Summary
Objective
The objective of this randomized equivalence trial was to determine the impact of consuming lean beef as part of a high protein (HP) weight‐reducing diet on changes in body weight, body composition and cardiometabolic health.
Methods
A total of 120 adults (99 female) with overweight or obesity (BMI: 35.7 ± 7.0 kg m−2) were randomly assigned to consume either a HP diet with ≥4 weekly servings of lean beef (B; n = 60) or a HP diet restricted in all red meats (NB; n = 60) during a 16‐week weight loss intervention.
Results
Body weight was reduced by 7.8 ± 5.9% in B and 7.7 ± 5.5% in NB (p < 0.01 for both). Changes in percent body weight were equivalent between B and NB (mean difference: 0.06%, 90% confidence interval: (−1.7, 1.8)). Fat mass was reduced in both groups (p < 0.01; B: 8.0 ± 0.6 kg, NB: 8.6 ± 0.6 kg), while lean mass was not reduced in either group. Improvements in markers of cardiometabolic health (total cholesterol, low‐density lipoprotein cholesterol, triglycerides and blood pressure) were not different between B and NB.
Conclusion
Results of this study demonstrate that HP diets – either rich or restricted in red meat intakes – are effective for decreasing body weight and improving body composition and cardiometabolic health.
Keywords: Body composition, lean body mass, obesity, red meats, weight loss
Introduction
While there are many available options for achieving weight loss 1, higher protein (HP) diets have garnered considerable attention within the general populous and scientific community due to potential beneficial effects on satiety, postprandial thermogenesis, resting energy expenditure, body composition and certain cardiometabolic risk factors 2. Further, evidence from multiple systematic reviews and meta‐analyses support modestly greater effects of HP compared to lower protein diets on weight/fat loss, lean mass retention, triglycerides and/or blood pressure 3, 4, 5. Although at least one meta‐analysis found no beneficial (or detrimental) effect of HP diets on these outcomes 6.
The widespread interest in HP diets has led to research to determine the importance of protein source/type (e.g. animal vs. vegetable (7, 8), soy 9, milk/dairy 10, red meats 8, 11, 12, 13) on weight loss and/or cardiometabolic outcomes. In particular, red meat (beef, pork, veal, lamb and mutton) has been the subject of substantial scientific debate 14, 15, 16. Recommendations to limit or restrict red meat consumption 17, 18 are common due to positive associations between its consumption and cardiovascular diseases 19, type 2 diabetes 20 and cancer 21, 22 in observational studies. According to the 2015 Dietary Guidelines for Americans, eating patterns that include lower intake of red meats are associated with reduced risk of obesity 18. However, findings from randomized controlled trials largely find no detrimental impact of lean red meat consumption on markers of cardiometabolic health during weight loss 8, 23 and weight maintenance 11, 12, 13. Red meat is a major contributor to overall protein intake and represents 58% of all meat consumption in the United States 24. Therefore, its exclusion from the diet represents a potential barrier to the long‐term adoption of HP diets.
Previous randomized clinical studies demonstrated that lean beef 11, 12 and pork 13 can be effectively incorporated into dietary patterns for improving cardiometabolic health during weight maintenance conditions. At least two randomized clinical trials found that including red meat in energy‐restricted diets does not negatively influence weight loss or improvements in cardiometabolic health 8, 23. However, previous weight loss intervention trials were limited by relatively small sample sizes, absence of a HP control diet with no red meat 8, 23 and the manipulation of multiple dietary components 23. Therefore, this randomized equivalence trial in 120 adults with overweight/obesity was conducted to determine the impact of consuming two HP diets (1: ≥4 weekly servings of lean beef [B] vs. 2: no red meat consumption [NB]) on weight loss, body composition and cardiometabolic health during a 16‐week weight loss intervention. It was hypothesized that B and NB would result in equivalent weight loss (primary aim) and that beneficial changes in body composition and cardiometabolic health would not differ between groups.
Methods
Participants
One‐hundred twenty individuals (99 female, 21 male) were recruited from the Denver, CO metropolitan area to participate in a behavioural weight loss study at the University of Colorado Anschutz Health and Wellness Center (AHWC; Figure 1). Inclusion criteria for the study were: male or female; age 18–50 years; BMI ≥ 27.0 kg m−2; weight stable (± 3 kg in previous 3 months); able to progress to 70 min day−1 of moderate intensity exercise; willing to comply with all study procedures including attendance to 16 weekly classes and three study visits. Individuals were excluded from the study if: pregnant or trying to become pregnant; diagnosis of diabetes; LDL cholesterol >160 mg dL−1; triglycerides >400 mg dL−1; untreated or unstable hypothyroidism; medication use that could cause weight loss or gain; following vegetarian or vegan diet; current eating disorder; any medical condition for which consuming a HP diet and/or engaging in 70 min of exercise daily would be inadvisable. All participants provided written informed consent and received a monetary stipend. The consent form and all study procedures and documents were approved for use by the Colorado Multiple Institutional Review Board. Of the 120 participants who provided consent for the study, 99 individuals (83 female, 16 male) completed the 16‐week intervention for a retention rate of 82.5% (Figure 1).
Figure 1.
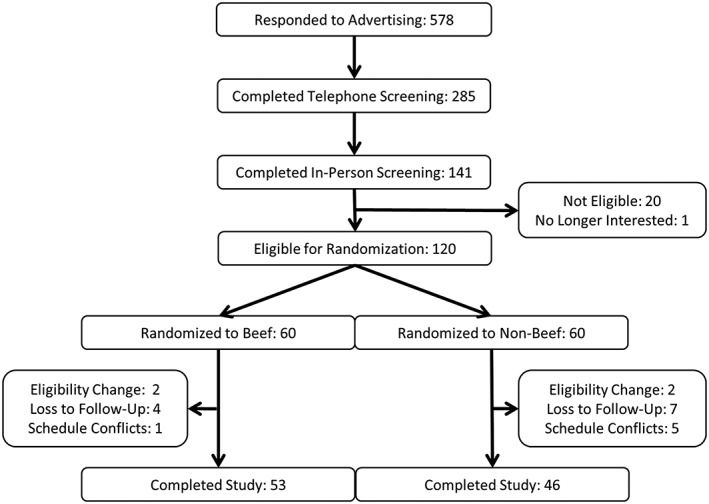
Participant recruitment diagram.
Experimental design
All participants participated in the State of Slim (SOS) weight management program, which is a 16‐week group‐based, lifestyle modification program 25. The program consisted of weekly classes of 20 participants that were stratified by diet assignment. A copy of the SOS book and access to the online SOS community were provided to all participants. Membership to the AHWC fitness facility was also provided to participants for the duration of their participation in the study. Participants were randomly assigned to one of two experimental diets; a HP diet with instructions to consume ≥4 weekly servings of lean beef as the only source of red meat (B) or a HP diet with instructions not to consume any red meat for the duration of the study (NB).
Body weight, body composition and indices of cardiometabolic health were measured at baseline and after completing the weight loss intervention (week 16). The study was registered on ClinicalTrials.gov (NCT02627105) and included a 2‐month follow‐up period following the 16‐week weight loss intervention. Because the primary objective of the current study was to assess the equivalence of B and NB for weight loss during the active intervention, results from the follow‐up period are not reported here.
Diet intervention
The SOS diet plan is HP, low in fat and emphasizes non‐starchy (i.e. vegetable) and whole‐grain carbohydrates (Table 1). The diet is plan is structured into three distinct phases with phase‐specific food choices from which participants can chose to eat in defined portions rather than counting calories. The SOS diet plan utilizes five ‘Diet Rules’ through all three phases of the diet to encourage weight loss: (i) Eat 6 times per day; (ii) Have breakfast within 1 h of waking; (iii) Don't count calories, measure portions; (iv) Have the right carbohydrate and protein mix at every meal (one carbohydrate and one protein at every meal, vegetables as only carbohydrate source at three meals); and (v) Eat a healthy fat twice a day.
Table 1.
recommended energy, macronutrient distribution and fibre intake of the published State of Slim diet plan*
| Nutrient | Phase 1 (Weeks 1–2) | Phase 2 (Weeks 3–8) | Phase 3 (Weeks 9–16) |
|---|---|---|---|
| Energy (kcals d−1) | 1,644 | 1,754 | 1,834 |
| Carbohydrate (%) | 26 | 28 | 32 |
| Protein (%) | 50 | 45 | 40 |
| Fat (%) | 24 | 27 | 28 |
| Fibre (g d−1) | 16 | 25 | 28 |
Nutrition Data System for Research (NDSR) was used to calculate approximate recommended energy, carbohydrate, protein, fat and fibre intakes during each phase of the SOS diet. The data for NDSR calculations were derived from phase‐specific food lists, recommended portion sizes and sample menus published in the SOS book 25. Group‐specific diet analyses (B vs. NB) were not completed as part of the study, but recommendations for total energy, carbohydrate, protein, fat and fibre intakes were the same for B and NB.
Participants completed daily food logs throughout the 16‐week weight loss intervention. However, the logs were not designed or intended as a measure total energy consumption or macronutrient distribution. Rather, the logs were used as a self‐monitoring tool to enhance weight loss 26 and as an indicator of beef consumption during the study. Self‐reported energy intake and macronutrient distribution were not tracked during the study because a principal aspect of the SOS program is to focus on portion sizes rather than counting calories (Diet Rule #3 above). A detailed food log would therefore be inconsistent with the goals and structure of the program. Further, self‐reported measures of food intake are highly unreliable, and their suitability for use in clinical research has been questioned 27.
Protein foods throughout the entire SOS program are lean and minimally processed (i.e. lean meat and poultry, fish, egg whites and fat‐free dairy). Lean beef is included in all three phases of the published diet plan. Prescribed food lists and portion sizes for the SOS diet plan (as published in the SOS book) are presented in Tables 2, 3, 4. All participants in the study were provided with a SOS book and instructed to select foods from the list with additional group‐specific instructions. Specifically, participants randomly assigned to B were instructed to consume ≥4 weekly servings of lean beef from the options included in the food lists. Participants assigned to NB were instructed not to consume any red meats (beef, pork, veal, lamb and mutton) for the duration of the study. Consuming four weekly servings of lean beef would result in ~20 ounces or 567 g (recommended portion size is 4–6 ounces) of total red meat consumption per week, which is comparable with total mean red meat consumption for US adults aged 20–49 years (80 grams per day) 24. Recommended sources of non‐beef dietary protein and total recommended protein consumption were the same between B and NB.
Table 2.
State of Slim diet plan, Phase 1 (weeks 1–2)*
| The leanest proteins (have one at every meal and snack) | ||
|---|---|---|
| Meat and poultry | ||
| Beef, ground, extra lean | 4–6 oz | |
| Beef, lean cuts | 4–6 oz | |
| Chicken breast, without skin | 4–6 oz | |
| Turkey breast, without skin | 4–6 oz | |
| Turkey breast, lean ground | 4–6 oz | |
| Fish | ||
| Cod | 4–6 oz | |
| Mahi mahi | 4–6 oz | |
| Salmon† | 4–6 oz | |
| Snapper | 4–6 oz | |
| Tilapia | 4–6 oz | |
| Tuna | 4–6 oz | |
| White fish | 4–6 oz | |
| Egg and high‐protein dairy | ||
| Cottage cheese, fat‐free | 8 oz | |
| Egg whites | 1 cup or 5–6 whites | |
| Greek yogurt, nonfat plain | 8 oz | |
| Other | ||
| Protein powder | 1 scoop | |
| Reignite carbohydrates (have one at a maximum of three meals and snacks | ||
| Grains | ||
| Oats, steel‐cut | ¼ cup dry | |
| Oats, old‐fashioned rolled | ½ cup dry | |
| Dairy and dairy substitutes | ||
| Almond milk, unsweetened | 1 cup | |
| Fat‐free milk | 1 cup | |
| Starchy vegetables | ||
| Pumpkin | 1 cup mashed | |
| Vegetable carbohydrates (only carbohydrate at three meals or snacks a day, unlimited portions) | ||
| Artichoke | Asparagus | Beets |
| Broccoli | Brussels sprouts | Cabbage and Chinese cabbage |
| Carrots | Cauliflower | Celery |
| Cucumbers | Dark leafy greens | Eggplant |
| Fennel | Green beans | Mushrooms |
| Onions and scallions | Parsnips | Peppers, sweet and hot |
| Salad greens, all varieties | Summer squash | Tomato and tomato sauce |
| Turnips and rutabagas | Zucchini | |
| Only the healthiest fats (include fats in two meals or snacks per day) | ||
| Nuts | ||
| Almonds | 15–18 | |
| Walnuts | 8–9 halves | |
| Oils | ||
| Canola oil | 1 tbsp | |
| Olive oil | 1 tbsp | |
This list is unedited from the published food list in the State of Slim book. All participants in the study were provided with a copy of the book and instructed to choose foods and portions from list and given additional group‐specific dietary instructions (B: ≥4 weekly servings of lean beef but no other sources of red meat, NB: no red meats).
Salmon also counts as 1 fat.
Abbreviations: oz, ounces; tbsp, tablespoon.
Table 3.
State of Slim diet plan, phase 2 (weeks 3–8)*
| The leanest proteins (have one at every meal and snack) | ||
|---|---|---|
| Meat and poultry | ||
| Beef, ground, extra lean | 4–6 oz | |
| Beef, lean cuts | 4–6 oz | |
| Buffalo, lean cuts † | 4–6 oz | |
| Canadian bacon | 4 oz | |
| Chicken breast, without skin | 4–6 oz | |
| Ostrich, lean cuts | 4–6 oz | |
| Pork tenderloin | 4–6 oz | |
| Turkey breast, without skin | 4–6 oz | |
| Turkey breast, lean ground | 4–6 oz | |
| Venison, lean cuts | 4–6 oz | |
| Fish | ||
| Cod | 4–6 oz | |
| Crab | 4–6 oz | |
| Lobster | 4–6 oz | |
| Mahi mahi | 4–6 oz | |
| Salmon‡ | 4–6 oz | |
| Scallops | 4–6 oz | |
| Shrimp | 4–6 oz | |
| Snapper | 4–6 oz | |
| Tilapia | 4–6 oz | |
| Tuna | 4–6 oz | |
| White fish | 4–6 oz | |
| Egg and high‐protein dairy | ||
| Cottage cheese, fat‐free | 8 oz | |
| Eggs, whole | 1, plus 3–4 whites | |
| Egg whites | 1 cup or 5–6 whites | |
| Greek yogurt, nonfat plain | 8 oz | |
| Other | ||
| Protein powder | 1 scoop | |
| Rebuild carbohydrates (have one at a maximum of three meals and snacks | ||
| Fruit | ||
| Apple | 1 medium | |
| Berries | 1 cup | |
| Grapefruit | 1 medium | |
| Breads | ||
| Ezekiel Bread | 1 slice | |
| Whole grain pita or tortilla | 1 | |
| Grains | ||
| Barley | ½ cup cooked | |
| Brown or wild rice | ½ cup cooked | |
| Oats, steel‐cut | ¼ cup dry | |
| Oats, old‐fashioned rolled | ½ cup dry | |
| Quinoa | ½ cup cooked | |
| Rice cakes | 2 | |
| Dairy and dairy substitutes | ||
| Almond milk, unsweetened | 1 cup | |
| Fat‐free milk | 1 cup | |
| Fat‐free or part‐skim ricotta cheese | ½ cup | |
| Reduced‐fat string cheese | 1–2 pieces | |
| Beans and starchy vegetables | ||
| Beans | ½ cup whole; ⅓ cup fat‐free refried | |
| Pumpkin | 1 cup mashed | |
| Sweet potato | 4 oz, ½ cup mashed | |
| Winter squash | 4 oz, ½ cup mashed | |
| Vegetable carbohydrates (only carbohydrate at three meals or snacks a day, unlimited portions) | ||
| Artichoke | Asparagus | Beets |
| Broccoli | Brussels sprouts | Cabbage and Chinese cabbage |
| Carrots | Cauliflower | Celery |
| Cucumbers | Dark leafy greens | Eggplant |
| Fennel | Green beans | Mushrooms |
| Onions and scallions | Parsnips | Peppers, sweet and hot |
| Salad greens, all varieties | Summer squash | Tomato and tomato sauce |
| Turnips and rutabagas | Zucchini | |
| Only the healthiest fats (include fats in two meals or snacks per day) | ||
| Nuts | ||
| Almonds | 15–18 | |
| Pistachios | 25 | |
| Walnuts | 8–9 halves | |
| Oils | ||
| Canola oil | 1 tbsp | |
| Olive oil | 1 tbsp | |
| Other | ||
| Avocado | ⅓ medium | |
| Olives | 10 small or 5 medium/large | |
This list is unedited from the published food list in the State of Slim book. All participants in the study were provided with a copy of the book and instructed to choose foods and portions from list and given additional group‐specific dietary instructions (B: ≥ 4 weekly servings of lean beef but no other sources of red meat, NB: no red meats).
Boldface foods added in Phase 2.
Salmon also counts as 1 fat.
Abbreviations: oz, ounces; tbsp, tablespoon.
Table 4.
State of Slim diet plan, phase 2 (weeks 9–16)*
| The leanest proteins (have one at every meal and snack) | ||
|---|---|---|
| Meat and poultry | ||
| Beef, ground, extra lean | 4–6 oz | |
| Beef, lean cuts | 4–6 oz | |
| Buffalo, lean cuts | 4–6 oz | |
| Canadian bacon | 4 oz | |
| Chicken breast, without skin | 4–6 oz | |
| Filet mignon † | 4–6 oz | |
| Lean deli meat | 4–6 oz | |
| Lean ham | 4–6 oz | |
| New York strip steak | 4–6 oz | |
| Ostrich, lean cuts | 4–6 oz | |
| Pork tenderloin | 4–6 oz | |
| Turkey bacon | 4 slices | |
| Turkey breast, without skin | 4–6 oz | |
| Turkey breast, lean ground | 4–6 oz | |
| Turkey sausage | ½–1 cup or 2 patties | |
| Venison, lean cuts | 4–6 oz | |
| Fish | ||
| Cod | 4–6 oz | |
| Crab | 4–6 oz | |
| Lobster | 4–6 oz | |
| Mahi mahi | 4–6 oz | |
| Salmon‡ | 4–6 oz | |
| Scallops | 4–6 oz | |
| Sea bass | 6–8 oz | |
| Shrimp | 4–6 oz | |
| Snapper | 4–6 oz | |
| Tilapia | 4–6 oz | |
| Trout | 6–8 oz | |
| Tuna | 4–6 oz | |
| White fish | 4–6 oz | |
| Egg and high‐protein dairy | ||
| Cottage cheese, fat‐free | 8 oz | |
| Eggs, whole | 1, plus 3–4 whites | |
| Egg whites | 1 cup or 5–6 whites | |
| Greek yogurt, nonfat plain | 8 oz | |
| Greek yogurt, nonfat or low‐fat, flavoured | 8 oz | |
| Other | ||
| Protein bars | 1 bar | |
| Protein powder | 1 scoop | |
| Reinforce carbohydrates (have one at a maximum of three meals and snacks | ||
| Fruit | ||
| Apple | 1 medium | |
| Apricots | 3 fruit or 1 cup | |
| Banana | 1 fruit or 1 cup | |
| Berries | 1 cup | |
| Cherries | 1 cup | |
| Dried cherries | 1 ½ tbsp | |
| Grapes | 1 cup | |
| Grapefruit | 1 medium | |
| Kiwifruit | 1 fruit or 1 cup | |
| Mango | 1 cup | |
| Orange | 1 fruit or 1 cup | |
| Peach | 1 fruit or 1 cup | |
| Pear | 1 fruit or 1 cup | |
| Plum | 1 fruit or 1 cup | |
| Breads | ||
| English muffin | 1 | |
| Ezekiel Bread | 1 slice | |
| Whole grain bagel thins | 1 | |
| Whole grain bread | 1 slice | |
| Whole grain pita or tortilla | 1 | |
| Grains | ||
| Barley | ½ cup cooked | |
| Brown or wild rice | ½ cup cooked | |
| Cereal, high‐fibre, low sugar | 1 cup | |
| Oats, steel‐cut | ¼ cup dry | |
| Oats, old‐fashioned rolled | ½ cup dry | |
| Quinoa | ½ cup cooked | |
| Rice cakes | 2 | |
| Whole grain couscous | ½–1 cup cooked | |
| Whole grain pasta | ½–1 cup cooked | |
| Dairy and dairy substitutes | ||
| Almond milk, unsweetened | 1 cup | |
| Fat‐free milk | 1 cup | |
| Fat‐free or part‐skim ricotta cheese | ½ cup | |
| Low‐fat or reduced‐fat cheeses | ¼ c grated or 1 oz | |
| Nonfat or low‐fat regular yogurt, fruit‐flavoured or plain | 6–8 oz | |
| Reduced‐fat string cheese | 1–2 pieces | |
| Beans and starchy vegetables | ||
| Beans | ½ cup whole; ⅓ cup fat‐free refried | |
| Baked potato | 1 medium, 6–8 oz | |
| Corn | 1 cup or 1 medium ear | |
| Edamame | ½ cup shelled | |
| Peas | 1 cup | |
| Pumpkin | 1 cup mashed | |
| Sweet potato | 4 oz, ½ cup mashed | |
| Winter squash | 4 oz, ½ cup mashed | |
| Vegetable carbohydrates (only carbohydrate at three meals or snacks a day, unlimited portions) | ||
| Artichoke | Asparagus | Beets |
| Broccoli | Brussels sprouts | Cabbage and Chinese cabbage |
| Carrots | Cauliflower | Celery |
| Cucumbers | Dark leafy greens | Eggplant |
| Fennel | Green beans | Mushrooms |
| Onions and scallions | Parsnips | Peppers, sweet and hot |
| Salad greens, all varieties | Summer squash | Tomato and tomato sauce |
| Turnips and rutabagas | Zucchini | |
| Only the healthiest fats (include fats in two meals or snacks per day) | ||
| Nuts | ||
| Almond butter | 1 tbsp | |
| Almonds | 15–18 | |
| Peanut butter | 1 tbsp | |
| Pistachios | 25 | |
| Walnuts | 8–9 halves | |
| Oils | ||
| Canola oil | 1 tbsp | |
| Olive oil | 1 tbsp | |
| Other | ||
| Avocado | ⅓ medium | |
| Hummus | ¼ cup | |
| Olives | 10 small or 5 medium/large | |
This list is unedited from the published food list in the State of Slim book. All participants in the study were provided with a copy of the book and instructed to choose foods and portions from list and given additional group‐specific dietary instructions (B: ≥4 weekly servings of lean beef but no other sources of red meat, NB: no red meats).
Boldface foods added in Phase 3.
Salmon also counts as 1 fat.
Abbreviations: oz, ounces; tbsp, tablespoon.
Anthropometric measurements
Body weight was measured using a digital platform scale (PS‐6600 ST, Befour, Inc., Saukville, WI, USA) at baseline and week 16 in a fasted‐state with the participant wearing light clothing and after voiding. Height was measured using a stadiometer at baseline. Body mass index (BMI; kg m−2) was calculated using these measurements. Body composition (fat and lean mass) was measured using dual x‐ray absorptiometry (Discovery QDR DXA System, APEX software version 4.5, Hologic, Inc., Marlborough, MA, 01752, USA). Waist circumference (WC) was measured at the midpoint between the lowest rib and iliac crest in accordance with recommendations from the World Health Organization 28.
Cardiometabolic health
Two blood samples were obtained from an antecubital vein by a trained phlebotomist after an overnight fast at baseline and week 16. One sample was processed to obtain plasma, and analyses for glucose, total cholesterol, low‐density lipoprotein cholesterol (LDL; calculated), high‐density lipoprotein cholesterol (HDL) and triglycerides were completed by the UC Health Clinical Laboratory within 24 h of collection. A whole‐blood sample was sent to the UC Health Clinical Laboratory and analysed for haemoglobin A1c (HbA1c) within 72 h of collection. Blood pressure (BP) was measured at the left upper arm by trained research staff using a manual sphygmomanometer after the participant rested quietly in a seated position for ≥5 min with his/her legs uncrossed and back and arms supported.
Statistical analysis
Study data were collected and managed using REDCap electronic data capture tools hosted at University of Colorado – Anschutz Medical Campus. REDCap (Research Electronic Data Capture) is a secure, web‐based application designed to support data capture for research studies, providing: (i) an intuitive interface for validated data entry; (ii) audit trails for tracking data manipulation and export procedures; (iii) automated export procedures for seamless data downloads to common statistical packages; and (iv) procedures for importing data from external sources 29.
This randomized equivalence clinical trial was powered on the intent‐to‐treat (ITT) analysis using two one‐sided t‐tests (TOST) to establish equivalence for percent weight loss (primary outcome) between the B and NB groups. An interval of −2.5 to 2.5% of the between‐group mean difference in percent weight loss over 16 weeks was considered clinically equivalent in this study. When delivered as a fee‐based program at the AHWC, average percent weight loss during the SOS program is 10.4 ± 4.6%. With these parameters and 60 participants per arm, a statistical power calculation indicated there was 81% power at 5% significance to establish clinical equivalence between two treatments using TOST or the between group difference in least square means (LSMEANS) plus a 90% confidence interval (CI). This power analysis assumed no expected difference between groups.
Demographic, baseline clinical and lab data were summarized by treatment groups (B vs. NB) using descriptive statistics and reported as mean ± SD. Imbalance in these data was examined using Student's t‐tests. Any significantly imbalanced confounding variables from the analysis of baseline data were adjusted using a linear regression model. Any participants with one or more observations after intervention were analysed, and baseline‐observation‐carried‐forward (BOCF) was used for missing data points at week 16. Equivalence was assessed a 90% CI of the mean between‐group difference in % weight loss between two groups, which is equivalent to using TOST. Changes in body weight are reported as % body weight loss (mean ± SD).
Changes in body composition and cardiometabolic health were secondary outcomes, and a priori statistical power calculations were not completed to determine equivalence in these outcomes. In order to assess the between‐ and within‐group differences, a linear mixed effects model was used to test effects of time (baseline vs. week 16), group (B vs. NB) and their interaction term on changes in body composition (fat mass, lean mass and WC) and cardiometabolic health (glucose, total cholesterol, LDL, HDL, triglycerides, HbA1c and BP). Changes in body composition and cardiometabolic health are reported as LSMEANS ± SE, and α = 0.05 was used to determine statistical significance.
Results
Participant characteristics
The mean age of participants was 37.6 ± 8.1 years with BMI of 35.7 ± 7.0 kg m−2 at baseline. Indices of cardiometabolic health were within normal reference ranges (Table 5). Participants randomly assigned to B were 3 years younger than participants assigned to NB (36.0 ± 8.3 years vs. 39.3 ± 7.8 years, p = 0.026). Compared to those who completed the study, participants who withdrew from the study were younger (33.3 ± 8.1 years vs. 38.5 ± 7.9 years, p = 0.010) and had lower fasting total (147.1 ± 23.3 mg dL−1 vs. 171.5 ± 34.4 mg dL−1, p = 0.0037) and LDL (82.1 ± 20 mg dL−1 vs. 103.3 ± 28.7 mg dL−1, p = 0.0027) cholesterol concentrations at baseline. More participants withdrew from NB (n = 14) than B (n = 7, Figure 1), but differences in attrition were not statistically significant (p = 0.22).
Table 5.
Baseline participant characteristics (n = 120)†
| Parameter | All | Beef | Non‐beef |
|---|---|---|---|
| Age (year) | 37.6 ± 8.1 | 36.0 ± 8.3 | 39.3 ± 7.8* |
| Body weight (kg) | 101.1 ± 22.8 | 100.8 ± 21.9 | 101.5 ± 24.0 |
| BMI (kg m−2) | 35.7 ± 7.0 | 35.9 ± 6.8 | 35.4 ± 7.1 |
| Glucose (mg dL−1) | 94.0 ± 9.7 | 94.0 ± 10.4 | 94.1 ± 9.0 |
| Total cholesterol (mg dL−1) | 167.6 ± 34.0 | 168.6 ± 35.6 | 166.6 ± 32.5 |
| LDL (mg dL−1) | 99.9 ± 28.5 | 101.4 ± 30.5 | 98.3 ± 26.5 |
| HDL (mg dL−1) | 46.6 ± 10.1 | 45.4 ± 9.2 | 47.9 ± 10.8 |
| Triglycerides (mg dL−1) | 103.6 ± 50.3 | 107.2 ± 49.0 | 100.0 ± 51.8 |
| Haemoglobin A1c (%) | 5.5 ± 0.4 | 5.4 ± 0.4 | 5.5 ± 0.4 |
| Systolic BP (mm Hg) | 116.5 ± 11.1 | 115.5 ± 10.3 | 117.4 ± 11.8 |
| Diastolic BP (mm Hg) | 76.4 ± 8.4 | 75.6 ± 8.6 | 77.2 ± 8.1 |
All values are mean ± SD.
Indicates significant difference (p < 0.05) between beef and non‐beef by unpaired t‐test (SAS Proc Ttest).
BMI, body mass index; BP, blood pressure; HDL, high‐density lipoprotein cholesterol; LDL, low‐density lipoprotein cholesterol.
Beef intake
Participants were instructed to complete daily food logs as a self‐monitoring tool and an indicator of beef consumption during the 16‐week intervention. Food log completion was the highest during Phase 1 (B: 6.5 ± 1.8 days of week 2, NB: 6.3 ± 1.8 days of week 2), but decreased over the course of the intervention ([Phase 2: B: 4.8 ± 3.2 days of week 8, NB: 4.6 ± 3.3 days of week 8], [Phase 3: B: 3.1 ± 3.5 days of week 16, NB: 4.0 ± 3.5 days of week 16]).
Participants assigned to B reported consuming 5.6 ± 2.0 weekly servings of lean beef during Phase 1 (week 2), 4.65 ± 1.7 weekly servings of lean beef during Phase 2 (week 8), and 5.75 ± 1.8 weekly servings of lean beef during Phase 3 (week 16) of the SOS diet, and reported no additional sources of red meat during any phase of the SOS diet. No participants assigned to NB reported beef or red meat consumption during any phase of the SOS diet.
Weight loss and body composition
Percent weight loss was equivalent in B and NB (B: 7.8 ± 5.9% vs. NB: 7.7 ± 5.5%, Figure 2 ). Total body mass and fat mass were significantly reduced at week 16 compared to baseline in B and NB with no differences between groups (Figure 3). Total lean mass was not different at the conclusion of the intervention compared to baseline (Figure 3). Waist circumference was reduced at the end of the intervention in both groups, but the reduction was greater in NB compared to B (10.6 ± 1.0 cm vs. 7.0 ± 1.0 cm, p = 0.034). However, reductions in trunk fat measured by DXA were not different between B (4.4 ± 0.4 kg) and NB (4.7 ± 0.4 kg; p = 0.55).
Figure 2.
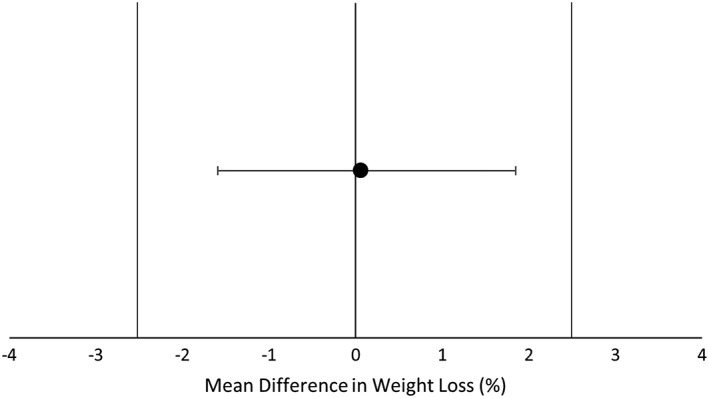
Mean difference in weight loss between Beef and Non‐beef groups. Equivalence was assessed using a 90% CI of the mean between‐group difference in % weight loss between two groups. An interval of −2.5% to 2.5% (vertical bars) of the between‐group mean difference in percent weight loss over 16 weeks was considered clinically equivalent. Changes in body weight were equivalent between Beef and Non‐beef groups.
Figure 3.
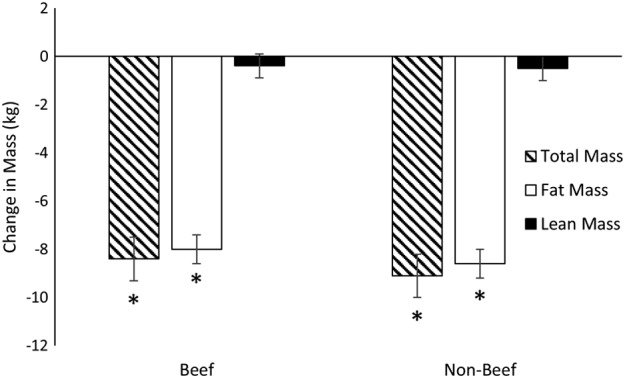
Changes in total, fat and lean mass. Linear mixed models (SAS, Proc Mixed) were used to assess changes in total, fat and lean between groups (Beef vs. Non‐beef) and over time (Baseline vs. Week 16). Significant reductions in total and fat mass were observed that did not differ between Beef and Non‐beef. Lean mass at Week 16 was not significantly different from Baseline in either group. Change in mass presented as LSMEANS ± SE from linear mixed model and * indicates a significant change from Baseline.
Cardiometabolic health
In both B and NB, total cholesterol, LDL, triglycerides, systolic BP and diastolic BP were reduced at week 16 compared to baseline with no differences between groups (Table 6). High‐density lipoprotein cholesterol, glucose and HbA1c did not change over 16 weeks (Table 6).
Table 6.
Parameters of cardiometabolic health*
| Parameter | Group | Baseline | Week 16 | Difference† | P‐value |
|---|---|---|---|---|---|
| Glucose (mg dL−1) | Beef | 94 (1) | 92 (1) | 2 (1) | 0.065 |
| Non‐beef | 94 (1) | 93 (1) | 1(1) | 0.272 | |
| Difference‡ | 0 (2) | −1 (2) | 1 (1) | 0.645 | |
| Cholesterol (mg dL−1) | Beef | 169 (4) | 156 (4) | 12 (3) | <.001 |
| Non‐beef | 167 (4) | 153 (4) | 14 (3) | <.001 | |
| Difference | 2 (6) | 3 (6) | −1 (4) | 0.711 | |
| LDL (mg dL−1) | Beef | 101 (3) | 93 (4) | 8 (2) | <.001 |
| Non‐beef | 98 (4) | 89 (4) | 9 (2) | <.001 | |
| Difference | 3 (5) | 4 (5) | −1 (3) | 0.851 | |
| HDL (mg dL−1) | Beef | 45 (1) | 46 (1) | 0 (1) | 0.576 |
| Non‐beef | 48 (1) | 47 (1) | 1 (1) | 0.328 | |
| Difference | −3 (2) | −1 (2) | −1 (1) | 0.273 | |
| Triglycerides (mg dL−1) | Beef | 107 (1) | 85 (6) | 22 (5) | <.001 |
| Non‐beef | 100 (6) | 82 (6) | 18 (5) | <.001 | |
| Difference | 7 (9) | 4 (9) | 3 (7) | 0.628 | |
| Haemoglobin A1c (%) | Beef | 5.39 (0.05) | 5.33 (0.05) | 0.06 (0.03) | 0.089 |
| Non‐beef | 5.52 (0.05) | 5.50 (0.05) | 0.03 (0.04) | 0.453 | |
| Difference | −0.13 (0.07) | −0.17 (0.07) | 0.03 (0.05) | 0.548 | |
| Systolic BP (mm Hg) | Beef | 116 (2) | 111 (2) | 5 (1) | <.001 |
| Non‐beef | 117 (2) | 109 (2) | 8 (1) | <.001 | |
| Difference | −2 (2) | 1 (2) | −3 (2) | 0.097 | |
| Diastolic BP (mm Hg) | Beef | 76 (1) | 72 (1) | 3 (1) | <.001 |
| Non‐beef | 77 (1) | 72 (1) | 5 (1) | <.001 | |
| Difference | −2 (2) | 0 (2) | −1 (1) | 0.327 |
Values are LSMEANS (SE) and rounded to the nearest whole number (except haemoglobin A1c).
Within group changes calculated as Baseline − Week 16 (positive numbers indicate reduction in parameter). Differences may not exactly reflect values for Baseline and Week 16 due to rounding.
Between group differences calculated as Beef − Non‐beef. Differences may not exactly reflect values for Beef and Non‐Beef due to rounding.
A linear mixed effects model (SAS, Proc Mixed) was used to test effects of time (Baseline vs. Week 16), group (Beef vs. Non‐beef), and their interaction term on changes in glucose, total cholesterol, LDL‐cholesterol, HDL‐cholesterol, triglycerides, haemoglobin A1c and blood pressure. Total cholesterol, LDL‐cholesterol and triglycerides were reduced at Week 16 vs. Baseline but there were no differences between Beef and Non‐beef.
BMI, body mass index; BP, blood pressure; HDL, high‐density lipoprotein cholesterol; LDL, low‐density lipoprotein cholesterol.
Discussion
Consistent with the hypotheses, the B and NB diets produced equivalent reductions in body weight and improvements in body composition and cardiometabolic health. While there was a greater decrease in WC in the NB group, no differences in amount of trunk fat assessed by DXA were observed. Findings from the current study indicate that lean beef can be effectively incorporated into a HP diet for weight loss and improving body composition and cardiometabolic health. Weight loss in the current study (~8%) was slightly lower than the average weight loss for SOS when delivered as a fee‐based program through the AHWC (~10%). This discrepancy in weight loss between SOS when delivered in research vs. commercial settings likely occurred due to the use of ITT analysis with BOCF for non‐completers in the current study. In addition, participants in the current study received the SOS program free‐of‐charge, and it is possible that paying for the program – as in the commercial program at the AHWC – could enhance motivation and result in greater weight loss.
A relative retention of lean mass during weight loss is a commonly cited benefit of high vs. standard protein diets 2 that is supported by results from meta‐analyses in young, middle‐aged 5 and older adults 3. While there was no standard protein group for comparison, the virtually complete retention of lean mass observed in the current study deserves mention. Approximately 95% of changes in total mass were due to changes in fat mass, and lean mass was not significantly reduced by the weight loss intervention in either group. Future research comparing the SOS weight loss program with a HP diet vs. a standard protein diet is warranted in light of past observations that ~25% of typically observed reductions in total body mass are due to a loss of lean mass 3, 30.
The upper age limit for the current study was 50 years, and the average age of participants was 38 years. The impact of the SOS weight loss program on lean mass retention should be tested in older adults, particularly those with or at risk for sarcopenic obesity. Weight loss is often discouraged in these individuals due to justifiable concerns regarding frailty, disability and loss of independence related to skeletal muscle loss 30. Thus, effective weight loss interventions that preferentially reduce body fat would substantially influence strategies for the prevention and treatment of sarcopenic obesity.
Results of the current study add further support to other evidence from randomized clinical trials demonstrating that consuming lean, minimally processed red meats does not adversely affect weight loss 8, 23 or improvements in indices of cardiometabolic health when consumed as part of ‘healthy’ dietary patterns 11, 12, 13, 31. The current study builds upon findings from past research by investigating the impact of lean beef consumption within the context of a HP diet for weight loss in a large randomized equivalence trial. Achieving ≥5% weight loss is widely recognized to elicit health benefits 32, 33, 34, and the current study demonstrates that regularly consuming lean beef for 16 weeks does not influence weight loss or the resultant improvements in cardiometabolic health. These findings are consistent with those of Ziegler et al. 23 and Hill et al. 8 indicating that weight loss improved cardiac vagal function and metabolic syndrome criteria, respectively, independent of red meat consumption. A recent meta‐analysis of randomized controlled trials also concluded that consuming ≥0.5 daily servings of red meat does not influence blood lipids/lipoproteins or BP compared to consuming <0.5 servings of red meat/d 35. Participants randomized to B in the current study were instructed to consume ≥4 weekly servings of lean beef, which is ~0.6 servings of red meat/d and further corroborates the results of the meta‐analysis.
The overall participant retention rate was 82.5% for the current study, but dropout rates differed by diet assignment. However, numerical differences in retention rates between B and NB were not confirmed statistically. Fifty‐three of 60 participants (88%) randomly assigned to B completed the study compared to 46 of 60 (77%) of those assigned to NB. Greater perceived diet/nutritional deprivation has been associated with poorer dietary adherence 36, 37. The SOS diet plan includes lean beef along with other protein sources that are low in fat and saturated fat 25, and both groups followed the same SOS plan except for the NB group was instructed to abstain from consuming beef. It is possible that the inclusion of lean beef in the published diet plan coupled with the broad popularity of beef 24 led to greater feelings of deprivation and diet inflexibility in the NB group leading to a greater dropout rate.
A major strength of the current study is the use of a randomized equivalence trial design 38. Previous work by our group indicated that the equivalence design was more conservative (least likely to show group‐level differences) than several alternative methods including linear mixed models, multiple imputation, ANCOVA and independent t‐tests 39. The use of a popular and evidence‐based weight loss program (SOS) 25 represents an additional strength of the current study. Participants assigned to B received the SOS program with very limited alterations (non‐beef sources of red meat were excluded) and is therefore available to the general public through the published book and/or participation in the commercial, fee‐based SOS program.
The current study is limited in some aspects including the lack of a standard defined protein control group, which would allow more definitive conclusions regarding the impact of the HP diets on study outcomes, especially the observed lack of changes in lean mass. Although no influence of sex was observed for any study outcomes, the majority of participants in the current study were women and results may not fully extrapolate to men. Last, the current study was of relatively short duration, and the results should not be extrapolated beyond the constraints of the study design (i.e. 16 weeks, majority of participants as women, age limited to 18–50 years). Future studies of longer duration and in diversified populations are required to fully evaluate the effectiveness of consuming red meat during weight loss and for long‐term weight loss maintenance.
In conclusion, consuming lean beef within the context of a HP weight‐reducing diet resulted in equivalent reductions in body weight and no difference in improvements of body composition and cardiometabolic health compared to a HP that was restricted in red meats. Results of this study demonstrate that HP diets – either rich or restricted in red meat intakes – are effective for decreasing body weight (especially body fat) and improving cardiometabolic health.
Funding
The Beef Checkoff, National Heart, Lung, and Blood Institute (grant #: T32 HL116276, an institutional postdoctoral training grant for Dr. Sayer), The National Center for Research Resources that supports the Colorado Clinical and Translational Science Institute (grant #: UL1 RR025780), and the National Institute of Diabetes and Digestive and Kidney Diseases, Colorado Nutrition Obesity Research Center (P30 DK48520).
Disclosures
Drs. Hill and Wyatt have received royalties from the book, State of Slim.
ClinicalTrials.gov Identifier: NCT02627105. https://clinicaltrials.gov
Author Contributions
J.O.H., H.R.W. and J.C.P. conceived the research project; R.D.S. and K.J.S. conducted the research; R.D.S. and Z.P. performed the statistical analyses; R.D.S. drafted the manuscript, and K.J.S., J.O.H., H.R.W., J.C.P. and Z.P. provided critical feedback and edits to the manuscript. All authors take responsibility for the final content of the manuscript.
Acknowledgements
The authors acknowledge Jeanne Anne Breen, Debbie Bochert, Hannah Nelson and Lisa Fryda for their substantial effort in participant recruitment/retention and for coordinating study visits. The financial supports had no role in the design and conduct of the study or collection, analysis and interpretation of the data.
Sayer, R. D. , Speaker, K. J. , Pan, Z. , Peters, J. C. , Wyatt, H. R. , and Hill, J. O. (2017) Equivalent reductions in body weight during the Beef WISE Study: beef's role in weight improvement, satisfaction and energy. Obesity Science & Practice, 3: 298–310. doi: 10.1002/osp4.118.
References
- 1. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014; 129: S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leidy HJ, Clifton PM, Astrup A, et al. The role of protein in weight loss and maintenance. Am J Clin Nutr 2015. pii: ajcn084038. [Epub ahead of print]. https://doi.org/10.3945/ajcn.114.084038 [DOI] [PubMed] [Google Scholar]
- 3. Kim JE, O'Connor LE, Sands LP, Slebodnik MB, Campbell WW. Effects of dietary protein intake on body composition changes after weight loss in older adults: a systematic review and meta‐analysis. Nutr Rev 2016; 74: 210–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Santesso N, Akl EA, Bianchi M, et al. Effects of higher‐ versus lower‐protein diets on health outcomes: a systematic review and meta‐analysis. Eur J Clin Nutr 2012; 66: 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy‐restricted high‐protein, low‐fat compared with standard‐protein, low‐fat diets: a meta‐analysis of randomized controlled trials. Am J Clin Nutr 2012; 96: 1281–1298. [DOI] [PubMed] [Google Scholar]
- 6. Schwingshackl L, Hoffmann G. Long‐term effects of low‐fat diets either low or high in protein on cardiovascular and metabolic risk factors: a systematic review and meta‐analysis. Nutr J 2013; 12: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li J, Armstrong CLH, Campbell WW. Effects of dietary protein source and quantity during weight loss on appetite, energy expenditure, and cardio‐metabolic responses. Nutr 2016; 8: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hill AM, Harris Jackson KA, Roussell MA, West SG, Kris‐Etherton PM. Type and amount of dietary protein in the treatment of metabolic syndrome: a randomized controlled trial. Am J Clin Nutr 2015; 102: 757–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Velasquez MT, Bhathena SJ. Role of dietary soy protein in obesity. Int J Med Sci 2007; 4: 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pasiakos SM. Metabolic advantages of higher protein diets and benefits of dairy foods on weight management, glycemic regulation, and bone. J Food Sci 2015; 80: A2–A7. [DOI] [PubMed] [Google Scholar]
- 11. Roussell MA, Hill AM, Gaugler TL, et al. Beef in an Optimal Lean Diet study: effects on lipids, lipoproteins, and apolipoproteins. Am J Clin Nutr 2012; 95: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roussell MA, Hill AM, Gaugler TL, et al. Effects of a DASH‐like diet containing lean beef on vascular health. J Hum Hypertens 2014; 28: 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sayer RD, Wright AJ, Chen N, Campbell WW. Dietary approaches to stop hypertension diet retains effectiveness to reduce blood pressure when lean pork is substituted for chicken and fish as the predominant source of protein. Am J Clin Nutr 2015; 102: 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alisson‐Silva F, Kawanishi K, Varki A. Human risk of diseases associated with red meat intake: analysis of current theories and proposed role for metabolic incorporation of a non‐human sialic acid. Mol Aspects Med 2016; 51: 16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boada LD, Henríquez‐Hernández LA, Luzardo OP. The impact of red and processed meat consumption on cancer and other health outcomes: epidemiological evidences. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc 2016; 92: 236–244. [DOI] [PubMed] [Google Scholar]
- 16. Wolk A. Potential health hazards of eating red meat. Journal of Internal Medicine 2017; 281: 106–122. [DOI] [PubMed] [Google Scholar]
- 17. Eckel RH, Jakicic JM, Ard JD, et al. AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129: S76–S99. [DOI] [PubMed] [Google Scholar]
- 18. Dietary guidelines for Americans 2015–2020, eighth edition.
- 19. Chen GC, Lv DB, Pang Z, Liu QF. Red and processed meat consumption and risk of stroke: a meta‐analysis of prospective cohort studies. Eur J Clin Nutr 2013; 67: 91–95. [DOI] [PubMed] [Google Scholar]
- 20. Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes – an updated review of the evidence. Curr Atheroscler Rep 2012; 14: 515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chan DS, Lau R, Aune D, et al. Red and processed meat and colorectal cancer incidence: meta‐analysis of prospective studies. PloS One 2011; 6: e20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu HC, Yang X, Xu LP, et al. Meat consumption is associated with esophageal cancer risk in a meat‐ and cancer‐histological‐type dependent manner. Dig Sci 2014; 59: 664–673. [DOI] [PubMed] [Google Scholar]
- 23. Ziegler D, Strom A, Nowotny B, et al. Effect of low‐energy diets differing in fiber, red meat, and coffee intake on cardiac autonomic function in obese individuals with type 2 diabetes. Diabetes Care 2015; 38: 1750–1757. [DOI] [PubMed] [Google Scholar]
- 24. Daniel CR, Cross AJ, Koebnick C, Sinha R. Trends in meat consumption in the USA. Public Health Nutr 2011; 14: 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hill JO, Wyatt H, Aschwanden C. State of Slim: Fix your Metabolism and Drop 20 Pounds in 8 Weeks on the Colorado Diet. Emmaus, Pennsylvania: Rodale, 2013. [Google Scholar]
- 26. Butryn ML, Phelan S, Hill JO, Wing RR. Consistent self‐monitoring of weight: a key component of successful weight loss maintenance. Obes Silver Spring 2007; 15: 3091–3096. [DOI] [PubMed] [Google Scholar]
- 27. Dhurandhar NV, Schoeller D, Brown AW, et al. Energy balance measurement: when something is not better than nothing. Int J Obes 2005 2015; 39: 1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anon . Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000; 894: i–xii. 1‐253. [PubMed] [Google Scholar]
- 29. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat‐free mass in middle‐aged and older adults: implications for sarcopenic obesity. Nutr Rev 2010; 68: 375–388. [DOI] [PubMed] [Google Scholar]
- 31. Nowson CA, Wattanapenpaiboon N, Pachett A. Low‐sodium dietary approaches to stop hypertension‐type diet including lean red meat lowers blood pressure in postmenopausal women. Nutr Res 2009; 29: 8–18. [DOI] [PubMed] [Google Scholar]
- 32. Blackburn G. Effect of degree of weight loss on health benefits. Obes Res 1995; 3: 211s–216s. [DOI] [PubMed] [Google Scholar]
- 33. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011; 34: 1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fayh APT, Lopes AL, da Silva AMV, Reischak‐Oliveira A, Friedman R. Effects of 5% weight loss through diet or diet plus exercise on cardiovascular parameters of obese: a randomized clinical trial. Eur J Nutr 2013; 52: 1443–1450. [DOI] [PubMed] [Google Scholar]
- 35. O'Connor LE, Kim JE, Campbell WW. Total red meat intake of ≥0.5 servings/d does not negatively influence cardiovascular disease risk factors: a systemically searched meta‐analysis of randomized controlled trials. Am J Clin Nutr 2017; 105: 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bruckert E, Pouchain D, Auboiron S, Mulet C. Cross‐analysis of dietary prescriptions and adherence in 356 hypercholesterolaemic patients. Arch Cardiovasc Dis 2012; 105: 557–565. [DOI] [PubMed] [Google Scholar]
- 37. Cheng L, Leung DY‐P, Sit JW‐H, et al. Factors associated with diet barriers in patients with poorly controlled type 2 diabetes. Patient Prefer Adherence 2016; 10: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med 2011; 26: 192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peters JC, Beck J, Cardel M, et al. The effects of water and non‐nutritive sweetened beverages on weight loss and weight maintenance: a randomized clinical trial. Obes Silver Spring Md 2016; 24: 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]


