Abstract
A viral etiology of cancer was first demonstrated in 1911 by Peyton Rous who injected cell-free filtrate from a chicken sarcoma into healthy chickens and found it induced a tumour. Since the discovery over 50 years ago of the Epstein-Barr virus as the cause of Burkitt lymphoma, seven other human viruses or groups of viruses—hepatitis B virus, hepatitis C virus, human immunodeficiency virus type 1, some human papillomaviruses, human T-cell lymphotropic virus type 1, Kaposi sarcoma-associated herpesvirus and Merkel cell polyomavirus—have been linked to human cancer. Collectively, these eight viruses cause over 20 different types of cancer and contribute to 10–12% of all cancer, with a greater burden in low- and middle-income countries. For many viruses, immunosuppression greatly increases the risks of persistent infection, development of chronic sequelae and cancer. Although several viruses share similar routes of transmission (especially sexual activity, injection drug use and mother-to-child transmission), the predominant route of transmission varies across viruses, and for the same virus can vary by geographical location. In general, vulnerable populations at the greatest risk for viral infections and their associated diseases include people, especially children, living in low- to middle-income countries, men who have sex with men, people who use injection drugs and female sex workers.
This article is part of the themed issue ‘Human oncogenic viruses’.
Keywords: cancer, viruses, epidemiology
1. Introduction
Epstein-Barr virus (EBV) was the first virus identified to cause cancer in humans; it was linked to Burkitt lymphoma in the early 1960s. In addition to EBV, the International Agency for Research on Cancer (IARC) as well as the USA' National Toxicology Program (NTP) also recognize seven other viruses or families of viruses as known or probable human carcinogens—hepatitis B virus (HBV) and hepatitis C virus (HCV), human immunodeficiency virus type 1 (HIV-1), some human papillomaviruses (HPV), human T-cell lymphotropic virus type 1 (HTLV-1), Kaposi sarcoma-associated herpesvirus (KSHV) and Merkel cell polyomavirus (MCV) [1–3].
2. Identifying tumour viruses
A common feature of these agents is their propensity to persist as chronic infections in their human hosts. Malignancy is an occasional outcome of the processes supporting infection persistence, but does not contribute substantially to continuation of infection in the individual host nor in the host population. Notably, the prevalence of these infections is generally orders of magnitude greater than the incidence of the associated cancers, which complicates recognition of causal relationships.
Viruses can cause cancer by direct (e.g. expression of viral oncogenes) and indirect (e.g. immunomodulation) modes of action. The frequency of many—but by no means all—of these malignant outcomes may be increased with various states of immune compromise, including inherited immunodeficiencies, the acquired immunodeficiency syndrome (AIDS), iatrogenic immunosuppression and perinatal acquisition. For example, HIV-1 infection impairs the body's immune system so that it cannot adequately suppress or destroy oncogenic viruses, resulting in an increased risk that these viruses will cause cancer in co-infected individuals. The concept of multi-causality, i.e. many determinants acting together to cause a disease, helps to explain the role that cofactors (such as immunosuppression and co-infection by multiple viruses) play in viral etiology and the fact that only a small fraction of virally infected individuals will develop cancer. Rothman & Greenland [4] defined a sufficient cause as a ‘complete causal mechanism’—not a single factor but a minimal set of factors (i.e. component causes)—that if present in an individual will cause disease. Most causes are neither necessary nor sufficient, in the absence of other factors, to produce the disease; however, a cause (such as viruses) does not have to be either necessary or sufficient for its removal to result in disease prevention [4,5].
The strongest evidence for identifying human carcinogens is from studies in humans; however, these are not limited to human epidemiology studies. For some virus-associated cancer endpoints (such as Kaposi sarcoma and nasopharyngeal cancer), there are numerous well-designed prospective epidemiology studies, and the Hill criteria may be used to evaluate causality [6]. However, for other cancer endpoints, available data are limited to cross-sectional and case-control studies, which hinder evaluation of temporality. In addition, some endpoints are rare (e.g. cutaneous T-cell lymphoma caused by HTLV-1), and the only available studies are case-comparisons and/or case-series, which have methodological limitations. In these instances, mechanistic studies from human tissues, in addition to epidemiological studies, are critical in the evaluation of causality. For example, mechanistic studies played an important role in the evaluation of MCV in Merkel cell carcinoma and of EBV in gastric cancer. Important factors for evaluating the mechanistic evidence from human tissues include the following [7]:
-
—
the proportion of virus-positive cases in a given tumour entity,
-
—
the proportion of tumour cells that carry the virus,
-
—
the presence and persistence of viral DNA in tumour biopsies and cell lines derived from the same tumour type,
-
—
the monoclonality of the virus,
-
—
integration of viral sequences into the tumour cell genome,
-
—
the expression of viral oncogenes or modification of host-cell genes containing viral sequences,
-
—
the expression of viral proteins linked to pathways of carcinogenesis.
In the evaluation of HIV-1 infection and cancer, mechanistic data supporting a link between immunosuppression (e.g. low numbers of CD4 cells) and cancer also contribute to the overall assessment of carcinogenicity [8]. In addition to studies in humans, mechanistic data from in vitro and animal models, such as data linking viral expression to events or endpoints, also inform cancer assessment.
3. Global burden of cancer due to viral infection
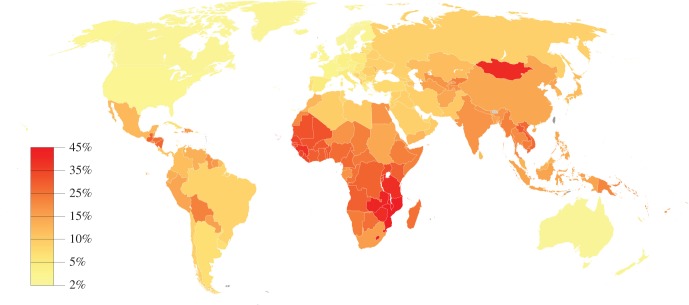
Collectively, the eight tumour viruses (or virus families) contribute to 10–12% of all cancers worldwide [9,10]. The attributable fraction varies inversely with level of economic development, ranging from 2% in North America and Western Europe to 45% in parts of sub-Saharan Africa (figure 1). Over 20 different types of cancers have been linked or possibly linked to viral infection [8]. Moreover, viral infections are the major cause of some types of cancer. For example, almost all cervical cancer, which accounts for 3.5% of the world burden of cancer, is caused by HPV. Most cases of liver cancer are caused by HBV (54% of all cases) or HCV (21% of all cases) and together these virus-associated cancers are estimated to account for another 4.1% of the world burden of cancer [11]. To date, vaccines are only available for HPV and HBV. Most of the eight viruses are common and/or disproportionately affect vulnerable subpopulations or people living in areas with limited resources, thus virus-induced cancer represents an important public health concern. Key features of these viruses are listed in table 1. An overview of viral epidemiology (e.g. seroprevalence patterns and transmission) for each of the tumour viruses follows this introduction.
Figure 1.
Attributable fraction of cancer related to viral infections, 2012. Adapted from data in Plummer et al. [10]. Map generated with GunnMap.
Table 1.
Summary characteristics of human tumour viruses. IARC, International Agency for Research on Cancer; MSM, men who have sex with men.
| HPV | HBV | EBV (HHV4) | HTLV-1 | HIV | HCV | KSHV (HHV8) | MCV | |
|---|---|---|---|---|---|---|---|---|
| discovery | 1984 | 1967 | 1964 | 1979 | 1983 | 1989 | 1994 | 2008 |
| carcinogenicity group (IARC) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2A |
| associated cancers | cervical/anogenital, oropharyngeal, epidermodysplasia verruciformis-associated skin | hepatocellular | lymphomas (endemic Burkitt, Hodgkin, NK/T-cell, immunosuppression-related non-Hodgkin), epithelial (nasopharyngeal and some gastric), pediatric leiomyosarcoma | adult T-cell leukaemia/lymphoma | Kaposi sarcoma, lymphomas (non-Hodgkin and Hodgkin), anogenital, pediatric leiomyosarcoma, conjunctival, Merkel cell | hepatocellular, non-Hodgkin lymphoma | Kaposi sarcoma, non-Hodgkin lymphoma (rare forms) | Merkel-cell carcinoma |
| global cancer cases/year | 640 000 | 420 000 | 200 000 | 3000 | a | 170 000 | 44 000 | ∼10 000 |
| global infection prevalence, % | ∼15 | ∼5 | >90 | <1 | <1 | ∼3 | <10 | 60–90 |
| major routes of transmission | sexual contact, skin-to-skin contact | perinatal, sexual contact, injection drug use, unscreened blood products, body piercing, medical waste | saliva | perinatal, breastfeeding, sexual contact, unscreened blood products | sexual contact, injection drug use, unscreened blood products, perinatal, breastfeeding | injection drug use, sexual contact among MSM, unscreened blood products, contaminated medical instruments, tattooing, body piercing, cosmetic procedures | poorly defined (possibly by saliva, blood products, intra-household, sexual contact among MSM) | poorly defined (possibly by skin-to-skin contact) |
| geographical areas with highest prevalence of infection | sub-Saharan Africa, Eastern Europe, Latin America | sub-Saharan Africa, East Asia, South America | global | southwestern Japan, sub-Saharan Africa, South America, Caribbean | sub-Saharan Africa | North Africa, South and East Asia, Eastern Mediterranean, West Pacific | Mediterranean, sub-Saharan Africa, northwestern China | global |
| recognized viral oncogenes | yes | yes | yes | yes | unknown | unknown | yes | unknown |
| integrated into the host genome in tumour cells | yes | yes | no | yes | no | no | no | yes |
| high-risk viral genotypes | yes | unknown | unknown | unknown | unknown | yes | unknown | unknown |
| mechanistic data in humans | yes | yes | yes | yes | yes | yes | yes | yes |
| animal models | yes | yes | yes | yes | yes | no | no | no |
| infection screening | yes | yes | no | yes | yes | yes | no | no |
| prophylactic vaccine | yes | yes | no | no | no | no | no | no |
aAssigned to other human oncogenic viruses representing frequent co-infections in HIV-associated cancer.
4. Human papillomaviruses
HPV are double-stranded DNA viruses that infect the basal layers of cutaneous and mucosal epithelia of the skin, anogenital tract and oropharynx. This family of human Papillomaviridae viruses include over 100 types and are classified by genus into genital-mucosal (alpha-papillomavirus) and cutaneous epidermodysplasia verruciformis (beta-papillomavirus) forms [12]. Over 40 types associated with genital-mucosal infections can result in cervical cancer in women and anogenital and oropharyngeal cancers in both sexes. Cancers that develop occur at local sites of infection corresponding with specific routes of exposure (e.g. cervical cancer with vaginal intercourse, anal cancer with receptive anal intercourse and oropharyngeal cancer with fellatio). Genital HPV types are classified as either high or low risk for the potential development of cervical cancer, which is the fourth most common cancer worldwide and the second most common in the developing world [13]. Over 13 types of high-risk HPV have been identified; the most common worldwide are HPV-16 and HPV-18, which are associated with 70–80% of cervical cancers and some anogenital and oropharyngeal cancers [14,15]. Approximately 75% of HPV infections in young children are caused by HPV-6 or HPV-11 and are mostly subclinical, except for the rare (four cases per 100 000 births) respiratory papillomatosis of infants associated with these low-risk types [16]. In infancy and childhood, HPV-associated clinical infections also include skin warts and genital warts; cervical squamous intraepithelial lesions have also been reported among adolescent girls [17]. Prevalence of genital warts in developed countries is 0.12–0.2% with a peak during teen to young adult ages; however, the majority of HPV infections have no overt clinical signs [12].
Immune surveillance is an important factor in clearance of HPV infection. Most women with a competent immune system will clear the infection within a few months after acquisition, and about 90% clear within 2 years [12]. Chronic immunosuppression, such as with HIV-1 infection, can result in HPV persistence and lesion progression [18]. Additional factors that can enhance HPV persistence and progression are smoking, co-infections such as with herpes simplex virus, frequency of sexual intercourse and number of sexual partners.
HPV is the most common sexually transmitted infection. Worldwide, the prevalence of HPV infection in women with no cervical abnormalities is 11–12% with higher rates in sub-Saharan Africa (24%), Eastern Europe (21%) and Latin America (16%) [19]. Age-specific prevalence generally peaks in women before 34 years of age, followed by a decline, although in many countries there is a second peak in women aged 45 years or older [1]. In the USA, epidemiological studies based on HPV DNA testing indicate that between 25 and 40% of sexually active women aged 15–25 are infected [20]. Recent sexual activity, the number of sex partners, frequency of sexual intercourse and the presence of genital warts on sex partners are strong predictors of HPV infection [1,21].
Infection may also be transmitted by non-sexual routes as shown by HPV DNA detection in infants and children, and in adults who have never had sexual intercourse. Non-sexual transmission can also occur when HPV from skin or fomites contacts injured skin surface [16]. Perinatal transmission of the virus is also possible. Newborns of HPV-infected mothers delivering vaginally have an increased rate of HPV detection (51.4%) compared with those delivered by Caesarian section (27.3%) [22]. While rare, evidence for horizontal transmission from mother-to-child has also been observed for both high- and low-risk HPV types [23].
HPV infection is detected by observation of visible lesions or microscopic changes in cells, by detection of HPV DNA or by detection of antibodies against HPV proteins in the blood. Papanicolaou (Pap) smear, which involves microscopic examination of stained exfoliated genital cells, detects koilocytosis (structural changes in squamous epithelium indicative of HPV infection) and other signs of cervical intraepithelial neoplasia (CIN); it is used as a screening test for prevention of invasive cervical cancer [24]. There are currently no US FDA-recommended screening methods similar to a Pap test for detecting cell changes caused by HPV infection in non-cervical tissues [14]. Clinical diagnosis of HPV is most commonly based on the Hybrid Capture 2 assay, which is specific for high- and intermediate-risk HPV genotypes; however, there are no FDA-approved tests to detect HPV infections in men.
Three vaccines are marketed in many countries for the prevention of infection by some genital-mucosal types of HPV [25]. All three vaccines are highly effective in preventing infection with high-risk HPV types 16 and 18 [26].
5. Hepatitis B virus
HBV is an enveloped DNA virus that is a member of the Hepadnaviridae family. It infects liver cells and can cause both acute and chronic hepatitis B, hepatic cirrhosis and hepatocellular carcinoma (HCC); it also is possibly linked to non-Hodgkin lymphoma and cholangiocarcinoma [1]. Worldwide, approximately 2 billion people are infected with HBV and over 350 million people are chronically infected. Each year, 50 million people will develop new infections [27], and more than one million people die from HBV-related liver cirrhosis and liver cancer [28]. The seroprevalence of chronic infection varies geographically, ranging from less than 1% in low endemic areas (Western Europe and North America), 2–8% in intermediate endemic areas (Middle East and the Indian subcontinent), and greater than 8% in high endemic areas (sub-Saharan Africa, East Asia and Southern America) [29]. HBV can be divided into 10 genotypes, whose relative distribution also varies geographically.
Chronic hepatitis B infection develops in individuals who are not able to clear the virus; infection rates are higher in HBV-infected infants (85–90%) than in children (30–50%) and adults (5%) [28]. The development of chronic infection from HBV acquired during the perinatal period, in which the immune system is learning to recognize and tolerate self, may be due to the recognition and tolerance of HBV antigens as self. Thus, mother-to-child transmission (MTCT), which is the major route of transmission in most endemic areas (such as East Asia), contributes significantly to the global burden of HBV-related diseases. Transmission of HBV can occur during pregnancy or during the postpartum period [27,30]. Maternal hepatitis B envelope antigen (HBeAg) positivity (a marker of active viral replication indicating that a person is infectious) and high circulating HBV DNA increase the risk of both perinatal transmission and the subsequent development of chronic HBV from infection acquired in infancy [30]. Other risk factors for chronic infection are male sex, close contact with mothers with high viral loads, HBV genotype and genetic susceptibility.
In intermediate and non-endemic areas, HBV infection is usually acquired during adolescence or as an adult; risk factors for infection include sexual activity and injection drug use [29]. In the USA and Western Europe, prevalence of chronic HBV infection among HIV-infected individuals is estimated to be 4–6% among heterosexuals, 9–17% among men who have sex with men (MSM) and 7–10% among people who inject drugs (PWID). Sexual risk behaviours associated with HBV infection include history of syphilis, number of sex partners, years of sexual activity and past or current sexually transmitted diseases [31]. Risk factors for HBV infection in MSM include number of lifetime sexual partners, seropositivity for HIV and syphilis, and receptive anal intercourse. HIV infection, which destroys CD4 cells and compromises the immune system, increases the risk of chronic hepatitis after acute HBV infection.
HBV can also be transmitted via healthcare practices including handling medical waste [32], body piercing [33] and transfused blood products or haemodialysis originating from hepatitis B carriers [34]. Screening practices using HBV nucleic acid tests have decreased risk of transmission by blood transfusion; however, the potential for blood transmission from occult infection (i.e. infection without detectable hepatitis B surface antigen, HbsAg), although very low, still remains [35].
HBV vaccine, which consists of a three-dose series, has been available since 1982 and is highly effective for preventing infection. Neonatal vaccination programmes have led to decreases in the incidence of acute HBV infection, lower prevalence of chronic infection, and declines in mortality from chronic liver disease and HCC [29]. Pregnant women who are positive for both HBsAg and HBeAg should receive antiviral therapy in the first or early second trimester in order to reduce HBV levels prior to delivery [36]. Despite interventions, breakthrough infections still occur, especially among mothers with high circulating HBV DNA [30,36]. Neonatal and catch-up vaccination programmes have been credited with decreasing the seroprevalence of HBV among HIV-infected MSM in Taiwan (20.3% for MSM born before 1984 versus 3.3% for those born after 1984).
6. Hepatitis C virus
HCV, a single-stranded enveloped RNA virus that belongs to the Flaviviridae family, was identified in the late 1980s/early 1990s as another virus that causes hepatitis (non-A, non-B) [37]. In the absence of treatment, approximately 55–85% people infected with HCV are not able to clear the virus within six months and develop chronic infection. Among people with chronic hepatitis C infection, 20–30% will develop liver cirrhosis, 1% of these people will develop HCC [38] and approximately 700 000 people will die from liver-related conditions each year [39]. The virus also causes non-Hodgkin lymphoma and possibly cholangiocarcinoma [1].
Worldwide, an estimated 180 million people are infected with HCV and approximately three to four million new infections occur each year [40]. Seroprevalence of HCV varies greatly both between countries (generally ranging from 0.5 to 15%) and within countries [38]. Hepatitis C infection is more common in low- and middle-income countries (approx. 5–10%), especially in countries in North Africa, South and East Asia, Eastern Mediterranean and the West Pacific. Prevalence is relatively low (1–2%) in the general population of high-income countries such as in North America, Australia, and Northern and Western Europe, but can be very elevated among subpopulations such as injection drug users. Seven genotypes and 67 subtypes of HCV have been identified [41], which vary by geographical location [39]. Genotypes 1 and 4 are particularly virulent although probably all HCV types can establish chronic hepatitis and subsequently HCC.
HCV is a blood-borne pathogen that is spread by parenteral transmission [40], primarily from sharing needles or receipt of contaminated blood or blood products. Globally, approximately half of PWID are infected with HCV and almost all people (98.7%) who have used injection drugs for greater than 30 years are infected with HCV. Moreover, in some European countries, seroprevalence of HCV infection among PWID is increasing, possibly due to switching to drugs (such as psychotropic substances) with higher infection frequency, decreases in syringe and needle availability, and low levels of opioid substitution coverage. HCV genotypes, which have important clinical consequences as they can influence progression of liver disease and susceptibility to antiviral therapies, differ between PWID and the general population [40].
In high-income countries, infection from blood products or transfusions is now extremely rare due to the screening of blood donors [42], decreasing from a high of 33% during the period between 1970 and 1998 [38]. However, blood safety, and infections from iatrogenic procedures such as medical injections, surgery or dental procedures are still a concern in many low-income countries in Africa and Asia. In particular, the high seroprevalence of HCV in Egypt may have been due in part to unsafe injection practices to treat schistosomiasis [37]. Examples of other activities associated with HCV transmission through contact with blood include tattooing, body piercing, cosmetic procedures and commercial barbering [38]. Higher rates of HCV are also observed among incarcerated and military populations.
In addition to injection drug use, sexual activity is also an important transmission route among MSM in high-income countries. In the last two decades, HCV infection rates among HIV-infected MSM have been increasing in North America; Europe and possibly Asia and Australia are showing similar patterns [43]. By contrast, HIV-negative MSM were 4–10 times less likely to acquire HCV than HIV-positive MSM. HIV-infected men may be more likely to engage in high-risk sexual practices, such as mucosa traumatizing activities, than HIV-negative men. Moreover, HCV infection is more persistent and associated with higher viral loads of HCV in blood and semen from HIV-infected men than HIV-negative men, which may also facilitate HCV transmission [43,44]. Among heterosexuals, sexual transmission is ineffective [43].
The seroprevalence of HCV infection in children ranges from 0.05 to 0.4% in high-income countries and 2–5% in some low- and middle-income countries [42]. Since the implementation of screening of blood products in the 1990s, MTCT is now the major cause of HCV infection in children living in high-income countries; risks of perinatal transmission have been reported to range between 3 and 10% in infants born to HCV antibody and RNA-positive mothers. The available pharmaceuticals for HCV infection are contraindicated during pregnancy because of teratogenic effects (ribavirin) or possible effects on fetal growth (interferon alpha) [42,45]. HIV is a major risk factor for perinatal transmission [46]. Treatment of HIV/HCV co-infected women with highly active antiretroviral therapy for HIV can reduce the risk of HCV transmission [42]. Other risk factors for MTCT include maternal injection drug use, female sex and delivery complications (premature rupture of membranes); mode of delivery and breast feeding do not increase the risk of HCV transmission.
There is no vaccine for HCV. Chronic hepatitis C is treated with a combination of antivirals such as interferon-based therapy (peginterferon) and direct-acting antiviral agents such as ribavirin, sofobuvir, ledipasvir and several protease inhibitors. The choice of treatment depends on the HCV genotype and costs. Access to the new, highly effective antiviral agents is generally cost-prohibitive outside high-income countries, particularly for economically disadvantaged populations such as PWID [40].
7. Epstein-Barr virus
EBV, also called HHV-4, is a double-stranded DNA gamma-1 herpesvirus. It was the first human cancer virus discovered when it was found to be associated with Burkitt lymphoma over 50 years ago [47] and, in 1984, EBV was the first human virus sequenced [48]. This work advanced molecular research on EBV infection and mechanisms as a causative factor in a diverse group of lymphoid cancers (endemic Burkitt, Hodgkin and NK/T-cell and immunosuppression-related non-Hodgkin lymphomas), epithelial cancers (nasopharyngeal and some forms of gastric cancers) [7] and pediatric leiomyosarcoma.
Over 90% of adults worldwide are infected with EBV and most have a lifelong dormant infection [7]. Infected cells, primarily resting memory B cells in peripheral blood, provide a permanent reservoir for the virus. Infection at an early age is mostly subclinical, but infections during adolescence and adulthood, which are more common in developed countries, can result in infectious mononucleosis in approximately 50% of the cases [15]. In people with severe immunosuppression such as that caused by HIV-1, EBV infection can result in oral hairy leukoplakia [7].
EBV is transmitted primarily through saliva and is shed intermittently in healthy carriers. Transmission through breast milk, genital secretions and blood transfusion have also been reported [7]. Primary EBV infection as a result of organ transplantation is a major risk factor for post-transplant lymphoproliferative disease [7,49].
Age at primary EBV infection varies geographically; a higher percentage (80%) of children is seropositive by 1 year of age in Uganda compared with rural areas of the USA (45%) [15]. The seroprevalence of EBV antibody in the USA based on NHANES data collected in 2009 and 2010 ranged from 50% in 6 to 8 year olds to 89% in 18 to 19 year olds [7]. Younger age, health insurance coverage, higher household income, and education level were significantly associated with a lower prevalence of EBV antibody within each race/ethnicity group. Genetics, race/ethnicity and family environment may also contribute to acquisition of EBV infection [50].
A healthy immune system holds the virus in a latent state with intermittent shedding of virions, and immune suppressive states increase EBV-related cancer vulnerability. For most EBV-related cancers, a weakened immune system can lead to the production of viral cancer-causing proteins. In addition, environmental factors, genetic make-up and early life exposure all can potentially have a role in the development of some EBV-related cancers [7].
8. Kaposi sarcoma-associated herpesvirus
KSHV, also called HHV-8, is an enveloped double-stranded DNA gamma-2 herpesvirus (rhadinovirus) that was first identified in humans in 1994 from spindle cells of Kaposi sarcoma lesions in association with AIDS [51]. KSHV has also been detected in circulating endothelial cells, B lymphocytes, macrophages, dendritic cells, keratinocytes, fibroblasts and prostate cells [1,52]. A lifelong latent reservoir of this virus is established in CD19+ B lymphocytes from which viral replication can occur.
Primary infection with KSHV is usually asymptomatic. In KSHV carriers, a healthy immune system tempers lytic viral reactivation and symptoms, although both latent and lytic phases have been shown to be important in some KSHV-associated diseases. Febrile illness and maculopapular rash have been described with primary infection of children. In primary infection in MSM, lymphadenopathy has been reported. Fever, lymphoid hyperplasia, splenomegaly and pancytopenia have been reported in organ transplant recipients [52]. Evidence from epidemiological and molecular studies show that KSHV causes Kaposi sarcoma, primary effusion lymphoma and a plasmablastic variant of multicentric Castleman disease.
Worldwide, seroprevalence of KSHV among adult populations varies from 2 to 3% in Northern Europe to over 50% in sub-Saharan African populations [1]. High-level endemic areas have seroprevalences between 30 and 70% among general adult populations and are found in many parts of Africa and the Middle East, e.g. Egypt [53]. Kaposi sarcoma is the most common cancer in many areas of sub-Saharan Africa due to high prevalence of HIV-1 infection in this KSHV endemic area [54,55] The Mediterranean coast with 10–30% seroprevalence is considered an intermediate-level endemic area [56]. In general, areas considered non-endemic with less than 10% seroprevalence are North, Central and South America, Northern Europe and Asia. In the USA, NHANES (1988–1994) reported a seroprevalence of approximately 7% for both sexes [57].
Across non-endemic areas, some subpopulations have higher seroprevalence. HIV-1-infected MSM have the highest seroprevalence (30–60%) followed by HIV-1-uninfected MSM with 20–30% seroprevalence. These high rates of infection are in marked contrast to less than 10% seroprevalence of women and non-homosexual men in these areas [52,58]. Ethnic groups with higher seroprevalence have also been reported in non-endemic areas; however, lifestyle, environmental and genetic factors contributing to this seroprevalence have not been resolved.
Intermittent reactivation of viral replication (lytic phase) results in spread of infection and secretion of the virus into the saliva [1]. KSHV can also be spread by sexual contact among infected adults, primarily among MSM, which may be a major factor in transmission in non-endemic areas [52,58]. KSHV has been identified in peripheral blood of carriers, and transmission has been reported in PWID and transfusion recipients, and from organ transplant donors to recipients [1,52]. In regions where KSHV infection is endemic, such as in some Mediterranean populations and sub-Saharan Africa, the virus is transmitted during childhood within families. It can be transmitted from mother to child with peak age of acquisition of infection between 6 and 10 years [52]. Risk factors for infection may include contact with infected family members and, in particular, HIV-1 infection [1,52]. Factors that increase the risk of HIV-1 infection such as number of sexual partners also increase KSHV infection risk. Because there is wide variation in an individual's antibody response to KSHV infection and poor interassay agreement, comparison of prevalence among different populations has been difficult [1,52].
9. Human immunodeficiency virus type 1
HIV-1, a single-stranded RNA retrovirus of the subfamily Orthoretrovirinae, was first identified as the virus that causes AIDS in 1983. HIV-1 infection destroys and impairs the function of immune cells, leading to low CD4 counts, immunodeficiency and susceptibility to a wide range of infections and diseases. AIDS, the most advanced stage of HIV-1 infection, is characterized by a series of diseases and cancers related to fungal, bacterial, parasitic and viral infections. HIV-1 increases the risk of several cancers (both AIDS-defining and others) caused by KSHV (Kaposi sarcoma), EBV (non-Hodgkin lymphoma, Hodgkin lymphoma, pediatric leiomyosarcoma), HPV (anogenital cancers and oral-related cancers), HBV/HCV (HCC) and MCV (Merkel cell carcinoma) [8]. It has also been associated with cancers not related to viral infections (such as conjunctival cancer).
HIV-1 infection and its associated diseases and mortality remain a major public health problem, especially among vulnerable populations such as economically disadvantaged people living in Africa, MSM, female sex workers and PWID. In 2015, over 35 million people worldwide were HIV-infected, 70% (25 million) of whom were in sub-Saharan Africa [59]. HIV seroprevalence varies geographically, with the highest rates found in East and Southern Africa, where 7.1% of adults aged 15 to 49 years were infected as of 2015. Seroprevalence rates were 2.2% in West and Central Africa, 0.9% in Eastern Europe and Central Asia, 0.5% in Latin America and the Caribbean, 0.3% in Western/Central Europe and North America, 0.2% in Asia and the Pacific, and 0.1% in the Middle East and North Africa [60]. Approximately 2.1 million people became newly infected with HIV in 2015.
To date, HIV infection has claimed the lives of more than 35 million people worldwide. With the development and implementation of antiretroviral therapy, annual AIDS-related mortality has decreased 45% from its former peak, but there were still 1.1 million associated deaths in 2015 [61].
HIV-1 may be transmitted from one individual to another by sexual activity, from direct blood contact and from mothers to children. Patterns and burden of all types of transmission vary geographically.
The AIDS epidemic was first recognized among previously healthy gay and bisexual men in large cities of the USA. Although HIV-1 in MSM peaked in the 1980s, to this day MSM remain a vulnerable population in many regions of the world, accounting for almost a third (in Latin America and the Caribbean) to half (in North America and Western and Central Europe) of all new infections [59]. Moreover, in the last 10 years, there is evidence that HIV-1 incidence among MSM has been increasing in both high- and low-middle income countries (such as China, Kenya and Thailand), especially among vulnerable subpopulations (e.g. minorities and young men) [62]. For example, in the USA, more than half of HIV-1-infected people are MSM and in the last 5 years, the number of new cases in gay and bisexual men has increased by 6% despite a 19% decrease of new cases of HIV-1 in the general population [63]. Risk factors for HIV-1 transmission include unprotected receptive anal intercourse, high numbers of recent and lifetime male partners and injection drug use. Other factors that affect HIV-1 transmission include viral load of the index partner, serosorting (i.e. choosing HIV-1-concordant sex partners) and social networks that result in exposure to varied sexual practices and HIV-1-positive partners [62].
Heterosexual transmission accounts for most (80%) of the HIV-1 epidemic in sub-Saharan Africa. In some low- and middle-income countries, 10% or more of female sex workers are infected with HIV-1 [64]. Risk factors for heterosexual transmission include uncircumcised male sex partners and sexually transmitted infections such as genital ulcers (chancroid) which may increase HIV-1 transmission by causing inflammation or breaks of mucous membranes in the vagina, vulva, penis or anus [65].
Approximately 3 million cases of HIV-1 infection occur annually among PWID, who account for 10% of all HIV-1 infections worldwide and 30% of cases outside Africa [66]. The greatest number of PWID infections are in Eastern Europe and Southeast Asia [65]; 51% of all HIV-1 infections in the geographical area that includes Europe and Central Asia occur in PWID [59]. In Western Europe, rates of HIV-1 in PWID have decreased in some countries (perhaps due to needle exchange programmes, opioid substitution and antiviral treatment) and increased in other countries (possibly due to changes in drug-use patterns with more frequent injections and sharing of injection equipment) [67]. Injection drug use is also a significant risk factor in the spread of HIV-1 in some parts of Asia, specifically the Philippines and Indonesia, where new cases of HIV-1 more than doubled between 2002 and 2012. In high-income countries, screening of blood supplies has almost eliminated transfusion-acquired HIV-1, although the risk remains in lower-income countries.
MTCT of HIV-1, either in utero, during birth, or through breast feeding, accounts for approximately 20% of the cases in sub-Saharan Africa. Since 2009, new HIV-1 cases have decreased by 45% (from 330 000 to 170 000) in 21 countries in Africa; these countries were part of the UN Global Plan to eliminate new HIV-1 infections through the provision of antiretroviral medicine to pregnant women [68].
Although there are no cures or vaccines to prevent HIV-1 infection, antiretroviral drug administration has been effective in stemming the HIV-1 epidemic in high-income countries and in decreasing MTCT in Africa. Approximately 17 million people, about half of the infected population, accessed treatment worldwide in 2015 [69]. The combination of different types of drugs, referred to as highly active antiretroviral therapy (HAART) or combination antiretroviral therapy (cART), is designed to block different steps in the HIV-1 replication cycle [70,71]. The United Nations Program on HIV-1/AIDS has set a goal of cART coverage reaching 90% of infected people globally by 2020 [69]. Pre-exposure prophylaxis (PreP), in which uninfected high-risk subgroups take antiretroviral drugs on a daily basis and are tested regularly, is now recommended for specific at-risk populations in the USA and other countries.
10. Human T-cell lymphotropic virus type 1
HTLV-1 was the first human retrovirus found to be associated with cancer after being isolated in 1979 from a patient with apparent cutaneous T-cell lymphoma [72]. The single-stranded RNA virus infects CD4+ T lymphocytes and to a lesser extent CD8+ T cells, undergoes reverse transcription, and incorporates into chromosomal DNA of the cell. HTLV-1 has also been detected in dendritic cells, monocytes/macrophages, fibroblasts, endothelial cells and B lymphocytes. Persistence of the virus in the carrier state is primarily through multiplication of infected cells.
HTLV-1 is a causal factor in adult T-cell leukaemia/lymphoma (ATLL) and is a part of the diagnostic criteria for this disease [72,73]. Chronic myelopathy (HTLV-1-associated myelopathy (HAM) also called tropical spastic paresis (TSP, or HAM/TSP)) and specific types of uveitis (HTLV-uveitis) are non-malignant complications associated with HTLV-1 infection.
Worldwide, HTLV-1 is estimated to infect approximately 15–20 million people [74] and it is estimated that 90 000–100 000 people in the USA are infected [72]. The virus is endemic to southern Japan, sub-Saharan Africa and the Seychelles. High infection rates also occur in Central and South America, Melanesia and southern Africa. Infection is rare in North America and Europe where it is associated primarily with immigrant populations from endemic areas.
HTLV-1 does not survive long outside the cell and cell-to-cell transmission is the primary means of initial infection; however, dendritic cells infected by cell-free virions can transmit the virus to T cells. HTLV-1 has been found in breast milk, blood, semen and cerebrospinal fluid [72] and disease transmission can be prevented by refraining from breastfeeding and practicing safe sex. Most carriers of HTLV-1 do not develop disease or cancer related to the infection, suggesting that there may be other factors in addition to the presence of the virus enabling cancer progression. ATLL has a long latency period of 40–60 years and 3–5% of carriers develop ATLL [75].
Infection in early childhood through breastfeeding from a carrier mother is a common route of transmission for HTLV-1. A cross-sectional study found that 97% of ATLL patients had seropositive mothers, whereas only 33% of HAM/TSP patients had seropositive mothers. These data suggest that ATLL primarily occurs with infection from early childhood [72,76] which is hypothesized to reflect relatively weak immunocompetence of the young child [15].
11. Merkel cell polyomavirus
Although MCV infection is common, the virus was only recently discovered in 2008 based on its link with Merkel cell carcinoma [77]. MCV primarily infects the skin and is also found elsewhere throughout the body including in saliva, mouth, respiratory tract, digestive tract, blood and urine, suggesting other sites of infections and systemic distribution. Most people are infected with this virus for life and usually do not develop any symptoms. Immunosuppression increases the likelihood that the viral DNA will mutate and integrate into the host cell, leading to the development of Merkel cell carcinoma [78].
Most adults have been infected with MCV and infection is acquired early in life after the disappearance of maternal antibodies [79]. Several studies in different geographical locations have reported steep increases in seroprevalence during early childhood (to 20–40%) and among children and teenagers (35–50%). Among adults, seroprevalence gradually increases from 60% to almost 90% in older adults [78].
The available data suggest that the virus may be spread from close contact or living in shared environments with infected individuals, especially between siblings, mother and child, or spouses [78,79]. Poor personal hygiene has also been suggested as risk factor for infection [80]. The modes of transmission have not been elucidated although potential routes include cutaneous, respiratory and fecal–oral. Healthy individuals have been shown to chronically shed MCV DNA from the skin surface [81]. Moreover, the virus is stable at temperatures up to 167° Fahrenheit and has been found on inanimate surfaces, which supports the potential for transmission from the environment. Several studies have detected MCV DNA in respiratory tissue of adults [82,83] suggesting that the virus can be spread via the respiratory route. Support for fecal–oral transmission comes from studies detecting MCV virus in urban sewage and MCV DNA in the aerodigestive and digestive tracts in humans [84]. At this time, there is no vaccine against MCV [78].
Acknowledgements
We acknowledge Alton Peters, Sanford Garner and Stanley Atwood for their contributions to relevant sections on the NTP/RoC monographs on EBV, KSHV, HIV-1, HTLV-1 and MCV, which served as a resource for this paper. We also thank Allan Hildesheim, Eric Engels, M. Constanza Camargo, Robert Biggar and James Goedert for providing expert reviews and suggestions for this manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
All authors contributed to the conception of the review, drafted or provided critical review of its intellectual content and approved the final draft.
Competing interests
We have no competing interests.
Funding
We received no funding for this study.
References
- 1.IARC. 2012. Biological agents. Lyon, France: International Agency for Research on Cancer. [Google Scholar]
- 2.IARC. 2013. Merkel cell polyomavirus. Malaria and some polyomaviruses (SV40, BK, JC, and merkel cell viruses). IARC monographs on the evaluation of carcinogenic risks to humans, vol. 104, pp. 309–350. Lyon, France: International Agency for Research on Cancer. [PMC free article] [PubMed] [Google Scholar]
- 3.NTP. 2016. Report on carcinogens, 14th edn Research Triangle Park, NC: National Toxicology Program. [Google Scholar]
- 4.Rothman KJ, Greenland S. 2005. Causation and causal inference in epidemiology. Am. J. Public Health 95(Suppl. 1), S144–S150. ( 10.2105/AJPH.2004.059204) [DOI] [PubMed] [Google Scholar]
- 5.zur Hausen H, de Villiers EM. 2014. Cancer ‘causation’ by infections—individual contributions and synergistic networks. Semin. Oncol. 41, 860–875. ( 10.1053/j.seminoncol.2014.10.003) [DOI] [PubMed] [Google Scholar]
- 6.Hill AB. 1965. The environment and disease: association or causation? Proc. R. Soc. Med. 58, 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NTP. 2016. Report on carcinogens monograph on Epstein-Barr virus. Research Triangle Park, NC: National Toxicology Program. [PubMed] [Google Scholar]
- 8.NTP. 2016. Report on carcinogens monograph on human immunodeficiency virus type 1. Research Triangle Park, NC: National Toxicology Program. [PubMed] [Google Scholar]
- 9.Parkin DM. 2006. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer. 118, 3030–3044. ( 10.1002/ijc.21731) [DOI] [PubMed] [Google Scholar]
- 10.Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. 2016. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob. Health 4, e609–e616. ( 10.1016/S2214-109X(16)30143-7) [DOI] [PubMed] [Google Scholar]
- 11.Beaglehole R, Bonita R, Magnusson R. 2011. Global cancer prevention: an important pathway to global health and development. Public Health 125, 821–831. ( 10.1016/j.puhe.2011.09.029) [DOI] [PubMed] [Google Scholar]
- 12.IARC. 2007. Human papillomaviruses. Lyon, France: International Agency for Research on Cancer. [Google Scholar]
- 13.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. 2015. Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108. ( 10.3322/caac.21262) [DOI] [PubMed] [Google Scholar]
- 14.NCI. 2015. HPV and Cancer: National Cancer Institute (updated 2/19/15). See http://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-fact-sheet-q7.
- 15.Vedham V, Verma M, Mahabir S. 2015. Early-life exposures to infectious agents and later cancer development. Cancer Med. 4, 1908–1922. ( 10.1002/cam4.538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacour DE, Trimble C. 2012. Human papillomavirus in infants: transmission, prevalence, and persistence. J. Pediatr. Adolesc. Gynecol. 25, 93–97. ( 10.1016/j.jpag.2011.03.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mammas IN, Sourvinos G, Spandidos DA. 2014. The paediatric story of human papillomavirus (review). Oncol. Lett. 8, 502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.zur Hausen H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer 2, 342–350. ( 10.1038/nrc798) [DOI] [PubMed] [Google Scholar]
- 19.Forman D, et al. 2012. Global burden of human papillomavirus and related diseases. Vaccine 30(Suppl. 5), F12–F23. ( 10.1016/j.vaccine.2012.07.055) [DOI] [PubMed] [Google Scholar]
- 20.Lowy DR, Howley PM. 2001. Papillomaviruses. In Fields virology (eds Knipe DM, Howley PM), pp. 2231–2264. Philadelphia, PA: Lippincott, Williams and Wilkins. [Google Scholar]
- 21.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. 1998. Natural history of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 338, 423–428. ( 10.1056/NEJM199802123380703) [DOI] [PubMed] [Google Scholar]
- 22.Tseng CJ, Liang CC, Soong YK, Pao CC. 1998. Perinatal transmission of human papillomavirus in infants: relationship between infection rate and mode of delivery. Obstet. Gynecol. 91, 92–96. ( 10.1016/S0029-7844(97)00593-0) [DOI] [PubMed] [Google Scholar]
- 23.Castellsague X, et al. 2009. Human Papillomavirus (HPV) infection in pregnant women and mother-to-child transmission of genital HPV genotypes: a prospective study in Spain. BMC. Infect. Dis. 9, 74 ( 10.1186/1471-2334-9-74) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trofatter KF., Jr 1997. Diagnosis of human papillomavirus genital tract infection. Am. J. Med. 102, 21–27. ( 10.1016/S0002-9343(97)00180-0) [DOI] [PubMed] [Google Scholar]
- 25.CDC. 2015. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. Morb. Mortal. Wkly Rep. 64, 300–304. [PMC free article] [PubMed] [Google Scholar]
- 26.Lowy DR, Schiller JT. 2012. Reducing HPV-associated cancer globally. Cancer Prev. Res. 5, 18–23. ( 10.1158/1940-6207.CAPR-11-0542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen E, Tran TT. 2016. Hepatitis B in the female population. Gastroenterol. Clin. North Am. 45, 359–370. ( 10.1016/j.gtc.2016.02.011) [DOI] [PubMed] [Google Scholar]
- 28.Voiculescu M. 2015. How far we are towards eradication of HBV infection. J. Gastrointestin. Liver Dis. 24, 473–479. ( 10.15403/jgld.2014.1121.244.hbv) [DOI] [PubMed] [Google Scholar]
- 29.Sun HY, Sheng WH, Tsai MS, Lee KY, Chang SY, Hung CC. 2014. Hepatitis B virus coinfection in human immunodeficiency virus-infected patients: a review. World J. Gastroenterol. 20, 14 598–14 614. ( 10.3748/wjg.v20.i40.14598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z, Hou X, Cao G. 2015. Is mother-to-infant transmission the most important factor for persistent HBV infection? Emerg. Microbes Infect. 4, e30 ( 10.1038/emi.2015.30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorgos L. 2013. Sexual transmission of viral hepatitis. Infect Dis. Clin. North Am. 27, 811–836. ( 10.1016/j.idc.2013.08.002) [DOI] [PubMed] [Google Scholar]
- 32.Amsalu A, Worku M, Tadesse E, Shimelis T. 2016. The exposure rate to hepatitis B and C viruses among medical waste handlers in three government hospitals, southern Ethiopia. Epidemiol. Health 38, e2016001 ( 10.4178/epih.e2016001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang S, et al. 2015. Transmission of hepatitis B and C virus infection through body piercing: a systematic review and meta-analysis. Medicine 94, e1893 ( 10.1097/MD.0000000000001893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fontenele AM, Filho NS, Ferreira AS. 2013. Occult hepatitis B in patients on hemodialysis: a review. Ann. Hepatol. 12, 359–363. [PubMed] [Google Scholar]
- 35.Seo DH, Whang DH, Song EY, Han KS. 2015. Occult hepatitis B virus infection and blood transfusion. World J. Hepatol. 7, 600–606. ( 10.4254/wjh.v7.i3.600) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thio CL, Guo N, Xie C, Nelson KE, Ehrhardt S. 2015. Global elimination of mother-to-child transmission of hepatitis B: revisiting the current strategy. Lancet Infect. Dis. 15, 981–985. ( 10.1016/S1473-3099(15)00158-9) [DOI] [PubMed] [Google Scholar]
- 37.Shire NJ, Sherman KE. 2015. Epidemiology of hepatitis C virus: a battle on new frontiers. Gastroenterol. Clin. North Am. 44, 699–716. ( 10.1016/j.gtc.2015.07.002) [DOI] [PubMed] [Google Scholar]
- 38.Lee MH, Yang HI, Yuan Y, L'Italien G, Chen CJ. 2014. Epidemiology and natural history of hepatitis C virus infection. World J. Gastroenterol. 20, 9270–9280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO. 2016. Hepatitis C Fact Sheet: World Health Organization (updated 7/16). See http://www.who.int/mediacentre/factsheets/fs164/en/.
- 40.Ruta S, Cernescu C. 2015. Injecting drug use: a vector for the introduction of new hepatitis C virus genotypes. World J. Gastroenterol. 21, 10 811–10 823. ( 10.3748/wjg.v21.i38.10811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lanini S, Easterbrook PJ, Zumla A, Ippolito G. 2016. Hepatitis C: global epidemiology and strategies for control. Clin. Microbiol. Infect. 22, 833–838. ( 10.1016/j.cmi.2016.07.035) [DOI] [PubMed] [Google Scholar]
- 42.Tovo PA, Calitri C, Scolfaro C, Gabiano C, Garazzino S. 2016. Vertically acquired hepatitis C virus infection: correlates of transmission and disease progression. World J. Gastroenterol. 22, 1382–1392. ( 10.3748/wjg.v22.i4.1382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan DP, Sun HY, Wong HT, Lee SS, Hung CC. 2016. Sexually acquired hepatitis C virus infection: a review. Int. J. Infect. Dis. 49, 47–58. ( 10.1016/j.ijid.2016.05.030) [DOI] [PubMed] [Google Scholar]
- 44.Jordan AE, Perlman DC, Neurer J, Smith DJ, Des Jarlais DC, Hagan H. 2016. Prevalence of hepatitis C virus infection among HIV+ men who have sex with men: a systematic review and meta-analysis. Int. J. STD AIDS 28, 145–169. ( 10.1177/0956462416630910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeung CY, Lee HC, Chan WT, Jiang CB, Chang SW, Chuang CK. 2014. Vertical transmission of hepatitis C virus: current knowledge and perspectives. World J. Hepatol. 6, 643–651. ( 10.4254/wjh.v6.i9.643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benova L, Mohamoud YA, Calvert C, Abu-Raddad LJ. 2014. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin. Infect. Dis. 59, 765–773. ( 10.1093/cid/ciu447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Epstein MA, Achong BG, Barr YM. 1964. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet 1, 702–703. ( 10.1016/S0140-6736(64)91524-7) [DOI] [PubMed] [Google Scholar]
- 48.Baer R, et al. 1984. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature 310, 207–211. ( 10.1038/310207a0) [DOI] [PubMed] [Google Scholar]
- 49.Hjalgrim H, Friborg J, Melbye M. 2007. Chapter 53. The epidemiology of EBV and its association with malignant disease. In Human herpesviruses: biology, therapy, and immunoprophylaxis (eds Arvin A, et al.). Cambridge, UK: Cambridge University Press; See https://www.ncbi.nlm.nih.gov/books/NBK47424/. [PubMed] [Google Scholar]
- 50.Condon LM, Cederberg LE, Rabinovitch MD, Liebo RV, Go JC, Delaney AS, Schmeling DO, Thomas W, Balfour HH Jr. 2014. Age-specific prevalence of Epstein-Barr virus infection among Minnesota children: effects of race/ethnicity and family environment. Clin. Infect. Dis. 59, 501–508. ( 10.1093/cid/ciu342) [DOI] [PubMed] [Google Scholar]
- 51.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266, 1865–1869. ( 10.1126/science.7997879) [DOI] [PubMed] [Google Scholar]
- 52.NTP. 2016. Report on carcinogens monograph on kaposi sarcoma-associated herpesvirus. Research Triangle Park, NC: National Toxicology Program. [PubMed] [Google Scholar]
- 53.Andreoni M, El-Sawaf G, Rezza G, Ensoli B, Nicastri E, Ventura L, Ercoli L, Sarmati L, Rocchi G. 1999. High seroprevalence of antibodies to human herpesvirus-8 in Egyptian children: evidence of nonsexual transmission. J. Natl Cancer Inst. 91, 465–469. ( 10.1093/jnci/91.5.465) [DOI] [PubMed] [Google Scholar]
- 54.Chokunonga E, Levy LM, Bassett MT, Mauchaza BG, Thomas DB, Parkin DM. 2000. Cancer incidence in the African population of Harare, Zimbabwe: second results from the cancer registry 1993–1995. Int. J. Cancer 85, 54–59. ( 10.1002/(SICI)1097-0215(20000101)85:1%3C54::AID-IJC10%3E3.0.CO;2-D) [DOI] [PubMed] [Google Scholar]
- 55.Wabinga HR, Parkin DM, Wabwire-Mangen F, Nambooze S. 2000. Trends in cancer incidence in Kyadondo County, Uganda, 1960–1997. Br. J. Cancer 82, 1585–1592. ( 10.1054/bjoc.1999.1071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whitby D, Luppi M, Barozzi P, Boshoff C, Weiss RA, Torelli G. 1998. Human herpesvirus 8 seroprevalence in blood donors and lymphoma patients from different regions of Italy. J. Natl Cancer Inst. 90, 395–397. ( 10.1093/jnci/90.5.395) [DOI] [PubMed] [Google Scholar]
- 57.Engels EA, et al. 2007. Risk factors for human herpesvirus 8 infection among adults in the United States and evidence for sexual transmission. J. Infect. Dis. 196, 199–207. ( 10.1086/518791) [DOI] [PubMed] [Google Scholar]
- 58.Martin JN. 2007. Chapter 54. The epidemiology of KSHV and its association with malignant disease. In Human herpesviruses: biology, therapy, and immunoprophylaxis (eds Arvin A, et al.). Cambridge, UK: Cambridge University Press; See https://www.ncbi.nlm.nih.gov/books/NBK47381/. [PubMed] [Google Scholar]
- 59.UNAIDS. 2016. AIDS by the numbers, 2016. Geneva, Switzerland: UNAIDS. [Google Scholar]
- 60.UNAIDS. 2016. HIV estimates with uncertainty bounds 1990–2015. See http://www.unaids.org/en/resources/documents/2016/HIV_estimates_with_uncertainty_bounds_1990–2015.
- 61.UNAIDS. 2015. AIDS by the numbers, 2015. Geneva, Switzerland: UNAIDS. [Google Scholar]
- 62.Beyrer C, et al. 2016. The global response to HIV in men who have sex with men. Lancet 388, 198–206. ( 10.1016/S0140-6736(16)30781-4) [DOI] [PubMed] [Google Scholar]
- 63.CDC. 2016. Social determinants of health among adults with diagnosed HIV infection in 11 states, the District of Columbia, and Puerto Rico, 2014. HIV. Surv. Suppl. Rep. 21, 1–38. [Google Scholar]
- 64.Shannon K, et al. 2015. Global epidemiology of HIV among female sex workers: influence of structural determinants. Lancet 385, 55–71. ( 10.1016/S0140-6736(14)60931-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Cock KM, Jaffe HW, Curran JW. 2012. The evolving epidemiology of HIV/AIDS. AIDS 26, 1205–1213. ( 10.1097/QAD.0b013e328354622a) [DOI] [PubMed] [Google Scholar]
- 66.WHO. 2016. People who inject drugs: World Health Organization (updated 5/16). See http://www.who.int/hiv/topics/idu/en/.
- 67.Nakagawa F, Phillips AN, Lundgren JD. 2014. Update on HIV in Western Europe. Curr. HIV/AIDS Rep. 11, 177–185. ( 10.1007/s11904-014-0198-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.UNAIDS. 2015. Progress report on the global plan towards the elimination of new HIV infections among children and keeping their mothers alive. UNAIDS report no. JC 2774/1/E. Geneva, Switzerland: UNAIDS.
- 69.UNAIDS. 2016. Global AIDS update. Geneva, Switzerland: UNAIDS. [Google Scholar]
- 70.AVERT. 2015. HIV drugs development. See http://www.avert.org/hiv-drugs-development.htm.
- 71.AVERT. 2015. Antiretroviral drugs. See http://www.avert.org/antiretroviral-drugs.htm.
- 72.NTP. 2016. Report on carcinogens monograph on human T-cell lymphotropic virus type 1. Research Triangle Park, NC: National Toxicology Program. [PubMed] [Google Scholar]
- 73.IARC. 2012. Human T-cell lymphotropic virus type 1. Biological agents. IARC monographs on the evaluation of carcinogenic risks to humans, vol. 100B, pp. 315–340. Lyon, France: International Agency for Research on Cancer. [Google Scholar]
- 74.Watanabe T. 2011. Current status of HTLV-1 infection. Int. J. Hematol. 94, 430–434. ( 10.1007/s12185-011-0934-4) [DOI] [PubMed] [Google Scholar]
- 75.Mortreux F, Gabet AS, Wattel E. 2003. Molecular and cellular aspects of HTLV-1 associated leukemogenesis in vivo. Leukemia 17, 26–38. ( 10.1038/sj.leu.2402777) [DOI] [PubMed] [Google Scholar]
- 76.Bartholomew C, Jack N, Edwards J, Charles W, Corbin D, Cleghorn FR, Blattner WA. 1998. HTLV-I serostatus of mothers of patients with adult T-cell leukemia and HTLV-I-associated myelopathy/tropical spastic paraparesis. J. Hum. Virol. 1, 302–305. [PubMed] [Google Scholar]
- 77.Feng H, Shuda M, Chang Y, Moore PS. 2008. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 319, 1096–1100. ( 10.1126/science.1152586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.NTP. 2016. Report on carcinogens monograph on Merkel cell polyomavirus. Research Triangle Park, NC: National Toxicology Program. [PubMed] [Google Scholar]
- 79.Martel-Jantin C, et al. 2013. Merkel cell polyomavirus infection occurs during early childhood and is transmitted between siblings. J. Clin. Virol. 58, 288–291. ( 10.1016/j.jcv.2013.06.004) [DOI] [PubMed] [Google Scholar]
- 80.Zhang C, et al. 2014. Seroprevalence of Merkel cell polyomavirus in the general rural population of Anyang, China. PLoS ONE 9, e106430 ( 10.1371/journal.pone.0106430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pastrana DV, Wieland U, Silling S, Buck CB, Pfister H. 2012. Positive correlation between Merkel cell polyomavirus viral load and capsid-specific antibody titer. Med. Microbiol. Immunol. 201, 17–23. ( 10.1007/s00430-011-0200-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goh S, Lindau C, Tiveljung-Lindell A, Allander T. 2009. Merkel cell polyomavirus in respiratory tract secretions. Emerg. Infect. Dis. 15, 489–491. ( 10.3201/eid1503.081206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kantola K, et al. 2009. Merkel cell polyomavirus DNA in tumor-free tonsillar tissues and upper respiratory tract samples: implications for respiratory transmission and latency. J. Clin. Virol. 45, 292–295. ( 10.1016/j.jcv.2009.04.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fratini M, Di Bonito P, La Rosa G. 2014. Oncogenic papillomavirus and polyomavirus in water environments: is there a potential for waterborne transmission? Food Environ. Virol. 6, 1–12. ( 10.1007/s12560-013-9134-0) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.