Abstract
Applying electrical stimulation over the prefrontal cortex can help nicotine dependents reduce cigarette craving. However, the underlying mechanism remains ambiguous. This study investigates this issue with functional magnetic resonance imaging. Thirty-two male chronic smokers received real and sham stimulation over dorsal lateral prefrontal cortex (DLPFC) separated by 1 week. The neuroimaging data of the resting state, the smoking cue-reactivity task and the emotion task after stimulation were collected. The craving across the cue-reactivity task was diminished during real stimulation as compared with sham stimulation. The whole-brain analysis on the cue-reactivity task revealed a significant interaction between the stimulation condition (real vs sham) and the cue type (smoking vs neutral) in the left superior frontal gyrus and the left middle frontal gyrus. The functional connectivity between the left DLPFC and the right parahippocampal gyrus, as revealed by both psychophysical interaction analysis and the resting state functional connectivity, is altered by electrical stimulation. Moreover, the craving change across the real and sham condition is predicted by alteration of functional connectivity revealed by psychophysical interaction analysis. The local and long-distance coupling, altered by the electrical stimulation, might be the underlying neural mechanism of craving regulation.
Keywords: nicotine dependence, addiction, craving, brain stimulation, tDCS, DLPFC
Introduction
Cigarette consumption is a worldwide health problem. A majority of smokers attempting traditional interventions, based on psychotherapy and pharmacotherapy, relapse within 1 year of quitting (Szasz et al., 2012). Craving, especially cue-elicited craving, is a trigger of relapse (Shiffman and Jarvik, 1976). Novel interventions are needed to improve therapeutic outcome. Recently, emerging evidence has shown that transcranial direct current stimulation (tDCS) can help reduce smoking-related behaviors, such as reported craving and number of cigarettes consumed (Fregni et al., 2008; Boggio et al., 2009; Jansen et al., 2013; Fecteau et al., 2014; Meng et al., 2014). Because of non-invasiveness, ease of use and low cost, tDCS may be a potential therapeutic alternative or augmentation in future smoking cessation programs (Wing et al., 2013). However, although the clinical value of tDCS in treating addiction is advocated, the underlying neurofunctional mechanism remains ambiguous, which restricts further clinical application.
The dorsal lateral prefrontal cortex (DLPFC) is a widely used target site in tDCS studies on nicotine addiction. As the DLPFC has been associated with executive function and salience attribution (Goldstein and Volkow, 2011), one possibility is that the local function of the DLPFC may be enhanced under tDCS in order to better control impulsive, risk-taking behavior and therefore allow for optimal decisions related to cigarette consumption (Fecteau et al., 2014).
The DLPFC is also connected with lots of brain areas, especially the orbitofrontal cortex, the thalamus, dorsal caudate nucleus and the hippocampus/parahippocampal gyrus (HPG) (Miller and Cohen, 2001). Thus, an alternative hypothesis is that tDCS alters the functional connectivity between the DLPFC and other addiction-related brain regions. Several fMRI studies on healthy adults have revealed that tDCS over the DLPFC induces widespread connectivity changes (Lindenberg et al., 2013; Park et al., 2013; Weber et al., 2014), which favor a network explanation of tDCS effects instead of a pure local modulation hypothesis(Fox et al., 2014).
One important pathway underlying addiction is the coupling between prefrontal cortex and the mesolimbic system composed of the striatum, the hippocampus, etc. The mesolimbic system is a brain center of motivation, adaptive learning and memory and is deemed a source of maladaptive salience attribution to addicted cues (Hyman, 2005). Regulation of cigarette craving through cognitive strategies depends on the prefrontal–striatal pathway (Kober et al., 2010) and the interaction between the DLPFC and the OFC (Hayashi et al., 2013), a neural hub anatomically associated with ventral-striatal brain areas. Although these studies indicate the potential mechanism of craving regulation, direct evidence of tDCS modulating the prefrontal–mesolimbic system pathway in craving reduction is still lacking, not only in nicotine addiction but also in other addictive disorders.
Based on previous works, this study directly investigates the neural mechanism of tDCS effects on cigarette craving with a combination of fMRI and tDCS. We hypothesized that stimulating the DLPFC decreases craving through modulation of local activation of the DLPFC and long-distance coupling between the DLPFC and the salience attribution system.
Materials and methods
Subjects
Subjects were recruited through advertisement from the city of Hefei in China and screened in a telephone interview, and met the following inclusion criteria: (1) The Fagerström Test for Nicotine Dependence score (FTND) ≥4; (2) consumed at least ten cigarettes per day in the past 2 years; (3) right-handed male; (4) did not receive pharmacotherapy or psychotherapy in the past 2 months; (5) never sought after nicotine addiction treatment. At the first visit, subjects were screened to exclude those who had neurological, psychiatric and other drug dependence (such as alcoholism) with the Mini-International Neuropsychiatric Interview (Sheehan et al., 1998). Forty male adult smokers participated in the study. Eight subjects were further excluded because of excessive head movements or dropping-out at the second fMRI session, leaving 32 subjects in the final analysis (see Table 1 for characteristics of the study sample). Informed written consent was obtained prior to study participation. Monetary compensation (400 RMB, equals 60 USD) was provided after the completion of all experimental sessions. The procedure was approved by the Institutional Review Board of Anhui Medical University in compliance with the Declaration of Helsinki.
Table 1.
Descriptive statistics of the smoker sample (n = 32)
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| Age (years) | 26.68 | 6.28 | 21 | 49 |
| Number of Cigarettes per day | 14.41 | 4.36 | 10 | 30 |
| Years of Smoking (years) | 8.11 | 7.02 | 3 | 32 |
| FTND | 5.03 | 1.4 | 4 | 9 |
Study design and procedure
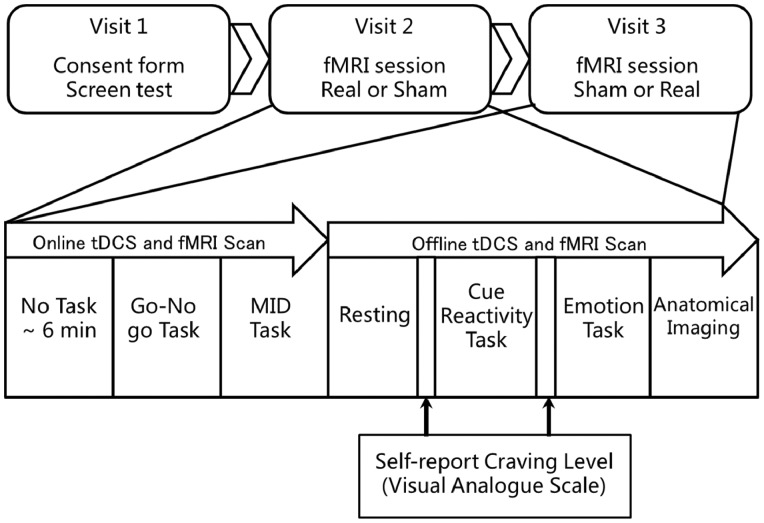
The study used a single blind sham controlled within-subject design (see Figure 1 for an illustration of the study procedures). The order of real and sham stimulation for each participants was generated by a homemade script using MATLAB before data collecting. Orders were produced at balanced permutations with equal number of real-sham order and sham-real order with block size of 10. In the first visit, subjects were screened for psychiatric condition and smoking history. At the second and third visits, all subjects attended a real tDCS session and a comparable sham session separated by 1 week, the order of which was counterbalanced between subjects. In each session, subjects received either real or sham stimulation for 30 min in the MRI scanner using a MRI compatible tDCS equipment (see details in “tDCS protocol” section), during which they also completed a go/no-go task and a monetary incentive delay task (data of these two tasks will be presented elsewhere). Then, subjects were scanned during a resting state, a cue-reactivity task and an emotion task. In addition, an anatomical scan was performed. The craving level prior to, and after, the cue-reactivity task was collected using a computerized visual analogue scale. Furthermore, participants completed two Positive and Negative Affective Scales in each session; one at the beginning of scanning and one at the end of scanning.
Fig. 1.
Overview of the study procedure. The go/no-go task and MID task (Monetary Incentive Delay task) belonged to another study on the online effect of tDCS.
tDCS protocol
Direct current was applied by a battery-driven and MRI compatible stimulator (DC-Stimulator Plus, neuroConn GmbH). The anode electrodes (size 5 × 7 cm2) and the cathode electrodes (size 10 × 10 cm2) were inserted into saline-soaked synthetic sponges. A symmetrical bilateral tDCS protocol was used with the anode over the F3 and the cathode over the F4 according to the 10–20 EEG system. Proper localization of the F3 and the F4 was aided by an elastic Quick EEG cap (Neuroscan Inc., USA). The stimulation started and ended in a ramp-like fashion over 30 s. Direct and constant current of 1 mA was delivered for 30 min in the real tDCS session. The sham tDCS only consisted of 30 s of ramp-up and 30 s of ramp-down to let subjects feel the itching sensation analogous to the real stimulation.
Tasks
Cue-reactivity task
The cue-reactivity task, adopted from a previous study (Luijten et al., 2011), was designed to measure neural responses to smoking cues. In each trial, a picture with either a smoking-related stimuli or a neutral stimuli was presented for 900 ms following a fixation cross (jittered from 1100 to 5100 ms). Two to five semi-randomly distributed lines were displayed within each picture. Participants were instructed to count the number of lines and to press the corresponding button as fast as possible. The picture content was not related to the number of lines. The task was composed of 150 trials.
Image acquisition
All images were collected on a Philips Achieva 3.0 Tesla scanner (Philips Medical Systems, Best, The Netherlands) with an eight-channel coil. Functional BOLD images in each task and resting scans were acquired using the T2-weighted echo planar imaging sequence (32 axial slices, slice thickness = 4 mm, TR = 2 s, TE = 30 ms, FA = 90°, Matrix = 64 × 64, FOV = 240 × 240). Individual high-resolution structural images were also obtained.
fMRI image processing
Task-based fMRI
Images were processed using AFNI (Cox, 1996). The functional 3D volume images were corrected for slice acquisition time differences and were registered to the last volume and spatially normalized to the Talairach space. Then the images were spatially smoothed (full width at half maximum = 6 mm) and each voxel time series was temporally normalized by scaling each run by its mean so that all runs had a mean signal of 100. In the first level GLM, head movements in the six directions were entered as covariates. The contrast between smoking pictures and neutral pictures (smoking-neutral) in the cue-reactivity task was the interest. A 2 × 2 ANOVA model was used to investigate the within subjects effects of tDCS (real vs sham) and cue type (smoking vs neutral) in the second level group analysis. The smoking-neutral contrast was used to define the main nodes of cue reactivity system. To correct for multiple comparisons, a cluster-wise threshold was determined using the Monte Carlo simulation implemented within the 3dClustSim of the AFNI (version: AFNI_16.2.06 released on July 25 2016). The results revealed that a voxel-wise threshold of P < 0.005 combined with a minimum volume of 1203 mm3 is needed to get a family-wise error corrected P < 0.05.
To explore whether the connectivity between prefrontal cortex and the cue reactivity system was modulated by tDCS, we performed the psychophysiological interaction (PPI) analysis. To avoid double dipping, the seed of lDLPFC was defined using a 5-mm-radius sphere with center at the corresponding cortex coordinate of F3 (Talairach space: −33.6, 27.2, 39.2) (Keeser et al., 2011) but not from the result of our cue-reactivity task (see Supplementary Figure S1 for an illustration). The time course of lDLPFC was extracted by averaging voxel seed region, with linear trends removed (3dDetrend) and deconvolved to get neural activity (3dTfitter). The PPI model included a psychological regressor comparing smoking vs neutral cue (coded as 1 and −1, respectively) convolved with a hemodynamic response function, a physiological regressor (the mean time course from lDLPFC) and the interaction between the two. Head motions in the six directions were also controlled as covariates. Whole brain PPI analysis was performed for each condition for each subject. Then the PPI value was extracted from the main nodes of the cue reactivity system (defined using significant clusters of smoking-neutral contrast) and averaged to represent the connectivity modulated by cue type between lDLPFC and a specific node. Whether the PPI value in each node of cue reactivity system was altered by tDCS was tested using pairwise t test for each node of the cue reactivity system.
Resting-state fMRI
Preprocessing of resting images was similar to task-based fMRI except for the addition of a 3dDespike step before time correction. In addition, signals originating from head motion, white matter and CSF were regressed out. Finally, the data were band-pass filtered (0.01–0.08 Hz). Then a seed-based connectivity analysis using lDLPFC (same as the PPI analysis) was performed. The correlation coefficients were fisher-transformed. Average correlation coefficients within the main nodes of cue-reactivity system (same as the PPI analysis) were computed to represent the connectivity between lDLPFC and a certain node. The definition of seed regions and regions of interest were the same as in the former PPI analysis of the cue-reactivity task. Then, a pairwise t-test employed to test whether the seed-based resting-state functional connectivity in each node of the cue reactivity system was altered by tDCS.
Results
fMRI results
Cue-reactivity task
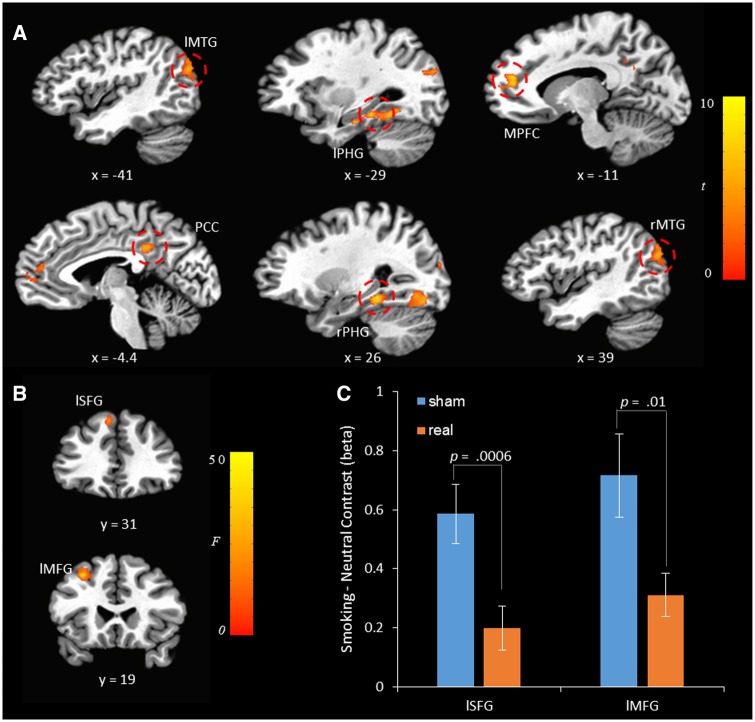
A 2 × 2 ANOVA model with tDCS (real vs sham) and cue type (smoking vs neutral) as two within-subject factors was performed (see Table 2 for detailed results). The smoking-neutral contrast was mainly manifested in the left/right middle temporal gyrus (MTG), the left/right HPG, medial prefrontal cortex (MPFC), and posterior cingulate cortex (PCC). Although no significant effect of tDCS was found, a significant tDCS × cue type interaction was revealed in the left superior frontal gyrus (SFG) and the left middle frontal gyrus (MFG) (Figure 2). As illustrated in Figure 2C, the smoking-neutral contrast was reduced in the real stimulation condition compared with sham stimulation condition in both the left SFG (t = 3.812, df = 31, P = 0.0006, Cohen’s d = 0.674) and the left MFG (t = 2.721, df = 31, P = 0.011, Cohen’s d = 0.481).
Table 2.
The whole-brain analysis of the cue-reactivity task
| Peak coordinates (Talairach space) |
Maximum | Volume size (mm3) | |||
|---|---|---|---|---|---|
| X | Y | Z | F | ||
| Smoking-neutral contrast | |||||
| rPHG/rFG | 26 | −36 | −9 | 26.51 | 4594 |
| lMTG | −41 | −74 | 26 | 19.74 | 3766 |
| lMFG | −11 | 44 | 19 | 25.10 | 3734 |
| rMTG | 39 | −76 | 11 | 17.02 | 2094 |
| lPHG | −29 | −31 | −11 | 25.09 | 2031 |
| PCC | 1 | −36 | 31 | 24.98 | 1719 |
| Real–sham contrast | |||||
| – | |||||
| tDCS × cue interaction | |||||
| lSFG | −6 | 31 | 46 | 22.41 | 1313 |
| lMPFC | −29 | 19 | 44 | 43.97 | 1218 |
Note: l, left; r, right; MFG, medial prefrontal cortex; PHG, para-hippocampal gyrus; FG, right fusiform gyrus; MTG, middle temporal gyrus; PCC, posterior cingulate cortex; SFG, superior frontal gyrus; MFG, middle frontal gyrus. The left, the posterior and the inferior directions are negative.
Fig. 2.
(A) The smoking-neutral contrast map in the cue-reactivity task. l: left; r: right; MPFC, medial prefrontal cortex; PHG, para-hippocampal gyrus; FG: right fusiform gyrus; MTG: middle temporal gyrus; PCC, posterior cingulate cortex; SFG: superior frontal gyrus; MFG: middle frontal gyrus. The left, the posterior, and the inferior directions are negative. The contrast map was thresholded with cluster-wise P values <0.05. (B) tDCS × cue type interaction. (C) A detailed illustration of the tDCS × cue interaction in the left SFG and the left MFG.
PPI analysis
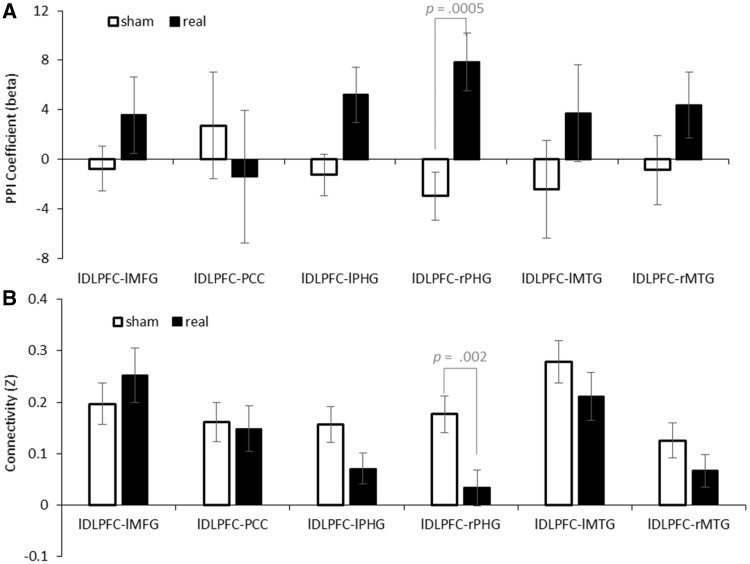
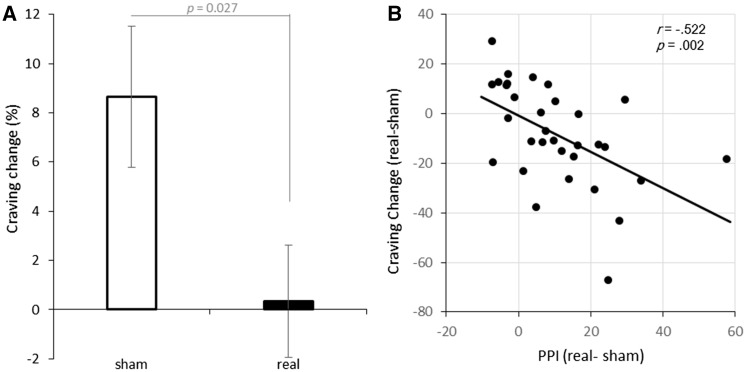
Whole brain PPI analysis (from lDLPFC) was performed for each tDCS condition for each subject. Then, we tested whether the PPIs were modulated by tDCS using the pairwise t-test for each node of the cue reactivity system. Results showed that the PPI between lDLPFC and rPHG was modulated by tDCS stimulation (t = 3.859, df = 31, P = 0.0005, Cohen’s d = 0.682). None of other PPI were significant (all Ps > 0.1). See Figure 3A for detail information. Besides, individual analysis suggested that the PPI between the lDLPFC and the rPHG was negatively correlated with craving change in the two tDCS sessions (r = −0.522, P = 0.002, Figure 4B).
Fig. 3.
(A) The tDCS effect on PPI between lDLPFC and cue-related brain regions. (B) The tDCS effect on resting state functional connectivity between lDLPFC and cue-related brain regions.
Fig. 4.
(A) Craving change (%) across cue-reactivity task in sham and real stimulation. The length of the error bar represents the standard error of the mean. (B) The changes of PPI between lDLPFC and rPHG across the two sessions (real–sham) correlated with the craving changes across the two sessions (real–sham).
Resting state fMRI
We also tested whether the seed-based resting-state functional connectivity between the lDLPFC and the rPHG was altered by tDCS. The definition of seed regions and regions of interest were the same as in the former PPI analysis of the cue-reactivity task. A pairwise t-test revealed that the resting-state functional connectivity between the lDLPFC and the rPHG was increased by real stimulation as compared with sham stimulation (t = 3.332, df = 31, P = 0.002, Cohen’s d = 0.589). None of connectivity in other cue-reactivity ROIs was significant (all Ps > 0.2). See Figure 4B for detail information.
Emotion task
A 2 × 2 ANOVA model with tDCS (real vs sham) and valence (negative vs neutral) as within-subject factors was performed. No significant main effect or interaction effect of tDCS was found in the task (see Supplementary Figure S2 and Table S1 for further information).
Behavior results
Response times (RTs) and accuracies in cue-reactivity task
Separate 2(tDCS condition) × 2(cue type) repeated measures Analysis of Variance (ANOVA) was performed on RTs and accuracy. No significant effect was found in any analysis (all P > 0.2).
Craving self-reports
Subjects reported a generally higher craving level after the cue-reactivity task (t = 2.369, df = 63, P = 0.021, Cohen’s d = 0.296). Despite this, the craving change across the cue-reactivity task was lower during real stimulation than during sham stimulation (t = −2.319, df = 31, P = 0.027, Cohen’s d = 0.410). See Figure 2 for an illustration. For analysis on absolute craving level, please refer to Supplementary Figure S3.
Positive and negative affective scale
No significant effect of tDCS was found on either change in positive affect or change in negative affect (all P > 0.2). See supplementary Material (Supplementary Figure S4) for further information.
Discussion
Neuroimaging studies have identified the DLPFC as one of the most important areas involved in the cue-associated anticipation and planning of drug use (Goldstein and Volkow, 2011). Although previous studies on cigarette craving assumed that local function of the prefrontal cortex might be affected when applying tDCS to it (Fregni et al., 2008; Boggio et al., 2009), direct support of this assumption is rare. We demonstrated that reactivity of the left SFG and the left MFG to smoking cues in the cue-reactivity task is modulated by tDCS; specifically, smoking cues elicited stronger activation than the neutral cues in the sham condition, while this pattern was reduced by real stimulation.
We identified several key cue reactivity brain regions including the MPFC, the left and right PHG, the left and right MTG and the PCC, which have also been reported in the literature of smoking-cue reactivity (Engelmann et al., 2012; Yalachkov et al., 2012; Jasinska et al., 2014). We found the coupling between the lDLPFC and the rPHG was relevant for smokers’ craving change and those couplings were altered both in the resting-state functional connectivity and the task-based functional connectivity after stimulation. Moreover, the lDLPFC–rPHG coupling correlated with smokers’ craving change as revealed by PPI analysis. Our results imply that tDCS over the DLPFC may change craving through the connection with other deep-brain neural circuits, which is consistent with the network explanation of non-invasive brain stimulation (Fox et al., 2014).
The PHG is associated with processing and representation of contextual associations, a core feature common to episodic memory (Aminoff et al., 2013). We found that the PHG was activated during the implicit cue-reactivity task, which is consistent with the viewpoint that strong desire, such as craving, reflects involuntary retrieval and processing of drug usage experience (Franken, 2003; Kavanagh et al., 2005). The tDCS effect on lDLPFC–rPHG coupling correlated with the tDCS effect on craving change individually, which indicates that tDCS may enhance the ability of the DLPFC in regulating retrieval of addiction memory. A noteworthy finding is that the lDLPFC–PHG coupling was altered even during the resting state after stimulation, which implies that tDCS may induce both intrinsic changes and task-specific effects on the lDLPFC network.
One alternative mechanism of tDCS effects on craving may due to the amelioration of negative emotions induced by applying electrical stimulation over the DLPFC (Lang et al., 1997). This possibility is supported by a recent study, in which a tDCS effect on negative emotion instead of craving was detected (Xu et al., 2013). However, our results do not support this hypothesis: On the one hand, negative emotions across real and sham stimulation were comparable; on the other hand, brain activation related to negative valence pictures was insensitive to the modulation of tDCS (Supplementary Table S1). Moreover, the lDLPFC–rPHG coupling, as revealed by a similar PPI analysis on an emotion task, was not affected by electrical stimulation (Supplementary Figure S5). However, as the emotion task always followed the cue-reactivity task, the null effect in the emotion task may originate from the weak effect failing to modulate brain activation after about 20 min (the scanning time for resting and cue-reactivity task). Despite this possibility, the current evidence, at least, does not favor the hypothesis that negative emotions are altered by tDCS.
We only recruited male chronic smokers in the study because of the asymmetry of number of male smokers and number of female smokers. According to a recent survey, two-thirds of men smoked in contrast with <1% smoking rate in females who were born after 1960s in China (Chen et al., 2015). Despite of this, it is interesting to focus the tiny population of Chinese female smokers in the future studies.
In summary, our results provide direct evidence that local smoking cue-elicited activation of the left DLPFC was altered by tDCS. Furthermore, we demonstrate that the coupling between the DLPFC and the PHG correlated with craving changes. The tDCS effect on the DLPFC–PHG coupling is even manifested in the resting state after stimulation. These findings imply that tDCS may help smokers to regulate involuntary memory retrieval and support the clinical application of tDCS in nicotine addiction.
Supplementary data
Supplementary data are available at SCAN online.
Supplementary Material
Acknowledgements
We thank Miss Yang Zhiyu for her assistance in the study process. We also thank the Supercomputing Centre of USTC for providing support of numerical calculations.
Funding
This research was funded by the National Natural Science Foundation of China. [31230032, 31471071, 31171083, 31500917]; The National Key Basic Research Program [2016YFA0400900]; The Fundamental Research Funds for the Central Universities of China; MOE-Microsoft Key Laboratory of USTC; and China Postdoctoral Science Foundation [2016M592051].
Conflict of interest. None declared.
References
- Aminoff E.M., Kveraga K., Bar M. (2013). The role of the parahippocampal cortex in cognition. Trends in Cognitive Sciences, 17(8), 379–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio P.S., Liguori P., Sultani N., Rezende L., Fecteau S., Fregni F. (2009). Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neuroscience Letters, 463(1), 82–6. [DOI] [PubMed] [Google Scholar]
- Chen Z.M., Peto R., Zhou M.G., et al. (2015). Contrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studies. Lancet, 386(10002), 1447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–73. [DOI] [PubMed] [Google Scholar]
- Engelmann J.M., Versace F., Robinson J.D., et al. (2012). Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. NeuroImage, 60(1), 252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S., Agosta S., Hone-Blanchet A., et al. (2014). Modulation of smoking and decision-making behaviors with transcranial direct current stimulation in tobacco smokers: a preliminary study. Drug and Alcohol Dependence, 140, 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Buckner R.L., Liu H.S., Chakravarty M.M., Lozano A.M., Pascual-Leone A. (2014). Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proceedings of the National Academy of Sciences of the United States of America, 111(41), E4367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken I.H.A. (2003). Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 27(4), 563–79. [DOI] [PubMed] [Google Scholar]
- Fregni F., Liguori P., Fecteau S., Nitsche M.A., Pascual-Leone A., Boggio P.S. (2008). Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. The Journal of Clinical Psychiatry, 69(1), 32–40. [DOI] [PubMed] [Google Scholar]
- Goldstein R.Z., Volkow N.D. (2011). Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature Reviews Neuroscience, 12(11), 652–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Ko J.H., Strafella A.P., Dagher A. (2013). Dorsolateral prefrontal and orbitofrontal cortex interactions during self-control of cigarette craving. Proceedings of the National Academy of Sciences of the United States of America, 110(11), 4422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman S.E. (2005). Addiction: a disease of learning and memory. American Journal of Psychiatry, 162(8), 1414–22. [DOI] [PubMed] [Google Scholar]
- Jansen J.M., Daams J.G., Koeter M.W., Veltman D.J., van den Brink W., Goudriaan A.E. (2013). Effects of non-invasive neurostimulation on craving: a meta-analysis. Neuroscience and Biobehavioral Reviews, 37(10 Pt 2), 2472–80. [DOI] [PubMed] [Google Scholar]
- Jasinska A.J., Stein E.A., Kaiser J., Naumer M.J., Yalachkov Y. (2014). Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neuroscience and Biobehavioral Reviews, 38, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh D.J., Andrade J., May J. (2005). Imaginary relish and exquisite torture: the elaborated intrusion theory of desire. Psychological Review, 112(2), 446–67. [DOI] [PubMed] [Google Scholar]
- Keeser D., Meindl T., Bor J., et al. (2011). Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. Journal of Neuroscience, 31(43), 15284–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H., Mende-Siedlecki P., Kross E.F., et al. (2010). Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences of the United States of America, 107(33), 14811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. (1997) International affective picture system (IAPS): Technical manual and affective ratings, University of Florida.
- Lindenberg R., Nachtigall L., Meinzer M., Sieg M.M., Floel A. (2013). Differential effects of dual and unihemispheric motor cortex stimulation in older adults. Journal of Neuroscience, 33(21), 9176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M., Veltman D.J., van den Brink W., et al. (2011). Neurobiological substrate of smoking-related attentional bias. NeuroImage, 54(3), 2374–81. [DOI] [PubMed] [Google Scholar]
- Meng Z., Liu C., Yu C., Ma Y. (2014). Transcranial direct current stimulation of the frontal-parietal-temporal area attenuates smoking behavior. Journal of Psychiatric Research, 54, 19–25. [DOI] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. [DOI] [PubMed] [Google Scholar]
- Park C.H., Chang W.H., Park J.Y., Shin Y.I., Kim S.T., Kim Y.H. (2013). Transcranial direct current stimulation increases resting state interhemispheric connectivity. Neuroscience Letters, 539, 7–10. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., et al. (1998). The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59, 22–33. [PubMed] [Google Scholar]
- Shiffman S.M., Jarvik M.E. (1976). Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology, 50(1), 35–9. [DOI] [PubMed] [Google Scholar]
- Szasz P.L., Szentagotai A., Hofmann S.G. (2012). Effects of emotion regulation strategies on smoking craving, attentional bias, and task persistence. Behaviour Research and Therapy, 50(5), 333–40. [DOI] [PubMed] [Google Scholar]
- Weber M.J., Messing S.B., Rao H.Y., Detre J.A., Thompson-Schill S.L. (2014). Prefrontal transcranial direct current stimulation alters activation and connectivity in cortical and subcortical reward systems: a tDCS-fMRI study. Human Brain Mapping, 35(8), 3673–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing V.C., Barr M.S., Wass C.E., et al. (2013). Brain stimulation methods to treat tobacco addiction. Brain Stimulation, 6(3), 221–30. [DOI] [PubMed] [Google Scholar]
- Xu J., Fregni F., Brody A.L., Rahman A.S. (2013). Transcranial direct current stimulation reduces negative affect but not cigarette craving in overnight abstinent smokers. Frontiers in Psychiatry, 4, 112.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalachkov Y., Kaiser J., Naumer M.J. (2012). Functional neuroimaging studies in addiction: multisensory drug stimuli and neural cue reactivity. Neuroscience and Biobehavioral Reviews, 36(2), 825–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.