Abstract
According to theories of emotion dynamics, emotions unfold across two phases in which different types of processes come to the fore: emotion onset and emotion offset. Differences in onset-bound processes are reflected by the degree of explosiveness or steepness of the response at onset, and differences in offset-bound processes by the degree of accumulation or intensification of the subsequent response. Whether onset- and offset-bound processes have distinctive neural correlates and, hence, whether the neural basis of emotions varies over time, still remains unknown. In the present fMRI study, we address this question using a recently developed paradigm that allows to disentangle explosiveness and accumulation. Thirty-one participants were exposed to neutral and negative social feedback, and asked to reflect on its contents. Emotional intensity while reading and thinking about the feedback was measured with an intensity profile tracking approach. Using non-negative matrix factorization, the resulting profile data were decomposed in explosiveness and accumulation components, which were subsequently entered as continuous regressors of the BOLD response. It was found that the neural basis of emotion intensity shifts as emotions unfold over time with emotion explosiveness and accumulation having distinctive neural correlates.
Keywords: affective neuroscience, emotion dynamics, fMRI, intensity profiles
Introduction
Emotions change over time. Consequently, developing an understanding of their dynamic nature is a prerequisite for reaching a thorough account of them (Frijda, 2007; Verduyn et al., 2012b). Research on the temporal unfolding of emotions is also important from an applied perspective as disturbances in emotion dynamics are key features of various mental health disorders, including depression, post-traumatic stress disorder, and borderline personality disorder (American Psychiatric Association, 2013).
However, for a long time, the inherent dynamic nature of emotions was ignored (Frijda, 2007). It was only in the nineties of the previous century that research on the way emotions unfold over time started to blossom (Frijda et al., 1991; Sonnemans and Frijda, 1994; Verduyn et al., 2015). Since then, researchers mainly used self-report methodologies to chart patterns of emotion unfolding, and more recently, neuroimaging tools to identify the neural regions or networks that underlie these dynamics.
Research on the temporal unfolding of emotions over time
Charting patterns of emotion unfolding
To chart changes in emotional experience over time, researchers typically either used an experience sampling or intensity profile tracking approach. In experience sampling, participants are asked to report on their emotional experience several times a day (Houben et al., 2015; Kuppens and Verduyn, 2015). This allows researchers to express patterns of emotion unfolding in dynamic features such as inertia (Suls et al., 1998; Kuppens et al., 2010), variability (Eid and Diener, 1999), or instability (Woyshville et al., 1999). A major advantage of experience sampling is that it allows to measure dynamics in emotional experience while minimizing memory biases. A disadvantage, however, is that it only provides a series of discrete measurements and, hence, this method does not allow to capture the continuous pattern of emotion unfolding.
A technique that does not suffer from this disadvantage is the intensity profile tracking approach, originally developed by Frijda and colleagues (Frijda et al., 1991; Sonnemans and Frijda, 1994). This validated self-report method (Hutcherson et al., 2005; Raz et al., 2012) consists of asking participants to draw curves reflecting continuous changes in the intensity of emotional experience over time. Using this approach, Verduyn et al. (2009) found evidence that the two most prominent sources of differences between intensity profiles are their degrees of explosiveness (i.e. profiles having a steep vs a gentle start) and accumulation (i.e. profiles increasing over time vs going back to baseline). These findings are consistent with theories on emotion dynamics claiming that emotions unfold across two stages: an onset stage (associated with explosiveness) and an offset stage (associated with accumulation) (Davidson, 1998; Koole, 2009; Verduyn et al., 2012a; Brans and Verduyn, 2014; Kuppens and Verduyn, 2015).
Multiple mechanisms have been proposed to underlie the onset and offset of emotional episodes. Onset-bound emotion processes may include event appraisals (Grandjean and Scherer, 2008; Moors, 2009), attentional biases (Waugh et al., 2015), emotion habituation (Tong et al., 2009), and reactive flexibility (Hollenstein, 2015). Offset-bound emotion processes may include emotion regulation (Koole, 2009), resilience (Schaefer et al., 2013), and sustained attention (Wadlinger and Isaacowitz, 2011).
Similar mechanisms likely underlie emotion explosiveness and accumulation as differences in onset- and offset-bound processes are reflected at the surface level by differences in explosiveness and accumulation, respectively. However, empirical research on the determinants of emotion explosiveness and accumulation is scarce (for a notable exception, see Verduyn et al., 2012a).
Identifying the neural basis of emotion dynamics
Early studies typically made use of EEG to examine the neural dynamics that occur during the milliseconds following exposure to an emotion-inducing stimulus (Schupp et al., 2000; Grandjean and Scherer, 2008; Weinberg and Hajcak, 2010). However, it is only recently that neuroimaging tools have been used to identify the neural mechanisms that underlie emotion dynamics across longer timescales. These studies are crucial to understand the neural basis of emotion unfolding as emotions typically last longer than a few (milli-)seconds (Waugh and Schirillo, 2012; Verduyn et al., 2015; Waugh et al., 2015).
Three approaches have been used in these studies on the neural basis of emotion unfolding. First, properties of the dynamics of the BOLD response following an emotion induction have been examined. These studies found evidence for several regions underlying emotion unfolding, including the medial prefrontal cortex (Lindquist et al., 2007; Haas et al., 2008; Wager et al., 2009), amygdala (Siegle et al., 2002; Walter et al., 2009; Davis et al., 2010; Lau et al., 2012; Mandell et al., 2014; Schuyler et al., 2014), insula (Waugh et al., 2008), striatum, and dorsolateral prefrontal cortex (Heller et al., 2013). However, these studies did not examine whether dynamics in neural activity were related to the time dynamics of the intensity of emotional experience.
Second, a number of studies have examined the relation between static (i.e. time-independent) measures of the intensity of emotional experience and dynamic features of the BOLD response following an emotion induction. Variability in intensity of emotional experience was found to be associated with activity in several regions, including the medial prefrontal cortex (Phan et al., 2003; Waugh et al., 2010, 2014), amygdala (Phan et al., 2003; Waugh et al., 2016), insula (Waugh et al., 2010, 2014), ventral striatum (Heller et al., 2015), nucleus accumbens (Heller et al., 2009), posterior cingulate cortex (Waugh et al., 2010), lateral prefrontal cortex (Waugh et al., 2014), thalamus, and midbrain (Waugh et al., 2016). However, as intensity was assessed in a static way these studies do not directly speak to neural activity underlying changes in emotional experience over time.
Third, a limited number of neuroimaging studies assessed the time dynamics of the intensity of emotional experience using an intensity profile tracking approach and examined the neural correlates of these changes. Evidence was found for the involvement of large neural networks underlying changes in emotional experience over time, with these networks including the medial prefrontal cortex, amygdala, and insula amongst others (Goldin et al., 2005; Hutcherson et al., 2005; Raz et al., 2014, 2016a,b).
In sum, recent studies increased our understanding of the neural basis of emotion dynamics. In each of the three approaches, the medial prefrontal cortex (mPFC), amygdala, and insula were identified as key regions involved in emotion unfolding. In particular, it has been argued that the medial prefrontal cortex remains active during the emotional episode to continuously assess and monitor the meaning of the stimulus for the self (Fossati et al., 2003; Northoff et al., 2006; Lindquist et al., 2012; Waugh and Schirillo, 2012). Similarly, the amygdala and insula remain active during the emotional episode, reflecting the monitoring of stimulus relevance (Ochsner and Gross, 2008; Lindquist et al., 2012; Schuyler et al., 2014) and visceral arousal (Lindquist et al., 2012; Waugh and Schirillo, 2012), respectively.
However, none of the reviewed studies took into account the possibility that the neural basis of changes in the intensity of emotional experience may change depending on the stage of the emotional response, with emotion onset (associated with explosiveness) and emotion offset (associated with accumulation) possibly having unique neural correlates. This is troublesome, as different mechanisms have been hypothesized to underlie both stages (Sonnemans and Frijda, 1995; Davidson, 1998; Verduyn et al., 2009) and, hence, it can be expected that the neural basis of emotion intensity varies over time.
The present study
The aim of the present study is to examine the neural basis of emotion explosiveness and accumulation following negative social feedback. The choice for this emotion-eliciting stimulus assures the ecological validity of our approach, as in daily life emotions are typically evoked by stimuli that are social and self-relevant in nature (Britton et al., 2006). Moreover, social feedback has been shown to generate emotional responses that often linger for some time (Wager et al., 2009), allowing us to study emotion dynamics while separating explosiveness from accumulation.
We hypothesize that emotion explosiveness and accumulation have distinctive neural correlates. Therefore, we first conducted whole brain voxel-based analyses to examine the neural correlates of explosiveness and accumulation in an exploratory way. Next, as the neural correlates likely include regions identified in earlier research on emotion dynamics, we additionally conducted region-of-interest (ROI) analyses on the medial prefrontal cortex, amygdala, and insula.
To examine the neural correlates of explosiveness and accumulation following social feedback, 31 participants were asked to write a number of brief essays on topics related to their personal dreams and aspirations. Participants were made to believe that these essays would be read by judges who would infer their personality from these texts. In reality, however, participants received the same negative and neutral personality assessments independent of the content of their essays. Feedback of this nature has been previously used in fMRI research to induce emotions (Somerville et al., 2006; Eisenberger et al., 2011). Participants were asked to read and think about the feedback for a total of 90 seconds, and reported on felt changes in emotional intensity during that period using an intensity profile tracking approach. The obtained profile data were decomposed in an explosiveness and accumulation component by means of non-negative matrix factorization (Lee and Seung, 1999). Next, their neural correlates were identified by first convolving them with the canonical hemodynamic response function and, subsequently, entering them as continuous regressors of the BOLD response.
Method
Sample
Thirty-one individuals (mean age = 28.68 years, s.d. age = 8.30, 13 females) gave informed consent for this study that was approved by University Paris VI’s institutional review board. All participants were right-handed native French language speakers and received 45 Euros for participating. A psychiatrist ensured that they did not suffer from any neurological or psychiatric illness, take medications or drugs, or have contraindications for MRI such as claustrophobia or metallic prostheses. One participant’s data were removed from all analyses as he did not believe the cover story. An additional 10 participants were excluded from all analyses (except for the manipulation check of the emotion induction): Four participants were excluded due to technical problems or excessive movements, and, similar to earlier neuroimaging research on emotion dynamics (Waugh et al., 2010), six participants were excluded because their responses showed a clear mismatch with the experimental design, resulting in a low number of either negative or neutral trials (i.e. experiencing less than three of the eight neutral trials as neutral or experiencing less than four of the eight negative trials as negative). The latter exclusion criterion was derived by subtracting the standard deviation of matching negative and neutral trials from their respective means. More conservative inclusion criteria led to very similar results. The final sample thus consisted of 20 individuals (mean age = 26.90 years, s.d. age = 7.33, 10 females).
Design
The study consisted of four phases. First, participants were asked to write four brief essays on prespecified topics reflecting their personal dreams and aspirations (25 min). The experimenter told participants that these essays would be read by four judges who would try to infer their personality from them. In reality, no other people were involved. To further strengthen the cover story, participants were explained that the supposed judges would be deceived by being told that each essay was written by someone else, which would allow us to study the stability of first impressions. Second, participants completed several questionnaires assessing personality traits, emotion regulation dispositions, and well-being (25 min). As these measures are not directly relevant to the present research questions, they will not be further discussed. While participants completed the mentioned questionnaires, their essays were supposedly being read and evaluated. Third, participants entered the scanner and were exposed to feedback on their essays, after which emotion dynamics were assessed (∼45 min, see Figure 1 for a visual representation of the structure of the scanner trials). Fourth, a funnelled debriefing was adopted to measure possible suspicion about the presence of evaluators in the study; this was followed by a full debriefing (10 min).
Fig. 1.
Time course of trials (in seconds). The scanner session was divided in two counterbalanced runs consisting of eight trials each. Each trial started with a screen notifying the participant that feedback was about to be shown. Subsequently, negative (eight trials) or neutral (eight trials) feedback was presented. Next, participants thought about the feedback while looking at a fixation cross. Then, they were asked to specify the emotion elicited by the feedback and draw an intensity profile reflecting the dynamics of the emotion they felt while reading and thinking about the feedback. To reduce carryover effects participants were asked to relax before a new trial started. sp, self-paced.
Social feedback
The feedback was based on earlier research using social feedback to induce emotions (Bushman and Baumeister, 1998; Harmon-Jones and Sigelman, 2001; Eisenberger et al., 2011). Feedback mainly consisted of ratings on positive and negative personality traits. In addition, participants read whether the essay-evaluator would like to have them as a friend. Negative feedback consisted of low and high scores on desirable and undesirable personality traits, respectively, as well as a low score on the item reflecting the judge’s desire to be friends. Neutral feedback consisted of ratings close to the neutral scale midpoint for all feedback items. The order of the trials was controlled such that no more than two consecutive trials were of the same type (negative or neutral) of feedback.
Emotion rating
Participants first indicated whether they experienced the feedback as positive, negative or neutral. Subsequently, they specified the nature of the negative (sadness, anger, shame, other negative emotion) or positive (joy, gratitude, pride, other positive emotion) emotion they felt. Finally, participants were asked to draw, using a trackball, a profile reflecting changes in the intensity of the selected positive or negative emotion they felt while reading and thinking about the feedback. For this purpose, a two-dimensional graph was displayed. The Y-axis represented emotional intensity and was divided into seven intervals ranging from ‘no emotion’ to ‘very intense’. The X-axis represented time and was divided into two parts corresponding to a period of reading and thinking about the feedback proportionally to their duration. Thus, even though the act of drawing was self-paced, the drawn profile reflected changes in emotion intensity for a fixed period that is identical across trials (i.e. 30 s of reading and 60 s of thinking). When participants experienced the feedback as neutral, they were asked to draw a horizontal line at the baseline level (‘no emotion’).
Task training
Before scanning, the experimenter walked participants through each screen of a feedback trial. First, the experimenter explained the social feedback items using a non-completed feedback form. Next, the experimenter urged participants to think about the feedback as long as a fixation cross appeared on the screen. Finally, the procedure to report emotion dynamics was explained and participants practiced until they felt capable to draw intensity profiles.
Functional MRI acquisition
Stimuli were generated and presented with E-Prime 2.0 and projected on a Plexiglas screen mounted at the end of the scanner bore. Two functional runs were acquired on a 3T Trio TIM MR-scanner (Siemens Medical Solutions, Erlangen, Germany) with Siemens standard 32-channel head coil. Participants’ head movements were restrained by foam paddings inside of the head coil. Functional images covering the whole brain were acquired using a T2*-weighted gradient echo, echo planar imaging (EPI) sequence, sensitive to blood oxygen level-dependent signal, employing the following parameters: repetition time: 2020 ms, echo time: 27 ms, flip angle: 78°, bandwidth: 2612 Hz, matrix: 66 × 66, field of view: 19.2 × 19.2 cm2, GRAPPA acceleration factor: 2. Forty sequential axial slices, with an isotropic voxel size of 3 × 3 × 3 mm3 were acquired parallel to the anteroposterior commissure plane. Each run lasted between 1036 and 1543 s (mean = 1197 s, s.d. = 94) resulting in between 513 and 764 images (mean = 593 images, s.d. = 47) depending on the time participants took to report on their emotion dynamics. Additional ‘dummy’ volumes were acquired at the beginning of each run to allow the magnetization to stabilize to a steady state before the first real volume. High-resolution three-dimensional T1-weighted sagittal images (3D fast gradient echo inversion recovery sequence, inversion time: 900 ms, repetition time: 2300 ms, echo time: 4.18 ms, bandwidth: 150 Hz, flip angle: 9°, matrix: 256 × 248, field of view: 256 × 256 mm2, voxel size: 1 × 1 × 1 mm3) were acquired for anatomical localization.
Analysis of intensity profiles
The intensity profiles of negative emotions following negative social feedback (i.e. matching negative trials) were decomposed using an optimally approximate Non-Negative Matrix Factorization (NNMF) procedure (Lee and Seung, 1999) as implemented in MATLAB 2013b that optimizes the Frobenius norm of the difference between the actual and reconstructed data. Approximate NNMF is a state-of-the-art dimension-reduction technique and provides component loadings and scores similar to, for instance, principal component analysis. However, this technique accommodates the inherently non-negative nature of subjective emotion intensity, as non-negative matrix factorization decomposes non-negative data X into a matrix of non-negative component scores A and a matrix of non-negative component loadings B, with the matrix product of scores and loadings optimally approximating the original data,
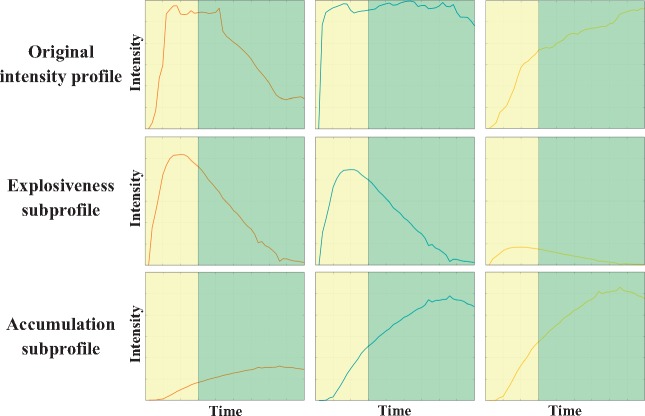
with representing the Frobenius norm. Within the present context, the loadings are profiles themselves that represent the component processes underlying the manifest profiles of subjective emotion experience. The scores, in turn, reflect the weights or importance values of the different processes for the different intensity profiles. As a result, each original intensity profile can be reconstructed by adding the component scores multiplied by the corresponding loadings (i.e. by adding reconstructed subprofiles). A visualization of this decomposition process is shown in Figure 2.
Fig. 2.
Original drawings (upper panel), explosiveness subprofiles (middle panel) and accumulation subprofiles (lower panel). Adding the reconstructed subprofiles closely approximates the original intensity profile. The reconstructed subprofiles convolved with the canonical hemodynamic response function were entered as regressors of the BOLD signal to examine the neural signature of emotion explosiveness and accumulation. Yellow (left) and green (right) backgrounds correspond to reading and thinking about the feedback, respectively.
Approximate NNMF can be considered the optimally suited analysis tool in the search for the neural signatures of the constituent processes underlying subjective emotional experience for two reasons. First, most if not all state-of-the-art analyses of hemodynamics are rooted in a linear systems account in which the BOLD response constitutes the output that results from convolving the system’s input with the system’s canonical response function. At this point, non-negative input values, as resulting from NNMF, can be given a most straightforward interpretation. Second, data on subjective emotional experience can be considered inherently noisy; the approximation implied by approximate NNMF allows the researcher to tell apart signal and noise.
Analysis of functional MRI data
Functional scans were preprocessed with SPM8, using slice-time correction, motion correction, spatial normalization to the MNI space, and spatial smoothing using a 8-mm full-width at half-maximum isotropic Gaussian kernel. Using other levels of smoothing (between 4 and 12 mm) led to very similar results. Spatial normalization was performed by first coregistering the high-resolution T1-weighted image to the mean functional image, normalizing the T1 to the MNI template applying the normalization parameters to the functional images.
Statistical analyses were conducted using the General Linear Model (GLM) framework implemented in SPM8. Boxcar regressors modelled the 5-s period during which feedback was announced, the 90-s period during which participants read and thought about negative feedback, the 90-s period during which participants read and thought about neutral feedback, the self-paced emotion rating period, and the self-paced emotion drawing period, with the relaxation period functioning as an implicit baseline. To examine the neural basis of emotion dynamics during the period that participants read and thought about the feedback, we further added the reconstructed subprofiles derived from the NNMF to the regression equation, reflecting the regressors of interest (explosiveness and accumulation). When participants reported not having experienced a negative emotion, intensity values were set at zero. All regressors were convolved with the canonical hemodynamic response function. A high-pass filter of 200 s was applied (on the basis of frequency domain plots of the time-series) similar to other studies using relatively long block-designs (Guo et al., 2012; Xu et al., 2014) and the motion realignment parameters were included as regressors of non-interest. Voxel-wise statistical parametric maps reflecting neural correlates of explosiveness and accumulation were calculated for each participant and then entered into random-effects group analyses using one sample t-tests. Statistical maps were thresholded at P<0.001 (uncorrected) combined with an extend threshold of 10 adjacent voxels as recommended by several scholars to balance Type I and Type II error rates (Lieberman and Cunningham, 2009; Woo et al., 2014) and as used in other research within the field of affective neuroscience (Baicy et al., 2007; Northoff et al., 2009; Zweynert et al., 2011). In addition to the mentioned threshold, we also provide FWER cluster-corrected and voxel-wise FDR corrected results. Resulting peaks were subsequently transformed into the Talairach space through the icbm2tal transform (Lancaster et al., 2007; Laird et al., 2010) and labelled using the Talairach atlas (Lancaster et al., 1997, 2000). When multiple peaks were located in the same Talairach region and Brodmann area, only the highest one was reported.
Regions of interest analysis
AAL’s (Tzourio-Mazoyer et al., 2002) structural masks included in the SPM8-compatible tool MarsBar (http://marsbar.sourceforge.net, Brett et al., 2002) were used to bilaterally define the amygdala, the mPFC, and the insula. The latter being usually divided into an anterior and a posterior part (Kross et al., 2011; Eisenberger, 2015), we subdivided AAL’s mask into an anterior (y > −10) and posterior (y < −10) insula, according to a functional clustering parcellation based on a meta-analysis (Chang et al., 2013). Using MarsBar, we subsequently calculated for each region of interest the mean value of the second level explosiveness and accumulation regression weights by aggregating across all voxels pertaining to the region of interest and tested for significance using one sample t-tests with Bonferroni correction.
Results
Emotion induction
First, we assessed the effectiveness of our emotion induction including all participants, except the one participant who did not believe the cover story (n = 30). Overall, participants reported feeling more often negative emotions following negative feedback compared to neutral feedback (χ2(2)=247.998, P<0.0001). In particular, following negative feedback, participants reported feeling negative in 82.92%, neutral in 14.58%, and positive in 2.50% of the cases. Following neutral feedback, participants reported feeling negative in 11.25%, neutral in 69.17%, and positive in 19.58% of the cases. When feeling negative, participants reported feeling anger in 53.98%, sadness in 23.45%, shame in 3.10%, and a non-specified other negative emotion in 19.47% of the cases. In sum, the negative emotion induction was successful.
Delineating emotion explosiveness and accumulation
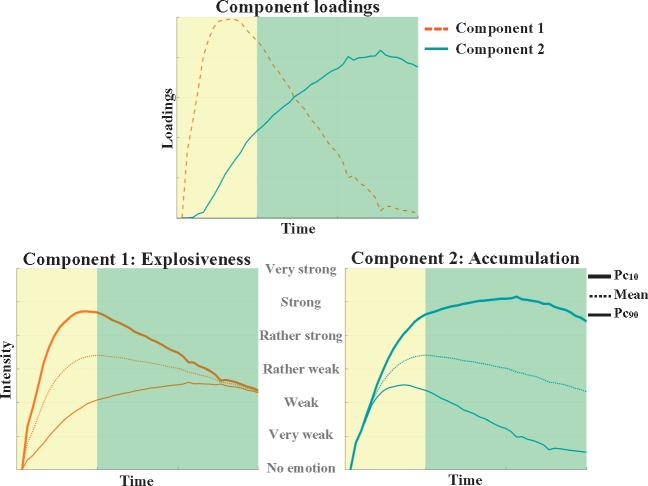
The profiles reflecting changes in intensity of negative emotion while reading and thinking about negative feedback were decomposed using non-negative matrix factorization. The appropriate number of components was determined by means of a scree plot, which suggested a two-component solution. The component loadings are depicted in Figure 3 as well as reconstructed profiles taking high (i.e. 90th percentile), average, or low (i.e. 10th percentile) scores on the component in question and mean scores on the other component to aid their substantive interpretation (Ramsay and Silverman, 2005). Below, the components in question will be presented according to the order of their peaks in the temporal process.
Fig. 3.
Two-component solution resulting from NNMF. Top: Component loadings of emotional intensity profiles over time. Bottom: Reconstructed profiles taking a high (90th percentile), average, or low (10th percentile) score on the component in question and a mean score on the other component. Bottom left panel: Reconstructed profiles differing in degree of explosiveness with the high (low) scoring profile showing high (low) levels of initial emotion intensity. Bottom right panel: Reconstructed profiles differing in degree of accumulation with the high (low) scoring profile showing an increase (decrease) in intensity over time following the initial response. Yellow (left) and green (right) backgrounds correspond to reading and thinking about the feedback, respectively.
As illustrated in Figure 3, the first component pertains to emotion explosiveness as reflected by initial high loadings followed by a steep decrease. The reconstructed intensity profile scoring high (low) on the first component consistently has an explosive (gentle) start. The second component pertains to emotion accumulation as reflected by increasing loadings after onset over time. This interpretation is corroborated by the reconstructed profile scoring high (low) on this component that shows an increase (decrease) in intensity over time following the initial emotional response. In sum, consistent with earlier research (Verduyn et al., 2009; Verduyn et al., 2012a), emotion explosiveness and accumulation were found to be the two main dimensions characterizing emotion unfolding.
Neural basis of emotion explosiveness and accumulation
The study’s main question concerned whether emotion explosiveness and accumulation have different neural correlates. To examine this, explosiveness and accumulation regressors were created for each participant by multiplying the component scores of intensity profiles by their corresponding loadings (i.e. reconstructed subprofiles), convolving them with the hemodynamic response function and entering them as regressors of the BOLD signal.
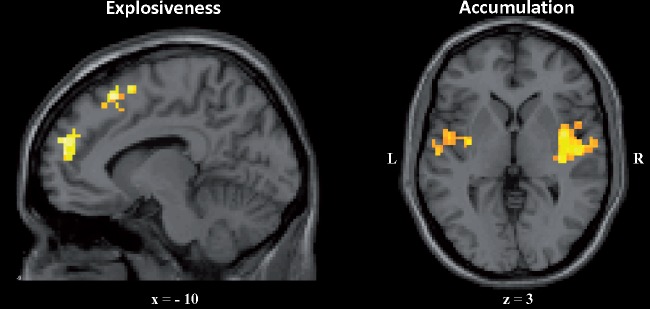
Emotion explosiveness appeared to be positively related to activity in the left mPFC, the left middle and superior frontal and temporal gyri, the left supramarginal gyrus, the right angular, superior temporal, lingual, and middle occipital gyri, and the right cerebellum (Table 1 and Figure 4).
Table 1.
Activations associated with explosiveness in whole-brain analysis
| Tal coordinates | ||||
|---|---|---|---|---|
| (mm) | ||||
| Region of activation | BA | T value | [x; y; z] | Vox. |
| 40 | ||||
| Superior Frontal Gyrus L | 6 | 5.35 | [−10;15;55] | |
| Medial Frontal Gyrus L | 6 | 4.87 | [−10;3;59] | |
| 42 | ||||
| Superior Frontal Gyrus L | 9 | 5.36 | [−12;49;26] | |
| 56 | ||||
| Middle Frontal Gyrus L | 9 | 5.16 | [−54;17;36] | |
| Middle Frontal Gyrus L | 6 | 4.17 | [−38;5;46] | |
| 45 | ||||
| Angular Gyrus R | 39 | 5.78 | [34;−62;32] | |
| Superior Temporal Gyrus R | 39 | 3.67 | [40;−54;33] | |
| 23 | ||||
| Superior Temporal Gyrus L | 39 | 5.46 | [−46;−53;26] | |
| Supramarginal Gyrus L | 40 | 4.27 | [−54;−52;24] | |
| 39 | ||||
| Middle Temporal Gyrus L | 21 | 4.60 | [−51;−18;−8] | |
| Superior Temporal Gyrus L | 21 | 3.86 | [−48;−24;−3] | |
| 11 | ||||
| Lingual Gyrus R | 18 | 4.59 | [15;−98;−1] | |
| Middle Occipital Gyrus R | 18 | 4.46 | [21;−96;5] | |
| 23 | ||||
| Cerebellum R | 4.52 | [35;−59;−27] |
Notes: All Ps < 0.001 uncorrected, number of voxels > 10 per cluster. BA, Brodmann’s areas; Vox, voxels in that cluster; L, left; R, right.
P<0.05 voxel-wise FDR-corrected.
P<0.05 FWE-corrected at cluster level.
Fig. 4.
Neural correlates of emotion explosiveness and accumulation. Left panel: Cortical midline regions associated with emotion explosiveness. Right panel: Insula activation associated with emotion accumulation. Coordinates in the Talairach space.
Emotion accumulation seemed to be positively related to bilateral activation in the insula (mid-posterior part) and cingulate cortex (mid-posterior part), the right claustrum and anterior cingulate cortex (dorsal part), the left middle frontal (dorsolateral part of the prefrontal cortex), pre/post-central, and superior temporal gyri, the left caudate body, and inferior parietal lobule (Table 2 and Figure 4).
Table 2.
Activations associated with accumulation in whole-brain analysis
| Tal coordinates | ||||
|---|---|---|---|---|
| (mm) | ||||
| Region of activation | BA | T value | [x; y; z] | Vox. |
| 804* | ||||
| Insula R | 13 | 6.06† | [54; −33;19] | |
| Claustrum R | 5.76† | [32; −15;10] | ||
| 318* | ||||
| Post-central Gyrus L | 40 | 5.36† | [−60; −24;18] | |
| Insula L | 13 | 4.68† | [−43; −3;10] | |
| Superior Temporal Gyrus L | 41 | 4.53† | [−54; −28;10] | |
| 27 | ||||
| Caudate L | 5.21† | [−18;16;20] | ||
| 453* | ||||
| Precentral Gyrus L | 4 | 5.16† | [−19; −29;70] | |
| Inferior Parietal Lobule L | 40 | 5.14† | [−35; −44;54] | |
| Post-central Gyrus L | 5 | 4.69† | [−8; −43;69] | |
| 25 | ||||
| Middle Frontal Gyrus L | 8 | 4.55† | [−21;25;34] | |
| 227* | ||||
| Cingulate Gyrus R | 31 | 4.94† | [4; −37;42] | |
| Cingulate Gyrus L | 31 | 4.66† | [−13; −34;40] | |
| 18 | ||||
| Anterior Cingulate R | 32 | 4.81† | [18;18;23] | |
| Cingulate Gyrus R | 32 | 3.74† | [21;17;31] | |
| 11 | ||||
| Posterior Cingulate R | 29 | 4.16† | [15;−44;20] |
Notes: All Ps < 0.001 uncorrected, number of voxels > 10 per cluster. BA, Brodmann’s areas; Vox, voxels in that cluster; L, left; R, right.
P<0.05 voxel-wise FDR-corrected.
P<0.05 FWE-corrected at cluster level.
Finally, the contrast comparing neural activity during the 90-s period during which participants watched and thought about the negative feedback with the 90-s period during which participants watched and thought about neutral feedback did not lead to suprathreshold voxels. Overall, it can be concluded that the main hypothesis was confirmed with emotion explosiveness and accumulation having distinctive neural correlates (Figure 4).
Regions of interest analyses
Aside from the whole brain voxel-based analyses, we also conducted ROI analyses to examine the role of the mPFC, amygdala and insula during emotion unfolding. The results (Table 3) are consistent with the whole brain analysis described earlier. In particular, emotion explosiveness was positively associated with activity in the mPFC (t(19)=3.00, P=0.01 Bonferroni corrected), whereas emotion accumulation was positively associated with activity in the posterior insula (t(19)=3.87, P=0.002 Bonferroni corrected). Finally, again the contrast comparing the neural activity during the 90-s period during which participants watched and thought about negative feedback with the 90-s period during which participants watched and thought about neutral feedback was not significantly related to activity in any of the ROIs.
Table 3.
Results of ROI analyses
| Explosiveness |
Accumulation |
|||
|---|---|---|---|---|
| Label | T | P | T | P |
| mPFC | 3.00 | 0.01 | −1.35 | 1.00 |
| Amygdala | −1.33 | 1.00 | 1.07 | 0.48 |
| Insula | ||||
| Anterior | −2.27 | 1.00 | −0.18 | 0.99 |
| Posterior | −2.14 | 1.00 | 3.87 | 0.002 |
Notes: mPFC, medial prefrontal cortex. Bonferroni-corrected P values are reported.
Discussion
This is the first study showing that the neural basis of emotional experience varies over time using a recently developed paradigm to disentangle processes underlying emotion unfolding. Consistent with theories on emotion dynamics (Davidson, 1998; Koole, 2009; Brans and Verduyn, 2014; Kuppens and Verduyn, 2015), onset- (associated with explosiveness) and offset-bound processes (associated with accumulation) were found to be the two main constituents underlying change in emotional experience over time. The present study shows that these two types of processes are associated with distinctive neural regions and illustrates that the dimension of time is a necessary ingredient in studies on the neural basis of emotional responding. This resonates with recent calls to put time on the research agenda of affective neuroscience (Davis et al., 2010; Waugh and Schirillo, 2012).
Specifically, it was found that differences in onset-bound responses (reflected by the degree of explosiveness of the response at onset) were related to activity in the mPFC. This result was found both when conducting whole brain analyses and ROI analyses. Even though the mPFC is involved in many tasks, activity in this region following self-relevant stimuli in emotional contexts is assumed to reflect self-referential processing (Fossati et al., 2003; Northoff et al., 2006). In the present experimental context, activity in the mPFC may reflect participants assessing the match or mismatch of the feedback with their self-perception. Although future studies are necessary to further investigate this interpretation, it is corroborated by the observed activity in inferior parietal areas (i.e. angular and supramarginal gyri), which together with the mPFC, constitute a large part of the default mode network, known to be involved in self-related processing (Andrews-Hanna et al., 2014). The observed activity of the mPFC during the onset period is also consistent with studies showing the role of the mPFC for meaning-making of stimuli (Waugh et al., 2010) and, more generally with appraisal theories (Scherer, 1984; Moors et al., 2013) according to which people initiate self-referential processing at the very start of the emotional episode to give meaning to emotion-provoking events. The appraisal outcome is then further assumed to influence the initial intensity of negative emotions depending on the degree of mismatch perceived between the event and one’s concerns (Sonnemans and Frijda, 1995). In the present experimental context, feedback that contrasted with participants’ concerns for a positive self-image may have required a higher degree of self-referential processing resulting in explosive emotional episodes.
In contrast, differences in offset-bound processes (reflected by the degree of emotion accumulation after onset) were found to be related to bilateral activity in the mid-posterior insula, which again was observed in both types of analyses (i.e. whole brain and ROI analyses). This is consistent with Hu et al. (2015) who found posterior insula activity during the offset period following sustained painful and non-painful stimuli. The insula has been shown to continuously monitor sustained visceral arousal (Waugh and Schirillo, 2012) and to be a key neural mechanism in the processing of emotional signals (Scherer, 2005; Lindquist et al., 2016; Nguyen et al., 2016), and especially of markers of social exclusion as when receiving negative feedback (Panksepp, 2003; Eisenberger, 2015). Information on sustained visceral arousal is monitored by the posterior insula and projected to the anterior insula where it gets integrated with exteroceptive information resulting in conscious experience of bodily arousal (Craig, 2002; Nguyen et al., 2016). This link between accumulation and sustained monitoring of interoceptive signals is substantiated by activity in the somatosensory cortices. However, it should be noted that no significant activity in the anterior insula was observed, and that further research is needed to examine the specific role of the (anterior and posterior) insula during emotion offset.
Even though onset- and offset-bound processes seem to have clearly distinct neural correlates, two cautionary notes have to be made. First, the identified neural basis of the two types of processes underlying emotional unfolding also suggests some functional overlap. In particular, accumulation was found to be related to the posterior cingulate cortex (PCC), which is another key node of the default mode network (Andrews-Hanna et al., 2014). This suggests that self-related processes may continue throughout the emotional episode, reflected by a shift from mPFC during emotion onset to PCC during emotion offset. This interpretation is substantiated by previous research revealing the importance of cortical midline regions for self-referential processing throughout emotional episodes (Waugh et al., 2010).
Second, when testing for the robustness either using FWER at the cluster level or voxel-wise FDR, we found the neural correlates of accumulation to be largely robust, while this was not the case for emotion explosiveness. As such, even though the present study indicates that neural correlates of emotion explosiveness and accumulation are largely distinct, future research is necessary to identify and assess the robustness of the specific neural correlates of the temporal features of emotion unfolding.
Additionally, future research using non-social or basic stimuli to induce emotions is needed to examine the generalizability of our findings to other contexts. In such studies, the amygdala may be identified as an additional key region in emotion unfolding, as amygdala activity is more likely to be observed when inducing emotions with stimuli that require a low degree of cognitive processing (Costafreda et al., 2008; Waugh et al., 2015). Moreover, future research is necessary to study the degree to which the neural process basis of emotion dynamics are open to external control. For this purpose, the effect of emotion regulation strategies on emotion onset- and offset-bound processes could be charted as well as the neural pathways mediating their effect. Finally, future research could extend the present fMRI approach by adding additional neurophysiological measures such as EEG, MEG, or peripheral activation.
In sum, the present study illustrates that it is crucial to take the dimension of time into account in order to reach an understanding of the neural basis of emotions. The neural regions that orchestrate the unfolding of an emotional response vary over time as revealed by distinctive neural correlates for emotion onset- and offset-bound processes following social exclusion.
Acknowledgements
The authors would like to thank the NeuroImaging Center at ICM (CENIR, Paris) and particularly Romain Valabregue for helping with the data collection and analysis.
Funding
The research leading to the results reported in this paper was supported in part by the Research Fund of KU Leuven (GOA/15/003) and by the Interuniversity Attraction Poles programme financed by the Belgian government (IAP/P7/06). P.V. is supported as a postdoctoral fellow of the Research Foundation – Flanders (FWO). P.F. is supported by the French National Research Agency (Agence Nationale pour la Recherche) [ANR SAMENTA2012 (Sante ´Mentale et Addictions, projet SENSO)].
Conflict of interest. None declared.
References
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorder (5th ed.). Washington, DC: American Psychiatric Publishing, Inc. DOI:10.1176/appi.books.9780890425596.744053.
- Andrews-Hanna J.R., Smallwood J., Spreng R.N. (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316(1), 29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baicy K., London E.D., Monterosso J., et al. (2007). Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proceedings of the National Academy of Sciences of the United States of America, 104(46), 18276–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brans, K., Verduyn, P. (2014). Intensity and duration of negative emotions: Comparing the role of appraisals and regulation strategies. PLoS ONE, 9(3), e92410. DOI:10.1371/journal.pone.0092410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M., Anton J.-L., Valabregue R., Poline J.-B. (2002). Region of interest analysis using the MarsBar toolbox for SPM 99. NeuroImage, 16(2), S497. [Google Scholar]
- Britton J.C., Phan K.L., Taylor S.F., Welsh R.C., Berridge K.C., Liberzon I. (2006). Neural correlates of social and nonsocial emotions: an fMRI study. NeuroImage, 31(1), 397–409. [DOI] [PubMed] [Google Scholar]
- Bushman B.J., Baumeister R.F. (1998). Threatened egotism, narcissism, self-esteem, and direct and displaced aggression: does self-love or self-hate lead to violence?. Journal of Personality and Social Psychology, 75(1), 219–29. [DOI] [PubMed] [Google Scholar]
- Chang L.J., Yarkoni T., Khaw M.W., Sanfey A.G. (2013). Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cerebral Cortex, 23(3), 739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda, S.G., Brammer, M.J., David, A.S., Fu, C.H.Y. (2008). Predictors of amygdala activation during the processing of emotional stimuli: A meta-analysis of 385 PET and fMRI studies. Brain Research Reviews, 58(1), 57–70. DOI:10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Craig A.D. (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience, 3(8), 655–66. [DOI] [PubMed] [Google Scholar]
- Davidson R.J. (1998). Affective style and affective disorders: perspectives from affective neuroscience. Cognition & Emotion, 12(3), 307–30. [Google Scholar]
- Davis M., Walker D.L., Miles L., Grillon C. (2010). Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology, 35(1), 105–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid M., Diener E. (1999). Intraindividual variability in affect: reliability, validity, and personality correlates. Journal of Personality and Social Psychology, 76(4), 662–76. [Google Scholar]
- Eisenberger N.I. (2015). Social pain and the brain: controversies, questions, and where to go from here. Annual Review of Psychology, 66(1), 601–29. [DOI] [PubMed] [Google Scholar]
- Eisenberger, N.I., Inagaki, T.K., Muscatell, K.A., Byrne Haltom, K.E., Leary, M.R. (2011). The neural sociometer: Brain mechanisms underlying state self-esteem. Journal of Cognitive Neuroscience, 23(11), 3448–55. DOI:10.1162/jocn_a_00027. [DOI] [PubMed] [Google Scholar]
- Fossati P., Hevenor S.J., Graham S.J., et al. (2003). In search of the emotional self: an fMRI study using positive and negative emotional words. American Journal of Psychiatry, 160(11), 1938–45. [DOI] [PubMed] [Google Scholar]
- Frijda N.H. (2007). The Laws of Emotion. Mahwah, NJ: Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Frijda N.H., Mesquita B., Sonnemans J., Van Goozen S. (1991). The duration of affective phenomena or emotions, sentiments and passions In Strongman K.T., editor. International Review of Studies on Emotion (pp. 187–225). Chichester: Wiley. [Google Scholar]
- Goldin P.R., Hutcherson C.A.C., Ochsner K.N., Glover G.H., Gabrieli J.D.E., Gross J.J. (2005). The neural bases of amusement and sadness: a comparison of block contrast and subject-specific emotion intensity regression approaches. NeuroImage, 27(1), 26–36. [DOI] [PubMed] [Google Scholar]
- Grandjean D., Scherer K.R. (2008). Unpacking the cognitive architecture of emotion processes. Emotion, 8(3), 341–51. [DOI] [PubMed] [Google Scholar]
- Guo X., Zheng L., Zhang W., et al. (2012). Empathic neural responses to others’ pain depend on monetary reward. Social Cognitive and Affective Neuroscience, 7(5), 535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas B.W., Constable R.T., Canli T. (2008). Stop the sadness: neuroticism is associated with sustained medial prefrontal cortex response to emotional facial expressions. NeuroImage, 42(1), 385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E., Sigelman J. (2001). State anger and prefrontal brain activity: evidence that insult-related relative left-prefrontal activation is associated with experienced anger and aggression. Journal of Personality and Social Psychology, 80(5), 797–803. [PubMed] [Google Scholar]
- Heller A.S., Fox A.S., Wing E.K., McQuisition K.M., Vack N.J., Davidson R.J. (2015). The neurodynamics of affect in the laboratory predicts persistence of real-world emotional responses. The Journal of Neuroscience, 35(29), 10503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller A.S., Johnstone T., Shackman A., et al. (2009). Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences of the United States of America, 106(52), 22445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller A.S., van Reekum C.M., Schaefer S.M., et al. (2013). Sustained striatal activity predicts eudaimonic well-being and cortisol output. Psychological Science, 24(11), 2191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenstein T. (2015). This time, it’s real: affective flexibility, time scales, feedback loops, and the regulation of emotion. Emotion Review, 7(4), 308–15. [Google Scholar]
- Houben M., Van Den Noortgate W., Kuppens P. (2015). The relation between short-term emotion dynamics and psychological well-being: a meta-analysis. Psychological Bulletin, 141(4), 901–30. [DOI] [PubMed] [Google Scholar]
- Hu L., Zhang L., Chen R., Yu H., Li H., Mouraux A. (2015). The Primary Somatosensory Cortex and the Insula Contribute Differently to the Processing of Transient and Sustained Nociceptive and Non-Nociceptive Somatosensory Inputs. Human Brain Mapping, 36(11), 4346–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcherson C.A.C., Goldin P.R., Ochsner K.N., Gabrieli J.D.E., Barrett L.F., Gross J.J. (2005). Attention and emotion: does rating emotion alter neural responses to amusing and sad films?. NeuroImage, 27(3), 656–68. [DOI] [PubMed] [Google Scholar]
- Koole S.L. (2009). The psychology of emotion regulation: an integrative review. Cognition & Emotion, 23(1), 4–41. [Google Scholar]
- Kross E., Berman M.G., Mischel W., Smith E.E., Wager T.D. (2011). Social rejection shares somatosensory representations with physical pain. Proceedings of the National Academy of Sciences of the United States of America, 108(15), 6270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppens P., Allen N.B., Sheeber L.B. (2010). Emotional inertia and psychological maladjustment. Psychological Science, 21(7), 984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppens P., Verduyn P. (2015). Looking at emotion regulation through the window of emotion dynamics. Psychological Inquiry, 26(1), 72–9. [Google Scholar]
- Laird A.R., Robinson J.L., McMillan K.M., et al. (2010). Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: validation of the Lancaster transform. NeuroImage, 51(2), 677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Rainey L.H., Summerlin J.L., et al. (1997). Automated labeling of the human brain: a preliminary report on the development and evalution of a forward-transform method. Human Brain Mapping, 5(4), 238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Tordesillas-Gutiérrez D., Martinez M., et al. (2007). Bias between MNI and talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping, 28(11), 1194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Woldorff M.G., Parsons L.M., et al. (2000). Automated Tailairach Atlas Labels for Functional Brain Mapping. Human Brain Mapping, 10(3), 120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J.Y.F., Guyer A.E., Tone E.B., et al. (2012). Neural responses to peer rejection in anxious adolescents: contributions from the amygdala-hippocampal complex. International Journal of Behavioral Development, 36(1), 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.D., Seung H.S. (1999). Learning the parts of objects by non-negative matrix factorization. Nature, 401(6755), 788–91. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Cunningham W.A. (2009). Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience, 4(4), 423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist K.A., Satpute A.B., Wager T.D., Weber J., Barrett L.F. (2016). The brain basis of positive and negative affect: evidence from a meta-analysis of the human neuroimaging literature. Cerebral Cortex, 26(5), 1910–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist K.A., Wager T.D., Kober H., Bliss-Moreau E., Barrett L.F. (2012). The brain basis of emotion: a meta-analytic review. The Behavioral and Brain Sciences, 35(3), 121–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist M.A., Waugh C., Wager T.D. (2007). Modeling state-related fMRI activity using change-point theory. NeuroImage, 35(3), 1125–41. [DOI] [PubMed] [Google Scholar]
- Mandell D., Siegle G.J., Shutt L., Feldmiller J., Thase M.E. (2014). Neural substrates of trait ruminations in depression. Journal of Abnormal Psychology, 123(1), 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moors, A. (2009). Theories of emotion causation: A review. Cognition & Emotion, 23(4), 625–62. DOI:10.1080/02699930802645739. [Google Scholar]
- Moors A., Ellsworth P.C., Scherer K.R., Frijda N.H. (2013). Appraisal theories of emotion: state of the art and future development. Emotion Review, 5(2), 119–24. [Google Scholar]
- Nguyen V.T., Breakspear M., Hu X., Guo C.C. (2016). The integration of the internal and external milieu in the insula during dynamic emotional experiences. NeuroImage, 124, 455–63. [DOI] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., de Greck M., Bermpohl F., Dobrowolny H., Panksepp J. (2006). Self-referential processing in our brain-A meta-analysis of imaging studies on the self. NeuroImage, 31(1), 440–57. [DOI] [PubMed] [Google Scholar]
- Northoff G., Schneider F., Rotte M., et al. (2009). Differential parametric modulation of self-relatedness and emotions in different brain regions. Human Brain Mapping, 30(2), 369–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. (2008). Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Current Directions in Psychological Science, 17(2), 153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J. (2003). Feeling the pain of social loss. Science, 302(5643), 237–9. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Taylor S.F., Welsh R.C., et al. (2003). Activation of the medial prefrontal cortex and extended amygdala by individual ratings of emotional arousal: a fMRI study. Biological Psychiatry, 53(3), 211–5. [DOI] [PubMed] [Google Scholar]
- Ramsay J.O., Silverman B.W. (2005). Functional Data Analysis, 2nd edn.New York, NY: Springer-Verlag. [Google Scholar]
- Raz G., Jacob Y., Gonen T., et al. (2014). Cry for her or cry with her: context-dependent dissociation of two modes of cinematic empathy reflected in network cohesion dynamics. Social Cognitive and Affective Neuroscience, 9(1), 30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz G., Shpigelman L., Jacob Y., Gonen T., Benjamini Y., Hendler T. (2016a). Psychophysiological whole-brain network clustering based on connectivity dynamics analysis in naturalistic conditions. Human Brain Mapping, 37(12), 4654–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz G., Touroutoglou A., Wilson-mendenhall C., et al. (2016b). Functional connectivity dynamics during film viewing reveal common networks for different emotional experiences. Cognitive, Affective & Behavioral Neuroscienceffective, & Behavioral Neuroscience, 16, 709–23. [DOI] [PubMed] [Google Scholar]
- Raz G., Winetraub Y., Jacob Y., et al. (2012). Portraying emotions at their unfolding: a multilayered approach for probing dynamics of neural networks. NeuroImage, 60(2), 1448–61. [DOI] [PubMed] [Google Scholar]
- Schaefer S.M., Boylan J.M., Van Reekum C.M., et al. (2013). Purpose in life predicts better emotional recovery from negative stimuli. PLoS One, 8(11), e80329.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer K.R. (2005). What are emotions? And how can they be measured? Social Science Information, 44(4), 695–729. [Google Scholar]
- Scherer, K. R. (1984). On the nature and function of emotion: A component process approach. In Scherer K. R. & Ekman P., editors. Approaches to emotion, pp. 293–317, Hillsdale, NJ: Erlbaum. [Google Scholar]
- Schupp H.T., Cuthbert B.N., Bradley M.M., Cacioppo J.T., Ito T., Lang P.J. (2000). Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology, 37(2), 257–61. [PubMed] [Google Scholar]
- Schuyler B.S., Kral T.R.A., Jacquart J., et al. (2014). Temporal dynamics of emotional responding: amygdala recovery predicts emotional traits. Social Cognitive and Affective Neuroscience, 9(2), 176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle G.J., Steinhauer S.R., Thase M.E., Stenger V.A., Carter C.S. (2002). Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry, 51(9), 693–707. [DOI] [PubMed] [Google Scholar]
- Somerville L.H., Heatherton T.F., Kelley W.M. (2006). Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience, 9(8), 1007–8. [DOI] [PubMed] [Google Scholar]
- Sonnemans J., Frijda N.H. (1994). The structure of subjective emotional intensity. Cognition & Emotion, 8(4), 329–50. [Google Scholar]
- Sonnemans J., Frijda N.H. (1995). The determinants of subjective emotional intensity. Cognition & Emotion, 9(5), 483–506. [Google Scholar]
- Suls J., Green P., Hillis S. (1998). Emotional reactivity to everyday problems, affective inertia, and neuroticism. Personality and Social Psychology Bulletin, 24(2), 127–36. [Google Scholar]
- Tong E.M.W., Bishop G.D., Enkelmann H.C., et al. (2009). Appraisal underpinnings of affective chronometry: the role of appraisals in emotion habituation. Journal of Personality, 77(4), 1103–36. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–89. [DOI] [PubMed] [Google Scholar]
- Verduyn P., Delaveau P., Rotgé J.-Y., Fossati P., Van Mechelen I. (2015). Determinants of emotion duration and underlying psychological and neural mechanisms. Emotion Review, 7(4), 330–5. [Google Scholar]
- Verduyn P., Van Mechelen I., Frederix E. (2012a). Determinants of the shape of emotion intensity profiles. Cognition & Emotion, 26(8), 1486–95. [DOI] [PubMed] [Google Scholar]
- Verduyn P., Van Mechelen I., Kross E., Chezzi C., Van Bever F. (2012b). The relationship between self-distancing and the duration of negative and positive emotional experiences in daily life. Emotion, 12(6), 1248–63. [DOI] [PubMed] [Google Scholar]
- Verduyn P., Van Mechelen I., Tuerlinckx F., Meers K., Van Coillie H. (2009). Intensity profiles of emotional experience over time. Cognition & Emotion, 23(7), 1427–43. [Google Scholar]
- Wadlinger H.A., Isaacowitz D.M. (2011). Fixing our focus: training attention to regulate emotion. Personality and Social Psychology Review, 15(1), 75–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Waugh C.E., Lindquist M.A., Noll D.C., Fredrickson B.L., Taylor S.F. (2009). Brain mediators of cardiovascular responses to social threat. Part I: reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. NeuroImage, 47(3), 821–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H., von Kalckreuth A., Schardt D., Stephan A., Goschke T., Erk S. (2009). The temporal dynamics of voluntary emotion regulation. PLoS One, 4(8), e6726.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh C.E., Hamilton J.P., Gotlib I.H. (2010). The neural temporal dynamics of the intensity of emotional experience. NeuroImage, 49(2), 1699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh C.E., Lemus M.G., Gotlib I.H. (2014). The role of the medial frontal cortex in the maintenance of emotional states. Social Cognitive and Affective Neuroscience, 9(12), 2001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh C.E., Schirillo J.A. (2012). Timing: a missing key ingredient in typical fMRI studies of emotion. Behavioral and Brain Sciences, 35(3), 170–1. [DOI] [PubMed] [Google Scholar]
- Waugh C.E., Shing E.Z., Avery B.M. (2015). Temporal dynamics of emotional processing in the brain. Emotion Review, 7(4), 323–9. [Google Scholar]
- Waugh C.E., Wager T.D., Fredrickson B.L., Noll D.C., Taylor S.F. (2008). The neural correlates of trait resilience when anticipating and recovering from threat. Social Cognitive and Affective Neuroscience, 3(4), 322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh C.E., Zarolia P., Mauss I.B., et al. (2016). Emotion regulation changes the duration of the BOLD response to emotional stimuli. Social Cognitive and Affective Neuroscience, 11(10), 1550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A., Hajcak G. (2010). Beyond good and evil: the time-course of neural activity elicited by specific picture content. Emotion, 10(6), 767–82. [DOI] [PubMed] [Google Scholar]
- Woo C.-W., Krishnan A., Wager T.D. (2014). Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. NeuroImage, 91, 412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woyshville M.J., Lackamp J.M., Eisengart J.A., Gilliland J.A.M. (1999). On the meaning and measurement of affective instability: clues from chaos theory. Biological Psychiatry, 45(3), 261–9. [DOI] [PubMed] [Google Scholar]
- Xu J., Vik A., Groote I.R., et al. (2014). Nondirective meditation activates default mode network and areas associated with memory retrieval and emotional processing. Frontiers in Human Neuroscience, 8(86), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweynert S., Pade J.P., Wüstenberg T., et al. (2011). Motivational salience modulates hippocampal repetition suppression and functional connectivity in humans. Frontiers in Human Neuroscience, 5(144), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]