Abstract
Kaposi sarcoma herpesvirus (KSHV), taxonomical name human gammaherpesvirus 8, is a phylogenetically old human virus that co-evolved with human populations, but is now only common (seroprevalence greater than 10%) in sub-Saharan Africa, around the Mediterranean Sea, parts of South America and in a few ethnic communities. KSHV causes three human malignancies, Kaposi sarcoma, primary effusion lymphoma, and many cases of the plasmablastic form of multicentric Castleman's disease (MCD) as well as occasional cases of plasmablastic lymphoma arising from MCD; it has also been linked to rare cases of bone marrow failure and hepatitis. As it has colonized humans physiologically for many thousand years, cofactors are needed to allow it to unfold its pathogenic potential. In most cases, these include immune defects of genetic, iatrogenic or infectious origin, and inflammation appears to play an important role in disease development. Our much improved understanding of its life cycle and its role in pathogenesis should now allow us to develop new therapeutic strategies directed against key viral proteins or intracellular pathways that are crucial for virus replication or persistence. Likewise, its limited (for a herpesvirus) distribution and transmission should offer an opportunity for the development and use of a vaccine to prevent transmission.
This article is part of the themed issue ‘Human oncogenic viruses’.
Keywords: KSHV, viral infection, KSHV-related diseases, aberrant angiogenesis, DNA damage response, innate immune evasion
1. History
First described by Moritz Kaposi in 1872 as ‘idiopathic multiple pigmented sarcoma of the skin’ occurring mainly in elderly males of Mediterranean origin [1], Kaposi sarcoma (KS) has long been considered an unusual neoplasm. Its clinical manifestations can range from slowly progressive and confined to the skin of the legs to aggressive, involving several visceral organs. In Africa, particularly East and Central Africa, it was first noted long before the arrival of HIV and reported to also occur in children [2,3]. Although a very rare tumour in Western countries before 1980, its incidence suddenly increased dramatically with the arrival of HIV/AIDS when it became one of the first AIDS-defining malignancies.
Careful epidemiological studies soon showed that not all people with HIV/AIDS were equally affected by this tumour. While most common among HIV-infected men who have sex with men, it hardly ever occurred in HIV-infected men suffering from haemophilia and who had contracted HIV through contaminated factor VIII preparation in the 1980s before regular testing of blood and plasma donations for HIV had begun. KS was also rare in those who had contracted HIV through blood donations and among HIV-infected women [4]. These observations, together with a further detailed epidemiological analysis of the behavioural risk factors among people with HIV/AIDS, led to the conclusion that KS was most likely caused by a new sexually transmissible infectious agent that appeared to be transmitted independently of HIV. These predictions led to attempts in several laboratories to identify the putative transmissible cause of KS and eventually to the discovery, by Yuan Chang, Patrick Moore and colleagues, of sequence fragments belonging to a new human herpesvirus in biopsies of AIDS-associated KS [5]. Termed Kaposi sarcoma-associated herpesvirus (KSHV) by its discoverers, and classified taxonomically as human gamma herpesvirus 8 and a member of the γ2-herpesviral lineage in the subfamily of γ-herpesviruses, this new herpesvirus was soon shown to also be the cause of two B-cell malignancies, primary effusion lymphoma (PEL) [6] and the plasma cell variant of multicentric Castleman's disease (MCD) [7]. In addition, some cases of plasmablastic lymphoma [8,9], and polyclonal plasmacytic lymphoproliferations, which can arise from MCD tumours [10–12], were also be found to be positive for KSHV. Furthermore, several cases of bone marrow failure, with or without accompanying haemophagocytic syndrome [13–18] as well KSHV-associated cases of hepatitis [14,19] have been described.
During the following years, a substantial body of epidemiological and experimental evidence quickly accumulated to prove the causative role of KSHV in KS, PEL and MCD. As a result, KSHV was classified as a class I carcinogen by IARC/WHO in 2009 [20,21].
2. Epidemiological aspects and origin of KSHV
A thorough review of the wealth of published epidemiological studies on the geographical distribution and transmission of KSHV is beyond the scope of this article and we therefore only highlight the most important aspects below. For a more extensive review of the epidemiology of KSHV, the reader is referred to reference [21].
(a). Geographical distribution
Compared with other human herpesviruses, which, with the exception of HSV-2, characteristically infect the majority of healthy individuals in most geographical regions, KSHV shows a very unusual geographical distribution. It is highly prevalent (greater than 50% seroprevalence rates) only in sub-Saharan Africa, moderately common around the Mediterranean basin (seroprevalence rates of 3–20%, depending on the geographical location sampled and the serological assays employed) and in some countries in South America, but infrequent (less than 10% seroprevalence) in most other parts of the world (for a summary of published studies, see [21]). There is, however, evidence of increased KSHV prevalence rates, or the presence of particular KSHV genotypes, in certain ethnic groups, such as the Uighur population in the Xinjiang region of China [22], people of Jewish descent [23], some native American populations [24,25] and the Buryat population in southern Siberia [26].
(b). Transmission
In the general population, KSHV appears to be transmitted mainly during childhood, with saliva being considered the most important vehicle of transmission [27–31]. Several studies show that KSHV can be transmitted within families, from mother to child as well as among siblings [30,32–35].
As originally predicted by epidemiological studies looking for risk factors for KS in patients with HIV/AIDS (see above), KSHV can also be sexually transmitted [36–39] (for further references, see [21]). This seems to play a particularly important role in individuals at increased risk for contracting other sexually transmitted diseases. It is thought that saliva also represents the most important vehicle during sexual transmission, but KSHV has also been detected, inconsistently and in low copy numbers, in semen [40,41].
There is also some evidence for KSHV transmission via blood transfusion [42–44] and through injecting drug use [45,46], although sexual transmission as a confounding factor has been difficult to eliminate for the latter. Transmission in the context of the transplantation of solid organs can occur [14,19,47–50], and in some instances cells in a KS tumour of a transplant recipients have been shown to be of organ donor origin, suggesting that KSHV-infected endothelial cells had been transmitted in the transplanted organ and grown into a KS tumour in the transplant recipient [51]. However, many cases of post-transplant KS occur in recipients who were already KSHV seropositive at the time of transplantation [47,49], suggesting that reactivation of a pre-existing KSHV infection is frequently responsible for the development of post-transplant KS.
(c). Origin and evolution of KSHV
KSHV (taxonomical name: human gammaherpesvirus 8) is classified as one of nine species in the genus Rhadinovirus of the subfamily Gammaherpesvirinae in the family Herpesviridae (www.ictvonline.org). The other ICTV-classified herpesvirus species in the genus Rhadinovirus are found in Old and New World monkeys, rodents and cattle. In addition, sequence fragments or nearly complete sequences of related rhadinoviruses have been documented in most Old World primate species, including the great apes gorilla and chimpanzee; these viruses appear to fall into two lineages, provisionally termed RV1 and RV2 [52–58]. These observations suggest that KSHV, like all other herpesviruses, has co-evolved with its host species over a very long time span. This notion is further supported by the fact that minor sequence variation (less than 3%) throughout most, and more extensive sequence variation in the two genes at either end, of the of the KSHV genome seem to have evolved in different geographical regions, suggesting coevolution with human populations [24,25,56,59–64]. As in the case of other herpesviruses, there is also evidence of recombination as a driving force in the evolution of KSHV genomes. This is most dramatically illustrated by the existence of three highly distant variants of the K15 gene at the ‘right’ end of the viral genome, which are thought to have resulted from recombination events with currently unknown KSHV precursors that could possibly have belonged to rare human or no longer existing hominid populations [25,60,63].
Given this coevolution of KSHV with human populations, the fact that it is today infrequent in many geographical areas still awaits a satisfactory explanation. Suggestions include host genetic polymorphisms that contribute to the maintenance of KSHV in endemic populations. In support of this possibility, the existence of a recessive gene on chromosome 3p22 predisposing to KSHV infection in childhood in an endemic population has been predicted [65,66]. Environmental factors such as plant chemicals that promote the reactivation of KSHV in infected individuals and thereby the spread in the community have also been invoked [67]. In addition, there is now increasing evidence that KSHV transmission in childhood in Africa is associated with parasitic infections, including malaria [68–70].
3. Pathogenesis
(a). KSHV-associated tumours depend on the presence/expression of KSHV
Clinical, histopathological and experimental observations indicate that the continued presence of KSHV in tumour cells is required to maintain tumour growth. The practical implication of this conclusion would be that targeting the virus, or some of its proteins, could represent a successful approach to therapy (see below).
In all KSHV-associated tumour entities (KS, PEL, MCD and plasmablastic lymphoma) tumour cells express the latency-associated nuclear antigen (LANA), and this is used for diagnostic purposes to show the presence of KSHV-infected cells in these tumours by immunohistochemistry (figure 1). In the case of KS, the number of LANA-expressing cells can be variable, with some tumours only showing a small proportion of LANA-expressing cells while in others most tumour cells express LANA. Immunohistochemistry staining for latent and lytic KSHV proteins as well as transcriptional profiling of KS tumours indicates that some tumours show a restricted pattern of viral gene expression, which is largely limited to the genes for LANA, the viral homologues of a D-type cyclin (vCYC), the viral FLICE inhibitory protein (vFLIP), and the viral micro-RNAs (miRNAs), in addition to K1, K15 and the viral G-protein-coupled receptor homologue (table 1), while in others additional viral genes belonging to the lytic (productive) replication cycle may be expressed [216–218]. KS tumours are in most cases not monoclonal and different KS nodules in the same individual may have independent clonal origins [219–221]. In addition, KS in transplant recipients can regress following the reduction of immunosuppressive therapy and KS lesions in AIDS patients often respond to antiretroviral combination therapy. However, in a proportion of AIDS patients KS may persist, or reappear, in those with well-controlled HIV viral loads (see below).
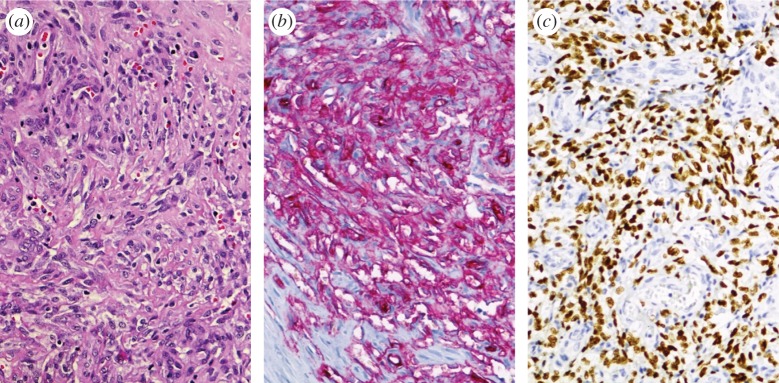
Figure 1.
Histology of a KS tumour infiltrating a lymph node (250× magnification). (a) HE staining showing the typical histological features such as elongated spindle cells, abnormal vessels with thinned epithelium and extravasated erythrocytes. (b) Immunohistochemistry staining for CD34 to indicate the endothelial origin of the spindle cells. (c) Immunohistochemistry staining for LANA, showing tumour cells with a latent KSHV infection.
Table 1.
Contribution of selected KSHV proteins or miRNAs to viral life cycle and pathogenesis.
| KSHV protein or RNA | function in viral life cycle | role in KSHV pathogenesis | references |
|---|---|---|---|
| K1 | regulation of KSHV lytic replication; activation of PI3 K/Akt and MEK/Erk pathways; modulation of B-cell antigen receptor | increases angiogenesis and invasiveness of KHSV-infected endothelial cells and may contribute to increased vascular permeability; overexpression in transgenic mice shows oncogenic/transforming properties | [71–83] |
| K3 | E3 ubiquitin ligase, downregulates MHC I | [84,85] | |
| K5 | E3 ubiquitin ligase, downregulates MHC I, ICAM-1, B7.2, BST/tetherin | [85–88] | |
| K15 | regulation of KSHV lytic replication; modulation of B-cell receptor-dependent signalling; activation of PLCγ1, MEK/Erk, JNK and NF-κB-dependent signalling | activation of angiogenesis and invasiveness in KSHV-infected endothelial cells; induction of inflammatory cytokines | [61,82,89–95] |
| kaposin A | promotion of proliferation of KSHV-infected endothelial cells; regulation of integrin-dependent cell adhesion and induction of glutamate receptor 1 expression | [96–98] | |
| kaposin B | modulation of p38 signalling cascade by interaction with MK2 to stabilize cytokines and Prox-1 mRNAs; promotion of STAT3 phosphorylation | promotion of RhoA-dependent stress fibre formation, motility and angiogenesis of endothelial cells | [99–102] |
| LANA | major viral protein for the persistence of KSHV infection, replication of latent episomes and their distribution to daughter cells during mitosis; promotion of KSHV lytic reactivation by non-canonical cytoplasmic isoforms | inhibition of p53, p73 and pRB activity; redistribution of GSK3β and regulation of c-Myc and β-catenin; extension of the lifespan of KSHV-infected cells | [103–116] |
| miRNAs | miR-K12–1 and miR-K12–3 modulate NF-κB signalling to repress lytic reactivation; miR-K12-1 targets p21; miR-K12-5 reduces Rta expression to maintain latency; miR-K12-7 reduces expression of natural killer (NK) cell ligand MICB; miR-K12-11 (orthologue of miR-155) attenuates TGF-β signalling and modulates B-cell maturation | contribution of miR-K12-11 to B-cell expansion and transformation of rat mesenchymal precursor cells; contribution of miR-K12-6 and miR-K12-11 to KSHV-induced endothelial cell differentiation by reduction of the lymphatic endothelial cell-specific transcription factor MAF | [117–129] |
| vCYC | regulation of cell cycle; involved in latency control | promotion of RB-dependent S phase entry; induction of DDR signalling and oncogene-induced senescence (OIS), abrogation of contact inhibition of latently infected cells | [130–136] |
| vFLIP | activation of NF-κB cascade; contribution to latency and persistence by inhibition of lytic reactivation; anti-apoptotic and anti-autophagy function; induction of interferon (IFN)-inducible cellular genes | contribution to KS spindle cell formation and PEL cell survival; antagonistic role on vCYC-induced OIS, induction of Notch signalling-dependent IL6 expression; involved in KSHV-induced differentiation of endothelial cells and in EndMT; involved in B-cell differentiation; induction of PRC2-complex-mediated epigenetic changes; contribution to inflammatory infiltrate in KS lesions | [110,137–154] |
| vGPCR | regulation of KSHV lytic reactivation; downmodulation of TLR4 expression | cancerogenic and angioproliferative properties in transgenic mice; contribution to KS development by promotion of VEGF and angiopoetin expression; activation of PI3 Kγ/Akt/mTOR pathway; modulation of Notch-mediated cascade; promotion of KSHV-induced endothelial cell differentiation and EndMT | [139,151,155–166] |
| vIL6 | activation of gp130 in the absence of IL6Rα; promotion of B-cell proliferation; induction of VEGF- and IL6-mediated angiogenesis; intracellular Notch signalling-dependent expression | promotion of PEL cell proliferation; contribution to VEGF- and IL6-mediated vascular permeability and to the pathogenesis of MCD and PEL; correlation of vIL6 levels with disease activity in MCD and inflammation; contribution to lymphatic endothelial cell differentiation of KSHV-infected blood vascular endothelial cells | [167–182] |
| vIRF1 | modulation of cGAS/STING pathway; inhibition of vIRF3-mediated CBP/p300 recruitment; repression of IFN-inducible genes and MHC expression | modulation of apoptotic signalling by inhibition of p53 function and interacting with Bim, Bid and GRIM19; suppression of TGF-β/Smad signalling pathway; inhibition activation-induced cell death (AICD) by modulation of CD95 pathway | [183–191] |
| vIRF2 | prevention of PKR activation and cellular IRF/STAT1-dependent IFN type I/III responses | inhibition of AICD by prevention of CD95 L expression | [192–194] |
| vIRF3 | modulation of cellular IRF-mediated IFN type I response; inhibition of NF-κB activation by interaction with IKKβ; disruption of PML nuclear bodies and decrease of MHC II expression | modulation of p53 function and inhibition of PKR-mediated apoptosis; prevention of interferon regulatory factor 3 (IRF3)-dependent apoptosis; enhancement of PEL cell survival by c-Myc activation; activation of HIF-1α and VEGF | [195–204] |
| vIRF4 | inhibition of Notch signalling by binding to CSL/CBF1; contribution to Rta-mediated lytic viral gene expression | promotion of p53 degradation by targeting Mdm2 and USP7 | [205–209] |
| vMIP I–III | vMIP-I (vCCL1): induction of angiogenesis via CCR8 activation; vMIP-II (vCCL2): inhibition of NK cells migration by modulation of CCR1-5/8 and CXCR4 activity; vMIP-I and -II promote KSHV lytic reactivation; vMIP-III (vCCL3): stimulation of angiogenesis and TH2 cells attraction via CCR4 and XCR1 activation | contribution to neutrophil and TH2 cell infiltration into KS lesions and to KSHV-promoted angiogenesis; promotion of PEL cell survival by vMIP-I and -II | [210–215] |
In a murine model of KS based on a mouse endothelial cell line transfected with a recombinant BAC genome, the loss of the viral genome or suppression of vGPCR (table 1) by small interfering RNA (siRNA), was associated with a loss in tumourigenicity in mice [155]. Together, these observations probably argue against a single ‘transformation event’ as tumour-initiating mechanism and in favour of a combination of KSHV-dependent effects on endothelial cell differentiation and proliferation with local inflammatory processes (see below for details). However, there is also evidence, summarized below, that KSHV has the potential to transform cells to independent growth. In the view of this heterogeneity of KS lesions, it appears possible that KS tumours differ with regard to their dependence on KSHV-induced effects and that in some a transforming event such as virus-induced DNA damage may have led to autonomously growing cell populations. This could potentially explain the variable response to currently used treatments (see below).
In the case of PEL, for which an experimental in vitro model is available in the form of PEL-derived lymphoma cell lines, silencing of the latent KSHV genes encoding the major latency protein LANA, vCYC and vFLIP [137,138], as well as the interferon regulatory factor homologue vIRF3 [195] inhibits the growth of PEL cell lines in tissue culture as well as (in the case of vFLIP) in a mouse xenograft model of PEL. The role of vFLIP in the survival of PEL cells may be linked to its ability to activate the NF-κB pathway, because a small molecule NF-κB inhibitor induces apoptosis in PEL cell lines [222]. Together, these observations suggest that the continued presence of the KSHV genome (mediated by LANA) and expression of the above proteins (which are part of the latency programme in B-cells) are required for the survival of PEL cells (further references in table 1). Similarly, inhibition of STAT3 signalling, which is activated by the KSHV interleukin 6 (IL6) homologue vIL6, results in apoptosis of PEL cells [223,224]. vIL6, although not considered a latent gene, is expressed in a substantial number of PEL cells [216].
Furthermore, mice double-transgenic for the KSHV latent locus (LANA, vCYC, K13, all KSHV miRNAs including the viral miR-155 orthologue, K12) and c-myc developed lymphoma at an increased rate [225].
No similar experimental evidence is available for KSHV-associated MCD, because of the lack of a suitable in vitro or in vivo experimental model. However, the clinical severity of MCD correlates with KSHV viral load in peripheral blood [167,226,227] as do the levels of cellular and viral IL6 and IL10 [167,228]. In addition, treatment attempts in individual cases with ganciclovir or valganciclovir [229], as well as a clinical trial using high-dose zidovudine and valganciclovir [230], reported the improvement of clinical symptoms accompanied by a decrease in KSHV viral load in peripheral blood.
(b). KSHV induces endothelial cell proliferation
In tissue culture, KSHV-infected primary human endothelial cells show evidence of increased proliferation after longer culture and a moderately extended lifespan, but do not outgrow their uninfected counterparts in the same culture, do not become fully transformed and lose the KSHV genomes after extended in vitro passage [89,130,231,232]. However, evidence of transformation (growth in soft agar and under low serum conditions, tumour formation in nude mice), could be seen in a telomerase-immortalized human endothelial cell line [233] and in primary rat mesenchymal precursor cells [234] infected with KSHV, as well as in a murine endothelial cell line transfected with a recombinant KSHV genome [155]. Whether KSHV-infected endothelial cells in KS tumours in vivo actually proliferate at an increased rate has however not been clearly established: in one study only 20% of KS spindle cells stained positive for the cellular proliferation marker PCNA [235], while an increased staining for TP53 and Ki-67, another proliferation marker, was reported in advanced KS lesions by another group [236]. This variability may reflect the fact that KS tumours differ with regard to their staining for LANA (figure 1) and their KSHV gene expression pattern [218].
Infection of primary human endothelial cells by KSHV activates the PI3 K/Akt/mTOR pathway. Several viral proteins, including K1, vIL6 and vGPCR have been shown to contribute to this activation [71,156,157,237]. A downstream target of this pathway, mTORC1, is activated in KSHV-infected lymphatic endothelial cells whose growth is inhibited by rapamycin, an mTOR inhibitor [238]. Interestingly, rapamycin has been shown to induce the regression of KS tumours that had developed in transplant recipients on an immunosuppressive regimen containing the cyclophilin inhibitor cyclosporin [239], suggesting that the PI3 K/Akt/mTOR pathway might represent a promising target to interfere with the proliferation of KSHV-infected endothelial cells and to inhibit the development of KS. This is illustrated schematically in figure 2. In addition to K1, vIL6 and vGPCR, several other KSHV proteins probably contribute to KSHV-induced cell proliferation. Among these are the three viral latent proteins LANA, vCYC and vFLIP, as well as the neighbouring cluster of latently expressed viral miRNAs and the viral interferon regulatory factor vIRF1 (table 1).
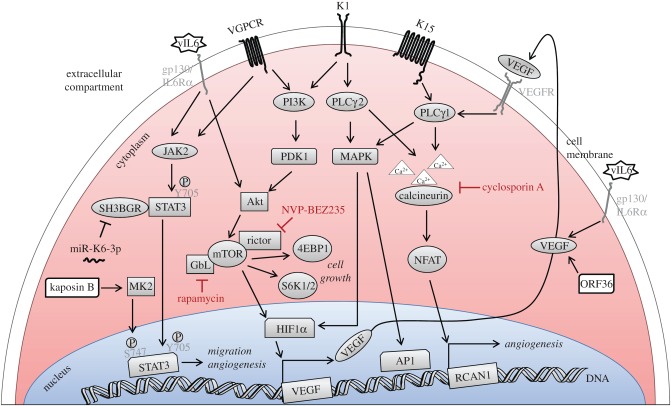
Figure 2.
Schematic of KSHV-promoted activation of STAT3, PI3 K/Akt, MAPK and PLCγ/NFAT pathways contributing to proliferation and migration of KSHV-infected endothelial cells. The transmembrane viral proteins vGPCR, K1 and K15 as well as the viral IL6 homologue vIL6, kaposin B and ORF36 activate PLCγ, Akt, MAPK and STAT3 pathways. Some of these could be promising targets to inhibit the development of Kaposi's sarcoma.
(c). KSHV alters the differentiation of infected endothelial cells
Long before the discovery of KSHV, it had been noted that the histological hallmark of advanced KS lesions, the elongated spindle cell, could display markers of both endothelial (e.g. podoplanin, VEGFR3, CD34) and mesenchymal (e.g. vimentin, PDGFRα) differentiation and its cellular origin was therefore controversial [240]. This can now be explained by the finding that KSHV can change the differentiation of blood vascular endothelial cells into lymphatic endothelial cells and vice versa [241,242] and also induce the aberrant expression of mesenchymal markers, referred to as ‘endothelial-to-mesenchymal transition’, EndMT [139,243]. Similar to the epithelial-to-mesenchymal transition frequently observed in some epithelial cancers, this differentiation towards a mesenchymal phenotype is accompanied by an increased invasiveness of KSHV-infected endothelial cells (figure 3), which is mediated by the viral proteins vFLIP and vGPCR and the cellular metalloproteinase MMT1-MMP and involves the cellular Notch pathway [139,243]. The latent viral protein vFLIP is also required for the development of the characteristic endothelial spindle cells found in advanced KS tumours [140–142]. The ability of KSHV to modulate blood vascular versus lymphatic endothelial cell differentiation involves the increased expression of Prox-1, an important regulator of lymphatic endothelial cell differentiation, as well as of inflammatory and angiogenic cytokines and their receptors [241,242]. In addition, activation of STAT3 signalling and the Akt pathway by the KSHV viral IL6 homologue vIL6 has been shown to contribute to the KSHV-induced lymphatic reprogramming of endothelial cells [168,237]. KSHV-infected lymphatic endothelial cells display a more ‘relaxed’ viral gene expression pattern compared with infected vascular endothelial cells [238]. This ‘relaxed’ latency transcriptome pattern involves the expression of several viral genes that have been linked to pathogenesis, e.g. K1, K15, vGPCR (table 1), some of which have also been shown to be expressed in KS tumours [218].
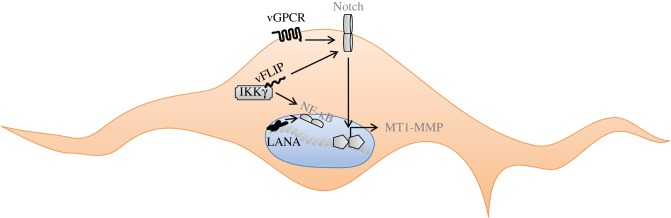
Figure 3.
KSHV-induced activation of NF-κB and Notch signalling pathways involved in atypical spindle cell differentiation and survival. The viral proteins vFLIP and vGPCR mediate the atypical differentiation of endothelial cells by triggering the Notch signalling pathway; in addition, the vFLIP–IKKγ interaction activates the pro-survival NF-κB pathway.
(d). KSHV promotes aberrant angiogenesis
In addition to promoting the proliferation of infected endothelial cells and altering their differentiation, KSHV can also confer additional angiogenic features on endothelial cells, which may explain some of the histological features of KS tumours, such as dilated abnormal vessels with thinned endothelium, leakage, erythrocyte extravasation and the presence of inflammatory cells (figure 1). In tissue culture, KSHV-infected primary endothelial cells form tubular structures on Matrigel in the absence of added growth factors, mimicking the effects of VEGF on (uninfected) primary endothelial cells. KSHV-infected primary and immortalized endothelial cells are also more invasive and migrate faster than their uninfected counterparts [89,90,139,232,244,245]. In addition, an increased secretion or expression of pro-angiogenic cytokines such as VEGF, Ang2, Il6, IL8, of several metalloproteinases, as well as of angiogenesis-promoting signalling components such as ephrin B2, emmprin, Hey1, PDGFRβ, c-kit has been noted [143,241,244–249]. KSHV-infected lymphatic endothelial cells grown in spheroids ‘sprout’ from the spheroid surface, reflecting their increased invasive potential [139].
(e). The involvement of DNA damage and repair in KSHV pathogenesis
The cellular DNA damage response/repair (DDR) machinery secures the genome integrity and the transmission of intact genomes to daughter cells during mitosis. As reviewed elsewhere in this series of articles [250], the activation of specific DNA damage sensors leads to the recruitment of downstream components of the DNA repair machinery, such as the kinases ATM, ATR, p38MAPK, MK2, Chk1 and Chk2, as well as the transcription factor p53, whose main function is to slow down the cell cycle and thereby allow the repair of the damaged DNA, or to induce apoptosis to avoid the propagation of a mutated genome. Activation of the DDR machinery in response to intracellular signalling pathways triggered by cellular oncogenes leads to cell cycle arrest and ‘oncogene-induced senescence’ (OIS) and therefore has a tumour-suppressing role. As several DNA viruses activate intracellular signalling pathways that are also triggered by cellular oncogenes, they have evolved mechanisms to antagonize the host DDR machinery to prevent these intracellular defence mechanisms from inducing OIS and thereby curtailing the replication of viral genomes (see accompanying review by M. Weitzman and colleagues [250]).
Infection of telomerase-immortalized human primary endothelial cells by KSHV initially leads to a p53-dependent growth arrest, which may be triggered by the action of the KSHV viral D-cyclin homologue vCYC; expressed on its own, vCYC can induce a DNA damage response and p53-dependent growth arrest of vCYC-expressing cells [130]. After extended passage of KSHV-infected endothelial cells in tissue culture, this growth arrest is overcome and their subsequent proliferation depends on the blocking of p53-dependent apoptosis pathways by viral proteins [130]. The proliferation of KSHV-infected endothelial cells or PEL cell lines can be inhibited by the small molecule inhibitor nutlin-3a, which antagonizes the p53 ubiquitin ligase Mdm2 and thereby restores p53 function [251]. Similarly, the small molecule inhibitor retra, which restores the function of the p53 homologue p73 in cells with a mutated p53, can induce apoptosis in p53 mutant PEL cell lines [103]. As nutlin-3a and retra can inhibit the binding of the major KSHV latency protein LANA to, respectively, p53 and p73, as well as the ability of LANA to antagonize p53- or p73-dependent transcriptional activation [103,251], it is likely that LANA is one of the viral proteins required for neutralizing the p53-dependent growth arrest [252–254], which is initially observed in KSHV-infected endothelial cells (figure 4). In PEL cells, the function of p53 or p73 can be restored, and apoptosis induced, either by disrupting the binding of p53 to LANA [103,251] or by silencing LANA by siRNA [137,138]. In addition, other KSHV proteins shown to interact with p53 and to antagonize its function, such as the viral interferon regulatory factor homologues vIRF1 and vIRF3, may be important in this context [195,196,255]. vIRF3, also termed LANA2, is expressed in latently infected B-cells, in particular PEL cells [196,256] and its silencing by siRNA in PEL cell lines induces a growth arrest [195].
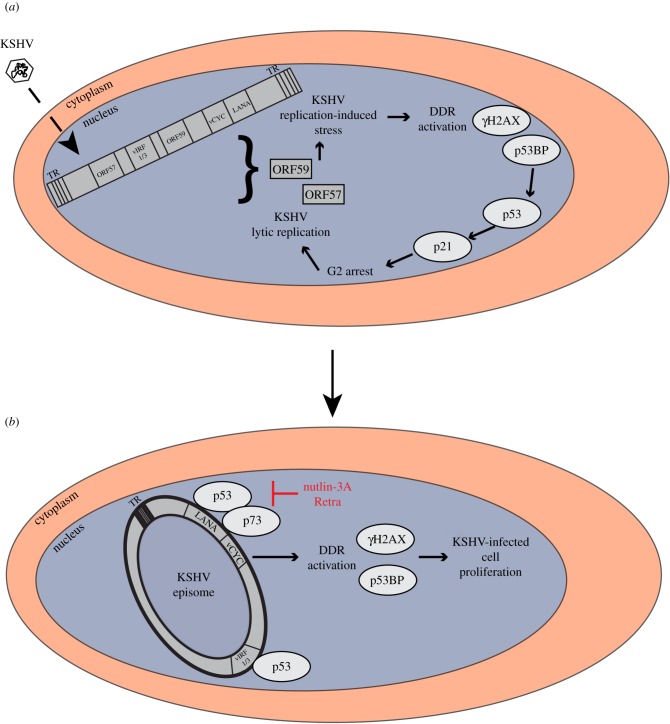
Figure 4.
The role of the DDR machinery in KSHV lytic (a) and latent (b) replication. DDR signalling induced by the sensing of viral DNA leads to a cell cycle block and ultimately favours KSHV lytic replication (a). Subsequently, the neutralization of p53 and p73 activity by viral proteins, e.g. LANA, removes the cell cycle block and allows KSHV latent replication (b). TR, terminal repeats.
During latent infection of cultured peripheral blood mononuclear or primary endothelial cells, γH2AX, the phosphorylated form of the histone variant H2AX and a key molecule in the DNA damage repair cascade activation, co-localizes with LANA and the KSHV genome [130,257,258]. In KS tumours, a proportion of KSHV-infected endothelial spindle cells show evidence of an activated DDR response (γH2AX and p53BP staining), particularly in early stage lesions [130]. Phosphorylation of H2AX may directly benefit KSHV replication or latent persistence. In analogy to the finding that the related γ-herpesviruses EBV and MHV-68 can directly phosphorylate H2AX by means of their ORF36/BGLF4 kinases and that ORF36-mediated phosphorylation is required for efficient MHV-68 replication [259], H2AX has been shown to be phosphorylated in KSHV vCYC- or LANA-transfected cells, and γH2AX to be deposited on the KSHV latent origin of replication and to be required for latent episome persistence [130,257,258]. Other DDR components to be recruited by LANA include the nuclear uracil-DNA glycosylase UNG2, which is involved in base excision repair. UNG2 depletion in PEL cells leads to a reduced number of viral genome copies and to a production of infection-deficient virus [260]. The LANA-mediated recruitment of ubiquitin-specific protease 7 (USP7), involved in the transcription-coupled nucleotide excision repair pathway, modulates KSHV latent replication [261]. The Ku70/Ku86 heterodimer phosphorylates LANA and thereby impairs LANA-dependent KSHV latent replication [262]. Together, these findings highlight the involvement of DDR proteins in the establishment and maintenance of KSHV latent infection.
A strong DDR response is also triggered by KSHV lytic replication [263]. The KSHV lytic cycle protein encoded by ORF57 promotes the nuclear export and translation of viral mRNAs at the expense of cellular mRNAs by recruiting the human transcription and export complex and thereby causes an aberrant accumulation of cellular newly transcribed mRNA in the so-called unstable R-loops which can cause double-strand breaks in cellular DNA [264]. In addition, another KSHV lytic protein, ORF59 or PF-8 (processivity factor-8), a DNA-binding protein supporting viral DNA synthesis and infectious virus production, has been reported to impair non-homologous end-joining repair activation by binding to the Ku70/Ku86 heterodimer, thereby inhibiting the subsequent recruitment of DNA-dependent protein kinases and causing double-strand break formation [265]. Activation of p53-dependent effectors, in particular transcription of the Cdk inhibitor p21, appears to be required for efficient lytic replication by arresting cells in the G2 phase of the cell cycle and thereby either providing access to the DDR machinery or preventing the condensation of chromatin on the viral genome during mitosis [266]. In line with the latter interpretation, TLK kinases, known to modulate the entry into mitosis and chromatin condensation, have been shown to suppress KSHV lytic replication [267].
These observations indicate that KSHV latent persistence as well as lytic replication may benefit from the activation of DDR pathways. Figure 4 shows a model that attempts to connect these observations. This model suggests that the DDR induced early after virus entry by the sensing of incoming viral DNA, activation of ATM [258] and perhaps the expression of vCYC [130] would lead to a p53-dependent cell cycle block that would favour lytic (productive) viral replication [266]. The subsequent neutralization of p53 and p73 by LANA and (in B-cells) vIRF3 would remove the cell cycle block and allow entry into latency, during which duplication of the viral episome happens during S phase and replicated episomes are partitioned to daughter cells during mitosis. Disruption, during latency, of the LANA-p53/p73 complex by the small molecule inhibitors nutlin-3a or retra restores a p53/p73-dependent cell cycle block and thereby lytic reactivation [266] (G Mariggiò, S Santag 2012, unpublished data). There may, however, also be an impact of the activated DDR on cellular DNA, as chromosomal abnormalities, in particular centrosome amplifications, have been noted in KSHV-infected and vCYC-overexpressing cultured endothelial cells [130,268,269].
(f). DDR response and innate immunity
As illustrated by the above examples, the activation of DDR signalling cascades by viral infections can be viewed as part of an ‘intrinsic’ intracellular defence against viral DNA. This role of the DDR is connected to ‘conventional’ mechanisms of innate immunity [270,271]. Here, the detection of ‘foreign’ DNA in the cytoplasm and/or nucleus by the DDR components Rad50, Mre11, interferon γ-inducible protein 16 (IFI16) or DNA-dependent protein kinases leads to the activation of innate immune response pathways such as the activation of interferon regulatory factor 3 (IRF3) and NF-κB, followed by the downstream expression of chemokines and inflammatory cytokines [104,272–282].
In the case of KSHV, the innate nuclear DNA sensor IFI16 recognizes latent viral episomes in a complex with the DNA damage repair sensor BRCA1 and induces the inflammasome and interferon β responses [280,281]. During lytic replication, IFI16 inhibits the expression of lytic viral genes but is itself degraded by late viral proteins to allow progression of the lytic replication cycle [282].
More recently, it has been reported that a sensor of cellular DNA, cGAS, is antagonized by several KSHV proteins, including the viral kinase encoded by ORF36, LANA, ORF57, vIRF1, ORF45, ORF52 and ORF55 [104,183,283,284]. A cytoplasmic form of LANA [285] directly interacts with cytoplasmic cGAS to restrict the cGAS-dependent activation of STING, TBK1, IRF3 and the induction of interferon gene expression in order to facilitate reactivation of KSHV from latency [104]. In an extension of this concept, we have recently found that the cellular DNA damage sensors Rad50 and Mre11, which are known to activate the STING/interferon and NF-κB pathways in response to cytoplasmic DNA [276,278], are also antagonized by cytoplasmic LANA variants to promote lytic reactivation [286].
(g). KSHV induces and benefits from inflammation
Several clinical observations, as well as histological and experimental evidence, suggest that KSHV reactivation is accompanied by inflammatory processes and that these may contribute to its pathology. KS tumours can arise in traumatized skin areas (Koebner phenomenon) [287], indicating that wound repair mechanisms support the development of this tumour. Inflammatory cellular infiltrates, consisting of monocytes, eosinophils and plasma cells, are frequently seen in KS lesions [288]. TH1 cytokines and interferons increase the KSHV viral load in peripheral blood B-cells and monocytes [289,290]. In HIV-infected patients, KS can develop within a few months after the start of combination antiretroviral therapy (cART), as part of the ‘immune reconstitution inflammatory syndrome’ (IRIS) [291–293].
In culture, KSHV-infected primary endothelial cells show an increased expression of inflammatory and angiogenic cytokines, such as IL6, IL8, IL15, IL16 and several chemokines [89,141,241], in addition to the viral IL6 homologue vIL6 and the three viral chemokines vCCL1–3. In KS lesions, a few KSHV-infected cells express vIL6 [216,217]. As mentioned earlier, vIL6 and vIL6-induced STAT3 activation are thought to play a role in the KSHV-induced lymphatic differentiation of infected vascular endothelial cells [168,237]. In addition, vIL6 is expressed in PEL and MCD, and vIL6, via vIL6-induced STAT3 activation, plays a crucial role in the continued proliferation of PEL cells (see above). In MCD, the variation of clinical symptoms in individual patients, as well as their KSHV viral load, is often mirrored by fluctuating vIL6, IL6 and IL10 levels [167,226–228].
The cellular inflammation mediator cyclooxygenase 2 (Cox-2) is expressed in KS tumours and induced by KSHV in infected cultured endothelial cells [245,294] through the action of several viral proteins, including vFLIP and K15 (table 1). The increased expression of Cox-2 in KSHV-infected endothelial cells contributes to the secretion of cellular chemokines (RANTES, MCP2, TARC, MIP1α, MDC) that modulate leucocyte trafficking and of pro-angiogenic factors (IGF1, PDGF, IL14, MCSF, GM-CSF, VEGF A, VEGF C, angiogenin, oncostatin M, TGFβ1); Cox-2 also enhances the activation of KSHV in latently infected endothelial cells as well as their invasiveness [245,294]. However, although a number of inflammatory signalling components have thus been experimentally linked to KSHV reactivation, their individual contribution to the development of KS remains to be established.
(h). Other cofactors contributing to disease development
In addition to the contribution of immune suppression and inflammation to KSHV pathogenesis (see above) it is very likely that other, as yet unidentified, cofactors play a role in disease development. For example, the much higher rates of classic KS in males, originally noted by M. Kaposi, still await a satisfactory explanation, as KSHV seroprevalence in southern Europe does not differ between men and women in most studies [21].
Genetic predisposition can increase the risk for a KSHV-infected individual to develop KS. Several cases of the extremely rare form of classic KS in children have been reported in consanguineous families and mutations in the genes for IFNR1, STIM, OX40/CD134 and WAS have been shown to be the likely underlying genetic cause [295–299]. The genetic defects all appear to compromise an efficient T-cell response to KSHV, indicating that genetically determined, as well as iatrogenic (for post-transplant KS) and infection-related (for AIDS KS) T-cell defects can predispose to KS development. In addition, a mutation altering the protein sequence of STAT4 was identified in a family in which five members, most of them women, developed classic KS at an advanced age [300]. Additional families with a high penetrance of classic KS have also been reported [301], again suggesting an underlying genetic contribution. In case–control studies, particular genetic variants of the genes encoding FCGR3A, CXCR2 and IL13 have been found to be associated with classic KS [302,303], although the functional significance of these variants has not yet been established. Altogether, these studies strongly indicate that genetically determined immunodeficiencies can predispose to the development of KS in a KSHV-infected individual at an early age and that other cellular genes may act as ‘disease modifiers’. There are thus interesting parallels to X-linked immunoproliferative disease following a primary Epein–Barr virus infection.
4. Implications for therapy and prevention
KSHV-associated diseases, KS, MCD and PEL, represent important and difficult to treat clinical problems. Although the success of cART has dramatically reduced the incidence of AIDS-KS in HIV-infected patients, this tumour can occur in patients on cART with low or undetectable HIV load and CD4 counts greater than 300 CD4 T-cells/mm3 [304]. In one report from the USA, about a third of HIV-associated KS cases still occurred in such a group of successfully treated HIV patients [305]. AIDS-KS is also seen as a first clinical symptom in patients with undiagnosed HIV infection and in advanced AIDS associated with cART failure. AIDS-KS can flare up after the initiation of cART as part of the IRIS (see above). In the transplant setting, KS is a significant cause of morbidity and mortality in countries with a higher KSHV prevalence (see above), e.g. in Italy, Turkey and Saudi Arabia. Transplant KS appears to be more common in kidney transplant recipients and has been estimated to occur in approximately 0.5–5% of solid organ transplant recipients, depending on the geographical region [18]. Its clinical severity ranges from isolated skin tumours to rapidly progressing visceral involvement in conjunction with severe haematological abnormalities such as thrombocytopenia, anaemia and bone marrow failure [18]. Lastly, endemic KS in sub-Saharan Africa, where it often occurs in children, awaits satisfactory treatment options.
Many of the treatments for KSHV-associated diseases were developed before the discovery of KSHV as the key aetiological agent. Recently, however, our increasing understanding of its role in pathogenesis has enabled the design of new treatment strategies, which target either KSHV itself, particular infected cells or key cellular pathways that have been shown to be important in KSHV biology.
Long established treatment options are based on cancer chemotherapy and involve the use of liposomal formulations of pegylated doxorubicin or daunorubicin, vincristine, bleomycin and etoposide as single agents or in combination, with taxol as a second line therapy option [306,307]. Radiotherapy or surgery is also sometimes used for isolated lesions. Reduction of immune suppression in transplant recipients will often improve post-transplant KS, and cART has made a huge impact on the incidence of AIDS-KS, with the exceptions noted above.
The only currently available ‘directly acting antivirals’ against KSHV are inhibitors of the KSHV DNA polymerase which were originally developed against other herpesviruses; those with activity against KSHV include ganciclovir, cidofovir and foscarnet [308–311]. Valganciclovir has been shown to reduce KSHV shedding in saliva of KSHV-infected individuals [312]. In KSHV-infected patients ganciclovir and foscarnet may lower the KSHV viral load in peripheral blood and occasionally improve KSHV-associated MCD or haemophagocytic syndrome [13,229,313,314]. Prophylactic treatment with ganciclovir for CMV disease is associated with a reduced incidence of KS in AIDS patients [315].
A combination of oral high-dose zidovudine and valganciclovir, which are, respectively, phosphorylated and thereby activated to become cytotoxic by the KSHV thymidine kinase (ORF21) and protein kinase (ORF36), achieved a high rate of clinical responses and a lowering of the inflammatory laboratory parameters (C-reactive protein, IL6, IL10) and KSHV viral load in HIV-infected patients with MCD [230]. While the success of this treatment regimen may be based on the activation of cytotoxic prodrugs in, and subsequent killing of, KSHV-infected B-cells, it is also conceivable that the inhibition of KSHV lytic replication by activated valganciclovir may also have contributed [230]. Rituximab, a monoclonal antibody to the B-cell surface protein CD20, has been used successfully, alone or in combination with liposomal daunorubicin, for the treatment of MCD in AIDS patients [316–319]. However, the use of rituximab alone has been reported to worsen KS, which is frequently found in patients with MCD [318]. Rituximab has also been found to be effective against PEL [320].
An example of a new treatment option based on a better understanding of KSHV pathogenesis is the observation that switching patients with post-transplant KS to an immunosuppressive regimen containing rapamycin can control their KS lesions [239,321]. Rapamycin binds to FK506-binding protein 12 (FKB12) and the rapamycin-FKB12 complex inhibits the activity of the cellular mTOR kinase [322] in the PI3 K/Akt pathway. As reviewed above, this pathway is now known to contribute to the proliferation and angiogenic properties of KSHV-infected endothelial cells (figure 2). Furthermore, the appreciation of the importance of receptor tyrosine kinases, such as PDGFR and c-kit in the life cycle of KSHV has led to clinical trials exploring the efficacy of imatinib, an inhibitor of Abl as well as of these two tyrosine kinases, against KS; these trials have reported a partial regression of KS tumours in about a third of treated patients [323,324]. Similarly, sorafenib, which targets receptors of VEGF and PDGF, has been tried [325]. Following the report that the KSHV thymidine kinase encoded by ORF21 serves as a protein tyrosine kinase [326], we recently showed that its tyrosine kinase activity is required for an efficient activation of the lytic replication cycle and identified additional US Food and Drug Administration approved tyrosine kinase inhibitors that potently inhibit KSHV lytic replication (G Beauclair, T Dubich, E Naimo, J Rückert, S Koch, A Dhingra, D Wirth, TF Schulz 2017, unpublished data).
With regard to prevention, from an epidemiological point of view KSHV seems to represent a case of a virus whose transmission should be preventable by vaccination. At least in middle-to-high-income countries, it is preferentially found in certain geographical regions or particular groups at risk for KSHV transmission (see above) and a vaccine could therefore be directed at specific populations. So far, only one successful vaccine for a herpesvirus (varicella zoster virus) is available. The success of this vaccine should stimulate efforts to develop an efficacious vaccine against KSHV. In order to be a candidate vaccine, it would have to be able to prevent primary infection, as KSHV-associated diseases, including clinical manifestations of primary infections, are too rare to be used as endpoints in a vaccine trial. Unfortunately, attempts to develop vaccines against the related γ-herpesvirus, Epstein-Barr virus have so far been limited, with only a gp350-based vaccine showing potential efficacy against the clinical manifestation of infectious mononucleosis, but not primary infection [327]. It is likely that novel approaches, such as the use of virus-like particles, will be necessary to develop an effective KSHV vaccine.
5. Conclusion and outlook
Nearly 150 years after the first description of KS and 22 years after the discovery of KSHV, the efforts of many investigators have laid the foundations for a much improved understanding of this virus and its role in the diseases that it is now known to be associated with. As in the case of its taxonomically close relative, Epstein–Barr virus, genetic, environmental or infectious cofactors are required for disease development. In the case of KSHV, inflammation and an insufficient T-cell surveillance, due to genetic predisposition, iatrogenic immune suppression or co-infections appear to be the most important cofactors. Our improved understanding of KSHV pathogenesis should now allow us to focus on key viral and cellular modulators of the viral life cycle in order to develop improved therapeutic modalities. Of particular interest could be the early phase of the lytic replication cycle, which appears to be adopted by the virus in lymphatic endothelial cells and in many KS biopsies, as well as in MCD and some PEL tumours, and during which key viral proteins with pathogenic properties are expressed. Targeting the activation of key cellular signalling cascades by one or several of these viral proteins could represent a promising approach to curtail the pathogenic effects of KSHV. In addition, the latency programme of KSHV, in particular LANA, which is essential for viral persistence, could represent a therapeutic target that would be worth pursuing. In view of the unusual distribution of KSHV and its potential for sexual transmission, there is probably a case for developing a KSHV vaccine that could be directed at groups who are at an increased risk of contracting this virus.
Acknowledgements
We gratefully acknowledge Dr Guntram Büsche for providing the images included in figure 1.
Authors' contributions
All authors contributed to writing the manuscript and gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This work was supported by an IRTG1273 PhD fellowship, funded by the Deutsche Forschungsgemeinschaft, DFG, to G.M., by DFG Collaborative Research Centre 900, project C1 to T.F.S., and by DFG grant SCHU-1668/3-1 to T.F.S.
References
- 1.Braun M. 1982. Classics in oncology. Idiopathic multiple pigmented sarcoma of the skin by Kaposi. CA Cancer J. Clin. 32, 340–347. ( 10.3322/canjclin.32.6.340) [DOI] [PubMed] [Google Scholar]
- 2.Maclean CM. 1963. Kaposi's sarcoma in Nigeria. Br. J. Cancer 17, 195–205. ( 10.1038/bjc.1963.28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slavin G, Cameron HM, Forbes C, Mitchell RM. 1970. Kaposi's sarcoma in East African children: a report of 51 cases. J. Pathol. 100, 187–199. ( 10.1002/path.1711000307) [DOI] [PubMed] [Google Scholar]
- 4.Beral V, Peterman TA, Berkelman RL, Jaffe HW. 1990. Kaposi's sarcoma among persons with AIDS: a sexually transmitted infection? Lancet 335, 123–128. ( 10.1016/0140-6736(90)90001-L) [DOI] [PubMed] [Google Scholar]
- 5.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266, 1865–1869. ( 10.1126/science.7997879) [DOI] [PubMed] [Google Scholar]
- 6.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332, 1186–1191. ( 10.1056/NEJM199505043321802) [DOI] [PubMed] [Google Scholar]
- 7.Soulier J, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86, 1276–1280. [PubMed] [Google Scholar]
- 8.Du MQ, et al. 2001. Kaposi sarcoma-associated herpesvirus infects monotypic (IgMλ) but polyclonal naive B cells in Castleman disease and associated lymphoproliferative disorders. Blood 97, 2130–2136. ( 10.1182/blood.V97.7.2130) [DOI] [PubMed] [Google Scholar]
- 9.Dupin N, Diss TL, Kellam P, Tulliez M, Du MQ, Sicard D, Weiss RA, Isaacson PG, Boshoff C. 2000. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood 95, 1406–1412. [PubMed] [Google Scholar]
- 10.Kapelushnik J, Ariad S, Benharroch D, Landau D, Moser A, Delsol G, Brousset P. 2001. Post renal transplantation human herpesvirus 8-associated lymphoproliferative disorder and Kaposi's sarcoma. Br. J. Haematol. 113, 425–428. ( 10.1046/j.1365-2141.2001.02740.x) [DOI] [PubMed] [Google Scholar]
- 11.Marcelin AG, et al. 2004. Fatal disseminated Kaposi's sarcoma following human herpesvirus 8 primary infections in liver-transplant recipients. Liver Transpl. 10, 295–300. ( 10.1002/lt.20058) [DOI] [PubMed] [Google Scholar]
- 12.Matsushima AY, et al. 1999. Posttransplantation plasmacytic proliferations related to Kaposi's sarcoma-associated herpesvirus. Am. J. Surg. Pathol. 23, 1393–1400. ( 10.1097/00000478-199911000-00010) [DOI] [PubMed] [Google Scholar]
- 13.Low P, Neipel F, Rascu A, Steininger H, Manger B, Fleckenstein B, Kalden JR, Harrer T. 1998. Suppression of HHV-8 viremia by foscarnet in an HIV-infected patient with Kaposi's sarcoma and HHV-8 associated hemophagocytic syndrome. Eur. J. Med. Res. 3, 461–464. [PubMed] [Google Scholar]
- 14.Luppi M, et al. 2000. Bone marrow failure associated with human herpesvirus 8 infection after transplantation. N. Engl. J. Med. 343, 1378–1385. ( 10.1056/nejm200011093431905) [DOI] [PubMed] [Google Scholar]
- 15.Luppi M, et al. 2002. Severe pancytopenia and hemophagocytosis after HHV-8 primary infection in a renal transplant patient successfully treated with foscarnet. Transplantation 74, 131–132. ( 10.1097/00007890-200207150-00023) [DOI] [PubMed] [Google Scholar]
- 16.Karras A, Thervet E, Legendre C. 2004. Hemophagocytic syndrome in renal transplant recipients: report of 17 cases and review of literature. Transplantation 77, 238–243. ( 10.1097/01.tp.0000107285.86939.37) [DOI] [PubMed] [Google Scholar]
- 17.Re A, et al. 2007. Fatal hemophagocytic syndrome related to active human herpesvirus-8/Kaposi sarcoma-associated herpesvirus infection in human immunodeficiency virus-negative, non-transplant patients without related malignancies. Eur. J. Haematol. 78, 361–364. ( 10.1111/j.1600-0609.2007.00828.x) [DOI] [PubMed] [Google Scholar]
- 18.Riva G, Luppi M, Barozzi P, Forghieri F, Potenza L. 2012. How I treat HHV8/KSHV-related diseases in posttransplant patients. Blood 120, 4150–4159. ( 10.1182/blood-2012-04-421412) [DOI] [PubMed] [Google Scholar]
- 19.Pietrosi G, et al. 2011. Primary and reactivated HHV8 infection and disease after liver transplantation: a prospective study. Am. J. Transplant. 11, 2715–2723. ( 10.1111/j.1600-6143.2011.03769.x) [DOI] [PubMed] [Google Scholar]
- 20.Bouvard V, et al. 2009. A review of human carcinogens—Part B: biological agents. Lancet Oncol. 10, 321–322. ( 10.1016/S1470-2045(09)70096-8) [DOI] [PubMed] [Google Scholar]
- 21.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 2012. Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr. Eval. Carcinog. Risks Hum. 100, 1–441. [PMC free article] [PubMed] [Google Scholar]
- 22.Fu B, et al. 2009. Seroprevalence of Kaposi's sarcoma-associated herpesvirus and risk factors in Xinjiang, China. J. Med. Virol. 81, 1422–1431. ( 10.1002/jmv.21550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davidovici B, et al. 2001. Seroepidemiology and molecular epidemiology of Kaposi's sarcoma-associated herpesvirus among Jewish population groups in Israel. J. Natl Cancer Inst. 93, 194–202. ( 10.1093/jnci/93.3.194) [DOI] [PubMed] [Google Scholar]
- 24.Biggar RJ, Whitby D, Marshall V, Linhares AC, Black F. 2000. Human herpesvirus 8 in Brazilian Amerindians: a hyperendemic population with a new subtype. J. Infect. Dis. 181, 1562–1568. ( 10.1086/315456) [DOI] [PubMed] [Google Scholar]
- 25.Whitby D, Marshall VA, Bagni RK, Wang CD, Gamache CJ, Guzman JR, Kron M, Ebbesen P, Biggar RJ. 2004. Genotypic characterization of Kaposi's sarcoma-associated herpesvirus in asymptomatic infected subjects from isolated populations. J. Gen. Virol. 85, 155–163. ( 10.1099/vir.0.19465-0) [DOI] [PubMed] [Google Scholar]
- 26.Cassar O, et al. 2010. Human herpesvirus 8, southern Siberia. Emerg. Infect. Dis. 16, 580–582. ( 10.3201/eid1603.091390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koelle DM, Huang ML, Chandran B, Vieira J, Piepkorn M, Corey L. 1997. Frequent detection of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) DNA in saliva of human immunodeficiency virus-infected men: clinical and immunologic correlates. J. Infect. Dis. 176, 94–102. ( 10.1086/514045) [DOI] [PubMed] [Google Scholar]
- 28.Blackbourn DJ, Lennette ET, Ambroziak J, Mourich DV, Levy JA. 1998. Human herpesvirus 8 detection in nasal secretions and saliva. J. Infect. Dis. 177, 213–216. ( 10.1086/517356) [DOI] [PubMed] [Google Scholar]
- 29.Cattani P, Capuano M, Cerimele F, La Parola IL, Santangelo R, Masini C, Cerimele D, Fadda G. 1999. Human herpesvirus 8 seroprevalence and evaluation of nonsexual transmission routes by detection of DNA in clinical specimens from human immunodeficiency virus-seronegative patients from central and southern Italy, with and without Kaposi's sarcoma. J. Clin. Microbiol. 37, 1150–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dedicoat M, et al. 2004. Mother-to-child transmission of human herpesvirus-8 in South Africa. J. Infect. Dis. 190, 1068–1075. ( 10.1086/423326) [DOI] [PubMed] [Google Scholar]
- 31.Mancuso R, et al. 2011. Intrafamiliar transmission of Kaposi's sarcoma-associated herpesvirus and seronegative infection in family members of classic Kaposi's sarcoma patients. J. Gen. Virol. 92, 744–751. ( 10.1099/vir.0.027847-0) [DOI] [PubMed] [Google Scholar]
- 32.Plancoulaine S, Abel L, van Beveren M, Tregouet DA, Joubert M, Tortevoye P, de The G, Gessain A. 2000. Human herpesvirus 8 transmission from mother to child and between siblings in an endemic population. Lancet 356, 1062–1065. ( 10.1016/s0140-6736(00)02729-x) [DOI] [PubMed] [Google Scholar]
- 33.Brayfield BP, Kankasa C, West JT, Muyanga J, Bhat G, Klaskala W, Mitchell CD, Wood C. 2004. Distribution of Kaposi sarcoma-associated herpesvirus/human herpesvirus 8 in maternal saliva and breast milk in Zambia: implications for transmission. J. Infect. Dis. 189, 2260–2270. ( 10.1086/421119) [DOI] [PubMed] [Google Scholar]
- 34.Mbulaiteye S, et al. 2006. Molecular evidence for mother-to-child transmission of Kaposi sarcoma-associated herpesvirus in Uganda and K1 gene evolution within the host. J. Infect. Dis. 193, 1250–1257. ( 10.1086/503052) [DOI] [PubMed] [Google Scholar]
- 35.Borges JD, et al. 2012. Transmission of human herpesvirus type 8 infection within families in American indigenous populations from the Brazilian Amazon. J. Infect. Dis. 205, 1869–1876. ( 10.1093/infdis/jis278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kedes DH, Operskalski E, Busch M, Kohn R, Flood J, Ganem D. 1996. The seroepidemiology of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat. Med. 2, 918–924. ( 10.1038/nm0896-918) [DOI] [PubMed] [Google Scholar]
- 37.Simpson GR, et al. 1996. Prevalence of Kaposi's sarcoma associated herpesvirus infection measured by antibodies to recombinant capsid protein and latent immunofluorescence antigen. Lancet 348, 1133–1138. ( 10.1016/s0140-6736(96)07560-5) [DOI] [PubMed] [Google Scholar]
- 38.Martin JN, Ganem DE, Osmond DH, Page-Shafer KA, Macrae D, Kedes DH. 1998. Sexual transmission and the natural history of human herpesvirus 8 infection. N. Engl. J. Med. 338, 948–954. ( 10.1056/nejm199804023381403) [DOI] [PubMed] [Google Scholar]
- 39.Dukers NH, Renwick N, Prins M, Geskus RB, Schulz TF, Weverling GJ, Coutinho RA, Goudsmit J. 2000. Risk factors for human herpesvirus 8 seropositivity and seroconversion in a cohort of homosexual men. Am. J. Epidemiol. 151, 213–224. ( 10.1093/oxfordjournals.aje.a010195) [DOI] [PubMed] [Google Scholar]
- 40.Howard MR, et al. 1997. Detection of human herpesvirus 8 DNA in semen from HIV-infected individuals but not healthy semen donors. AIDS 11, F15–F19. ( 10.1097/00002030-199702000-00001) [DOI] [PubMed] [Google Scholar]
- 41.Diamond C, et al. 1997. Absence of detectable human herpesvirus 8 in the semen of human immunodeficiency virus-infected men without Kaposi's sarcoma. J. Infect. Dis. 176, 775–777. ( 10.1086/517299) [DOI] [PubMed] [Google Scholar]
- 42.Blackbourn DJ, Ambroziak J, Lennette E, Adams M, Ramachandran B, Levy JA. 1997. Infectious human herpesvirus 8 in a healthy North American blood donor. Lancet 349, 609–611. ( 10.1016/s0140-6736(96)10004-0) [DOI] [PubMed] [Google Scholar]
- 43.Dollard SC, Nelson KE, Ness PM, Stambolis V, Kuehnert MJ, Pellett PE, Cannon MJ. 2005. Possible transmission of human herpesvirus-8 by blood transfusion in a historical United States cohort. Transfusion 45, 500–503. ( 10.1111/j.0041-1132.2005.04334.x) [DOI] [PubMed] [Google Scholar]
- 44.Hladik W, et al. 2006. Transmission of human herpesvirus 8 by blood transfusion. N. Engl. J. Med. 355, 1331–1338. ( 10.1056/NEJMoa055009) [DOI] [PubMed] [Google Scholar]
- 45.Cannon MJ, Dollard SC, Smith DK, Klein RS, Schuman P, Rich JD, Vlahov D, Pellett PE. 2001. Blood-borne and sexual transmission of human herpesvirus 8 in women with or at risk for human immunodeficiency virus infection. N. Engl. J. Med. 344, 637–643. ( 10.1056/nejm200103013440904) [DOI] [PubMed] [Google Scholar]
- 46.Atkinson J, Edlin BR, Engels EA, Kral AH, Seal K, Gamache CJ, Whitby D, O'Brien TR. 2003. Seroprevalence of human herpesvirus 8 among injection drug users in San Francisco. J. Infect. Dis. 187, 974–981. ( 10.1086/368332) [DOI] [PubMed] [Google Scholar]
- 47.Parravicini C, et al. 1997. Risk of Kaposi's sarcoma-associated herpes virus transmission from donor allografts among Italian posttransplant Kaposi's sarcoma patients. Blood 90, 2826–2829. [PubMed] [Google Scholar]
- 48.Regamey N, Tamm M, Wernli M, Witschi A, Thiel G, Cathomas G, Erb P. 1998. Transmission of human herpesvirus 8 infection from renal-transplant donors to recipients. N. Engl. J. Med. 339, 1358–1363. ( 10.1056/nejm199811053391903) [DOI] [PubMed] [Google Scholar]
- 49.Frances C, et al. 2009. The impact of preexisting or acquired Kaposi sarcoma herpesvirus infection in kidney transplant recipients on morbidity and survival. Am. J. Transplant. 9, 2580–2586. ( 10.1111/j.1600-6143.2009.02816.x) [DOI] [PubMed] [Google Scholar]
- 50.Lebbe C, et al. 2013. Human herpesvirus 8 (HHV8) transmission and related morbidity in organ recipients. Am. J. Transplant. 13, 207–213. ( 10.1111/j.1600-6143.2012.04290.x) [DOI] [PubMed] [Google Scholar]
- 51.Barozzi P, et al. 2003. Post-transplant Kaposi sarcoma originates from the seeding of donor-derived progenitors. Nat. Med. 9, 554–561. ( 10.1038/nm862) [DOI] [PubMed] [Google Scholar]
- 52.Rose TM, Strand KB, Schultz ER, Schaefer G, Rankin GW Jr, Thouless ME, Tsai CC, Bosch ML. 1997. Identification of two homologs of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in retroperitoneal fibromatosis of different macaque species. J. Virol. 71, 4138–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desrosiers RC, Sasseville VG, Czajak SC, Zhang X, Mansfield KG, Kaur A, Johnson RP, Lackner AA, Jung JU. 1997. A herpesvirus of rhesus monkeys related to the human Kaposi's sarcoma-associated herpesvirus. J. Virol. 71, 9764–9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greensill J, Sheldon JA, Renwick NM, Beer BE, Norley S, Goudsmit J, Schulz TF. 2000. Two distinct γ-2 herpesviruses in African green monkeys: a second γ-2 herpesvirus lineage among Old World primates? J. Virol. 74, 1572–1577. ( 10.1128/JVI.74.3.1572-1577.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greensill J, Sheldon JA, Murthy KK, Bessonette JS, Beer BE, Schulz TF. 2000. A chimpanzee rhadinovirus sequence related to Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8: increased detection after HIV-1 infection in the absence of disease. AIDS 14, F129–F135. ( 10.1097/00002030-200012010-00001) [DOI] [PubMed] [Google Scholar]
- 56.Lacoste V, Mauclere P, Dubreuil G, Lewis J, Georges-Courbot MC, Rigoulet J, Petit T, Gessain A. 2000. Simian homologues of human gamma-2 and betaherpesviruses in mandrill and drill monkeys. J. Virol. 74, 11 993–11 999. ( 10.1128/JVI.74.24.11993-11999.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whitby D, et al. 2003. Novel Kaposi's sarcoma-associated herpesvirus homolog in baboons. J. Virol. 77, 8159–8165. ( 10.1128/JVI.77.14.8159-8165.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duprez R, Boulanger E, Roman Y, Gessain A. 2004. Novel γ-2-herpesvirus of the Rhadinovirus 2 lineage in gibbons. Emerg. Infect. Dis. 10, 899–902. ( 10.3201/eid1005.030964) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zong JC, Metroka C, Reitz MS, Nicholas J, Hayward GS. 1997. Strain variability among Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genomes: evidence that a large cohort of United States AIDS patients may have been infected by a single common isolate. J. Virol. 71, 2505–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zong JC, et al. 1999. High-level variability in the ORF-K1 membrane protein gene at the left end of the Kaposi's sarcoma-associated herpesvirus genome defines four major virus subtypes and multiple variants or clades in different human populations. J. Virol. 73, 4156–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poole LJ, Zong JC, Ciufo DM, Alcendor DJ, Cannon JS, Ambinder R, Orenstein JM, Reitz MS, Hayward GS. 1999. Comparison of genetic variability at multiple loci across the genomes of the major subtypes of Kaposi's sarcoma-associated herpesvirus reveals evidence for recombination and for two distinct types of open reading frame K15 alleles at the right-hand end. J. Virol. 73, 6646–6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cook PM, et al. 1999. Variability and evolution of Kaposi's sarcoma-associated herpesvirus in Europe and Africa. AIDS 13, 1165–1176. ( 10.1097/00002030-199907090-00004) [DOI] [PubMed] [Google Scholar]
- 63.Kakoola DN, Sheldon J, Byabazaire N, Bowden RJ, Katongole-Mbidde E, Schulz TF, Davison AJ. 2001. Recombination in human herpesvirus-8 strains from Uganda and evolution of the K15 gene. J. Gen. Virol. 82, 2393–2404. ( 10.1099/0022-1317-82-10-2393) [DOI] [PubMed] [Google Scholar]
- 64.Kazanji M, et al. 2005. Serological and molecular evidence that human herpesvirus 8 is endemic among Amerindians in French Guiana. J. Infect. Dis. 192, 1525–1529. ( 10.1086/491744) [DOI] [PubMed] [Google Scholar]
- 65.Plancoulaine S, Gessain A, van Beveren M, Tortevoye P, Abel L. 2003. Evidence for a recessive major gene predisposing to human herpesvirus 8 (HHV-8) infection in a population in which HHV-8 is endemic. J. Infect. Dis. 187, 1944–1950. ( 10.1086/375345) [DOI] [PubMed] [Google Scholar]
- 66.Pedergnana V, Gessain A, Tortevoye P, Byun M, Bacq-Daian D, Boland A, Casanova JL, Abel L, Plancoulaine S. 2012. A major locus on chromosome 3p22 conferring predisposition to human herpesvirus 8 infection. Eur. J. Hum. Genet. 20, 690–695. ( 10.1038/ejhg.2011.260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whitby D, et al. 2007. Reactivation of Kaposi's sarcoma-associated herpesvirus by natural products from Kaposi's sarcoma endemic regions. Int. J. Cancer 120, 321–328. ( 10.1002/ijc.22205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nalwoga A, Cose S, Wakeham K, Miley W, Ndibazza J, Drakeley C, Elliott A, Whitby D, Newton R. 2015. Association between malaria exposure and Kaposi's sarcoma-associated herpes virus seropositivity in Uganda. Trop. Med. Int. Health 20, 665–672. ( 10.1111/tmi.12464) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wakeham K, et al. 2011. Parasite infection is associated with Kaposi's sarcoma associated herpesvirus (KSHV) in Ugandan women. Infect. Agents Cancer 6, 15 ( 10.1186/1750-9378-6-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wakeham K, et al. 2013. Risk factors for seropositivity to Kaposi sarcoma-associated herpesvirus among children in Uganda. J. Acquir. Immune Defic. Syndr. 63, 228–233. ( 10.1097/QAI.0b013e31828a7056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tomlinson CC, Damania B. 2004. The K1 protein of Kaposi's sarcoma-associated herpesvirus activates the Akt signaling pathway. J. Virol. 78, 1918–1927. ( 10.1128/JVI.78.4.1918-1927.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee H, Guo J, Li M, Choi JK, DeMaria M, Rosenzweig M, Jung JU. 1998. Identification of an immunoreceptor tyrosine-based activation motif of K1 transforming protein of Kaposi's sarcoma-associated herpesvirus. Mol. Cell. Biol. 18, 5219–5228. ( 10.1128/MCB.18.9.5219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lagunoff M, Majeti R, Weiss A, Ganem D. 1999. Deregulated signal transduction by the K1 gene product of Kaposi's sarcoma-associated herpesvirus. Proc. Natl Acad. Sci. USA 96, 5704–5709. ( 10.1073/pnas.96.10.5704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee BS, Alvarez X, Ishido S, Lackner AA, Jung JU. 2000. Inhibition of intracellular transport of B cell antigen receptor complexes by Kaposi's sarcoma-associated herpesvirus K1. J. Exp. Med. 192, 11–21. ( 10.1084/jem.192.1.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lagunoff M, Lukac DM, Ganem D. 2001. Immunoreceptor tyrosine-based activation motif-dependent signaling by Kaposi's sarcoma-associated herpesvirus K1 protein: effects on lytic viral replication. J. Virol. 75, 5891–5898. ( 10.1128/JVI.75.13.5891-5898.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prakash O, Tang ZY, Peng X, Coleman R, Gill J, Farr G, Samaniego F. 2002. Tumorigenesis and aberrant signaling in transgenic mice expressing the human herpesvirus-8 K1 gene. J. Natl Cancer Inst. 94, 926–935. ( 10.1093/jnci/94.12.926) [DOI] [PubMed] [Google Scholar]
- 77.Lee BS, Paulose-Murphy M, Chung YH, Connlole M, Zeichner S, Jung JU. 2002. Suppression of tetradecanoyl phorbol acetate-induced lytic reactivation of Kaposi's sarcoma-associated herpesvirus by K1 signal transduction. J. Virol. 76, 12 185–12 199. ( 10.1128/JVI.76.23.12185-12199.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang L, Wakisaka N, Tomlinson CC, DeWire SM, Krall S, Pagano JS, Damania B. 2004. The Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) K1 protein induces expression of angiogenic and invasion factors. Cancer Res. 64, 2774–2781. ( 10.1158/0008-5472.CAN-03-3653) [DOI] [PubMed] [Google Scholar]
- 79.Douglas J, Dutia B, Rhind S, Stewart JP, Talbot SJ. 2004. Expression in a recombinant murid herpesvirus 4 reveals the in vivo transforming potential of the K1 open reading frame of Kaposi's sarcoma-associated herpesvirus. J. Virol. 78, 8878–8884. ( 10.1128/JVI.78.16.8878-8884.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang L, Dittmer DP, Tomlinson CC, Fakhari FD, Damania B. 2006. Immortalization of primary endothelial cells by the K1 protein of Kaposi's sarcoma-associated herpesvirus. Cancer Res. 66, 3658–3666. ( 10.1158/0008-5472.CAN-05-3680) [DOI] [PubMed] [Google Scholar]
- 81.Guilluy C, Zhang Z, Bhende PM, Sharek L, Wang L, Burridge K, Damania B. 2011. Latent KSHV infection increases the vascular permeability of human endothelial cells. Blood 118, 5344–5354. ( 10.1182/blood-2011-03-341552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steinbruck L, Gustems M, Medele S, Schulz TF, Lutter D, Hammerschmidt W. 2015. K1 and K15 of Kaposi's sarcoma-associated herpesvirus are partial functional homologues of latent membrane protein 2A of Epstein-Barr virus. J. Virol. 89, 7248–7261. ( 10.1128/jvi.00839-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Z, Chen W, Sanders MK, Brulois KF, Dittmer DP, Damania B. 2016. The K1 protein of Kaposi's sarcoma-associated herpesvirus augments viral lytic replication. J. Virol. 90, 7657–7666. ( 10.1128/jvi.03102-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coscoy L, Ganem D. 2000. Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc. Natl Acad. Sci. USA 97, 8051–8056. ( 10.1073/pnas.140129797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ishido S, Wang C, Lee BS, Cohen GB, Jung JU. 2000. Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins. J. Virol. 74, 5300–5309. ( 10.1128/JVI.74.11.5300-5309.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ishido S, Choi JK, Lee BS, Wang C, DeMaria M, Johnson RP, Cohen GB, Jung JU. 2000. Inhibition of natural killer cell-mediated cytotoxicity by Kaposi's sarcoma-associated herpesvirus K5 protein. Immunity 13, 365–374. ( 10.1016/S1074-7613(00)00036-4) [DOI] [PubMed] [Google Scholar]
- 87.Coscoy L, Ganem D. 2001. A viral protein that selectively downregulates ICAM-1 and B7-2 and modulates T cell costimulation. J. Clin. Invest. 107, 1599–1606. ( 10.1172/JCI12432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mansouri M, Viswanathan K, Douglas JL, Hines J, Gustin J, Moses AV, Fruh K. 2009. Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of Kaposi's sarcoma-associated herpesvirus. J. Virol. 83, 9672–9681. ( 10.1128/JVI.00597-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bala K, et al. 2012. Kaposi's sarcoma herpesvirus K15 protein contributes to virus-induced angiogenesis by recruiting PLCγ1 and activating NFAT1-dependent RCAN1 expression. PLoS Pathog. 8, e1002927 ( 10.1371/journal.ppat.1002927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gramolelli S, Weidner-Glunde M, Abere B, Viejo-Borbolla A, Bala K, Ruckert J, Kremmer E, Schulz TF. 2015. Inhibiting the recruitment of PLCγ1 to Kaposi's sarcoma herpesvirus K15 protein reduces the invasiveness and angiogenesis of infected endothelial cells. PLoS Pathog. 11, e1005105 ( 10.1371/journal.ppat.1005105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Glenn M, Rainbow L, Aurade F, Davison A, Schulz TF. 1999. Identification of a spliced gene from Kaposi's sarcoma-associated herpesvirus encoding a protein with similarities to latent membrane proteins 1 and 2A of Epstein-Barr virus. J. Virol. 73, 6953–6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brinkmann MM, Glenn M, Rainbow L, Kieser A, Henke-Gendo C, Schulz TF. 2003. Activation of mitogen-activated protein kinase and NF-κB pathways by a Kaposi's sarcoma-associated herpesvirus K15 membrane protein. J. Virol. 77, 9346–9358. ( 10.1128/JVI.77.17.9346-9358.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brinkmann MM, Pietrek M, Dittrich-Breiholz O, Kracht M, Schulz TF. 2007. Modulation of host gene expression by the K15 protein of Kaposi's sarcoma-associated herpesvirus. J. Virol. 81, 42–58. ( 10.1128/JVI.00648-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pietrek M, et al. 2010. Role of the Kaposi's sarcoma-associated herpesvirus K15 SH3 binding site in inflammatory signaling and B-cell activation. J. Virol. 84, 8231–8240. ( 10.1128/JVI.01696-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Havemeier A, Gramolelli S, Pietrek M, Jochmann R, Sturzl M, Schulz TF. 2014. Activation of NF-κB by the Kaposi's sarcoma-associated herpesvirus K15 protein involves recruitment of the NF-κB-inducing kinase, IkB kinases, and phosphorylation of p65. J. Virol. 88, 13 161–13 172. ( 10.1128/JVI.01766-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muralidhar S, Pumfery AM, Hassani M, Sadaie MR, Kishishita M, Brady JN, Doniger J, Medveczky P, Rosenthal LJ. 1998. Identification of kaposin (open reading frame K12) as a human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus) transforming gene. J. Virol. 72, 4980–4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kliche S, Nagel W, Kremmer E, Atzler C, Ege A, Knorr T, Koszinowski U, Kolanus W, Haas J. 2001. Signaling by human herpesvirus 8 kaposin A through direct membrane recruitment of cytohesin-1. Mol. Cell 7, 833–843. ( 10.1016/S1097-2765(01)00227-1) [DOI] [PubMed] [Google Scholar]
- 98.Veettil MV, Bandyopadhyay C, Dutta D, Chandran B. 2014. Interaction of KSHV with host cell surface receptors and cell entry. Viruses 6, 4024–4046. ( 10.3390/v6104024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McCormick C, Ganem D. 2005. The kaposin B protein of KSHV activates the p38/MK2 pathway and stabilizes cytokine mRNAs. Science 307, 739–741. ( 10.1126/science.1105779) [DOI] [PubMed] [Google Scholar]
- 100.Yoo J, et al. 2010. Kaposin-B enhances the PROX1 mRNA stability during lymphatic reprogramming of vascular endothelial cells by Kaposi's sarcoma herpes virus. PLoS Pathog. 6, e1001046 ( 10.1371/journal.ppat.1001046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.King CA. 2013. Kaposi's sarcoma-associated herpesvirus kaposin B induces unique monophosphorylation of STAT3 at serine 727 and MK2-mediated inactivation of the STAT3 transcriptional repressor TRIM28. J. Virol. 87, 8779–8791. ( 10.1128/JVI.02976-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Corcoran JA, Johnston BP, McCormick C. 2015. Viral activation of MK2-hsp27-p115RhoGEF-RhoA signaling axis causes cytoskeletal rearrangements, P-body disruption and ARE-mRNA stabilization. PLoS Pathog. 11, e1004597 ( 10.1371/journal.ppat.1004597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Santag S, Jager W, Karsten CB, Kati S, Pietrek M, Steinemann D, Sarek G, Ojala PM, Schulz TF. 2013. Recruitment of the tumour suppressor protein p73 by Kaposi's sarcoma herpesvirus latent nuclear antigen contributes to the survival of primary effusion lymphoma cells. Oncogene 32, 3676–3685. ( 10.1038/onc.2012.385) [DOI] [PubMed] [Google Scholar]
- 104.Zhang G, Chan B, Samarina N, Abere B, Weidner-Glunde M, Buch A, Pich A, Brinkmann MM, Schulz TF. 2016. Cytoplasmic isoforms of Kaposi sarcoma herpesvirus LANA recruit and antagonize the innate immune DNA sensor cGAS. Proc. Natl Acad. Sci. USA 113, E1034–E1043. ( 10.1073/pnas.1516812113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rainbow L, Platt GM, Simpson GR, Sarid R, Gao SJ, Stoiber H, Herrington CS, Moore PS, Schulz TF. 1997. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J. Virol. 71, 5915–5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kedes DH, Lagunoff M, Renne R, Ganem D. 1997. Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi's sarcoma-associated herpesvirus. J. Clin. Invest. 100, 2606–2610. ( 10.1172/JCI119804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Radkov SA, Kellam P, Boshoff C. 2000. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat. Med. 6, 1121–1127. ( 10.1038/80459) [DOI] [PubMed] [Google Scholar]
- 108.Fujimuro M, Wu FY, apRhys C, Kajumbula H, Young DB, Hayward GS, Hayward SD. 2003. A novel viral mechanism for dysregulation of β-catenin in Kaposi's sarcoma-associated herpesvirus latency. Nat. Med. 9, 300–306. ( 10.1038/nm829) [DOI] [PubMed] [Google Scholar]
- 109.Watanabe T, Sugaya M, Atkins AM, Aquilino EA, Yang A, Borris DL, Brady J, Blauvelt A. 2003. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen prolongs the life span of primary human umbilical vein endothelial cells. J. Virol. 77, 6188–6196. ( 10.1128/JVI.77.11.6188-6196.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ye FC, Zhou FC, Yoo SM, Xie JP, Browning PJ, Gao SJ. 2004. Disruption of Kaposi's sarcoma-associated herpesvirus latent nuclear antigen leads to abortive episome persistence. J. Virol. 78, 11 121–11 129. ( 10.1128/JVI.78.20.11121-11129.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu J, Martin H, Shamay M, Woodard C, Tang QQ, Hayward SD. 2007. Kaposi's sarcoma-associated herpesvirus LANA protein downregulates nuclear glycogen synthase kinase 3 activity and consequently blocks differentiation. J. Virol. 81, 4722–4731. ( 10.1128/jvi.02548-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bubman D, Guasparri I, Cesarman E. 2007. Deregulation of c-Myc in primary effusion lymphoma by Kaposi's sarcoma herpesvirus latency-associated nuclear antigen. Oncogene 26, 4979–4986. ( 10.1038/sj.onc.1210299) [DOI] [PubMed] [Google Scholar]
- 113.Alkharsah KR, Schulz TF. 2012. A role for the internal repeat of the Kaposi's sarcoma-associated herpesvirus latent nuclear antigen in the persistence of an episomal viral genome. J. Virol. 86, 1883–1887. ( 10.1128/JVI.06029-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Domsic JF, Chen HS, Lu F, Marmorstein R, Lieberman PM. 2013. Molecular basis for oligomeric-DNA binding and episome maintenance by KSHV LANA. PLoS Pathog. 9, e1003672 ( 10.1371/journal.ppat.1003672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hellert J. et al 2013. A structural basis for BRD2/4-mediated host chromatin interaction and oligomer assembly of Kaposi sarcoma-associated herpesvirus and murine gammaherpesvirus LANA proteins. PLoS Pathog. 9, e1003640 ( 10.1371/journal.ppat.1003640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hellert J, Weidner-Glunde M, Krausze J, Lunsdorf H, Ritter C, Schulz TF, Luhrs T. 2015. The 3D structure of Kaposi sarcoma herpesvirus LANA C-terminal domain bound to DNA. Proc. Natl Acad. Sci. USA 112, 6694–6699. ( 10.1073/pnas.1421804112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Skalsky RL, Samols MA, Plaisance KB, Boss IW, Riva A, Lopez MC, Baker HV, Renne R. 2007. Kaposi's sarcoma-associated herpesvirus encodes an ortholog of miR-155. J. Virol. 81, 12 836–12 845. ( 10.1128/JVI.01804-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gottwein E, et al. 2007. A viral microRNA functions as an orthologue of cellular miR-155. Nature 450, 1096–1099. ( 10.1038/nature05992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nachmani D, Stern-Ginossar N, Sarid R, Mandelboim O. 2009. Diverse herpesvirus microRNAs target the stress-induced immune ligand MICB to escape recognition by natural killer cells. Cell Host Microbe 5, 376–385. ( 10.1016/j.chom.2009.03.003) [DOI] [PubMed] [Google Scholar]
- 120.Bellare P, Ganem D. 2009. Regulation of KSHV lytic switch protein expression by a virus-encoded microRNA: an evolutionary adaptation that fine-tunes lytic reactivation. Cell Host Microbe 6, 570–575. ( 10.1016/j.chom.2009.11.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lu CC, Li Z, Chu CY, Feng J, Feng J, Sun R, Rana TM. 2010. MicroRNAs encoded by Kaposi's sarcoma-associated herpesvirus regulate viral life cycle. EMBO Rep. 11, 784–790. ( 10.1038/embor.2010.132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lu F, Stedman W, Yousef M, Renne R, Lieberman PM. 2010. Epigenetic regulation of Kaposi's sarcoma-associated herpesvirus latency by virus-encoded microRNAs that target Rta and the cellular Rbl2-DNMT pathway. J. Virol. 84, 2697–2706. ( 10.1128/JVI.01997-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hansen A, et al. 2010. KSHV-encoded miRNAs target MAF to induce endothelial cell reprogramming. Genes Dev. 24, 195–205. ( 10.1101/gad.553410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gottwein E, Cullen BR. 2010. A human herpesvirus microRNA inhibits p21 expression and attenuates p21-mediated cell cycle arrest. J. Virol. 84, 5229–5237. ( 10.1128/JVI.00202-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lei X, Bai Z, Ye F, Xie J, Kim CG, Huang Y, Gao SJ. 2010. Regulation of NF-κB inhibitor IkBα and viral replication by a KSHV microRNA. Nat. Cell Biol. 12, 193–199. ( 10.1038/ncb2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Boss IW, Nadeau PE, Abbott JR, Yang Y, Mergia A, Renne R. 2011. A Kaposi's sarcoma-associated herpesvirus-encoded ortholog of microRNA miR-155 induces human splenic B-cell expansion in NOD/LtSz-scid IL2Rγnull mice. J. Virol. 85, 9877–9886. ( 10.1128/JVI.05558-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu Y, Sun R, Lin X, Liang D, Deng Q, Lan K. 2012. Kaposi's sarcoma-associated herpesvirus-encoded microRNA miR-K12-11 attenuates transforming growth factor beta signaling through suppression of SMAD5. J. Virol. 86, 1372–1381. ( 10.1128/JVI.06245-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dahlke C, Maul K, Christalla T, Walz N, Schult P, Stocking C, Grundhoff A. 2012. A microRNA encoded by Kaposi sarcoma-associated herpesvirus promotes B-cell expansion in vivo. PLoS ONE 7, e49435 ( 10.1371/journal.pone.0049435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moody R, Zhu Y, Huang Y, Cui X, Jones T, Bedolla R, Lei X, Bai Z, Gao SJ. 2013. KSHV microRNAs mediate cellular transformation and tumorigenesis by redundantly targeting cell growth and survival pathways. PLoS Pathog. 9, e1003857 ( 10.1371/journal.ppat.1003857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Koopal S, et al. 2007. Viral oncogene-induced DNA damage response is activated in Kaposi sarcoma tumorigenesis. PLoS Pathog. 3, 1348–1360. ( 10.1371/journal.ppat.0030140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chang Y, Moore PS, Talbot SJ, Boshoff CH, Zarkowska T, Godden K, Paterson H, Weiss RA, Mittnacht S. 1996. Cyclin encoded by KS herpesvirus. Nature 382, 410 ( 10.1038/382410a0) [DOI] [PubMed] [Google Scholar]
- 132.Godden-Kent D, Talbot SJ, Boshoff C, Chang Y, Moore P, Weiss RA, Mittnacht S. 1997. The cyclin encoded by Kaposi's sarcoma-associated herpesvirus stimulates Cdk6 to phosphorylate the retinoblastoma protein and histone H1. J. Virol. 71, 4193–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Swanton C, Mann DJ, Fleckenstein B, Neipel F, Peters G, Jones N. 1997. Herpes viral cyclin/Cdk6 complexes evade inhibition by CDK inhibitor proteins. Nature 390, 184–187. ( 10.1038/36606) [DOI] [PubMed] [Google Scholar]
- 134.Verschuren EW, Hodgson JG, Gray JW, Kogan S, Jones N, Evan GI. 2004. The role of p53 in suppression of KSHV cyclin-induced lymphomagenesis. Cancer Res. 64, 581–589. ( 10.1158/0008-5472.CAN-03-1863) [DOI] [PubMed] [Google Scholar]
- 135.Sarek G, Jarviluoma A, Moore HM, Tojkander S, Vartia S, Biberfeld P, Laiho M, Ojala PM. 2010. Nucleophosmin phosphorylation by v-cyclin-CDK6 controls KSHV latency. PLoS Pathog. 6, e1000818 ( 10.1371/journal.ppat.1000818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jones T, da Silva S Ramos, Bedolla R, Ye F, Zhou F, Gao SJ. 2014. Viral cyclin promotes KSHV-induced cellular transformation and tumorigenesis by overriding contact inhibition. Cell Cycle 13, 845–858. ( 10.4161/cc.27758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Guasparri I, Keller SA, Cesarman E. 2004. KSHV vFLIP is essential for the survival of infected lymphoma cells. J. Exp. Med. 199, 993–1003. ( 10.1084/jem.20031467) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 138.Godfrey A, Anderson J, Papanastasiou A, Takeuchi Y, Boshoff C. 2005. Inhibiting primary effusion lymphoma by lentiviral vectors encoding short hairpin RNA. Blood 105, 2510–2518. ( 10.1182/blood-2004-08-3052) [DOI] [PubMed] [Google Scholar]
- 139.Cheng F, et al. 2011. KSHV-initiated Notch activation leads to membrane-type-1 matrix metalloproteinase-dependent lymphatic endothelial-to-mesenchymal transition. Cell Host Microbe 10, 577–590. ( 10.1016/j.chom.2011.10.011) [DOI] [PubMed] [Google Scholar]
- 140.Grossmann C, Podgrabinska S, Skobe M, Ganem D. 2006. Activation of NF-κB by the latent vFLIP gene of Kaposi's sarcoma-associated herpesvirus is required for the spindle shape of virus-infected endothelial cells and contributes to their proinflammatory phenotype. J. Virol. 80, 7179–7185. ( 10.1128/JVI.01603-05) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alkharsah KR, et al. 2011. Deletion of Kaposi's sarcoma-associated herpesvirus FLICE inhibitory protein, vFLIP, from the viral genome compromises the activation of STAT1-responsive cellular genes and spindle cell formation in endothelial cells. J. Virol. 85, 10 375–10 388. ( 10.1128/JVI.00226-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ballon G, Akar G, Cesarman E. 2015. Systemic expression of Kaposi sarcoma herpesvirus (KSHV) Vflip in endothelial cells leads to a profound proinflammatory phenotype and myeloid lineage remodeling in vivo. PLoS Pathog. 11, e1004581 ( 10.1371/journal.ppat.1004581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.He M, Zhang W, Bakken T, Schutten M, Toth Z, Jung JU, Gill P, Cannon M, Gao SJ. 2012. Cancer angiogenesis induced by Kaposi sarcoma-associated herpesvirus is mediated by EZH2. Cancer Res. 72, 3582–3592. ( 10.1158/0008-5472.CAN-11-2876) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu L, Eby MT, Rathore N, Sinha SK, Kumar A, Chaudhary PM. 2002. The human herpes virus 8-encoded viral FLICE inhibitory protein physically associates with and persistently activates the IkB kinase complex. J. Biol. Chem. 277, 13 745–13 751. ( 10.1074/jbc.M110480200) [DOI] [PubMed] [Google Scholar]
- 145.An J, Sun Y, Sun R, Rettig MB. 2003. Kaposi's sarcoma-associated herpesvirus encoded vFLIP induces cellular IL-6 expression: the role of the NF-κB and JNK/AP1 pathways. Oncogene 22, 3371–3385. ( 10.1038/sj.onc.1206407) [DOI] [PubMed] [Google Scholar]
- 146.Field N, Low W, Daniels M, Howell S, Daviet L, Boshoff C, Collins M. 2003. KSHV vFLIP binds to IKK-γ to activate IKK. J. Cell Sci. 116, 3721–3728. ( 10.1242/jcs.00691) [DOI] [PubMed] [Google Scholar]
- 147.Chugh P, Matta H, Schamus S, Zachariah S, Kumar A, Richardson JA, Smith AL, Chaudhary PM. 2005. Constitutive NF-κB activation, normal Fas-induced apoptosis, and increased incidence of lymphoma in human herpes virus 8 K13 transgenic mice. Proc. Natl Acad. Sci. USA 102, 12 885–12 890. ( 10.1073/pnas.0408577102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ye FC, Zhou FC, Xie JP, Kang T, Greene W, Kuhne K, Lei XF, Li QH, Gao SJ. 2008. Kaposi's sarcoma-associated herpesvirus latent gene vFLIP inhibits viral lytic replication through NF-κB-mediated suppression of the AP-1 pathway: a novel mechanism of virus control of latency. J. Virol. 82, 4235–4249. ( 10.1128/JVI.02370-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bagneris C, Ageichik AV, Cronin N, Wallace B, Collins M, Boshoff C, Waksman G, Barrett T. 2008. Crystal structure of a vFlip-IKKγ complex: insights into viral activation of the IKK signalosome. Mol. Cell 30, 620–631. ( 10.1016/j.molcel.2008.04.029) [DOI] [PubMed] [Google Scholar]
- 150.Lee JS, et al. 2009. FLIP-mediated autophagy regulation in cell death control. Nat. Cell Biol. 11, 1355–1362. ( 10.1038/ncb1980) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Emuss V, Lagos D, Pizzey A, Gratrix F, Henderson SR, Boshoff C. 2009. KSHV manipulates Notch signaling by DLL4 and JAG1 to alter cell cycle genes in lymphatic endothelia. PLoS Pathog. 5, e1000616 ( 10.1371/journal.ppat.1000616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ballon G, Chen K, Perez R, Tam W, Cesarman E. 2011. Kaposi sarcoma herpesvirus (KSHV) vFLIP oncoprotein induces B cell transdifferentiation and tumorigenesis in mice. J. Clin. Invest. 121, 1141–1153. ( 10.1172/JCI44417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Leidal AM, Cyr DP, Hill RJ, Lee PW, McCormick C. 2012. Subversion of autophagy by Kaposi's sarcoma-associated herpesvirus impairs oncogene-induced senescence. Cell Host Microbe 11, 167–180. ( 10.1016/j.chom.2012.01.005) [DOI] [PubMed] [Google Scholar]
- 154.Singh VV, Kerur N, Bottero V, Dutta S, Chakraborty S, Ansari MA, Paudel N, Chikoti L, Chandran B. 2013. Kaposi's sarcoma-associated herpesvirus latency in endothelial and B cells activates gamma interferon-inducible protein 16-mediated inflammasomes. J. Virol. 87, 4417–4431. ( 10.1128/JVI.03282-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mutlu AD, et al. 2007. In vivo-restricted and reversible malignancy induced by human herpesvirus-8 KSHV: a cell and animal model of virally induced Kaposi's sarcoma. Cancer Cell 11, 245–258. ( 10.1016/j.ccr.2007.01.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Montaner S, Sodhi A, Molinolo A, Bugge TH, Sawai ET, He Y, Li Y, Ray PE, Gutkind JS. 2003. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi's sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell 3, 23–36. ( 10.1016/S1535-6108(02)00237-4) [DOI] [PubMed] [Google Scholar]
- 157.Sodhi A, et al. 2006. The TSC2/mTOR pathway drives endothelial cell transformation induced by the Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor. Cancer Cell 10, 133–143. ( 10.1016/j.ccr.2006.05.026) [DOI] [PubMed] [Google Scholar]
- 158.Yang TY, et al. 2000. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi's sarcoma. J. Exp. Med. 191, 445–454. ( 10.1084/jem.191.3.445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Montaner S, Sodhi A, Pece S, Mesri EA, Gutkind JS. 2001. The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer Res. 61, 2641–2648. [PubMed] [Google Scholar]
- 160.Bais C, Van Geelen A, Eroles P, Mutlu A, Chiozzini C, Dias S, Silverstein RL, Rafii S, Mesri EA. 2003. Kaposi's sarcoma associated herpesvirus G protein-coupled receptor immortalizes human endothelial cells by activation of the VEGF receptor-2/KDR. Cancer Cell 3, 131–143. ( 10.1016/S1535-6108(03)00024-2) [DOI] [PubMed] [Google Scholar]
- 161.Sodhi A, Montaner S, Patel V, Gomez-Roman JJ, Li Y, Sausville EA, Sawai ET, Gutkind JS. 2004. Akt plays a central role in sarcomagenesis induced by Kaposi's sarcoma herpesvirus-encoded G protein-coupled receptor. Proc. Natl Acad. Sci. USA 101, 4821–4826. ( 10.1073/pnas.0400835101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vart RJ, Nikitenko LL, Lagos D, Trotter MW, Cannon M, Bourboulia D, Gratrix F, Takeuchi Y, Boshoff C. 2007. Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6 and G-protein-coupled receptor regulate angiopoietin-2 expression in lymphatic endothelial cells. Cancer Res. 67, 4042–4051. ( 10.1158/0008-5472.CAN-06-3321) [DOI] [PubMed] [Google Scholar]
- 163.Lagos D, et al. 2008. Toll-like receptor 4 mediates innate immunity to Kaposi sarcoma herpesvirus. Cell Host Microbe 4, 470–483. ( 10.1016/j.chom.2008.09.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sandford G, Choi YB, Nicholas J. 2009. Role of ORF74-encoded viral G protein-coupled receptor in human herpesvirus 8 lytic replication. J. Virol. 83, 13 009–13 014. ( 10.1128/JVI.01399-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ma T, Jham BC, Hu J, Friedman ER, Basile JR, Molinolo A, Sodhi A, Montaner S. 2010. Viral G protein-coupled receptor up-regulates angiopoietin-like 4 promoting angiogenesis and vascular permeability in Kaposi's sarcoma. Proc. Natl Acad. Sci. USA 107, 14 363–14 368. ( 10.1073/pnas.1001065107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Martin D, Galisteo R, Molinolo AA, Wetzker R, Hirsch E, Gutkind JS. 2011. PI3 Kγ mediates Kaposi's sarcoma-associated herpesvirus vGPCR-induced sarcomagenesis. Cancer Cell 19, 805–813. ( 10.1016/j.ccr.2011.05.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Polizzotto MN, et al. 2013. Human and viral interleukin-6 and other cytokines in Kaposi sarcoma herpesvirus-associated multicentric Castleman disease. Blood 122, 4189–4198. ( 10.1182/blood-2013-08-519959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Morris VA, Punjabi AS, Wells RC, Wittkopp CJ, Vart R, Lagunoff M. 2012. The KSHV viral IL-6 homolog is sufficient to induce blood to lymphatic endothelial cell differentiation. Virology 428, 112–120. ( 10.1016/j.virol.2012.03.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Moore PS, Boshoff C, Weiss RA, Chang Y. 1996. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science 274, 1739–1744. ( 10.1126/science.274.5293.1739) [DOI] [PubMed] [Google Scholar]
- 170.Neipel F, Albrecht JC, Ensser A, Huang YQ, Li JJ, Friedman-Kien AE, Fleckenstein B. 1997. Human herpesvirus 8 encodes a homolog of interleukin-6. J. Virol. 71, 839–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nicholas J, Ruvolo V, Zong J, Ciufo D, Guo HG, Reitz MS, Hayward GS. 1997. A single 13-kilobase divergent locus in the Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genome contains nine open reading frames that are homologous to or related to cellular proteins. J. Virol. 71, 1963–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Molden J, Chang Y, You Y, Moore PS, Goldsmith MA. 1997. A Kaposi's sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J. Biol. Chem. 272, 19 625–19 631. ( 10.1074/jbc.272.31.19625) [DOI] [PubMed] [Google Scholar]
- 173.Parravicini C, Corbellino M, Paulli M, Magrini U, Lazzarino M, Moore PS, Chang Y. 1997. Expression of a virus-derived cytokine, KSHV vIL-6, in HIV-seronegative Castleman's disease. Am. J. Pathol. 151, 1517–1522. [PMC free article] [PubMed] [Google Scholar]
- 174.Burger R, Neipel F, Fleckenstein B, Savino R, Ciliberto G, Kalden JR, Gramatzki M. 1998. Human herpesvirus type 8 interleukin-6 homologue is functionally active on human myeloma cells. Blood 91, 1858–1863. [PubMed] [Google Scholar]
- 175.Aoki Y, Jaffe ES, Chang Y, Jones K, Teruya-Feldstein J, Moore PS, Tosato G. 1999. Angiogenesis and hematopoiesis induced by Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6. Blood 93, 4034–4043. [PubMed] [Google Scholar]
- 176.Wan X, Wang H, Nicholas J. 1999. Human herpesvirus 8 interleukin-6 (vIL-6) signals through gp130 but has structural and receptor-binding properties distinct from those of human IL-6. J. Virol. 73, 8268–8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jones KD, Aoki Y, Chang Y, Moore PS, Yarchoan R, Tosato G. 1999. Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi's sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood 94, 2871–2879. [PubMed] [Google Scholar]
- 178.Mori Y, Nishimoto N, Ohno M, Inagi R, Dhepakson P, Amou K, Yoshizaki K, Yamanishi K. 2000. Human herpesvirus 8-encoded interleukin-6 homologue (viral IL-6) induces endogenous human IL-6 secretion. J. Med. Virol. 61, 332–335. ( 10.1002/1096-9071(200007)61:3%3C332::AID-JMV8%3E3.0.CO;2-3) [DOI] [PubMed] [Google Scholar]
- 179.Suthaus J, Stuhlmann-Laeisz C, Tompkins VS, Rosean TR, Klapper W, Tosato G, Janz S, Scheller J, Rose-John S. 2012. HHV-8-encoded viral IL-6 collaborates with mouse IL-6 in the development of multicentric Castleman disease in mice. Blood 119, 5173–5181. ( 10.1182/blood-2011-09-377705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhu X, et al. 2013. Synergy between Kaposi's sarcoma-associated herpesvirus (KSHV) vIL-6 and HIV-1 Nef protein in promotion of angiogenesis and oncogenesis: role of the AKT signaling pathway. Oncogene 33, 1986–1996. ( 10.1038/onc.2013.136) [DOI] [PubMed] [Google Scholar]
- 181.Cousins E, Gao Y, Sandford G, Nicholas J. 2014. Human herpesvirus 8 viral interleukin-6 signaling through gp130 promotes virus replication in primary effusion lymphoma and endothelial cells. J. Virol. 88, 12 167–12 172. ( 10.1128/JVI.01751-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chatterjee M, Osborne J, Bestetti G, Chang Y, Moore PS. 2002. Viral IL-6-induced cell proliferation and immune evasion of interferon activity. Science 298, 1432–1435. ( 10.1126/science.1074883) [DOI] [PubMed] [Google Scholar]
- 183.Ma Z, et al. 2015. Modulation of the cGAS-STING DNA sensing pathway by gammaherpesviruses. Proc. Natl Acad. Sci. USA 112, E4306–E4315. ( 10.1073/pnas.1503831112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gao SJ, Boshoff C, Jayachandra S, Weiss RA, Chang Y, Moore PS. 1997. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene 15, 1979–1985. ( 10.1038/sj.onc.1201571) [DOI] [PubMed] [Google Scholar]
- 185.Lin R, Genin P, Mamane Y, Sgarbanti M, Battistini A, Harrington WJ Jr, Barber GN, Hiscott J. 2001. HHV-8 encoded vIRF-1 represses the interferon antiviral response by blocking IRF-3 recruitment of the CBP/p300 coactivators. Oncogene 20, 800–811. ( 10.1038/sj.onc.1204163) [DOI] [PubMed] [Google Scholar]
- 186.Seo T, Lee D, Shim YS, Angell JE, Chidambaram NV, Kalvakolanu DV, Choe J. 2002. Viral interferon regulatory factor 1 of Kaposi's sarcoma-associated herpesvirus interacts with a cell death regulator, GRIM19, and inhibits interferon/retinoic acid-induced cell death. J. Virol. 76, 8797–8807. ( 10.1128/JVI.76.17.8797-8807.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kirchhoff S, et al. 2002. Viral IFN-regulatory factors inhibit activation-induced cell death via two positive regulatory IFN-regulatory factor 1-dependent domains in the CD95 ligand promoter. J. Immunol. 168, 1226–1234. ( 10.4049/jimmunol.168.3.1226) [DOI] [PubMed] [Google Scholar]
- 188.Seo T, Park J, Choe J. 2005. Kaposi's sarcoma-associated herpesvirus viral IFN regulatory factor 1 inhibits transforming growth factor-β signaling. Cancer Res. 65, 1738–1747. ( 10.1158/0008-5472.CAN-04-2374) [DOI] [PubMed] [Google Scholar]
- 189.Shin YC, Nakamura H, Liang X, Feng P, Chang H, Kowalik TF, Jung JU. 2006. Inhibition of the ATM/p53 signal transduction pathway by Kaposi's sarcoma-associated herpesvirus interferon regulatory factor 1. J. Virol. 80, 2257–2266. ( 10.1128/JVI.80.5.2257-2266.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Choi YB, Nicholas J. 2010. Bim nuclear translocation and inactivation by viral interferon regulatory factor. PLoS Pathog. 6, e1001031 ( 10.1371/journal.ppat.1001031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Choi YB, Sandford G, Nicholas J. 2012. Human herpesvirus 8 interferon regulatory factor-mediated BH3-only protein inhibition via Bid BH3-B mimicry. PLoS Pathog. 8, e1002748 ( 10.1371/journal.ppat.1002748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Burysek L, Pitha PM. 2001. Latently expressed human herpesvirus 8-encoded interferon regulatory factor 2 inhibits double-stranded RNA-activated protein kinase. J. Virol. 75, 2345–2352. ( 10.1128/JVI.75.5.2345-2352.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Fuld S, Cunningham C, Klucher K, Davison AJ, Blackbourn DJ. 2006. Inhibition of interferon signaling by the Kaposi's sarcoma-associated herpesvirus full-length viral interferon regulatory factor 2 protein. J. Virol. 80, 3092–3097. ( 10.1128/JVI.80.6.3092-3097.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mutocheluh M, Hindle L, Areste C, Chanas SA, Butler LM, Lowry K, Shah K, Evans DJ, Blackbourn DJ. 2011. Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor-2 inhibits type 1 interferon signalling by targeting interferon-stimulated gene factor-3. J. Gen. Virol. 92, 2394–2398. ( 10.1099/vir.0.034322-0) [DOI] [PubMed] [Google Scholar]
- 195.Wies E, Mori Y, Hahn A, Kremmer E, Sturzl M, Fleckenstein B, Neipel F. 2008. The viral interferon-regulatory factor-3 is required for the survival of KSHV-infected primary effusion lymphoma cells. Blood 111, 320–327. ( 10.1182/blood-2007-05-092288) [DOI] [PubMed] [Google Scholar]
- 196.Rivas C, Thlick AE, Parravicini C, Moore PS, Chang Y. 2001. Kaposi's sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J. Virol. 75, 429–438. ( 10.1128/JVI.75.1.429-438.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Esteban M, Garcia MA, Domingo-Gil E, Arroyo J, Nombela C, Rivas C. 2003. The latency protein LANA2 from Kaposi's sarcoma-associated herpesvirus inhibits apoptosis induced by dsRNA-activated protein kinase but not RNase L activation. J. Gen. Virol. 84, 1463–1470. ( 10.1099/vir.0.19014-0) [DOI] [PubMed] [Google Scholar]
- 198.Seo T, Park J, Lim C, Choe J. 2004. Inhibition of nuclear factor κB activity by viral interferon regulatory factor 3 of Kaposi's sarcoma-associated herpesvirus. Oncogene 23, 6146–6155. ( 10.1038/sj.onc.1207807) [DOI] [PubMed] [Google Scholar]
- 199.Joo CH, Shin YC, Gack M, Wu L, Levy D, Jung JU. 2007. Inhibition of interferon regulatory factor 7 (IRF7)-mediated interferon signal transduction by the Kaposi's sarcoma-associated herpesvirus viral IRF homolog vIRF3. J. Virol. 81, 8282–8292. ( 10.1128/JVI.00235-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lubyova B, Kellum MJ, Frisancho JA, Pitha PM. 2007. Stimulation of c-Myc transcriptional activity by vIRF-3 of Kaposi sarcoma-associated herpesvirus. J. Biol. Chem. 282, 31 944–31 953. ( 10.1074/jbc.M706430200) [DOI] [PubMed] [Google Scholar]
- 201.Shin YC, Joo CH, Gack MU, Lee HR, Jung JU. 2008. Kaposi's sarcoma-associated herpesvirus viral IFN regulatory factor 3 stabilizes hypoxia-inducible factor-1α to induce vascular endothelial growth factor expression. Cancer Res. 68, 1751–1759. ( 10.1158/0008-5472.CAN-07-2766) [DOI] [PubMed] [Google Scholar]
- 202.Wies E, et al. 2009. The Kaposi's sarcoma-associated herpesvirus-encoded vIRF-3 inhibits cellular IRF-5. J. Biol. Chem. 284, 8525–8538. ( 10.1074/jbc.M809252200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Marcos-Villar L, Lopitz-Otsoa F, Gallego P, Munoz-Fontela C, Gonzalez-Santamaria J, Campagna M, Shou-Jiang G, Rodriguez MS, Rivas C. 2009. Kaposi's sarcoma-associated herpesvirus protein LANA2 disrupts PML oncogenic domains and inhibits PML-mediated transcriptional repression of the survivin gene. J. Virol. 83, 8849–8858. ( 10.1128/JVI.00339-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Schmidt K, Wies E, Neipel F. 2011. Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor 3 inhibits gamma interferon and major histocompatibility complex class II expression. J. Virol. 85, 4530–4537. ( 10.1128/JVI.02123-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Xi X, Persson LM, O'Brien MW, Mohr I, Wilson AC. 2012. Cooperation between viral interferon regulatory factor 4 and RTA to activate a subset of Kaposi's sarcoma-associated herpesvirus lytic promoters. J. Virol. 86, 1021–1033. ( 10.1128/JVI.00694-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lee HR, Toth Z, Shin YC, Lee JS, Chang H, Gu W, Oh TK, Kim MH, Jung JU. 2009. Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor 4 targets MDM2 to deregulate the p53 tumor suppressor pathway. J. Virol. 83, 6739–6747. ( 10.1128/JVI.02353-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Heinzelmann K, Scholz BA, Nowak A, Fossum E, Kremmer E, Haas J, Frank R, Kempkes B. 2010. Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor 4 (vIRF4/K10) is a novel interaction partner of CSL/CBF1, the major downstream effector of Notch signaling. J. Virol. 84, 12 255–12 264. ( 10.1128/JVI.01484-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lee HR, et al. 2011. Bilateral inhibition of HAUSP deubiquitinase by a viral interferon regulatory factor protein. Nat. Struct. Mol. Biol. 18, 1336–1344. ( 10.1038/nsmb.2142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Scholz BA, Harth-Hertle ML, Malterer G, Haas J, Ellwart J, Schulz TF, Kempkes B. 2013. Abortive lytic reactivation of KSHV in CBF1/CSL deficient human B cell lines. PLoS Pathog. 9, e1003336 ( 10.1371/journal.ppat.1003336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Boshoff C, et al. 1997. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science 278, 290–294. ( 10.1126/science.278.5336.290) [DOI] [PubMed] [Google Scholar]
- 211.Kledal TN, et al. 1997. A broad-spectrum chemokine antagonist encoded by Kaposi's sarcoma-associated herpesvirus. Science 277, 1656–1659. ( 10.1126/science.277.5332.1656) [DOI] [PubMed] [Google Scholar]
- 212.Endres MJ, Garlisi CG, Xiao H, Shan L, Hedrick JA. 1999. The Kaposi's sarcoma-related herpesvirus (KSHV)-encoded chemokine vMIP-I is a specific agonist for the CC chemokine receptor (CCR)8. J. Exp. Med. 189, 1993–1998. ( 10.1084/jem.189.12.1993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Weber KS, Grone HJ, Rocken M, Klier C, Gu S, Wank R, Proudfoot AE, Nelson PJ, Weber C. 2001. Selective recruitment of Th2-type cells and evasion from a cytotoxic immune response mediated by viral macrophage inhibitory protein-II. Eur. J. Immunol. 31, 2458–2466. ( 10.1002/1521-4141(200108)31:8less%20than%202458::AID-IMMU2458greater%20than%203.0.CO;2-L) [DOI] [PubMed] [Google Scholar]
- 214.Stine JT, et al. 2000. KSHV-encoded CC chemokine vMIP-III is a CCR4 agonist, stimulates angiogenesis, and selectively chemoattracts TH2 cells. Blood 95, 1151–1157. [PubMed] [Google Scholar]
- 215.Choi YB, Nicholas J. 2008. Autocrine and paracrine promotion of cell survival and virus replication by human herpesvirus 8 chemokines. J. Virol. 82, 6501–6513. ( 10.1128/JVI.02396-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Parravicini C, Chandran B, Corbellino M, Berti E, Paulli M, Moore PS, Chang Y. 2000. Differential viral protein expression in Kaposi's sarcoma-associated herpesvirus-infected diseases: Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Am. J. Pathol. 156, 743–749. ( 10.1016/S0002-9440(10)64940-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Katano H, Sato Y, Kurata T, Mori S, Sata T. 2000. Expression and localization of human herpesvirus 8-encoded proteins in primary effusion lymphoma, Kaposi's sarcoma, and multicentric Castleman's disease. Virology 269, 335–344. ( 10.1006/viro.2000.0196) [DOI] [PubMed] [Google Scholar]
- 218.Hosseinipour MC, et al. 2014. Viral profiling identifies multiple subtypes of Kaposi's sarcoma. MBio 5, e01633-14 ( 10.1128/mBio.01633-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Judde JG, et al. 2000. Monoclonality or oligoclonality of human herpesvirus 8 terminal repeat sequences in Kaposi's sarcoma and other diseases. J. Natl Cancer Inst. 92, 729–736. ( 10.1093/jnci/92.9.729) [DOI] [PubMed] [Google Scholar]
- 220.Delabesse E, Oksenhendler E, Lebbe C, Verola O, Varet B, Turhan AG. 1997. Molecular analysis of clonality in Kaposi's sarcoma. J. Clin. Pathol. 50, 664–668. ( 10.1136/jcp.50.8.664) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gill PS, Tsai YC, Rao AP, Spruck CH 3rd, Zheng T, Harrington WA Jr, Cheung T, Nathwani B, Jones PA. 1998. Evidence for multiclonality in multicentric Kaposi's sarcoma. Proc. Natl Acad. Sci. USA 95, 8257–8261. ( 10.1073/pnas.95.14.8257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Keller SA, Schattner EJ, Cesarman E. 2000. Inhibition of NF-κB induces apoptosis of KSHV-infected primary effusion lymphoma cells. Blood 96, 2537–2542. [PubMed] [Google Scholar]
- 223.Aoki Y, Feldman GM, Tosato G. 2003. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood 101, 1535–1542. ( 10.1182/blood-2002-07-2130) [DOI] [PubMed] [Google Scholar]
- 224.Cousins E, Nicholas J. 2013. Role of human herpesvirus 8 interleukin-6-activated gp130 signal transducer in primary effusion lymphoma cell growth and viability. J. Virol. 87, 10 816–10 827. ( 10.1128/jvi.02047-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sin SH, Kim Y, Eason A, Dittmer DP. 2015. KSHV latency locus cooperates with Myc to drive lymphoma in mice. PLoS Pathog. 11, e1005135 ( 10.1371/journal.ppat.1005135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Oksenhendler E, Carcelain G, Aoki Y, Boulanger E, Maillard A, Clauvel JP, Agbalika F. 2000. High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric Castleman disease in HIV-infected patients. Blood 96, 2069–2073. [PubMed] [Google Scholar]
- 227.Stebbing J, Adams C, Sanitt A, Mletzko S, Nelson M, Gazzard B, Newsom-Davis T, Bower M. 2011. Plasma HHV8 DNA predicts relapse in individuals with HIV-associated multicentric Castleman disease. Blood 118, 271–275. ( 10.1182/blood-2011-02-335620) [DOI] [PubMed] [Google Scholar]
- 228.Bower M, Newsom-Davis T, Naresh K, Merchant S, Lee B, Gazzard B, Stebbing J, Nelson M. 2011. Clinical features and outcome in HIV-associated multicentric Castleman's disease. J. Clin. Oncol. 29, 2481–2486. ( 10.1200/jco.2010.34.1909) [DOI] [PubMed] [Google Scholar]
- 229.Casper C, Nichols WG, Huang ML, Corey L, Wald A. 2004. Remission of HHV-8 and HIV-associated multicentric Castleman disease with ganciclovir treatment. Blood 103, 1632–1634. ( 10.1182/blood-2003-05-1721) [DOI] [PubMed] [Google Scholar]
- 230.Uldrick TS, et al. 2011. High-dose zidovudine plus valganciclovir for Kaposi sarcoma herpesvirus-associated multicentric Castleman disease: a pilot study of virus-activated cytotoxic therapy. Blood 117, 6977–6986. ( 10.1182/blood-2010-11-317610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Flore O, Rafii S, Ely S, O'Leary JJ, Hyjek EM, Cesarman E. 1998. Transformation of primary human endothelial cells by Kaposi's sarcoma-associated herpesvirus. Nature 394, 588–592. ( 10.1038/29093) [DOI] [PubMed] [Google Scholar]
- 232.Wang L, Damania B. 2008. Kaposi's sarcoma-associated herpesvirus confers a survival advantage to endothelial cells. Cancer Res. 68, 4640–4648. ( 10.1158/0008-5472.CAN-07-5988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.An FQ, Folarin HM, Compitello N, Roth J, Gerson SL, McCrae KR, Fakhari FD, Dittmer DP, Renne R. 2006. Long-term-infected telomerase-immortalized endothelial cells: a model for Kaposi's sarcoma-associated herpesvirus latency in vitro and in vivo. J. Virol. 80, 4833–4846. ( 10.1128/JVI.80.10.4833-4846.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Jones T, et al. 2012. Direct and efficient cellular transformation of primary rat mesenchymal precursor cells by KSHV. J. Clin. Invest. 122, 1076–1081. ( 10.1172/JCI58530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kaaya EE, Parravicini C, Sundelin B, Mgaya E, Kitinya J, Lema L, Luande J, Biberfeld P. 1992. Spindle cell ploidy and proliferation in endemic and epidemic African Kaposi's sarcoma. Eur. J. Cancer 28A, 1890–1894. ( 10.1016/0959-8049(92)90030-6) [DOI] [PubMed] [Google Scholar]
- 236.Hodak E, Hammel I, Feinmesser M, Zelinger A, Maron L, Sulkes J, David M. 1999. Differential expression of p53 and Ki-67 proteins in classic and iatrogenic Kaposi's sarcoma. Am. J. Dermatopathol. 21, 138–145. ( 10.1097/00000372-199904000-00005) [DOI] [PubMed] [Google Scholar]
- 237.Morris VA, Punjabi AS, Lagunoff M. 2008. Activation of Akt through gp130 receptor signaling is required for Kaposi's sarcoma-associated herpesvirus-induced lymphatic reprogramming of endothelial cells. J. Virol. 82, 8771–8779. ( 10.1128/JVI.00766-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chang HH, Ganem D. 2013. A unique herpesviral transcriptional program in KSHV-infected lymphatic endothelial cells leads to mTORC1 activation and rapamycin sensitivity. Cell Host Microbe 13, 429–440. ( 10.1016/j.chom.2013.03.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Stallone G, et al. 2005. Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N. Engl. J. Med. 352, 1317–1323. ( 10.1056/NEJMoa042831) [DOI] [PubMed] [Google Scholar]
- 240.Ojala PM, Schulz TF. 2014. Manipulation of endothelial cells by KSHV: implications for angiogenesis and aberrant vascular differentiation. Semin. Cancer Biol. 26, 69–77. ( 10.1016/j.semcancer.2014.01.008) [DOI] [PubMed] [Google Scholar]
- 241.Wang HW, et al. 2004. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat. Genet. 36, 687–693. ( 10.1038/ng1384) [DOI] [PubMed] [Google Scholar]
- 242.Hong YK, et al. 2004. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat. Genet. 36, 683–685. ( 10.1038/ng1383) [DOI] [PubMed] [Google Scholar]
- 243.Gasperini P, Espigol-Frigole G, McCormick PJ, Salvucci O, Maric D, Uldrick TS, Polizzotto MN, Yarchoan R, Tosato G. 2012. Kaposi sarcoma herpesvirus promotes endothelial-to-mesenchymal transition through Notch-dependent signaling. Cancer Res. 72, 1157–1169. ( 10.1158/0008-5472.CAN-11-3067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Qian LW, Xie J, Ye F, Gao SJ. 2007. Kaposi's sarcoma-associated herpesvirus infection promotes invasion of primary human umbilical vein endothelial cells by inducing matrix metalloproteinases. J. Virol. 81, 7001–7010. ( 10.1128/JVI.00016-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Haas DA, et al. 2013. The inflammatory kinase MAP4K4 promotes reactivation of Kaposi's sarcoma herpesvirus and enhances the invasiveness of infected endothelial cells. PLoS Pathog. 9, e1003737 ( 10.1371/journal.ppat.1003737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Masood R, Cesarman E, Smith DL, Gill PS, Flore O. 2002. Human herpesvirus-8-transformed endothelial cells have functionally activated vascular endothelial growth factor/vascular endothelial growth factor receptor. Am. J. Pathol. 160, 23–29. ( 10.1016/S0002-9440(10)64344-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Moses AV, et al. 2002. Kaposi's sarcoma-associated herpesvirus-induced upregulation of the c-kit proto-oncogene, as identified by gene expression profiling, is essential for the transformation of endothelial cells. J. Virol. 76, 8383–8399. ( 10.1128/JVI.76.16.8383-8399.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Qin Z, Dai L, Slomiany MG, Toole BP, Parsons C. 2012. Direct activation of emmprin and associated pathogenesis by an oncogenic herpesvirus. Cancer Res. 70, 3884–3889. ( 10.1158/0008-5472.CAN-09-4663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wang X, He Z, Xia T, Li X, Liang D, Lin X, Wen H, Lan K. 2014. Latency-associated nuclear antigen of Kaposi sarcoma-associated herpesvirus promotes angiogenesis through targeting Notch signaling effector Hey1. Cancer Res. 74, 2026–2037. ( 10.1158/0008-5472.CAN-13-1467) [DOI] [PubMed] [Google Scholar]
- 250.Pancholi NJ, Price AM, Weitzman MD. 2017. Take your PIKK: tumour viruses and DNA damage response pathways. Phil. Trans R. Soc. B 372, 20160269 ( 10.1098/rstb.2016.0269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sarek G, Kurki S, Enback J, Iotzova G, Haas J, Laakkonen P, Laiho M, Ojala PM. 2007. Reactivation of the p53 pathway as a treatment modality for KSHV-induced lymphomas. J. Clin. Invest. 117, 1019–1028. ( 10.1172/JCI30945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Friborg J Jr, Kong W, Hottiger MO, Nabel GJ. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402, 889–894. [DOI] [PubMed] [Google Scholar]
- 253.Si H, Robertson ES. 2006. Kaposi's sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen induces chromosomal instability through inhibition of p53 function. J. Virol. 80, 697–709. ( 10.1128/jvi.80.2.697-709.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Petre CE, Sin SH, Dittmer DP. 2007. Functional p53 signaling in Kaposi's sarcoma-associated herpesvirus lymphomas: implications for therapy. J. Virol. 81, 1912–1922. ( 10.1128/jvi.01757-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Nakamura H, Li M, Zarycki J, Jung JU. 2001. Inhibition of p53 tumor suppressor by viral interferon regulatory factor. J. Virol. 75, 7572–7582. ( 10.1128/jvi.75.16.7572-7582.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kati S, Tsao EH, Gunther T, Weidner-Glunde M, Rothamel T, Grundhoff A, Kellam P, Schulz TF. 2013. Activation of the B cell antigen receptor triggers reactivation of latent Kaposi's sarcoma-associated herpesvirus in B cells. J. Virol. 87, 8004–8016. ( 10.1128/jvi.00506-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Jha HC, Upadhyay SK M AJP, Lu J, Cai Q, Saha A, Robertson ES. 2013. H2AX phosphorylation is important for LANA-mediated Kaposi's sarcoma-associated herpesvirus episome persistence. J. Virol. 87, 5255–5269. ( 10.1128/jvi.03575-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Singh VV, Dutta D, Ansari MA, Dutta S, Chandran B. 2014. Kaposi's sarcoma-associated herpesvirus induces the ATM and H2AX DNA damage response early during de novo infection of primary endothelial cells, which play roles in latency establishment. J. Virol. 88, 2821–2834. ( 10.1128/jvi.03126-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Tarakanova VL, et al. 2007. Gamma-herpesvirus kinase actively initiates a DNA damage response by inducing phosphorylation of H2AX to foster viral replication. Cell Host Microbe 1, 275–286. ( 10.1016/j.chom.2007.05.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Verma SC, Bajaj BG, Cai Q, Si H, Seelhammer T, Robertson ES. 2006. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus recruits uracil DNA glycosylase 2 at the terminal repeats and is important for latent persistence of the virus. J. Virol. 80, 11 178–11 190. ( 10.1128/jvi.01334-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Jager W, Santag S, Weidner-Glunde M, Gellermann E, Kati S, Pietrek M, Viejo-Borbolla A, Schulz TF. 2012. The ubiquitin-specific protease USP7 modulates the replication of Kaposi's sarcoma-associated herpesvirus latent episomal DNA. J. Virol. 86, 6745–6757. ( 10.1128/JVI.06840-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Cha S, Lim C, Lee JY, Song YJ, Park J, Choe J, Seo T. 2010. DNA-PK/Ku complex binds to latency-associated nuclear antigen and negatively regulates Kaposi's sarcoma-associated herpesvirus latent replication. Biochem. Biophys. Res. Commun. 394, 934–939. ( 10.1016/j.bbrc.2010.03.086) [DOI] [PubMed] [Google Scholar]
- 263.Hollingworth R, Skalka GL, Stewart GS, Hislop AD, Blackbourn DJ, Grand RJ. 2015. Activation of DNA damage response pathways during lytic replication of KSHV. Viruses 7, 2908–2927. ( 10.3390/v7062752) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Jackson BR, Noerenberg M, Whitehouse A. 2014. A novel mechanism inducing genome instability in Kaposi's sarcoma-associated herpesvirus infected cells. PLoS Pathog. 10, e1004098 ( 10.1371/journal.ppat.1004098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Xiao Y, Chen J, Liao Q, Wu Y, Peng C, Chen X. 2013. Lytic infection of Kaposi's sarcoma-associated herpesvirus induces DNA double-strand breaks and impairs non-homologous end joining. J. Gen. Virol. 94, 1870–1875. ( 10.1099/vir.0.053033-0) [DOI] [PubMed] [Google Scholar]
- 266.Balistreri G, et al. 2016. Oncogenic herpesvirus utilizes stress-induced cell cycle checkpoints for efficient lytic replication. PLoS Pathog. 12, e1005424 ( 10.1371/journal.ppat.1005424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Dillon PJ, Gregory SM, Tamburro K, Sanders MK, Johnson GL, Raab-Traub N, Dittmer DP, Damania B. 2013. Tousled-like kinases modulate reactivation of gammaherpesviruses from latency. Cell Host Microbe 13, 204–214. ( 10.1016/j.chom.2012.12.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Verschuren EW, Klefstrom J, Evan GI, Jones N. 2002. The oncogenic potential of Kaposi's sarcoma-associated herpesvirus cyclin is exposed by p53 loss in vitro and in vivo. Cancer Cell 2, 229–241. ( 10.1016/S1535-6108(02)00123-X) [DOI] [PubMed] [Google Scholar]
- 269.Pan H, Zhou F, Gao SJ. 2004. Kaposi's sarcoma-associated herpesvirus induction of chromosome instability in primary human endothelial cells. Cancer Res. 64, 4064–4068. ( 10.1158/0008-5472.can-04-0657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Weitzman MD, Weitzman JB. 2014. What's the damage? The impact of pathogens on pathways that maintain host genome integrity. Cell Host Microbe 15, 283–294. ( 10.1016/j.chom.2014.02.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Trigg BJ, Ferguson BJ. 2015. Functions of DNA damage machinery in the innate immune response to DNA virus infection. Curr. Opin. Virol. 15, 56–62. ( 10.1016/j.coviro.2015.08.001) [DOI] [PubMed] [Google Scholar]
- 272.Kim T, Kim TY, Song YH, Min IM, Yim J, Kim TK. 1999. Activation of interferon regulatory factor 3 in response to DNA-damaging agents. J. Biol. Chem. 274, 30 686–30 689. ( 10.1074/jbc.274.43.30686) [DOI] [PubMed] [Google Scholar]
- 273.Brzostek-Racine S, Gordon C, Van Scoy S, Reich NC. 2011. The DNA damage response induces IFN. J. Immunol. 187, 5336–5345. ( 10.4049/jimmunol.1100040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. 2006. Molecular linkage between the kinase ATM and NF-κB signaling in response to genotoxic stimuli. Science 311, 1141–1146. ( 10.1126/science.1121513) [DOI] [PubMed] [Google Scholar]
- 275.Wenzel M, et al. 2012. Cytosolic DNA triggers mitochondrial apoptosis via DNA damage signaling proteins independently of AIM2 and RNA polymerase III. J. Immunol. 188, 394–403. ( 10.4049/jimmunol.1100523) [DOI] [PubMed] [Google Scholar]
- 276.Kondo T, Kobayashi J, Saitoh T, Maruyama K, Ishii KJ, Barber GN, Komatsu K, Akira S, Kawai T. 2013. DNA damage sensor MRE11 recognizes cytosolic double-stranded DNA and induces type I interferon by regulating STING trafficking. Proc. Natl Acad. Sci. USA 110, 2969–2974. ( 10.1073/pnas.1222694110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Peters NE, Ferguson BJ, Mazzon M, Fahy AS, Krysztofinska E, Arribas-Bosacoma R, Pearl LH, Ren H, Smith GL. 2013. A mechanism for the inhibition of DNA-PK-mediated DNA sensing by a virus. PLoS Pathog. 9, e1003649 ( 10.1371/journal.ppat.1003649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Roth S, et al. 2014. Rad50-CARD9 interactions link cytosolic DNA sensing to IL-1β production. Nat. Immunol. 15, 538–545. ( 10.1038/ni.2888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Monroe KM, Yang Z, Johnson JR, Geng X, Doitsh G, Krogan NJ, Greene WC. 2014. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science 343, 428–432. ( 10.1126/science.1243640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Dutta D, et al. 2015. BRCA1 regulates IFI16 mediated nuclear innate sensing of herpes viral DNA and subsequent induction of the innate inflammasome and interferon-β responses. PLoS Pathog. 11, e1005030 ( 10.1371/journal.ppat.1005030) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 281.Ansari MA, et al. 2015. Herpesvirus genome recognition induced acetylation of nuclear IFI16 is essential for its cytoplasmic translocation, inflammasome and IFN-β responses. PLoS Pathog. 11, e1005019 ( 10.1371/journal.ppat.1005019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Roy A, Dutta D, Iqbal J, Pisano G, Gjyshi O, Ansari MA, Kumar B, Chandran B. 2016. Nuclear innate immune DNA sensor IFI16 is degraded during lytic reactivation of Kaposi's sarcoma-associated herpesvirus (KSHV): role of IFI16 in maintenance of KSHV latency. J. Virol. 90, 8822–8841. ( 10.1128/jvi.01003-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Wu JJ, et al. 2015. Inhibition of cGAS DNA sensing by a herpesvirus virion protein. Cell Host Microbe 18, 333–344. ( 10.1016/j.chom.2015.07.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Li X, Bhaduri-McIntosh S. 2016. A central role for STAT3 in gammaherpesvirus-life cycle and -diseases. Front. Microbiol. 7, 1052 ( 10.3389/fmicb.2016.01052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Toptan T, Fonseca L, Kwun HJ, Chang Y, Moore PS. 2013. Complex alternative cytoplasmic protein isoforms of the Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 generated through noncanonical translation initiation. J. Virol. 87, 2744–2755. ( 10.1128/jvi.03061-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Mariggio G, Koch S, Zhang G, Weidner-Glunde M, Rückert J, Kati S, Santag S, Schulz TF. 2017. Kaposi sarcoma herpesvirus (KSHV) latency-associated nuclear antigen (LANA) recruits components of the MRN (Mre11-Rad50-NBS1) repair complex to modulate an innate immune signaling pathway and viral latency. PLoS Pathog. 13, e1006335 ( 10.1371/journal.ppat.1006335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Weiss G, Shemer A, Trau H. 2002. The Koebner phenomenon: review of the literature. J. Eur. Acad. Dermatol. Venereol. 16, 241–248. ( 10.1046/j.1473-2165.2002.00406.x) [DOI] [PubMed] [Google Scholar]
- 288.Grayson W, Pantanowitz L. 2008. Histological variants of cutaneous Kaposi sarcoma. Diagn. Pathol. 3, 31 ( 10.1186/1746-1596-3-31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Sirianni MC, et al. 1998. Gamma-interferon production in peripheral blood mononuclear cells and tumor infiltrating lymphocytes from Kaposi's sarcoma patients: correlation with the presence of human herpesvirus-8 in peripheral blood mononuclear cells and lesional macrophages. Blood 91, 968–976. [PubMed] [Google Scholar]
- 290.Monini P, et al. 1999. Reactivation and persistence of human herpesvirus-8 infection in B cells and monocytes by Th-1 cytokines increased in Kaposi's sarcoma. Blood 93, 4044–4058. [PubMed] [Google Scholar]
- 291.Connick E, Kane MA, White IE, Ryder J, Campbell TB. 2004. Immune reconstitution inflammatory syndrome associated with Kaposi sarcoma during potent antiretroviral therapy. Clin. Infect. Dis. 39, 1852–1855. ( 10.1086/426078) [DOI] [PubMed] [Google Scholar]
- 292.Bower M, et al. 2005. Immune reconstitution inflammatory syndrome associated with Kaposi's sarcoma. J. Clin. Oncol. 23, 5224–5228. ( 10.1200/jco.2005.14.597) [DOI] [PubMed] [Google Scholar]
- 293.Leidner RS, Aboulafia DM. 2005. Recrudescent Kaposi's sarcoma after initiation of HAART: a manifestation of immune reconstitution syndrome. AIDS Patient Care STDs 19, 635–644. ( 10.1089/apc.2005.19.635) [DOI] [PubMed] [Google Scholar]
- 294.Sharma-Walia N, Paul AG, Bottero V, Sadagopan S, Veettil MV, Kerur N, Chandran B. 2010. Kaposi's sarcoma associated herpes virus (KSHV) induced COX-2: a key factor in latency, inflammation, angiogenesis, cell survival and invasion. PLoS Pathog. 6, e1000777 ( 10.1371/journal.ppat.1000777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Camcioglu Y, et al. 2004. HHV-8-associated Kaposi sarcoma in a child with IFNγR1 deficiency. J. Pediatr. 144, 519–523. ( 10.1016/j.jpeds.2003.11.012) [DOI] [PubMed] [Google Scholar]
- 296.Picard C, et al. 2006. Kaposi's sarcoma in a child with Wiskott-Aldrich syndrome. Eur. J. Pediatr. 165, 453–457. ( 10.1007/s00431-006-0107-2) [DOI] [PubMed] [Google Scholar]
- 297.Sahin G, et al. 2010. Classic Kaposi sarcoma in 3 unrelated Turkish children born to consanguineous kindreds. Pediatrics 125, e704–e708. ( 10.1542/peds.2009-2224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Byun M, et al. 2010. Whole-exome sequencing-based discovery of STIM1 deficiency in a child with fatal classic Kaposi sarcoma. J. Exp. Med. 207, 2307–2312. ( 10.1084/jem.20101597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Byun M, et al. 2013. Inherited human OX40 deficiency underlying classic Kaposi sarcoma of childhood. J. Exp. Med. 210, 1743–1759. ( 10.1084/jem.20130592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Aavikko M, et al. 2015. Whole-genome sequencing identifies STAT4 as a putative susceptibility gene in classic Kaposi sarcoma. J. Infect. Dis. 211, 1842–1851. ( 10.1093/infdis/jiu667) [DOI] [PubMed] [Google Scholar]
- 301.Guttman-Yassky E, et al. 2004. Familial clustering of classic Kaposi sarcoma. J. Infect. Dis. 189, 2023–2026. ( 10.1086/386308) [DOI] [PubMed] [Google Scholar]
- 302.Brown EE, et al. 2005. Correlates of human herpesvirus-8 DNA detection among adults in Italy without Kaposi sarcoma. Int. J. Epidemiol. 34, 1110–1117. ( 10.1093/ije/dyi131) [DOI] [PubMed] [Google Scholar]
- 303.Brown EE, et al. 2006. Host immunogenetics and control of human herpesvirus-8 infection. J. Infect. Dis. 193, 1054–1062. ( 10.1086/501470) [DOI] [PubMed] [Google Scholar]
- 304.Maurer T, Ponte M, Leslie K. 2007. HIV-associated Kaposi's sarcoma with a high CD4 count and a low viral load. N. Engl. J. Med. 357, 1352–1353. ( 10.1056/NEJMc070508) [DOI] [PubMed] [Google Scholar]
- 305.Krown SE, Lee JY, Dittmer DP. 2008. More on HIV-associated Kaposi's sarcoma. N. Engl. J. Med. 358, 535–536; author reply 536 ( 10.1056/NEJMc072994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Krell J, Stebbing J. 2014. Broader implications of a stage-guided stratified therapeutic approach for AIDS-related Kaposi's sarcoma. J. Clin. Oncol. 32, 373–375. ( 10.1200/JCO.2013.53.7126) [DOI] [PubMed] [Google Scholar]
- 307.Bower M, Dalla Pria A, Coyle C, Andrews E, Tittle V, Dhoot S, Nelson M. 2014. Prospective stage-stratified approach to AIDS-related Kaposi's sarcoma. J. Clin. Oncol. 32, 409–414. ( 10.1200/JCO.2013.51.6757) [DOI] [PubMed] [Google Scholar]
- 308.Kedes DH, Ganem D. 1997. Sensitivity of Kaposi's sarcoma-associated herpesvirus replication to antiviral drugs. Implications for potential therapy. J. Clin. Invest. 99, 2082–2086. ( 10.1172/jci119380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Neyts J, De Clercq E. 1997. Antiviral drug susceptibility of human herpesvirus 8. Antimicrob. Agents Chemother. 41, 2754–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Sergerie Y, Boivin G. 2003. Evaluation of susceptibility of human herpesvirus 8 to antiviral drugs by quantitative real-time PCR. J. Clin. Microbiol. 41, 3897–3900. ( 10.1128/JCM.41.8.3897-3900.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Coen N, Duraffour S, Snoeck R, Andrei G. 2014. KSHV targeted therapy: an update on inhibitors of viral lytic replication. Viruses 6, 4731–4759. ( 10.3390/v6114731) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Casper C, Krantz EM, Corey L, Kuntz SR, Wang J, Selke S, Hamilton S, Huang ML, Wald A. 2008. Valganciclovir for suppression of human herpesvirus-8 replication: a randomized, double-blind, placebo-controlled, crossover trial. J. Infect. Dis. 198, 23–30. ( 10.1086/588820) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Boivin G, Gaudreau A, Toma E, Lalonde R, Routy JP, Murray G, Handfield J, Bergeron MG. 1999. Human herpesvirus 8 DNA load in leukocytes of human immunodeficiency virus-infected subjects: correlation with the presence of Kaposi's sarcoma and response to anticytomegalovirus therapy. Antimicrob. Agents Chemother. 43, 377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Robles R, Lugo D, Gee L, Jacobson MA. 1999. Effect of antiviral drugs used to treat cytomegalovirus end-organ disease on subsequent course of previously diagnosed Kaposi's sarcoma in patients with AIDS. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 20, 34–38. ( 10.1097/00042560-199901010-00005) [DOI] [PubMed] [Google Scholar]
- 315.Martin DF, Kuppermann BD, Wolitz RA, Palestine AG, Li H, Robinson CA, Roche Ganciclovir Study Group 1999. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. N. Engl. J. Med. 340, 1063–1070. ( 10.1056/nejm199904083401402) [DOI] [PubMed] [Google Scholar]
- 316.Corbellino M. eet al. 2001. Long-term remission of Kaposi sarcoma-associated herpesvirus-related multicentric Castleman disease with anti-CD20 monoclonal antibody therapy. Blood 98, 3473–3475. ( 10.1182/blood.V98.12.3473) [DOI] [PubMed] [Google Scholar]
- 317.Marcelin AG, Aaron L, Mateus C, Gyan E, Gorin I, Viard JP, Calvez V, Dupin N. 2003. Rituximab therapy for HIV-associated Castleman disease. Blood 102, 2786–2788. ( 10.1182/blood-2003-03-0951) [DOI] [PubMed] [Google Scholar]
- 318.Gerard L, et al. 2007. Prospective study of rituximab in chemotherapy-dependent human immunodeficiency virus associated multicentric Castleman's disease: ANRS 117 CastlemaB Trial. J. Clin. Oncol. 25, 3350–3356. ( 10.1200/jco.2007.10.6732) [DOI] [PubMed] [Google Scholar]
- 319.Uldrick TS, et al. 2014. Rituximab plus liposomal doxorubicin in HIV-infected patients with KSHV-associated multicentric Castleman disease. Blood 124, 3544–3552. ( 10.1182/blood-2014-07-586800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Lim ST, Rubin N, Said J, Levine AM. 2005. Primary effusion lymphoma: successful treatment with highly active antiretroviral therapy and rituximab. Ann. Hematol. 84, 551–552. ( 10.1007/s00277-005-1040-6) [DOI] [PubMed] [Google Scholar]
- 321.Yaich S, Charfeddine K, Zaghdane S, El Aoud N, Jarraya F, Kharrat M, Hachicha J. 2012. Sirolimus for the treatment of Kaposi sarcoma after renal transplantation: a series of 10 cases. Transplant. Proc. 44, 2824–2826. ( 10.1016/j.transproceed.2012.09.025) [DOI] [PubMed] [Google Scholar]
- 322.Sabers CJ, Martin MM, Brunn GJ, Williams JM, Dumont FJ, Wiederrecht G, Abraham RT. 1995. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J. Biol. Chem. 270, 815–822. ( 10.1074/jbc.270.2.815) [DOI] [PubMed] [Google Scholar]
- 323.Koon HB, et al. 2005. Imatinib-induced regression of AIDS-related Kaposi's sarcoma. J. Clin. Oncol. 23, 982–989. ( 10.1200/JCO.2005.06.079) [DOI] [PubMed] [Google Scholar]
- 324.Koon HB, et al. 2014. Phase II trial of imatinib in AIDS-associated Kaposi's sarcoma: AIDS Malignancy Consortium Protocol 042. J. Clin. Oncol. 32, 402–408. ( 10.1200/JCO.2012.48.6365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Ardavanis A, Doufexis D, Kountourakis P, Rigatos G. 2008. A Kaposi's sarcoma complete clinical response after sorafenib administration. Ann. Oncol. 19, 1658–1659. ( 10.1093/annonc/mdn528) [DOI] [PubMed] [Google Scholar]
- 326.Gill MB, Turner R, Stevenson PG, Way M. 2015. KSHV-TK is a tyrosine kinase that disrupts focal adhesions and induces Rho-mediated cell contraction. EMBO J. 34, 448–465. ( 10.15252/embj.201490358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Sokal EM, et al. 2007. Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein-Barr virus vaccine in healthy young adults. J. Infect. Dis. 196, 1749–1753. ( 10.1086/523813) [DOI] [PubMed] [Google Scholar]