Abstract
The dorsomedial prefrontal cortex (dmPFC) is a key hub of the ‘social brain’, but little is known about specific processes supported by this region. Using focal high-definition transcranial direct current stimulation (HD-tDCS) and a social cognitive battery with differing demands on self-other processing, we demonstrate specific involvement of the dmPFC in tasks placing high demands on self-other processing. Specifically, excitatory (anodal) HD-tDCS enhanced the integration of external information into the self for explicit higher-order socio-cognitive tasks across cognitive domains; i.e. visual perspective taking (VPT) and episodic memory. These effects were task specific, as no stimulation effects were found for attributing mental states from the eyes or implicit VPT. Inhibitory (cathodal) HD-tDCS had weaker effects in the opposite direction towards reduced integration of external information into the self. We thus demonstrate for the first time a specific and causal role of the dmPFC in integrating higher-order information from others/external source into that of the self across cognitive domains.
Keywords: high definition tDCS, perspective-taking, self-other processing, self-reference effect, theory of mind
Social cognition refers to a wide range of abilities that facilitate social functioning. A fundamental aspect of human social behaviour involves the ability to distinguish the self from others and attributing the behaviour and actions of others to underlying, unobservable mental states (Decety and Sommerville, 2003). At one level we must distinguish the ‘self’ from ‘other’ and understand that perspectives or beliefs differ accordingly. However, the notion of self and other are not necessarily independent, with some arguing that a mergence of these perspectives is necessary to empathise and attribute mental states to others (Cialdini et al., 1997). For example, if another person is present in a scene during perspective taking tasks, people implicitly judge a scene from the perspective of that person (Apperly and Butterfill, 2009; Samson et al., 2010; Tversky and Hard, 2009). This implicit, automatic representation of another’s perspective is a developmental foundation for explicit perspective taking, where subjects are able to inhibit the egocentric perspective (from the self) in order to adopt an allocentric perspective (in reference to an external position, Flavell, 1977; Gzesh and Surber, 1985; Moll and Tomasello, 2004). Moreover, perspective taking is a developmental precursor for other aspects of higher-order social cognition such as empathy and theory of mind (ToM, Hamilton et al., 2009). There is also evidence that the mental constructs of ‘self’ and ‘other’ bias cognitive processing in other domains. One such example is the biasing of memories for information related to the individual, which results in superior recall for items encoded in relation to or of importance to the ‘self’ (i.e. the self-referential effect, Symons and Johnson, 1997).
The core regions of the social brain have been well characterised and involve midline structures such as the medial prefrontal cortex (mPFC) and posterior cingulate cortex (pCC), bilateral temporoparietal junctions (TPJ), superior temporal lobes and temporal poles (Adolphs, 2009). However little is known about the specific processes supported by these regions. In particular, functional imaging studies have consistently shown activity in the dorsomedial prefrontal cortex (dmPFC) during tasks requiring higher order social judgements that incorporate several sources of information (Denny et al., 2012; Schurz et al., 2014). For example, it is involved in integrating information relevant for social judgements (Brosch et al., 2013; Ferrari et al., 2016) with a role in merging information pertaining to the self and other in order to guide decision-making (Wittmann et al., 2016). The mPFC, more generally, is activated during both ToM and visual perspective taking (VPT) tasks (Schurz et al., 2015), providing neural evidence to support a conceptual link (Hamilton et al., 2009). During VPT tasks, dmPFC activation was greatest during egocentric perspective tasks, but only when the allocentric perspective was incongruent (Schurz et al., 2015). Social cognitive measures that do not involve incorporating several sources of information, such as social animation judgments or the Reading the Mind in the Eyes Task (RMET), do not consistently involve the dmPFC (Schurz et al., 2014). A further distinction of the dmPFC is between the two levels of social cognitive processing, implicit and explicit (Frith and Frith, 2008). Only higher-order social tasks that require complex representations correlate with activity in the dmPFC (Amodio and Frith, 2006; Rilling et al., 2004; Volz et al., 2009) and although implicit processes also activate core mentalising regions, the dmPFC is only involved when intentional explicit judgements are required (Van Overwalle and Vandekerckhove, 2013).
However, mapping the ‘social brain’ by functional imaging techniques only provides correlational evidence for brain–behaviour relationships. Causal inferences can be achieved using non-invasive brain stimulation techniques such as transcranial direct current stimulation (tDCS; for review see Sellaro et al., 2016). However, previous studies that stimulated the social brain all relied on non-focal (‘conventional’) set-ups that preclude strong assumptions regarding the underlying neural effects. In contrast, novel high-definition tDCS (HD-tDCS) set-ups allow the administration of a current with higher spatial precision, resulting in focal neural modulation (for review see Alam et al., 2016) and regionally specific behavioural modulation (Gbadeyan et al., 2016b).
To assess the specific role of the dmPFC in social cognitive tasks requiring self and other distinction, we designed a battery consisting of, (i) an emotion recognition test—RMET that only required considering the mental state of another person, (ii) a VPT test that measured implicit and explicit perspective taking from both an egocentric (self) and allocentric (external) viewpoint (avatar or traffic light), across three levels of perspective taking (implicit, level one and level two) and (iii) a recognition episodic memory test for items encoded in relation to the subject or another person (Barack Obama; SRE, Figure 1 illustrates the design of the study). In two separate groups of participants we employed a sham-controlled, crossover, double-blinded design to assess the effects of either excitatory (‘anodal’) or inhibitory (‘cathodal’) HD-tDCS over the dmPFC. If the dmPFC is recruited when integrating social information from the other to the self, we expect to see significant stimulation effects during the VPT explicit, egocentric conditions such that anodal HD-tDCS will increase the integration of the allocentric perspective into that of the egocentric. During the episodic memory task, anodal HD-tDCS will result in greater salience for other encoded memories, removing or reducing the SRE. As the RMET does not involve integrating self with other to the same extent, we expect no differences on performance following anodal HD-tDCS. The opposite effects were expected for cathodal HD-tDCS.
Fig. 1.
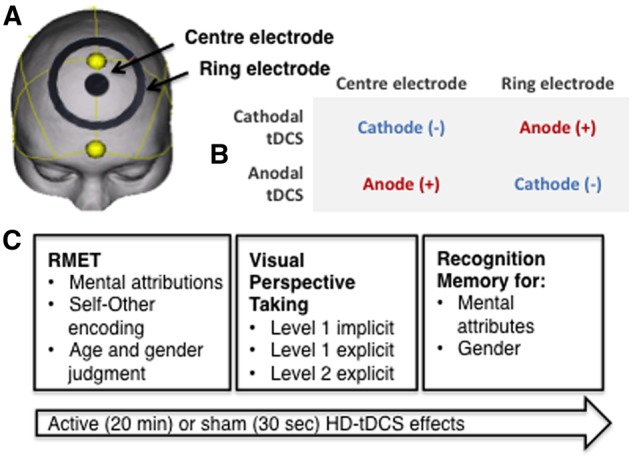
An overview of the study. (A) The HD-tDCS was administered to the dmPFC [MNI co-ordinates 0/54/33, taken from the peak activity associated with ToM in the meta-analysis (Schurz et al., 2014)]. (B) During anodal HD-tDCS the centre electrode was the anode and the ring electrode the cathode. The polarities were swapped during cathodal HD-tDCS. (C) An outline of the study procedure. After consent and safety screening, pre-stimulation Visual Analogue of Mood Scales (VAMS) was completed followed by the Autism Spectrum Quotient (ASQ). Then, subjects watched a 5 min documentary about Barack Obama. The tasks were then administered in the following order: Reading the Mind in the Eyes (RMET) mental attribution, self/other encoding, RMET age and gender judgments, VPT level one implicit, VPT level one explicit, VPT level two explicit. Self or other encoding of memory was manipulated by asking the subject ‘how often do you think or feel this way?’ or ‘how often does Barack Obama think or feel this way?’ The VPT task lasted ∼20 min. Subjects were then asked whether they previously saw the mental state in the eyes (1 = definitely did, 2 = probably did, 3 = probably not, 4 = definitely not). Source memory was measured by asking whether it was on a male or female face (1= definitely male, 2 = probably male, 3= probably female, 4= definitely female). Following testing subjects completed the post-stimulation VAMS, adverse effects questionnaire, and baseline cognitive testing.
Materials and methods
Study outline
The study timeline is presented in Figure 1. In brief, participants first completed the RMET and for each mental attribute selected participants were asked how often they (self) or Barack Obama (other) felt that way. This was followed by the visual perspective tasks in the order: level one implicit, level one explicit and level two explicit. Finally, the recognition episodic memory test for the selected mental attribute words and gender of the stimuli from the RMET. Prior to testing, all subjects completed a safety screening form outlining the project and any safety/risk issues pertaining to tDCS. Subjects completed a written consent form in accordance with Declaration of Helsinki (1991; p. 1194). The ethics committee of the University of Queensland granted ethical approval. In order to familiarise the subjects with Barack Obama, they initially viewed a short (∼5 min) documentary on his life and career. To engage them with the task, participants were instructed that their responses would be compared with those collected from people working with Barack Obama. The Visual Analogue of Mood Scales (VAMS, Folstein and Luria, 1973) was acquired before and after stimulation. The Autism Spectrum Quotient (ASQ, Baron-Cohen et al., 2001) was administered before the first session and the neuropsychological testing was divided between the two sessions and completed following the social cognitive testing.
Participants
40 healthy young adults aged between 18 and 35 were recruited for the study. 20 each were stratified by sex and assigned to the sham-controlled anodal or cathodal HD-tDCS, double-blinded, crossover studies. The groups were comparable on neuropsychological functioning, the ASQ and the Hospital Anxiety and Depression Scale (HADS, Zigmond and Snaith, 1983; see Table S3). All subjects were tDCS-naive, were not currently taking psychoactive medication, and had no diagnosis of neurological or psychiatric disorder. All participants provided written consent prior to inclusion, completed a safety-screening questionnaire and were compensated with AUD$50.
Apparatus
All tasks were presented on a 51 × 33 cm monitor using a standard keyboard. All tasks were presented using Cogent2000 v1.33.
tDCS
The stimulation was administered using a one-channel direct current stimulator (DC-Stimulator Plus®, NeuroConn) and two concentric rubber electrodes (Bortoletto et al., 2016; Gbadeyan et al., 2016c). A small centre electrode (diameter: 2.5 cm) and a ring-shaped return electrode (diameter inner/outer: 9.2/11.5 cm) were used (see Figure 1). The set-up is a variation of the ‘4 × 1’ HD-tDCS set-up, which constrains the current by using four return electrodes that are arranged in a circle around the centre electrode (Alam et al., 2016; Hogeveen et al., 2016; Kuo et al., 2013). Safety, effective behavioural modulation and focal current delivery have been demonstrated for both montages, but the concentric set-up was chosen because it does not require an expensive multi-channel stimulator (Bortoletto et al., 2016; Gbadeyan et al., 2016c). Electrodes were attached over the target region using an adhesive conductive gel (Weaver Ten20® conductive paste) and held in place with an EEG cap to ensure a stable conductive adhesion with the skin. The position of the centre electrode was determined using the 10–20 international EEG system. First FPz and Fz were located and the distance between measured. The scalp region overlying the dmPFC was located by measuring 15% of the distance from the Fz towards the FPz. This approximated the MNI coordinates (0/54/33), which corresponds to the peak activity in the ToM meta-analysis conducted by Schurz et al. (2014). The ring electrode was positioned symmetrically around the centre electrode.
In all stimulation conditions, the current was ramped up to 1 mA over 15 s prior to commencement of the experiment. In the active stimulation conditions (anodal & cathodal) HD-tDCS was administered for 20 min before ramping down. In the anodal HD-tDCS condition, the centre electrode was the anode and the ring electrode was the cathode. The polarities were switched for the cathodal HD-tDCS condition. During sham HD-tDCS, the current was ramped down after 15 s, which elicits a physical sensation on the scalp, mimicking that of the active stimulation, to assure participants were blinded to the experimental condition, without modulating neural function. Researchers were blinded to the experimental condition by using the ‘study-mode’ of the DC-stimulator (i.e. a pre-assigned code triggered the respective stimulation conditions).
Social cognitive tests
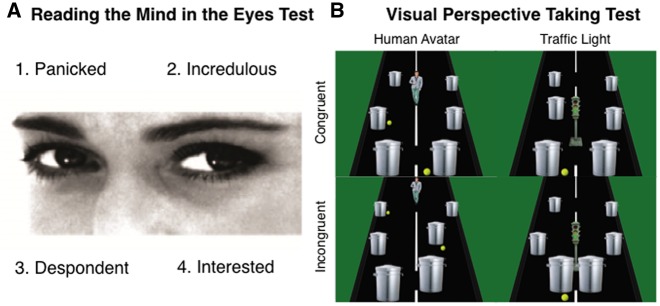
RMET. The RMET (Baron-Cohen et al., 2001) is considered a measure of affective ToM functioning, although recently conceptualised as a measure of emotion recognition (Oakley et al., 2016). A set of eyes were presented with four mental attribution words surrounding (see Figure 2). The RMET was modified so that each response only appeared once. Extra trials were included in order to balance out the gender ratio of the eyes. Subjects were instructed to choose the mental attribution that was best represented in the eyes. Following this, for the purposes of the later memory test, subjects were then asked, ‘how often they felt that way?’ (self-encoding) or ‘how often they thought Barack Obama felt or thought that way?’ (other-encoding). The responses were on a four-point scale (1 = very often, 2 = fairly often, 3 = rarely, 4 = very rarely). Subjects were then asked to judge the age and sex of the person in the picture, again on a four-point scale (1 = young male, 2 = young female, 3 = old man, 4 = old woman). Old was considered >50 years of age. There was no time limit on responding and the next stimulus was presented as soon as a response was recorded. Accuracy scores were computed for the mental attributions and age and sex judgments (total correct out of 38).
Fig. 2.
(A) Example from the Reading the Mind in the Eyes Test (RMET) and (B) the Visual Perspective Taking Test (VPT). Subjects were required to correctly identify the mental attribute displayed in the eyes and then how often they or Barack Obama would think or feel that way (1 = Very often, 2 = Often, 3 = Rarely, 4= Very Rarely) and finally the age and sex of the person (1 = Young Male, 2 = Young Female, 3 = Old Male, 4 = Old female). In the level one implicit task, subjects were instructed to answer as quickly as possible ‘how many tennis balls do you see?’ For the VPT level one explicit self-perspective condition, subjects were required to answer either ‘how many tennis balls can you see?’ (egocentric perspective) or ‘how many can the avatar see?’ or ‘how many does the light directly hit?’ (allocentric perspective). In the VPT level two explicit self-perspective condition, subjects were required to answer ‘can you see more tennis balls on the left or the left or on the right?’ (egocentric perspective) or ‘can the avatar see more on the left or the right of the road?’ or ‘does the light shine directly on more on the left or the right of the road?’ (allocentric perspective). Incongruent scenes were those where the number of tennis balls able to be seen differed between the egocentric and allocentric perspective. In the incongruent scenes, tennis balls were hidden behind a rubbish bin or placed behind the avatar/light.
VPT tasks. The VPT task involved three separate tests measuring level one VPT (implicit and explicit) and level two VPT (explicit). All tests involved a street scene with tennis balls, rubbish bins, and either a human avatar or a traffic light directly in front of the gaze of the subject at one of three positions on the street—far, middle or near (see Figure 2). The traffic light was used as a directional control that should direct attention in a similar manner to the human avatar, but crucially without the ability to hold a perspective of the scene, which was particularly of interest in the implicit VPT task (Apperly and Butterfill, 2009; Samson et al., 2010). The test consisted of 176 trials. In 50% of the trials (n = 88) a human avatar was present and in 50% of the trials a traffic light was present. The trials were further separated (50% each, resulting in 44 trials in each condition) by whether the number of balls seen by the subject was congruent or incongruent with that of the human avatar’s view or the number of tennis balls the light would directly hit. This resulted in four conditions; avatar congruent, avatar incongruent, light congruent, light incongruent (see Figure 2). All conditions were balanced for number and location of tennis balls. Each VPT had four counterbalanced versions and subjects were presented with different versions between sessions. All tests were completed in the order; level one implicit, level one explicit and level two explicit. Subjects were instructed to answer as quickly and as accurately as possible. The stimuli remained on the screen until a response was recorded. A fixation cross was presented for 500 ms prior to the stimuli. For the level one and level two VPT, the word ‘you’ or ‘other’ was presented for 750 ms prior to the presentation of the scene. Subjects were informed that tennis balls would be hidden from the avatar's view if a rubbish bin occluded the view or if the tennis ball was behind the avatar. If the traffic light was present, the subjects were instructed to imagine the light radiating out from the traffic light towards the subject and to answer how many tennis balls the light would directly hit. Again, if a bin occluded the light or if the ball was behind the traffic light then the light would not directly hit the ball.
Only the response times for the VPT tasks are of interest in the current study. The VPT tasks were designed to keep errors low. However, anodal or cathodal stimulation had no impact on error rates. This excludes the possibility that there was a speed-accuracy trade-off underlying the effects on response time.
Visual perspective task—level one implicit. In the first test subjects were instructed to respond as fast and accurately as possible with ‘how many tennis balls can you see?’ The answer was always between one and four with the response buttons clearly marked on the keyboard. The task was considered an implicit test, as subjects were not directed to consider the perspective from the perspective of the avatar in the scene and were only required to answer from the egocentric perspective.
Visual perspective task—level one explicit. In the level one explicit task, participants were required to take either an egocentric perspective or the allocentric perspective from the avatar or light and answer how many tennis balls could be seen/light would directly shine on. There were four possible responses for each condition, with one to four tennis balls for the egocentric judgements allocentric congruent conditions. In order to maintain four choices for the allocentric incongruent condition, without increasing the number of balls in the scene, scenes with zero balls visible to the avatar/light were included. Therefore, answers in this condition were from zero to three.
Visual perspective task—level two explicit. In the level two explicit VPT task, participants were again required to take either an egocentric perspective or the allocentric perspective of the avatar or light. However, this task required making a judgement on ‘how’ the subject or other avatar views the scene, by asking them ‘whether they/other could see/light would shine on, more balls on the left, right, or equal number on each side of the road?’ All conditions had three possible responses.
Self-referential memory task . Following the VPT, participants performed a recognition memory task for the mental attribution words from the RMET. The correct mental attribution words as well as 76 distractor words (38 incorrect choices from the RMET & 38 novel words not previously seen) were presented and subjects answered whether they had seen the mental attribution in the RMET task completed earlier. Responses were; 1 = Definitely did, 2 = Probably did, 3 = Probably not, 4 = Definitely not. Scoring was from 2 for a correct confident response through to -2 for a confident response that was incorrect. Words were divided according to whether they had been encoded in relation to the ‘self’ or to the ‘other’ (Barack Obama) and mean confidence scores were calculated. Prior to testing, all subjects viewed a short (∼5 min) documentary on the career of Barack Obama. To encourage engagement with the task, subjects were told that their responses would be compared against data collected from people who had worked with Barack Obama.
Source memory task . If subjects responded that they had seen the mental attribution in the eyes, they were asked a subsequent question ‘Was it on a male or a female face?’ Responses were, 1= Definitely male, 2= Probably male, 3= Probably female, 4= Definitely female. Scoring was identical to the mental attribution memory task. This was considered a source memory task, as it was a measure of a contextual memory not directly encoded in relation to the self or other.
Baseline cognitive measures
All subjects completed a battery of neurocognitive tests. After the first session subjects completed the Mini-mental State Examination (MMSE), The Stroop Test, The National Adult Reading Test (NART), The Boston Naming Task, Verbal fluency—phonemic and semantic, and the Hospital Anxiety and Depression Scale (HADS). Following the second session, subjects completed the following tests from the CogState: international shopping list, identification test, one and two back tests, set-shifting test, continuous paired associate learning test, social–emotional cognition test and the international shopping list—delayed recall.
Statistical analyses
All analyses were computed using JASP version 0.7.5.6. Blinding was tested using Chi-square tests. The VAMS was first collapsed into positive and negative valence scales. To assess potential effects on mood ratings (VAMS), differences between pre and post assessments were calculated for positive and negative scales and compared between sham and active HD-tDCS sessions. The VAMS and Adverse Effects were compared between the sham and active HD-tDCS conditions using paired t-tests. For the social cognitive tasks, the impact of HD-tDCS on the accuracy of emotion and age/sex judgements were analysed using paired sample t-tests. Cohen’s d provides an estimate of the effect size. For the VPT measures, response time differences were analysed using repeated-measures analysis of variance (RM-ANOVA) with congruency (congruent & incongruent), agent (avatar & traffic light), perspective (egocentric & allocentric) and stimulation (sham & active) as within-subject factors. If significant, separate analyses were performed for egocentric and allocentric perspectives. All VPT tasks were designed to be easy, therefore accuracy was not analysed. For the SRE task, a RM-ANOVA with agent (self & other) and stimulation (sham & active) as within-subject factors was used to analyse differences in the confidence scores for episodic memories. Eta squared provides an estimate of effect sizes for all ANOVAs. All figures represent the means and standard error of the mean, calculated using the Cousineau method for within-subject designs (Cousineau, 2005). For the VPT, individual trials that were >3 s.d. away from the mean were considered outliers and removed. Subjects who failed to get over 50% of the trials correct in any condition or stimulation session were excluded from the analysis as it was assumed they had failed to understand the task. This resulted in the removal of two participants from the anodal VPT level one, one from the anodal VPT level two, four from both the cathodal VPT level one and level two.
Results
Baseline cognitive measures
40 healthy participants in the anodal and cathodal crossover studies were comparable with regard to demographic variables (anodal, 10 men/10 women, mean age ± SD yrs = 22.9 ± 4.4; cathodal, 10 men/10 women, mean age ± SD yrs = 24.0 ± 4.1, P = 0.42) and cognitive profiles (see Table S1).
Social cognitive tasks
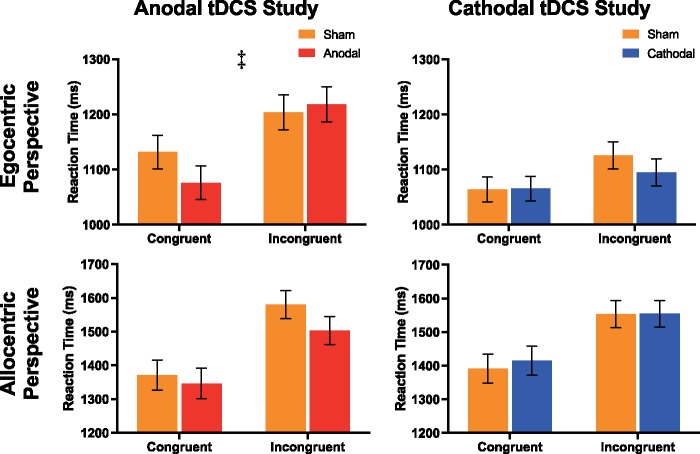
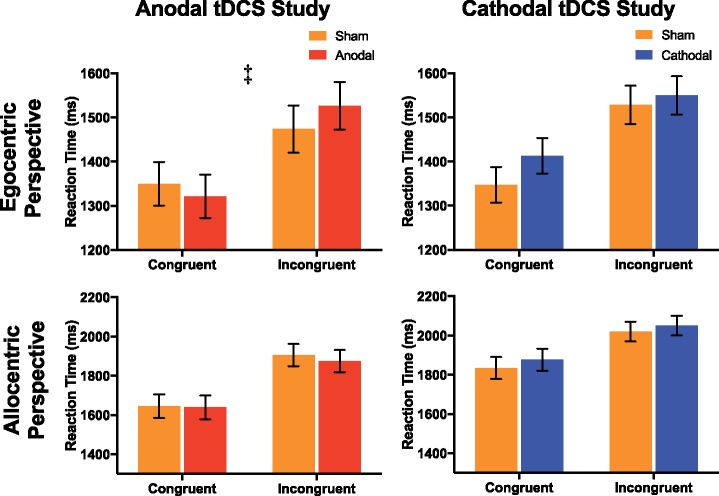
VPT: Anodal study. When required to make an egocentric judgement in both the level one and two explicit VPT tasks, subjects were slower when the allocentric perspective was incongruent with that of the egocentric perspective, M(sd) = 1211.1 (242.9) v 1103.7 (227.2) msecs, F(1, 17) = 29.23, P < 0.001, η2 = 0.63 and M(sd) = 1500.2 (260.7) v 1335.8 (217.3) msecs, F(1, 18)= 43.72, P < 0.001, η2 = 0.71, respectively. This demonstrates that when making an egocentric judgement, allocentric perspectives influence the response time of subjects. Similar to a previous study (Santiesteban et al., 2014), we failed to find a greater response time difference for scenes containing an avatar compared with a non-agential directional control (traffic light), for level one VPT, avatar v traffic light, mean difference (sd) = 62.3 (156.1) v 82.2 (115.7) msecs, F(1, 17) = 0.28, P = 0.60, η2 = 0.02, and level two VPT task, avatar v traffic light, mean difference (sd) = 80.5 (222.6) v 166.8 (168.4) msecs, F(1, 18) = 2.37, P = 0.14, η2 = 0.12; therefore we only tested stimulation effects for the allocentric perspective (i.e. both avatar and light combined). Likewise, when participants were required to take the allocentric perspective, subjects were slower when the egocentric perspective was incongruent for both level 1 VPT and level 2 VPT, M(sd) = 1545.1 (307.0) v 1359.1 (251.1), F(1, 17) = 57.59, P < 0.001, η2 = 0.77 and M(sd) = 1890.2 (375.8) v 1642.1 (305.3), F(1, 18) = 74.55, P < 0.001, η2 = 0.81.
A significant interaction was identified between stimulation, perspective and congruency, for both the level one VPT and level two VPT tasks, F(1, 17) = 4.65, P = 0.046, η2 = 0.22 and F(1, 18) = 4.82, P = 0.041, η2 = 0.21, respectively. Follow-up analysis demonstrated that anodal HD-tDCS to the dmPFC increased the integration of the allocentric and egocentric perspectives during egocentric judgements for level one VPT, F(1, 17) = 5.05, P = 0.038, η2 = 0.23 and level two VPT, F(1,18) = 4.49, P = 0.048, η2 = 0.20, as indexed by a greater difference between congruent and incongruent response times, sham v anodal-tDCS mean difference (sd) = 175.9 (223.7) v 287.6 (236.8), but not allocentric judgements, sham v anodal mean difference (sd) = 392.6 (203.9) v 351.5 (287.1), F(1, 17) = 0.41, P = 0.53, η2 = 0.02 and F(1, 18) = 0.35, P = 0.56, η2 = 0.02, (see Figures 3 and 4).
Fig. 3.
Reaction times for judgments based on self (egocentric) or other (allocentric) perspective in the level 1 VPT task in both the sham-controlled anodal-tDCS and sham-controlled cathodal-tDCS studies. Response times were slower in all conditions when the perspective was incongruent between self and other. A greater integration of the allocentric perspective was identified by a significant interaction between stimulation (sham & anodal-tDCS) and congruency (congruent & incongruent) during egocentric judgements only (‡ denotes significant interaction, P < 0.05).
Fig. 4.
Reaction times for judgments based on self (egocentric) or other (allocentric) perspective in the level 2 VPT task in both the sham-controlled anodal-tDCS and sham-controlled cathodal-tDCS studies. Response times were slower in all conditions when the perspective was incongruent between self and other. A greater integration of the allocentric perspective was identified by a significant interaction between stimulation (sham & anodal-tDCS) and congruency (congruent & incongruent) during egocentric judgments only (‡ denotes significant interaction, P < 0.05).
VPT: Cathodal study. In the cathodal study, in both the level one and level two VPT tasks, subjects were slower when the allocentric perspective was incongruent with that of the egocentric perspective, M(sd) = 1132.9 (224.9) v 1064.8 (206.0) msecs, F(1, 15) = 10.18, P = 0.006, η2 = 0.40 and F(1, 15) = 23.44, P < 0.001, η2 = 0.61, respectively. Likewise, when participants were required to take the allocentric perspective, subjects were slower when the egocentric perspective was incongruent for both level one VPT and level two VPT, M(sd) = 1553.6 (227.0) v 1403.1 (330.8) msecs, F(1, 15) = 12.47, P = 0.003, η2 = 0.45 and M(sd) = 2035.2 (377.6) v 1855.6 (462.4), F(1, 15) = 24.21, P < 0.001, η2 = 0.62, respectively.
No significant interaction was identified between stimulation, perspective and congruency, for the level one or two VPT tasks, F(1, 15) = 0.77, P = 0.4, η2 = 0.05 and F(1, 15) = 0.22, P = 0.65, η2 = 0.01. In the follow-up analysis for egocentric judgements only, cathodal HD-tDCS had no significant effect on the response time for scenes where the allocentric perspective was congruent or incongruent with that of the egocentric perspective for level one VPT, F(1, 17) = 0.14, P = 0.72, η2 = 0.01, and level two VPT, F(1, 17) = 2.02, P = 0.17, η2 = 0.11. Importantly, in the level two VPT task, cathodal HD-tDCS had an opposite, albeit weaker, effect compared with the anodal HD-tDCS study. This was highlighted by a significant interaction between the study setup (anodal and cathodal HD-tDCS) and the effect of active stimulation on the difference between response times for scenes congruent and incongruent with the egocentric and allocentric perspectives, with anodal increasing the difference and cathodal decreasing the difference during the egocentric judgements only, F(1, 36) = 4.92, P = 0.034, η2 = 0.13 (see Figures 3 and 4).
Implicit VPT. Prior to the explicit VPT tasks, subjects were initially instructed to only respond from the egocentric perspective. They were not made aware of the subsequent tasks and, therefore, had no reason to be salient of the congruency of the scene with the perspective of the avatar, hence an implicit VPT task. Previous studies have identified that subject’s response times are slower when the egocentric perspective is incongruent with the allocentric perspective, but only when an avatar is present and not a directional, non-agential figure such as a traffic light (Apperly and Butterfill, 2009; Samson et al., 2010). Replicating these results, we identified a significant difference in response times when the egocentric perspective was incongruent with the avatar M(sd) = 471.7(49.2) v 486.1(53.9) msec, but not the traffic light M(sd) = 485.8(55.6) v 474.2(56.9) msec, F(1, 19) = 38.1, P < 0.001, η2 = 0.67. If HD-tDCS over the dmPFC was able to influence the impact of this implicit, subconscious processing of other agents, we would show an increase in the response times between congruent and incongruent scenes only when the avatar was in the scene. However, anodal HD-tDCS had no impact on the implicit VPT effect avatar congruent v incongruent M(sd) = 483.4 (78.9) v 495.5 (74.3), traffic light congruent v incongruent = 500.9 (80.2) v 488.5 (73.1) , F(1, 19) = 0.04, P = 0.85, η2 = 0.00, or on congruency regardless of agent, F(1, 19) = 0.09, P = 0.77, η2 = 0.01. Similarly, cathodal HD-tDCS had no impact on implicit VPT compared with sham HD-tDCS, F(1, 19) = 2.26, P = 0.15, η2 = 0.11.
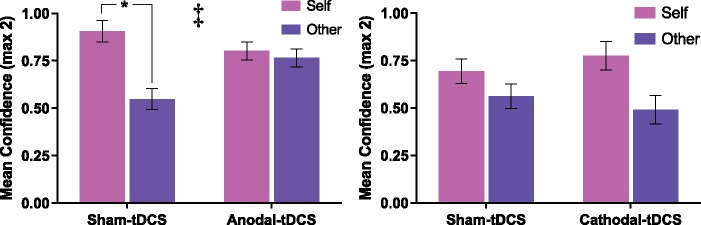
Self-referential memory task. Recognition for previously seen mental attributes (from the RMET) was measured using a four-point confidence score (1 = Definitely did, 2 = Probably did, 3 = Probably not, 4 = Definitely not). As predicted, a self-reference effect (SRE) in episodic memory was identified during the sham HD-tDCS condition, with greater confidence in the recognition of self-encoded memories in comparison to other-encoded memories, 0.90 (0.55) v 0.55 (0.75), t(19) = 3.18, P = 0.005, Cohen’s d = 0.77. Following anodal HD-tDCS this bias was removed, resulting in comparable recognition confidence for self and other encoded items, 0.80 (0.63) v 0.76 (0.62). This was demonstrated by a significant interaction between stimulation and agent, F(1, 19) = 4.73, P = 0.04, η2 = 0.20 (see Figure 5) and the removal of the SRE, t(19)=0.40, P = 0.70, Cohen’s d = 0.09. However, in the cathodal HD-tDCS study there was no effect of active stimulation, and a bias remained towards memories encoded in relation to the self, F(1, 19)=0.13, P = 0.72, η2 = 0.01. It is interesting to note that although not significant, the direction of effect moved in the opposite direction, with cathodal HD-tDCS resulting in a slightly greater bias towards self-encoded memories, sham: self 0.69 (0.59) other 0.56 (0.75); cathodal 0.78 (0.53) other 0.49 (0.64) (see Figure 5).
Fig. 5.
Mean confidence scores for items recognised in the memory test. A self-reference effect (SRE) was evident during sham stimulation (*P < 0.05). A significant interaction between stimulation (sham & anodal-tDCS) and encoding context (self & other) was identified, such that anodal tDCS removed the SRE in recognition confidence (‡ denotes significant interaction, P < 0.05). Cathodal-tDCS had no effect on the SRE in memory.
In the source memory condition, we assessed whether the effects were specific to items encoded in relation to the self or other (mental attributions) or whether other aspects of the memory were also affected (gender of the source). We demonstrate specificity to the mental attribution terms, as indicated by a lack of anodal HD-tDCS effects on confidence of gender scores sham: self 0.61 (0.36) other 0.65 (0.48); anodal: self 0.72 (0.39) other 0.72 (0.34); F(1, 19) = 0.08, P = 0.78, η2 = 0, nor for cathodal HD-tDCS sham: self 0.72 (0.48) other 0.38 (0.53); cathodal: self 0.58 (0.50) other 0.61 (0.60), F(1, 19) = 3.74, P = 0.07, η2 = 0.16.
RMET. As predicted there were no effects of anodal HD-tDCS on the accuracy of mental state attribution on the RMET (27.95 v 28.00), t(19) = -0.07, P = 0.95, Cohen’s d = -0.02, although a significant decrease in accuracy on the age and sex judgements was identified (33.45 v 32.3), t(19) = 2.44, P = 0.03, Cohen’s d = 0.55. Cathodal HD-tDCS had no impact on either the attribution of mental states (27.05 v 26.65), t(19) = 0.77, P = 0.45, Cohen’s d = 0.18, or the judgement of age and sex (32.45 v 31.55), t(19) = 1.02, P = 0.32, Cohen’s d = 0.23 (see Supplementary Figure S1).
Adverse effects and blinding
All participants tolerated the stimulation well with only minor physical sensations. Self-reported mild adverse effects and mood (Brunoni et al., 2011; Folstein and Luria, 1973) were comparable between the stimulation conditions (see Tables S2 and S3). Subjects guessed the stimulation order at chance level in both the anodal (number of correct guesses: 11/20, P = 0.65), and cathodal HD-tDCS studies (number of correct guesses: 7/20 P = 0.18). This demonstrates that our behavioural stimulation effects were not affected by those variables.
Discussion
The ability to integrate and also distinguish information pertaining to the self and other is fundamental in social cognition and thought to rely on the dmPFC. In the first study of its kind, we examined the impact of anodal and cathodal HD-tDCS over the dmPFC on a range of social cognitive tasks that pose different demands on self-other processing. Our results provide the first causal evidence that the dmPFC is involved in tasks requiring an explicit distinction between the self and other. Moreover, the results suggest that the dmPFC is specifically involved in higher-order social cognition involving the integration of information pertaining to others.
Integrating the perspective of the other into that of the self was apparent in both explicit VPT tasks. Specifically, anodal HD-tDCS increased the response time differences between congruent and incongruent scenes when the subject made a judgement from the egocentric perspective (self-perspective) in both level one and two explicit VPT tasks. Anodal HD-tDCS over the dmPFC also removed the SRE in memory. These distinct tasks both rely on weighting self and other information in order to judge a scene in the present or recall details from the past. Anodal HD-tDCS to the dmPFC integrated the other perspective into that of the self and removed the implicit bias for self-encoded items, suggesting a causal role in the integration of other oriented cognitive processes regardless of the cognitive domain. This blurring of the self-other divide has no impact on the ability to accurately attribute mental states from the eyes suggesting that other cognitive processes and underlying brain structures are involved (Schurz et al., 2014). The lack of any effect in the implicit VPT or on the source memory task, suggests that the dmPFC is only involved in explicit demands on self-other processing. Although cathodal HD-tDCS by itself had no significant effect on any of the tasks, overall we observed trends in the opposite direction. The weaker effects of cathodal stimulation are consistent with other cognitive tDCS studies and may reflect the contribution of compensatory systems buffering the impact of inhibitory stimulation to a specific region (Jacobson et al., 2012). Alternatively, reduced cognitive effects may be due to differences in the ability to affect neuronal activity, possibly due to the opposing polarisation of the soma and dendrites, tending to cancel out each other during cathodal stimulation (Lafon et al., 2016).
The dmPFC is a region associated with cognitive control, with consistent evidence of activation in tasks involving conflict detection or strategic control (Botvinick et al., 1999; Carter et al., 1998). Therefore, stimulating the dmPFC may exert greater cognitive control over a default implicit bias in favour of self-relevant information as observed in the SRE. The specific effects on the egocentric perspective judgments provide further evidence for dmPFC involvement in regulating self-processing. There is extensive functional and structural connectivity between the dmPFC and vmPFC (Price et al., 1996), a region associated with emotional, interoceptive and self processing (D'Argembeau, 2013; Northoff, 2012). As the dmPFC has also been implicated in higher-order processing such as metarepresentation and integration of information (Coricelli and Nagel, 2009; Hampton et al., 2008; Yoshida et al., 2010) stimulating the dmPFC may increase the integration of information that would otherwise be processed in parallel to those important for self-relevant processes (Nicolle et al., 2012). This embedding of information pertaining to the other could potentially explain how humans are able to represent the perspective of others and attribute observable behaviour to unobservable mental attributes.
In a previous study, anodal tDCS to the right TPJ increased the control of self and other representation using a VPT task and an inhibition of imitation task (Santiesteban et al., 2012). This suggests dissociation between the role of the TPJ and the dmPFC within the ‘social brain’. The results suggest that the TPJ is involved in controlling self and other representations and the dmPFC is involved in integrating information from the other into that of the self. Further studies directly comparing dmPFC and TPJ stimulation using the same tasks are required to test this dissociation. In regards to ToM, stimulating the left or right TPJ (Santiesteban et al., 2015) had no impact on the accuracy of attributing mental states and this was also the case with dmPFC stimulation. In the current study tDCS actually reduced performance on the lower level age and sex judgement component of the RMET. Although the dmPFC is associated with the RMET early in development, there is a significant reduction in activation by mid-adolescence (Overgaauw et al., 2015). This is likely due to the reduced demands on metarepresentational cognitive processes needed to integrate or distinguish other from self in the case of determining mental states from the eyes.
Biophysical models of current flow suggested that HD-compared with conventional tDCS set-ups results in more focal current flow (Bortoletto et al., 2016; Kuo et al., 2013) and impacts cognition in a regionally specific way (Gbadeyan et al., 2016a). However, aside from current modelling studies, little is known about the distribution and extent of HD-tDCS effects on brain physiology. Importantly, even more focal stimulation may result in modulation of functionally connected distant brain regions as suggested for conventional tDCS (Meinzer et al., 2012; Stagg et al., 2013). Similarly, the dmPFC refers to a large frontal region with both cortical and deeper midline components. The extent which HD-tDCS influences this region is currently unknown. As HD-tDCS is suitable for use simultaneously with fMRI (Gbadeyan et al., 2016c), future studies using this method will provide insight into the mechanisms underlying the cognitive effects demonstrated in the current study (Meinzer et al., 2013; Meinzer et al., 2014).
As well as furthering our understanding of the social brain and the contribution of the dmPFC to VPT and self-referential memory, the study has implications for other avenues of research. For example, HD-tDCS may provide a valuable technique for improving the outcome of cognitive training programs in clinical groups who experience specific social difficulties, such as schizophrenia and autism (Iacoboni, 2006; van der Weiden et al., 2015). It may also be applicable across the healthy lifespan (Perceval et al., 2016), with older adults having reduced social cognitive capacity coupled with alterations in dmPFC structure and function (Moran et al., 2012; Sowell et al., 2003). Simultaneous HD-tDCS and fMRI while completing tasks requiring self and other processing will allow for the direct analysis of the selective effects of HD-tDCS to the dmPFC and elaborate on the results of the current study, revealing a specific causal role for the dmPFC in integrating information from others into that of the self in human social cognition.
Supplementary data
Supplementary data are available at SCAN online.
Supplementary Material
Funding
This work was supported by a Future Fellowship grant [FT120100608] and a strategic seed-funding grant from the University of Queensland, awarded to Marcus Meinzer.
Conflict of interest. None declared.
Author contributions
A.K.M. & M.M. conceived the experiment. A.K.M., M.M. & I.D. designed the experiment. A.K.M. & S.R. collected the data. A.K.M. analysed the data. A.K.M. wrote the manuscript. I.D., S.R., & M.M. edited the manuscript.
References
- Adolphs R. (2009). The social brain: neural basis of social knowledge. Annual Review of Psychology, 60, 693–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M., Truong D.Q., Khadka N., Bikson M. (2016). Spatial and polarity precision of concentric high-definition transcranial direct current stimulation (HD-tDCS). Physics in Medicine and Biology, 61(12), 4506–21. [DOI] [PubMed] [Google Scholar]
- Amodio D.M., Frith C.D. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience, 7(4), 268–77. [DOI] [PubMed] [Google Scholar]
- Apperly I.A., Butterfill S.A. (2009). Do humans have two systems to track beliefs and belief-like states? Psychological Review, 116(4), 953–70. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Hill J., Raste Y., Plumb I. (2001). The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. Journal of Child Psychology and Psychiatry, 42(2), 241–51. [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. (2001). The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders, 31(1), 5–17. [DOI] [PubMed] [Google Scholar]
- Bortoletto M., Rodella C., Salvador R., Miranda P.C., Miniussi C. (2016). Reduced current spread by concentric electrodes in transcranial electrical stimulation (tES). Brain Stimulation, 9(4), 525–8. [DOI] [PubMed] [Google Scholar]
- Botvinick M., Nystrom L.E., Fissell K., Carter C.S., Cohen J.D. (1999). Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature, 402(6758), 179–81. [DOI] [PubMed] [Google Scholar]
- Brosch T., Schiller D., Mojdehbakhsh R., Uleman J.S., Phelps E.A. (2013). Neural mechanisms underlying the integration of situational information into attribution outcomes. Social Cognitive and Affective Neuroscience, 8(6), 640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni A.R., Amadera J., Berbel B., Volz M.S., Rizzerio B.G., Fregni F. (2011). A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. International Journal of Neuropsychopharmacology, 14(8), 1133–45. [DOI] [PubMed] [Google Scholar]
- Carter C.S., Braver T.S., Barch D.M., Botvinick M.M., Noll D., Cohen J.D. (1998). Anterior cingulate cortex, error detection, and the online monitoring of performance. Science, 280(5364), 747–9. [DOI] [PubMed] [Google Scholar]
- Cialdini R.B., Brown S.L., Lewis B.P., Luce C., Neuberg S.L. (1997). Reinterpreting the empathy-altruism relationship: when one into one equals oneness. Journal of Personality and Social Psychology, 73(3), 481–94. [PubMed] [Google Scholar]
- Coricelli G., Nagel R. (2009). Neural correlates of depth of strategic reasoning in medial prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 106(23), 9163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousineau D. (2005). Confidence intervals in within-subject designs: a simpler solution to Loftus and Masson’s method. Tutorials for Quantitative Methods for Psychology, 1(1), 42–5. [Google Scholar]
- D'Argembeau A. (2013). On the role of the ventromedial prefrontal cortex in self-processing: the valuation hypothesis. Frontiers in Human Neuroscience, 7, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J., Sommerville J.A. (2003). Shared representations between self and other: a social cognitive neuroscience view. Trends in Cognitive Sciences, 7(12), 527–33. [DOI] [PubMed] [Google Scholar]
- Denny B.T., Kober H., Wager T.D., Ochsner K.N. (2012). A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience, 24(8), 1742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari C., Lega C., Vernice M., et al. (2016). The dorsomedial prefrontal cortex plays a causal role in integrating social impressions from faces and verbal descriptions. Cerebral Cortex, 26(1), 156–65. [DOI] [PubMed] [Google Scholar]
- Flavell J.H. (1977). The development of knowledge about visual perception. Nebraska Symposium on Motivation, 25, 43–76. [PubMed] [Google Scholar]
- Folstein M.F., Luria R. (1973). Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychological Medicine, 3(4), 479–86. [DOI] [PubMed] [Google Scholar]
- Frith C.D., Frith U. (2008). Implicit and explicit processes in social cognition. Neuron, 60(3), 503–10. [DOI] [PubMed] [Google Scholar]
- Gbadeyan O., McMahon K., Steinhauser M., Meinzer M. (2016a). Stimulation of dorsolateral prefrontal cortex enhances adaptive cognitive control: a high-definition transcranial direct current stimulation study. Journal of Neuroscience, 36(50), 12530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbadeyan O., McMahon K., Steinhauser M., Meinzer M. (2016b). Stimulation of dorsolateral prefrontal cortex enhances adaptive cognitive control: a high-definition transcranial direct current stimulation study. Journal of Neuroscience, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbadeyan O., Steinhauser M., McMahon K., Meinzer M. (2016c). Safety, tolerability, blinding efficacy and behavioural effects of a novel MRI-compatible, high-definition tDCS set-up. Brain Stimulation, 9(4), 545–52. [DOI] [PubMed] [Google Scholar]
- Gzesh S.M., Surber C.F. (1985). Visual perspective-taking skills in children. Child Development, 56(5), 1204–13. [PubMed] [Google Scholar]
- Hamilton A.F., Brindley R., Frith U. (2009). Visual perspective taking impairment in children with autistic spectrum disorder. Cognition, 113(1), 37–44. [DOI] [PubMed] [Google Scholar]
- Hampton A.N., Bossaerts P., O'Doherty J.P. (2008). Neural correlates of mentalizing-related computations during strategic interactions in humans. Proceedings of the National Academy of Sciences of the United States of America, 105(18), 6741–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogeveen J., Grafman J., Aboseria M., David A., Bikson M., Hauner K.K. (2016). Effects of high-definition and conventional tDCS on response inhibition. Brain Stimulation, 9(5), 720–9 doi:10.1016/j.brs.2016.04.015. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. (2006). Failure to deactivate in autism: the co-constitution of self and other. Trends in Cognitive Sciences, 10(10), 431–3. [DOI] [PubMed] [Google Scholar]
- Jacobson L., Koslowsky M., Lavidor M. (2012). tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Experimental Brain Research, 216(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Kuo H.I., Bikson M., Datta A., et al. (2013). Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: a neurophysiological study. Brain Stimulation, 6(4), 644–8. [DOI] [PubMed] [Google Scholar]
- Lafon B., Rahman A., Biksom M., Parra L.C. (2016). Direct current stimulation alters neuronal input/output. Brain Stimulation, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M., Antonenko D., Lindenberg R., et al. (2012). Electrical brain stimulation improves cognitive performance by modulating functional connectivity and task-specific activation. Journal of Neuroscience, 32(5), 1859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M., Lindenberg R., Antonenko D., Flaisch T., Floel A. (2013). Anodal transcranial direct current stimulation temporarily reverses age-associated cognitive decline and functional brain activity changes. Journal of Neuroscience, 33(30), 12470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M., Lindenberg R., Darkow R., Ulm L., Copland D., Floel A. (2014). Transcranial direct current stimulation and simultaneous functional magnetic resonance imaging. Journal of Visualized Experiments 86, e51730. doi:10.3791/51730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll H., Tomasello M. (2004). 12- and 18-month-old infants follow gaze to spaces behind barriers. Developmental Science, 7(1), F1–9. [DOI] [PubMed] [Google Scholar]
- Moran J.M., Jolly E., Mitchell J.P. (2012). Social-cognitive deficits in normal aging. Journal of Neuroscience, 32(16), 5553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle A., Klein-Flugge M.C., Hunt L.T., Vlaev I., Dolan R.J., Behrens T.E. (2012). An agent independent axis for executed and modeled choice in medial prefrontal cortex. Neuron, 75(6), 1114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G. (2012). From emotions to consciousness – a neuro-phenomenal and neuro-relational approach. Frontiers in Psychology, 3, 303.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B.F., Brewer R., Bird G., Catmur C. (2016). Theory of mind is not theory of emotion: a cautionary note on the Reading the Mind in the Eyes Test. Journal of Abnormal Psychology, 125(6), 818–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaauw S., van Duijvenvoorde A.C., Gunther Moor B., Crone E.A. (2015). A longitudinal analysis of neural regions involved in reading the mind in the eyes. Social Cognitive and Affective Neuroscience, 10(5), 619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perceval G., Floel A., Meinzer M. (2016). Can transcranial direct current stimulation counteract age-associated functional impairment? Neuroscience & Biobehavioral Reviews, 65, 157–72. [DOI] [PubMed] [Google Scholar]
- Price J.L., Carmichael S.T., Drevets W.C. (1996). Networks related to the orbital and medial prefrontal cortex; a substrate for emotional behavior? Progress in Brain Research, 107, 523–36. [DOI] [PubMed] [Google Scholar]
- Rilling J.K., Sanfey A.G., Aronson J.A., Nystrom L.E., Cohen J.D. (2004). The neural correlates of theory of mind within interpersonal interactions. NeuroImage, 22(4), 1694–703. [DOI] [PubMed] [Google Scholar]
- Samson D., Apperly I.A., Braithwaite J.J., Andrews B.J., Bodley Scott S.E. (2010). Seeing it their way: evidence for rapid and involuntary computation of what other people see. Journal of Experimental Psychology:Human Perception & Performance, 36(5), 1255–66. [DOI] [PubMed] [Google Scholar]
- Santiesteban I., Banissy M.J., Catmur C., Bird G. (2012). Enhancing social ability by stimulating right temporoparietal junction. Current Biology, 22(23), 2274–7. [DOI] [PubMed] [Google Scholar]
- Santiesteban I., Banissy M.J., Catmur C., Bird G. (2015). Functional lateralization of temporoparietal junction – imitation inhibition, visual perspective-taking and theory of mind. European Journal of Neuroscience, 42(5), 2527–33. doi:10.1111/ejn.13036 [DOI] [PubMed] [Google Scholar]
- Santiesteban I., Catmur C., Hopkins S.C., Bird G., Heyes C. (2014). Avatars and arrows: implicit mentalizing or domain-general processing? Journal of Experimental Psychology: Human Perception & Performance, 40(3), 929–37. [DOI] [PubMed] [Google Scholar]
- Schurz M., Kronbichler M., Weissengruber S., Surtees A., Samson D., Perner J. (2015). Clarifying the role of theory of mind areas during visual perspective taking: issues of spontaneity and domain-specificity. NeuroImage, 117, 386–96. [DOI] [PubMed] [Google Scholar]
- Schurz M., Radua J., Aichhorn M., Richlan F., Perner J. (2014). Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neuroscience & Biobehavioral Reviews, 42, 9–34. [DOI] [PubMed] [Google Scholar]
- Sellaro R., Nitsche M.A., Colzato L.S. (2016). The stimulated social brain: effects of transcranial direct current stimulation on social cognition. Annals of the New York Academy of Sciences, 1369(1), 218–39. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Peterson B.S., Thompson P.M., Welcome S.E., Henkenius A.L., Toga A.W. (2003). Mapping cortical change across the human life span. Nature Neuroscience, 6(3), 309–15. [DOI] [PubMed] [Google Scholar]
- Stagg C.J., Lin R.L., Mezue M., et al. (2013). Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex. Journal of Neuroscience, 33(28), 11425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons C.S., Johnson B.T. (1997). The self-reference effect in memory: a meta-analysis. Psychological Bulletin, 121(3), 371–94. [DOI] [PubMed] [Google Scholar]
- Tversky B., Hard B.M. (2009). Embodied and disembodied cognition: spatial perspective-taking. Cognition, 110(1), 124–9. [DOI] [PubMed] [Google Scholar]
- van der Weiden A., Prikken M., van Haren N.E. (2015). Self-other integration and distinction in schizophrenia: a theoretical analysis and a review of the evidence. Neuroscience & Biobehavioral Reviews, 57, 220–37. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F., Vandekerckhove M. (2013). Implicit and explicit social mentalizing: dual processes driven by a shared neural network. Frontiers in Human Neuroscience, 7, 560.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz K.G., Kessler T., von Cramon D.Y. (2009). In-group as part of the self: in-group favoritism is mediated by medial prefrontal cortex activation. Society for Neuroscience, 4(3), 244–60. [DOI] [PubMed] [Google Scholar]
- Wittmann M.K., Kolling N., Faber N.S., Scholl J., Nelissen N., Rushworth M.F. (2016). Self-other mergence in the frontal cortex during cooperation and competition. Neuron, 91(2), 482–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida W., Seymour B., Friston K.J., Dolan R.J. (2010). Neural mechanisms of belief inference during cooperative games. Journal of Neuroscience, 30(32), 10744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond A.S., Snaith R.P. (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67(6), 361–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.