Abstract
Background
Compared to blood component safety, the administration of blood may not be as safe as intended. The German Interdisciplinary Task Force for Clinical Hemotherapy (IAKH) specialized registry for administration errors of blood products was chosen for a detailed analysis of reports.
Methods
Voluntarily submitted critical incident reports (n = 138) from 2009 to 2013 were analyzed.
Results
Incidents occurred in the operation room (34.1%), in the ICU (25.2%), and in the peripheral ward (18.5%). Procedural steps with errors were administration to the patient (27.2%), indication and blood order (17.1%), patient identification (17.1%), and blood sample withdrawal and tube labeling (18.0%). Bedside testing (BST) of blood groups avoided errors in only 2.6%. Associated factors were routine work conditions (66%), communication error (36%), emergency case (26%), night or weekend team (39%), untrained personnel (19%). Recommendations addressed process and quality (n = 479) as well as structure quality (n = 314). In 189 instances, an IT solution would have helped to avoid the error.
Conclusions
The administration process is prone to errors at the patient assessment for the need to transfuse and the application of blood products to patients. BST is only detecting a minority of handling errors. According to the expert recommendations for practice improvement, the potential to improve transfusion safety by a technical solution is considerable.
Keywords: Error, Critical incidents, Hemotherapy, Transfusion safety, Error reporting, Confusion, Right tube, Right patient, Administration safety
Introduction
The German transfusion law [1] and the cross-sectional guidelines for the use of blood and blood products of the German Medical Association [2] apply evidence-based recommendations for the use of each blood product. Their implementation and practical compliance are promoted by an obligatory structure of dedicated quality managers of hemotherapy, dedicated responsible physicians in every institution, and dedicated assigned physicians in each department for the correct use of blood and blood products. A transfusion committee meeting regularly discusses current problems to improve efficacy and safety of hemotherapy. Furthermore, the use of blood and blood product production are regulated by German pharmaceutical law since they are categorized pharmaceutical products. Documentation of internal or external audits has to be reported to regulatory bodies. Hemovigilance reports are published by the Paul-Ehrlich-Institut (PEI), reporting statistics of major transfusion reactions as well as blood product donation, use, and discard [3]. Bedside confirmation of recipient's identity and ABO blood group is undertaken prior to red cell transfusion. Bedside testing (BST) is obligatory in Germany for the administrating physician. Such confirmation testing for blood groups ABO and ABO+D is also used in a few other European countries. It confirms patient identity at the bedside immediately before a blood transfusion is started. In addition, BST is intended to confirm the recipient's ABO blood group antigens (of lesser vital importance D) previously determined and thus ensures that there is a match between the recipient's blood group and the blood product. Dependent on the producer of the little test cards, anti-serums for A and B (D) are either to be mixed with the drop of patient's blood (SERAFOL) or are in a liquid compartment where the blood must be injected into (MEDTRO). Agglutination of the red cells with the anti-serum becomes visible and should match the blood group of the blood product to be given. This identifies any incompatibility that may occur if the patient or the blood product had been confused.
Transfusion safety in Germany is believed to be sufficiently robust at state-of-the-art level. Underreporting of safety issues in Germany is not a major concern. Whereas fatal errors resulting in acute hemolytic transfusion reactions are documented in the PEI hemovigilance registry (ranging from 1 to 3 per million in recent years), an underreporting of incorrect allocation of products (risk of incorrectly transfused blood components 0.2 per million) must be assumed. In absence of hard data, incidences of non-fatal errors in the German system were estimated between 1:32,000 [4] and 1:36,000 [5], whereas data from the USA reported higher figures in the range from 1:12,000 to 1:19,000 [6].
To challenge and improve the blood transfusion safety standard in Germany, a dedicated critical incident reporting system (CIRS) for Germany was set up on a voluntary basis in 2009 [7] as a joint venture of the German Interdisciplinary Task Force for Clinical Hemotherapy (IAKH) [8] and the German Interdisciplinary Society of Critical Care and Emergency Medicine (DIVI). Free of charge and accessible for the public, error reporting in the IAKH error registry [9, 10] is encouraged for every user of autologous and allogeneic blood in every health care institution in Germany. As opposed to the PEI registry, any kind of error or critical incident associated with the administration of blood and coagulation products voluntarily can be reported by physicians and other medical staff. Reports were analyzed and categorized by an interdisciplinary committee of transfusion medicine specialists, internists, surgeons, and anesthesiologists. Critical incidents referring to transfusion issues from the German Anesthesiology Societies (DGAI and BDA) CIRS-AINS medical [11] were also surveyed on behalf of the BDA by the IAKH committee and included in the IAKH error registry database.
This article addresses the character of reported errors and critical incidents within the administration process of blood products after a 5-year period. Errors in blood product administration were analyzed for circumstances, relevance, risk of damage, and contributing factors. The publication of committee's recommendations may be of common interest for other than the reporting institutions - therefore, they are listed and grouped as proposed strategies of quality improvement.
Material and Methods
Database and Reporting Form
In 2009, the website went online, and the reporting was encouraged by information in the national medical press [7, 10]. In parallel, the German BDA started an anesthesia-based critical incident reporting system. All incidents concerning a blood product were sent to the IKAH committee for analysis and entry in the IAKH database. The multidisciplinary IAKH committee is constituted of experts from various clinical departments. All aspects of an error report were addressed by the respective specialist either in the committee's session or by requiring re-submittal after analysis.
To avoid a double entry of the same incident, each report is anonymized by coding the individual case with the third letter as well as the number of letters of the first and surname of the patient. The first letter of the area code of the reporting institution had to be given. In each type of entry form, general information such as the American Society of Anesthesiologists risk class (ASA) stratification of the patient according to the perioperative system by the American Society of Anesthesiologists, the location where the incident happened, and which profession detected the error is to be entered. The free accessible web page of error reporting initially had 5 entry forms to report a critical incident categorized by the process steps: 1) blood product order for each incident that happens before, after, or while ordering a blood product from the blood bank or depot including the assessment of the need of transfusion; 2) blood group sample withdrawal and tube labeling for each incident that happens before, after, or while blood withdrawal for type and screen, labeling and sending to the immunology laboratory; 3) laboratory handling of blood probes, type and screen, or release of ordered blood for all incidents in the laboratory, blood bank and depot from arrival of a blood probe until release of a blood or coagulation product to the user or transport system; 4) transport and handling of blood products for incidents happening while and associated to transport and storage of blood products, 5) blood product administration for all incidents happening immediately before, while, or after administration of the blood product to the recipient, identification of the patient, performance of BST, starting of the transfusion and documentation, 6) for other incidents such as coagulation management, autologous processing, or donation. The last form was introduced later and went active in January 2013. The shorter collection period of this type of error is considered in the analysis. All minor process steps covered by the 6 forms are given in table 1. Definition of errors and their categories are listed in table 2.
Table 1.
Process steps covered by the reporting form
| Form | Category description | Step |
|---|---|---|
| 1) Blood product order | Each incident that happens before, after, while ordering a blood product from the blood bank or depot including the assessment of the need of transfusion | a) Identification of the patient by entry in the hospital's PDMS during the admittance in administration, ICU or emergency unit Assessment of the need of transfusion in a clinical situation of an individual by clinical situations, symptoms, laboratory and history b) Order of the right number of units or products by delegation, by filling an electronic or analog order form for this patient c) Organization of process initiation by defining time, dose, clinical situation and urgency, responsibility |
| 2) Blood group sample withdrawal and tube labeling | Each incident that happens before, after, while blood withdrawal for type and screen, labeling and sending to the immunology laboratory | a) Preparation of blood containers, printing and labeling for the right individual b) Identification of the right patient for the blood withdrawal Blood withdrawal off the right patient in correct labeled containers at the right time |
| 3) Laboratory handling of blood probes and products | Each incident that happens before during and after blood probe arrival, screen and type, release of ordered blood for all incidents in the laboratory, blood bank and depot from arrival to release of a compatible product |
Errors of blood probe reception a) Acceptance of blood probes without correct labeling, in wrong tubes, insufficient volume, outdated since leaving the patient b) Mismatch at reception of blood probes for type and screen, confusion of attached documents Errors of blood group test either in an analyzer, gel cards, elution or other methods including c) Wrong results, no results despite antibody detectable, wrong diagnostic tool such as choose of wrong antibody panel, d) Delays in working on emergency cases associated with analysis, result reading, further diagnostic, and result output Errors associated to release of blood products while e) Retrieval of correct tested blood unit from storage f) Product checking out g) Releasing documentation and signature to the authorized person |
| 4) Transport and handling of blood products | Each incident that happens on and with transport of probe from patient to laboratory, from probe transport to analyzer, blood product transport and storage within the blood depot, blood product transport from blood depot to the sub-depot or to the patient | a) Incorrect transport conditions i.e. temperature, box, contamination, not approved storage condition, etc. b) Avoidable delays of urgent deliveries c) Mix-up and confusion of several products for various recipients d) Damage to or loss of products e) Inadequate handling of probe during transport and waiting period for application, i.e. no agitation and extended storage of platelets |
| 5) Blood product administration | Each incident that happens prior to product application to the recipient | a) Bedside test not done, wrong done, wrong interpreted, done from the wrong patient b) Mismatch of blood group documents of patients, blood group information on blood product and BST c) Inadequate preparation of products and transfusion sets, i.e. incomplete thawing, incomplete visual control of red cells for hemolysis d) Inadequate application condition, illegal delegation, inadequate venous access, wrong application mode or period, etc. e) Flawless documentation of application process f) No or insufficient monitoring during transfusion g) Any other administration error |
| 6) Patient identification | Each incident that happens while receiving, documenting, matching the identity of patients, blood products or probes | a) Wrong patient ID at hospital admission due to mix-up, wrong ID given, wrong or no wrist band, unconsciousness and later misallocation of emergency number to ID b) Wrong identification procedure prior to withdrawal of blood probe or application of blood product |
| 7) Others such as coagulation management, autologous processing or donation | Each incident that occurs in hemotherapy but does not fit the above classification within the process chain | a) Problems with or during production of autologous products by cell salvage and preoperative donation b) Problems with coagulation management, i.e. incorrect diagnostics, choosing the incorrect antidote or insufficient dosage, overdose, etc. c) Any other error not covered by any previous entry form |
PDMS = Patient data management system; ICU = intensive care unit; ID = identity.
Table 2.
Definition of error/incident categories (modified from SHOT categories)
| Error category | Subcategory | Error/incident definition |
|---|---|---|
| Avoidable, delayed or undertransfusion Near misses are not included |
Avoidable | Blood product unnecessary transfused according to guidelines with respect to clinical situation / laboratory values. Discarded blood products due to outdated or incorrect storage conditions, late return to the supply chain (reports of not indicated transfusions are not included- see Administration error, Indication below) |
| Delayed | Time delay by an error that happened earlier in the administration process | |
| Over transfusion/TACO | Transfusion associated circulatory overload, overdosing of blood product | |
| Undertransfusion | Ineffective dose of blood product administered | |
| Coagulation management and dosing error | Wrong dosing or error in coagulation management associated to the use of plasma and coagulation factors incl. concentrates, prothrombin complex, cryoprecipitate etc. | |
| Incorrect blood component transfused | General requirements not met | Wrong kind of blood product transfused (packed red cells, platelets, plasma, coagulation factors, outdated products, allogeneic instead of autologous etc.) |
| Unmatched | Unmatched or universal 0 negative red cells, not blood group matched platelets or AB fresh frozen plasma in elective cases, application of blood products unscreened for antibodies or compatibility | |
| Incompatible | Real administration (not near misses) of incompatible blood with respect of major blood group or allo-antibodies | |
| Compatible | Compatible blood but wrong recipient | |
| Specific requirements not met | Although necessary no irradiation, leukodepletion, washed, frozen, Jehovah witnesses transfused without consent etc. | |
| Errors related to cell salvage and preoperative donation | Production of autologous products | Use and production of autologous products including preoperative donation and operation of cell saver device Cell saver defect, technical malfunction Mismatch of products following disconnection or confusion of autologous products |
| Patient identification (ID) | Type and screen probe bedside test or laboratory | ID of patient not or wrong done, test withdrawn from wrong patient Laboratory test (hemoglobin levels, coagulation values or bedside test from wrong patient |
| Blood / coagulation product | Administration of blood /coagulation product to the wrong patient | |
| Handling and storage errors | ||
| Labeling | Labeling of blood samples / probes / tubes | Label not applied on tubes prior to withdrawal or wrong done (incorrect label form other subject) |
| Labeling of chart or other documents | Label not applied on chart or documents, incorrect label from other subject, wrong | |
| Labeling of blood products / coagulation products / drugs | Confused label on covers from other subjects, wrong content names or dosages | |
| Laboratory | Laboratory error | Wrong test results due to pre-analytic errors, diluted blood, test result issued for wrong subject, blood sample for antibody cross match expired, wrong blood sample sent, etc. |
| Testing error | analyzer defect or calibration missed, wrong POCT operation | |
| Storage | Storage error | Storage error of blood products (including ‘30-min rule’), extended storage in sub-depot location, extended non-agitated storage of platelets, unapproved storage in a food or drug refrigerator |
| Temperature deviation / cold chain error | Storage temperature deviations due to use of inappropriate storage i.e. warming or cooling devices | |
| Component expiry | Use of outdated products | |
| Product release /issue | Product / component issue / release | Mismatch or confusion at off-charge of product off the depot or blood bank, wrong or inappropriate component issued, wrong unit logged out, adjusting error, incomplete check out, documentation error |
| Transport | Transport error | Inadequate transport conditions, duration, products for multiple recipients in one box |
| Administration error | ||
| Indication | Indication | Erroneous indication for transfusion due to lab or pre-analytic error, missing guideline coverage due to misinterpretation of lab values, clinical context, circulatory symptoms, volume status or ignorance |
| Blood management | Uncorrected preoperative anemia | Uncorrected preoperative anemia in elective surgery |
| Transfusion trigger unrecognized | Restrictive transfusion strategy as suggested by guidelines | |
| Urgency of blood use | Not indicated emergency administration of not cross-matched, universal blood | |
| Volume status related to transfusion | Missing volume replacement for hyper-/hypovolemia, unsecure volume monitoring, blood products used for volume resuscitation | |
| Consent | Informed consent not done | Information about risks and complication of blood transfusion for conscious subjects and elective transfusion wrong or not done or not documented |
| Blood order | Blood order error | Missing or incorrect blood order, blood order for wrong patient, missing data on order form, etc. |
| Bedside test | Bedside test | Not or wrong done, wrong interpretation |
| Preparation for blood product administration | Type and screen, crossmatch | Missed/undone or wrong done type and screen, blood product reservation for elective transfusion or scheduled surgery, no antibody differentiation ordered |
| Pretransfusion procedure | immediately prior to blood product administration: lab value check, matching control of blood product ID blood group and patient blood group, antibody screen test, outdate of blood product, visual blood product control, transfusion system, venous access etc.) | |
| Timing | Inadequate timing of administration including premature timing of anesthesia before blood availability (induction of anesthesia without blood availability check) | |
| Delegation of transfusion | Start of transfusion cannot be delegated by German transfusion law | |
| Screen result expiry | Antibody screening test expires after 4 days | |
| Multiple transfusion | More than one transfusion process, more than one patient synchronously or massive transfusion as contributing factor or error | |
| Double unit administration | Ordering and transfusion of two units without review | |
| Monitoring | Monitoring during administration | Vitals and clinical symptoms monitoring for transfusion reaction not done |
| Documentation | Documentation of administration | Undone or wrong done documentation of administration of blood products |
| Inadequate timing of administration | For example at night time without urgency | |
| Errors related to IT | Wrong record selected | IT user based error: selection of wrong record and/or mismatch of order, test result or written entry to the wrong file/record. Attribution of diagnoses or diagnostic findings to the wrong chart/record by computer, analyzers or the operator manually, due to i.e. similar name, ID number or small font |
| Failure to consult or identify historical record | IT based or user based error: failure to retrieve a historical record in the data base with the search tool. For example, earlier blood group and antibodies cannot be compared with actual results for safety reasons | |
| Warning flag not updated or removed in error | IT user based error: Alert signs such as transfusion relevant antibodies are ignored, not updated or accidently removed | |
| Computer or other IT systems failure | Errors related to computer system such as no access possibility due to crash, updates or overhaul; errors related to electronic blood management system, access failure | |
| Incorrect entry | Incorrect or incomplete result or data entered or accessed manually, undetected or uncorrected wrong operation (copy, paste, delete) | |
| Blood order | Blood issued against wrong patient ID (sample or request form), error originates from electronic blood ordering system | |
| Administrator related error | Erroneous combination of records in data base, deletion of data, etc. | |
| Errors related to analog documentation paper-based forms/charts/documentation | Paper documentation related errors | Unreadable, wrong chart, misplaced, not filed in chart, torn, lacking, incomplete content etc. |
| Other equipment failure | Technical failure of used equipment | i.e. blood warming device for massive transfusion, blood gas analyzer, pressure device for rapid infusion, clotting of leukocyte depletion filters, etc. |
| Wrong or no equipment | Wrong or no equipment used, although available; monitoring, diagnostics, application technology not used, although first choice in a clinical situations, such as blood filter systems, plasmatherm for thawing of frozen blood components, hemoglobin analyzer≤ | |
| Understaffing | Reports, in which the error mainly is attributed to a lack of personnel | |
| Communication | Subject to subject | No or erroneous transmission of information from, i.e., surgeon to anesthesiologist, transfusion specialist to blood bank, nurse to physician, transport service to medical staff, etc. |
| Subject and technique including IT | Missing or erroneous exchange of relevant information from operator, reader or user to (hospital information system KIS, laboratory and monitoring IT or vice versa | |
Errors in categorizes and definition for the incidents. Near misses are not distinguished from real errors except when noted.
ID- = Identity check; IT = information technology, KIS = hospital information system; POCT = point of care testing; TACO = transfusion associated circulatory overload.
Analyses, Definitions, and Categories
In addition, during the work process with the registry, a frequent error located before the process chain even starts was recognized by the committee - the indication to transfuse frequently was not in congruence with the existing guidelines. Without creating a special entry form for that, the correctness of the transfusion indication was tested. The same applied for the patient identification and confusion error. This critical step occurs several times in the administration process - prior to type and screen, blood withdrawal, or blood product administration. The information about a correct or incorrect performance of the BST is required by the IAKH report form. The classification of an error into one or more process steps was done by the committee even if the incident was classified differently by the reporter in accordance to the given categorization of errors (table 2).
The incoming reports were edited and discussed among committee members in a secured phone conference weekly. Reports and categories of errors were entered in a database. Results are given in absolute counts or errors in the reported cases as well as in percentage of the 5 year's sum of all errors reported.
In an attempt to confirm the relevance of the critical incident to the reader, the expert group tried to estimate the frequency of the event in German practice with the help of published case reports and personal experience of committee members. Termed as ‘frequency estimate’, the estimated frequency of an error was classified according to the rate of serious adverse events of pharmaceutical drugs in approval studies as follows: extremely rare – 1:100,000, rare – 1:10,000, medium – 1:1,000, occasional – 1:100, frequent – 1:10. In a further attempt to categorize the potential risk for the recipient's integrity, which is also termed ‘damage potency’, the committee filed the error case in 5 damage groups from death risk to minor disabilities, similar to the serious adverse event grading in pharmaceutical approval studies: 1) no permanent or transient damage, 2) minor and/or weak transient damage, 3) minor and/or weak transient damage or light permanent disability, 4) considerable acute and/or considerable permanent disability, 5) death or permanent disability.
To assess the association between an erroneous blood administration process to the severity of pre-existing diseases, ASA physical statuses (perioperative risk stratification by the American Society of Anesthesiologists) were included in our analysis.
The committee edited and published each case on the IAKH website (www.iakh.de) together with recommendations to avoid the error both by changes in the process organization of the reporting institution and by changes of the institutional structure. The structure quality comprises the use of other or modern equipment, the increase in personnel, the use of computer technology, the change of existing equipment by the manufacturer, or a change of existing laws and guidelines by the respective authorities. Expert risk minimization recommendations were made based on a committee discussion where the potency for hindrance is either logic, self-explanatory, or based on the experience and knowledge of the experts.
Results
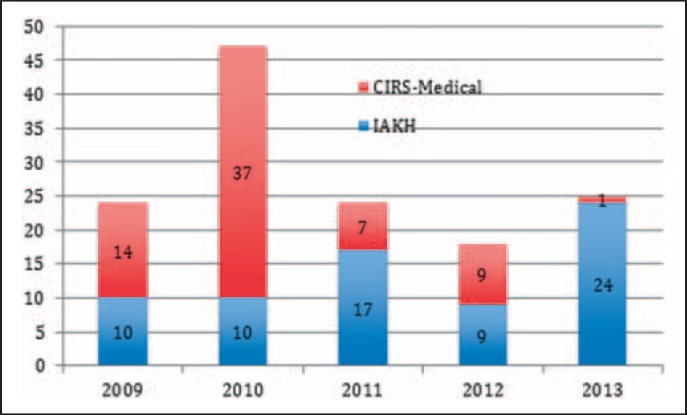
During the 5-year period since the start in 2009, 138 cases were reported via IAKH and CIRS-AINS. More than half of cases (55.9%) originated from the IAKH registry (fig. 1). The webpage-based anonymous entry of an incident is followed by a detailed assessment of the causes and an error analysis.
Fig. 1.
Chronicle and data base source of the reports. Critical incidents in hemotherapy were reported into two affiliated databases - the CIRS-Medical of the German Associations of Anesthesiologists (DGAI and BDA) and the IAKH database. Except for 2010, round 24 incident reports were posted per year.
In more than as half of the reports (52.7%), multiple errors in the application process could be identified. One report case contained 2.16 ± 1.6 errors (mean ± SD). Categories, numbers, and rates of reported errors are listed in table 3.
Table 3.
Reported errors by category – number and rates
| Error category | Subcategory | Number of reports | Rate of all errors, % |
|---|---|---|---|
| Avoidable, delayed or undertransfusion (no near misses) | Avoidable, discarded /wasted blood products | 6 | 2.01 |
| Delayed | 22 | 6.15 | |
| Overtransfusion / TACO | 9 | 3.02 | |
| Undertransfusion | 3 | 1.01 | |
| Coagulation management and dosing error | 20 | 6.71 | |
| Sum | 60 | 18.6 | |
| Incorrect blood component transfused | Wrong kind of blood product transfused | 8 | 2.68 |
| For mismatches during actual administrations or near misses see table 4 | |||
| Unmatched | 2 | 0.67 | |
| Wrong blood or recipient | Incompatible | 6 | 2.01 |
| Wrong blood or recipient | Compatible | 30 (for details see table 4) | 10.1 |
| Specific requirements for component not met | 1 | 0.34 | |
| Sum | 47 | 15.8 | |
| Errors related to preoperative donation or cell salvage | Use and production of autologous products | 11 | 3.69 |
| Cell saver defect (technical) | 1 | 0.34 | |
| Mismatch of products following disconnection or confusion of autologous products | 1 | 0.34 | |
| Sum | 13 | 4.36 | |
| Patient identification (ID) | Screen and/or type test probe from wrong patient | 9 | 3.02 |
| Laboratory test or bedside test from wrong patient | 2 | 0.67 | |
| Administration of blood /coagulation product | 5 | 1.68 | |
| Sum | 16 | 5.37 | |
| Handling and storage errors | |||
| Labeling | Labeling of blood samples/probes/ tubes | 7 | 2.35 |
| Labeling of chart, blood order forms or other documents | 2 | 0.67 | |
| Labeling of blood products /coagulation products /drugs | 5 | 1.68 | |
| Laboratory | Laboratory/POCT error including result output and pre-analytic errors | 10 | 3.36 |
| Testing error | 1 | 0.34 | |
| Storage | Storage errors | 4 | 1.34 |
| Temperature deviation | 3 | 1.01 | |
| Component expiry | 1 | 0.34 | |
| Product release | Unit booking-off from depot or blood bank | 5 | 1.68 |
| Transport | Transport error | 1 | 0.34 |
| Sum | 39 | 13.1 | |
| Administration error | |||
| Indication | Indication for transfusion erroneous due to lab or preanalytic error, missing guideline coverage etc. | 8 | 2.68 |
| Blood management | Uncorrected preoperative anemia | 1 | 0.34 |
| Transfusion trigger unrecognized | 5 | 1.68 | |
| Urgency of blood use | 3 | 1.01 | |
| Volume status assessment related to transfusion | 6 | 2.01 | |
| Consent | Informed consent not done | 1 | 0.34 |
| Blood order | Blood order error (missed, incorrect, wrong patient, missing data) | 5 | 1.68 |
| Bedside test | Bedside test not done | 1 | 0.34 |
| Bedside test wrongly interpreted / wrongly done | 4 | 1.34 | |
| Preparation of transfusion procedure administration | Type and Screen, crossmatch | 6 | 2.01 |
| Pretransfusion procedure | 12 | 4.03 | |
| Timing | 5 | 1.68 | |
| Delegation of transfusion | 2 | 0.67 | |
| Screening test result expiry | 2 | 0.67 | |
| Multiple transfusion processes for various patients synchronously or massive transfusion as contributing factor | 9 | 3.02 | |
| Double unit administration | 3 | 1.01 | |
| Monitoring | Monitoring during administration | 1 | 0.34 |
| Documentation | Documentation of administration | 1 | 0.34 |
| Sum | 75 | 25.2 | |
| Errors related to IT | |||
| Wrong record selected | 0 | 0 | |
| Failure to consult or identify historical record | 1 | 0.34 | |
| Warning flag not updated or removed in error | 0 | 0 | |
| Computer or other IT systems failure | 1 | 0.34 | |
| Incorrect entry | 2 | 0.67 | |
| Electronic blood order | 3 | 1.01 | |
| Administrator/system related error | 0 | 0 | |
| Sum | 7 | 2.35 | |
| Errors related to analog documentation paper based forms/charts/documentation | Unreadable, wrong chart, etc. | 4 | 1.34 |
| Sum | 4 | 1.34 | |
| Other equipment failure | Technical failure of used equipment | 4 | 1.34 |
| Wrong use of equipment or no equipment | 5 | 1.68 | |
| Understaffing | 3 | 1.01 | |
| Sum | 12 | 4.03 | |
| Communication | Subject to subject (interindividually/interinstitutionally) | 20 | 6.71 |
| Subject and technique including IT, laboratory and monitoring | 5 | 1.68 | |
| Sum | 25 | 8.39 | |
| Total sum | 298 | 100 | |
ID = Identity check; IT = information technology; KIS = hospital information system; POCT = point of care testing; TACO = transfusion associated circulatory overload.
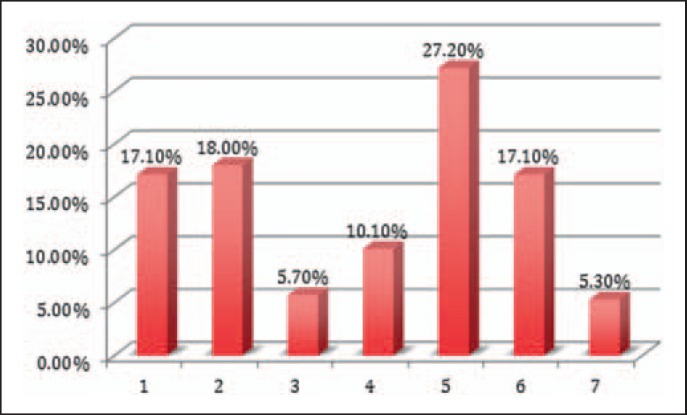
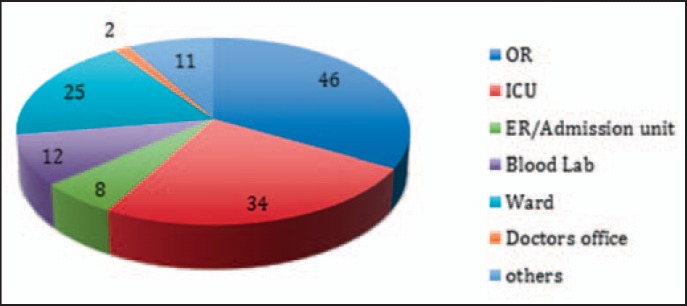
For all entries, the erroneous process step within the administration process was located (fig. 2). Thus, 5.3% of incidents occurred in steps beyond our predefined process steps, such as reports addressing surgical procedure and techniques, pre-donation, or blood product processing. In addition, in 135 cases, the functional unit in the medical institution could be located, i.e. the place where the error initiated (fig. 3). Most critical incidents occurred in operating rooms (OR) (34.1%), intensive care units (ICU) (25.2%) and peripheral wards (18.5%), and to a more minor degree in the laboratory (8.9%), emergency admission unit (5.9%), private practice office (1.5%) and others (e.g. recovery room of the blood donation unit 5.9%).
Fig. 2.
Error location within the administration process chain. Errors were located in various steps of the blood product donation process. Process steps: 1) blood product order; 2) blood group sample withdrawal and tube labeling; 3) laboratory handling of blood probes, screen and type, release of ordered blood; 4) transport and handling of blood products, 5) blood product administration, 6) patient identification, 7) others such as coagulation management, autologous processing, or donation (see table 1 for comparison). The administration process itself was by far the most frequent source of errors.
Fig. 3.
Institutional location of errors. Incidents occurred in various locations and surroundings (n = 138). However, from operating room (OR) and intensive care unit (ICU), 70% of incidents were reported. ER = emergency room.
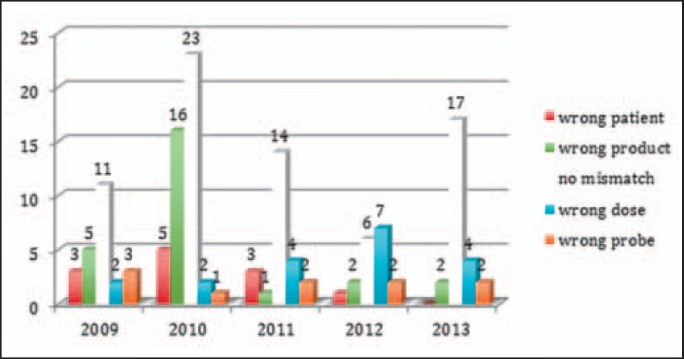
Both, near misses and true mismatches were reported in our critical incident reporting system. As true errors, we categorized the actual transfusion of wrong blood to the right recipient or vice versa. Whenever the blood product was administered to the patient, the error qualified for a true error instead of a near miss. Thus, transfusion of compatible but confused blood to a recipient was not categorized as near miss. Near misses were all detected errors where, as a consequence, no administration of the blood product to the patient had been occurred. For mismatch of recipient and blood product (wrong blood for right patient or wrong patient for wrong blood, near misses (46%) and true errors (53%) are given as absolute numbers in detail (table 4).
Table 4.
Details on mismatches
| Report IDs | Sum | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| True errors | ||||||||||||||
| Wrong blood Wrong patient |
2–20 09 Pasos 1 |
3–20 09 CM3745 |
8–20 09 32-20 11 |
Pasos 8 | CM53 11 | CM47 89 | CM35 07 | CM 34 49 | CM28 89 | CM28 11 | CM29 530 | 41-20 12 | 52-20 13 | 13 3 |
| Near misses | ||||||||||||||
| Wrong blood | Pasos5 | 7–20 09 | 13–20 10 | CM43 63 | CM42 39 | CM30 05 | CM15 711 | 61-20 13 | 62-20 13 | 9 | ||||
| Wrong patient | Pasos 11 | 11–20 10 | 20–20 10 | CM51 41 | CM27 278 | 5 | ||||||||
| True errors total (% of all errors) | 16 (54) | |||||||||||||
| Near misses total (%) | 14 (4.2) | |||||||||||||
Report IDs for mismatches of blood product and recipients. Counted is the number of errors as opposed the number of mismatched patients or blood products: The number of subjects concerned with this problem is different from the number of errors, i.e. one mismatch concerns frequently a second patient. Percentage given is the rate of all incidents (see table 3).
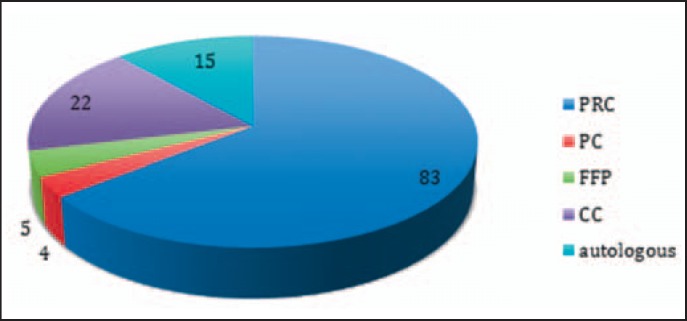
Identification of concerned blood products was possible in all cases. Blood type testing errors were assigned to red cell transfusion only. The administration error applied in 63% to packed red cells, in 17% to coagulation concentrates (eventually more if the reporting form for coagulation mismanagement would be active from the start in 2009, but this was set up in 2013), and in 11.6% to autologous products (pre-donation and cell salvage). Fresh frozen plasma and platelets played a minor role (3.9% and 3.1%, respectively) (fig. 4).
Fig. 4.
Blood product concerned. Administration incidents of all existing blood products were reported with the predominance of packed red cells (83 out of 129 reports). Nine reports were not referring to a specific product. The number of critical incidents for autologous product seems relatively high. PRC = Packed red cells; PC = platelet concentrates; FFP = fresh frozen plasma; CC = coagulation factor concentrate.
ASA classification of (intended) recipients was ASA III (46.3%) and IV (23.2%); minor groups were ASA II (17.1%), V (8.5%). and I (4.9%) (fig. 5).
Fig. 5.
ASA classification concerned. Incidents occurred in transfusion processes for all -from healthy to severely ill recipients (indicated by ASA physical status classification system), with a clear majority in ASA 3 (by definition patients with a severe systemic disease) and in ASA 4 (severe systemic disease that is a constant threat to life). Since the information of the ASA status is not obligatory in the report forms, 41 cases were not reported.
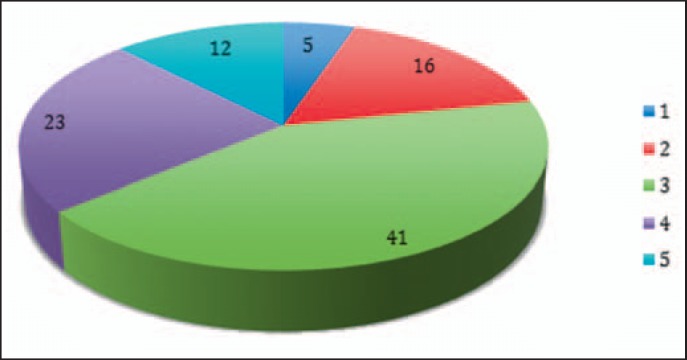
Estimated frequency of critical incidents was 1:10,000 (21%), 1:1,000 (43%), and 1:100 (25%) (fig. 6). Potential damage was mostly death or permanent disability in 58% of reports, considerable acute damage or considerable permanent disability in 25%, and minor and/or weak transient damage or light permanent disability in 13% (fig. 6).
Fig. 6.
Frequency and risk of damage estimate. Reported incidents were more than rare and inherited the risk of death for the recipient. Estimated frequency (blue columns) of reported critical incidents were classified by the experts as 1) extremely rare – 1: 100,000, 2) rare – 1:10,000, 3) medium – 1:1,000, 4) occasional – 1:100, 5) frequent – 1:10. The percentage of reports that was located in that classification is given on top of the column. The potential to harm the recipient (red columns) was classified as 1) No permanent or transient damage, 2) minor and/or weak transient damage, 3) minor and/or weak transient damage or light permanent disability, 4) considerable acute and/or considerable permanent disability, 5) death or permanent disability.
Process Quality
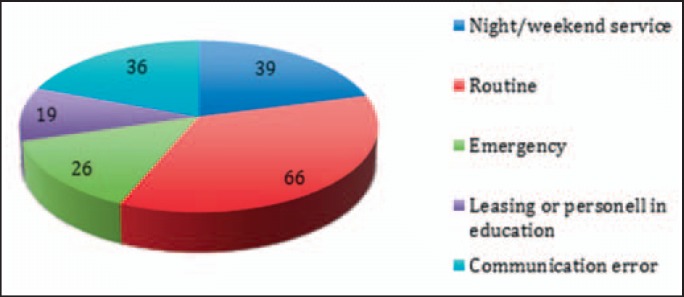
Critical incidents occurred in 35.5% of reported cases in a routine situation (35.5%), in 14% in emergency situations, and in 21% during irregular work hours at nighttime or weekends (fig. 7). Staff involved in education or only temporary at work was associated to the error in 10.2%. Miscommunication or incomplete information transfer as a major source of errors could be identified in 19.4%. However, communication errors among patients, health care personnel (physicians, nurses, ambulance drivers) and institutions (laboratory, wards, ICU, emergency department, OR) were an associated factor that played a role in the development of the incident in 55% of reported cases. Also the erroneous transmission of wrong or incomplete datasets of patients, blood orders, or blood products was defined as communication error. Only in 2 cases, the incidents were caused by acts of negligence. In total, team conflicts were obvious in 4 cases.
Fig. 7.
Circumstances and contributing factors. Incidents mainly occurred under routine circumstances. Emergency situation was a contributing factor only in 26%. For some reports, more than one contributing factor could be identified.
Indication of administration of blood products frequently was not in congruence with the existing cross-sectional guidelines for the use of blood and blood products as set by the German Medical Association [9]. It was correct in 29.0% but incorrect in 36.2%; for the rest of reports (34.8%), there were no indication if the given guidelines were followed or not.
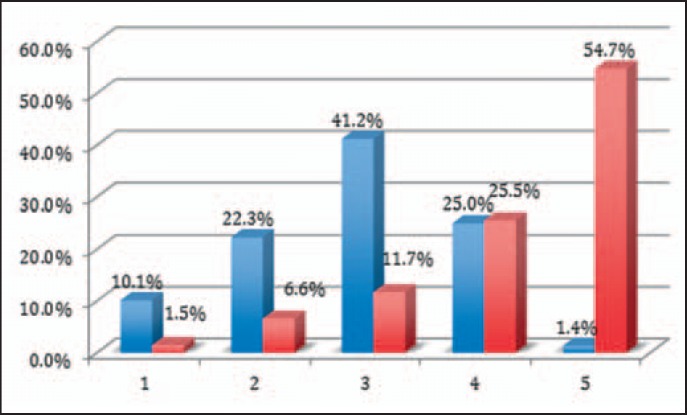
Confusions and mismatches as well as wrong dosages of blood products occurred in almost half of all reports (67 times in 138 reports, 48.6%) (fig. 8). Of all mismatches, blood product confusion contributed to 18.8%, patient confusion to 8.7%, and probe mismatch to 7.3%. Wrong doses of blood or coagulation factors were applied in 13.8%.
Fig. 8.
Confusion and mismatches, wrong doses. Incidents reported mismatches of blood product, of patients, and of patients. Also, administration of blood products in wrong dosage (according to efficacy or guidelines). White columns indicate incidents other than mismatch or wrong dosage.
Recipient's identity match to the blood group detected by obligatory BST (mostly done only for ABO without D) was tested with respect to its capability to avoid the incident reported or to detect a mismatch of either patient or blood unit. Such kind of errors were detected by BST in only 1.8%. BST was unable to avoid the reported mismatch in 29.7% or would have detected the confusion in 13.8%. BST was effective to avoid the confusion of a patient, blood sample, or product in 2.6% and would be suitable to detect it in 5.8%. However, it failed to avoid 66% of the incidents that have occurred. BST was performed in 96% of all reported administrations. It was done or interpreted wrongly in 4 of 142 cases. In 2 cases, the correct result did not come to the attention of the administrator, and once the BST was taken from the wrong patient. In 4 cases of identical ABO blood types, the BST was done but was unable to detect the error.
Administration process performance might be improved by detailed task descriptions (‘How is the task to be done in that institution, step by step?’) and standard operating procedures (SOPs) available for the field of hemotherapy. The committee gave 479 (3.7 ± 0.6 per report) recommendations to improve administration process quality in total. Among those were 151 (1.27 ± 0.5 per report) collective staff educational measures such as round tables, team coaching, lectures or clinic information events on a specific topic per report, and simulation training. Moreover, to create a permanent change, 214 (1.74 ± 0.5 per report) recommendations targeted to the establishment of a SOP, a written task description, or an algorithm. The measure to bring the report to the knowledge of the institutional transfusion committee was recommended in almost every case (and excluded from the listed recommendations in table 5).
Table 5.
Survey of committee's recommendations for structure quality
| Structure improvement by | Number | Percentage of all recommendations |
|---|---|---|
| Emergency box of a collection of blood and blood products for trauma and massive bleeding incl. sampling tubes | 5 | 1.57 |
| Establishment of clinical hemotherapy pathways* for patient blood management, bridging of coumarins and new oral anticoagulants, or for emergency revision surgery | 9 | 2.82 |
| Central coagulation and hemostasis consultant emergency phone | 8 | 2.51 |
| Staining dye for e.g. heparin that avoids confusion with another drug | 6 | 1.88 |
| Central IT registry for patients with multiple antibodies | 2 | 0.63 |
| Establish or install backup for defect POCT in OR, ICU (blood gas, coagulation, hemoglobin) | 13 | 4.08 |
| IT controlled storage and software check for the availability of blood products in the storage | 3 | 0.94 |
| Establish or use blood volume monitor (cardiac output and ejection fraction variability) | 8 | 2.51 |
| ID wrist bands for patient identification / scanner based ID check, bar code scanner for the administration of drugs and blood products | 17 | 5.33 |
| ID identity and compatibility check of blood product, probe and patient | 18 | 5.64 |
| Communication / compatibility of PDMS, laboratory and blood banking software (e.g. for plausibility check, also for documentation of a single patient history for all disciplines within the institution, match of type and screen with selected product, warning sign for drug orders and decreased liver or kidney function, documentation of type and screen test result, actual match of estimated blood loss with actual blood loss calculated from hemoglobin content) | 48 | 15.05 |
| IT control of electronic blood product and coagulation product order, feedback of blood product availability in storage, automated blood product order for blood loss surgery, indication check and match with guidelines, | 27 | 8.46 |
| Change or improve blood/coagulation product release of blood bank or depot and delivery (other than IT solutions) | 5 | 1.57 |
| Change medical device or drug / use other existing device or drug / introduce new/other/more drugs or medical devices | 19 | 5.96 |
| Indication check by the computerized order entry system | 7 | 2.19 |
| Use warning sign on devices or blood products | 9 | 2.82 |
| Establish / shut down satellite blood depot | 1/2 | 0.94 |
| GPS/RFID based tracking of blood products or blood samples or other form of transport and delivery documentation, IT based work status information | 7 | 2.19 |
| IT control of autologous blood donation and cell salvage (scale, printer, donor identity, quality, outcome, infection rate) | 6 | 1.88 |
| IT control of allogeneic blood product processing and production, document print | 2 | 0.63 |
| Change blood product label, package or concentration or Improve label printer software, bar code scanner based labeling and printing | 10 | 3.13 |
| Check personnel structure, Improve/increase staffing (quality, qualification or quantity, use shift or rotation models, prepare for emergency or mass transfusion, use documentation assistance on ward, use personnel scheduling software) | 14 | 4.39 |
| Cell saver software / warming device/ blood donation scale software or device improvement | 8 | 2.51 |
| Introduce new / change care structures (satellite pharmacy in the OR; genetic testing for coagulation disorder from the general practitioner; do not use tourniquets for knee surgery; unique patient ID number such as the social security number; dedicated venipuncture team; intermediate care ward, team coaching or communication training; enforce care in level 1 hospitals for rare diseases; use miniprobes for blood tests in adult ICU to minimize iatrogenic blood loss) | 12 (1, 1, 1, 1, 1, 2, 2, 1, 2 see details in brackets left) | 3.76 |
| Other IT or communication solutions (create unique ID for unknown subjects; check temperature of blood product itself in heating devices; use other paging device; communication of infusion pump with PDMS, text message for alarming laboratory values onto pager, handheld or cell phone; software for medical orders that considers GFR of recipient) | 6 (1, 1, 1, 2, 1 (see details in brackets left) | 1.88 |
| Change of laws and guidelines (erase blood group from autologous blood**; introduce obligatory user license for hemotherapy and cell salvage etc.) | 6 (3, 3 see details in brackets left) | 1.88 |
| Delta-check of laboratory values | 8 | 2.51 |
| Separate delivery and storage of various blood autologous and allogeneic products for same recipient, separation of same products for multiple recipients | 6 (4, 2 see details in brackets left) | 1.88 |
| Information chip about blood group, antibodies, coagulation disorder, or organ malfunction on electronic insurance card or in electronic data base | 13 | 4.08 |
| Improve blood product transport (temperature preservation and control) | 4 | 1.25 |
| Improve only transfusion documentation software (1 product for 2 subjects/kids) | 1 | 0.31 |
| Ensure information access about hemotherapy guidelines via intranet, handheld, etc. | 1 | 0.31 |
| Use electronic warnings or alert signs (other than for mismatches of KIS and laboratory / blood banking data: i.e., for organ malfunction on electronic chart; obligatory WHO checklist for or admission or or management software with alert warning function, in PDMS for discontinuation of coagulation drugs, …) | 8 | 2.51 |
Clinical pathways (CP) were considered structure quality. As opposed to a standard operating procedure or algorithm, CP requires special structures and personnel dedicated to the CP.
The deletion of the labeled blood group from autologous units has several advantages: 1- the match has to be by identity or signature instead of a universal blood group that fits many; 2- the autologous product is issued and labeled very different from allogeneic products. It works perfectly for cell saver blood and might improve administration safety for PAD blood, too.
ID = Identification document; GFR = glomerular filtration rate; KIS = hospital information system, PDMS = patient data management system; OR = operation room; ICU = intensive care unit; POCT = point of care testing; WHO = World Health Organization.
Structure Quality
Secondary to procedural aspects, safety of blood product administration processes is also dependent on the structure of the institution. Resources that may play a role are understaffing, equipment deficiency, and technical outdated data management. The latter has a potential to control the administration process if used as an obligatory measure. The committee addressed administration-associated structure 319 (2.6 ± 0.7 per incident) times. An IT solution (almost exclusively software replacement) had most likely avoided the critical incident 189 times (1.6 ± 0.4 per report).
Discussion
In summary, during the first 5 years of the registry, two error reports per month had been registered and discussed by the expert committee. This analysis predominantly aimed at the nature of the incidents, and not at the number. Obviously, the results of this 5-year review of a national CIRS specialized in hemotherapy must be interpreted very cautiously. In contrast to the voluntary hemovigilance study in the UK (SHOT - Serious Hazards of Transfusion Hemovigilance System) [12] which includes all participating health institutions in the UK, the IAKH voluntary web-based error registry cannot be used as a solid hemovigilance system. On the other hand, the German hemovigilance database of the PEI is not capable of giving information about non-fatal incidents as only transmission of infections, immunological transfusion reactions and transfusion-related acute lung injuries (TRALIs) have to be reported. There was no obligation until 2012 to report confusions or mismatches of blood products if they did not result in a lethal incompatibility reaction. Due to the lack of an obligation to report a critical incident to the IAKH error registry, the registry cannot be used to detect the absolute number of transfusion errors in Germany. Frequency estimates (fig. 6) given by the committee aim to demonstrate whether or not the reported incident is clinically relevant. To evaluate the benefit of these recommendations, further evaluations would be needed, e.g. comparison of the numbers of incorrectly labeled blood samples or comparison of patient misidentification before and after the implementation of risk minimization measures. However, a scientifically sound interpretation of the expert recommendation's efficacy is difficult and therefore remains speculative.
It is possible to evaluate cofactors such as disease severity or complexity of treatment given (e.g., ASA physical status (fig. 5), emergency transfusion, night call or untrained staff (fig. 7)) using the IAKH error registry, and the data obtained resembles those of administration statistics of blood products in Europe in general (for ASA status see [13]). However, two-thirds of errors happened during routine work hours and under regular conditions. This underlines the significance of improving routine administration since work processes under regular conditions can be better controlled and standardized.
Coagulation management-associated errors might be more important as reflected by our results since the special entry form to report such an error was added later. Furthermore, recommendations of experts for risk minimization cannot be considered valid tools to avoid such transfusion errors. However, any tool to improve patient safety is based on the exchange of methods and measures to avoid risks.
What can be concluded, however, is that the frequency and the variety of errors when administrating blood or blood products are much higher than with other drugs [14]. It appears that product safety is by far superior to administration safety. Moreover, it becomes obvious that all possible errors can actually occur every time and for every aspect of the transfusion process as was previously suspected. This circumstance is worrying for both recipients and physicians and thwarts the sense of a possibly false security generated by the presence of the strict German transfusion law with updated evidence based cross-sectional guidelines for the use of blood and blood products [9]. The question arises if administration safety can be guaranteed by laws, guidelines, code of conduct rules, obligatory reports to PEI every year, and the obligation for physicians to act in a prudent manner. The implementation of guidelines is delayed and incomplete [15], and the resistance to change daily practice should not be underestimated. Moreover, such an implementation is influenced by various interests at many different levels [16]. However, the knowledge of the frequency and variety of errors might contribute to overcome current implementation barriers. In this respect, the results of this analysis are of general interest.
In addition, the knowledge what causes an error might be used to develop strategies to avoid it. It is the opinion of the committee that a considerable part of the reported errors could be eliminated by changes of administration or IT solutions and eventually the implementation of strict SOPs (see three case reports and the respective recommendation for avoidance in the Amendment at http://content.karger.com/ProdukteDB/produkte.asp?doi=453320). Although the latter approach might be less effective in emergency situations, it should be kept in mind that the majority of reported incidents occurred in a routine situation, and, due to their compact and short form, the adherence to SOPs might be higher than that to laws or detailed guidelines [16]. However, even after implementation of SOPs the need for continued education of all faculty members remains. According to a recent prospective study, the ‘training methods varied with most perceived to have minimal effectiveness’ [17]. In this respect it is worth mentioning that almost 500 committee recommendations for 138 incidents (3.7 per incident) address improvements of education and process quality.
Another aspect of this analysis was to evaluate whether or not common techniques assumed to be crucial and effective, e.g., BSTare effective in avoiding transfusion errors. According to the results of the IAKH registry, BST was able to avoid the mismatch in only 2.6%. Most errors reported (66%) were unaffected by the BST although major incompatibility transfusion is still possible. Greinacher [4] retrospectively determined 50% of confusions occurring between BST and transfusion (16 of 32 cases). Thus there is reasonable suspicion that BST is overestimated in its efficacy to avoid confusions. BST fails if the probe for cross-match is flawlessly taken not from the recipient but from another person, if the recipient's identity is falsified but has the same blood group as the ID holder, if the BST is not done or misinterpreted, or if the match of blood groups and paperwork is done correctly but the transfusion started to an untested wrong recipient. This demonstrates that BST does not or only very marginally obviate handling errors such as a flawless comparison of ABO bedside results with all documents completed as well as with the products ready for transfusion. Moreover, it addresses only part of all administration safety issues. Based on this error analysis, the question arises if BST is worth the minimal cost as in most of the error reports it was ineffective as 66% of incidents were unchanged by the test result. In those reports concerned, the test was misinterpreted, was not done, the recipient was confused after performance of the test, or the wrong blood product was used for the test. The knowledge of the limitations of the BST should be kept in mind by administrators of red blood cells in Germany.
Technical solutions in general might be more effective since they both can lead the user through guideline-compliant administration of blood products and control the administration process [18]. Special IT-based safety features are proposed to erase user errors, e.g., the use of bar codes [19] and radiofrequency identification (RFID) tracking systems [20, 21, 22] from blood sampling to transfusion [23]. Using a computerized administration guide, the documentation of drug and blood administration can be improved. In a lot of German institutions blood product order via phone or paper forms is still active, not allowing a plausibility check and the data match to the patient's chart information and laboratory results. When considering the technical equipment of German hospitals, improvement of IT-based measures might a very promising approach to increase hemovigilance in Germany. However, current publications emphasize the potential of an end-to-end-control of blood product administration since SHOT lessons teach us that ‘the main risks remain human factors’ [12]. The suggestions by the IAKH committee comprised procedures - to mention only the most frequent recommendations - such as the delta check method of the laboratory software to detect implausible laboratory values or the compatibility of blood banking software with the institutional patient data management system (PDMS). Procedures and techniques not yet applied in Germany rarely were suggested by the committee, e.g. a central registry for patients with multiple antibodies (twice in table 4) as already recommended by Delaney and coauthors [24].
The most frequent suggestion (15% of all recommendations) of the expert committee was to establish compatible IT system for blood banking and PDMS. In the opinion of the committee, this change inherits the potential to avoid transfusion errors in multiple cases. On the other hand, it depicts a major problem in the IT structure of German transfusion medicine - the majority of blood banking software systems is incompatible with the PDMS of the treatment facilities. As a consequence, double data entry and double input errors occur, and information transfer and plausibility checks are missed. User errors remain undetected. The safety improvement with the implementation of compatible systems seems to be underestimated. The Eurocode International Blood Labeling System provides a quick solution. To date, the unique product ID, product type, and blood group are encoded eye-readable and in three linear barcodes at the product label but in the near future, the Eurocode system will provide a threefold match in one two-dimensional barcode format. The computer-generated match of data matrix and thus blood product information can easily be read in the PDMS by scanner cameras [25].
In addition, other IT-associated measures that more or less are based on the data management systems already in use were recommended, such as the electronic order of blood products [26] and identification checks [27, 28].
However, the question remains whether or not IT solutions will be able to increase the safety and performance in real practice. Current attempts to improve user performance by directing administration procesess and decision steps via computed guidance can be bypassed in some instances [29]. So it is questionable if a computer-guided order entry can be designed for better performance. Liberal practice is also reported elsewhere [30, 31, 32]. Therefore, the recommendation to establish an electronic order system is based on many theoretical but yet unproven advantages, such as adherence to guidelines, plausibility checks, data control, documentation, and storage-associated issues such as procurement. However, first attempts in Stanford, CA, and Minneapolis, MN, to install clinical decision support were promising [33, 34, 35, 36].
Among the IT solutions most frequently suggested by the committee (listed in table 1) was the scanner-based guided identification match from patient [37, 38], blood sample [39, 40], and blood products - the ‘vein-to-vein’ IT system. Its importance and potential benefit has only recently been proven [22, 41]. Mismatches in 1% of transfusion processes could be avoided by using a scanner-based system [19], although its implementation is not free of complications [42]. Even though this system or parts of it are tested by several institutions, only a few experienced users worldwide currently are using it today [43, 44]. Furthermore, its implementation is often restrained by the national data protection guidelines/laws. The present report, however, demonstrates the urgency to use and further develop those systems [27, 43, 45, 46, 47, 48, 49, 50, 51, 52] in order to markedly improve patient safety in spite of unsolved data safety issues. Encoding techniques still could be added and improved in the second implementation phase, but the benefit of a scanner-based system seems to be beyond question when considering the variety of incidents summarized in this report. Whether or not the technical refinement of vein-to-vein IT systems should be awaited before implementation is a matter of controversial debate. However, a number of problems are still unsolved, e.g., the choice of frequency and interference effects when applying RFID technology [22, 53, 54, 55]. Lastly, when considering the German hemovigilance report [3], it becomes obvious that administration safety is not well reported in Germany. The relation of In the IAKH registry, the erroneous administration of the wrong blood product to the wrong patient was reported as ‘near misses’ 14 times (nearly 10% of reports) even though the product had actually been administered. The actual rate of nationwide confusions is not known but clearly is much higher than the reported deaths due to transfusion errors. The ABO incompatibility frequency in Germany resulting in serious transfusion reactions amounts to 6.8% of all reported serious transfusion reactions per year (data from 1999 until 2009 [56]). To restrict the safety of blood administration onto fatalities might arouse patient safety concerns - especially when technical solutions to the problem are available and inexpensive, at least when compared to further reduction of infection transmission rates.
In summary, the analysis of errors occurring in the German transfusion system showed that BST covered only a minority of handling errors, that the adherence to the extensive German transfusion guidelines (particularly with regard to transfusion needs) is low, and that, according to the expert recommendations for practice improvement, transfusion safety might be improved by technical solutions such as barcode- or RFID-based scanner techniques.
Supported by
IAKH e.V., German Interdisciplinary Task Force for Clinical Hemotherapy
DIVI, German Interdisciplinary Association for Critical Care and Emergency Medicine, Section Hemotherapy and Hemostaseology
DGAI, German Society for Anesthesiology and Critical Care Medicine
BDA, Association of German Anesthesiologists.
Amendment
See supplemental material at http://content.karger.com/ProdukteDB/produkte.asp?doi=453320.
Disclosure Statement
All authors state that there is no conflict of interests.
Supplementary Material
Supplementary data
Acknowledgments
Prof. Dr. Volker Kretschmer founded the DIVI section for hemotherapy and hemostaseology. In this section, he planted the idea and organized and inaugurated this registry.
Dr. Arnulf Weiler-Lorentz constructed the entry forms and the connected data base.
The IAKH funded the remodeling and further development of the registry as well as this article.
We especially thank all current and previous committee members for their excellent work and for devoting their time, ideas, and thoughts.
References
- 1.Gesetz zur Regelung des Transfusionswesens (Transfusionsgesetz) in der Fassung der Bekanntmachung vom 28. August 2007 (BGBl. I S. 2169), zuletzt geändert durch Gesetz vom 17. Juli 2009 (BGBl. I S. 1990), pp 11.
- 2.Bundesärztekammer (German Medical Association) Cross-Sectional Guidelines for Therapy with Blood Components and Plasma Derivatives. 4th ed. www.bundesaerztekammer.de/fileadmin/user_upload/downloads/Querschnittsleitlinie_Gesamtdokument-englisch_07032011.pdf (last accessed February 15, 2017).
- 3.Funk M, Lohmann A, Spranger R.Hemovigilance Report of the Paul-Ehrlich-Institut 2013/2014. Assessment of the Reports of Serious Adverse Transfusion Reactions pursuant to Section 63i AMG (German Medicines Act). www.pei.de/SharedDocs/Downloads/vigilanz/haemovigilanz/publikationen/haemovigilance-report-2013–2014.pdf?_blob=publicationFile&v=6 (last accessed February 15, 2017).
- 4.Greinacher A. Technik der Bluttransfusion. In: Mueller-Eckhadrdt C, Kiefel V, editors. Transfusionsmedizin. Heidelberg: Springer; 2003. pp. 329–337. [Google Scholar]
- 5.Caspari G, Alpen U, Greinacher A. The risk of transfusion to the wrong patient in Germany. Transfusion. 2002;42:1238–1239. doi: 10.1046/j.1537-2995.2002.00230.x. [DOI] [PubMed] [Google Scholar]
- 6.Linden JV, Wagner K, Voytovich AE, Sheehan J. Transfusion errors in New York State: an analysis of 10 years' experience. Transfusion. 2000;40:1207–1213. doi: 10.1046/j.1537-2995.2000.40101207.x. [DOI] [PubMed] [Google Scholar]
- 7.Frietsch T, Weiler-Lorentz A, Schipplick M, Kretschmer V. The interdisciplinary work community for clinical hemotherapy start SHOT for a German haemovigilance system. Anasthesiol Intensivmed Notfallmed Schmerzther. 2009;44:626–628. doi: 10.1055/s-0029-1241166. [DOI] [PubMed] [Google Scholar]
- 8.IAKH Foundation in February 2002. Dtsch Arztebl. 2003;100 A-286 / B-254 / C-246. [Google Scholar]
- 9.German Association of Physicians Cross-Sectional Guidelines for Therapy with Blood Components and Plasma Derivatives. Transfus Med Hemother. 2009;36:347–481. [Google Scholar]
- 10.Frietsch T, Auswertekommission CID. CIRS transfusion report 2009. Anasthesiol Intensivmed. 2011;52:106–111. [Google Scholar]
- 11.Berufsverband Deutscher Anästhesisten, Deutsche Gesellschaft für Anästhesiologie und Intensivmedizin, Ärztliches Zentrum für Qualität in der Medizin: CIRS-AINS. www.dgai.de/projekte/cirs-ains (last accessed February 15, 2017)..
- 12.Bolton-Maggs PH, Cohen H. Serious hazards of transfusion (SHOT) haemovigilance and progress is improving transfusion safety. Br J Haematol. 2013;163:303–314. doi: 10.1111/bjh.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meier J, Filipescu D, Kozek-Langenecker S, Llau Pitarch J, Mallett S, Martus P, Matot I. Intraoperative transfusion practices in Europe. Br J Anaesth. 2016;116:255–261. doi: 10.1093/bja/aev456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau FY, Cheng G. To err is human nature. Can transfusion errors due to human factors ever be eliminated? Clin Chim Acta. 2001;313:59–67. doi: 10.1016/s0009-8981(01)00650-7. [DOI] [PubMed] [Google Scholar]
- 15.Napolitano LM, Kurek S, Luchette FA, Corwin HL, Barie PS, Tisherman SA, Hebert PC, Anderson GL, Bard MR, Bromberg W, Chiu WC, Cipolle MD, Clancy KD, Diebel L, Hoff WS, Hughes KM, Munshi I, Nayduch D, Sandhu R, Yelon JA. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med. 2009;37:3124–3157. doi: 10.1097/CCM.0b013e3181b39f1b. [DOI] [PubMed] [Google Scholar]
- 16.Bosse G, Breuer JP, Spies C. The resistance to changing guidelines - what are the challenges and how to meet them? Best Pract Res Clin Anaesthesiol. 2006;20:379–395. doi: 10.1016/j.bpa.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Heddle NM, Fung M, Hervig T, Szczepiorkowski ZM, Torretta L, Arnold E, Lane S, Murphy MF. Challenges and opportunities to prevent transfusion errors: a Qualitative Evaluation for Safer Transfusion (QUEST) Transfusion. 2012;52:1687–1695. doi: 10.1111/j.1537-2995.2011.03514.x. [DOI] [PubMed] [Google Scholar]
- 18.Nuttall GA, Stubbs JR, Oliver WC., Jr Transfusion errors: causes, incidence, and strategies for prevention. Curr Opin Anaesthesiol. 2014;27:657–659. doi: 10.1097/ACO.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 19.Askeland RW, McGrane S, Levitt JS, Dane SK, Greene DL, Vandeberg JA, Walker K, Porcella A, Herwaldt LA, Carmen LT, Kemp JD. Improving transfusion safety: implementation of a comprehensive computerized bar code-based tracking system for detecting and preventing errors. Transfusion. 2008;48:1308–1317. doi: 10.1111/j.1537-2995.2008.01668.x. [DOI] [PubMed] [Google Scholar]
- 20.Briggs L, Davis R, Gutierrez A, Kopetsky M, Young K, Veeramani R. RFID in the blood supply chain - increasing productivity, quality and patient safety. J Healthc Inf Manag. 2009;23:54–63. [PubMed] [Google Scholar]
- 21.Chao C, Jen W, Chi Y, Lin B. Improving patient safety with RFID and mobile technology. Int J Electr Healthcare. 2007;3:175–192. doi: 10.1504/IJEH.2007.013099. [DOI] [PubMed] [Google Scholar]
- 22.Dzik WH. Technology for enhanced transfusion safety. Hematology Am Soc Hematol Educ Program. 2005:476–482. doi: 10.1182/asheducation-2005.1.476. [DOI] [PubMed] [Google Scholar]
- 23.Bolton-Maggs PH, Wood EM, Wiersum-Osselton JC. Wrong blood in tube - potential for serious outcomes: can it be prevented? Br J Haematol. 2015;168:3–13. doi: 10.1111/bjh.13137. [DOI] [PubMed] [Google Scholar]
- 24.Delaney M, Dinwiddie S, Nester TN, Aubuchon JA. The immunohematologic and patient safety benefits of a centralized transfusion database. Transfusion. 2013;53:771–776. doi: 10.1111/j.1537-2995.2012.03789.x. [DOI] [PubMed] [Google Scholar]
- 25.Knels R, Schnurstein K, Redecker-Klein A, Hiller J. Labeling of blood products with eurocode. Anasthesiol Intensivmed. 2011;52:119–123. [Google Scholar]
- 26.Moore SB, Foss ML. Ordering blood for the wrong patient - getting inside the minds of ordering physicians. Mayo Clin Proc. 2003;78:1337–1339. doi: 10.4065/78.11.1337. [DOI] [PubMed] [Google Scholar]
- 27.Sandler SG, Langeberg A, Dohnalek L. Bar code technology improves positive patient identification and transfusion safety. Dev Biolog (Basel) 2005;120:19–24. [PubMed] [Google Scholar]
- 28.Lau FY, Wong R, Chui CH, Ng E, Cheng G. Improvement in transfusion safety using a specially designed transfusion wristband. Transfus Med. 2000;10:121–124. doi: 10.1046/j.1365-3148.2000.00241.x. [DOI] [PubMed] [Google Scholar]
- 29.Hibbs SP, Noel S, Miles D, Staves J, Murphy MF. The impact of electronic decision support and electronic remote blood issue on transfusion practice. Transfus Med. 2014;24:274–279. doi: 10.1111/tme.12149. [DOI] [PubMed] [Google Scholar]
- 30.Norgaard A, De Lichtenberg TH, Nielsen J, Johansson PI. Monitoring compliance with transfusion guidelines in hospital departments by electronic data capture. Blood Transfus. 2014;12:509–519. doi: 10.2450/2014.0282-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy DJ, Pronovost PJ, Lehmann CU, Gurses AP, Whitman GJ, Needham DM, Berenholtz SM. Red blood cell transfusion practices in two surgical intensive care units: A mixed methods assessment of barriers to evidence-based practice. Transfusion. 2014;54:2658–2667. doi: 10.1111/trf.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oddason KE, Gudbjartsson T, Gudmundsson S, Karason S, Hreinsson K, Sigurdsson GH. Inappropriate use of blood components in critical care? (in Icelandic) Laeknabladid. 2014;100:11–17. doi: 10.17992/lbl.2014.01.526. [DOI] [PubMed] [Google Scholar]
- 33.McKinney ZJ, Peters JM, Gorlin JB, Perry EH. Improving red blood cell orders, utilization, and management with point-of-care clinical decision support. Transfusion. 2015;55:2086–2094. doi: 10.1111/trf.13103. [DOI] [PubMed] [Google Scholar]
- 34.Goodnough LT, Shieh L, Hadhazy E, Cheng N, Khari P, Maggio P. Improved blood utilization using real-time clinical decision support. Transfusion. 2014;54:1358–1365. doi: 10.1111/trf.12445. [DOI] [PubMed] [Google Scholar]
- 35.Goodnough LT, Shah N. Is there a ‘magic’ hemoglobin number? Clinical decision support promoting restrictive blood transfusion practices. Am J Hematol. 2015;90:927–933. doi: 10.1002/ajh.24101. [DOI] [PubMed] [Google Scholar]
- 36.Goodnough LT, Shah N. The next chapter in patient blood management: real-time clinical decision support. Am J Clin Pathol. 2014;142:741–747. doi: 10.1309/AJCP4W5CCFOZUJFU. [DOI] [PubMed] [Google Scholar]
- 37.Murphy MF. Application of bar code technology at the bedside: the Oxford experience. Transfusion. 2007;47((2 suppl)):120S–124S. doi: 10.1111/j.1537-2995.2007.01366.x. discussion 130S-131S. [DOI] [PubMed] [Google Scholar]
- 38.Pagliaro P, Turdo R, Capuzzo E. Patients' positive identification systems. Blood Transfus. 2009;7:313–318. doi: 10.2450/2009.0001-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vuk T, Cipek V, Hecimovic A, Jukic I. Wrong blood in tube error: first study on donor blood samples. Transfusion. 2014;54:1200–1202. doi: 10.1111/trf.12549. [DOI] [PubMed] [Google Scholar]
- 40.Sindhulina C, Joseph NJ. Addressing sample identification errors in a multispecialty tertiary care hospital in Bangalore. Vox Sang. 2014;107:153–157. doi: 10.1111/vox.12139. [DOI] [PubMed] [Google Scholar]
- 41.Oldham J. Blood transfusion sampling and a greater role for error recovery. Br J Nurs. 2014;23:S28. doi: 10.12968/bjon.2014.23.Sup8.S28. S30-24. [DOI] [PubMed] [Google Scholar]
- 42.Chan JC, Chu RW, Young BW, Chan F, Chow CC, Pang WC, Chan C, Yeung SH, Chow PK, Lau J, Leung PM. Use of an electronic barcode system for patient identification during blood transfusion: 3-year experience in a regional hospital. Hong Kong Med J. 2004;10:166–171. [PubMed] [Google Scholar]
- 43.Nuttall GA, Abenstein JP, Stubbs JR, Santrach P, Ereth MH, Johnson PM, Douglas E, Oliver WC., Jr Computerized bar code-based blood identification systems and near-miss transfusion episodes and transfusion errors. Mayo Clin Proc. 2013;88:354–359. doi: 10.1016/j.mayocp.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Young D. Pittsburgh hospital combines RFID, bar codes to improve safety. Am J Health Syst Pharm. 2006;63:2431–2435. doi: 10.2146/news060030. [DOI] [PubMed] [Google Scholar]
- 45.Bertrand E, Schlatter J. Map of risks for the implementation of radio-frequency identification: application of ancillaries in the university hospital Jean Verdier. Pharmazie. 2010;65:64–68. [PubMed] [Google Scholar]
- 46.Hohberger C, Davis R, Briggs L, Gutierrez A, Veeramani D. Applying radio-frequency identification (RFID) technology in transfusion medicine. Biologicals. 2012;40:209–213. doi: 10.1016/j.biologicals.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Sun PR, Wang BH, Wu F. A new method to guard inpatient medication safety by the implementation of RFID. J Med Syst. 2008;32:327–332. doi: 10.1007/s10916-008-9137-9. [DOI] [PubMed] [Google Scholar]
- 48.Marconi M, Langeberg AF, Sirchia G, Sandler SG. Improving transfusion safety by electronic identification of patients, blood samples, and blood units. Immunohematology. 2000;16:82–85. [PubMed] [Google Scholar]
- 49.Murphy MF, Kay JD. Barcode identification for transfusion safety. Curr Opin Hematol. 2004;11:334–338. doi: 10.1097/01.moh.0000142801.38087.e5. [DOI] [PubMed] [Google Scholar]
- 50.Nichols JH, Bartholomew C, Brunton M, Cintron C, Elliott S, McGirr J, Morsi D, Scott S, Seipel J, Sinha D. Reducing medical errors through barcoding at the point of care. Clin Leadersh Manag Rev. 2004;18:328–334. [PubMed] [Google Scholar]
- 51.Sandler SG, Langeberg A, DeBandi L, Gibble J, Wilson C, Feldman CL. Radiofrequency identification technology can standardize and document blood collections and transfusions. Transfusion. 2007;47:763–770. doi: 10.1111/j.1537-2995.2007.01188.x. [DOI] [PubMed] [Google Scholar]
- 52.Traynor K. Details matter in bedside bar-code scanning. Am J Health Syst Pharm. 2004;61:1987–1988. doi: 10.1093/ajhp/61.19.1987. [DOI] [PubMed] [Google Scholar]
- 53.Knels R, Hans-Joachim M, Wittmann G, von Versen R, Pruss A. Coding of tissue preparations with eurocode in Germany. Transfus Med Hemother. 2012;39:409–415. doi: 10.1159/000345361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knels R, Ashford P, Bidet F, Bocker W, Briggs L, Bruce P, Csore M, Distler P, Gutierrez A, Henderson I, Hohberger C, Holcombe J, Holmberg J, Hulleman R, Marcel B, Messenger P, Mun I, Roberts S, Sandler G, Veeramani R, Wray B. Guidelines for the use of RFID technology in transfusion medicine. Vox Sang. 2010;98((suppl 2)):1–24. doi: 10.1111/j.1423-0410.2010.01324.x. [DOI] [PubMed] [Google Scholar]
- 55.Dzik WH. New technology for transfusion safety. Br J Haematol. 2007;136:181–190. doi: 10.1111/j.1365-2141.2006.06373.x. [DOI] [PubMed] [Google Scholar]
- 56.Funk M, Günay S.Haemovigilance Report of the Paul-Ehrlich-Institut 2010. Assessment of the Reports of Serious Adverse Transfusion Reactions pursuant to Section 63 c AMG (Arzneimittelgesetz, German Medicinal Products Act). www.pei.de/SharedDocs/Downloads/vigilanz/haemovigilanz/publikationen/haemovigilance-report-2010.pdf?_blob=publicationFile&v=5 (last accessed February 15, 2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data