Abstract
Host cells sense viral infection through pattern recognition receptors (PRRs), which detect pathogen-associated molecular patterns (PAMPs) and stimulate an innate immune response. PRRs are localized to several different cellular compartments and are stimulated by viral proteins and nucleic acids. PRR activation initiates signal transduction events that ultimately result in an inflammatory response. Human tumour viruses, which include Kaposi's sarcoma-associated herpesvirus, Epstein–Barr virus, human papillomavirus, hepatitis C virus, hepatitis B virus, human T-cell lymphotropic virus type 1 and Merkel cell polyomavirus, are detected by several different PRRs. These viruses engage in a variety of mechanisms to evade the innate immune response, including downregulating PRRs, inhibiting PRR signalling, and disrupting the activation of transcription factors critical for mediating the inflammatory response, among others. This review will describe tumour virus PAMPs and the PRRs responsible for detecting viral infection, PRR signalling pathways, and the mechanisms by which tumour viruses evade the host innate immune system.
This article is part of the themed issue ‘Human oncogenic viruses’.
Keywords: tumour virus, innate immunity, KSHV, EBV, HCV, HPV
1. Introduction
The detection of microbial pathogens is an essential first step in mounting an innate immune response to infection. Cells sense invading pathogens through germline-encoded pattern recognition receptors (PRRs) that recognize molecular signatures conserved across many microbes, known as pathogen-associated molecular patterns (PAMPs). Viral PAMPs include viral proteins and nucleic acids, such as single-stranded RNA, double-stranded RNA, CpG unmethylated DNA and 5′ triphosphorylated RNA.
PRR recognition of PAMPs triggers a signalling cascade that ultimately results in the activation of transcription factors, NF-κB and IRF3/7 (reviewed in [1]). NF-κB is responsible for upregulating pro-inflammatory cytokines and chemokines, which attract immune cells to the site of infection, while IRF3/7 upregulates the type I interferons (IFNs). Type I IFNs signal in an autocrine and paracrine manner to induce an antiviral state through the upregulation of interferon-stimulated genes (ISGs) such as PRRs, proteins involved in antigen presentation, transcription factors, pro-inflammatory cytokines and chemokines, and proteins that are directly antiviral. NF-κB activation occurs when PRR signalling results in degradation of IκB, a protein that sequesters NF-κB in the cytoplasm, which allows NF-κB to translocate to the nucleus. IRF3/7 activation also results in nuclear translocation, and occurs when PRR signalling activates kinases that phosphorylate these transcription factors.
2. Pattern recognition receptors and signalling
Toll-like receptors (TLRs) are one family of PRRs that sense PAMPs at the cell surface or in endosomes (reviewed in [2]). Eleven mammalian TLRs have been identified, and although their expression is cell-type dependent, most cells express a subset of these receptors. The plasma membrane localized TLRs that are relevant to viral infection are TLRs 2 and 4, which recognize viral proteins [3,4]. The endosomal TLRs recognize nucleic acid and include TLR3, which recognizes double-stranded RNA, TLR7/8, which recognizes single-stranded RNA, and TLR9, which recognizes CpG unmethylated DNA, a common motif in DNA virus genomes [5–9].
TLRs recognize their substrates through leucine-rich repeat (LRR) motifs in their Ig-like ectodomains (reviewed in [2]). TLRs signal through their Toll/interleukin-1 receptor (TIR) domains, found on the cytoplasmic side of the endosomal or plasma membrane, by interacting with the TIR domain-containing adaptor proteins MyD88 or TRIF. TLR3 is the only TRIF-dependent TLR, while TLRs 2, 7/8 and 9 signal through MyD88, and TLR4 can signal through either adaptor protein [10–20]. Signalling from these adaptors leads to IRF3/7 and NF-κB activation, resulting in upregulation of pro-inflammatory cytokines, chemokines and type I IFN. In some cell types, TLR activation can also result in upregulation of type III IFNs, the IFNλ family of cytokines [21]. Like type I IFNs, type III IFNs are upregulated by IRFs and combat viral infection by upregulating ISGs.
While some TLRs detect viral nucleic acid in endosomes, another family of PRRs called the RIG-I-like receptors (RLRs) sense non-self RNAs in the cytoplasm (reviewed in [22]). RLR signalling results in the activation of NF-κB and IRF3/7, and thus upregulation of pro-inflammatory cytokines, chemokines and type I IFN. The three RLR family members, RIG-I, MDA5 and LGP2, are expressed at a low level in most cell types, and are upregulated upon exposure of cells to IFN [23–26]. RIG-I and MDA5 are both activated by double-stranded RNAs with blunt ends, which are signatures of foreign RNA (reviewed in [27]). RIG-I is activated by short double-stranded RNAs bearing a 5′ triphosphate moiety, another signature of non-self RNAs, while MDA5 is activated by long double-stranded RNAs. RIG-I may also be activated by single-stranded RNAs bearing a 5′ triphosphate.
RLRs are composed of two N-terminal tandem caspase activation and recruitment (CARD) domains, a central DExD/H box RNA helicase domain, and a C-terminal repressor domain (reviewed in [22]). When no RNA is present, RIG-I remains in a closed conformation where the CARD domains are bound by the repressor domain and cannot signal [28]. RNA binding stimulates a conformational change that releases the CARD domains from the repressor domain, allowing for RIG-I multimerization and CARD domain association with the RLR adaptor protein, MAVS [29–32]. On the other hand, MDA5 lacks a functional repressor domain and does not shield its CARD domains, and instead relies on multimerization along double-stranded RNA for MAVS recruitment [33]. The third member of the RLR family, LGP2, lacks a CARD domain and may act as a regulator of RIG-I and MDA5 signalling [26].
The RLR adaptor protein, MAVS, is a CARD domain-containing transmembrane protein that is localized to the outer membrane of mitochondria, to mitochondria-associated membranes and to peroxisomes [29–32]. Upon RLR activation, relocalization to MAVS-containing membranes occurs, and CARD-CARD interactions between RLRs and MAVS leads to activation of kinases. These kinases can then phosphorylate and activate NF-κB and IRF3/7 to induce type I IFNs. Like TLRs, RLRs can also induce IFNλ.
While RLRs are cytosolic sensors of foreign RNA, there are also cytosolic sensors of foreign DNA. The central regulator of DNA sensing is stimulator of interferon genes (STING), an endoplasmic reticulum-resident transmembrane protein that also localizes to mitochondria and mitochondria-associated membranes [34–38]. Upon STING activation, either through direct sensing of DNA or by an upstream DNA sensor, STING dimerizes and translocates to the perinuclear region, where TBK1 is then recruited. TBK1 can then phosphorylate STING and IRF3, resulting in the induction of type I IFN.
There are multiple DNA sensors that can activate STING (reviewed in [39]). These include DAI, the first DNA sensor to be identified, DDX41, which is a dendritic cell-specific DNA sensor, and IFI16, which can recognize both cytoplasmic and nuclear DNA, among several others. cGAS is the most recently identified cytoplasmic DNA sensor, where binding of DNA to cGAS stimulates the catalysis of cGAMP from ATP and GTP [40–42]. cGAMP then acts as a second messenger to activate STING. cGAS can also be activated by RNA:DNA hybrid molecules, and there is some evidence that STING can be activated by fusion of viral and host membranes [43,44].
Nucleotide binding oligomerization domain (NOD)-like receptors (NLRs) are another class of PRRs that recognize PAMPs in the cytoplasm (reviewed in [45]). There are 22 mammalian NLRs, several of which can form inflammasomes, which are comprised of an NLR, procaspase 1 and ASC. NLR oligomerization results in activation of caspase 1, which can then cleave and mature pro-IL-1β and pro-IL18 to their active forms, IL-1β and IL18. These pro-inflammatory cytokines are secreted and can then bind their cognate receptors, resulting in downstream activation of NF-κB. Inflammasomes can also be formed by non-NLR proteins like AIM2 and RIG-I, an RLR.
NLRs consist of an N-terminal effector domain, a central NOD domain and variable numbers of LRR domains [46]. The effector domain can be either a CARD or PYD domain, which can form homotypic interactions with adaptor proteins and caspases. The NOD domain is responsible for NLR oligomerization, which is an ATP-dependent process. As is the case for TLRs, the LRR domains of NLRs are responsible for PAMP sensing. These domains may also function to auto regulate NLR activation by binding the NOD domain and preventing spontaneous NLR oligomerization [47,48]. NLRs can recognize several PAMPs, as well as damage-associated molecular patterns (DAMPs), and direct binding of NLRs to bacterial components has been demonstrated [49,50]. It is possible that NLRs sense cellular changes induced by viral infection, rather than viral proteins or nucleic acids [51,52].
3. Detection of tumour viruses by pattern recognition receptors
(a). DNA tumour viruses
(i). Kaposi's sarcoma-associated herpesvirus
Kaposi's sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8, is the etiologic agent of three human cancers, Kaposi's sarcoma, primary effusion lymphoma and multicentric Castleman's disease [53]. KSHV is a gammaherpesvirus with a large, double-stranded DNA genome. Like other herpesviruses, KSHV primarily establishes latent infection where only a few viral genes are expressed, but can be reactivated from latency to a lytic state where genome replication and production of progeny virions occur. KSHV infection is detected by a variety of PRRs. In primary monocytes, KSHV infection activates TLR3, which may be recognizing viral RNA transcripts packaged in the tegument, resulting in TLR3 upregulation and induction of IFN [54]. In plasmacytoid dendritic cells (pDCs), the major IFN-producing cells in the human body, KSHV DNA activates TLR9 [55,56]. TLR signalling can also stimulate reactivation from latency in infected B cells, as reactivation can be triggered by treatment with a TLR7/8 agonist or by infection with vesicular stomatitis virus [57]. Furthermore, de novo KSHV infection and reactivation from latency are detected by RIG-I and MAVS [58], as well as the cGAS-STING pathway [59]. Mice deficient for cGAS have higher viral titres following infection with MHV68, a virus related to KSHV, providing further evidence for the importance of the cGAS-STING pathway in detecting and suppressing KSHV infection [60]. KSHV infection is also detected by IFI16 and NLRP1, which form inflammasomes that stimulate IL-1β secretion [61,62].
(ii). Epstein–Barr virus
Epstein–Barr virus (EBV), also known as human herpesvirus 4, infects more than 90% of the world's population [63]. EBV is associated with Burkitt's lymphoma, Hodgkin lymphoma and post-transplant lymphoma, as well as nasopharyngeal carcinoma [63,64]. Like KSHV, EBV is a gammaherpesvirus with a large double-stranded DNA genome, and latent and lytic phases of the viral life cycle. EBV infection is detected by TLRs 2 and 7, which do not require viral replication for stimulation, as both wild-type and UV-inactivated virus can activate these TLRs [65]. Surprisingly, TLR2 can also be activated by a nonstructural protein, the EBV dUTPase [66]. In B cells, the natural reservoir of EBV, infection results in TLR7 upregulation and TLR9 activation [67–69]. Likewise, EBV stimulates TLRs 7 and 9 in pDCs [70,71]. TLR7 can be activated by EBV-encoded RNAs (EBERs), which may be released from infected cells in exosomes, activating PRRs in neighbouring cells and stimulating IFN production [71,72]. EBERs are also detected by RIG-I, as they are RNA polymerase III-derived transcripts which contain a 5′ triphosphate moiety [73].
(iii). Human papillomavirus
Human papillomavirus (HPV), a non-enveloped virus with a small, double-stranded DNA genome, infects the basal keratinocytes of skin or mucous membranes. High-risk HPV subtypes are the causative agents of cervical cancer and are also associated with anogenital, oropharyngeal, and head and neck cancers [74]. In keratinocytes, HPV DNA is detected by TLR9 [75]. Additionally, there is some circumstantial evidence to support a role for STING in detection of HPV in that HPV E2 downregulates STING [76], and HPV E7 interacts with and inhibits this sensor [77], although sensing of HPV primary infection by STING has not yet been demonstrated.
(iv). Hepatitis B virus
Hepatitis B virus (HBV) currently infects approximately 240 million people worldwide, and chronic HBV infection can lead to liver cancer [78]. An estimated 73% of liver cancer deaths worldwide are attributable to hepatitis viruses. HBV is an enveloped virus with a partially double-stranded DNA genome. When HBV-infected cells are transfected to overexpress the TLR adaptor proteins MyD88 or TRIF, the RLR adaptor protein MAVS, or the DNA sensor DAI, the quantity of HBV DNA and RNA in those cells decreases [79,80]. Treatment of HBV transgenic mice with TLR3, 4, 5, 7 or 9 agonists, but not TLR2 agonists, reduced HBV replication [81]. A TLR7 agonist also decreased HBV DNA in the liver of infected chimpanzees [82]. Additionally, in transfected cells or in mice hydroponically injected with HBV genomes, MDA5 is upregulated and is activated by HBV, and HBV-infected mice heterozygous for MDA5 have increased HBV DNA compared to wild-type mice [83]. Taken together, these data indicate that both TLRs and RLRs are responsible for detecting HBV infection.
(v). Merkel cell polyomavirus
Merkel cell polyomavirus (MCV) is a small, non-enveloped virus with a double-stranded DNA genome. MCV is the causative agent of Merkel cell carcinoma, a rare type of skin cancer [84]. The interaction between MCV and the innate immune system is largely unknown. However, TLR9 may play a role in the detection of MCV infection given that MCV large T antigen downregulates TLR9 expression [85].
(b). RNA tumour viruses
(i). Hepatitis C virus
Hepatitis C virus (HCV) is a flavivirus that currently infects approximately 170 million people worldwide [86]. HCV is capable of establishing chronic infection, which can result in liver damage and hepatocellular carcinoma [87]. HCV is an enveloped virus with a single-stranded, positive sense RNA genome. The HCV core protein and non-structural protein 3 activate TLR2 at the cell surface [88]. In endosomes, HCV RNA is detected by TLR3 and by TLR7/8 [89,90]. HCV RNA is also sensed in the cytoplasm by RIG-I, which recognizes several features of the HCV genome such as a 3′ polyU sequence, short regions of dsRNA, and the 5′ triphosphorylated end of the genome [91–93]. Surprisingly, the HCV 3′UTR can also stimulate STING [94]. HCV infection also activates NLRP3, which may sense cellular changes induced by infection, as the production of reactive oxygen species during infection is important for NLRP3 activation [95,96].
(ii). Human T-cell lymphotropic virus type 1
Human T-cell lymphotropic virus type 1 (HTLV-1) is a retrovirus that currently infects an estimated 15–20 million people worldwide [97]. HTLV-1 is associated with adult T-cell leukaemia/lymphoma, a proliferation of CD4+ T cells caused by integration of the HTLV-1 provirus. HTLV-1 is predominantly detected in CD4+ T cells, but has also been found in other immune cells including pDCs [98,99]. In pDCs, HTLV-1 RNA is detected by TLR7 [100]. Currently, no other PRRs have been described as important for innate immune detection of HTLV-1.
4. Evasion of host innate immunity by tumour viruses
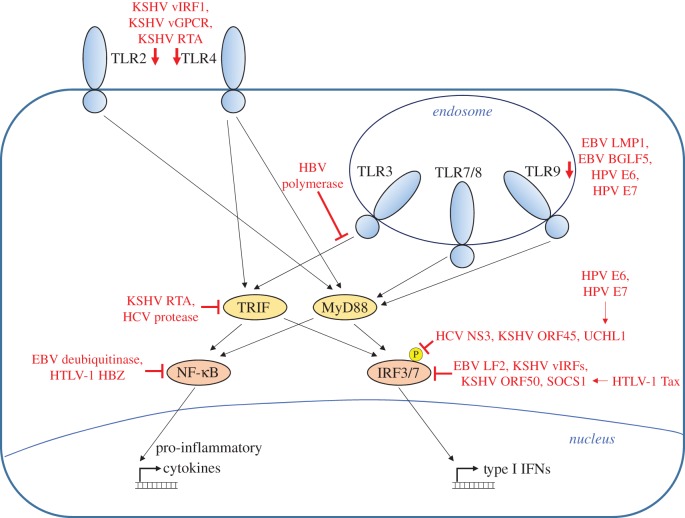
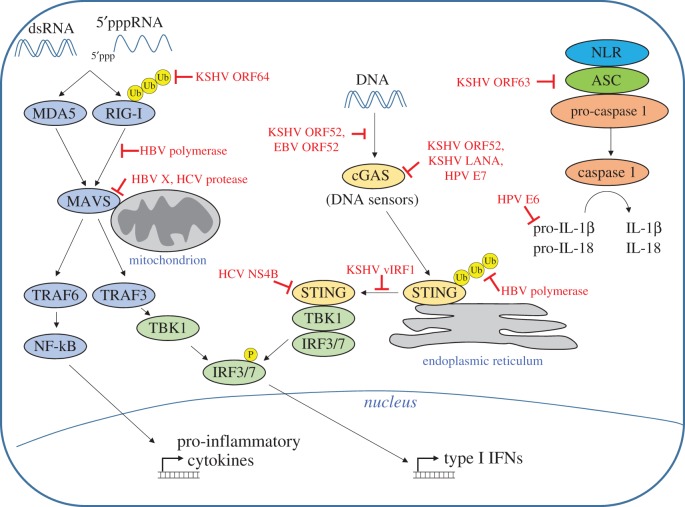
Tumour viruses employ a variety of mechanisms to evade the host innate immune response. These strategies include downregulation of sensors, inhibition of signal transduction pathways and disruption of transcription factor activation, among others. The following section will describe tumour virus-encoded proteins that antagonize the innate immune response. Figure 1 summarizes tumour virus inhibition of TLR signalling pathways, and figure 2 summarizes inhibition of cytoplasmic PRRs.
Figure 1.
Inhibition of TLR signalling by tumour viruses. TLRs 2 and 4, which are responsible for detecting viral proteins at the plasma membrane, are downregulated by KSHV vIRF1, vGPCR and RTA. The endosomal TLRs are responsible for detecting nucleic acid and include TLR3, TLR7/8 and TLR9. TLR9 is downregulated by EBV LMP1, EBV BGLF5, HPV E6 and HPV E7. TLRs signal through the adaptor proteins TRIF and MyD88. TLR3 signalling is inhibited by the HBV polymerase, and TRIF is inhibited by KSHV RTA and by the HCV protease. TRIF and MyD88 activate the transcription factors IRF3/7 and NF-κB, which is inhibited by the EBV deubiquitinase and by HTLV-1 HBZ. IRF phosphorylation is inhibited by HCV NS3, KSHV ORF45 and the cellular protein UCHL1, which is induced by HPV E6 and E7. IRFs are inhibited by EBV LF2, KSHV vIRFs, KSHV ORF50 and the cellular protein SOCS1, which is induced by HTLV-1 Tax.
Figure 2.
Inhibition of cytoplasmic PRRs by tumour viruses. Cytosolic double-stranded RNA is detected by the RLRs RIG-I and MDA5. RIG-I ubiquitination is inhibited by KSHV ORF64, and RIG-I signalling is inhibited by the HBV polymerase. RLRs signal through the adaptor protein MAVS, which is inhibited by HBV X and the HCV protease. Cytosolic DNA is detected by a variety of DNA sensors, including cGAS. Binding of DNA to cGAS is inhibited by KSHV ORF52 and EBV ORF52. cGAS activity is inhibited by KSHV ORF52, KSHV LANA and HPV E7. DNA sensors activate STING. STING ubiquitination is inhibited by the HBV polymerase, KSHV vIRF1 prevents STING association with TBK1, and HCV NS4B disrupts STING signalling complexes. The NLRP1 inflammasome is inhibited by KSHV ORF63, and HPV E6 mediates degradation of pro-IL-1β.
(a). DNA tumour viruses
(i). Kaposi's sarcoma-associated herpesvirus
KSHV encodes several proteins that antagonize the innate immune response. For example, KSHV vIRF1, vGPCR and RTA downregulate TLRs 2 and 4 [101,102]. Additionally, the adaptor protein responsible for mediating TLR3 and some TLR4 signalling, TRIF, is degraded by KSHV RTA [103]. RIG-I signalling is also disrupted by KSHV, as the KSHV deubiquitinase ORF64 prevents RIG-I ubiquitination, a modification essential for RIG-I activity [104]. In addition to inhibiting TLR and RLR signalling, KSHV blocks STING signalling. KSHV ORF52 disrupts cGAS binding to DNA and inhibits the enzymatic activity of cGAS to prevent STING activation [105]. Furthermore, there is evidence that cytoplasmic localized isoforms of LANA (latency-associated nuclear antigen) bind cGAS and prevent cGAS-mediated IFN production [106]. Additionally, KSHV vIRF1 blocks STING activation by disrupting STING interactions with TBK1 [59]. Inflammasome signalling can also be inhibited by KSHV ORF63, which blocks the NLRP1 inflammasome to prevent caspase activation and IL1β and IL18 processing, thereby reducing NLRP1-mediated cell death [62]. Finally, KSHV can disrupt transcription factor activation. Three of the four KSHV vIRF proteins interact with cellular IRFs and prevent their transcriptional activity (reviewed in [107]). KSHV ORF45 prevents IRF7 phosphorylation, and ORF50 can stimulate IRF7 ubiquitination and degradation [108,109]. Lastly, KSHV LANA competes with IRF3 to bind the IFN promoter, thereby reducing IFN transcription [110].
(ii). Epstein–Barr virus
Like KSHV, EBV inhibits the innate immune response at several points in PRR signalling pathways. The EBV proteins LMP1 and BGLF5 reduce TLR9 expression through inhibition of TLR9 transcription or degradation of TLR9 transcripts, respectively [69,111]. EBV encodes a deubiquitinase that prevents TLR signalling-mediated NF-κB activation [112]. Additionally, EBV ORF52 blocks cGAS binding to DNA [105]. Finally, the EBV tegument protein LF2 disrupts IRF7-mediated IFN expression [113], and EBV infection induces the expression of a dominant negative form of IRF5 [67].
(iii). Human papillomavirus
HPV employs similar innate immune evasion strategies to KSHV and EBV. For example, the E6 and E7 proteins from high-risk HPV subtypes reduce TLR9 expression, and the TLR3 adaptor, TICAM1, is downregulated in HPV-positive cells [75,114]. Additionally, HPV E7 blocks cGAS-STING signalling [77]. HPV-positive keratinocytes have reduced NLRP2 expression compared to uninfected cells, and the E6 protein from high-risk HPV subtypes can mediate degradation of the immature form of IL-1β, thereby reducing the impact of inflammasome signalling [114,115]. HPV also disrupts transcription factor activation, as the E6 and E7 proteins from high-risk HPVs downregulate genes involved in NF-κB activation, and upregulate UCHL1, a disruptor of TLR signalling that inhibits IRF3 phosphorylation [116]. UCHL1 also blunts NF-κB activation by destabilizing NF-κB essential modulator, a scaffolding protein that coordinates the degradation of IκB to allow for NF-κB nuclear translocation. HPV E2 downregulates IFNκ, a type of IFN secreted exclusively by epithelial cells [76]. Furthermore, the E6 protein from high-risk HPVs prevents phosphorylation of STAT transcription factors, which are critical for mediating signalling from the IFN receptor and upregulating ISGs [117]. This disruption is essential for HPV replication and long-term maintenance of HPV episomes. The E6 and E7 proteins from high-risk HPVs are also capable of downregulating some IFN-stimulated genes, and can counteract the effects of the ISG PKR, a kinase that blocks all translation by phosphorylating the translation initiation factor EIF2α [118,119]. E6 and E7 prevent PKR phosphorylation and activation, allowing translation to occur [119].
(iv). Hepatitis B virus
HBV is also capable of disrupting the innate immune response. Purified HBV virions inhibit signalling from TLRs 3 and 4, and the HBV polymerase inhibits both TLR3 and RIG-I signalling [120–122]. Signal transduction through the RLR adaptor protein MAVS is also disrupted by an interaction with the HBV X protein [123]. Additionally, the HBV polymerase interacts with STING and blocks STING K63-linked polyubiquitination, a modification essential for STING-mediated IFN induction [124].
(v). Merkel cell polyomavirus
The mechanisms of MCV evasion of the innate immune response remain largely unknown. However, it has been reported that MCV small T antigen binds to and disrupts NF-κB essential modulator, thereby inhibiting NF-κB activation and signalling [125].
(b). RNA tumour viruses
(i). Hepatitis C virus
HCV also encodes proteins that disrupt innate immune signalling. The HCV protease cleaves the TLR3 and TLR4 adaptor protein TRIF, as well as the RLR adaptor MAVS [30,126]. Additionally, the HCV NS4B protein is a STING homologue that interacts with STING and disrupts STING signalling complexes [94]. Finally, HCV NS3 prevents IRF3 phosphorylation [127].
(ii). Human T-cell lymphotropic virus type 1
HTLV-1 uses several mechanisms to disrupt IRF and NF-κB signalling. HTLV-1 induces expression of microRNAs that downregulate mediators of PRR signalling, like the kinase responsible for phosphorylating IRF3 [128,129]. Additionally, HTLV-1 Tax induces SOCS1 expression, a protein responsible for IRF3 ubiquitination and degradation [130,131]. Furthermore, the HTLV1 HBZ protein interacts with NF-κB and blocks NF-κB DNA binding activity, and can also stimulate NF-κB ubiquitination and degradation [132]. HTLV-1 also blunts STAT signalling, possibly through Tax competing with STAT2 for co-activating proteins [133,134].
In summary, cells encode an array of PRRs that can detect viral infection by recognizing PAMPs. These PRRs can sense viral proteins at the surface of the cell, or viral nucleic acid in endosomes, in the cytoplasm, or in the nucleus. Tumour virus infection is detected by these receptors, which results in activation of transcription factors that upregulate pro-inflammatory cytokines, chemokines and IFN. These molecules are responsible for attracting immune cells to the site of infection, and for inducing an antiviral state in infected and neighbouring cells. However, tumour viruses have several mechanisms for confounding the innate immune response, including downregulating PRRs, preventing PRR recognition of their substrates, blocking transcription factor activation, disrupting signalling from IFN receptors, and inhibiting the effects of antiviral proteins.
Acknowledgements
We apologize for not citing many publications due to limits on reference per journal policy.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
Our work is supported by public health service grants CA019014, AI109965, DE018281. S.E.H. is supported in part by Lineberger Cancer Center training grant no. T32-CA009156. B.D. is a Leukemia and Lymphoma Society Scholar, and a Burroughs Wellcome Fund Investigator in Infectious Disease.
References
- 1.Takeuchi O, Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140, 805–820. ( 10.1016/j.cell.2010.01.022) [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Takeda K. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511. ( 10.1038/nri1391) [DOI] [PubMed] [Google Scholar]
- 3.Kurt-Jones EA, et al. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1, 398–401. ( 10.1038/80833) [DOI] [PubMed] [Google Scholar]
- 4.Bieback K, et al. 2002. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J. Virol. 76, 8729–8736. ( 10.1128/JVI.76.17.8729-8736.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738. ( 10.1038/35099560) [DOI] [PubMed] [Google Scholar]
- 6.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531. ( 10.1126/science.1093616) [DOI] [PubMed] [Google Scholar]
- 7.Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl Acad. Sci. USA 101, 5598–5603. ( 10.1073/pnas.0400937101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemmi H, et al. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745. ( 10.1038/35047123) [DOI] [PubMed] [Google Scholar]
- 9.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. 2003. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198, 513–520. ( 10.1084/jem.20030162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. 1999. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11, 115–122. ( 10.1016/S1074-7613(00)80086-2) [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi O, Kaufmann A, Grote K, Kawai T, Hoshino K, Morr M, Muhlradt PF, Akira S. 2000. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a Toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 164, 554–557. ( 10.4049/jimmunol.164.2.554) [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi O, Takeda K, Hoshino K, Adachi O, Ogawa T, Akira S. 2000. Cellular responses to bacterial cell wall components are mediated through MyD88-dependent signaling cascades. Int. Immunol. 12, 113–117. ( 10.1093/intimm/12.1.113) [DOI] [PubMed] [Google Scholar]
- 13.Hacker H, Vabulas RM, Takeuchi O, Hoshino K, Akira S, Wagner H. 2000. Immune cell activation by bacterial CpG-DNA through myeloid differentiation marker 88 and tumor necrosis factor receptor-associated factor (TRAF)6. J. Exp. Med. 192, 595–600. ( 10.1084/jem.192.4.595) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnare M, Holt AC, Takeda K, Akira S, Medzhitov R. 2000. Recognition of CpG DNA is mediated by signaling pathways dependent on the adaptor protein MyD88. Curr. Biol. 10, 1139–1142. ( 10.1016/S0960-9822(00)00700-4) [DOI] [PubMed] [Google Scholar]
- 15.Hemmi H, et al. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3, 196–200. ( 10.1038/ni758) [DOI] [PubMed] [Google Scholar]
- 16.Hayashi F, et al. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103. ( 10.1038/35074106) [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. 2002. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J. Immunol. 169, 6668–6672. ( 10.4049/jimmunol.169.12.6668) [DOI] [PubMed] [Google Scholar]
- 18.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. 2003. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat. Immunol. 4, 161–167. ( 10.1038/ni886) [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto M, et al. 2003. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science 301, 640–643. ( 10.1126/science.1087262) [DOI] [PubMed] [Google Scholar]
- 20.Hoebe K, et al. 2003. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424, 743–748. ( 10.1038/nature01889) [DOI] [PubMed] [Google Scholar]
- 21.Coccia EM, Severa M, Giacomini E, Monneron D, Remoli ME, Julkunen I, Cella M, Lande R, Uze G. 2004. Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur. J. Immunol. 34, 796–805. ( 10.1002/eji.200324610) [DOI] [PubMed] [Google Scholar]
- 22.Loo YM, Gale M Jr. 2011. Immune signaling by RIG-I-like receptors. Immunity 34, 680–692. ( 10.1016/j.immuni.2011.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang DC, Gopalkrishnan RV, Lin L, Randolph A, Valerie K, Pestka S, Fisher PB. 2004. Expression analysis and genomic characterization of human melanoma differentiation associated gene-5, mda-5: a novel type I interferon-responsive apoptosis-inducing gene. Oncogene 23, 1789–1800. ( 10.1038/sj.onc.1207300) [DOI] [PubMed] [Google Scholar]
- 24.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, 730–737. ( 10.1038/ni1087) [DOI] [PubMed] [Google Scholar]
- 25.Imaizumi T, Kumagai M, Taima K, Fujita T, Yoshida H, Satoh K. 2005. Involvement of retinoic acid-inducible gene-I in the IFN-γ/STAT1 signalling pathway in BEAS-2B cells. Eur. Respir. J. 25, 1077–1083. ( 10.1183/09031936.05.00102104) [DOI] [PubMed] [Google Scholar]
- 26.Yoneyama M, et al. 2005. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 175, 2851–2858. ( 10.4049/jimmunol.175.5.2851) [DOI] [PubMed] [Google Scholar]
- 27.Schlee M, Hartmann G. 2010. The chase for the RIG-I ligand—recent advances. Mol. Ther. 18, 1254–1262. ( 10.1038/mt.2010.90) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saito T, Hirai R, Loo YM, Owen D, Johnson CL, Sinha SC, Akira S, Fujita T, Gale M Jr. 2007. Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc. Natl Acad. Sci. USA 104, 582–587. ( 10.1073/pnas.0606699104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6, 981–988. ( 10.1038/ni1243) [DOI] [PubMed] [Google Scholar]
- 30.Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437, 1167–1172. ( 10.1038/nature04193) [DOI] [PubMed] [Google Scholar]
- 31.Seth RB, Sun L, Ea CK, Chen ZJ. 2005. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF 3. Cell 122, 669–682. ( 10.1016/j.cell.2005.08.012) [DOI] [PubMed] [Google Scholar]
- 32.Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. 2005. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol. Cell 19, 727–740. ( 10.1016/j.molcel.2005.08.014) [DOI] [PubMed] [Google Scholar]
- 33.Wu B, Peisley A, Richards C, Yao H, Zeng X, Lin C, Chu F, Walz T, Hur S. 2013. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell 152, 276–289. ( 10.1016/j.cell.2012.11.048) [DOI] [PubMed] [Google Scholar]
- 34.Ishikawa H, Barber GN. 2008. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678. ( 10.1038/nature07317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin L, Waterman PM, Jonscher KR, Short CM, Reisdorph NA, Cambier JC. 2008. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol. Cell. Biol. 28, 5014–5026. ( 10.1128/MCB.00640-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun W, et al. 2009. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc. Natl Acad. Sci. USA 106, 8653–8658. ( 10.1073/pnas.0900850106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong B, et al. 2008. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 29, 538–550. ( 10.1016/j.immuni.2008.09.003) [DOI] [PubMed] [Google Scholar]
- 38.Ishikawa H, Ma Z, Barber GN. 2009. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792. ( 10.1038/nature08476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barber GN. 2014. STING-dependent cytosolic DNA sensing pathways. Trends Immunol. 35, 88–93. ( 10.1016/j.it.2013.10.010) [DOI] [PubMed] [Google Scholar]
- 40.Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Rohl I, Hopfner KP, Ludwig J, Hornung V. 2013. cGAS produces a 2'-5'-linked cyclic dinucleotide second messenger that activates STING. Nature 498, 380–384. ( 10.1038/nature12306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao D, Wu J, Wu YT, Du F, Aroh C, Yan N, Sun L, Chen ZJ. 2013. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 341, 903–906. ( 10.1126/science.1240933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun L, Wu J, Du F, Chen X, Chen ZJ. 2013. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791. ( 10.1126/science.1232458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mankan AK, et al. 2014. Cytosolic RNA:DNA hybrids activate the cGAS-STING axis. EMBO J. 33, 2937–2946. ( 10.15252/embj.201488726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holm CK, et al. 2012. Virus-cell fusion as a trigger of innate immunity dependent on the adaptor STING. Nat. Immunol. 13, 737–743. ( 10.1038/ni.2350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacobs SR, Damania B. 2012. NLRs, inflammasomes, and viral infection. J. Leukoc. Biol. 92, 469–477. ( 10.1189/jlb.0312132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ting JP, et al. 2008. The NLR gene family: a standard nomenclature. Immunity 28, 285–287. ( 10.1016/j.immuni.2008.02.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duncan JA, Bergstralh DT, Wang Y, Willingham SB, Ye Z, Zimmermann AG, Ting JP. 2007. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc. Natl Acad. Sci. USA 104, 8041–8046. ( 10.1073/pnas.0611496104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faustin B, et al. 2007. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol. Cell 25, 713–724. ( 10.1016/j.molcel.2007.01.032) [DOI] [PubMed] [Google Scholar]
- 49.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F. 2011. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477, 596–600. ( 10.1038/nature10510) [DOI] [PubMed] [Google Scholar]
- 50.Kofoed EM, Vance RE. 2011. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477, 592–595. ( 10.1038/nature10394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. 2007. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14, 1583–1589. ( 10.1038/sj.cdd.4402195) [DOI] [PubMed] [Google Scholar]
- 52.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. 2010. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11, 136–140. ( 10.1038/ni.1831) [DOI] [PubMed] [Google Scholar]
- 53.Ablashi DV, Chatlynne LG, Whitman JE Jr, Cesarman E. 2002. Spectrum of Kaposi's sarcoma-associated herpesvirus, or human herpesvirus 8, diseases. Clin. Microbiol. Rev. 15, 439–464. ( 10.1128/CMR.15.3.439-464.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.West J, Damania B. 2008. Upregulation of the TLR3 pathway by Kaposi's sarcoma-associated herpesvirus during primary infection. J. Virol. 82, 5440–5449. ( 10.1128/JVI.02590-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guggemoos S, Hangel D, Hamm S, Heit A, Bauer S, Adler H. 2008. TLR9 contributes to antiviral immunity during gammaherpesvirus infection. J. Immunol. 180, 438–443. ( 10.4049/jimmunol.180.1.438) [DOI] [PubMed] [Google Scholar]
- 56.West JA, Gregory SM, Sivaraman V, Su L, Damania B. 2011. Activation of plasmacytoid dendritic cells by Kaposi's sarcoma-associated herpesvirus. J. Virol. 85, 895–904. ( 10.1128/JVI.01007-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gregory SM, West JA, Dillon PJ, Hilscher C, Dittmer DP, Damania B. 2009. Toll-like receptor signaling controls reactivation of KSHV from latency. Proc. Natl Acad. Sci. USA 106, 11 725–11 730. ( 10.1073/pnas.0905316106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.West JA, Wicks M, Gregory SM, Chugh P, Jacobs SR, Zhang Z, Host KM, Dittmer DP, Damania B. 2014. An important role for mitochondrial antiviral signaling protein in the Kaposi's sarcoma-associated herpesvirus life cycle. J. Virol. 88, 5778–5787. ( 10.1128/JVI.03226-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma Z, et al. 2015. Modulation of the cGAS-STING DNA sensing pathway by gammaherpesviruses. Proc. Natl Acad. Sci. USA 112, E4306–E4315. ( 10.1073/pnas.1503831112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schoggins JW, et al. 2014. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 505, 691–695. ( 10.1038/nature12862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kerur N, Veettil MV, Sharma-Walia N, Bottero V, Sadagopan S, Otageri P, Chandran B. 2011. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe 9, 363–375. ( 10.1016/j.chom.2011.04.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gregory SM, Davis BK, West JA, Taxman DJ, Matsuzawa S, Reed JC, Ting JP, Damania B. 2011. Discovery of a viral NLR homolog that inhibits the inflammasome. Science 331, 330–334. ( 10.1126/science.1199478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cesarman E. 2011. Gammaherpesvirus and lymphoproliferative disorders in immunocompromised patients. Cancer Lett. 305, 163–174. ( 10.1016/j.canlet.2011.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raab-Traub N. 2015. Nasopharyngeal carcinoma: an evolving role for the Epstein-Barr virus. Curr. Top. Microbiol. Immunol. 390, 339–363. ( 10.1007/978-3-319-22822-8_14) [DOI] [PubMed] [Google Scholar]
- 65.Gaudreault E, Fiola S, Olivier M, Gosselin J. 2007. Epstein-Barr virus induces MCP-1 secretion by human monocytes via TLR2. J. Virol. 81, 8016–8024. ( 10.1128/JVI.00403-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ariza ME, Glaser R, Kaumaya PT, Jones C, Williams MV. 2009. The EBV-encoded dUTPase activates NF-κ B through the TLR2 and MyD88-dependent signaling pathway. J. Immunol. 182, 851–859. ( 10.4049/jimmunol.182.2.851) [DOI] [PubMed] [Google Scholar]
- 67.Martin HJ, Lee JM, Walls D, Hayward SD. 2007. Manipulation of the Toll-like receptor 7 signaling pathway by Epstein-Barr virus. J. Virol. 81, 9748–9758. ( 10.1128/JVI.01122-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lim WH, Kireta S, Russ GR, Coates PT. 2007. Human plasmacytoid dendritic cells regulate immune responses to Epstein-Barr virus (EBV) infection and delay EBV-related mortality in humanized NOD-SCID mice. Blood 109, 1043–1050. ( 10.1182/blood-2005-12-024802) [DOI] [PubMed] [Google Scholar]
- 69.van Gent M, et al. 2011. EBV lytic-phase protein BGLF5 contributes to TLR9 downregulation during productive infection. J. Immunol. 186, 1694–1702. ( 10.4049/jimmunol.0903120) [DOI] [PubMed] [Google Scholar]
- 70.Fiola S, Gosselin D, Takada K, Gosselin J. 2010. TLR9 contributes to the recognition of EBV by primary monocytes and plasmacytoid dendritic cells. J. Immunol. 185, 3620–3631. ( 10.4049/jimmunol.0903736) [DOI] [PubMed] [Google Scholar]
- 71.Quan TE, Roman RM, Rudenga BJ, Holers VM, Craft JE. 2010. Epstein-Barr virus promotes interferon-α production by plasmacytoid dendritic cells. Arthritis Rheum. 62, 1693–1701. ( 10.1002/art.27408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iwakiri D, et al. 2009. Epstein-Barr virus (EBV)-encoded small RNA is released from EBV-infected cells and activates signaling from Toll-like receptor 3. J. Exp. Med. 206, 2091–2099. ( 10.1084/jem.20081761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ablasser A, Bauernfeind F, Hartmann G, Latz E, Fitzgerald KA, Hornung V. 2009. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat. Immunol. 10, 1065–1072. ( 10.1038/ni.1779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.zur Hausen H. 2009. Papillomaviruses in the causation of human cancers—a brief historical account. Virology 384, 260–265. ( 10.1016/j.virol.2008.11.046) [DOI] [PubMed] [Google Scholar]
- 75.Hasan UA, et al. 2007. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J. Immunol. 178, 3186–3197. ( 10.4049/jimmunol.178.5.3186) [DOI] [PubMed] [Google Scholar]
- 76.Sunthamala N, Thierry F, Teissier S, Pientong C, Kongyingyoes B, Tangsiriwatthana T, Sangkomkamhang U, Ekalaksananan T. 2014. E2 proteins of high risk human papillomaviruses down-modulate STING and IFN-κ transcription in keratinocytes. PLoS ONE 9, e91473 ( 10.1371/journal.pone.0091473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lau L, Gray EE, Brunette RL, Stetson DB. 2015. DNA tumor virus oncogenes antagonize the cGAS-STING DNA-sensing pathway. Science 350, 568–571. ( 10.1126/science.aab3291) [DOI] [PubMed] [Google Scholar]
- 78.Ott JJ, Stevens GA, Groeger J, Wiersma ST. 2012. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 30, 2212–2219. ( 10.1016/j.vaccine.2011.12.116) [DOI] [PubMed] [Google Scholar]
- 79.Guo H, Jiang D, Ma D, Chang J, Dougherty AM, Cuconati A, Block TM, Guo JT. 2009. Activation of pattern recognition receptor-mediated innate immunity inhibits the replication of hepatitis B virus in human hepatocyte-derived cells. J. Virol. 83, 847–858. ( 10.1128/JVI.02008-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen QY, Liu YH, Li JH, Wang ZK, Liu JX, Yuan ZH. 2012. DNA-dependent activator of interferon-regulatory factors inhibits hepatitis B virus replication. World J. Gastroenterol. 18, 2850–2858. ( 10.3748/wjg.v18.i22.2850) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Isogawa M, Robek MD, Furuichi Y, Chisari FV. 2005. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J. Virol. 79, 7269–7272. ( 10.1128/JVI.79.11.7269-7272.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lanford RE, et al. 2013. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology 144, 1508–1517.e10. ( 10.1053/j.gastro.2013.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu HL, Liao F. 2013. Melanoma differentiation-associated gene 5 senses hepatitis B virus and activates innate immune signaling to suppress virus replication. J. Immunol. 191, 3264–3276. ( 10.4049/jimmunol.1300512) [DOI] [PubMed] [Google Scholar]
- 84.Chang Y, Moore PS. 2012. Merkel cell carcinoma: a virus-induced human cancer. Annu. Rev. Pathol. 7, 123–144. ( 10.1146/annurev-pathol-011110-130227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shahzad N, et al. 2013. The T antigen locus of Merkel cell polyomavirus downregulates human Toll-like receptor 9 expression. J. Virol. 87, 13 009–13 019. ( 10.1128/JVI.01786-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E. 2015. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 61, 77–87. ( 10.1002/hep.27259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brown RS. 2005. Hepatitis C and liver transplantation. Nature 436, 973–978. ( 10.1038/nature04083) [DOI] [PubMed] [Google Scholar]
- 88.Dolganiuc A, Oak S, Kodys K, Golenbock DT, Finberg RW, Kurt-Jones E, Szabo G. 2004. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology 127, 1513–1524. ( 10.1053/j.gastro.2004.08.067) [DOI] [PubMed] [Google Scholar]
- 89.Zhang YL, Guo YJ, Bin L, Sun SH. 2009. Hepatitis C virus single-stranded RNA induces innate immunity via Toll-like receptor 7. J. Hepatol. 51, 29–38. ( 10.1016/j.jhep.2009.03.012) [DOI] [PubMed] [Google Scholar]
- 90.Wang N, Liang Y, Devaraj S, Wang J, Lemon SM, Li K. 2009. Toll-like receptor 3 mediates establishment of an antiviral state against hepatitis C virus in hepatoma cells. J. Virol. 83, 9824–9834. ( 10.1128/JVI.01125-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saito T, Owen DM, Jiang F, Marcotrigiano J, Gale M Jr. 2008. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 454, 523–527. ( 10.1038/nature07106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Uzri D, Gehrke L. 2009. Nucleotide sequences and modifications that determine RIG-I/RNA binding and signaling activities. J. Virol. 83, 4174–4184. ( 10.1128/JVI.02449-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schnell G, Loo YM, Marcotrigiano J, Gale M Jr. 2012. Uridine composition of the poly-U/UC tract of HCV RNA defines non-self recognition by RIG-I. PLoS Pathog. 8, e1002839 ( 10.1371/journal.ppat.1002839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ding Q, Cao X, Lu J, Huang B, Liu YJ, Kato N, Shu HB, Zhong J. 2013. Hepatitis C virus NS4B blocks the interaction of STING and TBK1 to evade host innate immunity. J. Hepatol. 59, 52–58. ( 10.1016/j.jhep.2013.03.019) [DOI] [PubMed] [Google Scholar]
- 95.Burdette D, Haskett A, Presser L, McRae S, Iqbal J, Waris G. 2012. Hepatitis C virus activates interleukin-1beta via caspase-1-inflammasome complex. J. Gen. Virol. 93, 235–246. ( 10.1099/vir.0.034033-0) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.Negash AA, et al. 2013. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathogens 9, e1003330 ( 10.1371/journal.ppat.1003330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mueller N. 1991. The epidemiology of HTLV-I infection. Cancer Causes Control 2, 37–52. ( 10.1007/BF00052359) [DOI] [PubMed] [Google Scholar]
- 98.Richardson JH, Edwards AJ, Cruickshank JK, Rudge P, Dalgleish AG. 1990. In vivo cellular tropism of human T-cell leukemia virus type 1. J. Virol. 64, 5682–5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koyanagi Y, Itoyama Y, Nakamura N, Takamatsu K, Kira J, Iwamasa T, Goto I, Yamamoto N. 1993. In vivo infection of human T-cell leukemia virus type I in non-T cells. Virology 196, 25–33. ( 10.1006/viro.1993.1451) [DOI] [PubMed] [Google Scholar]
- 100.Colisson R, Barblu L, Gras C, Raynaud F, Hadj-Slimane R, Pique C, Hermine O, Lepelletier Y, Herbeuval JP. 2010. Free HTLV-1 induces TLR7-dependent innate immune response and TRAIL relocalization in killer plasmacytoid dendritic cells. Blood 115, 2177–2185. ( 10.1182/blood-2009-06-224741) [DOI] [PubMed] [Google Scholar]
- 101.Lagos D, et al. 2008. Toll-like receptor 4 mediates innate immunity to Kaposi sarcoma herpesvirus. Cell Host Microbe 4, 470–483. ( 10.1016/j.chom.2008.09.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bussey KA, et al. 2014. The gammaherpesviruses Kaposi's sarcoma-associated herpesvirus and murine gammaherpesvirus 68 modulate the Toll-like receptor-induced proinflammatory cytokine response. J. Virol. 88, 9245–9259. ( 10.1128/JVI.00841-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ahmad H, Gubbels R, Ehlers E, Meyer F, Waterbury T, Lin R, Zhang L. 2011. Kaposi sarcoma-associated herpesvirus degrades cellular Toll-interleukin-1 receptor domain-containing adaptor-inducing beta-interferon (TRIF). J. Biol. Chem. 286, 7865–7872. ( 10.1074/jbc.M110.191452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Inn KS, Lee SH, Rathbun JY, Wong LY, Toth Z, Machida K, Ou JH, Jung JU. 2011. Inhibition of RIG-I-mediated signaling by Kaposi's sarcoma-associated herpesvirus-encoded deubiquitinase ORF64. J. Virol. 85, 10 899–10 904. ( 10.1128/JVI.00690-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu JJ, et al. 2015. Inhibition of cGAS DNA sensing by a herpesvirus virion protein. Cell Host Microbe 18, 333–344. ( 10.1016/j.chom.2015.07.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang G, Chan B, Samarina N, Abere B, Weidner-Glunde M, Buch A, Pich A, Brinkmann MM, Schulz TF. 2016. Cytoplasmic isoforms of Kaposi sarcoma herpesvirus LANA recruit and antagonize the innate immune DNA sensor cGAS. Proc. Natl Acad. Sci. USA 113, E1034–E1043. ( 10.1073/pnas.1516812113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jacobs SR, Damania B. 2011. The viral interferon regulatory factors of KSHV: immunosuppressors or oncogenes? Front. Immunol. 2, 19 ( 10.3389/fimmu.2011.00019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu FX, King SM, Smith EJ, Levy DE, Yuan Y. 2002. A Kaposi's sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proc. Natl Acad. Sci. USA 99, 5573–5578. ( 10.1073/pnas.082420599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu Y, Wang SE, Hayward GS. 2005. The KSHV immediate-early transcription factor RTA encodes ubiquitin E3 ligase activity that targets IRF7 for proteosome-mediated degradation. Immunity 22, 59–70. ( 10.1016/j.immuni.2004.11.011) [DOI] [PubMed] [Google Scholar]
- 110.Cloutier N, Flamand L. 2010. Kaposi sarcoma-associated herpesvirus latency-associated nuclear antigen inhibits interferon (IFN) beta expression by competing with IFN regulatory factor-3 for binding to IFNB promoter. J. Biol. Chem. 285, 7208–7221. ( 10.1074/jbc.M109.018838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fathallah I, et al. 2010. EBV latent membrane protein 1 is a negative regulator of TLR9. J. Immunol. 185, 6439–6447. ( 10.4049/jimmunol.0903459) [DOI] [PubMed] [Google Scholar]
- 112.Saito S, Murata T, Kanda T, Isomura H, Narita Y, Sugimoto A, Kawashima D, Tsurumi T. 2013. Epstein-Barr virus deubiquitinase downregulates TRAF6-mediated NF-κB signaling during productive replication. J. Virol. 87, 4060–4070. ( 10.1128/JVI.02020-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu L, Fossum E, Joo CH, Inn KS, Shin YC, Johannsen E, Hutt-Fletcher LM, Hass J, Jung JU. 2009. Epstein-Barr virus LF2: an antagonist to type I interferon. J. Virol. 83, 1140–1146. ( 10.1128/JVI.00602-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Karim R, Meyers C, Backendorf C, Ludigs K, Offringa R, van Ommen GJ, Melief CJ, van der Burg SH, Boer JM. 2011. Human papillomavirus deregulates the response of a cellular network comprising of chemotactic and proinflammatory genes. PLoS ONE 6, e17848 ( 10.1371/journal.pone.0017848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Niebler M, et al. 2013. Post-translational control of IL-1β via the human papillomavirus type 16 E6 oncoprotein: a novel mechanism of innate immune escape mediated by the E3-ubiquitin ligase E6-AP and p53. PLoS Pathog. 9, e1003536 ( 10.1371/journal.ppat.1003536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Karim R, et al. 2013. Human papillomavirus (HPV) upregulates the cellular deubiquitinase UCHL1 to suppress the keratinocyte's innate immune response. PLoS Pathog. 9, e1003384 ( 10.1371/journal.ppat.1003384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hong S, Mehta KP, Laimins LA. 2011. Suppression of STAT-1 expression by human papillomaviruses is necessary for differentiation-dependent genome amplification and plasmid maintenance. J. Virol. 85, 9486–9494. ( 10.1128/JVI.05007-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nees M, Geoghegan JM, Hyman T, Frank S, Miller L, Woodworth CD. 2001. Papillomavirus type 16 oncogenes downregulate expression of interferon-responsive genes and upregulate proliferation-associated and NF-κB-responsive genes in cervical keratinocytes. J. Virol. 75, 4283–4296. ( 10.1128/JVI.75.9.4283-4296.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hebner CM, Wilson R, Rader J, Bidder M, Laimins LA. 2006. Human papillomaviruses target the double-stranded RNA protein kinase pathway. J. Gen. Virol. 87, 3183–3193. ( 10.1099/vir.0.82098-0) [DOI] [PubMed] [Google Scholar]
- 120.Wu J, et al. 2007. Toll-like receptor-mediated control of HBV replication by nonparenchymal liver cells in mice. Hepatology 46, 1769–1778. ( 10.1002/hep.21897) [DOI] [PubMed] [Google Scholar]
- 121.Yu S, Chen J, Wu M, Chen H, Kato N, Yuan Z. 2010. Hepatitis B virus polymerase inhibits RIG-I- and Toll-like receptor 3-mediated beta interferon induction in human hepatocytes through interference with interferon regulatory factor 3 activation and dampening of the interaction between TBK1/IKKepsilon and DDX3. J. Gen. Virol. 91, 2080–2090. ( 10.1099/vir.0.020552-0) [DOI] [PubMed] [Google Scholar]
- 122.Wang H, Ryu WS. 2010. Hepatitis B virus polymerase blocks pattern recognition receptor signaling via interaction with DDX3: implications for immune evasion. PLoS Pathog. 6, e1000986 ( 10.1371/journal.ppat.1000986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kumar M, Jung SY, Hodgson AJ, Madden CR, Qin J, Slagle BL. 2011. Hepatitis B virus regulatory HBx protein binds to adaptor protein IPS-1 and inhibits the activation of beta interferon. J. Virol. 85, 987–995. ( 10.1128/JVI.01825-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tsuchida T, Zou J, Saitoh T, Kumar H, Abe T, Matsuura Y, Kawai T, Akira S. 2010. The ubiquitin ligase TRIM56 regulates innate immune responses to intracellular double-stranded DNA. Immunity 33, 765–776. ( 10.1016/j.immuni.2010.10.013) [DOI] [PubMed] [Google Scholar]
- 125.Griffiths DA, et al. 2013. Merkel cell polyomavirus small T antigen targets the NEMO adaptor protein to disrupt inflammatory signaling. J. Virol. 87, 13 853–13 867. ( 10.1128/JVI.02159-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li K, Foy E, Ferreon JC, Nakamura M, Ferreon AC, Ikeda M, Ray SC, Gale M Jr, Lemon SM. 2005. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc. Natl Acad. Sci. USA 102, 2992–2997. ( 10.1073/pnas.0408824102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Foy E, Li K, Wang C, Sumpter R Jr, Ikeda M, Lemon SM, Gale M Jr. 2003. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science 300, 1145–1148. ( 10.1126/science.1082604) [DOI] [PubMed] [Google Scholar]
- 128.Pichler K, Schneider G, Grassmann R. 2008. MicroRNA miR-146a and further oncogenesis-related cellular microRNAs are dysregulated in HTLV-1-transformed T lymphocytes. Retrovirology 5, 100 ( 10.1186/1742-4690-5-100). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bellon M, Lepelletier Y, Hermine O, Nicot C. 2009. Deregulation of microRNA involved in hematopoiesis and the immune response in HTLV-I adult T-cell leukemia. Blood 113, 4914–4917. ( 10.1182/blood-2008-11-189845) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Charoenthongtrakul S, Zhou Q, Shembade N, Harhaj NS, Harhaj EW. 2011. Human T cell leukemia virus type 1 Tax inhibits innate antiviral signaling via NF-κB-dependent induction of SOCS1. J. Virol. 85, 6955–6962. ( 10.1128/JVI.00007-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Oliere S, et al. 2010. HTLV-1 evades type I interferon antiviral signaling by inducing the suppressor of cytokine signaling 1 (SOCS1). PLoS Pathog. 6, e1001177 ( 10.1371/journal.ppat.1001177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhao T, Yasunaga J, Satou Y, Nakao M, Takahashi M, Fujii M, Matsuoka M. 2009. Human T-cell leukemia virus type 1 bZIP factor selectively suppresses the classical pathway of NF-κB. Blood 113, 2755–2764. ( 10.1182/blood-2008-06-161729) [DOI] [PubMed] [Google Scholar]
- 133.Feng X, Ratner L. 2008. Human T-cell leukemia virus type 1 blunts signaling by interferon α. Virology 374, 210–216. ( 10.1016/j.virol.2007.12.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang J, Yamada O, Kawagishi K, Araki H, Yamaoka S, Hattori T, Shimotohno K. 2008. Human T-cell leukemia virus type 1 Tax modulates interferon-α signal transduction through competitive usage of the coactivator CBP/p300. Virology 379, 306–313. ( 10.1016/j.virol.2008.06.035) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.