Abstract
Both large-wildlife loss and climatic changes can independently influence the prevalence and distribution of zoonotic disease. Given growing evidence that wildlife loss often has stronger community-level effects in low-productivity areas, we hypothesized that these perturbations would have interactive effects on disease risk. We experimentally tested this hypothesis by measuring tick abundance and the prevalence of tick-borne pathogens (Coxiella burnetii and Rickettsia spp.) within long-term, size-selective, large-herbivore exclosures replicated across a precipitation gradient in East Africa. Total wildlife exclusion increased total tick abundance by 130% (mesic sites) to 225% (dry, low-productivity sites), demonstrating a significant interaction of defaunation and aridity on tick abundance. When differing degrees of exclusion were tested for a subset of months, total tick abundance increased from 170% (only mega-herbivores excluded) to 360% (all large wildlife excluded). Wildlife exclusion differentially affected the abundance of the three dominant tick species, and this effect varied strongly over time, likely due to differences among species in their host associations, seasonality, and other ecological characteristics. Pathogen prevalence did not differ across wildlife exclusion treatments, rainfall levels, or tick species, suggesting that exposure risk will respond to defaunation and climate change in proportion to total tick abundance. These findings demonstrate interacting effects of defaunation and aridity that increase disease risk, and they highlight the need to incorporate ecological context when predicting effects of wildlife loss on zoonotic disease dynamics.
Keywords: ticks, tick-borne disease, defaunation, climate, exclosure, Coxiella burnetii
1. Introduction
Zoonotic diseases are a rising concern worldwide [1–3]. Yet, amid rapidly declining wildlife populations and global climate change, there is no consensus on how these perturbations will independently and interactively affect zoonotic disease risk. Anthropogenic land-use change is likely to play a substantial role in facilitating outbreaks through a variety of mechanisms [2,4], including changes in wildlife host populations and communities [3–6]. Meanwhile, climate changes can have substantial and variable effects on zoonotic diseases [7,8], even when considered in isolation of changes to host populations. Thus, the combined effects of wildlife loss and climate change are likely to be complex [7,9], but data are lacking, especially for regions where medical resources and research efforts are low and zoonotic disease risk is highest [2]. Although there has been a widespread call for more research on the net effects of anthropogenic changes on disease and disease vectors globally [3–5], large-scale experimental tests remain scarce.
Ticks and tick-borne pathogens provide a salient system for examining the effects of wildlife loss and climate changes on disease risk. Globally, ticks are considered to be the most important disease vectors for wildlife and domestic animals [10], and are second only to mosquitoes among vectors affecting humans [11]. Estimated economic costs of ticks and tick-borne disease are variable [12], and although no recent estimate has been made, one study attributed annual losses of US$ 13.9 billion worldwide to tick-borne disease in cattle alone [13].
Globally, the pervasive decline in large-wildlife populations [14] is affecting a wide range of ecological functions and services, including disease control [15,16]. Ticks are also likely to be affected, considering their inextricable links to host population dynamics. While a substantial body of work demonstrates complex relationships among hosts, predators, and ticks (e.g. for the Lyme disease system in North America [17]), few studies have experimentally investigated how size-selective defaunation, which simulates the disproportionate vulnerability of larger animals to human disturbance [14], affects tick abundance and risk of tick-borne disease (but see [18]). Size-selective defaunation can directly affect tick abundance through the loss of hosts [19] and can also indirectly affect tick survival by altering vegetation structure [20–23] and the abundance and composition of small-vertebrate hosts [22,24]. Large-mammal loss often accompanies small-mammal abundance increases [22,24,25], leading to changes in host availability for different tick species. The relative importance of these sometimes opposing factors is poorly understood for most systems and likely depends on vector life cycles and host associations.
Climate can also affect the prevalence and distribution of zoonotic pathogens, particularly those limited by climate-sensitive vectors [7,26–28]. This topic has become increasingly relevant in the context of global climate changes [7,9,29]. As tick survival can depend on factors such as rainfall and temperature [21,30,31], several models have predicted shifting tick ranges that result in net range expansions under climate change scenarios, although this varies among tick species [32]. This experiment is one of few field studies that consider climatic effects on multiple tick species simultaneously, and is situated in a region where climate changes are already pervasive and will be challenging to mitigate [33].
While the independent effects of climate change and biodiversity loss on zoonotic disease have received considerable recent attention, their potential interaction has not been well explored. For tick-borne diseases, prior studies have been largely correlative, yielding mixed results on the relative importance of various climate metrics, host abundance, and their interaction in determining tick abundance [34–37], emphasizing the need for more data describing a range of interacting forces on tick biology. The indirect effects of large herbivores on other small consumers, from insects to birds and small mammals, are highly sensitive to variation in climate and productivity [22,38,39], but it is not known whether these results can be generalized to disease risk in particular.
East African savannahs are hotspots of tick and tick-borne pathogen diversity [40], and tick-borne pathogens such as Rickettsia, Coxiella, and Anaplasma are major regional economic and human health concerns [41–43]. For example, a recent study in Tanzania found that bacterial zoonoses caused 26% of acute fever cases; of these, 20% were Q fever, caused by Coxiella burnetii, and 30% were Rickettsiosis, caused by spotted fever group Rickettsia [44]. Accordingly, African savannahs offer an ideal system for testing the effects of varying degrees of defaunation on tick abundance, as hosts are diverse and abundant, ranging over six orders of magnitude in size and occupying diverse functional roles [22,45]. However, large wildlife are experiencing widespread and precipitous declines in many parts of this region [46,47], underscoring the importance of predicting effects across ecological communities. Furthermore, climate change is also likely to affect tick-borne disease in East Africa, due in part to shifting rainfall patterns [31]. While large-scale predictions for future rainfall regimes are mixed [33], much of the region has been affected by persistent reductions in the critical ‘long rains’ since 1970 [48], and localized rainfall prediction models indicate that this trend is likely to continue [49].
We used a replicated series of experimental large-herbivore exclosures to quantify the effects of size-selective defaunation, climatic context, and their interaction on tick abundance and prevalence of tick-borne pathogens. In the light of evidence that other consumer groups respond both numerically and behaviourally to an interaction between defaunation and primary productivity [38,39,50,51], we hypothesized that (i) large-herbivore removal has strong effects on ticks and their associated pathogens, (ii) tick species that use small-mammal hosts will increase in abundance when large mammals are excluded (and small-mammal densities increase), and (iii) the strength of these effects are contingent on climatic context and are strongest in more arid, low-productivity areas.
2. Material and methods
(a). Survey site and exclosures
Research was conducted in the Ungulate Herbivory Under Rainfall Uncertainty (UHURU) experimental plots [22,52,53], established in 2008 at Mpala Research Centre (MRC) in Laikipia County, Kenya (0°17′ N, 37°52′ E, 1 600 m elevation). MRC supports robust populations of wildlife including elephants (Loxodonta africana), giraffe (Giraffa camelopardalis), zebra (Equus grevyi and Equus quagga), impala (Aepyceros melampus), and dik-dik (Madoqua kirkii), among others. The UHURU plots consist of four 1 ha exclosure treatments replicated three times at each of three ‘levels’ of a rainfall and productivity gradient created by the rain shadow of Mt Kenya (i.e. nine total replicates of each treatment, 36 total plots; electronic supplementary material, table S1). The four treatments simulate different scenarios of size-selective species losses using different combinations of fencing. The treatments are as follows: (i) total exclusion of all ungulate herbivores (Total exclosure), (ii) exclusion of all herbivores greater than 15 kg (Meso exclosure), (iii) exclusion of only mega-herbivores (i.e. giraffe and elephant; ‘Mega exclosure’), and (iv) unfenced open plots (Control) [22]. Mean annual precipitation increases approximately 45% from the arid northern sites (440 mm yr−1), to the mesic southern sites (640 mm yr−1), with central sites intermediate (580 mm yr−1). Seasonal rains typically fall from March to May (long rains) and October to December (short rains) [54]. As in other semi-arid savannahs, primary productivity is tightly linked to precipitation across this gradient [22]. Although the Normalized Difference Vegetation Index (NDVI) has been used previously in studies of tick abundance [21], we used mean annual rainfall as the primary climatic variable in our analyses, both because NDVI increases in exclosure treatments due to decreased herbivory and trampling by large mammals [22] (and thus would not isolate climatic factors), and because climatic factors tend to outperform NDVI in predicting African tick distributions [31]. We also present a complementary analysis using a categorical ‘climatic level’ variable in lieu of the continuous precipitation variable; results are qualitatively similar (electronic supplementary material, tables S2 and S3).
(b). Ticks
The density of infected vectors is a common metric of vector-borne zoonotic disease risk [15,55,56] and is directly related to both vector density and pathogen infection rate. Thus, changes in tick density, infection rate, or a combination of the two can affect disease risk. To measure disease risk, we used tick drags and pathogen screening to quantify the density and infection rate of ticks.
(c). Tick drags
Ticks were collected in Total exclosure and Control plots each month for 13 months between October 2013 and November 2014. For each survey, a standard white canvas cloth was dragged throughout all passable portions of each plot, but areas of dense thicket areas were not sampled. Because exclosure plots often featured thick, thorny vegetation that precluded drags over fixed linear distances, we conducted drags for a 1 h period, with ticks collected every 5 min. We also surveyed the Mega and Meso exclosure plots for five months in 2014 (January, July, August, September, and November). To ensure that drags accurately estimated the tick species composition of each plot, the drags were complemented with CO2 traps [57] for two months.
Ticks were subsequently identified to species using microscopy and descriptions from [58]. We focused all analyses on three congeneric tick species—Rhipicephalus pravus, R. praetextatus, and R. pulchellus—that dominated the tick community. These tick species vary considerably in typical host preferences for each of their three distinct life stages (electronic supplementary material, figure S1). In general, immature stages of R. pravus and R. praetextatus feed upon small mammals (particularly rodents), which roughly double in abundance within total exclosures [22,53], whereas all stages of R. pulchellus feed on larger mammals [58,59]. Thus, the UHURU exclosure design alters the dominant host availability for each of these tick species (electronic supplementary material, figure S1 [22,53,58,59]).
(d). Pathogen screening
We extracted DNA and prepared double-indexed libraries for 136 ticks following [60]. Tick sample size was calculated to detect a 10% variation in pathogen prevalence across treatments while sampling across multiple species, treatments, and levels. Ticks with insufficient read data were excluded. Libraries were captured in pools of eight individuals (12.5 ng each library per capture; 100 ng total library per pool) using the Ectobaits protocol [60]. Double-indexed libraries were then amplified post capture with Illumina adapters by 18 cycles of PCR. Adapter multimers were removed prior to sequencing using QIAEX II Gel Extraction Kits (Qiagen). Captured products were sequenced on a MiSeq (Illumina, USA) using paired-end 150 bp reads. MiSeq library sequences underwent quality control as described in [60], except that the minimum average base quality score was 25. We differentiated between C. burnetii and Coxiella-like endosymbionts, as these groups are genetically similar, but endosymbionts are non-pathogenic and often have high infection rates [61]. We reanalysed five libraries (KenT11b–KenT15b) included in [60]. For a subset of ticks (n = 20), we confirmed Rickettsia, Coxiella, Ehrlichia, and Anaplasma infection and tick species using PCR assays following [60]. Positive PCR products were sequenced with an ABI 3130xl (Thermo Fisher Scientific, USA).
(e). Statistical analyses
We analysed the tick drag data with generalized linear mixed models (GLMMs), using counts of adult ticks per plot as our response variable [62]. Fixed effects included treatment (Total exclosure and Control for all months; all treatments for a subset of months), mean annual precipitation, and the treatment × rainfall interaction; random effects included replicate plot identity (three plots within each of three rainfall levels; n = 9) and time period (month; n = 12 for Total exclosure versus Control, n = 5 for all treatments). We ran two separate sets of GLMMs: one for Total exclosure and Control plots across all months, and another for all plots for the subset of five months. Candidate-model sets included all possible combinations of the two main effects and their interaction (the ‘full model’), along with a null model; all models included the random effects (table 1; electronic supplementary material, table S4). We analysed the combined total of all tick species and each species separately. As data were overdispersed and zero-inflated for individual tick species, we used zero-inflated negative-binomial distributions with log link functions in our GLMMs. For the two datasets that combined the three tick species, we used negative-binomial distributions with log link functions. All models were constructed using the glmmADMB package in R [63,64].
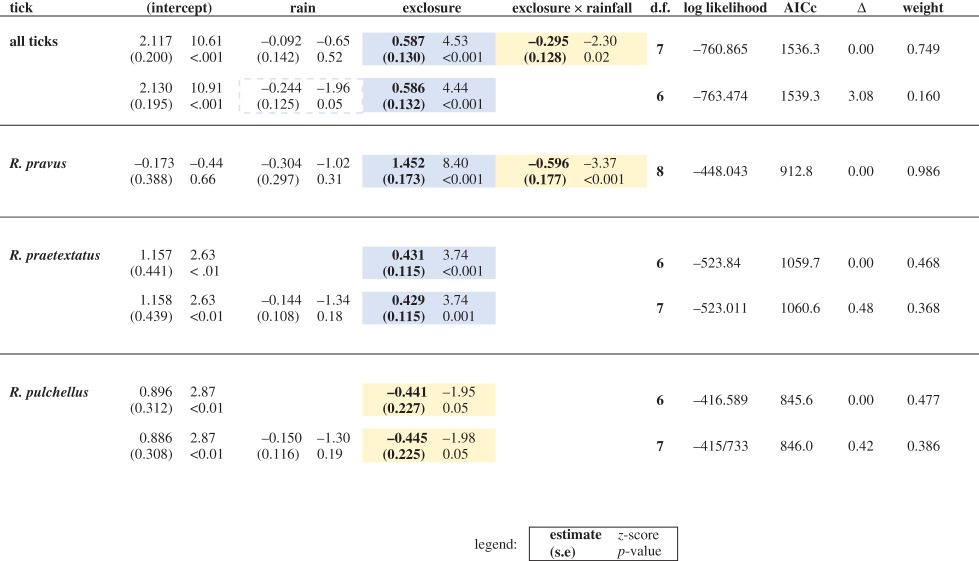
Table 1.
Effects of exclosure treatment, rainfall, and their interaction for all months (Control and Total exclosure plots only) from four GLMMs. Control plots are designated as the reference, and rainfall (millimetres) is scaled by standard error (84 mm) and centred at the mean (533 mm) for ease of interpretation. Significant relationships (p < 0.05) are in bold. Positive relationships are shaded in blue (darker); negative relationships are shaded in yellow (lighter). All estimates are shown with standard errors, z-score (upper right), and p-value (lower right). Full model sets and parameters are shown in electronic supplementary material, table S5. (Online version in colour.)
 |
All model combinations for each tick species and the combined total of ticks were ranked using the second-order Akaike's information criterion (AICc) [62] using the MuMIn package [65]. We investigated all models (reported in electronic supplementary material, S5 and S6) and present the 95% confidence interval set with individual parameter estimates and Akaike weights (wi) in tables 1 and 2.
Table 2.
Top models (95% CI) of exclosure treatment and rainfall on tick abundance (for a subset of months) from four GLMMs. Exclosure compares Control plots (all wildlife allowed), the reference, with plots that selectively exclude mega-herbivores (MEGA), mega and meso herbivores (MESO), and all herbivores greater than 5 kg (TOTAL). Rainfall (millimetres) is scaled by standard error (84 mm) and centred at the mean (533 mm) for ease of interpretation. Significant relationships (p < 0.05) are in bold, marginally significant relationships (p < 0.1) are bordered by a broken line, positive relationships are shaded in blue (darker), and negative relationships are shaded in yellow (lighter). All estimates are shown with standard errors, z-score (upper right), and p-value (lower right). Full model sets and parameters are shown in electronic supplementary material, table S6. (Online version in colour.)
 |
Coxiella burnetii and Rickettsia spp. were the only pathogens sufficiently prevalent to permit robust statistical analysis. We analysed the likelihood of infection using binomial GLMMs with logit link functions, with infection status of each tick (infected/uninfected) as the response. Experimental treatment, tick species, rainfall, and treatment × rainfall were fixed effects and plot replicate was a random effect.
All analyses were performed in R v. 3.3.0 [66]. Descriptive statistics are reported as mean number of ticks per ha ± 1 s.e.
3. Results
In total, we captured 5 677 ticks across all plots, including 4 180 via tick drags and 1 497 via traps. Of these, greater than 95% were adults of just three species: R. pravus (43%), R. praetextatus (36%), and R. pulchellus (17%). Adults were substantially more abundant than other life stages in both drag and trap collections, despite efforts to avoid undersampling juvenile ticks. Fewer than 3% of the ticks captured were nymphs, and no larvae were collected. Tick traps did not capture additional tick species; therefore, we used only drag data for all subsequent analyses (electronic supplementary material, S1, table S4 and figures S2, S3) and focused all analyses on adults of the three dominant species.
(a). Total abundance of the three dominant tick species
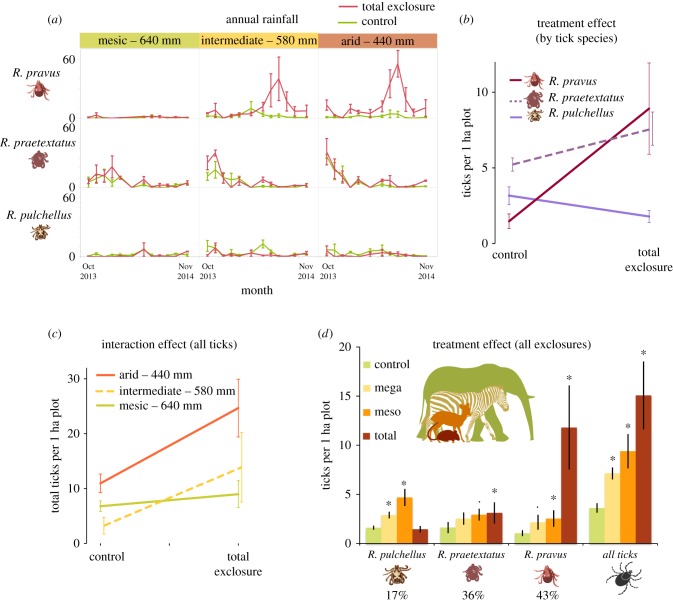
Total tick abundance varied seasonally over the 13-month sampling period, and the scale and timing of fluctuations differed among tick species (figure 1a). However, on average, total tick abundance doubled in Total exclosures (18.3 ± 1.9) relative to Control plots (9.9 ± 1.0) (figure 1a,b and table 1). Low-rainfall plots had 225% more ticks on average (17.8 ± 2.3) than mesic plots (7.9 ± 1.0). Total tick abundance was best explained by the GLMM that included exclosure treatment, precipitation, and their interaction (table 1; electronic supplementary material, table S5) (wi = 0.75). The interaction (z = −2.3, p = 0.02; table 1) reflected the increasing effect of wildlife exclusion on tick abundance as aridity increased (figure 1c and table 1; electronic supplementary material, table S5). We found some support (wi = 0.16) for a model with no interaction and a marginally negative relationship between rainfall and tick abundance (z = −1.96, p = 0.05). Net results were similar in the analysis that considered all four wildlife exclusion treatments for a subset of months: total tick abundance increased from 170% (only mega-herbivores excluded) to 360% (all large wildlife excluded) (figure 1d). The full model was again the best fit (wi = 0.99), with significant interactions between rainfall and the Total and Meso exclosure treatments (z = −3.61, p = 0.001, Total; z = −3.38, p = 0.001, Meso, table 2; electronic supplementary material, table S6).
Figure 1.
(a) Tick abundance varied over time, across rainfall levels, among species, and between treatments for the full 13-month dataset. (b) While total tick abundance increased in Total exclosures, the magnitude and direction of this effect varied by tick species for the 13-month dataset. (c) For all tick species summed together, exclusion interacted with annual rainfall, with stronger effects of exclusion in drier environments. (d) When all exclosures were surveyed for the five-month subset of data, tick species responded differently to varied wildlife loss levels. Asterisks indicate significant (p < 0.05) differences from Control plots (green; left-most column); dots indicate non-significant trends (p < 0.1). (Online version in colour.)
(b). Species-specific responses
Although R. pravus and R. praetextatus, two tick species that often parasitize smaller mammals, increased with large-mammal loss, only R. pravus abundance showed clear evidence of an interaction between exclosure and aridity. For the full 13 months of data, the best model for R. pravus included treatment, rainfall, and their interaction (wi = 0.99), whereas the best model for R. praetextatus included only treatment (wi = 0.47) and a second model (wi = 0.37) included the non-significant effect of rainfall (table 1). Both tick species increased in Total exclosures relative to Controls (z = 8.40, p < 0.001, R. pravus; z = 3.74, p < 0.001, R. praetextatus), and this effect was stronger in drier sites for R. pravus only (z = −3.37, p < 0.001). By contrast, rainfall had no detectable effect on tick abundance in Control plots (z = −1.02, p = 0.31). For the subset of data collected in all four wildlife exclusion treatments, the full model was the best fit for both tick species (wi = 0.93 and wi = 0.78, R. pravus and R. praetextatus, respectively). Both tick species increased in all exclosure treatments relative to Controls, and both increased significantly in Total exclosures (z = 7.22, p < 0.001; z = 4.07, p < 0.001, table 2). This effect was more pronounced in drier sites for both species, although this was only significant for R. praetextatus in Meso exclosures (z = −2.26, p = 0.02) and R. pravus in Total exclosures (z = −3.26, p < 0.001, table 2). A second model for R. praetextatus that included only treatment (wi = 0.13) received considerably less support.
For R. pulchellus, which often parasitize larger-bodied mammals, the best model for all months included only exclosure treatment (wi = 0.48), and a second model (wi = 0.39) included the non-significant effect of rainfall; but here Total wildlife exclusion caused a 43% decrease in abundance relative to Controls (z = −1.95, p = 0.05; table 1 and figure 1b). For the subset of data including all four treatments, the best model (wi = 0.46) again included only exclosure treatment, while a second model (wi = 0.37) included the non-significant effect of rainfall. However, this secondary analysis revealed that partial wildlife exclusion caused increases in tick abundance relative to controls (z = 4.72, p < 0.001, Meso; z = 2.44, p = 0.02, Mega, table 2 and figure 1d; electronic supplementary material, table S6), but total exclusion had no significant effect (z = −0.57, p = 0.57).
(c). Pathogens
The prevalence of C. burnetii isolates was 43% (n = 58 of 136 ticks screened), and the prevalence of Rickettsia spp. was 5% (n = 7 of 136 ticks; four of these were from the spotted fever group). We detected Ehrlichia in one adult tick and Anaplasma in one nymph (nymphs were not analysed due to the small sample size). We found a high prevalence of non-pathogenic Coxiella-like endosymbionts (57%; 46% of these were also present in ticks with confirmed C. burnetii isolates). Therefore, our analyses excluded ticks for which only a Coxiella-like endosymbiont was detected, but included ticks with both C. burnetii and Coxiella-like endosymbionts.
For the GLMM for C. burnetii, no combination of our predictors outperformed the null model (table 3). For Rickettsia spp., the best model included tick species and rainfall; however, neither estimate was significant (although rainfall marginally increased infection probability; table 3). In summary, there were no pronounced effects of treatment, tick species, or rainfall on pathogen prevalence (figure 2 and table 3; electronic supplementary material, table S7 and figure S4).
Table 3.
Results of GLMMs for Coxiella burnetii and Rickettsia sp. ‘Species’ compares the probability of tick infection with each pathogen for each tick species (R. pravus—RHPV and R. pulchellus—RHPU, when compared with R. praetextatus—RHPR). Marginally significant relationships (p < 0.1) are bordered by a broken line. All estimates are shown with standard errors, z-score (upper right), and p-value (lower right). (Online version in colour.)
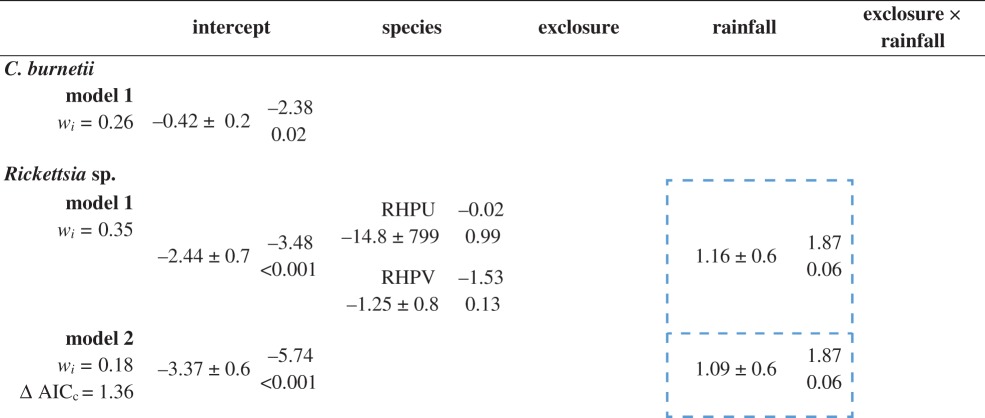 |
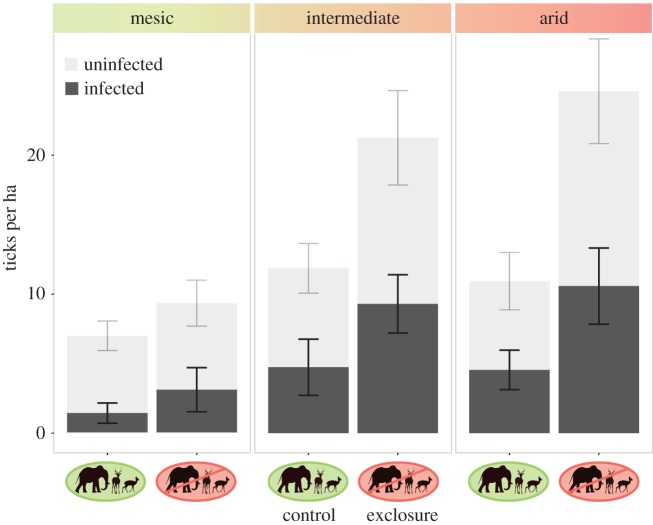
Figure 2.
The estimated number of infected and uninfected ticks increased in plots where large wildlife had been removed (Exclosure), and this was further increased in arid sites, regardless of tick-borne pathogen infection. (Online version in colour.)
4. Discussion
Our results support our hypothesis that defaunation and climate can interact to markedly affect the abundance of ticks and thus the risk of tick-borne disease exposure (although not necessarily the prevalence of these pathogens). Total exclusion of all large wildlife increased total tick abundance by 130% (mesic sites) to 225% (arid sites), showing a significant interaction with aridity. Tick abundance increased from 170% (only mega-herbivores excluded) to 360% (all large wildlife excluded) during the five-month period in which all exclosure plots were surveyed. We found no significant variation in pathogen prevalence across plots or tick species, suggesting that the risk of tick-borne pathogen exposure reflects observed tick abundance patterns.
However, this overall pattern masks strong differences in the magnitude and direction of effects of wildlife exclusion across tick species and over time. Tick species-specific responses show some overlap with expectations based on tick–host associations. Patterns in total tick abundance were driven by two dominant tick species, R. pravus and R. praetextatus, whose immature stages frequently feed upon small hosts, which also increase strongly following wildlife exclusion [22,50,51]. Although we do not expect changes in adult tick abundance to directly correlate with fluctuations in rodent abundance in these plots over time, a comparison of long-term rodent abundance and tick abundance within each plot produces positive correlations for R. pravus and R. praetextatus (z = 6.59, p < 0.001 and z = 3.17, p < 0.01, respectively; electronic supplementary material, table S8). By contrast, the third common tick species, R. pulchellus, whose adult stages primarily parasitize vertebrates larger than 15 kg [58], and whose immature stages are not found on rodents [59], decreased with the total absence of large wildlife for the 13-month dataset. However, for the five months for which all four exclosure treatments were surveyed, the abundance of this tick species in total exclosures was no different from that in controls, but we observed marked increases in abundance within partial wildlife exclosures (see electronic supplementary material, figure S1 for tick/host associations in exclosure plots). This discrepancy highlights temporal variation in exclosure effects: strong changes occur during months of peak tick abundance, which were not captured by the five-month dataset.
Other factors beyond the release of intermediate hosts may have also influenced the marked differences in adult tick abundance among experimental plots. Increases in small carnivores (potential hosts for all three tick species) in response to elevated rodent density in exclosure plots may increase total tick abundance [18]. Likewise, increases in understory vegetation cover following large-wildlife loss may increase tick survivorship (via lowered risk of desiccation) [22]. The relative importance of these factors may vary among tick species depending on their life histories. The complex pathways by which wildlife loss may affect the abundance of different tick species likely explains why the few previous studies on the effects of large-wildlife exclosure on tick abundance have produced mixed results [18,67].
Total tick abundance was greater in drier areas, although this pattern was largely driven by the most common tick species, R. pravus. R. praetextatus and R. pulchellus only increased modestly in these areas, and annual rainfall was not a major explanatory factor in models of their abundance. This is consistent with previous observations of climate preferences for these species, as R. pravus may particularly favour areas with extended dry seasons [61]. Notably, tick community composition varied considerably over seasons, and the most significant responses to exclosures occurred at months of peak abundance (figure 1a). These months of peak abundance drove overall patterns for each species and are likely to be a result of strong differences in tick phenology and responsiveness to rainfall.
Rhipicephalus pravus also drove an interaction between wildlife exclosure treatment and aridity on tick abundance, despite variation among tick species. This interaction and its variation are consistent with prior studies of the effects of defaunation on consumer communities, including a recent meta-analysis that found these effects are often context-dependent and mediated by site productivity [39,50,68]. In this region, rodent-borne pathogens have shown a similar response: anthropogenic disturbance tends to cause stronger increases in rodent-borne disease in drier climates with lower productivity [69]. However, consistent with our findings here, responses are variable across specific hosts and pathogens [69].
Both pathogens analysed in this study are globally important. C. burnetii, the causative agent of Q fever, is considered to be an emerging zoonotic disease [70], while rickettsial pathogens are responsible for a variety of spotted fevers—including African tick-bite fever (caused by Rickettsia africae) in our study location [42]. We observed no significant differences in the prevalence of either C. burnetii or Rickettsia spp. due to wildlife exclosure treatment, rainfall, or tick species. Larger sample sizes and screening over many seasons might reveal finer-scale dynamics; however, on a coarse level, this result suggests that tick-borne disease risk is likely to be well approximated by estimates of total tick abundance (figure 2). C. burnetii prevalence was surprisingly high. Although we excluded ticks for which only an endosymbiont was detected, 67% of the ticks infected with C. burnetii were also positive for the Coxiella-like endosymbiont. Endosymbionts may benefit some ticks [61], and recent work suggests that C. burnetii recently emerged from this group [71]. Thus, the genetic similarity between C. burnetii and Coxiella-like endosymbionts may have yielded some false positives given that the full Coxiella phylogeny is incomplete. However, we do not expect this to bias our results, given that the likelihood of false positives is consistent across all predictors.
Our study demonstrates the significant potential for size-selective defaunation to alter the risk of tick-borne disease. Substantial variation in tick abundance and species composition over time reflect the inherent complexity of a system that depends on host, environmental, and vector variables, but total effects suggest long-term patterns, especially when ticks peak in abundance. On average, when all large wildlife were excluded, the total number of ticks nearly doubled; and, when only Mega wildlife and Meso wildlife were excluded (perhaps a more realistic short-term defaunation scenario for much of the world), ticks of all three major species increased, suggesting that large-wildlife loss can contribute to an increased tick-borne disease risk that may be mitigated by conservation in many contexts. Furthermore, the costs of wildlife loss on tick-borne disease in this region may be intensified in drier, less productive areas that are likely to worsen with a changing climate [48], demonstrating interacting effects of wildlife loss and climate change on tick-borne disease risk. On a more global scale, our study highlights the challenge of predicting the effects of either biodiversity loss or climate change in isolation of other stressors on vector ecologies and infectious disease dynamics.
Supplementary Material
Acknowledgements
We thank the National Commission for Science, Technology and Innovation of the Kenyan Government, Kenya Wildlife Service, National Museums Kenya and Mpala Research Centre for their assistance.
Data accessibility
Datasets and R code for all analyses are available at: https://github.com/gtitcomb/Wildlife-loss-climate-ticks. Read data from this project are available in the BioProject Archive (accession PRJNA362357). Reanalysed library accessions are: SRS1133052, SRS1133057, SRS1133060, SRS1133069 and SRS1133099.
Authors' contributions
G.T. conducted fieldwork, performed analyses and wrote the first version of the manuscript; B.F.A. and T.H. identified ticks and contributed to the final report; T.A. conducted fieldwork, tick identification, sample logistics, and data entry; L.H. conducted all biomolecular laboratory work; R.M.P. and T.M.P. designed and provided access to experimental plots, contributed data, and assisted with data interpretation and writing the final report; L.N. assisted in obtaining Kenyan research permits and provided report feedback; M.G.C. conducted bioinformatic analyses and provided report feedback; R.F. coordinated and supervised molecular work; J.N.M. conducted fieldwork; H.S.Y. conceived the project and analyses, and assisted with data interpretation and writing the manuscript.
Competing interests
We declare no competing interests.
Funding
Financial support for this project came from the National Science Foundation Graduate Research Fellowship (1650114), the Morris Animal Foundation (D14ZO-308), the National Geographic Society (grant nos. 8846-10, 9291-13 and 9829-15), the National Science Foundation (DEB-1556786, DEB-1547679, DEB-1355122, DEB-09-09670 and CNH-1313822), the National Sciences and Engineering Research Council of Canada, the University of Wyoming, the University of Florida, and the Princeton Environmental Institute's Grand Challenges Initiative.
References
- 1.Smith KF, Goldberg M, Rosenthal S, Carlson L, Chen J, Chen C, Ramachandran S. 2014. Global rise in human infectious disease outbreaks. J. R. Soc. Interface 11, 20140950 ( 10.1098/rsif.2014.0950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451, 990–993. ( 10.1038/nature06536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottdenker NL, Streicker DG, Faust CL, Carroll CR. 2014. Anthropogenic land use change and infectious diseases: a review of the evidence. Ecohealth 11, 619–632. ( 10.1007/s10393-014-0941-z) [DOI] [PubMed] [Google Scholar]
- 4.Patz JA, et al. 2004. Unhealthy landscapes: policy recommendations on land use change and infectious disease emergence on JSTOR. Environ. Health Perspect. 112, 1092–1098. ( 10.1289/ehp.6877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Civitello DJ, et al. 2015. Biodiversity inhibits parasites: broad evidence for the dilution effect. Proc. Natl Acad. Sci. USA 112, 8667–8671. ( 10.1073/pnas.1506279112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keesing F, et al. 2010. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468, 647–652. ( 10.1038/nature09575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohr JR, Dobson AP, Johnson PTJ, Kilpatrick AM, Paull SH, Raffel TR, Ruiz-Moreno D, Thomas MB. 2011. Frontiers in climate change-disease research. Trends Ecol. Evol. 26, 270–277. ( 10.1016/j.tree.2011.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lafferty KD. 2009. The ecology of climate change and infectious diseases. Ecology 90, 888–900. ( 10.1890/08-0079.1) [DOI] [PubMed] [Google Scholar]
- 9.Patz JA, Olson SH, Uejio CK, Gibbs HK. 2008. Disease emergence from global climate and land use change. Med. Clin. N. Am. 92, 1473–1491. ( 10.1016/j.mcna.2008.07.007) [DOI] [PubMed] [Google Scholar]
- 10.Young AS, Groocock CM, Kariuki DP. 1988. Integrated control of ticks and tick-borne diseases of cattle in Africa. Parasitology 96, 403 ( 10.1017/S0031182000058388) [DOI] [PubMed] [Google Scholar]
- 11.de la Fuente J, Estrada-Peña A, Venzal JM, Kocan KM, Sonenshine DE. 2008. Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 13, 6938–6946. ( 10.2741/3200) [DOI] [PubMed] [Google Scholar]
- 12.Jongejan F, Uilenberg G. 2004. The global importance of ticks. Parasitology 129, S3–S14. ( 10.1017/S0031182004005967) [DOI] [PubMed] [Google Scholar]
- 13.de Castro JJ. 1997. Sustainable tick and tickborne disease control in livestock improvement in developing countries. Vet. Parasitol. 71, 77–97. ( 10.1016/S0304-4017(97)00033-2) [DOI] [PubMed] [Google Scholar]
- 14.Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJB, Collen B. 2014. Defaunation in the Anthropocene. Science 345, 401–406. ( 10.1126/science.1251817) [DOI] [PubMed] [Google Scholar]
- 15.Young HS, et al. 2014. Declines in large wildlife increase landscape-level prevalence of rodent-borne disease in Africa. Proc. Natl Acad. Sci. USA 111, 7036–7041. ( 10.1073/pnas.1404958111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estes JA, et al. 2011. Trophic downgrading of planet earth. Science 333, 301–306. ( 10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 17.Levi T, Kilpatrick AM, Mangel M, Wilmers CC. 2012. Deer, predators, and the emergence of Lyme disease. Proc. Natl Acad. Sci. USA 109, 10 942–10 947. ( 10.1073/pnas.1204536109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keesing F, Allan BF, Young TP, Ostfeld RS. 2013. Effects of wildlife and cattle on tick abundance in central Kenya. Ecol. Appl. 23, 1410–1418. ( 10.1890/12-1607.1) [DOI] [PubMed] [Google Scholar]
- 19.Wilson ML, Adler GH, Spielman A. 1985. Correlation between abundance of deer and that of the deer tick, Ixodes dammini (Acari: Ixodidae). Ann. Entomol. Soc. Am. 78, 172–176. ( 10.1093/aesa/78.2.172) [DOI] [Google Scholar]
- 20.Civitello DJ, Flory SL, Clay K. 2008. Exotic grass invasion reduces survival of Amblyomma americanum and Dermacentor variabilis ticks (Acari: Ixodidae). J. Med. Entomol. 45, 867–872. ( 10.1093/jmedent/45.5.867) [DOI] [PubMed] [Google Scholar]
- 21.Randolph SE. 2000. Ticks and tick-borne disease systems in space and from space. Adv. Parasitol. 47, 217–243. ( 10.1016/S0065-308X(00)47010-7) [DOI] [PubMed] [Google Scholar]
- 22.Goheen JR, Palmer TM, Charles GK, Helgen KM, Kinyua SN, Maclean JE, Turner BL, Young HS, Pringle RM. 2013. Piecewise disassembly of a large-herbivore community across a rainfall gradient: the UHURU experiment. PLoS ONE 8, e55192 ( 10.1371/journal.pone.0055192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pringle RM, Kimuyu DM, Sensenig RL, Palmer TM, Riginos C, Veblen KE, Young TP. 2015. Synergistic effects of fire and elephants on arboreal animals in an African savanna. J. Anim. Ecol. 84, 1637–1645. ( 10.1111/1365-2656.12404) [DOI] [PubMed] [Google Scholar]
- 24.Dirzo R, et al. 2015. KLEE: a long-term multi-species herbivore exclusion experiment in Laikipia, Kenya. Ecology 11, 205–215. ( 10.1038/38271) [DOI] [Google Scholar]
- 25.Mills JN. 2006. Biodiversity loss and emerging infectious disease: an example from the rodent-borne hemorrhagic fevers. Biodiversity 7, 9–17. ( 10.1080/14888386.2006.9712789) [DOI] [Google Scholar]
- 26.Estrada-Peña A, Ostfeld RS, Peterson AT, Poulin R, de la Fuente J. 2014. Effects of environmental change on zoonotic disease risk: an ecological primer. Trends Parasitol. 30, 205–214. ( 10.1016/j.pt.2014.02.003) [DOI] [PubMed] [Google Scholar]
- 27.Xu L, Schmid BV, Liu J, Si X, Stenseth NC, Zhang Z. 2015. The trophic responses of two different rodent–vector–plague systems to climate change. Proc. R. Soc. B 282, 20141846 ( 10.1098/rspb.2014.1846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilcox BA, Gubler DJ. 2005. Disease ecology and the global emergence of zoonotic pathogens. Environ. Health Prev. Med. 10, 263–272. ( 10.1007/BF02897701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisen L. 2008. Climate change and tick-borne diseases: a research field in need of long-term empirical field studies. Int. J. Med. Microbiol. 298, 12–18. ( 10.1016/j.ijmm.2007.10.004) [DOI] [Google Scholar]
- 30.Randolph SE. 1993. Climate, satellite imagery and the seasonal abundance of the tick Rhipicephalus appendiculatus in southern Africa: a new perspective. Med. Vet. Entomol. 7, 243–258. ( 10.1111/j.1365-2915.1993.tb00684.x) [DOI] [PubMed] [Google Scholar]
- 31.Cumming GS. 2002. Comparing climate and vegetation as limiting factors for species ranges of African ticks. Ecology 83, 255–268. ( 10.1890/0012-9658(2002)083%5B0255:CCAVAL%5D2.0.CO;2) [DOI] [Google Scholar]
- 32.Olwoch JM, Van Jaarsveld AS, Scholtz CH, Horak I. 2007. Climate change and the genus Rhipicephalus (Acari: Ixodidae) in Africa. Onderstepoort J. Vet. Res. 74, 45–72. ( 10.4102/ojvr.v74i1.139) [DOI] [PubMed] [Google Scholar]
- 33.IPCC. 2014. Climate Change 2014: Synthesis Report. In Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. Geneva, Switzerland: IPCC. [Google Scholar]
- 34.Ostfeld RS, Canham CD, Oggenfuss K, Winchcombe RJ, Keesing F. 2006. Climate, deer, rodents, and acorns as determinants of variation in Lyme-disease risk. PLoS Biol. 4, e145 ( 10.1371/journal.pbio.0040145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilbert L. 2009. Altitudinal patterns of tick and host abundance: a potential role for climate change in regulating tick-borne diseases? Oecologia 162, 217–225. ( 10.1007/s00442-009-1430-x) [DOI] [PubMed] [Google Scholar]
- 36.Gallivan G, Spickett A, Heyne H, Spickett A, Horak I. 2011. The dynamics of questing ticks collected for 164 consecutive months off the vegetation of two landscape zones in the Kruger National Park (1988-2002). Part III. The less commonly collected species. Onderstepoort J. Vet. Res. 78, 27–35. ( 10.4102/ojvr.v78i1.41) [DOI] [PubMed] [Google Scholar]
- 37.Oorebeek M, Kleindorfer S. 2008. Climate or host availability: what determines the seasonal abundance of ticks? Parasitol. Res. 103, 871–875. ( 10.1007/s00436-008-1071-8) [DOI] [PubMed] [Google Scholar]
- 38.Pringle RM, Young TP, Rubenstein DI, McCauley DJ. 2007. Herbivore-initiated interaction cascades and their modulation by productivity in an African savanna. Proc. Natl Acad. Sci. USA 104, 193–197. ( 10.1073/pnas.0609840104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daskin JH, Pringle RM. 2016. Does primary productivity modulate the indirect effects of large herbivores? A global meta-analysis. J. Anim. Ecol. 85, 857–868. ( 10.1111/1365-2656.12522) [DOI] [PubMed] [Google Scholar]
- 40.Cumming GS. 2000. Using habitat models to map diversity: pan-African species richness of ticks (Acari: Ixodida). J. Biogeogr. 27, 425–440. ( 10.1046/j.1365-2699.2000.00419.x) [DOI] [Google Scholar]
- 41.Minjauw B, McLeod A. 2003. Tick-borne diseases and poverty: the impact of ticks and tick-borne diseases on the livelihoods of small-scale and marginal livestock owners in India and eastern and southern Africa. Research report, DFID Animal Health Programme, Centre of Tropical Veterinary Medicine, University of Edinburgh.
- 42.Parola P, et al. 2013. Update on tick-borne rickettsioses around the world: a geographic approach. Clin. Microbiol. Rev. 26, 657–702. ( 10.1128/CMR.00032-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DePuy W, et al. 2014. Q fever risk across a dynamic, heterogeneous landscape in Laikipia County, Kenya. Ecohealth 11, 429–433. ( 10.1007/s10393-014-0924-0) [DOI] [PubMed] [Google Scholar]
- 44.Crump JA, et al. 2013. Etiology of severe non-malaria febrile illness in Northern Tanzania: a prospective cohort study. PLoS Neglect. Trop. Dis. 7, e2324 ( 10.1371/journal.pntd.0002324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young TP, Okello BD, Kinyua D, Palmer TM. 1997. KLEE: a long term multi species herbivore exclusion experiment in Laikipia, Kenya. Afr. J. Range Forage Sci. 14, 94–102. ( 10.1080/10220119.1997.9647929) [DOI] [Google Scholar]
- 46.Craigie ID, Baillie JEM, Balmford A, Carbone C, Collen B, Green RE, Hutton JM. 2010. Large mammal population declines in Africa's protected areas. Biol. Conserv. 143, 2221–2228. ( 10.1016/j.biocon.2010.06.007) [DOI] [Google Scholar]
- 47.Ogutu JO, Piepho H-P, Said MY, Ojwang GO, Njino LW, Kifugo SC, Wargute PW. 2016. Extreme wildlife declines and concurrent increase in livestock numbers in Kenya: what are the causes? PLoS ONE 11, e0163249 ( 10.1371/journal.pone.0163249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Camberlin P, Philippon N. 2002. The East African March–May rainy season: associated atmospheric dynamics and predictability over the 1968–97 period. J. Clim. 15, 1002–1019. ( 10.1175/1520-0442(2002)015%3C1002:TEAMMR%3E2.0.CO;2) [DOI] [Google Scholar]
- 49.Williams AP, Funk C. 2011. A westward extension of the warm pool leads to a westward extension of the walker circulation, drying eastern Africa. Clim. Dyn. 37, 2417–2435. ( 10.1007/s00382-010-0984-y) [DOI] [Google Scholar]
- 50.Young HS, et al. 2015. Context-dependent effects of large-wildlife declines on small-mammal communities in central Kenya. Ecol. Appl. 25, 348–360. ( 10.1890/14-0995.1) [DOI] [PubMed] [Google Scholar]
- 51.Long RA, Wambua A, Goheen JR, Palmer TM, Pringle RM. 2017. Climatic variation modulates the indirect effects of large herbivores on small-mammal habitat use. J. Anim. Ecol. 86, 739–748. ( 10.1111/1365-2656.12669) [DOI] [PubMed] [Google Scholar]
- 52.Pringle R. 2012. How to be manipulative. Am. Sci. 100, 30–37. [Google Scholar]
- 53.Kartzinel TR, Goheen JR, Charles GK, DeFranco E, Maclean JE, Otieno TO, Palmer TM, Pringle RM. 2014. Plant and small-mammal responses to large-herbivore exclusion in an African savanna: five years of the UHURU experiment. Ecology 95, 787 ( 10.1890/13-1023R.1) [DOI] [Google Scholar]
- 54.Schmocker J, Liniger HP, Ngeru JN, Brugnara Y, Auchmann R, Brönnimann S. 2016. Trends in mean and extreme precipitation in the Mount Kenya region from observations and reanalyses. Int. J. Climatol. 36, 1500–1514. ( 10.1002/joc.4438) [DOI] [Google Scholar]
- 55.Ostfeld RS, Keesing F. 2000. Biodiversity series: the function of biodiversity in the ecology of vector-borne zoonotic diseases. Can. J. Zool. 78, 2061–2078. ( 10.1139/z00-172) [DOI] [Google Scholar]
- 56.LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. 2003. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc. Natl Acad. Sci. USA 100, 567–571. ( 10.1073/pnas.0233733100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ginsberg HS, Ewing CP. 1989. Comparison of flagging, walking, trapping, and collecting from hosts as sampling methods for northern deer ticks, Ixodes dammini, and lone-star ticks, Amblyomma americanum (Acari: Ixodidae). Exp. Appl. Acarol. 7, 313–322. ( 10.1007/BF01197925) [DOI] [PubMed] [Google Scholar]
- 58.Walker JB, Keirans JE, Horak IG. 2000. The genus Rhipicephalus (Acari, Ixodidae): a guide to the brown ticks of the world. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 59.Guerra AS, et al. 2016. Host–parasite associations in small mammal communities in semiarid savanna ecosystems of East Africa. J. Med. Entomol. 53, 851–860. ( 10.1093/jme/tjw048) [DOI] [PubMed] [Google Scholar]
- 60.Campana MG, et al. 2016. Simultaneous identification of host, ectoparasite and pathogen DNA via in-solution capture. Mol. Ecol. Resour. 16, 1224–1239. ( 10.1111/1755-0998.12524) [DOI] [PubMed] [Google Scholar]
- 61.Zhong J. 2012. Coxiella-like endosymbionts. In Coxiella burnetii: recent advances and new perspectives in research of the Q fever bacterium (eds Toman R, Heinzen RA, Samuel JE, Mege J-L), pp. 365–379. Berlin, Germany: Springer. [Google Scholar]
- 62.Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer. [Google Scholar]
- 63.Fournier DA, Skaug HJ, Ancheta J, Ianelli J, Magnusson A, Maunder MN, Nielsen A, Sibert J. 2012. AD model builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim. Methods Softw. 27, 233–249. ( 10.1080/10556788.2011.597854) [DOI] [Google Scholar]
- 64.Skaug H, Bolker B, Magnusson A, Nielsen A. 2016. Generalized linear mixed models using ‘AD Model Builder’. R Package Version 0.8. 3.3.
- 65.Barton K. 2013. MuMIn: multi-model inference. R package version 1.15. 6. Vienna, Austria: R Proj. Stat. Comput. [Google Scholar]
- 66.R Core Team. 2012. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.r-project.org/.
- 67.Perkins SE, Cattadori IM, Tagliapietra V, Rizzoli AP, Hudson PJ. 2006. Localized deer absence leads to tick amplification. Ecology 87, 1981–1986. ( 10.1890/0012-9658(2006)87%5B1981:LDALTT%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 68.Augustine DJ, McNaughton SJ. 2007. Interactive effects of ungulate herbivores, soil fertility, and variable rainfall on ecosystem processes in a semi-arid savanna. Ecosystems 9, 1242–1256. ( 10.1007/s10021-005-0020-y) [DOI] [Google Scholar]
- 69.Young HS, et al. 2017. Interacting effects of land use and climate on rodent-borne pathogens in central Kenya. Phil. Trans. R. Soc. B 372, 20160131 ( 10.1098/rstb.2016.0131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Njeru J, Henning K, Pletz MW, Heller R, Neubauer H. 2016. Q fever is an old and neglected zoonotic disease in Kenya: a systematic review. BMC Public Health 16, 297 ( 10.1186/s12889-016-2929-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duron O, et al. 2015. The recent evolution of a maternally-inherited endosymbiont of ticks led to the emergence of the Q fever pathogen, Coxiella burnetii. PLoS Pathog. 11, e1004892 ( 10.1371/journal.ppat.1004892) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets and R code for all analyses are available at: https://github.com/gtitcomb/Wildlife-loss-climate-ticks. Read data from this project are available in the BioProject Archive (accession PRJNA362357). Reanalysed library accessions are: SRS1133052, SRS1133057, SRS1133060, SRS1133069 and SRS1133099.