Abstract
The late Ediacaran soft-bodied macroorganism Dickinsonia (age range approx. 560–550 Ma) has often been interpreted as an early animal, and is increasingly invoked in debate on the evolutionary assembly of eumetazoan body plans. However, conclusive positive evidence in support of such a phylogenetic affinity has not been forthcoming. Here we subject a collection of Dickinsonia specimens interpreted to represent multiple ontogenetic stages to a novel, quantitative method for studying growth and development in organisms with an iterative body plan. Our study demonstrates that Dickinsonia grew via pre-terminal ‘deltoidal’ insertion and inflation of constructional units, followed by a later inflation-dominated phase of growth. This growth model is contrary to the widely held assumption that Dickinsonia grew via terminal addition of units at the end of the organism bearing the smallest units. When considered alongside morphological and behavioural attributes, our developmental data phylogenetically constrain Dickinsonia to the Metazoa, specifically the Eumetazoa plus Placozoa total group. Our findings have implications for the use of Dickinsonia in developmental debates surrounding the metazoan acquisition of axis specification and metamerism.
Keywords: metazoan evolution, bilaterian, Ediacaran, development, ontogeny
1. Introduction
Ediacaran macrofossil assemblages document a variety of large, soft-bodied taxa that have been suggested to include both metazoan and non-metazoan organisms [1]. However, precise determination of the phylogenetic placement of many Ediacaran taxa can be problematic, owing to difficulties in identifying diagnostic morphological characters in available fossil material, and the likelihood that many taxa lie within stem groups to extant clades [2,3]. The resultant phylogenetic uncertainty surrounding Ediacaran macrofossils frustrates efforts to incorporate specific taxa into discussions of metazoan evolution and development (e.g. [4–6]), despite fossil assemblages of such organisms having the potential to yield abundant developmental data.
The iconic Ediacaran macrofossil Dickinsonia (figure 1) offers a prime example of these problems. Initially interpreted as a possible medusoid cnidarian [7,8], Dickinsonia has since been variously considered to represent an annelid worm close to the extant Spinther [9–11], a platyhelminth [12], a placozoan [4], a ctenophore [13], a polypoid organism [14], a stem-group bilaterian [5,15], an early-branching diploblastic metazoan [3], a lichen [16], a rhizopodan protist [17], or a member of an extinct clade [18]. Lichen and rhizopodan interpretations are refuted by observations of considerable flexibility in Dickinsonia specimens [19], as evidenced by twisted, folded [20] and contracted specimens [21], but other suggestions are yet to be categorically confirmed or disproven. Recent studies into growth in Dickinsonia costata [22], and arguments for a bilaterian affinity based on ancestral state reconstruction [5], rely on assumptions regarding growth in this taxon that we here argue are incorrect.
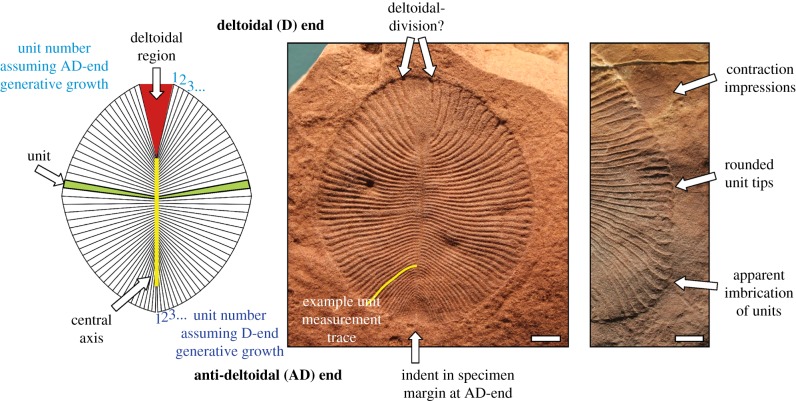
Figure 1.
The terminology used herein to describe Dickinsonia costata, and various morphological features discussed in the text. Images are of specimens SAM P40135 (D14, centre) and SAM P49355 (D17, right). Unit count is the total number of units counted within a specimen. Unit number denotes the order in which units were added in an individual specimen, assuming growth from a specific generative zone. Scale bars, 10 mm. (Online version in colour.)
The fossil record offers numerous assemblages of Dickinsonia specimens, most notably from the White Sea and Ural Mountains of Russia [23], and the Ediacara Member of South Australia [10]. Such assemblages include individuals exhibiting significant intra-specific variation in size and number of constructional units, and these are interpreted as recording a wide range of ontogenetic stages in the growth programme of this organism. Consideration of morphogenetic relationships between specimens in such assemblages can be used to infer developmental pattern in Dickinsonia, and ultimately inform phylogeny [5,24]. Here we characterize the morphogenesis of Dickinsonia, and show that its growth involved both pre-terminal serial addition, and inflation, of body units. This growth programme differs markedly from previous interpretations of growth in this taxon, which view the generative zone as being located in a truly terminal position ([5], figure 2), at the opposite end of the organism to that considered herein ([5,19,20,22]). Our new model reconciles Dickinsonia with a sub-set of metazoan ontogenetic growth programmes, and facilitates its incorporation into discussion of early animal evolution.
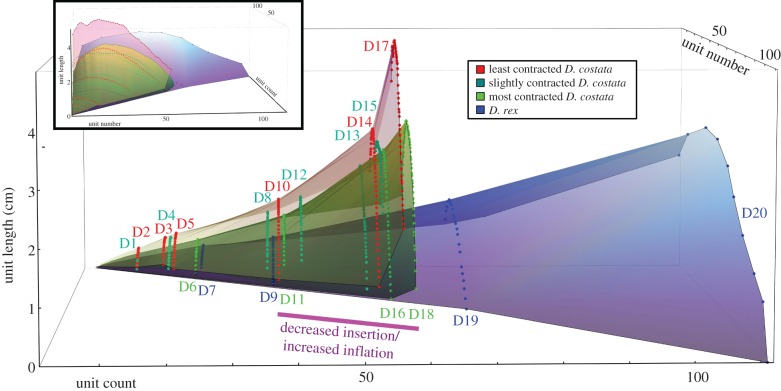
Figure 2.
Measurements of Dickinsonia unit length plotted against unit number and unit count (see text for definitions), assuming traditionally envisaged AD-end insertion. The measurements from individual specimens plot as arcs of points at a fixed unit count, with the unit number counted consecutively from the D-end. Inset: frontal view of the same plot. Specimens within different groups plot on distinct growth surfaces, with D. rex and D. costata clearly displaying different growth trajectories.
(a). Growth in Dickinsonia
Dickinsonia is inferred to have been a flattened, sheet-like organism (though see [11]) with a broadly ovate outline and a bilaterally symmetrical body constructed of multiple elongate units serially arranged along a central growth axis running down the longitudinal midline [5,20,22,25] (figure 1). There is a strong linear relationship between overall length and width of D. costata specimens, and a positive correlation between the overall length of the organism and the number of units within local populations [22,26]. Previous assessments of Dickinsonia have disagreed about whether the organism grew isometrically [22,25] or allometrically [21], and whether individual units initially expanded in volume before halting their growth after certain dimensions were reached [27], or inflated continuously throughout life [4]. All previous studies have assumed that new units are added terminally, at the end of the organism where the smallest units are located, but this assumption is untested. Early claims for a terminal ‘pygidium’ at this end of the organism, prior to which units may have been added in a sub-terminal position [10], have never been confirmed [28].
At one end of the midline there is often a broadly triangular area (here termed the deltoidal region, (cf. [20]), which has previously been interpreted as a ‘head’ (cf. [29]). This triangular region appears to be the most morphologically variable structure within the organism (comprising between 1% and 19% of the areal extent of the organism in our studied Australian material; electronic supplementary material, figure S2). We consider this variability to be inconsistent with the functioning of this region as a ‘head’, which we would expect would comprise a conserved anatomical component. At the opposite end of the midline, the units become progressively smaller in size. We use the neutral terms ‘deltoidal’ (D) and ‘anti-deltoidal’ (AD) to delineate the body axes in Dickinsonia (figure 1), and ‘units’ to describe the serially repeated structures that comprise the organism (see the electronic supplementary material for discussion of historical terminology for Dickinsonia morphology). Rarely, deltoidal regions are observed to exhibit grooves, oriented parallel to adjacent units, which extend in from the outer margins of the specimen but do not connect to the central axis to form complete units (figure 1; electronic supplementary material, figure S1, specimens D14 and D17). Such grooves were recognised by Wade [10, p. 174], and although they are relatively rare, can be observed in several well-preserved Dickinsonia specimens figured in the literature [e.g. [20], fig. 6; [22], fig. 2a–e). These features raise the possibility that the deltoidal region could be partially differentiated, and may imply D-end addition at a truly pre-terminal growth zone located at the margins of the deltoidal region, consistent with the pre-terminal growth of many extant bilaterian segmented taxa [28]. In assessing our data, we consider the possibility of both anti-deltoidal and deltoidal addition of new units.
Dickinsonia specimens may also exhibit faint, radially arranged, low-relief impressions around the outer margin of the organism, seemingly recording extensions of the positions of individual units (figure 1). These ‘rims’ have been interpreted as evidence for contraction resulting from either active muscular activity [9,10,25] or taphonomic contraction/deflation upon death and burial [19,21]. Contracted specimens are typically smaller than uncontracted specimens with a similar number of units [10], and the extent of contraction undergone by individual specimens was an important consideration in our interpretation of measurements taken from individual specimens. Contraction has not been accounted for in previous studies of growth in Dickinsonia (e.g. [22]).
2. Material and methods
Twenty Dickinsonia specimens (16 D. costata and 4 D. rex) from the Ediacara Member in the Flinders Ranges of South Australia were selected for study, each exhibiting a high quality of overall preservation. Specimens span a range of sizes, and are interpreted as snapshots of different ontogenetic stages within the life history of the two taxa. Dickinsonia costata specimens range from 7–134 mm in length, and possess 11–58 units. Contraction is recognized to vary in its extent within the studied population (electronic supplementary material, figure S1). Studied D. rex specimens range from 14–117 mm in length and possess 23–111 units. Uncertainties related to measurement protocols, taphonomic deformation and biological variation are discussed in the electronic supplementary material. Although we consider individual units to be connected to one another, we see no evidence for the presence of a membrane in any of our studied specimens (contra [22]).
All specimens were studied from either high resolution photographs, or casts (electronic supplementary material, table S1). Specimen and unit outlines were traced over images of the specimens in the vector-based graphics program Adobe Illustrator CS5. Measurements of unit length (measured from the axial midline to the margin of the specimen for every unit), unit count (total number of units), and unit number (progressive number of appearance of each unit, considering the possibility of generative zones at either the D-end or AD-end of the organism; figure 1; electronic supplementary material, table S2) were obtained for each specimen. Plotting these parameters against one another permits ready visualization of the data (figure 2; electronic supplementary material, figure S4) with individual specimens plotting as arcs of points at a specific unit count. In each specimen, individual unit lengths were measured from the best preserved side of the specimen, and document the distance from the central axis to the outer margin, following the natural curvature of the unit (figure 1). The length of the resulting curved lines was then calculated in Adobe Illustrator and calibrated to scales in the photographs to translate the measurements into millimetres. The lengths of individual units were indexed by unit number (counted continuously from both the deltoidal and anti-deltoidal terminal units). Our interpretation of growth in Dickinsonia assumes that: (i) units can increase or maintain their size, but cannot decrease in size (other than via contraction); (ii) units cannot be lost once they have been generated.
Wolfram Mathematica, version 9.0 was used for data analysis and programming of the growth model. To construct our model, we assume that in Dickinsonia: (i) units are added during ontogeny; (ii) units grow during ontogeny; (iii) all members of a species follow a similar growth plan, with similar unit lengths at a similar growth stage; (iv) units are added either at a terminal AD-end generative zone, or at a pre-terminal D-end generative zone.
3. Results
Our measurements of unit length, unit number and unit count (electronic supplementary material, table S1; figure 2) confirm that both D. costata and D. rex exhibit their shortest units at the anti-deltoidal tip of the organism (figure 3iii), while the longest units are near to the centre, being closer to the D-end in unit number (located at 33 ± 7% of the total number of units in D. costata, counted from the D-end, and at 35 ± 13% in D. rex; figure 3iii; electronic supplementary material, table S1). Larger specimens typically possess more units, which are longer at all positions within the organism, than smaller specimens (figures 2 and 3), though as expected [10], specimens showing signs of significant contraction have smaller unit lengths than uncontracted/less contracted specimens of a similar unit count (see the electronic supplementary material, figure S1 for details of the extent of contraction we interpret each specimen to have undergone). Plotting guiding surfaces to connect measurements from similar specimens demonstrates that Dickinsonia gradually increased its unit length with increasing unit count, but to varying degrees depending on the position of the unit within the organism (figure 2). We term these guiding surfaces ‘growth surfaces’, because they permit visualization of the pattern of morphogenesis in individual taxa. Dickinsonia rex specimens (figure 2, blue surface) plot a surface that lies beneath all D. costata specimens and extends to a higher unit count, because D. rex individuals possess a larger number of units relative to D. costata specimens of a comparable size. The red (least contracted specimens) and green (most contracted specimens) surfaces reveal variation within the D. costata population, with all specimens of that taxon lying on or between these surfaces.
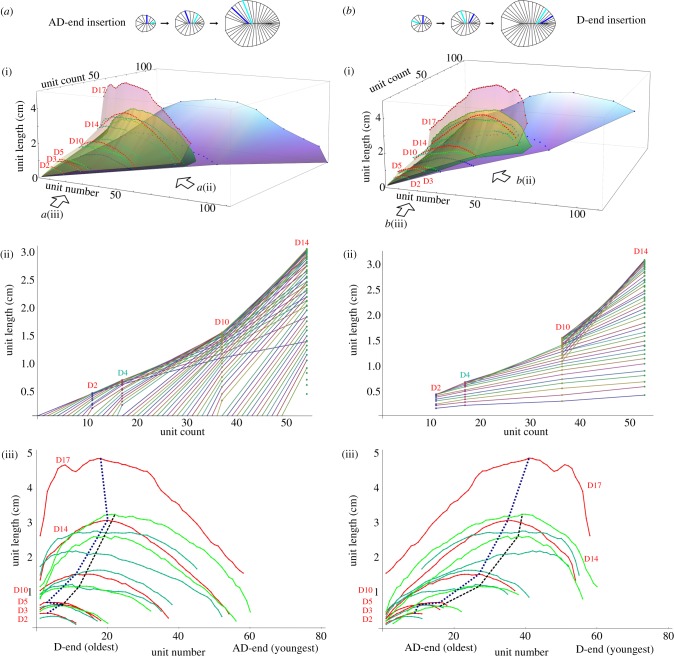
Figure 3.
Growth data for Dickinsonia specimens assuming (a) an anti-deltoidal (AD-end) and (b) a deltoidal (D-end) generative zone, plotted as (i) complete growth surfaces with units counted from the end interpreted as the oldest, (ii) growth lines of the lengths of individual units as a function of unit count for selected specimens, formed by connecting the measurements of units perceived to be homologous, and (iii) as plots of unit number against unit length, with each continuous line illustrating the measurements of a single specimen, and dotted lines connecting longest units of least and most contracted specimens. Legend for colour coding as in figure 2. The AD-end units of some specimens (particularly D15) could not be measured, so the number of missing units was estimated.
4. Discussion
Dickinsonia costata from South Australia is revealed to exhibit a consistent growth plan involving unit addition accompanied by concurrent extension of the body axis, and an increase in individual unit length, over the lifetime of the organism. The total number of units (unit count) broadly correlates with overall specimen size (though see [22]), with any variation consistent with that observed in natural populations of extant segmented organisms (cf. [30], figure 4). Our data are consistent with the suggestion that the number of units can be considered a proxy for relative age [25], but we note that other studies have considered the amount of variation in unit number to be more variable [22].
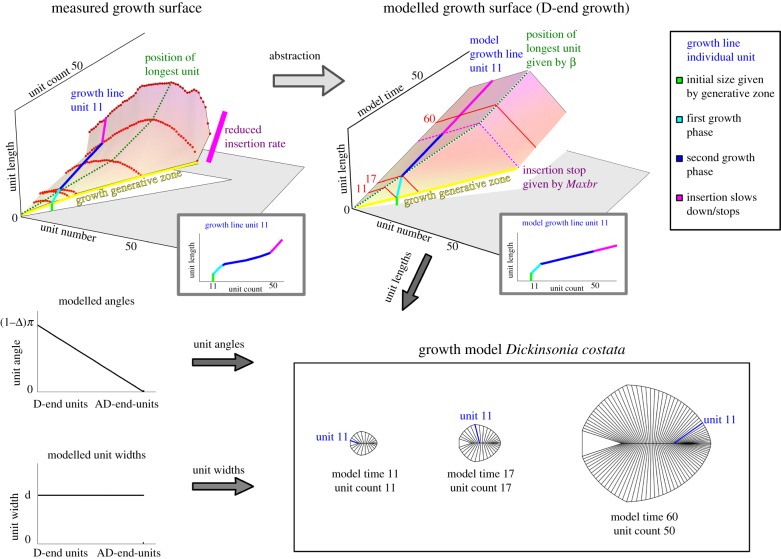
Figure 4.
Translation of the growth surface for D. costata specimen data (from uncontracted specimens) to a modelled growth surface, which renders unit lengths as a function of unit number and model time. Insets illustrate the measured and modelled growth of an individual unit, analogous to the growth lines in figure 3b. Each unit slows its relative growth after it has become the longest unit. With the additional input of unit angles and widths, this information can be used to render a model morphology at each point in time (see the interactive applet). (Online version in colour.)
The longest unit appears to maintain its position (as a proportion of unit count) along the axis throughout growth (figure 3iii). In order for this to happen, upon becoming the longest unit a unit must slow its growth rate relative to unit insertion to allow the next unit to overtake it in size. This organized growth programme implies that units did not grow independently, but rather adapted their growth based on their position in the body and the ontogenetic stage of the organism, resulting in a maintained gross morphology that is obtained via an allometric growth programme.
We find evidence for a shift in the relative rate of unit addition/inflation, reflected in changes in the gradient of the growth surfaces at approximately 35 and 50 units (figures 2 and 3) (apparent separation of these shifts is probably an artefact of irregular sampling intervals). Dickinsonia costata is rarely found with more than 50–60 units, suggesting that a reduction in the rate of unit addition is the most likely explanation for the observed shift, with unit insertion slowing and inflation becoming the dominant growth mechanism later in the growth programme (cf. [4]). This change in gradient is observed in other Dickinsonia studies (e.g. [22], figure 3b; [26], figure 2), but is here interpreted as an ontogenetic shift that may reflect a change in life history, for example, as part of a switch to a reproductive phase. However, without knowing the rate of unit insertion, it is not possible to derive an absolute sense of time from these data.
The D. rex growth surface exhibits a gentle gradient throughout, with little indication of an ontogenetic shift (figure 2), though we note that we do not possess data from sub-centimetre specimens as in D. costata. This seemingly stable growth rate with respect to unit insertion is consistent with the apparently indeterminate addition of units in D. rex. However, the small sample size for this taxon, and the possibility that ecophenotypic or intra-specific variation may exist within these populations (something that has not previously been investigated in Dickinsonia), precludes us from drawing conclusions regarding this species at present.
(a). Where was Dickinsonia's generative zone?
Growth in Dickinsonia has universally been assumed to have taken place at the anti-deltoidal end of the organism, because this is where the smallest, perceived to be the youngest, units are located [4,5,19,22,25]. However, our observations of potential deltoidal differentiation in some specimens raise the possibility of a deltoidal generative zone. We here interpret our growth data within both anti-deltoidal and deltoidal frameworks.
(i). An anti-deltoidal generative zone
If we assume an anti-deltoidal generative zone for Dickinsonia, our data can be plotted as in figure 3a, with the D-end units interpreted as the oldest. Growth curves for individual units, produced by connecting measurements from units perceived to be homologous across specimens (figure 3aii), exhibit variation in their slope. There is little consistency among the growth trajectories of the specimens (figure 3aiii), leading to significant overlap in the unit measurment arcs plotted by individuals. The oldest unit generally increases in size with increasing unit count, but the youngest unit, which would be expected to be of a similar size in all specimens if it represents the generative zone, appears to be variable in its length (figure 3aiii). When the relative position of the longest unit is investigated (figure 3aiii), the trend in our studied specimens is not unidirectional (as would be expected in an organism with a well-regulated growth programme), and must reverse if AD-end insertion is assumed. We do note that the grain size of the casting medium may limit our ability to observe the very smallest AD units [22].
(ii). A deltoidal generative zone
When a deltoidal generative zone is assumed for Dickinsonia, with new units being added by differentiation of the deltoidal unit, we see that new units neatly and consistently exhibit increasingly greater lengths as they are added (figure 3biii). Individual units grow at a relatively slower rate throughout life than when AD growth is assumed (compare the gentle and constant slopes in figure 3bii with those in figure 3aii). The AD-end regions of different specimens in figure 3biii (which would reflect the oldest units in this model) only slightly increase their size during growth. Unit length in general increases first relatively quickly and then gradually and consistently among the sampled specimens, as one would expect if the AD-end units were homologous (figure 3bii). D-end insertion further results in a consistent, unidirectional positive trend in the relative position of the longest unit (figure 3biii), in contrast to the reversed trend observed if AD-end insertion is assumed (figure 3aiii).
When combined with the aforementioned anatomical evidence for apparent differentiation within the deltoidal region (e.g. figure 1), and what appears to be a consistent decrease in the size of the deltoidal area relative to the total organism with increasing unit count (electronic supplementary material, figure S2), a deltoidal generative zone more parsimoniously explains the patterns observed in our data. We therefore conclude that, contrary to all previous interpretations of growth in this organism, D. costata added units at a D-end generative zone, with morphological evidence suggesting that this unit addition may have occurred in a pre-terminal position. These units inflated during life as part of an organized, intricate growth programme. Our study suggests the position of the smallest units alone may not be a robust indicator of the generative zone in this taxon; a finding with potential implications for developmental and phylogenetic studies into other Ediacaran taxa (e.g. Charnia [31]). Alternative suggestions that Dickinsonia might have been bipolar [21] are considered unlikely given the clear asymmetry of its termini.
Abstraction of our measurement data from D. costata enables construction of a simplified growth model that replicates its growth programme (figure 4; electronic supplementary material; see also our interactive downloadable applet: http://people.maths.ox.ac.uk/hoekzema/Applet/). The model illustrates that although different Dickinsonia species have disparate morphologies, they can be rationalized by a common morphogenetic model, substantiating their coherence as a natural group. Different reconstructed Dickinsonia species may look similar at an early stage of growth, but diverge in morphology during ontogeny. It is worth noting that ostensibly similar theoretical morphologies can be created by two quite different growth models (readers can compare AD-end and D-end growth in our applet), emphasizing that caution must be exercised when attempting to decipher biological growth programmes via modelling techniques (e.g. [32]).
(b). The phylogenetic affinity of Dickinsonia
The seemingly tightly constrained growth programme of Dickinsonia, whereby individual units change their growth rate in order to maintain the overall shape of the organism, reveals a growth programme with a greater level of organization than that observed in extant slime moulds. The combination of both additional and inflational growth in Dickinsonia [4] is confirmed by our data, and is incompatible with the insertion-only growth seen in extant foraminifera and xenophyophores [4]. The close spatial relationship and resemblance of Dickinsonia to the ichnotaxon Epibaion [20,29,33] implies that it was benthic and motile [4]. Such motility would refute fungal, algal and lichen biological affinities [4].
Evidence for putative biradial symmetry and internal structures was purported to demonstrate that Dickinsonia was a ctenophore [13], but relies heavily on a single, potentially unrepresentative, specimen. We note that no anatomical evidence has been presented to suggest that features inferred as meridional canals [13] connect to the ‘gut’—a characteristic of true meridional canals. Putative internal anatomy in Dickinsonia [34] shows more than eight ‘canals’ in total, and no evidence for any transverse canals. We, therefore, do not find the anatomical evidence in support of a ctenophoran affinity for Dickinsonia compelling. An alternative suggestion that the longitudinal axis of Dickinsonia is homologous to the oral–aboral axis of ctenophores is intriguing [3], but requires acceptance of a range of equivocal morphological similarities between Dickinsonia and radial taxa. Dickinsonia's axial growth contrasts starkly with the concentric isometric addition of units in corals such as Fungia, refuting some polypoid affinities [5]. However, given the developmental and morphological diversity exhibited by extant cnidarians, and the presence of a pre-terminal growth zone in some cnidarians [5], we consider it possible that Dickinsonia could potentially be allied with this group.
Interpretation of Epibaion traces as indicative of external digestion via the ventral surface of Dickinsonia [20,33] has been considered irreconcilable with poriferan or eumetazoan lineages [4], and consistent with a placozoan affinity. Impressions interpreted as trace fossils, such as Epibaion [29,33], imply that Dickinsonia lay static on the underlying microbial mat for long enough to remove the mat beneath it, leading to an interpretation as resting or feeding traces (e.g. [4]). However, in the absence of direct morphological evidence for feeding mechanisms, it is not yet possible to conclude with certainty whether such traces represent feeding by ventral sole digestion as in placozoans [4], cilia-driven grazing (e.g. [29]) or even passive reclining on the surface [35]. Modern placozoans have a poorly constrained, non-metameric body plan, but the derived nature of the placozoan crown-group leaves open the possibility that our developmental data may be compatible with a stem-group placozoan position for Dickinsonia.
Possible merging or branching of units in Dickinsonia specimens has been claimed to be incompatible with a bilaterian body plan [19], but we consider such observations to result from superposition of flexible, poorly (spatially) constrained individual units (figure 1). Rare morphological evidence for musculature [10] or internal organs [11,13,34] has largely been treated with caution, but would be consistent with a bilaterian affinity. Gold et al. [5] infer an anti-deltoidal, ‘terminal’ (i.e. pre-terminal sensu [28]) generative zone for Dickinsonia, which would support a bilaterian phylogenetic placement, because many bilaterian groups—and the anticipated bilaterian ancestor—are considered to grow in this way [36] (although certain derived bilaterian groups such as the Onychophora do possess truly terminal growth zones). However, the generative zone figured by Gold et al. appears truly terminal ([5], figure 2), a scenario that would inadvertently set Dickinsonia apart from most members of the Bilateria.
Our novel description of Dickinsonia possessing a deltoidal, pre-terminal growth zone would provide positive support for the potential assignment of Dickinsonia within the Bilateria. Indeed, our new model may actually facilitate polarization of Dickinsonia's growth axis, because growth via unit addition in serially repeated bilaterian taxa typically occurs at the posterior of the organism.
In summary, when combined with other evidence, our developmental data indicate that Dickinsonia was a metazoan, to the exclusion of all previously proposed alternative extant clades (electronic supplementary material, figure S7). More specifically, Dickinsonia is considered in light of developmental, behavioural and morphological information to have lain within the Eumetazoa plus Placozoa total-group. Although comparisons to the Bilateria are attractive in the absence of direct developmental evidence to ally Dickinsonia to the Placozoa or Cnidaria, on the basis of current data it would be premature to constrain its phylogenetic position more tightly.
(c). Implications for contemporaneous Ediacaran Dickinsonia-like organisms
There have been several attempts to resolve the phylogenetic relationships between Dickinsonia and its contemporary Ediacaran organisms, including consideration of the Kingdom Vendozoa [21], the Phylum Vendobionta [27] and the Proarticulata [37], the latter being a phylum characterized by a metameric body plan and glide symmetry (a pattern ostensibly similar to bilateral symmetry, but with a distinct offset along the midline) lying outside the Bilateria. Perhaps the most widely discussed grouping in recent years is the morphogroup Dickinsoniomorpha, a grouping of organisms considered to share a morphology constructed of featureless tubes and differentiation across a main body axis [38,39]. The precise taxonomic composition of this group is not yet agreed [23,38].
Taxa commonly considered to share close relationships to Dickinsonia include Andiva [40] and Yorgia [41], both of which differ in possessing a large and crescentic undifferentiated region of broadly consistent size at all ontogenetic stages relative to total body size, and distinct unit morphologies. We do not consider the observed morphological differences in unit form to be irreconcilable with our new model, nor do we consider the different symmetries across the dickinsoniomorphs (e.g. the bilateral symmetry of Dickinsonia versus the glide symmetry of Yorgia) to necessarily preclude a close phylogenetic relationship. Indeed, glide symmetry is known within several extant and extinct bilaterian taxa, including certain machaeridian worms (annelids), where external scales are organized in a glide-symmetrical fashion as a space filling response [28,42]. Different patterns of symmetry are only problematic for the coherence of the proposed dickinsoniomorph group if the units in the bilaterally symmetrical Dickinsonia reflect true segments that continue through the entire body, something that is yet to be determined [31]. If the units seen on the exterior of Dickinsonia are true segments, they cannot be homologous to the externally visible units in Yorgia, and so their growth programmes would not be amenable to comparison. In such a scenario we would regard it as unlikely that these organisms were closely related. If the units in Dickinsonia and Yorgia represent annulations, with internal anatomy not governed by the external patterning of the organism, then it is possible that such differences in symmetry could be compatible within a single clade.
The quantitative methodology presented in this study can be applied via our abstracted model and applet to investigate the growth plans of morphologically similar Ediacaran and non-Ediacaran taxa including other Dickinsoniomorphs (extended electronic supplementary material). This technique could open up new avenues through which to explore ontogenesis and development in taxa with iterative growth.
(d). The use of Dickinsonia in metazoan developmental studies
Resolution of Dickinsonia as a placozoan could imply an ancestral diversity of body plans, consistent with a rapidly growing body of genetic data that indicate considerable complexity in early metazoans [43]. The Placozoa, once considered sister group to the Bilateria [44], have more recently been interpreted as sister group to the Eumetazoa [45]. The presence of the homeotic gene Trox2 in the extant Placozoa [46] may suggest secondary simplification and a morphologically complex placozoan stem lineage ([47], though see [46]), implying that early total-group metazoans could potentially have included organisms with a Dickinsonia-like morphology. If Dickinsonia is alternatively resolved as lying within the Cnidaria, it would imply secondary loss of (or extinction of organisms showing) concomitant growth of the main body axis and serially repeated units (regardless of whether those units are regarded as metameres or segments).
If Dickinsonia is, as our ontogenetic data appear to suggest most strongly, resolvable within the total-group Bilateria, its implications for the evolution of the segmented body plan depend upon its precise position within the Bilateria. The serial anatomical organization of Dickinsonia is compatible with hypotheses of a complex metameric ancestral bilaterian, from which the segmentation mechanisms of chordates, annelids and arthropods were inherited [48]. However, while some authors consider segmentation to be a plesiomorphic bilaterian character [5], others consider simple external annulations to be a precursor to true metamerism [36]. There is increasing evidence that the urbilaterian may not have been a truly metameric organism: independent co-options of pre-existing gene regulatory networks (GRNs, involved in axial elongation) to form a segmentation cascade in the arthropods, annelids and chordates seems more parsimonious than invoking multiple independent losses of the segmented bauplan [36] in all non-metameric bilaterian groups. Metamerism in the chordates proceeds primarily from the mesoderm, rather than (typically) from the ectoderm in the annelids and arthropods [36], suggesting deep differences in the segmentation process (but see [48]). Recent studies propose Xenacoelomorpha (the group including the acoel flatworms and the xenoturbellids) as sister group to the Nephrozoa (protostomes plus deuterostomes) [45]. The xenacoelomorphs are considered to lack the metamerism apparent in some nephrozoan groups, but possess the true bilateral symmetry characteristic of the Bilateria as well as a suite of traits intermediate between the Cnidaria and the Nephrozoa (appearing to justify their position as sister to the Nephrozoa, though see [49]). Since the urbilaterian probably had the GRNs prerequisite to a metameric body plan, it is possible that stem-group xenacoelomorphs could have independently acquired, and subsequently lost, a metameric bauplan (in relation to other bilaterian groups). Future advances in xenacoelomorph ontogeny may allow for better discrimination here.
We do not attempt to resolve between true segmentation, annulation, or superficial metamerism in Dickinsonia, and nor do we attempt to resolve between placement within the Xenacoelomorpha and the Nephrozoa. However, there are currently no confidently identified apomorphies to tie Dickinsonia to any segmented Nephrozoan crown group, and we suggest that if Dickinsonia is resolved as belonging to the annelids, arthropods or, indeed, chordates, it would be in a stem-group capacity. If any of these scenarios are true, the apparent variation in unit count observed within the largest Dickinsonia specimens would support recent theoretical predictions suggesting that determinate addition of units evolved after both sequential segmentation and the evolution of posterior growth [50] (i.e. the level of flexibility in maximal unit count seen today only in annelids is plesiomorphic to the segmented state). Conversely, if Dickinsonia lies outside the segmented Nephrozoa [36], then it may represent an annulated ancestor from which disparate members of the Bilateria diverged to use metameric body organization in different ways [38].
5. Conclusion
Our data demonstrate that Dickinsonia grew by addition of serial units via differentiation at a probable pre-terminal (deltoidal) generative zone, concurrent with elongation of the main body axis as well as lateral and axial growth of those units. This study emphasizes that growth and development offer powerful tools with which to constrain the phylogenetic position of problematic fossil taxa. Assignment of Dickinsonia, a particularly enigmatic taxon, to the Placozoa plus Eumetazoa total group enables us to draw a line under previous suggestions of non-metazoan biological affinities, and move forward with more focused studies that can distinguish between remaining hypotheses; something that is imperative if we are to unlock this taxon's considerable potential in unravelling the origins of metamerism. Investigation of Dickinsonia's serially repeated body plan to determine whether it reflects annulation, metamerism or segmentation, represents the next key challenge in understanding this organism. We are confident that expansion of a developmental approach to the study of Ediacaran macro-organisms will enable palaeontological data to contribute substantial insights to developmental studies into early metazoan evolution.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank J. Gehling and C. Marshall for access to specimens, P. Donoghue and L. Parry for discussion and E. Sperling and one anonymous reviewer for helpful comments that have improved this manuscript.
Data accessibility
The datasets supporting this article are available in an accompanying electronic supplementary material. Our interactive applet is available for download at: http://people.maths.ox.ac.uk/hoekzema/Applet/.
Authors' contributions
R.S.H. and M.D.B. designed the project approach. R.S.H. carried out the research. All authors interpreted the data and R.S.H., F.S.D. and A.G.L. wrote the paper.
Competing interests
We have no competing interests.
Funding
R.S.H. thanks the Prins Bernhard Cultuurfonds, the Hendrik Mullerfonds, the Langerhuizen Stipendium, the VSBfonds, the Vreedefonds and the Genootschap Noorthey for financial support over the course of this project. A.G.L. and F.S.D. acknowledge funding from the Natural Environment Research Council (grant numbers NE/L011409/2 and NE/L002434/1, respectively).
References
- 1.Xiao S, Laflamme M. 2009. On the eve of animal radiation: phylogeny, ecology and evolution of the Ediacara biota. Trends Ecol. Evol. 24, 31–40. ( 10.1016/j.tree.2008.07.015) [DOI] [PubMed] [Google Scholar]
- 2.Cunningham JA, Liu AG, Bengtson S, Donoghue PC. 2017. The origin of animals: can molecular clocks and the fossil record be reconciled? Bioessays 39, 1–12. ( 10.1002/bies.201600120) [DOI] [PubMed] [Google Scholar]
- 3.Budd GE, Jensen S. 2017. The origin of the animals and a ‘Savannah’ hypothesis for early bilaterian evolution. Biol. Rev. 92, 446–473. ( 10.1111/brv.12239) [DOI] [PubMed] [Google Scholar]
- 4.Sperling EA, Vinther J. 2010. A placozoan affinity for Dickinsonia and the evolution of late Proterozoic metazoan feeding modes. Evol. Dev. 12, 201–209. ( 10.1111/j.1525-142X.2010.00404.x) [DOI] [PubMed] [Google Scholar]
- 5.Gold DA, Runnegar B, Gehling JG, Jacobs DK. 2015. Ancestral state reconstruction of ontogeny supports a bilaterian affinity for Dickinsonia. Evol. Dev. 17, 315–324. ( 10.1111/ede.12168) [DOI] [PubMed] [Google Scholar]
- 6.Martynov AV. 2012. Ontogeny, systematics, and phylogenetics: perspectives of future synthesis and a new model of the evolution of bilateria. Biol. Bull. 39, 393–401. ( 10.1134/S106235901205010X) [DOI] [PubMed] [Google Scholar]
- 7.Harrington HJ, Moore RC. 1956. Medusa of the Hydroidea. In Treatise on invertebrate paleontology, part F: Coelenterata (ed. Moore RC.), pp. 77–80. Lawrence, KS: GSA and University of Kansas Press. [Google Scholar]
- 8.Sprigg RC. 1947. Early Cambrian (?) jellyfishes from the Flinders Ranges, South Australia. Trans. R. Soc. South Australia 71, 212–224. [Google Scholar]
- 9.Glaessner MF, Wade M. 1966. The Late Precambrian fossils from Ediacara, South Australia. Palaeontology 9, 599–628. [Google Scholar]
- 10.Wade M. 1972. Dickinsonia: polychaete worms from the late Precambrian Ediacara fauna, South Australia. Memoirs Queensland Museum 16, 171–190. [Google Scholar]
- 11.Jenkins RJF. 1992. Functional and ecological aspects of Ediacaran assemblages. In Origin and early evolution of the Metazoa (eds Lipps JH, Signor PW), pp. 131–176. New York, NY: Plenum Press. [Google Scholar]
- 12.Fedonkin MA. 1981. Belomorskaya biota venda. Trudy Akademii Nauk SSSR 342, 1–100. [Google Scholar]
- 13.Zhang X, Reitner J. 2006. A fresh look at Dickinsonia: removing it from Vendobionta. Acta Geologica Sinica (English ed.) 80, 636–642. [Google Scholar]
- 14.Valentine JW. 1992. Dickinsonia as a polypoid organism. Paleobiology 18, 378–382. ( 10.1017/S0094837300010952) [DOI] [Google Scholar]
- 15.Fedonkin MA. 1990. Systematic description of Vendian metazoa. Vendian Syst. 1, 71–120. [Google Scholar]
- 16.Retallack GJ. 2007. Growth, decay and burial compaction of Dickinsonia, an iconic Ediacaran fossil. Alcheringa 31, 215–240. ( 10.1080/03115510701484705) [DOI] [Google Scholar]
- 17.Seilacher A, Grazhdankin D, Legouta A. 2003. Ediacaran biota: the dawn of animal life in the shadow of giant protists. Paleontol. Res. 7, 43–54. ( 10.2517/prpsj.7.43) [DOI] [Google Scholar]
- 18.Seilacher A. 1992. Vendobionta and Psammocorallia: lost constructions of Precambrian evolution. J. Geol. Soc. Lond. 149, 607–613. ( 10.1144/gsjgs.149.4.0607) [DOI] [Google Scholar]
- 19.Brasier MD, Antcliffe JB. 2008. Dickinsonia from Ediacara: a new look at morphology and body construction. Palaeogeogr. Palaeocl. Palaeoecol. 270, 311–323. ( 10.1016/j.palaeo.2008.07.018) [DOI] [Google Scholar]
- 20.Gehling JG, Droser ML, Jensen SR, Runnegar BN. 2005. Ediacara organisms: relating from to function. In Evolving form and function: fossils and development: proceedings of a symposium honoring Adolf Seilacher for his contributions to paleontology, in celebration of his 80th birthday (ed. Briggs DEG.), pp. 43–67. New Haven, CT: Peabody Museum of Natural History, Yale University. [Google Scholar]
- 21.Seilacher A. 1989. Vendozoa: organismal construction in the Proterozoic biosphere. Lethaia 22, 229–239. ( 10.1111/j.1502-3931.1989.tb01332.x) [DOI] [Google Scholar]
- 22.Evans SD, Droser ML, Gehling JG. 2017. Highly regulated growth and development of the Ediacara macrofossil Dickinsonia costata. PLoS ONE 12, e0176874 ( 10.1371/journal.pone.0176874) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grazhdankin D. 2014. Patterns of evolution of the Ediacaran soft-bodied biota. J. Paleontol. 88, 269–283. ( 10.1666/13-072) [DOI] [Google Scholar]
- 24.Brasier MD, Antcliffe JB. 2004. Decoding the Ediacaran enigma. Science 305, 1115–1117. ( 10.1126/science.1102673) [DOI] [PubMed] [Google Scholar]
- 25.Runnegar B. 1982. Oxygen requirements, biology and phylogenetic significance of the late Precambrian worm Dickinsonia, and the evolution of the burrowing habit. Alcheringa 6, 223–239. ( 10.1080/03115518208565415) [DOI] [Google Scholar]
- 26.Zakrevskaya MA, Ivantsov AYu. 2017. Dickinsonia costata: the first evidence of neoteny in Ediacaran organisms. Invert. Zool. 14, 92–98. [Google Scholar]
- 27.Seilacher A. 2007. The nature of Vendobionts. In The rise and fall of the Ediacaran biota. Geological Society, London, special publications, vol. 286 (eds Vickers-Rich P, Komarower P), pp. 387–397. London, UK: The Geological Society. [Google Scholar]
- 28.Jacobs DK, Hughes NC, Fitz-Gibbon ST, Winchell CJ. 2005. Terminal addition, the Cambrian radiation and the Phanerozoic evolution of bilaterian form. Evol. Dev. 7, 498–514. ( 10.1111/j.1525-142X.2005.05055.x) [DOI] [PubMed] [Google Scholar]
- 29.Ivantsov AYu. 2011. Feeding traces of Proarticulata - the Vendian metazoa. Paleontol. J. 45, 237–248. ( 10.1134/S0031030111030063) [DOI] [Google Scholar]
- 30.Parry LA, Wilson P, Sykes D, Edgecombe GC, Vinther J. 2015. A new fireworm (Amphinomidae) from the Cretaceous of Lebanon identified from three-dimensionally preserved myoanatomy. BMC Evol. Biol. 15, 256 ( 10.1186/s12862-015-0541-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunn FS, Liu AG, Donoghue PCJ. In press Ediacaran developmental biology. Biol. Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoyal Cuthill JF, Conway Morris S. 2014. Fractal branching organizations of Ediacaran rangeomorph fronds reveal a lost Proterozoic body plan. Proc. Natl Acad. Soc. USA 111, 13 122–13 126. ( 10.1073/pnas.1408542111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivantsov AYu, Malakhovskaya JE. 2002. Giant traces of the Vendian animals. Dokl. Akad. Nauk 385, 382–386. [Google Scholar]
- 34.Dzik J, Ivantsov AYu. 2002. Internal anatomy of a new Precambrian dickinsoniid dipleurozoan from northern Russia. N. Jb. Geol. Paläont. Mh. 7, 385–396. [Google Scholar]
- 35.McIlroy D, Brasier MD, Lang AS. 2009. Smothering of microbial mats by macrobiota: implications for the Ediacara biota. J. Geol. Soc. 166, 1117–1121. ( 10.1144/0016-76492009-073) [DOI] [Google Scholar]
- 36.Chipman AD. 2010. Parallel evolution of segmentation by co-option of ancestral gene regulatory networks. BioEssays 32, 60–70. ( 10.1002/bies.200900130) [DOI] [PubMed] [Google Scholar]
- 37.Fedonkin MA, Cope JCW. 1985. Precambrian metazoans: the problems of preservation, systematics and evolution [and Discussion]. Phil. Trans. R. Soc. Lond. B 311, 27–45. ( 10.1098/rstb.1985.0136) [DOI] [Google Scholar]
- 38.Erwin DH, Laflamme M, Tweedt SM, Sperling EA, Pisani D, Peterson KJ. 2011. The Cambrian conundrum: early divergence and later ecological success in the early history of animals. Science 334, 1091–1097. ( 10.1126/science.1206375) [DOI] [PubMed] [Google Scholar]
- 39.Laflamme M, Darroch SA, Tweedt SM, Peterson KJ, Erwin DH. 2013. The end of the Ediacara biota: extinction, biotic replacement, or Cheshire Cat? Gondwana Res. 23, 558–573. ( 10.1016/j.gr.2012.11.004) [DOI] [Google Scholar]
- 40.Fedonkin MA. 2002. Andiva ivantsovi gen. et sp. n. and related carapace-bearing Ediacaran fossils from the Vendian of the Winter Coast, White Sea, Russia. Ital. J. Zool. 69, 175–181. ( 10.1080/11250000209356456) [DOI] [Google Scholar]
- 41.Ivantsov AYu. 1999. A new representative of dikinsoniids from the Upper Vendian of the northern coast of the White Sea (Russia, Arkhangel'sk Region). Paleontol. Zh. 3, 3–11. [Google Scholar]
- 42.Parry L, Tanner A, Vinther J. 2014. The origin of annelids. Palaeontology 57, 1091–1103. ( 10.1111/pala.12129) [DOI] [Google Scholar]
- 43.Ferrier DEK. 2015. The origin of the Hox/ParaHox genes, the Ghost Locus hypothesis and the complexity of the first animal. Brief. Funct. Genom. 15, 333–341. ( 10.1093/bfgp/elv056) [DOI] [PubMed] [Google Scholar]
- 44.Pick K, et al. 2010. Improved phylogenomic taxon sampling noticeably affects nonbilaterian relationships. Mol. Biol. Evol. 27, 1983–1987. ( 10.1093/molbev/msq089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cannon JT, Vellutini BC, Smith J, Ronquist F, Jondelius U, Hejnol A. 2016. Xenacoelomorpha is the sister group to Nephrozoa. Nature 530, 89–93. ( 10.1038/nature16520) [DOI] [PubMed] [Google Scholar]
- 46.Dellaporta S, Holland P, Schierwater B, Jakob W, Sagasser S, Kuhn K. 2004. The Trox-2 Hox/ParaHox gene of Trichoplax (Placozoa) marks an epithelial boundary. Dev. Genes Evol. 214, 170–175. ( 10.1007/s00427-004-0390-8) [DOI] [PubMed] [Google Scholar]
- 47.Peterson KJ, Sperling EA. 2007. Poriferan ANTP genes: primitively simple or secondarily reduced? Evol. Dev. 9, 405–408. ( 10.1111/j.1525-142X.2007.00179.x) [DOI] [PubMed] [Google Scholar]
- 48.Budd GE. 2001. Why are arthropods segmented? Evol. Dev. 3, 332–342. ( 10.1046/j.1525-142X.2001.01041.x) [DOI] [PubMed] [Google Scholar]
- 49.Philippe H, Brinkmann H, Copley RR, Moroz LL, Nakano H, Poustka AJ, Wallberg A, Peterson KJ, Telford MJ. 2011. Acoelomorph flatworms are deuterostomes related to Xenoturbella. Nature 470, 255–258. ( 10.1038/nature09676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vroomans RMA, Hogeweg P, Tusscher KHWJ. 2016. In silico evo-devo: reconstructing stages in the evolution of animal segmentation. EvoDevo 7, 14 ( 10.1186/s13227-016-0052-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article are available in an accompanying electronic supplementary material. Our interactive applet is available for download at: http://people.maths.ox.ac.uk/hoekzema/Applet/.