Abstract
Background
Understanding the effects of oxygen levels on yeast xylose metabolism would benefit ethanol production. In this work, xylose fermentative capacity of Scheffersomyces stipitis, Spathaspora passalidarum, Spathaspora arborariae and Candida tenuis was systematically compared under aerobic, oxygen-limited and anaerobic conditions.
Results
Fermentative performances of the four yeasts were greatly influenced by oxygen availability. S. stipitis and S. passalidarum showed the highest ethanol yields (above 0.44 g g−1) under oxygen limitation. However, S. passalidarum produced 1.5 times more ethanol than S. stipitis under anaerobiosis. While C. tenuis showed the lowest xylose consumption rate and incapacity to produce ethanol, S. arborariae showed an intermediate fermentative performance among the yeasts. NAD(P)H xylose reductase (XR) activity in crude cell extracts correlated with xylose consumption rates and ethanol production.
Conclusions
Overall, the present work demonstrates that the availability of oxygen influences the production of ethanol by yeasts and indicates that the NADH-dependent XR activity is a limiting step on the xylose metabolism. S. stipitis and S. passalidarum have the greatest potential for ethanol production from xylose. Both yeasts showed similar ethanol yields near theoretical under oxygen-limited condition. Besides that, S. passalidarum showed the best xylose consumption and ethanol production under anaerobiosis.
Keywords: Xylose fermentation, Xylose reductase, Bioethanol, Yeast fermentation, Oxygen availability
Background
Conversion of all sugars present at cellulose and hemicellulose fractions of biomass would increase production and reduce cost of second-generation ethanol [1, 2]. Saccharomyces cerevisiae is the main yeast used for alcohol production worldwide, but it cannot produce ethanol from xylose, the second most abundant sugar in nature, unless when genetically engineered [3, 4]. Despite the relative success of engineered strains, recombinant S. cerevisiae strains show lower fermentation rates and less tolerance to fermentation inhibitors when fermenting xylose instead of glucose [5, 6]. Thus, the isolation, identification and characterization of native xylose-fermenting yeasts have received great attention in the past years [7–12].
Among the few naturally xylose-fermenting yeasts species, Scheffersomyces (Pichia) stipitis is one of the most studied [8, 12, 13]. It has been isolated from the gut of insects and its fermentation capability evaluated in different lignocellulosic hydrolysates [14]. More recently, yeasts from Spathaspora and Candida genera, as Spathaspora passalidarum, Spathaspora arborariae and Candida tenuis, have been isolated from rotting-wood samples or wood-boring insects and characterized as xylose fermenting yeasts [7, 9, 10]. Like S. stipitis, S. passalidarum showed xylose fermentation yields above 0.40 g ethanol g−1 sugar in both defined and lignocellulosic hydrolyzed medium Slininger [14, 15]. In general, naturally xylose-fermenting yeasts are able to ferment xylose only when the oxygen flow is tightly regulated. High oxygenation level leads to aerobic growth and low ethanol yield, whereas limited dissolved oxygen slows the fermentation rate, increases xylitol accumulation and causes poor ethanol productivity [1, 8, 15–17].
In yeasts, xylose is first reduced to xylitol, a reaction catalyzed by a NAD(P)H-dependent xylose reductase (XR). Then, a NAD+-dependent xylitol dehydrogenase (XDH) oxidises xylitol to xylulose [18–20]. Subsequently, xylulose enters into the pentose phosphate and glycolysis pathways, finally being converted to ethanol. Recently, it was shown that S. passalidarum exceptionally harbor two XRs, and one of them, preferentially uses NADH as cofactor [20].
As fermentative conditions like media composition, cell density and oxygen availability are usually different [1, 10, 20] a comparative assessment among xylose-consuming yeasts based on literature data becomes difficult. In addition, few studies on physiology of C. tenuis and S. arborariae are available [10, 20, 21]. Thus, a systematic comparison of fermentative physiology of S. stipitis, S. passalidarum, S. arborariae and C. tenuis is still missing and it might help elucidate important steps on xylose metabolism.
The aim of this study was to compare the alcoholic fermentative capacity of four native xylose-consuming yeasts under different oxygenation conditions. The physiology of S. stipitis, S. passalidarum, S. arborariae and C. tenuis in defined mineral medium containing xylose as sole carbon source was assessed under aerobic, oxygen-limited and anaerobic conditions. The results presented clearly distinguished the best performing yeast for each condition and highlights the importance of cofactor usage on ethanol production from xylose.
Methods
Strains
The yeasts employed in this study were Scheffersomyces (Pichia) stipitis NRRL Y-7124, S. passalidarum NRRL Y-27907, S. arborariae NRRL Y-48658 and C. tenuis NRRL Y-1498. All yeasts were preserved in 30% glycerol at −80 °C.
Xylose fermentations under different oxygen conditions
The xylose fermentation experiments were carried out in bioreactors (Multifors 2, Infors HT) with 500 mL working volume. Cells from −80 °C stock were initially grown in solid YPD medium (10 g L−1 yeast extract, 20 g L−1 peptone, 20 g L−1 glucose), overnight at 28 °C. One single colony was used to inoculate 50 mL of defined mineral medium [22] containing per litre: (NH4)2SO4, 12.5 g; KH2PO4, 7.5 g; MgSO4·7H2O, 1.25 g; EDTA, 37.5 mg; ZnSO4·7H2O, 11.25 mg; MnCl2·2H2O, 2.5 mg; CoCl2·6H2O, 0.75 mg; CuSO4·5H2O, 0.75 mg; Na2MoO4·H2O, 1.0 mg; CaCl2·2H2O, 11.25 mg; FeSO4·7H2O, 7.5 mg; H3BO3, 2.5 mg; KI, 0.25 mg. Filter-sterilized vitamins were added after heat sterilization of this medium. Final vitamin concentrations per litre were: biotin, 0.125 mg; Ca-pantothenate 2.5 mg; nicotinic acid 2.5 mg; inositol 62.5 mg; thiamin-HCl 2.5 mg; pyridoxine–HCl 2.5 mg; P-aminobenzoic acid 0.5 mg; riboflavin 0.5 g and; folic acid 0.005 g. The carbon source consisted of 40 g L−1 xylose.
The start culture at the bioreactor was OD600 nm of 0.5. Cultures were maintained with pH 5.5, by addition of KOH 3 M, under agitation—stirrer at 400 rpm, and temperature of 28 °C. First, yeast performance was evaluated under aerobic and oxygen-limited conditions. For aerobic experiments, synthetic air (20% pure oxygen and 80% pure nitrogen) was injected in the reactor at 0.5 L min−1. The dissolved oxygen measured in the reactor (Sensors METTLER TOLEDO) was above 60% during the entire fermentation period. For oxygen-limited experiments, the airflow of synthetic air was adjusted for 0.05 L min−1, which resulted in dissolved oxygen below 10% in the first 10 h of fermentation and zero afterwards. But the airflow was kept constant in a minimal rate, indicating that the entire oxygen that was entering the bioreactor was promptly consumed. All fermentations were carried out in biological triplicates.
Anaerobic fermentations with the four yeasts were performed in small cap vials sealed with a rubber stopper, equipped with a needle for carbon dioxide removal. Cells from −80 °C stock were initially grown in solid YPD medium, overnight at 28 °C. One single colony was used to inoculate 50 mL of defined mineral medium as described above. The culture started with a high cell density equal to OD600 nm of 2.0. The pH was adjusted for 5.5 and, the flasks incubated under agitation—stirrer at 400 rpm and temperature of 28 °C. All experiments were carried out in biological triplicates.
Analytical methods
To monitor yeast growth, samples were withdrawn regularly during fermentations and biomass was measured by optical density at 600 nm using a spectrophotometer (SpectraMax® M3, Molecular Devices). For cell dry weight (CDW) measurement, 5 mL of pre-inoculum culture and of the stationary phase of the growth during all fermentations were withdrawn and centrifuged (12,000×g, 5 min). Before weighing, the pellet was incubated in glass tube for at least 48 h at 60 °C. The cell dry weight was correlated with OD600 nm measured in the same time intervals. Each measurement was performed in duplicate.
Sugar consumption and products formed during fermentation experiments were measured using a high-pressure liquid chromatograph (HPLC) system. Initially, samples withdrawn regularly during fermentations were centrifuged (12,000×g, 5 min) and the supernatant was transferred to a new tube. Concentrations of xylose, xylitol, glycerol, acetate and ethanol in supernatants were measured using HPLC system (Acquity UPLC H Class, Waters) equipped with a refractive index detector and an Aminex® HPX-87H column (Bio-Rad) at 45 °C. The mobile phase was 5 mM sulfuric acid at a flow rate of 0.6 mL min−1. Results are shown as average ± standard deviations.
Enzymatic assays for XR and XDH
The enzymatic activity of xylose reductase (XR) and xylitol dehydrogenase (XDH) in crude-cell extracts was measured according to [23]. For this, 5 mL of cell suspension of S. stipitis, S. passalidarum, S. arborariae and C. tenuis were collected in the middle of the exponential growth phase during aerobic and oxygen-limited fermentations. Cells were pelleted by centrifugation, washed with sterile water, and lysed with Y-PER®—Yeast Protein Extraction Reagent (Pierce, Rockford, USA) to obtain cell-crude extracts. Protein concentrations in cell-free extracts were determined using Quick StartTM Bradford Protein Assay Kit (Bio-Rad Laboratories Ltda., USA), following the manufacture’s instruction.
XR reaction mixture contained 100 mM triethanolamine buffer (pH 7.0), 0.2 mM NADH or NADPH, 350 mM xylose. XDH reaction contained 100 mM triethanolamine buffer (pH 7.0), 0.3 mM NAD+, 300 mM xylitol. All reactions were started with addition of limiting substrates. The assays were performed at 30 °C and the oxidation of NADH/NADPH and reduction of NAD+ were followed as the change in absorbance at 340 nm. The value of 6.22 mL (μmol cm)−1 was used as the molar absorption coefficient of coenzymes per minute. The specific activities of XR and XDH were given in units per mg protein (U mg−1). Enzyme unit is defined as 1 μmol of cofactor reduced or oxidized per minute. All assays were performed in triplicate and the results are shown as means ± standard deviations.
Results and discussion
Xylose fermentation in defined mineral medium
The fermentative capacity of the four naturally xylose-consuming yeasts, S. stipitis, S. passalidarum, S. arborariae and C. tenuis were evaluated under aerobic, oxygen-limited and anaerobic conditions. Xylose consumption varied considerably among the four yeasts both under aerobic and oxygen-limited conditions (Fig. 1). Scheffersomyces stipitis, S. passalidarum and S. arborariae were able to consume xylose completely and showed at least 6 times higher specific xylose consumption rate than C. tenuis under aerobic condition (Fig. 1; Table 1). Even after more than 120 h of fermentation, C. tenuis consumed about 34 and 28 g L−1 of xylose under aerobic and oxygen-limited conditions, respectively (Fig. 1d). When cultivated under oxygen limitation, S. stipitis and S. passalidarum showed similar xylose consumption rates, however the rate was 2 times lower for S. arborariae (Table 1). As expected, biomass yields for all yeasts were 2 times higher under aerobic than oxygen-limited condition. However, biomass yield was twofolds higher for S. passalidarum than S. stipitis. This may explain the lower ethanol productivity rate for this yeast compared to S. stipitis under aerobic condition (Table 1).
Fig. 1.
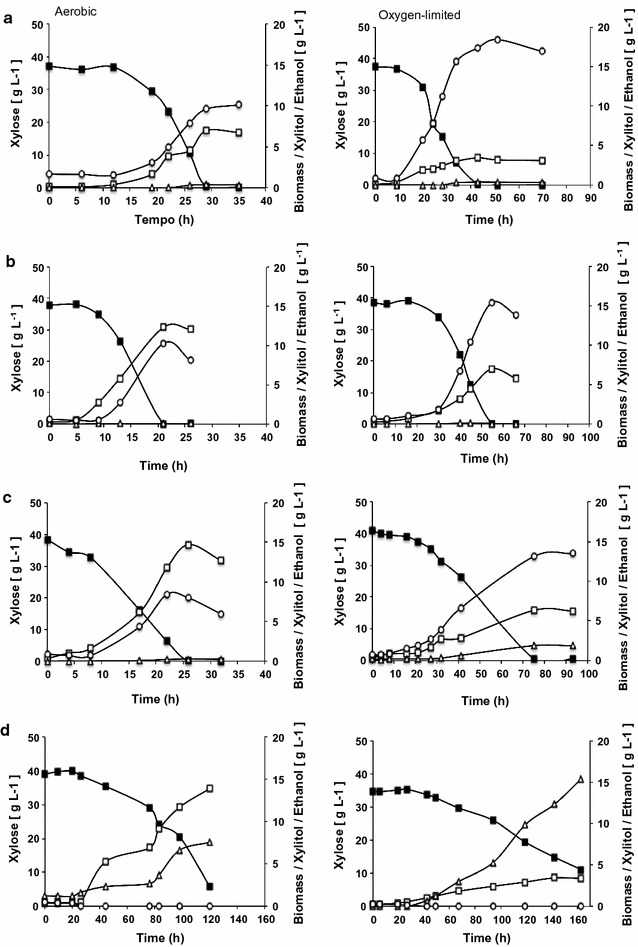
Xylose fermentation under different oxygen level conditions. Left: aerobic and right: oxygen-limited. S. stipitis (a); S. passalidarum (b); S. arborariae (c) and; C. tenuis (d). Xylose (closed square), biomass (open square), xylitol (open triangle) and ethanol (open circle). The different scales on x-axis highlight different fermentation rates
Table 1.
Parameters calculated for xylose fermentation
| Yeasts species | Oxygen condition | Xylitol (g L−1) | Ethanol (g L−1) | Xylitol yield [Yx/s (g g−1)] | Ethanol yield [Ye/s (g g−1)] | Biomass yield [Yb/s (g g−1)] | Specific xylose consumption [(g g−1cdw h−1)] | Specific ethanol productivity [(g g−1cdw h−1)] |
|---|---|---|---|---|---|---|---|---|
|
S. stipitis
S. passalidarum S. arborariae C. tenuis |
Aerobic | 0.41 ± 0.06 | 8.05 ± 0.91 | 0.01 ± 0.00 | 0.24 ± 0.02 | 0.16 ± 0.04 | 0.30 ± 0.09 | 0.08 ± 0.03 |
| 0.04 ± 0.00 | 10.06 ± 0.48 | 0.00 ± 0.00 | 0.28 ± 0.02 | 0.33 ± 0.02 | 0.13 ± 0.02 | 0.04 ± 0.01 | ||
| 0.27 ± 0.08 | 8.65 ± 1.16 | 0.01 ± 0.00 | 0.25 ± 0.02 | 0.31 ± 0.05 | 0.13 ± 0.02 | 0.03 ± 0.01 | ||
| 8.03 ± 1.48 | 0.00 ± 0.00 | 0.30 ± 0.06 | 0.00 ± 0.00 | 0.43 ± 0.06 | 0.02 ± 0.00 | 0.00 ± 0.00 | ||
|
S. stipitis
S. passalidarum S. arborariae C. tenuis |
Oxygen- limited |
0.37 ± 0.01 | 16.48 ± 0.83 | 0.01 ± 0.00 | 0.45 ± 0.04 | 0.09 ± 0.02 | 0.29 ± 0.09 | 0.10 ± 0.02 |
| 0.05 ± 0.02 | 16.36 ± 1.40 | 0.00 ± 0.01 | 0.44 ± 0.04 | 0.13 ± 0.04 | 0.22 ± 0.10 | 0.10 ± 0.05 | ||
| 1.82 ± 0.66 | 11.47 ± 2.37 | 0.04 ± 0.02 | 0.31 ± 0.02 | 0.15 ± 0.01 | 0.09 ± 0.01 | 0.03 ± 0.01 | ||
| 15.43 ± 1.90 | 0.00 ± 0.00 | 0.62 ± 0.04 | 0.00 ± 0.00 | 0.14 ± 0.01 | 0.04 ± 0.01 | 0.00 ± 0.00 |
The fermentative capacities were measurement under aerobic and oxygen-limited conditions. The values are calculated considering the exponential growth phase
Growth and ethanol production by these yeasts were strongly influenced by the oxygen availability. Biomass formation was favored in presence of oxygen whereas ethanol production was favored under more strictly oxygen availability. Produced biomass varied from a minimal of 6.7 g L−1 for S. stipitis to a maximum of 13.9 g L−1 to C. tenuis under aerobic condition (Fig. 1). Despite the variation in the total biomass, the specific growth rate of S. stipitis, S. passalidarum and S. arborariae ranged from 0.15 to 0.18 h−1. This is about 2 times higher than C. tenuis under aerobic conditions, which reached specific growth rate 0.08 h−1. The biomass formation was about 2 times lower under oxygen-limited condition than in aerobic one, varying from 3.0 to 7.0 g L−1 (Fig. 1). In anaerobic condition the maximum biomass formation reached was 3.5 g L−1 for S. stipitis, 2.4 g L−1 for S. passalidarum, 1.3 g L−1 for S. arborariae and 1.2 g L−1 for C. tenuis (Fig. 2).
Fig. 2.
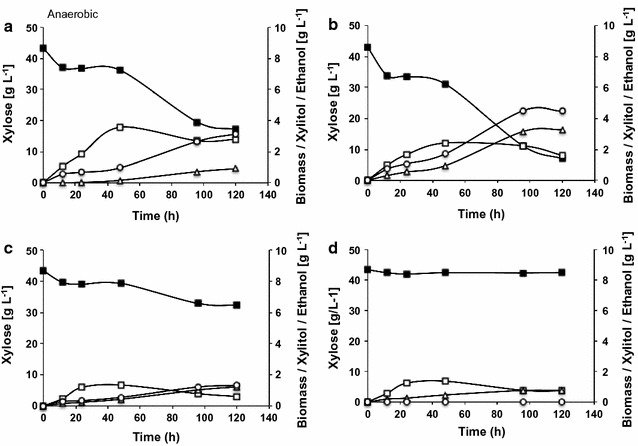
Xylose fermentation under anaerobic condition. S. stipitis (a); S. passalidarum (b); S. arborariae (c) and; C. tenuis (d). Xylose (closed square), biomass (open square), xylitol (open triangle) and ethanol (open circle)
Scheffersomyces stipitis, S. passalidarum and S. arborariae produced predominantly ethanol from xylose (Fig. 1a–c). Oxygen limitation increased ethanol production and yields by S. stipitis, S. passalidarum and S. arborariae. Indeed, the concentrations increased approximately twofold when compared to aerobic condition (Table 1). Scheffersomyces stipitis and S. passalidarum reached the highest ethanol yields (0.45 and 0.44 g g−1) among the four xylose-consuming yeasts employed in this study (Table 1). These values are in good agreement with previous studies, which showed ethanol yields varying from 0.40 to 0.48 g g−1 to S. stipitis [16, 24] and from 0.43 to 0.48 g g−1 to S. passalidarum [17, 20] under limited oxygenation levels. In turn, ethanol yield for S. arborariae was only of 0.31 g g−1 (Table 1), which was also observed previously in an independent study [20]. Xylitol, glycerol and acetate formation by S. stipitis, S. passalidarum and S. arborariae was minimal and did not show significant differences among them (Fig. 1; Table 1). On the other hand, C. tenuis did not produce ethanol under any condition evaluated. Indeed, it produced mainly xylitol during fermentation under oxygen-limited condition (0.62 g g−1) (Table 1).
The xylose consumption rate was lower under anaerobic condition in all evaluated yeasts (Fig. 2). Only S. stipitis and S. passalidarum were able to produce ethanol, and even so, xylitol formation also increased when compared to other aerobic and oxygen-limited conditions (Figs. 1a, b, 2a, b). Insufficient oxygen rate was reported to increase xylitol accumulation and to cause poor ethanol productivity in S. stipitis and S. passalidarum [17]. Despite the similar fermentative performances of S. stipitis and S. passalidarum under oxygen-limited condition, S. passalidarum consumed more xylose and produced 50% more ethanol than S. stipitis in anaerobic condition (Fig. 2a, b). These results are in agreement with those observed in previous work, when S. passalidarum showed efficient conversion of xylose into ethanol under anaerobic condition, while the S. stipitis almost did not ferment xylose [1]. Another study that assessed the aeration effect on xylose fermentation also showed that S. passalidarum (ethanol yield 0.43 g g−1) is a better native xylose-fermenting yeast than S. stipitis (ethanol yield 0.39 g g−1) when a smaller oxygen transfer rate is employed [17].
Although it has been proposed that C. tenuis is capable of fermenting xylose [10], it showed the poorest xylose consumption rates among the four yeasts assessed and it was not able to produce ethanol in any condition evaluated in this study (Figs. 1, 2). In the previous work, Wohlbach et al. [10] showed that C. tenuis produced approximately 2.0 g L−1 ethanol during microaerobic fermentation with 8% xylose and high initial cell density (OD600 nm of 10) in an Erlenmeyer flask. In our study, some change of parameters may have influenced the metabolism of C. tenuis, so the xylitol formation by this yeast was significant (up to 15.4 g L−1) and ethanol was not detected (Figs. 1, 2; Table 1). The approximately 20 times lower initial cell density (OD600 nm of 0.5, equal to 0.2 g L−1), the low flow air rate during oxygen-limited fermentation and the usage of defined mineral medium instead of yeast extract and peptone may have hampered ethanol detection in this work.
Xylose reductase (XR) and xylitol dehydrogenase (XDH) activities
Xylose reductase (XR) and xylitol dehydrogenase (XDH) activities were measured in crude-cell extracts of S. stipitis, S. passalidarum, S. arborariae and C. tenuis from cells growing under aerobic and oxygen-limited conditions. S. stipitis, S. passalidarum and S. arborariae presented NADH and NADPH-dependent XR activity, whereas C. tenuis XR were strictly NADPH-dependent (Table 2). While S. stipitis and S. arborariae showed higher NADPH-dependent XR activity, S. passalidarum showed approximately 1.5 times higher NADH-dependent XR activity.
Table 2.
Xylose reductase (XR) and xylitol dehydrogenase (XDH) specific activities in crude-cell extracts of S. stipitis, S. passalidarum, S. arborariae and C. tenuis
| Yeasts species | Oxygen conditions | XR (U mg−1) | XDH (U mg−1) | ||
|---|---|---|---|---|---|
| NADH | NAD(P)H | RatioNADH/NAD(P)H | NAD+ | ||
|
S. stipitis
S. passalidarum S. arborariae C. tenuis |
Aerobic | 0.17 ± 0.06 | 0.23 ± 0.05 | 0.74 ± 0.13 | 0.23 ± 0.11 |
| 2.96 ± 0.40 | 2.15 ± 0.12 | 1.38 ± 0.16 | 0.30 ± 0.05 | ||
| 0.88 ± 0.13 | 3.10 ± 0.29 | 0.29 ± 0.05 | 0.65 ± 0.07 | ||
| – | 0.35 ± 0.07 | – | 0.25 ± 0.05 | ||
|
S. stipitis
S. passalidarum S. arborariae C. tenuis |
Oxygen-limited | 0.26 ± 0.07 | 0.45 ± 0.11 | 0.59 ± 0.16 | 0.19 ± 0.08 |
| 0.60 ± 0.08 | 0.46 ± 0.04 | 1.29 ± 0.07 | 0.21 ± 0.03 | ||
| 0.77 ± 0.17 | 1.86 ± 0.25 | 0.43 ± 0.16 | 0.12 ± 0.04 | ||
| – | 0.27 ± 0.05 | – | 0.28 ± 0.06 | ||
Yeasts were grown under aerobic and oxygen-limited conditions and samples were withdrawn in the middle of exponential growth phase
The fermentative performances of yeasts under oxygen-limited condition could be directly correlated with the capability to use NADH on xylose reduction (Tables 1, 2). Indeed, S. passalidarum showed the highest ratio of NADH/NADPH XR activity (around 1.30) and the best fermentative performance, i.e. higher xylose consumption rate and higher concentration of ethanol, under anaerobic condition; followed by S. stipitis and S. arborariae with ratios around 0.6 and 0.4, respectively (Table 2). Candida tenuis, which XR prefers 33-fold NADPH over NADH [25] did not produce ethanol at all. Accordingly, it was recently shown that S. passalidarum possess two XR (genes XIL1.1 and XIL1.2) and one of them uses preferentially NADH as cofactor [20].
XR NADH-preference was previously correlated with improved ethanol production by engineered S. cerevisiae strains [18, 26, 27]. The usage of NADH on xylose reduction is advantageous because the redox balance in the xylose catabolic pathways is optimized, since XDH, the next enzyme in the pathway, is strictly NAD+-dependent (Table 2) [18]. The NAD+ surplus regenerated during xylose reduction would reduce xylitol formation due to higher xylose consumption rate, which impact positively the ethanol yield and productivity [20, 28]. Indeed, strategies aiming to increase NAD+ availability during fermentation increases xylose consumption rate and ethanol production. These include mutations to alter cofactor preference of XRs from NADPH to NADH [29], addition of external electron donor [30] or expression of additional reactions that generated increased NAD+ availability [31]. No enzymatic activity was performed in the anaerobic condition because the growth was very slow and there was no exponential growth phase. Thus, only fermentative capacity was compared.
Conclusion
The comparative assessment of the four-native xylose-consuming yeasts showed that the S. stipitis and S. passalidarum have the greatest potential for ethanol production from xylose. Both yeasts showed similar ethanol yields near theoretical under oxygen-limited condition. Besides that, S. passalidarum showed the best xylose consumption and ethanol production under anaerobiosis. The better performing yeasts, i.e. with higher xylose consumption rate and higher concentration of ethanol, during anaerobic xylose showed higher ratio of NADH/NADPH XR activity.
Authors’ contributions
HCTV participated in the design of the study, performed all experiments and analyzed the data and wrote the manuscript. NSP and JRMA participated in design of the study and commented the manuscript. All authors read and approved the manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This work was supported by funding from The National Council for Scientific and Technological Development (CNPQ), Brazilian Agricultural Research Corporation (EMBRAPA) and Coordination for the Improvement of Higher Education Personnel (CAPES).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Henrique César Teixeira Veras, Email: henrique.veras@colaborador.embrapa.br.
Nádia Skorupa Parachin, Email: nadiasp@gmail.com.
João Ricardo Moreira Almeida, Phone: +55 61 3448-2337, Email: joao.almeida@embrapa.br.
References
- 1.Hou X. Anaerobic xylose fermentation by Spathaspora passalidarum. Appl Microbiol Biotechnol. 2012;94:205–214. doi: 10.1007/s00253-011-3694-4. [DOI] [PubMed] [Google Scholar]
- 2.Arora R, Behera S, Kumar S. Bioprospecting thermophilic/thermotolerant microbes for production of lignocellulosic ethanol: a future perspective. Renew Sustain Energy Rev. 2015;51:699–717. doi: 10.1016/j.rser.2015.06.050. [DOI] [Google Scholar]
- 3.Parachin NS, Hahn-Hagerdal B, Bettiga M. A microbial perspective on ethanolic lignocellulose fermentation. Wastes from agriculture, forestry and food processing. 2011; 605–614.
- 4.Moyses DN, Reis VC, de Almeida JR, de Moraes LM, Torres FA. Xylose fermentation by Saccharomyces cerevisiae: challenges and prospects. Int J Mol Sci. 2016;17:207. doi: 10.3390/ijms17030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuyper M, Toirkens MJ, Diderich JA, Winkler AA, van Dijken JP, Pronk JT. Evolutionary engineering of mixed-sugar utilization by a xylose-fermenting Saccharomyces cerevisiae strain. FEMS Yeast Res. 2005;5:925–934. doi: 10.1016/j.femsyr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Wu M, Xu L, Hou J, Guo T, Bao X, Shen Y. Evaluation of industrial Saccharomyces cerevisiae strains as the chassis cell for second-generation bioethanol production. Microb Biotechnol. 2015;8:266–274. doi: 10.1111/1751-7915.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen NH, Suh SO, Marshall CJ, Blackwell M. Morphological and ecological similarities: wood-boring beetles associated with novel xylose-fermenting yeasts, Spathaspora passalidarum gen. sp. nov. and Candida jeffriesii sp. nov. Mycol Res. 2006;110:1232–1241. doi: 10.1016/j.mycres.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Jeffries TW, Grigoriev IV, Grimwood J, Laplaza JM, Aerts A, Salamov A, Schmutz J, Lindquist E, Dehal P, Shapiro H, et al. Genome sequence of the lignocellulose-bioconverting and xylose-fermenting yeast Pichia stipitis. Nat Biotechnol. 2007;25:319–326. doi: 10.1038/nbt1290. [DOI] [PubMed] [Google Scholar]
- 9.Cadete RM, Santos RO, Melo MA, Mouro A, Goncalves DL, Stambuk BU, Gomes FC, Lachance MA, Rosa CA. Spathaspora arborariae sp. nov., a d-xylose-fermenting yeast species isolated from rotting wood in Brazil. FEMS Yeast Res. 2009;9:1338–1342. doi: 10.1111/j.1567-1364.2009.00582.x. [DOI] [PubMed] [Google Scholar]
- 10.Wohlbach DJ, Kuo A, Sato TK, Potts KM, Salamov AA, Labutti KM, Sun H, Clum A, Pangilinan JL, Lindquist EA, et al. Comparative genomics of xylose-fermenting fungi for enhanced biofuel production. Proc Natl Acad Sci USA. 2011;108:13212–13217. doi: 10.1073/pnas.1103039108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cadete RM, Melo MA, Zilli JE, Vital MJ, Mouro A, Prompt AH, Gomes FC, Stambuk BU, Lachance MA, Rosa CA. Spathaspora brasiliensis sp. nov., Spathaspora suhii sp. nov., Spathaspora roraimanensis sp. nov. and Spathaspora xylofermentans sp. nov., four novel (d)-xylose-fermenting yeast species from Brazilian Amazonian forest. Antonie Van Leeuwenhoek. 2013;103:421–431. doi: 10.1007/s10482-012-9822-z. [DOI] [PubMed] [Google Scholar]
- 12.Krahulec S, Kratzer R, Longus K, Nidetzky B. Comparison of Scheffersomyces stipitis strains CBS 5773 and CBS 6054 with regard to their xylose metabolism: implications for xylose fermentation. MicrobiologyOpen. 2012;1:64–70. doi: 10.1002/mbo3.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parambil LK, Sarkar D. Probing the bioethanol production potential of Scheffersomyces (Pichia) stipitis using validated genome-scale model. Biotechnol Lett. 2014;36:2443–2451. doi: 10.1007/s10529-014-1629-8. [DOI] [PubMed] [Google Scholar]
- 14.Slininger PJ, Shea-Andersh MA, Thompson SR, Dien BS, Kurtzman CP, Balan V, Sousa LC, Uppugundla N, Dale BE, Cotta MA. Envolved strains of Scheffersomyces stipitis achieving high ethanol productivity on acid and base pretreated biomass hydrolyzate at high solids loading. Biotechnol Biofuels. 2015;8(60):1–27. doi: 10.1186/s13068-015-0239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long TM, Su YK, Headman J, Higbee A, Willis LB, Jeffries TW. Cofermentation of glucose, xylose, and cellobiose by the beetle-associated yeast Spathaspora passalidarum. Appl Environ Microbiol. 2012;78:5492–5500. doi: 10.1128/AEM.00374-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skoog K, Hahn-Hagerdal B. Effect of oxygenation on xylose fermentation by Pichia stipitis. Appl Environ Microbiol. 1990;56:3389–3394. doi: 10.1128/aem.56.11.3389-3394.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su Y, Willis LB, Jeffries TW. Effects of aeration on growth, ethanol and polyol accumulation by Spathaspora passalidarum NRRL Y-27907 and Scheffersomyces stipitis NRRL Y-7124. Biotechnol Bioeng. 2014;112:457–469. doi: 10.1002/bit.25445. [DOI] [PubMed] [Google Scholar]
- 18.Bruinenberg PM, de Bot PHM, van Dijken JP, Scheffers WA. NADH-linked aldose reductase—the key to anaerobic alcoholic fermentation of xylose by yeasts. Appl Microbiol Biotechnol. 1984;19:256–260. doi: 10.1007/BF00251847. [DOI] [Google Scholar]
- 19.Jeffries TW, Jin YS. Metabolic engineering for improved fermentation of pentoses by yeasts. Appl Microbiol Biotechnol. 2004;63:495–509. doi: 10.1007/s00253-003-1450-0. [DOI] [PubMed] [Google Scholar]
- 20.Cadete RM, de Las Heras AM, Sandstrom AG, Ferreira C, Girio F, Gorwa-Grauslund MF, Rosa CA, Fonseca C. Exploring xylose metabolism in Spathaspora species: XYL1.2 from Spathaspora passalidarum as the key for efficient anaerobic xylose fermentation in metabolic engineered Saccharomyces cerevisiae. Biotechnol Biofuels. 2016;9(167):1–14. doi: 10.1186/s13068-016-0570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cadete RM, Melo MA, Dussan KJ, Rodrigues RC, Silva SS, Zilli JE, Vital MJ, Gomes FC, Lachance MA, Rosa CA. Diversity and physiological characterization of d-xylose-fermenting yeasts isolated from the Brazilian Amazonian forest. PLoS ONE. 2012;7:e43135. doi: 10.1371/journal.pone.0043135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verduyn C, Postma E, Scheffers WA, van Dijken JP. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast. 1992;8:501–517. doi: 10.1002/yea.320080703. [DOI] [PubMed] [Google Scholar]
- 23.Smiley KL, Bolen PL. Demonstration of d-xylose reductase and d-xylitol dehydrogenase in Pachysolen tannophilus. Biotech Lett. 1982;4:607–620. doi: 10.1007/BF00127793. [DOI] [Google Scholar]
- 24.Papini M, Nookaew I, Uhlén M, Nielsen J. Scheffersomyces stipitis: a comparative systems biology study with the crabtree positive yeast Saccharomyces cerevisiae. Microb Cell Factories. 2012;11(136):1–16. doi: 10.1186/1475-2859-11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petschacher B, Leitgeb S, Kavanagh KL, Wilson DK, Nidetzky B. The coenzyme specificity of Candida tenuis xylose reductase (AKR2B5) explored by site-directed mutagenesis and X-ray crystallography. Biochem J. 2005;385:75–83. doi: 10.1042/BJ20040363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bengtsson O, Hahn-Hägerdal B, Gorwa-Grauslund MF. Xylose reductase from Pichia stipitis with altered coenzyme preference improves ethanolic xylose fermentation by recombinant Saccharomyces cerevisiae. Biotechnol Biofuels. 2009;2:9. doi: 10.1186/1754-6834-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeffries TW, Shi NQ. Genetic engineering for improved xylose fermentation by yeasts. Adv Biochem Eng Biotechnol. 1999;65:118–161. doi: 10.1007/3-540-49194-5_6. [DOI] [PubMed] [Google Scholar]
- 28.Hahn-Hägerdal B, Karhumaa K, Fonseca C, Spencer-Martins I, Gorwa-Grauslund MF. Towards industrial pentose-fermenting yeast strains. Appl Microbiol Biotechnol. 2007;74:937–953. doi: 10.1007/s00253-006-0827-2. [DOI] [PubMed] [Google Scholar]
- 29.Dasgupta D, Bandhu S, Adhikari DK, Ghosh D. Challenges and prospects of xylitol production with whole cell bio-catalysis: a review. Microbiol Res. 2017;197:9–21. doi: 10.1016/j.micres.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Wahlbom CF, Hahn-Hagerdal B. Furfural, 5-hydroxymethyl furfural, and acetoin act as external electron acceptors during anaerobic fermentation of xylose in recombinant Saccharomyces cerevisiae. Biotechnol Bioeng. 2002;78:172–178. doi: 10.1002/bit.10188. [DOI] [PubMed] [Google Scholar]
- 31.Almeida JR, Bertilsson M, Hahn-Hagerdal B, Liden G, Gorwa-Grauslund MF. Carbon fluxes of xylose-consuming Saccharomyces cerevisiae strains are affected differently by NADH and NADPH usage in HMF reduction. Appl Microbiol Biotechnol. 2009;84:751–761. doi: 10.1007/s00253-009-2053-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


