This study describes sockeye salmon heart rate recovery profiles after a fisheries catch-and-release simulation in 16°C, 19°C and 21°C water. Warmer temperatures increased the peak heart rate, scope for heart rate, factorial heart rate and the initial rate of energy expenditure, though the overall recovery duration was consistent across treatments.
Keywords: Cardiac, climate change, exhaustive exercise, fisheries, Pacific salmon, temperature
Abstract
Selective harvest policies have been implemented in North America to enhance the conservation of Pacific salmon (Oncorhynchus spp.) stocks, which has led to an increase in the capture and release of fish by all fishing sectors. Despite the immediate survival benefits, catch-and-release results in capture stress, particularly at high water temperatures, and this can result in delayed post-release mortality minutes to days later. The objective of this study was to evaluate how different water temperatures influenced heart rate disturbance and recovery of wild sockeye salmon (Oncorhynchus nerka) following fisheries interactions (i.e. exhaustive exercise). Heart rate loggers were implanted into Fraser River sockeye salmon prior to simulated catch-and-release events conducted at three water temperatures (16°C, 19°C and 21°C). The fisheries simulation involved chasing logger-implanted fish in tanks for 3 min, followed by a 1 min air exposure. Neither resting nor routine heart rate differed among temperature treatments. In response to the fisheries simulation, peak heart rate increased with temperature (16°C = 91.3 ± 1.3 beats min−1; 19°C = 104.9 ± 2.0 beats min−1 and 21°C = 117 ± 1.3 beats min−1). Factorial heart rate and scope for heart rate were highest at 21°C and lowest at 16°C, but did not differ between 19°C and 21°C. Temperature affected the initial rate of cardiac recovery but not the overall duration (~10 h) such that the rate of energy expenditure during recovery increased with temperature. These findings support the notion that in the face of climate change, efforts to reduce stress at warmer temperatures will be necessary if catch-and-release practices are to be an effective conservation strategy.
Introduction
Catch-and-release practices have been implemented by commercial, recreational and indigenous fisheries sectors to enhance the conservation of Pacific salmon (Onchorhynchus spp.) populations (Department of Fisheries and Oceans, 2005), whereby more plentiful stocks are harvested while at-risk and non-targeted species are released. Despite the potential conservation value of catch-and-release fisheries practices, capture is stressful to fish (Davis and Olla, 2001; Arlinghaus et al., 2007; Davis et al., 2011). The behavioral and physiological responses to fisheries interactions are highly context dependent, varying among species, populations and even sexes (reviewed in Raby et al., 2015a; Patterson et al., 2016). Stressors include air exposure, handling, physical injury and exhaustive exercise, for which the resulting physiological disturbances tend to be magnified at warmer temperatures (Chopin and Arimoto, 1995; Murphy et al., 1995; Olla et al., 1998; Davis and Olla, 2001; Cooke and Suski, 2005; Gale et al., 2013). These stressors can lead to ionic and osmotic dysregulation (Donaldson et al., 2010, 2011; Gale et al., 2011; Robinson et al., 2013), increased use of anaerobic metabolism (Raby et al., 2015b; Eliason et al., 2013a,b), an increase in energy mobilization and changes in cardiovascular performance (Cooke et al., 2003; Donaldson et al., 2010; Raby et al., 2015b). Negative impacts on post-release behavior include loss of equilibrium (Gale et al., 2011), impaired swimming performance (Cooke et al., 2000; Davis, 2005; Danylchuk et al., 2007; Schreer et al., 2005; Donaldson et al., 2012), and reduced predator avoidance abilities (Mesa et al., 1994; Lima and Dill, 1990). The magnitude of the stressor will ultimately determine its effects once fish are released, ranging from no immediate or long-term deleterious effects to lethal effects (Davis, 2002; Cooke and Schramm, 2007). Extreme temperatures have been implicated in the failure of salmon to complete migration (Farrell et al., 2008; Crossin et al., 2008) and has contributed to declines in spawner abundance across many populations (Martins et al., 2011; Hinch et al., 2012). As Pacific salmon are a semelparous species, the outcome of failed migration equals zero individual fitness and reduced population growth.
Previous studies investigating fish responses to catch-and-release have primarily focused on the immediate behavioral and physiological impacts (e.g. reflex impairment scores, blood parameters; reviewed in Cooke et al., 2013). Many of these studies explored the relationship between fisheries interactions and subsequent behavior and survival using electronic tracking techniques (Donaldson et al., 2008, 2011, 2012; Raby et al., 2013). In addition, studies have looked at snapshots of physiological recovery, using blood or gill samples collected 1–3 times per individual to avoid harming the fish (Patterson et al., 2004; Shrimpton et al., 2005; Gale et al., 2011). In contrast, the real time physiological stress response throughout the period of capture, release and recovery has not been well resolved. Fisheries capture induces an acute stress response, which is primarily mediated by the release of epinephrine, norepinephrine and cortisol from the adrenocortical tissues of the head kidney (Norris and Carr, 2006). Due to technical limitations, it is not possible to directly monitor the continual secretion of the stress hormones that mediate a fisheries interaction stress response. However, since one of the many effects of these hormones is to increase heart rate (fH) and energy mobilization, remote measurement of fH is a proxy for those effects (Cooke et al., 2002, 2016; Clark et al., 2010). In fish, fH alone cannot be used to calculate oxygen consumption (given that oxygen uptake = fH × stroke volume × arteriovenous oxygen extraction; Thorarensen et al., 1996; Priede and Tytler, 1997; Farrell, 1993). Nevertheless, past research conducted on various fish species, including salmonids, has demonstrated that fH is often correlated with metabolic rate (e.g. Armstrong, 1986; Campbell et al., 2004; Clark et al., 2005; Eliason et al., 2008, 2011), and thus provides an opportunity to understand the relative energetic consequences of different stressors (Cooke et al., 2016).
We are aware of three studies that have applied fH biotelemetry or biologger devices to quantify the energetic and physiological consequences of catch-and-release in salmonids. Anderson et al. (1998) monitored post-angling fH recovery in Atlantic salmon (Salmo salar) at various temperatures; Donaldson et al. (2010) reported the relationship between swimming intensity, fH and recovery in coho salmon (Oncorhynchus kisutch); and Raby et al. (2015b) measured fH in coho salmon under various temperatures and fisheries net entanglement durations. These studies have demonstrated that fisheries interactions inflict sufficient stress to trigger a 1–2-fold increase in fH, which requires a 15–16 h recovery period to return to routine fH. Hence, these studies have shown the value of remotely measuring fH as a proxy for metabolic rate, especially given the potential for metabolic rate dependent mortality arising from the collapse of aerobic scope (Priede, 1977), particularly at high water temperatures (Eliason et al., 2011, 2013a). fH can provide an indication of the magnitude of the fisheries stress through (1) the scope for fH, defined as the difference between peak fH and resting fH (2) the recovery duration, defined as the time for fH to reach routine fH, and (3) the excess post-exercise heart beats (EPHB), defined as the number of heart beats above routine fH that a fish spends during recovery (Raby et al., 2015b).
The current study investigated Fraser River sockeye salmon fH recovery after a simulated fisheries stress event (i.e. exhaustive exercise and air exposure) across a spectrum of ecologically relevant temperatures. This represents the first study of Pacific salmon to measure cardiac responses to exercise and air exposure intended to represent a general fisheries stressor (especially relevant to recreational fishing) and associated handling. Previous studies used a net to entangle fish (i.e. Raby et al. 2015b) or did not include an air exposure component nor vary temperature (i.e. Donaldson et al. 2010). The Fraser River is Canada’s most productive salmon migration river (Northcote and Larkin, 1989). However, recently, the average water temperature of the Fraser River has been increasing at a rate between 0.025°C and 0.044°C per year (Patterson et al., 2007), reaching up to 4°C above historical means (from 1971 to 2000) in July 2015, and approaching the upper thermal threshold of some sockeye salmon populations (21°C) (Eliason et al., 2011). We therefore selected Fraser River sockeye salmon as a model species due to their ecological and socio-economic relevance and the extensive research base already conducted on their physiology and temperature tolerance (i.e. Eliason et al., 2011; Patterson et al., 2016) that enabled us to contextualize our work. We surgically implanted fH loggers into adult sockeye salmon, subjected them to the fisheries stressor, then allowed them to recover at three temperature regimes, one close to the optimal temperature for aerobic scope (ToptAS, 16°C) and two that approached the upper functional thermal tolerance (Tcrit, 19°C, 21°C) for 48 h while fH was continuously monitored. We hypothesized that recovery from exhaustive exercise would be impeded at elevated temperatures. More specifically, we predicted that both the scope for fH and the duration of recovery would be greater at supraoptimal temperatures.
Methods
This experiment was conducted under Canadian Council on Animal Care guidelines in accordance with the standards set by Carleton University (AUP #103 128).
Animal collection and care
Between 14 and 15 August 2015, 80 summer-run sockeye salmon (mean mass of 2185 ± 8 g) were beach seined from the mainstem of the Fraser River near Hope, British Columbia, Canada (Peters Band Land; 49.3858°N, 121.4419°W). The summer run consists of co-migrating populations (e.g. Chilko, Quensel, Stellako and others) and the number of individuals per population in the migration run varies between years (Burgner, 1991; Hodgson et al., 2006; Lapointe et al., 2003). Stock composition was not determined for the individual fish in the present study. However, 20 individuals sampled concurrently with the present study on 13 August 2015, consisted predominantly of sockeye salmon from the Chilko stock (n = 11), and also included sockeye salmon from Great Central (n = 1), Harrison (n = 2), Nahatlatch (n = 1), Seymour (n = 2), Stellako (n = 2), and one that could not be identified. Furthermore, the DFO stock management records from 12 to 16 August 2015, which also provides DNA samples from the same cohort as the individuals used in the present study, show that the summer run sockeye salmon stock composition consisted of predominantly Chilko and Quesnel sockeye salmon. Specifically, the stock composition per day over the 4-day sampling period consisted of 4.75% ± 2% Harrison Widgeon stock, 13.75% ± 1% Late Stuart Stellako stock, 60.75% ± 1% Chilko Quesnel stock, and 2.75% ± 1% Raft North Thompson stock (samples taken from Cottonwood, which is 4 days downstream from Peters Band Land, and at Whonoock, which is 2 days downstream from Peters Band Land) (Department of Fisheries and Oceans, 2015).
Fish were transported less than 1 h by truck to the Department of Fisheries and Oceans Canada (DFO) Cultus Lake Laboratory using 1 250 L transportation containers. Salmon were then transferred into two 22 000 L tanks that were supplied by flow-through lake water (Cultus Lake; 12.5°C ± 1°C; DO = 85–100%) under a natural photoperiod (~14 h daylight; 10 h dark). Sockeye salmon remained in the holding tank for a minimum of 48 h before any surgical manipulations. Each tank held ~40 sockeye salmon.
Surgical procedures
A total of 67 sockeye salmon were implanted with commercially available fH loggers (DST milli HRT, 13 mm × 39.5 mm, Star-Oddi, Iceland; http://www.star-oddi.com/) programmed to record fH every 5 min at 200 Hz based on previous work (Clark et al., 2010; Eliason et al., 2013a). Raw electrocardiogram (ECG) traces were measured every 3 h to evaluate the quality of the fH measurements.
Prior to logger implantation, salmon were anesthetized with a buffered (NaHCO3; 200 mg/L) tricaine methanesulfonate (MS-222; 100 mg/L) solution (Raby et al., 2015b). Once equilibrium was lost, fish were promptly transferred to a surgical trough and maintained on a weaker anesthetic solution (70 mg/L MS-222; 140 mg/L NaHCO3), which was continuously pumped over the gills throughout the procedure.
A 5 cm incision was made on the mid-line of the ventral surface just posterior to the pectoral girdle. Loggers were inserted immediately posterior to the pericardial membrane and were sutured to the body wall (PDS II polydioxanone suture; violet monofilament, 2-0). The incision was then closed using three to four single interrupted sutures. A passive integrated transponder (PIT) tag (Oregon RFID 32 mm HDX) was injected into the dorsal musculature posterior to the dorsal fin. Fish recovery was aided by ventilating the gills with fresh water before returning it into the treatment tank (1200 L; DO = 80–100%; 12.5°C ± 1°C) for 48 h, alongside three other sockeye salmon that underwent the same procedure.
Experimental procedures: acclimating to treatment temperature (Day 3)
Beginning 48 h after logger implantation, the water in each tank was increased by 1°C per hour until treatment temperature was reached: 16°C optimal temperature for aerobic scope, 19°C, and 21°C upper functional thermal tolerance limit (Eliason et al., 2011). Temperatures were maintained at ±0.5°C from treatment target throughout the study. Since the 21°C treatment was decided later into the study, this treatment only began 13 days since the fish were brought to the lab. Otherwise, tank temperature was allocated such that treatments were distributed throughout the 3-week experimental period.
Salmon were then left to acclimate to the new temperature for 24 h. This brief acclimation period is ecologically relevant because Fraser River sockeye salmon often encounter warm water and fisheries pressure simultaneously, ~24 h after initiating upriver migration (Gale et al., 2011).
Simulated fisheries capture stress and terminal fish sampling (Day 4)
The fisheries simulation occurred ~80 h post-surgery, which exceeds the 40–72 h post-surgery recovery time reported in previous salmonid research (Anderson et al. 1998; Donaldson et al. 2010; Raby et al. 2015b). At mid-day (~80 h post-surgery), tagged acclimated fish were individually chased, in a separate sampling tank (500 L; doughnut-shaped) of the same temperature as their treatment tank. Salmon were dip netted from the treatment tank into the chase tank (~2 s air exposure) and chased for 3 min (Robinson et al., 2013). Chasing consisted of three to four experimenters leaning over the edge of the tank and alternating waving their hands at the fish, lightly pinching the caudal fin, or splashing vigorously whenever the sockeye salmon passed by. The goal of the chase was to exhaust the fish to a similar level that would occur during a seine net, gill net, or angling event. There was no injury aspect to this chase method, nor were the chasers attempting to harm the fish in any way besides exhausting it. Similar chase methods have been applied in previous work (Milligan, 1996; Robinson et al., 2013) and techniques have been refined to reasonably simulate fisheries exercise (Gale et al., 2011; Cooke et al., 2013). At the end of the 3 min, fish were dip netted and lifted out of the water for a 1 min air exposure (Robinson et al., 2013). During this time, the fish PIT tag ID (Oregon RFID PIT tag reader) was recorded. Sockeye salmon were then returned to the original treatment holding tank and left for 48 h. fH monitoring duration was selected based on past research stating that 24 h is insufficient for physiological recovery (Raby et al., 2015b), while monitoring for 48 h is sufficient for the recovery of blood plasma constituents (Gale et al., 2011). Temperature and dissolved oxygen (DO) were checked every 0.5–1 h using an Oxyguard dissolved oxygen meter (Handy Polaris), taking great care to minimize disturbance to the fish by making minimal noise and not standing within view of the fish.
At 48 h post-fisheries stressor, fish were sacrificed by cerebral concussion. A 2 ml blood sample was immediately taken via caudal puncture and stored on ice for hematocrit analysis. Fork length, weight, sex and physical condition (including an autopsy for internal condition) were recorded at that time.
Hematocrit was determined using heparinized capillary tubes (75 mm Drummond Hemato-Clad, ammonium heparin) and centrifuged (Clay-Adams, NJ) at 12 000 rpm for 5 min. Since this study targeted healthy sockeye salmon, only 35 sockeye salmon (52.2%) provided usable data; N = 13 in the 16°C treatment, N = 13 in the 19°C treatment and N = 9 in the 21°C treatment. Fish were removed from the sample size due to logger dislodgement producing false fH records (N = 11), logger failure (N = 4), internal hemorrhaging (N = 3), injured liver (N = 2), premature mortality (N = 6), treatment temperature deviating from the target temperature treatment by more than 1°C for more that 3 h over the course of the experiment (N = 4), and poor condition indicated by low hematocrit (i.e. ≤20% according to Gallaugher and Farrell, 1998; N = 2).
Data processing and statistical analysis
Routine, resting and peak fH were determined for each individual fish. Resting fH was calculated by taking the average of the lowest 10th percentile fH from the fH profile once the fish reached the experimental temperature. Routine fH and post-stress recovery time (duration of fH elevation until plateau) was calculated using breakpoint regression analysis (Schwarz, 2015) in RStudio (v. 3.2.3, RStudio Inc., Boston, MA, USA; https://www.rstudio.com/), starting from the fisheries capture simulation and ending 48 h post-fisheries stress simulation. We defined routine fH as the fH once the fish recovered from the fisheries stress (recovery curve plateaued). Peak fH was defined as the most elevated fH attained following the fisheries simulation. The time to reach peak fH was measured beginning from the start of the capture simulation (Raby et al., 2015b). Using these values, scope for fH (peak fH – resting fH) and factorial scope for fH (peak fH ÷ resting fH) were also determined. Mean treatment values were compared using one-way ANOVA (α = 0.05) in Sigmaplot (v. 11.0, Systat Software Inc., San Jose, CA, USA). Transformations including ln, reciprocal, and square-root were used to satisfy normality. Post-hoc analysis was conducted using Holm–Sidak non-parametric pairwise multiple comparison.
Finally, the integrals under the mean fH curve at every half-hour, starting from the chase start time and ending once routine fH was met (end of recovery time previously determined), provided the number of EPHB caused by the stressor over the recovery period (Raby et al., 2015b). Total post-exercise heart beats (TEPHB, the sum of all the EPHB from the fisheries simulation to the recovery point) provided an estimate of the extra energy, above routine levels, that the salmon allocated towards recovery after a stress event. TEPHB was compared between temperature treatments using a one-way ANOVA (α = 0.05). In addition, the cumulative increase in EPHB was calculated at every hour post-stress to describe the fH recovery profiles. This provided a quantifiable measurement for the comparison of the duration that temperature influences the rate of recovery and the added energetic cost of recovery induced by warmer water temperature after a fisheries stress event. Using RStudio and the nlme package (Pinheiro et al., 2013), a linear mixed effects model was used to compare the hourly cumulative EPHB increase during recovery, treating individual fish as a random effect to correct for repeated measures of fH. This described the shape of the fH recovery profile across temperature treatments. The model was compared using a one-way ANOVA (α = 0.05) and a Bonferroni adjusted Tukey post-hoc analysis.
Results
Capture simulations
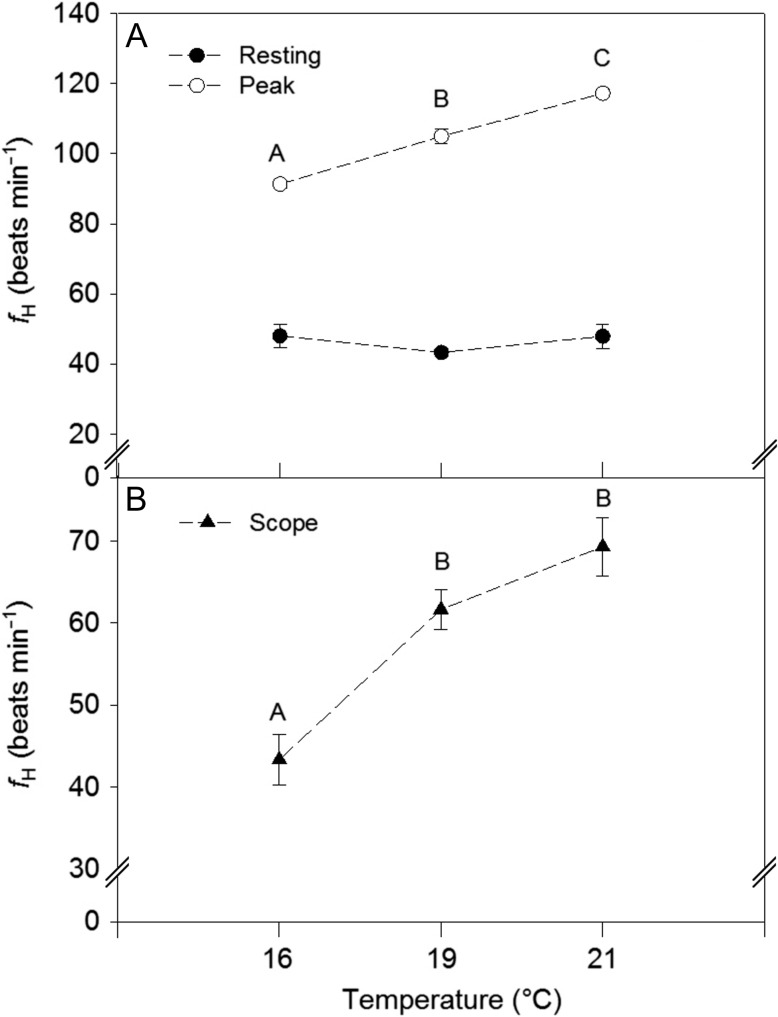
There was a significant effect of temperature on sockeye salmon fH during the fisheries capture simulation. Although resting fH did not differ between treatments (16°C = 48.0 ± 3.2 beats min−1; 19°C = 43.3 ± 1.2 beats min−1; 21°C = 47.9 ± 3.0 beats min−1; P-value = 0.461; Figs. 1 and 2A), fH traces varied with water temperature following the fisheries capture simulation. Peak fH increased with temperature between all three treatments (P-value = <0.001). Sockeye salmon in the 21°C treatment experienced the highest peak fH (117.2 ± 1.3 beats min−1) of the three treatments. The 19°C treatment sockeye salmon (104.9 ± 2.0 beats min−1) had an intermediate peak fH and the lowest peak fH occurred in the 16°C treatment group (91.3 ± 1.3 beats min−1). Similarly, scope for fH increased with temperature (P-value = <0.001; Fig. 2B). However, scope for fH did not differ significantly between the 21°C and 19°C treatments (69.3 ± 3.6 beats min−1 and 61.6 ± 6.0 beats min−1, respectively; P-value = 0.092), whereas scope for fH was ~20 beats min−1 less in the 16°C treatment compared to the other treatments (43.3 ± 3.1 beats min−1; P-value = <0.001 between 16°C versus 21°C and 16°C versus 19°C). The same trend arose in the factorial fH, revealing the magnitude of change in fH response induced by the fisheries stress (P-value = 0.004). Once again, there was no response difference between 19°C and 21°C treatments, with fH almost tripling in both water temperatures (factorial scope = 2.4 ± 0.1 and 2.5 ± 0.1 in 19°C and 21°C, respectively; P-value = 0.647). Sockeye salmon in the 16°C treatment exhibited ~20% less change in fH than fish in the 19°C and 21°C treatments (factorial scope = 1.99 ± 0.1, P-values = 0.003 and 0.005 comparing 16°C versus 21°C and 16°C versus 19°C, respectively).
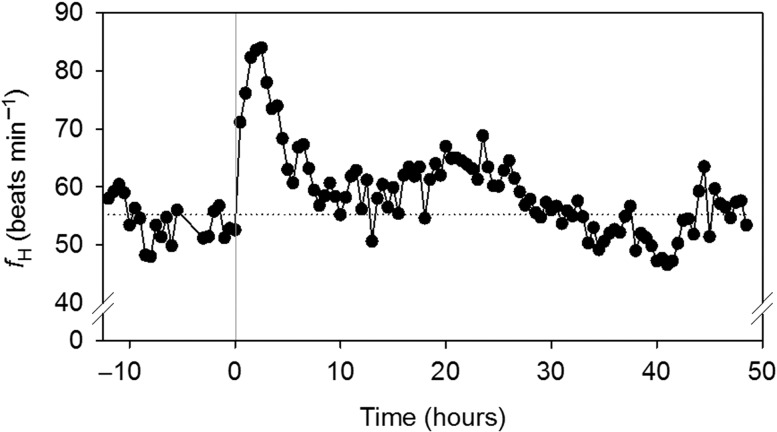
Figure 1:
Heart rate (fH) trace of an individual sockeye salmon before, during, and after fisheries pressure simulation. Sockeye salmon was exposed to the 16°C temperature treatment. Dashed line represents the routine fH. The gray line represents the fisheries capture simulation start at Time 0.
Figure 2:
Mean (±SE) (A) resting heart rate (fH) and peak fH and (B) scope for fH (peak fH – resting fH) of sockeye salmon during a fisheries capture simulation, while exposed to 16°C (N = 13), 19°C (N = 13) and 21°C (N = 9) water temperatures. Differing letters indicate significant differences among temperature treatments (One-way ANOVA; P-value <0.05).
Recovery
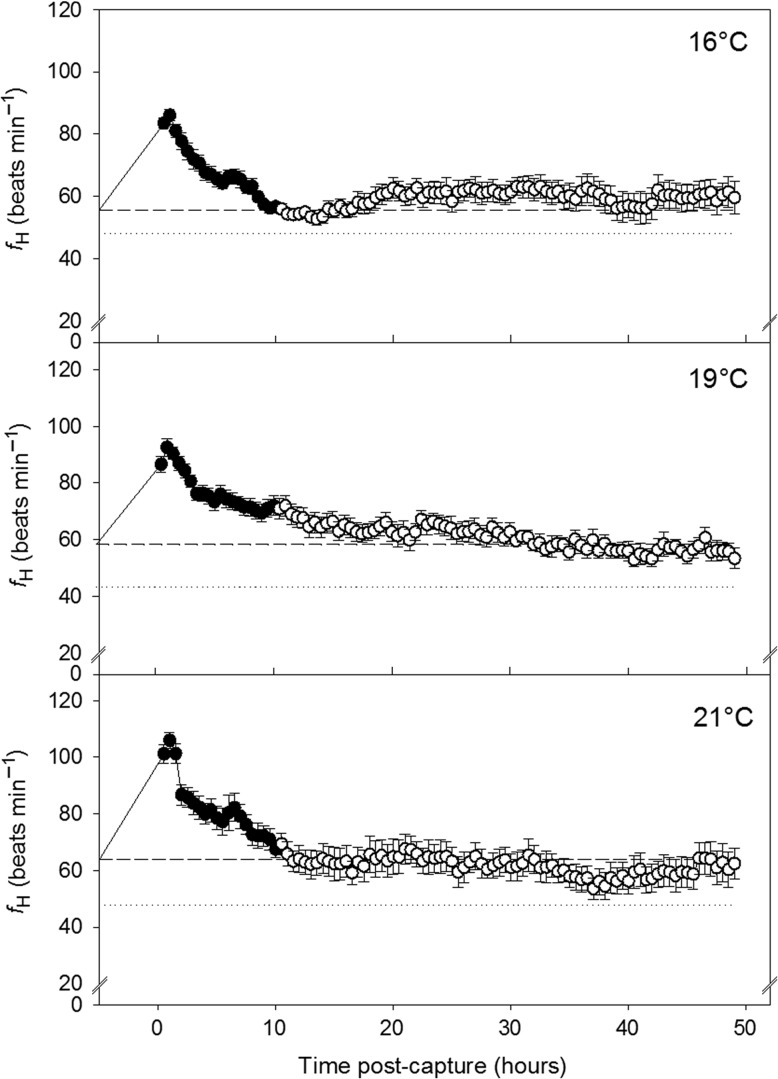
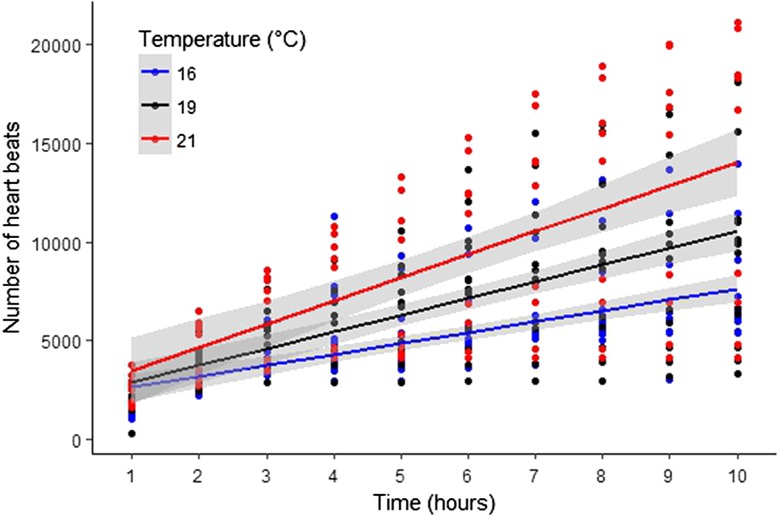
The fH recovery traces varied between treatment groups, where fH decreased at different rates before reaching a plateau, implying recovery (Figs 1 and 3). For all three temperature treatments, fH peaked at ~1 h post-capture simulation (P-value = 0.748), and recovered within ~10 h (P-value = 0.830; Fig. 3). TEPHB was highest in the 21°C treatment, but the difference was marginally non-significant at P-value = 0.085. Temperature strongly influenced the rate of change in EPHB during the first 10 h post-fisheries simulation (Fig. 4, time: P-value = <0.0001; temperature: P-value = 0.030). Specifically, EPHB was lower in the 16°C treatment group compared to EPHB in the 21°C treatment group (P-value = 0.015). The EPHB of fish exposed to 19°C did not significantly differ from either the 16°C (P-value = 0.470) nor the 21°C (P-value = 0.390) treatments. Details about when EPHB began to diverge between the 16°C and 21°C treatments remains unknown due to lack of significant interaction between temperature and time (P-value = 0.210).
Figure 3:
Sockeye salmon mean (±SE) heart rate (fH) recovery profiles (black circles), resting fH (mean lowest 10th percentile of fH from the entire experiment) (dotted line), and routine fH (mean recovery fH determined by breakpoint regression analysis) (dashed line) over time (hours) since fisheries capture simulation in 16°C (top), 19°C (middle) and 21°C (bottom) water. Black circles represent fH recovery during the first 10 h of recovery. White circles show fH recovery from 11 to 48 h post-fisheries simulation. Time 0 represents the fisheries capture simulation start time, and fH was monitored for the next 48 h.
Figure 4:
Regression plot showing the variability of cumulative excess post-exercise heart beats (EPHB) (beats) within temperature treatments, and the general relationship between temperature treatments according to the linear mixed effect model with individual fish as repeated measures, against the recovery time in hours post-fisheries capture simulation. The 16°C treatment (N = 13) is denoted by the blue circles and the blue regression line, the 19°C (N = 13) treatment is denoted by the black circles and black regression line, and the 21°C treatment (N = 9) is denoted by the red circles and red regression line. The shaded area around each regression line represents the 95% confidence region. Time 0 represents the start of the fisheries capture simulation.
Discussion
Using fH loggers, this study monitored fH in free-swimming sockeye salmon following a fisheries simulation event. We demonstrated that water temperature mediates the fH response to exhaustive exercise. Consistent with previous research using fH loggers in fish (Anderson et al., 1998; Donaldson et al., 2010; Raby et al., 2015b), fH increased 2.0–2.5 fold with anaerobic burst exercise and recovered back to routine levels within ~10 h. Exhaustive anaerobic exercise results in a depletion of energy and oxygen stores, the disruption of ionic, osmotic and biochemical balances, hypoxemia and acidosis (Gaesser and Brooks, 1984; Wood, 1991; Scarabello et al., 1992; Gale et al., 2011). Recovery is fueled by an increase in aerobic metabolism (excess post-exercise oxygen consumption, EPOC) (Gaesser and Brookes, 1984; Wood, 1991; Lee et al., 2003), which is supported by elevated cardiac output. The sockeye salmon in the current study exhibited the expected fH recovery profile after the fisheries capture simulation; in all three temperature treatments fH increased in response to the fisheries simulation and was followed by a prolonged recovery. However, the ‘rates' of fH recovery varied with temperature though the overall recovery ‘duration' did not differ.
Temperature effects on peak cardiac activity
Warmer water temperatures can limit cardiovascular performance and oxygen uptake (Fry, 1971). Based on the present results, fisheries interactions or exhaustive exercise occurring in higher temperatures likely influence fH recovery in several ways. Studies have shown that high water temperature directly mediates this process by increasing the intrinsic rate of the cardiac pacemaker cells (Randall, 1970; Farrell, 1991), resulting in an increase in fH and thus cardiac output, and thereby increasing oxygen delivery to the tissues. Yet, Eliason et al. (2013a) demonstrated that in swimming sockeye salmon, the rate of oxygen delivery to tissues decreases when water temperatures approach the critical maximum temperature. It has been suggested that fH determines the upper thermal tolerance limit in sockeye salmon, where supraoptimal temperatures correspond with the scope for fH decreasing to zero, at which point the fish experiences cardiac collapse (Brett, 1971; Eliason et al., 2013a). The present study found no difference in resting fH and routine fH across temperature treatments, and previous work (Eliason et al., 2011) suggests that all three temperature treatments could have been within the optimal thermal window for aerobic scope of these sockeye salmon (but see below). However, when high temperature was coupled with exhaustive exercise and air exposure, temperature did affect recovery. The increased peak fH, scope for fH, and factorial fH from the 16°C to the 21°C treatment show that overall, in warmer water, sockeye salmon must have a higher fH (and likely cardiac output and metabolic rate) to recover from exhaustive exercise (Figs 2 and 3). Similar trends have been observed in other salmonid (e.g. coho salmon; Raby et al., 2015b) and non-salmonid (e.g. largemouth bass, Micropterus salmoides; Cooke et al., 2003) fishes. Furthermore, at 21°C, sockeye salmon fH almost attained its upper limit, reaching a peak fH only 13 beats min−1 below the recorded maximum fH of ~130 beats min−1 (Eliason et al., 2013a). This raises concern as to whether sockeye salmon will have the metabolic and cardiac capacity to recover from exhaustive exercise (e.g. fisheries catch-and-release) in the future if water temperatures continue increasing as predicted (Patterson et al., 2007).
Factors influencing cardiac recovery
In response to the simulated catch-and-release event, sockeye salmon fH began increasing immediately once the exhaustive exercise began, but continued to increase and peaked 1 h later (Figs 1 and 3). This trend is similar to the response observed in other studies using different salmonid species (Schreer et al., 2001; Donaldson et al., 2010; Raby et al., 2015b). Changes in fH are a result of differences between the relative levels of adrenergic tone and cholinergic tone (Farrell, 1993; reviewed in Reid et al., 1998). Epinephrine acts on the ß-adrenoceptors on the heart, activating channels that increase the cycling of intracellular calcium, which increases the rate and force of contraction (Randall, 1970; Ask et al., 1981; Ask, 1983; Farrell, 1993). Cholinergic tone counteracts the adrenergic tone by activating muscarinic receptors in the pacemaker cells causing fH to decrease (Farrell, 1993). Furthermore, cholinergic tone increases at the start of a burst swimming event (chase) and during hypoxia (air exposure) before it becomes inhibited during exercise (Wood et al., 1979; Wood and Shelton, 1980; Farrell, 1993). Exhaustive exercise exposes the heart to hypoxia and acidosis (Kiceniuk and Jones, 1977; Wood, 1991; Gale et al., 2011), which may also hinder cardiac contractility (Hanson et al., 2006). Therefore, during the simulated catch-and-release stress, fH may have been initially supressed via a stronger contribution of cholinergic tone and via impaired contractility associated with a noxious venous blood environment (i.e. hypoxia and low pH). However, this response was likely followed by the release of epinephrine, increasing the contribution of adrenergic tone. Epinephrine is essential to improve cardiac contractility when the heart is exposed to hypoxia, acidosis and hyperkalemia (high [K+]) associated with exhaustive activity (Hanson et al., 2006). We propose that over time, as the venous blood returned to normoxic levels and the relative level of adrenergic tone outcompeted the cholinergic tone, fH increased, resulting in the observed steady increase in fH peaking ~1 h after the catch-and-release simulation (Figs 1 and 3). However, to our knowledge, the variation in epinephrine secretion and ß-adrenoceptor activities or in acetylcholine secretion and muscarinic receptor activities have never been quantified in sockeye salmon in response to exhaustive exercise and air exposure. Further studies are required to investigate the mechanism of the delay in attaining peak fH and the contribution of stroke volume during recovery. Given that the 1 h delay observed in the present study is consistent with exhaustive swimming experiments on Fraser summer run sockeye salmon (Eliason et al., 2013b) and coho salmon (Raby et al., 2015b), investigations of other Fraser River salmon, such as pink (Oncorhynchus gorbuscha), chum (Oncorhynchus keta) and Chinook salmon (Oncorhynchus tshawytscha), may offer insight as to whether this is a common response in all salmonids.
Although there was a significant effect of temperature treatment on fH, especially peak fH, temperature did not affect the duration of fH recovery; sockeye salmon from all treatment groups recovered ~10 h after the catch-and-release simulation. This is two-third the recovery duration observed in other species such as Atlantic salmon (15 h at 8°C and 16.5°C; Anderson et al., 1998) and coho salmon (15–16 h at 8°C Donaldson et al., 2010; 15°C Raby et al., 2015b). The difference in recovery duration is possibly because the salmon in this study were free-swimming. Nevertheless, reasons for the prolonged elevated fH remains unclear especially given that metabolic oxygen consumption (MO2) returns to baseline within ~1–2 h post-exhaustive exercise (Eliason et al., 2013b; Lee et al., 2003), and the energy invested to generate a faster fH is equivalent to the energy required for other functions, such as migrating >1 km upstream (Raby et al., 2015b). However, it is known that during exhaustive exercise, cortisol, muscle pH and muscle glycogen levels increase (Milligan, 1996), while lactate, produced during anaerobic exercise, is locally metabolized in the muscle and excess lactate accumulates in the blood (Wood, 1991; Scarabello et al., 1992). Past research found that rainbow trout require up to 12 h post-exercise to restore blood lactate, muscle pH and muscle glycogen concentrations back to routine levels (Stevens and Black, 1966, Milligan and Wood, 1987, Milligan, 1996), and a blood lactate threshold of 10–15 mmol L−1 has been proposed, beyond which repeat swim performance is impaired (Stevens and Black, 1966; discussed in Farrell et al., 2000). As such, we propose that the ~10 h recovery duration in the present study may have been associated with the duration required to restore muscle pH and to complete lactate, cortisol and glycogen clearance. Alternatively, this may also show that fH is more sensitive to exercise stress and that recovery processes are still ongoing despite MO2 returning to baseline, or that epinephrine remains elevated even after the exhaustive exercise event. It is also important to note that recovery duration may vary in the wild since activity (e.g. swimming against a current) promotes physiological recovery and decreases recovery duration (Farrell et al., 2001; Milligan et al., 2000). The fish holding tanks for our experiment had flow but not to the extent that fish had to actively swim to maintain position. Future studies are required to determine whether these trends hold in the wild.
Similar to peak fH, scope for fH and factorial fH, all of which increase with temperature (Fig. 2), the recovery profile (during the 10 h recovery period) also differs between temperature treatments. Higher EPHB indicates greater energy allocation towards recovery (e.g. a greater slope of the recovery curve; Fig. 3). TEPHB was marginally non-significant between treatment groups likely because, irrespective of treatment, there was considerable inter-individual variation in recovery times and TEPHB (Fig. 4), and unequal variance across treatment groups that may have masked differences. It is possible, though less likely, that some fish were still recovering from surgery and this may have contributed to inter-individual variability. Alternative reasons for the observed inter-individual variability include sex-specific differences (our sample size was too small to test for sex as a factor), behavioral differences in swimming performance during the chase (i.e. fast burst swimming versus slow swimming in response to the disturbance), and individual and population differences in physiological temperature tolerance. While stock composition was not determined in the present study, it is likely that most of the fish tested were from the Chilko population based on concurrent stock analysis conducted at time of fish collection (see Methods section). Eliason et al. (2011) demonstrated that physiological performance (e.g. aerobic scope) varies across sockeye salmon populations. Indeed, physiological capacities and tolerances (e.g. Tcrit) differ even within summer-run populations (e.g. Chilko, Quesnel, Stellako). Therefore, it is possible that the effects of water temperature on sockeye salmon fH following catch-and-release events varies between different summer-run sockeye salmon populations and this may have contributed to some of the observed variability. Future studies could investigate these possibilities further and would require significantly larger sample sizes, strategic sampling across a broader migration window, as well as appropriate budget for DNA analysis.
Regardless of the inter-individual variation, within the first 10 h of recovery, the rate of energy expenditure was highest in the 21°C group (Fig. 4). Several factors may have led to the temperature-dependent differences in EPHB rate. For example, mitochondrial oxygen demand increases with higher temperatures, necessitating a greater cardiac output to supply oxygen to the mitochondria (Farrell, 2009; Eliason et al., 2013b). Furthermore, salmon in warmer water may have accumulated a greater ionic, osmotic and biochemical imbalance relative to fish in colder water. This idea is supported by the previous observation that plasma lactate was significantly higher following exhaustive exercise in sockeye salmon swum at temperatures approaching their upper functional thermal tolerance (Jain and Farrell, 2003; Eliason et al., 2013b). Concurrently, oxygen uptake capacity is diminished after a simulated fisheries interaction in warmer water followed by air exposure, due to reductions in ventilation rates (Gale et al., 2011). This implies that salmon in warmer water must enhance oxygen delivery via increasing fH and stroke volume, such that EPHB is initially higher after exhaustive exercise. To a sockeye salmon migrating in the wild, it would be advantageous to minimize the duration of recovery, because swimming performance depends to a large extent on oxygen availability for aerobic scope (Brett, 1971; Omlin et al., 2014). In nature, when oxygen consumption is elevated after exhaustive exercise, the amount of aerobic scope remaining for activities such as continued upstream swimming, predator avoidance, and overcoming barriers to migration (waterfalls, rapids, etc.) would be reduced (Brett, 1971; Farrell, 2009), placing the fish at risk of migration failure (Farrell et al., 2008). To a semelparous, capital breeding fish like sockeye and other Pacific salmon, the implications of failed migration would equate no lifetime reproduction and to zero fitness.
Conclusions
The current study took an experimental approach using fH loggers to examine the combined effects of ecologically relevant temperatures and simulated fisheries capture and release on summer-run sockeye salmon physiological recovery. Although this study was framed around a fisheries event, our findings also apply to fish recovery after exhaustive exercise including burst swimming, ascending a fish ladder associated with dam passage, or crossing fast flow water, all of which are ecologically relevant situations for many salmonid and non-salmonid species of fish.
The results support the hypothesis that warm water temperatures increase physiological recovery effort after exhaustive exercise, such as a fisheries catch-and-release event. Although the overall recovery duration did not vary, warmer water temperature regimes induced a higher initial magnitude of change in fH (i.e. peak fH) and greater rate of EPHB during the initial 10 h of recovery. Therefore, it can be concluded that sockeye salmon invest more energy in the short term (i.e. first 10 h) to recover from a fisheries stress event at higher temperatures, even at temperatures that are suspected to be within the optimal thermal tolerance window for aerobic scope. This suggests that perhaps the true optimal temperature range for physiological performance in sockeye salmon is narrower than originally thought.
Finally, the energy required to overcome a fisheries event demands energy which could otherwise be used to overcome natural migration stressors such as predation, or to maximize reproductive investment and thus fitness. This study shows that if water temperature in the Fraser River continues to increase, as is predicted, it is possible that sockeye salmon will eventually be forced to invest more energy than they can afford to overcome stressors, such that catch-and-release practices prevent successful migration or result in pre-spawning mortality. These findings support the notion that in the face of climate change, efforts to reduce stress at warmer temperatures will be necessary if selective fishing practices are to be an effective conservation strategy.
Acknowledgments
We thank the staff of the DFO Cultus Lake Laboratory for logistical support. Environmental Watch (DFO), Sophia Jain-Schlaepfer, Alex Luscombe, Andrew Lotto, Erik Lotto and Steve Healy provided field and lab assistance. Members of the Peters Band assisted with fish collection.
Funding
This work was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) via Ocean Tracking Network (Dalhousie University), an NSERC Strategic Grant to S.G.H. and S.J.C., and the NSERC Discovery Grant program. Additional support for S.J.C. was provided by the Canada Research Chairs program and the E.W.R. Steacie Fellowship.
References
- Anderson WG, Booth TA, Beddow RS, McKinley RS, Finstad B, Økland F, Scruton D (1998) Remote monitoring of heart rate as a measure of recovery in angled Atlantic salmon, Salmo salar (L.). Hydrobiologia 371:233–240. [Google Scholar]
- Arlinghaus R, Cooke SJ, Lyman J, Policansky D, Schwab A, Suski C, Sutton SG, Thorstad EB (2007) Understanding the complexity of catch-and-release in recreational fishing: an integrative synthesis of global knowledge from historical, ethical, social, and biological perspectives. Rev Fish Sci 15:75–167. [Google Scholar]
- Armstrong JD. (1986) Heart rate as an indicator of activity, metabolic rate, food intake and digestion in pike, Esox lucius. J Fish Biol 29:207–221. [Google Scholar]
- Ask JA, Stene-Larsen G, Helle KB (1981) Temperature effects on the β2-adrenoreceptors of the trout atrium. J Comp Physiol 143:161–168. [Google Scholar]
- Ask JA. (1983) Comparative aspects of adrenergic receptors in the hearts of lower vertebrates. Comp Biochem Physiol A 76:543–552. [DOI] [PubMed] [Google Scholar]
- Brett JR. (1971) Energetic responses of salmon to temperature. A study of some thermal relations in the physiology and freshwater ecology of sockeye salmon (Onchorhynchus nerka). Am Zool 11:99–113. [Google Scholar]
- Burgner RL. (1991) Life history of sockeye salmon (Oncorhynchus nerka) In Groot C, Margolis L (eds). Pacific Salmon Life Histories. UBC Press, BC, Vancouver: 3–117. [Google Scholar]
- Campbell HA, Taylor EW, Egginton S (2004) The use of power spectral analysis to determine cardiorespiratory control in the short-horned sculpin Myoxocephalus scorpius. J Exp Biol 207:1969–1976. [DOI] [PubMed] [Google Scholar]
- Chopin FS, Arimoto T (1995) The condition of fish escaping from fishing gears – a review. Fish Res 21:315–327. [Google Scholar]
- Clark TD, Ryan T, Ingram BA, Woakes AJ, Butler PJ, Frappell PB (2005) Factorial aerobic scope is independent of temperature and primarily modulated by heart rate in exercising Murray cod (Maccullochella peelii peelii). Physiol Biochem Zool 78:347–355. [DOI] [PubMed] [Google Scholar]
- Clark TD, Sandblom E, Hinch SG, Patterson DA, Frappell PB, Farrell AP (2010) Simultaneous biologging of heart rate and acceleration, and their relationships with energy expenditure in free-swimming sockeye salmon (Oncorhynchus nerka). J Comp Physiol B 180:673–684. [DOI] [PubMed] [Google Scholar]
- Cooke SJ, Philipp DP, Schreer JF, McKinley RS (2000) Locomotory impairment of nesting largemouth bass following catch-and-release angling. N Am J Fish 20:968–977. [Google Scholar]
- Cooke SJ, Schreer JF, Wahl DH, Philipp DP (2002) Physiological impacts of catch-and-release angling practices on largemouth bass and smallmouth bass In Philipp DP, Ridgway MS, (eds), Black Bass 2000: ecology, conservation and management. American Fisheries Society Symposium 31, Bethesda, MD, pp 489–512. [Google Scholar]
- Cooke SJ, Ostrand KG, Schreer JF, Wahl DH, Philipp DA (2003) Cardiovascular responses of largemouth bass to exhaustive exercise and brief air exposure over a range of water temperatures. T Am Fish Soc 132:1154–1165. [Google Scholar]
- Cooke SJ, Suski CD (2005) Do we need species-specific guidelines for catch-and-release recreational angling to effectively conserve diverse fishery resources? Biodivers Conserv 14:1195–1209. [Google Scholar]
- Cooke SJ, Schramm HL (2007) Catch-and-release science and its application to conservation and management of recreational fisheries. Fisheries Manage Ecol 14:73–79. [Google Scholar]
- Cooke SJ, Donaldson MR, O’Connor CM, Raby GD, Arlinghaus R, Danylchuk AJ, Hanson KC, Clark TD, Patterson DA, Suski CD (2013) The physiological consequences of catch-and-release angling: perspectives on experimental design, interpretation, extrapolation and relevance to stakeholders. Fisheries Manage Ecol 20:268–287. [Google Scholar]
- Cooke SJ, Brownscombe JW, Raby GD, Broell F, Hinch SG, Clark TD, Semmens JM (2016) Remote bioenergetics measurements in wild fish: opportunities and challenges. Comp Biochem Physiol 202:23–37. [DOI] [PubMed] [Google Scholar]
- Crossin GT, Hinch SG, Cooke SJ, Welch DW, Patterson DA, Jones SRM, Lotto AG, Leggatt RA, Mathes MT, Shrimpton JM, Van Der Kraak G (2008) Exposure to high temperature influences the behaviour, physiology, and survival of sockeye salmon during spawning migration. Can J Zool 86:127–140. [Google Scholar]
- Danylchuk SE, Danylchuck AJ, Cooke SJ, Goldberg TL, Koppelman J, Philipp DP (2007) Effects of recreational angling on the post-release behaviour and predation of bonefish (Albula vulpes): the role of equilibrium status at the time of release. J Exp Mar Biol Ecol 346:127–133. [Google Scholar]
- Davis MW, Olla BL (2001) Stress and delayed mortality induced in Pacific halibut by exposure to hooking, net towing, elevated seawater temperature and air: implications for management of bycatch. N Am J Fish Manage 21:725–732. [Google Scholar]
- Davis MW. (2002) Key principles for understanding fish bycatch discard mortality. Can J Fish Aquat Sci 59:1834–1843. [Google Scholar]
- Davis MW. (2005) Behaviour impairment in captured and released sablefish: ecological consequences and possible substitute measures for delayed discard mortality. J Fish Biol 66:254–265. [Google Scholar]
- Davis MW, Olla BL, Schreck B (2011) Stress induced by hooking, net towing, elevated sea water temperature and air in sablefish: lack of concordance between mortality and physiological measures of stress. J Fish Biol 58:1–15. [Google Scholar]
- Department of Fisheries and Oceans (2005) Canada’s Policy for Conservation of Wild Pacific Salmon. Fisheries and Oceans Canada, British Columbia: 4–6. [Google Scholar]
- Department of Fisheries and Ocean (2015) Draft agenda Pacific salmon commission Fraser River Panel, file 71007. BC, Vancouver, pp 1–17.
- Donaldson MR, Arlinghaus R, Hanson KC, Cooke SJ (2008) Enhancing catch-and-release science with biotelemetry. Fish Fish 9:79–105. [Google Scholar]
- Donaldson MR, Clark TD, Hinch SG, Cooke SJ, Patterson DA, Gale MK, Frappell PB, Farrell AP (2010) Physiological responses of free-swimming adult coho salmon to simulated predator and fisheries encounters. Physiol Biochem Zool 83:973–983. [DOI] [PubMed] [Google Scholar]
- Donaldson MR, Hinch SG, Patterson DA, Hills J, Thomas JO, Cooke SJ, Raby GD, Thompson LA, Robichaud D, English KK, Farrell AP (2011) The consequences of angling, beach seining, and confinement on the physiology, post-release behaviour and survival of adult sockeye salmon during upriver migration. Fish Res 108:133–141. [Google Scholar]
- Donaldson MR, Hinch SG, Raby GD, Patterson DA, Farrell AP, Cooke SJ (2012) Population-specific consequences of fisheries-related stressors on adult sockeye salmon. Physiol Biochem Zool 85:729–739. [DOI] [PubMed] [Google Scholar]
- Eliason EJ, Higgs DA, Farrell AP (2008) Postprandial gastrointestinal blood flow, oxygen consumption and heart rate in rainbow trout (Oncorhynchus mykiss). Comp Biochem Phys A 149:380–388. [DOI] [PubMed] [Google Scholar]
- Eliason EJ, Clark TD, Hague MJ, Hanson LM, Gallagher ZS, Jeffries KM, Gale MK, Patterson DA, Hinch SG, Farrell AP (2011) Differences in thermal tolerance among sockeye salmon populations. Science 332:109–112. [DOI] [PubMed] [Google Scholar]
- Eliason EJ, Clark TD, Hinch SG, Farrell AP (2013. a) Cardiorespiratory collapse at high temperature in swimming adult sockeye salmon. Conserv Physiol 1:1 doi:10.10 93/conphys/cot008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliason EJ, Clark TD, Hinch SG, Farrell AP (2013. b) Cardiorespiratory performance and blood chemistry during swimming and recovery in three populations of elite swimmers: adult sockeye salmon. Comp Biochem Phys A 166:385–397. [DOI] [PubMed] [Google Scholar]
- Farrell AP. (1991) From hagfish to tuna: a perspective on cardiac function in fish. Physiol Zool 64:1137–1164. [Google Scholar]
- Farrell AP. (1993) Cardiovascular system In Evans DH (ed). The Physiology of Fishes. CRC Press Inc., Boca Raton: 219–250. [Google Scholar]
- Farrell AP, Gallaugher P, Clarke C, DeLury N, Kreiberg H, Parkhouse W, Routledge R (2000) Physiological status of coho salmon (Oncorhynchus kisutch) captured in commercial nonretention fisheries. Can J Fish Aquat Sci 57:1668–1678. [Google Scholar]
- Farrell AP, Gallaugher PE, Routledge R (2001) Rapid recovery of exhausted adult coho salmon after commercial capture by troll fishing. Can J Fish Aquat Sci 58:2319–2324. [Google Scholar]
- Farrell AP, Hinch SG, Cooke SJ, Patterson DA, Crossin GT, Lapointe M, Mathes MT (2008) Pacific Salmon in hot water: applying aerobic scope models and biotelemetry to predict the success of spawning migrations. Physiol Biochem Zool 81:697–709. [DOI] [PubMed] [Google Scholar]
- Farrell AP. (2009) Environment, antecedents and climate change: lessons from the study of temperature physiology and river migration of salmonids. J Exp Biol 212:3771–3780. [DOI] [PubMed] [Google Scholar]
- Fry FEJ. (1971) The effect of environmental factors on the physiology of fish. Fish Physiol 6:1–98. [Google Scholar]
- Gale MK, Hinch SG, Eliason EJ, Cooke SJ, Patterson DA (2011) Physiological impairment of adult sockeye salmon in fresh water after simulated capture-and-release across a range of temperatures. Fish Res 112:85–95. [Google Scholar]
- Gale MK, Hinch SG, Donaldson MR (2013) The role of temperature in the capture and release of fish. Fish Fish 141:1–33. [Google Scholar]
- Gallaugher P, Farrell AP (1998) Hematocrit and blood oxygen-carrying capacity In Perry SF, Tufts B (eds). Fish Respiration, Fish Physiology Series, Vol 17 Academic Press, San Diego: 185–227. [Google Scholar]
- Gaesser GA, Brooks GA (1984) Metabolic bases of excess post-exercise oxygen consumption: a review. Med Sci Sports Exerc 16:29–43. [PubMed] [Google Scholar]
- Hanson LM, Obradovich S, Mouniargi J, Farrell AP (2006) The role of adrenergic stimulation in maintaining maximum cardiac performance in rainbow trout (Oncorhynchus mykiss) during hypoxia, hyperkalemia and acidosis at 10 C. J Exp Biol 209:2442–2451. [DOI] [PubMed] [Google Scholar]
- Hinch SG, Cooke SJ, Farrell AP, Miller KM, Lapointe M, Patterson DA (2012) Dead fish swimming: a review of research on the early migration and high premature mortality in adult Fraser River sockeye salmon Oncorhynchus nerka. J Fish Biol 81:576–599. [DOI] [PubMed] [Google Scholar]
- Hodgson S, Quinn TP, Hilborn R, Francis RC, Rogers DE (2006) Marine and freshwater climatic factors affecting interannual variation in the timing of return migration to fresh water of sockeye salmon (Oncorhynchus nerka). Fish Oceanogr 15:1–24. [Google Scholar]
- Jain KE, Farrell AP (2003) Influence of seasonal temperature on the repeat swimming performance of rainbow trout Oncorhynchus mykiss. J Exp Biol 206:3569–3579. [DOI] [PubMed] [Google Scholar]
- Kiceniuk JW, Jones DR (1977) The oxygen transport system in trout (Salmo gairdneri) during sustained exercise. J Exp Biol 69:247–260. [Google Scholar]
- Lapointe MF, Cooke SJ, Hinch SG, Farrell AP, Jones S, MacDonald S, Patterson D, Healey MC, Van Der Kraak G (2003) Late-run sockeye salmon in the Fraser River, British Columbia are experiencing early upstream migration and unusually high rates of mortality: what is going on. In Proceedings of the 2003 Georgia Basin/Puget Sound Research Conference, Vancouver, BC, Vol 31, pp 1–14.
- Lee CG, Farrell AP, Lotto A, Hinch SG, Healey MC (2003) Excess post-exercise oxygen consumption in adult sockeye (Oncorhynchus nerka) and coho (O. kisutch) salmon following critical speed swimming. J Exp Biol 206:3253–3260. [DOI] [PubMed] [Google Scholar]
- Lima SL, Dill LM (1990) Behavioural decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640. [Google Scholar]
- Martins EG, Hinch SG, Patterson DA, Hague MJ, Cooke SJ, Miller KM, Lapointe MP, English KK, Farrell AP (2011) Effects of river temperature and climate warming on stock-specific survival of adult migrating Fraser River sockeye salmon (Oncorhynchus nerka). Glob Change Biol 17:99–114. [Google Scholar]
- Mesa MG, Poe TP, Gadomski DM, Petersen JH (1994) Are all prey created equal? A review and synthesis of differential predation on prey in substandard condition. J Fish Biol 45:81–96. [Google Scholar]
- Milligan CL, Wood CM (1987) Regulation of blood oxygen transport and red cell pHi after exhaustive activity in rainbow trout (Salmo gairdneri) and starry flounder (Platichthys stellatus). J Exp Biol 133:263–282. [DOI] [PubMed] [Google Scholar]
- Milligan CL. (1996) Metabolic recovery from exhaustive exercise in rainbow trout. Comp Biochem Phys 113:51–60. [Google Scholar]
- Milligan CL, Hooke GB, Johnson C (2000) Sustained swimming at low velocity following a bout of exhaustive exercise enhances metabolic recovery in rainbow trout. J Exp Biol 203:921–926. [DOI] [PubMed] [Google Scholar]
- Murphy MD, Heagey RF, Neugebauer VH, Gordon MD, Hintz JL (1995) Mortality of spotted seatrout released from gill-net or hook-and-line gear in Florida. N Am J Fish Manage 15:748–753. [Google Scholar]
- Norris DO, Carr JA (2006) Part I. Vertebrate Chemical Regulation In Norris DO, Carr JA, eds, The adrenal gland Endocrine Disruption: Biological Bases for health effects in wildlife and humans, Ed 5 Oxford University Press, New York, pp 3–161. [Google Scholar]
- Northcote TG, Larkin PA (1989) The Fraser river: a major salmonine production system. Canadian Special Publication of Fisheries and Aquatic Sciences/Publication speciale canadienne des sciences halieutiques et aquatiques.
- Olla BL, Davis MW, Schreck CB (1998) Temperature magnified postcapture mortality in adult sablefish after simulated trawling. J Fish Biol 53:743–751. [Google Scholar]
- Omlin T, Langevin K, Weber JM (2014) Exogenous lactate supply affects lactate kinetics of rainbow trout, not swimming performance. Am J Physiol Reg I 307:1018–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson DA, Macdonald JS, Hinch SG, Healey MC, Farrell AP (2004) The effect of exercise and captivity on energy partitioning, reproductive maturation and fertilization success in adult sockeye salmon. J Fish Biol 64:1039–1059. [Google Scholar]
- Patterson DA, Macdonald JS, Skibo KM, Barnes DP, Guthrie I, Hills J (2007) Reconstructing the summer thermal history for the lower Fraser River, 1941 to 2006, and implications for adult sockeye salmon (Oncorhynchus nerka) spawning migration. Can Tech Rep Fish Aquat Sci 2724:vii+43. [Google Scholar]
- Patterson DA, Cooke SJ, Hinch SG, Robinson KA, Young N, Farrell AP, Miller KM (2016) A perspective on physiological studies supporting the provision of scientific advice for the management of Fraser River sockeye salmon (Oncorhynchus nerka). Conserv Physiol 4:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, the R Development Core Team (2013) nlme: Linear and Nonlinear Mixed Effects Models. R package version 3:1–108.
- Priede IG. (1977) Natural selection for energetic efficiency and the relationship between activity level and mortality. Nature 267:610–611. [DOI] [PubMed] [Google Scholar]
- Priede IG, Tytler P (1997) Heart rate as a measure of metabolic rate in teleost fishes; Salmo gairdneri, Salmo trutta and Gadus morhua. J Fish Biol 10:231–242. [Google Scholar]
- Raby GD, Cooke SJ, Cook KV, McConnachie SH (2013) Resilience of Pink Salmon and Chum Salmon to simulated fisheries capture stress incurred upon arrival at spawning grounds. T Am Fish Soc 142:524–539. [Google Scholar]
- Raby GD, Donaldson MR, Hinch SG, Clark TD, Eliason EJ, Jeffries KM, Cook KV, Teffer A, Bass AL, Miller KM, Patterson DA (2015. a) Fishing for effective conservation: context and biotic variation are keys to understanding the survival of pacific salmon after catch-and-release. Integr Comp Biol 55:554–576. [DOI] [PubMed] [Google Scholar]
- Raby GD, Clark TD, Farrell AP, Patterson DA, Bett NN, Wilson SM, Willmore WG, Suski CD, Hinch SG, Cooke SJ (2015. b) Facing the river gauntlet: understanding the effects of fisheries capture and water temperature on the physiology of coho salmon. PLoS ONE 10:e0124023 doi:10.1371/journal.pone.0124023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall DJ. (1970) Chapter 4: The circulatory system In Hoar WS, Randall DJ, eds, Fish Physiology, Vol 4 Academic Press, California: 133–172. [Google Scholar]
- Reid SG, Bernier NJ, Perry SF (1998) The adrenergic stress response in fish: control of catecholamine storage and release. Comp Biochem Phys C 20:1–27. [DOI] [PubMed] [Google Scholar]
- Robinson KA, Hinch SG, Gale MK, Clark TD, Wilson SM, Donaldson MR, Farrell AP, Cooke SJ, Patterson DA (2013) Effects of post-capture ventilation assistance and elevated water temperature on sockeye salmon in a simulated capture-and-release experiment. Conserv Physiol 1, doi:10.1093/conphys/cot015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarabello M, Heigenhauser GJ, Wood CM (1992) Gas exchange, metabolite status and excess post-exercise oxygen consumption after repetitive bouts of exhaustive exercise in juvenile rainbow trout. J Exp Biol 167:155–169. [DOI] [PubMed] [Google Scholar]
- Schreer JF, Cooke SJ, McKinley RS (2001) Cardiac response to variable forced exercise at different temperatures: an angling simulation for smallmouth bass. T Am Fish Soc 130:783–795. [Google Scholar]
- Schreer JF, Resch DM, Gately LM, Cooke SJ (2005) Swimming performance of brook trout after simulated catch-and-release angling: looking for air exposure threasholds. N Am J Fish Manage 25:1513–1517. [Google Scholar]
- Shrimpton JM, Patterson DA, Richards JG, Cooke SJ, Schulte PM, Hinch SG, Farrell AP (2005) Ionoregulatory changes in different populations of maturing sockeye salmon Oncorhynchus nerka during ocean and river migration. J Exp Biol 208:4069–4078. [DOI] [PubMed] [Google Scholar]
- Schwarz CJ. (2015) Chapter 17: Regression—hockey sticks, broken sticks, piecewise, change points. In: The Big R Book, pp 1168–1184.
- Stevens ED, Black EC (1966) The effect of intermittent exercise on carbohydrate metabolism in rainbow trout, Salmo gairdneri. J Fisheries Res Board Can 23:471–485. [Google Scholar]
- Thorarensen H., Gallaugher PE, Farrell AP (1996) The limitations of heart rate as a predictor of metabolic rate in fish. J Fish Biol 49:226–236. [Google Scholar]
- Wood CM, McMahon BR, McDonald DG (1979) The influence of temperature and anemia on the adrenergic and cholinergic mechanisms controlling heart rate in the rainbow trout. Can J Zool 57:2440–2447. [Google Scholar]
- Wood CM, Shelton G (1980) The reflex control of heart rate and cardiac output in the rainbow trout: interactive influences of hypoxia, haemorrhage, and systemic vasomotor tone. J Exp Biol 87:271–284. [DOI] [PubMed] [Google Scholar]
- Wood CM. (1991) Acid-base and ion balance, metabolism, and their interactions, after exhaustive exercise in fish. J Exp Biol 160:285–308. [Google Scholar]