Wolbachia bacteria are intracellular bacteria present in the microbiome of a large fraction of insects and parasitic nematodes. They can block mosquitos’ ability to transmit several infectious disease-causing pathogens, including Zika, dengue, chikungunya, and West Nile viruses and malaria parasites. Certain extracellular bacteria present in the gut lumen of these insects can also block pathogen transmission. However, our understanding of interactions between Wolbachia and gut bacteria and how they influence each other is limited. Here we show that the presence of Wolbachia strain wMel changes the composition of gut commensal bacteria in the fruit fly. Our findings implicate interactions between bacterial species as a key factor in determining the overall composition of the microbiome and thus reveal new paradigms to consider in the development of disease control strategies.
KEYWORDS: Drosophila microbiome, gut microbiome, symbiosis, Wolbachia
ABSTRACT
Endosymbiotic Wolbachia bacteria and the gut microbiome have independently been shown to affect several aspects of insect biology, including reproduction, development, life span, stem cell activity, and resistance to human pathogens, in insect vectors. This work shows that Wolbachia bacteria, which reside mainly in the fly germline, affect the microbial species present in the fly gut in a lab-reared strain. Drosophila melanogaster hosts two main genera of commensal bacteria—Acetobacter and Lactobacillus. Wolbachia-infected flies have significantly reduced titers of Acetobacter. Sampling of the microbiome of axenic flies fed with equal proportions of both bacteria shows that the presence of Wolbachia bacteria is a significant determinant of the composition of the microbiome throughout fly development. However, this effect is host genotype dependent. To investigate the mechanism of microbiome modulation, the effect of Wolbachia bacteria on Imd and reactive oxygen species pathways, the main regulators of immune response in the fly gut, was measured. The presence of Wolbachia bacteria does not induce significant changes in the expression of the genes for the effector molecules in either pathway. Furthermore, microbiome modulation is not due to direct interaction between Wolbachia bacteria and gut microbes. Confocal analysis shows that Wolbachia bacteria are absent from the gut lumen. These results indicate that the mechanistic basis of the modulation of composition of the microbiome by Wolbachia bacteria is more complex than a direct bacterial interaction or the effect of Wolbachia bacteria on fly immunity. The findings reported here highlight the importance of considering the composition of the gut microbiome and host genetic background during Wolbachia-induced phenotypic studies and when formulating microbe-based disease vector control strategies.
IMPORTANCE Wolbachia bacteria are intracellular bacteria present in the microbiome of a large fraction of insects and parasitic nematodes. They can block mosquitos’ ability to transmit several infectious disease-causing pathogens, including Zika, dengue, chikungunya, and West Nile viruses and malaria parasites. Certain extracellular bacteria present in the gut lumen of these insects can also block pathogen transmission. However, our understanding of interactions between Wolbachia and gut bacteria and how they influence each other is limited. Here we show that the presence of Wolbachia strain wMel changes the composition of gut commensal bacteria in the fruit fly. Our findings implicate interactions between bacterial species as a key factor in determining the overall composition of the microbiome and thus reveal new paradigms to consider in the development of disease control strategies.
INTRODUCTION
All animals host a diverse and extensive microbial community, referred to as the microbiome. The microbiome plays a pivotal role in host development and growth (1). Insects provide a prime example of the relevance of microbes in the host, having a major impact on several aspects of host biology, including physiology, immunity, and evolution (2, 3). Microbes also play an important role in the reproductive capabilities of the insect host. In some cases, they hijack the insect’s cellular pathways to alter fecundity, sometimes evolving to become essential symbionts for host reproductive success (reviewed in references 2 and 4).
These reproductive symbionts can either be transmitted vertically by the parents or acquired horizontally from the environment. Wolbachia bacteria are obligate endosymbionts that are found in about 40% of all insect species and cause various types of reproductive phenotypes that generally favor their vertical transmission and spread in populations (4–6). Apart from reproductive manipulation, Wolbachia bacteria are also known to cause a plethora of other changes in their hosts, such as altering insulin signaling (7) and providing resistance to certain pathogens (reviewed in reference 8).
While Wolbachia bacteria usually infect reproductive tissues, other microbes infecting different tissues coexist in the insect host. The repertoire of the microbes associated with the host can vary on the basis of both environmental factors (9–12) and the host genotype (13). In Drosophila, the diversity of the microbiome is very low and depends greatly on the host diet, and lab-reared flies have comparatively fewer taxa than wild-caught flies (10). Acetobacter and Lactobacillus are the most commonly found genera of bacteria in Drosophila melanogaster, both in lab-reared flies and in nature (14, 15). They affect development, metabolism, and behavior (reviewed in reference 14).
Recent studies have explored the effects of various microbes on the composition of the insect microbiome, implicating interactions between microbes to play a significant role in the determination of the composition of the microbiome (16, 17). In particular, Hughes et al. showed that the presence of a specific bacterium, Asaia sp., prevents the stable transmission and maintenance of Wolbachia bacteria in the host. Upon antibiotic treatment and removal of Asaia bacteria, the mosquito host is able to successfully carry and vertically transmit Wolbachia bacteria.
Here we address the converse question of whether Wolbachia bacteria have a role in the determination of the composition of the microbiome. Sequencing-based approaches to the identification of the microbiome of Wolbachia-infected strains have been complicated because Wolbachia bacteria have been shown to be overrepresented in the microbiome of field-collected insects and valuable information about the composition of other microbes is lost. We solved this issue by restriction digestion of the Wolbachia 16S rRNA gene prior to profiling the composition of the microbiome. We then found that the presence of Wolbachia bacteria induces significant changes in the host microbiome. These changes persist throughout development in a stable lab stock. On further investigation with gnotobiotic organisms, we found that the Wolbachia-induced effect is independent of vertical transmission of the composition of the microbiome. The host genetic background contributes to the interaction of Wolbachia bacteria with the microbiota. Furthermore, transcriptional profiling of immune effector molecules controlled by the Imd and JAK/STAT pathways suggests that the mechanism of this Wolbachia-driven change in the gut microbiome is independent of classical host immunity pathways.
RESULTS
Previous study in our lab showed that Wolbachia bacteria affect the fecundity of Drosophila mauritiana flies (6). In a parallel study, we observed that the presence of Wolbachia bacteria in D. mauritiana changed the color of grape juice plates over time. The plates used for egg collection (see Materials and Methods) from Wolbachia-free flies were pink but displayed a yellowish tinge when exposed to Wolbachia-infected flies (Fig. 1A). Adding an acid or a base to the grape juice plates indicated that the color change is pH dependent (Fig. 1A). Since flies defecate on the agar medium, we suspected that the fecal microbiota-derived metabolites might contribute toward determining the final pH of the agar. In addition, if there is a difference in microbial composition between Wolbachia-free and -infected flies, this might contribute to the differential coloration of the grape juice agar plates. Since the D. mauritiana system does not have powerful genetic tools with which to further analyze this phenotype, we investigated the possibility that Wolbachia bacteria have an effect on the microbiome of D. melanogaster flies. Results obtained with D. melanogaster will be more broadly applicable than those obtained with D. mauritiana.
FIG 1 .
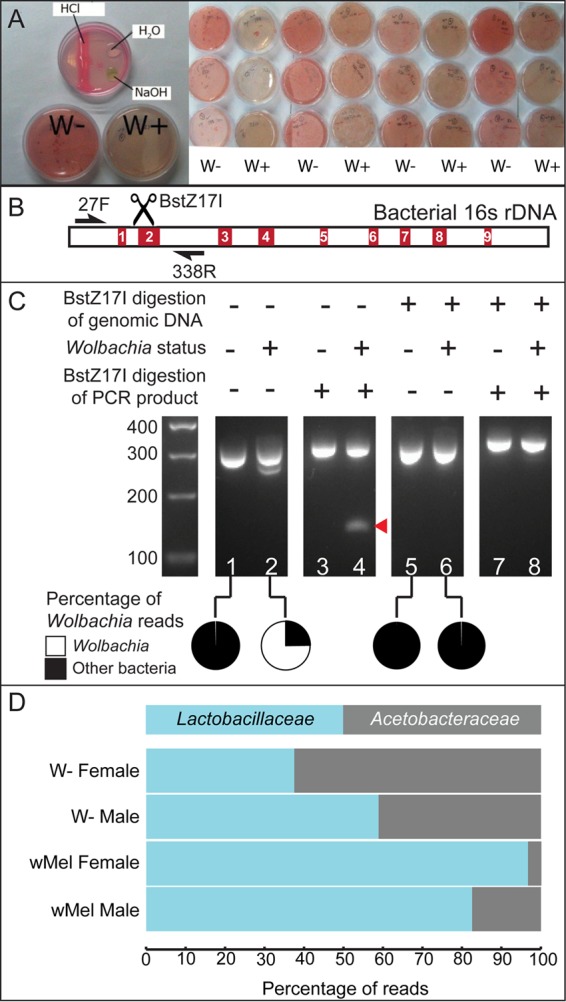
16S rRNA gene profiling of D. melanogaster shows reduction of Acetobacteraceae levels in Wolbachia-infected adult flies. (A) Grape juice agar plates used for D. mauritiana egg collection. Grape juice acts as a pH indicator, turning pink under acidic conditions and yellow under basic conditions. Over time, the microbial composition of the feces of Wolbachia-infected flies turns plates more yellowish than plates used by Wolbachia-free flies. (B) Schematic of the bacterial 16S rRNA gene. Hypervariable regions V1 to V9 are red. Primers (arrows, 27F and 338R) amplify regions V1 and V2. In BstZ17I restriction enzyme digestion of the total 16S rRNA gene pool, only the Wolbachia rRNA gene between V1 and V2 is selectively digested. (C) Agarose gel image showing the 16S rRNA gene PCR products from the microbiome of D. melanogaster and efficient digestion of the Wolbachia 16S rRNA gene amplicon by the BstZ17I restriction enzyme. The red arrowhead indicates the digested Wolbachia product. The pie charts indicate the percentages of Wolbachia reads before and after BstZ17I digestion. (D) 16S rRNA gene profiles of male and female wMel-free (W−) or -infected D. melanogaster. The proportions of Acetobacteraceae are significantly different under every pair of conditions (P < 0.0001, chi-square test with Yates correction).
For this analysis, we chose to examine D. melanogaster upd-Gal4; Cyo/Sco; P(UAS-hPF)B, here referred to as upd>hPABP-Flag flies. The hPABP-Flag transgene allows the isolation of mRNA from specific cell types for transcriptional profiling (18). We used the upd promoter, which is active in the stem cell niche (hub) of the testis, to drive the expression of hPABP-Flag. Since Wolbachia bacteria show tropism to the hubs in males (19), this construct allows for the isolation of RNA for studying gene expression patterns that are altered because of Wolbachia infection. The upd>hPABP-Flag strain was isogenized and used for molecular characterization of the effects of Wolbachia bacteria on stem cells and their niches (see Materials and Methods). Since the microbiome has been shown to affect host phenotypes such as fecundity (20, 21), which is a direct result of stem cell activity (6), we considered testing of the effects of Wolbachia bacteria on the microbiome to be important especially in this specific genotype.
Wolbachia 16S rRNA gene sequences can be efficiently removed prior to sequencing.
To determine the microbial content of upd>hPABP-Flag flies, we used high-throughput sequencing of the 16S rRNA gene amplicons from total genomic DNA isolated from flies. A major hurdle in the sequencing of 16S rRNA gene sequences from flies infected with Wolbachia bacteria is the overrepresentation of Wolbachia sequences (10). BstZ17I digestion of the total genomic DNA specifically prevents amplification of the Wolbachia 16S rRNA gene, as the restriction enzyme cleaves between the V1 and V2 regions. Microbes normally found in Drosophila flies do not contain the BstZ17I restriction sites. Other bacteria containing this site are present in the orders Rhizobiales and Myxococcales and non-Wolbachia members of the order Rickettsiales that have been reported to be absent or occur at very low numbers in Drosophila flies (22). We found that BstZ17I digestion prior to 16S rRNA gene PCR of the V1 and V2 regions effectively removed most of the Wolbachia amplicons (Fig. 1B and C; see Table S1 in the supplemental material). While more than 70% of the reads originated from Wolbachia bacteria in the case of undigested genomic DNA, the BstZ7I-digested genomic DNA produced less than 1% Wolbachia reads (Fig. 1C, lane 6). We also confirmed the Wolbachia strain present to be wMel by variable-number tandem-repeat (VNTR) analysis (Fig. S1).
VNTR analysis to determine the Wolbachia strain. Different Wolbachia strains have different numbers of repeats in some VNTRs, specifically, 105 and 141. The expected sizes in wMel are 1,347 bp for VNTR 105 and 1,330 bp for VNTR 141; in wMelCS, they are 1,241 bp for VNTR 105 and 1,189 bp for VNTR 141 (67). Lanes 1 and 2 in both gels are the Wolbachia-free and wMel-infected flies used in this study, respectively. Lanes 3 are flies infected with wMelCS (not used in this study) for comparison. Download FIG S1, TIF file, 0.2 MB (167.8KB, tif) .
Copyright © 2017 Simhadri et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Number of reads for each sample and effectiveness of Bstz17I digestion. Sequencing of the 16S rRNA gene PCR products of either BstZ17I-digested or undigested total genomic DNA of flies shows that the BstZ17I enzyme is capable of eliminating the amplification of the Wolbachia 16S rRNA gene. Download TABLE S1, DOCX file, 0.01 MB (12.3KB, docx) .
Copyright © 2017 Simhadri et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Primers used for amplification and sequencing. The different regions of the primers are Illumina adaptor (red), indexing barcode (black), primer pad and linker (green), and 16S rRNA gene annealing primer (blue) sequences. Download TABLE S2, DOCX file, 0.01 MB (12.2KB, docx) .
Copyright © 2017 Simhadri et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Wolbachia bacteria reduce the proportion of Acetobacteraceae.
To assess the effect of Wolbachia on bacterial composition associated with these flies, we sequenced the non-Wolbachia 16S rRNA gene from 0- to 2-week-old adult male and female flies separately (n = 5 flies per sample, 20 flies total). We then grouped the 16S rRNA gene sequences into 97% identical operational taxonomic units. We found that the vast majority (>99.8%) of the microbiome of both Wolbachia-free and -infected flies is restricted to only two families—Acetobacteraceae and Lactobacillaceae (Fig. 1D; Table S3). Intriguingly, there was a striking contrast between the proportions of the two families of bacteria in Wolbachia-free and -infected flies. Members of the family Acetobacteraceae make up less than 20% (17% in males and 3% in females) of the microbes in wMel-infected flies and more than 40% (41% in males and 62% in females) of those in Wolbachia-free flies (Fig. 1D).
Percentages of (non-Wolbachia) bacterial taxa found in each of the samples by 16S rRNA gene sequencing. The two most abundant families of bacteria are Acetobacteraceae and Lactobacillaceae, which constitute more than 99.8% of the reads in any sample. Download TABLE S3, DOCX file, 0.01 MB (12.9KB, docx) .
Copyright © 2017 Simhadri et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To determine the specific bacterial species present in these flies, we used culture-based techniques, followed by Sanger sequencing of the 16S rRNA gene of the bacterial isolates and whole-genome sequencing with the PacBio sequencing platform (see Materials and Methods). Consistent with our 16S rRNA sequencing data, we found that Acetobacter pasteurianus and Lactobacillus plantarum are the only two species residing in this fly strain. We then designed species-specific primers against their glmS gene for further analysis with PCR-based assays (see Fig. S2 and Materials and Methods).
Species-specific primers designed for qPCR. Primers were designed against the glmS gene of both species. The PCR results show that the primers are specific, having no cross-species targets. A. p is A. pasteurianus, and L. p is L. plantarum. Download FIG S2, TIF file, 0.1 MB (116.7KB, tif) .
Copyright © 2017 Simhadri et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
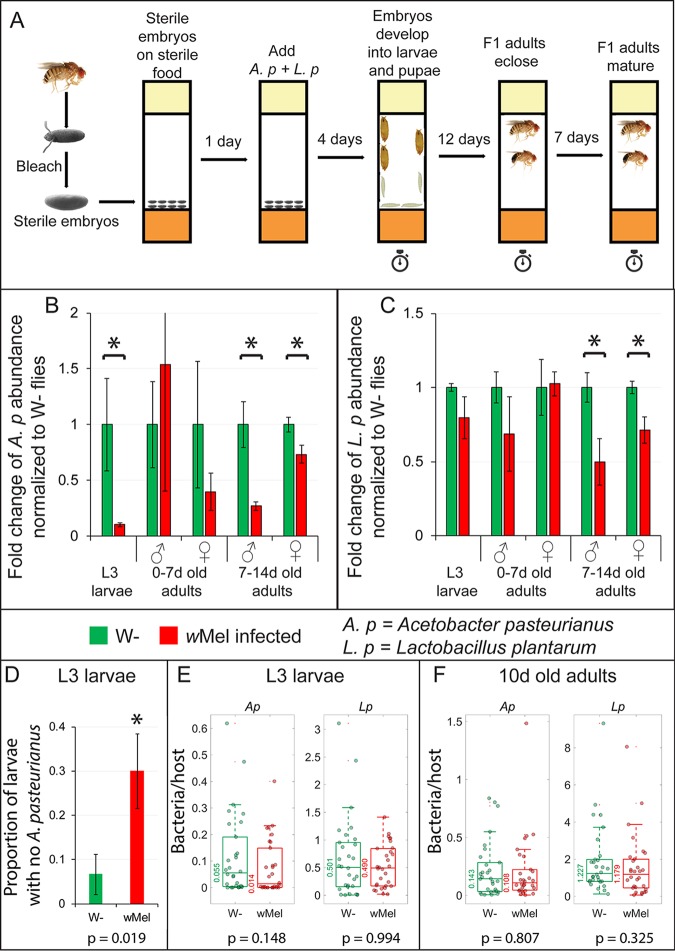
Wolbachia-induced reduction of A. pasteurianus levels persists throughout development.
To further corroborate the differences in the composition of the microbiome shown by the deep sequencing results, we performed PCR of Wolbachia-free and -infected Drosophila stocks with species-specific primers. We also analyzed the effect of Wolbachia bacteria on the microbiome during development (see the diagram of the experiment in Fig. 2A); 35 adult males and 35 adult females were used as the parental generation for the experiment. To analyze the composition of the parental microbiota, five males and females each were surface sterilized and DNA was extracted. The remaining 30 males and females each were split into three replicates of 10 male and 10 female parents for both the wMel-free and -infected flies. After 7 days of egg laying, these F0 adults were removed from the vials. Five individuals each from the next generation (F1) were collected in triplicate at multiple life stages—L3 larvae, 0- to 7-day-old adults, and 7- to 14-day-old adults. DNA was extracted from surface-sterilized organisms and digested with BstZ17I to exclude Wolbachia 16S rRNA gene amplification. On performing PCR with species-specific primers against the two isolated species, we found that A. pasteurianus was not detected in the majority of the Wolbachia-infected samples. There was no detectable A. pasteurianus in the parent flies, two out of three replicates of the F1 L3 larvae, and both the adult stages. That is, in 12 out of a total of 17 Wolbachia-infected samples, A. pasteurianus was absent, compared to its detection in 17 out of 17 Wolbachia-free flies. The lack of A. pasteurianus was observed in all of the life stages sampled (Fig. 2B to E). In the samples that did have A. pasteurianus, quantification by quantitative PCR (qPCR) showed that the levels of the bacteria were about 10-fold lower in the Wolbachia-infected organisms than in their Wolbachia-free counterparts (Fig. S3). These results confirm that the presence of Wolbachia bacteria consistently reduces the levels of A. pasteurianus.
FIG 2 .
Wolbachia bacteria suppress A. pasteurianus across various life stages of D. melanogaster. (A) Schematic of the experimental setup used. Stopwatches indicate sample collection times. (B to E) PCR products obtained with A. pasteurianus and L. plantarum species-specific primers from BstZ17I-digested total genomic DNA from wMel-free (W−) or -infected D. melanogaster. (B) The parental flies are F0 0- to 7-day-old males and females. (C to E) Experiments were done in triplicate, and each sample consisted of five adults (B, D, E) or five larvae (C). Vial numbers are above the gel lanes. (C) F1 unsexed L3 larvae (n = 3), (D) F1 0- to 7-day-old male and female flies (n = 3). (E) F1 7- to 14-day-old male and female flies (n = 3).
Quantification of effects of Wolbachia bacteria on A. pasteurianus levels in flies during development. (A to C) Relative levels of A. pasteurianus and L. plantarum in wMel-infected flies and those in Wolbachia-free flies. (A) F1 unsexed L3 larvae. (B) F1 0- to 7-day-old male and female flies. (C) F1 7- to 14-day-old male and female flies. (A to C) A. pasteurianus was absent from two out of the three replicates of Wolbachia-infected vials tested, hence the absence of error bars in the A. pasteurianus bars. Bar graphs show mean values (n = 3 when the qPCR produced amplicons), and error bars show standard deviations. Download FIG S3, TIF file, 0.6 MB (603.1KB, tif) .
Copyright © 2017 Simhadri et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
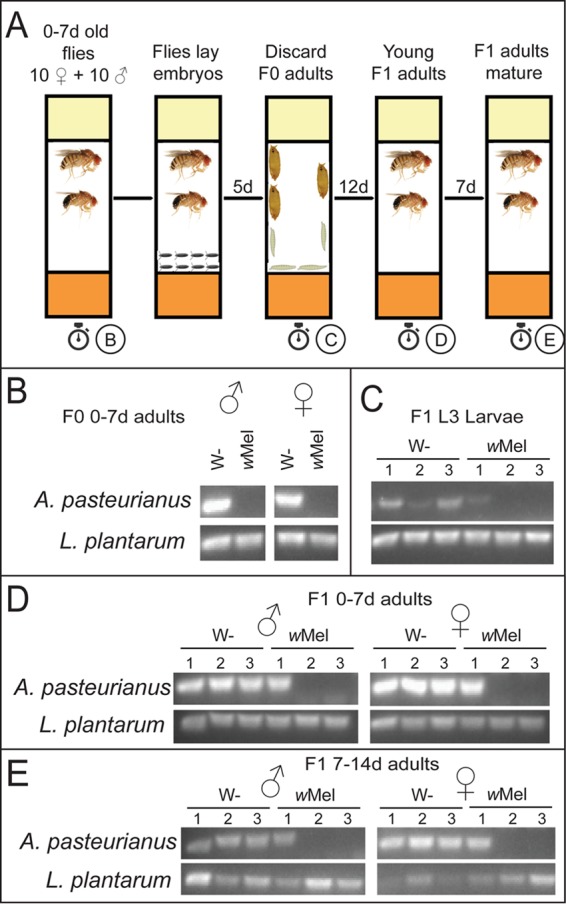
Wolbachia bacteria also reduce A. pasteurianus levels in gnotobiotic organisms.
Besides the presence of Wolbachia bacteria, another factor that could influence the microbiome is the difference in the relative abundance of each bacterium passed on by the parents. The general absence of A. pasteurianus in the F1 generation (Fig. 2) could have been due to the low abundance of this bacterium in the parent flies and not necessarily caused by Wolbachia infection. To test this possibility, we eliminated the variability in the quantities of the bacterial species imparted by the parent flies between the various samples. We produced gnotobiotic organisms that were infected with equal quantities of A. pasteurianus and L. plantarum (Fig. 3A). Ten-microliter volumes of bleach-sterilized eggs of wMel-free and -infected flies were seeded on sterile fly food in triplicate. To minimize growth rate and survival differences between the bacteria in the fly food, 1,000 CFU of each of the bacteria were added to the food after a majority of the eggs hatched (see Materials and Methods). Five individuals each from various life stages—L3 larvae, 0- to 7-day-old adults, and 7- to 14-day-old adults—were then collected from each biological replicate. Upon performing qPCR for each of the bacteria, we found that the levels of A. pasteurianus were indeed about 10-fold higher (P < 0.05) in the Wolbachia-free larvae than in the wMel-infected larvae (Fig. 3B). However, the 0- to 7-day-old adults, either Wolbachia free or infected, had no statistically significant difference in the levels of A. pasteurianus (Fig. 3B). Finally, the 7- to 14-day-old wMel-infected adults had lower levels of both A. pasteurianus and L. plantarum than the Wolbachia-free flies (Fig. 3B and C).
FIG 3 .
Wolbachia infection reduces A. pasteurianus levels in L3 larvae of gnotobiotic flies. (A) Schematic of the experimental setup used. Stopwatches indicate sample collection times. (B, C) qPCR of A. pasteurianus (B) and L. plantarum (C) titers in wMel-infected gnotobiotic flies compared to those in Wolbachia-free (W−) gnotobiotic flies, normalized to 16S rRNA gene levels, in L3 larvae, 0- to 7-day-old adults, and 7- to 14-day-old adults. Bar graphs show mean values (three biological replicates of a sample of five individuals per replicate), and error bars show standard deviations. Asterisks indicate statistically significant differences (P < 0.05, Student t test). (D) Proportion of individual L3 larvae that had no A. pasteurianus in the gut (n = 30, chi-square test; error bars show confidence intervals). (E, F) Box-and-whisker plots of levels of bacteria in L3 larvae (E) and 10-day-old adults (F) (n = 30). Median values are shown next to the boxes, and P values (two-sided Wilcoxon rank sum test) are reported.
To elucidate if this reduction of A. pasteurianus levels is due to an overall reduction of the bacteria in all Wolbachia-infected flies or to the complete lack of A. pasteurianus in large fractions of Wolbachia-infected flies, we sampled individual organisms to assay the levels of each gut bacterium relative to the host. Gnotobiotic organisms were produced as described above. Three separate experiments were performed, and 10 individuals (L3 larvae and 10-day-old adults) were sampled from each experiment. On performing qPCRs with species-specific primers and comparing the results to the host DNA, we found that about 30% of the Wolbachia-infected L3 larvae did not harbor any A. pasteurianus, compared to 6% of Wolbachia-free flies (P = 0.019) (Fig. 3D). Further, the median levels of A. pasteurianus in Wolbachia-infected flies were 4-fold lower than in Wolbachia-free flies (P = 0.148), while the levels of L. plantarum were unaffected by Wolbachia bacteria (Fig. 3E). In the 10-day-old adult flies, neither A. pasteurianus nor L. plantarum levels relative to the host were affected by the presence of Wolbachia bacteria (Fig. 3F). To ensure that this was not an artifact of a lack of A. pasteurianus in the food, we sampled the bacterial levels in the food (see Materials and Methods), and the levels of both bacteria were comparable in bottles of Wolbachia-free and -infected L3 larvae and 10-day-old adults (Fig. S4).
Bacterial levels in the food of upd>hPABP-Flag flies. Relative levels of each bacterial species per nanogram of DNA obtained from food, normalized to the levels in food inhabited by Wolbachia-free flies are shown. Bar graphs represent the mean values of three experiments, and error bars show standard deviations. P values were determined by Student t tests. Download FIG S4, TIF file, 0.2 MB (211.3KB, tif) .
Copyright © 2017 Simhadri et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
From these results, the greatest species-specific effect of Wolbachia bacteria on the microbiome appears to occur at an earlier developmental stage but, importantly, is independent of the transmission of a specific microbial composition from the parents.
Wolbachia-related microbiome changes are host genotype dependent.
We tested another, unrelated, genotype to assess if Wolbachia-induced microbiome changes are sensitive to the host genotype. We used isogenized white homozygous flies (see Materials and Methods) and performed the aforementioned assays with individual organisms (three experiments, 10 individuals in each) to measure microbial levels and variation in L3 larvae and adult flies (Fig. S5). The fractions of L3 larvae that harbored no A. pasteurianus were comparable between Wolbachia-free and -infected flies (20% versus 17%, respectively, P = 0.741) (Fig. S5A). There was also no Wolbachia-dependent effect on the level of either A. pasteurianus or L. plantarum relative to that in the host (Fig. S5B and C). The bacterial levels on the food were similar in Wolbachia-free and -infected bottles at both the L3 larval and 10-day-old adult stages (Fig. S6). However, the levels of bacteria in this genotype are, notably, at least an order of magnitude lower than in the genotype where the presence of Wolbachia bacteria affected their levels, even though similar amounts of bacteria were seeded to produce the gnotobiotic organisms. These results show that the effect of Wolbachia bacteria on the gut microbiota can be sensitive to the host genotype. Further assessment of a wide range of isogenized host genotypes will shed light on specific genetic factors that influence host-Wolbachia interactions that, in turn, can affect commensal microbes.
The effect of Wolbachia infection on the microbiome is genotype dependent. A. pasteurianus levels in flies with a different genetic background are shown (white). (A) Proportions of individual L3 larvae with a different genetic background (white) that had no A. pasteurianus in the gut (n = 30, chi-square test; error bars show confidence intervals). (B, C) Box-and-whisker plots of levels of bacteria in L3 larvae (B) and 10-day adults (C) (n = 30, median values are shown next to the boxes, and P values obtained with the two-sided Wilcoxon rank sum test are reported). (D) Relative expression of Imd pathway signal transducer imd, transcription factor Rel, AMPs, Nox, and AMPs downstream of JAK/STAT signaling in both axenic and gnotobiotic L3 larval guts in the presence or absence of Wolbachia infection as determined by RT-qPCR. All conditions are normalized to Wolbachia-free gnotobiotic L3 larval guts. Bar graphs show mean values (three biological replicates of 10 larvae each), and error bars show standard deviations. Asterisks indicate statistically significant differences (P < 0.05, Student t test). Download FIG S5, TIF file, 0.8 MB (822.6KB, tif) .
Copyright © 2017 Simhadri et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Bacterial levels in the food of w1118 (white-eyed) flies. Levels of each bacterial species per nanogram of DNA obtained from the food, normalized to the levels in food inhabited by Wolbachia-free flies, are shown. Bar graphs represent the mean values of three experiments, and error bars show standard deviations. P values were determined by Student t tests. Download FIG S6, TIF file, 0.2 MB (201.9KB, tif) .
Copyright © 2017 Simhadri et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Wolbachia bacteria are present intracellularly in the gut epithelium but absent from the lumen.
We investigated the stock (upd>hPABP-Flag) where Wolbachia bacteria reduced the levels of A. pasteurianus for possible causes of this phenotype. Toward determining the mechanistic basis for this Wolbachia-induced microbiome differences, we characterized the distribution of Wolbachia bacteria and A. pasteurianus in the gut by fluorescent in situ hybridization (FISH) (see Materials and Methods).
In five out of six Wolbachia-free guts that had A. pasteurianus, we observed that the bacteria were predominantly present only in the anterior midgut but not in the posterior regions or the hindgut (Fig. 4B and C). We then looked at the Wolbachia-infected guts to analyze if Wolbachia and A. pasteurianus bacteria were spatially exclusive. However, this was not possible, as most of the guts analyzed (three out of four) did not have any A. pasteurianus (Fig. 4C to E). This trend of absence of A. pasteurianus (P = 0.0325, one-tailed chi-square test) is in agreement with the previous result, where it was absent from a larger fraction of Wolbachia-infected than Wolbachia-free guts (Fig. 3D).
FIG 4 .
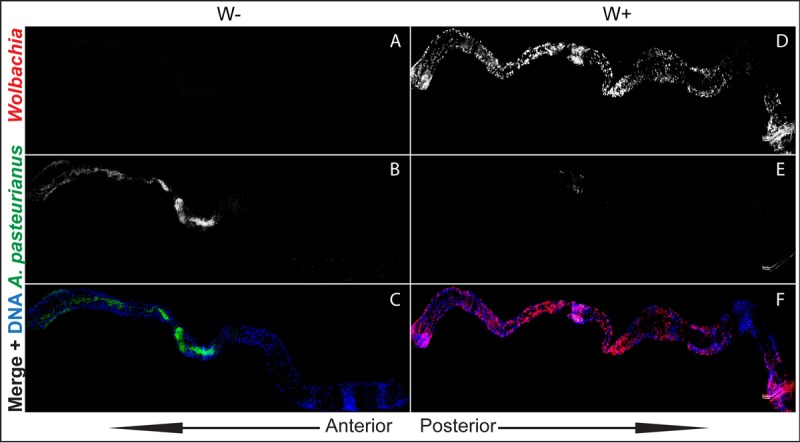
A. pasteurianus is absent from Wolbachia-infected (W+) L3 larval guts. (A to F) Composites of z-stack projections of confocal images of gnotobiotic L3 larval midguts. (A to C) Wolbachia-free (W−) guts. (D to F) Wolbachia-infected guts. (A, D) Wolbachia channel. (B, E) A. pasteurianus channel. (C, F) Merged images of Wolbachia (red), A. pasteurianus (green), and DNA (blue).
Even though Wolbachia bacteria are primarily reproductive symbionts found in the gonads, we found them in a majority of the gut cells. Wolbachia bacteria did not show any preference for a specific region of the gut (Fig. 5). We also assessed the possibility of Wolbachia bacteria in the lumen directly affecting the gut commensal bacteria. Higher-magnification confocal images (Fig. 5B to E) showed that Wolbachia bacteria were present only in the gut epithelium and absent from the lumen. Wolbachia bacteria do not occupy the same niche as the gut microbiome, in agreement with the previously reported absence of Wolbachia bacteria from fecal matter (23).
FIG 5 .
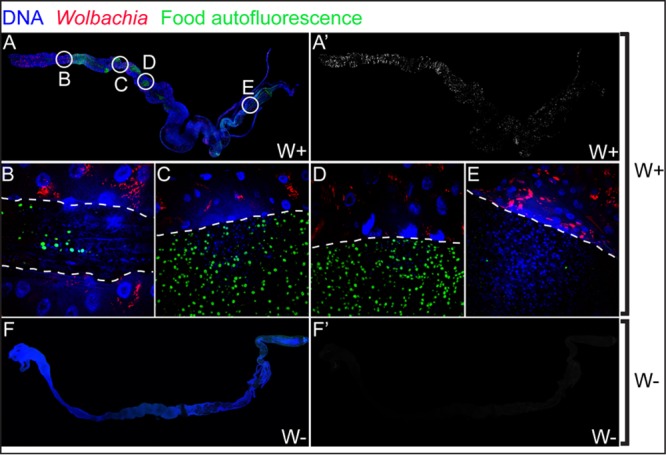
Wolbachia bacteria are present in gut cells but absent from the lumen. Composite confocal images of the whole midgut and hindgut of Wolbachia-infected (W+) flies (A) and Wolbachia-free (W−) flies (F) and the respective Wolbachia channels (A′ and F′). Magnification (×60) of the midgut (B to D) and hindgut (E) regions shows that Wolbachia bacteria are present intracellularly in gut cells but absent from the lumen of the gut (the lumen is marked by green autofluorescence).
The presence of Wolbachia bacteria in flies does not alter the gut immune response.
Since direct interaction between Wolbachia bacteria and gut microbes is unlikely, we hypothesized that the presence of Wolbachia bacteria in flies might elicit a gut-specific immune response that could alter the composition of the gut microbiota. To examine this possibility, we first generated axenic organisms that were either Wolbachia-free or infected in triplicate. Since the greatest difference in the composition of the microbiome was observed at the L3 larval stage, we isolated whole guts of 10 axenic L3 larvae for each replicate, extracted whole RNA, and performed reverse transcription (RT)-qPCRs of several immunity related genes. Two major signaling pathways, Toll and Imd, control Drosophila systemic immunity. However, in the Drosophila midgut, only Imd signaling induces an immune response (15, 24). We first tested for the expression of the immune deficiency (imd) and Relish (Rel) genes, which are the key components of signal transduction in the major immunity pathway in the gut tissue, and found that there were no significant differences due to the presence of Wolbachia bacteria (Fig. 6A). Since antimicrobial peptide (AMP) expression is a well-characterized immune response readout, we measured the expression levels of all of the AMPs downstream of the Imd pathway. The expression of AttB, AttD, CecA2, CecC, DptB, and Dro was not different between Wolbachia-free and -infected L3 larval gut tissues (Fig. 6B). Other AMPs downstream of Imd, such as Atta, AttC, CecA1, CecB, and Dpt, were either not expressed or expressed at very low levels. These results indicate that under the axenic conditions used, Wolbachia bacteria alone do not activate Imd signaling.
FIG 6 .
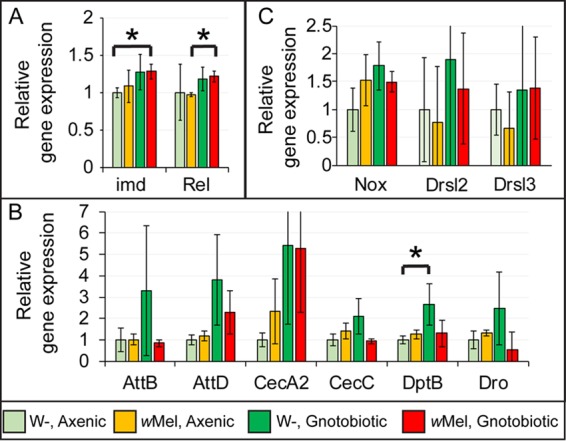
Wolbachia bacterial infection does not affect the expression of Imd pathway components and ROS-producing oxidases. The relative expression of Imd pathway signal transducer imd (A), the transcription factor Rel (A), AMPs (B), Nox (C), and AMPs downstream of JAK/STAT signaling in both axenic and gnotobiotic L3 larval guts in the presence or absence of Wolbachia infection was determined by RT-qPCR. All conditions are normalized to Wolbachia-free (W−) axenic L3 larval guts. Bar graphs show mean values (three biological replicates of 10 larvae each), and error bars show standard deviations. Asterisks indicate statistically significant differences (P < 0.05, Student t test).
To quantify the expression of Imd pathway genes in the presence of gut bacteria, we generated gnotobiotic larvae by seeding germfree larvae with A. pasteurianus and L. plantarum (see Materials and Methods). Compared to those in axenic larvae, the expression levels of imd and Rel were moderately upregulated in the presence of gut microbes. Importantly, this upregulation was independent of Wolbachia infection status. In gnotobiotic flies, imd and Relish expression is similar in Wolbachia-free and -infected larval guts (Fig. 6B). Regarding AMP production, again, Atta, AttC, CecA1, CecB, and Dpt were either not expressed or expressed at very low levels. For expressed AMPs, the introduction of commensal bacteria caused an overall increase in the levels of AMPs expressed. Because of the high variability in expression levels, only Diptericin B (DptB) was statistically significantly different. The presence of Wolbachia bacteria did not result in significant changes in AMP gene expression in gnotobiotic flies.
In addition, the JAK/STAT pathway is also relevant to gut innate immunity and can regulate the expression of some AMPs (25, 26). We tested Drsl2 and Drsl3 (also known as dro2 and dro3) transcription levels in larvae with different Wolbachia and microbiome statuses. Again, we found no significant differences due to the presence of Wolbachia bacteria in axenic or gnotobiotic L3 larvae (Fig. 6C).
Another key defense mechanism in the gut is the production of reactive oxygen species (ROS). A key readout of their immune activation is upregulation of the Nox and Duox genes (27). Regardless of Wolbachia infection status, levels of Duox expression were negligible and levels of Nox expression were not modified (Fig. 6C). Together, these results suggest that Wolbachia modulation of the microbiome is independent of AMP production or Nox expression in the gut.
DISCUSSION
The interaction between the resident microbes can be a key determinant of the microbial species present in a host. In the insect gut, pathogenic organisms can be excluded by resident microbes (reviewed in reference 28). The insect microbiome can also influence symbiont vertical transmission. A recent study by Hughes and collaborators showed that in Anopheles mosquitos, intracellular Wolbachia bacteria are robustly excluded from vertical transmission by Asaia sp., an acetic acid bacterium (17). Here, we show for the first time a reciprocal aspect of commensal microbiome bacteria and symbionts; our data indicate that Wolbachia bacteria in D. melanogaster are capable of altering the composition of resident microbes compared to that in the Wolbachia-free host.
We initially surveyed the composition of the resident bacterial species in a Drosophila stock reared in our lab—upd>hPABP-Flag—and found that A. pasteurianus and L. plantarum are the only two species in this strain of flies. This is in general agreement with reports from other groups that list Acetobacter and Lactobacillus as genera commonly associated with Drosophila flies reared in the lab and also wild-caught fly strains (10, 12, 29). We compared the microbial composition of another stock with the same genetic background that was not infected with Wolbachia bacteria. Surprisingly, the levels of A. pasteurianus are lower in both male and female Wolbachia-infected flies than in Wolbachia-free flies. By validating this result by PCR with species-specific primers, we also showed that the levels of A. pasteurianus are consistently reduced in adults and larvae.
These differences in the microbiome could be due to the presence of Wolbachia bacteria or could reflect an event unrelated to Wolbachia bacteria that is maintained across generations. Since the microbiome is transmitted vertically via fly feces to the next generation, the difference in the levels of bacteria that we observed could be due to just lower seeding from previous generations. To eliminate the effect of parental transmission of microbes to offspring, we studied the effects of Wolbachia bacteria on flies raised from gnotobiotic embryos. The results show that Wolbachia infection is sufficient to reduce the levels of A. pasteurianus at certain developmental stages. Specifically, the A. pasteurianus level is considerably lower in Wolbachia-infected larvae than in their Wolbachia-free counterparts. We show that this reduction is due to a complete lack of A. pasteurianus in a significant fraction of the Wolbachia-infected L3 larvae compared to Wolbachia-free larvae. Further, by using FISH to probe for A. pasteurianus in L3 larval guts, we observed a marked absence of A. pasteurianus in a majority of Wolbachia-infected guts but not in Wolbachia-free guts. Recent work with Anopheles stephensi mosquitos also shows variation of Wolbachia-induced differences in the mosquito microbiome. Directly after a blood meal, Wolbachia-infected mosquitos had a smaller proportion of gammaproteobacteria than Wolbachia-cured mosquitos did (see Fig. 2 in reference 30). A week after a blood meal, Wolbachia-infected mosquitos have a significantly more diverse microbiota than Wolbachia-free insects. However, when fed just a sugar meal or immediately after a blood meal, there are no differences between Wolbachia-free and -infected mosquitos (see Table 1 in reference 30). Another study of D. melanogaster showed that the presence of Wolbachia reduced the diversity of the gut microbiome (see Fig. 2B in reference 32), and also reduced the abundance of Acetobacter genus (see Table 2 in reference 32).
In contrast to the results obtained with larvae, the relative levels of A. pasteurianus in young gnotobiotic adult flies were not significantly different from those in Wolbachia-free adult flies. This could be a result of loss of the microbiome during histolysis of the larval gut during pupation. In concordance, several studies showed that newly eclosed adults have extremely low densities of resident bacteria and are recolonized by feeding (9, 31). Therefore, any Wolbachia-induced reduction of A. pasteurianus in the larval stages is lost and must be reestablished over time. This explains the large variability in the relative quantities of the gut microbes in both Wolbachia-free and -infected young adults. On the other hand, conventionally reared Wolbachia-infected adults have greatly reduced levels of A. pasteurianus since the bacteria are not externally introduced into the food. In the case of conventionally reared flies, the only source of A. pasteurianus bacteria available to offspring is adults, and from our data, they are not detected or greatly reduced.
We used another genotype, w1118, to address whether the host genotype plays a role in this Wolbachia-induced microbial change. We did not detect any significant effect of Wolbachia bacteria on either A. pasteurianus or L. plantarum levels at the L3 larval stage or in 10-day-old adult flies. Since the levels of A. pasteurianus in flies of the w1118 genotype are significantly lower than in those of the upd>hPABP-Flag genotype, Wolbachia-induced effects on A. pasteurianus, if there are any, might be harder to detect. Further, the two genotypes used here have quite a few dissimilarities, including a balancer chromosome and the UAS-GAL4 system in upd>hPABP-Flag flies, which is absent from w1118 flies. Thus, the host genotype can play a role in determining the outcome of microbial populations in the gut. Findings presented here are in accordance with mounting evidence showing a complex interaction of Wolbachia bacteria and commensal microbial composition influenced by several variables, including host genotypes (17, 30, 32–34). A thorough future investigation of host and microbial genetic and epigenetic determinants that can alter the tolerance and carrying capacity of the host for each of the colonizing microbes across a wide range of insect species is needed. Taking this into account, we investigated the upd>hPABP-Flag genotype, in which Wolbachia bacteria play a role in the determination of the microbial composition, for a possible mechanism of this phenotype.
There are several possible mechanisms for modulation of the microbiome by Wolbachia bacteria. A. pasteurianus levels could be reduced because of direct competition for nutrients; however, this is unlikely since ingested nutrients are immediately available to A. pasteurianus, which is present in the lumen, while Wolbachia bacteria are intracellular. Additionally, factors derived from either Wolbachia bacteria or the host could inhibit A. pasteurianus.
Regarding Wolbachia-derived factors, Wolbachia bacteria contain a type 4 secretion system (35), which can be used to secrete factors that subvert host cell biology to favor bacterial survival and growth. However, there is no evidence of a Wolbachia-derived factor that could directly influence the presence of other bacteria. Such a factor, to be effective against A. pasteurianus, would have to be exported into the gut lumen. Since both the host genotypes tested harbor the same strain of Wolbachia, and yet do not produce the same microbiome composition, Wolbachia secreted factors are unlikely to play a role. Therefore, we favor host-derived factors as the most likely mechanism.
A second possibility is indirect inhibition of A. pasteurianus by Wolbachia bacteria via the host. The host immune system, specifically AMPs, could play a role in altering the microbiome in response to Wolbachia infection. Previous studies of host immunity showing that Wolbachia bacteria upregulate the immune response were performed with nonnative hosts that were transinfected with Wolbachia bacteria from another host (36–39). However, similar studies performed with a host such as D. melanogaster natively infected with Wolbachia strain wMel did not show any systemic upregulation of immunity (39, 40). Though both native and nonnative Wolbachia-infected hosts exhibit a robust antiviral response to single-stranded RNA viruses (37, 41–44), the native host (Drosophila, in this case) did not show any antibacterial activity when infected with pathogenic strains of bacteria via injury (40, 45). No previous study addressed Wolbachia-induced antimicrobial effects on commensal gut microbes or Wolbachia putative immune regulation specifically in the digestive tract. Regulation of the immune response in the fly gut differs in several aspects of systemic immunity. It is possible that Wolbachia infection could be generating an intestine-specific immune response acting to destabilize the microbiome. We tested this hypothesis in both axenic and gnotobiotic organisms that were either Wolbachia free or infected. There were no significant changes in the expression of AMPs of the Imd or JAK/STAT pathway between Wolbachia-infected and Wolbachia-free L3 larval gut tissues under both axenic and gnotobiotic conditions.
Finally, ROS could also play a role in modulation of the microbiome in response to infection with intracellular bacteria like those of the genus Wolbachia. There are several papers indicating that Wolbachia bacteria upregulate ROS in their insect host (46–50). Most of the measurements were done by using the whole organism. The amplitude of the response varies according to the tissue; data from naive hosts suggest that while changes in the major ROS effector genes Nox and Duox can be significantly affected by Wolbachia bacteria at the systemic level, in the gut, there are no significant differences (47). In agreement, we also did not observe Wolbachia-driven changes in the levels of Nox or Duox expression in the fly gut. These results suggest that the mechanism of Wolbachia modulation of the microbiome does not operate through simple changes in the expression of genes for the two major classes of key effectors of gut immunity, the genes for AMPs and Nox. These findings highlight the complexity of the interaction of Wolbachia bacteria with their hosts.
Our results do not rule out the possibility of the involvement of these pathways at the posttranscriptional level. Besides changes at the translational and posttranslational control levels, these pathways affect gut physiology beyond a simple immune response (51–53). For instance, it is well established that ROS (53) and JAK/STAT signaling (51) affects stem cell proliferation and regulates turnover of the gut epithelium. Other physiological aspects, such as the gut pH, have been shown to be affected by commensal microbiota (52). Differences in the gut pH due to the presence of Wolbachia bacteria could selectively influence the presence of A. pasteurianus and L. plantarum. Future studies should provide further insight into the mechanisms of Wolbachia-induced changes in the composition of the microbiome.
Our data show that the presence of Wolbachia bacteria has a significant effect on the composition of the microbiome of bacterial species in certain host genotypes under lab conditions. Though this effect might not be generalizable to every fly genotype and host species, these finding are relevant for studies investigating the phenotypic consequences of Wolbachia infection on the host. Wolbachia bacteria have been shown to be capable of altering many phenotypes in insects, such as fecundity (6, 7), insulin signaling and metabolism (7), immunity and resistance to pathogens (36–39, 41, 42, 54, 55), stem cell activity (6), and life span (56). With growing evidence that resident gut microbes are also capable of altering many of these phenotypes (16, 57–62), it is important to delineate the relative contributions of each of the bacteria to the phenotypic changes seen.
Modulation of the microbiome by Wolbachia bacteria may have dramatic effects on host fitness. For example, in terms of immunity, it is known that a certain composition of the microbiome confers protection against pathogens on several organisms, from plants to humans (63, 64). In Drosophila, it has been reported that higher relative levels of L. plantarum promote protection against Serratia marcescens and Pseudomonas aeruginosa, two known Drosophila pathogens that also cause opportunistic infections in humans (31). These findings raise the possibility that Wolbachia bacteria change the host defense indirectly by affecting the composition of the microbiome. Altered immune competence can play a key role in the survival of host populations in nature.
The results shown here are also relevant for the development of bacterium-based approaches to vector control. Several studies have shown that gut and Wolbachia bacteria inhibit the presence of human pathogens in insect vectors, including Plasmodium falciparum and the dengue, West Nile, and chikungunya viruses (reviewed in references 8, 28, 36–39, 41, 42, 54, and 55). Therefore, it is important to understand the interactions of Wolbachia bacteria with other bacteria that inhibit disease transmission in order to determine the effects of synergistic or antagonistic interactions on vector control.
MATERIALS AND METHODS
Fly strains, Wolbachia typing, and husbandry.
The D. mauritiana fly stocks used in this study were from Fast et. al. (6). The D. melanogaster fly stocks used in this study were upd-Gal4; Cyo/Sco; P(UAS-hPF)B (upd>hPABP-Flag in short) and white mutant (w1118). upd>hPABP-Flag fly stocks with and without Wolbachia bacteria have similar genetic backgrounds through introgression backcrosses, as previously described (according to reference 65), and white mutant (w) flies with and without Wolbachia bacteria were a generous gift from Luis Teixeira (66). The fly food used was of the typical cornmeal-yeast-molasses-agar type (agar at 9.7 g/liter, cornmeal at 65.7 g/liter, yeast at 27 g/liter, molasses at 65.7 ml/liter) with preservatives (propionic acid at 6.9 ml/liter and tegosept at 4 ml/liter). All stocks and experiments were maintained in a 25°C incubator with 60% humidity.
Wolbachia typing was performed by PCR against VNTRs 141 and 105 (67).
Egg collection and grape juice agar plates.
Fifteen newly eclosed Wolbachia-free and -infected D. mauritiana flies were collected and kept in bottles containing yeasted grape juice agar plates. Bottles were maintained at 25°C with 60% humidity. Plates were changed every 24 h and stored at 4°C.
DNA extraction from flies and food.
Genomic DNA was isolated by using a modified form of the protocol for the Qiagen DNeasy Blood and Tissue kit (catalog no. 69506). We surface sterilized flies by vortexing them with a 50% household bleach solution (4% sodium hypochlorite) for 5 min and washing them three times with sterile water. Effective removal of external bacteria was confirmed as previously described, with modifications (10, 62). The efficiency of this procedure was confirmed by PCR assay of the water from the final wash with universal 16S rRNA gene primers. Flies were then homogenized in 200 µl of lysis buffer (20 mM Tris [pH 8.0], 2 mM EDTA, 1.2% Triton X-100) with lysozyme (MP Biomedicals, catalog no. 210083401) at 20 mg/ml and incubated for 90 min at 37°C. A 200-µl volume of AL buffer (Qiagen Blood and Tissue kit) and 20 µl of proteinase K were then added to the mixture, which was incubated further for 90 min at 56°C. Subsequent extraction with the columns was performed as recommended by the kit’s manufacturer.
To isolate DNA from the food, 50 to 100 mg of food was collected from the bottles and the protocol described above without bleach treatment was used.
Elimination of Wolbachia 16S rRNA gene sequences from samples.
Total genomic DNA was extracted and digested with the NEB BstZ17I restriction enzyme (catalog no. R0594S) for 1 h at 37°C, followed by 10 min at 65°C to prevent amplification of the Wolbachia V1 and V2 regions of the 16S rRNA gene prior to high-throughput sequencing of the bacterial 16S rRNA gene on the MiSeq platform.
16S rRNA gene library preparation and sequencing.
A 500-ng portion of BstZ17I-digested total genomic DNA was used per sample for each library. All PCRs were performed with Platinum Taq DNA Polymerase High Fidelity from Life Technologies, Inc. (catalog no. 10790-020), and appropriate primers (Table S1). 16S rRNA gene amplicons from PCRs were separated by agarose gel electrophoresis and subsequently extracted with the Qiagen QIAquick gel extraction kit (catalog no. 28706). Kappa Biosystems DNA standards (catalog no. KK4903) were used for calibration of the DNA concentration used for sequencing by qPCR with Illumina adaptors (Table S1). qPCRs were performed with SYBR GreenER qPCR SuperMix Universal from Life Technologies, Inc. (catalog no. 11790-01k). Sequencing was performed with the Illumina MiSeq platform by using 250-bp paired-end reads. Analysis of the reads was performed with the QIIME 1.8.0 package. Default parameters were used for the analysis, and the Greengenes database was used to assign taxonomy.
Isolation and identification of bacteria from flies.
Twenty flies were homogenized in 500 µl of MRS medium (catalog no. 288130; BD), fly debris was removed by brief centrifugation in a tabletop centrifuge, and 20 µl of the supernatant was plated on MRS agar. The plates were incubated at either 37 or 30°C. Sixteen colonies were sampled, and colony PCR was performed with the universal bacterial 16S rRNA gene primers. The PCR amplicons were Sanger sequenced with both forward and reverse universal 16S rRNA gene primers.
Bacterial cultures.
All bacterial cultures were grown in MRS liquid medium or agar (catalog no. 288130; BD). L. plantarum cultures were grown at 37°C, and A. pasteurianus cultures were grown at 30°C.
Bacterial whole-genome sequencing.
DNA was extracted from overnight cultures of L. plantarum and A. pasteurianus by a modified version of the protocol for the Qiagen blood and tissue kit as described above. DNA was sheared with Covaris spin tubes (catalog no. 520079). Genome libraries were prepared in accordance with the PacBio Template Preparation and Sequencing Guide selecting for approximately 10-kb genome fragments. DNA quality and size were confirmed on a Bioanalyzer, followed by sequencing with a PacBio RS II sequencer. Raw reads were assembled de novo by SMRT analysis software. Manual curation and closing of the genome were done by NCBI alignment. The A. pasteurianus chromosome (3.12 Mb) and plasmid (140 kb) were annotated by using a database of closed Acetobacter strains (CP012111 and NC_013209). The L. plantarum (3.32 Mb) genome was annotated by rapid annotations using subsystems technology (68, 69).
Gnotobiotic flies.
Gnotobiotic flies were generated by exposing sterile embryos to 1,000 CFU of the required bacteria on sterile fly food. Embryos were sterilized by three washes in 50% bleach (Clorox diluted in sterile water to a final concentration of approximately 4% sodium hypochlorite) for 2 min per wash. Subsequently, the dechorionated sterile embryos were washed three times with a sterile Triton salt solution (4 g/liter NaCl, 300 µl/liter Triton X-100) to prevent sticking to surfaces. After removal of all the Triton salt solution, an equal volume of fresh Triton salt solution was added to the embryos. A 10-µl volume of this mixture was added to autoclave-sterilized fly food. Sterilized fly food was prepared similarly to regular fly food without preservatives after autoclaving. A correlation of the optical density (OD) at 600 nm and the CFU count was generated for each species; L. plantarum was 1.11 × 109 CFU/ml/OD unit, and A. pasteurianus was 2.6 × 108 CFU/ml/OD unit. By using appropriate dilutions of log-phase cultures whose ODs were measured, 1,000 CFU each of A. pasteurianus and L. plantarum were added to sterile hatching L1 larvae. L3 larvae and adult flies were collected under sterile conditions, and their DNA was extracted after surface sterilization with 50% bleach.
Wolbachia and A. pasteurianus immunocytochemistry.
Wolbachia in situ localization in the gut was determined by FISH as previously described (65). A. pasteurianus probes were designed and tested for specificity. The probe used in this study was 5′ 6-carboxyfluorescein--AGAGTGCCCAGCCCAACCTGA from IDT DNA. Both Wolbachia and A. pasteurianus probes were used at 1 ng/µl. To perform FISH of gut contents, the fly food was modified by replacing yeast with yeast extract and sugars to eliminate the autofluorescence of yeast. The composition of the modified fly food was dextrose at 50 g/liter, sucrose at 25 g/liter, yeast extract at 15 g/liter, cornmeal at 60 g/liter, agar at 6.5 g/liter, tryptone at 30 g/liter, and molasses at 65 g/liter. L3 larval guts were dissected and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 1 h and then in 50% ethanol with PBS for 30 min at −20°C. Hybridization was performed as previously described (65). Image acquisition was performed with an Olympus FluoView 1000 confocal microscope. Full-gut images were assembled with Microsoft Image Composite Editor and the MosaicJ package in FIJI from individual images collected at ×40 (for Fig. 4) or ×60 (for Fig. 5) magnification. Images were processed with Photoshop to eliminate pixels outside the gut and equalize channel intensity within the same composite image.
qPCR.
qPCRs were performed with SYBR GreenER qPCR SuperMix Universal from Life Technologies, Inc. (catalog no. 11790-01k). BstZ17I was used to digest 200 ng of total genomic DNA for 1 h at 37°C, followed by 10 min at 65°C. A 5-ng sample of the digested DNA was amplified with the following species-specific primers: A. pasteurianus, forward primer 5′ GCACCCTCATGGTACCGAGC 3′ and reverse primer 5′ ACCAGCAGGGCGATGGTTTC 3′; L. plantarum, forward primer 5′ ACGTTAGGGCTACTCGGCCA 3′ and reverse primer 5′ GCCTTCGCCGACCCCAATTA 3′. Universal 16S rRNA gene primers or D. melanogaster 14-3-3 gene forward primer 5′ CATGAACGATCTGCCACCAAC 3′ and reverse primer 5′ CTCTTCGCTCAGTGTATCCAAC 3′ were used for normalization.
RT-qPCRs for transcriptional profiling.
Ten guts of axenic or gnotobiotic L3 larvae were dissected in Grace’s insect medium (Lonza catalog no. 04-457F), and the RNA was immediately extracted with the Qiagen miRNeasy minikit (catalog no. 217004) in accordance with the manufacturer’s protocol. For quantification of the mRNA levels of the genes in the Imd pathway, the EXPRESS one-Step SYBR GreenER kit with premixed ROX from Life Technologies, Inc., was used (catalog no. 1179001k). Twenty nanograms of RNA was used as the input for each reaction, and the conditions used were those recommended by the kit’s manufacturer.
Accession number(s).
The accession numbers of the BioProjects containing the raw reads from this study in the NCBI database are PRJNA381361 (16S rRNA gene raw reads) and PRJNA384998 (whole-genome sequencing of A. pasteurianus and L. plantarum).
ACKNOWLEDGMENTS
We thank K. McCall, D. Segre, J. Talbot, and members of the Frydman lab for suggestions and comments on the manuscript. C. Dally (Harvard Sequencing Facility) assisted with 16S rRNA gene profiling. Isogenized w flies with and without Wolbachia bacteria were a generous gift from Luis Teixeira.
Work in the Frydman lab is supported by Boston University funds, National Institute of Allergy and Infectious Diseases grant 1R56AI97589-01A1, and NSF 1225360.
R.K.S. and H.M.F. designed the study. R.K.S. and H.M.F. wrote the manuscript. R.K.S. performed all of the experiments and analysis, unless stated otherwise. R.G. performed FISH with A. pasteurianus. E.M.F. performed the grape juice plate pH assays with D. mauritiana. R.K.S., M.J.S., N.V., J.B., and B.E.S. contributed to the sequencing and assembly of the bacterial genomes. L.O. contributed to the preparation of 16S rRNA gene libraries from flies. B.E.S and H.M.F. contributed material and reagents.
REFERENCES
- 1.McFall-Ngai M, Hadfield MG, Bosch TC, Carey HV, Domazet-Lošo T, Douglas AE, Dubilier N, Eberl G, Fukami T, Gilbert SF, Hentschel U, King N, Kjelleberg S, Knoll AH, Kremer N, Mazmanian SK, Metcalf JL, Nealson K, Pierce NE, Rawls JF, Reid A, Ruby EG, Rumpho M, Sanders JG, Tautz D, Wernegreen JJ. 2013. Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci U S A 110:3229–3236. doi: 10.1073/pnas.1218525110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serbus LR, Casper-Lindley C, Landmann F, Sullivan W. 2008. The genetics and cell biology of Wolbachia-host interactions. Annu Rev Genet 42:683–707. doi: 10.1146/annurev.genet.41.110306.130354. [DOI] [PubMed] [Google Scholar]
- 3.Dale C, Moran NA. 2006. Molecular interactions between bacterial symbionts and their hosts. Cell 126:453–465. doi: 10.1016/j.cell.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Werren JH, Baldo L, Clark ME. 2008. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 5.Zug R, Hammerstein P. 2012. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One 7:e38544. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fast EM, Toomey ME, Panaram K, Desjardins D, Kolaczyk ED, Frydman HM. 2011. Wolbachia enhance Drosophila stem cell proliferation and target the germline stem cell niche. Science 334:990–992. doi: 10.1126/science.1209609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikeya T, Broughton S, Alic N, Grandison R, Partridge L. 2009. The endosymbiont Wolbachia increases insulin/IGF-like signalling in Drosophila. Proc Biol Sci 276:3799–3807. doi: 10.1098/rspb.2009.0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourtzis K, Dobson SL, Xi Z, Rasgon JL, Calvitti M, Moreira LA, Bossin HC, Moretti R, Baton LA, Hughes GL, Mavingui P, Gilles JR. 2014. Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop 132(Suppl):S150–S163. doi: 10.1016/j.actatropica.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Wong CN, Ng P, Douglas AE. 2011. Low-diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environ Microbiol 13:1889–1900. doi: 10.1111/j.1462-2920.2011.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandler JA, Lang JM, Bhatnagar S, Eisen JA, Kopp A. 2011. Bacterial communities of diverse Drosophila species: ecological context of a host-microbe model system. PLoS Genet 7:e1002272. doi: 10.1371/journal.pgen.1002272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox CR, Gilmore MS. 2007. Native microbial colonization of Drosophila melanogaster and its use as a model of Enterococcus faecalis pathogenesis. Infect Immun 75:1565–1576. doi: 10.1128/IAI.01496-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corby-Harris V, Pontaroli AC, Shimkets LJ, Bennetzen JL, Habel KE, Promislow DE. 2007. Geographical distribution and diversity of bacteria associated with natural populations of Drosophila melanogaster. Appl Environ Microbiol 73:3470–3479. doi: 10.1128/AEM.02120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryu JH, Kim SH, Lee HY, Bai JY, Nam YD, Bae JW, Lee DG, Shin SC, Ha EM, Lee WJ. 2008. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science 319:777–782. doi: 10.1126/science.1149357. [DOI] [PubMed] [Google Scholar]
- 14.Lee WJ, Brey PT. 2013. How microbiomes influence metazoan development: insights from history and Drosophila modeling of gut-microbe interactions. Annu Rev Cell Dev Biol 29:571–592. doi: 10.1146/annurev-cellbio-101512-122333. [DOI] [PubMed] [Google Scholar]
- 15.Buchon N, Broderick NA, Lemaitre B. 2013. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nat Rev Microbiol 11:615–626. doi: 10.1038/nrmicro3074. [DOI] [PubMed] [Google Scholar]
- 16.Newell PD, Douglas AE. 2014. Interspecies interactions determine the impact of the gut microbiota on nutrient allocation in Drosophila melanogaster. Appl Environ Microbiol 80:788–796. doi: 10.1128/AEM.02742-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes GL, Dodson BL, Johnson RM, Murdock CC, Tsujimoto H, Suzuki Y, Patt AA, Cui L, Nossa CW, Barry RM, Sakamoto JM, Hornett EA, Rasgon JL. 2014. Native microbiome impedes vertical transmission of Wolbachia in Anopheles mosquitoes. Proc Natl Acad Sci U S A 111:12498–12503. doi: 10.1073/pnas.1408888111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Z, Edenberg HJ, Davis RL. 2005. Isolation of mRNA from specific tissues of Drosophila by mRNA tagging. Nucleic Acids Res 33:e148. doi: 10.1093/nar/gni149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toomey ME, Frydman HM. 2014. Extreme divergence of Wolbachia tropism for the stem-cell-niche in the Drosophila testis. PLoS Pathog 10:e1004577. doi: 10.1371/journal.ppat.1004577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elgart M, Stern S, Salton O, Gnainsky Y, Heifetz Y, Soen Y. 2016. Impact of gut microbiota on the fly’s germ line. Nat Commun 7:11280. doi: 10.1038/ncomms11280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coon KL, Brown MR, Strand MR. 2016. Gut bacteria differentially affect egg production in the anautogenous mosquito Aedes aegypti and facultatively autogenous mosquito Aedes atropalpus (Diptera: Culicidae). Parasit Vectors 9:375. doi: 10.1186/s13071-016-1660-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staubach F, Baines JF, Künzel S, Bik EM, Petrov DA. 2013. Host species and environmental effects on bacterial communities associated with Drosophila in the laboratory and in the natural environment. PLoS One 8:e70749. doi: 10.1371/journal.pone.0070749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fink C, Staubach F, Kuenzel S, Baines JF, Roeder T. 2013. Noninvasive analysis of microbiome dynamics in the fruit fly Drosophila melanogaster. Appl Environ Microbiol 79:6984–6988. doi: 10.1128/AEM.01903-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzou P, Ohresser S, Ferrandon D, Capovilla M, Reichhart JM, Lemaitre B, Hoffmann JA, Imler JL. 2000. Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity 13:737–748. doi: 10.1016/S1074-7613(00)00072-8. [DOI] [PubMed] [Google Scholar]
- 25.Osman D, Buchon N, Chakrabarti S, Huang YT, Su WC, Poidevin M, Tsai YC, Lemaitre B. 2012. Autocrine and paracrine unpaired signaling regulate intestinal stem cell maintenance and division. J Cell Sci 125:5944–5949. doi: 10.1242/jcs.113100. [DOI] [PubMed] [Google Scholar]
- 26.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. 2009. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe 5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Bae YS, Choi MK, Lee WJ. 2010. Dual oxidase in mucosal immunity and host-microbe homeostasis. Trends Immunol 31:278–287. doi: 10.1016/j.it.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Cirimotich CM, Ramirez JL, Dimopoulos G. 2011. Native microbiota shape insect vector competence for human pathogens. Cell Host Microbe 10:307–310. doi: 10.1016/j.chom.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren C, Webster P, Finkel SE, Tower J. 2007. Increased internal and external bacterial load during Drosophila aging without life span trade-off. Cell Metab 6:144–152. doi: 10.1016/j.cmet.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Zhao J, Joshi D, Xi Z, Norman B, Walker ED. 2016. Persistent infection by Wolbachia wAlbB has no effect on composition of the gut microbiota in adult female Anopheles stephensi. Front Microbiol 7:1485. doi: 10.3389/fmicb.2016.01485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blum JE, Fischer CN, Miles J, Handelsman J. 2013. Frequent replenishment sustains the beneficial microbiome of Drosophila melanogaster. mBio 4:e00860-13. doi: 10.1128/mBio.00860-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye YH, Seleznev A, Flores HA, Woolfit M, McGraw EA. 2017. Gut microbiota in Drosophila melanogaster interacts with Wolbachia but does not contribute to Wolbachia-mediated antiviral protection. J Invertebr Pathol 143:18–25. doi: 10.1016/j.jip.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Dittmer J, Beltran-Bech S, Lesobre J, Raimond M, Johnson M, Bouchon D. 2014. Host tissues as microhabitats for Wolbachia and quantitative insights into the bacterial community in terrestrial isopods. Mol Ecol 23:2619–2635. doi: 10.1111/mec.12760. [DOI] [PubMed] [Google Scholar]
- 34.Rossi P, Ricci I, Cappelli A, Damiani C, Ulissi U, Mancini MV, Valzano M, Capone A, Epis S, Crotti E, Chouaia B, Scuppa P, Joshi D, Xi Z, Mandrioli M, Sacchi L, O’Neill SL, Favia G. 2015. Mutual exclusion of Asaia and Wolbachia in the reproductive organs of mosquito vectors. Parasit Vectors 8:278. doi: 10.1186/s13071-015-0888-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu M, Sun LV, Vamathevan J, Riegler M, Deboy R, Brownlie JC, McGraw EA, Martin W, Esser C, Ahmadinejad N, Wiegand C, Madupu R, Beanan MJ, Brinkac LM, Daugherty SC, Durkin AS, Kolonay JF, Nelson WC, Mohamoud Y, Lee P, Berry K, Young MB, Utterback T, Weidman J, Nierman WC, Paulsen IT, Nelson KE, Tettelin H, O’Neill SL, Eisen JA. 2004. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol 2:E69. doi: 10.1371/journal.pbio.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kambris Z, Cook PE, Phuc HK, Sinkins SP. 2009. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 326:134–136. doi: 10.1126/science.1177531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, Rocha BC, Hall-Mendelin S, Day A, Riegler M, Hugo LE, Johnson KN, Kay BH, McGraw EA, van den Hurk AF, Ryan PA, O’Neill SL. 2009. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell 139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 38.Bian G, Xu Y, Lu P, Xie Y, Xi Z. 2010. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog 6:e1000833. doi: 10.1371/journal.ppat.1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rancès E, Ye YH, Woolfit M, McGraw EA, O’Neill SL. 2012. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog 8:e1002548. doi: 10.1371/journal.ppat.1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong ZS, Hedges LM, Brownlie JC, Johnson KN. 2011. Wolbachia-mediated antibacterial protection and immune gene regulation in Drosophila. PLoS One 6:e25430. doi: 10.1371/journal.pone.0025430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teixeira L, Ferreira A, Ashburner M. 2008. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6:e2. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hedges LM, Brownlie JC, O’Neill SL, Johnson KN. 2008. Wolbachia and virus protection in insects. Science 322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- 43.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, Leong YS, Dong Y, Axford J, Kriesner P, Lloyd AL, Ritchie SA, O’Neill SL, Hoffmann AA. 2011. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 44.Glaser RL, Meola MA. 2010. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS One 5:e11977. doi: 10.1371/journal.pone.0011977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rottschaefer SM, Lazzaro BP. 2012. No effect of Wolbachia on resistance to intracellular infection by pathogenic bacteria in Drosophila melanogaster. PLoS One 7:e40500. doi: 10.1371/journal.pone.0040500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andrews ES, Crain PR, Fu Y, Howe DK, Dobson SL. 2012. Reactive oxygen species production and Brugia pahangi survivorship in Aedes polynesiensis with artificial Wolbachia infection types. PLoS Pathog 8:e1003075. doi: 10.1371/journal.ppat.1003075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan X, Zhou G, Wu J, Bian G, Lu P, Raikhel AS, Xi Z. 2012. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A 109:E23–E31. doi: 10.1073/pnas.1116932108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brennan LJ, Haukedal JA, Earle JC, Keddie B, Harris HL. 2012. Disruption of redox homeostasis leads to oxidative DNA damage in spermatocytes of Wolbachia-infected Drosophila simulans. Insect Mol Biol 21:510–520. doi: 10.1111/j.1365-2583.2012.01155.x. [DOI] [PubMed] [Google Scholar]
- 49.Brennan LJ, Keddie BA, Braig HR, Harris HL. 2008. The endosymbiont Wolbachia pipientis induces the expression of host antioxidant proteins in an Aedes albopictus cell line. PLoS One 3:e2083. doi: 10.1371/journal.pone.0002083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bian G, Joshi D, Dong Y, Lu P, Zhou G, Pan X, Xu Y, Dimopoulos G, Xi Z. 2013. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science 340:748–751. doi: 10.1126/science.1236192. [DOI] [PubMed] [Google Scholar]
- 51.Broderick NA, Buchon N, Lemaitre B. 2014. Microbiota-induced changes in Drosophila melanogaster host gene expression and gut morphology. mBio 5:e01117-14. doi: 10.1128/mBio.01117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Overend G, Luo Y, Henderson L, Douglas AE, Davies SA, Dow JA. 2016. Molecular mechanism and functional significance of acid generation in the Drosophila midgut. Sci Rep 6:27242. doi: 10.1038/srep27242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones RM, Luo L, Ardita CS, Richardson AN, Kwon YM, Mercante JW, Alam A, Gates CL, Wu H, Swanson PA, Lambeth JD, Denning PW, Neish AS. 2013. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. EMBO J 32:3017–3028. doi: 10.1038/emboj.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hughes GL, Ren X, Ramirez JL, Sakamoto JM, Bailey JA, Jedlicka AE, Rasgon JL. 2011. Wolbachia infections in Anopheles gambiae cells: transcriptomic characterization of a novel host-symbiont interaction. PLoS Pathog 7:e1001296. doi: 10.1371/journal.ppat.1001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kambris Z, Blagborough AM, Pinto SB, Blagrove MS, Godfray HC, Sinden RE, Sinkins SP. 2010. Wolbachia stimulates immune gene expression and inhibits Plasmodium development in Anopheles gambiae. PLoS Pathog 6:e1001143. doi: 10.1371/journal.ppat.1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Min KT, Benzer S. 1997. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc Natl Acad Sci U S A 94:10792–10796. doi: 10.1073/pnas.94.20.10792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brummel T, Ching A, Seroude L, Simon AF, Benzer S. 2004. Drosophila lifespan enhancement by exogenous bacteria. Proc Natl Acad Sci U S A 101:12974–12979. doi: 10.1073/pnas.0405207101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joseph RM, Devineni AV, King IF, Heberlein U. 2009. Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila. Proc Natl Acad Sci U S A 106:11352–11357. doi: 10.1073/pnas.0901419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharon G, Segal D, Ringo JM, Hefetz A, Zilber-Rosenberg I, Rosenberg E. 2010. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc Natl Acad Sci U S A 107:20051–20056. doi: 10.1073/pnas.1009906107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Storelli G, Defaye A, Erkosar B, Hols P, Royet J, Leulier F. 2011. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab 14:403–414. doi: 10.1016/j.cmet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 61.Shin SC, Kim SH, You H, Kim B, Kim AC, Lee KA, Yoon JH, Ryu JH, Lee WJ. 2011. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 334:670–674. doi: 10.1126/science.1212782. [DOI] [PubMed] [Google Scholar]
- 62.Ridley EV, Wong AC, Westmiller S, Douglas AE. 2012. Impact of the resident microbiota on the nutritional phenotype of Drosophila melanogaster. PLoS One 7:e36765. doi: 10.1371/journal.pone.0036765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mazmanian SK, Round JL, Kasper DL. 2008. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 64.Emmert EA, Handelsman J. 1999. Biocontrol of plant disease: a (gram-) positive perspective. FEMS Microbiol Lett 171:1–9. doi: 10.1111/j.1574-6968.1999.tb13405.x. [DOI] [PubMed] [Google Scholar]
- 65.Toomey ME, Panaram K, Fast EM, Beatty C, Frydman HM. 2013. Evolutionarily conserved Wolbachia-encoded factors control pattern of stem-cell niche tropism in Drosophila ovaries and favor infection. Proc Natl Acad Sci U S A 110:10788–10793. doi: 10.1073/pnas.1301524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chrostek E, Marialva MS, Esteves SS, Weinert LA, Martinez J, Jiggins FM, Teixeira L. 2013. Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: a phenotypic and phylogenomic analysis. PLoS Genet 9:e1003896. doi: 10.1371/journal.pgen.1003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riegler M, Iturbe-Ormaetxe I, Woolfit M, Miller WJ, O’Neill SL. 2012. Tandem repeat markers as novel diagnostic tools for high resolution fingerprinting of Wolbachia. BMC Microbiol 12(Suppl 1):S12. doi: 10.1186/1471-2180-12-S1-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O. 2008. The RAST server: rapid annotations using subsystems technology. BMC Genomics 9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, Edwards RA, Gerdes S, Parrello B, Shukla M, Vonstein V, Wattam AR, Xia F, Stevens R. 2014. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res 42:D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
VNTR analysis to determine the Wolbachia strain. Different Wolbachia strains have different numbers of repeats in some VNTRs, specifically, 105 and 141. The expected sizes in wMel are 1,347 bp for VNTR 105 and 1,330 bp for VNTR 141; in wMelCS, they are 1,241 bp for VNTR 105 and 1,189 bp for VNTR 141 (67). Lanes 1 and 2 in both gels are the Wolbachia-free and wMel-infected flies used in this study, respectively. Lanes 3 are flies infected with wMelCS (not used in this study) for comparison. Download FIG S1, TIF file, 0.2 MB (167.8KB, tif) .
Copyright © 2017 Simhadri et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Number of reads for each sample and effectiveness of Bstz17I digestion. Sequencing of the 16S rRNA gene PCR products of either BstZ17I-digested or undigested total genomic DNA of flies shows that the BstZ17I enzyme is capable of eliminating the amplification of the Wolbachia 16S rRNA gene. Download TABLE S1, DOCX file, 0.01 MB (12.3KB, docx) .
Copyright © 2017 Simhadri et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Primers used for amplification and sequencing. The different regions of the primers are Illumina adaptor (red), indexing barcode (black), primer pad and linker (green), and 16S rRNA gene annealing primer (blue) sequences. Download TABLE S2, DOCX file, 0.01 MB (12.2KB, docx) .
Copyright © 2017 Simhadri et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Percentages of (non-Wolbachia) bacterial taxa found in each of the samples by 16S rRNA gene sequencing. The two most abundant families of bacteria are Acetobacteraceae and Lactobacillaceae, which constitute more than 99.8% of the reads in any sample. Download TABLE S3, DOCX file, 0.01 MB (12.9KB, docx) .
Copyright © 2017 Simhadri et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Species-specific primers designed for qPCR. Primers were designed against the glmS gene of both species. The PCR results show that the primers are specific, having no cross-species targets. A. p is A. pasteurianus, and L. p is L. plantarum. Download FIG S2, TIF file, 0.1 MB (116.7KB, tif) .
Copyright © 2017 Simhadri et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Quantification of effects of Wolbachia bacteria on A. pasteurianus levels in flies during development. (A to C) Relative levels of A. pasteurianus and L. plantarum in wMel-infected flies and those in Wolbachia-free flies. (A) F1 unsexed L3 larvae. (B) F1 0- to 7-day-old male and female flies. (C) F1 7- to 14-day-old male and female flies. (A to C) A. pasteurianus was absent from two out of the three replicates of Wolbachia-infected vials tested, hence the absence of error bars in the A. pasteurianus bars. Bar graphs show mean values (n = 3 when the qPCR produced amplicons), and error bars show standard deviations. Download FIG S3, TIF file, 0.6 MB (603.1KB, tif) .
Copyright © 2017 Simhadri et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Bacterial levels in the food of upd>hPABP-Flag flies. Relative levels of each bacterial species per nanogram of DNA obtained from food, normalized to the levels in food inhabited by Wolbachia-free flies are shown. Bar graphs represent the mean values of three experiments, and error bars show standard deviations. P values were determined by Student t tests. Download FIG S4, TIF file, 0.2 MB (211.3KB, tif) .
Copyright © 2017 Simhadri et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The effect of Wolbachia infection on the microbiome is genotype dependent. A. pasteurianus levels in flies with a different genetic background are shown (white). (A) Proportions of individual L3 larvae with a different genetic background (white) that had no A. pasteurianus in the gut (n = 30, chi-square test; error bars show confidence intervals). (B, C) Box-and-whisker plots of levels of bacteria in L3 larvae (B) and 10-day adults (C) (n = 30, median values are shown next to the boxes, and P values obtained with the two-sided Wilcoxon rank sum test are reported). (D) Relative expression of Imd pathway signal transducer imd, transcription factor Rel, AMPs, Nox, and AMPs downstream of JAK/STAT signaling in both axenic and gnotobiotic L3 larval guts in the presence or absence of Wolbachia infection as determined by RT-qPCR. All conditions are normalized to Wolbachia-free gnotobiotic L3 larval guts. Bar graphs show mean values (three biological replicates of 10 larvae each), and error bars show standard deviations. Asterisks indicate statistically significant differences (P < 0.05, Student t test). Download FIG S5, TIF file, 0.8 MB (822.6KB, tif) .
Copyright © 2017 Simhadri et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Bacterial levels in the food of w1118 (white-eyed) flies. Levels of each bacterial species per nanogram of DNA obtained from the food, normalized to the levels in food inhabited by Wolbachia-free flies, are shown. Bar graphs represent the mean values of three experiments, and error bars show standard deviations. P values were determined by Student t tests. Download FIG S6, TIF file, 0.2 MB (201.9KB, tif) .
Copyright © 2017 Simhadri et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.