Abstract
Nutrients in breeding sites are critical for the survival and development of malaria mosquitoes, having a direct impact on vectorial capacity. Yet, there is a limited understanding about the natural larval diet and its impact on the individual fitness of mosquitoes. Recent studies have shown that gravid Anopheles arabiensis Patton (Diptera: Culicidae) are attracted by and oviposit in grass-associated habitats. The pollen provided by these grasses is a potential source of nutrients for the larvae. Here, we assess the effect of Typha latifolia L. (Poales: Typhaceae), Echinochloa pyramidalis Lamarck, Pennisetum setaceum Forsskål, and Zea mays L. pollen on larval survival and rate of development in An. arabiensis under laboratory conditions. In addition, we characterize the carbon to nitrogen ratio and the size of pollen grains as a measure of diet quality. Carbon-rich pollen with a small grain size (T. latifolia and P. setaceum; 9.7 ± 0.3 × 103 and 5.5 ± 0.2 × 104 µm3, respectively) resulted in enhanced rates of development of An. arabiensis. In contrast, the larva fed on the nitrogen-rich control diet (TetraMin) was slower to develop, but demonstrated the highest larval survival. Larvae fed on carbon-rich and large-grained Z. mays pollen (4.1 ± 0.2 × 105 µm3) survived at similar levels as those fed on the control diet and also took a longer time to develop compared with larvae fed on the other pollens. While males and females did not appear to develop differently on the different pollen diets, males consistently emerged faster than their female counterparts. These results are discussed in relation to integrated vector management.
Keywords: Carbon to nitrogen ratio, pollen grain, nutrient, mosquito, malaria
Nutrients in breeding habitats are essential for the survival and development of mosquitoes (Araújo et al., 2012, Takken et al. 2013, Moller-Jacobs et al. 2014, Shapiro et al. 2016). Nutrition during larval development determines the size and metabolic reserves of adult mosquitoes upon emergence (Merritt et al. 1992; Ye-Ebiyo et al. 2000, 2003a; Kivuyo et al. 2014; Moller-Jacobs et al. 2014). In addition, and in relation to the body size, the number of eggs produced by female mosquitoes is governed by the size of the preceding blood meal (Lyimo and Takken 1993, Ameneshewa and Service 1996, Roitberg and Gordon 2005). Consequently, the paucity of knowledge concerning the natural diet of malaria mosquito larvae affects efforts to understand the dynamics of vector populations and thus to effectively plan management programs in sub-Saharan Africa (Merritt et al. 1992, Kebede et al. 2005, Garros et al. 2008).
The most productive natural larval habitat types for Anopheles arabiensis Patton, a primary vector of malaria in sub-Saharan Africa, are transient puddles (Ndenga et al. 2011), often found amidst wild and domesticated grasses (Bøgh et al. 2003; Ye-Ebiyo et al. 2003a; Minakawa et al. 2004, 2005; Mwangangi et al. 2007, 2010; Fillinger et al. 2009; Gouagna et al. 2012). Grasses are potential sources of mosquito larval nutrients, derived directly from shed pollen (Ye-Ebiyo et al. 2000, 2003a) and indirectly from associated microorganisms and accumulated detritus (Merritt et al. 1992, Garros et al. 2008, Chukalo and Abato 2017). The importance of grasses on the nutritional ecology of malaria mosquitoes is reflected in that gravid An. arabiensis are attracted by and discriminate among grass-associated habitats (Wondwosen et al. 2016, 2017; Wondwosen et al. submitted; Asmare et al. 2017), in which they lay their eggs and thereby provide their offspring with access to these resources. The pollen from maize enhances larval development, vector density, and mosquito longevity (Ye-Ebiyo et al. 2000, 2003a). Although feeding on maize pollen is a recent adaptation for An. arabiensis (Ye-Ebiyo et al. 2000, 2003a), their larvae readily ingest whole pollen grains, suggesting that this behavior has evolved from feeding on other pollens, which occur in breeding sites.
Particle size is a major factor influencing mosquito larval diet. Mosquito larvae are limited in the particulate matter that they ingest, with the maximum size reported being 50 µm, and most studies reporting that smaller particles are more highly represented (Merritt et al. 1992). Morphology and physiology associated with the mouthparts of the larvae limit the size of the diet particle and regulate their feeding behaviors to optimize ingestion and nutrient assimilation rates (Merritt 1987, Merritt et al. 1992). One of the important nutrient requirements of an insect for its survival and development is the carbon: nitrogen ratio of a diet (Raubenheimer and Simpson 1995, 1999; Patt et al. 2003; Mayntz et al. 2005; Raubenheimer et al. 2009). While proteins have been found to be essential for growth in mosquito larval diets, carbohydrates have not, although carbohydrates greatly increase larval growth rate within an optimal range (Sneller and Dadd 1981, Timmerman and Briegel 1999).
The carbohydrate and protein content of pollen have been the focus of many studies, predominantly in the context of plant-animal interactions (Roulston et al. 2000). Pollen is a major food source for many flower visiting insects, such as honey bees and pollen beetles (Cook et al. 2004, Keller et al. 2005). The variable nature of the protein content of pollen among plants (15–50% of the dry mass) influences plant-insect interactions, with a tendency for insect-pollinated plants to produce pollen richer in protein content (Roulston et al. 2000). The protein content of grass pollens investigated, including Cyperaceae, Typha, and Poaceae, ranges from 17 to 25% of the dry mass (Roulston et al. 2000). Whether the protein content of Zea mays pollen is a key driver of the observed enhanced development and survival of An. arabiensis larvae (Ye-Ebiyo et al. 2000, 2003a), or if carbohydrate content influences these processes, is yet unknown.
In this study, the association between grasses, particularly their pollen, and the malaria vector An. arabiensis is further characterized in order to assess the impact of pollen quality on larval development and survival. The nutrient composition of grass pollen, specifically the carbohydrate to protein ratio, and pollen grain size, in Typha latifolia, Echinochloa pyramidalis, Pennisetum setaceum, and Z. mays differentially affected larval performance. The potential evolutionary impact of the observed association is discussed.
Materials and Methods
Pollen Collection
Pollen was collected from broadleaf cattail, T. latifolia L. (Typhaceae), and Antelope grass, E. pyramidalis Lam. (Poaceae), at the edge of the lakeshore of Lake Tana, Ethiopia (11°37′N, 37°21′E), from crimson fountain grass, P. setaceum Forssk. (Poaceae), in the adjacent inland area of the lake, and from maize, Z. mays L. (BH660 cultivar; Poaceae), in the farm land area of Merawi (11°30′N, 37°15′E). The rationale for selecting the pollen from T. latifolia and E. pyramidalis was that these grasses previously have been shown to be associated with An. arabiensis breeding sites and differentially support the production of larvae under natural conditions (Asmare et al. 2017). While P. setaceum has not previously been shown to be associated with breeding sites, it is a common grass species within the landscape containing prolific breeding sites and is wind pollinated. The pollens were collected by covering mature flowers with a paper bag and then agitating. The estimated weight of pollen released by individual inflorescences of T. latifolia, E. pyramidalis, P. setaceum, and Z. mays was ca. 0.100 mg, 0.020 mg, 0.025 mg, and 1.500 mg, respectively. The collected pollen was sterilized within 24 h of collection using UV light overnight for ca. 12 h and then stored on silica gel until used for further experiments. In total, approximately 10 g of pollen grains were collected from each species.
Pollen Nutrient Analysis
A combustion CHNS/O analyzer (Flash 2000, Thermo Fisher Scientific, Waltham, MA) was used to determine the carbohydrate and protein content of the grass pollen, according to the manufacturer’s protocol. Approximately 5 mg of sample pollen from each grass species was enclosed in tin capsules (8 mm × 5 mm). The capsule was inserted into the combustion chamber using the autosampler at a temperature of 1,050ºC. In the presence of a catalyst and in a high oxygen environment, the temperature was elevated to 1,800°C, and all carbon and nitrogen in the samples were oxidized forming carbon dioxide and NOx. The gasses were carried by helium through a reduction column where NOx was reduced to N2. The gasses were then transported to a gas chromatograph column (CHNS separation column, Thermo Fisher Scientific), where N2 and CO2 were separated and then detected by a thermal conductivity detector. The signal was transferred from the detector via an A/D converter to a computer and analyzed (Eager Exerience, version 1.2, Thermo Fisher Scientific). The nitrogen values were converted to crude protein by a multiplier of 6.25 (Roulston et al. 2000). Thereafter, the C:N ratio of the grass pollen was determined by %C/%N × 6.25. The C:N ratios were log transformed to meet the assumption for normal distribution, prior to analysis using a univariate general linear model (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.). Significant differences between C:N ratios were determined at α = 0.05, and post hoc comparisons among the grass pollens were made using Tukey’s honest significant difference test.
Pollen Grain Size Analysis
The pollen grain sizes were measured according to Roulston et al. (2000). Pollen grains were mounted in paraffin oil and measured at 400× with an ocular micrometer. Polar axis measurements were taken for all pollen grains. Volumes were calculated using volume equations for spheres (4/3πp3). Ten randomly selected grains per slide were measured, and these values were averaged for calculating volumes. Significant differences between pollen grain sizes were determined on log transformed volumes at α = 0.05 and using post hoc pairwise comparisons with Tukey’s HSD test.
Mosquito Rearing
An. arabiensis were maintained at 27 ± 2°C, 80% relative humidity, and at a 12 h:12 h light:dark photoperiod in the insectary of the Ethiopian Public Health Institute (EPHI), Addis Ababa. Larvae were reared in plastic trays (22 cm × 34 cm × 10 cm) filled with 1 liter distilled water and fed powdered Phoenix Koy Pellets (Armitage Pet Products Ltd., Nottingham, United Kingdom) daily. Pupae were removed from the rearing trays and placed in insect cages (30 cm × 30 cm × 30 cm) for adult emergence. Adult males and females were kept together and provided ad libitum access to 10% sucrose solution. For colony maintenance, female mosquitoes were blood fed on a live rabbit at 3- to 4-d intervals. Ethical approval for feeding on live rabbits was obtained from the EPHI Research Ethical Review Committee (FMST 2014, EPHI 2017). Eggs were laid in 30-ml plastic cups filled with distilled water and then transferred to larval trays for hatching. The third instar larval stages of the An. arabiensis mosquitoes were used at the start of all experimental bioassays. To ensure that all larvae were, indeed, third instars, the larvae were carefully monitored from emergence from the egg and fed consistently to regulate variation in size.
Larval Development and Survival Analyses
Development and survival of individual An. arabiensis larvae in 150 ml plastic cups (Dongyang City Plastics Co., Shanghai, China) filled with 50 ml of distilled water were investigated using artificial and pollen-containing larval diets. To determine the baseline for comparison across pollen and pollen-supplemented diets, the ED50 denoting the effective dose for 50% survival to adulthood on the artificial diet, TetraMin fish food (Tetrawerke, Melle, Germany), was established using 0.50, 0.75, 1.00, and 1.50 mg of the TetraMin alone. The ED50 (0.75 mg) was then used as the dose for each pollen diet, as well as for the TetraMin control. A second control, a double dose (1.50 mg) of the TetraMin fish food, was also included in the pollen-supplemented diet experiments to ensure one diet with 100% survival to adulthood. Two sets of experiments were done to determine the dietary effect of the grass pollen alone and the supplementation of the ED50 of a standard larval diet with pollen on survival and development of larval An. arabiensis. For all experiments, 10 replicates were performed on three separate occasions. Treatment and control cups were paired, to buffer for any day effect. The number of larvae surviving, pupating, and emerging as adults were recorded each day until all larvae had either died or emerged. Of the adults, the number of male and female adults that emerged were also recorded.
Data Analyses of Larval Development and Survival
A fully parametric form of survival analysis was used to model the data and to produce a measure of the probable rate of larval mortality, pupation, and adult emergence in third instar An. arabiensis larvae fed on treatment and control diets (JMP Pro 12.0.1. SAS Institute Inc., Cary, NC). The model was based on the Weibull distribution. For both pollen alone diets and supplemented diets, an overall model of the effects of both sex and diet on larval development was performed for pupation and adult emergence; censoring those that died before the end of the experiment. Similarly, an overall model of the effect of diets on larval survival was also performed; censoring those that emerged before the end of the experiment. The effect of sex on larval survival was not assessed. Wald’s test was used to determine significant differences between diets based on χ2 and P-values. A priori pairwise analyses of the probability models, using nonlinear variable slope curve fits (Hill slope, top and bottom of the curve) based on Akaike information criterion (AICc) and percent probabilities, were conducted among the pollen diets and the TetraMin controls (0.75 mg and 1.50 mg, respectively; Prism, version 5a, GraphPad Software, Inc, La Jolla, CA).
Results
Pollen Size and Nutrient Content
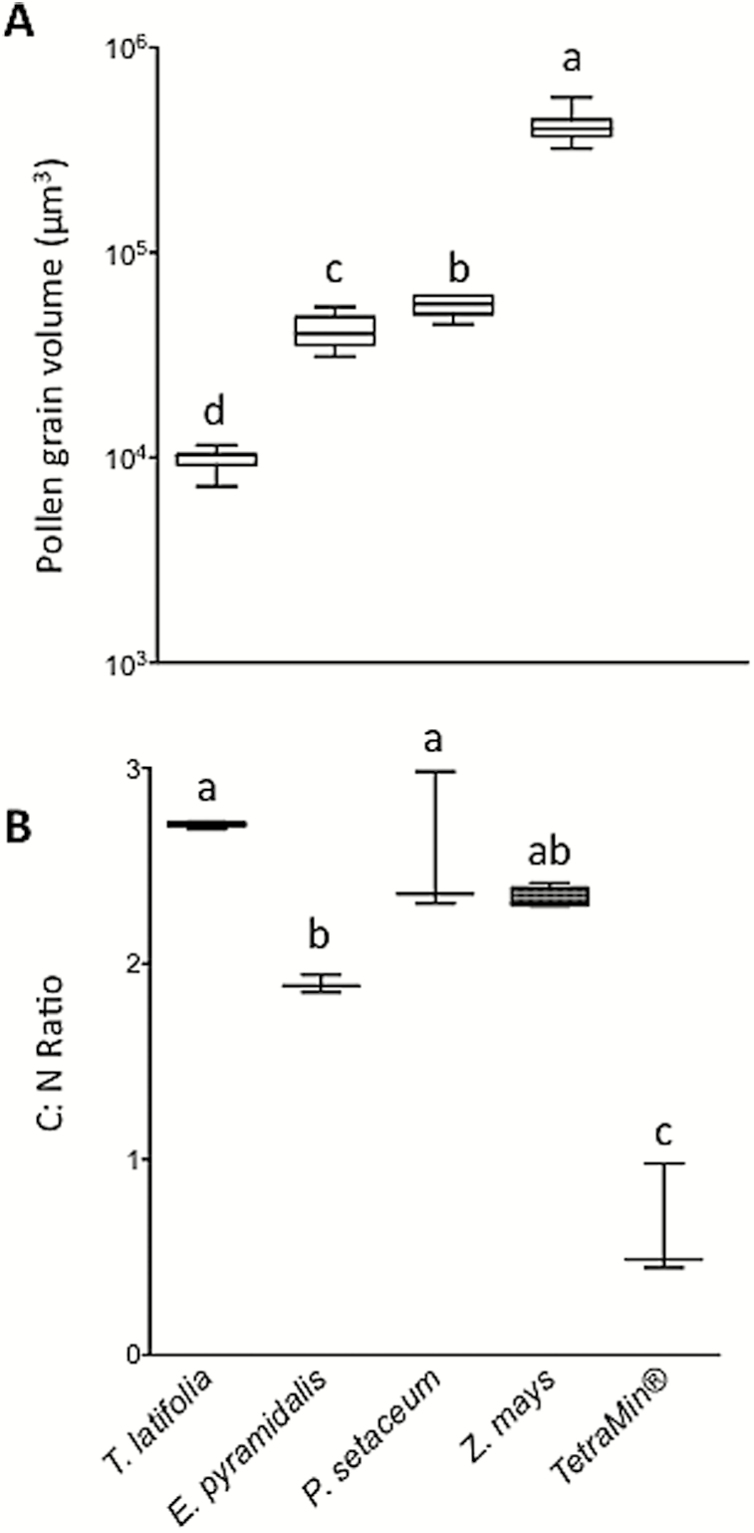
All pollen grains were spherical in shape, with smooth surfaces. The pollen grain volume differed significantly between grass species (Fig. 1A), with Z. mays (mean diameter 92.2 ± 1.65 µm) > P. setaceum (47.1 ± 0.57 µm) > E. pyramidalis (42.8 ± 0.84 µm) > T. latifolia (26.5 ± 0.34 µm) (F = 1033, df = 3, P < 0.0001). The C:N ratios of pollen differed between grass species with the pollen C:N ratios of T. latifolia > Z. mays = P. setaceum > E. pyramidalis (Fig. 1B). T. latifolia had a higher proportion of carbon (52%), compared with the three other species (44%).
Fig. 1.
Size (A) and carbon to nitrogen (C:N) ratios (B) of Typha latifolia, Echinochloa pyramidalis, Pennisetum setaceum, and Zea mays pollen are described in box plots (whiskers ± maximum and minimum values). The C:N ratio of the TetraMin control is also shown. Means with different letter designations are significantly different from one another (analysis of variance with Tukey’s HSD post hoc analysis; P < 0.05).
Larval Survival
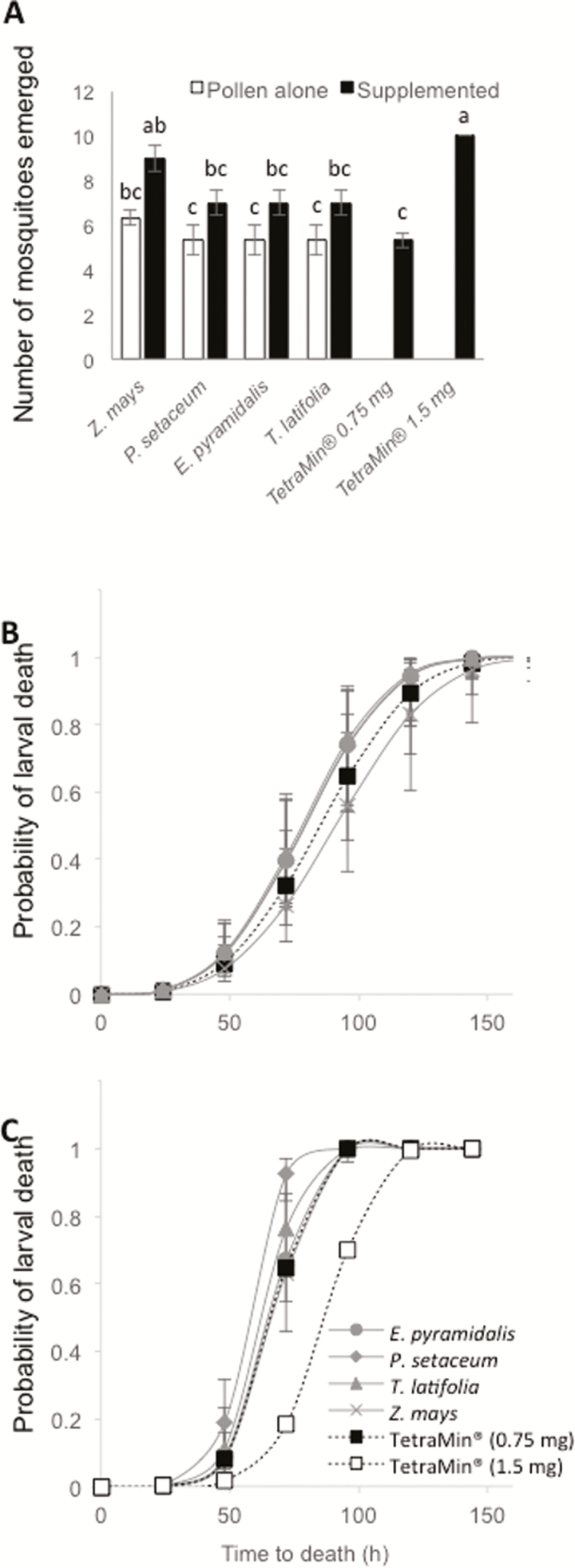
The overall larval survival differed significantly among the diets tested (analysis of variance F = 9.235, df = 9, P < 0.0001). Post-hoc pairwise comparisons revealed that larval survival on the pollen alone diets was lower than that on the 2× ED50 TetraMin diet (Fig. 2A). Larval survival on the pollen-supplemented diets was also lower than the 2× ED50 TetraMin diet, with one exception. The Z. mays pollen-supplemented diet did not differ from either the 2× ED50 TetraMin diet or the other pollen-supplemented diets, but the larval survival was significantly higher than on the other pollen alone diets (Fig. 2A). Interestingly, both Z. mays pollen diets did not differ from each other (Fig. 2A).
Fig. 2.
The survival to adulthood of Anopheles arabiensis, presented as number of mosquitoes emerged (A), and the probable life expectancy of the larvae in response to Echinochloa pyramidalis, Pennisetum setaceum, Typha latifolia, and Zea mays pollen alone (B), and pollen-supplemented TetraMin fish food (C) diets. Control diets include the effective dose for 50% survival (ED50) and 2× ED50 TetraMin fish food (0.75 mg and 1.5 mg, respectively). The error bars represent the standard error of the mean (A) and whiskers represent the upper and lower limits of variation (B and C). Means with different letter designations are significantly different from one another (analysis of variance with Tukey’s HSD post hoc analysis; P < 0.05) (A).
There was no overall effect of diet on the probability of larval survival over time in An. arabiensis when fed on any of the treatments (1.50 mg) (Wald’s χ2 = 2.5808, df = 4, P = 0.6302; Fig. 2B). However, in subsequent pairwise comparisons among each pollen diet and the TetraMin control, T. latifolia pollen was shown to significantly decrease the probability of survival (AICc = 2.935, P = 81.26%), while the probability of survival on the other pollen diets did not differ from the control. Z. mays pollen was shown to significantly increase the probability of survival compared to each of the other grass pollens (E. pyramidalis, AICc = 16.66, P = 99.98%; P. setaceum, AICc = 16.01, P = 99.97%; T. latifolia, AICc = 18.89, P = 99.99%). Moreover, the probability of larvae dying when the basic diet, TetraMin (0.75 mg), was supplemented with pollen (0.75 mg), was found to be significantly different from the overall model (χ2 = 19.14, df = 4, P = 0.0007; Fig. 2C). All of the larvae fed on the 2× ED50 TetraMin diet survived to adulthood, i.e., larvae had a longer probable life expectancy when feeding on 2× ED50 TetraMin diet than on grass pollen-supplemented diets (Fig. 2C; E. pyramidalis, AICc = 30.28, P > 99.99%; P. setaceum, AICc = 46.80, P > 99.99%; T. latifolia, AICc = 38.17, P > 99.99%; Z. mays, AICc = 26.77, P > 99.99%). Only larvae fed on P. setaceum supplemented diets were found to have a shorter probable life expectancy than those fed on the 0.75 mg TetraMin diet (AICc = 22.20, P > 99.99%).
Effect of Pollen on Pupation
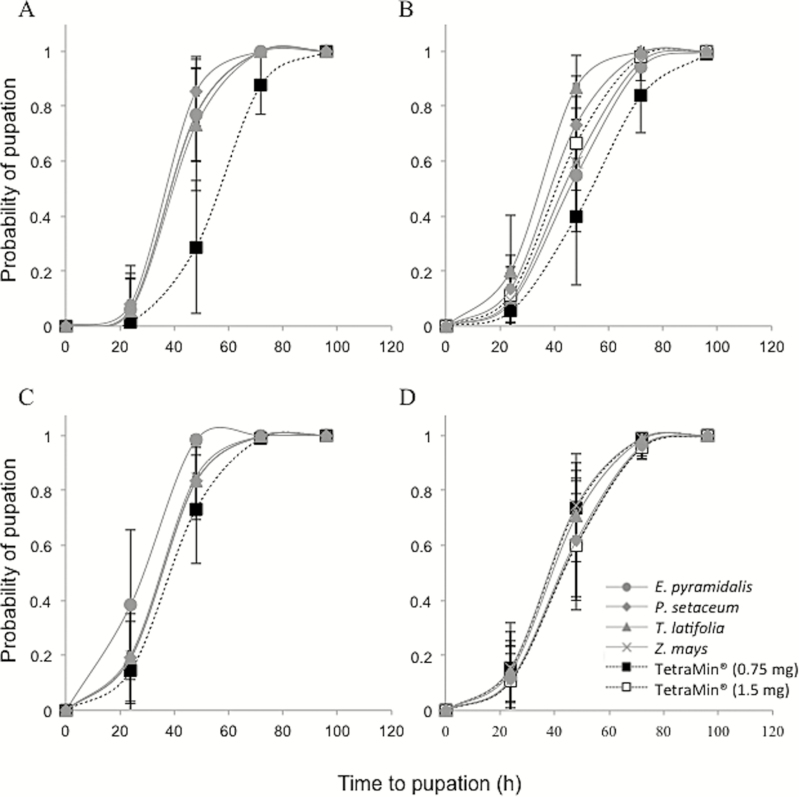
The overall model analysis of the effect of grass pollen diets on developmental time from third instar to pupation in An. arabiensis showed significant differences between the diets (χ2 = 90.31, df = 14, P < 0.0001), with both food (χ2 = 12.92, df = 4, P = 0.0117) and sex (χ2 = 69.41, df = 2, P < 0.0001) contributing to the significant deviation from the overall model. As there was no interaction between food and sex (χ2 = 4.723, df = 9, P = 0.7969), each of these factors were analyzed separately. This analysis showed that female larvae fed on pollen had a higher probability of a reduced developmental time between third instar and pupation compared to when they were fed on TetraMin alone (χ2 = 15.21, df = 4, P = 0.0043; Fig. 3A); most third instar female larvae (70–80%) fed on pollen generally pupated by 48 h after the start of the experiment whereas only ca. 30% of the third instar female larvae fed on TetraMin were likely to have pupated within this time frame. Moreover, larvae fed on each of the pollens were found to take less time from third instar to pupation compared to those fed on the 0.75 mg TetraMin diet (E. pyramidalis, AICc = 84.08, P > 99.99%; P. setaceum, AICc = 81.53, P > 99.99%; T. latifolia, AICc = 69.33, P > 99.99%; Z. mays, AICc = 77.28, P > 99.99%). In contrast, the males maintained a consistent developmental time from third instar to pupation in response to all diets (χ2 = 5.225, df = 4, P = 0.2650; Fig. 3B).
Fig. 3.
The probability of pupation of female (A and B) and male (C and D) mosquitoes over time in response to Echinochloa pyramidalis, Pennisetum setaceum, Typha latifolia, and Zea mays pollen alone (A and C) and pollen-supplemented TetraMin fish food (B and D) diets. Control diets include the effective dose for 50% survival (ED50) and 2× ED50 TetraMin fish food (0.75 mg and 1.5 mg, respectively). The whiskers represent the upper and lower limits of variation.
When pollen was supplemented on top of the basic TetraMin diet, the overall model analysis of the effect of combination diets on the development time from third instar to pupation in the mosquito showed significant differences between diets (χ2 = 89.71, df = 16, P < 0.0001), however there was no significant deviation from the overall model by either food (χ2 = 6.138, df = 5, P = 0.2930) or sex (χ2 = 4.199, df = 2, P = 0.1225). No effect of the interaction between food and sex was observed (χ2 = 11.43, df = 9, P = 0.2473). Thus, for combination diets, both females (χ2 = 9.056, df = 4, P = 0.1068) and males (χ2 = 1.980, df = 4, P = 0.8520) maintained a consistent developmental time from third instar to pupation in response to all diets.
Effect of Grass Pollen in the Larval Diet on Adult Emergence
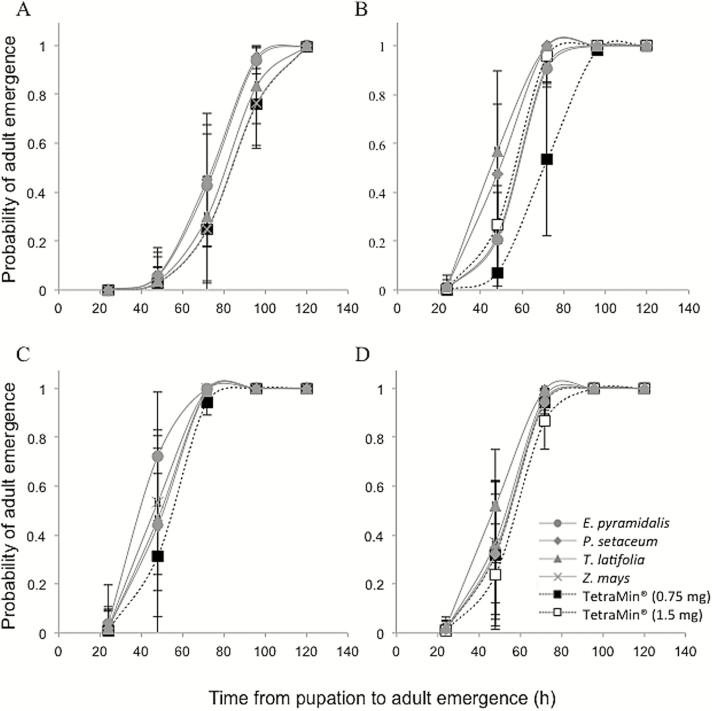
The overall model analysis of the effect of grass pollen diets on development time from pupation to emergence in An. arabiensis revealed significant differences between the diets (χ2 = 24.34, df = 14, P = 0.0417) with sex contributing significantly to the deviation from the overall model (χ2 = 15.90, df = 2, P = 0.0004), whereas there was no effect of food (χ2 = 2.53, df =4, P = 0.6396). As there was no interaction between food and sex (χ2 = 4.367, df = 8, P = 0.8226), each of these factors were analyzed separately. This analysis showed that both female (χ2 = 4.224, df = 4, P = 0.3765) and male (χ2 = 2.870, df = 4, P = 0.5798) mosquitoes maintained a consistent developmental time from pupation to emergence in response to all diets (Fig. 4). Subsequent pairwise analyses revealed that there were no differences in the probability of time to emergence among the pollen diets within each sex (P > 0.05). This indicates that the males were emerging as adult sooner than the females, regardless of the diet ingested.
Fig. 4.
The probability of adult emergence of female (A and B) and male (C and D) mosquitoes over time in response to Echinochloa pyramidalis, Pennisetum setaceum, Typha latifolia, and Zea mays pollen alone (A and C) and pollen-supplemented TetraMin fish food (B and D) diets. Control diets include the effective dose for 50% survival (ED50) and 2× ED50 TetraMin fish food (0.75 mg and 1.5 mg, respectively). The whiskers represent the upper and lower limits of variation.
Similarly, the overall model analysis of the effect of pollen-supplemented diets on the development time from pupation to emergence in the mosquito revealed significant differences between diets (χ2 = 24.08, df = 14, P = 0.0449) with sex contributing significantly to the deviation from the overall model (χ2 = 8.462, df = 2, P = 0.0145), whereas there was no effect of diet (χ2 = 5.921, df = 4, P = 0.2051). There was no interaction found between food and sex for pollen-supplemented diets (χ2 = 9.022, df = 8, P = 0.3404). When analyzed separately, the probability of adult emergence in females fed on pollen-supplemented diets differed significantly when compared to the overall model (χ2 = 15.16, df = 5, P = 0.0097), whereas that of the males did not (χ2 = 3.993, df = 5, P = 0.5504). Pairwise comparisons among the grass pollens indicated that T. latifolia pollen supplemented with TetraMin emerged as adult females faster than E. pyramidalis (AICc = 0.2315, P = 52.89%) and Z. mays (AICc = 0.03921, P = 50.49%).
Discussion
Pollen is a nutrient rich food source, enhancing growth, development, and survival for larvae from many insect orders (Cook et al. 2004, Keller et al. 2005). Here, we show that grass pollen in the diet of An. arabiensis larvae significantly affected larval survival, as well as the time to pupation and adult emergence in females. In line with Ye-Ebiyo et al. (2000, 2003a) and Kivuyo et al. (2014), Z. mays pollen significantly increased larval survival, both alone and in combination with an equal weight of TetraMin fish food (2× ED50), compared to the other diets tested. The other grass pollens were able to maintain a similar rate of survival among the larvae, when provided alone, with the P. setaceum diet demonstrating the shortest time to larval death. Interestingly, time to pupation decreased in females, but not in males, that fed on pollen alone when compared to the TetraMin diet; an effect that was not observed when the pollen was supplemented with TetraMin. Time to adult emergence in females was differentially affected by feeding on the different pollens, with mosquitoes feeding on Z. mays and E. pyramidalis taking a significantly longer time to emerge compared with those feeding on T. latifolia. This is in line with previous studies, in which it is demonstrated that male anophelines develop faster and emerge sooner than females (Lehmann et al. 2006, Mwangangi et al. 2006).
Previous studies indicate that the amount of carbon in the diet of mosquito larvae does not influence the overall survival but does increase the growth rate (Sneller and Dadd 1981, Grieco et al. 2007). In support of this, the larvae fed on the pollen of all the wild grass species had similar ultimate survival, however T. latifolia and P. setaceum, those containing the higher relative amounts of carbon, demonstrated shorter times to pupation and adult emergence and in the case of P. setaceum, death. In line with other studies in mosquitoes (Golberg and De Meillon 1948; Sneller and Dadd 1977, 1981; Kivuyo et al. 2014), larvae fed on the diet containing higher amounts of nitrogen, in our case TetraMin fish food (Timmerman and Briegel 1999, Dennis et al. 2010), demonstrated the highest larval survival but were slower to develop to pupae and to emerge as adults. Thus, these findings indicate that the balance between carbon and nitrogen in the diet plays a significant role in the development and survival of An. arabiensis larvae, above and beyond the contribution of each nutrient alone, which is in line with that which has been reported in other insects (Raubenheimer and Simpson 1997, 1998, 1999; Raubenheimer et al. 2009). However, other pollen characteristics, such as size, may also play a role in regulating development and survival. Z. mays has a carbon to nitrogen ratio that is similar to T. latifolia and P. setaceum, however, the demonstrated growth rate of larvae fed on this diet was prolonged. This is likely due to the larger size of the Z. mays pollen grains, which may reduce the rate of access to the nutrients contained within (Pucat 1965, Merritt et al. 1978). However, while Z. mays demonstrates a C:N ratio and a pollen size that appear to be that of a lower quality diet (Merritt et al. 1992, Young et al. 2014), the combination of these factors act synergistically, enhancing the development and survival of the malaria mosquito larvae.
Breeding habitats close to maize plantations, where the common food sources, such as detritus and microorganisms, are supplemented with shed pollen, have been shown to increase the survival and development of larval An. arabiensis, resulting in increased larval abundance (Ye-Ebiyo et al. 2000, 2003a). In addition, the presence of Z. mays pollen in the larval diet enhances the size of pupae and emerged adults, as well as prolonging adult survival (Ye-Ebiyo et al. 2000, 2003a). This prolific nutrient source provides an abundant source of nitrogen in an otherwise nitrogen resource poor environment, as the C:N ratios of microorganisms and detritus range from 6 to 9 (Redfield 1963, Hamilton et al. 1992, Baird and Middleton 2004).
The association between An. arabiensis and maize may have evolved out of the fitness benefits gained from feeding on grass pollen. This association is reinforced by the female Anopheles gambiae sensu lato preference to select and oviposit in grass habitats (Wondwosen et al. 2016, 2017; Asmare et al. 2017); in fact, the odors emanating from grass pollen alone are sufficient to attract and stimulate females to oviposit (Wondwosen et al. 2017, Wondwosen et al. submitted). We argue that the preadaptation to be stimulated by (Ye-Ebiyo et al. 2003b) and feed on grass pollen by An. gambiae sensu lato has been instrumental in establishing and strengthening the association of malaria mosquitoes with grass crops. Intensified cultivation of these crops has helped to create suitable conditions for increased vector populations, which are linked to greater malaria transmission (Kebede et al. 2005).
Conclusions
Continued investigation into the natural diet of malaria mosquito larvae will enhance our ability to construct effective Integrated Vector Management programs. For example, investigations into genetically modified grasses, such as Bacillus thuringiensis maize, expressing the delta-endotoxin, Cry, of the bacterium B. thuringiensis in the pollen grains could identify a new tool for use in mosquito larval control. Further characterizing the sources of the natural larval diet will help us to identify the likely and most prolific larval habitats and to engage in larval control through, e.g., the physical disturbance of such sites on a regular basis, which is already being used to good effect in many communities throughout Ethiopia. In addition, being able to correlate the larval food source with what insecticides the larvae have ingested will inform our decisions concerning which insecticides to use and the development of resistance in the adults. Moreover, future identification of pollen-associated phagostimulants may have applications to increase ingestion of larvicides (Schorkorpf et al. 2016). Thus, an understanding of malaria mosquito larval diet will aid us to further intervene and suppress malaria transmission and to plan and implement improved vector management programs in sub-Saharan Africa.
References Cited
- Ameneshewa B., and Service M. W.. 1996. The relationship between female body size and survival rate of the malaria vector Anopheles arabiensis in Ethiopia. Med. Vet. Entomol. 10: 170–172. [DOI] [PubMed] [Google Scholar]
- Araújo M. da-S., Gil L. H. S., and e Silva A. A.. 2012. Larval food quantity affects development time, survival and adult biological traits that influence the vectorial capacity of Anopheles darlingi under laboratory conditions. Malar. J. 11: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmare Y., Hill S. R., Hopkins R. J., Tekie H., and Ignell R.. 2017. The role of grass volatiles on oviposition site selection by Anopheles arabiensis and Anopheles coluzzii. Malar. J. 16: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird M. E., and Middleton J. H.. 2004. On relating physical limits to the carbon: nitrogen ratio of unicellular algae and benthic plants. J. Marine Syst. 49: 169–175. [Google Scholar]
- Bøgh C., Clarke S. E., Jawara M., Thomas C. J., and Lindsay S. W.. 2003. Localized breeding of the Anopheles gambiae complex (Diptera: Culicidae) along the River Gambia, West Africa. Bull. Entomol. Res. 93: 279–287. [DOI] [PubMed] [Google Scholar]
- Chukalo E., and Abate D.. 2017. Bacterial populations of mosquito breeding habitats in relation to maize pollen in Asendabo, south western Ethiopia. African J Microbiol Res. 11: 55–64. [Google Scholar]
- Cook S. M., Murray D. A., and Williams I. H.. 2004. Do pollen beetles need pollen? The effect of pollen on oviposition, survival, and development of a flower feeding herbivore. Ecol. Entomol. 29: 164–173. [Google Scholar]
- Dennis C. A., MacNeil M. A., Rosati J. Y., Pitcher T. E., and Fisk A. T.. 2010. Diet discrimination factors are inversely related to δ15N and δ13C values of food for fish under controlled conditions. Rapid Commun. Mass Sp. 24: 3515–3520. [DOI] [PubMed] [Google Scholar]
- EPHI (Ethiopian Public Health Institute). . 2017. Anopheles mosquito rearing and insectary handling guideline, Addis Ababa, Ethiopia. [Google Scholar]
- Fillinger U., Sombroek H., Majambere S., van Loon E., Takken W., and Lindsay S. W.. 2009. Identifying the most productive breeding sites for malaria mosquitoes in the Gambia. Malar. J. 8: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FMST (Federal Ministry of Science and Technology) 2014. National research ethics review guideline, FMST, Fifth Edition, Addis Ababa, Ethiopia. [Google Scholar]
- Garros C., Ngugi N., Githeko A. E., Tuno N., and Yan G.. 2008. Gut content identification of larvae of the Anopheles gambiae complex in western Kenya using a barcoding approach. Mol. Ecol. Res. 8: 512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golberg L., and De Meillon B.. 1948. The nutrition of the larva of Aëdes aegypti Linneus. 4. Protein and amino acid requirements. Biochem. J. 43: 379–387. [PMC free article] [PubMed] [Google Scholar]
- Gouagna L. C., Rakotondranary M., Boyer S., Lempérière G., Dehecq J. S., and Fontenille D.. 2012. Abiotic and biotic factors associated with the presence of Anopheles arabiensis immatures and their abundance in naturally occurring and man-made aquatic habitats. Parasit Vectors. 5: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco J. P., Rejmánková E., Achee N. L., Klein C. N., Andre R., and Roberts D.. 2007. Habitat suitability for three species of Anopheles mosquitoes: Larval growth and survival in reciprocal placement experiments. J Vector Ecol. 32: 176–187. [DOI] [PubMed] [Google Scholar]
- Hamilton S. K., Lewis W. M., and Sippel S. J.. 1992. Energy sources for aquatic animals in the Orinoco River flood plain: evidence from stable isotopes. Oecol. 89: 324–330. [DOI] [PubMed] [Google Scholar]
- Kebede A., McCann J. C., Kiszewski A. E., and Ye-Ebiyo Y.. 2005. New evidence of the effects of agro-ecologic change on malaria transmission. Am. J. Trop. Med. Hyg. 73: 676–680. [PubMed] [Google Scholar]
- Keller I., Fluri P., and Imdorf A.. 2005. Pollen nutrition and colony development in honey bees: part 1. Bee World. 86: 3–10. [Google Scholar]
- Kivuyo H. S., Mbazi P. H., Kisika D. S., Munga S., Rumisha S. F., Urasa F. M., and Kweka E. J.. 2014. Performance of five food regimes on Anopheles gambiae senso stricto larval rearing to adult emergence in insectary. PLoS One. 9: e110671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann T., Dalton R., Kim E. H., Dahl E., Diabate A., Dabire R., and Dujardin J. P.. 2006. Genetic contribution to variation in larval development time, adult size, and longevity of starved adults of Anopheles gambiae. Infect. Genet. Evol. 6: 410–416. [DOI] [PubMed] [Google Scholar]
- Lyimo E. O., and Takken W.. 1993. Effects of adult body size on fecundity and the pre-gravid rate of Anopheles gambiae females in Tanzania. Med. Vet. Entomol. 7: 328–332. [DOI] [PubMed] [Google Scholar]
- Mayntz D., Raubenheimer D., Salomon M., Toft S., and Simpson S. J.. 2005. Nutrient-specific foraging in invertebrate predators. Science. 307: 111–113. [DOI] [PubMed] [Google Scholar]
- Merritt R. W. 1987. Do different instars of Aedes triseriatus feed on particles of the same size? J. Am. Mosq. Control Assoc. 3: 94–96. [PubMed] [Google Scholar]
- Merritt R. W., Mortland M. M., Gersabeck E. R., and Ross D. H.. 1978. X-ray diffraction analysis of particles ingested by filter-feeding animals. Entomol. Exp. Appl. 24: 27–34. [Google Scholar]
- Merritt R. W., Dadd R. H., and Walker E. D.. 1992. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu. Rev. Entomol. 37: 349–376. [DOI] [PubMed] [Google Scholar]
- Minakawa N., Sonye G., Mogi M., and Yan G.. 2004. Habitat characteristics of Anopheles gambiae ss larvae in a Kenyan highland. Med. Vet. Entomol. 18: 301–305. [DOI] [PubMed] [Google Scholar]
- Minakawa N., Munga S., Atieli F., Mushinzimana E., Zhou G., Githeko A.K., and Yan G.. 2005. Spatial distribution of anopheline larval habitats in Western Kenyan highlands: effects of land cover types and topography. Am. J. Trop. Med. Hyg. 73: 157–165. [PubMed] [Google Scholar]
- Moller-Jacobs L., Murdock C. C., and Thomas M. B.. 2014. Capacity of mosquitoes to transmit malaria depends on larval environment. Parasit. Vectors. 7: 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwangangi J. M., Muturi E. J., Shililu J., Muriu S. M., Jacob B., Kabiru E. W., Mbogo C. M., Githure J., and Novak R.. 2006. Survival of immature Anopheles arabiensis (Diptera: Culicidae) in aquatic habitats in Mwea rice irrigation scheme, central Kenya. Malar. J. 5: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwangangi J. M., Mbogo C. M., Muturi E. J., Nzovu J. G., Githure J. I., Yan G., Minakawa N., Novak R., and Beier J. C.. 2007. Spatial distribution and habitat characterisation of Anopheles larvae along the Kenyan coast. J. Vector Dis. 44: 44. [PMC free article] [PubMed] [Google Scholar]
- Mwangangi J. M., Shililu J., Muturi E. J., Muriu S., Jacob B., Kabiru E. W., Mbogo C. M., Githure J., and Novak R. J.. 2010. Anopheles larval abundance and diversity in three rice agro-village complexes, Mwea irrigation scheme, central Kenya. Malar. J. 9: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndenga B. A., Simbauni J. A., Mbugi J. P., Githeko A. K., and Fillinger U.. 2011. Productivity of malaria vectors from different habitat types in the western Kenya highlands. PLoS One. 6: e19473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patt J. M., Wainright S. C., Hamilton G. C., Whittinghill D., Bosley K., Dietrick J., and Lashomb J. H.. 2003. Assimilation of carbon and nitrogen from pollen and nectar by a predaceous larva and its effects on growth and development. Ecol. Entomol. 28: 717–728. [Google Scholar]
- Pucat A. M. 1965. The functional morphology of the mouth parts of some mosquito larvae. Quaest. Entomol. 1: 41–86. [Google Scholar]
- Raubenheimer D., and Simpson S. J.. 1995. Constructing nutrient budgets. Entomol. Exp. Appl. 77: 99–104. [Google Scholar]
- Raubenheimer D., and Simpson S. J.. 1997. Integrative models of nutrient balancing: application to insects and vertebrates. Nutr. Res. Rev. 10: 151–179. [DOI] [PubMed] [Google Scholar]
- Raubenheimer D., and Simpson S. J.. 1998. Nutrient transfer functions: the site of integration between feeding behavior and nutritional physiology. Chemoecology. 8: 61–68. [Google Scholar]
- Raubenheimer D., and Simpson S. J.. 1999. Integrating nutrition: a geometrical approach. Entomol. Exp. Appl. 91: 67–82. [Google Scholar]
- Raubenheimer D., Simpson S. J., and Mayntz D.. 2009. Nutrition, ecology and nutritional ecology: toward an integrated framework. Funct. Ecol. 1: 4–16. [Google Scholar]
- Redfield A. C. 1963. The influence of organisms on the composition of sea water. The sea. 2: 26–77. [Google Scholar]
- Roitberg B. D., and Gordon I.. 2005. Does the Anopheles blood meal - fecundity curve, curve? J. Vector Ecol. 30: 83–86. [PubMed] [Google Scholar]
- Roulston T. H., Cane J. H., and Buchmann S. L.. 2000. What governs protein content of pollen: pollinator preferences, pollen pistil interactions, or phylogeny? Ecol. Monogr. 70: 617–643. [Google Scholar]
- Schorkorpf D. L. P., Spanoudis C. G., Mboera L. E. G., Mafra-Neto A., Ignell R., and Dekker T.. 2016. Combining attractants and larvicides in biodegradable matrices for sustainable mosquito vector control. PloS Neg. Trop. Dis. 10: e0005043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L. L., Murdock C. C., Jacobs G. R., Thomas R. J., and Thomas M. B.. 2016. Larval food quantity affects the capacity of adult mosquitoes to transmit human malaria. Proc. R. Soc. B. 283: 20160298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneller V. P. and Dadd R. H.. 1977. Requirement for sugar in a chemically defined diet for larval Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 14: 387–392. [DOI] [PubMed] [Google Scholar]
- Sneller V. P., and Dadd R. H.. 1981. Interaction of amino acids and glucose on growth of Aedes aegypti (Diptera: Culicidae) in a synthetic rearing medium. J. Med. Entomol. 18: 235–239. [Google Scholar]
- Takken W., Smallegange R. C., Vigneau A. J., Johnston V., Brown M., Mordue-Luntz A. J., and Billingsley P. F.. 2013. Larval nutrition differentially affects adult fitness and Plasmodium development in the malaria vectors Anopheles gambiae and Anopheles stephensi. Parasit. Vectors. 6: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman S. E., and Briegel H.. 1999. Larval growth and biosynthesis of reserves in mosquitoes. J. Insect. Physiol. 45: 461–470. [DOI] [PubMed] [Google Scholar]
- Wondwosen B., Birgersson G., Seyoum E., Tekie H., Torto B., Fillinger U., Hill S. R., and Ignell R.. 2016. Rice volatiles lure gravid malaria mosquitoes, Anopheles arabiensis. Sci. Rep. 6: 37930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wondwosen B., Hill S. R., Birgersson G., Seyoum E., Tekie H., and Ignell R.. 2017. A (maize)ing attraction: gravid Anopheles arabiensis are attracted and oviposit in response to maize pollen odours. Malar. J. 16: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye-Ebiyo Y., Pollack R. J., and Spielman A.. 2000. Enhanced development in nature of larval Anopheles arabiensis mosquitoes feeding on maize pollen. Am. J. Trop. Med. Hyg. 63: 90–93. [DOI] [PubMed] [Google Scholar]
- Ye-Ebiyo Y., Pollack R. J., Kiszewski A., and Spielman A.. 2003a. Enhancement of development of larval Anopheles arabiensis by proximity to flowering maize (Zea mays) in turbid water and when crowded. Am. J. Trop. Med. Hyg. 68: 748–752. [PubMed] [Google Scholar]
- Ye-Ebiyo Y., Pollack R. J., Kiszewski A., and Spielman A.. 2003b. A component of maize pollen that stimulates larval mosquitoes (Diptera: Culicidae) to feed and increases toxicity of microbial larvicides. J. Med. Entomol. 40: 860–864. [DOI] [PubMed] [Google Scholar]
- Young G. B., Golladay S., Covich A., and Blackmore M.. 2014. Stable isotope analysis of larval mosquito diets in agricultural wetlands in the coastal plain of Georgia, USA. J. Vector Ecol. 39: 288–297. [DOI] [PubMed] [Google Scholar]