Abstract
The constantly increasing requests for the measurement of serum 25-hydroxyvitamin D over the last years has led reagent manufacturers to market different automated and semi-automated methods, that being unfortunately not fully harmonized, yield different results. Liquid chromatography coupled to tandem mass spectrometry (LC/MS2) has more recently been introduced. This approach allows the distinction between the two forms of 25-hydroxyvitamin D and to measure other metabolites. This approach also requires harmonization to curtail the differences between the different analytical methods. To meet this requirement, the American National Institutes of Health (NIH), the Centre for Disease Control and Prevention (CDC) in Atlanta, the National Institute of Standards and Technology (NIST) and the vitamin D Reference laboratory of Ghent University have pooled their expertise to develop a standardization program.
This article reviews the main elements and the difficulties of the automated and semi-automated methods for 25-hydroxyvitamin D, from sample preparation to the analytical phase, as well as those related to mass spectrometry. It also emphasizes the need for standardization to better define the clinical decision thresholds of vitamin D nutritional status.
Keywords: Vitamin D, 25-Hydroxycholecalciferol, 25-Hydroxyergocalciferol, HPLC, Mass spectrometry, Immunoassays
Highlights
-
•
We review elements of the published methods for serum 25OHD measurement.
-
•
We emphasize the inter-method differences in assessing serum 25OHD concentrations.
-
•
We illustrate the lack of standardized reference material in past methods.
-
•
We question the use of the historical thresholds defining vitamin D status.
-
•
We encourage clinical laboratories to adhere to Vitamin D Standardization Program.
1. Introduction
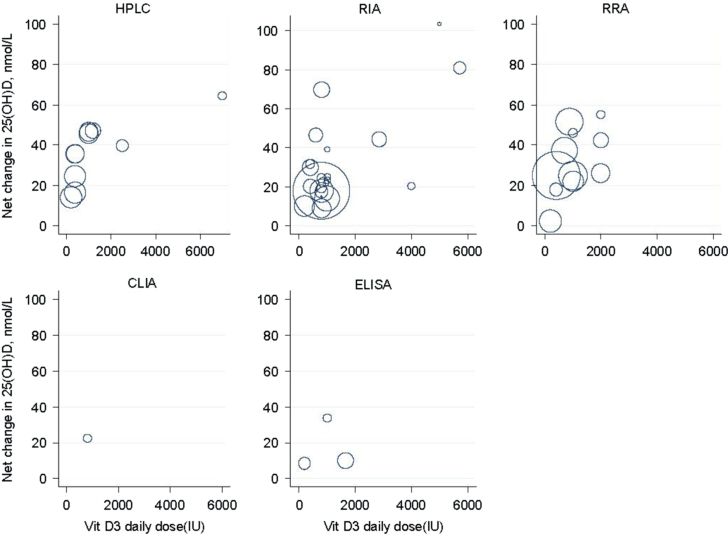
The role of cholecalciferol or vitamin D3 in growth and bone metabolism is well established [1]. Its effects in the prevention and treatment of diseases as varied as diabetes, multiple sclerosis and cancer have also been reported, but are still matter of debate [2], [3], [4], [5], [6]. Both the Institute of Medicine (IoM) [7] and the Agency for Healthcare Research and Quality (AHRQ) [8] have published extensive documents dampening the optimism aroused by these reports. The AHRQ report [8] makes the case that studies (observational, randomised controlled interventions) and systematic reviews or meta-analyses based on those, involved different types of assays that, except for the most recently published, did not use appropriate reference material. It also shows, as a series of bubble plots, that there was an important variation in responses to vitamin D supplementation (Fig. 1). This apparent variation is multifactorial. The individual response to sun exposure and the formulation of the vitamin D supplement are parts of the equation. However, inter-laboratory variations also contribute to this observation as they hinder comparison between results. Indeed, the inter-laboratory differences between the mean serum 25-hydroxyvitamin D (25OHD) values, that reached almost 32%, according to a Vitamin D External Quality Assessment Scheme (DEQAS) survey in 1994, could have lead to misclassification of patients in terms of vitamin D nutritional status. Since then, the standardization process has improved, and in 2009, the inter-laboratory imprecision had dramatically decreased [9], and thus if similar experiments were conducted today, the vitamin D dose-response relationship should be stricter. However at the present time, the observed wide-spread difference in circulating 25OHD concentrations restrain the conclusions of past epidemiological studies on the circulating 25OHD concentrations required for optimal health status, and confuse the efforts in developing international evidence-based public health guidelines. To solve this challenge, the NIH Office of Dietary Supplements (ODS), jointly with the Center for Disease Control (CDC) National Center for Environmental Health (NCEH), the National Institute of Standards and Technology (NIST) and Ghent University, established in 2010 the Vitamin D Standardization Program (VDSP) with the main goal of promoting consistency in the methods for the measurement of 25OHD [10]. This consortium is thus advocating, based on the recommendations of Stöckl et al. [11], an imprecision (CV) of ≤10% and a bias ≤5% as current goals for the analytical performance of vitamin D assays in routine clinical laboratories [12]. This initiative has resolved the imprecision issue. However, the trueness or accuracy although improved, remains a work in progress.
Fig. 1.
Relationship between doses of vitamin D3 supplementation and net changes in serum 25OHD concentrations in RCTs by assay type. Legends: Each empty circle represents one study. The area of the circle is proportional to the inverse of the within-study variances. The larger the bubble is, the larger the sample size and the smaller the standard error of the changes in 25OHD.
Reprinted with permission from Newberry et al. [8].
As it has often been mentioned, the number requests for the measurement of circulating 25OHD, the accepted biomarker for the vitamin D nutritional status [13], [14], has constantly increased over the last 3 decades, imposing structural and financial burdens on laboratory facilities and public funding. The Ontario Health Technology Advisory Committee (OHTAC) has reported that, the volume of laboratory vitamin D tests had increased from approximately 30,000 in 2004 to over 730,000 in 2009 [15]. Similar observations were made worldwide. This increased request load has lead most of the clinical laboratories to abandon manual binding-protein assays and radio-immunoassays (RIAs), the methods mostly utilized clinical laboratories in the 1980s and early 1990s, in favor of automated competitive binding-protein assays (CBPA), enzyme-linked immunoassays (ELISAs) or chemiluminescent immunoassays (CLIA). Techniques based on high-performance liquid chromatography (HPLC), coupled or not tandem mass spectrometry (LCMS-MS), while more exact, are still the privilege of specialised and research laboratories. These physicochemical approaches are however indispensible when one realizes that vitamin D is not a single entity. Indeed, there are 2 common forms, vitamin D3 (endogenously produced or dietary) and vitamin D2 from plant origin or from supplements. Vitamin D2 differs from its D3 homolog by having a double bond at C22–C23 and by being methylated at C24. These 2 structural modifications are reported, although not unanimously, to induce metabolic and functional alterations. For example, Biancuzzo et al. [16] have shown that orange juice supplemented with vitamin D2 or vitamin D3 was as effective in maintaining vitamin D status in adults, To the contrary Armas et al. [17] have shown that vitamin D2 was 1/3 less potent than vitamin D3 in maintaining serum total 25OHD concentrations after a single 50,000 IU oral dose, and that the difference was essentially due to a more rapid clearance/metabolism of vitamin D2. In terms of biological function, Tsugawa et al. [18] have shown in a variety of in vitro and ex vivo models, that binding affinity for the vitamin D receptor (VDR), bone-resorbing activity and cell-differentiating effects of 1α,25(OH)2D2 were almost comparable to 1α,25(OH)2D3. The picture is furthermore complexified as the 2 vitamin D precursors exist as A-ring diastereoisomers or epimers at carbon 3 (3α and 3β) that are hydroxylated to their metabolites, which respective physiological functions remain a matter of debate. While in vitro, the downstream metabolite of C3-epi-25OHD3 (3-epi-25-OHD3, 3β25-OHD3), 3-epi-1,25(OH)2D3 (3β,1,25(OH)2D3) displays less potent gene-regulatory effects on some vitamin D receptor-responsive genes involved in bone metabolism than 1α,25(OH)2D3; it is as potent with regard to the suppression of the transcription of the PTH gene [19], [20]. These data support the need for, further research and for distinguishing vitamin D metabolite epimers.
Despite the recent technological advances, the variety of circulating vitamin D metabolites, and the complex nature of the biological matrix in which they bathe, make the measurement of 25OHD difficult. Many important issues have still to be resolved to obtain an accurate measure of serum 25OHD concentration. Each phase of the process will be reviewed in order to provide clinical laboratories with information on the difficulties they have to overcome.
2. The sample preparation phase
In order to understand the problems related to the recovery of 25OHD during the extraction procedures, one must have some knowledge of the physiological processes involved in its transport. Due to their lipophilicity, vitamins D3 and D2, as well as their respective hydroxylated metabolites (ligands), must be transported by amphoteric carriers. Although vitamin D binding-protein (DBP) is their predominant transporter, albumin and lipoproteins are also important components. Whereas vitamin D synthesized in the skin is preferentially transported by DBP to be hydroxylated in the liver, lymphatic chylomicrons and lipoproteins mediate its transport and hepatic uptake [21], [22], [23], [24].
Each ligand–vitamin D carrier complex possesses its own affinity constant. For example 25OHD binds DBP with high affinity (Ka=5×10−8 M), whereas 1α,25(OH)2D3, the hormonal form of vitamin D3, exhibits a lower affinity (Ka=4×10−7 M) [25], [26]. In both cases the carrier being in large excess, <5% of the DBP sites are occupied, and the free concentrations of the metabolites are extremely low. The other transporters have similar kinetics at however different orders of magnitude. It becomes apparent that the dissociation of 25OHD from the collection of the carriers must be highly efficient in order to obtain an accurate total quantitation. The problem is not so much for binding-protein assays, radio-immunoassays, high performance liquid chromatography, coupled or not to mass spectrometry, that all require an organic extraction step destroying the binding capacity of the carriers, but for automated non-extracting assays for which organic solvents are not compatible, and in which alternative releasing agents with proprietary protection are used. Indeed the varying serum DBP concentration with physiological and pathological conditions, such as pregnancy, estrogen therapy or renal failure [27], [28], [29], affects the dissociation of vitamin D metabolites from the carrier and the competition kinetics involved in methods relying on pH changes or blocking agents. In support of this statement, several reports have highlighted the inaccuracy of total 25OHD measurement by automated immunoassays and competitive binding-protein assays performed in populations with different levels of DBP [30], [31], [32], [33]. Evaluation of the recovery of 25OHD3 and 25OHD2 added to serum or plasma samples is customary in evaluating the efficiency of the on-line dissociation step from the binding components. The validity of such in vitro recovery experiments is founded on the proviso that exogenous and endogenous vitamin D metabolites fully equilibrate with and bind equally to all serum binding components. In practice, this may however not occur. The rise in serum pH during storage, decreasing the affinity of binding proteins for vitamin D metabolites, might stimulate the sequestration of exogenous 25OHD by serum components, such as lipids or lipoproteins. Carter et al. [34] and Horst [35] have reported this artefact showing an under-recovery of exogenously added 25OHD in automated assays. This has been extended to methods based on HPLC-tandem-mass spectrometry, when Lankes et al. [36] have shown that the recovery of 25OHD was affected by suboptimal extraction conditions. These observations, that elude complete understanding, question the present process of recovery experiments, and warrant caution in interpreting reported data.
3. The analytical phase
Dietary supplements currently provide 2 forms of vitamin D: vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol). It is therefore essential that the analytical methods be able to measure the 2 forms equally in order to avoid an underestimation of the circulating total 25OHD in vitamin D2 supplemented individuals [37], [38], [39], [40]. On the other hand, they must be able to distinguish C3-epi-25OHD3 and 24,25(OH)2D, present in different proportions and to avoid an overestimation of circulating 25OHD. This is particularly important for samples from infants under the age of 1 year [41], [42] in which C3-epi-25OHD3 constitutes the major proportion of the total 25OHD. A number of assays have been published and marketed, certain of which claim to achieve these goals. The following paragraphs address their characteristics.
4. Binding-protein assays and immunoassays
Table 1a summarizes some of the characteristics of the CBPAs and immunoassays. A limited number of CBPAs have been reported and used clinically between 1971 and 1980. Haddad et al. [43] reported first a manual CBPA for the measurement of serum 25OHD. The method was based on the displacement of 3H-labeled 25OHD3 from post-microsomal kidney supernatants of rachitic rats by 25OHD extracted from human serum and purified by chromatography on silicic acid columns. The authors suggested that this assay recognized equally 25OHD3 and 25OHD2. The assay analytical sensitivity was 10 nmol/L. Almost 10 years later, Delvin et al. [44] published a simplified CBPA using a commercially available bovine α-globulin enriched fraction (Cohn fraction IV). The serum samples, spiked with purified 3H-25OHD3, for recovery calculation purposes, were chromatographed on silicic acid columns after lipoprotein precipitation with heparin/MnCl2. The analytical sensitivity was 5 nmol/L. Although both 25OHD3 and 25OHD2 were equally recognized, contrary to the rat kidney extracts, the α-globulin fraction showed no affinity for 24,25(OH)2D. These assays requiring chromatographic purification on silicic acid and Sephadex LH-20 column after organic extraction were time-consuming and could not be implemented in routine clinical laboratories. In 1984, Bouillon et al. [45] described a non-chromatographic direct assay for 25OHD using rachitic rat serum as the source of DBP, after extraction with ethylacetate and cyclohexane. It measured 25OHD3 and 25OHD2 equally and exhibited a 100% cross-reactivity for 24,25(OH)2D. Parviainen et al. [46] published in 1981, a method based on HPLC separation of vitamin D metabolites and their subsequent measurement with a CBPA for 25OHD and 24,25(OH)2D, and a vitamin D-receptor assay for 1α,25(OH)2D. Although the recovery of the labeled metabolites was relatively low, the coefficient of variation (CV) was <10% for 25OHD. This method proved to be time-consuming and hence was not applied for routine purposes by other groups. Although the above assays exhibited clinically acceptable analytical sensitivity and imprecision, the development of polyclonal antibodies directed against 25OHD that lead to RIAs, and the simplification of HPLC equipment made them obsolete and allowed the introduction of these novel technologies in clinical laboratories.
Table 1a.
Characteristics for in-house manual competitive binding-protein and radioimmunological 25OHD assays.
| In-house and commercial manual assays | |||||||
|---|---|---|---|---|---|---|---|
| Reference | Extraction and purification procedures | Vehicle for assay solubilization | Assay principle | Equivalence for 25OHD2/25OHD3, Cross-reactivity C3-epi/24,25(OH)2D | Traceability, recovery | LOQ/(LOD) (nmol/L) | Precision, intra-assay, inter-assay, CV |
| Haddad et al. [43] | Plasma, 1 ml diethyl ether, silicic acid chromatography | Absolute ethanol | Rachitic rat kidney extracts CBPA, 3H-25OHD as tracer | Equivalence: NR, cross-reaction: NR | Traceability NR, 25OHD3 64.1±10.9% | NR/(10) | 14% at 40 nmol/L, NR |
| Delvin et al. [44] | Serum 500 μl, lipoprotein precipitation (NaHep/MnCl2) diethyl ether, silicic acid chromatography | Absolute ethanol | Bovine □-globulin CBPA, 3H-25OHD as tracer | Equivalence: 74%/100%, No cross-reactivity for 24,25(OH)2D | Traceability NR, 25OHD3 90±1.6% | NR | 8.9% at 54 nmol/l, 8.4% at 37 nmol/L |
| Bouillon et al. [45] | Serum 100 μl, EtAc/cycloHexane (1:1 v/v) | Absolute ethanol | Rachitic rat serum CBPA, 3H-25OHD as tracer | Equivalence: Yes, 100% cross-reactivity for 24,25(OH)2D | Traceability NR, 25OHD3 107±8.9% | NR/(2.5) | 5.6% at 45 nmol/L, NR |
| Parviainen et al. [46] | Serum 100–500 μl, EtOH 150 μl, 2-propanol/hexane, 2:1 vol/vol hexane, silicic acid chromatography | Absolute ethanol | Human serum CBPA, 3H-25OHD as tracer | Equivalence: Yes, 100% cross-reactivity for 24,25(OH)2D | Traceability NR, 25OHD3 77±7% | NR/NR | NR 8%, Conc. NR |
| Hummer et al. [47] | Serum 500 μl MeCN SPE | Absolute ethanol | RIA, 3H-25OHD3 as tracer | Equivalence: 2.2%/100%, 10% cross-reactivity for 24,25(OH)2D | Traceability NR, 25OHD3 93.7–115.1% | NR/(4.3) | 4.5% at 54 nmol/l, 10.4% at 32 nmol/L |
| Hollis et al. [48] | Plasma 25 μl MeCN | Absolute ethanol | RIA, 3H-25OHD3 as tracer | Equivalence: Yes, 100% cross-reactivity for 24,25(OH)2D | Traceability NR, 25OHD3 108±18% | NR/(7.5) | <13% Conc. NR |
| Hollis et al. [50] | Plasma/serum 25 μl MeCN | Absolute ethanol | RIA, 125I-CC derivative | Equivalence: Yes, 100% cross-reactivity for 24,25(OH)2D | Traceability NR, 25OHD3 97±10% | NR/(7.0) | 5.6% at 23 nmol/L, 15.9% at 23 nmol/L |
NR: Not reported.
5. Radio-immunoassays
RIAs, developed early in the 1980s, constitute the next generation of assay methods. In 1984, Bouillon et al. [45] first described a simplified non-chromatographic RIA, based on the production of rabbit polyclonal antibodies directed against BSA-25OHD3-hemisuccinate conjugate and the competition of the serum-extracted 25OHD for [26(27)-methyl-3H]-25OHD3 as tracer. Although the assay was analytically as sensitive as the CBPA, the 2 anti-sera produced had widely different characteristics in terms of specificity: the cross-reactivity varying between 0% and 11% for 25OHD2 and 40 to 270% for 1α,25(OH)2D3. The second, developed by Hummer et al. [47], required a preliminary chromatography step. As neither assays measured 25OHD2 their utility was limited in assessing total vitamin D nutritional status, at a time when vitamin D2 was widely used as dietary supplement. The next year, Hollis et al. [48] described and validated a non-chromatographic radioimmunoassay based on an anti-serum raised against the 23,24,25,26,27-pentanor-C-(22)-carboxylic acid vitamin D-BSA conjugate. [26,27-methyl-3H]-25OHD3 was also used as tracer. Although the antibody had little affinity for both 1α,25(OH)2D3 and 1α,25(OH)2D2 (±5%) or for vitamin D3 or D2 (±10%), it had a 100% cross-reactivity for 25OHD2 and other known vitamin D metabolites. The radically different recovery of labeled 25OHD3 depending whether the tracer was added to the sample before or after the addition of acetonitrile was of concern. In order to obtain a quantitative recovery, the tracer had to be added after the addition of acetonitrile. When added to the native sample and equilibrated before the extraction step, the recovery dropped to ±53%. One may therefore question whether the endogenous 25OHD was quantitatively recovered. To further confuse matters, in the above-mentioned assays, only 3H-25OHD3 was used to monitor recover. This was a limitation, as Stryd et al. [49] had emphasized as soon as 1978, total 25OHD could be underestimated since the recovery of the 2 vitamin D isomers may not necessarily be identical in the extraction processes.
Eight years later Hollis et al. [50] described a RIA based on goat anti 23,24,25,26,27-pentanor-C(22)-carboxylic acid of vitamin D-BSA conjugate and 125I-vitamin D-23,24,25,26,27-pentanor-C(22)-carboxylic-amide-3-aminopropyl as the tracer. As in the former assay [48] this antibody had little affinity for 1α,25(OH)2D3 and 1β,25(OH)2D2 (±2.5%) or for vitamin D3 or D2 (<1%), and had a 100% cross-reactivity for 25OHD2 and other vitamin D metabolites. Despite the fact that collectively these metabolites account for a small percentage, the assays probably over-quantified the “true” 25OHD concentration. Nevertheless this RIA gave a better estimate of the total vitamin D status as both 25OHD3 and 25OHD2 could be measured equally, on the proviso that 25OHD was quantitatively recovered during the extraction procedure. This assay probably led to the 1st commercial radioimmunoassay for the measurement of 25OHD marketed by DiaSorin (Stillwater, MN, USA).
Table 1b summarizes the characteristics of the marketed RIAs and automated non-radioactive immunoassays. It can be appreciated that the 2 RIAs listed differ in their performance claimed by the respective manufacturers. The DiaSorin assay measures 25OHD2 and 25OHD3 equally whereas the IDS RIA underestimates 25OHD2 by 25%. The different affinity of the antibodies may be due to the difference in the vitamin D analog used to raise the polyclonal antibodies. DiaSorin use a vitamin D analog that lacks the side-chain while retaining the open B-ring cis-triene structure common to both vitamins D2 and D3 as the hapten, thus ensuring that the antibodies would only recognize this structure. It should be noted that neither assay kits are standardised with reference material, thereby diminishing their accuracy. In both cases the lower limit of detection (LoD) is in the range of 3 nmol/L, although, to our knowledge, there are no independent data to support this claim. DiaSorin and IDS claimed 100% 25OHD recovery from spiked samples. However, for exogenous 25OHD3 and 25OHD2 respectively a 2005 DEQAS survey reported a mean recovery of 82% and 83% for the DiaSorin assay, and 45% and 25% for the IDS RIA kit [34]. Both methods used an acetonitrile extraction of vitamin D metabolites. Addition of NaOH in the initial denaturation-extraction procedure of the IDS RIA has been suggested as the source of the difference. This hypothesis can however be dismissed as both the DiaSorin and IDS assays gave similar results for the specimen containing only endogenous vitamin D. The discrepancy can be explained, at least in part, by the lower affinity of the IDS primary antibody for 25OHD2 [51]. On the other hand, Glendenning et al. [52] have reported that the DiaSorin RIA overestimates total 25OHD within the range of 40–60 nmol/L when compared to a HPLC method. The assays also differ in their imprecision, DiaSorin reporting an intra-assay CV of 11.7% at 21.5 nmol/L and IDS of 5.3% at 26 nmol/L.
Table 1b.
Characteristics for manual and automated commercial 25OHD assays according to insertrs.
| Platform vendor | Extraction and purification procedures | Assay principle | Equivalence 25OHD2/25OHD3, Cross-reactivity (C3-epi/24,25(OH)2D | Traceability, recovery (%) | LOQ/(LOD) (nmol/L) | Precision, intra-assay, inter-assay, CV (%) |
|---|---|---|---|---|---|---|
| DiaSorin | S/P acetonitrile | RIA, 125I-CC, derivative goat polyclonal Ab | Equivalence: Yes, cross-reactivity: Yes NR/100% | Calibrators traceable to a pure preparation of the 25OHD Ag calculated by spectrophotometry | 6.25a/(4.0) | 11.7% at 21.5 nmol/L, 9.4% at 21.5 nmol/L |
| Immuno Diagnostics Ltd. | S/P 50 μl, NaOH, acetonitrile | RIA, 125I-25OHD | Equivalence: 75%/100%, cross-reactivity: NR/≥100% | Calibrators standardised by UV quantitation, 89–102 at 20 nmol/L | NR/(3.0) | 5.3% at 26 nmol/L, 8.2% at 20 nmol/L |
| Immuno Diagnostic Systems Ltd. | S/P 25 μl, 2-step procedure w/o extraction | ELISA, immobilized anti-25OHD, sheep polyclonal Ab, 25OHD-labeled with biotin HRP/TMB | Equivalence: 75%/≥100%, cross-reactivity NR/≥100% | Calibrators standardised by UV quantitation, 97–105 | NR/(5.0) | 5.3% at 39 nmol/L, 4.6% at 40 nmol/L |
| Immuno Diagnostic Systems Ltd. | S 10 μl, 2-step procedure, Denaturation DBP+NaOH | CLIA, acridinium-labeled anti-25OHD, sheep polyclonal Ab | Equivalence: Yes, cross-reactivity: 1%/NR | Calibrators standardised to ID-LC-/MS/MS) 25OHD RMP; traceable to the NIST SRM 2972, recovery not reported | 17.5/(6.0) | 6.2% at 30 nmol/L, 11.6% at 30 nmol/L |
| DiaSorin, Liaison, Total DiaSorin | CLIA, HRP – isoluminol derivative | Equivalence: Yes, cross-reactivity: 1.3%/NR | Calibrators traceable to UV spectrophotometric analysis | 10.0/(NR) | 3.8% at 20 nmol/L, 12.2% at nmol/L | |
| Advia, Centaur, Siemens | S/P 20 μl, buffered releasing agent | CLIA, acridinium-labeled mouse mAb, fluorescein vitamin D analog, anti-fluorescein mAb, PMP 1-anilinonaphthalene-8-sulfonic | Equivalence: Yes 104%/100%, cross-reactivity: 1.1%/NR | Calibrators standardised to ID-LC-/MS/MS, 25OHD RMP; traceable to the NIST SRM 2972, recovery not reported | 10.5(8.0) | 4.7% at 34 nmol/L, 11.9% at 34 nmol/L |
| Architect 1, Abbott | S/P 60 μl, 2 step procedure, EtOH/triethanolamine/ANSA | CLIA, Sheep polyclonal Ab-anti-25OHD, acridinium-labeled biotinylated anti-biotin IgG complex | Equivalence: 82%/100%, cross-reactivity: 2.7%/112% | NR, no mention of traceability, recovery not reported | 20 (7.8) | 3.1% at 58 nmol/L, 4.0% at 58 nmol/L |
| Roche Elecsys, Roche Diagnostics | S/P 15 μl, 2 step procedure, Dithiothreitol pH 5.5, then NaOH | ECL, CBPA, Ruthenium | Equivalence: 92%/100%, cross-reactivity: 91%/149% | Standardized against in house LC-MS/MS standardized to the NIST standard, recovery not reported | 10 (7.5) | 7.8% at 17 nmol/L |
| Vitros 5600, Vitros | S 60 μl, 1 step procedure, acid pH | CLIA, Sheep mcAB-anti-25OHD, Horseradish peroxidase – Luminol | Equivalence: Yes, cross-reactivity: Yes 37.4%/34.3% | In house reference calibrators, Correlation to LC/MS/MS, recovery not reported | 32 (21.6) | 7.4% at 56nmol/L, 14.0% at 56 nmol/L |
| Beckman Dxi, Beckman–Coulter | S/P 30 μl, 1 step procedure, Tris buffered saline | CLIA, Sheep mcAB-anti-25OHD 25OHD, analog AP-conjugate, Lumi-Phos* 530 | Equivalence: Yes, cross-reactivity: 65%/0% | Calibrators standardised to ID-LC-/MS/MS, 25OHD RMP; traceable to the NIST SRM 2972, recovery not reported | 11 (3.7) | 4.6% at 39 nmol/L, 8.1% at 39 nmol/L |
Unless otherwise specified, the characteristics of the commercial assays are derived from the information given in the respective inserts. Recovery refers to the % of the exogenously added 25OHD3 (nmol/L) before extraction recovered at completion of the assay. RIA: radio immnuno assay; EIA: enzyme-linked immuno assay; CLIA: chemi luminescent immuno assay, CBPA: competitive binding-protein assay; ELISA: enzyme-linked immuno sorbent assay; CLIA: chemi luminesent immuno assay; ECL: electro chemiluminescence S: serum; P: plasma; LOQ: lower limit of quantification defined as a measure with a CV<20%; LOD: lower limit of detection defined as the lowest concentration that can be defined with a confidence of 95%; NR: not reported; CV: coefficient of variation at the lowest concentration tested. EtOH: Ethanol; 3H-25OHD2: [23,24(n)-3H]-25-hydroxyvitamin D3 or [26(27)-methyl-3H]-25-hydroxyvitamin D3; 125I-CC: vitamin D-23,24,25,26,27-pentanor-C(22)-carboxylic-amide-3-aminopropyl; ANSA: 8-anilino-1-naphthalene sulfonic acid; IgG: immunoglobulin G; mcAB: monoclonal antibody; BSA: bovine serum albumin; AP: alkaline phosphatase; Lumi-Phos* 530: trademark of Lumigen Inc. (Southfield, MI); ID-LC-/MS/MS: isotope dilution-liquid chromatography/tandem mass spectrometry; RMP: reference method procedure; NIST: National Institute of Standards and Technology; SRM: standard reference material
Personal communication (E Cavalier).
6. Automated immunoassays
RIAs gradually gave way to automated enzyme-linked immunoassays (ELISAs), CLIAs or CBPAs. Characteristics of the direct automated methods found in the manufacturers׳ information inserts are summarized in Table 1b. As can been appreciated, according to the manufacturers׳ respective inserts, 5 out of 6 automated CLIA-based methods measured 25OHD2 and 25OHD3 equivalently (IDS, DiaSorin, Advia Centaur, Vitros, Beckman) whereas the IDS ELISA assay underestimated 25OHD2 by 25%, the Abbott CLIA by 18% and Roche ECL by 8%. However in the case of the Advia Centaur, Le Goff et al. [53] using native clinical samples reported a 30% mean overestimation (4–59%) for 25OHD2. These assays exhibited, when reported, variable cross-reactivity for 24,25(OH)2D3 (0% for Beckman to 149% for Roche) and C3-epi-25OHD3 (1% for the IDS CLIA to 91% for the Roche CBPA). Interestingly, van den Ouweland et al. [54] demonstrated recently, that when present endogenously, C3-epi-25OHD3 is not recognized in the Roche CPB assay and urge for caution in interpreting recovery data.
It is interesting to note that 4 out of 8 automated assays were directly or indirectly standardized against a NIST Standard Reference Material (SRM), however none provide information on recovery of exogenous 25OHD3 or 25OHD2. Automated immunoassays, as well as CBPAs, are based on delicate non-denaturing conditions to free 25OHD from DBP and other serum binding components to allow its binding either to the kit antibodies or DBP. This step, sensitive to matrix effects, may yield varying results [55], [56].
The performance of different commercial assays has recently been reported in independent investigations. Su et al. [57] have reported that, compared to a Liquid Chromatography Tandem Mass spectrometry method (LC/MS–MS), a CBPA (Diazyme, Poway, CA) exhibited a positive bias when samples contained only 25OHD3 and negative biases as the 25OHD2/25OHD3 ratios increased (10.8%, −23.6%, −38.4%). As the DBP in all likelihood recognizes the 25OHD isomers equally, the bias could be explained by the inefficient recovery of 25OHD2. Holmes et al. [58], compared total 25OHD results in 163 clinical specimens obtained by 3 direct immunoassays, (DiaSorin Liaison assay, Siemens Centaur, Abbott Architect), to those obtained after extraction and followed by LC/MS–MS and RIA. Their data revealed high degrees of random variability and bias relative to LC/MS–MS and RIA results. Importantly, the magnitude of the biases and random errors exceeded the criterion for the total allowable error of a 25OHD test [11] in almost ½ of the clinical specimens and led to misclassify an appreciable number of study patients as vitamin D deficient. Cavalier et al. [59] also reported a concordance between methods varying between 65% and 82% when comparing 6 automated platforms to the NIST/NIH VDSP-accredited LC/MS–MS method. As Sempos et al. [10] have emphasized, this inter-assay variability could lead to misleading conclusions in epidemiological studies aiming at evaluating the vitamin D status and to limiting the comparability between national surveys. All assays have satisfactory precision, although defined at variable concentrations.
7. High performance liquid chromatography
Table 2 lists the different HPLC methods published the past 35 years. Eisman et al. [60] published the 1st HPLC method for the measurement of 25OHD in 1978, followed within a year by Gilbertson et al. [61] and Jones [62]. Variants of these initial methods have been published until very recently [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76]. As can be appreciated, although the HPLC-based methods were able to separate 25OHD2 from 25OHD3, the authors used either a single in-house or commercially available labeled 25OHD3 as internal standard or even surrogate molecules (retinyl acetate, docecanophenone, derivatised 25-hydroxydehydrocholesterol, 1α-OHD) to monitor the recovery of 25OHD, although reporting in most case concentrations for both isomers. However, as mentioned earlier Stryd et al. [49], questioned in 1978 the accepted notion that 25OHD2 and 25OHD3 behaved identically during the extraction and chromatographic procedures, and therefore held that using the recovery of the tracer 3H-25OHD3 to calculate the concentration of the 2 isomers was an error. This led them to report values only for 25OHD3 contrary to others. This premise can be extended to the proxy tracers. Among variants reported, Shimada et al. [67] used 2 internal standards: 25OHD2 (IS1) and derivatized 25-hydroxy-7-dehydrocholesterol (IS2) to assess 25OHD3 recovery. However the methodology used requires clarification. To start with, they added the 1st internal standard after precipitation of plasma proteins with ethanol, thereby removing an important step that could lead to misinterpretation. They also performed experiments to evaluate the “absolute” recovery of 25OHD3. For this part, they added 25OHD3 standards to 7% buffered bovine serum albumin together with the IS1 and performed the extraction. They then added the IS2 after the HPLC process they calculated the peak-height ratios between the 25OHD3, the IS1 and IS2. It is difficult to conceive how this maneuver allowed the accurate assessment of the endogenous 25OHD. The recovery studies vary in their structure (labeled or not-labeled tracer, 25OHD or surrogate molecules). Hence it is difficult to assess accurately the performance of the methods. The accuracy of the methods described is ill-defined, as in most cases no calibrator traceable to a standard reference material was available. However Hymøller et al. [75] have shown that their method yielded results within acceptable boundaries, in terms of accuracy and precision, for 25OHD2 and 25OHD3 using the NIST standard reference material 972.
Table 2.
Physical separation and detection methods.
| Reference | Sample volume, extraction procedure, chromatographic procedure, detection wavelength | Internal standards, analyte measured | Recovery | LOQ (nmol/L) | Precision, Intra-assay CV, Intra-assay CV |
|---|---|---|---|---|---|
| Eisman et al. [60] | Plasma 4 ml, extraction: MeOH:CHCl3 (50:50 v/v), pre-treatment: Sephadex LH-20, SkellySolve B: CHCl3 (50:50 v/v), SkellySolve B: CHCl3:MeOH (18:2:1 v/v), HPLC: porasil silicic acid column, 2-propanol:hexane (2.5:97.5 v/v), detection: 254 nm | In-house IS [26], [27], 3H-25OHD3, [3α]3H-25OHD2, 25OHD2, 25OHD3 | 3H-25OHD3: 72.2±10% | NR | NR |
| Gilbertson et al. [61] | Serum 1 ml, extraction: CHCl2:MeOH (2:1 v/v), pre-treatment: silicic acid CH2Cl2:EtOH (98:2 v/v) then n-hexane, HPLC: porasil silicic acid column, EtOH:hexane (5:95 v/v), detector: 254 nm | Commercial IS [24], [25], 3H-25OHD3, 25OHD3 | 3H-25OHD3: 60.8±14.4% | NR | 25OHD3, 5.2% at 28 nmol/L, 5.5% at 28 nmol/L |
| Jones [62] | Plasma or serum 2 ml, extraction: MeOH:CHCl3 (2:1v/v), 2-propanol:hexane (4.5:95.5 v/v), HPLC: Zorbax-SIL MeOH:H2O (98.5:1.5 v/v) followed by MeOH:H2O (91.0:9.0 v/v), Zorbax-ODS MeOH:H2O (98.5:1.5 v/v), detection: 254 nm | Commercial IS [26], [27], 3H-25OHD3, In-house IS, [3α]3H-25OHD2, 25OHD2, 25OHD3 | 3H-25OHD3: 68.8±6.5% | NR | 25OHD3, 9.0% at 30 nmol/L, 16% at 30 nmol/L |
| Babek et al. [63] | Plasma 0.5–3.0 ml, pre-treatment: SPE: Sep-pak C18 MeOH:H2O (69:31 then 80:20 v/v), silicic acid HPLC n-hexane-propane-2-ol (100:2.4 v/v), detection: 254 nm | Commercial IS [23], [24], 3H-25OHD3, 25OHD3, 25OHD2 | 3H-25OHD3: 93% | NR | 25OHD3: 5%, 25OHD2: 5%, concentrations not mentioned |
| Turnbull et al. [64] | Plasma 2.0–3.0 ml, extraction: MeCN pre-treatment: SPE: Sep-pak C18 MeOH:H2O (70:30 v/v) then MeCN, derivatisation to isotachysterols Zorbax-Sil n-hexane-propane-2-ol (95:5 v/v), detection: 301 nm | Commercial IS [23], [24], 3H-25OHD3, 25OHD3, 25OHD2 | 3H-25OHD3: 54.9±2.5% | NR | 25OHD3: 5.9% at 57 nmol/L, 25OHD2: 6.8% at 14 nmol/L, 25OHD3: 8.0% at 62 nmol/L, 25OHD2: 7.1% at 16 nmol/L |
| Loo et al. [65] | Plasma 1.0 ml PP: MeOH, extraction: n-hexane 1st HPLC: Li-Chrosorb-Si n-hexane-EtOH (90:10 v/v), 2nd HPLC: Ultraspher-Octyl C-8 MeCN:H2O (80:20 v/v), detection 254 nm | Commercial IS, [26], [27], 3H-25OHD3, 25OHD3, 25OHD2 | 3H-25OHD3: 74.7±3.4% | NR | NR |
| Norris et al. [66] | Plasma/serum 2.0 ml PP: MeOH, pre-treatment: SPE: Sep-pak C18 (MeOH), 1st HPLC: Li-Chrosorb-Si n-hexane-propane-2-ol (91:9 v/v), 2nd HPLC: Spherisorb-ODS MeOH:H2O (88:12 v/v), detection 285 nm | Commercial IS [23], [24], 3H-25OHD3, 25OHD3, 25OHD2 | 3H-25OHD3: 54.9±2.5% | 25OHD3: 7.5, 25OHD2: 7.5 | 25OHD3: 7.3% at 28 nmol/L, 25OHD2: 6.4% at 16 nmol/L |
| Shimada et al. [67] | 500 μl Plasma PP: EtOH, extraction: EtOH/KOH followed by Et2O, pre-treatment: silicic acid column n-hexane-propane-2-ol (98.5:1.5 v/v) n-hexane-propane-2-ol (84:16 v/v) HPLC:J’sphere ODS-HS0, MeCN:H2O (70:30 v/v), detection 265 nm | In-house IS, 25OHD2, MBPTD-25OHdC, 25OHD3 | 25OHD2 55.2±3.3%, 25OHD3: 59.3±4.2% | 12.5 | 4.0% at 43.6 nmol/L (average of 4 determinations), 8.2% at 65.0 nmol/L (average of 4 determinations) |
| Masuda et al. [68] | 100 μl, plasma, extraction MeCl2/MeOH HPLC: nucleosil 5-C18 column MeCN:MeOH (95:5 v/v)/HClO4, detection: ECD at +0.60 V | IS: NR, 25OHD3 | 25OHD3: 81.5±5.8%, | NR | 5.3% at 76 nmol/L, 9.7% at 76 nmol/L |
| Alvarez et al. [69] | 500 μl Plasma PP: EtOH, extraction: n-Hexane/MeCl2, HPLC: Lichrospher 100 RP-18 MeCN:MeOH:H2O (90:4:6 v/v), gradient to MeCN:MeOH (40:60 v/v), detection 267 nm | Commercial IS, 1α-OHD3, 25OHD2, 25OHD3 | 1α-OHD3 93.0±7.9%, 25OHD2: 81.5±4.7%, 25OHD3: 88.0±5.1% | 25OHD2: 12.5, 25OHD3: 12.5 | 25OHD2: 6.1% at 15 nmol/L, 25OHD3: 7.7% at 22.5 nmol/L, 25OHD2: 10.8% at 15 nmol/L, 25OHD3: 11.8% at 22.5 nmol/L |
| Brunetto et al. [70] | 1 ml Plasma, extraction: EtOH:MeCN HPLC: Spherisorb C18, gradient: MeCN:phosphate buffer pH6.5 (20:80 v/v) to MeOH:MeCN:THF (65:20:15 v/v), detection: 265 nm | No IS, 25OHD3 | Spiked sample, 25OHD3: 91% at 20 nmol | 25OHD3: 7.5 | 25OHD3: 2% at 17.5 nmol/L, 25OHD3: 2% at 17.5 nmol/L |
| Quesada et al. [71] | 1 ml Serum PP: EtOH, extraction: n-hexane:MeCl2 HPLC: Ultrabase C18 column, gradient from MeOH:H20 (90:10 v/v) to MeOH:propane-2-ol (90:10 v/v), detection: 265 nm | Commercial IS, retinyl acetate, 25OHD3 | NR | 25OHD3: 0.75 | 25OHD3: 4.3%, concentration: NR 25OHD3: 9.2%, concentration: NR |
| Lensmeyer et al. [72] | Serum 1 ml PP (MeCN), extraction: HPLC: SB-CN column, MeOH:H2O (67:33 v/v), detection: 275 nm | Commercial IS, laurophenone (dodecanophenone) 25OHD3, 25OHD2 | Exogenous 25OHD2: 101.2±9.4% (8–253 nmol/L), 25OHD3: 95.1±7.6% (11–260 nmol/L) | 25OHD2: 12.5, 25OHD3: 12.5 | 25OHD2: 13% at 11.0 nmol/L, 25OHD3: 8.5% at 28.9 nmol/L |
| Granado-Lorencio et al. [73] | 1 ml Serum PP: EtOH, extraction: n-hexane:MeCl2 HPLC: Spheri-5-ODS column, gradient from MeCN:MeOH (85:15 v/v) to MeCN:MeCl2:MeOH (70:20:10 v/v/v), detection: 267 nm | Commercial IS, retinyl acetate 25OHD (no distinction between 25OHD3 and 25OHD2) | 25OHD: >85% (no details given) | NR | <10% concentration: NR, <10% concentration: NR |
| Kand’ár et al. [74] | 500 μl Plasma PP: EtOH, extraction: SPE Discovery DSC-18 MeOH:H2O (2:3 v/v), MeOH. HPLC: Purospher STAR-RP-18e MeOH/H2O (95:5 v/v), detection: 265 nm | Commercial IS, retinyl acetate 25OHD3 | Spiked samples, 25OHD3: 96.9±7.6% from 5 to 100 nm/L | 10 nmol/L (2.5 nmol/L) | 25OHD3: 5.3% at 57 nmol/L, 25OHD3: 8.7% at 67 nmol/L |
| Hymøller et al. [75] | 1.5 ml Plasma saponification: MeOH/KOH/ASC, extraction: heptane HPLC: YMC-C30 RP column, gradient: H2O:EtOH (95:5 v/v), H2O:EtOH (60:40 v/v); H2O:EtOH (10:90 v/v), detection: 265 nm | Commercial IS, 1αOHD3, 25OHD2, 25OHD3 | 25OHD2: 101.0% at 75 nmol/L, 25OHD3: 100.3% at 75 nmol/L | 1.3 nmol/L (Metabolite not specified) | 25OHD2:0.2% at 150 nmol/L, 25OHD3: 0.6% at 150 nmol/L |
| Nurmi et al. [76] | 500 μl Serum PP: MeOH:propane-2-ol (80:20 v/v), extraction: n-hexane HPLC: Supelco Discovery HS F5, gradient: 60 mM NaClO4/HClO4/MeOH/MeCN (30:50:20 v/v/v), NaClO4/HClO4/MeCN, (10:90 v/v), detection: CEAD 630 mV | No IS, 25OHD2, 25OHD3 | 25OHD2: 72% at 24 nmol/L, 25OHD3: 61% at 24 nmol/L | 25OHD2: 12, 25OHD3: 12 | 25OHD3: 6.2% at 27.5 nmol/L |
PP: protein precipitation; SPE: solid phase extraction; LLE: liquid–liquid extraction; OLTFE: on line turboflow extraction; ECD: electrochemical detection; CEAD: coulometric electrode array detector. 25OHdC: 25-Hydroxy-7-dehydrocholesterol; 1α-OHD3: 1-alpha-hydroxyvitamin D3; MBPTD: 4-[4-(6-methoxy-2-benzoxazolyl)phenyl]-1,2,4-triazoline-3,5-dione; MeNH2: methyl amine; MeOH: methanol; EtOH: ethanol; NH4Ac: ammonium acetate; MeCN: acetonitrile: Et2O: diethyl-ether; KOH: potassium hydroxide; MeCl2: dichloromethane; HClO4: perchloric acid; THF: tetrahydrofuran; ASC: 20% ascorbic acid water solution; IS: internal standard; NR: not reported.
Some investigators have proposed a coulometric electrochemical detection system [68], [76] based on the oxidation potential of the conjugated-diene structure of vitamin D metabolites to quantitate 25OHD after the HPLC step. Although this detection method is as efficient as methods based on UV, it is not widely adopted by clinical laboratories. This may be due to the demanding maintenance of the detectors.
8. Mass spectrometry
Watson et al. [77] were among the first to describe a LC/MS–MS method for the measurement of vitamin D2, vitamin D3, and their respective mono- and di-hydroxylated metabolites. The clinical use of LC/MS–MS has since steadily grown, especially for the quantitation of low molecular weight analytes such as vitamins, hormones and steroids. According to the October 2013 DEQAS (www.deqas.org), 25% of the participants reported using this technology. Vogeser [78] and van den Ouweland et al. [79] have published extensive reviews on the subject. Table 3 highlights, in a chronological order, the methodology and performance characteristics of methods published during the last 15 years [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103]. Methods that include derivatization of the vitamin D metabolites with Cookson-like reagents are worth mentioning. Although sensitive and specific, they are not transposable for routine analysis in clinical laboratories but should be considered in clinical studies for vitamin D metabolite profiling [80], [81], [89], [103]. The advantages of the addition of a nitrophenyl group to the conjugated-diene portion of the secosteroids are two-fold. It increases the ionization efficiency and the analytical sensitivity by moving molecular masses of the parent ions to a region where there is reduced background noise thereby increasing the signal/noise ratio. The LC/MS–MS methods cited in Table 3 have all quantitation limits below 10 nmol/L well below the concentration considered as severe hypovitaminosis (25 nmol/L) [83], [84], [85], [86], [87], [88], [90], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102].
Table 3.
Mass spectrometric methods applicable to clinical laboratories.
| Ref | Sample volume, extraction procedure, chromatographic procedure, ionization, mode of monitoring | Internal standards, analyte measured, acquisition settings, m/z | Recoverya | LOQ nmol/L, LOD nmol/L | Precision (CV) Intra-assay Inter-assay |
|---|---|---|---|---|---|
| Higashi et al. [80] | Plasma 20 μl, PP: MeCN, extraction: LLE (AcOEt), derivatisation (DMEQ-TAD) HPLC: J׳sphere ODS H-80 MeCN/H2O (3/2 v/v) TMS: APCI+ SIM | In-house IS: 25OHD4: 760.1, 25OHD3: 746.1, 25OHD2: 758.1 | 25OHD3: 98.8–109.8% (12.5 nmol/L), 25OHD2: 101.1–104.2% (12.5 nmol/L) | 25OHD3: 7.5, 25OHD2: 7.5, 25OHD3: 1.3 | 25OHD3: 3.24% at 21.9 nmol/L, 25OHD2: 3.17% at 12.5 nmol/L |
| Higashi et al. [81] | Plasma 20 μl, PP: MeCN, extraction: LLE (AcOEt), derivatisation (NPTAD) HPLC: J׳sphere ODS H-80 MeOH/H2O (7/1 v/v) TMS: APCI− SIM | In-house IS: 25OHD4: 634.2, 25OHD3: 620.2 | Analytical recovery: NR | 25OHD3: 7.5, 25OHD3: 1.3 | 25OHD3: 8.2% at 7.5 nmol/L |
| Vogeser et al. [82] | Serum 200 μl, NaOH, PP: MeCN, extraction: on-line SPE: Oasis HLB® HPLC: LiCrospher® 100 RP-18 MeOH/NH4Ac:0.5mM (90/10 v/v) TMS: ESI+ | In-house IS: 2H3, 13C1-25OHD3: 405>159, 25OHD3: 401>159 | 25OHD3: 91±1.6% IS (325 nmol/L), injected into TMS/IS, extracted+TMS | NR | 25OHD3: 12% at 14.5 nmol/L |
| Tsugawa et al. [83] | Serum 100 μl, PP: MeOH, extraction: SPE: Bond-Elute C18® HPLC: CapCell PAK C-18 UG120® MeOH/H2O (95/5 v/v) TMS: APCI+ MRM | In-house IS: 2H6-25OHD3: 407>263, 25OHD3: 401>257, 25OHD2: 413>255 | 25OHD3: 103.8% (50 nmol/L), 25OHD2: 98.8% (7.5 nmol/L) | 25OHD3: 2.5, 25OHD2: 2.5 | 25OHD3: 5.7% at 50 nmol/L, 25OHD2: 4.5% at 7.5 nmol/L, 25OHD3: 2.5% at 47.5 nmol/L, 25OHD2: 5.1% at 8.0 nmol/L |
| Maunsell et al. [84] | Serum 100 μl, PP: MeOH:Propanol (80:20 v/v), Extraction: LLE: n-Hexane HPLC: BDS C8® ThermoHypersil MeOH>H2O+0.05% CHO2H Gradient TMS: ESI+ MRM | In-house IS: 2H6-25OHD3: 407.2>389.4, 25OHD3: 401.8>383.5, 25OHD2: 413.5>395.4 | 25OHD3: 91–110% at 128–256 nmol/L, 25OHD2: 94–108% at 158–317 nmol/L | 25OHD3: <4.0, 25OHD2: <5.0 | 25OHD3: 6.2% at 16 nmol/L, 25OHD3: 5.1% at 55 nmol/L, 25OHD2: 9.5% at 52 nmol/L |
| Chen et al. [85] | Serum 200 μl, PP: MeCN Extraction: SPE: Oasis HLB® MeOH/H2O (30/70 v/v); MeCN/MeOH (50/50 v/v), HPLC: SupelCosil®LC-18-DB EtOH:H2O (83:17 v/v) TMS: APCI+ MRM | Commercial IS: 2H6-25OHD3: 407.7>389.7, 25OHD3: 401.4>383.4, 25OHD2: 413.4>395.4 | 25OHD3: 99±2% at 34.2–132.8 nmol/L, 25OHD2: 95±0.8% at 32.2–115.5 nmol/L | 25OHD3: 4.0, 25OHD2: 15.5, 25OHD3: 1.2, 25OHD2: 4.6 | 25OHD3: 6.2% at 34 nmol/L, 25OHD2: 8.7% at 23 nmol/L, 25OHD3: 11% at 34 nmol/L, 25OHD2: 16% at 23 nmol/L |
| Bunch et al. [86] | Serum 100 μl, PP: MeOH Extraction: OLTFE HPLC: Hypersil Gold aQ® MeOH/H2O (95/5 v/v) TMS: APCI+ MRM | In-house IS 2H6-25OHD3: 407.2>389.4, 25OHD2: 413.5>395.4, 25OHD3: 401.8>383.5 | 25OHD3: 3.0, 25OHD2: 4.6 | ||
| Hojskov et al. [87] | Serum 100 μl, PP: MeCN, extraction: automated LLE: 96-well Isolute HM-N plate®/diatomaceous earth; Heptane HPLC: Synergi MAX-RP® MeOH/2.0 mM NH4Ac (85/15 v/v) TMS: APCI+ MRM | Commercial IS 2H6-25OHD3: 407.4>371.4 25OHD3: 401.4>365.2, 25OHD2: 413.4>395.4 | NR | 25OHD3: <10, 25OHD2: <10 | 25OHD3: 9.4% at 32 nmol/L, 25OHD2: 8.6% at23.4 nmol/L |
| Hermann et al. [88] | Serum 100 μl, PP: MeCN HPLC: Supelcosil LC-8® H2O>MeOH>H2O/MeOH (98/2 v/v)>Toluene APPI+ MRM | Commercial IS 2H6-25OHD3: 389>371, 2H6-25OHD2 401>383, 25OHD3: 395>377, 25OHD2: 413.5>395.4 | 108–113%, expressed as total 25OHD added (45–90 nmol/L) | 25OHD3: 1.3, 25OHD2: 1.3 | 25OHD: 5.7% at 17 nmol/L, 25OHD: 8.7% at 17 nmol/L |
| Ding et al. [89] | Serum 200 μl, PP: MeCN, extraction: SPE Oasis HLB® MeCN; EtOAc Derivatisation (PTAD)/MeCN HPLC: ACQUITY BEH C18® 0.1% CHO2H/H2O/MeNH2; CHO2H/MeOH gradient TMS: ESI+ MRM | Commercial IS 2H6-25OHD3: 613>298, 2H6-25OHD2 625>298, 25OHD3: 607>298, 25OHD2: 619>298 | 2H6-25OHD3: 84.9±2.4%b, 2H6-25OHD2: 79.3±14.4%b | #25OHD3: 0.025, #25OHD2: 0.025 | #25OHD3: 3.8% at 0.025 nmol/L, #25OHD2: 1.6% at 0.025 nmol/L |
| Van den Ouweland et al. [90] | Serum 250 μl, PP: NaOH-MeCN/MeOH (9/1 v/v) SPE: Strata C18-E® H2O-MeOH/H2O (60/40 v/v)-MeOH HPLC: ACQUITY UPLC BEH C18® 0.1% CHO2H/2 mM NH4Ac; MeOH/CHO2H (99.7:0.3 v/v) gradient, TMS: AP-ESI+ SRM | Commercial IS 2H6-25OHD3: 407.5>159.2, 25OHD3: 401.5>159.2, 25OHD2: 413.4>83.1 | 25OHD3: 94.9–106.9% at 49.9–99.9 nmol/L, 25OHD2: 82.7–100.3% at 54.3–108.6 nmol/L | 25OHD3: 3.5, 25OHD2: 2.0, 25OHD3: 1.5, 25OHD2: 1.2 | 25OHD3: 2.7% at 64.9 nmol/L, 25OHD2: 4.2% at 33.3 nmol/L, 25OHD3: 6.0% at 64.9 nmol/L, 25OHD2: 3.8% at 33.3 nmol/L |
| Tai et al. [91] | Serum 2 g pH adjusted to 9.8 (Na2CO3) LLE, extraction: n-hexane/EtAc (50/50 v/v), Residue dissolved in MeOH HPLC: Zorbax CB-CN column H2O/MeOH (34/66 v/v) TMS: APCI+ MRM | Commercial IS 2H3-25OHD3 404>386 2H3-25OHD2 416>398, 25OHD3 C3-epi-25OHD3: 401>383, 25OHD2 C3-epi-25OHD2: 413>395, Stds traceable to NIST | 25OHD3: 100.0–10%, 25OHD2: 98.0–100.1% | 25OHD3: 0.15 ng/g, 25OHD2: 0.1 ng/g | 25OHD3: 0.4% at 6.31 ng/g, 25OHD2: 0.9% 0.86 ng/g, 25OHD3: 0.6% at 6.31 ng/g, 25OHD2: 0.86% 0.86 ng/g |
| Stepman et al. [92] | Serum 250 μl, extraction: LLE: NaOH/n-hexane Sephadex LH-20 chromatography MeOH/CHCl3/cC6H14 (1/4/8, v/v/v) 2-dimensional UPLC Chromatography 1:Acquity BEH 300C4®, column 2: Acquity BEH C18® column-25OHD2 2: Zorbax SB-CN® column-25OHD3, step gradients MeOH/H2O/CHO2H (50/50/0.025) MeOH/H2O/CHO2H (95/5/0.025) TMS: ESI+ SIM | Commercial IS 2H6-25OHD3 407.3>159.3, 2H6-25OHD2 419.4>159.4, 25OHD3: 401.3>159.3, 25OHD2: 413.4>159.4C3-epi-25OHD3 401.3>159.3, Stds Traceable to NIST | 25OHD3: 71%±4%c, 25OHD2: 70%±8%c | 25OHD3: 1.12±0.05, 25OHD2: 1.22±0.05 | 25OHD3: 1.4% at 30.8 nmol/L, 25OHD2: 2.0% at 64.1 nmol/L, 25OHD3: 1.7% at 30.8 nmol/L, 25OHD2: 1.1% at 64.1 nmol/L |
| Adamec et al. [93] | Serum 100 μl, extraction: LLE: Acetone HPLC: ACE3C8® column, gradient: H2O/MeOH+1% toluene TMS: APPI+ MRM | Commercial IS 2H6-25OHD3 407.3>263.3, 2H6-25OHD2 419.3>401.2, 25OHD3: 401.2>257.2, 25OHD2: 413.3>337.2, Stds traceable to NIST | 25OHD3: NR, 25OHD2: NR | 25OHD3: 2.0, 25OHD2: 2.0 | 25OHD3: 3.7% at 5 nmol/L, 25OHD2: 16.7% at 5.0 nmol/L, 25OHD3: 15.4% at 5.0 nmol/L, 25OHD2: 14.0% at 5.0 nmol/L |
| Wang et al. [94] | Plasma 1 ml, PP: MeCN LLE: EtOAc, derivatisation: PTAD HPLC: Hypersil Gold® column MeCN/H2O+0.1% CHO2H gradient (40/60; 60/40; 90/10, 40/60 v/v) TMS: ESI+ MRM | Commercial IS 2H6-25OHD3 564>298, 25OHD3: 558>298 | 25OHD3: 73%±2% (BSA matrix) | 25OHD3: 0.125, 25OHD3: 0.01 | 25OHD3: 2.1% at 25 nmol/L, 25OHD3: 7.0% at 25.0 nmol/L |
| Bogusz et al. [95] | Serum 100 μl, PP: MeOH/MeCN/0.05 M ZnSO4 (6.5/1/2 v/v/v) HPLC: Kinetex C18 NH4CHO2H/MeOH Gradient (70/30; 90/10; 70/30) TMS: APCI+ MRM | Commercial IS 2H6-25OHD3 389>371 389>211, 2H6-25OHD2 401> 383 401>209, 25OHD3: 383>365 383>211, 25OHD2: 395>209 395>269, Stds traceable to NIST | 25OHD3: 98%, 25OHD2: 97% | 25OHD3: 3.0, 25OHD2: 1.5, 25OHD3: 1.5, 25OHD2: 0.5 | 25OHD3: 3% at 41.7 nmol/L, 25OHD2: 4% at 42.1 nmol/L |
| Baecher et al. [96] | Serum 200 μl, PP: MeCN On-line SPE: LiChrospher® column MeOH/H2O (5/95 v/v) HPLC: Kinetex® PFP column MeOH/0.5 mM NH4Ac (75/25 v/v) TMS: APCI+ MRM | Commercial IS 2H6-25OHD3 407.3>263.2, 407.3>159.2, 25OHD3: 401.3>257.2, 401.3>159.2, 25OHD2: 413.4>159.2C3-epi25OHD3 401.3>257.2, 401.3>159.2, NIST SRM 2972 (levels 1-4) used for comparison | 25OHD3: NR, 25OHD2: NR, C3-epi25OHD3, 95.5% at 5.05 nmol/L | 25OHD3: 4.0, 25OHD2: 3.9C3-epi25,OHD3: 2.0 | 25OHD3: 3.1% at 39.8 nmol/L, 25OHD2: 4.9% at 27.5 nmol/L, C3-epi25O,HD3: 4.2% at 20.1 nmol/L 25OHD3: 3.8% at 39.8 nmol/L, 25OHD2: 3.4% at 27.5 nmol/L, C3-epi25O, HD3: 3.4% at 20.1 nmol/L |
| Farrell et al. [97] | Serum 150 μl, PP: 2.0 M ZnSO4/MeOH TMS: 0.2 M/MeOH, SPE:Oasis μElution HLB plate MeOH/H2O (60/40 v/v) 2 mM NH4Ac+0.1% CHO2H/MeOH/2 mM NH4Ac+0.1% CHO2H (27/73 v/v) UPLC: ACQUITY BEH C8® 2 mM NH4Ac+0.1% CHO2H/MeOH/2 mM NH4Ac+0.1% CHO2H Gradient from (27/73 v/v) to (98/2 v/v) ESI+ MRM | Commercial IS 2H6-25OHD3 407.3>159.1, 2H3-25OHD2 416.3>398.3, 25OHD3: 401.3>383.5, 401.3>159.1, 25OHD2: 413.3>83.1, 413.3>395.3 | 25OHD3: NR, 25OHD2: NR | 25OHD3: 2.0, 25OHD2: 2.0, 25OHD3: 0.5, 25OHD2: 0.5 | 25OHD: 1.6% at 79 nmol/L, 25OHD: 2.0% at 79 nmol/L |
| Lensmeyer et al. [98] | Serum 300 μl, PP: MeCN/2 mM ZnSO4 (87/13 v/v)/MeOH, extraction: SPE Strata C18E® MeCN/H2O (45/55 v/v), Acetone/MeCN (20/80 v/v) HPLC: Zorbax cyanopropyl column MeOH/H2O (67/33 v/v), TMS: APCI+ MRM | IS: NR 25OHD3: 383.3>211.1, 25OHD2: 395.3>209.1C3-epi, 25OHD3 383.3>211.1 | NR | NR | NR |
| Thibault et al. [99] | Serum 200 μl, PP: MeCN On-line SPE: X-Terra C18 MeOH/0.1% CHO2H+2 mM NH4Ac in H2O (98/2 v/v)/0.1% CHO2H+2 mM NH4Ac in H2O (68/32 v/v) HPLC: Sunfire C18 MeOH/0.1% CHO2H+2 mM NH4Ac in H2O (98/2 v/v)/ 0.1% CHO2H+2 mM NH4Ac in H2O (85/15 v/v), TMS: ESI+ MRM | Commercial IS [2H6]25-OHD3 407.5>371.3, [2H6]25-OHD2 419.4>355.2, 25OHD3: 401.4>365.3, 25OHD2: 413.4>355.3 | 25OHD3: 4, 25OHD2: 3 | 25OHD3: 3.4% at 59.8 nmol/L, 25OHD2: 1.8% at 99.5 nmol/L, 25OHD3: 5.9% at 66.7 nmol/L, 25OHD2: 5.9% at 101.3 nmol/L | |
| Strathmann et al. [100] | Serum 200 μl extraction: 1 M NaOH/n-heptane HPLC: XTerra MS C8+Restek columns, NH4Ac/0.1% CHO2H in MeOH/H20 (95/5 v/v), TMS: APCI+ MRM | Commercial IS [2H6]25-OHD3 407.3>371.3, [2H6]25-OHD2 419.4>355.2, 25OHD3: 401.3>355.3, 25OHD2: 413.4>355.3, Stds traceable to NIST | 25OHD3: 80–116% (23.4 nmol/L), 25OHD2: 94–115% (23.4 nmol/L) | 25OHD3: 1.95, 25OHD2: 0.6 | 25OHD3: 2.9% at 58 nmol/L, 25OHD2: 2.8% at 85 nmol/L, 25OHD3: 9.6% at 63 nmol/L, 25OHD2: 6.2% at 95 nmol/L |
| Mochizuki et al. [101] | Serum or plasma 25 μl, PP: MeCN 2-dimension HPLC: SPE: Turboflow XL C18-P® column, step gradient 0.1% CHO2H; MeCN/propanol-2ol/acetone (44/40/20 v/v/v); MeOH/0.1% CHO2H HPLC: Hypersil Gold® column 0.1% CHO2H; MeOH/0.1% CHO2H TMS: APCI+ SRM | Commercial IS [2H6]25-OHD3 389.3>263.2 25OHD3: 383.3>365.2 25OHD2: 395.3>377.4 Stds traceable to NIST | 25OHD3: 102.6–106% (36.9–59.8 nmol/L), 25OHD2: NR | 25OHD3: 2.2, 25OHD2: 3.5, 25OHD3: 0.8, 25OHD2:2.2 | 25OHD3: 5.2% at 18 nmol/L, 25OHD2: 10.6% at 18 nmol/L, 25OHD3: 7.2% at 18 nmol/L, 25OHD2: NR |
| Zhang et al. [102] | Serum 200 μl, PP: MeOH, extraction: n-heptane HPLC: Zorbax SB-C18, Step Gradient: 2 mM NH4Ac/0.1% CHO2H–H2O; 2 mM NH4Ac/0.1% CHO2H–MeOH, TMS: ESI+ MRM | Commercial IS [2H3]-25OHD3 404.3>368.2, [2H3]-25OHD2 416.3>358.2, 25OHD3: 401.3>365.2, 25OHD2: 413.3>355.2 | 25OHD3: ≥62% (125–200 nmol/L), 25OHD2: ≥72% (18–200 nmol/L) | 25OHD3: 6.2, 25OHD2: 6.2, 25OHD3: NR, 25OHD2: NR | 25OHD3: 2.2% at 18 nmol/L, 25OHD2: 2.1% at 18 nmol/L, 25OHD3: 4.4% at 18 nmol/L, 25OHD2: 5.0 at 18 nmol/L |
| Kaufmann et al. [103] | Serum 100 μl, PP: 0.1 M HCl/0.2 M ZnSO4/MeOH, extraction: n-hexane/t-butyl ether (1/1 v/v), derivatisation (DMEQ-TAD)/AcOEt UPLC: BEH-Phenyl column, MeOH/H2O gradient, TMS: ESI+ MRM | Commercial IS 2H3-25OHD3: 613>298, 2H3-25OHD2 625>298 25OHD3: 746.6>468, 25OHD2: 758.6>468 | 25OHD3: NR, 25OHD2: NR | 25OHD3: 0.25, 25OHD2: 0.25, 25OHD3: 0.10, 25OHD2: 0.10 | 25OHD3: 3–4% at 55 nmol/L, 25OHD2: 3–4% at 83 nmol/L, 25OHD3: 4–7% at 55 nmol/L, 25OHD2: 4–7% at 83 nmol/L |
HPLC: high performance liquid chromatography; UPLC: UlLC: performance liquid chromatography; MS: mass spectrometry; TMS: tandem-mass spectrometry; AP: atmospheric pressure; ESI: electron spray ionization; APCI: atmospheric pressure chemical ionization; APPI: atmospheric pressure photo-ionization; ID: isotope dilution; MRM: multiple reaction monitoring; SRM: selected reaction monitoring; PP: protein precipitation; SPE: solid phase extraction; LLE: liquid/liquid extraction; OLTFE: on-line turboflow extraction; DMEQ-TAD: 4-[2-(6,7-dimethoxy-4-methyl-3-oxo-3,4-dihydroquinoxalyl)ethyl]-1,2,4-triazoline-3,5-dione; NPTAD: 4-(4-Nitrophenyl)-1,2,4-triazoline-3,5-dione; PTAD: 4-phenyl-1,2,4-triazoline-3,5-dione; EAD: enzyme-assisted derivatisation; GP: Girard reagent P reagent (1-(carboxymethyl)pyridinium chloride hydrazide); 25OHdC: 25-Hydroxy-7-dehydrocholesterol; 1α–OHD3. 1-alpha-hydroxyvitamin D3 AcOEt: ethyl acetate; MeNH2: methyl amine; MeOH: methanol; EtOH: ethanol; NH4Ac: ammonium acetate; MeCN: acetonitrile: Et2O: diethyl-ether; KOH: potassium hydroxide; MeCl2: dichloromethane; HClO4: perchloric acid; THF: tetrahydrofuran; ASC: 20% ascorbic acid water solution; CHO2H: formic acid; IS: internal standard; NR: not reported; NIST: National Institute of Standards and Technology (Gaithersburg, USA); SRM: standard reference material; levels 1–4: level 1: human serum; level 2: human serum diluted with horse serum to achieve a lower 25(OH)Dx concentration; level 3: human serum fortified with 25(OH)D2; and level 4: human serum fortified with 3-epi-25(OH)D3.
Recovery: exogenously added vitamin D metabolite.
% Recovery±SD for the 2 deuterated compounds at a 50 fmol/μl fortification level.
Expressed as % recovery of the NIST-certified values.
Three candidate reference methods have been proposed in the last 10 years. In 2004, Vogeser et al. [82] published the 1st candidate reference method for the measurement of 25OHD3 by stable isotope-dilution LC/MS–MS applicable to clinical laboratory practice. Their method involved a protein denaturation process to release the bound vitamin D metabolites, and on-line solid-phase extraction before the reverse-phase HPLC coupled to the MS–MS with the detector set in the electrospray atmospheric pressure ionization in the positive mode. In 2010 and 2011, Tai et al. [91] and Stepman et al. [92] proposed each a candidate method that differed from that of Voseger et al. [82] and from each other in a number of ways. Whereas Vogeser et al. [82] utilized a 25OHD3 internal standard containing 3 Deuterium atoms and 1 13C atom, Tai et al. [91] used tri-deuterated 25OHD3 and 25OHD2, and Stepman et al. [92] hexa-deuterated hydroxylated vitamins D2 and D3. Differences lied also in the sample volume (200 μl to 2 ml), sample preparation (liquid–liquid or solid-phase extraction), HPLC conditions and detection process [APCI+ or ESI+ and multiple reaction monitoring (MRM) or single ion monitoring (SIM)]. Despite their differences, the IFCC Joint Committee for Traceability in Laboratory Medicine (JCTLM) recognized Tai et al.׳s [90] and Stepmans et al.׳s [92] as reference method procedures (RMP). Furthermore, the NIST has used Tai et al.׳s [91] candidate RMP to certify the concentrations of 25(OH)D3 and 25(OH)D2 in their Standard Reference Material for Vitamin D in human serum to validate the accuracy for the methods used in clinical laboratories.
9. Problems related to LC/MS–MS
Undoubtedly, LC/MS–MS methods offer many advantages. First they have the potential of measuring simultaneously all species of the 25-hydroxylated vitamin D as well as downstream dihydroxylated metabolites. Second, they are not bound to conditions imposed by the manufacturers, although commercial “turn-key” tandem-mass spectrometry methods are now available. Gervasoni et al. [104] have recently reported a comparison between 2 such methods suitable for application in clinical laboratories. Third, the technological progress has allowed LC/MS–MS, using Single Ion Monitoring (SIM), to reach high analytical specificity and sensitivity while resorting to relatively short chromatography run time, essential when considering a clinical application.
The development of advanced informatics coupled to the simplified use of LC/MS–MS equipment have led users to underestimate the complexity of the analytical processes involved in the quantitation of vitamin D metabolites and hence to undervalue limitations that may compromise the dependability of the data. Indeed, LC/MS–MS is not devoid of hindrance when considering clinical laboratories. Firstly, the instruments are costly, and their complexity requires well-trained personnel for their operation and maintenance. Secondly, matrix effects may be a significant drawback when Electrospray (ESI) or Atmospheric Pressure Chemical Ionization (APCI) sources are employed. Hence, better sample clean-up and lengthier chromatography are required. Thirdly, high sensitivity and high specificity may be mutually exclusive in the SIM mode when interfering compounds with identical precursor and product ions co-elute with vitamin D metabolites [78], [105], [106]. The example of 1α-OHD and 7α-OH-4-cholestene-dione (a marker of bile acid malabsorption) as being potential interfering substances in the LC/MS–MS analysis, but resolved by a more elaborate HPLC step illustrates this point [84]. The selection of a second or third product ion that does not interfere also helps solving specificity-related problems. For example 25OHD2, 25OHD3 and their respective C3-epimers, present in high concentration in infants’ serum [41], [42], may be distinguished using different SIM transitions although sharing the same product ions [41], [87], [89], [108], [109]. Knox et al. [107], recognizing that the purification steps are time-consuming in the perspective of clinical laboratories, proposed a procedure that involves protein precipitation with methanol and a robotized 6-step solid-phase extraction, that could handle up to 300 samples per day. This procedure should yield cleaner extracts before injection on the LC/MS–MS instrument, decrease background noise and increase sensitivity.
10. The present and the future
As specific as LC-TMS may be for the measurement of vitamin D metabolites, accuracy and precision depend on strict standardization procedures. These aspects were until recently Achilles’ heel of this field and discredited the threshold definition for the vitamin D nutritional status. The coefficients of variation in a 2013 DEQAS survey, varying between 11 to 25% for all tested laboratory methods (437 participants) and between 9.7% and 11.3% for MS–MS-based methods (147 laboratories), illustrate the inter-laboratory differences. Carter et al. [110] have reported in a detailed study of analytical performance of the laboratories using LC-TMS, an 11% positive bias with respect to the RMP and suggested that it was due to the inclusion of the C3-epimer, that most laboratories could not separate from 25OHD3. At that time, the lack of a RMP and SRM prohibited the evaluation of the accuracy. The recent SRM 972a and calibration solutions developed by the NIST [111], [112] will improve the analytical performance of all methods, as Cavalier et al. [113] have shown for automated methods.
11. Conclusions
The different serum 25OHD values obtained through the years with different methods may have lead to misclassification of patients in terms of the vitamin D nutritional status. The historical thresholds defining vitamin D sufficiency, insufficiency and deficiency, upon which supplementation decisions are taken, should to be employed with caution. Cavalier et al. [114] have made the point that for assuring the “optimal” serum 25OHD concentration (75 nmol/L), the measured value could vary from 50 to 100 nmol/L, and that the threshold should be method-specific. The C3-epi-25OHD3 present in high concentration in infants׳ serum and to a lesser extent in adults, remains an issue as there are diverging opinions on the biological action of C3-epi-1α,25(OH)2D3 [19], [20], [115], [116]. Whatever the answer is, C3-epi-25OHD3 should be quantified when evaluating the vitamin D nutritional status. The recently developed reference method procedures and certified reference and calibration solutions developed by the NIST, to which all laboratories performing 25OHD assays are urged to adhere, will improve the analytical performance of all methods.
Conflicts of interest
The authors declare to have no conflicts of interest related to the present review subject.
All authors contributed to the writing and to the revision of the manuscript.
References
- 1.Morris H.A. Vitamin D activities for health outcomes. Ann Lab Med. 2014;34:181–186. doi: 10.3343/alm.2014.34.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holick M.F. Medical progress: vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Joergensen C., Hovind P., Schmedes A., Parving H.H., Rossing P. Vitamin D levels, microvascular complications, and mortality in type 1 diabetes. Diabetes Care. 2011;34:1081–1085. doi: 10.2337/dc10-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim Y., Franke A.A., Shvetsov Y.B., Wilkens L.R., Cooney R.V., Lurie G. Plasma 25-hydroxyvitamin D3 is associated with decreased risk of postmenopausal breast cancer in whites: a nested case-control study in the multi-ethnic cohort study. BMC Cancer. 2014;14:29–36. doi: 10.1186/1471-2407-14-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W.L., Tenniswood M. Vitamin D, intermediary metabolism and prostate cancer tumor progression. Front Physiol. 2014;5:1–9. doi: 10.3389/fphys.2014.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierrot-Deseilligny C., Souberbielle J.-C. Is hypovitaminosis one of the environmental risk factors for multiple sclerosis? Brain. 2010;133:1869–1888. doi: 10.1093/brain/awq147. [DOI] [PubMed] [Google Scholar]
- 7.Ross A.C., Taylor C.L., Yaktine A.L., Del Valle H.B. The National Academies Press; Washington, DC: 2011. Committee to Review Dietary Reference Intakes (DRI) for Vitamin D and Calcium. IOM (Institute of Medicine)〈http://www.nap.edu〉 [PubMed] [Google Scholar]
- 8.Newberry S.J., Chung M., Shekelle P.G., Booth M.S., Liu J.L., Maher A.R. Agency for Healthcare Research and Quality; Rockville, MD: 2014. Vitamin D and Calcium: A Systematic Review of Health Outcomes (Update). Evidence Report/Technology Assessment No. 217. (Prepared by the Southern California Evidence-based Practice Centre under Contract No. 290-2012-00006-I.) AHRQ Publication No. 14-E004-EF. 〈www.effectivehealthcare.ahrq.gov/reports/final.cfm〉. [Google Scholar]
- 9.Carter G.D., Berry J.L., Gunter E., Jones G., Jones J.C., Makin H.J. Proficiency testing of 25-hydroxyvitain D (25OHD) assays. J Steroid Biochem Mol Biol. 2010;121:176–179. doi: 10.1016/j.jsbmb.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 10.Sempos C.T., Vesper H.W., Phinney K.W., Thienpont L.M., Coates P.M., the Vitamin D Standardization Program (VDSP) Vitamin D status as international issue: national surveys and the problem of standardization. Scand J Clin Lab Investig. 2012;72(Suppl. 243):S32–S40. doi: 10.3109/00365513.2012.681935. [DOI] [PubMed] [Google Scholar]
- 11.Stöckl D., Sluss P.M., Thienpont L.M. Specifications for trueness and precision of a reference measurement system for serum/plasma 25-hydroxyvitamin D analysis. Clin Chim Acta. 2009;408:8–13. doi: 10.1016/j.cca.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Binkley N., Sempos C.T., for the Vitamin D Standardization Program (VDSP) Standardizing vitamin D assays: the way forward. J Bone Miner Res. 2014;29:1709–1714. doi: 10.1002/jbmr.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 14.Hiljer J., Friedel A., Herr R., Rausch T., Roos F., Wahl D.A. A systematic review of the vitamin D status worldwide. Br J Nutr. 2014;111:23–45. doi: 10.1017/S0007114513001840. [DOI] [PubMed] [Google Scholar]
- 15.Medical Advisory Secretariat Clinical utility of vitamin D testing: an evidence-based analysis. Ont Health Technol Assess Ser. 2010;10(2):1–95. 〈http://www.health.gov.on.ca/english/providers/program/mas/tech/reviews/pdf/rev_vitamin d_201002.pdf〉 [Internet]. 2010 Feb [cited 01.01.15] [PMC free article] [PubMed] [Google Scholar]
- 16.Biancuzzo R.M., Young A., Bibuld D., Cai M.H., Winter M.R., Klein E.K. Fortification of orange juince with vitamin D2 or vitamin D3 is as effective as an oral supplement in maintaining vitamin D status in aults. Am J Clin Nutr. 2010;91:1621–1626. doi: 10.3945/ajcn.2009.27972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armas Lag L., Hollis B.W., Heaney R.P. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–5391. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- 18.Tsugawa N., Nakagawa K., Kawamoto Y., Tachibana Y., Hayashi Y., Ozono K. Biological activity profiles of 1alpha,25-dihydroxyvitamin D2, D3, D4, D7, and 24-epi-1alpha,25-dihydroxyvitamin D2. Biol Pharm Bull. 1999;22:371–377. doi: 10.1248/bpb.22.371. [DOI] [PubMed] [Google Scholar]
- 19.Molnár F., Sigüeiro R., Sato Y., Araujo C., Schuster I., Antony P. 1a,25(OH)2-3-epi-Vitamin D3, a natural physiological metabolite of vitamin D3: Its synthesis, biological activity and crystal structure with its receptor. PLoS One. 2011;6:e18124. doi: 10.1371/journal.pone.0018124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown A.J., Ritter C., Slatopolsky E., Muralidharan K.R., Okamura W.H., Reddy G.S. 1Alpha,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of 1alpha,25-dihydroxyvitamin D3, is a potent suppressor of parathyroid hormone secretion. J Cell Biochem. 1999;73:106–113. [PubMed] [Google Scholar]
- 21.Dueland S., Helgerud P., Pedersen J.I., Berg T., Drevon C.A. Plasma clearance, transfer and distribution of vitamin D3 from intestinal lymph. Am J Physiol. 1983;245:E326–E331. doi: 10.1152/ajpendo.1983.245.4.E326. [DOI] [PubMed] [Google Scholar]
- 22.Haddad J.G., Matsuoka L.Y., Hollis B.W., Hu Y.Z., Worstman J. Human plasma transport of vitamin D after endogenous synthesis. J Clin Investig. 1993;91:2552–2555. doi: 10.1172/JCI116492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francheschi R.T., Simpson R.U., DeLuca H.F. Binding proteins for vitamin D metabolites: serum carriers and intracellular receptors. Arch Biochem Biophys. 1981;210:1–13. doi: 10.1016/0003-9861(81)90157-0. [DOI] [PubMed] [Google Scholar]
- 24.Whyte M.P., Haddad J.G., Walters D.D., Stamp T.C.B. Vitamin D bioavailability: serum 25-hydroxyvitamin D levels in man after oral subcutaneous, intramuscular and intravenous vitamin D administration. J Clin Endocrinol Metab. 1979;48:906–911. doi: 10.1210/jcem-48-6-906. [DOI] [PubMed] [Google Scholar]
- 25.White P., Cooke N. The multifunctional properties and characteristics of vitamin D-binding protein. Trends Endocrinol Metab. 2000;11:320–327. doi: 10.1016/s1043-2760(00)00317-9. [DOI] [PubMed] [Google Scholar]
- 26.Chun R.F., Peercy B.E., Orwoll E.S., Nielsen C.M., Adams J.S., Hewison M. Vitamin D and DBP: The free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2014;144:132–137. doi: 10.1016/j.jsbmb.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray T.K., Lowe W., Lester G.E. Vitamin D and pregnancy: the maternal–fetal metabolism of vitamin D. Endocr Rev. 1981;2:264–271. doi: 10.1210/edrv-2-3-264. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz J.B., Lai J., Lizaola B., Kane L., Weyland P., Terrault N.A. Variability in free 25(OH) vitamin D in clinical populations. J Steroid Biochem Mol Biol. 2014;144:156–158. doi: 10.1016/j.jsbmb.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhan I., Powe C.E., Berg A.H., Ankers E., Wenger J.B., Karumanchi S.A. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int. 2012;82:84–89. doi: 10.1038/ki.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Depreter B., Heijboer A.C., Langlois M.R. Accuracy of three automated 25-hydroxyvitamin D assays in hemodialysis patients. Clin Chim Acta. 2013;415:255–260. doi: 10.1016/j.cca.2012.10.056. [DOI] [PubMed] [Google Scholar]
- 31.Freeman J., Wilson K., Spears R., Shalhoub V., Sibley P. Influence of vitamin D binding protein on accuracy of 25-hydroxyvitamin D measurement using the ADVIA Centaur Vitamin D total assay. Int J Endocrinol. 2014:691679. doi: 10.1155/2014/691679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heijboer A.C., Blankenstein M.A., Kema I.P., Buijs M.M. Accuracy of 6 routine 25 hydroxyvitamin D assays: influence of vitamin D binding protein concentration. Clin Chem. 2012;58:543–548. doi: 10.1373/clinchem.2011.176545. [DOI] [PubMed] [Google Scholar]
- 33.Cavalier E., Wallace A.M., Knox S., Mistretta V.I., Cormier C., Souberbielle J.-C. Serum vitamin D measurement may not reflect what you give to your patients. Bone Miner Res. 2008;23:1864–1865. doi: 10.1359/jbmr.080608. [DOI] [PubMed] [Google Scholar]
- 34.Carter G.D., Jones J.C., Berry J.L. The anomalous behaviour of 25-hydroxyvitamin D in competitive binding assays. J Steroid Biochem Mol Biol. 2007;103:480–482. doi: 10.1016/j.jsbmb.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Horst R.L. Exogenous versus endogenous recovery of 25-hydroxyvitamin D2 and D3 in human samples using high-performance liquid chromatography and the DiaSorin Liaison Total-D assay. J Steroid Biochem Mol Biol. 2010;121:180–182. doi: 10.1016/j.jsbmb.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Lankes U., Elder P.A., Lewis J.G., George P. Differential extraction of endogenous and exogenous 25-OH-vitamin D from serum makes the accurate quantification in liquid-chromatography-tandem mass spectrometry assays challenging. Ann Clin Biochem. 2014;XX:1–10. doi: 10.1177/0004563214533316. [DOI] [PubMed] [Google Scholar]
- 37.Wallace A.M., Gibson S., de la Hunty A., Lamberg-Allardt C., Ashwell M. Measurement of 25-hydroxyvitamin D in the clinical laboratory: current procedures, performance characteristics and limitations. Steroids. 2010;75:477–488. doi: 10.1016/j.steroids.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Ross A.C., Manson J.E., Abrams S.A., Aloia J.F., Brannon P.M., Clinton S.K. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salle B., Duhamel J.F., Souberbielle J.C. Rapport de l’Académie nationale de médecine sur la vitamine D. Bull Acad Nat Med. 2012;196:1011–1015. [Google Scholar]
- 40.Cavalier E., Wallace A.M., Carlisi A., Chapelle J.P., Delanaye P., Souberbielle J.C. Cross- reactivity of 25-hydroxyvitamin D2 from different commercial immunoassays for 25-hydroxyvitamin D: an evaluation without spiked samples. Clin Chem Lab Med. 2011;49:555–558. doi: 10.1515/CCLM.2011.072. [DOI] [PubMed] [Google Scholar]
- 41.Bailey D., Veljkovic K., Yazdanpanah M., Adeli K. Analytical measurement and clinical relevance of vitamin D3 C3-epimer. Clin Biochem. 2013;46:190–196. doi: 10.1016/j.clinbiochem.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 42.Bailey D., Perumal N., Yadzanpanah M., Al Mahmud A., Baqui A.H., Adeli K. Maternal–fetal–infant dynamics of the C3-epimer of 25-hydroxyvitamin D. Clin Biochem. 2014;47:816–822. doi: 10.1016/j.clinbiochem.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 43.Haddad J.G., Chuy K.J. Competitive protein binding radioassay for 25-hydroxycholecalciferol. J Clin Endocrinol Metab. 1971;33:992–995. doi: 10.1210/jcem-33-6-992. [DOI] [PubMed] [Google Scholar]
- 44.Delvin E.E., Dussault M., Glorieux F.H. A simplified assay for serum 25-cholecalciferol. Clin Biochem. 1980;13:10608. doi: 10.1016/s0009-9120(80)90735-3. [DOI] [PubMed] [Google Scholar]
- 45.Bouillon R., Van Heck E., Jans I., Tan B.K., Van Baelen H., De Moor P. Two direct (nonchromatographic) assays for 25-hydroxyvitamin D. Clin Chem. 1984;30:1731–1736. [PubMed] [Google Scholar]
- 46.Parviainen M.T., Savolainen K.E., Korhonen P.H., Alhava E.M., Visakorpi J.K. An improved method for routine determination of vitamin D and its hydroxylated metabolites in serum from children and adults. Clin Chim Acta. 1981;114:233–247. doi: 10.1016/0009-8981(81)90396-x. [DOI] [PubMed] [Google Scholar]
- 47.Hummer L., Nilas L., Tjellesen L., Christiansen C. A selective and simplified radioimmunoassay of 25-hydroxyvitamin D3. Scand J Clin Lab Investig. 1984;44:163–167. doi: 10.3109/00365518409161399. [DOI] [PubMed] [Google Scholar]
- 48.Hollis B.W., Napoli J.L. Improved radioimmunoassay for vitamin D and its use in assessing vitamin D status. Clin Chem. 1985;31:1815–1819. [PubMed] [Google Scholar]
- 49.Stryd R.P., Gilbertson T.J. Some problems in development of a high-performance liquid chromatographic assay to measure 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 simultaneously in human serum. Clin Chem. 1978;24:927–930. [PubMed] [Google Scholar]
- 50.Hollis B.W., Kamerud J.Q., Selvaag S.R., Lorenz J.D., Napoli J.L. Determination of vitamin D status with a 125I-labeled tracer. Clin Chem. 1993;39:529–533. [PubMed] [Google Scholar]
- 51.Hollis B.W. Comparison of commercially available 125I-based RIA methods for the determination of circulating 25-hydroxyvitamin D. Clin Chem. 2000;46:1657–1661. [PubMed] [Google Scholar]
- 52.Glendenning P., Taranto M., Noble J.M., Musk A.A., Hammond C., Goldswain P.R. Current assays overestimate 25-hydroxyvitamin D3 and underestimate 25-hydroxyvitamin D2 compared to HPLC: need for assay-specific decision limits and metabolite-specific assays. Ann Clin Biochem. 2006;43:23–30. doi: 10.1258/000456306775141650. [DOI] [PubMed] [Google Scholar]
- 53.Le Goff C., Peeters S., Crine Y., Lukas P., Souberbielle J.-C., Cavalier E. Evaluation of the cross-reactivity of 25-hydroxyvitamin D2 on seven commercial immunoassays on native samples. Clin Chem Lab Med. 2012;50:2031–2032. doi: 10.1515/cclm-2012-0164. [DOI] [PubMed] [Google Scholar]
- 54.Van den Ouweland J.M.W., Beijers A.M., van Daal H., Elisen M.G., Steen G., Wielders J.P. Absence of 3-epi-25-hydroxyvitamin D3 cross-reactivity in the Roche Elecsys vitamin D total protein-binding assay. Clin Chem Lab Med. 2014;52:373–380. doi: 10.1515/cclm-2013-0702. [DOI] [PubMed] [Google Scholar]
- 55.Farrell C.J., Martin S., McWhinney B., Straub I., Williams P., Herrmann M. State-of-the-art vitamin D assays: a comparison of automated immunoassays with liquid chromatography tandem mass spectrometry methods. Clin Chem. 2012;58:531–542. doi: 10.1373/clinchem.2011.172155. [DOI] [PubMed] [Google Scholar]
- 56.Farrell C., Soldo J., Williams P., Herrmann M. 25-hydroxyvitamin D testing: challenging the performance of current immunoassays. Clin Chem Lab Med. 2012;50:1953–1963. doi: 10.1515/cclm-2012-0522. [DOI] [PubMed] [Google Scholar]
- 57.Su Z., Slay B.R., Carr R., Zhu Y. The recognition of 25-hydroxyvitamin D2 and D3 by a new binding protein based 25-hydroxyvitamin D assay. Clin Chim Acta. 2013;417:62–66. doi: 10.1016/j.cca.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 58.Holmes E.W., Garbincius J., McKenna K.M. Analytical variability among methods for the measurement od 25-hydroxyvitamin D. Am J Clin Pathol. 2013;140:550–560. doi: 10.1309/AJCPU2SKW1TFKSWY. [DOI] [PubMed] [Google Scholar]
- 59.Cavalier E., Rousselle O., Ferrante N., Carlisi A., Le Goff C., Souberbielle J.-C. Technical and clinical evaluation of the Vitros® immunodiagnostic products 25-OHVitamin D Total assay – comparison with marketed automated immunoassays and a liquid-chromatography-tandem mass spectrometry method. Clin Chem Lab Med. 2013;51:1983–1989. doi: 10.1515/cclm-2013-0138. [DOI] [PubMed] [Google Scholar]
- 60.Eisman J.A., Shepard R.M., DeLuca H.F. Determination of 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 in human plasma using high pressure liquid chromatography. Anal Biochem. 1977;80:298–305. doi: 10.1016/0003-2697(77)90648-0. [DOI] [PubMed] [Google Scholar]
- 61.Gilbertson T.J., Stryd R.P. High-performance liquid chromatographic assay for 25-hydroxyvtamin D3 in serum. Clin Chem. 1977;23:1700–1704. [PubMed] [Google Scholar]
- 62.Jones G. Assay of vitamin D2 and D3, and 25-hydroxyvitamins D2 and D3 in human plasma by high-performance liquid chromatography. Clin Chem. 1978;24:287–298. [PubMed] [Google Scholar]
- 63.Babek J.T., Härkönen M., Wahlroos Ö., Adlercreutz H. Assay of plasma 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 by liquid “high-performance” liquid chromatography. Clin Chem. 1981;27:1346–1351. [PubMed] [Google Scholar]
- 64.Turnbull H., Trafford D.J.H., Makin H.L.J. A rapid and simple method for the measurement of plasma 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 using Sep-Pak C18 cartridges and a single high-performance liquid chromatographic step. Clin Chim Acta. 1982;120:65–76. doi: 10.1016/0009-8981(82)90078-x. [DOI] [PubMed] [Google Scholar]
- 65.Loo J.C.K., Brien R. Analysis of 25-hydroxyvitamin D3 in plasma by high-performance liquid chromatography. Res Commun Chem Pathol Pharmacol. 1983;41:139–148. [PubMed] [Google Scholar]
- 66.Norris R.L.G., Thomas M.J., Craswell P.W. Assessment of a two-step high-performance liquid chromatographic assay using dual-wavelength ultraviolet monitoring for 25-hydroxyergocalciferol and 25-hydroxycholchalciferol in human serum or plasma. J Chromatogr. 1986;381:53–61. doi: 10.1016/s0378-4347(00)83564-1. [DOI] [PubMed] [Google Scholar]
- 67.Shimada K., Mitamura K., Kitarna N., Kawasaki M. Determination of 25-hydroxyvitamin D3 in human plasma by reverse-phase high-performance liquid chromatography with ultraviolet detection. J Chromatogr. 1997;689:409–414. doi: 10.1016/s0378-4347(96)00324-6. [DOI] [PubMed] [Google Scholar]
- 68.Masuda S., Okano T., Kamao M., Kanedai Y., Kobayashi T. A novel high-performance liquid chromatographic assay for vitamin D metabolites using a coulometric electrochemical detector. J Pharm Biomed Anal. 1997;15:1497–1502. doi: 10.1016/s0731-7085(96)02005-5. [DOI] [PubMed] [Google Scholar]
- 69.Alvarez J.-C., De Mazancourt P. Rapid and sensitive high-performance liquid chromatographic method for simultaneous determination of retinol, α-tocopherol, 25-hydroxyvitamin D3, and 25-hydroxyvitamin D2 in human plasma with photodiode-array ultraviolet detection. J Chromatogr. 2001;755:129–135. doi: 10.1016/s0378-4347(01)00047-0. [DOI] [PubMed] [Google Scholar]
- 70.Brunetto M.R., Obando M.A., Gallignani M., Alarcón O.M., Nieto E., Salinas R. HPLC determination of vitamin D3 and its metabolite in human plasma with on-line sample clean-up. Talanta. 2004;64:1364–1370. doi: 10.1016/j.talanta.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 71.Quesada J.M., Mata-Granados J.M., Luque de Castro M.D. Automated method for the determination of fat-soluble vitamins in serum. J Steroid Biochem Mol Biol. 2004;89–90:473–477. doi: 10.1016/j.jsbmb.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 72.Lensmeyer G.L., Wiebe D.A., Binkley N., Drezner M.K. HPLC method for 25-hydroxyvitamin D measurement: comparison with contemporary assays. Clin Chem. 2006;52:1120–1126. doi: 10.1373/clinchem.2005.064956. [DOI] [PubMed] [Google Scholar]
- 73.Granado-Lorencio F., Olmedilla-Alonso B., Herrero-Barbudo C., Blanco-Navarro I., Blázquez-García S., Pérez-Sacristán B. Simultaneous determination of vitamins A, E and 25-OH-vitamin D: application in clinical assessments. Clin Biochem. 2006;39:180–182. doi: 10.1016/j.clinbiochem.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 74.Kan’ár R., Záková P. Determination of 25-hydroxyvitamin D3 in human plasma using HPLC UV detection based on SPE sample preparation. J Sep Sci. 2009;32:2953–2957. doi: 10.1002/jssc.200900213. [DOI] [PubMed] [Google Scholar]
- 75.Hymøller L., Krogh Jensen S. Vitamin D analysis in plasma by high-performance liquid chromatography (HPLC) with C30 reverse phase column and UV detection – easy and acetonitrile-free. J Chromatogr. 2011;1218:1835–1841. doi: 10.1016/j.chroma.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 76.Nurmi T., Tuomainen T.-P., Virtanen J., Mursu J., Voutilainen S. High-performance liquid chromatography and coulometric electrode array detector in serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 analyses. Anal Biochem. 2013;435:1–9. doi: 10.1016/j.ab.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 77.Watson D., Setchell K.D., Ross R. Analysis of vitamin D and its metabolites using thermospray liquid chromatography/mass spectrometry. Biomed Chromatogr. 1991;5:153–160. doi: 10.1002/bmc.1130050404. [DOI] [PubMed] [Google Scholar]
- 78.Vogeser M. Quantification of circulating 25-hydroxyvitamin D by liquid chromatography-tandem mass spectrometry. J Steroid Biochem Mol Biol. 2010;121:565–573. doi: 10.1016/j.jsbmb.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 79.Van den Ouweland J.M.W., Vogeser M., Bächer S. Vitamin D and metabolites measurement by tandem mass spectrometry. Rev Endocr Metab Disord. 2013;14:159–184. doi: 10.1007/s11154-013-9241-0. [DOI] [PubMed] [Google Scholar]
- 80.Higashi T., Awada D., Shimada K. Simultaneous determination of 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 in human plasma by liquid chromatography-tandem mass spectrometry employing derivatization with a Cookson-type reagent. Biol Pharm Bull. 2001;24:738–743. doi: 10.1248/bpb.24.738. [DOI] [PubMed] [Google Scholar]
- 81.Higashi T., Yamauchi A., Shimada K. Application of 4-(4-nitrophenyl)-1,2,4-triazoline-3,5-dione to analysis of 25-hydroxyvitamin D3 in human plasma by liquid chromatography/electron capture atmospheric pressure chemical ionization-mass spectrometry. Anal Sci. 2003;19:941–943. doi: 10.2116/analsci.19.941. [DOI] [PubMed] [Google Scholar]
- 82.Vogeser M., Kyriatsoulis A., Huber E., Kobold U. Candidate reference method for the quantification of circulating 25-hydroxyvitamin D3 by liquid chromatography-tandem mass spectrometry. Clin Chem. 2004;50:1415–1417. doi: 10.1373/clinchem.2004.031831. [DOI] [PubMed] [Google Scholar]
- 83.Tsugawa N., Suhara Y., Kamao M., Okano T. Determination of 25-hydroxyvitamin D in human plasma using high-performance liquid chromatography-tandem mass spectrometry. Anal Chem. 2005;77:3001–3007. doi: 10.1021/ac048249c. [DOI] [PubMed] [Google Scholar]
- 84.Maunsell Z., Wright D.J., Rainbow S.J. Routine isotope-dilution liquid chromatography-tandem mass spectrometry assay for 25-hydroxy metabolites of vitamins D2 and D3. Clin Chem. 2005;51:1683–1690. doi: 10.1373/clinchem.2005.052936. [DOI] [PubMed] [Google Scholar]
- 85.Chen H., McCoy L.F., Schleicher R.L., Pfeiffer C.M. Measurement of 25-hydroxyvitamin D3 (25OHD3) and 25-hydroxyvitamin D2 (25OHD2) in human serum using liquid chromatography-tandem mass spectrometry and its comparison to a radioimmunoassay. Clin Chim Acta. 2008;391:6–12. doi: 10.1016/j.cca.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 86.Bunch D.R., Miller A.Y., Wang S. Development and validation of a liquid chromatography-tandem mass spectrometry assay for serum 25-hydroxyvitamin D2/D3 using a turbulent flow line extraction technology. Clin Chem Lab Med. 2009;47:1565–1572. doi: 10.1515/CCLM.2009.342. [DOI] [PubMed] [Google Scholar]
- 87.Højskov C.S., Heickendorff L., Møller H.J. High-throughput liquid/liquid extraction and LC/MS–MS assay for determination of circulating 25(OH)vitamin D3 and D2 in the routine clinical laboratory. Clin Chim Acta. 2010;411:114–116. doi: 10.1016/j.cca.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 88.Herrmann M., Harwood T., Gaston-Parry O., Kouzios D., Wong T., Lih A. A new quantitative LC tandem mass spectrometry assay for serum 25-hydroxyvitamin D. Steroids. 2010;75:1106–1112. doi: 10.1016/j.steroids.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 89.Ding S., Schoenmakers I., Jones K., Koulman A., Prentice A., Volmer D.A. Quantitative determination of vitamin D metabolites in plasma using UHPLC–MS/MS. Anal Bional Chem. 2010;398:779–789. doi: 10.1007/s00216-010-3993-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Van den Ouweland J.M.W., Beijers A.M., Demacker P.N.M., van Daal H. Measurement of 25-OH-vitamin D in human serum using liquid chromatography tandem-mass spectrometry with comparison to radioimmunoassay and automated immunoassay. J Chromatogr B. 2010;878:1163–1168. doi: 10.1016/j.jchromb.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 91.Tai S.S., Bedner M., Phinney K.W. Development of a candidate reference measurement procedure for the determination of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem. 2010;82:1942–1948. doi: 10.1021/ac9026862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stepman H.C., Vanderroost A., van Uytfanghe K., Thienpont L.M. Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotope-dilution liquid chromatography-tandem mass spectrometry. Clin Chem. 2011;57:441–448. doi: 10.1373/clinchem.2010.152553. [DOI] [PubMed] [Google Scholar]
- 93.Adamec J., Jannasch A., Huang J., Hohman E., Fleet J.C., Peacock M. Development and optimization of an LC-MS/MS-based method for simultaneous quantification of vitamin D2, vitamin D3, 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3. J Sep Sci. 2011;34:11–20. doi: 10.1002/jssc.201000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Z., Senn T., Kalhorn T., Zheng X.I., Zheng S., Davis C.L. Simultaneous measurement of plasma vitamin D3 metabolites including 4β,25-dihydroxyvitamin D3 using liquid chromatography-tandem mass spectrometry. Anal Biochem. 2011;418:126–133. doi: 10.1016/j.ab.2011.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bogusz M.J., Al Enazi E., Tahtamoni M., Jawaad J.A., Al Tufail M. Determination of serum vitamins 25-OH-D2 and 25-OH-D3 with liquid chromatography-tandem mass spectrometry using atmospheric pressure chemical ionization or electrospray source and core-shell or sub-2 μm particle columns: a comparative study. Clin Biochem. 2011;44:1329–1337. doi: 10.1016/j.clinbiochem.2011.08.1134. [DOI] [PubMed] [Google Scholar]
- 96.Baecher S., Lienenbach A., Wright J.A., Pongratz S., Kobold U., Thiele R. Simultaneous quantification of four vitamin metabolites in human serum using high performance liquid chromatography tandem mass spectrometry for vitamin D profiling. Clin Biochem. 2012;45:1491–1496. doi: 10.1016/j.clinbiochem.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 97.Farrell C.J., Martin S., McWhinney B., Straub I., Williams P., Herrmann M. State-of-the-Art vitamin D assays: a comparison of automated immunoassays with liquid chromatography–tandem mass spectrometry methods. Clin Chem. 2012;58:531–542. doi: 10.1373/clinchem.2011.172155. [DOI] [PubMed] [Google Scholar]
- 98.Lensmeyer G., Poquette M., Wiebe D., Binkley N. The C-3 epimer of 25-hydroxyvitamin D3 is present in adult serum. J Clin Endocrinol Metab. 2012;97(1):163–168. doi: 10.1210/jc.2011-0584. [DOI] [PubMed] [Google Scholar]
- 99.Thibeault D., Caron N., Djiana R., Kremer R., Blank D. Development and optimization of simplified LC–MS/MS quantification of 25-hydroxyvitamin D using protein precipitation with on-line solid phase extraction (SPE) J Chromatogr B. 2012;883–884:120–127. doi: 10.1016/j.jchromb.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 100.Strathmann F.G., Sadilkova K., Laha T.J., Lesourd S.E., Bornhorst J.A., Hoofnagle A.N. 3-epi-25 hydroxyvitamin D concentrations are not correlated with age in a cohort of infants and adults. Clin Chim Acta. 2012;413:203–206. doi: 10.1016/j.cca.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mochizuchi A., Kodera Y., Saito T., Satoh M., Sogawa K., Nishimura M. preanalytical evaluation of serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 measurement using LC–MS/MS. Clin Chim Acta. 2013;420:114–120. doi: 10.1016/j.cca.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 102.Zhang S., Jian W., Sullivan S., Sankaran B., Edom R.W., Weng N. Development and validation of an LC–MS/MS based method for quantification of 25 hydroxyvitamin D2 and 25 hydroxyvitamin D3 in human serum and plasma. J Chromatogr B. 2014;961:22–70. doi: 10.1016/j.jchromb.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 103.Kaufmann M., Callagher J.C., Peacock M., Schlingmann K.-P., Konrad M., DeLuca H.F. Clinical utility of simultaneous quantitation of 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D by LC–MS/MS involving derivatization with DMEQ-TAD. J Clin Endocrinol Metab. 2014;99:2567–2574. doi: 10.1210/jc.2013-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gervasoni J., Cocci A., Zuppi C., Persichilli S. Total 25-hydroxyvitamin D determination by an entry level triple quadrupole instrument: Comparison between two commercial kits. BioMed Res Int. 2013 doi: 10.1155/2013/270426. [ID 270426] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vogeser M., Seger C. Pitfalls associated with the use of liquid chromatography–tandem mass spectrometry in the clinical laboratory. Clin Chem. 2010;56:1234–1244. doi: 10.1373/clinchem.2009.138602. [DOI] [PubMed] [Google Scholar]
- 106.Musteata M.L., Musteata F.M. Overview of extraction methods for analysis of vitamin D and its metabolites in biological samples. Bioanalysis. 2011;3(17):1987–2002. doi: 10.4155/bio.11.195. [DOI] [PubMed] [Google Scholar]
- 107.Knox S., Harris L., Calton A.M., Wallace A.M. A simple automated solid-phase extraction procedure for measurement of 25-hydroxyvitamin D3 and D2 by liquid chromatography–tandem mass spectrometry. Ann Clin Biochem. 2009;46:226–230. doi: 10.1258/acb.2009.008206. [DOI] [PubMed] [Google Scholar]
- 108.Singh R.J., Taylor R.L., Reddy G.S., Grebe S.K. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab. 2006;91:3055–3061. doi: 10.1210/jc.2006-0710. [DOI] [PubMed] [Google Scholar]
- 109.Granado-Lorencio F., Blanco-Navarro I., Pérez-Sacristan B., Donoso-Navarro E., Silvestre-Mardomingo R. Serum levels of 3-epi-25-OH-D3 during hypervitaminosis D in clinical practice. J Clin Endocrinol Metab. 2012;97:E2266–E2270. doi: 10.1210/jc.2012-2627. [DOI] [PubMed] [Google Scholar]
- 110.Carter G.D. Accuracy of 25-hydroxyvitamin D assays: confronting the issues. Curr Drug Targets. 2011;12:19–28. doi: 10.2174/138945011793591608. [DOI] [PubMed] [Google Scholar]
- 111.Phinney K., Bedner M., Tai S.S., Vamathevan V., Sander L.C., Sharpless K.E. Development and certification of a standard reference material for vitamin D metabolites in human serum. Anal Chem. 2012;84:956–962. doi: 10.1021/ac202047n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Certificate of Analysis. Standard Reference Material 2972: 25-hydroxyvitamin D2 and D3 calibration solutions. Gaithersburg, MD: Standard Reference Materials Program, NIST. 〈http://www.nist.gov/index.html〉; 2009.
- 113.Cavalier E., Lukas P., Crine Y., Peeters S., Carlisi A., Le Goff C. Evaluation of automated immunoassays for 25(OH)-vitamin D determination in different critical populations before and after standardization of the assays. Clin Chim Acta. 2014;431:60–65. doi: 10.1016/j.cca.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 114.Cavalier E., Wallace A.M., Knox S., Mistrella V.I., Cormier C., Souberbielle J.-C. Serum vitamin D measurement may not reflect what you give to your patients. J Bone Miner Res. 2008;23:1865. doi: 10.1359/jbmr.080608. [DOI] [PubMed] [Google Scholar]
- 115.Fleet J.C., Bradley J., Reddy G.S., Ray R., Wood R.J. 1-Alpha,25-(OH)2-vitamin D3 analogs with minimal in vivo calcemic activity can stimulate significant transepithelial calcium transport and mRNA expression in vitro. Arch Biochem Biophys. 1996;329:228–234. doi: 10.1006/abbi.1996.0213. [DOI] [PubMed] [Google Scholar]
- 116.Brown A.J., Ritter C., Slatopolsky E., Muralidharan K.R., Okumura W.H., Reddy G.S. 1-Alpha,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of 1-alpha,25-dihydroxyvitamin D3, is a potent suppressor of parathyroid hormone secretion. J Cell Biochem. 1999;73:1–13. [PubMed] [Google Scholar]