–C) forming a regular pattern of one non-rotating (C=C) and two rotating bonds (C–C). This arrangement reduces the energy of rotation that increases the fluidity of the lipid membrane [3]. It is also a double bond (C=C) of the chromophore that is subject to cis–trans isomerization. Lipids of the photoreceptor outer segment are prone to oxidation. Reactive oxygen species (ROS) drive lipid peroxidation, a chain reaction, in which one ROS can induce the oxidation of a large number of lipid molecules-containing PUFA [4]. Fatty acid hydroperoxides are finally decomposed into reactive aldehydes, such as 4-hydroxy-2-nonenal (HNE) and malondialdehyde (MDA). Monounsaturated and saturated fatty acids are much less reactive and do not usually participate in lipid peroxidation. The end-products of lipid peroxidation (MDA and HNE) cause protein damage by reacting with chemical groups within certain amino acids as cysteines, lysines, and histidines [5] (Fig. 2a). The nucleophilic thiol side chain in cysteine participates in many enzymatic reactions and the irreversible formation of HNE adduct with photoreceptor proteins is detrimental to their function [6] (Fig. 2b). Photoreceptors are post-mitotic neurons that do not regenerate, at least in mammals. The damaged lipids are eliminated from vertebrate photoreceptors by phagocytosis of disks by the retinal pigmented epithelium (RPE). This process is regulated by the circadian clock, so that 10 % of rod photoreceptor outer segment is daily engulfed in phagosomes of RPE cells. Phagosomes are moved from the apical membrane toward the basal membrane where their contained in proteins and lipids are degraded [7]. To maintain its length, the photoreceptor outer segment is renewed from its base, a process that involves protein and lipid synthesis in the inner segment of photoreceptor, a cellular substructure just beneath the outer segment. Contrarily to the cones, the rod outer segments are made up of a stack of individualized membranal disks unconnected to the plasma membrane of the inner segment. Consequently, lipids are transferred from the plasma membrane to the disks [8–10].
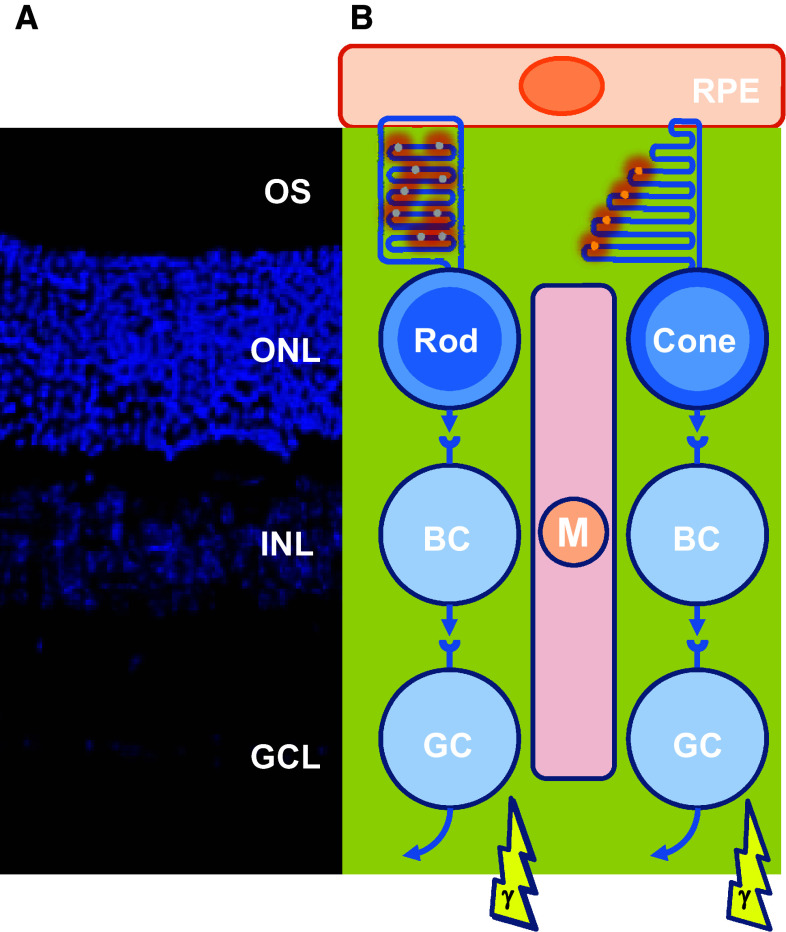
Fig. 1.
Architecture of the retina of vertebrates. a Mouse adult retinal section with nuclei labeled with 4′,6-diamidino-2-phenylindole (DAPI). OS outers segment, ONL outer nuclear layer, INL inner nuclear layer, GCL ganglion cell layer. b Schematic drawing of the retinal cells and their functional relations. RPE retinal pigmented epithelium, BC bipolar cell, GC ganglion cell, M Muller glial cell
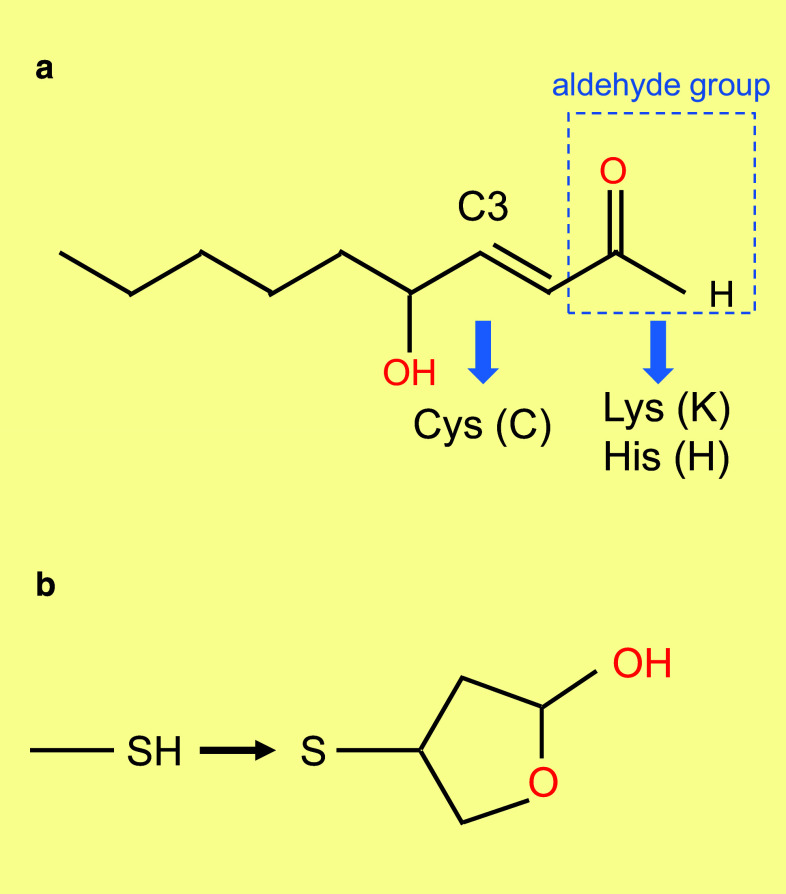
Fig. 2.
Lipid peroxidation chain reaction. a Chemical structure of 4-hydroxy-2-nonenal (HNE). The carbon at position C3 targets cysteine modification. The aldehydes group targets lysine and histidine modifications. b Modification of a cysteine residue (SH) in a protein by HNE through thiol Michael addition at position C3
The renewal of rod outer segments was elegantly discovered by Young [11]. In rods, most of the new proteins are first concentrated at their base, where they are used in the assembly of new disk membranes. After a single injection of 3H-methionine, he observed by autoradiography, labeled disks progressively displaced along the outer segment due to the repeated formation of newer disks. A similar observation was made using tritium-labeled fatty acids [12]. Most likely, it is this mechanism of disk shedding and renewal that imposed the upside down positioning of the photoreceptors and their outer segments in the most distal part of the retina from the incident light. One could argue that this is not the only possible organization of photoreceptors in the eye, since cephalopods have an everted retina, so that the distal end of rhabdomeric photoreceptors is pointing toward incoming light [13].
The life of photoreceptors: a challenging task
The renewal of photoreceptor outer segment in vertebrate retina is energetically demanding and biologically challenging. The outer retina formed by photoreceptors is avascular, in contrast to the inner retina. Exchanges between the retina and the circulation are controlled at two levels: the blood-retinal barrier in the inner retina, made up of retinal vessels surrounded by pericytes and glial cells, and the outer retinal barrier, which is constituted by the RPE adherent junctions (Fig. 3). All nutrients, including glucose, vitamin A, and fatty acids, are provided to photoreceptors by choroidal blood flow behind the RPE, one of the highest rates of blood flow of the whole body [14]. The blood supply to photoreceptors must be transported through RPE cells that form an epithelium with adherent junctions.
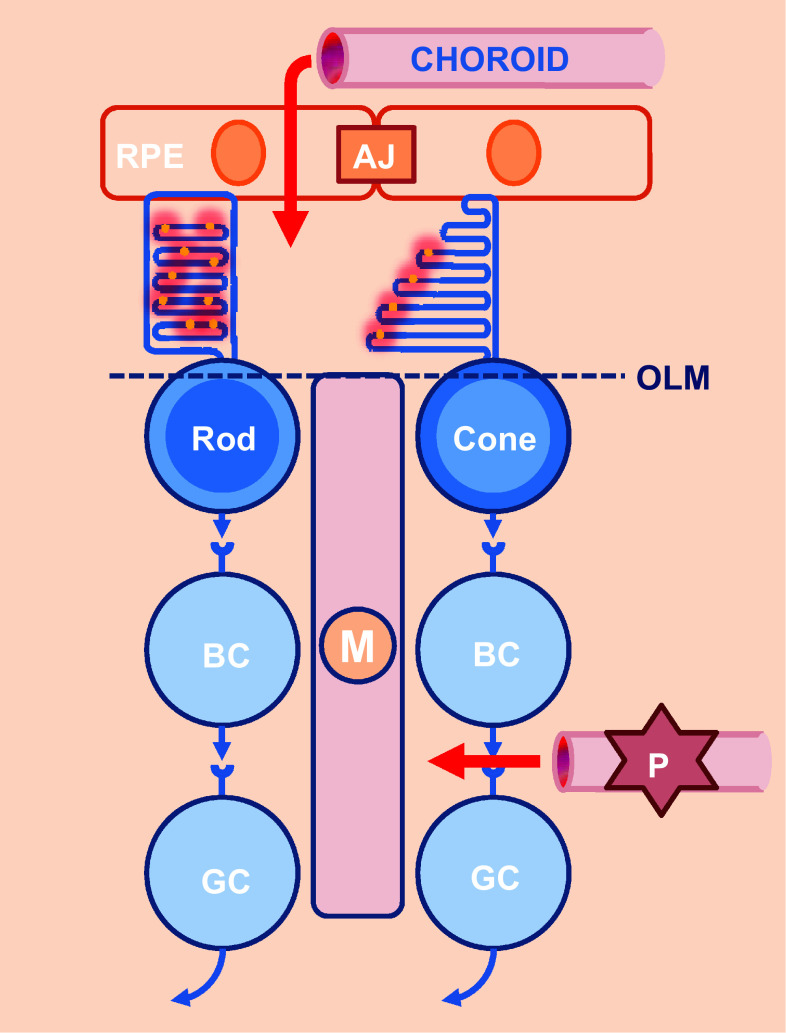
Fig. 3.
Exchanges between the retina and the blood circulation. Blood circulation is controlled at two levels (red arrows): a blood-retinal barrier in the inner retina and an outer retinal barrier, which is constituted by the RPE. RPE retinal pigmented epithelium, BC bipolar cell, GC ganglion cell, M Muller glial cell, AJ adherent junction, OLM outer limiting membrane, P pericyte
Good fats: supplying fatty acids to photoreceptor cells
It is broadly accepted that in mammals, unsaturated fatty acids are not synthesized in the retina but originate from blood supply. The essential fatty acids, among which DHA (C22:6, n-3) and its precursor, α-linolenic acid (18:3, n-3) are hydrophobic molecules transported by serum albumin from the liver to the basal side of the RPE cells where they are transferred to photoreceptors through the apical side (Fig. 4). Two distinct molecules have been implicated in the transport of DHA through the RPE, the major facilitator superfamily domain-containing protein 2a (MFSD2A) and the adiponectin receptor 1 (ADIPOR1) [15–17]. MFSD2A is a typical 12 transmembrane domains transporter, while ADIPOR1 is an atypical 7 transmembrane domains receptor of adiponectin, an essential hormone secreted by adipocytes that regulates glucose and fatty acid metabolism. The mechanisms that link the two transporter proteins are unknown.
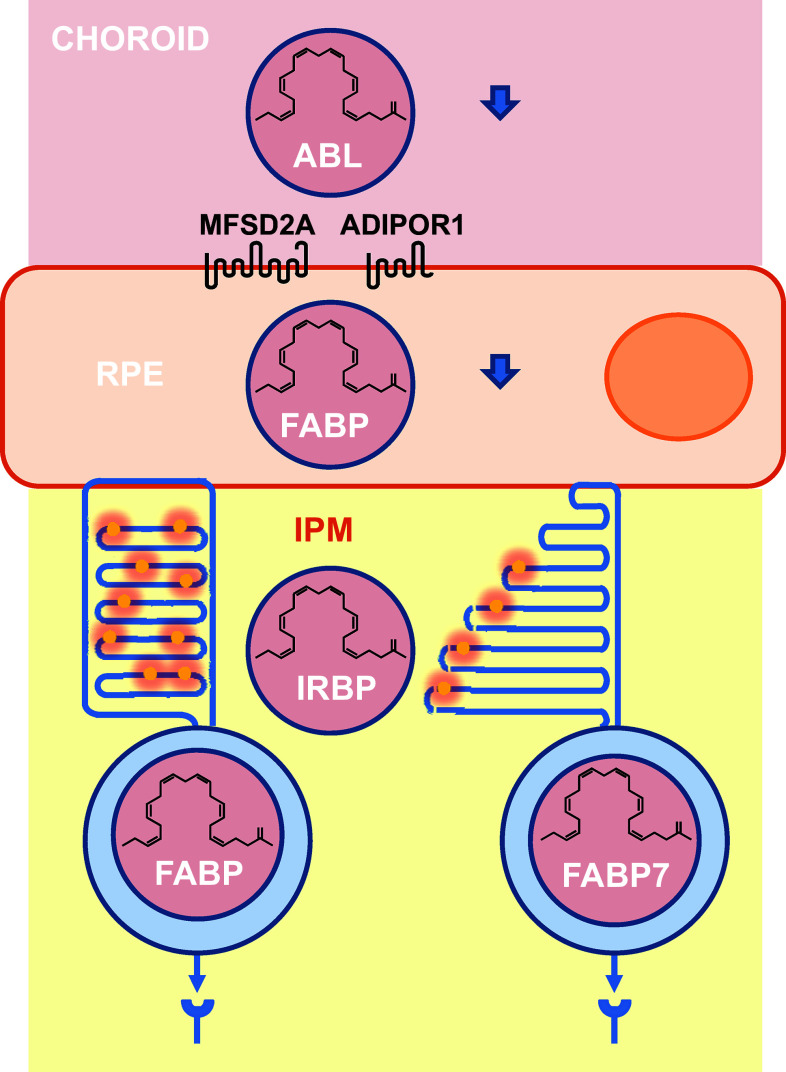
Fig. 4.
Transport of essential fatty acids from the blood circulation to photoreceptors. RPE retinal pigmented epithelium, ABL albumin, ADIPOR1 adiponectin receptor 1, MFSD2A fatty acid transporter, IPM inter-photoreceptor matrix, FABP fatty acid-binding protein, IRBP inter-photoreceptor retinoid-binding protein
Transport of insoluble fatty acids from basal to apical surfaces of the RPE certainly involves transient interactions with fatty acid-binding proteins (FABP) to allow intra-cellular translocation of hydrophobic molecules in the aqueous cytosol.
Essential fatty acids as DHA retrieved from shed photoreceptor apical disk membranes are recycled back to the photoreceptor inner segment and further incorporated into phospholipids of renewed photoreceptor outer segments. The recycled fatty acids are transferred from the RPE to photoreceptors through the inter-photoreceptor matrix (IPM), in the extracellular space between the photoreceptor outer segments and the RPE, in the absence of albumin, the inter-photoreceptor retinoid-binding protein (IRBP) binds to fatty acids [18, 19]. Since the expression of MFSD2A by photoreceptors was not reported, it is still unclear how essential fatty acids are taken up by photoreceptors, but pulsed-labeling experiments with radiotracers demonstrated that this happens in vitro and in vivo [20]. To be incorporated into the renewing photoreceptor outer segments, non-polar fatty or aliphatic acid tails part of the amphiphilic phospholipid must be linked to a polar head derived from glycerol-3-phosphate for glycerophospholipids [21]. This is taking place at the surface of the endoplasmic reticulum in the inner segment of photoreceptors. Intra-cellular fatty acid transport proceeds through binding to FABP.
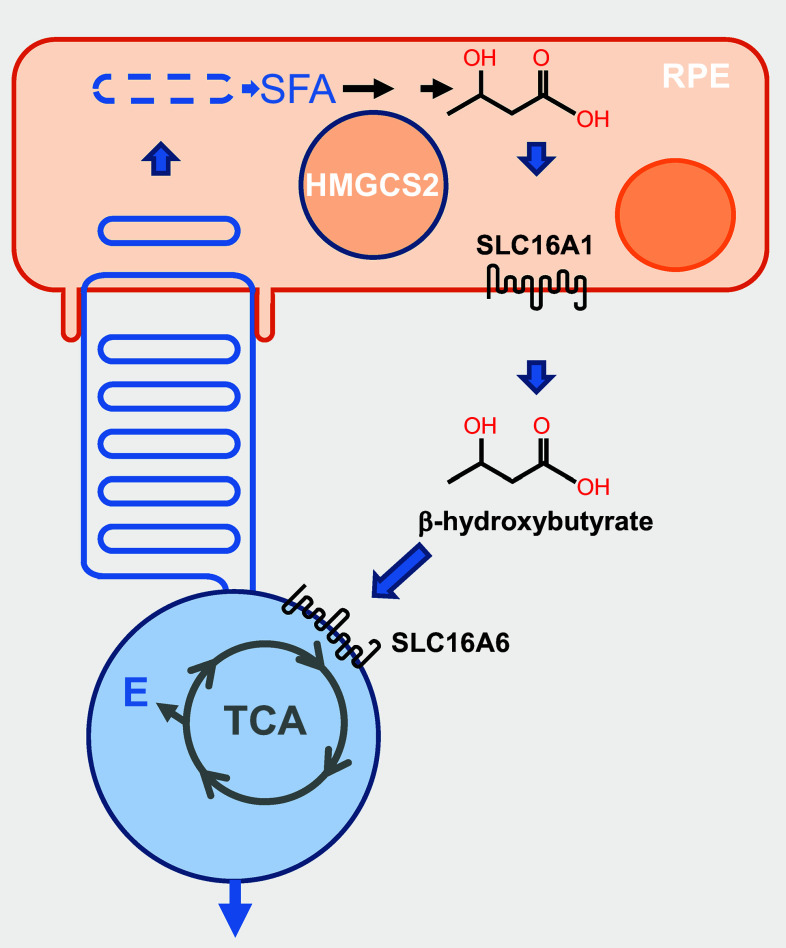
Quite interestingly, a yet unknown fraction of the saturated fatty acids from photoreceptor outer segments is recycled to provide energy instead of structural components to photoreceptors. They are metabolized by RPE cells from the phagosome by the high levels of mitochondrial HMG-coenzyme A (CoA)-synthase 2 (HMGCS2) into ketone derivatives (C=O), then enzymatically processed into β-hydroxybutyrate by fatty acid β-oxidation pathway [22] (Fig. 5). β-Hydroxybutyrate is then released preferentially into the apical compartment through the monocarboxylate transporter isoform 1 (SLC16A1), facing photoreceptor cells that internalize it through the monocarboxylate transporter isoform 7 (SLC16A6). β-Hydroxybutyrate is oxidized by the tricarboxylic acid (TCA) cycle to produce energy and glutamate (E), a neurotransmitter involved in the vertical transmission of the signal from photoreceptors to bipolar cells [23]. The contribution of this cycle to the enrichment of photoreceptor outer segments into PUFAs is presently unknown.
Fig. 5.
Recycling of saturated fatty acids by photoreceptors. RPE retinal pigmented epithelium, SFA saturated fatty acid, HMGCS2 mitochondrial HMG-coenzyme A (CoA)-synthase 2, SLC16A1 monocarboxylate transporter isoform 1, SLC16A6 monocarboxylate transporter isoform 7, TCA tricarboxylic acid, E glutamic acid
Renewing proteins of photoreceptor outer segments
Rhodopsin represents 80 % of total protein content in rod outer segments and a density of 25,000 molecule/mm2 on the disk membrane forming a supramolecular organization of tracks of rhodopsin dimers [24–26]. The pace of renewal of photoreceptor outer segment imposes that a high level of protein biosynthesis occurs on daily basis. Disk assembly at the base rod outer segment is estimated to be 80 disks per day, which requires the synthesis of ~1000 rhodopsin molecules per minute [27]. This requires efficient mechanism of transcription and translation. Integral transmembrane proteins and peripheral membrane proteins are then crossing the connecting cilium to reach photoreceptor outer segment. Transport of proteins and lipids through the cilium is mediated by the intra-flagellar transport (IFT) machinery.
Natural history of cones and rods
As explained previously, in pulse-chase experiments, newly radiolabeled proteins migrate toward the connecting cilium and are incorporated into nascent disks of rod photoreceptors that move progressively toward the RPE [28]. In the cones, the radiolabeled proteins diffuse throughout the entire outer segment, because of the absence of disks; the outer segment membrane of cones is in direct continuation of the plasma membrane [29]. The difference in the morphology of the two classes of vertebrate photoreceptors was originally described by Max Schultze in 1866 [30]. In most vertebrates, vision is based on a dual system of photoreceptors. The rods are responsible for scotopic vision, in conditions of low luminosity, and the cones are responsible for photopic vision, in conditions of high luminosity, for color vision and high-acuity. Color vision is the process by which information is extracted from a visual stimulus based on its wavelength composition. It is based on differences in spectral sensitivities of visual pigments, or opsins. In general, these opsins are expressed in a specific type of photoreceptors, in accordance with the principle of one receptor–one neuron that applies to most sensory systems. The different groups of cone opsins are defined in terms of the spectral sensitivity: cluster S (blue, ultraviolet <440 nm), clusters M1 and M2 (440–510 nm), and cluster L (red >500 nm) [31]. Most mammals have a retina, in which rods predominate. Nonetheless, despite this predominance of photoreceptors designed for night vision, many mammals have developed a diurnal lifestyle, in which vision is essentially dependent on cone activity. This paradox of a rod-dominated retina in animals adapted to diurnal activity applies to humans and other primates. High-acuity vision, in particular, is dependent on the presence of the fovea, a specialized region in the centre of the retina constituted exclusively of cones in its most central part. The absence of M1 and M2 opsin genes in all sequenced mammalian genomes led to the hypothesis that the common ancestor of all mammals probably had a nocturnal lifestyle not requiring complex color vision. The selection pressure exerted on the first mammals by contemporary diurnal sauropsids forced primitive mammals to adopt a nocturnal lifestyle relying on scotopic vision and explains the loss of M1 and M2 opsin genes. The sudden extinction of the dinosaurs enabled mammals to colonize the vacated diurnal ecological niches, a mechanism known as nocturnal bottleneck [32]. Contemporary birds, which belong to the sauropsids, have a retina dominated by cones as presumably that of dinosaurs and contrarily to mammals [33, 34]. Primitive primates (prosimians) with a nocturnal life style, such as many lemurs, have only dichromatic vision. Humans and other old world apes (Cercopithecidae) have trichromatic vision, due to duplication of the L-opsin gene on the X chromosome. Humans thus have four visual pigments: rhodopsin (RHO), S-(OPN1SW, 425 nm), M-(OPN1MW, 530 nm), and L-opsins (OPN1LW, 560 nm), expressed by rods and the blue, green and red cones, respectively. Nevertheless, L-opsin duplication is not specific to Cercopithecidae, it also occurred in a family of platyrrhines, the howler monkeys (Alouatta caraya) [35]. This duplication is an independent event and more recent than that in the Cercopithecidae. The evolutionary history of color vision of primates illustrates the importance of the cone in the acquisition of complex behaviors.
The most ancient ciliary photoreceptors in Cnidarians (corals, sea anemones and jellyfishes) share with vertebrate cones a low sensitivity to light and are adapted primarily for diurnal vision [36]. This observation asks the question of the origin of the rods. A major step in the evolution of the vertebrate eye was the emergence of rods in addition to cones to produce a duplex retina [37]. In a duplex retina, rods are functional for dim light vision with great sensitivity, and when the light intensity increases, the rods are saturated and turned off, leaving the cones to function in bright light, greatly reducing energy required for vision. Functional rods evolved before the split between the jawed and jawless vertebrates. Sea lamprey (Petromyzon marinus) has two types of photoreceptors, the short and the long both with a cone-like morphology of outer segment, but the short photoreceptors have a single-photon sensitivity similar to that of rods in other vertebrates [38–40]. The typical rod outer segment morphology with segmented disks was acquired later during evolution, probably because it allows only the removal of the oldest macromolecules during outer segment disk shedding contrarily to cones. An increased metabolic rate, along with changes in energy allocation, was crucial in the evolution of human brain size and life history [41]. One could speculate that the reduction in energy requirement for vision had permitted energy allocations in favor of cephalization and cognitive functions during evolution of jawed vertebrates [42]. Citing Spinoza “Living organisms are designed with an ability to react emotionally to different objects and events”, we could propose that the light perception by photoreceptors and cognitive functions are parallel attributes of the same substance [43]. Intriguingly, in all conditions where the rods are destroyed, the cones degenerate secondarily. This is particularly well established in rod-dominated mammalian retina, but was also observed in retina with an equal proportion of rods and cones [44]. Ablation of rod photoreceptors in Xenopus laevis results in outer segment degeneration and cone cell death. In such retina, there are evidences that rods also need cones to survive [45]. A mutation in the cone-specific phosphodiesterase zebrafish gene (pde6c) triggers cone death followed by rod degeneration in areas of the retina that was originally rich in cones. In mouse models of rod-to-cone degeneration, it was proposed that glucose uptake by cones and/or its intra-cellular concentration in cones may be compromised [46]. The cones need the rods to survive [47].
Cones got married to rods for life
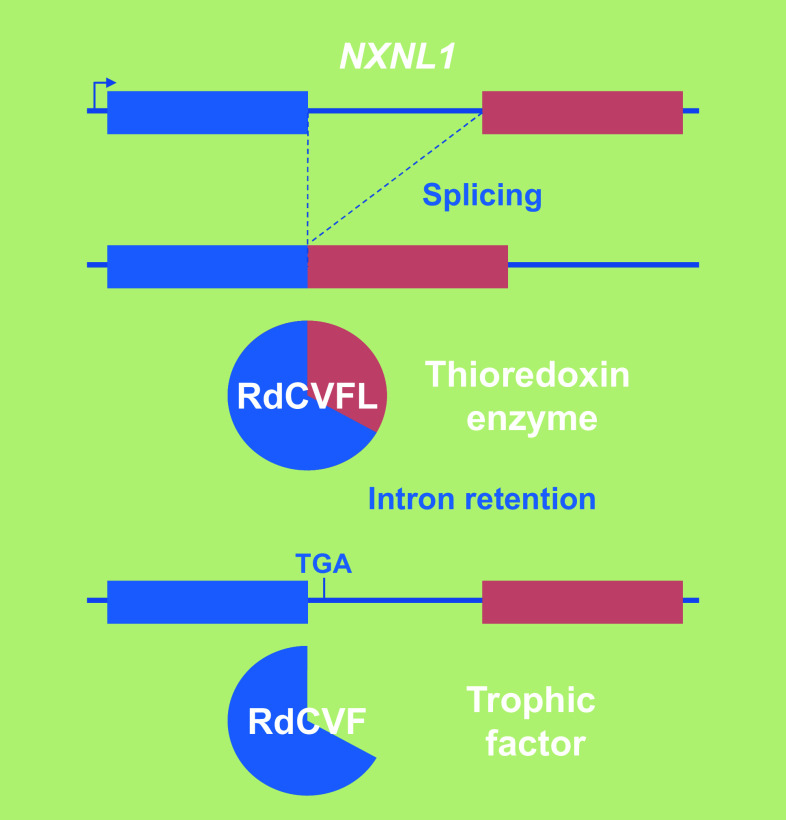
In patients suffering from retinitis pigmentosa, the most common form of inherited retinal degeneration, the vision loss develops in two successive steps. Early in their adult life, these patients lose ability to see in dim light conditions that refers to a night vision lost and corresponds to the loss of function and degeneration of rod photoreceptors. This is felt as a minor handicap, especially in individuals affected by congenital stationary night blindness, another type of inherited retinal disease characterized exclusively by lack of rod function; in our current well-illuminated environment, these people retain an almost normal way of life [48]. For those patients affected with rod-cone dystrophy, the disease then progresses through another debilitating step resulting from loss of function and degeneration of the second class of photoreceptors, the cones that dominate at the centre of the retina. Cones represent only 3–5 % of all photoreceptors in most mammals, but their role for vision is essential. This secondary event leads to central vision loss and potentially complete blindness. Because the cones underlie all visual functions in lighted environment, cone rescue was deemed to be a clinically relevant target [49, 50]. Widespread cone death in the naturally occurring rd1 mutant mouse, a model of retinitis pigmentosa, is well described [51]. The degeneration does not arise in this model through a mutation within cone photoreceptor cells, but as a result of a recessive mutation in the rod photoreceptor-specific cGMP phosphodiesterase-β subunit (PDE6B), and is consequently non-cell autonomous [52]. This mutation also leads to rod-cone degeneration in humans [53]. Grafting normal photoreceptors (97 % rods) into the eye of the rod-less rd1 mouse before the cones degenerate exerts a positive effect on the host retina cones [54]. Co-culture studies demonstrated that such trophic effect on cone photoreceptors might be mediated through a diffusible factor [55]. Rod death in the first phase of the disease is triggered by the loss of expression of rod-derived cone viability factor (RdCVF), a truncated thioredoxin-like protein encoded by the nucleoredoxin-like-1 gene (NXNL1) [56, 57]. The inactivation of the Nxnl1 gene in the mouse triggers an age-dependent loss of cones even with the potential complementation of its paralog Nxnl2 [58–60]. RdCVF is a translation product made from an alternatively spliced mRNA encoding the exon 1 and retaining the following intron that contains an in-frame stop codon (Fig. 6). The other product (RdCVFL), made by splicing intron 1 of the NXNL1 gene, is an active thioredoxin enzyme that protects rod and cone photoreceptors against photo-oxidative stress [61–64]. NXNL2, the paralog of NXNL1, also encodes for at least two proteins: RdCVF2, a trophic factor produced by the rods and active on cones, and the thioredoxin protein RdCVF2L by alternative splicing [60, 65]. The administration of RdCVF protein would restore rod-to-cone signaling preventing the secondary degeneration of cones and thus transforming retinitis pigmentosa in a type of night blindness associated with moderate visual impairment, independent from the causative mutations in any of the 60 known genes [66–68]. Injection of RdCVF in animal models of retinitis pigmentosa prevents the shortening of cone outer segments, which precedes cone loss [69]. The secondary degeneration of cones in retinitis pigmentosa patients occurs over a period of more than a decade with an average loss of 4 % of visual acuity per year [70]. Preventing the secondary loss of cone by the administration of RdCVF is medically rational, since most patients that consult an ophthalmologist have already lost most of the rods, while visual acuity is only reduced when 50 % of the cones become non-functional [71].
Fig. 6.
Genomic organization of the bifunctional gene nucleoredoxin-like 1. NXNL1 nucleoredoxin-like 1, RdCVFL the thioredoxin enzyme rod-derived cone viability factor long, RdCVF the trophic factor rod-derived cone viability factor, TGA stop codon
Rods feed the cones
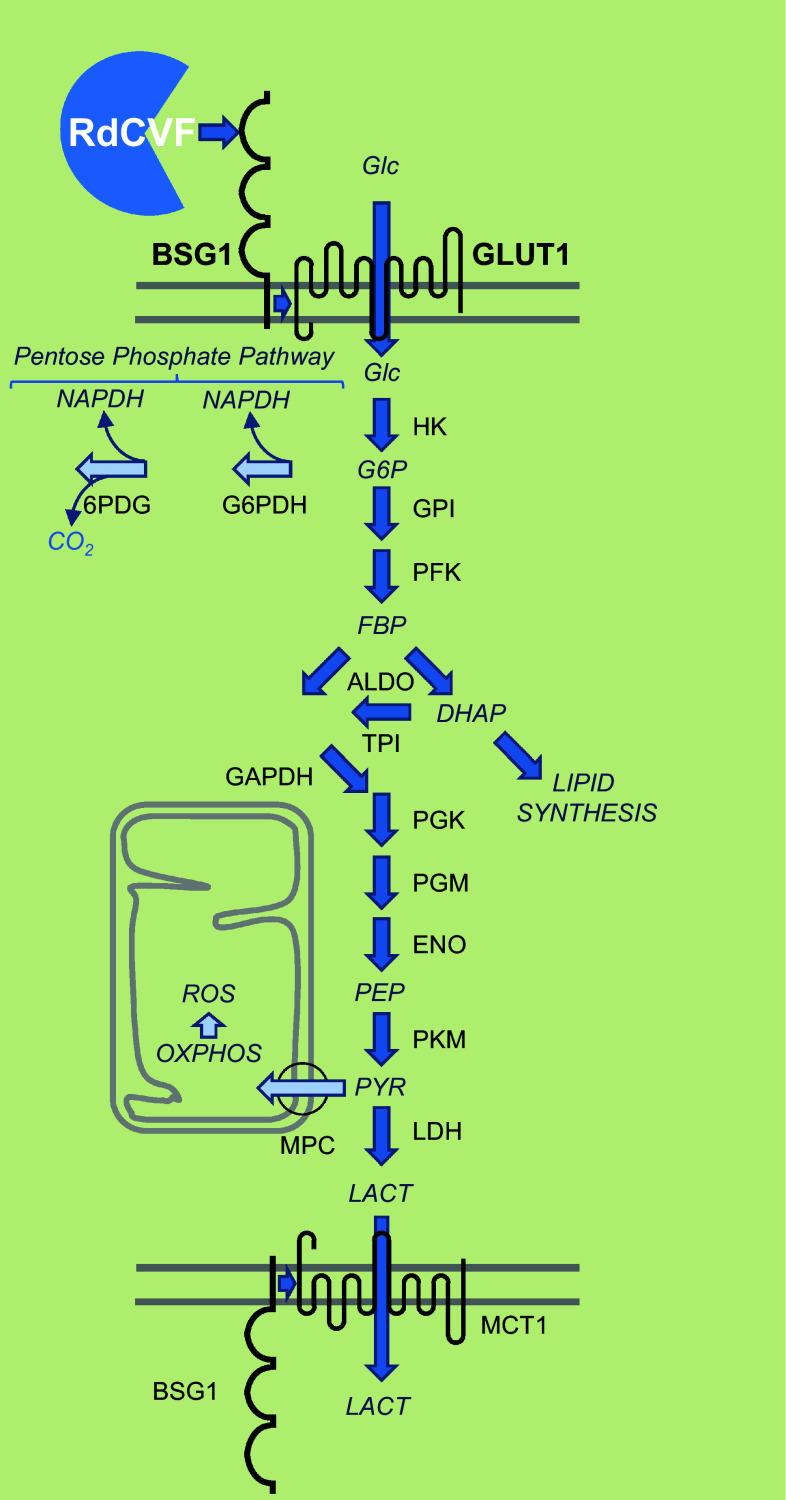
RdCVF binds basigin-1 (BSG1), its cell-surface receptor on cones [72]. BSG1 is a photoreceptor-specific alternative spliced isoform of the BSG gene, which has three extracellular immunoglobulin (Ig) domains contrarily to BSG2, the other product of the same gene more broadly expressed that possesses only two Ig domains [73]. RdCVF binds to BSG1, but not to BSG2. BSG1 forms a complex with the glucose transporter GLUT1 at the cone surface, whose transport activity is increased by RdCVF binding. Glucose is metabolized by cones via aerobic glycolysis to produce metabolites necessary for renewing cone outer segments (Fig. 7). Cone survival relies on the ability of RdCVF to stimulate aerobic glycolysis [74, 75]. GLUT1 catalyses the rate-limiting step in supplying cells of the central nervous system. The trophic effect of RdCVF via aerobic glycolysis is thus mediated by a three proteins complex-containing RdCVF, basigin-1, and GLUT1. This is distinct from the effect of insulin on cones that is mediated by the insulin receptor [46, 76–79]. GLUT1 exists in equilibrium between homodimeric and homotetrameric forms [80]. Each subunit of GLUT1 contains an extracellular disulfide bridge (C347 and C421) that stabilizes the tetrameric structure and thereby accelerates transport function by increasing the Vmax of transport and decreasing the dissociation constant, Km [81]. GLUT1 reduction causes GLUT1 tetramers to dissociate into dimers. RdCVF binding to basigin-1 may somehow displace the equilibrium toward the tetramer, accelerating GLUT1 transport function and stimulating glucose uptake by cones acting in this scheme as an allosteric modulator of GLUT1. When Otto Warburg described aerobic glycolysis as a hallmark of cancer cells in 1922, he also identified the retina as an exception to this observation even if he thought that could be an artifact of tissue preparation [82]. Aerobic glycolysis in mammalian retina is providing carbohydrates metabolite used for the daily renewal of 10 % of the outer segments of photoreceptors [83]. Similarly, cancer cells proliferate and rely on the production of carbohydrate intermediates at a high rate [84, 85]. Metabolic reprogramming of cancer cells to Warburg effect is a primary transformation occurring prior to the activation of proto-oncogenes and inactivation of tumor suppressor genes [86]. Pyruvate is at a crucial metabolic branch point [87]. When transported into mitochondria by the mitochondrial pyruvate carrier, a heterodimer composed of MPC1 and MPC2, pyruvate is oxidized to Acetyl-CoA by the multi-subunit pyruvate dehydrogenase complex localized in the mitochondrial matrix. Acetyl-CoA then enters the TCA cycle, where it is further oxidized. Following glycolysis and oxidative phosphorylation, each fully oxidized molecule of glucose to CO2 produces 30 molecules of ATP. Alternatively, pyruvate can also be reduced to lactate in the cytosol by lactate dehydrogenase. This reaction allows the regeneration of NAD+ from the nicotinamide adenine dinucleotide (NADH) that is produced by glycolysis. Aerobic glycolysis departs from oxidative phosphorylation in the glycolytic part of the reaction by the involvement of hexokinase 2 (HK2) instead of hexokinase 1 and pyruvate kinase isoform M2 (PKM2) instead of PKM1. Hexokinase 2 (HK2) is highly activated in cancer cells and is located on the outer membrane of mitochondria [88]. Both HK2 and PKM2 are expressed preferentially by photoreceptors [72, 89–91]. Hk2 expression is increased during the maturation of photoreceptor in the mouse retina. RdCVF does not activate the expression of HK2, nor does it promote a switch from oxidative phosphorylation to aerobic glycolysis [72]. Hexokinases are responsible for a rate-limiting step of glycolysis phosphorylating glucose to glucose-6-phosphate (G6P), which is maintained in the cytoplasm. G6P is the branch point for proceeding to glycolysis or the pentose phosphate pathway (PPP) (Fig. 7). The PPP shunt is the major contributor of reducing equivalents in the form of reduced nicotinamide adenine dinucleotide phosphate (NADPH). Pyruvate kinases catalyze an ATP-generating step of glycolysis, in which phosphoenolpyruvate (PEP) is converted to pyruvate. Pyruvate kinase exists in two M isoforms, differentiated by alternative splicing of exons 9 and 10, which in PKM2 codes for a specific allosteric pocket, absent in PKM1 that allows the binding of the activating glycolytic intermediate, fructose-1,6-bisphosphate (FBP) [92]. PKM2 controls the final step of glycolysis, and its regulation serves to integrate intra-cellular signaling inputs with the metabolic state of the cell. Down-regulation of PKM2 activity and up-regulation of other enzymes committing glucose to glycolysis will lead to the accumulation of phosphorylated glycolytic intermediates that spill into branching biosynthetic pathways, as the production of glycerol-3-phosphate from dihydroxyacetone phosphate (DHAP) or the production of NADPH through the pentose phosphate pathway [93] (Fig. 7). The carbon flux must be diverted, since if each six-carbon glucose is entirely transformed producing two molecules of three-carbon lactate, no carbon would be incorporated from glucose into phospholipids of the cone outer segments. Injection of glucose in a pig model of retinitis pigmentosa was sufficient to promote cone outer segment regrowth, which is consistent with the mode of action of RdCVF [94].
Fig. 7.
Metabolic signaling regulated by rod-derived cone viability factor. Top-to-bottom RdCVF rod-derived cone viability factor, BSG1 basigin-1, GLUT1 glucose transporter SLC2A1, Glc glucose, G6P glucose-6-phosphate, FBP fructose biphosphate, DHAP dihydroxyacetone phosphate, PEP phosphoenol pyruvate, PYR pyruvate, LACT lactate, MPC mitochondrial pyruvate carrier, HK hexokinase, GPI glucose-6-phosphate isomerase, PFK phosphofructokinase, ALDO aldolase, TPI triosephosphate isomerase, PGK phosphoglycerate kinase, PGM phosphoglycerate mutase, ENO enolase, PKM pyruvate kinase M, LDH lactate dehydrogenase, MCT1 lactate transporter SLC16A, NADPH nicotinamide adenine dinucleotide phosphate, G6PDH glucose-6-phosphate dehydrogenase, 6PDG 6-phosphogluconate dehydrogenase, OXPHO oxidative phosphorylation, ROS reactive oxygen species
For retinitis pigmentosa patients, in the midterm of their disease, the regrowth of cone outer segments, which ultimately will reverse the disease by restoring central vision can be theoretically obtained by stimulating the rate of aerobic glycolysis by increased glucose entry and by increasing the expression and/or the activity of key glycolytic enzymes [72, 77]. Cone outer segment regrowth is a real possibility, as it was observed in patients suffering from acute idiopathic blind spot enlargement and after retinal detachment [95–97].
Antioxidants versus redox signaling
According to the free radical theory of aging, first proposed by Harman in 1956 [98], free radicals are continuously produced in the cell as a product of aerobic life and induce oxidative damage while aging [99]. Caloric restriction, which lower glucose metabolism, is the only unequivocally effective scheme to extend life span significantly for most organisms and is known to increased resistance to various oxidative stresses in many animals [100]. More than 90 % of total cellular oxygen is reduced to water stepwise via electron carriers of the mitochondrial respiratory chain [101]. Presently, it is virtually impossible to identify which specific respiratory complex or mitochondrial enzyme is producing reactive oxygen species by leakage [102]. Reactive nitrogen species (RNS) are produced when the superoxide ion O·−2 reacts with nitric oxide (NO) produced by NO synthases (NOS1–3). To avoid the damage of macromolecules by ROS and RNS, proper redox conditions must be maintained within the intra-cellular environment. Therefore, aerobic organisms have developed several antioxidant systems. Antioxidant molecules, such as uric acid, glutathione (GSH), and vitamins C and E, scavenge ROS and RNS to prevent oxidative damage. Antioxidant enzymes detoxify ROS/RNS into less reactive species. Paradoxically, the protective role of antioxidants has been challenged by the observation that antioxidants prevent health-promoting effects of physical exercise in humans [103].
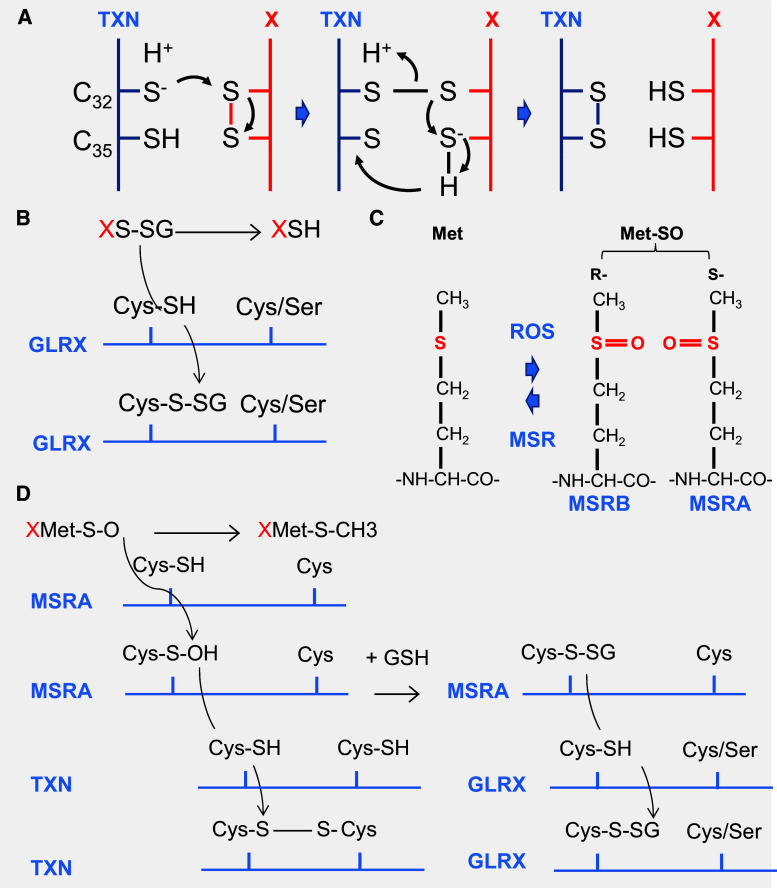
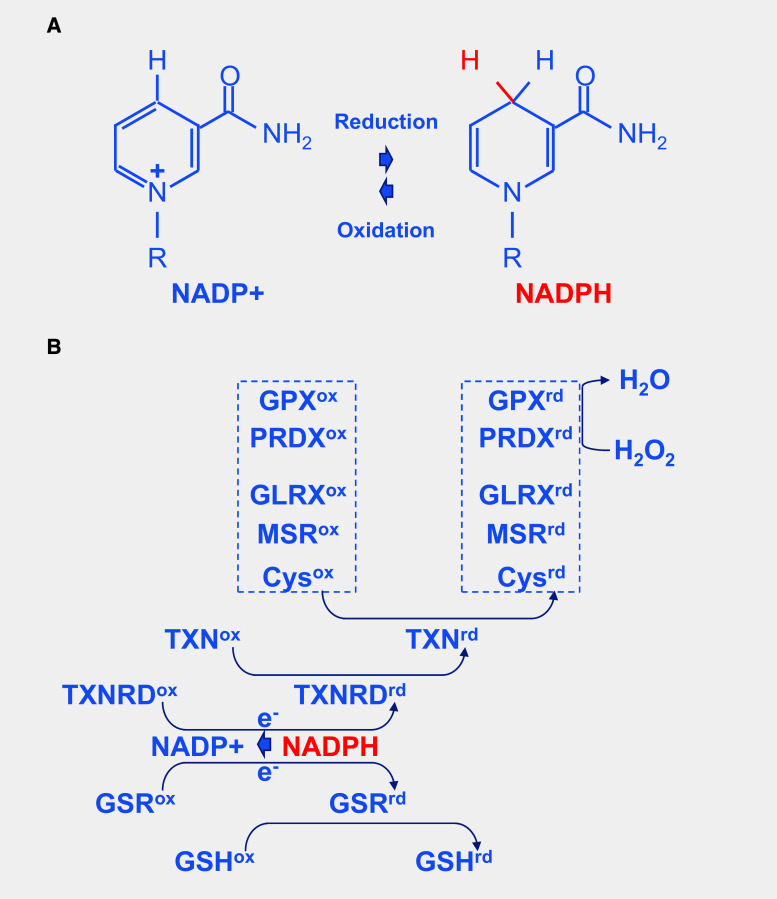
Alternatively, repairing enzymes reduces the oxidized groups in macromolecules. Importantly, the reversible oxidation of the thiol group in cysteines and methionines is the rational of redox signaling [104]. Cysteine is a rarely used amino acid that accounts for about 2 % of the amino acids in eukaryotic proteins. ROS and RNS can induce redox signals by means of oxidative modifications of cysteine residues. The large, polarizable sulfur atom in a thiol group is electron-rich and highly nucleophilic; hence, cysteines can undergo a broad range of chemical reactions. The sulfhydryl group (C–SH) is in equilibrium with C–S− and to the disulfide (S–S), can be oxidized by ROS to sulfenic (C–SOH), sulfinic (SO2H), and sulfonic (SO3H), or S-nitrosylated by RNS to C–SNO, and finally, in the presence of glutathione (GSH), S-thiolated to C–S–SG [6]. Sulfonic acid modifications, the most oxidized form of the thiol group, are irreversible and are thus deleterious oxidative damage, much as HNE adducts [105]. Cysteines differ in their reactivity properties depending of the protein microenvironment, and not all cysteines are susceptible to modifications. Most, but not all, of these modifications are reversible through reduction catalyzed by oxidoreductases, such as thioredoxins (TXN1 and 2), glutaredoxins (GLRX1-3 and 5), and sulfiredoxin (SRXN1). The prototype of thioredoxin proteins, TXN1, is a 12 kDa protein with a redox active conserved disulfide/dithiol group C32GPC35 (Fig. 8a). Reduced TXN1 catalyses the reduction of disulfide bounds in many proteins, and oxidized TXN1 is reversibly reduced by the action of thioredoxin reductases (TXNRD1–3) and NADPH [106]. TXN is secreted from cells by a hitherto unknown mechanism that is not dependent on a signal peptide, as for RdCVF [107]. Transgenic mice overexpressing human TXN1 have a statistically significant increase in life span [108]. Under oxidative conditions, heterologous disulfides can be formed non-enzymatically between proteins and the tripeptide glutathione (GSH), one of the most prevalent and important thiol buffers in the cell. Reduced GSH is oxidized to GSSG or in protein-S–SG (XS–SG). This reaction is termed S-glutathionylation [109]. S-Glutathionylation is a protection of protein thiols under oxidative conditions, since it can be reversed [110]. It prevents the sequential oxidation of thiol groups to sulfenic, sulfinic, and sulfonic acids; the latter is irreparably damaged [111]. Deglutathionylation is catalyzed by glutaredoxins (GLRX) through a monothiol reaction that depends only on the N-terminal active site cysteine residue (Fig. 8b). GSSG is reduced by the mitochondrial glutathione reductase (GSR). GSR requires riboflavin (vitamin B2) in the flavin adenine dinucleotide (FAD) coenzyme form to perform the reduction of GSH: FADH2 + GSSG > FAD + GSH [112].
Fig. 8.
Thioredoxin/glutaredoxin system. a The oxidoreduction reaction between the thioredoxin (TXN) and its substrate (X protein). Reduced thioredoxin TXN-SH2 binds to a target protein X via its hydrophobic surface area. Nucleophilic attack by the thiolate of Cys32 results in the formation of a transient mixed disulfide, which is followed by a nucleophilic attack of the deprotonated Cys35 generating oxidized TXN-S2 and the reduced protein, X-SH2. b Deglutathionylation reaction by glutaredoxin (GLRX). c The oxidation of methionine generates a diastereomeric mixture of two stereoisomers methionine S-sulfoxide and methionine R-sulfoxide. Met-SO methionine sulfoxide MSRA and MSRB methionine sulfoxide reductase A and B, ROS reactive oxygen species. d Methionine sulfoxide reductase A reaction. MSRA methionine sulfoxide reductase A, TXN thioredoxin, GLRX glutaredoxin reductase, GSH glutathione
Methionine is also sensitive to non-enzymatic oxidation by ROS. Methionine sulfoxide (Met-SO) can be further oxidized to sulfone (Met-SO2) by an irreversible oxidation. The oxidation of methionine generates a diastereomeric mixture of two stereoisomers methionine, S-sulfoxide and methionine R-sulfoxide, due to the asymmetry of sulfur atom that is repaired by methionine sulfoxide reductases: MSRA and MSRB1-3, respectively (Fig. 8c). Most MSR possess two cysteine residues (2-Cys-MSR), a catalytic cysteine and a recycling cysteine. For both MSRA and MSRB, the catalytic cysteine of the MSR first attacks the sulfur atom of Met-SO in a nucleophilic reaction. The catalytic cysteine forms a disulfide bond with the recycling cysteine, MSR is oxidized, and Met-SO is reduced to Met in the reaction (Fig. 8d). The recycling of MSR involves either TXN or GLRX.
The pentose phosphate pathway links glucose metabolism to redox signaling
The reduction of NADP+ to NADPH is particularly important, because it provides reducing power for most antioxidant and redox regulatory enzymes controlling cell redox homeostasis. Nicotinamide is derived from ATP [113] (Fig. 9a). In response to oxidative stress, glucose metabolism is diverted from energy formation to reductive biosynthesis [114]. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) harbors a strictly conserved catalytic cysteine, which is susceptible to a variety of thiol modifications, including inhibitory but reversible S-glutathionylation [115]. Oxidation of PKM at cysteine 358 (C358) stops the production of pyruvate and results in the accumulation of phosphoenol pyruvate (PEP) [93]. PEP, an allosteric inhibitor of triose phosphate isomerase (TPI), interrupts glycolysis, which produces an elevation of the intra-cellular concentration of glucose-6-phosphate (G6P). G6P, produced by the action of hexokinases on glucose entry to the cell via glucose transporters, is diverted to produce ribulose-5-phosphate (Ru5P) by the oxidative part of the pentose phosphate pathway, the enzymatic steps catalyzed by glucose-6-phosphate dehydrogenase (G6PDH) and 6-phosphogluconate dehydrogenase (6PGD), the later producing also CO2 (Fig. 7). Both G6PDH and PGD use NADP+ as a co-factor that is reduced to NADPH during the reaction. The electrons derived from NADPH are transferred to the glutathione and thioredoxin systems via thioredoxins reductases (TXNRD1–3) and glutathione reductase (GSR), respectively, to reduce ROS and to reduce oxidized proteins (Fig. 9b). Thus, the cellular antioxidant machineries are maintained with energy provided by glucose catabolism through NADPH-mediated electron transport [116]. G6PDH activity is regulated by the NADP+/NADPH ratio, NADPH inhibits its activity, whereas NADP+ is required for its proper active conformation [114]. In the non-oxidative part of the pentose phosphate pathway, Ru5P is converted to ribose-5-phosphate (R5P) by ribulose-5-phosphate isomerase (RPIA), and R5P might reenter the glycolytic pathway when converted in fructose-6-phosphate (F6P) or glyceraldehyde-3-phosphate (G3P) [117]. Increased flux of glucose through the pentose phosphate pathways can have a neuroprotective function [118]. The conversion of G6P to Ru5P generates two moles of NADPH and one of CO2, so that under conditions of excess oxidative stress requiring the maximal amount of NADPH, G6P is completely oxidized to CO2 by six complete cycles. The complete oxidation of glucose by cells to CO2 is likely to be detrimental to the organism, but in non-pathological conditions, the production of redox power through NADPH should balance the negative effect of oxidation of glycolytic enzymes, such as GAPDH and PKM and restore redox homeostasis (Fig. 10).
Fig. 9.
Redox power is regulated by the production of NADPH by the pentose phosphate pathway. a Oxidoreduction of NADP+ and NADPH. b Schematic drawing of the thioredoxin/glutaredoxin system. TXNRD thioredoxin reductase, GSR glutathione reductase, GSH glutathione, TXN thioredoxin, Cys cysteine, MSR methionine sulfoxide reductase, GLRX glutaredoxin, PRDX peroxiredoxin, GPX glutathione peroxidase. The suffix ox and rd represent the oxidized and reduced forms, respectively
Fig. 10.
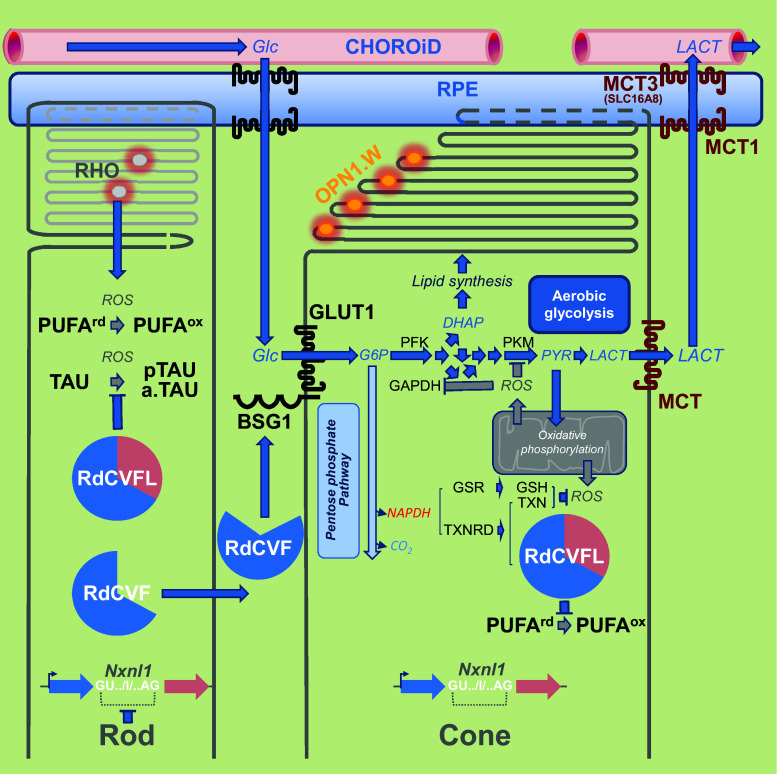
Metabolic and redox signaling of the NXNL1 gene products. Rods produce the thioredoxin RdCVFL and the trophic factor RdCVF by alternative splicing. Cones exclusively produce the thioredoxin RdCVFL. RPE: retinal pigmented epithelium. Left to right RHO rhodopsin, PUFA polyunsaturated fatty acid, TAU microtubule-associated protein TAU, BSG1 basigin-1, GLUT1 glucose transporter SLC2A1, Glc glucose, G6P glucose-6-phsphate, DHAP dihydroxyacetone phosphate, PYR pyruvate, LACT lactate, PFK phosphofructokinase, PKM pyruvate kinase M, GAPDH glyceraldehyde-3-phosphate dehydrogenase, ROS reactive oxygen species, NADPH nicotinamide adenine dinucleotide phosphate, TXNRD thioredoxin reductase, GSR glutathione reductase, TXN thioredoxin, GSH glutathione, MCT1 lactate transporter 1 (SLC16A1), MCT3 lactate transporter 3 (SLC16A8). The suffix p and a. represent, respectively, the phosphorylated and the aggregated forms. Framing the coding intron I of the NXNL1 genes, GU.. and ..AG, is the splicing donor and acceptor sites, respectively. The suffix ox and suffix rd represent the oxidized and reduced forms, respectively
Oxidative stress promotes retinal diseases
Epidemiological studies have suggested that elderly patients who consumed diets rich in antioxidants throughout their life are less likely to be afflicted with age-related macular degeneration (AMD) [119]. In animal models, the endogenous antioxidant molecule taurine and its derivatives are able to prevent photoreceptor degeneration following exposure to damaging light [120, 121]. There is also an attenuation of retinal photo-oxidative damage in thioredoxin transgenic mice [122]. There is an additional cause of oxidative stress to photoreceptors in the course of normal life or in inherited diseases with rod-specific mutations. A problem with the choroidal circulation lies in inefficient self-regulatory mechanisms that fail to meet the demands of the tissue it serves. One consequence is that when the oxygen consumption of the photoreceptors falls, oxygen tension in the outer retina rises sharply [123]. Consequently, the retinal oxygen pressure reaches values that approximate hyperoxia. Moreover, cone death can be delayed by reducing oxidative stress during disease [124, 125].
The disruption of the rod-derived cone viability gene leads to photoreceptor dysfunction and susceptibility to oxidative stress [58]. Cone function is normal in young Nxnl1 −/− mice, but deteriorates month-by-month as the mice age, indicating that the gene is involved in the late (but not early) developmental stages, or aging. Specifically, RdCVFL interacts with the microtubule-associated protein TAU (TAU) and prevents its oxidation in vitro. It also prevents its phosphorylation and aggregation in the retina [58, 126]. RdCVFL protects rod photoreceptors against photo-oxidative damage [64] and reduces the oxidation of polyunsaturated fatty acids induced by photoreceptor degeneration in the rd10 mouse [66]. RdCVFL is normally expressed by cones as well as rods in the mouse retina, while the trophic factor RdCVF is specifically expressed by rods: alternative splicing of the Nxnl1 gene is restricted to rods [127]. Differences between splicing pattern between rods and cones were revealed by the study of the PRPH2 gene [128]. The cones and their outer segments composed of polyunsaturated fatty acids are damaged by reactive oxygen species produced from leakage of the respiratory chain. In the absence of Nxnl1, the retina shows the signs of lipid peroxidation, as HNE adducts [58]. After selective recombination of the Nxnl1 gene in the cones, the retina also displayed signs of oxidative damage. Nevertheless, in the retina, the thiol-oxidoreductase activity of RdCVFL depends on its cycling between a reduced and an oxidized status as any other thioredoxin. Once oxidized, RdCVFL must be reduced by thioredoxin reductases [106]. The co-factor of thioredoxin reductases, NADPH, is produced mostly from glucose through the pentose phosphate pathway. In that respect, in cones, RdCVF likely acts upstream of RdCVFL by providing reducing power (Figs. 7 and 10). Reduced RdCVFL could reestablish aerobic glycolysis in cones after oxidative stress, and we have identified a potential interaction between RdCVFL and PKM [126]. One could speculate that in patients suffering from retinitis pigmentosa after the loss of expression of RdCVF produced by rods, cones become non-functional and die within several years as a result of the loss RdCVFL expression and subsequent oxidative damage in cones. Thus, a therapy aimed at preventing secondary cone degeneration should be pursued using both RdCVF and RdCVFL.
Perspectives opened by metabolic and redox signaling in the retina
The retina is a biological model system that was at the origin of many breakthroughs in biology and the metabolic and redox signaling revealed by the study of the mechanism of secondary degeneration of cones in retinitis pigmentosa might be part of these scientific founding principles. Nevertheless, we are perfectly aware that our report will become obsolete in the near future, since the research on the retina is a very active field. We simply intend to draw here the big scene that could both incorporate the numerous new findings that can be anticipated and evolve accordingly.
Acknowledgments
Funding was provided by the Institut National de la Santé et de la Recherche Médicale (INSERM). We thank A. Carruthers, A. Holmgren, M. Perluigi, and A. van Dorsselaer for their inspiring scientific comments during the first workshop on metabolic and redox signaling in the retina organized at the Collège de France on March 16th 2016. Video recording of this event can be downloaded at http://www.college-de-france.fr/site/jose-alain-sahel/studyday-2016-03-16-14h15.htm on the Collège de France Web site.
Abbreviations
- 6PGD
6-Phosphogluconate dehydrogenase
- ADIPOR1
Adiponectin receptor protein 1
- AMD
Age-related macular degeneration
- BSG1
Basigin-1
- BSG2
Basigin-2
- DHA
Docosahexaenoic acid
- DHAP
Dihydroxyacetone phosphate
- F6P
Fructose-6-phosphate
- FABP
Fatty acid-binding proteins
- FAD
Flavin adenine dinucleotide
- FBP
Fructose-1,6-bisphosphate
- G3P
Glyceraldehyde-3-phosphate
- G6P
Glucose-6-phosphate
- G6PDH
Glucose-6-phosphate dehydrogenase
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- GLRX
Glutaredoxin
- GLUT1
Facilitated glucose transporter SLC2A1
- GPX
Glutathione peroxidase
- GSH
Glutathione
- GSR
Glutathione reductase
- HK
Hexokinase
- HMGCS2
HMG-coenzyme A-synthase 2
- HNE
Hydroxy-2-nonenal
- IFT
Intra-flagellar transport
- Ig
Immunoglobulin domain
- IPM
Inter-photoreceptor matrix
- IRBP
Inter-photoreceptor retinoid-binding protein
- MCT1
Monocarboxylate transporter 1 SLC16A1
- MCT3
Monocarboxylate transporter 3 SLC16A8
- MDA
Malondialdehyde
- Met-SO
Methionine sulfoxide
- MFSD2A
Sodium-dependent lysophosphatidylcholine symporter 1
- MSR
Methionine sulfoxide reductases
- NADH
Nicotinamide adenine dinucleotide
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NO
Nitric oxide
- NXNL1
Nucleoredoxin-like-1 gene
- OPN1LW
Long-wave-sensitive opsin 1
- OPN1MW
Medium-wave-sensitive opsin 1
- OPN1SW
Short-wave-sensitive opsin 1
- PDE6B
Phosphodiesterase-b subunit
- PEP
Phosphoenolpyruvate
- PKM
Pyruvate kinase isoform M
- PPP
Pentose phosphate pathway
- PRDX
Peroxiredoxin
- PRPH2
Peripherin-2
- PUFA
Polyunsaturated fatty acids
- R5P
Ribose-5-phosphate
- rd1
Retinal degeneration 1 mouse
- RdCVF
Rod-derived cone viability factor
- RdCVF2
Rod-derived cone viability factor 2
- RdCVF2L
Rod-derived cone viability factor 2 long
- RdCVFL
Rod-derived cone viability factor long
- RHO
Rhodopsin
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- RPE
Retinal pigmented epithelium
- RPIA
Ribulose-5-phosphate isomerase
- Ru5P
Ribulose-5-phosphate
- SLC
Solute carrier family of transporters
- SLC16A
Monocarboxylate transporter family 16
- SRXN
Sulfiredoxin
- TAU
Microtubule-associated protein TAU
- TCA
Tricarboxylic acid
- TPI
Triose phosphate isomerase
- TXN
Thioredoxin
- TXNRD
Thioredoxin reductase
References
- 1.Kennedy B, Malicki J. What drives cell morphogenesis: a look inside the vertebrate photoreceptor. Dev Dyn. 2009;238(9):2115–2138. doi: 10.1002/dvdy.22010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giusto NM, Pasquare SJ, Salvador GA, Castagnet PI, Roque ME, de Boschero MGI. Lipid metabolism in vertebrate retinal rod outer segments. Prog Lipid Res. 2000;39(4):315–391. doi: 10.1016/S0163-7827(00)00009-6. [DOI] [PubMed] [Google Scholar]
- 3.Antonny B, Vanni S, Shindou H, Ferreira T. From zero to six double bonds: phospholipid unsaturation and organelle function. Trends Cell Biol. 2015;25(7):427–436. doi: 10.1016/j.tcb.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Catala A. An overview of lipid peroxidation with emphasis in outer segments of photoreceptors and the chemiluminescence assay. Int J Biochem Cell Biol. 2006;38(9):1482–1495. doi: 10.1016/j.biocel.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Tanito M, Elliott MH, Kotake Y, Anderson RE. Protein modifications by 4-hydroxynonenal and 4-hydroxyhexenal in light-exposed rat retina. Invest Ophthalmol Vis Sci. 2005;46(10):3859–3868. doi: 10.1167/iovs.05-0672. [DOI] [PubMed] [Google Scholar]
- 6.Kim HJ, Ha S, Lee HY, Lee KJ. ROSics: chemistry and proteomics of cysteine modifications in redox biology. Mass Spectrom Rev. 2015;34(2):184–208. doi: 10.1002/mas.21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology (Bethesda) 2010;25(1):8–15. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuang JZ, Zhao Y, Sung CH. SARA-regulated vesicular targeting underlies formation of the light-sensing organelle in mammalian rods. Cell. 2007;130(3):535–547. doi: 10.1016/j.cell.2007.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgoyne T, Meschede IP, Burden JJ, Bailly M, Seabra MC, Futter CE. Rod disc renewal occurs by evagination of the ciliary plasma membrane that makes cadherin-based contacts with the inner segment. Proc Natl Acad Sci USA. 2015;112(52):15922–15927. doi: 10.1073/pnas.1509285113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volland S, Hughes LC, Kong C, Burgess BL, Linberg KA, Luna G, Zhou ZH, Fisher SK, Williams DS. Three-dimensional organization of nascent rod outer segment disk membranes. Proc Natl Acad Sci USA. 2015;112(48):14870–14875. doi: 10.1073/pnas.1516309112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33(1):61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bibb C, Young RW. Renewal of fatty acids in the membranes of visual cell outer segments. J Cell Biol. 1974;61(2):327–343. doi: 10.1083/jcb.61.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Packard A. Cephalopods and fish: the limits of convergence. Biol Rev. 1972;47(2):241–307. doi: 10.1111/j.1469-185X.1972.tb00975.x. [DOI] [Google Scholar]
- 14.Ryan SJ. Retina. 4. Philadelphia: Elsevier Mosby; 2006. [Google Scholar]
- 15.Nguyen LN, Ma DL, Shui GH, Wong PY, Cazenave-Gassiot A, Zhang XD, Wenk MR, Goh ELK, Silver DL. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509(7501):503. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 16.Rice DS, Calandria JM, Gordon WC, Jun B, Zhou YD, Gelfman CM, Li SH, Jin MH, Knott EJ, Chang B, Abuin A, Issa T, Potter D, Platt KA, Bazan NG. Adiponectin receptor 1 conserves docosahexaenoic acid and promotes photoreceptor cell survival. Nature Commun. 2015;6:6228. doi: 10.1038/ncomms7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong BH, Chan JP, Cazenave-Gassiot A, Poh RW, Foo JC, Galam DLA, Ghosh S, Nguyen LN, Barathi VA, Wey YS, Luu CD, Wenk MR, Silver DL. Mfsd2a is a transporter for the essential omega-3 fatty acid DHA in eye and important for photoreceptor cell development. J Biol Chem. 2016;291(20):10501–10514. doi: 10.1074/jbc.M116.721340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Fernandez F. Interphotoreceptor retinoid-binding protein—an old gene for new eyes. Vis Res. 2003;43(28):3021–3036. doi: 10.1016/j.visres.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Fernandez F, Ghosh D. Focus on molecules: interphotoreceptor retinoid-binding protein (IRBP) Exp Eye Res. 2008;86(2):169–170. doi: 10.1016/j.exer.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Deturco EBR, Gordon WC, Bazan NG. Docosahexaenoic acid is taken up by the inner segment of frog photoreceptors leading to an active synthesis of docosahexaenoyl-inositol lipids—similarities in metabolism in-vivo and in-vitro. Curr Eye Res. 1994;13(1):21–28. doi: 10.3109/02713689409042394. [DOI] [PubMed] [Google Scholar]
- 21.Berg JM, Tymoczko JL, Stryer L. Biochemistry. 5. New York: W.H. Freeman; 2002. [Google Scholar]
- 22.Adijanto J, Du J, Moffat C, Seifert EL, Hurle JB, Philp NJ. The retinal pigment epithelium utilizes fatty acids for ketogenesis. J Biol Chem. 2014;289(30):20570–20582. doi: 10.1074/jbc.M114.565457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tom Dieck S, Brandstatter JH. Ribbon synapses of the retina. Cell Tissue Res. 2006;326(2):339–346. doi: 10.1007/s00441-006-0234-0. [DOI] [PubMed] [Google Scholar]
- 24.Papermaster DS, Dreyer WJ. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 1974;13(11):2438–2444. doi: 10.1021/bi00708a031. [DOI] [PubMed] [Google Scholar]
- 25.Koch KW, Dell’Orco D. Protein and signaling networks in vertebrate photoreceptor cells. Front Mol Neurosci. 2015;8:67. doi: 10.3389/fnmol.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006;75:743–767. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baehr W. Membrane protein transport in photoreceptors: the function of PDE delta the proctor lecture. Invest Ophthalmol Vis Sci. 2014;55(12):8653–8666. doi: 10.1167/iovs.14-16066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42(2):392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young RW. A difference between rods and cones in the renewal of outer segment protein. Investig Ophthalmol. 1969;8(2):222–231. [PubMed] [Google Scholar]
- 30.Schultze M. Zur Anatomie und Physiologie der Retina. Archiv für Mikroskopische Anatomie. 1866;2(1):175–286. doi: 10.1007/BF02962033. [DOI] [Google Scholar]
- 31.Okano T, Fukada Y, Yoshizawa T. Molecular basis for tetrachromatic color vision. Comp Biochem Physiol B Biochem Mol Biol. 1995;112(3):405–414. doi: 10.1016/0305-0491(95)00085-2. [DOI] [PubMed] [Google Scholar]
- 32.Hall MI, Kamilar JM, Kirk EC. Eye shape and the nocturnal bottleneck of mammals. Proc Biol Sci R Soc. 2012;279(1749):4962–4968. doi: 10.1098/rspb.2012.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godefroit P, Sinitsa SM, Dhouailly D, Bolotsky YL, Sizov AV, McNamara ME, Benton MJ, Spagna P. A Jurassic ornithischian dinosaur from Siberia with both feathers and scales. Science. 2014;345(6195):451–455. doi: 10.1126/science.1253351. [DOI] [PubMed] [Google Scholar]
- 34.Bhullar BAS, Marugan-Lobon J, Racimo F, Bever GS, Rowe TB, Norell MA, Abzhanov A. Birds have paedomorphic dinosaur skulls. Nature. 2012;487(7406):223–226. doi: 10.1038/nature11146. [DOI] [PubMed] [Google Scholar]
- 35.Araujo AC, Didonet JJ, Araujo CS, Saletti PG, Borges TR, Pessoa VF. Color vision in the black howler monkey (Alouatta caraya) Vis Neurosci. 2008;25(3):243–248. doi: 10.1017/S0952523808080292. [DOI] [PubMed] [Google Scholar]
- 36.Lamb TD. Evolution of phototransduction, vertebrate photoreceptors and retina. Prog Retinal Eye Res. 2013;36:52–119. doi: 10.1016/j.preteyeres.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Fain GL, Hardie R, Laughlin SB. Phototransduction and the evolution of photoreceptors. Curr Biol. 2010;20(3):R114–R124. doi: 10.1016/j.cub.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morshedian A, Fain GL. Single-photon sensitivity of lamprey rods with cone-like outer segments. Curr Biol. 2015;25(4):484–487. doi: 10.1016/j.cub.2014.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clements T, Dolocan A, Martin P, Purnell MA, Vinther J, Gabbott SE. The eyes of Tullimonstrum reveal a vertebrate affinity. Nature. 2016;532(7600):500–503. doi: 10.1038/nature17647. [DOI] [PubMed] [Google Scholar]
- 40.Warrant EJ. Photoreceptor evolution: ancient ‘cones’ turn out to be rods. Curr Biol. 2015;25(4):R148–R151. doi: 10.1016/j.cub.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Pontzer H, Brown MH, Raichlen DA, Dunsworth H, Hare B, Walker K, Luke A, Dugas LR, Durazo-Arvizu R, Schoeller D, Plange-Rhule J, Bovet P, Forrester TE, Lambert EV, Thompson ME, Shumaker RW, Ross SR. Metabolic acceleration and the evolution of human brain size and life history. Nature. 2016;533(7603):390–392. doi: 10.1038/nature17654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isler K, van Schaik CP. The expensive brain: a framework for explaining evolutionary changes in brain size. J Hum Evol. 2009;57(4):392–400. doi: 10.1016/j.jhevol.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 43.Damasio AR. Looking for Spinoza joy, sorrow and the feeling brain. London: Vintage; 1998. [Google Scholar]
- 44.Choi RY, Engbretson GA, Solessio EC, Jones GA, Coughlin A, Aleksic I, Zuber ME. Cone degeneration following rod ablation in a reversible model of retinal degeneration. Invest Ophthalmol Vis Sci. 2011;52(1):364–373. doi: 10.1167/iovs.10-5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stearns G, Evangelista M, Fadool JM, Brockerhoff SE. A mutation in the cone-specific pde6 gene causes rapid cone photoreceptor degeneration in zebrafish. J Neurosci. 2007;27(50):13866–13874. doi: 10.1523/JNEUROSCI.3136-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Punzo C, Kornacker K, Cepko CL. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci. 2009;12(1):44–52. doi: 10.1038/nn.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCall MA, Gregg RG, Merriman K, Goto Y, Peachey NS, Stanford LR. Morphological and physiological consequences of the selective elimination of rod photoreceptors in transgenic mice. Exp Eye Res. 1996;63(1):35–50. doi: 10.1006/exer.1996.0089. [DOI] [PubMed] [Google Scholar]
- 48.Wright AF. A searchlight through the fog. Nat Genet. 1997;17(2):132–134. doi: 10.1038/ng1097-132. [DOI] [PubMed] [Google Scholar]
- 49.Leveillard T, Sahel JA. Rod-derived cone viability factor for treating blinding diseases: from clinic to redox signaling. Sci Transl Med. 2010;2(26):26ps16. doi: 10.1126/scitranslmed.3000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leveillard T. Spare the rod, spoil the degeneration. J Pediatr Ophthalmol Strabismus. 2014;51(2):74. doi: 10.3928/01913913-20140220-06. [DOI] [PubMed] [Google Scholar]
- 51.Carter-Dawson LD, LaVail MM, Sidman RL. Differential effect of the rd mutation on rods and cones in the mouse retina. Invest Ophthalmol Vis Sci. 1978;17(6):489–498. [PubMed] [Google Scholar]
- 52.Bowes C, Li T, Danciger M, Baxter LC, Applebury ML, Farber DB. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990;347(6294):677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- 53.McLaughlin ME, Sandberg MA, Berson EL, Dryja TP. Recessive mutations in the gene encoding the beta-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nat Genet. 1993;4(2):130–134. doi: 10.1038/ng0693-130. [DOI] [PubMed] [Google Scholar]
- 54.Mohand-Said S, Hicks D, Simonutti M, Tran-Minh D, Deudon-Combe A, Dreyfus H, Silverman MS, Ogilvie JM, Tenkova T, Sahel J. Photoreceptor transplants increase host cone survival in the retinal degeneration (rd) mouse. Ophthalmic Res. 1997;29(5):290–297. doi: 10.1159/000268027. [DOI] [PubMed] [Google Scholar]
- 55.Mohand-Said S, Deudon-Combe A, Hicks D, Simonutti M, Forster V, Fintz AC, Leveillard T, Dreyfus H, Sahel JA. Normal retina releases a diffusible factor stimulating cone survival in the retinal degeneration mouse. Proc Natl Acad Sci USA. 1998;95(14):8357–8362. doi: 10.1073/pnas.95.14.8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leveillard T, Mohand-Said S, Lorentz O, Hicks D, Fintz AC, Clerin E, Simonutti M, Forster V, Cavusoglu N, Chalmel F, Dolle P, Poch O, Lambrou G, Sahel JA. Identification and characterization of rod-derived cone viability factor. Nat Genet. 2004;36(7):755–759. doi: 10.1038/ng1386. [DOI] [PubMed] [Google Scholar]
- 57.Reichman S, Kalathur RK, Lambard S, Ait-Ali N, Yang Y, Lardenois A, Ripp R, Poch O, Zack DJ, Sahel JA, Leveillard T. The homeobox gene CHX10/VSX2 regulates RdCVF promoter activity in the inner retina. Hum Mol Genet. 2010;19(2):250–261. doi: 10.1093/hmg/ddp484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cronin T, Raffelsberger W, Lee-Rivera I, Jaillard C, Niepon ML, Kinzel B, Clerin E, Petrosian A, Picaud S, Poch O, Sahel JA, Leveillard T. The disruption of the rod-derived cone viability gene leads to photoreceptor dysfunction and susceptibility to oxidative stress. Cell Death Differ. 2010;17(7):1199–1210. doi: 10.1038/cdd.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clerin E, Wicker N, Mohand-Said S, Poch O, Sahel JA, Leveillard T. e-conome: an automated tissue counting platform of cone photoreceptors for rodent models of retinitis pigmentosa. BMC Ophthalmol. 2011;11:38. doi: 10.1186/1471-2415-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jaillard C, Mouret A, Niepon ML, Clerin E, Yang Y, Lee-Rivera I, Ait-Ali N, Millet-Puel G, Cronin T, Sedmak T, Raffelsberger W, Kinzel B, Trembleau A, Poch O, Bennett J, Wolfrum U, Lledo PM, Sahel JA, Leveillard T. Nxnl2 splicing results in dual functions in neuronal cell survival and maintenance of cell integrity. Hum Mol Genet. 2012;21(10):2298–2311. doi: 10.1093/hmg/dds050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang XW, Liou YC, Ho B, Ding JL. An evolutionarily conserved 16-kDa thioredoxin-related protein is an antioxidant which regulates the NF-kappaB signaling pathway. Free Radic Biol Med. 2007;42(2):247–259. doi: 10.1016/j.freeradbiomed.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 62.Wang XW, Tan BZ, Sun M, Ho B, Ding JL. Thioredoxin-like 6 protects retinal cell line from photooxidative damage by upregulating NF-kappaB activity. Free Radic Biol Med. 2008;45(3):336–344. doi: 10.1016/j.freeradbiomed.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 63.Brennan LA, Lee W, Kantorow M. TXNL6 is a novel oxidative stress-induced reducing system for methionine sulfoxide reductase a repair of alpha-crystallin and cytochrome C in the eye lens. PLoS One. 2010;5(11):e15421. doi: 10.1371/journal.pone.0015421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elachouri G, Lee-Rivera I, Clerin E, Argentini M, Fridlich R, Blond F, Ferracane V, Yang Y, Raffelsberger W, Wan J, Bennett J, Sahel JA, Zack DJ, Leveillard T. Thioredoxin rod-derived cone viability factor protects against photooxidative retinal damage. Free Radic Biol Med. 2015;81C:22–29. doi: 10.1016/j.freeradbiomed.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Chalmel F, Leveillard T, Jaillard C, Lardenois A, Berdugo N, Morel E, Koehl P, Lambrou G, Holmgren A, Sahel JA, Poch O. Rod-derived cone viability factor-2 is a novel bifunctional-thioredoxin-like protein with therapeutic potential. BMC Mol Biol. 2007;8:74. doi: 10.1186/1471-2199-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Byrne LC, Dalkara D, Luna G, Fisher SK, Clerin E, Sahel JA, Leveillard T, Flannery JG. Viral-mediated RdCVF and RdCVFL expression protects cone and rod photoreceptors in retinal degeneration. J Clin Investig. 2015;125(1):105–116. doi: 10.1172/JCI65654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang Y, Mohand-Said S, Danan A, Simonutti M, Fontaine V, Clerin E, Picaud S, Leveillard T, Sahel JA. Functional cone rescue by RdCVF protein in a dominant model of retinitis pigmentosa. Mol Ther J Am Soc Gene Ther. 2009;17(5):787–795. doi: 10.1038/mt.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.RetNet (2016). https://sph.uth.edu/retnet/sum-dis.htm
- 69.Yang Y, Mohand-Said S, Danan A, Simonutti M, Fontaine V, Clerin E, Picaud S, Leveillard T, Sahel JA. Functional cone rescue by RdCVF protein in a dominant model of retinitis pigmentosa. Mol Ther. 2009;17(5):787–795. doi: 10.1038/mt.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sandberg MA, Rosner B, Weigel-DiFranco C, Dryja TP, Berson EL. Disease course of patients with X-linked retinitis pigmentosa due to RPGR gene mutations. Invest Ophthalmol Vis Sci. 2007;48(3):1298–1304. doi: 10.1167/iovs.06-0971. [DOI] [PubMed] [Google Scholar]
- 71.Geller AM, Sieving PA. Assessment of foveal cone photoreceptors in Stargardt’s macular dystrophy using a small dot detection task. Vis Res. 1993;33(11):1509–1524. doi: 10.1016/0042-6989(93)90144-L. [DOI] [PubMed] [Google Scholar]
- 72.Ait-Ali N, Fridlich R, Millet-Puel G, Clerin E, Delalande F, Jaillard C, Blond F, Perrocheau L, Reichman S, Byrne LC, Olivier-Bandini A, Bellalou J, Moyse E, Bouillaud F, Nicol X, Dalkara D, van Dorsselaer A, Sahel JA, Leveillard T. Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell. 2015;161(4):817–832. doi: 10.1016/j.cell.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 73.Ochrietor JD, Moroz TP, van Ekeris L, Clamp MF, Jefferson SC, deCarvalho AC, Fadool JM, Wistow G, Muramatsu T, Linser PJ. Retina-specific expression of 5A11/Basigin-2, a member of the immunoglobulin gene superfamily. Invest Ophthalmol Vis Sci. 2003;44(9):4086–4096. doi: 10.1167/iovs.02-0995. [DOI] [PubMed] [Google Scholar]
- 74.Cepko C, Punzo C. Cell metabolism: sugar for sight. Nature. 2015;522(7557):428–429. doi: 10.1038/522428a. [DOI] [PubMed] [Google Scholar]
- 75.Krol J, Roska B. Rods feed cones to keep them alive. Cell. 2015;161(4):706–708. doi: 10.1016/j.cell.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 76.Ma S, Venkatesh A, Langellotto F, Le YZ, Hall MN, Ruegg MA, Punzo C. Loss of mTOR signaling affects cone function, cone structure and expression of cone specific proteins without affecting cone survival. Exp Eye Res. 2015;135:1–13. doi: 10.1016/j.exer.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Venkatesh A, Ma S, Le YZ, Hall MN, Ruegg MA, Punzo C. Activated mTORC1 promotes long-term cone survival in retinitis pigmentosa mice. J Clin Investig. 2015;125(4):1446–1458. doi: 10.1172/JCI79766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rajala A, Dighe R, Agbaga MP, Anderson RE, Rajala RV. Insulin receptor signaling in cones. J Biol Chem. 2013;288(27):19503–19515. doi: 10.1074/jbc.M113.469064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ivanovic I, Anderson RE, Le YZ, Fliesler SJ, Sherry DM, Rajala RV. Deletion of the p85alpha regulatory subunit of phosphoinositide 3-kinase in cone photoreceptor cells results in cone photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2011;52(6):3775–3783. doi: 10.1167/iovs.10-7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Zutter JK, Levine KB, Deng D, Carruthers A. Sequence determinants of GLUT1 oligomerization: analysis by homology-scanning mutagenesis. J Biol Chem. 2013;288(28):20734–20744. doi: 10.1074/jbc.M113.469023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hebert DN, Carruthers A. Glucose transporter oligomeric structure determines transporter function. Reversible redox-dependent interconversions of tetrameric and dimeric GLUT1. J Biol Chem. 1992;267(33):23829–23838. [PubMed] [Google Scholar]
- 82.Krebs HA. The Pasteur effect and the relations between respiration and fermentation. Essays Biochem. 1972;8:1–34. [PubMed] [Google Scholar]
- 83.Casson RJ, Chidlow G, Han G, Wood JP. An explanation for the Warburg effect in the adult mammalian retina. Clin Exp Ophthalmol. 2013;41(5):517. doi: 10.1111/ceo.12050. [DOI] [PubMed] [Google Scholar]
- 84.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leveillard T. Cancer metabolism of cone photoreceptors. Oncotarget. 2015;6(32):32285–32286. doi: 10.18632/oncotarget.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sebastian C, Zwaans BM, Silberman DM, Gymrek M, Goren A, Zhong L, Ram O, Truelove J, Guimaraes AR, Toiber D, Cosentino C, Greenson JK, Macdonald AI, McGlynn L, Maxwell F, Edwards J, Giacosa S, Guccione E, Weissleder R, Bernstein BE, Regev A, Shiels PG, Lombard DB, Mostoslavsky R. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell. 2012;151(6):1185–1199. doi: 10.1016/j.cell.2012.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bender T, Martinou JC. The mitochondrial pyruvate carrier in health and disease: to carry or not to carry? Biochim Biophys Acta. 2016;1863(10):2436–2442. doi: 10.1016/j.bbamcr.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 88.Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206(Pt 12):2049–2057. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- 89.Casson J, Wood JPM, Han G, Kittipassorn T, Peet DJ, Chidlow G. M-type pyruvate kinase isoforms and lactate dehydrogenase a in the mammalian retina: metabolic implications. Investig Ophthalmol Vis Sci. 2016;57(1):66–80. doi: 10.1167/iovs.15-17962. [DOI] [PubMed] [Google Scholar]
- 90.Lindsay KJ, Du J, Sloat SR, Contreras L, Linton JD, Turner SJ, Sadilek M, Satrustegui J, Hurley JB. Pyruvate kinase and aspartate-glutamate carrier distributions reveal key metabolic links between neurons and glia in retina. Proc Natl Acad Sci USA. 2014;111(43):15579–15584. doi: 10.1073/pnas.1412441111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reidel B, Thompson JW, Farsiu S, Moseley MA, Skiba NP, Arshavsky VY. Proteomic profiling of a layered tissue reveals unique glycolytic specializations of photoreceptor cells. Mol Cell Proteom. 2011;10(3):M110-002469. doi: 10.1074/mcp.M110.002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Israelsen WJ, Vander Heiden MG. Pyruvate kinase: function, regulation and role in cancer. Semin Cell Dev Biol. 2015;43:43–51. doi: 10.1016/j.semcdb.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, Thomas CJ, Vander Heiden MG, Cantley LC. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334(6060):1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang W, Lee SJ, Scott PA, Lu X, Emery D, Liu Y, Ezashi T, Roberts MR, Ross JW, Kaplan HJ, Dean DC. Two-step reactivation of dormant cones in retinitis pigmentosa. Cell Rep. 2016;15(2):372–385. doi: 10.1016/j.celrep.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Horton JC, Parker AB, Botelho JV, Duncan JL. Spontaneous regeneration of human photoreceptor outer segments. Sci Rep. 2015;5:12364. doi: 10.1038/srep12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guerin CJ, Anderson DH, Fariss RN, Fisher SK. Retinal reattachment of the primate macula. Photoreceptor recovery after short-term detachment. Invest Ophthalmol Vis Sci. 1989;30(8):1708–1725. [PubMed] [Google Scholar]
- 97.Terauchi G, Shinoda K, Matsumoto CS, Watanabe E, Matsumoto H, Mizota A. Recovery of photoreceptor inner and outer segment layer thickness after reattachment of rhegmatogenous retinal detachment. Brit J Ophthalmol. 2015;99(10):1323–1327. doi: 10.1136/bjophthalmol-2014-306252. [DOI] [PubMed] [Google Scholar]
- 98.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 99.Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mech Ageing Dev. 2004;125(10–11):811–826. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 100.Finkel T. The metabolic regulation of aging. Nat Med. 2015;21(12):1416–1423. doi: 10.1038/nm.3998. [DOI] [PubMed] [Google Scholar]
- 101.Nohl H, Gille L, Staniek K. Intracellular generation of reactive oxygen species by mitochondria. Biochem Pharmacol. 2005;69(5):719–723. doi: 10.1016/j.bcp.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 102.Sanz A. Mitochondrial reactive oxygen species: do they extend or shorten animal lifespan? Bioch Biophys Acta. 2016;1857(8):1116–1126. doi: 10.1016/j.bbabio.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 103.Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA. 2009;106(21):8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pillay CS, Eagling BD, Driscoll SR, Rohwer JM. Quantitative measures for redox signaling. Free Radic Biol Med. 2016;96:290–303. doi: 10.1016/j.freeradbiomed.2016.04.199. [DOI] [PubMed] [Google Scholar]
- 105.Chung HS, Wang SB, Venkatraman V, Murray CI, Van Eyk JE. Cysteine oxidative posttranslational modifications: emerging regulation in the cardiovascular system. Circ Res. 2013;112(2):382–392. doi: 10.1161/CIRCRESAHA.112.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 107.Rubartelli A, Bajetto A, Allavena G, Wollman E, Sitia R. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J Biol Chem. 1992;267(34):24161–24164. [PubMed] [Google Scholar]
- 108.Mitsui A, Hamuro J, Nakamura H, Kondo N, Hirabayashi Y, Ishizaki-Koizumi S, Hirakawa T, Inoue T, Yodoi J (2002) Overexpression of human thioredoxin in transgenic mice controls oxidative stress and life span. Antioxid redox signal 4(4):693–696 [DOI] [PubMed]
- 109.Grek CL, Zhang J, Manevich Y, Townsend DM, Tew KD. Causes and consequences of cysteine S-glutathionylation. J Biol Chem. 2013;288(37):26497–26504. doi: 10.1074/jbc.R113.461368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Herrero E, de la Torre-Ruiz MA. Monothiol glutaredoxins: a common domain for multiple functions. Cell Mol Life Sci. 2007;64(12):1518–1530. doi: 10.1007/s00018-007-6554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xiong Y, Uys JD, Tew KD, Townsend DM. S-Glutathionylation: from molecular mechanisms to health outcomes. Antioxid Redox Signal. 2011;15(1):233–270. doi: 10.1089/ars.2010.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ashoori M, Saedisomeolia A. Riboflavin (vitamin B2) and oxidative stress: a review. Brit J Nutr. 2014;111(11):1985–1991. doi: 10.1017/S0007114514000178. [DOI] [PubMed] [Google Scholar]
- 113.Berg JM, Tymoczko JL, Stryer L (2002) Biochemistry, 5th ed. In: Berg J, Tymoczko J, Stryer L/web content by Clarke ND (eds). W.H. Freeman and Co., New York [Basingstoke: Palgrave] [distributor], 2001
- 114.Patra KC, Hay N. The pentose phosphate pathway and cancer. Trends Biochem Sci. 2014;39(8):347–354. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hildebrandt T, Knuesting J, Berndt C, Morgan B, Scheibe R. Cytosolic thiol switches regulating basic cellular functions: GAPDH as an information hub? Biol Chem. 2015;396(5):523–537. doi: 10.1515/hsz-2014-0295. [DOI] [PubMed] [Google Scholar]
- 116.Oka S, Hsu CP, Sadoshima J. Regulation of cell survival and death by pyridine nucleotides. Circ Res. 2012;111(5):611–627. doi: 10.1161/CIRCRESAHA.111.247932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bouzier-Sore AK, Bolanos JP. Uncertainties in pentose-phosphate pathway flux assessment underestimate its contribution to neuronal glucose consumption: relevance for neurodegeneration and aging. Front Aging Neurosci. 2015;7:89. doi: 10.3389/fnagi.2015.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schubert D. Glucose metabolism and Alzheimer’s disease. Ageing Res Rev. 2005;4(2):240–257. doi: 10.1016/j.arr.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 119.Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- 120.Pasantes-Morales H, Cruz C. Taurine and hypotaurine inhibit light-induced lipid peroxidation and protect rod outer segment structure. Brain Res. 1985;330(1):154–157. doi: 10.1016/0006-8993(85)90018-6. [DOI] [PubMed] [Google Scholar]
- 121.Boatright JH, Moring AG, McElroy C, Phillips MJ, Do VT, Chang B, Hawes NL, Boyd AP, Sidney SS, Stewart RE, Minear SC, Chaudhury R, Ciavatta VT, Rodrigues CM, Steer CJ, Nickerson JM, Pardue MT. Tool from ancient pharmacopoeia prevents vision loss. Mol Vis. 2006;12:1706–1714. [PubMed] [Google Scholar]
- 122.Tanito M, Masutani H, Nakamura H, Oka S, Ohira A, Yodoi J. Attenuation of retinal photooxidative damage in thioredoxin transgenic mice. Neurosci Lett. 2002;326(2):142–146. doi: 10.1016/S0304-3940(02)00314-2. [DOI] [PubMed] [Google Scholar]
- 123.Stone J, Maslim J, Valter-Kocsi K, Mervin K, Bowers F, Chu Y, Barnett N, Provis J, Lewis G, Fisher SK, Bisti S, Gargini C, Cervetto L, Merin S, Peer J. Mechanisms of photoreceptor death and survival in mammalian retina. Prog Retinal Eye Res. 1999;18(6):689–735. doi: 10.1016/S1350-9462(98)00032-9. [DOI] [PubMed] [Google Scholar]
- 124.Komeima K, Rogers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci USA. 2006;103(30):11300–11305. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xiong W, MacColl Garfinkel AE, Li Y, Benowitz LI, Cepko CL. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J Clin Investig. 2015;125(4):1433–1445. doi: 10.1172/JCI79735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fridlich R, Delalande F, Jaillard C, Lu J, Poidevin L, Cronin T, Perrocheau L, Millet-Puel G, Niepon ML, Poch O, Holmgren A, Van Dorsselaer A, Sahel JA, Leveillard T. The thioredoxin-like protein rod-derived cone viability factor (RdCVFL) interacts with TAU and inhibits its phosphorylation in the retina. Mol Cell Proteom. 2009;8(6):1206–1218. doi: 10.1074/mcp.M800406-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mei X, Chaffiol A, Kole C, Yang Y, Millet-Puel G, Clerin E, Ait-Ali N, Bennett J, Dalkara D, Sahel JA, Duebel J, Leveillard T. The thioredoxin encoded by the rod-derived cone viability factor gene protects cone photoreceptors against oxidative stress. Antioxid Redox Signal. 2016;24(16):909–923. doi: 10.1089/ars.2015.6509. [DOI] [PubMed] [Google Scholar]
- 128.Becirovic E, Bohm S, Nguyen ON, Riedmayr LM, Koch MA, Schulze E, Kohl S, Borsch O, Santos-Ferreira T, Ader M, Michalakis S, Biel M. In vivo analysis of disease-associated point mutations unveils profound differences in mRNA splicing of peripherin-2 in rod and cone photoreceptors. PLoS Genet. 2016;12(1):e1005811. doi: 10.1371/journal.pgen.1005811. [DOI] [PMC free article] [PubMed] [Google Scholar]