Abstract
Synchrony in social groups may confer significant evolutionary advantages by improving group cohesion and social interaction. However, the neurobiological mechanisms translating social synchrony into refined social information transmission between interacting individuals are still elusive. In two successively conducted experiments involving a total of 306 healthy volunteers, we explored the involvement of the neuropeptide oxytocin (OXT) in reciprocal social interaction. First, we show that synchronous social interactions evoke heightened endogenous OXT release in dyadic partners. In a second step, we examined the consequences of elevated OXT concentrations on emotion transmission by intranasally administering synthetic OXT before recording emotional expressions. Intriguingly, our data demonstrate that the subjects’ facial and vocal expressiveness of fear and happiness is enhanced after OXT compared with placebo administration. Collectively, our findings point to a central role of social synchrony in facilitating reciprocal communication between individuals via heightened OXT signaling. Elevated OXT concentrations among synchronized individuals seem to augment the partners’ emotional expressiveness, thereby contributing to improved transmission of emotional information in social communication.
Keywords: emotion expression, imitation, oxytocin, social synchrony
Introduction
Social synchrony, or the temporal concordance of behavioral and physiological processes among individuals, is thought to confer evolutionary advantages in various social species. For example, groups of insects, birds and fish synchronize their moves and speeds, a social phenomenon called swarming, flocking, schooling or herding (Xuan and Filkov, 2013). In humans, social synchrony has been documented across a wide range of settings and contexts, ranging from choir singing during mass to the concerted behavior of stock market traders (Xuan and Filkov, 2013). Current anthropological and psychological perspectives on social synchrony emphasize the strengthening of group cohesion and synergy, which may underlie collective intelligence (Woolley et al., 2010). Furthermore, social synchrony may be central to the development of affiliative bonds, including maternal and partner bonds (Feldman, 2012; Atzil et al., 2014). While the evolutionary benefits of social synchrony may be most prominent in the reproductive domain, it appears that synchronized action can ease interpersonal communication and collaboration more generally. For instance, synchronizing one’s body movements with those of another person during dyadic interactions elicits a broad range of positive interpersonal behaviors in the imitated person, such as generosity (van Baaren et al., 2003), rapport (Bernieri, 1988), feelings of closeness (Catmur and Heyes, 2013), cooperation (Kirschner and Tomasello, 2010) and affiliation (Hove and Risen, 2009; Cacioppo et al., 2014). Moreover, therapeutic interventions aiming at refined kinesthetic synchronization are believed to foster emotional empathic processes (Koehne et al., 2016; Behrends et al., 2012). Likewise, interpersonal coordination in body movement during psychotherapy sessions reflects the emotional quality of the therapeutic relationship (Ramseyer and Tschacher, 2011). Thus, interpersonal synchronization at the sensorimotor level seems to be closely linked to processes of cognitive (e.g. social conformity) and emotional alignment (e.g. emotional contagion) with others. Consistent with this notion, evidence from neuroimaging studies suggests that social synchrony is orchestrated by the interplay of various brain regions involved in the detection and sharing of emotions and mind states (Atzil et al., 2014). Yet, the neuroendocrine substrates of social synchrony and the neurobiological mechanisms translating social synchrony into refined socio-cognitive information transmission have received limited attention.
In recent years, the hypothalamic peptide oxytocin (OXT) has been identified as a key modulator of social group living and emotional processing. A plethora of human intranasal studies have emphasized OXT’s involvement in various forms of higher order interpersonal behaviors such as cooperation (Declerck et al., 2014), trust (Kosfeld et al., 2005; Baumgartner et al., 2008) and romantic relationships (Scheele et al., 2012; Scheele et al., 2013; Hurlemann and Scheele, 2016). Intriguingly, there is converging evidence from studies in non-human and human primates that both receiving OXT and experiencing synchrony (i.e. being imitated by an interacting partner) yield similar behavioral and neural outcomes (Simpson et al., 2014; Paukner et al., 2009; Andari et al., 2010; Guastella et al., 2010; Anagnostou et al., 2012; Field et al., 2001). For example, exogenous OXT increases infant macaques’ affiliative gestures towards a human caregiver, and peripheral OXT levels positively correlate with the time spent in close proximity to the caregiver (Simpson et al., 2014). Likewise, capuchin monkeys show a visual preference for and spend more time in proximity to caregivers who imitate them compared with an equally familiar, non-imitating partner (Paukner et al., 2009).
In fact, Aoki and Yamasue (2015) have put forward the hypothesis that ‘being imitated by others may act similarly to OXT administration through enhanced secretion of endogenous OXT’. In line with this hypothesis, peripheral OXT levels have been associated with synchronized behavior in parent–infant bonds (Feldman, 2012; Feldman et al., 2013) and new lovers (Schneiderman et al., 2012). Interestingly, elevated OXT levels following experienced synchrony may induce a self-reinforcing loop by concomitantly increasing social synchrony. For instance, intranasal OXT has been found to modulate the neural response to highly synchronous stimuli (Levy et al., 2016). Empirical evidence from a computerized drawing task further indicates that OXT administration improves cooperative performance (Arueti et al., 2013). In this task, coordination of behavior was required for cooperation and OXT selectively improved the synchronized performance of pairs, but had no effect on individual performance. Furthermore, a recent study reported that OXT increases rhythmical counting and the inter-brain coupling of alpha-band oscillations during a coordination task (Mu et al., 2016). Regarding social emotion transmission, numerous studies have documented that intranasal OXT enhances emotion recognition from static (Domes et al., 2007) and dynamic facial expressions (Lischke et al., 2012). Importantly, however, social communication relies not only on the precision of emotion recognition in perceivers but also on senders’ ability to accurately convey their emotions. This bidirectional nature of social interactions points to a potential involvement of OXT in both the recognition and expression of emotions that has been largely neglected so far. Tops et al. (2007) reported a positive correlation between cortisol-induced increases in plasma OXT levels and self-reported emotional expression. However, as yet, no study has directly examined the hormone’s influence on the ability to facially or vocally express emotions. We propose here that social synchrony triggers the release of endogenous OXT, which in turn may improve bidirectional social emotion transmission by enhancing emotional expressiveness. This augmentation of affective sharing may subsequently contribute to better synchronized interactions, thus enabling the fine-tuning of social communication.
Experiment 1 of the present study was therefore aimed at determining whether social synchrony, operationalized as being imitated by another person, evokes the release of endogenous OXT in either the sender or the receiver of non-verbal signals. To control for the effect of mere social interaction, we randomly assigned 164 healthy participants (98 women) to a synchronous or an asynchronous interaction condition. In Experiment 2, we examined the influence of exogenously elevated OXT concentrations on emotion expression, as a core prerequisite of social communication. Thirty-five healthy male participants were recorded while producing facial and vocal expressions of happiness, fear and anger after intranasal administration of synthetic OXT (24 IU) and placebo (PLC). Subsequently, an independent sample of 106 women and men rated the salience of all emotional expressions. We hypothesized that (i) being imitated results in an increase of peripheral OXT levels and that (ii) elevated OXT levels foster the social transmission of emotions by enhancing the subject’s emotional expressiveness.
Methods and results
Ethics and enrollment
The present study was approved by the local ethics committee of the Medical Faculty of the University of Bonn, Germany. All subjects gave their written, informed consent in accordance with the latest revision of the Helsinki Declaration.
Screening
Enrollment in the experiments was preceded by sociodemographic data acquisition and clinical questionnaires. In Experiment 1, we administered the ‘Saarbrücker Persönlichkeitsfragebogen’, a German version of the Interpersonal Reactivity Index (Davis, 1983). In Experiment 2, we assessed trait anxiety (State Trait Anxiety Inventory, STAI; Spielberger, 1983), depressive symptoms (Beck Depression Inventory, BDI-II; Beck et al., 1996), alexithymia symptoms (Toronto Alexithymia Scale, TAS; Taylor et al., 2003) and autistic-like traits (Autism-Spectrum Quotient; Baron-Cohen et al., 2001). Additionally, mood (The Positive and Negative Affect Schedule, PANAS; Watson et al., 1988) and state anxiety (State-Trait Anxiety Inventory, STAI; Spielberger, 1983) were measured at the start and end of each visit. Furthermore, we conducted a comprehensive psychological screening, ensuring that all subjects were free of any current or past physical or psychiatric illness as assessed by medical history and the Mini-International Neuropsychiatric Interview (MINI; Sheehan et al., 1998). Moreover, subjects were naive to prescription-strength psychoactive medication and had not taken any over-the-counter psychoactive medication in the preceding four weeks.
Neuroendocrine parameter extraction
To asses peripheral OXT levels, all study participants were asked to submit two (Experiment 1) or three (Experiment 2) saliva samples throughout the testing session, using commercial sampling devices (Salivettes, Sarstedt). Salivettes were immediately centrifuged at 4000 rpm for 2 min and stored at -80 °C until assayed. Two additional saliva samples were obtained before and after task completion from participants of Experiment 2, to allow for a comprehensive assessment of peripheral cortisol levels.
Saliva OXT concentrations were extracted and quantified using a highly sensitive and specific radioimmunoassay (RIAgnosis, Munich, Germany) (Kagerbauer et al., 2013).The limit of detection was 0.1–0.5 pg, depending on the age of the tracer. Intra-assay and inter-assay coefficients of variability were < 10%. All samples to be compared were assayed in the same batch, i.e. under intra-assay conditions. Cortisol concentrations were determined using an electrochemiluminescence immunoassay (Elecsys Cortisol Test, Roche, Mannheim). The sensitivity of the assay was set at 0.054–63.4 μg/dl. The mean inter- and intra-assay coefficients of variation for the assays were 6.1% and 11.8%, respectively.
Statistical analysis
Demographic, neuropsychological and behavioural data were analyzed using IBM SPSS Statistics 22 (IBM, New York, NY, USA). Main effects and interactions were identified via repeated measures analysis of variance (ANOVA) and paired t-tests. The assumption of sphericity was assessed with Mauchly’s test and Greenhouse–Geisser’s correction was applied for significant violations. Pearson’s chi-squared tests were used for qualitative variables. Correlation analyses were performed via Pearson’s product-moment correlation. Due to the directed hypothesis, we applied one-tailed P-values in Experiment 1, expecting increased OXT levels after synchronous interaction. Having less clear hypotheses regarding the effect of intranasal OXT on the various facets of emotional expression we applied two-tailed P-values in Experiment 2. All reported P-values are corrected for multiple testing, using the Bonferroni correction method. Effect sizes are given as measures of Eta-squared and Cohen’s d.
Experiment 1
In Experiment 1, we wanted to examine whether synchronous interactions elicit OXT release in dyadic pairs of participants compared with asynchronous interactions. Facing each other, one participant (‘sender’) made hand gestures, while the other (‘receiver’) was either instructed to mirror these gestures (synchronous condition) or to perform different gestures (asynchronous condition).
Methods
Subjects and protocol
One hundred sixty-four healthy volunteers participated in the study after giving their written, informed consent. Saliva samples were not available for five participants. Thus, the data from 159 participants (98 women; mean age ± SD: 21.84 ± 2.94 years) were analyzed. Sixty-four women were using hormonal contraception.
Design and procedure
All participants were tested in groups, which were then randomly assigned to either a synchronous (n = 90) or an asynchronous interaction condition (n = 69). There were no a priori differences between participants in the synchronous and asynchronous conditions (cf. Supplementary Table S1). In the synchronous condition, random pairs of participants were instructed to perform a synchronous non-verbal interaction task for 5 min. Actor A (i.e. the sender) made hand gestures and Actor B (i.e. the imitator or receiver) mirrored these hand gestures. Before the experiment, short videos of five different hand gestures were shown to the participants who could choose any of these hand gestures to imitate (moving the right hand to the left ear, moving the right hand to the head, turning the head, turning the hands or moving the right hand towards the other actor). To facilitate the imitation, each hand gesture was repeated five times before a new hand gesture could be presented in the interaction task. In the asynchronous interaction, Actor B was instructed to make hand gestures that were different from the hand gestures shown by Actor A. All participants were asked to keep a neutral facial expression during the interaction task. After the task, all participants rated the likeability of the interaction partner on a Likert scale (1 = very unlikable; 7 = very likeable). In the synchronous condition, Actor A also rated the quality of the imitation (1 = very low; 7 = very high). Saliva samples were collected at the start (i.e. before the task) and after completion of the dyadic interaction (i.e. after the task).
Results
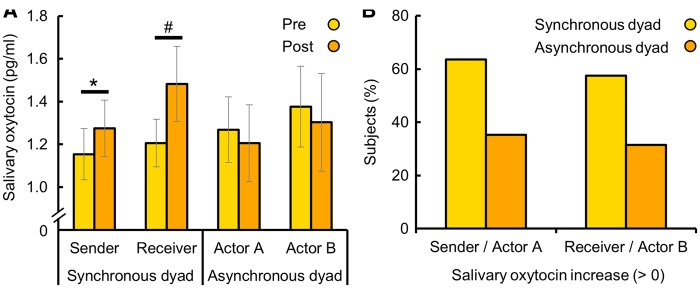
A repeated measures ANOVA with ‘time point of saliva sampling’ (before the task, after the task) as within-subject factor, ‘synchrony’ (synchronous, asynchronous), ‘actor’ (sender, receiver) and ‘use of hormonal contraception’ (hormonal contraception use, freely cycling) as between-subject variables, and the salivary OXT concentration as dependent variable revealed a significant interaction of ‘time point’ and ‘synchrony’ (F(1, 151) = 5.55, P = 0.02, η2 = 0.04), but no further main or interaction effects. Post hoc paired t-tests revealed that the synchronous interaction evoked the release of endogenous OXT (sender: t(42) = 2.19, P = 0.04, d = 0.13; receiver: t(47) = 1.91, P = 0.06, d = 0.27; cf. Figure 1A), while there was no effect in the asynchronous condition (P ≥ 0.41). In fact, the number of participants who showed an increase in OXT concentration (> 0 pg/ml) during the task was significantly larger in the synchronous than in the asynchronous condition (χ2(1) = 11.12, P < 0.01, cf. Figure 1B). This pattern of results did not change when we included gender as an additional between-subject variable (cf. Supplementary Information). The likability ratings of the interaction partner were comparable between the synchronous and the asynchronous conditions (t(157) = 0.59, P = 0.56, d = 0.09). Furthermore, there was no association between likeability and imitation quality ratings and OXT concentrations (before the task, after the task or in the difference ‘after minus before’; all Ps ≥ 0.14).
Fig. 1.
Effect of imitation on endogenous oxytocin concentrations. The imitation of hand gestures induced the release of endogenous oxytocin (OXT) in both the sender and the receiver (i.e. imitator) of synchronously interacting dyads (A). Likewise, the majority of subjects in synchronous dyads (n = 90) showed an increase of salivary OXT concentrations during the imitation (B). There was no significant change in the OXT concentrations of non-synchronous dyads (n = 69). Error bars indicate the standard error of the mean (SEM). OXT, oxytocin; Pre, before imitation; Post, after imitation. *P < 0.05, #P < 0.10.
Collectively, we demonstrate increased OXT release after synchronous but not asynchronous interactions in both the sender and receiver of non-verbal signals, which was not driven by differences in gender, likability or imitation quality.
Experiment 2
In Experiment 1 we showed that synchrony increases peripheral OXT levels; in Experiment 2 we wanted to follow up and ask whether increased OXT levels then impact social–emotional communication. Here we moved to an experimental paradigm to elucidate causal effect of heightened OT on emotion expression in the sender of social information. To this end, we separately recorded male participants while they expressed happy, fearful, and angry facial and vocal expressions. All senders conducted the emotion expression task twice on two different days, after having received OXT or PLC, respectively (see ‘Emotion expression recording’). An independent sample of receivers rated the emotional intensity by quantifying the intensity of happiness, fear and anger they perceived in every expression (see ‘Emotion expression rating’).
Methods
Emotion expression recording
Subjects and protocol . The expression task included 35 healthy male senders. Following a double-blind, placebo-controlled, within-subject design, participants performed the emotion expression task twice (once after intranasal OXT and once after intranasal PLC administration). The order of OXT and PLC application was randomized across testing days and the mean latency between application of treatment and task onset was 52 min. During the waiting time, senders filled out questionnaires and participated in an unrelated task. Study appointments were scheduled at least 24 h apart. Data from three subjects were lost due to technical problems, resulting in a final sample size of 32 participants (age: 24.5 ± 4.1 years).
Intranasal treatment . Participants self-administered nasal sprays containing either synthetic OXT or PLC at the beginning of the testing session. The administration instructions were in accordance with the latest standardization guidelines (Guastella et al., 2013) and administration was supervised by a trained research assistant. Participants received an OXT dose of 24 IU (three puffs per nostril, each with 4 IU OXT). The synthetic OXT and the PLC solutions were provided by Sigma-Tau Pharmaceuticals, Inc. (Pomezia, Italy). The PLC solution contained the identical ingredients except for the peptide itself.
Accumulating evidence indicates that intranasal OXT leads to elevated OXT concentrations in the cerebrospinal fluid (CSF) in macaques (Dal Monte et al., 2014; Lee et al., 2017) and humans (Striepens et al., 2013). CSF penetration by intranasal OXT may be much lower than penetration into the cerebral tissue, as only a small fraction of intranasal OXT reaches the CSF, where it has to accumulate (over 60–75 min) in order to produce a signal (Lee et al., 2017; Striepens et al., 2013). Experiments in mice have shown that intranasal OXT quickly permeates into specific areas of the brain (Smith et al., 2016), thus underlining OXT’s central mode of action (Kanat et al., 2014) and the utility of intranasal OXT as a pharmacological means to alter central OXT signaling.
Task procedure . The emotion expression task comprised two runs, requiring emotion expression in the facial and vocal modalities, respectively. Specifically, participants were recorded while producing angry, fearful and happy facial expressions (with a neutral face in between emotions) and vocalizing pseudowords with angry, fearful, happy and neutral prosodies on demand. Randomly presented, one-word instructions on a computer screen indicated the emotion to be expressed next, complemented by a disyllabic pseudoword to be read aloud in the prosody condition. The here used pseudowords (i.e. phonologically legal but semantically meaningless letter strings, such as ‘hultin’ or ‘galeng’) were retrieved from the validated test battery TEPP© (Wendt and Scheich, 2002; Wendt, 2007). Stimuli from the TEPP© have already been applied in multiple experimental and clinical studies on prosody (Edwards et al., 2002; Quadflieg et al., 2007; Thönnessen et al., 2010). Two beeps of defined audible frequencies (one sharp and one deep tone) 3 s apart marked the start and end of an emotional expression. The interstimulus interval between emotional expression and instruction was 3 s. To prevent interference between two consecutively displayed facial expressions, non-emotional control blocks (yawning, opening and closing one’s mouth, puffing one’s cheeks) were alternated with the emotions of interest, but were not further considered in the analysis. The vocal and facial expressions were recorded twice. The first expression served as a practice trial to prevent confounds due to surprise or inattention effects. Thus, only the second emotion expression was further processed and evaluated by the independent sample of raters. The order of trials was randomized across all subjects in both the facial and vocal recording conditions. Facial expressions were recorded via a camcorder (SONY HD-Camcorder HXR-MC2000E) that constantly filmed the sender from the shoulders up against a grey backdrop. Senders were asked to directly face the camera after the presentation of instructional cue words. Vocal expressions were recorded using a high quality recording device, with the microphone placed 5 cm away from senders’ mouths. During all recordings, senders were alone in the testing room and sat on a fixed chair.
Each run lasted ∼7 min, resulting in a total task duration of 14 min. Finally, senders were asked to rate their stress levels during the recording procedure on a scale ranging from 0 (‘not at all stressful’) to 10 (‘very stressful’) and to guess whether they had received OXT or PLC at the current testing day. Saliva samples were collected before nasal spray administration (baseline), and at the start and after completion of the emotion expression task.
Data processing . After completion of the experiment, a blinded assistant who was not involved in the experiments evaluated the video and audio recordings for recording quality and processed them with iMovie (Version 10.0.5) on a Mac G5 tower running OSX 10.9, resulting in single, 3-s long video/audio files for each emotion expression. Videos were standardized in height and width and adjusted to comparable brightness. Audio files were adjusted to comparable volume. This standardized editing procedure eventually resulted in a final stimulus pool of 256 facial and 256 vocal expressions (128 taken after OXT and 128 after PLC administration).
Emotion expression ratings
Subjects . For the subsequently conducted emotional expressions ratings, we recruited an independent sample of 106 subjects (referred to as ‘receivers’ from here on). All receivers were randomly assigned to quantify the emotional intensity of either vocal or facial expressions. Five subjects had to be excluded from analysis as they failed to complete the task. Thus, the final sample comprised 56 receivers for the facial expressions (21 male, 25.42 ± 4.22 years) and 45 receivers for vocal expressions (22 male, 25.67 ± 4.36 years). There were no a priori differences between sender and receiver samples (cf. Supplementary Table S2).
Rating procedure . All receivers read standardized instructions that explained the rating procedure and were then seated in front of a computer screen. Receivers assigned to judge vocal expressions were additionally equipped with noise-canceling headphones. In each of the 256 randomly ordered trials (32 senders displaying four emotions; once after OXT, once after PLC treatment), receivers saw or heard one of the previously recorded happy, fearful, angry or neutral expressions. After each trial, participants were asked to quantify the intensity of happiness, fear and anger in the precedent emotion expression using visual analogue scales ranging from 1 (minimal intensity) to 100 (maximal intensity). Hence, the final data pool for each receiver consisted of three intensity ratings per stimulus (e.g. for happy expressions there was one intensity rating for the instructed emotion category, happiness, and two for the remaining alternative categories).
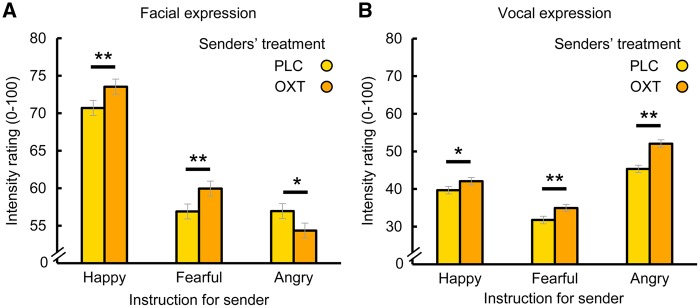
Results . To evaluate the impact of the intranasal OXT administration on facial expressiveness, we computed a repeated measures ANOVA with ‘senders’ emotion’ (happy, fearful and angry) and ‘senders’ treatment’ (OXT, PLC) as within-subject factors and the ‘mean intensity rating for the depicted facial emotion’ as dependent variable. This ANOVA yielded main effects of ‘senders’ emotion’ (F(2, 110) = 70.67, P < 0.01, η2 = 0.56) and ‘senders’ treatment’ (F(1, 55) = 5.23, P = 0.03, η2 = 0.09) as well as a significant interaction of both factors (F(2, 110) = 14.91, P < 0.01, η2 = 0.21; cf. Figure 2A). Post hoc comparisons revealed that OXT enhanced the emotional intensity of expressions of happiness (t(55) = 4.5, P < 0.01, d = 0.16) and fear (t(55) = 3.32, P < 0.01, d = 0.11), whereas angry expressions were perceived as less intense after OXT administration (t(55) = -2.91, P = 0.02, d = -0.14).
Fig. 2.
Effects of elevated oxytocin levels on the salience of facial and vocal emotional expressions. Following the intranasal administration of oxytocin (OXT; 24 IU) or placebo (PLC), 32 male participants (senders) were instructed to produce facial and vocal expressions of anger, fear and happiness. Two independent samples of women and men (receivers, n = 56 and n = 45) rated the intensity of the facial and vocal expressions. OXT enhanced the perceived intensity of happy and fearful facial expressions but reduced the perceived intensity of the facial display of anger (A). In the auditory domain, OXT increased the emotional expressions’ intensity unequivocally across valences (B). Error bars indicate the standard error of the mean (SEM). OXT, oxytocin; PLC, placebo. *P < 0.05, **P < 0.01.
A repeated measures ANOVA with the ‘mean intensity rating for the depicted vocal emotion’ as the dependent variable also showed main effects of ‘senders’ emotion’ (F(2, 88) = 43.14, P < 0.01, η2 = 0.50) and ‘senders’ treatment’ (F(1, 44) = 52.07, P < 0.01, η2 = 0.54) as well as a significant interaction effect (F(2, 88) = 7.6, P = 0.01, η2 = 0.15; cf. Figure 2B). In contrast to facial expression, OXT treatment led to increased intensity of vocal expressions in all emotion categories. However, this effect was more pronounced for angry expressions (t(44) = 6.64, P < 0.01, d = 0.39) than for fearful (t(44) = 3.51, P < 0.01, d = 0.18) or happy (t(44) = 2.64, P = 0.03, d = 0.13) expressions (for details on task validation and emotion-specific recognition patterns see Supplementary Figure S1 and Supplementary Information). Treatment had no effect on subjective stressfulness ratings or cortisol levels as a surrogate marker of stress axis activity (for details see Supplementary Information).
Collectively, our results provide evidence for a key role of OXT in modulating emotional expressiveness. Specifically, OXT increased the intensity of expression of happiness and fear across sensory modalities, while differentially modulating angry expressions.
Discussion
In accordance with our initial hypotheses, in Experiment 1 we found that the experience of social synchrony evokes the release of endogenous OXT in both the sender and the receiver of non-verbal signals. Intriguingly, the results of Experiment 2 indicate that heightened OXT levels enhance emotion transmission of social information. Specifically, intranasal OXT made signals of happiness and fear more salient in both facial and vocal expressions. Thus, OXT seems to augment the sender’s expressiveness, yielding a bidirectional enhancement of social communication.
Overall, our findings point to a central role of social synchrony in facilitating reciprocal communication between individuals via heightened OXT signaling. By means of a bio-behavioral feedback loop, elevated OXT levels among highly synchronized interacting partners may contribute to a bidirectional refinement of emotion transmission, which in turn yields further interpersonal synchronization and concomitant OXT release in both the sender and the receiver of social information. How can enhanced emotional expressiveness promote social synchrony? First, more salient facial and vocal expressions of emotions facilitate the communication of nuanced information about the nature of any dyadic relationship (Niedenthal and Brauer, 2012). Affective displays convey information about the sender’s underlying motives and character (Ames and Johar, 2009) and modulate the likelihood of cooperation (Brown et al., 2003). Second, built on mechanisms of simulation and embodiment (Niedenthal, 2007), more pronounced expressiveness may support vicarious emotional responding. Thus, it seems likely that the improved transmission of emotional signals may foster coordinated behavior in both timing and form (Duranton and Gaunet, 2016). Collectively, our results highlight the involvement of the oxytocinergic system in this circular amplification of dyadic attunement, which may also explain why synchrony can increase cooperation (Wiltermuth and Heath, 2009).
Our observation of increased OXT levels after synchronous, but not asynchronous, interaction in Experiment 1 is congruent with the finding that intranasal OXT enhances automatic imitative behavior (De Coster et al., 2014) and supports the notion that OXT mediates the effects of imitation on social behavior (Aoki and Yamasue, 2015). Accordingly, heightened generosity (van Baaren et al., 2003) and visual preference or affiliative behaviors towards an imitating person (Field et al., 2001) may thus depend on increased OXT secretion in the imitated person. In fact, it has been reported that a social interaction pretreatment has a similar effect as the intranasal administration of OXT on the processing of negative emotional faces (Kis et al., 2013). Against this background, therapeutic interventions fostering imitation or synchronization in general (Koehne et al., 2016), may constitute an efficient non-pharmacological probe to increase OXT levels and improve social skills.
While numerous studies have documented that intranasal OXT enhances emotion recognition from both static (Domes et al., 2007) and dynamic facial expressions (Lischke et al., 2012), evidence for an effect of OXT on emotion expression generation has been scarce to date. Here, we demonstrate OXT’s positive influence on expressiveness in the facial and vocal domains. These findings resonate well with previous observations that individual differences in the OXT receptor gene are associated with behavioral manifestations of prosociality (Kogan et al., 2011) and that cortisol-induced increases in plasma OXT levels correlate with self-reported emotional expressiveness (Tops et al., 2007). Of note, the observed OXT effect on the expression of happiness and fear is also remarkably congruent with the OXT effect on emotion recognition. A recent meta-analysis concluded that OXT specifically affects the recognition accuracy of happiness and fear (Shahrestani et al., 2013). In contrast to anger expressions, both happy and fearful facial expressions elicit approach behavior (Marsh et al., 2005). As such, the enhancing effect of OXT on happy and fearful expressions in both the facial and vocal domain lends further support to theoretical models assuming that OXT facilitates approach behavior (Kemp and Guastella, 2011). An alternative explanation for the increased intensity of facial expressions might be that the neuropeptide facilitates the control of facial muscles. However, the fact that OXT produced similar effects in the vocal domain speaks against a simple muscular mechanism and rather points to global effects of OXT on emotional expressiveness.
OXT thus seems to attenuate the ambiguity of emotional expressions, thereby enhancing the salience, and reducing uncertainty about the predictive value, of communicative signals (Meyer-Lindenberg et al., 2011). An improved signal-to-noise ratio in emotion transmission under conditions of OXT-mediated social synchrony may lie at the core of feelings of closeness and familiarity. This notion has strong implications for a wide range of social settings and contexts, ranging from gestures of encouragement in team sports and the communication of empathic concern during patient–caregiver interactions to attachment bonds between parents and infants. For instance, we have previously shown that OXT promotes the romantic bond between men and women by enhancing reward system responses to the partner’s face (Scheele et al., 2013; Scheele et al., 2016). Findings that marital satisfaction positively correlates with emotional expressiveness (King, 1993), could thus be driven in part by an OXT-induced increase in behavioral synchrony between partners in addition to other changes in communicative style (Ditzen et al., 2009; Ditzen et al., 2013).
Several aspects of our study deserve further comment. First, only male participants were tested in Experiment 2. As a substantial body of evidence now points to sexual-dimorphic effects of OXT (Preckel et al., 2014; Rilling et al., 2014; Scheele et al., 2014), we cannot extrapolate our findings to women. Therefore, future studies are warranted to replicate the current findings in women and explore possible interactions with gonadal steroids. Second, in the present study, we focused on instructed imitation and posed expressions. It remains to be determined how spontaneous social synchrony affects OXT levels in the sender and receiver and whether the effects of OXT are comparable between spontaneous and instructed production of emotion expressions (Motley and Camden, 1988). Third, we applied a consecutive study design to standardize the social imitation and interaction and did not assess emotion transmission in a real-life setting.
In conclusion, we here propose a comprehensive model that sheds new light on the role of the OXT signaling in mediating emotion transmission between individuals by facilitating communicative reciprocity. In the presence of social synchrony, OXT may enhance the salience of emotional expressions towards an interacting partner, thus improving the signal-to-noise ratio in social communication and promoting social cohesion.
Supplementary data
Supplementary data are available at SCAN online.
Supplementary Material
Acknowledgements
The present work was supported by a German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) Grant (HU1202/4-1 and BE 5465/2-1 to R.H. and D.S.). F.B.S. was supported by a PhD fellowship by the German National Academic Foundation (Studienstiftung des Deutschen Volkes e.V.). The authors wish to thank Paul Jung for programming assistance and Alexandra Patin for proofreading of the manuscript.
Conflict of interest. None declared.
Author contributions
F.B.S. and D.S contributed equally to this work (shared 1st authorship); F.B.S., D.S. and R.H. designed the experiments; F.B.S., D.S., C.K., A.F. and S.S. conducted the experiments; F.B.S., D.S., C.K., A.F., S.S. and R.H. analyzed the data; B.S. contributed new reagents/analytic tools; F.B.S., D.S., N.M., C.K., A.F., S.S., B.S., W.M. and R.H. wrote the paper.
References
- Ames D.R., Johar G.V. (2009). I’ll know what you’re like when I see how you feel. Psychological Science, 20, 586–93. [DOI] [PubMed] [Google Scholar]
- Anagnostou E., Soorya L., Chaplin W., et al. (2012). Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: a randomized controlled trial. Molecular Autism, 3, 1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andari E., Duhamel J.-R., Zalla T., Herbrecht E., Leboyer M., Sirigu A. (2010). Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proceedings of the National Academy of Sciences, 107, 4389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y., Yamasue H. (2015). Reply: does imitation act as an oxytocin nebulizer in autism spectrum disorder? Brain, 138, e361.. [DOI] [PubMed] [Google Scholar]
- Arueti M., Perach-Barzilay N., Tsoory M.M., Berger B., Getter N., Shamay-Tsoory S.G. (2013). When two become one: the role of oxytocin in interpersonal coordination and cooperation. Journal of Cognitive Neuroscience, 25, 1418–27. [DOI] [PubMed] [Google Scholar]
- Atzil S., Hendler T., Feldman R. (2014). The brain basis of social synchrony. Social Cognitive and Affective Neuroscience, 9, 1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. (2001). The autism-spectrum quotient (AQ): evidence from asperger syndrome/high-functioning autism, malesand females, scientists and mathematicians. Journal of Autism and Developmental Disorders, 31, 5–17. [DOI] [PubMed] [Google Scholar]
- Baumgartner T., Heinrichs M., Vonlanthen A., Fischbacher U., Fehr E. (2008). Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron, 58, 639–50. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. (1996) Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Behrends A., Müller S., Dziobek I. (2012). Moving in and out of synchrony: a concept for a new intervention fostering empathy through interactional movement and dance. The Arts in Psychotherapy, 39, 107–16. [Google Scholar]
- Bernieri F.J. (1988). Coordinated movement and rapport in teacher–student interactions. Journal of Nonverbal Behavior, 12, 120–38. [Google Scholar]
- Brown W.M., Palameta B., Moore C. (2003). Are there nonverbal cues to commitment? An exploratory study using the zero-acquaintance video presentation paradigm. Evolutionary Psychology, 1, 42–69. [Google Scholar]
- Cacioppo S., Zhou H., Monteleone G., et al. (2014). You are in sync with me: neural correlates of interpersonal synchrony with a partner. Neuroscience, 277, 842–58. [DOI] [PubMed] [Google Scholar]
- Catmur C., Heyes C. (2013). Is it what you do, or when you do it? The roles of contingency and similarity in pro-social effects of imitation. Cognitive Science, 37, 1541–52. [DOI] [PubMed] [Google Scholar]
- Dal Monte O., Noble P.L., Turchi J., Cummins A., Averbeck B.B. (2014). CSF and blood oxytocin concentration changes following intranasal delivery in macaque. PLoS ONE, 9, e103677.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.H. (1983). Measuring individual differences in empathy: evidence for a multidimensional approach. Journal of Personality and Social Psychology, 44, 113. [Google Scholar]
- De Coster L., Mueller S.C., T'Sjoen G., De Saedeleer L., Brass M. (2014). The influence of Oxytocin on automatic motor simulation. Psychoneuroendocrinology, 50, 220–6. [DOI] [PubMed] [Google Scholar]
- Declerck C.H., Boone C., Kiyonari T. (2014). The effect of oxytocin on cooperation in a prisoner’s dilemma depends on the social context and a person’s social value orientation. Social Cognitive and Affective Neuroscience, 9, 802–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzen B., Nater U.M., Schaer M., et al. (2013). Sex-specific effects of intranasal oxytocin on autonomic nervous system and emotional responses to couple conflict. Social Cognitive and Affective Neuroscience, 8, 897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzen B., Schaer M., Gabriel B., Bodenmann G., Ehlert U., Heinrichs M. (2009). Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological Psychiatry, 65, 728–31. [DOI] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Michel A., Berger C., Herpertz S.C. (2007). Oxytocin improves “mind-reading” in humans. Biological Psychiatry, 61, 731–3. [DOI] [PubMed] [Google Scholar]
- Duranton C., Gaunet F. (2016). Behavioural synchronization from an ethological perspective: overview of its adaptive value. Adaptive Behavior, 24, 1–11. [Google Scholar]
- Edwards J., Jackson H.J., Pattison P.E. (2002). Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clinical Psychology Review, 22, 789–832. [DOI] [PubMed] [Google Scholar]
- Feldman R. (2012). Parent–infant synchrony: a biobehavioral model of mutual influences in the formation of affiliative bonds. Monographs of the Society for Research in Child Development, 77, 42–51. [Google Scholar]
- Feldman R., Gordon I., Influs M., Gutbir T., Ebstein R.P. (2013). Parental oxytocin and early caregiving jointly shape children’s oxytocin response and social reciprocity. Neuropsychopharmacology, 38, 1154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T., Field T., Sanders C., Nadel J. (2001). Children with autism display more social behaviors after repeated imitation sessions. Autism, 5, 317–23. [DOI] [PubMed] [Google Scholar]
- Guastella A.J., Einfeld S.L., Gray K.M., et al. (2010). Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biological Psychiatry, 67, 692–4. [DOI] [PubMed] [Google Scholar]
- Guastella A.J., Hickie I.B., McGuinness M.M., et al. (2013). Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology, 38, 612–25. [DOI] [PubMed] [Google Scholar]
- Hove M.J., Risen J.L. (2009). It's all in the timing: interpersonal synchrony increases affiliation. Social Cognition, 27, 949–60. [Google Scholar]
- Hurlemann R., Scheele D. (2016). Dissecting the role of oxytocin in the formation and loss of social relationships. Biological Psychiatry, 79, 185–93. [DOI] [PubMed] [Google Scholar]
- Kagerbauer S., Martin J., Schuster T., Blobner M., Kochs E., Landgraf R. (2013). Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. Journal of Neuroendocrinology, 25, 668–73. [DOI] [PubMed] [Google Scholar]
- Kanat M., Heinrichs M., Domes G. (2014). Oxytocin and the social brain: neural mechanisms and perspectives in human research. Brain Research, 11, 160–71. [DOI] [PubMed] [Google Scholar]
- Kemp A.H., Guastella A.J. (2011). The role of oxytocin in human affect a Novel Hypothesis. Current Directions in Psychological Science, 20, 222–31. [Google Scholar]
- King L.A. (1993). Emotional expression, ambivalence over expression, and marital satisfaction. Journal of Social and Personal Relationships, 10, 601–7. [Google Scholar]
- Kirschner S., Tomasello M. (2010). Joint music making promotes prosocial behavior in 4-year-old children. Evolution and Human Behavior, 31, 354–64. [Google Scholar]
- Kis A., Kemerle K., Hernadi A., Topal J. (2013). Oxytocin and social pretreatment have similar effects on processing of negative emotional faces in healthy adult males. Frontiers in Psychology, 4, 532.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehne S., Behrends A., Fairhurst M.T., Dziobek I. (2016). Fostering social cognition through an imitation- and synchronization-based dance/movement intervention in adults with autism spectrum disorder: a controlled proof-of-concept study. Psychotherapy and Psychosomatics, 85, 27–35. [DOI] [PubMed] [Google Scholar]
- Kogan A., Saslow L.R., Impett E.A., Oveis C., Keltner D., Saturn S.R. (2011). Thin-slicing study of the oxytocin receptor (OXTR) gene and the evaluation and expression of the prosocial disposition. Proceedings of the National Academy of Sciences, 108, 19189–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M., Heinrichs M., Zak P.J., Fischbacher U., Fehr E. (2005). Oxytocin increases trust in humans. Nature, 435, 673–6. [DOI] [PubMed] [Google Scholar]
- Lee M.R., Scheidweiler K.B., Diao X.X., et al. (2017). Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: determination using a novel oxytocin assay. Molecular Psychiatry, doi:10.1038/mp.2017.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J., Goldstein A., Zagoory-Sharon O., et al. (2016). Oxytocin selectively modulates brain response to stimuli probing social synchrony. NeuroImage, 124, 923–30. [DOI] [PubMed] [Google Scholar]
- Lischke A., Berger C., Prehn K., Heinrichs M., Herpertz S.C., Domes G. (2012). Intranasal oxytocin enhances emotion recognition from dynamic facial expressions and leaves eye-gaze unaffected. Psychoneuroendocrinology, 37, 475–81. [DOI] [PubMed] [Google Scholar]
- Marsh A.A., Ambady N., Kleck R.E. (2005). The effects of fear and anger facial expressions on approach- and avoidance-related behaviors. Emotion, 5, 119–24. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A., Domes G., Kirsch P., Heinrichs M. (2011). Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews Neuroscience, 12, 524–38. [DOI] [PubMed] [Google Scholar]
- Motley M.T., Camden C.T. (1988). Facial expression of emotion: A comparison of posed expressions versus spontaneous expressions in an interpersonal communication setting. Western Journal of Communication, 52, 1–22. [Google Scholar]
- Mu Y., Guo C., Han S. (2016). Oxytocin enhances inter-brain synchrony during social coordination in male adults. Social Cognitive and Affective Neuroscience, 11, 1882–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedenthal P.M. (2007). Embodying emotion. Science, 36, 1002–5. [DOI] [PubMed] [Google Scholar]
- Niedenthal P.M., Brauer M. (2012). Social functionality of human emotion. Annual Reviews of Psychology, 63, 259–85. [DOI] [PubMed] [Google Scholar]
- Paukner A., Suomi S.J., Visalberghi E., Ferrari P.F. (2009). Capuchin monkeys display affiliation toward humans who imitate them. Science, 325, 880–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preckel K., Scheele D., Kendrick K.M., Maier W., Hurlemann R. (2014). Oxytocin facilitates social approach behavior in women. Frontiers in Behavioral Neuroscience, 8, 191.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadflieg S., Wendt B., Mohr A., Miltner W.H., Straube T. (2007). Recognition and evaluation of emotional prosody in individuals with generalized social phobia: a pilot study. Behaviour Research and Therapy, 45, 3096–103. [DOI] [PubMed] [Google Scholar]
- Ramseyer F., Tschacher W. (2011). Nonverbal synchrony in psychotherapy: coordinated body movement reflects relationship quality and outcome. Journal of Consulting and Clinical Psychology, 79, 284.. [DOI] [PubMed] [Google Scholar]
- Rilling J.K., Demarco A.C., Hackett P.D., et al. (2014). Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology, 39, 237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D., Plota J., Stoffel-Wagner B., Maier W., Hurlemann R. (2016). Hormonal contraceptives suppress oxytocin-induced brain reward responses to the partner's face. Social Cognitive and Affective Neuroscience, 11, 767–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D., Striepens N., Güntürkün O., et al. (2012). Oxytocin modulates social distance between males and females. The Journal of Neuroscience, 32, 16074–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D., Striepens N., Kendrick K.M., et al. (2014). Opposing effects of oxytocin on moral judgment in males and females. Human Brain Mapping, 35, 6067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D., Wille A., Kendrick K.M., et al. (2013). Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proceedings of the National Academy of Sciences, 110, 20308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman I., Zagoory-Sharon O., Leckman J.F., Feldman R. (2012). Oxytocin during the initial stages of romantic attachment: relations to couples’ interactive reciprocity. Psychoneuroendocrinology, 37, 1277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrestani S., Kemp A.H., Guastella A.J. (2013). The impact of a single administration of intranasal oxytocin on the recognition of basic emotions in humans: a meta-analysis. Neuropsychopharmacology, 38, 1929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D. V., Lecrubier Y., Sheehan K. H., et al. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59, 22–33. [PubMed] [Google Scholar]
- Striepens N., Kendrick K.M., Hanking V., et al. (2013). Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Scientific Reports, 3, 3440.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E.A., Sclafani V., Paukner A., et al. (2014). Inhaled oxytocin increases positive social behaviors in newborn macaques. Proceedings of the National Academy of Sciences, 111, 6922–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.S., Korgan A.C., Fastman J., Granovetter M.C., Song J., Young W.S. (2016) Nasal oxytocin in a genetic knockout: pharmacokinetics and behavior. In: Poster presented at the Society for Neuroscience conference, San Diego, CA.
- Spielberger, C. D., Gorsuch, R. L., Lushene, R., Vagg, P. R., Jacobs, G. A. (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press.
- Taylor G.J., Bagby R.M., Parker J.D. (2003). The 20-item Toronto Alexithymia Scale: IV. Reliability and factorial validity in different languages and cultures. Journal of Psychosomatic Research, 55, 277–83. [DOI] [PubMed] [Google Scholar]
- Thönnessen H., Boers F., Dammers J., Chen Y.-H., Norra C., Mathiak K. (2010). Early sensory encoding of affective prosody: neuromagnetic tomography of emotional category changes. Neuroimage, 50, 250–9. [DOI] [PubMed] [Google Scholar]
- Tops M., van Peer J.M., Korf J. (2007). Individual differences in emotional expressivity predict oxytocin responses to cortisol administration: Relevance to breast cancer? Biological Psychology, 75, 119–23. [DOI] [PubMed] [Google Scholar]
- van Baaren R.B., Holland R.W., Steenaert B., van Knippenberg A. (2003). Mimicry for money: behavioral consequences of imitation. Journal of Experimental Social Psychology, 39, 393–8. [Google Scholar]
- Watson D., Clark L.A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology, 54, 1063.. [DOI] [PubMed] [Google Scholar]
- Wendt B. (2007) Analysen Emotionaler Prosodie, Hallesche Schriften zur Sprechwissenschaft und Phonetik, Vol. 20. Peter Lang Internationaler Verlag der Wissenschaften.
- Wendt B., Scheich H. (2002) The “Magdeburger Prosodie-Korpus”. In: Speech Prosody 2002, International Conference.
- Wiltermuth S.S., Heath C. (2009). Synchrony and cooperation. Psychological Science, 20, 1–5. [DOI] [PubMed] [Google Scholar]
- Woolley A.W., Chabris C.F., Pentland A., Hashmi N., Malone T.W. (2010). Evidence for a collective intelligence factor in the performance of human groups. Science, 330, 686–8. [DOI] [PubMed] [Google Scholar]
- Xuan, Q., Filkov, V. (2013). Synchrony in social groups and its benefits. In: Michelucci P., editors. Handbook of Human Computation. New York: Springer. pp. 791–802. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.