Abstract
Adequate maternal iodine consumption during pregnancy and lactation guarantees normal thyroid hormones (TH) production, which is crucial to the development of the fetus. Indeed, iodine deficiency is clearly related to maternal hypothyroidism and deleterious effects in the fetal development. Conversely, the effects of iodine excess (IE) consumption on maternal thyroid function are still controversial. Therefore, this study aimed to investigate the impact of IE exposure during pregnancy and lactation periods on maternal hypothalamus–pituitary–thyroid axis. IE-exposed dams presented reduced serum TH concentration and increased serum thyrotropin (TSH) levels. Moreover, maternal IE exposure increased the hypothalamic expression of Trh and the pituitary expression of Trhr, Dio2, Tsha and Tshb mRNA, while reduced the Gh mRNA content. Additionally, IE-exposed dams presented thyroid morphological alterations, increased thyroid oxidative stress and decreased expression of thyroid genes/proteins involved in TH synthesis, secretion and metabolism. Furthermore, Dio1 mRNA expression and D1 activity were reduced in the liver and the kidney of IE-treated animals. Finally, the mRNA expression of Slc5a5 and Slc26a4 were reduced in the mammary gland of IE-exposed rats. The latter results are in accordance with the reduction of prolactin expression and serum levels in IE-treated dams. In summary, our study indicates that the exposure to IE during pregnancy and lactation induces primary hypothyroidism in rat dams and impairs iodide transfer to the milk.
Keywords: iodine excess, pregnancy, lactation, hypothalamus–pituitary–thyroid axis, oxidative stress
Introduction
Iodine is essential for thyroxine (T4) and triiodothyronine (T3) synthesis, which are the only iodine-containing hormones in vertebrates and exert crucial effects on the regulation of development, metabolism and growth (1). Thyroid hormones (TH) are also important for fetal development and maturation of central nervous system (2).
TH production depends on a complex process that initiates with the absorption of iodide by the gastrointestinal tract (3). Thereafter, iodide is actively transported into thyrocytes through the activity of the sodium-iodide symporter (NIS) (4). In the lumen, iodide is oxidized to iodine and incorporated into the tyrosine residues of the thyroglobulin (TG) molecules by the activity of the thyroid peroxidase (TPO), which is also required for coupling iodotyrosines that generates TH within the TG molecules (5). TPO activity depends on the production of hydrogen peroxide by the activity of the thyroid dual oxidase (DUOX or ThOX) (6). TH production is mainly regulated by thyroid-stimulating hormone (TSH) action through its binding to the TSH receptor (TSHR) that is expressed in the basolateral membrane of thyrocytes (7). TH secretion is also stimulated by TSH, which stimulates the endocytosis of colloid and the activity of lysosomal enzymes that digest TG molecules and release T4 and T3. Thereafter, TH are secreted into the bloodstream through the monocarboxylate transporter 8 (MCT8) (8). In summary, TH synthesis and secretion depend on the expression and activity of several proteins, but iodide uptake is the first and limiting step of this process.
In agreement, the World Health Organization (WHO), which is responsible for monitoring the iodine status worldwide, suggests a daily consumption of 150 μg of iodine to guarantee an adequate TH production. Even so, iodine deficiency (ID) is still a public health problem in several countries and salt iodine supplementation represents the main global effort to prevent thyroid disorders associated with this issue (9). Conversely, some reports have shown that iodine excess (IE) consumption due to extensive environmental iodine exposure, such as elevated salt ingestion or exaggerated use of kelp supplements is also related to thyroid disorders (10, 11, 12).
It is worth noting that the daily requirement of iodine consumption increases to 200–250 µg during pregnancy and lactation, in order to guarantee normal maternal thyroid function (13). Indeed, adequate maternal TH production is essential to the initial steps of fetal development that depend on TH action, especially because the fetal thyroid gland is fully developed only in the second trimester of gestation. The increased requirement of iodine intake during pregnancy and lactation are mainly related to the increased kidney clearance of iodide, the augmented levels of T4-binding proteins, the stimulatory effects of chorionic gonadotropin on thyroid as well as the placental transfer of TH to the fetus (14, 15). Therefore, several physiological changes occur in the maternal hypothalamus–pituitary–thyroid axis to successfully achieve both maternal and fetal TH serum levels requirements (16).
ID is commonly associated with maternal hypothyroidism and several disorders in the fetal development (17, 18, 19). However, recent studies have reported that IE consumption during pregnancy and/or lactation also increases the maternal susceptibility to develop hypothyroidism, subclinical hypothyroidism or hypothyroxinemia (20, 21, 22). Taken together, these data suggest that ID and IE should be carefully monitored since both conditions significantly alter maternal thyroid function (23).
Even so, the molecular mechanisms involved in the impairment of maternal thyroid function by IE exposure have never been described before. Therefore, this study aimed to further characterize these mechanisms by evaluating the effects of maternal exposure to IE during pregnancy and lactation on TH production, secretion and peripheral metabolism using female Wistar rats.
Materials and methods
Ethical approval
The experimental protocol was approved by the Institute of Biomedical Sciences/University of São Paulo Ethical Committee for Animal Research (protocol number 155, page 156, 2012). The protocols are in accordance with the ethics principles in animal research adopted by the National Council for the Control of Animal Experimentation.
Animals and treatments
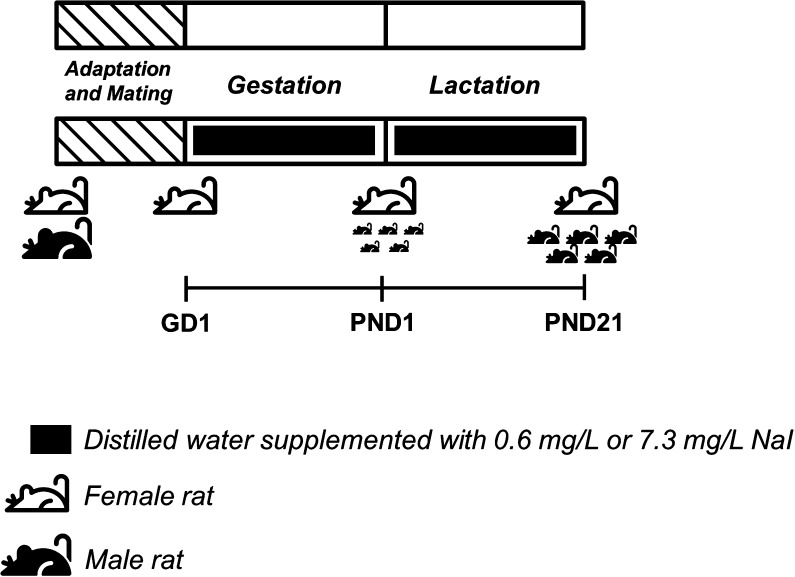
Virgin male and female Wistar rats were obtained from the Animal Breeding Centre at the Institute of Biomedical Sciences, University of Sao Paulo. The animals were maintained at constant temperature (23 ± 1°C), 12:12-h light-dark cycle schedule and on rat chow and water ad libitum. After an adaptation period, at 8 weeks of age, the female rats were mated with male rats (two female rats with one male rat per cage). The presence of spermatozoa in the vaginal smear was defined as first day of gestation (GD1). Then, the pregnant rats were isolated in separated cages and randomly divided into the following groups (15 animals per group):
Control (C): Dams supplied with distilled water during pregnancy and lactation periods.
Iodine 0.6 mg/L (5×): Dams supplied with distilled water supplemented with 0.6 mg/L NaI during pregnancy and lactation periods.
Iodine 7.3 mg/L (50×): Dams supplied with distilled water supplemented with 7.3 mg/L NaI during pregnancy and lactation periods (Fig. 1).
Figure 1.
Schematic representation of experimental protocol. Male and female Wistar rats were mated and after confirming the pregnancy through the presence of spermatozoa in the vaginal smear, the pregnant rats (GD1) were randomly treated or not with distillated water supplemented with 0.6 mg/L or 7.3 mg/L NaI during pregnancy and lactation periods. At post-natal day 21 (PND21) the rat dams were anesthetized and killed by decapitation and several tissues were collected for performing molecular and biochemical analysis. The rat icons used in this figure were designed by Freepik (www.freepik.com) from Flaticon (www.flaticon.com).
Iodine treatment doses were chosen based on the normal daily exposure of rats to iodine through rat chow (~200 µg/Kg) and on a previous study (24). Therefore, the iodine supplementation in the water has guaranteed an increment of 5 and 50 times the normal exposure of rats to iodine.
After the IE treatment during pregnancy and lactation, maternal urine was collected to determine the urinary concentration of iodine, as previously described (25). Thereafter, the rats were anesthetized and killed by decapitation (Fig. 1). Hypothalamus, pituitary, thyroid, kidney, liver and mammary gland were rapidly excised for total RNA and/or protein extraction, as described below. Thyroid gland lobes were also prepared for histological analysis. Blood samples were collected to evaluate TSH, T4, T3 and prolactin serum levels. Hearts were excised to determine the wet (WHW) and dry heart weight (DHW) as well as the ratio between DHW and body weight (DHW/BW).
Real-time PCR analysis
Hypothalamus, pituitary, thyroid, kidney, liver and mammary gland total RNA extraction was performed using TRIzol, following the manufacturer's recommendations (Life Technologies). The expression of thyrotropin-releasing hormone (Trh), Trh receptor (Trhr), type 2 iodothyronine deiodinase (Dio2), alpha subunit of thyrotropin (Tsha), beta subunit of thyrotropin (Tshb), thyrotropin receptor (Tshr), sodium-iodide symporter (Slc5a5), thyroid peroxidase (Tpo), thyroglobulin (Tg), type 1 iodothyronine deiodinase (Dio1), dual oxidase (Duox), monocarboxilate transporter 8 (Mct8), Megalin, Prolactin and pendrin (Slc26a4) expression were evaluated by real-time PCR by using Platinum SYBR Green qPCR Super Mix-UDG, according to the manufacturer’s instructions (Invitrogen). Gene expression alterations were evaluated by the 2−ΔΔCt method using Rpl19 as a housekeeping gene. Primer sequences are described in Table 1.
Table 1.
Primers used for gene expression analysis through Real-Time PCR.
| Forward | Reverse | |
|---|---|---|
| Trh | CGGTGCTGCCTTAGACTCCTGGA | GCCGGGGTGCTGTCGTTTGT |
| Tshb | GGCAAACTGTTTCTTCCCAA | GTTGGTTTTGACAGCCTCGT |
| Tsha | CACTCTGGCATTTCCCATTA | GCCAGGTCCAAGAAGACAAT |
| Trhr | TGGCCACTGTGCTTTACGGG | CAACCACTGCAAGCATCTTGG |
| Dio2 | GGACCGATGTGCTGCAGCCC | GGCGTGAGCTTCTTCAATGTA |
| Gh | TCAAGAAGGACCTGCACAAG | GTGGCAGTTGCCAGAGTACA |
| Prolactin | GCCAAAATGTGCAGACCCTG | AGTCATTGATGGCCTTGGCA |
| Slc5a5 | AGCCTCGCTCAGAACCATTC | GTGTACCGGCTCCGAGGAT |
| Tshr | GGCTGCTGGCTGCTTCTTTT | TCAGACGCATGATCAAAATGAAA |
| Tg | CTCAGGACGATGGGCTTATCA | GTTCGGCCTTGGCTTTCTTC |
| Tpo | ACAGTTCTCCACGGATGCACTA | GGCAAGCATCCTGACAGGTT |
| Mct8 | AGCCTGCGCTACTTCACCTA | GGCCAGCTTGATTCTGTCTC |
| Megalin | CATGGACATCGGTGTGTCTC | GGCCACTTTGGAAGTGTTGT |
| Duox | TGCTCTCAACCCCAAAGTG | TCTCAAACCAGTAGCGATCAC |
| Dio1 | ATTTGACCAGTTCAAGAGACTCG | GGCGTGAGCTTCTTCAATGTA |
| Slc26a4 | TCCTCTTGAACTGATGGAAGCA | CCAGGTTCTGCCTAGCAGTC |
| Rpl19 | CCAATGAAACCAACGAAATCG | TCAGGCCATCTTTGATCAGCTT |
Protein expression
Total protein extraction of thyroid and pituitary as well as Western blotting analysis were performed as previously described (25). Briefly, membranes were blocked with 3% BSA solution and incubated with specific primary antibodies against TSHA, TSHB, TSHR, NIS, TPO, TG, PAX8 and NKX2.1. Equal loading was evaluated by incubating the membranes with a primary antibody against GAPDH. The brands and the concentration of the primary antibodies are described in Table 2. Blots were developed using the enhanced chemiluminescence (ECL) kit (Amersham Biosciences). Blot densitometry was analyzed by using the ImageJ software (National Institutes of Health).
Table 2.
Primary antibodies list.
| Peptide/Protein target | Antibody name | Manufacter and catalog | Species raised in; monoclonal or polyclonal | Dilution |
|---|---|---|---|---|
| TSHB | NIDDK-anti-rat bTSH-IC-1 | NHPP reagents | Rabbit; polyclonal | 1 3000 |
| TSHA | Anti-rat glycoprotein hormone alpha subunit | NHPP reagents | Rabbit; polyclonal | 1 3000 |
| NIS | Anti-rNIS | Dr Nancy Carrasco | Rabbit; polyclonal | 1 3000 |
| TPO | Anti-Thyroperoxidase (MoAb47) | Santa Cruz (sc-58432) | Mouse; monoclonal | 1 1000 |
| TSHR | Anti-TSHR antibody | Sigma Aldrich (SAB2102588) | Rabbit; polyclonal | 1 1000 |
| TG | Anti-Thyroglobulin antibody | Abcam (ab80783) | Mouse; monoclonal | 1 5000 |
| PAX8 | Anti-PAX8 antibody | Santa Cruz (sc-81353) | Mouse; monoclonal | 1 500 |
| NKX2.1 | Anti-NKX2.1 antibody | Santa Cruz (sc-13040) | Rabbit; polyclonal | 1 5000 |
| GAPDH | Anti-GAPDH antibody | Santa Cruz (sc-32233) | Mouse; monoclonal | 1 1000 |
Thyroid histological analysis
Thyroid histology was performed as previously described (25). Thyroid sections were stained with hematoxylin-eosin and examined by using a Nikon Eclipse E600 microscope and a digital camera (Roper Scientific, Trenton, NJ, USA).
Protein carbonylation analysis
Thyroid protein carbonylation status was analyzed by using the OxyBlot Protein Oxidation Detection Kit (EMD Millipore Headquarters), as previously described (26). Briefly, the proteins were extracted with a specific buffer. Western blotting analysis was performed and membranes were incubated with a specific primary antibody to detect the presence of carbonyl groups. Thereafter, the membranes were incubated with a peroxidase-conjugated secondary antibody and the blots were developed using the ECL kit (Amersham Biosciences). Blot densitometry was analyzed by using the ImageJ software (National Institutes of Health). Equal loading was analyzed by staining the membranes with Ponceau S solution. Results are presented as percentage in comparison to the control group.
D1 activity assay
Liver and kidney D1 activity were determined as described before (27). Briefly, liver and kidney were homogenized and incubated with 1 mM rT3 (Sigma Chemical Co), purified tracer [125I]rT3 (Perkin–Elmer Life Sciences) and 10 mM dithiothreitol in 100 mM potassium phosphate buffer. The reactions were stopped at 4°C-bath and by adding fetal bovine serum and trichloroacetic acid (50%, v/v) to the samples. The liberated 125I− was measured in a gamma counter and D1 activity was expressed as picomols rT3/min/mg.
Determination of TSH, T3, T4and prolactin serum levels
TSH, T4, and T3 rat serum concentrations were determined by the Milliplex Luminex kit #RTHY-30K (EMD Millipore Headquarters). Prolactin serum levels were assessed by the Milliplex Luminex kit #RPTMAG-86K (EMD Millipore Headquarters).
Statistical analysis
All data are reported as means ± s.e.m. Statistical analysis was performed using the GraphPad Prism Software. Data were subjected to unpaired one-way ANOVA followed by Student–Newman–Keuls post hoc test. Differences were considered statistically significant at P < 0.05.
Results
Evaluation of IE treatment effectiveness
The effectiveness of rat dams’ treatment with both doses of IE throughout pregnancy and lactation was confirmed by the increased concentration of iodine in the urine of these animals in comparison to the control group. As expected, the urinary iodine concentration of the rats exposed to the 50× dose of treatment was significantly higher than the one detected in the rats exposed to the lowest dose of treatment (5×) (Table 3).
Table 3.
Urinary iodine concentration, body weight, thyroid lobe weight, wet heart weight, dry heart weight and dry heart to body weight ratio of rat dams exposed to high iodine concentration during pregnancy and lactation periods.
| Groups | UIC (µg/dL) | BW (g) | TLW (mg) | WHW (g) | DHW (g) | DHW/BWa |
|---|---|---|---|---|---|---|
| Control | 42.0 ± 1.9 | 309.1±7.0 | 20.02±0.5 | 0.98±0.03 | 0.25±0.01 | 0.79±0.01 |
| 5× | 160.0 ± 7.9*** | 299.1± 8.8 | 19.9±0.7 | 0.84±0.03* | 0.20±0.01** | 0.67±0.01*** |
| 50× | 510.0 ± 20.6***/••• | 305.1±9.7 | 20.21±0.9 | 0.87±0.03* | 0.21±0.01** | 0.68±0.01*** |
Values multiplied by 1000. Results are expressed by means ± s.e.m., n = 10 per group. **P < 0.01; ***P < 0.001 vs Control. •••P < 0.001 vs 5×.
UIC, urinary iodine concentration; BW, body weight; TLW, thyroid lobe weight; WHW, wet heart weight; DHW, dry heart weight; DHW/BW, dry heart weight to body weight ratio.
Effects of maternal IE exposure on body, thyroid and heart weights
As presented in Table 3, maternal IE exposure during pregnancy and lactation has not altered body and thyroid lobe weight in comparison to the control animals. Conversely, wet heart weight (WHW), dry heart weight (DHW) and the ratio between dry heart weight and body weight (DHW/BW) were significantly reduced in the rat dams exposed to both doses of IE treatment.
Effects of maternal IE exposure on TH and TSH serum levels
The exposure of rat dams during pregnancy and lactation to both doses of IE significantly reduced serum T3 and T4 concentrations in comparison to the control group. In accordance, serum TSH levels were significantly increased in the rat dams exposed to both iodine studied doses (Table 4).
Table 4.
Serum T3, T4 and TSH concentrations of rat dams exposed to high iodine concentration during pregnancy and lactation periods.
| Groups | Total serum T3 concentration (ng/dL) | Total serum T4concentration (μg/dL) | Serum TSH concentration (ng/mL) |
|---|---|---|---|
| Control | 27.0 ± 2.3 | 6.72 ± 0.18 | 0.75 ± 0.09 |
| 5× | 16.8 ± 1.3** | 5.87 ± 0.19* | 2.0 ± 0.4** |
| 50× | 15.1 ± 1.6* | 5.51 ± 0.20** | 1.9 ± 0.2*** |
Results are expressed by means ± s.e.m., n = 6 per group. *P < 0.05, **P < 0.01, ***P < 0.01 vs Control.
Effects of maternal IE exposure on hypothalamus and pituitary gene expression
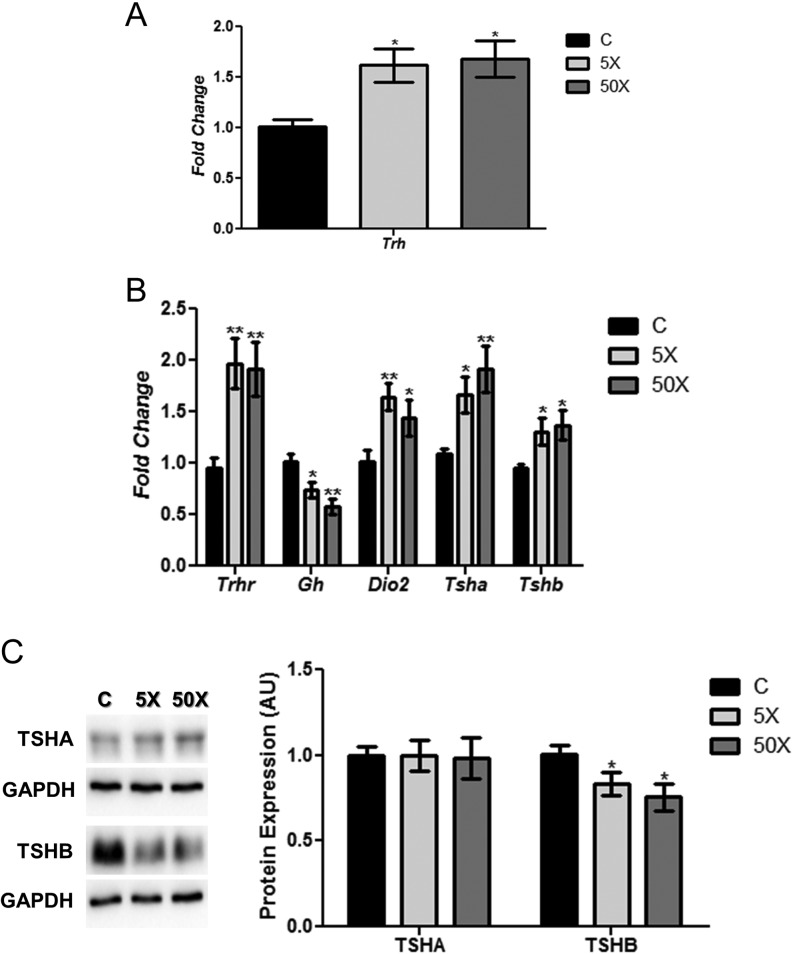
Rat dams exposed to IE treatment presented a significant increase in the hypothalamic expression of Trh mRNA (Fig. 2A). Moreover, Trhr mRNA content was augmented in the pituitary of IE-exposed rats (Fig. 2B). Interestingly, maternal pituitary Gh mRNA expression was decreased, whereas Dio2 mRNA content was increased by both doses of IE treatment (Fig. 2B). The mRNA content of both subunits of TSH were also increased in the pituitary of rat dams exposed to IE treatment (Fig. 2B). In contrast, no significant alterations were observed in the TSHA protein content (Fig. 2C). Even so, IE significantly reduced the protein content of the beta subunit of TSH in the pituitary of the exposed dams in comparison to the control animals (Fig. 2C).
Figure 2.
Maternal IE exposure alters gene expression in the hypothalamus and the pituitary. Rat dams received distilled water (Control Group; C); distilled water supplemented with 0.6 mg/L (iodine excess group; 5×) or distilled water supplemented with 7.3 mg/L (iodine excess group; 50×) throughout pregnancy and lactation. Thereafter, hypothalamic Trh mRNA content (A) and pituitary Trhr, Gh, Dio2, Tsha and Tshb mRNA contents (B) were analyzed by Real-Time PCR and normalized to Rpl19 mRNA content. Total pituitary TSHA and TSHB protein content (C) were analyzed through Western blotting analysis, using GAPDH as loading control. Results are expressed as means ± s.e.m. as fold change or arbitrary units (AU) (n = 10–12/group). *P < 0.05, **P < 0.01 vs C (ANOVA, Student–Newman–Keuls).
Effects of maternal exposure to IE treatment on thyroid morphology
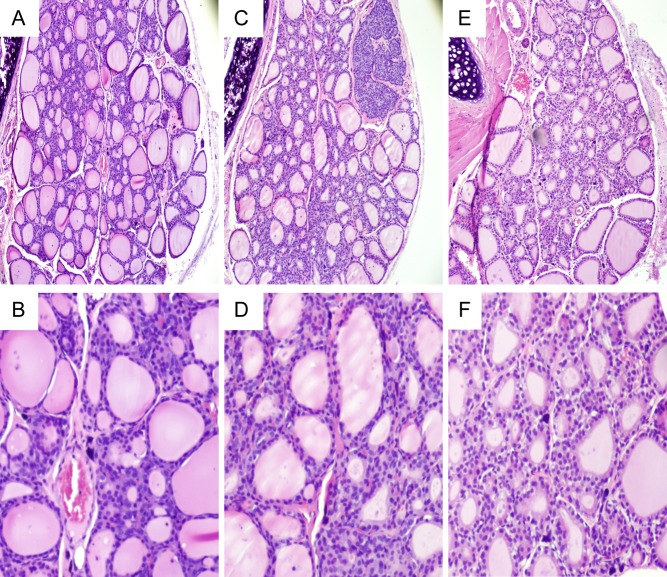
The macroscopic appearance of thyroid lobes was not significantly changed by IE exposure during pregnancy and lactation (data not shown). Even so, the histological analysis has shown some differences among the groups (Fig. 3). Control and 5×-IE-treated animals presented follicles with various size and shape (Fig. 3A, B, C and D). Conversely, rat dams exposed to the highest dose of IE treatment (50×) presented thyroid follicles with slightly decreased diameter filled with lower content of TG (Fig. 3E and F). No infiltration of immune cells was observed in any of the studied groups.
Figure 3.
Effects of maternal IE exposure on thyroid morphology. Light microscope view of the thyroid follicles of rat dams that received distilled water (Control Group; C) (A and B); distilled water supplemented with 0.6 mg/L (Iodine excess group; 5×) (C and D) or distilled water supplemented with 7.3 mg/L (Iodine excess group; 50×) (E and F) throughout pregnancy and lactation (n = 4/group). The highest dose of IE treatment slightly decreased the diameter of thyroid follicles and the TG content in the lumen in comparison to animals of control and 5× groups. Lymphocytic infiltration was not observed. Hematoxylin and eosin. Magnification 100× (A, C and E) and digital zoom (B, D and F).
Effects of maternal exposure to IE treatment on thyroid gene expression
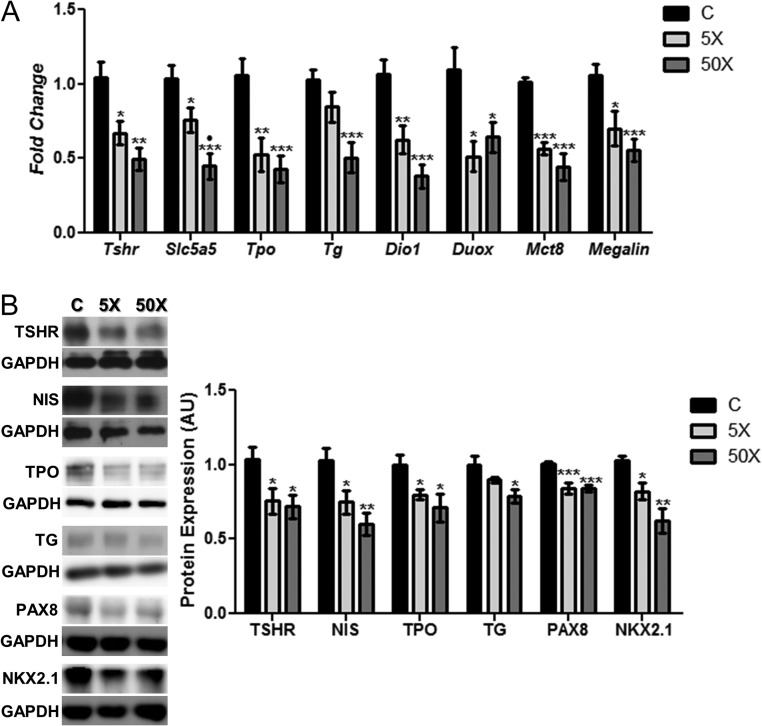
As demonstrated in Fig. 4, the expression of genes and/or proteins involved in the TH synthesis, secretion and metabolism were significantly altered in the thyroid of rat dams exposed to IE treatment during pregnancy and lactation. Indeed, the Tshr, Slc5a5, Tpo and Tg mRNA expression (Fig. 4A) and protein content (Fig. 4B) were reduced in the thyroid of IE-treated rats. Moreover, Dio1, Duox, Mct8 and Megalin mRNA expression were also reduced by both doses of IE treatment (Fig. 4A). In accordance, the protein expression of PAX8 and NKX2.1 were significantly reduced in the thyroid of rat dams exposed to IE in comparison to the control group (Fig. 4B).
Figure 4.
Maternal IE exposure reduces thyroid gene and protein expression. (A) Rat dams received distilled water (control group; C); distilled water supplemented with 0.6 mg/L (iodine excess group; 5×) or distilled water supplemented with 7.3 mg/L (iodine excess group; 50×) throughout pregnancy and lactation. Thyroid Tshr, Slc5a5, Tpo, Tg, Dio1, Duox, Mct8 and Megalin mRNA content was analyzed by real-time PCR and normalized to Rpl19 mRNA content. (B) Total thyroid TSHR, NIS, TPO, TG, PAX8 and NKX2.1 protein content was analyzed through Western blotting analysis, using GAPDH as loading control. Results are expressed as means ± s.e.m. as fold change or arbitrary units (AU) (n = 10–12/group). *P < 0.05, **P < 0.01; ***P < 0.001 vs C; •P < 0.05 vs 5× (ANOVA, Student–Newman–Keuls).
Effects of maternal IE exposure on thyroid oxidative stress
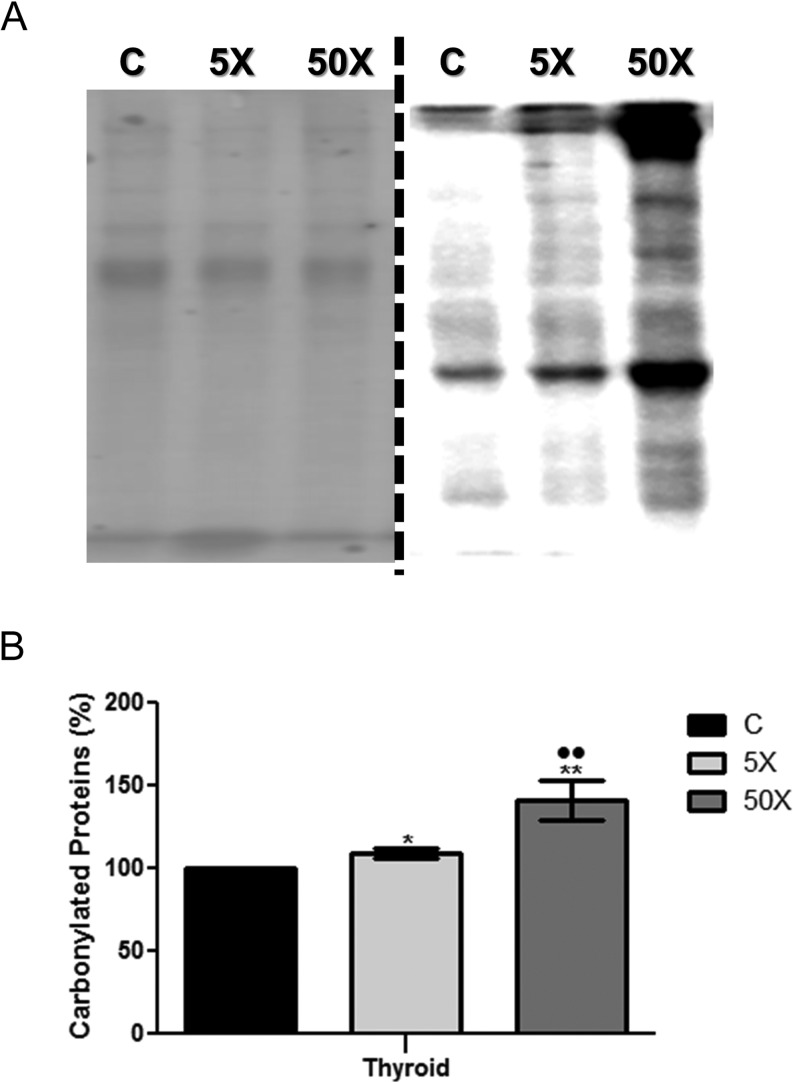
Both doses of IE treatment (5× and 50×) augmented the oxidative stress in the thyroid gland of exposed rat dams. Indeed, as shown in Fig. 5, there was a significant increase in the content of carbonylated proteins in the thyroid of IE-treated rat dams in comparison to the control animals. Moreover, the thyroid of the 50×-IE-exposed rats presented higher amounts of carbonylated proteins in comparison to the 5×-IE-exposed animals.
Figure 5.
Maternal IE exposure increases thyroid oxidative stress. Rat dams received distilled water (Control Group; C); distilled water supplemented with 0.6 mg/L (Iodine excess group; 5×) or distilled water supplemented with 7.3 mg/L (Iodine excess group; 50×) throughout pregnancy and lactation. Protein carbonylation measurement was performed through Western blotting analysis, using Ponceau staining as loading control. (A) Left panel: Ponceau S staining of the nitrocellulose membrane. Right panel: Immunoblots obtained after the incubation with primary and secondary specific antibodies to detect carbonylated proteins. (B) The bar graph represents the quantification of blot densitometry analysis normalized to Ponceau S staining. Results are expressed as means ± s.e.m. in percentage (%) (n = 6 per group). *P < 0.05, **P < 0.01 vs C; ••P < 0.01 vs 5× (ANOVA, Student–Newman–Keuls).
Effects of maternal IE exposure on Dio1 expression and D1 activity
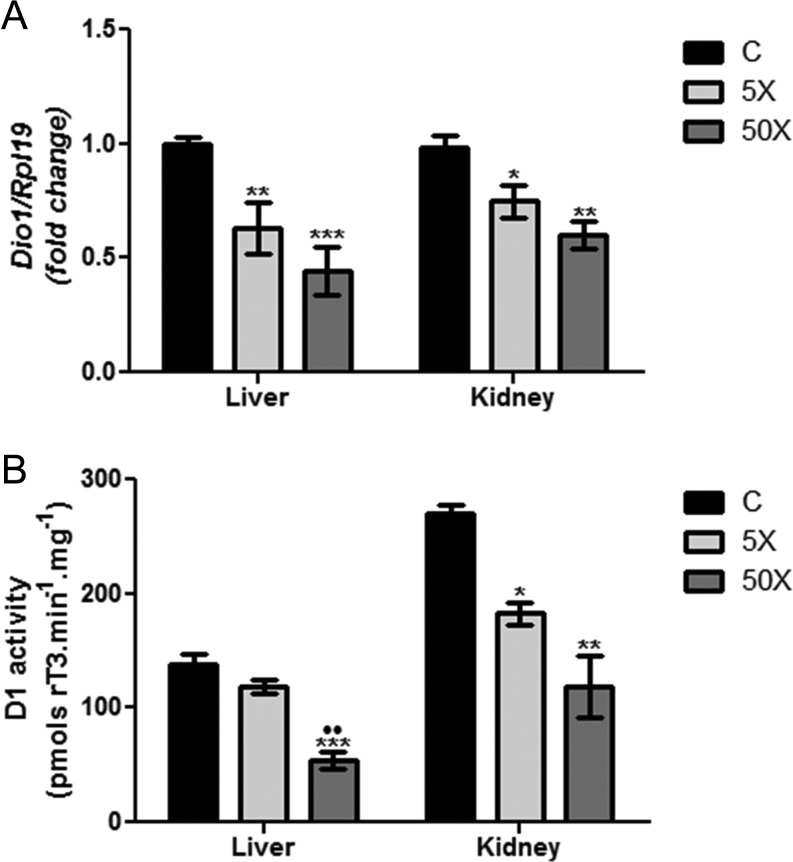
Dio1 mRNA expression and D1 activity were evaluated both in the liver and the kidney of control and IE-exposed rat dams. As demonstrated in Fig. 6, both doses of IE treatment (5× and 50×) significantly reduced the mRNA expression of Dio1 in the liver (Fig. 6A), whereas only the highest IE dose (50×) was able to reduce liver D1 activity in comparison to the other groups (Fig. 6B). In contrast, both studied IE doses significantly reduced Dio1 mRNA content and D1 activity in the kidney of exposed rat dams in comparison to the control group (Fig. 6A and B).
Figure 6.
Maternal IE exposure reduces liver and kidney Dio1 mRNA expression and D1 activity. Rat dams received distilled water (Control Group; C); distilled water supplemented with 0.6 mg/L (Iodine excess group; 5×) or distilled water supplemented with 7.3 mg/L (Iodine excess group; 50×) throughout pregnancy and lactation. (A) Dio1 mRNA expression was analyzed by Real-Time PCR in the liver and kidney by Real-Time PCR and normalized to Rpl19 mRNA content (n = 8/group). Results are expressed as means ± s.e.m. as fold change. (B) D1 activity was evaluated in the microsomes from liver and kidney of control and IE-exposed rat dams (n = 3/group). Results are expressed as means ± s.e.m. as picomols rT3/min/mg. *P < 0.05, **P < 0.01; ***P < 0.01 vs C; ••P < 0.01 vs 5× (ANOVA, Student–Newman–Keuls).
Effects of maternal IE exposure on mammary gland gene expression and pituitary prolactin expression
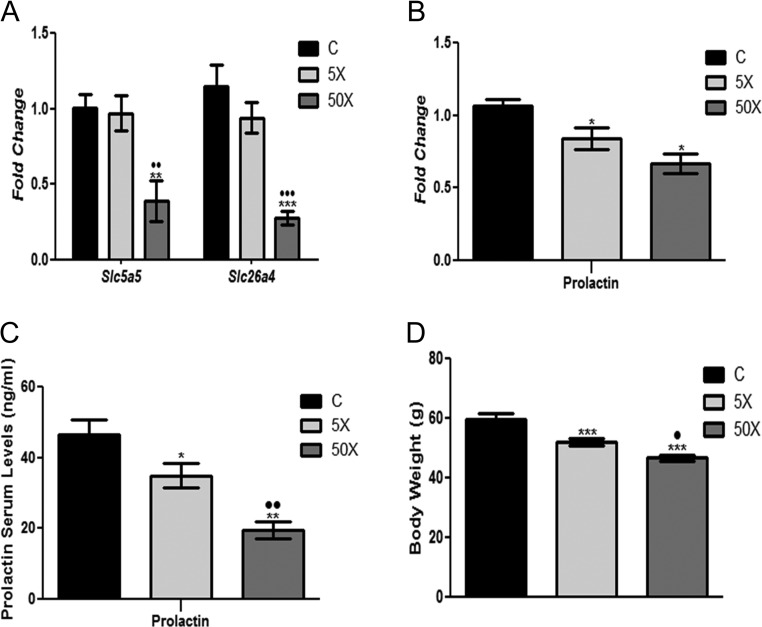
The dams exposed to the 50×-IE dose presented a significant reduction of the mammary gland Slc5a5 and Slc26a4 mRNA content in comparison to the control and 5×-IE-exposed animals (Fig. 7A). Interestingly, rat dams exposed to both doses of IE treatment presented a significant reduction of prolactin mRNA expression in the pituitary and reduced prolactin serum levels in comparison to the control group (Fig. 7B and C). It is worth noting that there was a dose-dependent effect in the regulation of prolactin serum levels. Indeed, rat dams exposed to the 50×-IE dose presented a greater reduction of prolactin in the serum than those animals exposed to the lowest dose of treatment (5×). Finally, the pups of the rat dams exposed to IE presented lower body weight at the end of weaning in comparison to the pups of the control animals (Fig. 7D). Additionally, there was a dose–response relationship in this parameter, since the highest dose of treatment (50×) induced a higher reduction of body weight in comparison to the 5×-IE dose.
Figure 7.
Maternal IE exposure diminishes NIS and Pendrin expression in the mammary gland, prolactin content in the pituitary/serum and body weight of the offspring at weaning. Rat dams received distilled water (Control Group; C); distilled water supplemented with 0.6 mg/L (Iodine excess group; 5×) or distilled water supplemented with 7.3 mg/L (Iodine excess group; 50×) throughout pregnancy and lactation. Slc5a5 and Slc26a4 mRNA expression in the mammary gland (A) and prolactin mRNA content in the pituitary (B) were analyzed by Real-Time PCR and normalized to Rpl19 mRNA content (n = 8/group). Results are expressed as means ± s.e.m. as fold change. (C) Prolactin serum levels were measured through Multiplex assays and results are expressed as means ± s.e.m. in nanograms per milliliter (ng/mL). (D) Body weight of the pups at weaning. Results are expressed as means ± s.e.m. in grams (g). *P < 0.05, **P < 0.01; ***P < 0.01 vs C; •P < 0.05; ••P < 0.01; •••P < 0.01 vs 5×(ANOVA, Student–Newman–Keuls).
Discussion
The present study indicates that the exposure of rat dams to IE during pregnancy and lactation induces maternal primary hypothyroidism by the impairment of TH production, secretion and peripheral metabolism.
The data presented herein clearly indicated that IE exposure during pregnancy and lactation reduces TH serum levels in rat dams. This result is coherent with previous data that demonstrated the impairment of thyroid function by IE in rats, mice and humans exposed to high doses of iodine (25, 28, 29). Even so, it is important to stress that the periods of exposure to IE in these studies differ from those investigated in the present study. In agreement, recent and large epidemiological studies have demonstrated that IE exposure during early pregnancy or during lactation are associated with a significant higher risk for developing maternal subclinical hypothyroidism and hypothyroxinemia (20, 22). Nevertheless, by using an animal experimental model, our study was the first to elucidate the molecular mechanisms associated with maternal thyroid dysfunction induced by IE exposure during pregnancy and lactation.
The reduction of maternal TH serum levels is alarming, especially because gestational hypothyroidism has been previously associated with anemia, preeclampsia, preterm births, intrauterine growth restriction, miscarriage, congenital hypothyroidism and cognitive deficits in children (30, 31). Interestingly, TH are detected in embryonic fetal tissues even before the full development of fetal thyroid gland, indicating the important role of maternal TH transfer to the fetal development (14, 32). Therefore, the reduced maternal serum TH levels observed in our study could also be associated with impaired placental transfer of TH to the fetus and contribute to the neurodevelopment impairment of the offspring of IE-exposed rat dams, as previously described (33).
The decreased serum TH levels were consistent with the observed reduction of the WHW, DHW and DHW/BW ratio, which suggest a maternal hypothyroid condition. Indeed, hypothyroidism is commonly associated with the reduction of heart weight in animal models, since this organ is a classical target of TH action (34, 35).
Besides that, IE-exposed rat dams presented higher serum TSH levels, which is an additional evidence of maternal hypothyroid condition. This result could be related to at least three different effects: increased secretion and action of TRH, reduced effect of TH on the negative regulation of TSH expression and/or a direct effect of IE in the pituitary.
The first hypothesis is strengthened by the increased hypothalamic expression of Trh mRNA and pituitary expression of Trhr mRNA in IE-exposed rat dams. These results strongly suggest an increased action of TRH in the pituitary of these animals (36). Moreover, since TH downregulate the expression of Trh and Trhr mRNA (37), our data also suggest a reduced effect of TH in the negative feedback loop in the hypothalamus–pituitary axis of IE-exposed rats. Additionally, TH also reduce the expression and secretion of TSH by thyrotropes (38, 39). This effect was confirmed by the increased mRNA expression of both subunits of TSH in the pituitary of IE-treated dams. However, despite the increased Tshb mRNA content, there was a significant reduction of the TSHB protein content in these animals. This result could be justified by the increased secretion rate of TSH in IE-exposed dams, as confirmed by the increased serum TSH levels in IE-treated animals. The reduced action of TH in the pituitary of IE-exposed dams was also evidenced by the reduction of Gh mRNA content and the increased expression of Dio2 mRNA; genes that are known to be regulated by TH levels (40, 41).
Taken together, our data indicate the integrity of the hypothalamus–pituitary–thyroid axis in the IE-treated rat dams, since the reduction of TH levels increased the TSH serum levels in these animals. It is worth noting that besides the effects of TH in the hypothalamus and pituitary, a direct role of iodine in the regulation of gene expression and/or deiodinases activity in these organs cannot be ruled out.
Interestingly, IE-treated rat dams presented significant alterations on thyroid morphology and in the thyroid gene/protein expression in comparison to the control animals. Indeed, the 50×-IE dose of treatment has decreased the thyroid follicles diameter as well as reduced the content of TG within the lumen of the follicles. These results concur with the reduction on Tg mRNA expression and TG content only observed in rat dams exposed to the highest dose of IE treatment. Furthermore, maternal IE exposure also downregulated the expression other genes and/or proteins involved in TH synthesis (TSHR, NIS, TPO), TH secretion (Megalin and MCT8) (8, 42) and TH metabolism (D1) (43). These results are in accordance with previous reports that demonstrated the impairment of thyroid gene expression by IE treatment (25, 29, 44). Even so, this was the first study that associated maternal IE exposure with thyroid gene/protein expression repression. The decreased content of PAX8 and NKX2.1 in IE-exposed rat dams could be involved in the reduction of thyroid gene expression as the role of these transcription factors in the maintenance of thyroid differentiation is fairly well known (45). In addition, a previous in vitro study has shown that IE treatment diminishes the binding of thyroid transcription factors to the promoter of thyroid genes (44). Therefore, the inhibitory effects of IE on thyroid gene expression could be mediated by iodide per se. However, the impairment of TSH signaling pathway by reduced TSHR expression or by IE-induced iodolipids production cannot be excluded (44, 46).
Additionally, the data presented herein indicated that the exposure of rat dams to IE increased the thyroid content of carbonylated proteins, which are directly associated with tissue oxidative stress (47). In this context, it has been previously reported that hypothyroid condition increases the amount of carbonylated proteins in the testis of male rats (48). Furthermore, previous studies have associated IE with increased thyroid production of reactive oxygen species (ROS) (44, 49, 50). Therefore, increased protein carbonylation in the maternal thyroid gland could support the hypothyroid condition and/or the increased production of ROS induced by IE exposure during pregnancy and lactation. Importantly, the increased oxidative stress in the thyroid of IE-treated dams might also be involved in the reduction of thyroid gene expression. This hypothesis is strengthened by the inhibitory effect of oxidative stress on the binding of transcription factors to the promoters of thyroid differentiation genes (44, 51).
IE treatment has also significantly decreased the peripheral metabolism of TH, since we observed reduced expression/activity of D1 in the liver and kidney of IE-treated dams. D1 is essential for the conversion of T4 to T3, and it is transcriptionally upregulated by TH (43, 52). Therefore, the diminished Dio1 mRNA expression, and consequently, the reduction of D1 activity, could be related to the reduction of serum TH levels in IE-exposed rat dams. Nevertheless, the role of iodide per se on the regulation of D1 expression/activity cannot be ruled out, since previous reports have suggested a direct regulation of deiodinases by this trace element (24, 53).
Our data suggest that the exposure to IE also impairs the maternal transfer of iodide to the milk, at least in the highest dose of treatment. This hypothesis is reinforced by the significant reduction of Slc5a5 and Slc26a4 mRNA expression that was observed in the mammary gland of rat dams exposed to the 50× dose of IE treatment. It is worth noting that the reduction of NIS and pendrin in the mammary gland of IE-exposed animals could represent an adaptive response to protect the offspring from the deleterious effects of IE. Indeed, NIS and pendrin are involved in the transport of iodide to the milk and their expression are upregulated by prolactin (54, 55, 56, 57). Therefore, the reduced expression of these transporters in IE-exposed dams might be related to the decreased expression of prolactin in the pituitary as well as to the reduction of serum prolactin levels, as observed herein. The reduction of prolactin in the IE-exposed animals may also be involved in the reduction of the body weight of the pups at weaning, since this hormone is essential for milk production (58).
The absence of a dose-dependent response in several parameters of this study suggests the existence of adaptive responses that protect the rat dams from the deleterious effects of IE exposure. Indeed, previous studies have shown that iodide controls its own absorption through the downregulation of the NIS in the intestine (3). In addition, we cannot rule out the participation of other mechanisms that protect the mother from the exposure to extremely high doses of iodine (e.g. increased renal clearance of iodine) (15). Even so, some IE-induced alterations were more prominent in the highest dose of treatment (50×), e.g. thyroid protein carbonylation, D1 activity, NIS and pendrin expression in the mammary gland, serum concentration of prolactin, body weight of the pups at weaning. These results suggest that different tissues may have different mechanisms of protection against IE exposure.
The doses of iodine treatment used herein might be a limitation of this study, since they are higher than those commonly used for human supplementation. Even so, these doses were chosen based on the daily iodine intake by rats as well as in a previous report that tried to mimic the human exposure to IE by using an animal experimental model (24). Furthermore, another study has shown that some prenatal multivitamins have higher concentrations of iodine than those described on the label, potentiating the deleterious effects of IE exposure in the thyroid function of pregnant/lactating women (59). Finally, although we agree that it is difficult to extrapolate animal data to human situation, this experimental model is usually used to clarify several molecular aspects of human physiology and pathology.
In summary, the data presented herein elucidated the molecular mechanisms involved in the alterations of the hypothalamus–pituitary–thyroid axis in rat dams exposed to IE during pregnancy and lactation. It is important to mention that our data do not refute the importance of maternal iodine supplementation during pregnancy/lactation in iodine-deficient areas. In conclusion, our results demonstrated that IE exposure impairs rat dams’ thyroid function during important periods of development.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) 2012/24391-6 (CSN) and 2013/05629-4 (MTN).
Acknowledgement
The authors are grateful to Leonice Lourenço Poyares for excellent technical support. The authors would like to thank Freepik from Flaticon (www.flaticon.com) for designing the rat icons that were used in Fig. 1.
References
- 1.Carvalho DP, Dupuy C. Thyroid hormone biosynthesis and release. Molecular and Cellular Endocrinology 2017. [in press]. ( 10.1016/j.mce.2017.01.038) [DOI] [PubMed] [Google Scholar]
- 2.Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. Journal of Neuroendocrinology 2004. 16 809–818. ( 10.1111/j.1365-2826.2004.01243.x) [DOI] [PubMed] [Google Scholar]
- 3.Nicola JP, Basquin C, Portulano C, Reyna-Neyra A, Paroder M, Carrasco N. The Na+/I− symporter mediates active iodide uptake in the intestine. American Journal of Physiology – Cell Physiology 2009. 296 C654–C662. ( 10.1152/ajpcell.00509.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature 1996. 379 458–460. ( 10.1038/379458a0) [DOI] [PubMed] [Google Scholar]
- 5.Deme D, Pommier J, Nunez J. Specificity of thyroid hormone synthesis. The role of thyroid peroxidase. Biochimica et Biophysica Acta 1978. 540 73–82. ( 10.1016/0304-4165(78)90436-1) [DOI] [PubMed] [Google Scholar]
- 6.Dupuy C, Pomerance M, Ohayon R, Noël-Hudson MS, Dème D, Chaaraoui M, Francon J, Virion A. Thyroid oxidase (THOX2) gene expression in the rat thyroid cell line FRTL-5. Biochemical and Biophysical Research Communications 2000. 277 287–292. ( 10.1006/bbrc.2000.3671) [DOI] [PubMed] [Google Scholar]
- 7.Vassart G, Dumont JE. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocrine Reviews 1992. 13 596–611. ( 10.1210/edrv-13-3-596) [DOI] [PubMed] [Google Scholar]
- 8.Di Cosmo C, Liao XH, Dumitrescu AM, Philp NJ, Weiss RE, Refetoff S. Mice deficient in MCT8 reveal a mechanism regulating thyroid hormone secretion. Journal of Clinical Investigation 2010. 120 3377–3388. ( 10.1172/JCI42113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmermann MB, Andersson M. Update on iodine status worldwide. Current Opinion in Endocrinology, Diabetes and Obesity 2012. 19 382–387. ( 10.1097/MED.0b013e328357271a) [DOI] [PubMed] [Google Scholar]
- 10.Nepal AK, Suwal R, Gautam S, Shah GS, Baral N, Andersson M, Zimmermann MB. Subclinical hypothyroidism and elevated thyroglobulin in infants with chronic excess iodine intake. Thyroid 2015. 25 851–859. ( 10.1089/thy.2015.0153) [DOI] [PubMed] [Google Scholar]
- 11.Luo Y, Kawashima A, Ishido Y, Yoshihara A, Oda K, Hiroi N, Ito T, Ishii N, Suzuki K. Iodine excess as an environmental risk factor for autoimmune thyroid disease. International Journal of Molecular Sciences 2014. 15 12895–12912. ( 10.3390/ijms150712895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zava TT, Zava DT. Assessment of Japanese iodine intake based on seaweed consumption in Japan: a literature-based analysis. Thyroid Research 2011. 4 14 ( 10.1186/1756-6614-4-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delange F. Iodine requirements during pregnancy, lactation and the neonatal period and indicators of optimal iodine nutrition. Public Health Nutrition 2007. 10 1571–1580. ( 10.1017/S1368980007360941) [DOI] [PubMed] [Google Scholar]
- 14.Chan SY, Vasilopoulou E, Kilby MD. The role of the placenta in thyroid hormone delivery to the fetus. Nature Clinical Practice: Endocrinology and Metabolism 2009. 5 45–54. ( 10.1038/ncpendmet1026) [DOI] [PubMed] [Google Scholar]
- 15.Burrow GN, Fisher DA, Larsen PR. Maternal and fetal thyroid function. New England Journal of Medicine 1994. 331 1072–1078. ( 10.1056/NEJM199410203311608) [DOI] [PubMed] [Google Scholar]
- 16.Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocrine Reviews 1997. 18 404–433. ( 10.1210/edrv.18.3.0300) [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann MB. The effects of iodine deficiency in pregnancy and infancy. Paediatric and Perinatal Epidemiology 2012. 26 (Supplement 1) 108–117. ( 10.1111/j.1365-3016.2012.01275.x) [DOI] [PubMed] [Google Scholar]
- 18.Pearce EN. Iodine deficiency in children. Endocrine Development 2014. 26 130–138. ( 10.1159/000363160) [DOI] [PubMed] [Google Scholar]
- 19.Wei W, Wang Y, Dong J, Wang Y, Min H, Song B, Shan Z, Teng W, Xi Q, Che J. Hypothyroxinemia induced by mild iodine deficiency deregulats thyroid proteins during gestation and lactation in dams. International Journal of Environmental Research and Public Health 2013. 10 3233–3245. ( 10.3390/ijerph10083233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L, Wang D, Liu P, Meng F, Wen D, Jia Q, Liu J, Zhang X, Jiang P, Shen H. The relationship between iodine nutrition and thyroid disease in lactating women with different iodine intakes. British Journal of Nutrition 2015. 114 1487–1495. ( 10.1017/S0007114515003128) [DOI] [PubMed] [Google Scholar]
- 21.Orito Y, Oku H, Kubota S, Amino N, Shimogaki K, Hata M, Manki K, Tanaka Y, Sugino S, Ueta M, et al. Thyroid function in early pregnancy in Japanese healthy women: relation to urinary iodine excretion, emesis, and fetal and child development. Journal of Clinical Endocrinology and Metabolism 2009. 94 1683–1688. ( 10.1210/jc.2008-2111) [DOI] [PubMed] [Google Scholar]
- 22.Shi X, Han C, Li C, Mao J, Wang W, Xie X, Li C, Xu B, Meng T, Du J, et al. Optimal and safe upper limits of iodine intake for early pregnancy in iodine-sufficient regions: a cross-sectional study of 7190 pregnant women in China. Journal of Clinical Endocrinology and Metabolism 2015. 100 1630–1638. ( 10.1210/jc.2014-3704) [DOI] [PubMed] [Google Scholar]
- 23.Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel SJ, et al. 2016 guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017. 27 315–389. ( 10.1089/thy.2016.0457) [DOI] [PubMed] [Google Scholar]
- 24.Li N, Jiang Y, Shan Z, Teng W. Prolonged high iodine intake is associated with inhibition of type 2 deiodinase activity in pituitary and elevation of serum thyrotropin levels. British Journal of Nutrition 2012. 107 674–682. ( 10.1017/S0007114511003552) [DOI] [PubMed] [Google Scholar]
- 25.Calil-Silveira J, Serrano-Nascimento C, Laconca RC, Schmiedecke L, Salgueiro RB, Kondo AK, Nunes MT. Underlying mechanisms of pituitary-thyroid axis function disruption by chronic iodine excess in rats. Thyroid 2016. 26 1488–1498. ( 10.1089/thy.2015.0338) [DOI] [PubMed] [Google Scholar]
- 26.Cunha TF, Bacurau AV, Moreira JB, Paixão NA, Campos JC, Ferreira JC, Leal ML, Negrão CE, Moriscot AS, Wisløff U, et al. Exercise training prevents oxidative stress and ubiquitin-proteasome system overactivity and reverse skeletal muscle atrophy in heart failure. PLoS ONE 2012. 7 e41701 ( 10.1371/journal.pone.0041701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marassi MP, Fortunato RS, Matos da Silva AC, Pereira VS, Carvalho DP, Rosenthal D, Corrêa da Costa VM. Sexual dimorphism in thyroid function and type 1 iodothyronine deiodinase activity in pre-pubertal and adult rats. Journal of Endocrinology 2007. 192 121–130. ( 10.1677/joe.1.06901) [DOI] [PubMed] [Google Scholar]
- 28.Miyai K, Tokushige T, Kondo M. Suppression of thyroid function during ingestion of seaweed ‘Kombu’ (Laminaria japonoca) in normal Japanese adults. Endocrine Journal 2008. 55 1103–1108. ( 10.1507/endocrj.K08E-125) [DOI] [PubMed] [Google Scholar]
- 29.Li HS, Carayanniotis G. Induction of goitrous hypothyroidism by dietary iodide in SJL mice. Endocrinology 2007. 148 2747–2752. ( 10.1210/en.2007-0082) [DOI] [PubMed] [Google Scholar]
- 30.Saki F, Dabbaghmanesh MH Ghaemi SZ Forouhari S Ranjbar Omrani G Bakhshayeshkaram M. Thyroid function in pregnancy and its influences on maternal and fetal outcomes. International Journal of Endocrinology and Metabolism 2014. 12 e19378. ( 10.5812/ijem.19378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert ME, Rovet J Chen Z Koibuchi N. Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology 2012. 33 842–852. ( 10.1016/j.neuro.2011.11.005) [DOI] [PubMed] [Google Scholar]
- 32.Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Fetal and maternal thyroid hormones. Hormone Research 1987. 26 12–27. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Teng W Liu Y Li J Mao J Fan C Wang H Zhang H Shan Z. Effect of maternal excessive iodine intake on neurodevelopment and cognitive function in rat offspring. BMC Neuroscience 2012. 13 121 ( 10.1186/1471-2202-13-121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carneiro-Ramos MS, Diniz GP Almeida J Vieira RL Pinheiro SV Santos RA Barreto-Chaves ML. Cardiac angiotensin II type I and type II receptors are increased in rats submitted to experimental hypothyroidism. Journal of Physiology 2007. 583 213–223. ( 10.1113/jphysiol.2007.134080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocrine Reviews 2005. 26 704–728. ( 10.1210/er.2003-0033) [DOI] [PubMed] [Google Scholar]
- 36.Taylor T, Wondisford FE Blaine T Weintraub BD. The paraventricular nucleus of the hypothalamus has a major role in thyroid hormone feedback regulation of thyrotropin synthesis and secretion. Endocrinology 1990. 126 317–324. ( 10.1210/endo-126-1-317) [DOI] [PubMed] [Google Scholar]
- 37.Chiamolera MI, Wondisford FE. Minireview: thyrotropin-releasing hormone and the thyroid hormone feedback mechanism. Endocrinology 2009. 150 1091–1096. ( 10.1210/en.2008-1795) [DOI] [PubMed] [Google Scholar]
- 38.Shupnik MA, Chin WW Habener JF Ridgway EC. Transcriptional regulation of the thyrotropin subunit genes by thyroid hormone. Journal of Biological Chemistry 1985. 260 2900–2903. [PubMed] [Google Scholar]
- 39.Bargi-Souza P, Romano RM Salgado Rde M Goulart-Silva F Brunetto EL Zorn TM Nunes MT. Triiodothyronine rapidly alters the TSH content and the secretory granules distribution in male rat thyrotrophs by a cytoskeleton rearrangement-independent mechanism. Endocrinology 2013. 154 4908–4918. ( 10.1210/en.2013-1508) [DOI] [PubMed] [Google Scholar]
- 40.Silva FG, Giannocco G Santos MF Nunes MT. Thyroid hormone induction of actin polymerization in somatotrophs of hypothyroid rats: potential repercussions in growth hormone synthesis and secretion. Endocrinology 2006. 147 5777–5785. ( 10.1210/en.2006-0110) [DOI] [PubMed] [Google Scholar]
- 41.Leonard JL, Siegrist-Kaiser CA, Zuckerman CJ. Regulation of type II iodothyronine 5′-deiodinase by thyroid hormone. Inhibition of actin polymerization blocks enzyme inactivation in cAMP-stimulated glial cells. Journal of Biological Chemistry 1990. 265 940–946. [PubMed] [Google Scholar]
- 42.Lisi S, Segnani C Mattii L Botta R Marcocci C Dolfi A McCluskey RT Pinchera A Bernardini N Marinò M. Thyroid dysfunction in megalin deficient mice. Molecular and Cellular Endocrinology 2005. 236 43–47. ( 10.1016/j.mce.2005.03.009) [DOI] [PubMed] [Google Scholar]
- 43.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocrine Reviews 2002. 23 38–89. ( 10.1210/edrv.23.1.0455) [DOI] [PubMed] [Google Scholar]
- 44.Serrano-Nascimento C, Nicola JP, Teixeira Sda S, Poyares LL, Lellis-Santos C, Bordin S, Masini-Repiso AM, Nunes MT. Excess iodide downregulates Na(+)/I(−) symporter gene transcription through activation of PI3K/Akt pathway. Molecular and Cellular Endocrinology 2016. 426 73–90. ( 10.1016/j.mce.2016.02.006) [DOI] [PubMed] [Google Scholar]
- 45.Fernandez LP, Lopez-Marquez A, Santisteban P. Thyroid transcription factors in development, differentiation and disease. Nature Reviews Endocrinology 2015. 11 29–42. ( 10.1210/mend.10.7.8813721) [DOI] [PubMed] [Google Scholar]
- 46.Rossich LE, Thomasz L Nicola JP Nazar M Salvarredi LA Pisarev M Masini-Repiso AM Christophe-Hobertus C Christophe D Juvenal GJ. Effects of 2-iodohexadecanal in the physiology of thyroid cells. Molecular and Cellular Endocrinology 2016. 437 292–301. ( 10.1016/j.mce.2016.08.036) [DOI] [PubMed] [Google Scholar]
- 47.Suzuki YJ, Carini M, Butterfield DA. Protein carbonylation. Antioxidants and Redox Signaling 2010. 12 323–325. ( 10.1089/ars.2009.2887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sahoo DK, Roy A Bhanja S Chainy GB. Hypothyroidism impairs antioxidant defence system and testicular physiology during development and maturation. General and Comparative Endocrinology 2008. 156 63–70. ( 10.1016/j.ygcen.2007.11.007) [DOI] [PubMed] [Google Scholar]
- 49.Serrano-Nascimento C, da Silva Teixeira S, Nicola JP, Nachbar RT, Masini-Repiso AM, Nunes MT. The acute inhibitory effect of iodide excess on sodium/iodide symporter expression and activity involves the PI3K/Akt signaling pathway. Endocrinology 2014. 155 1145–1156. ( 10.1210/en.2013-1665) [DOI] [PubMed] [Google Scholar]
- 50.Burek CL, Rose NR. Autoimmune thyroiditis and ROS. Autoimmunity Reviews 2008. 7 530–537. ( 10.1016/j.autrev.2008.04.006) [DOI] [PubMed] [Google Scholar]
- 51.Kambe F, Nomura Y, Okamoto T, Seo H. Redox regulation of thyroid-transcription factors, Pax-8 and TTF-1, is involved in their increased DNA-binding activities by thyrotropin in rat thyroid FRTL-5 cells. Molecular Endocrinology 1996. 10 801–812. ( 10.1210/mend.10.7.8813721) [DOI] [PubMed] [Google Scholar]
- 52.Maia AL, Kieffer JD, Harney JW, Larsen PR. Effect of 3,5,3′-Triiodothyronine (T3) administration on dio1 gene expression and T3 metabolism in normal and type 1 deiodinase-deficient mice. Endocrinology 1995. 136 4842–4849. ( 10.1210/endo.136.11.7588215) [DOI] [PubMed] [Google Scholar]
- 53.Wang K, Wang K, Sun YN, Liu JY, Zhang L, Ye Y, Lin LX, Yan YQ, Chen ZP. The impact of iodine excess on thyroid hormone biosynthesis and metabolism in rats. Biological Trace Element Research 2009. 130 72–85. ( 10.1007/s12011-009-8315-z) [DOI] [PubMed] [Google Scholar]
- 54.Tazebay UH, Wapnir IL, Levy O, Dohan O, Zuckier LS, Zhao QH, Deng HF, Amenta PS, Fineberg S, Pestell RG, et al. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nature Medicine 2000. 6 871–878. ( 10.1038/78630) [DOI] [PubMed] [Google Scholar]
- 55.Rillema JA, Hill MA. Pendrin transporter carries out iodide uptake into MCF-7 human mammary cancer cells. Experimental Biology and Medicine 2003. 228 1078–1082. ( 10.1177/153537020322800915) [DOI] [PubMed] [Google Scholar]
- 56.Rillema JA, Hill MA. Prolactin regulation of the pendrin-iodide transporter in the mammary gland. American Journal of Physiology – Endocrinology and Metabolism 2003. 284 E25–E28. ( 10.1152/ajpendo.00383.2002) [DOI] [PubMed] [Google Scholar]
- 57.Rillema JA, Yu TX, Jhiang SM. Effect of prolactin on sodium iodide symporter expression in mouse mammary gland explants. American Journal of Physiology – Endocrinology and Metabolism 2000. 279 E769–E772. [DOI] [PubMed] [Google Scholar]
- 58.Crowley WR. Neuroendocrine regulation of lactation and milk production. Comprehensive Physiology 2015. 5 255–291. ( 10.1002/cphy.c140029) [DOI] [PubMed] [Google Scholar]
- 59.Leung AM, Pearce EN, Braverman LE. Iodine content of prenatal multivitamins in the United States. New England Journal of Medicine 2009. 360 939–40. ( 10.1056/NEJMbib807851) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a