Abstract
Environmental and occupational exposures to respirable ultrafine fractions of particulate matter (PM) have been implicated in the initiation and exacerbation of lung diseases. However, the precise mechanisms underlying production of cell damage and death attributed to nanoparticles (NP) on human airway epithelium are not fully understood. This study examined the role of neurotrophic pathways in NP-induced airway toxicity. Size and agglomeration of TiO2 nano (TiO2-NP) and fine (TiO2-FP) particles were measured by dynamic light scattering. Expression and signaling of key neurotrophic factors and receptors were assessed by real time polymerase chain reaction, flow cytometry, immunostaining, and Western blot in various respiratory epithelial cells after exposure to TiO2-NP or TiO2-FP. Particle-induced cell death was measured by flow cytometry after annexin V/propidium iodide staining. The role of neurotrophin-dependent apoptotic pathways was analyzed with specific blocking antibodies or siRNAs. Exposure of human epithelial cells to TiO2-NP enhanced interleukin (IL)-1α synthesis, as well as nerve growth factor (NGF) gene expression and protein levels, specifically the precursor form (proNGF). TiO2-NP exposure also increased expression of p75NRF receptor genes. These neurotropic factor and receptor responses were stimulated by IL-1α and abolished by its specific receptor antagonist (IL-1-ra). TiO2-NP also increased JNK phosphorylation and apoptosis, which was prevented by anti-p75NRF or NGFsiRNA. Data demonstrated that TiO2-NP exerted adverse effects in the respiratory tract by inducing unbalanced overexpression of immature neurotrophins, which led to apoptotic death of epithelial cells signaled through the death receptor p75NTR. This may result in airway inflammation and hyperreactivity after exposure to TiO2–NP.
Keywords: Air pollution, Asthma, Lung injury, Neurotrophins, Particulate matter
INTRODUCTION
Because of their size (≤100 nm diameter), engineered nanoparticles (NP) and the ultrafine fraction of ambient air pollution display greater ability to be inhaled and deposited in the fragile epithelial structures of the distal airways compared to larger particles (Tsuda et al. 2009; Kermanizadeh et al. 2016; Oberdorster et al. 2015). Indeed, a number of epidemiologic studies reported that exposure to ambient particulate matter, which includes a NP component, exerts deleterious effects on human lungs leading to obstructive and fibrotic disease, exacerbation of pre-existing airway disease, and increased risk of respiratory infections (Chen et al. 2015; Costa et al. 2014; de Oliveira et al. 2014; Nel 2005; Pietropaoli et al. 2004; Tsai et al. 2015). In particular, the toxicology of inhalable titanium dioxide (TiO2) NP has drawn significant attention because of its extensive commercial use (Kermanizadeh et al. 2016; Xia et al. 2006), with most studies focusing on TiO2-induced oxidative stress mediated by reactive oxygen species (ROS) (Gurr et al. 2005; Olmedo et al. 2005).
Previously, Scuri et al (2010a) reported that exposure to NP upregulates the expression of lung neurotrophins which subsequently leads to airway hyperresponsiveness and inflammation. The neurotrophin family includes the nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophins (NT) 3 and 4 (Robinson et al. 1999). By binding to high-affinity tropomyosin receptor kinases (TrkA, TrkB, TrkC) or to the pan-neurotrophin receptor p75NTR, neurotrophins not only play a key role in neuronal survival, development, and function (Scuri et al 2010b), but also exert direct and indirect (i.e., nerve-mediated) effects on innate and adaptive immune pathways involved in allergic inflammation (Braun et al. 1999). In addition, mature NGF non-specifically modulate cell death in a variety of tissues by increasing expression of the anti-apoptotic Bcl-2 family members (Othumpangat et al. 2009).
The aim of this study was to determine the role played by neurotrophic pathways in the interactions between TiO2-NP and human airways. To this end, the effects of nano-size (21 nm primary particle diameter) and fine-size (<5 μm) TiO2 particles were first compared in vitro on viability of human epithelial cells sampled from different anatomical levels of the respiratory tract (nasal, tracheal, bronchial, and alveolar). Therefore, the influence of these particles on gene and protein expression of key neurotrophic factors and receptors in the airway epithelium were systematically analyzed. These initial experiments directed attention to the immature dimeric precursor of the NGF protein (proNGF), which binds with high affinity to p75NTR death receptor and initiates apoptosis in target cells by signaling through downstream stress-activated protein kinase/c-Jun-amino-terminal kinase (SAPK/JNK). Finally, specific inhibitors were used to determine the contribution of this pathway in the apoptotic or necrotic death of primary human epithelial cells exposed to titanium dioxide nanoparticles (TiO2-NP).
MATERIALS and METHODS
Materials
Commercial grade titanium dioxide nanoparticles (TiO2-NP) and titanium(IV) oxide fine particles (TiO2-FP) were kindly provided by the National Institute for Occupational Safety and Health (NIOSH). MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] cell proliferation assay kits were purchased from Cayman Chemical (Ann Arbor, MI); FITC Annexin-V Apoptosis Detection kits from BD Biosciences (San Jose, CA); and RNeasy kits for total RNA isolation from Qiagen (Valencia, CA). Primers for human NGF, BDNF, TrkA, TrkB and p75NTR were purchased from SABiosciences (Valencia, CA) and primers for the β2 microglobulin (B2M) housekeeping gene from RealTimePrimers (Elkins Park, PA).
The following primary antibodies, secondary antibodies, and isotype controls were used for immunostaining: NGF, BDNF, p75NTR, GAPDH and JNK (Santa Cruz Biotechnology, Santa Cruz, CA); phospho-SAPK/JNK (Cell Signaling, Danvers, MA); pro-NGF (Millipore, Billerica, MA); TrkA and TrkB (R&D Systems, Minneapolis, MN); AlexaFluor 488-conjugated secondary antibodies (Invitrogen, Carlsbad, CA); and horseradish peroxidase (HRP)-conjugated secondary antibodies (Santa Cruz Biotechnology). Western blotting reagents were purchased from Amersham Life Science (Buckinghamshire, United Kingdom). Interleukin 1 alpha (IL-1α) and IL-1 receptor antagonist (IL1-ra) were purchased from Santa Cruz Biotechnology.
Preparation of particles
Stock solutions (10 mg/ml) of TiO2-NP or TiO2-FP were prepared in aseptic phosphate-buffered saline (PBS) by sonicating for 30 sec (Misonix Sonicator XL-2000; Qsonica, Newtown, CT) and cooling on ice for 15 sec for a total of 3 min. Before use, particles were diluted under aseptic conditions to 0–100 μg/ml in PBS, and then sonicated for another 1 min. Particle size distribution was measured with a Nanotrac Particle Size Analyzer (Microtrac, Montgomeryville, PA) performing dynamic light scattering (DLS) analysis of 100 μg/ml of particles suspended in PBS and sonicated for 2 min (TiO2-FP) or 1 min (TiO2-NP) in a Vibra-Cell ultrasonic processor (Sonics & Materials, Newtown, CT). Sonication of TiO2-NP for a longer than 1 min resulted in increased agglomeration. To monitor structure size and agglomeration pattern of the particles as delivered in cell culture medium, 10 μg/ml of sonicated particle suspensions were added to the medium and then reanalyzed by DLS.
Cell culture
Human nasal epithelial cells from PromoCell GmbH (Heidelberg, Germany); human tracheal and bronchial epithelial cells from Cell Applications (San Diego, CA); and Type I alveolar cells from ScienCell Research Laboratories (Carlsbad, CA) were used. All these primary human epithelial cells were grown with their respective medium at 37°C in 5% CO2 atmosphere. All experiments were performed when the cells reached 70–80% confluence and within 5–6 passages, except alveolar cells that were used within 3–4 passages.
Cytotoxicity assay
Epithelial cells were plated at a density of 5x103 per well in flat bottom 96-well plates (CellStar; Greiner Bio-One, Frickenhausen, Germany). Once cells reached approximately 70% confluence, medium was replaced with 200 μl fresh medium containing different concentrations of TiO2-FP or TiO2-NP (0–100 μg/ml) and incubated for 24 hr. Following exposure, cell viability was measured by adding 10 μl MTT reagent and incubating for 3 hr at 37°C. Crystallized MTT was dissolved, and its optical density was measured at 575 nm with a reference wavelength of 690 nm (PowerWave XS ELISA plate reader; BioTek, Winooski, VT). Viability was calculated as the ratio of the average OD obtained for each treatment to that of untreated cells using the Gen5TM data analysis software (BioTek). Data shown are means of 3 separate experiments.
Reverse transcription-polymerase chain reaction (RT-PCR)
Gene expression of neurotrophic factors and receptors was analyzed by RT-PCR. Total RNA (100 ng) isolated with the RNeasy kit was amplified with QuantiTect One-step SYBR Green and specific primers using an Applied Biosystems 7500 real-time thermocycler (Foster City, CA). Fold changes in neurotrophins expression were normalized to B2M and calculated with the ΔΔCt method (Livak and Schmittgen 2001).
Fluorescence-activated cell sorting (FACS)
Neurotrophins protein expression after 24 hr exposure to TiO2 particles was measured using FACS. NGF, BDNF and their TrkA, TrkB and p75NTR receptors were stained with appropriate primary antibodies. Approximately 1x105 airway epithelial cells were isolated by trypsinization, fixed in 4% paraformaldehyde, and permeabilized with 0.5% Triton X-100 in PBS. Nonspecific binding was removed by adding human IgG on ice for 20 min. Cells were incubated separately with primary antibodies for NGF, BDNF, TrkA, TrkB, or p75NTR followed by the respective fluorescent conjugated secondary antibodies, and analyzed using FACSCalibur with CellPro software (BD Biosciences). Data were further analyzed with the Windows Multiple Document Interface (WinMDI) software version 2.9 (Scripps Research Institute, La Jolla, CA).
Immunostaining
Imaging studies were performed on 1x103 human airway epithelial cells grown on poly-L-lysine coated glass coverslips in 6-well plates and exposed to 10 μg/ml of TiO2-FP or TiO2-NP for 24 hr. Cells were then rinsed in PBS, fixed with 4% paraformaldehyde, and immunostained with the anti-NGF antibody followed by AlexaFluor 488-labeled secondary antibody. Coverslips were mounted with Prolong Gold anti-fade reagent containing 4,6-diamidino-2-phenylindole (DAPI). Cells were visualized using EC Plan-Neofluar 40x1.30 oil DIC M27 objective and photographed using a confocal microscope (LSM510; Carl Zeiss, Jena, Germany) with 405-diode laser to excite DAPI and Argon laser to excite the AlexaFluor 488-labeled secondary antibody.
Immunoprecipitation/Immunoblot
Protein was extracted from control and TiO2-treated cell pellets using RIPA lysis buffer (50 mM Tris-HCl at pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) containing a cocktail of protease inhibitors (Calbiochem, San Diego, CA). Protein concentration was determined using bicinchoninic acid (Pierce, Rockford, IL). Equal amounts of protein (50 μg) were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to a nitrocellulose membrane at 4°C. Membranes containing the protein samples were blocked with 5% milk in TBS-T buffer (50 mM Tris at pH 7.5, 150 mM NaCl, 0.05% Tween 20) followed by incubation with anti-NGF antibody or with phospho-JNK. Protein bands were visualized by chemiluminescence detection (ECL, Amersham Life Sciences, United Kingdom). Immunoblots were subsequently stripped, blocked and re-probed with antibodies specific for β-actin, GAPDH, or total JNK and quantified using Image J1.43u chemiluminescent densitometry software (National Institutes of Health, Bethesda, MD).
To study the role of p75NTR-mediated activation of the JNK cell death pathway, 200 μg of protein from cells exposed to TiO2 particles or untreated controls was incubated overnight at 4°C with anti-p75NTR mouse monoclonal antibody (sc-56448). Antigen-antibody complexes were precipitated by binding to A/G plus-agarose beads (sc-2003) for 2 hr at 4°C, and once eluted from the beads were subjected to immunoblotting as described earlier. Membranes were then incubated overnight at 4°C with rabbit polyclonal anti-NGF antibody (sc-33602) in blocking buffer, and chemiluminescent signals were developed as detailed above.
Role of IL-1α
To study the role of IL-1α in modulating NGF expression in bronchial epithelial cells exposed to TiO2-NP, confluent (80%) plates of bronchial epithelial cells were exposed to varying concentrations of recombinant IL-1α (0–5 ng/ml) or were pre-treated with IL1-ra (0–100 μM) for 3 hr followed by TiO2-NP (10 μg/ml) for an additional 24 hr. Following incubation, cells were either processed for RNA extraction and stored at −80°C, or processed immediately for Western blot and immunofluorescence assays.
To confirm the role of p75NTR in mediating neurotrophin-induced death, a specific blocking antibody (anti-human NGFR p75NTR, SPM299) was employed. Bronchial and alveolar epithelial cells were pre-incubated for 24 hr with 1 μg/ml antibody before exposure to TiO2-NP for another 24 hr. To assess specificity of the blocking antibody, an isotype IgG antibody was used as control. Cell death was measured by FACS as described previously.
Apoptosis/necrosis assay
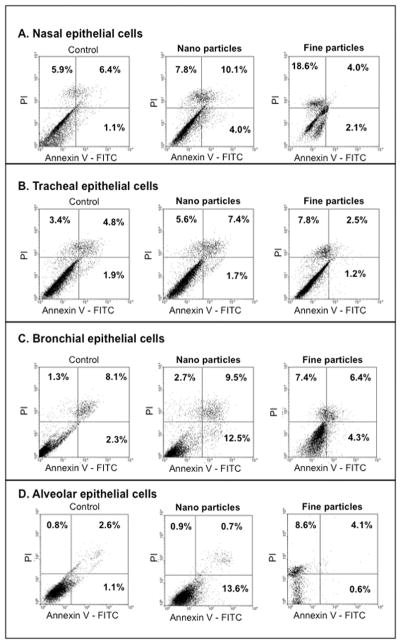
To understand the mode of TiO2-induced cell death, the annexin V/propidium iodide (PI) assay was utilized. Airway epithelial cells were grown in 6-well plates and exposed to TiO2-NP or TiO2-FP for 24 hr. All floating cells were collected by centrifugation and adherent cells were detached by trypsinization. These cells (106 cells/ml) were incubated with fluorescein isothiocyanate (FITC)-conjugated annexin V and PI for 15 min at room temperature in the dark and analyzed with a FACSCalibur flow cytometer and CellQuest Pro software. Cells in the right lower quadrant represented early apoptosis, while cells in the right upper quadrant represented late apoptosis or post-apoptotic necrosis. Necrosis is represented by the left upper quadrant. A total of 20,000 events were acquired for each sample, and data expressed as means of 3 separate experiments.
NGF silencing
To silence NGF gene expression, a specific siRNA (Dharmacon, Chicago, IL) with Lipofectamine™2000 as transfection reagent (Invitrogen, Carlsbad, CA) was used. Silencing efficiency was measured by RT-PCR using scrambled siRNA to control for both transfection and gene silencing. NGF-deficient bronchial epithelial cells were then exposed to TiO2 particles (10 μg/ml) for another 24 hr. Cells were collected by trypsinization. Apoptosis and necrosis were measured by FACS. To further confirm the role of p75NTR in proNGF-induced apoptosis, confluent bronchial epithelial cells were pre-treated with 1 μg/ml anti-human p75NTR antibody for 24 hr and then exposed to TiO2-NP for an additional 24 hr. Anti-human IgG antibody was used as the isotype control. Cells were harvested after incubation and apoptosis was measured by FACS.
Statistical analysis
All data are expressed as means ± standard deviations of the mean (SD). Data were analyzed by ANOVA for multiple comparisons and t-test for comparisons within a group using SigmaStat 3.5 (Systat Software, San Jose, CA). Post-hoc comparisons between groups were analyzed with the Holm-Sidak method or the Tukey-Kramer test. P-value <0.05 was considered significant.
RESULTS
TiO2-induced cytotoxicity
Size and distribution pattern of TiO2 particles in PBS and cell culture medium were measured by DLS. According to the manufacturer, TiO2-NP (80/20% anatase/rutile) consisted of small aggregates with primary particles having a mean diameter of approximately 21 nm and specific surface of 50 m2/g, while TiO2-FP (100% rutile) had an average diameter <5 μm with specific surface area of 2–3 m2/g. However, TiO2-NP agglomerated in suspension with mean structure diameters of 2.6 μm in PBS and 0.7 μm in cell culture medium (Table 1), whereas TiO2-FP showed less agglomeration with a mean structure size of 1 μm in PBS and 1.2 μm in medium.
Table 1.
Physical properties of nano and fine TiO2 particles
| Description | Nano particles Titanium Oxide P25 | Fine particles Titanium[IV] oxide |
|---|---|---|
| Purity | 99% | 99.9% |
| Primary particle diameter | 21 nm | <5 μm |
| Specific surface area (m2/g) | 50 ± 15 | 2–3 |
| Particle form (crystal phase) | 80/20 anatase/rutile | rutile |
| Average size in PBS (μm) | 2.6 ± 0.3 (2.3–3.16) | 1.0 ± 2.4(0.8–6.2) |
| Average size in medium (μm) | 0.7 ± 0.1 (0.4–0.9) | 1.2 ± 0.1 (0.9–1.5) |
| Suspension in medium | 100% | 100% |
Physical properties provided by nanomaterial manufacturers.
Specific surface area measured with the Brunauer-Emmett-Teller (BET) method.
Particles suspended in PBS or cell culture medium by sonication.
Agglomerate structure diameter measured by dynamic light scattering (DLS).
The viability of nasal, tracheal, bronchial, and alveolar human type I epithelial cells was measured after exposure to increasing concentrations of TiO2-NP at 37°C for 24 hr (Figure 1). Alveolar and bronchial cells tended to be more susceptible to TiO2 toxicity than tracheal and nasal cells. The MTT assay also showed numerically higher fall in cell viability after exposure to TiO2-NP than to TiO2-FP using equal mass concentrations. Specifically, 69.9% of bronchial cells remained viable after exposure to 10-μg/ml TiO2-NP in comparison to 79.4% of cells exposed to TiO2-FP.
Figure 1. Viability of TiO2-exposed airway epithelia.
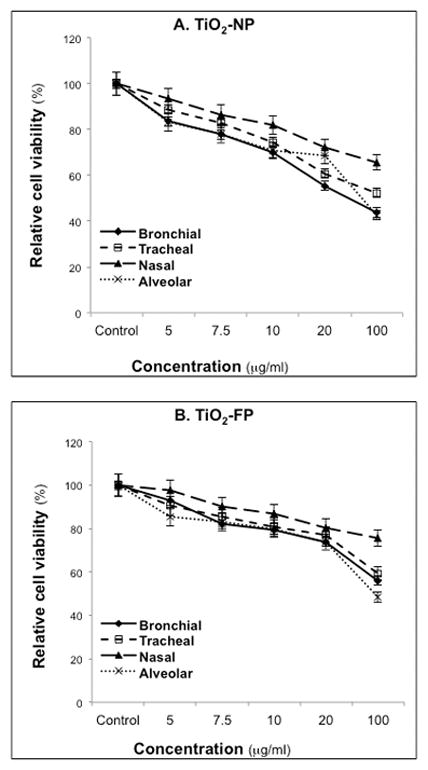
Human nasal, tracheal, bronchial and alveolar epithelial cells were exposed to various concentrations of TiO2-NP (A) or TiO2-FP (B) for 24 hr. Relative cell viability was measured by the MTT assay. Data are expressed as means ± SDs of 3 independent experiments.
TiO2-induced neurotrophins expression
Exposure to TiO2-NP resulted in elevated neurotrophins synthesis and surface receptors expression in airway epithelial cells, with important differences dependent on airway type (Figure 2). TiO2-NP exposure significantly enhanced expression of NGF and p75NTR receptor genes in nasal, bronchial, and alveolar epithelial cells, whereas TrkA receptor expression was only increased in alveolar epithelial cells. In contrast, BDNF and TrkB gene expression remained near basal levels in all cell types except for TrkB expression in nasal epithelial cells. Further, tracheal epithelial cells were the least responsive to TiO2-NP with no significant changes in neurotrophin gene expressions.
Figure 2. Neurotrophins gene expression in TiO2-exposed airway epithelia.
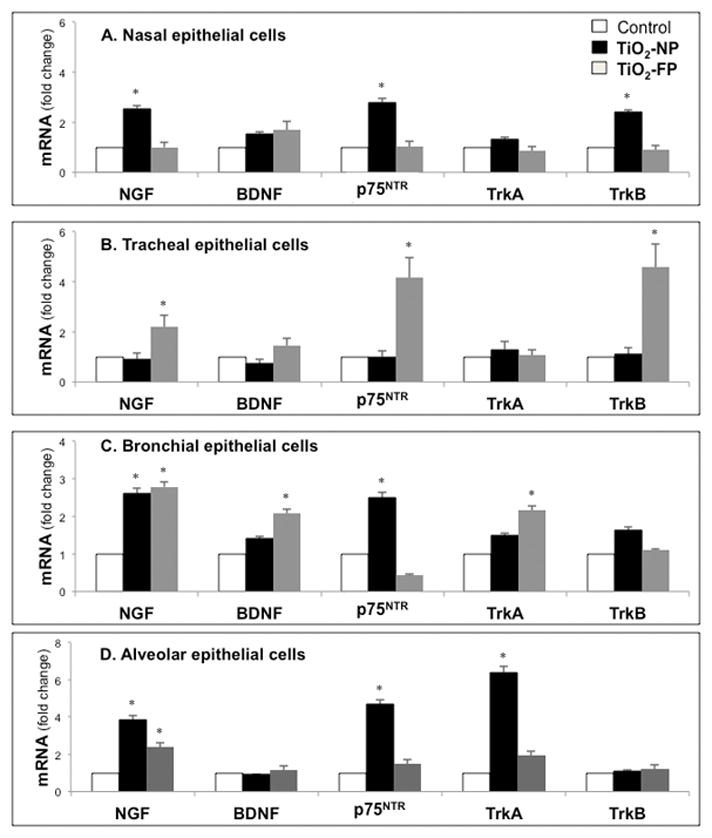
Total cellular RNA was isolated from human nasal (A), tracheal (B), bronchial (C), and alveolar (D) epithelial cells after exposure to 10 μg/ml of TiO2-NP or TiO2-FP for 24 hr. Gene expression was analyzed by real-time PCR and cycle threshold values were normalized to the housekeeping gene B2M. Data are expressed as means ± SDs of 3 independent experiments. *p<0.05 compared to control cells not exposed to TiO2.
TiO2-FP at the same mass concentrations increased NGF gene expressions in tracheal, bronchial, and alveolar epithelial cells, together with a marked rise in p75NTR in tracheal epithelial cells and elevated TrkA in bronchial epithelial cells. In general, the lower respiratory tract epithelium, particularly alveolar and bronchial epithelial cells, exhibited the greatest rise in transcripts encoding NGF and its cognate receptors, without associated significant changes in the BDNF-TrkB axis.
Whether increased levels of NGF transcripts in epithelial cells exposed to TiO2-NP translated into higher protein concentrations was subsequently examined at the single cell level by FACS (Figure 3). Alveolar epithelial cells exhibited significant upregulation of NGF protein synthesis after exposure to10 μg/ml TiO2-NP or TiO2-FP compared to non-exposed controls. Nasal epithelial cells also expressed markedly higher NGF protein concentration when exposed to TiO2-NP, whereas tracheal epithelial cells did not display any significant change in NGF compared to controls independent from particle size.
Figure 3. NGF protein expression in TiO2-exposed airway epithelia.
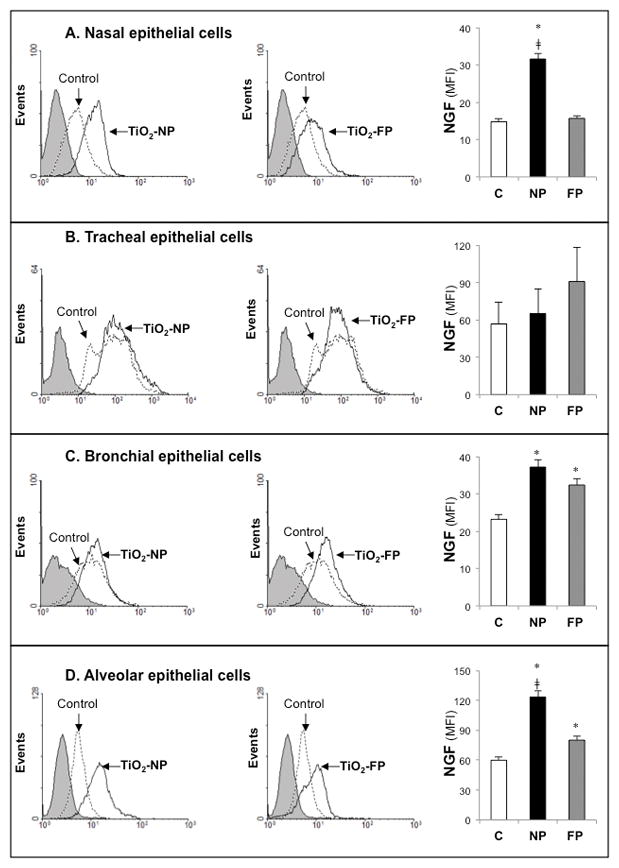
Human nasal (A), tracheal (B), bronchial (C), and alveolar (D) epithelial cells exposed to TiO2-NP or TiO2-FP were stained with anti-human rabbit polyclonal NGF primary antibody and anti-rabbit secondary antibody, and changes in intracellular protein expression were measured by FACS. Mean fluorescent activity (MFI) data are expressed as means ± SDs of 3 independent experiments. *p<0.05; compared to control cells not exposed to TiO2. ‡p<0.05 compared to cells exposed to TiO2-FP.
RT-PCR and FACS data were further confirmed by confocal immunostaining analysis. Nasal and alveolar cells treated with TiO2-NP demonstrated significant increase in intracellular staining for NGF as opposed to near-baseline levels following TiO2-FP exposure (Figure 4). However, bronchial epithelial cells significantly upregulated endogenous NGF when exposed to either TiO2-NP or TiO2-FP.
Figure 4. NGF immunoreactivity in TiO2-exposed airway epithelia.
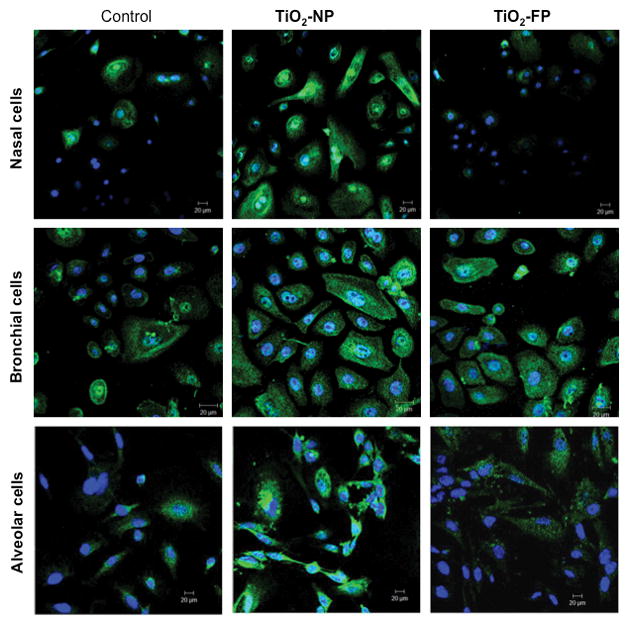
NGF immunoreactivity increased in human nasal, bronchial, and alveolar epithelial cells exposed to 10 μg/ml of TiO2-NP for 24 hr. Immunostaining was done with rabbit polyclonal NGF antibody and Alexa-Fluor 488 conjugated secondary anti-rabbit antibody. Nuclei were stained with DAPI. Cells were visualized using an EC Plan-Neofluar 1.30 oil DIC M27 objective. Magnification = 40X.
As in preliminary studies, IL-1α synthesis was elevated in cells exposed to TiO2-NP. Therefore, experiments were conducted to determine whether this pro-inflammatory cytokine played a role in modulation of NGF gene expression in bronchial epithelial cells exposed to TiO2-NP. IL-1α protein concentration rose significantly in cell supernatants after exposure to either TiO2-NP or TiO2-FP (Figure 5A). Exogenous IL-1α increased NGF gene expression to levels similar to TiO2-NP, and pretreatment with a specific receptor antagonist (IL1-ra) abolished the effect of TiO2-NP exposure on NGF gene expression. IL1-ra exerted a similar inhibitory effect on TiO2-NP induced NGF protein synthesis measured by either Western blot (Figure 5B) or FACS (Figure 5C) analysis of bronchial epithelial cells.
Figure 5. IL-1α modulation of NGF expression.
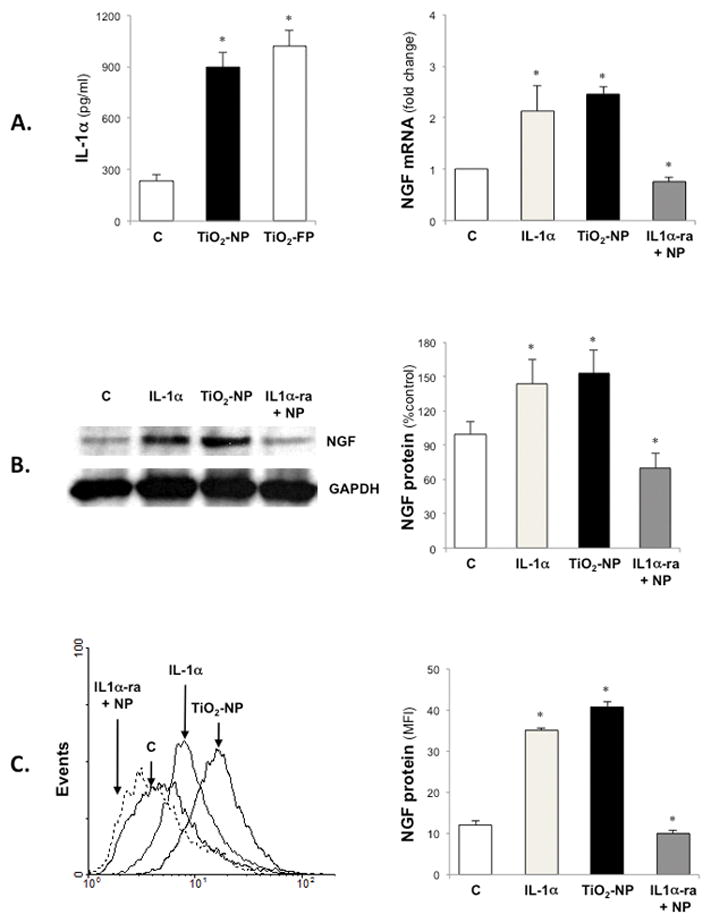
TiO2–FP exposure increased IL-1α synthesis in bronchial epithelial cells and exogenous IL-1α increased NGF gene expression to levels similar to TiO2-NP, while pretreatment with a specific receptor antagonist (IL1α-ra) abolished the effect of TiO2-NP exposure on NGF gene expression (A). Similar results were obtained by analyzing the expression of proNGF protein by Western blot (B) or FACS (C). Data are expressed as means ± SDs of 3 independent experiments. *p<0.05 compared to control cells not exposed to TiO2.
ProNGF/p75NTR mediated cell death
Immunoblot studies revealed a dimeric form of NGF (proNGF, 27 kDa) with highest concentrations in bronchial epithelial cells following TiO2-NP exposure (Figures 6A–B-C). Immunoprecipitation showed that proNGF binds with high affinity to its p75NTR receptor (Figure 6D). Western blot analysis demonstrated significant increase in JNK phosphorylation following TiO2-NP exposure in bronchial epithelial cells (Figure 6E), suggesting that this pathway may play a critical role in cell death initiated by signaling from the proNGF-p75NTR axis.
Figure 6. ProNGF/p75NTR signaling.
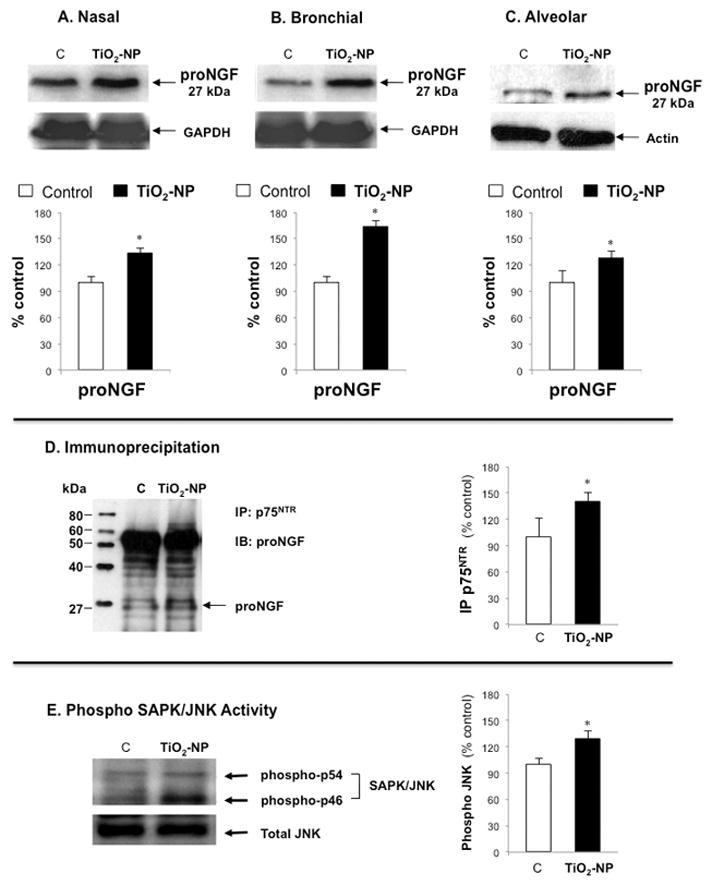
Top panel shows Western blot and densitometry measurements of dimeric forms of NGF (proNGF, 27kDA) in nasal (A), bronchial (B), and alveolar (C) epithelial cells exposed to 10 μg/ml of TiO2-NP for 24 hr. Middle panel (D) shows the interaction between proNGF and its p75NTR receptor in bronchial epithelial cells exposed to TiO2-NP with the corresponding densitometry analysis. Lower panel (E) shows the Western blot analysis of downstream stress-associated protein kinase/Jun-amino-terminal kinase (SAPK/JNK) in bronchial epithelial cells. Blots were probed with phospho SAPK/JNK [Thr183/Tyr185] and re-probed with JNK antibody as an internal control. Densitometry data are expressed as percentage of non-exposed controls after normalizing with GAPDH for NGF or with total JNK for p-JNK in corresponding graphs. Data shown are the means ± SDs of 3 independent experiments. *p<0.05 compared to non-exposed control cells (C).
TiO2-NP induced marked airway epithelial cell death primarily by apoptosis, particularly among bronchial epithelial cells (22 ± 5.6% vs. 10.4 ± 0.7%; Figure 7). Similarly, an increased proportion of early apoptotic cells (lower right quadrant) was noted following treatment with TiO2-NP in alveolar>bronchial>nasal epithelial cells. In contrast, exposure to TiO2-FP induced cell death primarily via necrosis (upper left quadrant) in nasal>alveolar>tracheal>bronchial epithelial cells.
Figure 7. Nano vs. fine particles-induced cell death.
FACS data for nasal (A), tracheal (B), bronchial (C) and alveolar (D) epithelial cells are shown. Airway epithelial cells were exposed to 10 μg/ml of TiO2-NP or TiO2-FP, then stained with annexin V-FITC and propidium iodide (PI), and cytotoxicity was measured by FACS. Bronchial and alveolar epithelial cells showed increased fractions of early apoptotic cells after TiO2-NP exposure, while TiO2-FP diverted cells to necrosis. Data shown are representative of 3 independent experiments.
To further understand the role of p75NTR in proNGF-mediated cell death, bronchial and alveolar epithelial cells were exposed to TiO2-NP after pretreatment with anti-p75NTR blocking antibody (Figure 8). This inhibitor prevented TiO2-NP induced cell death and significantly enhanced survival of both bronchial and alveolar epithelial cells, confirming that signaling through the proNGF-p75NTR axis is essential for NP-induced apoptosis. Further, bronchial cells transfected with NGF-specific siRNA (NGFsiRNA) before exposure to TiO2-NP for 24 hr displayed significant reduction in early apoptosis along with increased necrosis compared to controls transfected with scrambled siRNA (scRNA), although apoptosis remained significantly higher than in non-exposed cells (Figure 9). Viability of non-exposed scrambled siRNA transfectants was similar to untreated cells (89.3 vs. 90.9%).
Figure 8. Anti-apoptotic effect of anti-p75NTR.
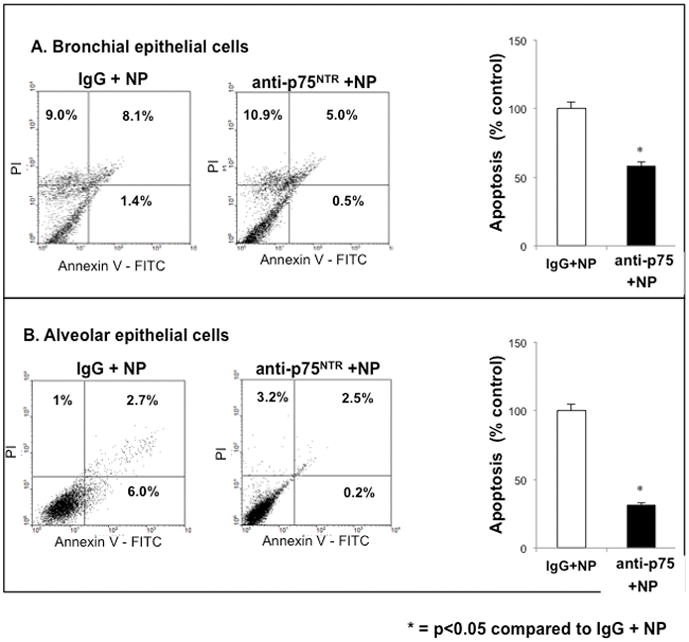
Selective immunologic blockade of the cell death receptor p75NTR inhibited TiO2-NP induced apoptosis. Lower respiratory tract bronchial (A) and alveolar (B) epithelial cells were pretreated anti-human p75NTR antibody for 24 h before exposure to 10 μg/ml of TiO2-NP. Control group was treated with human IgG antibody. Cells were harvested, stained with annexin V-FITC and propidium iodide (PI), and viability was analyzed by FACS. Data shown are the means ± SDs of 3 independent experiments. *p<0.05 compared to control cells.
Figure 9. Anti-apoptotic effect of NGF gene silencing.
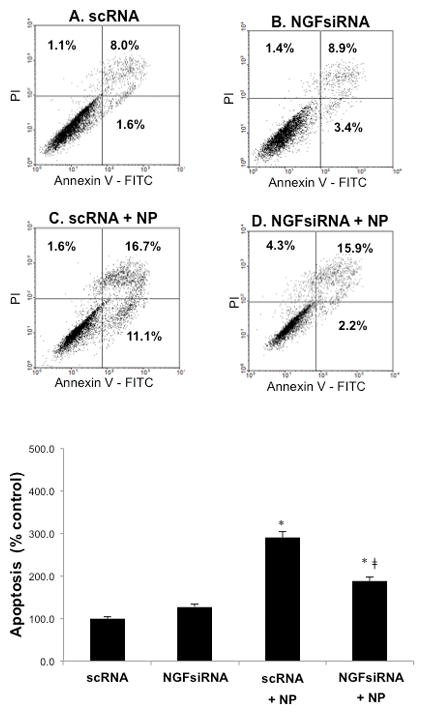
NGF gene knockdown by siRNA reduced TiO2-NP mediated apoptosis. After transfection with either scrambled siRNA (scRNA) or NGF-specific siRNA (NGFsiRNA) for 48 hr, bronchial epithelial cells were exposed to 10 μg/ml TiO2-NP, stained with annexin V-FITC and propidium iodide (PI), and analyzed by FACS. Each experiment included: unexposed cells treated with scRNA (A); unexposed cells treated with NGFsiRNA (B); cells transfected with scRNA before exposure to TiO2-NP (C); and cells transfected with NGFsiRNA before exposure to TiO2-NP (D). Data shown are the means ± SDs of 3 independent experiments. *p<0.05 compared to scRNA; ‡p<0.05 compared to scRNA + TiO2-NP.
DISCUSSION
In humans, it can generally be considered that inhaled particles with diameter 5–10 μm are deposited in the large conducting airways and those <5 μm in the small airways and alveoli; thus, particles with diameter <5 μm constitute the breathable fraction of an aerosol (Oberdorster et al, 2015). As the epithelial lining of the respiratory tract is the primary target of inhaled particles, our study sought to clarify the cytotoxic mechanisms activated by TiO2 in the form of NP or FP after deposition onto human airway epithelium.
In vitro exposure to both particle sizes induced concentration-dependent cytotoxicity, and cells derived from more distal segments of the respiratory tract tended in general to be more susceptible than those from more proximal segments. Some previous studies reported that NP are more toxic than FP on a mass dose basis (Oberdorster 1996; Okuda-Shimazaki et al. 2010). Oberdorster (1996) proposed that surface area increases as particle size becomes smaller, and thus NP tend to be more toxic when compared with the same mass of FP. However, in the present study, the difference in potency of TiO2 –NP vs. TiO2 –FP was slight. Shvedova et al. (2007) noted that degree of agglomeration markedly impacts the bioactivity of NP. In the present study, TiO2-NP tended to agglomerate in serum-free culture media generating submicronic structures (0.7 μm) that were only slightly smaller than agglomerates derived from FP (1.2 μm). Several studies suggested that singlet NP are more active than agglomerates in their ability to evoke a response (Oberdorster 1996; Oberdorster et al 2005; Shvedova et al. 2007; Hartmann et al. 2015). Therefore, agglomeration of TiO2-NP in the present study likely decreased their bioactivity.
Although NP pose an emergent challenge to toxicologists, no apparent limit for their average ambient air concentration has been set by any regulatory agency (OSHA or EPA). The American Conference of Governmental Industrial Hygienists (ACGIH, Cincinnati, OH, 1992) assigned to FP a threshold limit value of 10 mg/m3 (total dust) as a time-weighted average for a normal 8-hr workday and a 40-hr workweek, although data suggest that high-risk individuals (e.g., patients with asthma or COPD) may be exposed to ambient levels of atmospheric particles close to 20 μg/cm2 (Phalen et al 2006). More recently the National Institute for Occupational Safety and Health (NIOSH) proposed size-dependent exposure limits, i.e. 0.3 mg/m3 for TiO2-NP vs. 2.4 mg/m3 for TiO2-FP.
Another source of concern is that novel therapeutics in the form of nanosize preparations of molecules, proteins, DNA or small inhibitory RNAs are advancing at a rapid rate toward the clinic. Indeed, NP derived from a variety of different molecular approaches provide a means to deliver drugs to target organs and cells, lowering dose requirements and potentially overcoming problems associated with systemic drug delivery. Because of their accessibility, the respiratory and digestive systems are natural targets for NP therapies. On the other hand, data such as those reported in the present study raise awareness of potential and previously unknown side effects that may limit the use of otherwise promising strategies.
Neurotrophins
Exposure to TiO2-NP or TiO2-FP resulted in complex changes in the pattern of neurotrophins expression in airway epithelial cells. Specifically, exposure to 10 μg/ml TiO2-NP for 24 hr upregulated expression of genes encoding NGF and its low-affinity p75NTR receptor in all respiratory tract epithelial cell types, except for tracheal cells. Further, TiO2-NP selectively co-upregulated TrkA and p75NTR surface expressions in alveolar epithelial cells. In contrast, TiO2-FP upregulated the NGF-p75NTR axis only in tracheal epithelial cells, and no cell type expressed a coordinated BDNF-TrkB upregulation after exposure to either particle size. Data indicate responses of airway epithelium to fine and ultrafine particles are consistent with the upregulated expression of lung neurotrophins previously observed in murine airways exposed to TiO2-NP in vivo (Scuri et al. 2010a).
Previous in vivo studies showed IL-1α-dependent pro-inflammatory activity of TiO2-NP in lung and peritoneum of murine inflammation models (Yazdi et al. 2010), and the same cytokine is known to potently induce NGF expression (Pons et al. 2001). IL-1α precursor is constitutively expressed in airway epithelial cells and immediately released if these constituents become damaged, thereby initiating a cascade of cytokines and chemokines that play a key role in innate immunity and inflammation. In the present study, bronchial epithelial cells incubated with low concentrations of recombinant human IL-1α showed NGF upregulation similar to that observed when treated with TiO2-NP, and pretreatment with IL-1 receptor antagonist (IL-1ra) virtually abolished the NGF upregulation in response to TiO2-NP.
ProNGF/p75NTR
After defining the early events triggering neurotrophins synthesis, the present study focused attention on the signaling pathways triggered by TiO2. Results demonstrated that TiO2-NP modulate airway epithelial cell death predominantly through proNGF binding to its p75NTR receptor. Blocking p75NTR with a selective antibody increased significantly the viability of bronchial epithelial cells and - to a lesser extent – alveolar epithelial cells. Similarly, inhibiting endogenous NGF production by siRNA interference also significantly lowered the rate of apoptosis in TiO2-NP treated bronchial epithelial cells, but with a concomitant rise in the proportion of necrotic cells.
Data are consistent with the notion that neurotrophins elicit cell survival or death depending on which receptor and signaling pathway is activated. In many neuronal and non-neuronal cell types, binding and activation of the Trk family of tyrosine kinase receptors regulates survival and differentiation (Barde 1994; Snider and Silos-Santiago 1996). In contrast, when expressed in the relative absence of Trk receptors, NGF binds p75NTR and initiates apoptotic cell death (Frade and Barde 1998; Majdan et al. 2001) via downstream activation of the mitochondrial stress kinase JNK (Verheij et al. 1996). Similarly, TiO2-NP exposed bronchial epithelial cells exhibited enhanced expression of p75NTR without any change in TrkA receptors, which resulted in upregulated p-JNK activity and significantly higher proportion of apoptotic cells. A lower apoptotic rate in TiO2-NP exposed alveolar epithelial cells may be due to co-expression of p75NTR with TrkA receptors, as co-expression may modify neurotrophin binding (Barker and Shooter 1994; Hempstead et al. 1991), ligand discrimination (Chao 1994), retrograde transport (Curtis et al. 1995), and signal transduction (Verdi et al. 1994).
All neurotrophins are initially synthesized as precursors consisting of an N-terminal pro-domain and a C-terminal mature domain. Pro-neurotrophins are normally cleaved by furin in the trans-Golgi, and the mature domain is then trafficked by secretory vesicles to the extracellular space. However, immature isoforms might also reach the extracellular space, where they might be degraded by matrix metalloprotease-7 (MMP-7) or converted by plasmin into mature neurotrophins (Bruno and Cuello 2006). Binding of mature neurotrophins to p75NTR expressing cells induces apoptosis but only at high concentrations. Whereas, proNGF binds with high affinity to a complex consisting of p75NTR and the transmembrane protein sortilin to induce apoptosis at subnanomolar concentrations (Lee et al. 2001). Thus, proNGF is a distinct, biologically active ligand that might induce actions opposing the anti-apoptotic effects of mature NGF binding to its TrkA receptor.
ProNGF is constitutionally expressed at low levels in the central and peripheral nervous systems of children and young adults, but it is markedly upregulated in adults of advanced age or with Alzheimer’s disease, suggesting that imbalance of the proNGF:mature NGF ratio in the CNS is responsible for the cholinergic neuron loss seen in aging and dementia (Lee et al. 2001). This imbalance implies that proteolysis of extracellular proNGF is impaired in pathological states and may result from the coordinate induction of inhibitors of matrix metalloproteinases (MMPs) and plasmin, such as tissue inhibitors of metalloproteinase (TIMPs), neuroserpin, and alpha-2 macroglobulin (Bruno and Cuello 2006). Similarly, dysregulation of NGF expression or impaired conversion of proNGF to mature NGF might contribute to pathology in other organs. Data from the present study suggest that chronic inhalation of NP in highly polluted areas may generate “premature senescence” of the lower airways by shifting balance between pro-apoptotic and pro-survival neurotrophic pathways.
Conclusions
Data demonstrated that exposure to TiO2-NP produce complex changes in expression pattern of key neurotrophic proteins and their cognate receptors in the respiratory tract epithelium with differences dependent on airway depth in the respiratory tract. In particular, NGF precursors are overexpressed in bronchial epithelial cells exposed to TiO2-NP, but fail to convert into mature neurotrophins and therefore bind predominantly the p75NTR death receptor. In the same cells, p75NTR is significantly overexpressed in parallel to its protein ligand and vis-a-vis of unchanged expression of the anti-apoptotic TrkA receptor. The resulting increase in proNGF/p75NTR signaling activates downstream the SAPK-JNK pathway, resulting in apoptotic death and premature aging of the bronchial epithelium. Thus, imbalance between immature NGF precursors and its mature form, paired to preferential expression of receptors favoring cell death, contributes to the adverse effects of airborne NP on the respiratory tract.
Acknowledgments
This work was supported in part by the U.S. National Institutes of Health grants RO1-HL61007 to Dr. Giovanni Piedimonte. Image acquisition and data analysis were performed at the West Virginia University Imaging Core Facility, which was supported in part by the NIH grant P20RR016440. FACS experiments were performed in the WVU Flow Cytometry Core Facility, which was supported in part by NIH grants RR106440 and RR020866. We also would like to thank Drs. Min Ding and Sreekumar Othumpangat (NIOSH, Morgantown, WV), as well as Lennie Samsell and Cheryl Walton (WVU Pediatric Research Institute, Morgantown, WV) for providing the titanium dioxide particles and technical help.
References
- Barde YA. Neurotrophins: A family of proteins supporting the survival of neurons. Prog Clin Biol Res. 1994;390:45–56. [PubMed] [Google Scholar]
- Barker PA, Shooter EM. Disruption of NGF binding to the low affinity neurotrophin receptor p75LNTR reduces NGF binding to TrkA on PC12 cells. Neuron. 1994;13:203–215. doi: 10.1016/0896-6273(94)90470-7. [DOI] [PubMed] [Google Scholar]
- Braun A, Lommatzsch M, Lewin GR, Virchow JC, Renz H. Neurotrophins: A link between airway inflammation and airway smooth muscle contractility in asthma? Int Arch Allergy Immunol. 1999;118:163–165. doi: 10.1159/000024056. [DOI] [PubMed] [Google Scholar]
- Bruno MA, Cuello AC. Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc Nat Acad Sci USA. 2006;103:6735–6740. doi: 10.1073/pnas.0510645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV. The p75 neurotrophin receptor. J Neurobiol. 1994;25:1373–1385. doi: 10.1002/neu.480251106. [DOI] [PubMed] [Google Scholar]
- Chen Y-C, Weng YH, Chiu YW, Yang CY. Short-term effects of coarse particulate matter on hospital admissions for cardiovascular diseases: A case-crossover study in a tropical city. J Toxicol Environ Health A. 2015;78:1241–1253. doi: 10.1080/15287394.2015.1083520. [DOI] [PubMed] [Google Scholar]
- Costa S, Ferreira J, Silveira C, Costa C, Lopes D, Relvas H, Borrego C, Roebeling P, Miranda AI, Teixeira JP. Integrating health on air quality assessment - Review report on health risks of two major European outdoor air pollutants: PM and NO. J Toxicol Environ Health B. 2014;17:307–340. doi: 10.1080/10937404.2014.946164. [DOI] [PubMed] [Google Scholar]
- Curtis R, Adryan KM, Stark JL, Park JS, Compton DL, Weskamp G, Huber LJ, Chao MV, Jaenisch R, Lee KF. Differential role of the low affinity neurotrophin receptor (p75) in retrograde axonal transport of the neurotrophins. Neuron. 1995;14:1201–1211. doi: 10.1016/0896-6273(95)90267-8. [DOI] [PubMed] [Google Scholar]
- de Oliveira BF, Chacra AP, Frauches TS, Vallochi A, Hacon S. A curated review of recent literature of biomarkers used for assessing air pollution exposures and effects in humans. J Toxicol Environ Health B. 2014;17:369–410. doi: 10.1080/10937404.2014.976893. [DOI] [PubMed] [Google Scholar]
- Frade JM, Barde YA. Nerve growth factor: Two receptors, multiple functions. Bioessays. 1998;20:137–145. doi: 10.1002/(SICI)1521-1878(199802)20:2<137::AID-BIES6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Gurr JR, Wang AS, Chen CH, Jan KY. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology. 2005;213:66–73. doi: 10.1016/j.tox.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Hartmann NB, Jensen KA, Baun A, Rasmussen K, Rauscher H, Tantra R, Cupi D, Gilliland D, Pianella F, Riego Sintes JM. Techniques and protocols for dispersing nanoparticle powders in aqueous media - Is there a rationale for harmonization? J Toxicol Environ Health B. 2015;18:299–326. doi: 10.1080/10937404.2015.1074969. [DOI] [PubMed] [Google Scholar]
- Hempstead BL, Martin-Zanca D, Kaplan DR, Parada LF, Chao MV. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991;350:678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Kermanizadeh A, Gosens I, MacCalman L, Johnston H, Danielsen PH, Jacobsen NR, Lenz AG, Fernandes T, Schins RP, Cassee FR, Wallin H, Kreyling W, Stoeger T, Loft S, Møller P, Tran L, Stone V. A multilaboratory toxicological assessment of a panel of 10 engineered nanomaterials to human health - ENPRA project - The highlights, limitations, and current and future challenges. J Toxicol Environ Health B. 2016;19:1–28. doi: 10.1080/10937404.2015.1126210. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted pro-neurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Majdan M, Walsh GS, Aloyz R, Miller FD. TrkA mediates developmental sympathetic neuron survival in vivo by silencing an ongoing p75NTR-mediated death signal. J Cell Biol. 2001;155:1275–1285. doi: 10.1083/jcb.200110017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A. Atmosphere. Air pollution-related illness: Effects of particles. Science. 2005;308:804–806. doi: 10.1126/science.1108752. [DOI] [PubMed] [Google Scholar]
- Oberdorster G. Significance of particle parameters in the evaluation of exposure-dose-response relationships of inhaled particles. Inhal Toxicol. 1996;(8 Suppl):73–89. [PubMed] [Google Scholar]
- Oberdorster G, Castranova V, Asgharian B, Sayre P. Inhalation exposure to carbon nanotubes (CNT) and carbon nanofibers (CNF): Methodology and dosimetry. J Toxicol Environ Health B. 2015;18:121–212. doi: 10.1080/10937404.2015.1051611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ Health Persp. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda-Shimazaki J, Takaku S, Kanehira K, Sonezaki S, Taniguchi A. Effects of titanium dioxide nanoparticle aggregate size on gene expression. Int J Mol Sci. 2010;11:2383–2392. doi: 10.3390/ijms11062383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmedo DG, Tasat DR, Guglielmotti MB, Cabrini RL. Effect of titanium dioxide on the oxidative metabolism of alveolar macrophages: An experimental study in rats. J Biomed Mater Res A. 2005;73:142–149. doi: 10.1002/jbm.a.30230. [DOI] [PubMed] [Google Scholar]
- Othumpangat S, Gibson LF, Samsell L, Piedimonte G. NGF is an essential survival factor for bronchial epithelial cells during respiratory syncytial virus infection. PLoS One. 2009;4:e6444. doi: 10.1371/journal.pone.0006444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phalen RF, Oldham MJ, Nel AE. Tracheobronchial particle dose considerations for in vitro toxicology studies. Toxicol Sci. 2006;92:126–132. doi: 10.1093/toxsci/kfj182. [DOI] [PubMed] [Google Scholar]
- Pietropaoli AP, Frampton MW, Hyde RW, Morrow PE, Oberdorster G, Cox C, Speers DM, Frasier LM, Chalupa DC, Huang LS, Utell MJ. Pulmonary function, diffusing capacity, and inflammation in healthy and asthmatic subjects exposed to ultrafine particles. Inhal Toxicol. 2004;16(Suppl 1):59–72. doi: 10.1080/08958370490443079. [DOI] [PubMed] [Google Scholar]
- Pons F, Freund V, Kuissu H, Mathieu E, Olgart C, Frossard N. Nerve growth factor secretion by human lung epithelial A549 cells in pro- and anti-inflammatory conditions. Eur J Pharmacol. 2001;428:365–369. doi: 10.1016/s0014-2999(01)01280-8. [DOI] [PubMed] [Google Scholar]
- Robinson RC, Radziejewski C, Spraggon G, Greenwald J, Kostura MR, Burtnick LD, Stuart DI, Choe S, Jones EY. The structures of the neurotrophin 4 homodimer and the brain-derived neurotrophic factor/neurotrophin 4 heterodimer reveal a common Trk-binding site. Protein Sci. 1999;8:2589–2597. doi: 10.1110/ps.8.12.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuri M, Chen BT, Castranova V, Reynolds JS, Johnson VJ, Samsell L, Walton C, Piedimonte G. Effects of titanium dioxide nanoparticle exposure on neuroimmune responses in rat airways. J Toxicol Environ Health A. 2010a;73:1353–1369. doi: 10.1080/15287394.2010.497436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuri M, Samsell L, Piedimonte G. The role of neurotrophins in inflammation and allergy. Inflamm Allergy Drug Targets. 2010b;9:173–178. doi: 10.2174/187152810792231913. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Sager T, Murray A, Kisin E, Porter DW, Leonard SS, Schwegler-Berry D, Robinson VA, Castranova V. Nanotechnology: Characterization, Dosing and Health Effects. Philadelphia: Informa Healthcare; 2007. Critical issues in the evaluation of possible effects resulting from airborn nanoparticles; pp. 221–232. [Google Scholar]
- Snider WD, Silos-Santiago I. Dorsal root ganglion neurons require functional neurotrophin receptors for survival during development. Phil Trans R Soc Lond B Biol Sci. 1996;351:395–403. doi: 10.1098/rstb.1996.0034. [DOI] [PubMed] [Google Scholar]
- Tsai SS, Weng YH, Chiu YW, Yang CY. Short-term effect of coarse particles on daily mortality rate in a tropical city, Kaohsiung, Taiwan. J Toxicol Environ Health A. 2015;78:1409–1420. doi: 10.1080/15287394.2015.1093674. [DOI] [PubMed] [Google Scholar]
- Tsuda H, Xu J, Sakai Y, Futakuchi M, Fukamachi K. Toxicology of engineered nanomaterials - A review of carcinogenic potential. Asian Pac J Cancer Prev. 2009;10:975–980. [PubMed] [Google Scholar]
- Verdi JM, Birren SJ, Ibanez CF, Persson H, Kaplan DR, Benedetti M, Chao MV, Anderson DJ. p75LNGFR regulates Trk signal transduction and NGF-induced neuronal differentiation in MAH cells. Neuron. 1994;12:733–745. doi: 10.1016/0896-6273(94)90327-1. [DOI] [PubMed] [Google Scholar]
- Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, Haimovitz-Friedman A, Fuks Z, Kolesnick RN. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, Sioutas C, Yeh JI, Wiesner MR, Nel AE. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6:1794–1807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- Yazdi AS, Guarda G, Riteau N, Drexler SK, Tardivel A, Couillin I, Tschopp J. Nanoparticles activate the NLR pyrin domain containing 3 (NLRP3) inflammasome and cause pulmonary inflammation through release of IL-1alpha and IL-1beta. Proc Natl Acad Sci USA. 2010;107:19449–19454. doi: 10.1073/pnas.1008155107. [DOI] [PMC free article] [PubMed] [Google Scholar]