Abstract
Time-dependent variability in mood and anxiety suggest that related neural phenotypes, such as threat-related amygdala reactivity, may also follow a diurnal pattern. Here, using data from 1,043 young adult volunteers, we found that threat-related amygdala reactivity was negatively coupled with time of day, an effect which was stronger in the left hemisphere (β = −0.1083, p-fdr = 0.0012). This effect was moderated by subjective sleep quality (β = −0.0715, p-fdr = 0.0387); participants who reported average and poor sleep quality had relatively increased left amygdala reactivity in the morning. Bootstrapped simulations suggest that similar cross-sectional samples with at least 300 participants would be able to detect associations between amygdala reactivity and time of scan. In control analyses, we found no associations between time and V1 activation. Our results provide initial evidence that threat-related amygdala reactivity may vary diurnally, and that this effect is potentiated among individuals with average to low sleep quality. More broadly, our results suggest that considering time of scan in study design or modeling time of scan in analyses, as well as collecting additional measures of circadian variation, may be useful for understanding threat-related neural phenotypes and their associations with behavior, such as fear conditioning, mood and anxiety symptoms, and related phenotypes.
Keywords: amygdala, diurnal, anxiety, time, sleep
Introduction
Despite widespread evidence that time of day is a strong modulator of behavior across species [from basic physiology to complex cognitive function (Herzog, 2007)], neuroimaging studies do not typically consider the potential effects of time of day on behaviorally-relevant neural phenotypes. This may be especially important for neural phenotypes that have been linked to forms of psychopathology characterized by diurnal disruption [e.g. sleep disruption in anxiety and unipolar and bipolar depression (American Psychiatric Association, 2013), diurnal cortisol dysregulation in depression (Doane et al., 2013)]. Considering time of day in neuroimaging studies may provide more precise estimates of neural function, enabling the detection of brain-behavior relationship with greater accuracy. Further, it potentially may even yield unique time-dependent associations with behavior and psychopathology risk that inform not only our understanding of basic biological processes regulating behavior, but also efforts to treat dysregulated mood and anxiety.
The amygdala is critical for learning the emotional significance of stimuli and effecting adaptive changes in behavioral vigilance and physiological arousal in response to environmental triggers including threat (Phelps and LeDoux, 2005). Circumstantial evidence suggests that threat-related amygdala function may vary diurnally. First, heightened amygdala reactivity has been linked to neuroticism, depression, and anxiety disorders, which are characterized by circadian disruption (Etkin and Wager, 2007; Chen et al., 2011; Binelli et al., 2014; Hilbert et al., 2014). Moreover, there is evidence that human mood varies diurnally, with negative affect peaking in the morning and declining over the course of the day, while positive affect shows an opposing relationship (Clark et al., 1989; Geraci and Uhde, 1992; Wirz-Justice, 2008; Ayuso-Mateos et al., 2013). Second, amygdala reactivity is positively correlated with circadian changes in physiology, particularly circulating cortisol concentrations, which peak during morning hours (Urry et al., 2006; van Stegeren et al., 2007; Merz et al., 2013; Weldon et al., 2015; Henckens et al., 2016). Third, amygdala-dependent behaviors, such as fear conditioning and extinction, are potentiated during species-specific active-phases (i.e. morning for humans, evening for rodents) of the circadian cycle (Valentinuzzi et al., 2001; Chaudhury and Colwell, 2002; Pace-Schott et al., 2013, 2015; Woodruff et al., 2015). Fourth, in rodents, threat exposure (i.e. predator odor) produces greater amygdala activity (i.e. Fos expression) during the active relative to inactive phase of the circadian cycle (Funk and Amir, 2000). Fifth, circadian rhythmicity of amygdala activation has recently been observed during a sustained attention task under conditions of extended (e.g. 42 hours) wakefulness (Muto et al., 2016). Sixth, bright light intervention, a promising treatment of depression and anxiety, normalizes circadian disruption and leads to reduced amygdala reactivity to emotional faces (McClung, 2007; Dodson et al., 2010; Fisher et al., 2014; Lam et al., 2015; Nussbaumer et al., 2015).
Here, we sought to extend this prior work by examining whether threat-related amygdala reactivity varies according to time of day among 1043 young adult volunteers. We further explored whether sleep quality (Balbo et al., 2010; Hosseini et al., 2014; Ly et al., 2015), moderates associations between time of day and amygdala reactivity. Based on evidence that negative affect and cortisol concentrations peak in the morning and that rodent amygdala reactivity to threat and related behaviors (e.g. fear conditioning) are potentiated during the active phase of the circadian cycle, we hypothesized that amygdala reactivity would be highest during the morning and lowest during the afternoon and evening. Further in light of evidence that sleep deprivation enhances amygdala reactivity to threat (Yoo et al., 2007; Motomura et al., 2013, 2014; Simon et al., 2015; Reidy et al., 2016), we predicted that temporally-dependent amygdala reactivity would be moderated by subjective sleep quality.
Materials and methods
Participants
Neuroimaging data that were fully processed by 06 January 2015 were available from 1156 participants who completed the ongoing Duke Neurogenetics Study (DNS). The DNS assesses a wide range of behavioral, experiential, and biological phenotypes among young-adult (i.e. 18- to 22-year old) college students. Each participant provided informed written consent prior to participation in accord with the guidelines of the Duke University Medical Center Institutional Review Board and received $120 remuneration. All participants were in good general health and free of DNS exclusion criteria: (i) medical diagnosis of cancer, stroke, diabetes requiring insulin treatment, chronic kidney or liver disease or lifetime psychotic symptoms; (ii) use of psychotropic, glucocorticoid or hypolipidemic medication, and (iii) conditions affecting cerebral blood flow and metabolism (e.g. hypertension). Current DSM-IV Axis I and select Axis II disorders (Antisocial Personality Disorder and Borderline Personality Disorder) were assessed with the electronic Mini International Neuropsychiatric Interview (Sheehan et al., 1998) and Structured Clinical Interview for the DSM-IV Axis II (SCID-II) (First et al., 2002). These disorders are not exclusionary as the DNS seeks to establish broad variability in multiple behavioral phenotypes related to psychopathology.
The final sample consisted of 1043 participants after quality assurance (age = 19.71 ± 1.25; 477 males; 209 with a DSM-IV Axis I disorder; Supplementary Table S1; 478 European Americans, 119 African–Americans, 275 Asians, 62 Latinos, and 109 of Other/Multiple racial origins according to self-report). Participants were excluded (n = 113) for scanner-related artifacts in fMRI data (n = 26), problems with task presentation (n = 6), incidental structural brain abnormalities (n = 4), a large number of movement outliers in fMRI data (n = 34; see ART below), poor behavioral performance (n = 36), scanner malfunction (n = 2), study non-completion or incomplete data (n = 5).
Sleep quality, state anxiety and time of scan
The Pittsburgh Sleep Quality Inventory (PSQI; ×=4.94, σ = 2.50, α = 0.612, min = 0, max = 12.7304, skewness = 0.694) was used to measure global sleep quality and sleep-related symptoms over the past month (Buysse et al., 1989). Time of scan (TOS) was logged as the time that the scan began (8 am–7 pm; ×= 12:36 pm, σ = 2.3 h). A subset of participants (n = 730) additionally completed the state version of the State-Trait Anxiety Inventory immediately prior to the scanning session (STAI; ×=29.29, σ = 6.91, α = 0.614, min = 15, max = 67, skewness = 1.236) which was used to measure the participant’s current anxiety state (Spielberger et al., 1983).
BOLD fMRI paradigm
Our amygdala reactivity paradigm has been described in detail previously (Carré et al., 2012; Demers et al., 2016). Briefly, this task consists of four blocks of a face-processing task interleaved with five blocks of a sensorimotor control task. In emotion-specific (i.e. anger, fear, neutral, surprise) face matching blocks, participants view a trio of faces and indicate which of the two faces presented on the bottom, matches the target face displayed on top. Within face matching blocks, six face trios were presented for 4 s, with a variable inter-stimulus interval of 2–6 s, for a total block length of 48 s. During control blocks, participants match geometric shapes (ellipses). Each sensorimotor control block had six different shape trios, each presented for 4 s, with a fixed inter-stimulus interval of 2 s, for a total block length of 36 s. Our contrast of interest was all face blocks relative to control blocks (Faces > Shapes), which reflects broad threat-related activity of the amygdala (Yoon and Zinbarg, 2008; Mattavelli et al., 2014).
BOLD fMRI data acquisition
Each participant was scanned using a research-dedicated GE MR750 3T scanner equipped with high-power high-duty-cycle 50-mT/m gradients at 200 T/m/s slew rate, and an eight-channel head coil for parallel imaging at high bandwidth up to 1 MHz at the Duke-UNC Brain Imaging and Analysis Center. A semi-automated high-order shimming program was used to ensure global field homogeneity. A series of 34 interleaved axial functional slices aligned with the anterior commissure-posterior commissure (AC-PC) plane were acquired for full-brain coverage using an inverse-spiral pulse sequence to reduce susceptibility artifact (TR/TE/flip angle = 2000 ms/30 ms/60; FOV = 240 mm; 3.75 × 3.75 × 4 mm voxels; interslice skip = 0). Four initial RF excitations were performed (and discarded) to achieve steady-state equilibrium. To allow for spatial registration of each participant’s data to a standard coordinate system, high-resolution three-dimensional structural images were acquired in 34 axial slices co-planar with the functional scans (TR/TE/flip angle = 7.7 s/3.0 ms/12; voxel size = 0.9 × 0.9 × 4 mm; FOV = 240 mm, interslice skip = 0).
BOLD fMRI data preprocessing
Images for each subject were realigned to the first volume in the time series to correct for head motion, spatially normalized into a standard stereotactic space (Montreal Neurological Institute template) using a 12-parameter affine model (final resolution of functional images = 2 mm isotropic voxels), and smoothed to minimize noise and residual difference in gyral anatomy with a Gaussian filter, set at 6-mm full-width at half-maximum (Fonov et al., 2011). Voxel-wise signal intensities were ratio normalized to the whole-brain global mean.
Movement in single-subject whole-brain functional volumes was determined using the Artifact Recognition Toolbox (http://www.nitrc.org/projects/artifact_detect). Individual whole-brain BOLD fMRI volumes meeting at least one of two criteria were flagged and regressed out when determining task-specific effects: (i) significant mean-volume signal intensity variation (i.e. within volume mean signal greater or less than 4 s.d. of mean signal of all volumes in time series), and (ii) individual volumes where scan-to-scan movement exceeded 2 mm translation or 2° rotation in any direction. Participants with 5% or more flagged volumes per task run were excluded from analysis (n = 34, see Participants section for a full listing of all exclusionary criteria).
BOLD fMRI analysis
The general linear model of SPM8 (http://www.fil.ion.ucl.ac.uk/spm) was used for fMRI data analyses. Linear contrasts employing canonical hemodynamic response functions estimated amygdala reactivity from the Faces > Shapes contrast for each individual. Individual contrast images were used in second-level random effects models to determine mean condition-specific responses using one-sample t-tests (Faces > Shapes). A statistical threshold of P <0.05 FWE and ≥10 contiguous voxels was applied to our amygdala regions of interest (ROI), defined by the automatic anatomical labeling option in the Wake Forest University PickAtlas (Lancaster et al., 2000; Maldjian et al., 2003). Consistent with prior work (Bogdan et al., 2012) and recent recommendations (Tong et al., 2016) we extracted parameter estimates from amygdala ROIs arising as a main effect of task to preclude the possibility of correlation coefficient inflation that may result when an explanatory covariate is used to select a region of interest. Amygdala reactivity data were winsorized to 3 s.d. reduce the influence of extreme outliers. As a control analysis, task-related parameter estimates were extracted from primary visual cortex ROIs, defined by 7 mm spheres centered on the peak voxels of activation within V1 (18, −90, −6 and −18, −95, −2) to examine whether time of day effects were non-specific to our ROI of interest.
Statistical analyses
Regression-based models were tested using the R (3.1.2) ‘Stats’ package (R Development Core Team, 2013). Non-parametric bias-corrected boot-strapped 95% (i.e. 2.5% and 97.5%) confidence-intervals (CI) were calculated with 10 000 replicates using the R ‘boot’ package (Canty and Ripley, 2012). Because of high positive skew, STAI scores were log-transformed for all regression analyses. All models included age, sex, ethnicity (dummy-coded as separate binary variables for self-reported Caucasian, African–American, Asian, Hispanic, and Multi-racial), and season as covariates. Given evidence that human brain function varies seasonally, with sinusoidal phases of activity that peak during either the summer or autumn equinoxes, depending on the task (Meyer et al., 2016), season was controlled for with 2-year-long period sine and cosine functions.
We first tested whether Time of Scan (TOS) predicted bilateral amygdala reactivity. As a control analysis, we tested whether TOS is associated with bilateral V1 activity. Because follow-up tests demonstrated that TOS more strongly associated with left amygdala reactivity (see Results), we evaluated whether sleep quality (PSQI) moderates the effect of TOS on left and right amygdala reactivity separately. All moderation analyses included main effects and an additional 12 terms for all bivariate interactions between covariates and variables-of-interest (e.g. TOS x Sex and PSQI x Age), to better account for potential confounds (Keller, 2014; Baranger et al., 2016). All variables were mean centered prior to the computation of interaction terms. Posthoc Johnson-Neyman and simple-slopes analyses were used to characterize the PSQI x TOS interaction. Plots were generated with the R packages ‘ggplot2’ and ‘ColorBrewer’ (Wickham, 2009; Neuwirth and Brewer, 2014). Posthoc false discovery rate (FDR) correction for multiple tests (6 total: i.e. TOS predicting bilateral, left, and right amygdala reactivity, TOS x sleep problems predicting left and right amygdala, and TOS predicting bilateral V1 activity) was applied in R (Benjamini and Hochberg, 1995).
Two additional posthoc analyses were conducted. First, we repeated the above analyses in the subset of participants for whom state-related anxiety data (STAI) were available, with STAI as an additional covariate to ensure that any associations between TOS and amygdala function were not accounted for by state-dependent anxiety. Second, we tested whether the effect of TOS on amygdala reactivity remained when TOS was recoded as the cosine-transformed amount of time since participants reported, on the PSQI questionnaire, that they have usually awoken over the prior month.
Simulations were conducted to estimate the sample-size needed to observe the TOS effect on amygdala reactivity and to identify the time-periods during which the effect is observed. Bootstrapped linear regression models were computed at sample-sizes from n = 50 to n = 1000, at intervals of 50 (e.g. 50, 100, 150 and 200). For each sample-size interval, 10 000 linear regression replicates were computed by randomly selecting, with replacement, a subsample of the given size (e.g. n = 300). R ‘quantile’ was used to estimate the 95% CI. Sample sizes in which both CI boundaries were <0 (consistent with the negative association observed) were considered sufficient size to detect the observed effect, given the TOS distributions of our data. A sliding-window regression analysis was conducted to evaluate whether TOS effects were observed during particular times of day. Non-parametric bias-corrected boot-strapped 95% CIs were calculated with 10 000 replicates. Windows of 4 h were advanced in 30-min increments. Four hour windows were chosen to allow for morning and afternoon specific windows and to allow for the minimum sample, needed to observe an overall TOS effect in our sample within each window, n = 300–350 according to the bootstrapped sample size analyses.
Results
Main effect of task and associations with sample demographics
All ROIs showed robust activation across participants (Figure 1). Comparison of ethnicities found significant differences in self-reported sleep quality (PSQI), bilateral amygdala reactivity, prevalence of psychiatric diagnoses, and gender (PSQI: F = 4.3694, P = 0.0130; Psychiatric Diagnosis: χ2=12.7487, P = 0.0126; Gender: χ2=15.559, P = 0.0037; see “self-report ethnicity” section in Table 1). European/European–American participants had greater amygdala reactivity relative to African/African–American and Asian/Asian–American participants. Asian/Asian–American participants had fewer diagnoses and worse sleep quality than all other groups. Hispanic participants had more diagnoses than all other groups. Proportionally fewer European/EuropeanAmerican participants identified as female, and more African–American participants identified as female
Fig. 1.
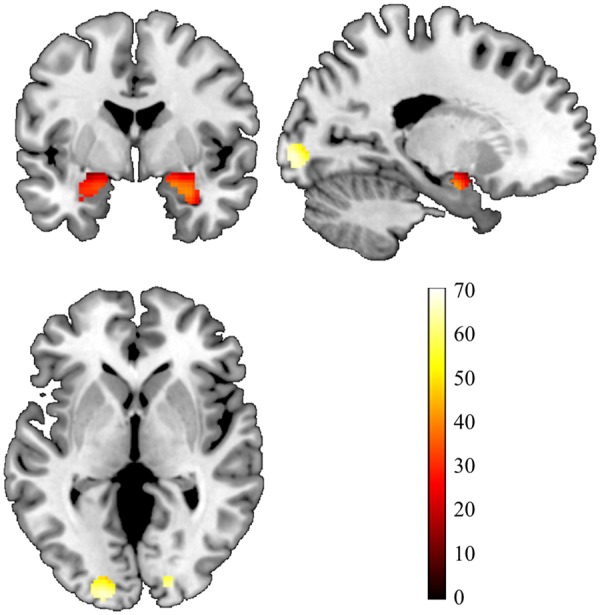
Amygdala and primary visual cortex from the Faces > Shapes contrast of the fMRI task. Statistical parametric map illustrating bilateral amygdala and primary visual cortex (V1) activation clusters for the contrast ‘Faces > Shapes’ with bilateral ROIs for the amygdala (defined by the automatic anatomical labeling option in the Wake Forest University PickAtlas) and bilateral V1 [defined by 7 mm spheres centered on the peak voxels of activation within V1 (18, −90, −6 and −18, −95, −2)], overlaid onto a canonical structural brain image Montreal Neurological Institute coordinates and statistics (P<0.05, family-wise error whole-brain corrected and ≥10 contiguous voxels). The displayed image is centered on MNI coordinates [−20, −1, 0].
Table 1.
Associations of self-report ethnicity and sex with time of scan, self-report variables, amygdala reactivity, season of study participation, and presence of psychiatric diagnosis
| Self-report ethnicity | Caucasian (s.d.) n=459 | African–American (s.d.) n=117 | Asian (s.d.) n=266 | Hispanic (s.d.) n=56 | Multi-racial (s.d.) n=103 | F/χ2 | P |
|---|---|---|---|---|---|---|---|
| Time of scan | 12.5617 (2.3198) | 12.8277 (2.1168) | 12.5436 (2.2036) | 13.0968 (2.424) | 12.4954 (2.4641) | 1.1488 | 0.3321 |
| Age | 19.795 (1.2488) | 19.6555 (1.182) | 19.6145 (1.3085) | 19.7258 (1.2825) | 19.6789 (1.1617) | 1.0232 | 0.3941 |
| PSQI | 4.8623 (2.3841) | 5.8083 (2.5487) | 4.7052 (2.499) | 4.9473 (2.8185) | 4.9308 (2.5963) | 4.3694 | 0.0017 |
| State STAI | 28.9878 (6.8017) | 30.425 (8.3738) | 29.2284 (6.574) | 29.6744 (6.221) | 29.3373 (6.9234) | 0.7335 | 0.5693 |
| n=327 | n=80 | n=197 | n=43 | n=83 | |||
| Bilateral AMY reactivity | 0.2491 (0.1884) | 0.1956 (0.1583) | 0.2023 (0.198) | 0.2451 (0.2094) | 0.2313 (0.1614) | 3.8757 | 0.0039 |
| Cosine-season | −0.0775 (0.5958) | −0.1592 (0.5618) | −0.0483 (0.5952) | −0.0083 (0.6086) | −0.005 (0.6236) | 1.2643 | 0.2822 |
| Sine-season | −0.1462 (0.7872) | −0.1005 (0.8106) | −0.2147 (0.7751) | 0.1579 (0.7878) | −0.1385 (0.7753) | 0.5615 | 0.6906 |
| Psychiatric diagnosis* | n=110 (23.4%) | n=28 (23.9%) | n=37 (13.9%) | n=17 (30.4%) | n=24 (23.3%) | 12.7487 | 0.0126 |
| Gender (# women)* | n=251 (54.7%) | n=85 (72.6%) | n=155 (58.3%) | n=37 (66.1%) | n=68 (66.0%) | 15.559 | 0.0037 |
| Sex | Men (s.d.) n=447 | Women (s.d.) n=596 | t/χ2 | P | |||
| Time of scan | 12.6454 (2.173) | 12.5872 (2.3755) | 0.411 | 0.6812 | |||
| Age | 19.745 (1.2993) | 19.693 (1.2139) | 0.658 | 0.5107 | |||
| PSQI | 4.7979 (2.3984) | 5.0484 (2.5693) | −1.6189 | 0.1058 | |||
| State STAI | 28.9811 (6.9427) | 29.5291 (6.88) | −1.0616 | 0.2888 | |||
| n=318 | n=412 | ||||||
| Cosine-season | −0.084 (0.6051) | −0.055 (0.5892) | −0.7746 | 0.4388 | |||
| Sine-season | −0.1495 (0.7789) | −0.166 (0.7899) | 0.3361 | 0.7369 | |||
| Bilateral AMY reactivity | 0.2604 (0.2117) | 0.2047 (0.1633) | 4.6288 | 4.3x10−6 | |||
| Psychiatric diagnosis* | n=108 (24.2%) | n=108 (18.1%) | 5.3134 | 0.0212 | |||
PSQI, Pittsburgh Sleep Quality Inventory; STAI, State-Trait Anxiety Inventory; AMY, Amygdala.
Analyses were run as a chi-squared test. All others were run as t-test.
In support of prior observations (Newhoff et al., 2015), men had greater bilateral amygdala reactivity than women (t = 4.6288, P = 4.3×10−6; see “sex” section Table 1). More male participants qualified for a psychiatric diagnosis (24.2%, χ2=5.3134, P = 0.0212), which was driven by elevated alcohol abuse and dependence (15%, χ2=11.2320, P = 0.0008). Samples with and without STAI data available differed by Time of Scan (TOS), wherein those participants who did not have STAI data were scanned, on an average, slightly later (28.74 min; t = 3.0357, P = 0.0025; Supplementary Table S2). These samples additionally differed by season; pre-scan STAI was added after data collection had commenced, and thus participants without STAI data are more likely to have completed the study in the winter (data collection began in January 2010; cosine: t=−1.9766, P = 0.0485; sine: t=−2.3459, P = 0.0193). Importantly, in addition to not being associated with ethnicity or gender, TOS was not associated with age (r=−0.0471, P = 0.1282), sleep quality (r=−0.0144, P = 0.6432), season of participation (cosine: r=−0.0488, P = 0.1155; sine: r = 0.0376, P = 0.2255), self-reported state anxiety (r=−0.0075, P = 0.8388), or psychiatric status (t = 0.3408, P = 0.7334), suggesting that these variables were not confounded with TOS scheduling. Additionally, psychiatric status itself was not associated with bilateral amygdala reactivity (t=−1.3583, P = 0.1753), but was associated with worse sleep quality (t=−6.5722, P = 2.2×10−10).
Earlier time of scan is associated with greater threat-related amygdala activity
Time of Scan (TOS) was negatively associated with bilateral amygdala reactivity (β = −0.1083, t=−3.550, P = 0.0004, LLCI=−0.1660, ULCI=−0.0500, p-fdr = 0.0012; Figure 2,Supplementary Table S3). This effect was consistent across hemispheres, but stronger in the left amygdala (Left: β = −0.1137, t=−3.722, P = 0.0002, LLCI=−0.1745, ULCI=−0.0547, p-fdr = 0.0012; Right: β = −0.0909, t=−2.968, P = 0.0031, LLCI=−0.1480, ULCI=−0.0313, p-fdr = 0.0062). Control analyses revealed that TOS was not associated with bilateral V1 activation suggesting that time dependent modulation of threat-related processing may be restricted to a corticolimbic network implicated in behavioral vigilance (β = −0.0471, t=−1.534, P = 0.1254, LLCI=−0.1084, ULCI = 0.0136, p-fdr = 0.1505; Supplementary Table S4).
Fig. 2.
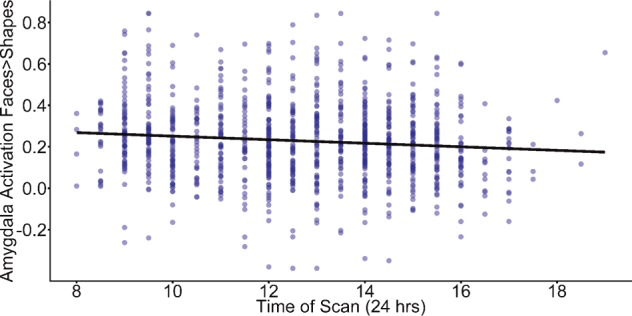
An earlier time of scan (TOS) is associated with increased bilateral amygdala reactivity. There is a negative association between time of day and threat-related amygdala reactivity.
Results in the subset of participants with pre-scan STAI data available (n = 730), including STAI as a covariate, were consistent with results in the full sample (Bilateral Amygdala: β = −0.1184, t=−3.209, P = 0.0014, LLCI=−0.1893, ULCI=−0.0461; Left Amygdala: β = −0.1162, t=−3.145, P = 0.0017, LLCI=−0.1902, ULCI=−0.0449; Right Amygdala: β = −0.1082, t=−2.923, P = 0.0036, LLCI=−0.1791, ULCI=−0.0359; Bilateral V1: β = −0.0552, t=−1.496, P = 0.1351, LLCI=−0.1285, ULCI = 0.0187). Additionally, pre-scan STAI was not a significant covariate in any analysis (all P > 0.4). Inclusion of psychiatric status (i.e. the presence of any DSM-IV Axis I psychopathology) as a covariate did not alter any results, was not a significant covariate in any analysis (all P > 0.3), and did moderate the association of TOS with bilateral amygdala reactivity (P > 0.24). Recoding TOS based on the cosine-transformed difference between when the scan took place and the time participants report habitually waking up resulted in a similar association with bilateral amygdala reactivity (β = −0.1132, t=−3.694, P = 0.0002, LLCI=−0.1718, ULCI=−0.0538), and including both did not significantly improve model fit (F = 1.5411, P = 0.2147).
Sleep quality moderates the association between amygdala reactivity and time of scan
We next examined whether sleep quality (i.e. PSQI) (Buysse et al., 1989; Grutsch et al., 2011), moderates the association between TOS and amygdala reactivity. Sleep quality interacted with TOS to predict left, but not right, amygdala reactivity (Left: β = −0.0715, t= −2.232, P = 0.0258, LLCI= −0.1330, ULCI=−0.0067, p-fdr = 0.0387; Figure 3; Right: β = −0.0418, t=−1.306, P = 0.1917, LLCI=−0.1006, ULCI = 0.0172, p-fdr = 0.1917; Supplementary Table S5). Posthoc analyses revealed that relatively worse sleep quality (a higher PSQI score) was associated with increased left amygdala activity during morning hours (Johnson-Neyman significance for TOS ≤ 11:30 am). Further, partitioning participants into three equally-sized groups based on the distribution of sleep problems in this sample (low = 0–4, medium = 5–6, high = 7–12.7), revealed that participants with relatively low and average sleep quality displayed a negative association between amygdala activity and TOS (Medium: β = −0.0162, t=−3.105, P = 0.0021; High: β = −0.0189, t=−2.826, P = 0.0051), while no significant association was observed among those reporting high sleep quality (β = −0.0032, t=−0.787, P = 0.4319). Including psychiatric diagnosis as well as its interaction with sleep quality and TOS, did not alter the significance of the observed TOS x PSQI interaction (all P > 0.09).
Fig. 3.
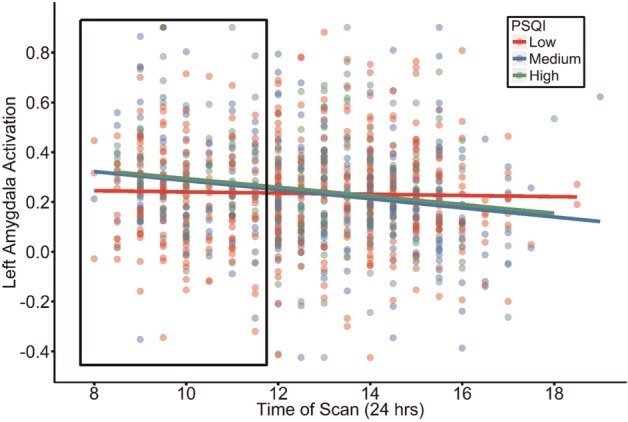
Time of Scan interacts with sleep problems to predict left amygdala reactivity. Participants reporting elevated (i.e. medium or high) sleep problems (PSQI: Pittsburgh Sleep Quality Inventory) had heightened amygdala reactivity during morning scans (i.e. before 11:30 am). Data were analyzed in a continuous fashion to determine Johnson-Neyman regions of significance (represented by the boxed region) to identify where the association between TOS and amygdala activity significantly diverges as a function of sleep quality. Individuals were partitioned into three equally-sized groups according to self-reported sleep problems for display-purposes and simple slope comparisons.
Sample sizes and restricted times where the effect of time of scan is detected
Bootstrapped regressions revealed that the effect of TOS on bilateral amygdala reactivity can be detected in samples ≥ 350 given the distribution of TOS in this study (LLCI=−0.0171, ULCI=−0.0007; Table 2). The effect could be observed in the left and right amygdala in samples ≥ 300 (LLCI=−0.0211, ULCI=−0.0001), and 450 (LLCI=−0.0145, ULCI=−0.0002), respectively. Bootstrapped regressions found that the negative effect of TOS on bilateral amygdala reactivity can be reliably detected in the morning and afternoon (i.e. 8:30 am–12:30 pm and 1:30 pm–6 pm), but not within windows which include the early afternoon (12:30–1:30; Table 3). Analyses in the left and right amygdala found that this effect was largely driven by the left amygdala, with a reliable association in the right amygdala detected only between 2:30 pm and 5:30 pm.
Table 2.
Bootstrapped confidence intervals for the sample-size at which the effect of time of scan on amygdala reactivity becomes reliably negative
| Sample size | Bilateral amygdala |
Left amygdala |
Right amygdala |
|||
|---|---|---|---|---|---|---|
| LLCI | ULCI | LLCI | ULCI | LLCI | ULCI | |
| 50 | −0.0323 | 0.0162 | −0.0390 | 0.0184 | −0.0312 | 0.0178 |
| 100 | −0.0245 | 0.0078 | −0.0297 | 0.0087 | −0.0230 | 0.0089 |
| 150 | −0.0218 | 0.0042 | −0.0260 | 0.0049 | −0.0201 | 0.0055 |
| 200 | −0.0202 | 0.0023 | −0.0238 | 0.0022 | −0.0179 | 0.0040 |
| 250 | −0.0189 | 0.0013 | −0.0222 | 0.0011 | −0.0169 | 0.0023 |
| 300 | −0.0177 | 0.0002 | −0.0211 | −0.0001 | −0.0161 | 0.0016 |
| 350 | −0.0171 | −0.0007 | −0.0203 | −0.0008 | −0.0154 | 0.0010 |
| 400 | −0.0166 | −0.0009 | −0.0198 | −0.0013 | −0.0150 | 0.0003 |
| 450 | −0.0161 | −0.0014 | −0.0191 | −0.0021 | −0.0145 | −0.0002 |
| 500 | −0.0159 | −0.0021 | −0.0187 | −0.0023 | −0.0141 | −0.0004 |
| 550 | −0.0154 | −0.0020 | −0.0183 | −0.0030 | −0.0138 | −0.0009 |
| 600 | −0.0150 | −0.0024 | −0.0182 | −0.0033 | −0.0135 | −0.0012 |
| 650 | −0.0151 | −0.0027 | −0.0176 | −0.0036 | −0.0134 | −0.0013 |
| 700 | −0.0146 | −0.0030 | −0.0175 | −0.0036 | −0.0131 | −0.0015 |
| 750 | −0.0146 | −0.0033 | −0.0171 | −0.0040 | −0.0127 | −0.0018 |
| 800 | −0.0143 | −0.0035 | −0.0168 | −0.0043 | −0.0126 | −0.0019 |
| 850 | −0.0142 | −0.0037 | −0.0167 | −0.0045 | −0.0124 | −0.0023 |
| 900 | −0.0140 | −0.0038 | −0.0166 | −0.0047 | −0.0123 | −0.0023 |
| 950 | −0.0138 | −0.0038 | −0.0164 | −0.0048 | −0.0122 | −0.0023 |
| 1000 | −0.0137 | −0.0039 | −0.0164 | −0.0049 | −0.0121 | −0.0025 |
LLCI, Lower Limit Confidence Interval; ULCI, Upper Limit Confidence Interval.
Table 3.
Bootstrapped confidence intervals for time-windows at which the effect of time of scan on amygdala reactivity is reliably negative
| Bilateral |
Left |
Right |
|||||
|---|---|---|---|---|---|---|---|
| Window (24 h) | N | LLCI | ULCI | LLCI | ULCI | LLCI | ULCI |
| 8–11 | 315 | −0.1608 | 0.0486 | −0.1697 | 0.0319 | −0.1432 | 0.0755 |
| 8.5–11.5 | 352 | −0.2092 | −0.0046 | −0.2053 | 0.0019 | −0.1985 | 0.0085 |
| 9–12 | 425 | −0.2083 | −0.0202 | −0.2350 | −0.0422 | −0.1700 | 0.0171 |
| 9.5–12.5 | 425 | −0.1980 | −0.0021 | −0.2273 | −0.0280 | −0.1558 | 0.0352 |
| 10–13 | 437 | −0.0755 | 0.1078 | −0.0978 | 0.0845 | −0.0567 | 0.1289 |
| 10.5–13.5 | 432 | −0.0680 | 0.1171 | −0.0649 | 0.1207 | −0.0771 | 0.1093 |
| 11–14 | 505 | −0.0876 | 0.0796 | −0.0593 | 0.1042 | −0.1175 | 0.0547 |
| 11.5–14.5 | 519 | −0.0678 | 0.0986 | −0.0581 | 0.1110 | −0.0818 | 0.0843 |
| 12–15 | 548 | −0.0858 | 0.0751 | −0.0605 | 0.0991 | −0.1065 | 0.0531 |
| 12.5–15.5 | 514 | −0.1074 | 0.0723 | −0.0909 | 0.0902 | −0.1222 | 0.0539 |
| 13–16 | 483 | −0.1790 | 0.0123 | −0.1882 | −0.0002 | −0.1582 | 0.0331 |
| 13.5–16.5 | 419 | −0.2123 | −0.0177 | −0.2347 | −0.0343 | −0.1760 | 0.0093 |
| 14–17 | 380 | −0.2196 | −0.0280 | −0.2579 | −0.0594 | −0.1762 | 0.0128 |
| 14.5–17.5 | 276 | −0.2552 | −0.0408 | −0.2782 | −0.0563 | −0.2241 | −0.0033 |
| 15–18 | 206 | −0.2782 | −0.0095 | −0.3189 | −0.0342 | −0.2295 | 0.0302 |
| 15.5–18.5 | 138 | −0.3102 | 0.0127 | −0.3296 | 0.0222 | −0.2765 | 0.0344 |
| 16–19 | 75 | −0.1120 | 0.4271 | −0.1128 | 0.3791 | −0.1358 | 0.4413 |
LLCI, Lower Limit Confidence Interval; ULCI, Upper Limit Confidence Interval.
Discussion
We examined whether threat-related amygdala reactivity differs depending on time of day, and whether such variation is moderated by sleep quality. Two primary findings suggest that threat-related amygdala reactivity may vary diurnally. First, consistent with our primary hypothesis, amygdala reactivity was highest in the morning and decreased over the course of the day. Second, sleep quality moderated this effect; there was a larger effect of time of scan among participants who reported an average or high level of sleep problems. More broadly, these findings suggest that neuroimaging studies of threat-related neural reactivity, much like investigations of other phenotypes that vary diurnally (e.g. cortisol (Dickerson and Kemeny, 2004), may wish to consider TOS in study design and analyses, and further may wish to include measurements of circadian rhythms, to improve their ability to elucidate brain-behavior relationships.
Diurnal variation of cognition and related neuroimaging phenotypes
A growing neuroimaging literature suggests that time-dependent effects on behavior may arise from diurnal variation of neural phenotypes. Behavioral circadian rhythms have been observed since the beginning of the 19th century in psychometric tasks assaying attention, executive function, and memory (Blatter and Cajochen, 2007; Schmidt et al., 2007; Valdez et al., 2012; Gaggioni et al., 2014). Similarly, affect and anxiety vary diurnally, with negative affect and anxiety peaking in the morning (Clark et al., 1989; Murray, 2007; Wirz-Justice, 2008; Ayuso-Mateos et al., 2013). Given abundant evidence that human cognition and emotion vary diurnally, it is unsurprising that there are several reports of circadian effects on task-related fMRI activation. These studies have generally found that peaks in activation coincide alongside related behavioral/cognitive function peaks. For instance, reward-related activity peaks mid-day, when positive affect is at its highest (Hasler et al., 2014; Masterson et al., 2015). Similarly, diurnal variation in cognitive control and working memory is moderated by age and chronotype, of which similar effects have been observed in fMRI studies of these constructs (Marek et al., 2010; Schmidt et al., 2012, 2015; Anderson et al., 2014). A recent study found evidence for circadian variation of fMRI activation across the brain, in a sustained attention task under conditions of prolonged wakefulness, and notably found that different regions peaked at different times of day (Muto et al., 2016).
The current study is particularly notable as the first study to identify that one of the major neural underpinnings of human emotion, amygdala reactivity, displays diurnal variation in the context of an emotional face task. This finding is consistent with evidence that fear conditioning, an amygdala-dependent behavior, as well as rodent threat-related amygdala activity, are potentiated during the active phase of the circadian cycle (Funk and Amir, 2000; Valentinuzzi et al., 2001; Chaudhury and Colwell, 2002; Woodruff et al., 2015). Moreover, our control analyses of V1 suggest that this effect does not reflect more widespread diurnal variation of brain function in response to our task more generally.
We observed no correlation between pre-scan state-anxiety symptoms (STAI) and time of scan, which runs counter to a well-established literature showing diurnal variation of affect and anxiety (Clark et al., 1989; Murray, 2007; Wirz-Justice, 2008; Ayuso-Mateos et al., 2013). However, it is important to consider these findings in the context of the current study. Unlike other studies of diurnal affect which typically ask participants to report emotional experience throughout their daily life, the present study asked participants about state-related affect directly before undergoing an MRI session, which may be conceptualized as a challenge. Indeed, it is not uncommon for participants to experience anxiety related to the MRI session, with 14% of hospital patients requiring sedation prior to an MRI session (Kieran and Brunbergz, 1997). Thus, our data may be best interpreted as providing evidence that state anxiety before an impending MRI scan does not vary diurnally. Further, we found that pre-scan STAI was not a significant predictor of amygdala reactivity in any analysis. While there are numerous reports of associations between trait anxiety and amygdala function (Etkin et al., 2004; Etkin and Wager, 2007; Ewbank et al., 2009;Ball et al., 2012; Laeger et al., 2012; Toki et al., 2013; Binelli et al., 2014; Hilbert et al., 2014), this result suggests that the amygdala reactivity phenotype is less influenced by the participant’s current anxiety state (but see (Bishop et al., 2004; Somerville et al., 2004) for counter examples in smaller samples).
Possible mechanisms of time of day variation in amygdala function
Given the data available within this study, we are unable to probe potential biological mechanisms through which time of day may influence amygdala function. However, an emerging literature, as well as findings from the present study, points to diurnal variation of the hypothalamic-pituitary-adrenal (HPA) axis, or the circadian factors that regulate it, as a likely candidate. HPA axis activation and cortisol production follow a diurnal rhythm, wherein cortisol levels peak shortly after awakening and steadily decrease over the course of the day (Fries et al., 2009). Notably, amygdala activation is sensitive to levels of cortisol. Basal, stress-induced, and pharmacologically increased cortisol levels predict elevated amygdala activation, both while processing emotional stimuli (van Stegeren et al., 2007; Weldon et al., 2015; Henckens et al., 2016), as well as during the regulation of negative affect (Urry et al., 2006).
Two findings from the present study support this interpretation. First, while cortisol levels ultimately decrease over the day, smaller cortisol increases occur, particularly during times when meals are regularly consumed, even if the meal is missed on that particular day (Follenius et al., 1982). We found that the association between TOS and amygdala reactivity is strongest between 8:30–12:30 pm and 1:30–6 pm. That is, no effect of TOS was observed in any 4-hour window which included 12:30–1:30 pm. This effect may be related to lunch, which is often consumed during these hours, and which may have a larger impact on cortisol-dependent physiological processes than earlier meals, as basal cortisol levels are lower in the afternoon than the morning. Had more participants been scanned in the evening we would have expected to see similar associations with dinner consumption/expectation. Second, poor sleep quality, which is associated with disrupted circadian rhythmicity, is predictive of elevated morning cortisol (Hatzinger et al., 2008, 2010; Balbo et al., 2010; Bostock and Steptoe, 2013; Abell et al., 2016) and we found that self-reported poor sleep quality was associated with increased amygdala reactivity among participants scanned in the morning. Notably, this finding is further congruent with prior evidence that sleep deprivation is associated with increased amygdala reactivity (Yoo et al., 2007; Motomura et al., 2013, 2014; Simon et al., 2015; Reidy et al., 2016).
Our interpretation that TOS effects may be driven by diurnal variation of the HPA axis is also congruent with the aforementioned observation that pre-scan state-anxiety symptoms (STAI) were not associated with TOS or amygdala reactivity. Indeed, while there is strong evidence that trait anxiety is linked to an elevated cortisol awakening response (Mantella et al., 2008; Adam et al., 2014), associations between state anxiety and cortisol are mixed, and have typically been examined in the context of stress-related cortisol reactivity. While some studies have reported no association between state anxiety and cortisol (Noto et al., 2005; Polk et al., 2005; Brooks and Robles, 2009; Keulers et al., 2014), there is also evidence that the association between state anxiety and cortisol is moderated by trait anxiety (Shackman et al., 2016; Villada et al., 2016), familiarity with the stressor (Carré et al., 2006; Hare et al., 2013; Federenko et al., 2004), and physical fitness (Rimmele et al., 2007, 2009; Klaperski et al., 2013).
A linear model best fit our data, despite evidence that circadian rhythms follow sinusoidal patterns of variation (Herzog, 2007). Given our hypothesis that HPA axis activation is a strong contributor to diurnal variation of amygdala reactivity, we anticipate that activation peaks soon after wakening. Thus, a linear fit may simply be due to the relatively restricted times of scan used in this study. We would expect that sinusoidal variation would be observed if extended scanning throughout the day, including just after awakening and immediately before sleeping, were employed (Muto et al., 2016).
It should be noted that there are many other potential biological mechanisms which may also contribute to diurnal variation in amygdala reactivity. Indeed, as human physiology is inherently circadian, due to adaptation to the day/night cycles of the Earth, an exhaustive list is impossible (Herzog, 2007). Here we briefly list three strong additional candidates. First, circadian variation of gene expression is a likely candidate, as many gene transcripts vary in their expression in a circadian-dependent fashion throughout the postmortem human brain, including in the amygdala (Li et al., 2013; Bunney et al., 2015; Chen et al., 2015). Second, circadian variation of neurotransmitter release, including dopamine, serotonin, and opioids, which has been primarily noted in rodent studies, likely contributes to diurnal variation of human brain function (Asai et al., 1998; Monnet, 2002; Castañeda et al., 2004; Ferris et al., 2014). Third, inflammatory processes, which have been shown to affect amygdala function (Inagaki et al., 2012; Muscatell et al., 2015, 2016), also follow circadian patterns of variation (Keller et al., 2009; Narasimamurthy et al., 2012; Gibbs et al., 2014). It is likely that these three processes act in concert with the HPA axis, as well as other systems, to influence diurnal variation of human brain function.
Left amygdala laterality
Across TOS analyses, activation in the left amygdala produced consistently stronger effects than activation in the right (though the right amygdala always showed the same direction of effect as the left amygdala when the left amygdala showed a significant association). Meta-analyses have found that blocked design tasks more robustly recruit the left relative to the right amygdala, and that left amygdala reactivity is more commonly associated with behavior (Baas et al., 2004; Costafreda et al., 2008; Sergerie et al., 2008). While the reason for such lateralization is unclear, some evidence suggests that the left amygdala plays a greater role in conscious emotional processing (Gainotti, 2012). As a result, it is possible that our blocked task design with overt emotional stimuli may result in relatively potentiated associations in the left, relative to right, amygdala. Notably, while some additional evidence suggests that the right amygdala habituates more quickly (Wright et al., 2001), we observed no habituation differences across blocks between hemispheres (see Supplementary Data).
Implications for study design and analysis
Prior studies of amygdala activation to threatening faces have found a range of test–retest reliability intra-class correlation coefficients, with ICC values from −0.02 to 0.79 (Johnstone et al., 2005; Manuck et al., 2007; Plichta et al., 2012; Sauder et al., 2013). Such a wide range of reported values may be explained by the presence of additional confounding factors. Indeed, despite the relatively small effect size observed in the present, cross-sectional, sample (β = −0.1083), our results suggest that controlling for time of scan would increase the test–retest reliability of amygdala reactivity. Time of scan may also be a useful covariate in studies designed to detect relatively small effects (e.g. genetic analyses). Moreover, we detect an effect of TOS even though additional (and potentially more sensitive) markers of circadian rhythms were not collected [e.g. cortisol, melatonin, chronotype, or actigraphy (Emens et al., 2009; Fries et al., 2009; Grutsch et al., 2011; Schmidt et al., 2015)]. Including these measurements in future studies will most likely further improve power in studies of amygdala function.
Limitations
The present study should be interpreted in the context of limitations. First, as this is an analysis of an archival dataset, several measures that would have been particularly relevant to our speculation that circadian differences may mediate associations between time of scan and amygdala function were not available. These include diurnal salivary melatonin and cortisol, diurnal levels of activity via actigraphy, self-report of chronotype, and circadian (e.g. PER1, PER2) gene expression. Second, while our models imply a direction of effect, we cannot determine if variability in one phenotype precedes variability in another (e.g. it is possible that trait-related elevations in amygdala activity led individuals to schedule an earlier scan time). Notably, we found no association of time of scan with demographic factors, state anxiety symptoms, or the presence of a psychiatric disorder, suggesting that between-participant differences on these phenotypes did not confound scan time scheduling. A follow-up within-subject study, with extensive time of scan counterbalancing across multiple days, due to known effects of repeated exposure on amygdala activation (i.e. habituation (Plichta et al., 2014), would be especially informative. A third limitation is that self-report measures of sleep quality are poor predictors of objective measures of sleep quality, which were not collected in the present study (Grandner et al., 2006; Lemola et al., 2013; Landry et al., 2015). However, it should be noted that, while the interaction of time of scan with self-report sleep quality (PSQI) was analyzed in a continuous fashion, posthoc comparisons suggest that this interaction was primarily driven by a difference between participants with relatively good sleep quality (e.g. PSQI < 5) and all others (including those with average sleep quality). Thus, even accounting for the low correlation between subjective and objective sleep quality measures, it is plausible that this group of low sleep-disruption participants may truly have higher sleep quality. Finally, it should be noted that a previous study identified amygdala activation which peaked later in the day than the peak observed in the present study (Muto et al., 2016), in the context of a sustained attention task under conditions of prolonged wakefulness. This result suggests that diurnal peaks in brain activation may be context-specific, and that the peak amygdala reactivity observed in the present study may not generalize to other paradigms (e.g. positive affect).
Conclusions
Limitations notwithstanding, we provide initial evidence that amygdala reactivity may vary in a diurnal manner, decreasing over the course of the day. Future studies with samples greater than n = 300 might be able to replicate this effect, depending on when scanning was conducted. Further, self-reported sleep disruption was associated with amplified associations between TOS and amygdala function. More broadly, these results suggest that time of scan scheduling should be considered in studies of emotional-related neural circuitry or potentially included as a covariate or moderating factor.
Supplementary data
Supplementary data are available at SCAN online.
Funding
The Duke Neurogenetics Study is supported by Duke University and the National Institutes of Health (NIDA DA033369). A.R.H. receives additional support from the National Institutes of Health (NIDA DA031579). D.A.A.B. was supported by National Institutes of Health (T32-GM008151) and National Science Foundation (DGE-1143954). R.B. was supported by the Klingenstein Third Generation Foundation and receives additional support from the National Institutes of Health (R01-AG045231, R01-HD083614, U01-AG052564).
Conflict of interest. None declared.
Supplementary Material
References
- Abell J.G., Shipley M.J., Ferrie J.E., Kivimäki M., Kumari M. (2016). Recurrent short sleep, chronic insomnia symptoms and salivary cortisol: a 10-year follow-up in the Whitehall II study. Psychoneuroendocrinology, 68, 91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam E.K., Vrshek-Schallhorn S., Kendall A.D., Mineka S., Zinbarg R.E., Craske M.G. (2014). Prospective associations between the cortisol awakening response and first onsets of anxiety disorders over a six-year follow-up – 2013 Curt Richter Award Winner. Psychoneuroendocrinology, 44, 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th edn. Washington, DC: American Journal of Psychiatry.
- Anderson J.A.E., Campbell K.L., Amer T., Grady C.L., Hasher L. (2014). Timing is everything: age differences in the cognitive control network are modulated by time of day. Psychology and Aging, 29, 648–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai M., Zubieta M., Matamoros-Trejo G., Linares G., Agustin P. (1998). Diurnal variations of opioid peptides and synenkephalin in vitro release in the amygdala of kindled rats. Neuropeptides, 32(3), 293–9. [DOI] [PubMed] [Google Scholar]
- Ayuso-Mateos J.L., Miret M., Caballero F.F., et al. (2013). Multi-country evaluation of affective experience: validation of an abbreviated version of the day reconstruction method in seven countries. PLoS One, 8(4), e61534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas D., Aleman A., Kahn R.S. (2004). Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Research Reviews, 45(2), 96–103. [DOI] [PubMed] [Google Scholar]
- Balbo M., Leproult R., Van Cauter E. (2010). Impact of sleep and its disturbances on hypothalamo-pituitary-adrenal axis activity. International Journal of Endocrinology, 2010, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball T.M., Sullivan S., Flagan T., et al. (2012). Selective effects of social anxiety, anxiety sensitivity, and negative affectivity on the neural bases of emotional face processing. NeuroImage, 59(2), 1879–87. [DOI] [PubMed] [Google Scholar]
- Baranger D.A.A., Ifrah C., Prather A.A., et al. (2016). PER1 rs3027172 genotype interacts with early life stress to predict problematic alcohol use, but not reward-related ventral striatum activity. Frontiers in Psychology, 7(March), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57(1), 289–300. [Google Scholar]
- Binelli C., Subira S., Batalla A., et al. (2014). Common and distinct neural correlates of facial emotion processing in social anxiety disorder and Williams syndrome: a systematic review and voxel-based meta-analysis of functional resonance imaging studies. Neuropsychologia, 64, 205–17. [DOI] [PubMed] [Google Scholar]
- Bishop S.J., Duncan J., Lawrence A.D. (2004). State anxiety modulation of the amygdala response to unattended threat-related stimuli. The Journal of Neuroscience, 24(46), 10364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter K., Cajochen C. (2007). Circadian rhythms in cognitive performance: methodological constraints, protocols, theoretical underpinnings. Physiology and Behavior, 90(2–3), 196–208. [DOI] [PubMed] [Google Scholar]
- Bogdan R., Williamson D.E., Hariri A.R. (2012). Mineralocorticoid receptor Iso/Val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. American Journal of Psychiatry, 169(5), 515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock S., Steptoe A. (2013). Influences of early shift work on the diurnal cortisol rhythm, mood and sleep: within-subject variation in male airline pilots. Psychoneuroendocrinology, 38(4), 533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks K.P., Robles T.F. (2009). Recent depressive and anxious symptoms predict cortisol responses to stress in men. Psychoneuroendocrinology, 34(7), 1041–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney B.G., Li J.Z., Walsh D.M., et al. (2015). Circadian dysregulation of clock genes: clues to rapid treatments in major depressive disorder. Molecular Psychiatry, 20, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse D.J., Reynolds C.F., Monk T.H., Berman S.R., Kupfer D.J. (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Canty A., Ripley B. (2012). boot: Bootstrap R (S-Plus) Functions. R Package Version, 3–7.
- Carré J., Muir C., Belanger J., Putnam S.K. (2006). Pre-competition hormonal and psychological levels of elite hockey players: relationship to the “home advantage”. Physiology and Behavior, 89(3), 392–8. [DOI] [PubMed] [Google Scholar]
- Carré J.M., Fisher P.M., Manuck S.B., Hariri A.R. (2012). Interaction between trait anxiety and trait anger predict amygdala reactivity to angry facial expressions in men but not women. Social Cognitive and Affective Neuroscience, 7(2), 213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañeda T.R., de Prado B.M., Prieto D., Mora F. (2004). Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. Journal of Pineal Research, 36(3), 177–85. [DOI] [PubMed] [Google Scholar]
- Chaudhury D., Colwell C.S. (2002). Circadian modulation of learning and memory in fear-conditioned mice. Behavioural Brain Research, 133(1), 95–108. [DOI] [PubMed] [Google Scholar]
- Chen C.H., Suckling J., Lennox B.R., Ooi C., Bullmore E.T. (2011). A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disorders, 13(1), 1–15. [DOI] [PubMed] [Google Scholar]
- Chen C.-Y., Logan R.W., Ma T., et al. (2015). Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 113(1), 206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L.A., Watson D., Leeka J. (1989). Diurnal variation in the positive affects. Motivation and Emotion, 13(3), 205–34. [Google Scholar]
- Costafreda S.G., Brammer M.J., David A.S., Fu C.H.Y. (2008). Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Research Reviews, 58(1), 57–70. [DOI] [PubMed] [Google Scholar]
- Demers C.H., Conley E.D., Bogdan R., Hariri A.R. (2016). Interactions between anandamide and corticotropin-releasing hormone signaling modulate human amygdala function and risk for anxiety disorders: an imaging genetics strategy for modeling molecular interactions. Biological Psychiatry, 80(5), 356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson S.S., Kemeny M.E. (2004). Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–91. [DOI] [PubMed] [Google Scholar]
- Doane L.D., Mineka S., Zinbarg R.E., Craske M., Griffith J.W., Adam E.K. (2013). Are flatter diurnal cortisol rhythms associated with major depression and anxiety disorders in late adolescence? The role of life stress and daily negative emotion. Development and Psychopathology, 25(3), 629–42. [DOI] [PubMed] [Google Scholar]
- Dodson E.R., Zee P.C., Manuscript A. (2010). Therapeutics for circadian rhythm sleep disorders. Sleep Medicine, 5(4), 701–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emens J., Lewy A., Kinzie J.M., Arntz D., Rough J. (2009). Circadian misalignment in major depressive disorder. Psychiatry Research, 168(3), 259–61. [DOI] [PubMed] [Google Scholar]
- Etkin A., Klemenhagen K.C., Dudman J.T., et al. (2004). Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron, 44(6), 1043–55. [DOI] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American Journal of Psychiatry, 164(October), 1476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewbank M.P., Lawrence A.D., Passamonti L., Keane J., Peers P.V., Calder A.J. (2009). Anxiety predicts a differential neural response to attended and unattended facial signals of anger and fear. NeuroImage, 44(3), 1144–51. [DOI] [PubMed] [Google Scholar]
- Federenko I.S., Nagamine M., Hellhammer D.H., Wadhwa P.D. and Wüst, S. (2004). The heritability of hypothalamus pituitary adrenal axis responses to psychosocial stress is context dependent. The Journal of Clinical Endocrinology & Metabolism, 89(12), 6244–50. [DOI] [PubMed] [Google Scholar]
- Ferris M.J., España R. a., Locke J.L., et al. (2014). Dopamine transporters govern diurnal variation in extracellular dopamine tone. Proceedings of the National Academy of Sciences of the United States of America, 111(26), E2751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Miriam G., Williams J.B.W. (2002). Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Non-Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute.
- Fisher P.M., Madsen M.K., Mc Mahon B., et al. (2014). Three-week bright-light intervention has dose-related effects on threat-related corticolimbic reactivity and functional coupling. Biological Psychiatry, 76(4), 332–9. [DOI] [PubMed] [Google Scholar]
- Follenius M., Brandenberger G., Hietter B., Siméoni M. and Reinhardt. (1982). Diurnal cortisol peaks and their relationships to meals. Journal of Clinical Endocrinology & Metabolism, 55(4), 757–61. [DOI] [PubMed] [Google Scholar]
- Fonov V., Evans A.C., Botteron K., Almli C.R., McKinstry R.C., Collins D.L. (2011). Unbiased average age-appropriate atlases for pediatric studies. NeuroImage, 54(1), 313–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E., Dettenborn L., Kirschbaum C. (2009). The cortisol awakening response (CAR): facts and future directions. International Journal of Psychophysiology, 72(1), 67–73. [DOI] [PubMed] [Google Scholar]
- Funk D., Amir S. (2000). Circadian modulation of Fos responses to odor of the red fox, a rodent predator, in the rat olfactory system. Brain Research, 866(1–2), 262–7. [DOI] [PubMed] [Google Scholar]
- Gaggioni G., Maquet P., Schmidt C., Dijk D., Vandewalle G. (2014). Neuroimaging, cognition, light and circadian rhythms. Frontiers in Systems Neuroscience, 8(July), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainotti G. (2012). Unconscious processing of emotions and the right hemisphere. Neuropsychologia, 50(2), 205–18. [DOI] [PubMed] [Google Scholar]
- Geraci M.F., Uhde T.W. (1992). Diurnal rhythms and symptom severity in panic disorder. A preliminary study of 24-hour changes in panic attacks, generalised anxiety, and avoidance behaviour. British Journal of Psychiatry, 161(OCT), 512–6. [DOI] [PubMed] [Google Scholar]
- Gibbs J., Ince L., Matthews L., et al. (2014). An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nature Medicine, 20(8), 919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner M.A., Kripke D.F., Yoon I.Y., Youngstedt S.D. (2006). Criterion validity of the Pittsburgh Sleep Quality Index: investigation in a non-clinical sample. Sleep and Biological Rhythms, 4(2), 129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutsch J.F., Wood P. a., Du-Quiton J., et al. (2011). Validation of actigraphy to assess circadian organization and sleep quality in patients with advanced lung cancer. Journal of Circadian Rhythms, 9(1), 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare O.A., Wetherell M.A., Smith M.A. (2013). State anxiety and cortisol reactivity to skydiving in novice versus experienced skydivers. Physiology and Behavior, 118, 40–4. [DOI] [PubMed] [Google Scholar]
- Hasler B.P., Forbes E.E., Franzen P.L. (2014). Time-of-day differences and short-term stability of the neural response to monetary reward: a pilot study. Psychiatry Research: Neuroimaging, 224(1), 22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzinger M., Brand S., Perren S., et al. (2008). Electroencephalographic sleep profiles and hypothalamic-pituitary-adrenocortical (HPA)-activity in kindergarten children: early indication of poor sleep quality associated with increased cortisol secretion. Journal of Psychiatric Research, 42(7), 532–43. [DOI] [PubMed] [Google Scholar]
- Hatzinger M., Brand S., Perren S., et al. (2010). Sleep actigraphy pattern and behavioral/emotional difficulties in kindergarten children: association with hypothalamic-pituitary-adrenocortical (HPA) activity. Journal of Psychiatric Research, 44(4), 253–61. [DOI] [PubMed] [Google Scholar]
- Henckens M.J.A.G., Klumpers F., Everaerd D., Kooijman S.C., Van Wingen G.A., Fernández G. (2016). Inter-individual differences in stress sensitivity: basal and stress-induced cortisol levels differentially predict neural vigilance processing under stress. Social Cognitive and Affective Neuroscience, 11(4), 667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog E.D. (2007). Neurons and networks in daily rhythms. Nature Reviews Neuroscience, 8(10), 790–802. [DOI] [PubMed] [Google Scholar]
- Hilbert K., Lueken U., Beesdo-Baum K. (2014). Neural structures, functioning and connectivity in Generalized Anxiety Disorder and interaction with neuroendocrine systems: a systematic review. Journal of Affective Disorders, 158, 114–26. [DOI] [PubMed] [Google Scholar]
- Hosseini F., Adha N., Zainol R., Isahak M., Nemati N. (2014). Neighborhood-level stress and circadian cortisol: a systematic review and meta-analysis. Iranian Journal of Public Health, 43(10), 1324–34. [PMC free article] [PubMed] [Google Scholar]
- Inagaki T.K., Muscatell K.A., Irwin M.R., Cole S.W., Eisenberger N.I. (2012). Inflammation selectively enhances amygdala activity to socially threatening images. NeuroImage, 59(4), 3222–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T., Somerville L.H., Alexander A.L., et al. (2005). Stability of amygdala BOLD response to fearful faces over multiple scan sessions. NeuroImage, 25(4), 1112–23. [DOI] [PubMed] [Google Scholar]
- Keller M., Mazuch J., Abraham U., et al. (2009). A circadian clock in macrophages controls inflammatory immune responses. Proceedings of the National Academy of Sciences of the United States of America, 106(50), 21407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M.C. (2014). Gene × environment interaction studies have not properly controlled for potential confounders: the problem and the (simple) solution. Biological Psychiatry, 75(1), 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keulers E.H.H., Stiers P., Nicolson N.A., Jolles J. (2014). The association between cortisol and the BOLD response in male adolescents undergoing fMRI. Brain Research, 1598, 1–11. [DOI] [PubMed] [Google Scholar]
- Kieran K.J., Brunbergz J.A. (1997). Adult claustrophobia, anxiety and sedation in MRI. Magnetic Resonance Imaging, 15(1), 51–4. [DOI] [PubMed] [Google Scholar]
- Klaperski S., von Dawans B., Heinrichs M., Fuchs R. (2013). Does the level of physical exercise affect physiological and psychological responses to psychosocial stress in women? Psychology of Sport and Exercise, 14(2), 266–74. [Google Scholar]
- Laeger I., Dobel C., Dannlowski U., et al. (2012). Amygdala responsiveness to emotional words is modulated by subclinical anxiety and depression. Behavioural Brain Research, 233(2), 508–16. [DOI] [PubMed] [Google Scholar]
- Lam R.W., Levitt A.J., Levitan R.D., et al. (2015). Efficacy of bright light treatment, fluoxetine, and the combination in patients with nonseasonal major depressive disorder. JAMA Psychiatry, 73(1), 56–63. [DOI] [PubMed] [Google Scholar]
- Lancaster J.L., Woldorff M.G., Parsons L.M., et al. (2000). Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping, 10, 120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry G.J., Best J.R., Liu-Ambrose T. (2015). Measuring sleep quality in older adults: a comparison using subjective and objective methods. Frontiers in Aging Neuroscience, 7(SEP), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemola S., Ledermann T., Friedman E.M. (2013). Variability of sleep duration is related to subjective sleep quality and subjective well-being: an actigraphy study. PLoS One, 8(8), e71292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.Z., Bunney B.G., Meng F., Hagenauer M.H., Walsh D.M., Vawter M.P. (2013). Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proceedings of the National Academy of Sciences, 11(24), 9950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly J., McGrath J.J., Gouin J.P. (2015). Poor sleep as a pathophysiological pathway underlying the association between stressful experiences and the diurnal cortisol profile among children and adolescents. Psychoneuroendocrinology, 57, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19, 1233–9. [DOI] [PubMed] [Google Scholar]
- Mantella R.C., Butters M.A., Amico J.A., et al. (2008). Salivary cortisol is associated with diagnosis and severity of late-life generalized anxiety disorder. Psychoneuroendocrinology, 33(6), 773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck S.B., Brown S.M., Forbes E.E., Hariri A.R. (2007). Temporal stability of individual differences in amygdala reactivity. American Journal of Psychiatry, 164(10), 1613–4. [DOI] [PubMed] [Google Scholar]
- Marek T., Fafrowicz M., Golonka K., et al. (2010). Diurnal patterns of activity of the orienting and executive attention neuronal networks in stubjects performing a stroop-like task: a functional magnetic resonance imaging study. Chronobiology International, 27(5), 945–58. [DOI] [PubMed] [Google Scholar]
- Masterson T.D., Kirwan C.B., Davidson L.E., LeCheminant J.D. (2015). Neural reactivity to visual food stimuli is reduced in some areas of the brain during evening hours compared to morning hours: an fMRI study in women. Brain Imaging and Behavior, 10(1), 68–78. [DOI] [PubMed] [Google Scholar]
- Mattavelli G., Sormaz M., Flack T., et al. (2014). Neural responses to facial expressions support the role of the amygdala in processing threat. Social Cognitive and Affective Neuroscience, 9(11), 1684–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung C.A. (2007). Circadian genes, rhythms and the biology of mood disorders. Pharmacology and Therapeutics, 114(2), 222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz C.J., Stark R., Vaitl D., Tabbert K., Wolf O.T. (2013). Stress hormones are associated with the neuronal correlates of instructed fear conditioning. Biological Psychology, 92(1), 82–9. [DOI] [PubMed] [Google Scholar]
- Meyer C., Muto V., Jaspar M., et al. (2016). Seasonality in human cognitive brain responses. Proceedings of the National Academy of Sciences of the United States of America, 113(11), 3066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnet F.P. (2002). Melatonin modulates [3h]serotonin release in the rat hippocampus: effects of circadian rhythm. Journal of Neuroendocrinology, 14(3), 194–9. [DOI] [PubMed] [Google Scholar]
- Motomura Y., Kitamura S., Oba K., et al. (2013). Sleep debt elicits negative emotional reaction through diminished amygdala-anterior cingulate functional connectivity. PLoS One, 8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motomura Y., Kitamura S., Oba K., et al. (2014). Sleepiness induced by sleep-debt enhanced amygdala activity for subliminal signals of fear. BMC Neuroscience, 15(1), 97.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray G. (2007). Diurnal mood variation in depression: a signal of disturbed circadian function? Journal of Affective Disorders, 102(1–3), 47–53. [DOI] [PubMed] [Google Scholar]
- Muscatell K.A., Dedovic K., Slavich G.M., et al. (2015). Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain, Behavior, and Immunity, 43, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell K.A., Eisenberger N.I., Dutcher J.M., Cole S.W., Bower J.E. (2016). Links between inflammation, amygdala reactivity, and social support in breast cancer survivors. Brain, Behavior, and Immunity, 53, 34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto V., Jaspar M., Meyer C., et al. (2016). Local modulation of human brain responses by circadian rhythmicity and sleep debt. Science, 353(6300), 687–90. [DOI] [PubMed] [Google Scholar]
- Narasimamurthy R., Hatori M., Nayak S.K., Liu F., Panda S., Verma I.M. (2012). Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proceedings of the National Academy of Sciences of the United States of America, 109(31), 12662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwirth E. (2014). RColorBrewer: ColorBrewer Palettes. R Package Version 1.1-2.
- Newhoff M., Treiman D.M., Smith K.A., Steinmetz P.N. (2015). Gender differences in human single neuron responses to male emotional faces. Frontiers in Human Neuroscience, 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noto Y., Sato T., Kudo M., Kurata K., Hirota K. (2005). The relationship between salivary biomarkers and state-trait anxiety inventory score under mental arithmetic stress: a pilot study. Anesthesia and Analgesia, 101(6), 1873–6. [DOI] [PubMed] [Google Scholar]
- Nussbaumer B., Ca F., Lc M., et al. (2015). Light therapy for preventing seasonal affective disorder. Cochrane Database of Systematic Reviews, 11, Art.No.: CD011269. [DOI] [PubMed] [Google Scholar]
- Pace-Schott E.F., Rubin Z.S., Tracy L.E., Spencer R.M.C., Orr S.P., Verga P.W. (2015). Emotional trait and memory associates of sleep timing and quality. Psychiatry Research, 229(3):, 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott E.F., Spencer R.M.C., Vijayakumar S., et al. (2013). Extinction of conditioned fear is better learned and recalled in the morning than in the evening. Journal of Psychiatric Research, 47(11), 1776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J.E. (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron, 48(2), 175–87. [DOI] [PubMed] [Google Scholar]
- Plichta M.M., Grimm O., Morgen K., et al. (2014). Amygdala habituation: a reliable fMRI phenotype. NeuroImage, 103, 383–90. [DOI] [PubMed] [Google Scholar]
- Plichta M.M., Schwarz A.J., Grimm O., et al. (2012). Test-retest reliability of evoked BOLD signals from a cognitive-emotive fMRI test battery. NeuroImage, 60(3), 1746–58. [DOI] [PubMed] [Google Scholar]
- Polk D.E., Cohen S., Doyle W.J., Skoner D.P., Kirschbaum C. (2005). State and trait affect as predictors of salivary cortisol in healthy adults. Psychoneuroendocrinology, 30(3), 261–72. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. (2013). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
- Reidy B.L., Hamann S., Inman C., Johnson K.C., Brennan P.A. (2016). Decreased sleep duration is associated with increased fMRI responses to emotional faces in children. Neuropsychologia, 84, 54–62. [DOI] [PubMed] [Google Scholar]
- Rimmele U., Seiler R., Marti B., Wirtz P.H., Ehlert U., Heinrichs M. (2009). The level of physical activity affects adrenal and cardiovascular reactivity to psychosocial stress. Psychoneuroendocrinology, 34(2), 190–8. [DOI] [PubMed] [Google Scholar]
- Rimmele U., Zellweger B.C., Marti B., et al. (2007). Trained men show lower cortisol, heart rate and psychological responses to psychosocial stress compared with untrained men. Psychoneuroendocrinology, 32(6), 627–35. [DOI] [PubMed] [Google Scholar]
- Sauder C.L., Hajcak G., Angstadt M., Phan K.L. (2013). Test-retest reliability of amygdala response to emotional faces. Psychophysiology, 50(11), 1147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C., Collette F., Cajochen C., Peigneux P. (2007). A time to think: circadian rhythms in human cognition. Cognitive Neuropsychology, 24(7), 755–89. [DOI] [PubMed] [Google Scholar]
- Schmidt C., Collette F., Reichert C.F., et al. (2015). Pushing the limits: chronotype and time of day modulate working memory-dependent cerebral activity. Frontiers in Neurology, 6(SEP), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C., Peigneux P., Leclercq Y., et al. (2012). Circadian preference modulates the neural substrate of conflict processing across the day. PLoS One, 7(1), e29658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie K., Chochol C., Armony J.L. (2008). The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 32(4), 811–30. [DOI] [PubMed] [Google Scholar]
- Shackman A.J., Tromp D.P.M., Stockbridge M.D., Kaplan C.M., Tillman R.M., Fox A.S. (2016). Dispositional negativity: an integrative psychological and neurobiological perspective. Psychological Bulletin, 142(12), 1275–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., et al. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry, 59(Suppl. 20), 22–33. [PubMed] [Google Scholar]
- Simon E.B., Oren N., Sharon H., et al. (2015). Losing neutrality: the neural basis of impaired emotional control without sleep. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 35(38), 13194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Kim H., Johnstone T., Alexander A.L., Whalen P.J. (2004). Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biological Psychiatry, 55(9), 897–903. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene P.R., Vagg P.R., Jacobs A.G. (1983). ManualfortheState-TraitAnxietyInventory Pal Alto, CA: Manual for the Statetrait Anxiety Inventory STAI. [Google Scholar]
- Toki S., Okamoto Y., Onoda K., et al. (2013). Automatic and intentional brain responses during evaluation of face approachability: correlations with trait anxiety. Neuropsychobiology, 68(3), 156–67. [DOI] [PubMed] [Google Scholar]
- Tong Y., Chen Q., Nichols T.E., et al. (2016). Seeking optimal region-of-interest (ROI) single-value summary measures for fMRI studies in imaging genetics. Plos One, 11(3), e0151391.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry H.L., van Reekum C.M., Johnstone T., et al. (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26(16), 4415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez P., Ramirez C., Garcia A. (2012). Circadian rhythms in cognitive performance: implications for neuropsychological assessment. ChronoPhysiology and Therapy, 2, 81–92. [Google Scholar]
- Valentinuzzi V., Kolker D., Vitaterna M., Ferrari E., Takahashi J., Turek F. (2001). Effect of circadian phase on context\rand cued fear conditioning in C57BL/6J mice. Animal Learning & Behavior, 29(2), 133–42. [Google Scholar]
- van Stegeren A.H., Wolf O.T., Everaerd W., Rombouts S. a R B. (2007). Interaction of endogenous cortisol and noradrenaline in the human amygdala. Progress in Brain Research, 167, 263–8. [DOI] [PubMed] [Google Scholar]
- Villada C., Hidalgo V., Almela M., Salvador A. (2016). Individual differences in the psychobiological response to psychosocial stress (Trier Social Stress Test): the relevance of trait anxiety and coping styles. Stress and Health, 32(2), 90–9. [DOI] [PubMed] [Google Scholar]
- Weldon A.L., Hagan M., Van Meter A., et al. (2015). Stress response to the functional magnetic resonance imaging environment in healthy adults relates to the degree of limbic reactivity during emotion processing. Neuropsychobiology, 71(2), 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. (2009). ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer-Verlag. [Google Scholar]
- Wirz-Justice A. (2008). Diurnal variation of depressive symptoms. Dialogues in Clinical Neuroscience, 10(3), 337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff E.R., Greenwood B.N., Chun L.E., Fardi S., Hinds L.R., Spencer R.L. (2015). Adrenal-dependent diurnal modulation of conditioned fear extinction learning. Behavioural Brain Research, 286, 249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C.I., Fischer H., Whalen P.J., McInerney S.C., Shin L.M., Rauch S.L. (2001). Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport, 12(2), 379–83. [DOI] [PubMed] [Google Scholar]
- Yoo S.S., Gujar N., Hu P., Jolesz F. A., Walker M.P. (2007). The human emotional brain without sleep – a prefrontal amygdala disconnect. Current Biology, 17(20), 877–8. [DOI] [PubMed] [Google Scholar]
- Yoon K.L., Zinbarg R.E. (2008). Interpreting neutral faces as threatening is a default mode for socially anxious individuals. Journal of Abnormal Psychology, 117(3), 680–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


