Abstract

Studies on serotonin-selective reuptake inhibitors have established that disturbances in the ascending 5-HT neuron systems and their 5-HT receptor subtypes and collateral networks to the forebrain contribute to the etiology of major depression and are targets for treatment. The therapeutic action of serotonin-selective reuptake inhibitors is of proven effectiveness, but the mechanisms underlying their effect are still unclear. There are many 5-HT subtypes involved; some need to be blocked (e.g., 5-HT2A, 5-HT3, and 5-HT7), whereas others need to be activated (e.g., postjunctional 5-HT1A and 5-HT4). These state-of-the-art developments are in line with the hypothesis that the development of major depression can involve an imbalance of the activity between different types of 5-HT isoreceptors. In the current study, using in situ proximity ligation assay (PLA), we report evidence for the existence of brain 5-HT1A–5-HT2A isoreceptor complexes validated in cellular models with bioluminescence resonance energy transfer (BRET2) assay. A high density of PLA-positive clusters visualizing 5-HT1A–5-HT2A isoreceptor complexes was demonstrated in the pyramidal cell layer of the CA1–CA3 regions of the dorsal hippocampus. A marked reduction in the density of PLA-positive clusters was observed in the CA1 and CA2 regions 24 h after a forced swim test session, indicating the dynamics of this 5-HT isoreceptor complex. Using a bioinformatic approach, previous work indicates that receptors forming heterodimers demonstrate triplet amino acid homologies. The receptor interface of the 5-HT1A–5-HT2A isoreceptor dimer was shown to contain the LLG and QNA protriplets in the transmembrane and intracellular domain, respectively. The 5-HT2A agonist TCB2 markedly reduced the affinity of the 5-HT1A agonist ipsapirone for the 5-HT1A agonist binding sites in the frontal lobe using the 5-HT1A radioligand binding assay. This action was blocked by the 5-HT2A antagonist ketanserin. It is proposed that the demonstrated 5-HT1A–5-HT2A isoreceptor complexes may play a role in depression through integration of 5-HT recognition, signaling and trafficking in the plasma membrane in two major 5-HT receptor subtypes known to be involved in depression. Antagonistic allosteric receptor–receptor interactions appear to be involved in this integrative process.
Introduction
A central role for serotonin 5-HT1A receptors was proposed in the pathophysiology of depression and in the mechanism of action of antidepressant drugs.1−6 Their activation inhibits neuronal firing in limbic regions, which are hyperactive in depression.4 However, treatment with 5-HT1A agonists is complicated by the fact that their activation of 5-HT1A autoreceptors inhibits the firing of the ascending serotonin (5-HT) neurons and can contribute to depression development.1,5 Chronic antidepressant treatment differentially desensitizes 5-HT1A autoreceptors, explaining the delayed development of antidepressant effects with SSRIs.1,4,5 For treatment of depression, it is therefore of interest to develop 5-HT1A agonists selective for the postjunctional 5-HT1A receptors,2,5 which seems possible in view of the differential regional development of 5-HT1A homo- and heteroreceptor complexes in forebrain versus midbrain raphe.6,7
A functional brain analysis of the role of 5-HT in depression employed functional magnetic resonance imaging and magnetoencephalography. 5-HT was found to differentially regulate reward-predictive activities at different time scales in the striatum–prefrontal cortex network.8 5-HT may adjust the rate of delayed reward discounting. The existence was proposed of a parallel organization of reward prediction at different time scales in the striatum, which is under the differential modulation by 5-HT.9 This work may help understand the role of 5-HT in the reward networks of the human brain, but it is not known if the 5-HT1A receptor is involved in these actions of 5-HT on the reward networks.
GALR1–5-HT1A heteroreceptor complexes were found10 with allosteric receptor–receptor interactions inhibiting 5-HT1A recognition and an exaggerated activation of Gi/o-mediated signaling in 5-HT1AR.10−12 Galanin peptide (Gal (1–15)) given alone instead acts at GalR1–GalR2 heteroreceptor complexes in the raphe-limbic 5-HT system to exert its strong depression-like and anxiogenic effects.13 In contrast, Gal (1–15) enhances the antidepressant effects in the forced swim test (FST) induced by the 5-HT1AR agonist 8-OH-DPAT acting on postjunctional and somatodendritic 5-HT1AR of the mesolimbic 5-HT neurons.13 The results obtained suggest the existence of GalR1–GalR2–5-HT1A heteroreceptor complexes14 in balance inter alia with GalR1–5-HT1A complexes, where upon coactivation of the former with Gal1–15 and 5-HT1A agonists, differential allosteric receptor–receptor interactions develop in the two regions, leading to antidepressant-like actions.
The serotonin and neurotrophic factor hypotheses of depression are recognized. The discovery of brain fibroblast growth factor receptor 1 (FGFR1)–5-HT1A heteroreceptor complexes, as well as their enhancement of neuroplasticity, allows an integration of these two hypotheses.15 FGFR1–5-HT1A heteroreceptor complexes were discovered in both the midbrain 5-HT neurons and the hippocampus.15,16 Coactivation of FGFR1 and 5-HT1A protomers in the hippocampus may contribute to more rapid and robust antidepressant actions. Prolonged combined agonist treatment was postulated to counteract hippocampal atrophy in depression.
Six families of G protein-coupled 5-HT receptors exist, namely, 5-HT1, 5-HT2, 5-HT4, 5-HT5, 5-HT6, and 5-HT7 receptors.17 It is of high interest that 5-HT1A–5-HT7 heteroreceptor complexes were found in 2012 in balance with 5-HT1A homodimers and 5-HT7 homodimers and the corresponding monomers.18 The 5-HT7 protomer upon agonist activation inhibits the 5-HT1A-mediated Gi/o signaling, which results in a reduction of the ability of the 5-HT1A receptor protomer to activate GIRK channels. The 5-HT7 protomer also enhances the internalization of the 5-HT1A receptor protomer.18 It should be noted that one of the triplet amino acid homologies in its interface is used in the interface of the 5-HT1A–FGFR1 heteroreceptor complex.6 According to the triplet puzzle theory, such homologies in the receptor interface will help guide the receptors toward each other and facilitate the heterodimer formation.19
In the continuation of this search for amino acid homologies in putative 5-HT1A isoreceptor complexes, they were observed in putative 5-HT1A–5-HT2A isoreceptor complexes (current study). In line with these results, it was found in 2004 that 5-HT1A and 5-HT2A receptors are frequently coexpressed in pyramidal cells of the prefrontal cortex.20 However, a clear overlap between 5-HT1A and 5-HT2A immunoreactivities (IRs) in the pyramidal cells remains to be established.21−23
In the current article, using in situ proximity ligation assay (PLA), we report evidence for the existence of brain 5-HT1A–5-HT2A isoreceptor complexes validated in cellular models with bioluminescence resonance energy transfer (BRET). We also study their interface using the triplet puzzle theory19,24 and how it compares with the interface in other 5-HT isoreceptor and heteroreceptor complexes.6,10,25,26 Antagonistic allosteric receptor–receptor interactions in these isoreceptor complexes were established in the frontal lobe, by which 5-HT2A agonist-induced activation of the 5-HT2A protomer strongly reduced the affinity of the 5-HT1A agonist binding sites of the 5-HT1A protomer. The current results open up a new molecular mechanism for how the function of inhibitory 5-HT1A and excitatory 5-HT2A isoreceptors20,27,28 can become integrated in the brain.
Combined Results and Discussion
5-HT1A and 5-HT2A are two major 5-HT receptor subtypes in the brain, with 5-HT1A having inhibitory actions via Gi/o and 5-HT2A, excitatory actions via Gq/11.1,29 It is therefore of high interest that in the current study it was possible to demonstrate the existence of 5-HT1A–5-HT2A isoreceptor complexes in the dorsal hippocampus and the anterior cingulate cortex using in situ PLA assay.
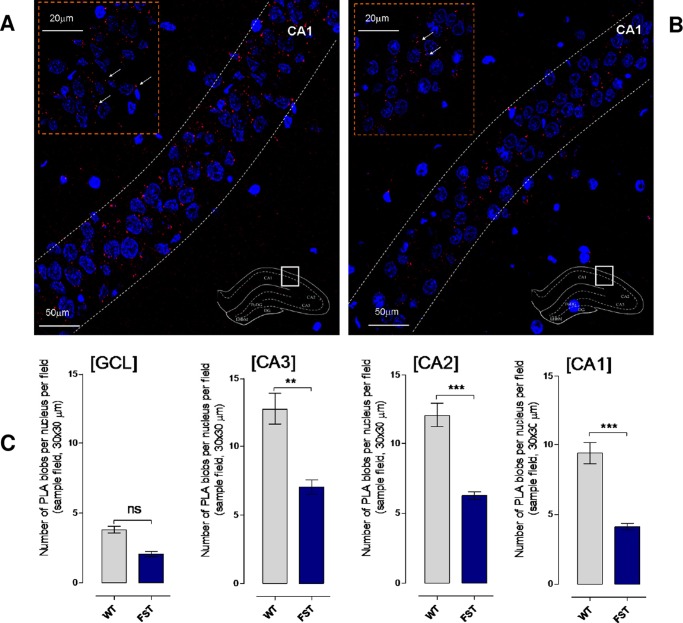
In the dorsal hippocampus of untreated Sprague–Dawley (SD) rats, a high density of PLA-positive clusters (5-HT1A–5-HT2A isoreceptor complexes) was found in the pyramidal cell layer of the CA1, CA2, and CA3 regions, whereas only a few were found in the stratum oriens and radiatum of these areas, which was similar to the background found in negative controls (Figure 1A). A single z-scan confocal microscopy photograph with a higher magnification of the high density of PLA-positive clusters is shown in the upper left part of Figure 1A. The quantitative data are shown in Figure 1C as mean ± SEM (five rats/group). The columns in gray are from the untreated rats and show the number of PLA clusters (blobs) per nucleus per sampled field (30 × 30 μm2). They range mainly from 10 to 15 PLA clusters in the CA1, CA2, and CA3 regions and reflect the high density in the pyramidal cell layer. There is only a low density of the PLA clusters in the granular cell layer of the dentate gyrus (GCL).
Figure 1.
Illustration of the 5-HT1A–5-HT2A isoreceptor complexes in the dorsal hippocampus of rat brain. (A) Microphotographs of transverse sections of the rat dorsal hippocampus (bregma level: −3.6 mm) showing the distribution of the 5-HT1A–5-HT2A isoreceptor complexes in CA1 using the in situ PLA technique.15,31,39 They are shown as red PLA blobs (clusters) found in high densities per cell in a large number of nerves cells in the pyramidal cell layer using confocal laser microscopy. No specific PLA blobs were found in the stratum moleculare and radiatum of the CA1–CA3 regions. The nuclei are shown in blue by 4’,6-diamidino-2-phenylindole. In the upper left part, the PLA blobs in the pyramidal cell layer are shown in a higher magnification. In the lower right part, the different parts of the dorsal hippocampus are shown in a transverse section. The square outlines the CA1 area from which the picture was taken. Abbreviations: CA1–3: region I–III of hippocampus proper is a portion of the hippocampal formation. CA stands for the latin cornus ammonis. (B) These panels give representative examples of the significant reduction of the density of PLA blobs in the CA1 subregion after the FST sessions (2 h) (B) vs unexposed controls (B). (C) SD rats show a significant reduction in 5-HT1A–5-HT2A isoreceptor complexes (PLA blobs) in CA3–CA1 subregions of the hippocampus after the FST sessions. All animals were euthanized by a lethal dose of pentobarbital (200 mg/kg) followed by formalin perfusion. PLA was quantified as PLA per nucleus per sampled field by an experimenter blind to treatment conditions. 5-HT1A–5-HT2A isoreceptor complexes remain unchanged in SD rats after FST session in the granular cell layer of the dentate gyrus (CGL) subregion of hippocampus (no significance, mean ± SEM. Five rats per group, unpaired t-test). CA3 subregion of the hippocampus (**p < 0.01, mean ± SEM, five rats per group, unpaired t-test), CA2 and CA1 subregions of the hippocampus (***p < 0.001, mean ± SEM, five rats per group, unpaired t-test). The number of PLA-positive cells in percent of the total number of nuclei per sampled field did not change in any region (data not shown). The 5-HT1A–5-HT2A isoreceptor complexes are stress sensitive.
In the rats exposed to a FST session 24 h earlier (Figure 1C, dark blue columns), a significant reduction in the density of PLA-positive clusters was observed in the CA1 and CA2 regions, whereas only a reduction was noted in the CA3 region (Figure 1C). No significant reduction was observed in the granular cell layer. An example of the reduction of the PLA clusters is given in the CA1 region (Figure 1B).
Double immunolabeling demonstrated a partial colocalization of 5-HT1A immunoreactivity in red and 5-HT2A immunoreactivity in green, as seen in Figure 2A–D. The CA1 pyramidal cell layer is shown; by merging the 5-HT1A and 5-HT2A images, the partial colocalization is shown in yellow-orange around the nerve cell bodies (Figure 2A). In higher-magnification images shown in Figure 2B, the arrows give examples of the partial colocalization of 5-HT1A and 5-HT2A IRs. In Figure 3C,D, images of 5-HT1A and 5-HT2A IRs are also shown in the pyramidal cell layer of the CA1. In these panels, colocalization is also indicated in putative dendrites, as pointed out by the arrows.
Figure 2.
Illustration of the 5-HT1A–5-HT2A double-immunolabeling studies in the dorsal hippocampus of rat brain. Microphotographs from transverse sections of the rat dorsal hippocampus (bregma level: −3.6 mm) showing the distribution of the 5-HT1A and 5-HT2A IRs in CA1 pyramidal cell layer. In the lower panel, a higher magnification of selected area is given. A high density of 5-HT1A (red) and 5-HT2A (in green) immunofluorescences is observed in nerve cells using double immunolabeling. (A) The IRs are shown to partially collocate (yellow-orange upon merging) within the CA1 pyramidal cell layer. Orange demonstrates the partial collocation of the two IRs in the surfaces of the pyramidal cells, indicated by arrows in the high-magnification images in (B). It is also shown that the two IRs can be partially collocated in putative dendrite processes in the CA1 region located in the pyramidal cell layer and immediate surrounding (C, D). CA stands for the latin cornus ammonis.
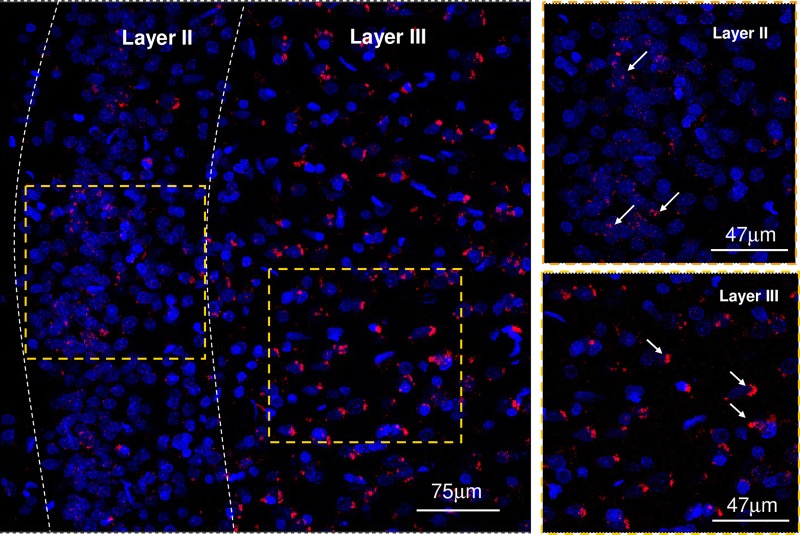
Figure 3.
Distribution of PLA-positive clusters in layers II and III from the anterior cingulate cortex. Microphotographs from transverse sections of the rat anterior cingulate cortex (bregma level: 1.2 mm) showing the distribution of the 5-HT1A–5-HT2A isoreceptor complexes using the in situ PLA technique.15,31,39 They are shown as red PLA blobs (clusters) found in high densities in layer III and in low to moderate densities in layer II. Layer III represents the external pyramidal cell layer in which large PLA-positive clusters are found and may be located in the endoplasmatic reticulum of the pyramidal cells, whereas the small circular clusters found may be located in the plasma membrane. Layer II represents the external granular layer in which mainly small circular clusters were found. High-magnification images of these layers are shown in the right panels.
In the anterior cingulate cortex (bregma level: 1.2 mm), a high density of PLA-positive clusters (5-HT1A–5-HT2A isoreceptor complexes) is found in layer III, and a low to moderate number of PLA clusters in layer II are shown in low and high magnifications in Figure 3.
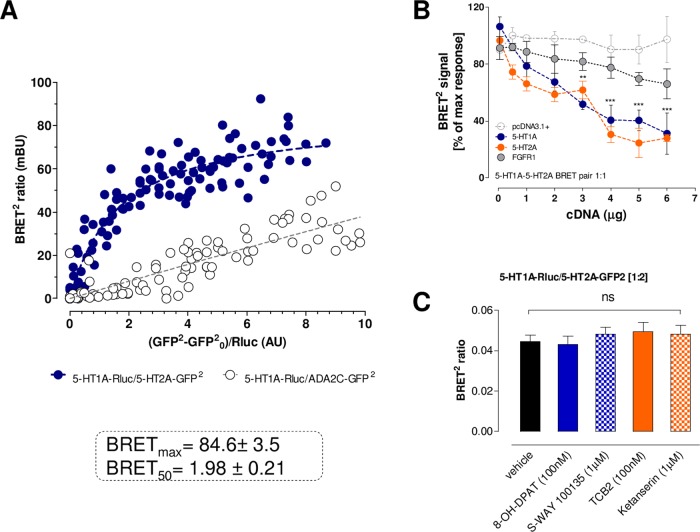
The findings were supported by the demonstration of these complexes in cellular models using a BRET2 saturation assay. A saturable and strong BRET2 signal was found in the HEK293T cells after cotransfection with 5-HT1ARluc and 5-HT2AGFP2 (Figure 4A). BRET2 signaling demonstrated a hyperbolic function in response to increasing amounts of transfected 5-HT2AGFP2, reaching saturation at the highest concentrations obtained. The specificity of the saturation obtained for the 5-HT1ARluc cells and 5-HT2AGFP2 pair was demonstrated because the negative controls25 with coexpressing 5-HT1ARluc and ADA2CGFP2 receptors only produced quasilinear curves (Figure 4A). The specificity was further supported by the demonstration that BRET2 experiments on cells coexpressing TASR14GFP2 and 5-HT1ARluc receptors only produced quasilinear curves as observed with the ADA2C receptor (data not shown). The specificity is again shown by the observation that FGFR1 unlike 5-HT1A and 5-HT2A also failed to compete with the formation of the 5-HT1A–5-HT2A isoreceptor complex, as seen from a nonreduced BRET2 ratio in competition experiments (Figure 4B). It is true that FGFR1 can form heteroreceptor complexes with 5-HT1A.15,16,30 However, in a heterotrimer complex of 5-HT1A–5-HT2A–FGFR1, FGFR1 may not interact with the 5-HT1A–5-HT2A interface because the interface can be different. Therefore, the 5-HT2A and FGFR1 may not compete with each other. In addition, FGFR1 with a single transmembrane (TM) domain can have a higher plasticity with regard to the interface interaction, leading to an improved accommodation. Agonist and antagonists of the 5-HT1A and 5-HT2A receptors did not modulate the BRET2 ratio (Figure 4C).
Figure 4.
(A) BRET2 saturation curves for the 5-HT1A–5-HT2A isoreceptor complexes, and cells coexpressing 5-HT1ARluc and ADA2CGFP2 were used as negative controls. Plotted on the X axis is the fluorescence value obtained from the GFP2, normalized with the luminescence value of 5-HT1ARluc expression 10 min after coelenterazine incubation. The 5-HT1A–5-HT2A curve fitted better to a saturation curve than to a linear regression, as found with the negative control (F test (p < 0.001). Data are mean ± SEM; n = 10–21. (B) BRET2 competition experiment for the 5-HT1A–5-HT2A isoreceptor complexes. A fixed ratio (1:1) of expression levels of the 5-HT1ARluc- or 5-HT2AGFP2-tagged receptors was used in the presence of increasing concentrations of wild-type receptors, pcDNA3.1+ vector, and the FGFR1. Plotted on the X axis is the concentration of cDNA transfected per competitor. Mean ± SEM; n = 14 in triplicate. ***: Significantly different from pcDNA3+ in the range of 4–6 μg cDNA) (p < 0.001) by two-way analysis of variance (ANOVA). (C) Agonists and antagonists effect in the BRET2 assay. A fixed ratio (1:2) of expression levels of the 5-HT1ARluc- or 5-HT2AGFP2-tagged receptors was used in the presence of specific agonists and antagonists for each receptor protomer. Mean ± SEM; n = 6 in triplicate. None of the ligands tested produced a significant change in the BRET ratio signal, as indicated by one-way ANOVA.
The selective 5-HT2A agonist TCB2 at 30 nM produced a marked shift to the right in the competition curve obtained with the 5-HT1A receptor agonist ipsapirone from [3H]-8-OH-DPAT binding sites (Figure 5A,C) in both areas of the brain. A significant increase in the mean Ki value was obtained with TCB2 (five independent experiments), demonstrating a reduction in the affinity of the high-affinity 5-HT1A agonist binding sites by the 5-HT2A agonist in both regions. The specificity is shown by the ability of the 5-HT2A antagonist ketanserin (1 μM) to significantly block the action of TCB2 in frontal cortex membranes, as indicated by statistical analysis using one-way ANOVA followed by Tukey’s multiple comparison post test (*p < 0.05) (Figure 5D). In the case of hippocampus, there are also indications for antagonistic effects of ketanserin (Figure 5B). It indicates the existence of inhibitory allosteric receptor–receptor interactions in the 5-HT1A–5-HT2A isoreceptor complexes by which agonist activation of the 5-HT2A protomer can reduce affinity and thus recognition of the 5-HT1A protomer. Thus, the current study strongly suggests that the existence of the 5-HT1A–5-HT2A isoreceptor complexes, so far demonstrated in the anterior cingulate cortex and in the hippocampus, allows a fine-tuned antagonistic modulation of 5-HT1A recognition and likely signaling through the 5-HT2A protomer. It should be pointed out however that many 5-HT1A and 5-HT2A receptors are not colocated but can also operate independently of each other.
Figure 5.
Modulation by serotonin 5-HT2A agonist TCB2 (30 nM) of the 5-HT1A affinity is based on 3H-8-OH-DPAT/ipsipirone competition experiments in membrane preparations from the hippocampus (A, B) and frontal lobe (C, D). Thus, competition experiments involving 5-HT1A receptor agonist [3H]-8-OH-DPAT binding vs increasing concentrations of ipsipirone were performed in hippocampus (A) and frontal lobe (C) membranes in the presence or absence of the 5-HT2A agonist TCB2 (30 nM) as indicated. Nonspecific binding was defined as the binding in the presence of 200 μM serotonin. The curves are based on the mean ± SEM of five rats, each one performed in triplicate. The binding values are given in percent of specific binding at the lowest concentration of ipsipirone employed. A histogram of the Ki values (nM) obtained from the competition curves is shown for both the hippocampus (B) and frontal lobe (D). TCB2 (30 nM) produces a marked change in the reduction of the Ki values (p < 0.05) in the hippocampus (B) and frontal lobe (D). This change in the reduction of the Ki value is blocked by ketanserin (1 μM) (p < 0.05) in membrane preparation from the frontal lobe (D), one-way ANOVA followed by post hoc Turkey’s multiple comparison test. In the hippocampus, the ketanserin-treated group was not significant from control.
It should be noted that the stoichiometry and composition of the 5-HT1A–5-HT2A isoreceptor complexes are unknown. There could exist a dynamic balance between dimeric, trimeric, tetrameric, and even higher-order heteromeric receptor complexes and their associated adapter proteins depending inter alia on the agonist activity at the different receptors in the complexes.7,31 The G protein signaling likely involves mainly 5-HT1A-mediated Galphai signaling and 5-HT2A-mediated Galphaq signaling also in a dynamic balance with each other through the allosteric receptor–receptor interactions and the structure of the heteroreceptor complex and the degree of coactivation. With changes in the allosteric mechanisms, the 5-HT1A and 5-HT2A receptor protomers can develop diversity and bias in their signaling through recruiting different types of G proteins and/or other proteins like β-arrestin2. The current observations indicate that activation of the 5-HT2A protomer reduces the affinity of the 5-HT1A protomer agonist binding site. It therefore seems likely that an antagonistic allosteric receptor–receptor interaction may exist in this receptor complex as well as in 5-HT1A protomer signaling exerted by the 5-HT2A protomer upon its agonist activation.
In agreement with the demonstration of 5-HT1A–5-HT2A isoreceptor complexes in the anterior cingulate cortex and the hippocampus, coexpression of 5-HT1A and 5-HT2A mRNAs were found in a high number of pyramidal neurons of the rodent prefrontal cortex.20 There is no general agreement on the overlap of 5-HT1A and 5-HT2A imunoreactivities in the pyramidal cells, but a partial overlap seems likely in the somatodendritic regions based on several publications21−23 in line with the current studies in the hippocampus (Figure 2). It is proposed that in a large number of pyramidal nerve cells there exist 5-HT1A homoreceptor complexes, 5-HT2A homoreceptor complexes, and 5-HT1A–5-HT2A isoreceptor complexes, the latter found in the overlap zones of the two IRs (Figure 1 and 2). The 5-HT nerve terminal networks mainly operate via extrasynaptic volume transmission with extracellular diffusion of 5-HT in the range of micrometers,23,32−34 reaching inter alia different types of high-affinity 5-HT1A and 5-HT2A homo- and isoreceptor complexes. On the basis of this view, there is a highly dynamic decoding of the serotonin signal as the diffusing serotonin in the micrometer range reaches a panorama of dynamic 5-HT homo- and heteroreceptor complexes, in which the 5-HT1A and 5-HT2A protomers play a major role. The demonstrated 5-HT1A–5-HT2A isoreceptor complexes make possible a fine-tuning of these two major 5-HT isoreceptors already in the plasma membrane.
These isoreceptor complexes may represent import targets for novel antidepressant drugs. It was early found that some classical antidepressant drugs can block certain types of 5-HT receptors35,36 now known under the name of 5-HT2A receptors, which appear to enhance depression.1 Instead, postjunctional 5-HT1A receptors possess antidepressant activity.1 It therefore seems possible that a heterobivalent compound built up of a 5-HT1A agonist pharmacophor linked to a 5-HT2A antagonist pharacophor may specifically target the discovered 5-HT1A–5-HT2A isoreceptor complex and be a novel type of antidepressant drugs.
In line with this view, it was demonstrated that acute exposure to a FST session produced a marked reduction in the density of 5-HT1A–5-HT2A isoreceptor complexes in the pyramidal cell layers in the dorsal hippocampus. Thus, stress can acutely reduce their formation or increase their internalization, which may lead to disturbances in the activity of the pyramidal nerve cells and in their projections to cortical areas and to the ventral striatum, with consequences for neuronal networks involved in reward and antireward.
Previously, the 5-HT1A–5-HT7 isodimer was elegantly demonstrated.18 5-HT7 was shown to reduce the Gi signaling of the 5-HT1A receptor protomer although enhancing 5-HT1A signaling over the mitogen-activated protein kinase. An analysis of the 5-HT1A–5-HT2A receptor interface using the triplet puzzle theory and comparison with the interface of other 5-HT isoreceptor and heteroreceptor complexes revealed four sets of protriplet amino acid homologies, namely, LLG, TLG, QNA, and RNA (Figure 6). The receptor interface of the 5-HT1A–5-HT2A isoreceptor dimer may operate via the LLG and QNA protriplets in the transmembrane and the intracellular C-tail (5-HT1A) and ic1 (5-HT2A)) domains, respectively (Figure 6). It is possible that the 5-HT1A–5-HT7 isoreceptor dimer also may use the QNA protriplet localized in the C-tail of both receptor protomers. The other protriplet is TLG located in both receptor protomers in the border zone between ic3 and TM6.
Figure 6.
Example of triplets LLG, TLG, QNA, and RNA in the protomers of human receptor heteromers. #: yes in both receptors and may mediate their interaction; +: yes in both receptors; and −: no in any receptor. Abbreviations: TM, transmembrane helix; ic, intracellular domains; c-tail, C-terminal tail. Dark red-shaded R (Arg) and K (Lys) are positively charged, whereas dark blue-shaded D (Asp) and E (Glu) are negatively charged amino acid residues. Black-shaded Y (Tyr) is a possible binding site. Bold red L (leu), orange I (Ile) and V (Val), and green C (Cys) and N (Asn) are main players of leucine-rich motifs. In bold S (Ser) and T (Thr) are negatively charged amino acid upon phosphorylation.
For comparison, the 5-HT1A–GalR1 heterodimer may use the RNA protriplet homology located in ic3 (5-HT1A) and in the zone between ic3 and TM4 (GalR1) besides the LLG homology also present in the 5-HT1A–5-HT2A isoreceptor dimer (Figure 6). Instead, the 5-HT1A–FGFR1 heterodimer6 shows a TLG homology located in intracellular domains also found in the 5-HT1A–5-HT7 isorecepor dimer. A different type of isoreceptor dimer GalR1–GalR2 may also use the RNA protriplet located in a similar position to that found in GalR1 and again an LLG homology may be involved as in the 5-HT1A–5-HT2A isoreceptor dimer (Figure 6). The role of these protriplets in guiding these receptor protomers toward each other19 is also indicated by the findings that the nonheterodimer 5-HT1A–ADRA2C25 lacked these four protriplet homologies.
Overall, a new 5-HT1A isoreceptor complex, composed of a 5-HT1A–5-HT2A isomer, was found in cellular models and in the dorsal hippocampus and the anterior cingulate cortex of the rat brain. Also, their receptor interface may involve the QNA and LLG protriplet homologies. Antagonistic allosteric receptor–receptor interactions likely exist in this isoreceptor complex because a standard 5-HT2A agonist significantly and markedly reduced the affinity of the 5-HT1A agonist binding sites. Its dynamics was shown by the significant reduction of the PLA clusters visualizing the 5-HT1A–5-HT2A complex in the dorsal hippocampus upon exposure to FST sessions.
Material and Methods
Reagents
[3H]-8-hydroxy-2-(di-n-propylamino)-tetralin ([3H]-8-OH-DPAT) (141 Ci/mmole) was obtained from PerkinElmer. Serotonin as well as other basic chemicals used in buffer preparation were obtained from Sigma-Aldrich. 4-Bromo-3,6-dimethoxybenzocyclobuten-1-yl)methylamine hydrobromide (TCB2), 8-OH-DPAT, ipsapirone, and ketanserin were purchased from Tocris Cookson Inc. Dulbecco’s modified Eagle’s medium, penicillin/streptomycin, and fetal bovine serum (FBS) were purchased from Invitrogen.
Animals
All experiments were performed using male SD rats (Scanbur, Sweden). The rats were 3–4 months of age at the time of behavioral testing. The animals were group-housed under standard laboratory conditions (20–22 °C, 50–60% humidity). Food and water were available ad libitum. For the behavioral testing, the rats were handled for a minimum of 6 days before testing to minimize stress effects. Each animal was used for one test only. To understand the dynamics of the 5-HT1A–5-HT2A heteroreceptor complexes in the dorsal hippocampus, the SD rats were exposed to a FST session37 2 h before decapitation and compared with a control group (five rats/group). All studies involving animals were performed in accordance with the Institutional Animal Ethics Committee of the University of Málaga, the Stockholm North Committee on Ethics of Animal Experimentation, the Swedish National Board for Laboratory Animal, the Spanish Directive (Real Decretory 53/2013), and the European Communities Council Directive (Cons 123/2006/3) guidelines for accommodation and care of laboratory animals.
Plasmid Constructs, Cell Culture, and Transfection
The constructs presented herein were made using standard molecular biology as described previously.10,26 HEK293T cells were grown and transiently transfected as depicted in Borroto-Escuela et al.15
Double-Immunolabeling Histochemistry
The experiments were performed as described previously.38 Adult age-matched male SD rats (n = 3) were anesthetized and perfused intracardially with 4% (w/v) paraformaldehyde in saline. Brains were removed, postfixed by immersion overnight in 4% paraformaldehyde in phosphate-buffered saline (PBS), and coronal sections (20 μm) were cut on a cryostat and processed for free-floating immunohistochemistry. The sections were permeabilized with buffer A containing 0.2% Triton X-100 for 5 min and then preincubated in a blocking buffer containing 0.3% (w/v) triton and 4% (w/v) bovine serum albumin. After 1 h at room temperature, the sections were labeled with the indicated primary antibodies for 1 h, extensively washed, and stained with the indicated fluorescence-labeled secondary antibodies. The samples were rinsed and visualized employing a Leica SP2 confocal microscope. The primary antibodies used were as follows: mouse monoclonal anti-5-HT1A (1 μg/mL, Millipore, Stockholm, Sweden) and rabbit monoclonal anti-5-HT2A (SAB4301791, 1 μg/mL; Sigma-Aldrich, Stockholm, Sweden). The secondary antibodies used were Alexa Fluor 488-conjugated goat antimouse IgG (1:2000; Invitrogen, Stockholm, Sweden) and Alexa Fluor 594-conjugated goat antirabbit IgG (1:2000; Invitrogen, Stockholm, Sweden).
In Situ PLA
To study the 5-HT1A–5-HT2A isoreceptor complexes, the in situ PLA was performed as described previously.15,16,31,39 Free-floating formalin-fixed brain sections (30 μm) at a bregma level of −3.6 mm from untreated SD rats were employed using the following primary antibodies: mouse monoclonal anti-5-HT1A (1 μg/mL, Millipore, Stockholm, Sweden) and rabbit monoclonal anti-5-HT2A (SAB4301791, 1 μg/mL; Sigma-Aldrich, Stockholm, Sweden). Control experiments employed only one primary antibody or cells transfected with cDNAs encoding only one type of receptor. The PLA signal was visualized and quantified using a Leica TCS–SL confocal microscope (Leica) and the Duolink ImageTool software. Briefly, fixed free-floating rat brain sections (storage at −20 °C in Hoffman solution) were washed four times with PBS and quenched with 10 mM glycine buffer for 20 min at room temperature. Then, after three PBS washes, incubation took place with a permeabilization buffer (10% FBS and 0.5% Triton X-100 or Tween 20 in Tris-buffered saline, pH 7.4) for 30 min at room temperature. Again, the sections were washed twice, 5 min each, with PBS at room temperature and incubated with the blocking buffer (0.2% BSA in PBS) for 30 min at room temperature. The brain sections were then incubated with the primary antibodies diluted in a suitable concentration in the blocking solution for 1–2 h at 37 °C or at 4 °C overnight. The day after, the sections were washed twice and the proximity probe mixture was applied to the sample and incubated for 1 h at 37 °C in a humidity chamber. The unbound proximity probes were removed by washing the slides twice, 5 min each, with the blocking solution at room temperature under gentle agitation, and the sections were incubated with the hybridization–ligation solution (BSA (250 g/mL), T4 DNA ligase (final concentration, 0.05 U/μL), Tween 20 (0.05%), NaCl (250 mM), adenosine 5′-triphosphate (1 mM), and the circularization or connector oligonucleotides (125–250 nM)) and incubated in a humidity chamber at 37 °C for 30 min. The excess of connector oligonucleotides was removed by washing twice, 5 min each, with the washing buffer A (Sigma-Aldrich; Duolink Buffer A (8.8 g of NaCl, 1.2 g of Tris base, and 0.5 mL of Tween 20 dissolved in 800 mL of high-purity water at pH 7.4) at room temperature under gentle agitation, and the rolling circle amplification mixture was added to the slices and incubated in a humidity chamber for 100 min at 37 °C. Then, the sections were incubated with the detection solution in a humidity chamber at 37 °C for 30 min. In a last step, the sections were washed twice in the dark, 10 min each, with the washing buffer B (Sigma-Aldrich; Duolink Buffer B (5.84 g of NaCl, 4.24 g of Tris base, 26.0 g of Tris–HCl dissolved in 500 mL of high-purity water at pH 7.5) at room temperature under gentle agitation. The free-floating sections were put on a microscope slide, and a drop of appropriate mounting medium (e.g., VectaShield or Dako) was applied. The coverslip was placed on the section and sealed with nail polish. The sections were protected against light and stored for several days at −20 °C before confocal microscope analysis.
BRET2 Saturation Assay
The BRET2 saturation experiment was performed as described previously.40,41 HEK293T cells, 48 h after transfection, transiently transfected with constant (1 μg) or increasing amounts (0.12–5 μg) of plasmids encoding for 5-HT1ARluc and 5-HT2AGFP2, respectively, were rapidly washed twice in PBS, detached, and resuspended in the same buffer. Cell suspensions (20 μg protein) were put in duplicates into the 96-well microplate black plates with a transparent bottom (Corning 3651) (Corning, Stockholm, Sweden) for fluorescence measurement or white plates with a white bottom (Corning 3600) for BRET determination. For BRET2 measurements, coelenterazine 400a also called DeepBlueC substrate (VWR, Sweden) was used at a final concentration of 5 μM. The readings were made 1 min after using the POLARstar Optima plate reader (BMG Labtech, Offenburg, Germany) that allows the sequential integration of the signals observed with two filter settings [410 nm (80 nm bandwidth) and 515 nm (30 nm bandwidth)]. The BRET2 ratio is defined as previously described by Borroto-Escuela et al.42
5-HT1A Radioligand Binding Assay
Membrane Preparation
Frozen frontal lobe and hippocampus tissue was homogenized with an Ultra-Turrax in 5 mL of ice-cold preparation buffer containing 50 mM Tris–HCl/Tris base and 2.5 mM ethylenediaminetetraacetic acid (pH 7.4). The membranes were precipitated by centrifugation at 20 000 rpm and 4 °C for 10 min. The resulting pellet was resuspended in the same volume of preparation buffer, preincubated at 37 °C for 10 min, and centrifuged at 20 000 rpm and 4 °C for 10 min three times. The final pellet was resuspended in preparation buffer and sonicated for 10 s (Soniprep 150, U.K.), and the total protein concentration of homogenates was determined by BCA Protein Assay (Pierce, Sweden). Membranes were prepared on the day of radioligand binding assay.
5-HT1A Receptor Binding
Competition binding experiments for the 5-HT1A receptor were performed with the 5-HT1A agonist (ipsapirone, 12 concentrations from 0.01 nM to 100 μM (TOCRIS, catalog 1869, batch 2) using a 96-well microplate with GF/B filter (UniFilter GF/B, PerkinElmer), and a 1 nM concentration of [3H]-8-OH-DPAT. The modulation of the 5-HT1A competition curve by the 5-HT2A agonist TCB2 was tested at a concentration of 30 nM in view of its Ki value around 10 nM.26 The assay mixture (total volume, 300 μL) contained the membrane suspension (120 μg of protein per reaction), [3H]-8-OH-DPAT in the buffer containing 50 mM Tris–HCl, 10 μM pargyline, 4 mM CaCl2, and 0.1% ascorbic acid. For nonspecific binding, 200 μM serotonin was used. The reaction mixture was incubated at room temperature for 60 min with gentle shaking. The binding was terminated by a rapid filtration, followed by three washes with 200 μL of cold washing buffer (50 mM Tris–HCl, pH 7.4). The filters were dried and immersed in 2 mL of scintillation liquid (Ultima Gold MV, PerkinElmer). The bound ligand was determined by WALLAC 1409 DSA liquid scintillation counter. The results were calculated in GraphPad Prism 6.0 (GraphPad Software Inc.).
Bioinformatic Analysis
Using a bioinformatics approach, indications were obtained that receptors forming heterodimers demonstrated triplet amino acid homologies. This was not true for pairs of receptors that fail to form heterodimers.19 These triplet homologies can therefore participate in the receptor interface and give a code that helps the formation of the heterodimer. It was called the triplet puzzle theory.19,43 The 5-HT1A–5-HT2A isodimer will be analyzed with this mathematical approach in the current study and compared with other 5-HT isoreceptor and heteroreceptor complexes. The compiled data for the bioinformatic analysis was obtained from the GPCR Hetnet database (www.gpcr-hetnet.com).25
Statistical Analysis
The number of samples (n) under each experimental condition is indicated in figure legends. Data from competition experiments were analyzed by nonlinear regression analysis using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA). The inhibition constants of the high- and low-affinity states of the receptor (pKiH and pKiL, respectively) from several independent replications were averaged allowing statistical comparisons using a one-way ANOVA. Group differences after ANOVAs were measured by post hoc Turkey’s multiple comparison test. A p value of 0.05 and lower was considered significant. BRET2 isotherms were fitted using a nonlinear regression equation assuming a single binding site, which provided BRETmax and BRET50 values.
Acknowledgments
The authors are supported by grants from the Swedish Medical Research Council (04X-715 and VR-link) to K.F., by Hjärnfonden (FO2016-0302) to D.O.B.-E., and by AFA Försäkring (130328) to K.F. and D.O.B.-E. D.O.B.-E belongs to Academia de Biólogos Cubanos. A.O.T. has not received any support for this work. The authors thank Professor Graham Budd, Uppsala University, for correction and improvement of the text.
Author Contributions
We confirm and declare that all authors meet the criteria for authorship according to the ICMJE, including approval of the final manuscript, and they take public responsibility for the work and have full confidence in the accuracy and integrity of the work of other group authors. They have substantially contributed to the conception or design of the work. Also they have participated in the acquisition, analysis, and interpretation of data for the current review version. They have also helped revising it critically for important intellectual content and in the publication of its final approved version. In addition, they have contributed to this final version of the manuscript in terms of assistance in writing, technical editing, language editing, and proofreading. D.O.B.-E., X.L., A.O.T., D.S., K.S., Y.A.T., A.J.B., M.N., B.P., Z.D.-C., R.C., P.A., M.L., and K.F. designed methods and experiments, carried out the laboratory experiments, analyzed the data, and interpreted the results. D.O.B.-E., X.L., B.P., and K.F. codesigned and co-worked on the radioligand binding experiments. D.O.B.-E., K.S., and A.J.B. codesigned and co-worked on the double-immunolabeling experiments. D.O.B.-E., D.S., M.N., Y.A.T., Z.D.-C., R.C., P.A., M.L., and K.F. codesigned; discussed analyses, interpretation, and presentation of all immunohistochemistry; and conducted in situ PLA experiments. D.O.B.-E., D.S., M.N., Y.A.T., Z.D.-C., R.C., P.A., M.L., and K.F. designed and directed its implementation of the quality assurance and control of each used antibody. A.O.T. designed, analyzed the data, and interpreted all of the bioinformatics studies. K.F. and D.O.B.-E. wrote the paper. All authors have contributed to, seen, and approved the manuscript.
The authors declare no competing financial interest.
References
- Artigas F. Serotonin receptors involved in antidepressant effects. Pharmacol. Ther. 2013, 137, 119–131. 10.1016/j.pharmthera.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Artigas F. Developments in the field of antidepressants, where do we go now?. Eur. Neuropsychopharmacol. 2015, 25, 657–670. 10.1016/j.euroneuro.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Mansari M. E.; Manta S.; Oosterhof C.; El Iskandrani K. S.; Chenu F.; Shim S.; Blier P. Restoration of serotonin neuronal firing following long-term administration of bupropion but not paroxetine in olfactory bulbectomized rats. Int. J. Neuropsychopharmacol. 2015, 18, pyu050 10.1093/ijnp/pyu050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P.; El Mansari M. Serotonin and beyond: therapeutics for major depression. Philos. Trans. R. Soc., B 2013, 368, 20120536 10.1098/rstb.2012.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P.; Bortolozzi A.; Artigas F. Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: rationale and current status of research. CNS Drugs 2013, 27, 703–716. 10.1007/s40263-013-0071-0. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D. O.; Tarakanov A. O.; Fuxe K. FGFR1-5-HT1A Heteroreceptor Complexes: Implications for Understanding and Treating Major Depression. Trends Neurosci. 2016, 39, 5–15. 10.1016/j.tins.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Fuxe K.; Borroto-Escuela D. O. Heteroreceptor Complexes and their Allosteric Receptor-Receptor Interactions as a Novel Biological Principle for Integration of Communication in the CNS: Targets for Drug Development. Neuropsychopharmacology 2016, 41, 380–382. 10.1038/npp.2015.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y.; Okada G.; Shishida K.; Fukumoto T.; Machino A.; Yamashita H.; Tanaka S. C.; Doya K.; Yamawaki S. Effects of serotonin on delay discounting for rewards--an application for understanding of pathophysiology in psychiatric disorders. Seishin Shinkeigaku Zasshi 2012, 114, 108–114. [PubMed] [Google Scholar]
- Demoto Y.; Okada G.; Okamoto Y.; Kunisato Y.; Aoyama S.; Onoda K.; Munakata A.; Nomura M.; Tanaka S. C.; Schweighofer N.; Doya K.; Yamawaki S. Neural and personality correlates of individual differences related to the effects of acute tryptophan depletion on future reward evaluation. Neuropsychobiology 2012, 65, 55–64. 10.1159/000328990. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D. O.; Narvaez M.; Marcellino D.; Parrado C.; Narvaez J. A.; Tarakanov A. O.; Agnati L. F.; Diaz-Cabiale Z.; Fuxe K. Galanin receptor-1 modulates 5-hydroxtryptamine-1A signaling via heterodimerization. Biochem. Biophys. Res. Commun. 2010, 393, 767–772. 10.1016/j.bbrc.2010.02.078. [DOI] [PubMed] [Google Scholar]
- Fuxe K.; Borroto-Escuela D. O.; Romero-Fernandez W.; Tarakanov A. O.; Calvo F.; Garriga P.; Tena M.; Narvaez M.; Millon C.; Parrado C.; Ciruela F.; Agnati L. F.; Narvaez J. A.; Diaz-Cabiale Z. On the existence and function of galanin receptor heteromers in the central nervous system. Front. Endocrinol. 2012, 3, 127. 10.3389/fendo.2012.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K.; von Euler G.; Agnati L. F.; Ogren S. O. Galanin selectively modulates 5-hydroxytryptamine 1A receptors in the rat ventral limbic cortex. Neurosci. Lett. 1988, 85, 163–167. 10.1016/0304-3940(88)90448-X. [DOI] [PubMed] [Google Scholar]
- Millón C.; Flores-Burgess A.; Narvaez M.; Borroto-Escuela D. O.; Santin L.; Parrado C.; Narvaez J. A.; Fuxe K.; Diaz-Cabiale Z. A role for galanin N-terminal fragment (1–15) in anxiety- and depression-related behaviors in rats. Int. J. Neuropsychopharmacol. 2014, 18, 1–13. 10.1093/ijnp/pyu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millón C.; Flores-Burgess A.; Narvaez M.; Borroto-Escuela D. O.; Santin L.; Gago B.; Narvaez J. A.; Fuxe K.; Diaz-Cabiale Z. Galanin (1–15) enhances the antidepressant effects of the 5-HT1A receptor agonist 8-OH-DPAT: involvement of the raphe-hippocampal 5-HT neuron system. Brain Struct. Funct. 2016, 221, 4491–4504. 10.1007/s00429-015-1180-y. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D. O.; Romero-Fernandez W.; Mudo G.; Perez-Alea M.; Ciruela F.; Tarakanov A. O.; Narvaez M.; Di Liberto V.; Agnati L. F.; Belluardo N.; Fuxe K. Fibroblast growth factor receptor 1- 5-hydroxytryptamine 1A heteroreceptor complexes and their enhancement of hippocampal plasticity. Biol. Psychiatry 2012, 71, 84–91. 10.1016/j.biopsych.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D. O.; Narvaez M.; Perez-Alea M.; Tarakanov A. O.; Jimenez-Beristain A.; Mudo G.; Agnati L. F.; Ciruela F.; Belluardo N.; Fuxe K. Evidence for the existence of FGFR1-5-HT1A heteroreceptor complexes in the midbrain raphe 5-HT system. Biochem. Biophys. Res. Commun. 2015, 456, 489–493. 10.1016/j.bbrc.2014.11.112. [DOI] [PubMed] [Google Scholar]
- Barnes N. M.; Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology 1999, 38, 1083–1152. 10.1016/S0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Renner U.; Zeug A.; Woehler A.; Niebert M.; Dityatev A.; Dityateva G.; Gorinski N.; Guseva D.; Abdel-Galil D.; Frohlich M.; Doring F.; Wischmeyer E.; Richter D. W.; Neher E.; Ponimaskin E. G. Heterodimerization of serotonin receptors 5-HT1A and 5-HT7 differentially regulates receptor signalling and trafficking. J. Cell Sci. 2012, 125, 2486–2499. 10.1242/jcs.101337. [DOI] [PubMed] [Google Scholar]
- Tarakanov A. O.; Fuxe K. G. Triplet puzzle: homologies of receptor heteromers. J. Mol. Neurosci. 2010, 41, 294–303. 10.1007/s12031-009-9313-5. [DOI] [PubMed] [Google Scholar]
- Amargós-Bosch M.; Bortolozzi A.; Puig M. V.; Serrats J.; Adell A.; Celada P.; Toth M.; Mengod G.; Artigas F. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb. Cortex 2004, 14, 281–299. 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- Willins D. L.; Deutch A. Y.; Roth B. L. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse 1997, 27, 79–82. . [DOI] [PubMed] [Google Scholar]
- Riad M.; Garcia S.; Watkins K. C.; Jodoin N.; Doucet E.; Langlois X.; el Mestikawy S.; Hamon M.; Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J. Comp. Neurol. 2000, 417, 181–194. . [DOI] [PubMed] [Google Scholar]
- Jansson A.; Tinner B.; Bancila M.; Verge D.; Steinbusch H. W.; Agnati L. F.; Fuxe K. Relationships of 5-hydroxytryptamine immunoreactive terminal-like varicosities to 5-hydroxytryptamine-2A receptor-immunoreactive neuronal processes in the rat forebrain. J. Chem. Neuroanat. 2001, 22, 185–203. 10.1016/S0891-0618(01)00133-8. [DOI] [PubMed] [Google Scholar]
- Tarakanov A. O.; Fuxe K. The triplet puzzle theory indicates extensive formation of heteromers between opioid and chemokine receptor subtypes. J. Neural Transm. 2015, 122, 1509–1514. 10.1007/s00702-015-1421-5. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D. O.; Brito I.; Romero-Fernandez W.; Di Palma M.; Oflijan J.; Skieterska K.; Duchou J.; Van Craenenbroeck K.; Suarez-Boomgaard D.; Rivera A.; Guidolin D.; Agnati L. F.; Fuxe K. The G protein-coupled receptor heterodimer network (GPCR-HetNet) and its hub components. Int. J. Mol. Sci. 2014, 15, 8570–8590. 10.3390/ijms15058570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroto-Escuela D. O.; Romero-Fernandez W.; Narvaez M.; Oflijan J.; Agnati L. F.; Fuxe K. Hallucinogenic 5-HT2AR agonists LSD and DOI enhance dopamine D2R protomer recognition and signaling of D2-5-HT2A heteroreceptor complexes. Biochem. Biophys. Res. Commun. 2014, 443, 278–284. 10.1016/j.bbrc.2013.11.104. [DOI] [PubMed] [Google Scholar]
- Mengod G.; Palacios J. M.; Cortes R. Cartography of 5-HT1A and 5-HT2A Receptor Subtypes in Prefrontal Cortex and Its Projections. ACS Chem. Neurosci. 2015, 6, 1089–1098. 10.1021/acschemneuro.5b00023. [DOI] [PubMed] [Google Scholar]
- Leiser S. C.; Li Y.; Pehrson A. L.; Dale E.; Smagin G.; Sanchez C. Serotonergic Regulation of Prefrontal Cortical Circuitries Involved in Cognitive Processing: A Review of Individual 5-HT Receptor Mechanisms and Concerted Effects of 5-HT Receptors Exemplified by the Multimodal Antidepressant Vortioxetine. ACS Chem. Neurosci. 2015, 6, 970–986. 10.1021/cn500340j. [DOI] [PubMed] [Google Scholar]
- Hamon M.; Blier P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 45, 54–63. 10.1016/j.pnpbp.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D. O.; Perez-Alea M.; Narvaez M.; Tarakanov A. O.; Mudo G.; Jimenez-Beristain A.; Agnati L. F.; Ciruela F.; Belluardo N.; Fuxe K. Enhancement of the FGFR1 signaling in the FGFR1-5-HT1A heteroreceptor complex in midbrain raphe 5-HT neuron systems. Relevance for neuroplasticity and depression. Biochem. Biophys. Res. Commun. 2015, 463, 180–186. 10.1016/j.bbrc.2015.04.133. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D. O.; Hagman B.; Woolfenden M.; Pinton L.; Jiménez-Beristain A.; Oflijan J.; Narvaez M.; Di Palma M.; Feltmann K.; Sartini S.; Ambrogini P.; Ciruela F.; Cuppini R.; Fuxe K.. In Situ Proximity Ligation Assay to Study and Understand the Distribution and Balance of GPCR Homo- and Heteroreceptor Complexes in the Brain. In Receptor and Ion Channel Detection in the Brain; Lujan R., Ciruela F., Eds.; Springer: Berlin, 2016; Vol. 110, pp 109–126. [Google Scholar]
- Fuxe K.; Agnati L. F.. Volume Transmission in the Brain, Novel Mechanisms for Neural Transmission, 1st ed.; Raven Press: New York, 1991; Vol. 1, pp 1–624. [Google Scholar]
- Fuxe K.; Dahlstrom A.; Hoistad M.; Marcellino D.; Jansson A.; Rivera A.; Diaz-Cabiale Z.; Jacobsen K.; Tinner-Staines B.; Hagman B.; Leo G.; Staines W.; Guidolin D.; Kehr J.; Genedani S.; Belluardo N.; Agnati L. F. From the Golgi-Cajal mapping to the transmitter-based characterization of the neuronal networks leading to two modes of brain communication: wiring and volume transmission. Brain Res. Rev. 2007, 55, 17–54. 10.1016/j.brainresrev.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Fuxe K.; Dahlstrom A. B.; Jonsson G.; Marcellino D.; Guescini M.; Dam M.; Manger P.; Agnati L. The discovery of central monoamine neurons gave volume transmission to the wired brain. Prog. Neurobiol. 2010, 90, 82–100. 10.1016/j.pneurobio.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Fuxe K.; Ogren S. O.; Agnati L.; Gustafsson J. A.; Jonsson G. On the mechanism of action of the antidepressant drugs amitriptyline and nortriptyline. Evidence for 5-hydroxytryptamine receptor blocking activity. Neurosci. Lett. 1977, 6, 339–343. 10.1016/0304-3940(77)90095-7. [DOI] [PubMed] [Google Scholar]
- Ogren S. O.; Fuxe K.; Agnati L. F.; Gustafsson J. A.; Jonsson G.; Holm A. C. Reevaluation of the indoleamine hypothesis of depression. Evidence for a reduction of functional activity of central 5-HT systems by antidepressant drugs. J. Neural Transm. 1979, 46, 85–103. 10.1007/BF01250331. [DOI] [PubMed] [Google Scholar]
- Overstreet D. H.; Wegener G. The flinders sensitive line rat model of depression--25 years and still producing. Pharmacol. Rev. 2013, 65, 143–155. 10.1124/pr.111.005397. [DOI] [PubMed] [Google Scholar]
- Di Liberto V.; Borroto-Escuela D. O.; Frinchi M.; Verdi V.; Fuxe K.; Belluardo N.; Mudo G. Existence of muscarinic acetylcholine receptor (mAChR) and fibroblast growth factor receptor (FGFR) heteroreceptor complexes and their enhancement of neurite outgrowth in neural hippocampal cultures. Biochim. Biophys. Acta 2017, 1861, 235–245. 10.1016/j.bbagen.2016.10.026. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D. O.; Romero-Fernandez W.; Garriga P.; Ciruela F.; Narvaez M.; Tarakanov A. O.; Palkovits M.; Agnati L. F.; Fuxe K. G protein-coupled receptor heterodimerization in the brain. Methods Enzymol. 2013, 521, 281–294. 10.1016/B978-0-12-391862-8.00015-6. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D. O.; Romero-Fernandez W.; Tarakanov A. O.; Marcellino D.; Ciruela F.; Agnati L. F.; Fuxe K. Dopamine D2 and 5-hydroxytryptamine 5-HT((2)A) receptors assemble into functionally interacting heteromers. Biochem. Biophys. Res. Commun. 2010, 401, 605–610. 10.1016/j.bbrc.2010.09.110. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D. O.; Narvaez M.; Di Palma M.; Calvo F.; Rodriguez D.; Millon C.; Carlsson J.; Agnati L. F.; Garriga P.; Diaz-Cabiale Z.; Fuxe K. Preferential activation by galanin 1–15 fragment of the GalR1 protomer of a GalR1-GalR2 heteroreceptor complex. Biochem. Biophys. Res. Commun. 2014, 452, 347–353. 10.1016/j.bbrc.2014.08.061. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D. O.; Flajolet M.; Agnati L. F.; Greengard P.; Fuxe K. Bioluminescence resonance energy transfer methods to study G protein-coupled receptor-receptor tyrosine kinase heteroreceptor complexes. Methods Cell Biol. 2013, 117, 141–164. 10.1016/B978-0-12-408143-7.00008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarakanov A. O.; Fuxe K. G. Integrin triplets of marine sponges in the murine and human MHCI-CD8 interface and in the interface of human neural receptor heteromers and subunits. SpringerPlus 2013, 2, 128. 10.1186/2193-1801-2-128. [DOI] [PMC free article] [PubMed] [Google Scholar]