Abstract
Background
Many patients hospitalized with pneumonia are treated with combination macrolide/cephalosporin therapy. Macrolides have immunomodulatory effects and do not directly cause bacterial lysis. These effects suggest the possibility that initial treatment with a macrolide before a cephalosporin could improve patient outcomes by preventing the inflammatory response to rapid bacterial lysis that can be caused by cephalosporin treatment. This study explores whether initial treatment for pneumonia with a macrolide before a cephalosporin is associated with better patient outcomes than treatment with a cephalosporin before a macrolide.
Methods
This is a retrospective cohort study using a clinically rich database derived from electronic health records of 71 hospitals. We compared outcomes for pneumonia patients who received intravenous treatment with a macrolide at least 1 hour before a cephalosporin, versus patients who received a cephalosporin at least 1 hour before a macrolide. Propensity matching was performed for 527 patients in each group.
Results
Among the propensity-matched cohorts, for the macrolide first group, in-hospital mortality was 4.2% vs 5.5% for the cephalosporin first group (P = .31), combined in-hospital mortality/hospice discharge was 6.3% vs 9.3% (P = .06), median hospital length of stay was 101.5 hours vs 109.5 hours (P = .09), and 30-day readmission was 12.9% vs 10.6% (P = .27).
Conclusions
Treatment of pneumonia with a macrolide before a cephalosporin was not associated with significantly improved outcomes when compared with treatment with a cephalosporin first; however, the lower rate of mortality/discharge to hospice and the large confidence intervals allow for the possibility of a clinically significant benefit.
Keywords: immunomodulatory, macrolides, outcomes, pneumonia
Pneumonia remains a common cause of morbidity and mortality, with approximately 1 million US hospital admissions in 2013 [1] and 30-day mortality remaining at 11.5% for Medicare patients [2]. Deterioration after antibiotic treatment begins is common [3–6] and is associated with very high mortality [7, 8].
It has been theorized that bacterial lysis by antibiotics that are active against the bacterial cell wall, with resulting release of proinflammatory substances, may contribute to the poor outcomes seen in some patients with pneumonia [9, 10]. Several lines of evidence support this theory. First, it is well established that bacterial lysis leads directly to a surge in the release of bacterial inflammatory mediators, including endotoxin, lipotechoic acid, and peptidoglycan [11, 12]. Furthermore, corticosteroids reduce the risk of mortality and deterioration in patients with severe pneumonia, possibly by minimizing the inflammation associated with antibiotic treatment [13]. Animal studies of combined influenza/bacterial pneumonia demonstrate that treatment with antibiotics that do not directly result in bacterial lysis, such as macrolides, are associated with a lessened inflammatory response and greater survival [14].
Treatment of human bacterial infections with macrolides or fluoroquinolones, which are not active on the cell wall, might prevent the massive bacterial lysis and resulting inflammation that can be caused by β-lactam exposure [9, 10]. Several observational studies [15] and a randomized trial [16] have suggested improved outcomes when antibiotic therapy for community-acquired pneumonia (CAP) includes a macrolide. However, there is also evidence that macrolides have potent direct immunomodulatory effects on the host, unrelated to any antibacterial effect [17]. This might explain why some studies and a meta-analysis have suggested better outcomes in patients with pneumonia who are treated with macrolides compared with fluoroquinolones [18–20].
Many patients hospitalized with pneumonia are treated with both a macrolide and a cephalosporin antibiotic, according to current guidelines [21]. Given the possibility that macrolide antibiotics may improve pneumonia patient outcomes by either preventing rapid bacterial lysis or by a beneficial immunomodulatory effect that blunts the inflammatory response to products released by bacterial lysis, or by both effects, we theorized that patients treated with a macrolide antibiotic before a cephalosporin would have better outcomes than patients given cephalosporin treatment followed by a macrolide. To our knowledge, no studies have tested this hypothesis. Working with a clinically rich database drawn from the electronic medical records of a large sample of United States hospitals, we compared the outcomes of patients hospitalized for pneumonia who received a macrolide before a cephalosporin to those who received a cephalosporin before a macrolide. Our a priori hypothesis was that patients who received a macrolide first would have better outcomes than patients who received a cephalosporin first.
METHODS
Design, Setting, and Subjects
We conducted a retrospective cohort study of patients hospitalized for pneumonia at 70 hospitals that contribute electronic health record data to HealthFacts (Cerner Corporation, Kansas City, MO). This database is a deidentified Health Insurance Portability and Accountability Act (HIPAA)-compliant comprehensive source containing time-stamped pharmacy, laboratory, and clinical results along with traditional hospital claims data [22–24].
We included discharges from an acute care hospital between January 1, 2009 and December 31 2012, age 18 years or older with a principal diagnosis of pneumonia (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes: 481, 482.x, 483.x, 484.x, 485, 486, 487.x, 488, 507.x, 136.3) or a principal diagnosis of sepsis (ICD-9-CM codes: 038.x, 790.7, 995.91, 995.92, 785.52), or acute respiratory failure (ICD-9-CM codes: 518.81, 518.82, 518.84, 786.09) with a secondary diagnosis of pneumonia. We included patients who had initial treatment with both an intravenous macrolide (erythromycin or azithromycin) and a third- or fourth-generation cephalosporin (cefotaxime, ceftizoxime, ceftriaxone, cefepime). Antibiotic timing was based on the time the infusion began. We included only patients in whom the 2 antibiotics were given 1 hour or more apart. Exclusion criteria are provided in the online Supplementary Data.
Patient and Hospital Characteristics
Patient characteristics used for risk adjustment and propensity matching included age, gender, race/ethnicity, payer, type of pneumonia, healthcare-associated pneumonia (HCAP) vs CAP, Gagne combined score [25], Elixhauser comorbidities, smoking, obesity, receipt of invasive or noninvasive mechanical ventilation in a prior admission, and prior hospitalization. Patients were characterized as having HCAP if they were admitted from a skilled or intermediate care facility, were hospitalized during the prior 90 days, were on dialysis, or receiving immune-suppressing medications. Other patients were designated as having CAP. The primary analyses were performed on the entire cohort, whereas secondary analyses were done on the CAP cohort. Disease severity was assessed using the laboratory acute physiology score (LAPS), a validated tool that assesses severity of illness at the time of hospital admission using data extractable from an electronic health record database to predict hospital mortality [26, 27] as well as several other methods, as described in the online Supplementary Data. Vital sign data were not available, precluding calculation of pneumonia-specific mortality risk measures. Hospital characteristics were also used in the models.
Outcomes
The primary outcome of the study was in-hospital mortality. Given the limitations of in-hospital mortality as an outcome, we defined a second mortality outcome that also included patients discharged to hospice. Secondary outcomes were hospital length of stay and all-cause readmission within 30 days among survivors.
Analyses
Summary statistics describing the study cohort are presented as (1) frequencies and proportions for categorical variables and medians and (2) interquartile ranges (IQRs) for continuous variables. Associations between patient or hospital characteristics and antibiotic treatment were assessed via generalized estimating equation (GEE) models accounting for patients clustering within hospitals. Association of treatment with outcomes in the propensity-matched cohort was assessed using conditional logistic regression models accounting for matching. We developed a nonparsimonious GEE model accounting for patient clustering within hospitals to predict receipt of macrolide first. This model included patient age, gender, insurance payer, comorbidity score, selected Elixhauser comorbidities, HCAP vs CAP, LAPS, initial care venue, receipt of invasive or noninvasive mechanical ventilation in prior year, number of admissions in prior year, receipt of early vasopressors, invasive or noninvasive mechanical ventilation, hospital size, teaching status, region, and selected significant interactions between these variables. We then matched each macrolide first-treated patient with a patient who was treated first with cephalosporin with a similar propensity score via the Greedy match algorithm. Additional detail on the analytic methods is provided in the online Supplementary Data.
We performed several sensitivity analyses; exclusion of HCAP patients, only including patients whose initial macrolide and cephalosporin treatment were at least 2 hours or more apart, defining receipt of ventilator support or vasopressor support that was initiated more than 2 hours after initial antibiotics as an outcome (as opposed to more than 12 hours for the primary analyses). In an effort to preferentially study patients more likely to have a higher bacterial load, or otherwise at higher risk, we performed (1) a series of stratified analyses based on the presence of bacteremia and (2) LAPS. We also examined the order of antibiotic administration in patients who died within 3 days of presentation.
RESULTS
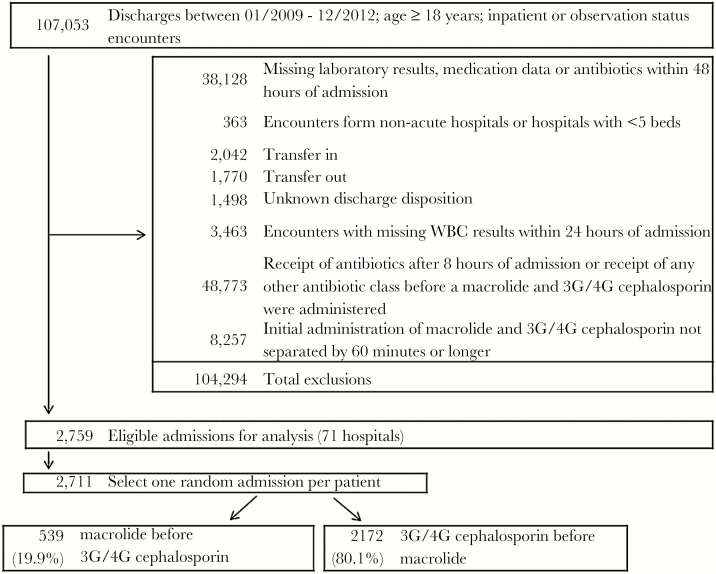
The database included 107 701 patients admitted to the hospital with pneumonia. Figure 1 demonstrates the application of inclusion and exclusion criteria to derive the study population. After exclusions, 59 789 patients were potentially eligible for inclusion. Among these, 2711 patients received a β-lactam and a macrolide more than 1 hour apart, but within 8 hours of each other, 539 (19.9%) received a macrolide first and 2172 (80.1%) received a cephalosporin first. For the macrolide first group, the median time (IQR) between the 2 antibiotics was 2.3 hours (IQR, 1.4–4.4 hours). For the cephalosporin first group, the median time between the 2 antibiotics was 2.1 hours (IQR, 1.3–4.0 hours).
Figure 1.
Flowchart of inclusions and exclusions. Abbreviations: G, generation; WBC, white blood cells.
The patient and hospital characteristics and unadjusted outcomes are shown in Table 1. Patients who received a macrolide first were somewhat younger (median age, 68 vs 72 years), more likely to have chronic obstructive pulmonary disease (48.1% vs 41.6%), less likely to be admitted to an intensive (9.5% vs 12.6%) or intermediate care unit (3.3% vs 4.8%), but were more likely to require vasopressors (2.4% vs 0.5%) and noninvasive ventilation (2.8% vs 2.2%) before initial antibiotic therapy. Patients who received macrolide treatment first were more likely to be cared for in teaching hospitals (70.3% vs 56.8%). Patients who received macrolide treatment first had a lower hospital mortality (4.1% vs 5.9%) and lower rate of hospital mortality/hospice discharge (6.1% vs 8.4%), although these differences were not statistically significant (P = .07 and P = 0.09). Hospital length of stay was statistically lower in patients who received a macrolide first, but the magnitude of difference was small (0.4 hours, P = .05).
Table 1.
Association of Observed Patient and Hospital Characteristics and Outcomes With Initial Antibiotic Treatment
| Characteristics | Total | Macrolide Before Cephalosporin | Cephalosporin Before Macrolide | P Valuea |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| N | 2711 (100) | 539 (19.9) | 2172 (80.1) | |
| Age, median (IQR), years | 71 (55–83) | 68 (53–81) | 72 (56–83) | .03 |
| Gender | .17 | |||
| Male | 1296 (47.8) | 246 (45.6) | 1050 (48.3) | |
| Female | 1415 (52.2) | 293 (54.4) | 1122 (51.7) | |
| Race/Ethnicity | .85 | |||
| White | 2145 (79.1) | 414 (76.8) | 1731 (79.7) | |
| Black | 351 (12.9) | 80 (14.8) | 271 (12.5) | |
| Hispanic | 61 (2.3) | 15 (2.8) | 46 (2.1) | |
| Other | 154 (5.7) | 30 (5.6) | 124 (5.7) | |
| Insurance Payer | .14 | |||
| Medicare | 1209 (44.6) | 200 (37.1) | 1009 (46.5) | |
| Medicaid | 132 (4.9) | 28 (5.2) | 104 (4.8) | |
| Other | 1370 (50.5) | 311 (57.7) | 1059 (48.8) | |
| LAPS, median (IQR) | 41 (29–55) | 40 (28–53) | 41 (30–55) | .22 |
| Principal Diagnosis | .04 | |||
| Pneumonia | 2109 (77.8) | 437 (81.1) | 1672 (77) | |
| ARF | 161 (5.9) | 28 (5.2) | 133 (6.1) | |
| Sepsis | 441 (16.3) | 74 (13.7) | 367 (16.9) | |
| Type of Pneumonia | ||||
| CAP | 1864 (68.8) | 359 (66.6) | 1505 (69.3) | |
| HCAP | 847 (31.2) | 180 (33.4) | 667 (30.7) | |
| Gagne combined score, median (IQR) | 2 (1–4) | 2 (1–3) | 2 (1–4) | .55 |
| Comorbiditiesb | ||||
| Hypertension | 1255 (46.3) | 239 (44.3) | 1016 (46.8) | .74 |
| Chronic pulmonary disease | 1163 (42.9) | 259 (48.1) | 904 (41.6) | .005 |
| Smoking | 758 (28.0) | 156 (28.9) | 602 (27.7) | .54 |
| Diabetes | 737 (27.2) | 155 (28.8) | 582 (26.8) | .08 |
| Chronic blood loss anemia/deficiency anemias | 669 (24.7) | 110 (20.4) | 559 (25.7) | .04 |
| Congestive heart failure | 620 (22.9) | 118 (21.9) | 502 (23.1) | .99 |
| Renal failure | 381 (14.1) | 76 (14.1) | 305 (14.0) | .74 |
| Other neurological disorders | 345 (12.7) | 64 (11.9) | 281 (12.9) | .95 |
| Depression/psychoses | 333 (12.3) | 66 (12.2) | 267 (12.3) | .63 |
| Hypothyroidism | 314 (11.6) | 54 (10.0) | 260 (12.0) | .24 |
| Obesity | 246 (9.1) | 59 (10.9) | 187 (8.6) | .07 |
| Valvular disease | 204 (7.5) | 37 (6.9) | 167 (7.7) | .92 |
| Metastatic cancer/solid tumor without metastasis | 167 (6.2) | 34 (6.3) | 133 (6.1) | .84 |
| Weight loss | 169 (6.2) | 33 (6.1) | 136 (6.3) | .61 |
| Peripheral vascular disease | 138 (5.1) | 21 (3.9) | 117 (5.4) | .35 |
| Pulmonary circulation disease | 137 (5.1) | 28 (5.2) | 109 (5.0) | .61 |
| Prior Year Receipt of IMV | ||||
| 0 | 2668 (98.4) | 530 (98.3) | 2138 (98.4) | |
| 1 time | 38 (1.4) | 9 (1.7) | 29 (1.3) | |
| 2 or more times | 5 (0.2) | 5 (0.2) | ||
| Prior Year Receipt of NIV | .03 | |||
| 0 | 2676 (98.7) | 528 (98) | 2148 (98.9) | |
| 1 time | 21 (0.8) | 5 (0.9) | 16 (0.7) | |
| 2 or more times | 14 (0.5) | 6 (1.1) | 8 (0.4) | |
| Prior Year Admissions | .19 | |||
| 0 | 1610 (59.4) | 309 (57.3) | 1301 (59.9) | |
| 1 time | 560 (20.7) | 115 (21.3) | 445 (20.5) | |
| 2 or more times | 541 (20) | 115 (21.3) | 426 (19.6) | |
| Initial Care Venue | .09 | |||
| Ward | 2265 (83.5) | 470 (87.2) | 1795 (82.6) | |
| Intensive Care Unit | 324 (12) | 51 (9.5) | 273 (12.6) | |
| Intermediate care | 122 (4.5) | 18 (3.3) | 104 (4.8) | |
| Vasopressors (before 1st abx) | 23 (0.8) | 13 (2.4) | 10 (0.5) | <.001 |
| Vasopressors (before 1st abx or ≤12 hrs after 1st abx) | 111 (4.1) | 22 (4.1) | 89 (4.1) | .83 |
| NIV (before 1st abx) | 62 (2.3) | 15 (2.8) | 47 (2.2) | .04 |
| NIV (before 1st abx or ≤12 hrs after 1st abx) | 78 (2.9) | 17 (3.2) | 61 (2.8) | .1 |
| IMV (before 1st abx) | 97 (3.6) | 22 (4.1) | 75 (3.5) | .5 |
| IMV (before 1st abx or ≤12 hrs after 1st abx) | 128 (4.7) | 26 (4.8) | 102 (4.7) | .81 |
| Hospital Region | .07 | |||
| Midwest | 465 (17.2) | 108 (20) | 357 (16.4) | |
| Northeast | 1093 (40.3) | 216 (40.1) | 877 (40.4) | |
| South | 894 (33) | 166 (30.8) | 728 (33.5) | |
| West | 259 (9.6) | 49 (9.1) | 210 (9.7) | |
| Hospital size | .51 | |||
| 6–199 beds | 848 (31.3) | 144 (26.7) | 704 (32.4) | |
| 200–499 beds | 1419 (52.3) | 296 (54.9) | 1123 (51.7) | |
| 500 and more beds | 444 (16.4) | 99 (18.4) | 345 (15.9) | |
| Hospital Teaching Status | .001 | |||
| Nonteaching | 1099 (40.5) | 160 (29.7) | 939 (43.2) | |
| Teaching | 1612 (59.5) | 379 (70.3) | 1233 (56.8) | |
| Outcomes | ||||
| Clinical Deterioration | ||||
| Late NIV | 25 (0.9) | 3 (0.6) | 22 (1) | .35 |
| Late IMV | 64 (2.5) | 13 (2.5) | 51 (2.5) | .9 |
| Late vasopressors | 90 (3.5) | 23 (4.4) | 67 (3.2) | .29 |
| In-hospital mortality | 151 (5.6) | 22 (4.1) | 129 (5.9) | .07 |
| In-hospital mortality/hospice discharge | 216 (8.0) | 33 (6.1) | 183 (8.4) | .09 |
| LOS, median (IQR), hours | 102.8 (67.9–166.0) | 102.6 (70.0–169.6) | 103.2 (67.2–164.4) | .05 |
| Readmission within 30 days (among survivors) | 319 (12.5) | 65 (12.6) | 254 (12.4) | .97 |
| Discharge disposition (among survivors, nonhospice discharges, n = 2150) | .11 | |||
| Home/home-health | 1795 (71.9) | 379 (74.9) | 1416 (70.2) | |
| SNF/ICF | 627 (25.1) | 118 (23.3) | 509 (25.6) | |
| Other | 73 (2.9) | 9 (1.8) | 64 (3.2) | |
Abbreviations: abx, antibiotic; ARF, acute respiratory failure; CAP, community acquired pneumonia; GEE, generalized estimating equation; HCAP, healthcare-associated pneumonia; ICF, intermediate care facility; IMV, invasive mechanical ventilation; IQR, interquartile range; LAPS, Laboratory Acute Physiology Score; LOS, length of stay; NIV, noninvasive mechanical ventilation; SNF, skilled nursing facility.
a P value from GEE models accounting for patient clustering within hospitals.
bComorbidities with at least 5% prevalence are presented.
We matched 527 patients in each group for the propensity analysis. Table 2 shows the patient and hospital characteristics and outcomes among this cohort. Patient characteristics in the initial macrolide and initial cephalosporin groups were very similar, although there was a lower percentage of Medicare patients in the initial macrolide group and differences in the percentage of patients treated at teaching hospitals. Mortality was 4.2% in the macrolide first group and 5.5% in the matched cephalosporin group (P = .31), and mortality/hospice discharge was 6.3% for the macrolide first group and 9.3% for the cephalosporin first group (P = .06). The hospital length of stay was nonsignificantly lower in patients who received a macrolide first (P = .09), whereas the 30-day readmission rate was nonsignificantly higher in the macrolide first group (12.9% vs 10.6%, P = .27).
Table 2.
Observed Patient and Hospital Characteristics and Outcomes Among Propensity-Matched Cohort
| Characteristics | Macrolide Before Cephalosporin | Cephalosporin Before Macrolide | P Valuea |
|---|---|---|---|
| N (%) | N (%) | ||
| 527 (50) | 527 (50) | ||
| Age, median (IQR), yearsb | 68 (53–81) | 70 (54–81) | .61 |
| Gender | .90 | ||
| Male | 240 (45.5) | 238 (45.2) | |
| Female | 287 (54.5) | 289 (54.8) | |
| Race/Ethnicity | .63 | ||
| White | 404 (76.7) | 415 (78.7) | |
| Black | 79 (15) | 79 (15) | |
| Hispanic | 14 (2.7) | 11 (2.1) | |
| Other | 30 (5.7) | 22 (4.2) | |
| Insurance Payer | .03 | ||
| Medicare | 197 (37.4) | 237 (45) | |
| Medicaid | 26 (4.9) | 28 (5.3) | |
| Other | 304 (57.7) | 262 (49.7) | |
| LAPS, median (IQR)b | 40 (27–53) | 40 (30–55) | .22 |
| Principal Diagnosis | .28 | ||
| Pneumonia | 430 (81.6) | 415 (78.7) | |
| ARF | 26 (4.9) | 38 (7.2) | |
| Sepsis | 71 (13.5) | 74 (14) | |
| Type of Pneumonia | .290 | ||
| CAP | 351 (66.6) | 367 (69.6) | |
| HCAP | 176 (33.4) | 160 (30.4) | |
| Gagne combined score, median (IQR)b | 2 (1–3) | 2 (0–4) | .68 |
| Comorbiditiesc | |||
| Chronic pulmonary disease | 251 (47.6) | 224 (42.5) | .09 |
| Hypertension | 231 (43.8) | 240 (45.5) | .58 |
| Smoking | 151 (28.7) | 154 (29.2) | .84 |
| Diabetes | 151 (28.7) | 150 (28.5) | .95 |
| Congestive heart failure | 117 (22.2) | 111 (21.1) | .65 |
| Chronic blood loss anemia/deficiency anemias | 110 (20.9) | 111 (21.1) | .94 |
| Renal failure | 75 (14.2) | 72 (13.7) | .79 |
| Depression/psychoses | 65 (12.3) | 69 (13.1) | .71 |
| Other neurological disorders | 62 (11.8) | 72 (13.7) | .36 |
| Hypothyroidism | 53 (10.1) | 59 (11.2) | .55 |
| Obesity | 54 (10.2) | 54 (10.2) | 1 |
| Valvular disease | 37 (7.0) | 30 (5.7) | .38 |
| Metastatic cancer/solid tumor without metastasis | 34 (6.5) | 37 (7.0) | .71 |
| Weight loss | 33 (6.3) | 26 (4.9) | .35 |
| Pulmonary circulation disease | 27 (5.1) | 27 (5.1) | 1 |
| Prior Year Receipt of IMV | |||
| 0 | 519 (98.5) | 514 (97.5) | |
| 1 time | 8 (1.5) | 10 (1.9) | |
| 2 or more times | 3 (0.6) | ||
| Prior Year Receipt of NIV | |||
| 0 | 519 (98.5) | 520 (98.7) | |
| 1 time | 5 (0.9) | 5 (0.9) | |
| 2 or more times | 3 (0.6) | 2 (0.4) | |
| Prior Year Admissions | .71 | ||
| 0 | 305 (57.9) | 298 (56.5) | |
| 1 time | 110 (20.9) | 121 (23) | |
| 2 or more times | 112 (21.3) | 108 (20.5) | |
| Initial Care Venue | .46 | ||
| Ward | 458 (86.9) | 452 (85.8) | |
| Intensive care unit | 51 (9.7) | 49 (9.3) | |
| Intermediate care | 18 (3.4) | 26 (4.9) | |
| Vasopressors (before 1st abx or ≤12 hrs after 1st abx) | 22 (4.2) | 23 (4.4) | .88 |
| NIV (before 1st abx or ≤12 hrs after 1st abx) | 15 (2.8) | 18 (3.4) | .60 |
| IMV (before 1st abx or ≤12 hrs after 1st abx) | 26 (4.9) | 22 (4.2) | .55 |
| Hospital Region | .02 | ||
| Midwest | 106 (20.1) | 83 (15.7) | |
| Northeast | 208 (39.5) | 254 (48.2) | |
| South | 165 (31.3) | 157 (29.8) | |
| West | 48 (9.1) | 33 (6.3) | |
| Hospital Size | .05 | ||
| 6–199 beds | 143 (27.1) | 110 (20.9) | |
| 200–499 beds | 286 (54.3) | 319 (60.5) | |
| 500 and more beds | 98 (18.6) | 98 (18.6) | |
| Hospital Teaching Status | .46 | ||
| Nonteaching | 160 (30.4) | 149 (28.3) | |
| Teaching | 367 (69.6) | 378 (71.7) | |
| Outcomesc | |||
| Clinical Deterioration | |||
| Late NIV | 3 (0.6) | 5 (1) | .48 |
| Late IMV | 12 (2.4) | 17 (3.4) | .34 |
| Late vasopressors | 23 (4.6) | 22 (4.4) | .88 |
| In-hospital mortality | 22 (4.2) | 29 (5.5) | .31 |
| In-hospital mortality/hospice discharge | 33 (6.3) | 49 (9.3) | .06 |
| LOS, median (IQR), hours | 101.5 (70.0–170.1) | 109.5 (67.2–166.0) | .09 |
| Readmission within 30 days (survivors) | 65 (12.9) | 53 (10.6) | .27 |
| Discharge disposition (among survivors, nonhospice discharges, n = 972) | .17 | ||
| Home/home-health | 369 (74.7) | 339 (70.9) | |
| SNF/ICF | 116 (23.5) | 125 (26.1) | |
| Other | 9 (1.8) | 14 (2.9) | |
Abbreviations: abx, antibiotic; ARF, acute respiratory failure; CAP, community-acquired pneumonia; HCAP, healthcare-associated pneumonia; ICF, intermediate care facility; IMV, invasive mechanical ventilation; IQR, interquartile range; LAPS, Laboratory Acute Physiology Score; LOS, length of stay; NIV, noninvasive mechanical ventilation; SNF, skilled nursing facility.
aχ2 test.
bKruskal-Wallis test.
cComorbidities with at least 5% prevalence are presented.
c P values account for propensity score matching.
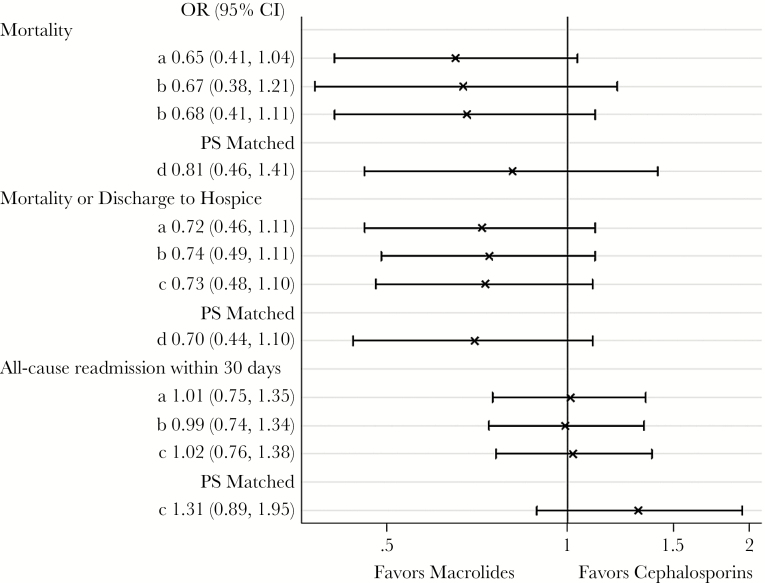
Figure 2 is a Forest plot demonstrating the adjusted risk of mortality and 30-day readmission, as described above. The adjusted mortality and combined mortality/hospice discharge rates were approximately 30% lower in the initial macrolide groups than the initial cephalosporin groups, although statistical significance was not reached. For most of the models, we found no difference in the adjusted risk of readmission among patients who received initial treatment with a macrolide compared with those initially treated with a cephalosporin.
Figure 2.
Forest plot demonstrating observed and adjusted patient outcomes for entire cohort and propensity-matched cohort. (a) Unadjusted. (b) Adjusted for age, laboratory acute physiology score (LAPS), combined comorbidity score, healthcare-associated pneumonia (HCAP) vs community-acquired pneumonia (CAP). (c) Adjusted for age, LAPS, combined comorbidity score, HCAP vs CAP, propensity score. (d) Conditional logistic regression adjusting for unbalanced covariates. Models a, b, and c account for patient clustering within hospitals (generalized estimating equation models). Abbreviations: CI, confidence interval; OR, odds ratio; PS, propensity score.
Several sensitivity analyses were performed. Among patients with less than the median LAPS (lower risk patients), mortality was 1.4% in both treatment groups. In contrast, among the patients with higher LAPS, mortality was 7.0% in those who received a macrolide first, compared with 10.2% in patients who received a cephalosporin first (P = .11). The analysis of CAP patients only (Supplemental Tables 1 and 2) and the analysis that was limited to patients whose antibiotics were at least 2 hours apart (Supplemental Table 3) demonstrated results very similar to those of the primary cohort. The analyses limited to bacteremic patients and patients who died within 3 days of presentation and with the alternative definition of late deterioration (receipt of ventilator support or vasopressors starting 2 or more hours after initial antibiotics) were limited by low numbers of events and are therefore not presented. No significant differences were seen between the 2 treatment groups in these analyses.
DISCUSSION
In this study of 2711 patients admitted to the hospital with pneumonia, we found that patients who received treatment with a macrolide antibiotic at least 1 hour before a cephalosporin had an approximately 30% lower adjusted risk of the combined outcome of in-hospital death or discharge to hospice than those in whom the sequence of antibiotic therapy was reversed. This difference did not reach statistical significance, possibly due to low numbers of events in the macrolide first group. However, the magnitude and direction of effect was consistent in several sensitivity analyses. The observed mortality benefit appeared to be limited to patients with more severe illness. There did not appear to be a meaningful association between the order of antibiotic treatment and 30-day readmission or hospital length of stay.
There are several mechanisms by which macrolide antibiotics might improve mortality in patients with bacterial pneumonia compared with other antibiotics. Macrolides seem to have direct immunomodulatory effects upon the host. They have been shown to decrease the release of inflammatory cytokines, adhesion molecule expression, production of reactive oxygen species, and inhibit polymorphonuclear cell chemotaxis [17]. Furthermore, macrolides might indirectly decrease the inflammatory response to bacterial infection by inhibiting bacterial quorum sensing, bacterial toxin production, or by causing less rapid bacterial lysis than cell wall active antibiotics, including cephalosporins [17]. Some of these mechanisms would likely not depend upon when the macrolide was given in relation to a cephalosporin. However, avoidance of rapid bacterial lysis and an immunomodulatory effect of macrolides on the host (especially by muting the inflammatory response to the liberation of inflammatory mediators from lysed bacteria) could theoretically depend on the timing of the 2 types of antibiotics. With both of these mechanisms, it is reasonable to think that treatment with a macrolide before a cephalosporin could improve outcomes by preventing rapid bacterial lysis and by blunting the resulting host inflammatory response. To our knowledge, there are no prior human studies of this issue to which our results can be compared.
The approximate 30% lower adjusted mortality seen in pneumonia patients who received macrolide therapy before cephalosporin therapy suggests the possibility that providing macrolide therapy initially might result in improved outcomes, despite the lack of statistically significant results. Based on our sensitivity analysis, it is possible that such therapy might have benefit only for a subgroup of pneumonia patients who are at higher risk for worsened outcomes, perhaps in part related to rapid bacterial lysis. These might include patients with severe pneumonia, those without clinical markers of severe pneumonia who have a high bacterial load [28, 29], and those with markedly elevated inflammatory markers [13].
If these findings are replicated in other observational studies and clinical trials, they could have a large clinical impact. Providing a macrolide antibiotic before a cephalosporin is an intervention that would be easy to implement, would have essentially no additional cost, and would likely be associated with minimal potential for risk. A delay in nonmacrolide therapy for only 2–3 hours would be unlikely to result in worse outcomes related to a delay in appropriate therapy [30]. Furthermore, the most common pathogens causing CAP are usually sensitive to azithromycin. The similar results seen in the primary study group and the CAP group suggests that there is not a significant risk associated with delaying cephalosporin therapy even in patients at highest risk of being infected with nonmacrolide sensitive organisms (HCAP patients). Therefore, even if such a strategy would only benefit a subpopulation of patients with more severe pneumonia, it could be appropriate to apply the strategy to all pneumonia patients, if identifying the highest risk patients imposed increased burden or could lead to a delay in therapy (eg, testing for bacterial load or inflammatory markers or other laboratory results).
The discordant results with respect to mortality, in which there was a strong signal suggesting benefit versus 30-day readmission rates, for which there was no suggestion of benefit, is worthy of comment. Of course, one explanation could be that the mortality benefit would not be seen with a prospective randomized study. However, lower mortality is not always associated with lower readmission rates or lower hospital length of stay [31, 32]. It has been suggested that interventions that improve mortality can salvage the most vulnerable patients, who are then prone to longer hospital stays and higher risk for readmission [33].
Strengths of this study include the large number of patients drawn from a geographically and structurally diverse sample of US hospitals, the broad set of ICD-9-CM codes used to define pneumonia—reducing the possibility of bias due to differences in coding across hospitals—and access to clinically rich data obtained directly from electronic medical records at participating institutions that enabled us to adjust for severity of illness in ways that are superior than those using only claims data. Our study should be interpreted in light of several limitations. As is the case in any observational comparative effectiveness research study, our findings are subject to the possibility of residual confounding due to unmeasured factors. However, our analyses included a large number of measures of comorbidity and disease severity, including the LAPS; our propensity models achieved good covariate balance; and our results were consistent across a variety of analytic methods and sensitivity analyses. The finding of a possible mortality benefit only among the patients with higher LAPS (higher risk patients) has biologic plausibility and provides further support that the observed differences are unrelated to unmeasured confounders, because such confounding would not likely be seen in only the more severely ill patients. Moreover, on the face of it, our research question—focused on the impact of the order of antibiotic delivery—would seem to have little risk of confounding by indication that often arises in observational studies of intended treatment effects, because the timing of antibiotics is more likely related to chance as opposed to a specific choice of the treating clinician. The number of patients with poor clinical outcomes was fairly low in the macrolide first group, introducing the possibility of a type II statistical error as the explanation for the lack of statistical significance of the outcome differences we found between the 2 patient groups. Furthermore, we relied on documentation in the record to define antibiotic administration time; there is a possibility that these times were not accurate. However, there is little reason to believe that there would be differential misclassification; therefore, any inaccuracies would likely bias our results toward the null hypothesis.
CONCLUSIONS
In summary, we performed a retrospective cohort study using electronic health record data from a large number of US hospitals to investigate the association of the order of macrolide therapy and cephalosporin therapy to patients with pneumonia, in which we adjusted for differences in patient characteristics using propensity matching and multivariate modeling. We found that treatment with a macrolide before a cephalosporin was associated with an approximate 30% lower risk of adjusted mortality than treatment in the reverse order, although this difference fell short of achieving statistical significance. These results suggest that providing a macrolide before a cephalosporin may lower mortality in patients admitted to the hospital with pneumonia. Further investigations of this concept should be undertaken, given the potential for achieving improved outcomes, likely with minimal risk and minimal resource use. Such investigations could include the use of a larger study sample, which would allow a more precise estimate of the effect (or lack of effect) of this strategy and analyses in subpopulations, such as those with severe pneumonia. Depending on the results of such analyses, a prospective randomized trial may be appropriate.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
Author contributions. M. L. M. contributed to study conception and design, interpretation of data, drafting manuscript, and final approval. A. P. contributed to study design, acquisition and interpretation of data, drafting manuscript, and final approval. E. M. M. contributed to study design, interpretation of data, critical revision, and final approval. P. K. L. contributed to study design, acquisition of data, drafting manuscript, and final approval. All authors are accountable for the content of this work.
Financial support. P. K. L. received funding from the National Heart Lung and Blood Institute of the National Institutes of Health (Grant K24 HL132008).
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Agency for Healthcare Quality and Research. HCUPnet Available at: https://hcupnet.ahrq.gov/?JS=Y#setup. Accessed 28 July 2017.
- 2. Centers for Medicare and Medicaid ServicesMedicare.gov. Hospital Compare: Readmissions & Deaths Available at: https://www.medicare.gov/hospitalcompare/profile.html#vwgrph=1&profTab=4&ID=070036&cmprID=070036&dist=25&loc=06001&lat=41.7918396&lng=-72.8633635&cmprDist=7.1&Distn=7.1. Accessed 26 December 2016.
- 3. Menendez R, Torres A. Treatment failure in community-acquired pneumonia. Chest 2007; 132:1348–55. [DOI] [PubMed] [Google Scholar]
- 4. Arancibia F, Ewig S, Martinez JA et al. Antimicrobial treatment failures in patients with community-acquired pneumonia: causes and prognostic implications. Am J Respir Crit Care Med 2000; 162:154–60. [DOI] [PubMed] [Google Scholar]
- 5. Rosón B, Carratalà J, Fernández-Sabé N et al. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med 2004; 164:502–8. [DOI] [PubMed] [Google Scholar]
- 6. Hoogewerf M, Oosterheert JJ, Hak E et al. Prognostic factors for early clinical failure in patients with severe community-acquired pneumonia. Clin Microbiol Infect 2006; 12:1097–104. [DOI] [PubMed] [Google Scholar]
- 7. Kolditz M, Ewig S, Klapdor B et al. Community-acquired pneumonia as medical emergency: predictors of early deterioration. Thorax 2015; 70:551–8. [DOI] [PubMed] [Google Scholar]
- 8. Restrepo MI, Mortensen EM, Rello J et al. Late admission to the ICU in patients with community-acquired pneumonia is associated with higher mortality. Chest 2010; 137:552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Waterer G, Rello J. Why should we measure bacterial load when treating community-acquired pneumonia? Curr Opin Infect Dis 2011; 24:137–41. [DOI] [PubMed] [Google Scholar]
- 10. McCullers JA, English BK. Improving therapeutic strategies for secondary bacterial pneumonia following influenza. Future Microbiol 2008; 3:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holzheimer RG. Antibiotic induced endotoxin release and clinical sepsis: a review. J Chemother 2001; 13 Spec No 1:159–72. [DOI] [PubMed] [Google Scholar]
- 12. Nau R, Eiffert H. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 2002; 15:95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Torres A, Sibila O, Ferrer M et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015; 313:677–86. [DOI] [PubMed] [Google Scholar]
- 14. Karlström A, Boyd KL, English BK, McCullers JA. Treatment with protein synthesis inhibitors improves outcomes of secondary bacterial pneumonia after influenza. J Infect Dis 2009; 199:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee JS, Giesler DL, Gellad WF, Fine MJ. Antibiotic therapy for adults hospitalized with community-acquired pneumonia: a systematic review. JAMA 2016; 315:593–602. [DOI] [PubMed] [Google Scholar]
- 16. Garin N, Genné D, Carballo S et al. β-lactam monotherapy vs β-lactam-macrolide combination treatment in moderately severe community-acquired pneumonia: a randomized noninferiority trial. JAMA Intern Med 2014; 174:1894–901. [DOI] [PubMed] [Google Scholar]
- 17. Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev 2010; 23:590–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Metersky ML, Ma A, Houck PM, Bratzler DW. Antibiotics for bacteremic pneumonia: improved outcomes with macrolides but not fluoroquinolones. Chest 2007; 131:466–73. [DOI] [PubMed] [Google Scholar]
- 19. Wilson BZ, Anzueto A, Restrepo MI et al. Comparison of two guideline-concordant antimicrobial combinations in elderly patients hospitalized with severe community-acquired pneumonia. Crit Care Med 2012; 40:2310–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sligl WI, Asadi L, Eurich DT et al. Macrolides and mortality in critically ill patients with community-acquired pneumonia: a systematic review and meta-analysis. Crit Care Med 2014; 42:420–32. [DOI] [PubMed] [Google Scholar]
- 21. Mandell LA, Wunderink RG, Anzueto A et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44Suppl 2:S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amin AP, Salisbury AC, McCullough PA et al. Trends in the incidence of acute kidney injury in patients hospitalized with acute myocardial infarction. Arch Intern Med 2012; 172:246–53. [DOI] [PubMed] [Google Scholar]
- 23. Kosiborod M. Blood glucose and its prognostic implications in patients hospitalised with acute myocardial infarction. Diab Vasc Dis Res 2008; 5:269–75. [DOI] [PubMed] [Google Scholar]
- 24. Kosiborod M, Inzucchi SE, Krumholz HM et al. Glucose normalization and outcomes in patients with acute myocardial infarction. Arch Intern Med 2009; 169:438–46. [DOI] [PubMed] [Google Scholar]
- 25. Gagne JJ, Glynn RJ, Avorn J et al. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol 2011; 64:749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Escobar GJ, Greene JD, Scheirer P et al. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care 2008; 46:232–9. [DOI] [PubMed] [Google Scholar]
- 27. van Walraven C, Escobar GJ, Greene JD, Forster AJ. The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population. J Clin Epidemiol 2010; 63:798–803. [DOI] [PubMed] [Google Scholar]
- 28. Rello J, Lisboa T, Lujan M et al. Severity of pneumococcal pneumonia associated with genomic bacterial load. Chest 2009; 136:832–40. [DOI] [PubMed] [Google Scholar]
- 29. Werno AM, Anderson TP, Murdoch DR. Association between pneumococcal load and disease severity in adults with pneumonia. J Med Microbiol 2012; 61:1129–35. [DOI] [PubMed] [Google Scholar]
- 30. Meehan TP, Fine MJ, Krumholz HM et al. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA 1997; 278:2080–4. [PubMed] [Google Scholar]
- 31. Lipitz-Snyderman A, Steinwachs D, Needham DM et al. Impact of a statewide intensive care unit quality improvement initiative on hospital mortality and length of stay: retrospective comparative analysis. BMJ 2011; 342:d219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gorodeski EZ, Starling RC, Blackstone EH. Are all readmissions bad readmissions? N Engl J Med 2010; 363:297–8. [DOI] [PubMed] [Google Scholar]
- 33. Krumholz HM, Lin Z, Keenan PS et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA 2013; 309:587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.